Abstract
Simple Summary
Diabetes increases the risk of certain types of cancer. However, the literature regarding the incidence of diabetes after cancer diagnosis is inconsistent. We aimed to assess whether there was a higher incidence of diabetes among cancer patients by performing a systematic review and meta-analysis of results from cohort studies. To the best of our knowledge, this review conducted the largest and most up-to-date meta-analysis to compare the incidence of diabetes between cancer patients and the cancer-free population. Our results suggest that new-onset diabetes is positively associated with cancer.
Abstract
Background: Diabetes increases the risk of certain types of cancer. However, the literature regarding the incidence of diabetes after cancer diagnosis is inconsistent. We aimed to assess whether there was a higher incidence of diabetes among cancer patients by performing a systematic review and meta-analysis of results from cohort studies. Methods: A systematic electronic literature search was carried out from cohort studies regarding the incidence of diabetes in cancer patients, using the databases PubMed (MEDLINE), Embase, Web of Science, and the Cochrane Library. Random-effects meta-analyses were conducted to pool the estimates. Results: A total of 34 articles involving 360,971 cancer patients and 1,819,451 cancer-free controls were included in the meta-analysis. An increased pooled relative risk (RR) of 1.42 (95% confidence interval (CI): 1.30–1.54, I2 = 95, τ2 = 0.0551, p < 0.01) for diabetes in cancer patients was found compared with the cancer-free population. The highest relative risk was observed in the first year after cancer diagnosis (RR = 2.06; 95% CI 1.63–2.60). Conclusions: New-onset diabetes is positively associated with cancer, but this association varies according to cancer type. More prospective studies with large sample sizes and longer follow-up times are advocated to further examine the association and the underlying mechanisms.
Keywords: cancer survivors, cancer patients, diabetes, cohort studies, systematic review, meta-analysis
1. Introduction
Cancer today is considered a major public health problem globally. It is estimated that around 19.3 million new cancer cases were diagnosed in 2020 worldwide [1]. Due to improvements in cancer screening, diagnosis, and therapy as well as demographic aging [2,3,4], the number of cancer patients (including long-term cancer survivors who have survived for at least 5 years [5]) is increasing worldwide [6].
Previous studies have suggested that comorbid diabetes among cancer patients could be common [7]. Diabetes is found to be more prevalent in cancer patients than in the cancer-free population [8] which may be due to several reasons. For instance, cancer and comorbid diabetes could share common risk factors, such as older age, smoking, obesity, unhealthy diet, physical inactivity, and higher alcohol consumption [9]. Diabetes might also increase the risk for certain types of cancer such as breast cancer and colorectal cancer, and doubles the risk of liver, pancreas, and endometrial cancer [10]. In addition to the cancer disease, cancer treatments such as radiotherapy, glucocorticoids, targeted therapy, hematopoietic cell transplantation (HCT), and androgen deprivation therapy (ADT) may also result in an increased risk for diabetes [11,12,13,14,15,16,17,18,19].
In cancer patients, diabetes is associated with a poorer health-related quality of life (HRQOL) [20], higher healthcare utilization [21], and an increased risk of cancer progression and mortality [22,23,24], which highlights the clinical importance of knowing whether cancer patients are more likely to develop diabetes.
Currently, inconsistent results have been reported on the incidence of diabetes among cancer survivors compared with the cancer-free population. Several studies demonstrated a positive association between developing subsequent diabetes and cancer [25,26], while other studies did not [27]. A recent systematic review reported an overall positive association between cancer and incident diabetes by pooling 13 population-based cohort studies with time-to-event results, but that review did not include non-population-based studies and population-based studies without time-to-event data [28]. Therefore, in this study, we aimed to assess whether there was a higher incidence of diabetes among cancer patients and survivors by performing a systematic review and meta-analysis from the results of population-based and non-population-based cohort studies.
2. Materials and Methods
2.1. Electronic Searches
A comprehensive search was carried out for studies published from 1 January 2010 to 20 April 2021, using the databases PubMed (MEDLINE), Embase, Web of Science, and the Cochrane Library. The search was restricted to start from 2010, as there is a trend in increasing number of publications related to the aim of our study in recent years. Free-text words combined with the MeSH (for Pubmed and the Cochrane Library) and Emtree (for Embase) terms related to the review question were used: patients (cancer patients/survivors), controls (cancer-free population), outcomes (diabetes/comorbidity), and study type (cohort study). The detailed search strategy and specific terms are listed in Text S1 in Supplementary Material. In addition, a manual check on reference lists was also conducted to retrieve additional potentially relevant publications. Before data analyses, we conducted a second electronic search with an identical search strategy on 1 September 2021, to retrieve articles published since the first searching time point.
2.2. Inclusion Criteria
We included primary studies that met the following criteria: (1) conceptualized as prospective or retrospective cohort studies; (2) included a cancer-free control group; (3) included incident diabetes in the outcomes; (4) provided relative risk (RR), incidence rate ratio (IRR), hazard ratio (HR), or odds ratio (OR) with 95% confidence intervals (CI), or reported sufficient data to calculate these estimates; (5) were written in English; (6) were published as full-text in peer-reviewed journals. When more than one publication was reported on the same sample, the study with the most up-to-date or the most complete data was included.
2.3. Study Selection and Data Extraction
A consecutive process was conducted by KY to select primary articles according to the eligibility criteria: (1) duplicate check with Citavi 6® (version 6.4.0.35, Swiss Academic Software, Wädenswil, Switzerland); (2) titles and abstracts screening; (3) full-text reading. After that, data were extracted by KY and ZL independently by using Microsoft Excel 2016 for Windows®. When there was a disagreement that could not be resolved after double checking between KY and ZL, MT was involved. Every step was confirmed by VA. Information was extracted from each of the included studies as follows: (1) basic information about the study (year of publication, first author, country of study, study design, study period, sample size, sample source, index date); (2) demographic characteristics of the case and control group (age at survey and diagnosis, and gender); (3) cancer site and method of diagnosis; (4) type of diabetes and method of diagnosis; (5) risk estimates with 95% CIs, confounders and adjustments.
2.4. Quality Assessment
Two reviewers (KY and ZL) independently used the nine-point Newcastle–Ottawa quality assessment scale (NOS) for cohort studies [29] to estimate the quality of each study included. The NOS is composed of eight items relating to three major domains: selection, comparability of cohorts, and the assessment of outcomes and follow-up. Each study could be awarded a maximum score of nine, and a score of 8 or 9 was defined as having a low risk of bias [30].
2.5. Statistical Analysis
The main outcome was the pooled RR in cancer patients compared with the cancer-free population. The HR, IRR, and OR were regarded as approximations of the estimates of the RR.
To obtain the pooled estimates, random-effects meta-analyses were conducted in this study. The inverse variance method was used for pooling, and the DerSimonian-Laird method was used to estimate the between-study variance (τ2). When no RR for the entire follow-up period was provided in a study, we first pooled the RR of each time interval using the method recommended by Tierney et al. [31]. Then the pooled estimate was used for the meta-analysis. When a study only contained several estimates from different subgroups but no overall RR, the estimate of each subgroup was directly added to the main analysis as the RR of an independent cohort if the case and controls had been matched within each subgroup. Otherwise, we calculated a combined RR for the subgroups using random-effects models and then added the combined RR into the main analysis.
The I² was calculated to estimate the proportion of the total variability that was due to between-study heterogeneity. We also calculated the 95% prediction interval which accounts for the uncertainty of the pooled estimate. Subgroup analyses stratified by study design (population-based or not), follow-up duration, gender, age at diagnosis, geographic regions, cancer type, diabetes type, specific factors controlled for (e.g., age, gender, prevalent comorbidities, body mass index (BMI)), and methodologic quality were carried out to explore the potential sources of heterogeneity. Sensitivity analyses were conducted by both omitting each one of the studies from the main analysis and including only studies reporting HRs, to examine whether results would significantly change. We also assessed potential publication bias using funnel plots combined with tests developed by Egger [32] and Begg [33]. A p-value < 0.1 in either Egger’s or Begg’s test would indicate the presence of publication bias.
The protocol of this study was predefined in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [34] and registered on PROSPERO (registration number CRD42021236041). All statistical analyses were performed using R software (version 4.1.1, R Core Team (2021), Vienna, Austria). All tests were two-tailed and p-values < 0.05 were considered statistically significant.
3. Results
3.1. Search and Identification of Studies
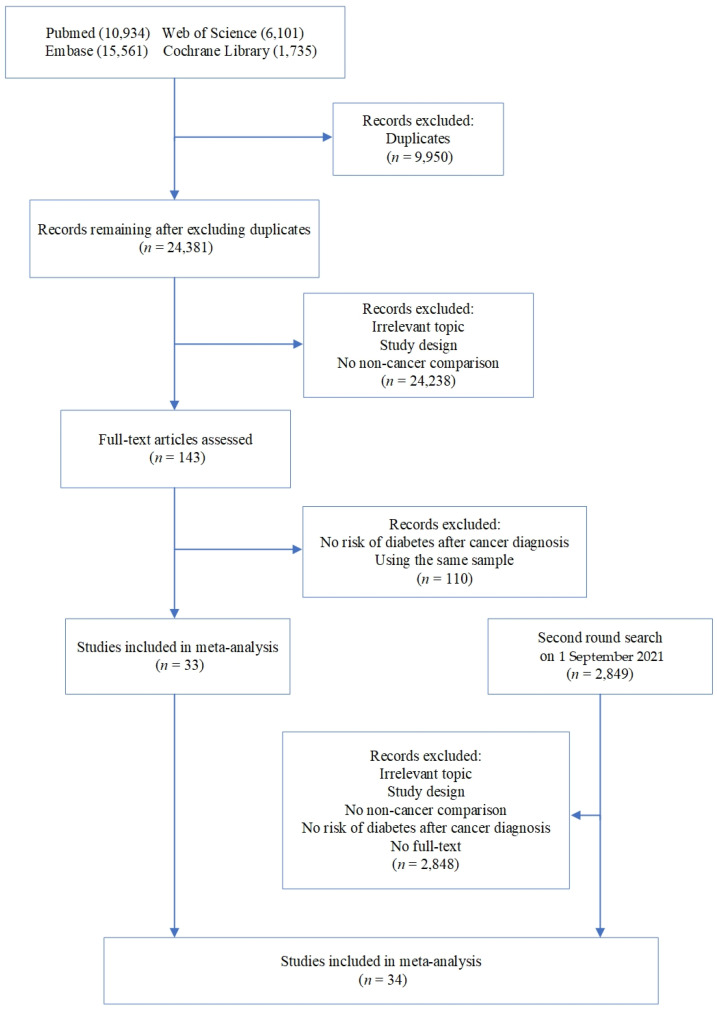
A total of 34,331 articles were identified from initial electronic search. We excluded 9950 duplicates, and after title and abstract screening, the number of remaining references was reduced to 143. We then reviewed the full texts of the remaining literature to further rule out those that did not meet the inclusion criteria. The most frequent reasons for exclusion were irrelevant topics and the absence of cancer-free control groups. Details are shown in Figure 1. A second search was conducted shortly before the start of data analyses, and an additional 2849 articles were found. Finally, after a cross-reference check and screening, 34 articles were included in the meta-analysis.
Figure 1.
Flow chart for identification of studies.
3.2. Characteristics of Included Studies
The general characteristics of the 34 studies included are shown in Table 1 and Table S1 (in Supplementary Materials). The studies included were published between 2010 and 2021, with a total of 360,971 cancer patients (sample sizes ranged from 153 to 51,950) and 1,819,451 cancer-free controls (sample sizes ranged from 138 to 479,059) who reported no prevalent diabetes at the start of the study and were enrolled between 1991 and 2015. Over 60% of all the participants were female and most of the published papers were on breast cancer. These studies were performed in North America (18 of 34), Asia (7 of 34), Europe (6 of 34), and Australia (3 of 34). Almost all of the included studies were based on population-based cohorts (32 of 34). The information of the diagnosis of cancer and diabetes was obtained from cancer registries, public health organizations, or medical records. Six of the studies explicitly focused on type two diabetes mellitus (T2DM), and only one of the remaining studies differentiated between type one diabetes mellitus (T1DM) and T2DM in the analyses.
Table 1.
Summary of the characteristics of studies included in meta-analysis (studies are sorted by year of publication and first author).
| Study (First Author, Year of Publication, and Country) | Study Design | Study Period | Cancer Site | Participants | Association | |
|---|---|---|---|---|---|---|
| Cancer | Control | |||||
| Khan, 2011 (UK) [35] | PBC | 2003–2006 | BC, CRC, PC | 26,276 | 104,486; 4:1, matched by age, gender and primary care practice | HR |
| Landis, 2011 (UK) [36] | PBC | 2000–2007 | SCCHN | 1499 | 5996; 4:1, matched by age and gender | HR |
| Danese, 2012 (USA) [37] | PBC | 1998–2002 a | BC | 51,950 | 51,950; 1:1, matched by time of diagnosis and geographic area | IRR b |
| Li, 2012 (USA) [38] | PBC | 1998–2008 | PC | 2616 | 2616; 1:1, matched by age, region of practice, length of follow-up, and observation period | OR |
| Lipscombe, 2012 (Canada) [39] | PBC | 1996–2008 | BC | 24,976 | 124,880; 5:1, matched by age | HR |
| Stålberg, 2012 (Sweden) [40] | PBC | 1993–2006 | OC | 11,139 | 55,687; up to 5:1, matched by birth year | HR |
| van Herk-Sukel, 2012 (The Netherlands) [41] |
PBC | 2000–2008 | STS | 533 | 5330; 10:1, matched by age and gender | HR |
| Chia, 2013 (USA) [42] | PBC | 1998–2005 | OC | 5087 | 5087; 1:1, matched by the county of residence | IRR b |
| Jordan, 2014 (USA) [43] | PBC | 1990–2005 | BC | 1361 | 1361; 1:1, matched by age | HR |
| Sun, 2014 (Taiwan, China) [44] | PBC | 2000–2011 | BC | 22,257 | 89,028; 4:1, matched by age and index year | HR |
| Li, 2015 (China) [45] | MCC | 2008–2014 | BC | 1283 | 1361; not matched | RR b |
| Ording, 2015 (Denmark) [46] | PBC | 1999–2013 | BC | 32,403 | 162,015; 5:1, matched by year of birth and calendar date | RR b |
| Chang, 2016 (China) [47] | PBC | 2000–2012 | AML | 440 | 4400; 10:1, matched by age, gender, and index year | HR |
| Chao, 2016 (USA) [48] | PBC | 1995–2010 | Leukemia, Lymphoma, et al. | 652 | 6520; 10:1, matched by age, gender, and calendar year of the index date | IRR |
| Crawley, 2016 (UK) [49] | PBC | 2006–2013 | PC | 34,031 | 167,205; 5:1, matched by birth year and county of residence | HR |
| Hashibe, 2016 (USA) [50] | PBC | 1991–2007 a. | TeC | 785 | 3323; 4-5:1, matched by birth year, birth region and the date of last residence in Utah | HR |
| Lowe, 2016 (USA) [51] | PBC | 2000–2009 | GC | 12,612 | 12,612; 1:1, matched by year of enrollment in Medicare and county of primary residence | IRR b |
| Santorell, 2016 (USA) [27] | PBC | 2007–2009 | BC | 2678 | 10,712; 4:1, matched by age | HR |
| Singh, 2016 (Canada) [52] | PBC | 2002–2012 | CRC | 39,707 | 198,535; 5:1, matched by age on the date of diagnosis and gender | HR |
| Blackburn, 2017 (USA) [53] | PBC | 1997–2012 a | ThC | 3706 | 15,587; up to 5:1, matched by birth year, gender, and birth state | HR |
| Hwangbo, 2018 (South Korea) [25] | PBC | 2002–2013 | Pancreas, Kidney, et al. | 15,130 | 479,059; not matched | HR |
| Lega, 2018 (Canada) [54] | PBC | 1991–2015 | Leukemia, Lymphoma, et al. | 10,438 | 52,190; 5:1, matched by year of birth and gender | HR |
| Ng (BC), 2018 (Australia) [55] | PBC | 2004–2014 | BC | 3799 | 37,990; 10:1, matched by age and gender | HR |
| Ng (PC), 2018 (Australia) [56] | PBC | 2004–2014 | PC | 3176 | 31,760; 10:1, matched by age | HR |
| Dhopeshwarkar, 2019 (USA) [57] | PBC | 2003-2013 | AML | 3911 | 3911; 1:1, matched by year of birth, gender, ethnicity, and geographic region | HR |
| Hawkins, 2019 (USA) [58] | PBC | 1997–2013 a | CRC | 7114 | 25,979; up to 5:1, matched by birth year, gender, and birth state | HR |
| Ng, 2019 (Australia) [59] | PBC | 2005–2013 | BC | 10,321 | 20,642; 2:1, matched by birth year | HR |
| Accordino, 2020 (USA) [60] | PBC | 2005–2013 | BC (including male BC) | 13,529 | 13,529; 1:1, matched by date of birth and race | RR b |
| Bigelow, 2020 (USA) [61] | PBC | 2003–2013 | SCCHN | 2497 | 4994; 2:1, matched by age, race, gender, and SEER region | HR |
| Chao, 2020 (USA) [62] | PBC | 2002–2014 | Lymphoma, Melanoma, et al. | 6778 | 87,737; 13:1, matched by age, gender, and calendar year | IRR |
| Kim, 2020 (USA) [63] | PBC | 1997–2012 a | EC | 2314 | 8583; up to 5:1, matched by birth year, and birth state | HR |
| Markus, 2020 (Israel) [64] | SCC | 2008–2015 | MM (including SMM) | 153 | 138; matched by age, gender, and length of follow-up | HR |
| Wang, 2020 (Taiwan, China) [65] | PBC | 2000–2015 | BC | 4607 | 23,035; 5:1, matched by age | HR |
| Lin, 2021 (Taiwan, China) [26] | PBC | 2000–2013 | PC | 1213 | 1213; 1:1, matched by index year, demographic variables, and comorbidities by propensity score matching | HR |
| Total | - | 1991–2015 | - | 359,472 | 1,819,451 | - |
a Participants were diagnosed with cancer during the listed time period. A time point for the end of the study was not given. b The estimate was not given directly, thus it was calculated with data provided in the primary article.
3.3. Quality Assessment of Included Studies
The risk of bias in each study included is shown in Table 2. In summary, most of the studies had a low risk of bias (27/34). Only one study indicated four areas of potential biases [45]. No clear statement of subjects lost to follow-up (13/34) was the most frequent source of potential bias, followed by insufficient length of follow-up (9/34).
Table 2.
Quality assessment of included studies using the Newcastle–Ottawa Scale (NOS) for cohort studies a (studies are sorted by year of publication and first author).
| First Author | Selection | Comparability | Outcome | Total Score | Risk of Bias | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of Cohort | Selection of Control Cohort | Ascertainment of Exposure | Outcome Not Present at Start | Comparability of Cohorts | Assessment of Outcome | Length of Follow-up | Adequacy of Follow-up b | Maximum Score: 9 | High (0–7) or Low (8–9) |
|
| Khan, 2011 [35] | * | * | * | * | ** | * | 7 | High | ||
| Landis, 2011 [36] | * | * | * | * | ** | * | * | 8 | Low | |
| Danese, 2012 [37] | * | * | * | * | ** | * | * | 8 | Low | |
| Li, 2012 [38] | * | * | * | * | * | * | * | 7 | High | |
| Lipscombe, 2012 [39] | * | * | * | * | ** | * | * | 8 | Low | |
| Stålberg, 2012 [40] | * | * | * | * | * | * | 6 | High | ||
| van Herk-Sukel, 2012 [41] | * | * | * | * | ** | * | * | 8 | Low | |
| Chia, 2013 [42] | * | * | * | * | * | * | * | 7 | High | |
| Jordan, 2014 [43] | * | * | * | * | ** | * | * | * | 9 | Low |
| Sun, 2014 [44] | * | * | * | * | ** | * | * | * | 9 | Low |
| Li, 2015 [45] | * | * | * | * | 4 | High | ||||
| Ording, 2015 [46] | * | * | * | * | ** | * | * | * | 9 | Low |
| Chang, 2016 [47] | * | * | * | * | ** | * | * | * | 9 | Low |
| Chao, 2016 [48] | * | * | * | * | ** | * | * | * | 9 | Low |
| Crawley, 2016 [49] | * | * | * | * | ** | * | * | * | 9 | Low |
| Hashibe, 2016 [50] | * | * | * | * | * | * | * | * | 8 | Low |
| Lowe, 2016 [51] | * | * | * | * | * | * | 6 | High | ||
| Santorell, 2016 [27] | * | * | * | * | ** | * | 7 | High | ||
| Singh, 2016 [52] | * | * | * | * | ** | * | * | 8 | Low | |
| Blackburn, 2017 [53] | * | * | * | * | ** | * | * | * | 9 | Low |
| Hwangbo, 2018 [25] | * | * | * | * | ** | * | * | 8 | Low | |
| Lega, 2018 [54] | * | * | * | * | * | * | * | * | 8 | Low |
| Ng (BC), 2018 [55] | * | * | * | * | * | * | * | * | 8 | Low |
| Ng (PC), 2018 [56] | * | * | * | * | * | * | * | * | 8 | Low |
| Dhopeshwarkar, 2019 [57] | * | * | * | * | ** | * | * | 8 | Low | |
| Hawkins, 2019 [58] | * | * | * | * | ** | * | * | 8 | Low | |
| Ng, 2019 [59] | * | * | * | * | ** | * | * | * | 9 | Low |
| Accordino, 2020 [60] | * | * | * | * | ** | * | * | 8 | Low | |
| Bigelow, 2020 [61] | * | * | * | * | ** | * | * | 8 | Low | |
| Chao, 2020 [62] | * | * | * | * | ** | * | * | * | 9 | Low |
| Kim, 2020 [63] | * | * | * | * | ** | * | * | * | 9 | Low |
| Markus, 2020 [64] | * | * | * | * | ** | * | * | 8 | Low | |
| Wang, 2020 [65] | * | * | * | * | ** | * | * | 8 | Low | |
| Lin, 2021 [26] | * | * | * | * | ** | * | * | 8 | Low | |
a A study can be awarded a maximum of one point (*) for each item within the Selection and Outcome categories. A maximum of two points (**) can be given for Comparability. b We regarded a minimum of 5 years of follow-up time as sufficient length for follow-up.
3.4. Cancer Patients and the Association between Cancer and New-Onset Diabetes
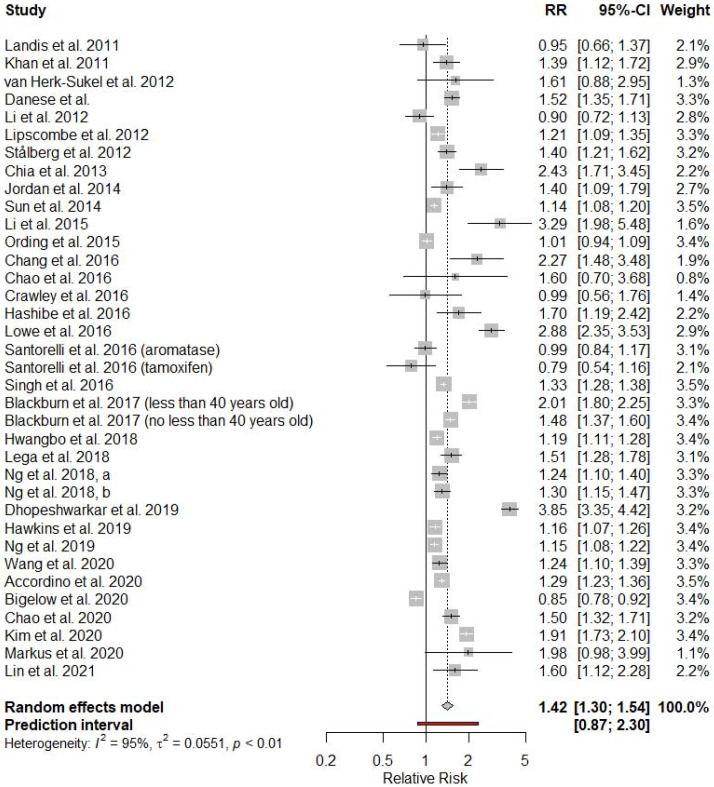
A total of 36 independent cancer cohorts from 34 studies were included in the main analysis. The pooled RR was 1.42 with a 95% confidence interval (CI) of 1.30 to 1.54. However, the between-study heterogeneity was high (τ2 = 0.0551, I2 = 95%, p < 0.01), and the 95% prediction interval was 0.87 to 2.30. In nine cohorts from eight individual studies, statistically nonsignificant results were reported, one study reported statistically significant inverse association between cancer and incident diabetes (HR = 0.85; 95% CI 0.78–0.92), and in all the other 26 cohorts from the other 25 studies, statistically significant positive associations were reported (Figure 2).
Figure 2.
Overall relative risk of diabetes among cancer patients compared with cancer-free controls. The 95% prediction interval provides a predicted range for the true association between cancer and incident diabetes.
3.5. Subgroup Analyses and Sensitivity Analysis
We conducted subgroup meta-analyses stratified by various factors to further understand the association between cancer and incident diabetes in cancer patients and to investigate sources of heterogeneity. The results are shown in Table 3.
Table 3.
Subgroup analysis.
| Subgroup | No. Studies | No. Estimates Pooled | RR (95% CI) | I2 (%) | Difference between Subgroups (p) |
|---|---|---|---|---|---|
| Overall | 34 | 36 | 1.42 (1.30, 1.54) | 95 | - |
| Study design [sampling frame] | 34 | 36 | 0.008 | ||
| Population-based cohort | 32 | 34 | 1.39 (1.28, 1.52) | 95 | |
| Non-population-based cohort | 2 | 2 | 2.71 (1.67, 4.39) | 24 | |
| Gender | 21 | 23 | 0.9169 | ||
| Male | - | 7 | 1.32 (1.09, 1.60) | 63 | |
| Female | - | 16 | 1.31 (1.18, 1.45) | 92 | |
| Age at diagnosis | 31 | 33 | 0.074 | ||
| <50 | - | 8 | 1.64 (1.40, 1.93) | 88 | |
| ≥50 | - | 25 | 1.36 (1.20, 1.55) | 96 | |
| Study region | 34 | 36 | 0.0327 | ||
| North America | 18 | 20 | 1.49 (1.30, 1.70) | 97 | |
| Europe | 6 | 6 | 1.19 (0.98, 1.45) | 78 | |
| Australia | 3 | 3 | 1.21 (1.12, 1.31) | 45 | |
| Asia | 7 | 7 | 1.38 (1.20, 1.58) | 81 | |
| Matched or adjusted for age and gender | 34 | 36 | 0.1506 | ||
| Yes | 31 | 33 | 1.37 (1.25, 1.50) | 95 | |
| No | 3 | 3 | 2.25 (1.15, 4.39) | 97 | |
| Matched or adjusted for status of prevalent comorbidity | 34 | 36 | 0.2517 | ||
| Yes | 18 | 20 | 1.36 (1.22, 1.52) | 96 | |
| No | 16 | 16 | 1.52 (1.30, 1.79) | 93 | |
| Matched or adjusted for BMI | 34 | 36 | 0.5732 | ||
| Yes | 5 | 6 | 1.49 (1.23, 1.80) | 96 | |
| No | 29 | 30 | 1.40 (1.27, 1.54) | 95 | |
| Follow-up length | 34 | 36 | 0.7334 | ||
| <5 years | 5 | 6 | 1.51 (1.08, 2.12) | 94 | |
| 5-10 years | 14 | 14 | 1.35 (1.17, 1.56) | 97 | |
| >10 years | 15 | 16 | 1.44 (1.27, 1.63) | 93 | |
| Time period post cancer diagnosis | 15 | 29 | 0.0011 | ||
| 0–1 years | - | 12 | 2.06 (1.63, 2.60) | 95 | |
| 1–5 years | - | 6 | 1.49 (1.31, 1.71) | 90 | |
| 5–10 years | - | 7 | 1.22 (1.08, 1.39) | 76 | |
| >10 years | - | 4 | 1.19 (0.83, 1.72) | 74 | |
| Cancer entity | 32 | 55 | <0.0001 | ||
| Pancreas | - | 1 | 5.15 (3.32, 7.99) | - | |
| Hematologic | - | 6 | 2.21 (1.52, 3.23) | 87 | |
| Kidney | - | 1 | 2.06 (1.34, 3.16) | - | |
| Liver | - | 1 | 1.94 (1.73, 2.10) | - | |
| Gallbladder | - | 1 | 1.79 (1.08, 2.98) | - | |
| Testicular | - | 2 | 1.74 (1.23, 2.46) | 0 | |
| Lung | - | 1 | 1.72 (1.27, 2.32) | - | |
| Ovarian | - | 3 | 1.63 (1.05, 2.53) | 77 | |
| Bladder | - | 1 | 1.61 (1.08, 2.40) | - | |
| Thyroid | - | 3 | 1.59 (1.26, 2.00) | 92 | |
| Breast | - | 13 | 1.23 (1.14, 1.33) | 86 | |
| Colorectal | - | 4 | 1.23 (1.10, 1.38) | 79 | |
| Gastric | - | 2 | 1.97 (0.94, 4.13) | 97 | |
| Germ cell tumors | - | 1 | 1.68 (0.98, 2.88) | - | |
| Soft tissue sarcoma | - | 1 | 1.61 (0.88, 2.96) | - | |
| Uterus | - | 2 | 1.56 (0.97, 2.50) | 80 | |
| Central nervous system | 2 | 1.23 (0.80, 1.88) | 0 | ||
| Prostate | - | 5 | 1.10 (0.87, 1.41) | 73 | |
| Esophagus | - | 1 | 0.83 (0.34, 1.99) | - | |
| Head and neck | - | 4 | 0.86 (0.80, 0.93) | 0 | |
| Type of diabetes | 34 | 37 | 0.1874 | ||
| T1DM | - | 1 | 3.93 (1.03, 14.94) | ||
| T2DM | - | 7 | 1.63 (1.16, 2.30) | 98 | |
| Not differentiated | - | 29 | 1.37 (1.26, 1.48) | 92 | |
| Cancer therapy | 11 | 15 | 0.0111 | ||
| Chemotherapy | - | 5 | 1.82 (1.22, 2.73) | 92 | |
| No chemotherapy | - | 3 | 1.73 (1.24, 2.42) | 96 | |
| ADT | - | 3 | 1.32 (1.15, 1.51) | 7 | |
| Endocrine therapy for breast cancer | - | 4 | 1.11 (0.99, 1.26) | 65 | |
| Methodologic quality of study (NOS) | 34 | 36 | 0.644 | ||
| High (low risk of bias) | 27 | 28 | 1.40 (1.28, 1.53) | 96 | |
| Low (high risk of bias) | 7 | 8 | 1.51 (1.10, 2.08) | 94 |
Consistent results were observed when stratified by study design (population-based or non-population-based), gender, age at diagnosis, study period, type of diabetes, risk of bias, and whether controlled for age, gender, prevalent comorbidity, or BMI. In subgroup analyses by cancer type, a statistically significant positive association between cancer and diabetes was found in most cancer types, except for tumors in the head and neck, central nervous system (CNS), prostate, gastric, uterus, esophagus, germ cell, and soft tissue sarcoma. Moreover, in the head and neck cancer subgroup, incident diabetes was inversely associated with cancer (RR = 0.86; 95% CI 0.80–0.93; I2 = 0), indicating that the head and neck cancer patients were less likely to have incident diabetes. Statistically nonsignificant associations were observed in some subgroups stratified by geographic regions (Europe, RR = 1.19; 95% CI 0.98–1.45), the time period post cancer diagnosis (>10 years, RR = 1.19; 95% CI 0.83–1.72), and treatment (endocrine therapy for breast cancer, RR = 1.11; 95% CI 0.99–1.26). I2s were not significantly reduced in most subgroup analyses. However, I2 = 0 was observed in some cancer type subgroups (head and neck, CNS, testicular). When stratified by therapy, the heterogeneity within the ADT subgroup was also small (I2 = 7).
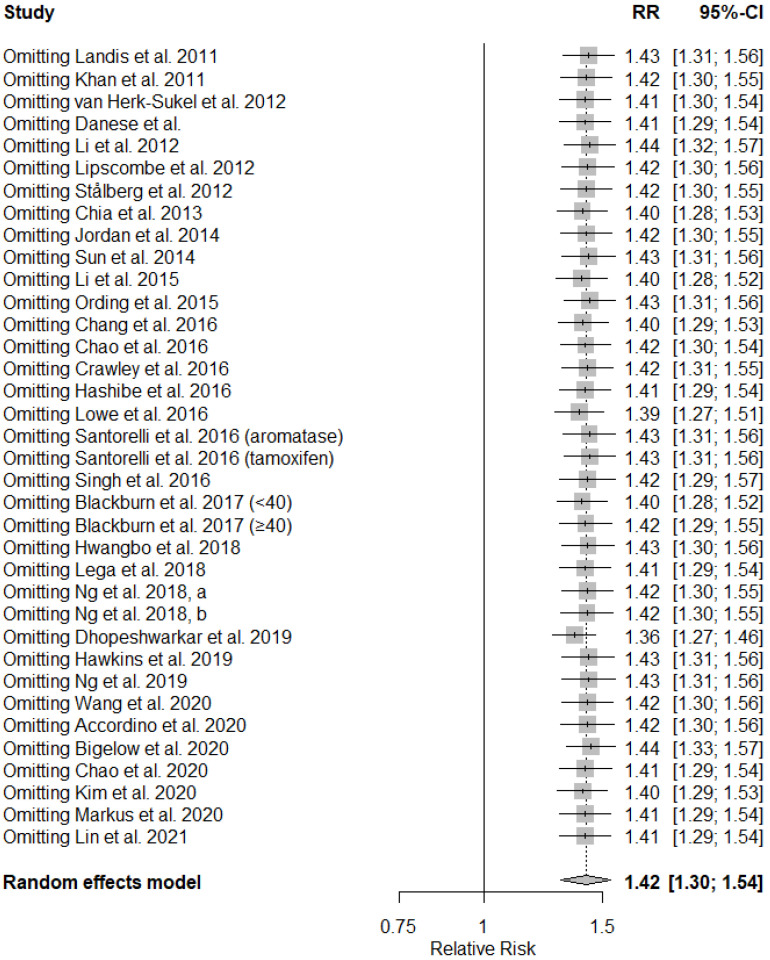
In sensitivity analyses, the pooled RRs did not significantly alter when we omitted any of the studies from the main analysis, even those studies with a high risk of bias (Figure 3). When only the studies that reported HRs were included (n = 27), the results were similar to the original results (RR = 1.38; 95% CI 1.25–1.52). No statistically significant difference (p = 0.25) was observed when the pooled estimate of these 27 studies was compared with the pooled estimate of the non-time-to-event studies (n = 7; RR = 1.60; 95% CI 1.27–2.02).
Figure 3.
Sensitivity analysis.
3.6. Publication Bias
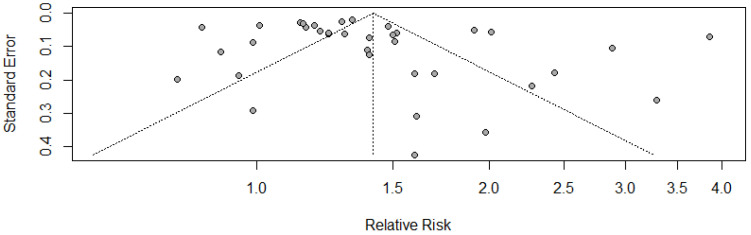
The Egger’s test (p = 0.13) and the Begg’s test (p = 0.24) both indicated the absence of publication bias. However, the funnel plot seemed asymmetric (Figure 4), which might be attributed to the inherent heterogeneity between the included studies.
Figure 4.
Funnel plot for publication bias.
4. Discussion
To the best of our knowledge, this review conducted the largest and most up-to-date meta-analysis to compare the incidence of diabetes between cancer patients and the cancer-free population. In this review, we found that new-onset diabetes in general was positively associated with cancer during cancer survival, and there was a stronger association in the first year after cancer diagnosis. Xiao et al. [28] reported in their study an overall 1.39-fold increased risk of diabetes in cancer survivors when compared with the general population, with the pooled HR peaking in the first year post diagnosis (HR = 2.09; 95% CI 1.32–3.32), which is similar to our results. Consistent findings were also reported by other studies [8,11,66,67]. In a meta-analysis looking at the prevalence of metabolic syndrome in cancer patients compared with a cancer free population, a pooled OR of 1.08 (95% CI 0.57–2.03) for high glucose level was reported when stratified by individual components of metabolic syndrome. However, our meta-analysis included a greater number of, mostly large population-based cohort studies, and focused specifically on the incidence of diabetes. Therefore, our study is more likely to reveal the potential association between cancer and subsequently developed diabetes. Moreover, since diabetes could imply an increased risk for cardiovascular conditions such as coronary heart disease and stroke [68], our results are also consonant with findings that cancer patients are at a higher risk of cardiovascular disease (CVD) [69,70].
Although there is currently no clear explanation on the underlying mechanism for the association between cancer and diabetes, there are still some clues to be tracked. According to previous studies, most cancer treatment modalities could be positively associated with new-onset diabetes. When our body is not able to produce sufficient insulin or cannot use it effectively, diabetes occurs [71]. Interestingly, varying mechanisms for diabetes can be observed in different therapeutic methods according to cancer type. Surgery and radiotherapy involving the pancreas can result in pancreatic insufficiency, which is one of the important reasons for diabetes development [12]. Cranial irradiation and total body irradiation can influence the hypothalamic–pituitary axis, leading to changes in the body composition (e.g., overweight) and insulin resistance [13,14]. Although no evidence has been found that chemotherapeutic drugs could directly impinge on glucose metabolism, in classical chemotherapy regimens, they are often used in conjunction with glucocorticoids. Glucocorticoids can enhance the efficacy of chemotherapy, treat swelling, intracranial hypertension, pain, nausea, or be used as antitumor drugs in hematological malignancies [15]. Glucocorticoids affect several insulin-signaling pathways, leading to reduced insulin sensitivity, inducing insulin resistance, and increasing the risk of diabetes [15]. Targeted therapy drugs are also often used alone or in combination with chemotherapy. Some inhibitors block the pathways that are also involved in glucose regulation, such as the tyrosine kinase receptors insulin growth factor receptor 1 (IGF-1R) which could lead to insulin resistance [16]. Patients with hematologic malignancies are at high risk of exposure to glucocorticoids. They are also likely to receive HCT. HCT can contribute to the release of several proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) [17,18], and previous studies have shown that the latter could contribute to insulin resistance by influencing the insulin signaling pathway [19]. ADT is widely used in prostate cancer [72]. A reduction in testosterone levels can be observed during the application of ADT drugs, however, decreased testosterone is directly associated with insulin resistance in men [73]. As for hormone therapy for breast cancer patients, we observed no significant association in a subgroup analysis involving only patients having received hormone therapy. This might be due to the favorable changes on the lipid profile by aromatase inhibitors (AIs) and tamoxifen [27,55].
In our current study, we found that the incidence of diabetes may be higher in the first year after cancer diagnosis. Closer contact between care providers and patients and a potential higher risk of detection bias in the initial years after cancer diagnosis might be possible explanations for this observation. Furthermore, some limitations in the underlying studies such as information bias regarding the correct assessment of the timing of the DM diagnosis and the potential survival bias during long-term follow-up could also explain these results. Moreover, since there is an age-related increase in diabetes risk in both cancer patients and controls [74], a reduced relative risk might be observed with the passing of time.
However, a statistically significant positive association was also observed during years one to ten. Several reasons could partly explain the fact that incident diabetes was still positively associated with cancer five or more years post diagnosis, when the effects of most treatments would most likely have subsided. First, in cancer patients, particularly those who suffer from cancer cachexia, insulin resistance often occurs as a result of the secretion and activation of several proinflammatory cytokines induced by cancer itself, such as TNF-α [75,76,77]. Some psychological consequences of cancer, such as depression, may also make the survivors more vulnerable to diabetes due to integrated mechanisms such as hypothalamic abnormality and an unhealthy lifestyle [78]. Moreover, the alteration of the glucose metabolism can start to appear more than ten years before the diagnosis of diabetes [79]. Furthermore, cancer and diabetes share a number of common risk factors, such as obesity, tobacco abuse, and alcohol consumption [9] and cancer survivors are more likely to be physically inactive compared with the general population [80]. A positive association was also observed ten or more years post diagnosis (Table 3). Although this result was not statistically significant (RR = 1.19, 95% CI 0.83–1.72), the potential positive association should not be ignored, and future studies with sufficient power and a sufficiently long follow-up post cancer diagnosis are recommended. Furthermore, extension of surveillance for incident diabetes to beyond ten years post diagnosis could be of clinical relevance.
In subgroup analyses, we found that the association between cancer and new-onset diabetes varied by cancer type. The highest relative risk of diabetes was found in pancreatic cancer, which was not surprising. This association in patients of hematologic malignancies was also strong, which was comparable with another study [81], possibly due to intensive and comprehensive therapy such as total body irradiation, chemotherapy with glucocorticoids, and HCT. However, we observed a statistically significant inverse association in the head and neck cancer patients. One possible explanation could be that cancer patients whose salivary glands and oral cavity were included in the treatment field, whether surgery or radiotherapy, could experience an unpleasant dietary intake thus would be less likely to become obese [82]. Furthermore, increased glucose-disposal rates were found in head and neck cancer patients indicating that the cancer cells played the role of a glucose drain [83]. However, the results were not always consistent. Future studies conducting research on head and neck cancer and diabetes should also assess covariates such as diet.
An increased risk of breast, colorectal, liver, and endometrial cancer has been detected among adults with diabetes [84]. In our study, new-onset diabetes was positively associated with these types of cancer. However, no statistical significance was found for uterine cancer based on two studies (RR = 1.56, 95% CI 0.97–2.50) (Table 3), which might be due to the small number of cases, the inclusion of other rare forms of cancer that arise in uterus (e.g., uterine sarcoma), and a possible bias from the exclusion of patients with prevalent diabetes at cancer diagnosis in the primary articles. For endometrial cancer alone, a statistically significant positive association was found in the primary article (HR = 1.91, 95% CI 1.73–2.10) [63].
Our study had some limitations. First, we focused on various types of cancer patients with no restriction on cancer treatment, age, gender, and follow-up duration, which could have contributed to the high heterogeneity of the included studies. In our study, reduced I2s were observed in some cancer type subgroups (head and neck, CNS, testicular) and the ADT subgroup (Table 3), which suggested that heterogeneity was generated by complex factors, and that cancer type and therapy could be two potential sources of heterogeneity. Since the study size in our meta-analysis was highly variable, and I2 is highly influenced by the size of the included studies [85], this could be another important reason for the high I2 reported. We also calculated the between-study variance (τ2 = 0.0551) and the 95% prediction interval (0.87–2.30) for the main analysis to analyze the heterogeneity. This indicated that there was substantial uncertainty about the significant association. It also provided a range for the association in a potential new original study on the association between new-onset diabetes and cancer overall. Furthermore, it offered a reference for clinicians to make more informed clinical decision making [86,87]. Second, apart from large population-based studies, we also included non-population-based studies with relatively smaller sample sizes. Nevertheless, the results in the main analysis, sensitivity analyses and subgroup analyses were mostly consistent, suggesting that our results of the underlying association are valid despite the high heterogeneity. Third, we could not perform more comprehensive subgroup analyses stratified by types of diabetes, treatment method, and other important factors such as BMI, physical activity, diet, smoking status, and alcohol consumption, due to the absence of individual patient data and due to a lack of studies that had differentiated relevant subgroups of cancer patients.
5. Conclusions
In summary, our meta-analyses of cohort studies revealed that new-onset diabetes was positively associated with cancer. This association was stronger during the first years after cancer diagnosis and varied according to cancer type. In this context, integration and coordination of healthcare should be applied for cancer patients. More prospective studies with large sample sizes and longer follow-up times (over ten years post diagnosis) are advocated to further examine the association and the underlying mechanisms.
Acknowledgments
We are grateful to Andrea Heppert from Central Library, German Cancer Research Center (DKFZ), for her contribution to this work.
Abbreviations
| ADT | Androgen deprivation therapy |
| AML | Acute myeloid leukemia |
| BC | Breast cancer |
| BMI | Body mass index |
| CI | Confidence interval |
| CNS | Central nervous system |
| CRC | Colorectal cancer |
| CVD | Cardiovascular disease |
| EC | Endometrial cancer |
| GC | Gastric cancer |
| HCT | Hematopoietic cell transplantation |
| HR | Hazard ratio |
| HRQOL | Health-related quality of life |
| IGF-1R | Insulin growth factor receptor 1 |
| IL-6 | Interleukin-6 |
| IRR | Incidence rate ratio |
| MCC | Multi-center cohort |
| MM | Multiple myeloma |
| n.a. | not available |
| NOS | the Newcastle-Ottawa Scale for cohort studies |
| OC | Ovarian cancer |
| OR | Odds ratio |
| PBC | Population-based cohort |
| PC | Prostate cancer |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RR | Relative risk |
| SCC | Single center cohort |
| SCCHN | Squamous cell carcinoma of the head and neck |
| SMM | Smoldering multiple myeloma |
| STS | Soft tissue sarcoma |
| T1DM | Type 1 diabetes mellitus |
| T1DM | Type 2 diabetes mellitus |
| TeC | Testicular cancer |
| ThC | Primary thyroid cancer |
| TNF-α | Tumor necrosis factor-α |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14071808/s1, Text S1: Searching strategies; Table S1: Summary of additional characteristics of studies included in meta-analysis.
Author Contributions
Conceptualization, material preparation, data collection, methodology, data analysis, original draft, writing-review and editing, K.Y.; conceptualization, data collection, methodology, writing-review and editing, Z.L.; conceptualization, data collection, methodology, writing-review and editing, M.S.Y.T.; conceptualization, methodology, writing-review and editing, D.D.; conceptualization, data collection, methodology, writing-review and editing, supervision, V.A. All authors have read and agreed to the published version of the manuscript.
Funding
K.Y. is supported by the China Scholarship Council PhD program (ID number: 202006240060). Z.L. is supported by the China Scholarship Council PhD program (ID number: 201808320419). The China Scholarship Council had no role in the study design, collection, analysis, or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Derksen J.W.G., Beijer S., Koopman M., Verkooijen H.M., van de Poll-Franse L.V., May A.M. Monitoring potentially modifiable lifestyle factors in cancer survivors: A narrative review on currently available methodologies and innovations for large-scale surveillance. Eur. J. Cancer. 2018;103:327–340. doi: 10.1016/j.ejca.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Malvezzi M., Bertuccio P., Rosso T., Rota M., Levi F., La Vecchia C., Negri E. European cancer mortality predictions for the year 2015: Does lung cancer have the highest death rate in EU women. Ann. Oncol. 2015;26:779–786. doi: 10.1093/annonc/mdv001. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 5.Aziz N.M. Cancer survivorship research: State of knowledge, challenges and opportunities. Acta Oncol. 2007;46:417–432. doi: 10.1080/02841860701367878. [DOI] [PubMed] [Google Scholar]
- 6.Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Nikšić M., Bonaventure A., Valkov M., Johnson C.J., Estève J., et al. Global surveillance of trends in cancer survival 2000–2014 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards B.K., Noone A.-M., Mariotto A.B., Simard E.P., Boscoe F.P., Henley S.J., Jemal A., Cho H., Anderson R.N., Kohler B.A., et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S.S., Nadarajah A., Wang K.-W., Kay S., Banfield L., Foroutan F., Johnston D., Zelcer S., Rassekh S.R., Thabane L., et al. The Prevalence of Diabetes Mellitus in Childhood Cancer Survivors: A Systematic Review and Meta-Analysis. Can. J. Diabetes. 2020;44:S19. doi: 10.1016/j.jcjd.2020.08.048. [DOI] [Google Scholar]
- 9.Extermann M. Interaction between comorbidity and cancer. Cancer Control. 2007;14:13–22. doi: 10.1177/107327480701400103. [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E., Harlan D.M., Archer M.C., Bergenstal R.M., Gapstur S.M., Habel L.A., Pollak M., Regensteiner J.G., Yee D. Diabetes and cancer: A consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H., Sun X., Zhao L., Chen X., Zhao J. Androgen deprivation therapy is associated with diabetes: Evidence from meta-analysis. J. Diabetes Investig. 2016;7:629–636. doi: 10.1111/jdi.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vathaire F., El-Fayech C., Ben Ayed F.F., Haddy N., Guibout C., Winter D., Thomas-Teinturier C., Veres C., Jackson A., Pacquement H., et al. Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: A retrospective cohort study. Lancet Oncol. 2012;13:1002–1010. doi: 10.1016/S1470-2045(12)70323-6. [DOI] [PubMed] [Google Scholar]
- 13.Friedman D.N., Tonorezos E.S., Cohen P. Diabetes and Metabolic Syndrome in Survivors of Childhood Cancer. Horm. Res. Paediatr. 2019;91:118–127. doi: 10.1159/000495698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sklar C.A., Mertens A.C., Walter A., Mitchell D., Nesbit M.E., O’Leary M., Hutchinson R., Meadows A.T., Robison L.L. Changes in body mass index and prevalence of overweight in survivors of childhood acute lymphoblastic leukemia: Role of cranial irradiation. Med. Pediatr. Oncol. 2000;35:91–95. doi: 10.1002/1096-911X(200008)35:2<91::AID-MPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 15.Ariaans G., de Jong S., Gietema J.A., Lefrandt J.D., de Vries E.G.E., Jalving M. Cancer-drug induced insulin resistance: Innocent bystander or unusual suspect. Cancer Treat. Rev. 2015;41:376–384. doi: 10.1016/j.ctrv.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Goldman J.W., Mendenhall M.A., Rettinger S.R. Hyperglycemia Associated with Targeted Oncologic Treatment: Mechanisms and Management. Oncologist. 2016;21:1326–1336. doi: 10.1634/theoncologist.2015-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakata N., Yasui M., Okamura T., Inoue M., Yumura-Yagi K., Kawa K. Kinetics of plasma cytokines after hematopoietic stem cell transplantation from unrelated donors: The ratio of plasma IL-10/sTNFR level as a potential prognostic marker in severe acute graft-versus-host disease. Bone Marrow Transplant. 2001;27:1153–1161. doi: 10.1038/sj.bmt.1703060. [DOI] [PubMed] [Google Scholar]
- 18.Schots R., Kaufman L., van Riet I., Ben Othman T., de Waele M., van Camp B., Demanet C. Proinflammatory cytokines and their role in the development of major transplant-related complications in the early phase after allogeneic bone marrow transplantation. Leukemia. 2003;17:1150–1156. doi: 10.1038/sj.leu.2402946. [DOI] [PubMed] [Google Scholar]
- 19.Hotamisligil G.S. Mechanisms of TNF-alpha-induced insulin resistance. Exp. Clin. Endocrinol. Diabetes. 1999;107:119–125. doi: 10.1055/s-0029-1212086. [DOI] [PubMed] [Google Scholar]
- 20.Thong M.S.Y., van de Poll-Franse L., Hoffman R.M., Albertsen P.C., Hamilton A.S., Stanford J.L., Penson D.F. Diabetes mellitus and health-related quality of life in prostate cancer: 5-year results from the Prostate Cancer Outcomes Study. BJU Int. 2011;107:1223–1231. doi: 10.1111/j.1464-410X.2010.09861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zylla D., Gilmore G., Eklund J., Richter S., Carlson A. Impact of diabetes and hyperglycemia on health care utilization, infection risk, and survival in patients with cancer receiving glucocorticoids with chemotherapy. J. Diabetes Complicat. 2019;33:335–339. doi: 10.1016/j.jdiacomp.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Brown J.C., Zhang S., Ou F.-S., Venook A.P., Niedzwiecki D., Lenz H.-J., Innocenti F., O’Neil B.H., Shaw J.E., Polite B.N., et al. Diabetes and Clinical Outcome in Patients with Metastatic Colorectal Cancer: CALGB 80405 (Alliance) JNCI Cancer Spectr. 2020;4:pkz078. doi: 10.1093/jncics/pkz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ederaine S.A., Dominguez J.L., Harvey J.A., Mangold A.R., Cook C.B., Kosiorek H., Buras M., Coppola K., Karlin N.J. Survival and glycemic control in patients with co-existing squamous cell carcinoma and diabetes mellitus. Future Sci. OA. 2021;7:FSO683. doi: 10.2144/fsoa-2020-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X.-B., Ren G.-S. Diabetes mellitus and prognosis in women with breast cancer: A systematic review and meta-analysis. Medicine. 2016;95:e5602. doi: 10.1097/MD.0000000000005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwangbo Y., Kang D., Kang M., Kim S., Lee E.K., Kim Y.A., Chang Y.J., Choi K.S., Jung S.-Y., Woo S.M., et al. Incidence of Diabetes After Cancer Development: A Korean National Cohort Study. JAMA Oncol. 2018;4:1099–1105. doi: 10.1001/jamaoncol.2018.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin S.-Y., Lin C.-L., Chang S.-S., Chang Y.-H., Hsu W.-H., Lin C.-C., Kao C.-H. Risk of diabetes mellitus in patients with prostate cancer receiving injection therapy: A nationwide population-based propensity score-matched study. Int. J. Clin. Pract. 2021;75:e14416. doi: 10.1111/ijcp.14416. [DOI] [PubMed] [Google Scholar]
- 27.Santorelli M.L., Hirshfield K.M., Steinberg M.B., Rhoads G.G., Lin Y., Demissie K. Hormonal therapy for breast cancer and diabetes incidence among postmenopausal women. Ann. Epidemiol. 2016;26:436–440. doi: 10.1016/j.annepidem.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Xiao Y., Wang H., Tang Y., Yan J., Cao L., Chen Z., Shao Z., Mei Z., Jiang Z. Increased risk of diabetes in cancer survivors: A pooled analysis of 13 population-based cohort studies. ESMO Open. 2021;6:100218. doi: 10.1016/j.esmoop.2021.100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 28 January 2021)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 30.Zhang F., Wang K., Du P., Yang W., He Y., Li T., Mei Z. Risk of Stroke in Cancer Survivors: A Meta-analysis of Population-Based Cohort Studies. Neurology. 2021;96:e513–e526. doi: 10.1212/WNL.0000000000011264. [DOI] [PubMed] [Google Scholar]
- 31.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 34.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Khan N.F., Mant D., Carpenter L., Forman D., Rose P.W. Long-term health outcomes in a British cohort of breast, colorectal and prostate cancer survivors: A database study. Br. J. Cancer. 2011;105((Suppl. S1)):S29–S37. doi: 10.1038/bjc.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landis S.H., El-Hariry I.A., van Herk-Sukel M.P.P., van den Haak P., Janssen-Heijnen M.L.G., Penning-van Beest F.J.A., Herings R.M.C. Prevalence and incidence of acute and chronic comorbidity in patients with squamous cell carcinoma of the head and neck. Head Neck. 2012;34:238–244. doi: 10.1002/hed.21720. [DOI] [PubMed] [Google Scholar]
- 37.Danese M.D., O’Malley C., Lindquist K., Gleeson M., Griffiths R.I. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann. Oncol. 2012;23:1756–1765. doi: 10.1093/annonc/mdr486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H., Hodgson E., Watson L., Shukla A., Nelson J.J. Comorbidities and Concomitant Medication Use in Men with Prostate Cancer or High Levels of PSA Compared to Matched Controls: A GPRD Analysis. J. Cancer Epidemiol. 2012;2012:291704. doi: 10.1155/2012/291704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipscombe L.L., Chan W.W., Yun L., Austin P.C., Anderson G.M., Rochon P.A. Incidence of diabetes among postmenopausal breast cancer survivors. Diabetologia. 2013;56:476–483. doi: 10.1007/s00125-012-2793-9. [DOI] [PubMed] [Google Scholar]
- 40.Stålberg K., Svensson T., Granath F., Kieler H., Tholander B., Lönn S. Evaluation of prevalent and incident ovarian cancer co-morbidity. Br. J. Cancer. 2012;106:1860–1865. doi: 10.1038/bjc.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Herk-Sukel M.P.P., Shantakumar S., Overbeek L.I.H., van Boven H., Penning-Van Beest F.J.A., Herings R.M.C. Occurrence of comorbidities before and after soft tissue sarcoma diagnosis. Sarcoma. 2012;2012:402109. doi: 10.1155/2012/402109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chia V.M., O’Malley C.D., Danese M.D., Lindquist K.J., Gleeson M.L., Kelsh M.A., Griffiths R.I. Prevalence and incidence of comorbidities in elderly women with ovarian cancer. Gynecol. Oncol. 2013;129:346–352. doi: 10.1016/j.ygyno.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 43.Jordan J.H., Thwin S.S., Lash T.L., Buist D.S.M., Field T.S., Haque R., Pawloski P.A., Petersen H.V., Prout M.N., Quinn V.P., et al. Incident comorbidities and all-cause mortality among 5-year survivors of Stage I and II breast cancer diagnosed at age 65 or older: A prospective-matched cohort study. Breast Cancer Res. Treat. 2014;146:401–409. doi: 10.1007/s10549-014-3021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun L.-M., Chen H.-J., Liang J.-A., Li T.-C., Kao C.-H. Association of tamoxifen use and increased diabetes among Asian women diagnosed with breast cancer. Br. J. Cancer. 2014;111:1836–1842. doi: 10.1038/bjc.2014.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J., Wei W., Liu Z., Chen C., Cheng J., Gong Y., Wang C., Yu D., Sun S. Clinical pathological characteristics of breast cancer patients with secondary diabetes after systemic therapy: A retrospective multicenter study. Tumour Biol. 2015;36:6939–6947. doi: 10.1007/s13277-015-3380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ording A.G., Boffetta P., Garne J.P., Nyström P.M.W., Cronin-Fenton D., Frøslev T., Silliman R., Sørensen H.T., Lash T.L. Relative mortality rates from incident chronic diseases among breast cancer survivors—A 14 year follow-up of five-year survivors diagnosed in Denmark between 1994 and 2007. Eur. J. Cancer. 2015;51:767–775. doi: 10.1016/j.ejca.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Chang K.-H., Hwang W.-L., Muo C.-H., Hsu C.Y., Teng C.-L.J. Outcome and late effects among acute myeloid leukemia survivors: A nationwide population-based study. Support. Care Cancer. 2016;24:4993–5000. doi: 10.1007/s00520-016-3361-5. [DOI] [PubMed] [Google Scholar]
- 48.Chao C., Xu L., Bell E., Cooper R., Mueller L. Long-term Health Outcomes in Survivors of Childhood Cancer Diagnosed Between 1990 and 2000 in a Large US Integrated Health Care System. J. Pediatr. Hematol. Oncol. 2016;38:123–130. doi: 10.1097/MPH.0000000000000492. [DOI] [PubMed] [Google Scholar]
- 49.Crawley D., Garmo H., Rudman S., Stattin P., Häggström C., Zethelius B., Holmberg L., Adolfsson J., van Hemelrijck M. Association between duration and type of androgen deprivation therapy and risk of diabetes in men with prostate cancer. Int. J. Cancer. 2016;139:2698–2704. doi: 10.1002/ijc.30403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashibe M., Abdelaziz S., Al-Temimi M., Fraser A., Boucher K.M., Smith K., Lee Y.-C.A., Rowe K., Rowley B., Daurelle M., et al. Long-term health effects among testicular cancer survivors. J. Cancer Surviv. 2016;10:1051–1057. doi: 10.1007/s11764-016-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lowe K.A., Danese M.D., Gleeson M.L., Langeberg W.J., Ke J., Kelsh M.A. Racial and Ethnic Variability in the Prevalence and Incidence of Comorbidities Associated with Gastric Cancer in the United States. J. Gastrointest. Cancer. 2016;47:168–181. doi: 10.1007/s12029-016-9809-5. [DOI] [PubMed] [Google Scholar]
- 52.Singh S., Earle C.C., Bae S.J., Fischer H.D., Yun L., Austin P.C., Rochon P.A., Anderson G.M., Lipscombe L. Incidence of Diabetes in Colorectal Cancer Survivors. J. Natl. Cancer Inst. 2016;108:djv402. doi: 10.1093/jnci/djv402. [DOI] [PubMed] [Google Scholar]
- 53.Blackburn B.E., Ganz P.A., Rowe K., Snyder J., Wan Y., Deshmukh V., Newman M., Fraser A., Smith K., Herget K., et al. Aging-related disease risks among young thyroid cancer survivors. Cancer Epidemiol. Biomark. Prev. 2017;26:1695–1704. doi: 10.1158/1055-9965.EPI-17-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lega I.C., Pole J.D., Austin P.C., Lau C., Nathan P.C., Baxter N.N. Diabetes Risk in Childhood Cancer Survivors: A Population-Based Study. Can. J. Diabetes. 2018;42:533–539. doi: 10.1016/j.jcjd.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Ng H.S., Koczwara B., Roder D.M., Niyonsenga T., Vitry A.I. Comorbidities in Australian women with hormone-dependent breast cancer: A population-based analysis. Med. J. Aust. 2018;208:24–28. doi: 10.5694/mja17.00006. [DOI] [PubMed] [Google Scholar]
- 56.Ng H.S., Koczwara B., Roder D., Vitry A. Development of comorbidities in men with prostate cancer treated with androgen deprivation therapy: An Australian population-based cohort study. Prostate Cancer Prostatic Dis. 2018;21:403–410. doi: 10.1038/s41391-018-0036-y. [DOI] [PubMed] [Google Scholar]
- 57.Dhopeshwarkar N., Iqbal S., Wang X., Salas M. A Retrospective Study of Comorbidities and Complications in Elderly Acute Myeloid Leukemia Patients in the United States. Clin. Lymphoma Myeloma Leuk. 2019;19:e436–e456. doi: 10.1016/j.clml.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 58.Hawkins M.L., Blackburn B.E., Rowe K., Snyder J., Deshmukh V.G., Newman M., Fraser A., Smith K., Herget K., Ganz P.A., et al. Endocrine and Metabolic Diseases Among Colorectal Cancer Survivors in a Population-Based Cohort. J. Natl. Cancer Inst. 2020;112:78–86. doi: 10.1093/jnci/djz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ng H.S., Vitry A., Koczwara B., Roder D., McBride M.L. Patterns of comorbidities in women with breast cancer: A Canadian population-based study. Cancer Causes Control. 2019;30:931–941. doi: 10.1007/s10552-019-01203-0. [DOI] [PubMed] [Google Scholar]
- 60.Accordino M.K., Wright J.D., Buono D., Lin A., Huang Y., Neugut A.I., Hillyer G.C., Hershman D.L. Incidence and Predictors of Diabetes Mellitus after a Diagnosis of Early-Stage Breast Cancer in the Elderly Using Real-World Data. Breast Cancer Res. Treat. 2020;183:201–211. doi: 10.1007/s10549-020-05756-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bigelow E.O., Blackford A.L., Eytan D.F., Eisele D.W., Fakhry C. Burden of comorbidities is higher among elderly survivors of oropharyngeal cancer compared with controls. Cancer. 2020;126:1793–1803. doi: 10.1002/cncr.32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chao C., Bhatia S., Xu L., Cannavale K.L., Wong F.L., Huang P.-Y.S., Cooper R., Armenian S.H. Chronic Comorbidities Among Survivors of Adolescent and Young Adult Cancer. J. Clin. Oncol. 2020;38:3161–3174. doi: 10.1200/JCO.20.00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim S., Park J., Chen Y., Rowe K., Snyder J., Fraser A., Smith K., Deshmukh V.G., Newman M., Herget K., et al. Long-term diabetes risk among endometrial cancer survivors in a population-based cohort study. Gynecol. Oncol. 2020;156:185–193. doi: 10.1016/j.ygyno.2019.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markus E., Trestman S., Cohen Y., Angel Y., Sofer Y., Mittelman M., Avivi I., Stern N., Izkhakov E. Components of metabolic syndrome in patients with multiple myeloma and smoldering multiple myeloma. BMC Cancer. 2020;20:489. doi: 10.1186/s12885-020-06976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang C.-Y., Shih S.-R., Huang K.-C. Increasing risk of diabetes mellitus in postmenopausal women with newly diagnosed primary breast cancer. J. Diabetes Investig. 2020;11:490–498. doi: 10.1111/jdi.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jo A., Scarton L., O’Neal L.J., Larson S., Schafer N., George T.J., Munoz Pena J.M. New onset of type 2 diabetes as a complication after cancer diagnosis: A systematic review. Cancer Med. 2021;10:439–446. doi: 10.1002/cam4.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ye F., Wen J., Yang A., Wang Y., Li N., Yu P., Wei W., Tang J. The Influence of Hormone Therapy on secondary diabetes mellitus in Breast Cancer: A Meta-analysis. Clin. Breast Cancer. 2021;22:e48–e58. doi: 10.1016/j.clbc.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 68.Sarwar N., Gao P., Seshasai S.R.K., Gobin R., Kaptoge S., Di Angelantonio E., Ingelsson E., Lawlor D.A., Selvin E., Stampfer M., et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saylor P.J., Keating N.L., Freedland S.J., Smith M.R. Gonadotropin-releasing hormone agonists and the risks of type 2 diabetes and cardiovascular disease in men with prostate cancer. Drugs. 2011;71:255–261. doi: 10.2165/11588930-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zöller B., Ji J., Sundquist J., Sundquist K. Risk of coronary heart disease in patients with cancer: A nationwide follow-up study from Sweden. Eur. J. Cancer. 2012;48:121–128. doi: 10.1016/j.ejca.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 71.International Diabetes Federation . IDF Diabetes Atlas. 9th ed. International Diabetes Federation; Brussels, Belgium: 2019. [Google Scholar]
- 72.Basaria S. Androgen deprivation therapy, insulin resistance, and cardiovascular mortality: An inconvenient truth. J. Androl. 2008;29:534–539. doi: 10.2164/jandrol.108.005454. [DOI] [PubMed] [Google Scholar]
- 73.Pitteloud N., Hardin M., Dwyer A.A., Valassi E., Yialamas M., Elahi D., Hayes F.J. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J. Clin. Endocrinol. Metab. 2005;90:2636–2641. doi: 10.1210/jc.2004-2190. [DOI] [PubMed] [Google Scholar]
- 74.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 75.Fearon K.C.H., Glass D.J., Guttridge D.C. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 76.Porporato P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5:e200. doi: 10.1038/oncsis.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shoelson S.E., Lee J., Goldfine A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu M., Zhang X., Lu F., Fang L. Depression and Risk for Diabetes: A Meta-Analysis. Can. J. Diabetes. 2015;39:266–272. doi: 10.1016/j.jcjd.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 79.Tabák A.G., Jokela M., Akbaraly T.N., Brunner E.J., Kivimäki M., Witte D.R. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: An analysis from the Whitehall II study. Lancet. 2009;373:2215–2221. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hawkins M.L., Buys S.S., Gren L.H., Simonsen S.E., Kirchhoff A.C., Hashibe M. Do cancer survivors develop healthier lifestyle behaviors than the cancer-free population in the PLCO study. J. Cancer Surviv. 2017;11:233–245. doi: 10.1007/s11764-016-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jung H.-S., Myung S.-K., Kim B.-S., Seo H.G. Metabolic syndrome in adult cancer survivors: A meta-analysis. Diabetes Res. Clin. Pract. 2012;95:275–282. doi: 10.1016/j.diabres.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 82.Munshi A., Pandey M.B., Durga T., Pandey K.C., Bahadur S., Mohanti B.K. Weight loss during radiotherapy for head and neck malignancies: What factors impact it. Nutr. Cancer. 2003;47:136–140. doi: 10.1207/s15327914nc4702_5. [DOI] [PubMed] [Google Scholar]
- 83.Byerley L.O., Heber D., Bergman R.N., Dubria M., Chi J. Insulin action and metabolism in patients with head and neck cancer. Cancer. 1991;67:2900–2906. doi: 10.1002/1097-0142(19910601)67:11<2900::AID-CNCR2820671132>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 84.Tsilidis K.K., Kasimis J.C., Lopez D.S., Ntzani E.E., Ioannidis J.P.A. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 85.Rücker G., Schwarzer G., Carpenter J.R., Schumacher M. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med. Res. Methodol. 2008;8:79. doi: 10.1186/1471-2288-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riley R.D., Higgins J.P.T., Deeks J.J. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 87.IntHout J., Ioannidis J.P.A., Rovers M.M., Goeman J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.