Abstract
During osteoarthritis (OA), hypertrophy-like chondrocytes contribute to the disease process. TGF-β’s signaling pathways can contribute to a hypertrophy(-like) phenotype in chondrocytes, especially at high doses of TGF-β. In this study, we examine which transcription factors (TFs) are activated and involved in TGF-β-dependent induction of a hypertrophy-like phenotype in human OA chondrocytes. We found that TGF-β, at levels found in synovial fluid in OA patients, induces hypertrophic differentiation, as characterized by increased expression of RUNX2, COL10A1, COL1A1, VEGFA and IHH. Using luciferase-based TF activity assays, we observed that the expression of these hypertrophy genes positively correlated to SMAD3:4, STAT3 and AP1 activity. Blocking these TFs using specific inhibitors for ALK-5-induced SMAD signaling (5 µM SB-505124), JAK-STAT signaling (1 µM Tofacitinib) and JNK signaling (10 µM SP-600125) led to the striking observation that only SB-505124 repressed the expression of hypertrophy factors in TGF-β-stimulated chondrocytes. Therefore, we conclude that ALK5 kinase activity is essential for TGF-β-induced expression of crucial hypertrophy factors in chondrocytes.
Keywords: chondrocyte hypertrophy, TGF-β, osteoarthritis, transcription factors, ALK5
1. Introduction
Osteoarthritis (OA) is the result of a disturbance in joint homeostasis, leading to a loss of articular cartilage. In articular chondrocytes, this disturbance is associated with a switch towards a hypertrophy-like phenotype, which resembles the terminal differentiation route in growth plate chondrocytes [1,2,3]. Such hypertrophy-like cells express factors such as collagen type 10 (COL10A1), runt-related transcription factor 2 (RUNX2), Indian hedgehog (IHH) and vascular endothelial growth factor A (VEGFA) [2,4,5]. The presence of hypertrophic chondrocytes is thought to contribute to the disease process by production of cartilage-degrading enzymes such as metalloproteinases (MMPs) and aggrecanases (ADAMTS) [4,5].
In healthy cartilage, transforming growth factor-β (TGF-β) prevents chondrocyte hypertrophic differentiation and maintains chondrocyte homeostasis [6,7,8]. It does this via activation of the intracellular transcription factors (TFs) SMAD2 and SMAD3 [9,10]. At the same time, the literature indicates that TGF-β can be a driver of chondrocyte hypertrophy under OA conditions. For example, TGF-β can signal via its alternative detrimental SMAD1/5/9 signaling route. For example, SMAD1/5 can interact with RUNX2 to induce COL10A1 expression, an important hypertrophy gene [6,11,12,13]. In addition, TGF-β can induce many other SMAD-independent signaling routes, such as mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) pathways [6]. It is unknown if activation of these SMAD-independent pathways and subsequent activation of corresponding TFs contribute to induction of hypertrophy during osteoarthritis [14,15,16,17,18,19]. Previous research in experimental OA models [20,21,22] and also recent large-scale gene expression data confirm that TGF-β pathways can contribute to OA development and chondrocyte hypertrophy, since expression of TGFB1, the gene encoding for TGF-β1, and other TGF-β-related genes is found to be strongly correlated with chondrocytes in pre-hypertrophic and late-OA stages [23,24,25,26].
Importantly, in OA, active TGF-β levels are elevated compared to levels in healthy joints where these are low and only locally load-activated [27,28,29,30,31]. During OA, such elevated TGF-β levels activate different TFs and signaling pathways than in a healthy joint because of the use of different receptors and co-receptors [9,32,33]. Improving our understanding of TGF-β-mediated activation of TFs and signaling pathways in this context will help us to develop new treatment strategies for OA. Therefore, in this study, we examine which transcription factors (TFs) are activated in human chondrocytes, that are stimulated to undergo hypertrophy, by levels of active TGF-β comparable to those found in the synovial fluid in OA joints.
2. Materials and Methods
2.1. Patient Material
Primary human chondrocytes were isolated from macroscopically intact cartilage obtained from 30 anonymous OA donors (12 males and 18 females, ages 56–84; median 71 years) undergoing total knee arthroplasty (Sint Maartenskliniek, Nijmegen, the Netherlands). Patients were informed, and permission was requested for the anonymized use of this material for research (File CMO 2018-4319). These donors were randomly distributed over the different experiments.
2.2. Primary Chondrocyte Cell Culture and Stimulation
To isolate chondrocytes, cartilage slices were digested by incubation with 1.0 mg/mL pronase (Roche Diagnostics, Basel, Switzerland) for 30 min at 37 °C followed by overnight incubation in 1.5 mg/mL collagenase B (Roche Diagnostics) in DMEM/F12 medium (1:1) (Gibco) at 37 °C. The next day, chondrocyte suspension was spun down at 300× g for 10 min, washed three times using saline and resuspended in DMEM/F12 (ThermoFisher, Carlsbad, CA, USA) containing 10% fetal calf serum (FCS; Sigma-Aldrich, Saint Louis, Missouri, USA), 100 mg/L sodium pyruvate, 100 U/mL penicillin and 100 µg/mL streptomycin (Gibco, Carlsbad, CA, USA) (complete culture medium). Chondrocytes were plated at a density of 4.7 × 104 cells/cm2 in white polystyrene 96-well plates for luciferase reporter assays and in 24-well plates for gene expression experiments and cultured for one week prior to experiments at 37 °C and 5% CO2. Medium was refreshed every three days. Before start of experiments, chondrocytes were serum-starved (0% FCS) overnight. Chondrocytes were stimulated with rhTGF-β1 (Peprotech, Cranbury, NJ, USA) for time periods and dosages indicated in figure legends. Inhibitors to block TGF-β-induced signaling were 5 µM SB-505124 (ALK4/5/7 inhibitor, Sigma-Aldrich), 1 µM Tofacitinib (JAK inhibitor, LC laboratories, Woburn, MA, USA) and 10 µM SP-600125 (JNK inhibitor, Calbiochem, San Diego, CA, USA), all dissolved in dimethyl sulfoxide (DMSO) and pre-incubated for 1 h before TGF-β stimulation.
2.3. Construction Reporter Plasmids, Virus Production and Transduction
Binding sequences specific to 15 different transcription factors known to activate chondrocyte signaling pathways (Table 1) [8,26,32,33,34] were extended with a spacer and minimal promoter (sequence: AGAGGGTATATAATGGAAGCTCGACTTCCAG) (Full sequences in Supplementary Table S1) were derived from Promega (Madison, WI, USA) and synthesized by Genecust (Boynes, France). These binding sequences were directionally cloned into the pNL1.2 Nanoluciferase-PEST (NlucP) vector (Promega). NlucP is an unstable luciferase and was chosen because it has very low background signal and is therefore able to detect minimal responses [35]. Subsequently, lentiviral constructs were generated by re-cloning pNL1.2 reporters with the In-Fusing Cloning method (TakaraBio, Kusatsu, Shiga, Japan) in the ClaI restriction site of the pLVX-EF1α-IRES-Puro producer vector (TakaraBio). Viral supernatants were generated in Lenti-X 293T cells (TakaraBio) and the 4th generation lentiviral production system (TakaraBio) using 1 mg/mL polyethylenimine (PEI; Polysciences, Warrington, USA). Viral supernatant was concentrated using Lenti-X concentrator (TakaraBio) according to manufactures’ protocol. Lentiviral concentration was determined using p24 INNOTEST ELISA assay (Fujirebio, Gent, Belgium). Primary OA chondrocytes, cultured for a week to form a monolayer, were transduced for 6–8 h with 500 ng of virus particles per 62,500 cells with 8 µg/mL polybrene in DMEM/F12 medium without FCS, penicillin or streptomycin (protocol was optimized: Supplementary Figure S1A). A selection pressure of 2 µg/mL puromycin (Sigma-Aldrich) for 7 days was used to ensure transgene expression (± 90% efficient transduction using 500 ng of lentivirus per 62,500 cells, results not shown).
Table 1.
Transcription factor (TF) elements used for luciferase TF activity constructs, each of them known to activate relevant chondrocyte signaling pathways.
| Pathway | TF(s) | TF Element (Abbreviation Construct) | Positive Control | References |
|---|---|---|---|---|
| MAPK/JNK | c-FOS:c-JUN | Activator Protein 1 response element (AP1) | FCS (20%) | [36,37,38,39] |
| SOX9 | SOX9 | SRY-box transcription factor 9 response element (SOX9) | Forskolin (10 µM) | [40,41,42] |
| NFκB | NFκB:p65 | Nuclear factor κ B response element (NFκB) | IL-1β (1 ng/mL) | [43,44,45] |
| Notch | CSL | CBF1/RBPJκ/Suppressor of Hairless/Lag-1 response element (CSL) | FCS (20%) | [46,47,48,49] |
| cAMP/PKA | CREB | Cyclic AMP response element (CRE) | Forskolin (10 µM) | [41,50,51] |
| Glucocorticoid signaling | Glucocorticoid receptor | Glucocorticoid receptor response element (GRE) | Dexamethasone (10 µM) | [50,52,53,54] |
| TGF-β | SMAD3:SMAD4 | SMAD binding element (SBE) | TGF-β1 (1 ng/mL) | [8,9,55] |
| Wnt | TCF/LEF | T-cell factor/lymphoid enhancer factor family response element (TCF/LEF) | Wnt3a (200 ng/mL) | [51,56,57] |
| INF-α | STAT1:STAT2 | Interferon-stimulated response element (ISRE) | IFN-α (100 ng/mL) | [58,59] |
| IL-6 | STAT3:STAT3 | Sis-inducible element (SIE) | IL-6 (10 ng/mL) | [55,60,61,62] |
| MAPK/ERK | ELK-1:SRF | Serum response element (SRE) | FCS (20%) | [63,64] |
| RhoA | SRF | Serum response factor (SRF) | FCS (20%) | [63,65] |
| Oxidative stress | NRF2 | Antioxidant response element (ARE) | H2O2 (50 µM) | [66,67,68] |
| Calcium/calcineurin: hyperosmotic signaling | NFAT5 | Nuclear factor of activated T-cells 5 response element (NFAT5) | +100 milliosmole | [69,70,71] |
| PPARγ | PPARγ | Peroxisome Proliferator activated receptor-γ response element (PPRE) | Rosiglitazone (20 µM) | [72,73,74] |
2.4. Stimulation and Reporter Gene Assays
After lentiviral transduction for 48 h, cells were serum-starved overnight in 0% FCS supplemented DMEM/F12. Transduced and serum-starved cells were stimulated for 6 h with 5 ng/mL TGF-β (with or without 1 h pre-incubation of inhibitors) or an appropriate positive control (Table 1), which is a compound known to activate the construct in a chondrocyte cell line. Cells were lysed 6 h post-stimulation using 30 µl of ultra-pure water. An equal amount of Nano-Glo luciferase reagent (Promega) was added, and luminescence was determined at 470–480 nm at room temperature (CLARIOstar, BMG Labtech, Ortenberg, Germany). Each condition was performed in quadruplicate, and the mean per donor is depicted.
2.5. RNA Isolation and Quantitative Real-Time PCR
mRNA was isolated using 500 µL of TRIzol (Sigma-Aldrich), according to manufacturer’s protocol. After isolation, a maximum of 1 µg of mRNA was treated with 1 µL of DNAse (Life Technologies, Carlsbad, CA, USA) for 15 min at room temperature to remove possible genomic DNA, followed by 10 min of inactivation by incubation at 65 °C with 1 µL of 25 mM EDTA (Life Technologies). mRNA was reverse-transcribed to complementary DNA using 1.9 µL of ultrapure water, 2.4 µL of 10× DNAse buffer, 2.0 µL of 0.1M dithiothreitol, 0.8 µL of 25mM dNTP, 0.4 µg of oligo dT primer, 200U M-MLV reverse transcriptase (all Life Technologies) and 0.5 µL of 40 U/mL RNAsin (Promega) and incubated for 5 min at 25 °C, 60 min at 39 °C and 5 min at 95 °C using a thermocycler. Gene expression was measured using SYBR Green Master Mix (Applied Biosystems, Waltham, MA, USA) and 0.25 mM primers (Biolegio, Nijmegen, the Netherlands: see Table 2) with a StepOnePlus real-time PCR system (Applied Biosystems). The amplification protocol was 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Melting curves were analyzed to confirm product specificity. To calculate the relative gene expression (−ΔCt), the average of the three reference genes GAPDH, RPL22 and RPS27A was used.
Table 2.
Primer sequences as used in this study.
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| GAPDH | ATCTTCTTTTGCGTCGCCAG | TTCCCCATGGTGTCTGAGC |
| RPL22 | TCGCTCACCTCCCTTTCTAA | TCACGGTGATCTTGCTCTTG |
| RPS27A | TGGCTGTCCTGAAATATTATAAGGT | CCCCAGCACCACATTCATCA |
| RUNX2 | GCAAGGTTCAACGATCTGAGA | TTCCCGAGGTCCATCTACTG |
| COL10A1 | TTTTACGCTGAACGATACCAAATG | CTGTGTCTTGGTGTTGGGTAGTG |
| COL1A1 | AGATCGAGAACATCCGGAG | AGTACTCTCCACTCTTCCAG |
| VEGFA | CAGGGAAGAGGAGGAGATGAGA | GCTGGGTTTGTCGGTGTTC |
| IHH | CAATTACAATCCAGACATCATCTTCA | CGAGATAGCCAGCGAGTTCAG |
2.6. Statistical Analysis
Quantitative data of gene expression analysis are expressed as column scatter graphs, and one dot displays the mean value of a technical triplicate sample per donor. Results are represented as mean of multiple donors with corresponding 95% confidence interval (CI). For TF-reporter assays, conditions were investigated in quadruple, and one dot displays the mean of one donor. The graphs show the mean of multiple experiments with corresponding 95% confidence interval (CI). Differences were tested using displayed means with analysis of variance (ANOVA) followed by Bonferroni’s post-test to take multiple comparisons into account. Statistical differences were considered as significant if the p-value was below 0.05. All analyses were performed using Graph Pad Prism version 9.0 (GraphPad Software).
3. Results
3.1. TGF-β Drives a Hypertrophy-like Phenotype in Primary Human OA Chondrocytes
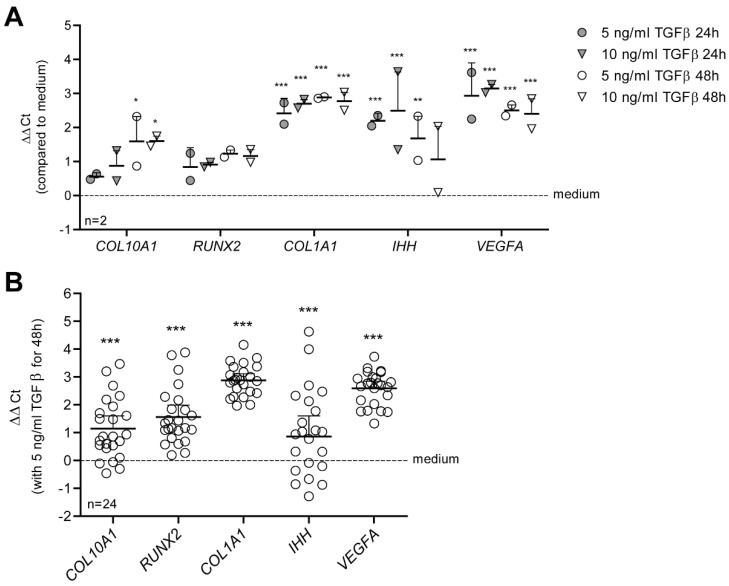
The growth factor TGF-β is important for chondrocyte homeostasis, but can be anti- or pro-hypertrophic, depending on its concentration and on the chondrocyte responsiveness. We established an in vitro model in which TGF-β drives a hypertrophy-like phenotype in primary human OA chondrocytes, which we defined as increased expression of characteristic markers of chondrocyte hypertrophy RUNX2, COL10A1, VEGFA and IHH [2], and increased expression of dedifferentiation marker COL1A1, which is also related to an OA phenotype [75,76]. For this, we used a concentration of 5 or 10 ng/mL TGF-β, levels comparable to those found in the synovial fluid of OA patients [27,28,29,30,31], and tested whether these concentrations of TGF-β were able to increase expression of these hypertrophy genes. After stimulation of OA chondrocytes of two donors with 5 or 10 ng/mL TGF-β1 for 24 and 48 h, expression of COL1A1, IHH and VEGFA were increased significantly both at 24 and 48 h (Figure 1A). COL10A1 was only significantly upregulated when stimulated for 48 h with TGF-β1, and RUNX2 was not significantly upregulated. Because we found no difference between a concentration of 5 or 10 ng/mL TGF-β and the hypertrophy-like phenotype was best induced after 48 h, we continued with 5 ng/mL TGF-β for 48 h for our hypertrophy-like model. We verified the model in OA chondrocytes of another 24 donors, incubated for 48 h with 5 ng/mL TGF-β1 (Figure 1B). mRNA expression of COL10A1 (+1.147 ∆∆Ct, p < 0.001), RUNX2 (+1.564 ∆∆Ct, p < 0.001), COL1A1 (+2.882 ∆∆Ct, p < 0.001), VEGFA (+1.189 ∆∆Ct, p < 0.001) and IHH (+2.592 ∆∆Ct p < 0.001) was strongly upregulated, confirming a hypertrophy-like phenotype in OA chondrocytes induced by TGF-β.
Figure 1.
TGF-β1 drives a hypertrophy-like phenotype in primary human OA chondrocytes. Elevated levels of TGF-β that are comparable to those in synovial fluid of OA patients increase expression of crucial hypertrophy genes. (A) Human chondrocytes of two donors were cultured in monolayer and stimulated with 5 or 10 ng/mL TGF-β1 for 24 or 48 h. This resulted in induction of hypertrophy-like differentiation, as measured by increased expression of relative collagen type 10 (COL10A1), runt-related transcription factor 2 (RUNX2), alpha-1 collagen type 1 (COL1A1), Indian hedgehog (IHH) and vascular endothelial growth factor A (VEGFA) using qPCR. (B) These results were verified in chondrocytes of another 24 donors, stimulated for 48 h with 5 ng/mL TGF-β1. Data are plotted as mean ± 95% CI with each dot representing the average of three technical replicates in one chondrocyte donor. Statistical analysis was performed using a repeated measures two-way (A) or one-way (B) ANOVA with Bonferroni’s post hoc test: * p ≤ 0.05; ** p ≤ 0.01; *** p < 0.001.
3.2. TGF-β Activates SMAD3:4, STAT3 and AP1 Signaling in Human OA Chondrocytes
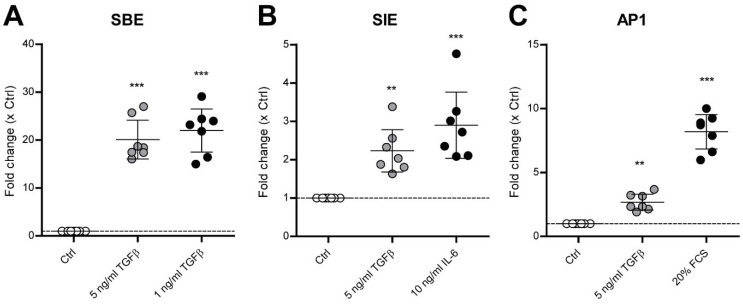
To identify which TFs were activated by TGF-β in OA chondrocytes undergoing hypertrophy, we made use of luciferase-based TF activity assays. Using lentiviral transduction, 15 different TF-based luciferase constructs (Table 1), each of them known to be downstream of chondrocyte signaling pathways [8,26,32,33,34], were transduced into human OA chondrocytes. Importantly, this lentiviral transduction did not affect our TGF-β-induced hypertrophy model in these cells (Supplementary Figure S1B). For every TF-based construct, activation upon TGF-β treatment and a positive control (a compound known to activate the construct in a chondrocyte cell line: Table 1) was assessed in multiple OA chondrocyte donors (Supplementary Figure S2). Stimulation for 6 h with an appropriate positive control induced SBE-, SIE-, AP1-, NFκB-, CRE-, SRF-, GRE-, SRE-, ISRE- and NFAT5-controlled pathways in human OA chondrocytes. TCF/LEF, PPRE, SOX9, CSL and ARE signaling were not activated by a positive stimulus or were already fully activated in control condition.
TGF-β in a concentration of 5 ng/mL for 6 h did not activate NFκB-, CRE-, SRF-, GRE-, SRE-, ISRE-, NFAT5-, TCF/LEF-, PPRE-, SOX9-, CSL- or ARE-controlled pathways (Supplementary Figure S2). Importantly, TGF-β did activate SBE, SIE and AP1 signaling (Figure 2 and Supplementary Figure S3). In all seven donors, 5 ng/mL TGF-β significantly increased SBE-luciferase (Supplementary Figure S3A) activity with an average of a 20.09-fold increase (p < 0.001) (Figure 2A). SIE signaling was significantly increased in two out of seven donors (Supplementary Figure S3B), however with a significant average 2.23-fold increase (p = 0.0039) (Figure 2B). In six out of seven donors, AP1 luciferase signaling was significantly increased (Supplementary Figure S3C) with a 2.70-fold average increase (p = 0.0059) (Figure 2C). These results indicate that these pathways are potentially involved in TGF-β-induced OA pathogenesis and induction of hypertrophy-like differentiation in OA chondrocytes.
Figure 2.
TGF-β1 activates SMAD3:4, STAT3 and AP1 signaling in human OA chondrocytes. Primary human OA chondrocytes, cultured for a week to form a monolayer, were transduced for 6–8 h with 500 ng of lentivirus per 62,500 cells. After 48 h, the transduced cells were serum-starved overnight and then stimulated with a positive control (black dots) or with 5 ng/mL TGF-β1 (gray dots) for 6 h. Luminescence was determined at 470–480 nm, and fold change compared to medium-stimulated cells (Ctrl, white dots) was calculated. TGF-β1 (gray dots) activated cell signaling regulated by the transcription factors (A)SMAD3:4 (SBE: SMAD binding element), (B) STAT3 (SIE: Interleukin(IL)-6 sis-inducible element or STAT3 response element) and (C) AP1 (Activation Protein 1). Data are plotted as mean ± 95% CI, with each dot representing the average of a quadruple sample in one chondrocyte donor (total n = 7). Additionally, the induction of the pathways with positive controls is reported (black dots), which were 1 ng/mL TGF-β1 for the SBE pathway, 10 ng/mL IL-6 for the SIE pathway and 20% fetal calf serum (FCS) for the AP1 pathway. Statistical analysis was performed using one-way ANOVA with Bonferroni’s post hoc test: ** p ≤ 0.01; *** p < 0.001.
3.3. TGF-β Activated SMAD3:4, STAT3 and AP1 Signaling Is Blocked by Specific Inhibitors
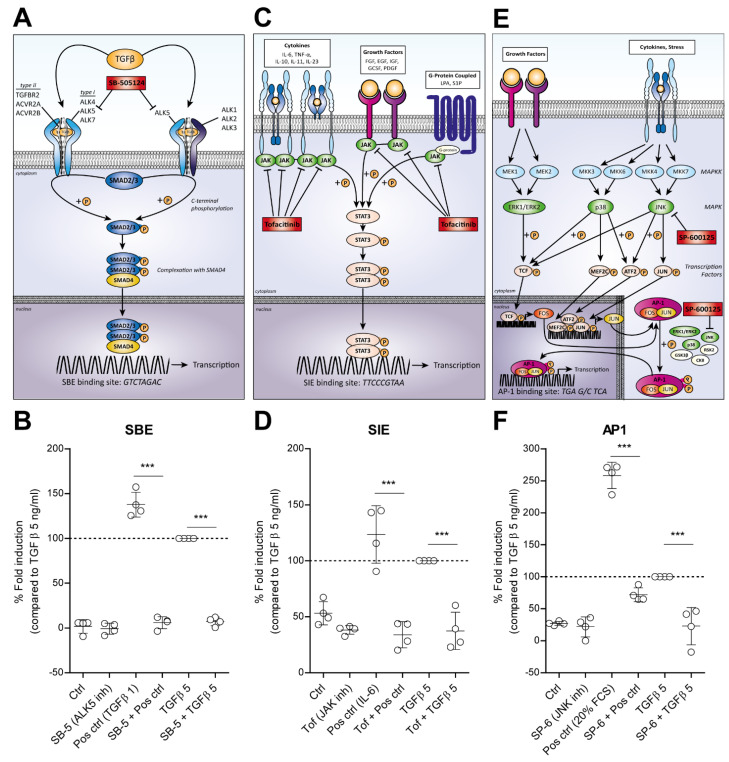
Specific pathway inhibitors were used to probe the involvement of the SBE, SIE and AP1 pathways in the involvement of the TGF-β-driven hypertrophy-like phenotype. The SMAD binding element (SBE: GTCTAGAC) in DNA binds a phosphorylated complex of R-SMAD3 and co-SMAD4, which is formed and transported to the nucleus upon TGF-β receptor type I activation, i.e., ALK4, ALK5 and ALK7 (Figure 3A) [6]. These receptors can be inhibited with the SB-505124 inhibitor, thereby blocking SMAD3 activation [77]. We pre-incubated human chondrocytes transduced with the SBE-luciferase reporter construct for 1 h with 5 µM SB-505124 before stimulation with TGF-β, showing significant inhibition of luciferase induction with both 1 and 5 ng/mL TGF-β (p < 0.0001) (Figure 3B).
Figure 3.
TGF-β1-stimulated SMAD3:4, STAT3 and AP1 signaling are blocked with pathway inhibitors. Specific pathway inhibitors were used to target the SBE, SIE and AP1 pathways. (A) The SBE pathway starts with TGF-β ligand binding, which induces formation of a receptor complex containing type I (ALK4/5/7) and type II receptors. Receptor-SMAD3 is recruited and activated by C-terminal phosphorylation (P). A complex is formed with the common SMAD4 and this translocates to the nucleus where it binds the DNA to the SMAD3:4 binding element (SBE: GTCTAGAC), activating transcription and luciferase signal. SB-505124 (SB-5) is an ALK-4/5/7 inhibitor interfering with SMAD-dependent signaling. (B) Pre-incubation of human chondrocytes (n = 4) transduced with the SBE-luciferase reporter construct for 1 h with 5 µM SB-5 before stimulation with TGF-β1 for 6 h, results in significant inhibition of luciferase induction with both 1 (Pos ctrl) and 5 ng/mL TGF-β. (C) The SIE pathway consists of repeats of IL-6 sis-inducible elements (SIE: TTCCCGTA) and is also called STAT3 response element since it is activated by STAT3. STAT3 is phosphorylated (P) by receptor-associated Janus kinases (JAK) in response to many different ligands. Tofacitinib (Tof) is an inhibitor of JAK1 and JAK3, thereby interfering with this JAK-STAT pathway. (D) Pre-incubation of human chondrocytes (n = 4), transduced with the SIE-luciferase reporter construct, with 1 µM of completely blocked IL-6 (Pos ctrl) and TGF-β1 (5 ng/mL) induced SIE signaling. (E) The AP1 pathway is regulated by transcription factor Activator Protein 1 (AP1: TGA G/C TCA), which is a heterodimer composed of FOS, JUN and ATF protein families. These are activated by MAPKs pathways, consisting of the ERKs, p38s and JNKs. SP-600125 (SP-6) is an inhibitor of all JNK isoforms, thereby interfering with this AP1 pathway. (F) Pre-incubation of primary human OA chondrocytes, transduced with the AP1-luciferase reporter construct with 10 µM SP-6 blocked luciferase induction with 20% FCS (Pos ctrl) or 5 ng/mL TGF-β1. Data are plotted as mean ± 95% CI with each dot representing the average of four technical replicates in one chondrocyte donor; in total, chondrocytes from four different donors were used, as seen in (B,D,F). Luciferase activity was measured relative to experimental condition stimulated with 5 ng/mL TGF-β, as set at 100%. Statistical analysis was performed using one-way ANOVA with Bonferroni’s post hoc test: *** p < 0.001. ALK = ALK tyrosine kinase receptor, ACVR2 = Activin type-2 receptor, GF = growth factor, IL = Interleukin, JAK = janus kinase, JNK = c-JUN N-terminal kinase, MAPK = mitogen-activated protein kinase, STAT3 = Signal Transducer and Activator of Transcription 3, TGFBR = TGF-β receptor.
TGF-β also induced luciferase activation of the SIE-responsive element, which consists of repeats of Interleukin (IL)-6 Sis-Inducible Elements (SIE: TTCCCGTAA), also called STAT3 response elements, since it is activated by STAT3 [78]. STAT3 is phosphorylated by receptor-associated Janus kinases (JAKs) in response to many different ligands as IL-6, interferons and fibroblast growth factor (FGF) (Figure 3C). Tofacitinib is an inhibitor of JAK1 and JAK3, thereby interfering with this JAK-STAT pathway [79]. Pre-incubation with 1 µM Tofacitinib completely blocked IL-6-induced SIE signaling (p < 0.0001), which we used as a positive control to activate this pathway. In addition, Tofacitinib completely blocked TGF-β-induced SIE signaling (p < 0.0001) (Figure 3D).
The other pathway we found to be induced by TGF-β in OA chondrocytes was regulated by the transcription factor Activator Protein 1 (AP1: TGA G/C TCA), which is induced in response to a variety of stimuli, including cytokines, growth factors and stress. AP1 is a heterodimer composed of proteins belonging to the c-FOS, c-JUN and activation transcription factor (ATF) protein families, which are activated by mitogen-activated protein kinases (MAPKs) pathways [80,81]. The three separate groups of the MAPKs consist of the ERKs, p38s and the c-JUN N-terminal kinases (JNKs), all activated by different MAPK kinases (MAPKKs) (Figure 3E). We used the JNK inhibitor SP-600125, inhibiting all isoforms JNK1, JNK2 and JNK3, as a possible strategy to interfere with the AP1 pathway. Pre-incubation of 10 µM SP-600125 before stimulation with 20% FCS, which we used as a positive control to activate AP1-targeted signaling, inhibited the AP1-induced luciferase signal for 80,5% (p < 0.0001). The TGF-β-induced AP1-luciferase signal was also fully blocked by SP-600125 (p < 0.0001) (Figure 3F).
3.4. TGF-β-Driven Hypertrophy-like Phenotype in OA Chondrocytes Is Dependent on ALK5 and Is Not Reliant on JAK and JNK
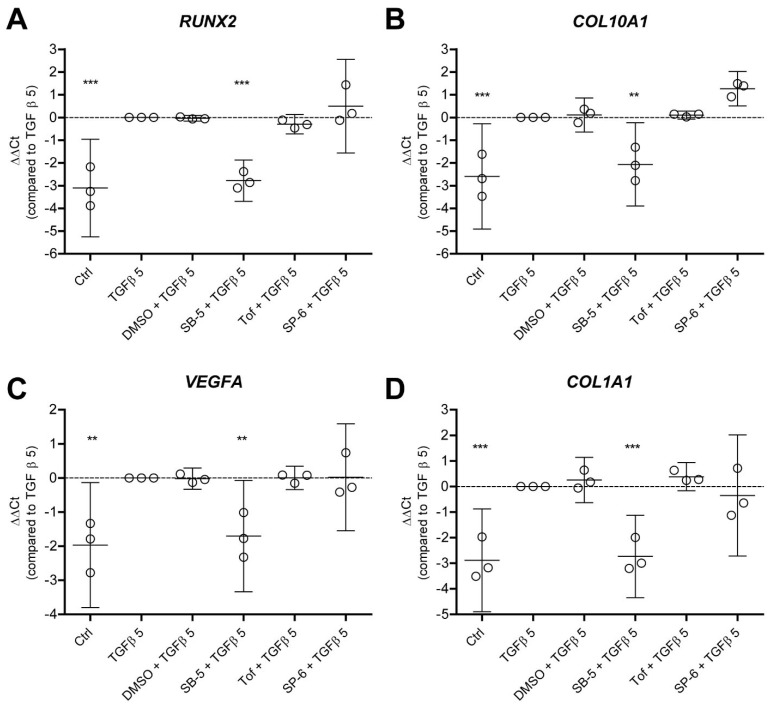
Finally, we wanted to investigate whether blocking these TGF-β-activated pathways can also prevent the TGF-β-driven hypertrophy-like phenotype in human OA chondrocytes, again defined by expression of crucial hypertrophy and dedifferentiation genes. Before stimulation with 5 ng/mL TGF-β, we pre-incubated it with one of the three different pathway inhibitors (and DMSO as the vehicle control) and measured the expression of crucial hypertrophy genes RUNX2, COL10A1 and IHH and dedifferentiation gene COL1A1. Inhibition of the SBE pathway by blocking ALK4/5/7 kinase activity with SB-505124 abolished the effect of TGF-β on the genes RUNX2 (−2.782 ∆∆Ct, p = 0.0005) (Figure 4A), COL10A1 (−2.064 ∆∆Ct, p = 0.0002) (Figure 4B), VEGFA (−2.614 ∆∆Ct, p < 0.0001) (Figure 4C) and COL1A1 (−1.703 ∆∆Ct, p = 0.0007) (Figure 4D). This suggests that TGF-β-induced ALK5 kinase activity, which is responsible for SMAD phosphorylation, is involved in the development of a hypertrophy-like phenotype by TGF-β. On the other hand, the JAK-STAT pathway and AP1 signaling via JNK are not involved in induction of TGF-β-driven hypertrophy. Blocking these pathways did not affect the upregulation of hypertrophy and dedifferentiation factors by TGF-β (Figure 4A–D).
Figure 4.
A hypertrophy-like phenotype in OA chondrocytes, induced by TGF-β, is dependent on ALK5 and is not reliant on JAK and JNK. Elevated levels of TGF-β that are comparable to those in synovial fluid of OA patients induce expression of hypertrophic factors in human OA chondrocytes of three different donors. This is defined by increased mRNA expression of (A) runt-related transcription factor 2 (RUNX2), (B) relative collagen type 10 (COL10A1), (C) vascular endothelial growth factor A (VEGFA) and (D) alpha-1 collagen type 1 (COL1A1) after stimulation with 5 ng/mL TGF-β1 for 48 h. Specific pathway inhibitors were used to target the SBE, SIE and AP1 pathways, which were also activated by these levels of TGF-β. Cells were pre-incubated with 5 µM SB-505124 (SB-5: ALK5 kinase activity inhibitor), 1 µM Tofacitinib (Tof: JAK inhibitor) or 10 µM SP-600125 (SP-6: JNK inhibitor) before stimulation with 5 ng/mL TGF-β1. Only blocking ALK5 kinase activity with SB-5 prevented the effect of TGF-β on hypertrophy genes, whereas inhibition of JAK and JNK did not. Data are plotted as mean ± 95% CI with each dot representing the average of three technical replicates in one chondrocyte donor (total of 3 different chondrocyte donors were used). Statistical analysis was performed using repeated measures one-way ANOVA with Bonferroni’s post hoc test: ** p ≤ 0.01; *** p < 0.001.
4. Discussion
Chondrocyte hypertrophy is a hallmark of OA development, and under healthy conditions, tightly regulated by the growth factor TGF-β [6,7,8]. Due to inflammation, cartilage degradation and high levels of proteases, levels of active TGF-β increase in synovial fluid of OA joints, changing the role of this growth factor [27,28]. In this study, we showed that levels of TGF-β similar to those in OA synovial fluid can induce a hypertrophy-like phenotype in human OA chondrocytes, which we defined as increased expression of crucial hypertrophy factors RUNX2, COL10A1, VEGFA and IHH and dedifferentiation factor COL1A1. We demonstrated that TGF-β activates signaling through the TFs SMAD3:4, STAT3 and AP1 in OA chondrocytes. Although activated, STAT3 and AP1 signaling did not contribute to TGF-β-induced hypertrophic factors, whereas blockage of ALK-5 kinase activity did prevent a hypertrophy-like phenotype induced by TGF-β in OA chondrocytes.
To study the potency of TGF-β in inducing chondrocyte hypertrophy, we made use of primary end-stage OA chondrocytes originating from patients receiving total knee replacement. We stimulated these cells with TGF-β1, which is the predominant form of TGF-β in articular cartilage [7]. Under physiological conditions, TGF-β levels in the joint are predominantly present in a latent form (>98% of total), and TGF-β’s activity requires activation [82]. However, during OA levels of active TGF-β are significantly elevated due to the presence of inflammation in over 50% of all OA patients [83,84]. The activation of macrophages and fibroblasts, cartilage degradation and high levels of proteases in osteoarthritic joints result in increased production and activation of TGF-β [9,28,29]. Unfortunately, most studies have only reported total TGF-β concentrations in synovial fluid without distinguishing between the active and inactive form, which makes it difficult to make a statement about actual active TGF-β levels in situ (overall estimation of 5–10 ng/mL active TGF-β in OA joints) [27,28,29,30,31]. The availability of receptors and co-receptors and differential activation of them with ageing and during OA conditions will also influence sensibility and responsiveness of the chondrocytes to TGF-β [85,86]. These factors combined will contribute to further elevated levels of active TGF-β and different subsequent downstream signaling in OA versus healthy chondrocytes.
In healthy chondrocytes, TGF-β acts as an inhibitor of chondrocyte hypertrophic differentiation and reduces expression of COL10A1 and MMP13 via SMAD3 activation. However, during OA a shift in receptor use and disturbed downstream TGF-β signaling causes a switch of the chondrocyte phenotype [87,88]. Our results illustrate that stimulation of primary human OA chondrocytes with representative levels of TGF-β in the synovial fluid of OA patients causes a hypertrophy-like phenotype in these cells. In this study, we defined a hypertrophy-like phenotype caused by increased expression of the genes COL10A1, RUNX2, IHH and VEGFA, which are factors that are associated with calcification of the articular cartilage and characteristic of chondrocyte hypertrophy [2] and increased expression of dedifferentiation marker COL1A1, which is also correlated to OA severity [75,76]. Allas et al., who used the same experimental set-up, demonstrated these pro-hypertrophic effects of TGF-β before, also at the protein level [89]. Other late-hypertrophic genes, such as alkaline phosphatase (ALPL), MMP13 and ADAMTS5, were not upregulated after 48 h of stimulation with TGF-β (Supplementary Figure S4). The short time period of 48 h of stimulation with TGF-β limited us in the study of expression of these late-hypertrophic catabolic genes and calcification of the extracellular matrix [90,91,92].
COL10A1 is considered as the standard marker and RUNX2 as the master transcription factor of chondrocyte hypertrophy [2,34,93,94,95,96,97]. IHH is a key regulator of endochondral ossification and is mainly produced and secreted by pre-hypertrophic chondrocytes [98,99,100], while VEGFA is mainly found in the late hypertrophic phase, correlating with osteoblast formation [95,101,102,103]. Lastly, TGF-β increased COL1A1 expression, which is associated with dedifferentiation towards fibrotic chondrocytes. It is present in the superficial layer of the cartilage or near cartilage lesions. Since it is found to be correlated with OA severity and also upregulated by TGF-β levels comparable to those found in OA patients, we included this factor in our study [75,76,104,105,106,107]. Because we saw induction of these genes in our model, we think our model is appropriate for use to study the TGF-β-induced hypertrophy-like phenotype and that the use of this easy and quick in vitro model of the TGF-β-induced OA phenotype could be useful to screen OA treatments.
A number of TFs have been identified to regulate chondrocyte differentiation towards hypertrophy, of which many could also be activated by TGF-β [2,3]. We identified which TFs are activated by TGF-β levels comparable to those in OA patients and whether they have a role in induction of chondrocyte hypertrophy in our in vitro hypertrophy-like model in primary human OA chondrocytes. We used luciferase-based TF activity assays; all of these TFs are known to be downstream of relevant chondrocyte signaling pathways [8,26,32,33,34]. Luciferase assays have been efficiently used to define and quantify the functional connection between a protein (in our case TGF-β) and transcription activation or repression by many TF complexes [35]. Of note, we studied early activation ofTFs by TGF-β stimulation, because we wanted to exclude indirect activation of the TFs, which could occur when TGF-β activates or represses a distinct protein that itself affects transcription. In human OA chondrocytes, we did not observe NFκB-, CRE-, SRF-, GRE-, SRE-, ISRE-, NFAT5- TCF/LEF-, PPRE-, SOX9-, CSL- and ARE-controlled pathways to be activated by prolonged TGF-β stimulation. However, this does not mean that these pathways do not play a role in other OA pathology or even chondrocyte hypertrophy—merely that their direct role in TGF-β-induced hypertrophy is possibly limited. The molecular control of the chondrocyte phenotype is kept in check by dozens of TFs and chondrogenic transcriptional networks, not only by TGF-β [108]. There are various ways that could lead to chondrocyte hypertrophy. For example, SOX9, TCF/LEF (Wnt signaling), NFAT5 and CREB are also found to be associated with chondrocyte differentiation [97,108].
In this study, we found the SIE pathway to be activated by TGF-β in OA chondrocytes. The SIE pathway runs via activation of STAT3 [78]. We reported earlier that TGF-β activates STAT3 signaling itself, and also that TGF-β stimulates IL-6 production by articular chondrocytes, which then also activates STAT3 [109]. In this study, SIE signaling could be completely blocked when pre-stimulated with Tofacitinib, which is an inhibitor that targets JAK kinases upstream of STAT3. Tofacitinib is already clinically effective in rheumatoid arthritis but has not yet been tested in OA patients [110,111,112]. There are indications that JAK-STAT inhibition could be promising to target OA pathology. For instance, Tofacitinib blocked cytokine-induced proteoglycan loss and restored COL2 synthesis in bovine cartilage [113]. Additionally, in experimental OA mouse models, JAK-STAT inhibition appears to have protective effects [65,114,115]. On the other hand, our results demonstrate that Tofacitinib was not able to block TGF-β-induced expression of RUNX2, COL10A1, VEGFA and COL1A1 in OA chondrocytes, suggesting a limited role for JAK-STAT signaling in TGF-β-driven hypertrophy. It would be interesting to elucidate if targeting JAK-STAT has beneficial roles in joint biology and OA development [116].
Previous research established that the transcription factor AP1 can directly interact with SOX9 and RUNX2, thereby promoting hypertrophic gene expression [117,118]. Therefore, AP-1 signaling was also included in this study, and it was found to be activated by TGF-β stimulation in OA chondrocytes as well. AP1 consists of the subunits c-JUN and c-FOS, which were formed after MAPKs activation. The three separate groups of the MAPKs consist of the ERKs, p38s and the JNKs, which in turn are all activated by different MAPKKS (MEK1-7) [80,81]. Despite major progress in understanding AP1 signaling and function, as well as the identification of small molecules integrating with AP1 signaling pathways, no effective AP1 inhibitors have yet been approved for clinical use [36,119]. In our study, we completely blocked the AP1 pathway by inhibiting JNK with the inhibitor SP-600125, which suggests that AP1 signaling in human OA chondrocytes is dependent on JNK signaling. Nevertheless, we found that blocking JNK did not inhibit the TGF-β-induced hypertrophy-like phenotype in OA chondrocytes. According to the literature, JNK activation is associated with decreased proteoglycan synthesis and enhanced MMP13 expression and is linked to osteoblast proliferation and development of osteosarcoma [39,120,121]. A possible role for AP1 signaling in induction of chondrocyte hypertrophy in OA could still exists, although not in the TGF-β-induced hypertrophy-like phenotype of chondrocytes.
As expected, we found the SBE signaling pathway to be activated by TGF-β in OA chondrocytes, which is regulated by the TFs SMAD3 and SMAD4. Moreover, blocking ALK4/5/7 kinase activity with the inhibitor SB-505124 abolished the effect of TGF-β on hypertrophy-related genes. The type I receptor ALK5 is known to be specific for TGF-β ligands, and ALK4 and ALK7 are thought to mediate signaling via the ligands activin and nodal[114,115,122]. This suggests that receptor SMADs, activated by TGF-β-induced ALK5 phosphorylation, are involved in TGF-β-driven hypertrophy. These receptor SMADs include the homeostatic SMAD2 and SMAD3, as well as SMAD1 and SMAD5. Activation of SMAD3, the transcription factor of the SBE construct, is associated with inhibition of chondrocyte hypertrophy [9,10]. Meanwhile, signaling via SMAD1/5 promotes pro-hypertrophic signaling via RUNX2 and expression of COL10A1 [6,11,12,13]. The detrimental SMAD1/5 signaling route, for which activation was not reflected using our SBE construct, is mostly activated by bone morphogenetic proteins (BMPs) [6]. However, we reported earlier that TGF-β is also able to induce SMAD1/5 signaling, via activation of the ALK5:ALK1 receptor complex, and this activation is also inhibited using SB-505124 [123]. Previous studies have shown that this TGF-β-induced SMAD1/5 signaling does not act via BMP response elements (BREs), since TGF-β failed to induce BRE-luciferase activity [124,125], and therefore we have not measured these SMADs with a BRE-TF construct. The importance of ALK5 kinase activity in SMAD1/5 signaling was already demonstrated by Goumans et al. [120] Of note, it is known that high levels of TGF-β during OA conditions skew more to ALK1/SMAD1/5 signaling in chondrocytes, and ageing a shift towards ALK1 is observed, leading to increased MMP13 expression [85]. Additionally, Qu et al. demonstrated that high doses of TGF-β contribute to degeneration of nucleus pulposus cells in the intervertebral disc via upregulation of ALK1 and SMAD1/5 [121]. Moreover, gene expression analysis in experimental OA models and human OA samples showed upregulation of the TGF-β co-receptor Cripto, which participates in a TGF-β-ALK1-Cripto receptor complex, thereby inducing SMAD1/5 signaling in chondrocytes and induction of hypertrophy [126]. Thus, the impact of increased TGF-β activation in articular cartilage may move from homeostatic SMAD2/3 signaling towards pathological SMAD1/5 signaling, depending on its levels, context and receptor usage.
In conclusion, the TGF-β-induced hypertrophy-like phenotype in human OA chondrocytes is dependent on ALK5 kinase activity, of which the SMADs are a prominent target. Although STAT3 and AP1 signaling are also activated by levels of TGF-β similar to those present in OA patients, these appear not to contribute to TGF-β-induced expression of hypertrophic and dedifferentiation genes. Future studies should indicate whether this increased expression of OA genes due to high levels of TGF-β is a result of increased SMAD1/5 signaling activated by ALK5 kinase. TGF-β is of great importance for chondrocyte homeostasis in healthy cartilage but at the same time also a driver for hypertrophy and dedifferentiation in OA circumstances. Therefore, improvement of our understanding of the different subsets of TGF-β-mediated signaling and how they function differently in healthy versus OA chondrocytes is crucial. Inhibition of chondrocyte hypertrophy-like changes by use of inhibitors targeting specific hypertrophy-inducing pathways in TGF-β-signaling might be a promising therapeutic target for slowing down OA progression.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cells11071232/s1, Table S1: Full promoter sequences containing transcription factor elements (bold) driven by a minimal promoter including a TATA-box (underlined), Figure S1: Primary human OA chondrocytes can be efficiently transduced with lentivirus, thereby not affecting TGF-β1-induced signaling, Figure S2: Investigation of transcription factor activation by 5 ng/ml TGF-β1 in primary human OA chondrocytes, Figure S3: TGF-β1 activates SMAD3:4, STAT3 and AP1 signaling in human OA chondrocytes, Figure S4: Late-hypertrophic genes ALPL, MMP13 and ADAMTS5 are not increased by TGF-β1 in primary human OA chondrocytes.
Author Contributions
Conceptualization, N.G.M.T., A.P.M.v.C. and P.M.v.d.K.; methodology, N.G.M.T., A.P.M.v.C. and P.M.v.d.K.; validation, N.G.M.T., M.N., E.L.V., H.M.v.B. and A.P.M.v.C.; formal analysis, N.G.M.T.; investigation, N.G.M.T., M.N., E.L.V., H.M.v.B. and A.P.M.v.C.; data curation, N.G.M.T.; writing—original draft preparation, N.G.M.T.; writing—review and editing, N.G.M.T., M.N., E.L.V., H.M.v.B., A.B.B., M.I.K., P.L.E.M.v.L., F.A.J.v.d.L., E.N.B.D., A.P.M.v.C. and P.M.v.d.K.; visualization, N.G.M.T.; supervision, E.N.B.D., A.P.M.v.C. and P.M.v.d.K.; project administration, P.M.v.d.K.; funding acquisition, P.M.v.d.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from the Dutch Arthritis Foundation (ReumaNederland), under grant number 16-1-40.
Institutional Review Board Statement
The study was approved by the local ethics review board (CMO region Arnhem–Nijmegen, the Netherlands), approval number 2018-4319.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mariani E., Pulsatelli L., Facchini A. Signaling Pathways in Cartilage Repair. Int. J. Mol. Sci. 2014;15:8667–8698. doi: 10.3390/ijms15058667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van der Kraan P.M., Van den Berg W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012;20:223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Ripmeester E.G.J., Timur U.T., Caron M.M.J., Welting T.J.M. Recent Insights into the Contribution of the Changing Hypertrophic Chondrocyte Phenotype in the Development and Progression of Osteoarthritis. Front. Bioeng. Biotechnol. 2018;6:18. doi: 10.3389/fbioe.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh P., Marcu K.B., Goldring M.B., Otero M. Phenotypic instability of chondrocytes in osteoarthritis: On a path to hypertrophy. Ann. New York Acad. Sci. 2018;1442:17–34. doi: 10.1111/nyas.13930. [DOI] [PubMed] [Google Scholar]
- 5.Jung Y.K., Park H.-R., Cho H.-J., Jang J.-A., Lee E.-J., Han M.-S., Kim G.-W., Han S. Degrading products of chondroitin sulfate can induce hypertrophy-like changes and MMP-13/ADAMTS5 production in chondrocytes. Sci. Rep. 2019;9:15846. doi: 10.1038/s41598-019-52358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thielen N.G.M., van der Kraan P.M., van Caam A.P.M. TGFbeta/BMP Signaling Pathway in Cartilage Homeostasis. Cells. 2019;8:969. doi: 10.3390/cells8090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Kraan P.M. Differential Role of Transforming Growth Factor-beta in an Osteoarthritic or a Healthy Joint. J. Bone Metab. 2018;25:65–72. doi: 10.11005/jbm.2018.25.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong L., Huang X., Karperien M., Post J.N. The Regulatory Role of Signaling Crosstalk in Hypertrophy of MSCs and Human Articular Chondrocytes. Int. J. Mol. Sci. 2015;16:19225–19247. doi: 10.3390/ijms160819225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C.G., Thuillier D., Chin E.N., Alliston T. Chondrocyte-intrinsic Smad3 represses Runx2-inducible matrix metalloproteinase 13 expression to maintain articular cartilage and prevent osteoarthritis. Arthritis Care Res. 2012;64:3278–3289. doi: 10.1002/art.34566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim K.O., Sampson E.R., Maynard R.D., O’Keefe R.J., Chen D., Drissi H., Rosier R.N., Hilton M.J., Zuscik M.J. Ski inhibits TGF-beta/phospho-Smad3 signaling and accelerates hypertrophic differentiation in chondrocytes. J. Cell Biochem. 2012;113:2156–2166. doi: 10.1002/jcb.24089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horiki M., Imamura T., Okamoto M., Hayashi M., Murai J., Myoui A., Ochi T., Miyazono K., Yoshikawa H., Tsumaki N. Smad6/Smurf1 overexpression in cartilage delays chondrocyte hypertrophy and causes dwarfism with osteopenia. J. Cell Biol. 2004;165:433–445. doi: 10.1083/jcb.200311015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javed A., Bae J.-S., Afzal F., Gutierrez S., Pratap J., Zaidi S.K., Lou Y., van Wijnen A.J., Stein J.L., Stein G.S., et al. Structural Coupling of Smad and Runx2 for Execution of the BMP2 Osteogenic Signal. J. Biol. Chem. 2008;283:8412–8422. doi: 10.1074/jbc.M705578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leboy P., Grasso-Knight G., D’Angelo M., Volk S.W., Lian J.V., Drissi H., Stein G.S., Adams S.L. Smad-Runx interactions during chondrocyte maturation. Pt 1J. Bone. Joint. Surg. Am. 2001;83-A((Suppl. 1)):S15–S22. doi: 10.2106/00004623-200100001-00003. [DOI] [PubMed] [Google Scholar]
- 14.Li T.F., O’Keefe R.J., Chen D. TGF-beta signaling in chondrocytes. Front. Biosci. 2005;10:681–688. doi: 10.2741/1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiao B., Padilla S.R., Benya P.D. Transforming growth factor (TGF)-beta-activated kinase 1 mimics and mediates TGF-beta-induced stimulation of type II collagen synthesis in chondrocytes independent of Col2a1 transcription and Smad3 signaling. J. Biol. Chem. 2005;280:17562–17571. doi: 10.1074/jbc.M500646200. [DOI] [PubMed] [Google Scholar]
- 16.Coricor G., Serra R. TGF-beta regulates phosphorylation and stabilization of Sox9 protein in chondrocytes through p38 and Smad dependent mechanisms. Sci. Rep. 2016;6:38616. doi: 10.1038/srep38616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong Y.F., Soung D.Y., Chang Y., Enomoto-Iwamoto M., Paris M., O’Keefe R.J., Schwarz E.M., Drissi H. Transforming growth factor-beta and Wnt signals regulate chondrocyte differentiation through Twist1 in a stage-specific manner. Mol. Endocrinol. 2007;21:2805–2820. doi: 10.1210/me.2007-0199. [DOI] [PubMed] [Google Scholar]
- 18.Ionescu A.M., Schwarz E.M., Zuscik M.J., Drissi H., Puzas J.E., Rosier R.N., O’Keefe R.J. ATF-2 cooperates with Smad3 to mediate TGF-beta effects on chondrocyte maturation. Exp. Cell. Res. 2003;288:198–207. doi: 10.1016/S0014-4827(03)00181-2. [DOI] [PubMed] [Google Scholar]
- 19.Van den Bosch M.H., Blom A.B., van Lent P.L., van Beuningen H.M., Davidson E.N.B., van der Kraan P.M., van den Berg W.B. Canonical Wnt signaling skews TGF-beta signaling in chondrocytes towards signaling via ALK1 and Smad 1/5/8. Cell. Signal. 2014;26:951–958. doi: 10.1016/j.cellsig.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Van Beuningen H.M., Van Der Kraan P.M., Arntz O.J., Berg W.B.V.D. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab. Investig. 1994;71:279–290. [PubMed] [Google Scholar]
- 21.Bakker A., van de Loo F., van Beuningen H., Sime P., van Lent P., van der Kraan P., Richards C., Berg W.V.D. Overexpression of active TGF-beta-1 in the murine knee joint: Evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthr. Cartil. 2001;9:128–136. doi: 10.1053/joca.2000.0368. [DOI] [PubMed] [Google Scholar]
- 22.Scharstuhl A., Glansbeek H.L., van Beuningen H.M., Vitters E.L., van der Kraan P.M., van den Berg W.B. Inhibition of endogenous TGF-beta during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J. Immunol. 2002;169:507–514. doi: 10.4049/jimmunol.169.1.507. [DOI] [PubMed] [Google Scholar]
- 23.Ji Q., Zheng Y., Zhang G., Hu Y., Fan X., Hou Y., Wen L., Li L., Xu Y., Wang Y., et al. Single-cell RNA-seq analysis reveals the progression of human osteoarthritis. Ann. Rheum. Dis. 2019;78:100–110. doi: 10.1136/annrheumdis-2017-212863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen J., Li S., Chen D. TGF-beta signaling and the development of osteoarthritis. Bone Res. 2014;2:14002. doi: 10.1038/boneres.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi S., Wan F., Zhou Z., Tao R., Lu Y., Zhou M., Liu F., Liu Y. Identification of key regulators responsible for dysregulated networks in osteoarthritis by large-scale expression analysis. J. Orthop. Surg. Res. 2021;16:259. doi: 10.1186/s13018-021-02402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boer C.G., Hatzikotoulas K., Southam L., Stefánsdóttir L., Zhang Y., Coutinho de Almeida R., Wu T.T., Zheng J., Hartley A., Teder-Laving M., et al. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell. 2021;184:4784–4818.e17. doi: 10.1016/j.cell.2021.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fava R., Olsen N., Keski-Oja J., Moses H., Pincus T. Active and latent forms of transforming growth factor beta activity in synovial effusions. J. Exp. Med. 1989;169:291–296. doi: 10.1084/jem.169.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koli K., Saharinen J., Hyytiäinen M., Penttinen C., Keski-Oja J. Latency, activation, and binding proteins of TGF-beta. Microsc. Res. Tech. 2001;52:354–362. doi: 10.1002/1097-0029(20010215)52:4<354::AID-JEMT1020>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Q., Qiu Y.-T., Chen M.-J., Zhang Z.-Y., Yang C. Synovial TGF-beta1 and MMP-3 levels and their correlation with the progression of temporomandibular joint osteoarthritis combined with disc displacement: A preliminary study. Biomed. Rep. 2013;1:218–222. doi: 10.3892/br.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandy J., Chan D., Trevino R., Wimmer M., Plaas A. Human genome-wide expression analysis reorients the study of inflammatory mediators and biomechanics in osteoarthritis. Osteoarthr. Cartil. 2015;23:1939–1945. doi: 10.1016/j.joca.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zielinski S., Bartels K., Cebulski K., Kühne C., Kekow J. Evidence of proteolytic activation of transforming growth factor beta in synovial fluid. Adv. Exp. Med. Biol. 2000;477:477–481. doi: 10.1007/0-306-46826-3_48. [DOI] [PubMed] [Google Scholar]
- 32.Gao X., Sun Y., Li X. Identification of key gene modules and transcription factors for human osteoarthritis by weighted gene co-expression network analysis. Exp. Ther. Med. 2019;18:2479–2490. doi: 10.3892/etm.2019.7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisch K., Gamini R., Alvarez-Garcia O., Akagi R., Saito M., Muramatsu Y., Sasho T., Koziol J., Su A.I., Lotz M. Identification of transcription factors responsible for dysregulated networks in human osteoarthritis cartilage by global gene expression analysis. Osteoarthr. Cartil. 2018;26:1531–1538. doi: 10.1016/j.joca.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neefjes M., van Caam A.P.M., van der Kraan P.M. Transcription Factors in Cartilage Homeostasis and Osteoarthritis. Biology. 2020;9:290. doi: 10.3390/biology9090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neefjes M., Housmans B.A.C., van den Akker G.G.H., van Rhijn L.W., Welting T.J.M., van der Kraan P.M. Reporter gene comparison demonstrates interference of complex body fluids with secreted luciferase activity. Sci. Rep. 2021;11:1359. doi: 10.1038/s41598-020-80451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ge H.-X., Zou F.-M., Li Y., Liu A.-M., Tu M. JNK pathway in osteoarthritis: Pathological and therapeutic aspects. J. Recept. Signal Transduct. 2017;37:431–436. doi: 10.1080/10799893.2017.1360353. [DOI] [PubMed] [Google Scholar]
- 37.Kameda T., Watanabe H., Iba H. C-Jun and JunD suppress maturation of chondrocytes. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 1997;8:495–503. [PubMed] [Google Scholar]
- 38.Ionescu A.M., Schwarz E.M., Vinson C., Puzas J.E., Rosier R., Reynolds P.R., O’Keefe R.J. PTHrP Modulates Chondrocyte Differentiation through AP-1 and CREB Signaling. J. Biol. Chem. 2001;276:11639–11647. doi: 10.1074/jbc.M006564200. [DOI] [PubMed] [Google Scholar]
- 39.Moritani N.H., Kubota S., Eguchi T., Fukunaga T., Yamashiro T., Takano-Yamamoto T., Tahara H., Ohyama K., Sugahara T., Takigawa M. Interaction of AP-1 and the ctgf gene: A possible driver of chondrocyte hypertrophy in growth cartilage. J. Bone Miner. Metab. 2003;21:205–210. doi: 10.1007/s00774-003-0410-1. [DOI] [PubMed] [Google Scholar]
- 40.Haseeb A., Kc R., Angelozzi M., de Charleroy C., Rux D., Tower R.J., Yao L., da Silva R.P., Pacifici M., Qin L., et al. SOX9 keeps growth plates and articular cartilage healthy by inhibiting chondrocyte dedifferentiation/osteoblastic redifferentiation. Proc. Natl. Acad. Sci. USA. 2021;118:e2019152118. doi: 10.1073/pnas.2019152118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang B., Mamidi M.K., Samsa W.E., Chen Y., Lee B., Zheng Q., Zhou G. Targeted and sustained Sox9 expression in mouse hypertrophic chondrocytes causes severe and spontaneous osteoarthritis by perturbing cartilage homeostasis. Am. J. Transl. Res. 2020;12:1056–1069. [PMC free article] [PubMed] [Google Scholar]
- 42.Ikegami D., Akiyama H., Suzuki A., Nakamura T., Nakano T., Yoshikawa H., Tsumaki N. Sox9 sustains chondrocyte survival and hypertrophy in part through Pik3ca-Akt pathways. Development. 2011;138:1507–1519. doi: 10.1242/dev.057802. [DOI] [PubMed] [Google Scholar]
- 43.Choi M.C., Jo J., Park J., Kang H.K., Park Y. NF-kappaB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells. 2019;8:734. doi: 10.3390/cells8070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito T., Tanaka S. Molecular mechanisms underlying osteoarthritis development: Notch and NF-kappaB. Arthritis Res. Ther. 2017;19:94. doi: 10.1186/s13075-017-1296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olivotto E., Otero M., Marcu K.B., Goldring M.B. Pathophysiology of osteoarthritis: Canonical NF-kappaB/IKKbeta-dependent and kinase-independent effects of IKKalpha in cartilage degradation and chondrocyte differentiation. RMD Open. 2015;1((Suppl. 1)):e000061. doi: 10.1136/rmdopen-2015-000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sassi N., Gadgadi N., Laadhar L., Allouche M., Mourali S., Zandieh-Doulabi B., Hamdoun M., Nulend J.K., Makni S., Sellami S. Notch signaling is involved in human articular chondrocytes de-differentiation during osteoarthritis. J. Recept. Signal Transduct. 2013;34:48–57. doi: 10.3109/10799893.2013.856920. [DOI] [PubMed] [Google Scholar]
- 47.Sassi N., Laadhar L., Driss M., Kallel-Sellami M., Sellami S., Makni S. The role of the Notch pathway in healthy and osteoarthritic articular cartilage: From experimental models to ex vivo studies. Arthritis Res. Ther. 2011;13:208. doi: 10.1186/ar3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao D., Bi R., Liu X., Mei J., Jiang N., Zhu S. Notch Signaling Regulates MMP-13 Expression via Runx2 in Chondrocytes. Sci. Rep. 2019;9:15596. doi: 10.1038/s41598-019-52125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zieba J.T., Chen Y.-T., Lee B.H., Bae Y. Notch Signaling in Skeletal Development, Homeostasis and Pathogenesis. Biomolecules. 2020;10:332. doi: 10.3390/biom10020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macfarlane E., Seibel M.J., Zhou H. Arthritis and the role of endogenous glucocorticoids. Bone Res. 2020;8:33. doi: 10.1038/s41413-020-00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nalesso G., Sherwood J., Bertrand J., Pap T., Ramachandran M., De Bari C., Pitzalis C., Dell’Accio F. WNT-3A modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. J. Cell Biol. 2011;193:551–564. doi: 10.1083/jcb.201011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Savvidou O., Milonaki M., Goumenos S., Flevas D., Papagelopoulos P., Moutsatsou P. Glucocorticoid signaling and osteoarthritis. Mol. Cell. Endocrinol. 2019;480:153–166. doi: 10.1016/j.mce.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Hartmann K., Koenen M., Schauer S., Wittig-Blaich S., Ahmad M., Baschant U., Tuckermann J.P. Molecular Actions of Glucocorticoids in Cartilage and Bone During Health, Disease, and Steroid Therapy. Physiol. Rev. 2016;96:409–447. doi: 10.1152/physrev.00011.2015. [DOI] [PubMed] [Google Scholar]
- 54.Tu J., Zhang P., Ji Z., Henneicke H., Li J., Kim S., Swarbrick M.M., Wu Y., Little C.B., Seibel M.J., et al. Disruption of glucocorticoid signalling in osteoblasts attenuates age-related surgically induced osteoarthritis. Osteoarthr. Cartil. 2019;27:1518–1525. doi: 10.1016/j.joca.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Latourte A., Cherifi C., Maillet J., Ea H.-K., Bouaziz W., Funck-Brentano T., Cohen-Solal M., Hay E., Richette P. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann. Rheum. Dis. 2017;76:748–755. doi: 10.1136/annrheumdis-2016-209757. [DOI] [PubMed] [Google Scholar]
- 56.Held A., Glas A., Dietrich L., Bollmann M., Brandstädter K., Grossmann T.N., Lohmann C.H., Pap T., Bertrand J. Targeting beta-catenin dependent Wnt signaling via peptidomimetic inhibitors in murine chondrocytes and OA cartilage. Osteoarthr. Cartil. 2018;26:818–823. doi: 10.1016/j.joca.2018.02.908. [DOI] [PubMed] [Google Scholar]
- 57.Hwang S.-G., Yu S.-S., Lee S.-W., Chun J.-S. Wnt-3a regulates chondrocyte differentiation via c-Jun/AP-1 pathway. FEBS Lett. 2005;579:4837–4842. doi: 10.1016/j.febslet.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 58.Kim S., Koga T., Isobe M., Kern B.E., Yokochi T., Chin Y.E., Karsenty G., Taniguchi T., Takayanagi H. Stat1 functions as a cytoplasmic attenuator of Runx2 in the transcriptional program of osteoblast differentiation. Genes Dev. 2003;17:1979–1991. doi: 10.1101/gad.1119303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Damerau A., Gaber T., Ohrndorf S., Hoff P. JAK/STAT Activation: A General Mechanism for Bone Development, Homeostasis, and Regeneration. Int. J. Mol. Sci. 2020;21:9004. doi: 10.3390/ijms21239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kondo M., Yamaoka K., Sakata K., Sonomoto K., Lin L., Nakano K., Tanaka Y. Contribution of the Interleukin-6/STAT-3 Signaling Pathway to Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Arthritis Rheumatol. 2015;67:1250–1260. doi: 10.1002/art.39036. [DOI] [PubMed] [Google Scholar]
- 61.Litherland G.J., Elias M.S., Hui W., Macdonald C.D., Catterall J.B., Barter M.J., Farren M.J., Jefferson M., Rowan A.D. Protein kinase C isoforms zeta and iota mediate collagenase expression and cartilage destruction via STAT3- and ERK-dependent c-fos induction. J. Biol. Chem. 2010;285:22414–22425. doi: 10.1074/jbc.M110.120121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu N.Q., Lin Y., Li L., Lu J., Geng D., Zhang J., Jashashvili T., Buser Z., Magallanes J., Tassey J., et al. gp130/STAT3 signaling is required for homeostatic proliferation and anabolism in postnatal growth plate and articular chondrocytes. Commun. Biol. 2022;5:64. doi: 10.1038/s42003-021-02944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Housmans B., Neefjes M., Surtel D., Vitík M., Cremers A., van Rhijn L., van der Kraan P., Akker G.v.D., Welting T. Synovial fluid from end-stage osteoarthritis induces proliferation and fibrosis of articular chondrocytes via MAPK and RhoGTPase signaling. Osteoarthr. Cartil. 2022 doi: 10.1016/j.joca.2021.12.015. in press. [DOI] [PubMed] [Google Scholar]
- 64.Prasadam I., van Gennip S., Friis T., Shi W., Crawford R., Xiao Y. ERK-1/2 and p38 in the regulation of hypertrophic changes of normal articular cartilage chondrocytes induced by osteoarthritic subchondral osteoblasts. Arthritis Rheum. 2010;62:1349–1360. doi: 10.1002/art.27397. [DOI] [PubMed] [Google Scholar]
- 65.Öztürk E., Despot-Slade E., Pichler M., Zenobi-Wong M. RhoA activation and nuclearization marks loss of chondrocyte phenotype in crosstalk with Wnt pathway. Exp. Cell Res. 2017;360:113–124. doi: 10.1016/j.yexcr.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 66.Kubo Y., Beckmann R., Fragoulis A., Conrads C., Pavanram P., Nebelung S., Wolf M., Wruck C.J., Jahr H., Pufe T. Nrf2/ARE Signaling Directly Regulates SOX9 to Potentially Alter Age-Dependent Cartilage Degeneration. Antioxidants. 2022;11:263. doi: 10.3390/antiox11020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morita K., Miyamoto T., Fujita N., Kubota Y., Ito K., Takubo K., Miyamoto K., Ninomiya K., Suzuki T., Iwasaki R., et al. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J. Exp. Med. 2007;204:1613–1623. doi: 10.1084/jem.20062525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolduc J.A., Collins J.A., Loeser R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019;132:73–82. doi: 10.1016/j.freeradbiomed.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erickson G.R., Alexopoulos L.G., Guilak F. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. J. Biomech. 2001;34:1527–1535. doi: 10.1016/S0021-9290(01)00156-7. [DOI] [PubMed] [Google Scholar]
- 70.Van der Windt A.E., Haak E., Das R.H.J., Kops N., Welting T.J.M., Caron M.M.J., van Til N.P., Verhaar J.A.N., Weinans H., Jahr H. Physiological tonicity improves human chondrogenic marker expression through nuclear factor of activated T-cells 5 in vitro. Arthritis Res. Ther. 2010;12:R100. doi: 10.1186/ar3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson Z.I., Shapiro I.M., Risbud M.V. Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: Evolving role of TonEBP. Matrix Biol. 2014;40:10–16. doi: 10.1016/j.matbio.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Francois M., Richette P., Tsagris L., Raymondjean M., Fulchignoni-Lataud M.-C., Forest C., Savouret J.-F., Corvol M.-T. Peroxisome proliferator-activated receptor-gamma down-regulates chondrocyte matrix metalloproteinase-1 via a novel composite element. J. Biol. Chem. 2004;279:28411–28418. doi: 10.1074/jbc.M312708200. [DOI] [PubMed] [Google Scholar]
- 73.Poleni P.E., Bianchi A., Etienne S., Koufany M., Sebillaud S., Netter P., Terlain B., Jouzeau J.Y. Agonists of peroxisome proliferators-activated receptors (PPAR) alpha, beta/delta or gamma reduce transforming growth factor (TGF)-beta-induced proteoglycans’ production in chondrocytes. Osteoarthr. Cartil. 2007;15:493–505. doi: 10.1016/j.joca.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 74.Poleni P.E., Etienne S., Velot E., Netter P., Bianch A. Activation of PPARs alpha, beta/delta, and gamma Impairs TGF-beta1-Induced Collagens’ Production and Modulates the TIMP-1/MMPs Balance in Three-Dimensional Cultured Chondrocytes. PPAR Res. 2010;2010:635912. doi: 10.1155/2010/635912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhong L., Huang X., Karperien M., Post J.N. Correlation between Gene Expression and Osteoarthritis Progression in Human. Int. J. Mol. Sci. 2016;17:1126. doi: 10.3390/ijms17071126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Truong L.-H., Kuliwaba J.S., Tsangari H., Fazzalari N.L. Differential gene expression of bone anabolic factors and trabecular bone architectural changes in the proximal femoral shaft of primary hip osteoarthritis patients. Arthritis Res. Ther. 2006;8:R188. doi: 10.1186/ar2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DaCosta Byfield S., Major C., Laping N.J., Roberts A.B. SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2004;65:744–752. doi: 10.1124/mol.65.3.744. [DOI] [PubMed] [Google Scholar]
- 78.Lau Y.-T.K., Ramaiyer M., Johnson D.E., Grandis J.R. Targeting STAT3 in Cancer with Nucleotide Therapeutics. Cancers. 2019;11:1681. doi: 10.3390/cancers11111681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adis Editorial Tofacitinib. Drugs R. D. 2010;10:271–284. doi: 10.2165/11588080-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gazon H., Barbeau B., Mesnard J.-M., Peloponese J.-M., Jr. Hijacking of the AP-1 Signaling Pathway during Development of ATL. Front. Microbiol. 2017;8:2686. doi: 10.3389/fmicb.2017.02686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eferl R., Wagner E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 82.Albro M.B., Nims R.J., Cigan A.D., Yeroushalmi K.J., Shim J.J., Hung C.T., Ateshian G.A. Dynamic mechanical compression of devitalized articular cartilage does not activate latent TGF-beta. J. Biomech. 2013;46:1433–1439. doi: 10.1016/j.jbiomech.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sokolove J., Lepus C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013;5:77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robinson W.H., Lepus C.M., Wang Q., Raghu H., Mao R., Lindstrom T.M., Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016;12:580–592. doi: 10.1038/nrrheum.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blaney Davidson E.N., Remst D.F.G., Vitters E.L., van Beuningen H.M., Blom A.B., Goumans M.-J., van den Berg W.B., van der Kraan P.M. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J. Immunol. 2009;182:7937–7945. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- 86.Davidson E.N.B., Scharstuhl A., Vitters E.L., Van Der Kraan P.M., Berg W.B.V.D. Reduced transforming growth factor-beta signaling in cartilage of old mice: Role in impaired repair capacity. Arthritis Res. Ther. 2005;7:R1338–R1347. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cherifi C., Monteagudo S., Lories R.J. Promising targets for therapy of osteoarthritis: A review on the Wnt and TGF-beta signalling pathways. Ther. Adv. Musculoskelet. Dis. 2021;13:1759720X211006959. doi: 10.1177/1759720X211006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van der Kraan P.M. The changing role of TGFbeta in healthy, ageing and osteoarthritic joints. Nat. Rev. Rheumatol. 2017;13:155–163. doi: 10.1038/nrrheum.2016.219. [DOI] [PubMed] [Google Scholar]
- 89.Allas L., Rochoux Q., Leclercq S., Boumediene K., Baugé C. Development of a simple osteoarthritis model useful to predict in vitro the anti-hypertrophic action of drugs. Lab. Investig. 2020;100:64–71. doi: 10.1038/s41374-019-0303-0. [DOI] [PubMed] [Google Scholar]
- 90.Babur B.K., Futrega K., Lott W., Klein T., Cooper-White J., Doran M.R. High-throughput bone and cartilage micropellet manufacture, followed by assembly of micropellets into biphasic osteochondral tissue. Cell Tissue Res. 2015;361:755–768. doi: 10.1007/s00441-015-2159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Antoniou J., Wang H.T., Alaseem A.M., Haglund L., Roughley P.J., Mwale F. The effect of Link N on differentiation of human bone marrow-derived mesenchymal stem cells. Arthritis Res. Ther. 2012;14:R267. doi: 10.1186/ar4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mueller M.B., Tuan R.S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Care Res. 2008;58:1377–1388. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kirsch T., von der Mark K. Remodelling of collagen types I, II and X and calcification of human fetal cartilage. Bone Miner. 1992;18:107–117. doi: 10.1016/0169-6009(92)90851-4. [DOI] [PubMed] [Google Scholar]
- 94.Nurminskaya M., Linsenmayer T.F. Identification and characterization of up-regulated genes during chondrocyte hypertrophy. Dev. Dyn. 1996;206:260–271. doi: 10.1002/(SICI)1097-0177(199607)206:3<260::AID-AJA4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 95.Li J., Dong S. The Signaling Pathways Involved in Chondrocyte Differentiation and Hypertrophic Differentiation. Stem Cells Int. 2016;2016:2470351. doi: 10.1155/2016/2470351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rashid H., Chen H., Javed A. Runx2 is required for hypertrophic chondrocyte mediated degradation of cartilage matrix during endochondral ossification. Matrix Biol. Plus. 2021;12:10008. doi: 10.1016/j.mbplus.2021.100088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Solomon L.A., Bérubé N.G., Beier F. Transcriptional regulators of chondrocyte hypertrophy. Birth Defects Res. Part C Embryo Today Rev. 2008;84:123–130. doi: 10.1002/bdrc.20124. [DOI] [PubMed] [Google Scholar]
- 98.Amano K., Densmore M., Nishimura R., Lanske B. Indian hedgehog signaling regulates transcription and expression of collagen type X via Runx2/Smads interactions. J. Biol. Chem. 2014;289:24898–24910. doi: 10.1074/jbc.M114.570507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou J., Wei X., Wei L. Indian Hedgehog, a critical modulator in osteoarthritis, could be a potential therapeutic target for attenuating cartilage degeneration disease. Connect. Tissue Res. 2014;55:257–261. doi: 10.3109/03008207.2014.925885. [DOI] [PubMed] [Google Scholar]
- 100.Vortkamp A., Lee K., Lanske B., Segre G.V., Kronenberg H.M., Tabin C.J. Regulation of Rate of Cartilage Differentiation by Indian Hedgehog and PTH-Related Protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 101.Gerber H.-P., Vu T.H., Ryan A.M., Kowalski J., Werb Z., Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 102.Nagao M., Hamilton J.L., Kc R., Berendsen A.D., Duan X., Cheong C.W., Li X., Im H.-J., Olsen B.R. Vascular Endothelial Growth Factor in Cartilage Development and Osteoarthritis. Sci. Rep. 2017;7:13027. doi: 10.1038/s41598-017-13417-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takano S., Uchida K., Shoji S., Itakura M., Iwase D., Aikawa J., Mukai M., Sekiguchi H., Inoue G., Takaso M. Vascular Endothelial Growth Factor Is Regulated by the Canonical and Noncanonical Transforming Growth Factor-beta Pathway in Synovial Fibroblasts Derived from Osteoarthritis Patients. Biomed. Res. Int. 2019;2019:6959056. doi: 10.1155/2019/6959056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Charlier E., Deroyer C., Ciregia F., Malaise O., Neuville S., Plener Z., Malaise M., de Seny D. Chondrocyte dedifferentiation and osteoarthritis (OA) Biochem. Pharmacol. 2019;165:49–65. doi: 10.1016/j.bcp.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 105.Rim Y.A., Ju J.H. The Role of Fibrosis in Osteoarthritis Progression. Life. 2020;11:3. doi: 10.3390/life11010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deroyer C., Charlier E., Neuville S., Malaise O., Gillet P., Kurth W., Chariot A., Malaise M., De Seny D. CEMIP (KIAA1199) induces a fibrosis-like process in osteoarthritic chondrocytes. Cell Death Dis. 2019;10:103. doi: 10.1038/s41419-019-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miosge N., Hartmann M., Maelicke C., Herken R. Expression of collagen type I and type II in consecutive stages of human osteoarthritis. Histochem. Cell Biol. 2004;122:229–236. doi: 10.1007/s00418-004-0697-6. [DOI] [PubMed] [Google Scholar]
- 108.Liu C.F., Samsa W.E., Zhou G., Lefebvre V. Transcriptional control of chondrocyte specification and differentiation. Semin. Cell. Dev. Biol. 2017;62:34–49. doi: 10.1016/j.semcdb.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wiegertjes R., van Caam A., van Beuningen H., Koenders M., van Lent P., van der Kraan P., van de Loo F., Davidson E.B. TGF-beta dampens IL-6 signaling in articular chondrocytes by decreasing IL-6 receptor expression. Osteoarthr. Cartil. 2019;27:1197–1207. doi: 10.1016/j.joca.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 110.Wiegertjes R., Loo F.A.J.V.D., Davidson E.N.B. A roadmap to target interleukin-6 in osteoarthritis. Rheumatology. 2020;59:2681–2694. doi: 10.1093/rheumatology/keaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kang S., Tanaka T., Narazaki M., Kishimoto T. Targeting Interleukin-6 Signaling in Clinic. Immunity. 2019;50:1007–1023. doi: 10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 112.O’Shea J.J., Kontzias A., Yamaoka K., Tanaka Y., Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann. Rheum. Dis. 2013;72:ii111–ii115. doi: 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kjelgaard-Petersen C.F., Sharma N., Kayed A., Karsdal M.A., Mobasheri A., Hägglund P., Bay-Jensen A.-C., Thudium C.S. Tofacitinib and TPCA-1 exert chondroprotective effects on extracellular matrix turnover in bovine articular cartilage ex vivo. Biochem. Pharmacol. 2019;165:91–98. doi: 10.1016/j.bcp.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 114.Heldin C.H., Miyazono K., ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 115.Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- 116.Blanco M.F., Garcia H.D., Legeai-Mallet L., van Osch G. Tyrosine kinases regulate chondrocyte hypertrophy: Promising drug targets for Osteoarthritis. Osteoarthr. Cartil. 2021;29:1389–1398. doi: 10.1016/j.joca.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 117.He X., Ohba S., Hojo H., McMahon A.P. AP-1 family members act with Sox9 to promote chondrocyte hypertrophy. Development. 2016;143:3012–3023. doi: 10.1242/dev.134502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hess J., Porte D., Munz C., Ange P. AP-1 and Cbfa/runt physically interact and regulate parathyroid hormone-dependent MMP13 expression in osteoblasts through a new osteoblast-specific element 2/AP-1 composite element. J. Biol. Chem. 2001;276:20029–20038. doi: 10.1074/jbc.M010601200. [DOI] [PubMed] [Google Scholar]
- 119.Ye N., Ding Y., Wild C., Shen Q., Zhou J. Small Molecule Inhibitors Targeting Activator Protein 1 (AP-1) J. Med. Chem. 2014;57:6930–6948. doi: 10.1021/jm5004733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goumans M.J., Valdimarsdottir G., Itoh S., Lebrin F., Larsson J., Mummery C., Karlsson S., ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol. Cell. 2003;12:817–828. doi: 10.1016/S1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 121.Qu Z., Zhang F., Chen W., Lin T., Sun Y. High-dose TGF-beta1 degrades human nucleus pulposus cells via ALK1-Smad1/5/8 activation. Exp. Ther. Med. 2020;20:3661–3668. doi: 10.3892/etm.2020.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schmierer B., Hill C.S. TGFbeta-SMAD signal transduction: Molecular specificity and functional flexibility. Nat. Rev. Mol. Cell. Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 123.Van Caam A., Madej W., de Vinuesa A.G., Goumans M.-J., ten Dijke P., Blaney Davidson E., van der Kraan P. TGFbeta1-induced SMAD2/3 and SMAD1/5 phosphorylation are both ALK5-kinase-dependent in primary chondrocytes and mediated by TAK1 kinase activity. Arthritis. Res. Ther. 2017;19:112. doi: 10.1186/s13075-017-1302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Korchynskyi O., ten Dijke P. Identification and Functional Characterization of Distinct Critically Important Bone Morphogenetic Protein-specific Response Elements in the Id1 Promoter. J. Biol. Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- 125.Zilberberg L., ten Dijke P., Sakai L.Y., Rifkin D.B. A rapid and sensitive bioassay to measure bone morphogenetic protein activity. BMC Cell Biol. 2007;8:41. doi: 10.1186/1471-2121-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garcia de Vinuesa A., Sanchez-Duffhues G., Blaney-Davidson E., van Caam A., Lodder K., Ramos Y., Kloppenburg M., Meulenbelt I., van der Kraan P., Goumans M.-J., et al. Cripto favors chondrocyte hypertrophy via TGF-beta SMAD1/5 signaling during development of osteoarthritis. J. Pathol. 2021;255:330–342. doi: 10.1002/path.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.