Abstract
Simple Summary
Knowledge on the real-world outcomes of patients with advanced melanoma and the value of different endpoints for evaluating survival benefits is limited. We investigated the outcomes and different surrogate endpoints for overall survival (OS) in 664 pembrolizumab-treated patients with advanced melanoma in Germany. Our findings support the effectiveness of pembrolizumab in real-world clinical practice. The real-world time to next treatment was most strongly correlated with OS, suggesting it as a valuable surrogate endpoint to assess treatment effectiveness. Real-world studies assessing time to next treatment could support clinical and payer decision making.
Abstract
Knowledge on the real-world characteristics and outcomes of pembrolizumab-treated advanced melanoma patients in Germany and on the value of different real-world endpoints as surrogates for overall survival (OS) is limited. A sample of 664 pembrolizumab-treated patients with advanced melanoma from the German registry ADOReg was used. We examined OS, real-world progression-free survival (rwPFS), real-world time to next treatment (rwTtNT), and real-world time on treatment (rwToT). Spearman’s rank and iterative multiple imputation (IMI)-based correlation coefficients were computed between the OS and the rwPFS, rwTtNT, and rwToT and reported for the first line of therapy and the overall sample. The median OS was 30.5 (95%CI 25.0–35.4) months, the rwPFS was 3.9 months (95%CI 3.5–4.9), the rwTtNT was 10.7 months (95%CI 9.0–12.9), and the rwToT was 6.2 months (95%CI 5.1–6.8). The rwTtNT showed the highest correlation with the OS based on the IMI (rIMI = 0.83), Spearman rank correlations (rs = 0.74), followed by the rwToT (rIMI = 0.74 and rs = 0.65) and rwPFS (rIMI = 0.69 and rs = 0.56). The estimates for the outcomes and correlations were similar for the overall sample and those in first-line therapy. The median OS was higher compared to recent real-world studies, supporting the effectiveness of pembrolizumab in regular clinical practice. The rwTtNT may be a valuable OS surrogate, considering the highest correlation was observed with the OS among the investigated real-world endpoints.
Keywords: advanced melanoma, surrogate endpoint, real-world evidence, overall survival (OS), time to next treatment (rwTtNT), pembrolizumab
1. Introduction
The last decade has seen a revolution in the treatment of advanced (unresectable stage III and stage IV) melanoma with the advent of immunotherapies [1]. Immune checkpoint inhibitors, such as the programmed cell death protein 1 (PD-1) inhibitors, pembrolizumab and nivolumab, have improved the overall survival (OS) of patients with advanced melanoma compared to common chemotherapy regimens [2,3,4,5]. PD-1 inhibitors showed higher response rates and lower toxicity levels than ipilimumab [6] and are now considered a standard of care for advanced melanoma patients in the first-line setting [7,8]. Randomized clinical trials (RCTs) on pembrolizumab have yielded extensive evidence of its efficacy in advanced melanoma [4,9,10]. RCTs have high internal validity but are conducted on highly selected populations and tightly controlled care settings [11,12], making their generalizability limited with respect to real-world populations [13]. Real-world data sources, such as electronic health records (EHR) and claims databases, can provide additional meaningful insights into the outcomes of representative and heterogeneous patient populations, which contribute to a better understanding of the clinical benefits in real-world patients [14,15].
Real-world evidence (RWE) on pembrolizumab in US samples has shown median OS rates between 19.4 and 30 months [16,17,18,19] and a median real-world progression-free survival (rwPFS) of 4.2 months [19]. Recent RWE data on pembrolizumab from Slovenia showed a comparable median OS of 25.1 months, with a median rwPFS of 10.7 months [20]. Improvements in OS have been reported after the introduction of PD-1 inhibitors in Germany [21]; however, detailed insights on real-world outcomes related to pembrolizumab are still lacking.
Crucially, RWE is sourced from information collected for routine clinical practice. Therefore, challenges in data completeness and frequency of assessments to capture clinical endpoints may exist in real-world data. Research has shown that real-world effectiveness is comparable to RCT-based treatment efficacy when OS is used as an endpoint [22]. Yet, there can be gaps in real-world death data as EHRs and claims data do not routinely capture death date or cause [23]. Moreover, the timing of progression and progression-free survival may be difficult to assess precisely in the real-world setting because it can only be measured at evaluations of clinical parameters, such as imaging, biomarkers, or patient-reported outcomes, and these evaluations may occur at irregular intervals based on a range of factors, for example, patient availability [24,25]. The unavailability of the precise date of progression in structured EHRs and in tumor registries, the lack of consistent timing of tumor assessments, along with subjective assessment, pseudo-progression, and a lack of high-quality structured data are all potential limitations that may be encountered with respect to measuring progression in real-world data sources. Due to these limitations, we separately refer to real-world outcomes with the prefix “rw” to distinguish them from the stringent outcomes measured in clinical trials. Because OS can be definitively assessed, the real-world prefix is not applied.
Furthermore, rwPFS may not comprehensively reflect the outcomes with PD-1 inhibitors, where treatment beyond progression can be important for preserving tumor control and ensuring long-term efficacy [26,27]. For immunotherapies, such as pembrolizumab, proxy measures such as real-world time to next treatment (rwTtNT) and real-world time on treatment (rwToT) might, therefore, offer valuable alternative endpoints that can often be derived with higher certainty. The rwTtNT captures the interval from the initiation of a therapy to the commencement of the next line of therapy, thereby indicating the period of therapeutic benefit. rwToT captures the time interval between the index date and the date of the last dose of a therapy [28]. Evidence for the applicability of rwTtNT and rwToT to evaluate the real-world efficacy of PD-1 inhibitors in advanced melanoma patients is emerging [16]. However, more evidence for the associations between different surrogate endpoints for treatment effectiveness and OS is needed [29], including a better understanding of how time on treatment or treatment discontinuation rates correlate with OS [27]. It is, therefore, important that RWE studies systematically consider different real-world endpoints and their correlations, as recently proposed by the Friends of Cancer Research (FoCR) group [27].
ADOReg is the largest clinical registry in Germany in the field of dermatological oncology. It is maintained by the German Working Group on Dermatological Oncology (ADO), also known as the German Dermatologic Cooperative Oncology Group (DeCOG), and IQVIA. It comprises high-quality data and is, therefore, well suited for a comparative investigation of different real-world endpoints. The objectives of this study were to examine real-world clinical outcomes and to evaluate the performance of rwPFS, rwToT, and rwTtNT as surrogate endpoints of OS associated with pembrolizumab treatment in advanced melanoma patients in the German ADOReg database.
2. Materials and Methods
2.1. Study Design and Data Source
This observational retrospective study used the data of eligible patients from the skin cancer registry ADOReg of the German Dermatologic Cooperative Oncology Group [30]. The ADOReg platform was developed in 2014 and collects healthcare data on melanoma patients from 59 geographically diverse skin cancer centers or practice-based dermato-oncologists certified by the German Cancer Society (DKG), 35 of which contributed to the current study. Details on the treatment of dermatological oncology in everyday clinical practice were recorded in an unidentifiable, pseudonymized form at the patient level. The median lag time for clinical updates to the data was approximately 3 to 6 months. The ADOReg registry was approved by the ethics committee of the University Duisburg-Essen (14-5921-BO). Patient consent was obtained for inclusion in the registry, and institutional review board (IRB) approval for the ADOReg database includes the use of data for research purposes.
2.2. Study Population
The study included the data of adults aged ≥18 years with a confirmed diagnosis of advanced melanoma and who received ≥1 dose of pembrolizumab at the index date. The index date was defined as the date of pembrolizumab treatment initiation in a study period between 1 August 2015 and 30 June 2019. The data cut-off date was September 2019. Patients were excluded if they received pembrolizumab in a clinical trial, simultaneously received any other systemic therapy, or were treated with pembrolizumab for an indication other than advanced melanoma.
2.3. Study Variables
We included patient demographics (i.e., age, gender) and clinical characteristics (i.e., melanoma stage III or IV, Eastern Cooperative Oncology Group (ECOG) score, presence of brain metastasis, lactate dehydrogenase (LDH) level, chronic steroid use, autoimmune diseases, and BRAF status) at the index date to describe the study population. The time-to-event endpoints that were computed for this study were the OS, rwPFS, rwTtNT, and rwToT. Additional secondary endpoints, the real-world tumor response rate (rwTRR) and the real-world tumor control rate (rwTCR), were also examined and are reported in the Supplementary Materials. The definitions of the endpoints are presented in Table 1.
Table 1.
Definition of real-world endpoints used in this study.
| Endpoint | Definition |
|---|---|
| Primary | |
| Overall survival (OS) | The time interval from index date to date of death. Patients alive at the date of last contact were censored. |
| Real-world progression-free survival (rwPFS) |
The time interval from index date to physician-reported date of progression, death date or start date of a new treatment due to progression of disease (whichever came first). Patients without a progression event or date of death were censored at the date of last contact. |
| Real-world time to next treatment (rwTtNT) | The time interval between index date and date of
censored at the date of last recorded encounter in the database. |
| Real-world time on treatment (rwToT) | The time interval between index date and the date of last dose of pembrolizumab within the same line of therapy (last dose date minus first dose date +1 day) at or before decision to discontinue * treatment or date of death if the patient died during treatment. Patients with ongoing pembrolizumab treatment or lost to follow-up were censored at the date of last contact. |
| Secondary | |
| Real-world tumor response rate (rwTRR) | The proportion of patients with a complete response or partial response based on real-world response assessments# relative to all patients initiating treatment. (The best therapy response using both the clinical assessments in the medical record and the radiological assessment in the staging findings are captured within the ADOReg database). |
| Real-world tumor control rate (rwTCR) | The proportion of patients who had a complete response, partial response, or stable disease based on real-world response assessments †. (The best therapy response using both the clinical assessments in the medical record and the radiological assessment in the staging findings are captured within the ADOReg database). |
Note. * Complete discontinuation refers to a treatment discontinuation for at least 120 days or if subsequent therapy line was initiated. † Complete response: complete resolution of all visible disease; partial response: disease still present, with partial reduction in size of visible disease in some or all areas without any areas of increase in visible disease; stable disease: no change in overall size of visible disease or mixed response.
2.4. Statistical Analysis
The data were analyzed using the SAS v9.3 (SAS Institute Inc., Cary, NC, USA) and R v3.3 software packages. The descriptive statistics calculated included the frequencies and percentages of categorical variables, while the means, medians, standard deviations (SD), and ranges of continuous variables were determined for the demographic and clinical characteristics. The median follow-up times were estimated overall and by line of treatment using the reverse Kaplan–Meier estimator [31].
The time-to-event endpoints (OS, rwPFS, rwTtNT, and rwToT) were examined using the Kaplan–Meier (KM) method, and the OS and rwPFS rates at 6, 12, 18, and 24 months of follow-up were estimated. The OS and rwPFS were estimated in patients with at least 6 months of follow-up post-index or a survival and/or progression event if less than 6 months of follow-up. In addition, for the rwTtNT estimated rates for “not on subsequent treatment” are reported at 6, 12, 18, and 24 months. For rwToT, the restricted mean at 24, 30, and 36 months and estimates for being on treatment at 12, 24, 30, and 36 months of follow-up are reported. For rwTRR and rwTCR, the percentage response is reported. We report these endpoints for the overall sample in the main manuscript and separately by the first, second, and third+ lines of therapy in the Supplementary Materials, Tables S2–S5 and Figure S1a–c.
To investigate the relationship between the real-world endpoints, correlations of the rwTtNT, rwPFS, and rwToT to the OS were assessed at the patient level by estimating the Spearman rank correlation coefficients (0 = no association and 1 = perfect association) and the corresponding 95% CIs [23]. Spearman rank correlations are frequently used but do not take into account censoring, which can lead to biases in the estimated correlations. We, therefore, additionally applied a copula-based approach with an iterative multiple imputation method for the estimation of the correlation coefficients to account for censoring [32,33]. Correlations are reported for the overall sample and for the first line of therapy only, as there were only slight differences in terms of outcomes by therapy line.
3. Results
3.1. Patient Characteristics
Data were abstracted for 664 eligible patients (Table 2). The majority of patients were male (59.9%), and more than 90% of all enrolled patients had stage IV melanoma, assessed according to the 2018 (Eighth Edition) American Joint Committee on Cancer (AJCC) Melanoma staging criteria [34]. The median patient age was 70 years (range 22 to 96), and ECOG scores of 0 and 1 were reported for 40.8% and 21.4% of patients, respectively. Nearly a quarter (23.0%) of all included patients had a history of brain metastases. Furthermore, 39.0% of the patients had elevated LDH levels, with 6.5% of patients with LDH values greater than or equal to twice the upper limit of normal (ULN). Of those with available data, only 3.2% were on long-term steroid treatment, and 0.9% of patients had an autoimmune disease at the index date. A BRAF mutation was present in 32.4% of the patients. Sixty-four percent of the patients received pembrolizumab as the first line of therapy, 21.5% in the second line, and 18.1% in the third or higher lines of therapy. Regarding treatment history, 35.7% of the patients in the second line and 47.5 of patients in the third or higher lines were previously treated with BRAF/MEK inhibitors. Furthermore, prior treatment with ipilimumab was observed in 44.1% of patients in the second line and 60.8% of patients in the third or higher lines (Supplementary Materials, Table S1). The median (95% CI) follow-up time was 36.1 (33.5 to 38.3) months, 32.8 (29.4 to 38.3) months, 36.1 (32.8 to 40.2) months, and 46.4 (41.5 to 49.6) months, for overall, and the first, second, and third or higher lines of therapy, respectively.
Table 2.
Baseline characteristics of patients (N = 664) at the index date (initiation of pembrolizumab treatment).
| Characteristic | |
|---|---|
| Age (years) | |
| Mean (SD) | 67.4 (13.2) |
| Median (min–max) | 70 (22–96) |
| Gender, n (%) | |
| Male | 398 (59.9) |
| Female | 266 (40.1) |
|
Stage (2018 AJCC Melanoma
Staging), n (%) |
|
| Stage III | 62 (9.3) |
| Stage IV | 602 (90.7) |
| Origin of primary melanoma, n (%) | |
| Cutaneous | 537 (80.9) |
| Mucosal | 17 (2.6) |
| Ocular | 30 (4.5) |
| Unknown primary | 80 (12.0) |
| ECOG score, n (%) | |
| 0 | 271 (40.8) |
| 1 | 142 (21.4) |
| 2 | 30 (4.5) |
| 3 | 7 (1.1) |
| Missing | 214 (32.2) |
| Line of therapy, n (%) | |
| 1st | 401 (60.4) |
| 2nd | 143 (21.5) |
| 3rd+ | 120 (18.1) |
| Brain metastasis, n (%) | |
| Present | 154 (23.2) |
| Absent | 510 (76.8) |
| LDH level, n (%) | |
| WNL | 402 (60.5) |
| >1-2X ULN | 216 (32.5) |
| >2X ULN | 43 (6.5) |
| Missing | 3 (0.5) |
| Chronic Steroid use, n (%) | |
| Yes | 21 (3.2) |
|
History of autoimmune disease
at index, n (%) |
|
| Yes | 6 (0.9) |
| BRAF status, n (%) | |
| Wildtype (negative) | 360 (54.2) |
| Positive | 215 (32.4) |
| Missing/Unknown | 89 (13.4) |
Note. AJCC: American Joint Committee on Cancer; ECOG: Eastern Cooperative Oncology Group; LDH: lactate dehydrogenase; SD: standard deviation; ULN: upper limit of normal; WNL: within normal limit.
3.2. Clinical Outcomes
The median (95% CI) OS for the overall sample was 30.5 (25.0 to 35.4) months, and the estimated probabilities of survival at 6, 12, 18, and 24 months after treatment initiation were 84.3%, 71.0%, 62.2%, and 55.4%, respectively (Supplementary Materials Figure S1a and Table S2). The median (95% CI) rwPFS for all included patients was 3.9 (3.5 to 4.9) months from the date of initiation of pembrolizumab. The estimated probability of remaining progression-free at 6, 12, 18, and 24 months was 42.8%, 30.7%, 24.5%, and 22.5%, respectively (Supplementary Materials, Figure S1b and Table S2). The median rwTtNT was 10.7 months (95% CI, 9.0 to 12.9) for the overall study population treated with pembrolizumab. The estimates for rates of not on subsequent treatment were 65.5%, 47.6%, 37.5%, and 33.8% at 6, 12, 18, and 24 months (Supplementary Materials, Figure S1c and Table S3). For OS, the rwPFS and rwTtNT outcomes were comparable for patients in the first and third+ therapy lines and slightly shorter in the second line compared to the first and third+ lines (e.g., first, third+, and second therapy line OS medians were 30.9, 33.5 and 26.5 months, respectively).
Across the entire study population, the median rwToT was 6.2 months (95% CI, 5.1 to 6.8) (Supplementary Materials, Figure S1d and Table S4), and the 2-year restricted mean rwToT was 9.3 months. The estimates for the restricted mean rwToT at 30 and 36 months were 9.9 and 10.4 months, respectively. The estimates for the restricted mean rwToT by line of therapy at 24 to 36 months ranged from 9.4 to 10.5 months for the first line, 8.2 to 9.2 months for the second line, and 10.2 to 11.5 months for the third+ lines of therapy.
The results for the secondary clinical endpoints of rwTRR and rwTCR are presented in the Supplementary Materials, Table S5. For the overall study population, the rwTRR (95% CI) was 24.4% (21.1 to 27.7), with 24.9% (20.7 to 29.2), 21.0% (14.3 to 27.7), and 26.7% (18.8 to 34.6) for the first, second and third+ lines of therapy, respectively. The rwTCR (95% CI) was 40.2% (36.5 to 43.9) for the overall study population and 43.4% (38.5 to 48.2), 35.0% (27.1 to 42.8), and 35.8% (27.3 to 44.4) for those in the first, second and third+ lines of therapy, respectively.
3.3. Correlations among Real-World Endpoints
3.3.1. Spearman’s Rank Correlations
All real-world endpoints (rwTtNT, rwToT, rwPFS) showed significant correlations with the OS in the complete study population and the first line of therapy. Across the entire sample, the highest correlation (95% CI) with OS was present for rwTtNT (0.74 (0.70 to 0.78)) followed by rwToT (0.65 (0.61 to 0.70)). The rwPFS showed the lowest correlation with the OS (0.56 (0.50 to 0.61)) (Supplementary Materials, Figure S2a–c). Correlations were marginally lower for the first line of therapy (Supplementary Materials, Figure S3a–c) compared to the overall sample (see Table 3).
Table 3.
Correlations among real-world outcome endpoints.
| Comparison | Spearman’s Rank Correlation (95% CI) | IMI Correlation (95% CI) | ||
|---|---|---|---|---|
| Overall | 1st Line | Overall | 1st Line | |
| OS vs. rwPFS | 0.56 (0.50, 0.61) | 0.52 (0.44, 0.59) | 0.69 (0.62, 0.74) | 0.68 (0.59, 0.74) |
| OS vs. rwTtNT | 0.74 (0.70, 0.78) | 0.70 (0.65, 0.75) | 0.83 (0.79, 0.86) | 0.83 (0.77, 0.87) |
| OS vs. rwToT | 0.65 (0.61, 0.70) | 0.62 (0.55, 0.68) | 0.74 (0.69, 0.79) | 0.75 (0.68, 0.80) |
Note. IMI: iterative multiple imputation; CI: confidence interval; OS: real-world overall survival; rwPFS: real-world progression; rwToT: real-world time on treatment; rwTtNT: real-world time to next treatment.
3.3.2. Iterative Multiple Imputation (IMI) Correlation
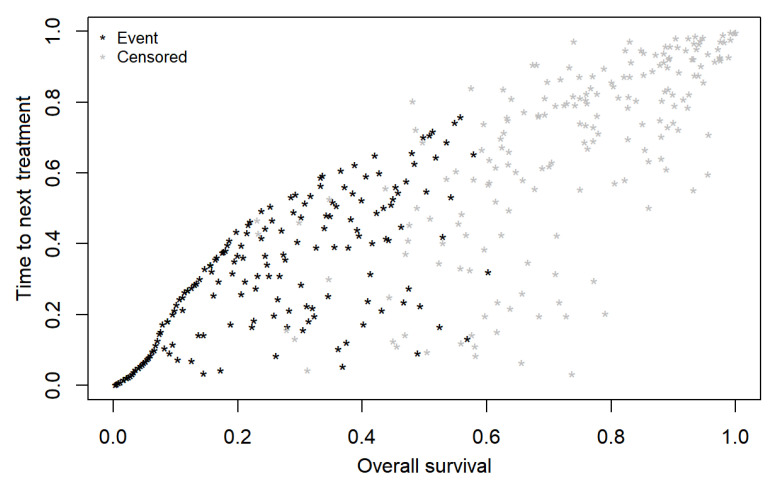
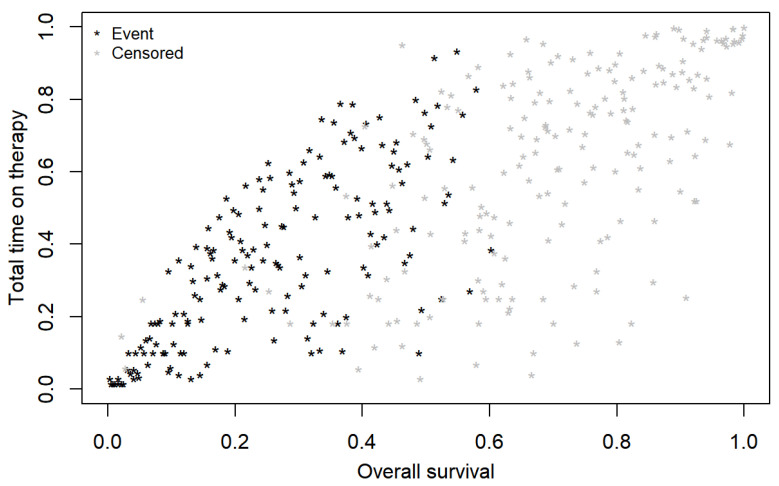
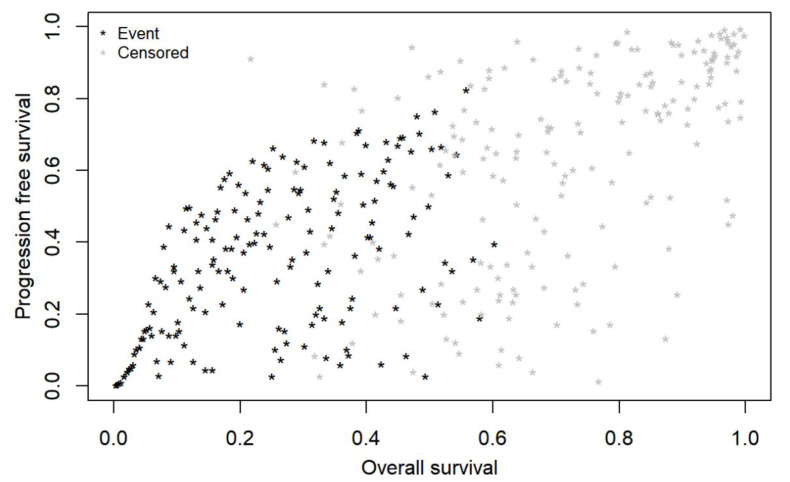
All real-world endpoints (rwPFS, rwTtNT, rwToT) showed higher IMI correlations with the OS compared to those derived with the Spearman rank method. The highest correlation with OS emerged for rwTtNT (0.83 (0.79 to 0.86)), followed by rwToT (0.74 (0.69 to 0.79)), and rwPFS with the lowest correlation (0.69 (0.62 to 0.74)) (see Figure 1, Figure 2 and Figure 3). Correlations were only slightly lower for patients in the first line of therapy than for the overall sample (see Table 3).
Figure 1.
Bivariate copula plot depicting correlation between real-world overall survival (rwOS) and real-world time to next treatment (rwTtNT) of patients with advanced melanoma treated with first-line pembrolizumab.
Figure 2.
Bivariate copula plot depicting correlation between real-world overall survival (rwOS) and real-world time to next treatment (rwToT) of patients with advanced melanoma treated with first-line pembrolizumab.
Figure 3.
Bivariate copula plot depicting correlation between real-world overall survival (rwOS) and real-world progression-free survival (rwPFS) of patients with advanced melanoma treated with first-line pembrolizumab.
Correlations between the OS and the rwPFS, rwTtNT, and rwToT for both methods in the overall study population and for patients who received pembrolizumab as first-line of therapy, along with their 95% CIs, are reported in Table 3.
4. Discussion
This study reported real-world clinical outcomes for advanced melanoma patients receiving pembrolizumab treatment and examined the associations between the OS and the rwPFS, rwTtNT, and rwToT in advanced melanoma patients treated with pembrolizumab in Germany to evaluate the outcomes and different surrogate endpoints for OS in real-world clinical practice. The median survival was 30.5 months for the overall patient sample. Consistently, the highest correlations were found between the OS and rwTtNT using both the IMI and Spearman rank-based methods, suggesting that rwTtNT may be a useful surrogate endpoint.
4.1. Clinical Outcomes
The median OS (30.5 months) and 2-year survival rate (55.4%) were found to be higher in this study compared to previous reports of the median OS, ranging from 19.4 to 30 months [16,18,20], and the 2-year survival rates (48% and 44%) reported by others [16,18]. A lower proportion of patients with a poor ECOG score in the present study may explain these differences from RWE studies with poorer OS outcomes. However, when compared to RCTs, the median OS in the present study is substantially shorter [10]. The more advanced illness of patients in our real-world sample (e.g., ECOG score >1, prevalence of brain metastases), some of whom would have been excluded from RCTs, could explain this difference. Moreover, the RWE sample was substantially older than the sample included in the RCT (median age 70 vs. 61).
The median rwPFS in the current study is comparable to the median rwPFS (3.9 vs. 4.2 months) reported by a recent US RWE study [18]. In addition, the result for the rwTtNT in the current study is similar to what has recently been reported for advanced melanoma of cutaneous origin patients in a US sample (10.7 vs. 11.2 months) [16]. The median rwToT of 6.2 months (2-year restricted mean rwToT of 9.3 months) in the current study is slightly longer than the previously reported rwToT by others (median rwToT of 4.9 months, 2-year restricted mean treatment duration of 8.0 months) [16].
The clinical outcomes identified in this sample appear to be similar among lines of therapy, which may be due to the relatively short length of follow-up in some patients. An update of the clinical outcomes will be completed in a larger sample with longer follow-up in the near future.
4.2. Evaluation of Relationships among Real-World Outcome Endpoints
In real-world practice, capturing outcomes such as OS and rwPFS can be challenging due to, for example, recording mechanisms or treatment that continues beyond progression to preserve tumor control. For the purpose of this study, we have, therefore, evaluated the correlation between different possible surrogate endpoints and the OS in alignment with the FoCR group’s framework to evaluate real-world endpoints [27]. The FoCR framework used Spearman’s rank correlation coefficient, which is among the most often used statistical methods in medical research. However, it ignores censoring, thus leading to biased estimates, particularly if censoring is prevalent. We, therefore, additionally used the IMI method, which iteratively augments normal deviates related to censored times [32,33]. We found high correlations between all the investigated surrogate endpoints and OS. Correlations were higher with the copula-based IMI than the more widely used Spearman rank method, indicating that accounting for censoring using the IMI-based method may affect correlation estimates. The rwTtNT had a higher correlation with OS (rIMI = 0.83) than the rwPFS (rIMI = 0.69) and rwToT (rIMI = 0.74). The magnitude of the correlations differed only slightly by line of therapy. The findings suggest that rwTtNT as defined (initiation of next line of therapy or death) in this study is a reliable reflection of OS and clinical benefit in this setting, supporting its application as a clinically meaningful surrogate endpoint. Using the rwTtNT as the endpoint has additional advantages. It can be estimated with shorter follow-up than OS and avoids challenges associated with pin-pointing the exact date of progression. It also offers an assessment of the duration of therapeutic benefit as well as a better reflection of the patients’ treatment experiences than more conventional endpoints, such as rwPFS [28].
4.3. Strengths and Limitations of the Study
Our study also provides novel insights into the surrogate endpoints for OS and the outcomes observed in pembrolizumab-treated patients with advanced melanoma in Germany. We used a copula-based approach that accounts for censoring, thus yielding more robust results than the more widely used Spearman rank correlations. The well-maintained ADOReg database allowed for the inclusion of a large sample of a heterogeneous patient population. Patients with a poor ECOG score (>1), primary melanoma of other than cutaneous origin, and co-morbidities, who are generally excluded or under-represented in RCTs, were included in this study, making it possible to present a more comprehensive picture of real-world clinical outcomes. Most patients were male and elderly and had an ECOG score between 0 and 1. Almost one-third of all evaluated melanomas were positive for a BRAF mutation. The demographic and clinical characteristics of patients in this study are in line with earlier published real-world advanced melanoma studies [16,18,19].
While novel and important, this study’s findings need to be interpreted in light of several important methodological considerations. First, while the ADOReg database is the largest and most complete melanoma registry in Germany, the data for this observational retrospective study was not primarily recorded for research purposes but based on physician reports from clinical practice. As a result, the dataset was not always complete, with missing data on real-world outcomes for some individuals (e.g., ~9% for OS and ~6% for rwPFS). Second, the generalizability of the results beyond Germany and those being treated with immunotherapy may be limited due to differing prescribing practices, approaches to routine treatment, and treatment effects. Additional prospective investigations of pembrolizumab and advanced melanoma real-world outcomes are, therefore, needed to corroborate and extend our findings.
5. Conclusions
Our study yielded important new insights into the outcomes of patients with advanced melanoma and surrogate endpoints for OS for real-world studies. Our findings support the effectiveness of pembrolizumab in patients with advanced melanoma and that all investigated real-world endpoints are suitable surrogates for OS. However, rwTtNT had the highest correlation with OS information, suggesting it as the most reliable indicator of OS among the investigated endpoints.
Acknowledgments
The authors gratefully acknowledge the efforts of Anne Fett in preparation of this manuscript. The authors further acknowledge additional clinicians and sites for their assistance in data collection for ADOReg; these include but are not limited to Ingrid Feldmann-Böddeker, Anca Sindrilaru (Ulm), Martin Kaatz, and Vesna Alar.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14071804/s1, Figure S1: Kaplan–Meier curves showing probability estimates for rwOS (a) rwPFS (b), rwTtNT (c), and rwToT (d) of patients with advanced melanoma treated with pembrolizumab by line of therapy; Figure S2: Scatter plots depicting correlations between rwOS and rwPFS (a), rwOS and rwTtNT (b), and rwOS and rwToT (c) of patients with advanced melanoma treated with pembrolizumab, irrespective of therapy line; Figure S3: Scatter plots depicting correlations between rwOS and rwPFS (a), rwOS and rwTtNT t (b), and rwOS and rwToT (c) of patients with advanced melanoma treated with first-line pembrolizumab. Table S1: Treatment history by pembrolizumab line of therapy; Table S2: Kaplan–Meier estimate for median rwOS and rwPFS (from index date), overall and by line of therapy; Table S3: Kaplan–Meier estimate for median rwTtNT (from index date) and on treatment rates, overall, and by line of therapy; Table S4: Kaplan–Meier estimate for median rwToT (from index date), overall and by line of therapy; Table S5: Real-world tumor response rate (rwTRR) and real-world tumor control rate (rwTCR), overall and by line of therapy.
Author Contributions
Conceptualization: M.W., P.M., S.C., E.S., D.S.; data curation: P.M., C.A., I.G., A.H., K.C.K., A.R., M.S., U.L., S.U., D.S., K.C.K., R.G., C.P., P.T., J.U. (Jochen Utikal), A.K., S.H., J.C.H., C.B., J.U. (Jens Ulrich), T.E., R.H., C.L., F.M., M.B., M.W., C.W., J.W., G.S.; formal analysis: M.W., M.S., A.R.; methodology: M.W., P.M., S.U., U.L., A.R., S.C., E.S., R.J.; project administration: M.S., S.C., A.R., P.M., M.W., R.J.; supervision: M.W., E.S., R.J., M.S., A.R., D.S.; writing—original draft: M.W., M.S., A.R.; writing—review and editing: P.M., E.S., S.C., A.R., M.S., U.L., S.U., D.S., K.C.K., R.G., C.P., P.T., J.U. (Jochen Utikal), A.K., S.H., J.C.H., C.B., J.U. (Jens Ulrich), T.E., R.H., C.L., F.M., M.B., C.K., R.J., M.W., C.A., I.G., A.H., C.W., J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Institutional Review Board Statement
The ADOReg registry was approved by the Ethics Committee of the University Duisburg-Essen (14-5921-BO).
Informed Consent Statement
Patient consent was obtained for inclusion in the registry, IRB approval for the ADOReg database includes the use of data for research purposes.
Data Availability Statement
Data is not publicly available and cannot be shared.
Conflicts of Interest
Carola Berking declares speaker and advisory board honoraria from Bristol-Myers Squibb, Immunocore, Merck Sharp & Dohme, Merck Serono, Novartis, Pierre Fabre, Regeneron, Roche, and Sanofi-Aventis and Sanofi-Genzyme. Thomas Eigentler declares research support from Bristol-Myers Squibb and Merck Sharp & Dohme; speaker and advisory board honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Roche/Genentech, Novartis, Amgen, Pierre Fabre, and Sanofi. Ralf Gutzmer reports grants from Merck Sharp & Dohme during the conduct of the study; personal fees and non-financial support from Bristol-Myers Squibb; personal fees and non-financial support from Roche Pharma; personal fees and non-financial support from Merck Serono; grants, personal fees, and non-financial support from Amgen; personal fees and non-financial support from Pierre Fabre; personal fees and non-financial support from Sanofi Regeneron; personal fees from Merck Sharp & Dohme; grants, personal fees, and non-financial support from Novartis; personal fees and non-financial support from Almirall Hermal; grants and personal fees from Pfizer; personal fees from Bayer; personal fees from Immunocore; personal fees from SUN Pharma; personal fees from 4SC; grants from Johnson & Johnson, outside the submitted work. Sebastian Haferkamp declares research support from Bristol-Myers Squibb; speaker and advisory board honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Amgen, Pierre Fabre, and Sanofi. Jessica Hassel declares research support from Bristol-Myers Squibb; advisory board honoraria from Pierre Fabre, Sanofi, Sun Pharma, and Merck Sharp & Dome; speaker honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Roche, Sanofi, and Almirall, and travel support from Pierre Fabre. Rudolf Herbst declares speaker and advisory board honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, Roche, and SUN-Pharma. Katharina C. Kähler served as a consultant and/or has received honoraria from Amgen, Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Medac, Sanofi, and Sun Pharma, and travel support from Amgen, Merck Sharp & Dohme, Bristol-Myers Squibb, Amgen, Pierre Fabre, Sun Pharma, and Novartis, outside the submitted work. Ulrike Leiter declares research support from Merck Sharp & Dohme; speaker and advisory board honoraria from Merck Sharp & Dohme, Almirall Herma, Sun Pharma, Novartis, Bristol-Myers Squibb, and Roche. Carmen Loquai declares advisory board honoraria from MSD, Merck, BMS, Roche, Pierre Fabre, Novartis, Biontech, Almirall Hermal, Kyowa Kirin, Sun Pharma, and Sanofi; speaker fee from MSD, Merck, BMs, Roche, Pierre Fabre, Novartis, Biontech, Almirall Hermal, Kyowa Kirin, Sun Pharma, and Sanofi; travel reimbursement from MSD, Merck, BMS, Roche, Pierre Fabre, Novartis, Biontech, Almirall Hermal, Kyowa Kirin, Sun Pharma, and Sanofi. Friedegund Meier has received travel support and/or speaker’s fees and/or advisor’s honoraria by Novartis, Roche, BMS, MSD, and Pierre Fabre and research funding from Novartis and Roche. Peter Mohr declares research support from Bristol-Myers Squibb and Merck Sharp & Dohme; speaker and advisory board honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Roche/Genentech, Novartis, Amgen, GlaxoSmithKline, Pierre Fabre, Merck, Sanofi, and Sun Pharma, and travel support from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, Amgen, and Roche; consulting or advisory role: Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Amgen, Roche, GlaxoSmithKline, Merck, Pierre Fabre, and Sanofi. Claudia Pföhler received honoraria (speaker honoraria or honoraria as a consultant) and travel support from Novartis, BMS, Roche, Merck Serono, MSD, Celgene, Amgen, Sun Pharma, Allery Therapeutics, AbbVie, and LEO. Dirk Schadendorf declares research support from Bristol-Myers Squibb, Novartis, and Amgen; speaker and advisory board honoraria from Array, Bristol-Myers Squibb, Helsinn, Merck Sharp & Dohme, Merck Serono, Immunocore, InFlarX, Nektar, Neracare, Novartis, OncoSec, Pfizer, Philogen, Pierre-Fabre, Replimune, Roche, Sandoz, Sanofi, and Sun Pharma, and travel support from Bristol-Myers Squibb, Merck Sharp & Dohme, Merck-Serono, Nektar, Novartis, Pierre-Fabre, Roche, and Sanofi. Patrick Terheyden received speaker honoraria from BMS, Novartis, MSD, Pierre-Fabre, CureVac, and Roche, consultant honoraria from BMS, Novartis, Pierre-Fabre, Merck Serono, Sanofi, and Roche, and travel support from BMS, Pierre-Fabre, and Roche. Selma Ugurel declares research support from Bristol-Myers Squibb, and Merck Serono; speaker and advisory board honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Merck Serono, Novartis, and Roche, and travel support from Bristol-Myers Squibb, and Merck Sharp & Dohme. Jens Ulrich declares research support from Novartis; speaker and advisory board honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, Sun Pharma, and Roche, and travel support from Bristol-Myers Squibb, Medac, and SUN Pharma. Jochen Utikal is on the advisory board or has received honoraria and travel support from Amgen, Bristol-Myers Squibb, GSK, LeoPharma, Merck Sharp and Dohme, Novartis, Pierre Fabre, Roche, and Sanofi. Michael Weichenthal declares speaker and advisory board honoraria from Merck, Bristol-Myers Squibb, Roche, Novartis, Sanofi, Pierre Fabre, Beiersdorf, Sun Pharma, and Takeda. All other authors declare no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harries M., Malvehy J., Lebbe C., Heron L., Amelio J., Szabo Z., Schadendorf D. Treatment patterns of advanced malignant melanoma (stage III–IV)—A review of current standards in Europe. Eur. J. Cancer. 2016;60:179–189. doi: 10.1016/j.ejca.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Robert C., Thomas L., Bondarenko I., O’Day S. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Robert C., Long G.V., Brady B., Dutriaix C. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 4.Hamid O., Puzanov I., Dummer R., Schachter J., Daud A., Schadendorf D., Blank C., Cranmer L.D., Robert C., Pavlick A.C., et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur. J. Cancer. 2017;86:37–45. doi: 10.1016/j.ejca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Eggermont A.M., Robert C., Ribas A. The new era of adjuvant therapies for melanoma. Nat. Rev. Clin. Oncol. 2018;15:535–536. doi: 10.1038/s41571-018-0048-5. [DOI] [PubMed] [Google Scholar]
- 6.Franklin C., Livingstone E., Roesch A., Schilling B., Schadendorf D. Immunotherapy in melanoma: Recent advances and future directions. Eur. J. Surg. Oncol. 2017;43:604–611. doi: 10.1016/j.ejso.2016.07.145. [DOI] [PubMed] [Google Scholar]
- 7.Michielin O., van Akkooi A.C.J., Ascierto P.A., Dummer R., Keilholz U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2019;30:1884–1901. doi: 10.1093/annonc/mdz411. [DOI] [PubMed] [Google Scholar]
- 8.Michielin O., Atkins M.B., Koon H.B., Dummer R., Ascierto P.A. Evolving impact of long-term survival results on metastatic melanoma treatment. J. Immunother. Cancer. 2020;8:e000948. doi: 10.1136/jitc-2020-000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribas A., Puzanov I., Dummer R., Schadendorf D., Hamid O., Robert C., Hodi F.S., Schachter J., Pavlick A.C., Lewis K.D., et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schachter J., Ribas A., Long G.V., Arance A., Grob J.-J., Mortier L., Daud A., Carlino M.S., McNeil C., Lotem M., et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390:1853–1862. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 11.Booth C.M., Tannock I.F. Randomised controlled trials and population-based observational research: Partners in the evolution of medical evidence. Br. J. Cancer. 2014;110:551–555. doi: 10.1038/bjc.2013.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherman R.E., Anderson S.A., Pan G.J.D., Gray G.W., Gross T., Hunter N.L., LaVange L., Marinac-Dabic D., Marks P.W., Robb M.A., et al. Real-world evidence—What is it and what can it tell us? N. Engl. J. Med. 2016;375:2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 13.Franklin J.M., Schneeweiss S. When and how can real world data analyses substitute for Randomized Controlled Trials? Clin. Pharm. Therap. 2017;102:924–933. doi: 10.1002/cpt.857. [DOI] [PubMed] [Google Scholar]
- 14.Booth C.M., Karim S., Mackillop W.J. Real-world data: Towards achieving the achievable in cancer care. Nat. Rev. Clin. Oncol. 2019;16:312–325. doi: 10.1038/s41571-019-0167-7. [DOI] [PubMed] [Google Scholar]
- 15.Di Maio M., Perrone F., Conte P. Real-World Evidence in Oncology: Opportunities and Limitations. Oncologist. 2020;25:e746–e752. doi: 10.1634/theoncologist.2019-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu F.X., Ou W., Diede S.J., Whitman E.D. Real-world experience with pembrolizumab in patients with advanced melanoma: A large retrospective observational study. Medicine. 2019;98:e16542. doi: 10.1097/MD.0000000000016542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitman E., Scherrer E., Ou W., Krepler C. Outcomes of retreatment with anti-PD-1 monotherapy after response to first course in patients with cutaneous melanoma. Future Oncol. 2020;16:1441–1453. doi: 10.2217/fon-2020-0314. [DOI] [PubMed] [Google Scholar]
- 18.Cowey C.L., Liu F.X., Black-Shinn J., Stevinson K., Boyd M., Frytak J.R., Ebbinghaus S.W. Pembrolizumab utilization and outcomes for advanced melanoma in US community oncology practices. J. Immunother. 2018;41:86–95. doi: 10.1097/CJI.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowey C.L., Liu F.X., Boyd M., Aguilar K.M., Krepler C. Real-world treatment patterns and clinical outcomes among patients with advanced melanoma: A retrospective, community oncology-based cohort study (A STROBE-compliant article) Medicine. 2019;98:e16328. doi: 10.1097/MD.0000000000016328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hribernik N., Boc M., Ocvirk J., Knez-Arbeiter J., Mesti T., Ignjatovic M., Rebersek M. Retrospective analysis of treatment-naive Slovenian patients with metastatic melanoma treated with pembrolizumab—Real-world experience. Radiol. Oncol. 2020;54:119–127. doi: 10.2478/raon-2020-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forschner A., Eichner F., Amaral T., Keim U., Garbe C., Eigentler T.K. Improvement of overall survival in stage IV melanoma patients during 2011–2014: Analysis of real-world data in 441 patients of the German Central Malignant Melanoma Registry (CMMR) J. Cancer Res. Clin. Oncol. 2017;143:533–540. doi: 10.1007/s00432-016-2309-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakdawalla D.N., Shafrin J., Hou N., Peneva D., Vine S., Park J., Zhang J., Brookmeyer R., Figlin R.A. Predicting real-world effectiveness of cancer therapies using overall survival and progression-free survival from clinical trials: Empirical evidence for the ASCO value framework. Value Health. 2017;20:866–875. doi: 10.1016/j.jval.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Stewart M., Norden A.D., Dreyer N., Henk H.J., Abernethy A.P., Chrischilles E., Kushi L., Mansfield A.S., Khozin S., Sharon E. An exploratory analysis of real-world end points for assessing outcomes among immunotherapy-treated patients with advanced non–small-cell lung cancer. JCO Clin. Cancer Inform. 2019;3:1–15. doi: 10.1200/CCI.18.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Driscoll J.J., Rixe O. Overall survival: Still the gold standard: Why overall survival remains the definitive end point in cancer clinical trials. Cancer J. 2009;15:401–405. doi: 10.1097/PPO.0b013e3181bdc2e0. [DOI] [PubMed] [Google Scholar]
- 25.Griffith S.D., Tucker M., Bowser B., Calkins G., Chang C.J., Guardino E., Khozin S., Kraut J., You P., Schrag D., et al. Generating real-world tumor burden endpoints from electronic health record data: Comparison of RECIST, radiology-anchored, and clinician-anchored approaches for abstracting real-world progression in non-small cell lung cancer. Adv. Ther. 2019;36:2122–2136. doi: 10.1007/s12325-019-00970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regan M.M., Werner L., Rao S., Gupte-Singh K., Hodi F.S., Kirkwood J.M., Kluger H.M., Larkin J., Postow M.A., Ritchings C., et al. Treatment-free survival: A novel outcome measure of the effects of immune checkpoint inhibition-a pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 2019;37:3350–3358. doi: 10.1200/JCO.19.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friends of Cancer Research . Establishing a Framework to Evaluate Real-World Endpoints. Friends of Cancer Research; Washington, DC, USA: 2018. [Google Scholar]
- 28.Campbell B.A., Scarisbrick J.J., Kim Y.H., Wilcox R.A., McCormack C., Prince H.M.J.C. Time to next treatment as a meaningful endpoint for trials of primary cutaneous lymphoma. Cancers. 2020;12:2311. doi: 10.3390/cancers12082311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritchie G., Gasper H., Man J., Lord S., Marschner I., Friedlander M., Lee C.K. Defining the most appropriate primary end point in phase 2 trials of immune checkpoint inhibitors for advanced solid cancers: A systematic review and meta-analysis. JAMA Oncol. 2018;4:522–528. doi: 10.1001/jamaoncol.2017.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leiter U., Weichenthal M. ADOReg—Wissenschaftliches Register der Arbeitsgemeinschaft Dermatologische Onkologie. J. Dtsch. Dermatol. Ges. 2014;12:1156–1157. doi: 10.1111/ddg.12556. [DOI] [PubMed] [Google Scholar]
- 31.Schemper M., Smith T.L. A note on quantifying follow-up in studies of failure time. Control Clin. Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-X. [DOI] [PubMed] [Google Scholar]
- 32.Schemper M., Kaider A., Wakounig S., Heinze G. Estimating the correlation of bivariate failure times under censoring. Stat. Med. 2013;32:4781–4790. doi: 10.1002/sim.5874. [DOI] [PubMed] [Google Scholar]
- 33.Savina M., Le Cesne A., Blay J.-Y., Ray-Coquard I., Mir O., Toulmonde M., Cousin S., Terrier P., Ranchere-Vince D., Meeus P., et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: The METASARC observational study. BMC Med. 2017;15:78. doi: 10.1186/s12916-017-0831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gershenwald J.E., Scolyer R.A. Melanoma Staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Ann. Surg. Oncol. 2018;25:2105–2110. doi: 10.1245/s10434-018-6513-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is not publicly available and cannot be shared.