Abstract
Systemic and superficial fungal infections are a major problem among immunocompromised patients with hematological malignancy. A double-blind, double-placebo, randomized, multicenter trial was performed to compare the efficacy and safety of itraconazole oral solution (2.5 mg/kg of body weight twice a day) with amphotericin B capsules (500 mg orally four times a day) for prophylaxis of systemic and superficial fungal infection. Prophylactic treatment was initiated on the first day of chemotherapy and was continued until the end of the neutropenic period (>0.5 × 109 neutrophils/liter) or up to a maximum of 3 days following the end of neutropenia, unless a systemic fungal infection was documented or suspected. The maximum treatment duration was 56 days. In the intent-to-treat population, invasive aspergillosis was noted in 5 (1.8%) of the 281 patients assigned to itraconazole oral solution and in 9 (3.3%) of the 276 patients assigned to oral amphotericin B; of these, 1 and 4 patients died, respectively. Proven systemic fungal infection (including invasive aspergillosis) occurred in 8 patients (2.8%) who received itraconazole, compared with 13 (4.7%) who received oral amphotericin B. Itraconazole significantly reduced the incidence of superficial fungal infections as compared to oral amphotericin B (2 [1%] versus 13 [5%]; P = 0.004). Although the incidences of suspected fungal infection (including fever of unknown origin) were not different between the groups, fewer patients were administered intravenous systemic antifungals (mainly intravenous amphotericin B) in the group receiving itraconazole than in the group receiving oral amphotericin B (114 [41%] versus 132 [48%]; P = 0.066). Adequate plasma itraconazole levels were achieved in about 80% of the patients from 1 week after the start of treatment. In both groups, the trial medication was safe and well tolerated. Prophylactic administration of itraconazole oral solution significantly reduces superficial fungal infection in patients with hematological malignancies and neutropenia. The incidence of proven systemic fungal infections, the number of deaths due to deep fungal infections, and the use of systemic antifungals tended to be lower in the itraconazole-treated group than in the amphotericin B-treated group, without statistical significance. Itraconazole oral solution is a broad-spectrum systemic antifungal agent with prophylactic activity in neutropenic patients, especially for those at high risk of prolonged neutropenia.
Among neutropenic patients with hematological malignancy, infections remain a major problem. Nowadays, the majority of bacterial infections can be treated or prevented successfully by using broad-spectrum antibiotics. Fungal infections, on the other hand, remain a common cause of morbidity and mortality in patients at risk of neutropenia (1). Moreover, during the past two decades, the incidence of fungal infections in this patient population has increased dramatically, the major pathogens being Candida spp. and Aspergillus spp.
Systemic fungal infections remain the major cause of death in neutropenic patients with hematological malignancy, and optimal management of these opportunistic infections is a major challenge for clinicians. Among the measures that can be taken to prevent fungal infections, environmental and hygienic control and elimination of well-known sources of infection are important.
Early studies of the prophylactic use of oral drugs like amphotericin B, nystatin, clotrimazole, miconazole, and ketoconazole yielded unsatisfactory results because of poor absorption, a narrow spectrum, poor compliance, and toxicity (4; E. J. Bow, M. Laverdiere, N. Lussier, C. Rotstein, and S. Ioannou, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. LM-88, p. 381, 1997). Neutropenic patients with persistent fever refractory to antibiotic treatment are often treated empirically with intravenous amphotericin B, despite its toxicity. Prophylactic treatment with fluconazole, an azole antifungal agent (15), is widely used nowadays. However, fluconazole, either oral or intravenous, offers no protection against Aspergillus spp., and several pathogenic Candida spp. are either resistant (C. krusei) or less sensitive (C. glabrata) to this compound (8, 17, 18). Intranasal administration of amphotericin B did not show a significant benefit over placebo as prophylaxis against aspergillosis, and although the liposomal formulations of amphotericin B seem to be safer, the clinical efficacy has not yet been proven, and few data are available with regard to prophylaxis (14). Moreover, prophylactic treatment with these liposomal formulations is expensive, thereby limiting its potential as a commonly used prophylactic agent (6). Itraconazole is a broad-spectrum triazole antifungal agent currently available as a capsule formulation for the treatment of systemic mycoses such as aspergillosis, candidosis, cryptococcosis, histoplasmosis, and several endemic mycoses; it has demonstrated clinical activity against Aspergillus infections (3). The newer oral solution formulation (10% itraconazole in a 40% hydroxypropyl-β-cyclodextrin [HDβCD] solution) has overcome the limitations of the capsule formulation in that both adequate plasma drug concentrations are reached in the neutropenic host (12, 13) and it can be administered with or without food (2, 16). The prophylactic use of itraconazole oral solution offers an alternative at least as effective as fluconazole (10). Treatment with this cyclodextrin-containing solution is well tolerated (11).
This trial was initiated to compare the efficacy of itraconazole oral solution with nonabsorbable oral amphotericin B in the prevention of systemic and superficial fungal infections in patients with hematological malignancy and profound neutropenia. Furthermore, the frequency of and the time to initiation of intravenous systemic antifungals (e.g., amphotericin B) were compared.
MATERIALS AND METHODS
Patients.
Male or female patients of at least 18 years of age, with a life expectancy of at least 14 days, and who had one of the following underlying conditions were eligible for this trial: acute leukemia scheduled for remission or induction or consolidation or reinduction chemotherapy; autologous bone marrow transplantation; myelodysplastic syndrome and scheduled to receive leukemia-like cytostatic therapy; chemotherapy for the blast crisis of chronic myelogenous leukemia; or lymphoma or myeloma and undergoing aggressive chemotherapy (e.g., platinum-based salvage regimen). In addition, their neutrophil count was expected to be <500/μl for at least 14 days, and they were not to have signs or symptoms of fungal infection (fungal colonization was allowed). Informed consent was obtained from each patient or from the patient's relative, guardian, or representative. Patients were excluded from the trial if they had been diagnosed with a proven or suspected deep fungal infection (including all cases without mycological sampling) during previous episodes of neutropenia, presented with a chest X ray suggestive of fungal infection, or with a fever of unknown origin (>38.5°C, rectal temperature), had received systemic antifungal therapy within 2 weeks before trial entry or topical intraoral antifungal therapy within 1 week before trial entry, were receiving terfenadine, astemizole, phenytoin, phenobarbital, rifampin, warfarin, loratadine, oral midazolam, triazolam, or cisapride or had received these medications in the 2-week period prior to trial entry or were expected to receive granulocyte-macrophage-colony-stimulating factor (GM-CSF), M-CSF, interleukin 3 (IL-3) or other growth factors (except for G-CSF, which was allowed). Women who were known to be pregnant or breast feeding, or who were of childbearing potential and not practicing adequate birth control were also excluded, as were patients who were unable to take oral medication, had liver disease defined as liver enzyme levels (alanine aminotransferase [ALAT] or aspartate aminotransferase [ASAT]) ≥4 times the upper normal limit at trial entry, were receiving investigational drugs other than anticancer regimens concurrently or within 1 month prior to trial entry, had previously shown hypersensitivity to azole antifungals or to oral amphotericin B, were known to be human immunodeficiency virus positive, had profuse diarrhea on presentation, or were entered into the trial previously.
Independent Ethics Committees approved the trial protocol and the amendments.
Treatments.
Patients were, on the basis of a computer-generated randomization list, grouped in blocks of four, assigned to receive double-blind, double-placebo treatment with one of the two active trial substances. Because of the double-blind, double-placebo nature of the trial, patients received both the solution and the capsule regimen: they received either active itraconazole solution together with placebo capsules or active amphotericin B capsules together with placebo solution. Prophylaxis was started on the first day of chemotherapy. Itraconazole oral solution (2.5 mg/kg of body weight twice a day) was provided as 100-ml bottles containing 100 mg of itraconazole per 10 ml of HPβCD solution or HPβCD-identical placebo. Oral amphotericin B (500 mg four times a day) was administered as 250-mg (Fungizone) capsules or identical placebo capsules. The trial medications were preferably to be taken without a meal. The oral solution was to remain in contact with the oral mucosa for at least 10 s and then was swallowed; it was not to be diluted.
Treatment was continued up to the end of neutropenia or up to a maximum of 3 days following the end of neutropenia, unless a trial end point had been reached earlier. The maximum treatment duration was 8 weeks (56 days).
Effectiveness assessments.
The leukocyte count and the neutrophil count were recorded at baseline and during prophylaxis, daily, or at least three times weekly. All changes in urinary and intravenous catheters were to be recorded. Removed catheters were examined for the presence of yeast or molds in case of a fungal trial end point. Identification of the isolated yeast or mold was always to be done up to the species level. A chest X ray was to be carried out at baseline and weekly thereafter during prophylaxis. Signs and symptoms potentially attributable to superficial or deep fungal infection were recorded. Both during and after prophylaxis, additional tests (biopsy, computed tomography [CT] scan, chest X ray, and bronchoalveolar lavage [BAL]) were to be carried out whenever possible and acceptable in terms of the clinical condition of the patient. In addition, BAL was to be carried out in case of pulmonary infiltrates. In the case of the CT scan, high-resolution scanning was to be utilized.
Trial end point.
Prophylactic treatment was stopped at the end of the neutropenic period, when a fungal infection trial end point was reached earlier, or when the investigator considered it, for safety reasons, in the best interest of the patient that or he/she be withdrawn. The end of neutropenia was defined as at least one neutrophil count higher than 500/μl. The fungal infection trial end point was classified according to one of the following criteria.
(i) Proven deep fungal infections were defined as positive histology on biopsy from deep tissue, at least one positive blood culture for yeasts, or clinical signs and radiological lesions in combination with the presence of Aspergillus spp. or other filamentous fungi in BAL fluid.
(ii) Suspected deep fungal infections were defined as clinical signs and symptoms (with or without radiological lesions) with fever of unknown origin unresponsive to broad-spectrum antibacterials, highly suggestive radiological lesions (X ray and CT scan) for deep fungal infection without mycological evidence by culture or histology (e.g., hepatosplenic candidosis and some types of pulmonary aspergillosis), or clinical signs and symptoms (with or without radiological lesions) not highly suggestive of fungal infection, but associated with suggestive fungal isolation (e.g., from sputum or nasal cavities for aspergillosis).
(iii) Fever of unknown origin requiring empiric treatment with intravenous amphotericin B was defined as fever of unknown origin for at least 48 h on broad-spectrum antibiotics without clinical signs and symptoms.
(iv) Superficial fungal infections were defined as clinical signs and symptoms of oral, esophageal, or vaginal candidosis combined with positive mycology at the site of infection; documented oral or vaginal candidosis only resulted in stopping the trial prophylaxis in case systemic antifungal treatment was required.
The prophylaxis end point was recorded when the trial medication was discontinued. The postprophylaxis end point was recorded within 4 weeks after the prophylaxis end point. At this administrative visit, all available evidence for further characterization of a fungal infection was collected. If the postprophylaxis end point was not recorded, the prophylaxis end point and the postprophylaxis end point were considered to be identical.
Assessments by the independent Expert Committee.
The Expert Committee classification was a reclassification of the investigator's postprophylaxis end point. The Expert Committee, which was composed of three members with specific expertise on fungal infections during neutropenia and not participating into the trial, was provided with copies of all test results used to substantiate a diagnosis, the original CT scan or X rays providing evidence of fungal infection, as well as data on autopsy or immediate postmortem biopsies in order to enable them to reevaluate the classification of all trial end points prior to analysis. This final overall assessment was carried out blinded for the prophylactic trial medication.
Plasma drug concentrations.
A 5-ml blood sample was taken immediately before the morning intake once weekly during prophylaxis and at the end of prophylaxis for the measurement of plasma itraconazole and hydroxyitraconazole concentrations. Date and hour of sampling and of the intake of study medication in the 24-h period before sampling were recorded. Concentrations of itraconazole and hydroxyitraconazole in plasma were determined by high-performance liquid chromatography with UV detection. The quantification limit of the assay was 10 ng/ml.
Safety assessments.
The date of onset and duration of any adverse event that had occurred during the trial period (including any intercurrent disease) were noted, as well as their intensity, frequency, action taken, presumed drug relatedness, and outcome. The occurrence of serious adverse events was documented. Laboratory parameters (potassium, sodium, calcium, ALAT, ASAT, alkaline phosphatase, γ-glutamyltransferase, total cholesterol, creatinine, urea, and total bilirubin) were determined at selection and on a twice-weekly basis during the trial.
Statistical analysis.
The statistical analysis of all data was performed by the Janssen Research Foundation. The primary population was the intent-to-treat population, which included all randomized patients with at least one drug administration. The subgroup of patients with neutropenia of <0.5 × 109/liter for at least 14 days (i.e., high-risk patients for fungal infection) was also analyzed. The comparability among the trial groups was evaluated relative to demographic and baseline variables.
The incidence of invasive aspergillosis according to the classification of the Expert Committee was the primary efficacy parameter. A reduction from 5% in the amphotericin B arm to 1% in the itraconazole arm was postulated. Therefore, data from 250 neutropenic patients were required in each group to achieve the 5% significance level (two sided) with 80% power. Based on a 20% dropout rate, 640 subjects were to be randomized. An interim analysis was planned for the 400 patients first randomized. Based on the results of this blinded interim analysis, it became evident that the required number of documented cases of aspergillosis could not be obtained by recruiting all 640 patients as stipulated in the sample size calculation. Therefore, the trial was stopped at the time of the interim analysis, when 559 patients were randomized to treatment.
Dichotomous parameters were analyzed with the Pearson chi-square test, time-to-an-event parameters were graphically presented using the Kaplan-Meier estimate and compared between the treatment groups by using the log-rank test for censored data, and continuous and ordinal categorical data were compared by means of the Mann-Whitney U test. Additionally, a Cox proportional hazards model with factors for treatment and country was applied for time to first use of amphotericin B. To investigate possible country effects, the Cochran-Mantel-Haenszel test controlling for country was applied.
Descriptive statistics (mean ± standard deviation, mean, minimum to maximum) were calculated for the minimum concentration of drug in serum of itraconazole and hydroxyitraconazole for the plasma samples at specified intervals after the start of treatment.
The type and incidence of adverse events were tabulated per treatment group. Special attention was paid to drug-related adverse events, serious adverse events, and other significant adverse events (i.e., those leading to the patient's withdrawal). For the clinical laboratory data, descriptive statistics and pre- versus within- and posttreatment cross-tabulations (with classes for below, within, and above the normal range of the particular laboratory) were generated for all tests performed. In addition, important abnormalities were tabulated.
RESULTS
Investigators from 49 centers in seven different countries (Austria, Belgium, France, Greece, The Netherlands, Portugal, and Spain) recruited 557 patients (from July 1994 to April 1997) who were randomized to treatment: 281 received itraconazole, and 276 were given amphotericin B (intent-to-treat population).
There were no significant intergroup differences in baseline characteristics; data on sex, age, underlying disease, therapy of the underlying disease, duration of neutropenia, and duration of treatment are summarized in Table 1. The median (minimum to maximum) durations of neutropenia were 18 (0 to 84) days in itraconazole-treated patients and 20 (0 to 88) days in amphotericin B-treated patients. In line with these results, the median durations of treatment were 19 (1 to 56) days in the itraconazole group and 18 (2 to 56) days in the amphotericin B group. The main underlying pathology was leukemia (71% of the patients in each group).
TABLE 1.
Patient and disease characteristics at the start of treatment, duration of neutropenia, and duration of prophylactic treatment
| Intent-to-treat population parameter | Result with:
|
|
|---|---|---|
| Itraconazole | Amphotericin B | |
| Patient and disease characteristics at the start of treatment | ||
| No. of patients randomized (male/female) | 281 (167/114) | 276 (147/129) |
| Age; median (minimum–maximum) yr | 48 (15–75) | 49.5 (17–82) |
| Underlying disease; n (%) | ||
| Acute myeloid leukemia | 166 (59) | 153 (55) |
| Acute lymphatic leukemia | 33 (12) | 42 (15) |
| Other | 83 (29) | 81 (29) |
| Therapy of underlying disease; n (%) | ||
| Chemotherapy | 232 (89) | 221 (88) |
| Bone marrow transplant | 14 (5) | 13 (5) |
| Other | 14 (5) | 16 (6) |
| Not recorded | 21 | 26 |
| Status of disease; n (%) | ||
| First treatment | 188 (79) | 184 (76) |
| Other | 51 (21) | 58 (24) |
| Not recorded | 42 | 34 |
| Duration of neutropenia and duration of prophylactic treatment | ||
| Duration of neutropenia of <0.5 × 109/liter; median (minimum–maximum) days | 18 (0–84) | 20 (0–88) |
| Use of G-CSF | 83 | 81 |
| Duration of prophylactic treatment; median (minimum–maximum) days | 19 (1–56) | 18 (2–56) |
Invasive aspergillosis.
Invasive aspergillosis was documented in five (1.8%) itraconazole-treated patients and in nine (3.3%) amphotericin B-treated patients (P = 0.264) (Table 2); of these, only one patient in the itraconazole group compared with four patients in the amphotericin B group died (Table 3). The pathogens that were identified were Aspergillus fumigatus in all cases, except for one in the amphotericin B group (Aspergillus terreus) (Table 3).
TABLE 2.
Kind and incidence of fungal postprophylaxis end points according to the classification of the Expert Committee
| Intent-to-treat population | No. (% of patients with end point
|
Pa | |
|---|---|---|---|
| Itraconazole (n = 281) | Amphotericin B (n = 276) | ||
| Invasive aspergillosis | 5 (1.8)b | 9 (3.3)c | 0.264 |
| Proven deep fungal infection (including invasive aspergillosis) | 8 (2.8)b | 13 (4.7)d | 0.248 |
| Superficial fungal infection | 2 (0.7) | 13 (4.7) | 0.004 |
| Total proven fungal infection | 10 (3.6) | 26 (9.4) | 0.005 |
| Suspected deep fungal infection (including fever of unknown origin) | 83 (29.5) | 80 (29.0) | 0.886 |
Intergroup comparison, chi-square test.
One died.
Four died.
Five died.
TABLE 3.
Pathogens identified in patients with proven deep fungal infections after prophylactic treatment with itraconazole or amphotericin B
| Pathogens | No. of patients with pathogen detected posttreatment
|
|
|---|---|---|
| Itraconazole | Amphotericin B | |
| Aspergillus fumigatus | 5a | 8b |
| Aspergillus terreus | 0 | 1a |
| Candida albicans | 1c | 0 |
| Candida glabrata | 1 | 0 |
| Candida krusei | 0 | 1c |
| Candida parapsilosis | 0 | 1c |
| Cryptococcus neoformans | 1 | 0 |
| Fusarium nygamai | 0 | 1c |
| Geotrichum spp. | 0 | 1ac |
| Total | 8 | 13 |
One died.
Three died.
Detected in blood.
In the amphotericin B group, all nine patients were positive for Aspergillus spp. in BAL fluid, whereas this was the case in only two out of five patients in the itraconazole group. Of the three remaining itraconazole-treated patients, one had positive Aspergillus cultures from the nose and sputum and was diagnosed by the Expert Committee as having invasive pulmonary aspergillosis (biopsy and CT scans), one patient had positive cultures obtained from nose and stools (X ray, CT scan, and biopsy), and the remaining patient was graded by the Expert Committee as having a proven aspergillosis infection based on a very typical halo sign on serial X rays and the CT scan. In the majority of the cases, classification of aspergillosis was further supported by CT scan, X rays, or both.
Other fungal infections.
Apart from invasive aspergillosis, other proven deep fungal infections were noted in three (1.1%) patients in the itraconazole group and in four (1.4%) patients in the amphotericin B group (Table 2; P is not significant); of these, none of the itraconazole patients and one amphotericin B patient died (Table 3). In the amphotericin B group, all four fungal infections were documented in the blood. (Candida krusei, Candida parapsilosis, Geotrichium spp., and Fusarium nygamai were each noted in one patient.) In the itraconazole group, one patient had a Candida albicans infection of the blood, one had a skin biopsy positive for Cryptococcus neoformans (maculopapular eruptions), and one had a positive culture for Candida glabrata (pus and an abscess in the right iliac fossa).
The incidence of superficial fungal infections was significantly higher in the amphotericin B group than in the itraconazole group: 13 (5%) versus 2 (1%; P = 0.004). Also, the total number of patients with a proven fungal infection (i.e., proven deep fungal infection including invasive aspergillosis and superficial fungal infection) was significantly higher in the amphotericin B group (26 [9.4%]) than in the itraconazole group (10 [3.6%]) (P = 0.005). Suspected deep fungal infections (including fever of unknown origin) were noted in a comparable number of patients in the two groups: i.e., 83 (30%) in the itraconazole group and 80 (29%) in the amphotericin B group.
Initiation of systemic antifungal treatment.
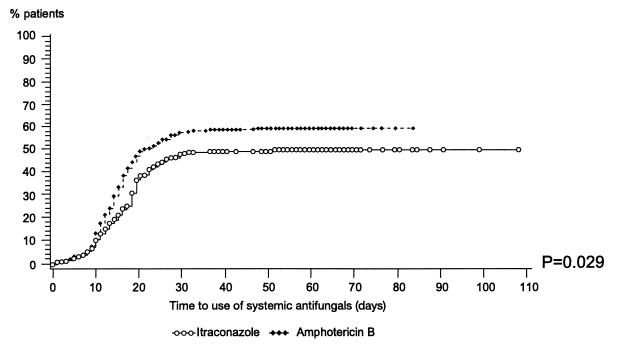
During the trial, systemic antifungal treatments (intravenous amphotericin B [any formulation], fluconazole, and voriconazole) were initiated in 114 (41%) itraconazole-treated patients and in 132 (48%) oral amphotericin B-treated patients. The Kaplan-Meier analysis of time to an event showed that, in the first quartile of patients, the times to first administration of systemic antifungals were 18 days in the itraconazole group and 15 days in the amphotericin B group; this intergroup difference tended to be significant (P = 0.055). In the analysis of high-risk patients, these times were 18 and 14 days, respectively; this intergroup difference was statistically significant (P = 0.029) (Fig. 1).
FIG. 1.
Time to use of systemic antifungals for eligible patient analysis (neutrophil count of <0.5 × 109/liter for at least 14 days).
According to the Cox proportional hazard model, the instantaneous risk of administration of amphotericin B was 33% lower in the itraconazole group than in the amphotericin B group (i.e., risk ratio, 1.326; 95% confidence limits, 0.998 and 1.761; P = 0.052).
Survival.
The overall mortalities were 6% (n = 18) in the itraconazole group and 8% (n = 23) in the oral amphotericin B group (P = 0.384). Of those, five patients died with a proven deep fungal infection in the oral amphotericin B group versus only one in the itraconazole group.
Plasma itraconazole concentrations.
Mean concentrations of itraconazole in plasma were 512 ng/ml after 1 week of treatment and increased further to 764, 1,028, and 1,253 ng/ml after 2, 3, and 4 weeks of treatment, respectively (Table 4). The efficacy levels were achieved shortly after initiation of treatment. From 1 week after the start of treatment onwards, about 80% of the patients had predose concentrations of itraconazole in plasma greater than 250 ng/ml, which is considered to be the minimum level required for efficacy. After 4 weeks of treatment, these levels were attained in 96% of the patients (Table 4).
TABLE 4.
Plasma itraconazole and hydroxyitraconazole concentrations
| Time (n) | Itraconazole
|
Hydroxyitraconazole median concn; ng/ml (minimum; maximum) | |
|---|---|---|---|
| Median concn; ng/ml (minimum; maximum) | Concn in plasma >250 ng/ml (% of total) | ||
| After 1 wk (122) | 472 (NQa; 2,359) | 77.0 | 1,136 (NQ; 2,708) |
| After 2 wk (88) | 690 (NQ; 2,236) | 84.1 | 1,411 (NQ; 3,488) |
| After 3 wk (55) | 961 (59; 3,069) | 87.3 | 1,694 (NQ; 4,108) |
| After 4 wk (30) | 1,225 (NQ; 3,460) | 86.7 | 2,018 (NQ; 4,207) |
| After >4 wk (25) | 1,957 (193; 5,165) | 96.0 | 2,677 (695; 6,762) |
NQ, not quantifiable.
Two proven fungal infections were diagnosed in the 20% group of patients who did not have a plasma drug level >250 ng/ml at the week 1 evaluation. Five proven infections were diagnosed in the 80% group of patients who did reach the 250-ng/ml level at week 1. No valid statistical conclusion can be made with regard to this interrelationship.
Subanalysis of high-risk patients.
As outlined in the protocol, we analyzed the subgroup of all patients with neutropenia of <0.5 × 109/liter for at least 14 days (201 itraconazole patients and 186 amphotericin B patients). Due to the combination of depth and duration of neutropenia, this group is considered to be a high-risk population for fungal infection.
The analysis confirmed the findings of the main analysis: all cases of invasive aspergillosis (i.e., five [2.5%] in the itraconazole group and nine [4.8%] in the amphotericin B group) were identified in this group (Table 5; P = 0.216); also, all other cases of proven deep fungal infections (three in each group), except for one in the amphotericin B group, were identified in this group. Overall, the incidence of all proven fungal infections (i.e., proven deep fungal infections, including aspergillosis and superficial fungal infections) was significantly lower in the itraconazole group (10 patients [5.0%]) than in the amphotericin B group (23 patients [12.4%]; P = 0.009). Significantly fewer itraconazole patients (2 [1%]) than amphotericin B patients (11 [6%]) developed superficial fungal infections (P = 0.007). The incidences of suspected deep fungal infections were similar in the two groups (37% for itraconazole and 39% for amphotericin B versus 29.5 and 29%, respectively, in the total patient sample).
TABLE 5.
Kind and incidence of fungal postprophylaxis end points according to the classification of the Expert Committee for eligible patients
| Eligible patient analysis parametera | No. % of patients with end point posttreatment
|
Pb | |
|---|---|---|---|
| Itraconazole (n = 201) | Amphotericin B (n = 186) | ||
| Invasive aspergillosis | 5 (2.5)c | 9 (4.8)d | 0.216 |
| Proven deep fungal infection (including invasive aspergillosis) | 8 (4.0)c | 12 (6.5)e | 0.273 |
| Superficial fungal infection | 2 (1.0) | 11 (5.9) | 0.007 |
| Total proven fungal infection | 10 (5.0) | 23 (12.4) | 0.009 |
| Suspected deep fungal infection (including fever of unknown origin) | 75 (37.3) | 73 (39.2) | 0.696 |
Patients were classified as eligible with a neutrophil count of <0.5 × 109/liter for at least 14 days.
Intergroup comparison, chi-square test.
One died.
Four died.
Five died.
Safety.
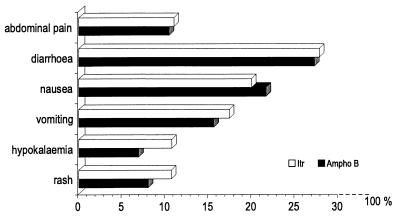
Adverse events were reported in 222 (79%) itraconazole-treated patients and in 205 (74%) amphotericin B-treated patients. The most frequently reported adverse events were of gastrointestinal origin (Fig. 2). Rash (17 and 13%, respectively) and hypokalemia (11% for itraconazole and 7% for amphotericin B) were the other most frequently reported adverse events. The incidences of adverse events were similar in the two groups.
FIG. 2.
Most frequently reported adverse events for the patients in this study treated with itraconazole (Itr) or amphotericin B (Ampho B).
The investigators considered one or more adverse events to be definitely drug related in 13 (5%) patients in each group. The main drug-related adverse events were local intolerability (five patients in each group), nausea (two itraconazole-treated patients and seven amphotericin B-treated patients), and vomiting (three and six patients, respectively).
Overall, comparable numbers of patients in both treatment groups permanently stopped the treatment because of adverse events (including death): i.e., 75 (27%) in the itraconazole group and 78 (28%) in the oral amphotericin B group; the median (minimum to maximum) durations of treatment in these patients were 10 (1 to 45) days in the itraconazole group and 12 (2 to 28) days in the amphotericin B group. Nausea (9 and 11%, respectively) and vomiting (8 and 7%, respectively) were the most frequently reported adverse events leading to withdrawal.
Serious adverse events were reported in 26 (9%) patients in the itraconazole group and in 32 (12%) of the patients in the amphotericin B group. Of these, 18 (6%) itraconazole-treated patients and 23 (8%) amphotericin B-treated patients died. The most frequently reported adverse events with the outcome of death were aggravated underlying condition (four and five patients, respectively), circulatory failure (four and two patients, respectively), sepsis (three subjects in each group), diarrhea (one patient in the itraconazole group and four in the amphotericin B group), gastrointestinal hemorrhage (four amphotericin B-treated patients), fungal infection (two patients in each group), and respiratory failure (one patient in the itraconazole group and three in the amphotericin B group). Changes in biochemical laboratory parameters were comparable in the two groups. There were relatively many important laboratory abnormalities, but considering this patient population, these could be accounted for.
DISCUSSION
The current trial was set up to establish the potential role of the broad-spectrum azole antifungal itraconazole in preventing fungal infections in patients with a hematological malignancy including autologous bone marrow transplant patients expected to be neutropenic for at least 14 days. Attitudes towards antifungal prophylaxis are different between countries and between centers. As a remnant of the gastrointestinal decontamination approach, the still widely used oral nonabsorbable agent amphotericin B was selected as the comparative agent.
Collection of adequately documented fungal end point data has been recognized as a serious difficulty for prophylactic evaluation, since confirmation is often obtained only after initiation of intravenous amphotericin B or at autopsy. Therefore, the 4-week postprophylaxis visit was considered essential to collect additional postprophylaxis evidence of suspected and deep fungal infection. An overall and blinded assessment by the Expert Committee harmonized the interpretation for a limited number of difficult cases.
The incidences of aspergillosis (1.8%) and proven deep fungal infection (2.8%, including invasive aspergillosis) observed in the itraconazole recipients were very similar to those observed previously. In two large prophylactic trials with itraconazole in neutropenic patients (9, 10), Aspergillus infection has been reported in 0 and 2% and proven deep fungal infections have been reported in 0.5 and 2.5% of the itraconazole-treated patients, respectively. In the present and both previous trials combined, a clinically relevant reduction in the number of cases of deep fungal infection including Aspergillus infection was obtained in favor of itraconazole oral solution compared with the use of oral amphotericin B, placebo, and fluconazole (14 versus 28 cases, respectively). Related to this observation is the number of patients dying with proven deep fungal infections, mostly aspergillosis: 2 in the itraconazole arms versus 14 in the comparative arms (oral amphotericin B, n = 5; placebo, n = 5; and fluconazole, n = 4). Based on the blinded interim analysis, it became evident that the required number of documented cases of aspergillosis in the present trial could not be obtained by recruiting all patients as stipulated in the sample size calculation. Statistical conclusions on very small incidences are difficult to interpret. The majority of the patient population in this trial mainly consisted of neutropenic patients with a lower risk for mold infections. The risk for aspergillosis is higher for patients undergoing allogeneic bone marrow transplantation, for patients with acute myeloid leukemia in second relapse, or patients with graft-versus-host disease treated with high-dose steroids. Only a few high-risk patients were included in this study.
All cases of invasive aspergillosis were identified in France, except for one in the itraconazole group, which was observed in The Netherlands. An explanation for the difference could be that the incidence of aspergillosis varies strongly between different hospitals, even in the same country. Furthermore, 46% of the total patient sample was recruited in France (129 of 281 patients in the itraconazole group and 125 of 276 patients in the oral amphotericin B group).
As expected from the trial comparison versus amphotericin B capsules, a statistically significant (P = 0.004) higher incidence of superficial candidosis, mainly oropharyngeal candidosis, was documented. Also, for itraconazole oral solution, which is effective in the treatment of oral and esophageal candidosis (5), this effect is not surprising. The clinical relevance is, however, less clear. A significant relationship with deep candidosis could not be established in this trial. The administration of empiric or therapeutic intravenous amphotericin B is preferably to be avoided due to the potential of severe renal toxicity and other adverse experience. In the itraconazole oral solution arm of the trial, the instantaneous risk of administration of intravenous amphotericin B was 33% lower than that in the oral amphotericin B arm. This difference was marginally statistically significant (P = 0.052) and is considered to be of clinical relevance. More than in therapeutic trials, the safety evaluation of a proposed drug for prophylaxis is very important. Interpreting safety aspects of one component of a multidrug regimen in highly immunocompromised patients is difficult. Even though HPβCD was also used in the placebo formulation, the double-blind, double-placebo design of the trial and the standard methodology to evaluate adverse events and to classify biochemical parameters have been instrumental in this assessment.
In general, treatment with the itraconazole oral solution is well tolerated (11). Any diarrhea induced by treatment with itraconazole oral solution is most likely due to the HPβCD carrier, which is known to induce soft stools and diarrhea from a daily dose of 16 g onwards. This dose was administered in the placebo as well as in the active formulation. However, nausea and vomiting, which both belong to the adverse event profile of both itraconazole capsules and oral solution, did occur to similar extents in both groups. In the population studied, the overall reporting of gastrointestinal adverse events mirrors the clinical day-by-day observations which occur independent of a clinical trial setting. Other adverse events for itraconazole are rashes, transient increases in liver function tests, and hypokalemia, but again, no relevant differences were observed.
Biochemistry abnormalities are inherent in the patient population studied. All observed increases and decreases were similar in both groups, pointing at the background laboratory abnormalities in this population. The hypokalemia confirmed findings in the adverse event analysis.
In conclusion, prophylactic administration of itraconazole oral solution at 5 mg/kg daily in patients with hematological malignancies and neutropenia significantly reduced the incidence of superficial fungal infections. The number of systemic fungal infections, the number of deaths due to proven deep fungal infections, the incidence of administration of systemic antifungals, and the time prior to this administration were influenced positively by the administration of itraconazole oral solution.
ACKNOWLEDGMENTS
We are indebted to our patients and health care professionals at various medical centers, without whose willing participation this trial would not have been possible. We thank the following additional participants in the trial: in Austria, M. Eibl and P. Neumeister (Medizinische Universitätsklinik, Graz); D. Lutz and M. Girschikofsky (KH. D. Elisabethinen, Linz); and P. Kalhs (AKH, Vienna); in France, G. Auzanneau, T. De Revel, and G. Nedellec (Hôpital du Val de Grace, Paris); J. Abgrall, C. Berthou, and L. Sensebe (CHU Morvan, Brest); F. Dreyfus and D. Bouscary (L'Hôpital Cochin, Paris); D. Caillot (CHU Hôpital d'enfants, Dijon); D. Fiere, C. Cartel, and J. Trency (CHU Edouard Herriot, Lyon); C. Martin and B. Corront (Centre Hospitalier d'Annessy, Annecy); J. Cahn and E. De Coninck (CHU de Besançon, Besançon); B. Desablens (CHU d'Amiens, Amiens); H. Dombret and S. Glaisner (Hôpital Saint-Louis, Paris); J. Rossi and N. Fegueux (Hôpital Lapeyronie, Montpellier); J. Pico and C. Fuertes (Institut Gustave Roussey, Villejuif); F. Guilhot and E. Randriamalala (Centre Hospitalier Universitaire, Poitiers); A. Stoppa (Institut Paoli, Calmettes, Marseille); R. Zittoun and A. Vekhoff (Hôpital Dieu, Paris); J. Sotto and F. Viret (CHU Grenoble, Grenoble); B. Witz (Hôpital de Brabois, Nancy); R. Hebrecht (Hôpital Hautepierre, Strasbourg); J. Pris and F. Huguet (Hôpital Purpan, Toulouse); A. Najman and F. Isnard (Hôpital Saint-Antoine, Paris); V. Leblond, C. Soussain, and H. Zouabi (Hôpital Pitie-Salpetriere, Paris); O. Lertholary (Hôpital Avicenne, Bobigny); J. Sotto and L. Melina (Hôpital Michallon, Grenoble); D. Guyetat and P. Criel (Hôpital Nord, Saint Etienne); B. Pignon (CHU de Reims, Reims); H. Leporrier and O. Reman (Hôpital Clemenceau, Caen); and M. Renoux (Hôpital de Bayonne, Bayonne); in Greece, H. Bassaris (Pathologic Clinic, Rio-Patras); G. Petrikkos (Laiko General Hospital of Athens, Athens); and B. Seitanidis (Metaxa Hospital, Athens); in Portugal, (F. Campilho, A. Junior, and P. Pimentel (Instituto Portugues de Oncologia, Porto); J. Fernandes (Hospital dos Capuchos, Lisbon); F. Principe and C. Granato (Hospital de Sao Joao, Porto); and P. Garcia and I. Sousa (Hospital da Universidade de Coimbra, Coimbra); in Spain, F. Hernandez, M. Alvarez, M. Canales, M. Jimenez, V. Jimenez, A. Lafuente, M. Martin, P. Matecs, M. Merado, M. Rodriguez, and J. Sevilla (Hospital La Paz, Madrid); J. San Miguel, C. Canizo, and L. Vazquez (Hospital Universitario de Salamanca, F. Cobo and E. Montserrat (Hospital Clínic IDIBAPS, Barcelona); A. Rodriguez Noriega, C. Grande, and M. Lizascain (Hospital 12 de Octubre, Madrid); and M. Sanz and I. Jarque (Hospital La Fe, Valencia); and in The Netherlands, B. Daenen (Academisch Ziekenhuis Groningen, Groningin); B. De Pauw (Sint-Radboud Ziekenhuis, Nijmegen); W. Gerrits (Ziekenhuis Leyenburg, Amsterdam); and J. Van der Lelie (Academic Medical Center, Amsterdam).
REFERENCES
- 1.Anaissie E. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin Infect Dis. 1992;14(Suppl.1):S43–S53. doi: 10.1093/clinids/14.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- 2.De Beule K L. Itraconazole: pharmacology, clinical experience and future development. Int J Antimicrob Agents Chemother. 1996;6:175–181. doi: 10.1016/0924-8579(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 3.Denning D W, Lee J Y, Hostetler J S, Pappas P, Kauffman C A, Dewsnup D H, Galgiani J N, Graybill J R, Sugar A M, Catanzaro A, Gallis H, Perfect J R, Dockery B, Dismukes W E, Stevens D A. NIAID mycoses study group multicenter trial of oral itraconazole therapy of invasive aspergillosis. Am J Med. 1994;97:135–144. doi: 10.1016/0002-9343(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 4.Gotzsche P C, Johansen H K. Meta-analysis of prophylactic or empirical antifungal treatment in patients with cancer complicated by neutropenia. Br Med J. 1997;314:1238–1244. doi: 10.1136/bmj.314.7089.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graybill J R, Vazquez J, Darouiche R O, Morhart R, Greenspan D, Tuazon C, Wheat L J, Carey J, Leviton I, Hewitt R G, MacGregor R R, Valenti W, Respredo M, Moskovitz B L. Randomized trial of itraconazole oral solution for oropharyngeal candidiasis in HIV/AIDS patients. Am J Med. 1998;104:33–39. doi: 10.1016/s0002-9343(97)00307-0. [DOI] [PubMed] [Google Scholar]
- 6.Kelsey S M, Goldman J M, McCann S, Newland A C, Scarffe J H, Oppenheim B A, Mufti G J. Liposomal amphotericine (AmBisome) in the prophylaxis of fungal infections in neutropenic patients: a randomised, double-blind, placebo-controlled study. Bone Marrow Transplant. 1999;23:163–168. doi: 10.1038/sj.bmt.1701543. [DOI] [PubMed] [Google Scholar]
- 7.Lippert H, Lehman H P, editors. SI units in medicine. Baltimore, Md: Urban & Schwarzenberg; 1978. [Google Scholar]
- 8.Menichetti F, Del Favero A, Martino P, Bucaneve G, Micozzi A, D'Antonio D, Ricci P, Carotenuto M, Liso V, Nosari A M, Barbui T, Fasola G, Mandelli F the GIMEMA Infection Program. Preventing fungal infection in neutropenic patients with acute leukemia: fluconazole compared with oral amphotericin B. Ann Intern Med. 1994;120:913–918. doi: 10.7326/0003-4819-120-11-199406010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Menichetti F, Del Favero A, Martino P, Bucaneve G, Micozzi A, Girmenia C, Barbabietola G, Pagano L, Leoni P, Specchia G, Caiozzo A, Raimondi R, Mandelli F. Itraconazole oral solution as prophylaxis for fungal infections in neutropenic patients with hematologic malignancies: a randomized, placebo-controlled, double-blind, multicenter trial. Clin Infect Dis. 1999;28:250–255. doi: 10.1086/515129. [DOI] [PubMed] [Google Scholar]
- 10.Morgenstern G R, Prentice A G, Prentice H G, Ropner J E, Schey S A, Warnock D W. A randomised controlled trial of itraconazole versus fluconazole for the prevention of fungal infections in patients with haematological malignancies. Br J Haematol. 1999;105:901–911. doi: 10.1046/j.1365-2141.1999.01465.x. [DOI] [PubMed] [Google Scholar]
- 11.Phillips, P., K. De Beule, G. Frechette, S. Tchmouroff, B. Vandercam, L. Weitner, A. Hoepelman, G. Stingl, and B. Clotet. 1998. A double-blind comparison of itraconazole oral solution and fluconazole capsules for the treatment of oropharyngeal candidiasis in patients with AIDS. 26:1368–1373. [DOI] [PubMed]
- 12.Prentice A G, Warnock D W, Johnson S A N, Phillips M J, Oliver D A. Multiple dose pharmacokinetics of an oral solution of itraconazole in autologous bone marrow transplant recipients. J Antimicrob Chemother. 1994;34:247–252. doi: 10.1093/jac/34.2.247. [DOI] [PubMed] [Google Scholar]
- 13.Prentice A G, Warnock D W, Johnson S A N, Taylor P C, Oliver D A. Multiple dose pharmacokinetics of an oral solution of itraconazole in patients receiving chemotherapy for acute myeloid leukaemia. J Antimicrob Chemother. 1995;36:657–663. doi: 10.1093/jac/36.4.657. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz, S., G. Behre, V. Heinemann, H. Wandt, E. Schilling, M. Arning, A. Trittin, W. V. Kern, O. Boenisch, D. Bosse, K. Lenz, W. D. Ludwig, W. Hiddemann, W. Siegert, and J. Beyer. 1999. Aerosolized amphotericin B inhalations as prophylaxis of invasive aspergillus infections during prolonged neutropenia: results of a prospective randomized multicenter trial. 93:3654–3661. [PubMed]
- 15.Sheehan D J, Hitchcock C A, Sibley C M. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40–79. doi: 10.1128/cmr.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van de Velde V J, Van Peer A P, Heykants J J, Woestenborghs R J, Van Rooy P, De Beule K L, Cauwenbergh G F. Effect of food on the pharmacokinetics of a new hydroxypropyl-beta-cyclodextrin formulation of itraconazole. Pharmacotherapy. 1996;16:424–428. [PubMed] [Google Scholar]
- 17.Wingard J R, Merz W G, Rinaldi M G, Johnson T R, Kapp E J, Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991;325:1274–1277. doi: 10.1056/NEJM199110313251803. [DOI] [PubMed] [Google Scholar]
- 18.Winston D J, Chandrasekar P H, Lazarus H M, Goodman J L, Silber J L, Horowitz H, Shadduck R K, Rosenfeld C S, Ho W G, Winston G H, Islam M Z, Buell D N. Fluconazole prophylaxis of fungal infections in patients with acute leukemia. Ann Intern Med. 1993;118:495–501. doi: 10.7326/0003-4819-118-7-199304010-00003. [DOI] [PubMed] [Google Scholar]