Abstract
Inappropriate use of antibiotics during the COVID-19 pandemic has the potential to increase the burden of antimicrobial resistance. In this study, we report on the prevalence of antibiotic use and its associated factors among suspected and confirmed COVID-19 patients admitted to 35 health facilities in Sierra Leone from March 2020–March 2021. This was a cross-sectional study using routinely collected patient data. Of 700 confirmed COVID-19 patients, 47% received antibiotics. The majority (73%) of the antibiotics belonged to the ’WATCH’ group of antibiotics, which are highly toxic and prone to resistance. The most frequently prescribed antibiotics were azithromycin, ceftriaxone, amoxicillin, metronidazole, and amoxicillin-clavulanic acid. Antibiotic use was significantly higher in patients aged 25–34 years than in those with severe disease. Of 755 suspected COVID-19 patients, 61% received antibiotics, of which the majority (58%) belonged to the ‘WATCH’ category. The most frequently prescribed antibiotics were ceftriaxone, metronidazole, azithromycin, ciprofloxacin, and amoxycillin. The prevalence of antibiotic use among suspected and confirmed COVID-19 patients admitted to healthcare facilities in Sierra Leone was high and not in line with national and WHO case management guidelines. Training of health care providers, strengthening of antimicrobial stewardship programs, and microbiological laboratory capacity are urgently needed.
Keywords: antimicrobial resistance, COVID-19, AWaRe classification, antimicrobial stewardship, antibiotic use, SORT IT, operational research, Sierra Leone
1. Introduction
Antimicrobial resistance (AMR) is a global public health concern that has the potential to reverse decades of progress in decreasing the morbidity and mortality from infectious disease outbreaks and pandemics [1,2,3]. In 2019 alone, 1.27 million deaths were attributed to bacterial AMR, with the highest age-adjusted death rates in western sub-Saharan Africa. This mortality burden is similar to the global HIV deaths (680,000) and malaria deaths (627,000) combined and ranks behind only the coronavirus disease 2019 (COVID-19) and tuberculosis in terms of global deaths from infection [4]. The number of deaths due to AMR is estimated to increase to about 10 million annually by 2050 if no actions are taken [5].
The use of antimicrobial agents (including appropriate use, inappropriate use, overuse, misuse, and underuse) drives the development and spread of AMR [6,7,8]. Several studies have confirmed that AMR rates are higher in countries that use antimicrobial drugs more often [9,10].
It has been documented that approximately one-third of patients admitted to healthcare facilities receive antimicrobial agents during their hospital stay [11,12]. More alarming is the fact that up to 50% of all courses of antimicrobial therapy are deemed unnecessary [13,14,15]. The misuse of antimicrobial agents (antibiotics) will increase multi-drug resistance (MDR) with higher mortality, longer hospital stays, and increased costs to both the patients and the hospital management [16]. This is a growing threat to the effective treatment of an increasing range of infectious disease outbreaks, such as the ongoing COVID-19 pandemic [17,18,19].
COVID-19 was declared by the World Health Organization (WHO) to be a global pandemic on 11 March 2020, and since then the disease has had devastating health and economic consequences [20,21]. As of date, there are limited treatment options for COVID-19 patients. The treatment options available include supportive care, invasive and non-invasive oxygen support, anticoagulants, and the use of systemic corticosteroids in severe and critical patients, which has been shown to prevent deaths due to COVID-19 [22,23]. While many vaccines are now approved for use, equitable access remains a challenge.
At the start of the COVID-19 outbreak in Wuhan city, Hubei province, China, in December 2019, 90% of hospitalized COVID-19 patients at their healthcare facilities received antibiotics despite little supporting evidence of associated bacterial infections [24]. The realization of the inappropriate management of COVID-19 patients led the WHO to develop clinical guidelines for managing COVID-19 patients. The WHO case management clinical guidelines recommend that people with suspected COVID-19 (while being investigated) should not be treated with antibiotics. Similarly, people with laboratory-confirmed COVID-19 who are asymptomatic or have mild symptoms should not receive antibiotic treatment or prophylaxis. Patients with moderate or severe COVID-19 should not be given antibiotics unless there is clinical suspicion of a bacterial infection, while critically ill COVID-19 patients should receive antibiotics within an hour of admission [23]. It is further recommended that the choice of antibiotic be based on clinical diagnosis, local epidemiology, antibiotic susceptibility data, and national guidelines. It is preferable to use an antibiotic with the least ecological impact, such as from the ‘access’ group of the WHO AWaRe classification of antibiotics [23]. However, despite the presence of WHO case management clinical guidelines, there is growing concern about the misuse of antibiotics in the treatment of COVID-19 patients [19,25,26].
A recent rapid review and meta-analysis that involved 154 studies globally reported that the prevalence of antibiotic use among COVID-19 patients was 74.6%, while only 8.6% had bacterial co-infection—indicating high rates of unnecessary antibiotic use [27]. Antibiotic use was higher in adults (compared to children) and in those with more severe illnesses, such as those hospitalized and requiring mechanical ventilation. The most common antibiotic classes prescribed were fluoroquinolones, macrolides, β-lactam/β-lactamase inhibitors, and cephalosporins. The prevalence of antibiotic use was higher in the Southeast Asia region and the Middle East compared to the Americas and Europe [25].
Despite the need to monitor and document antibiotic use in COVID-19 patients, there is limited evidence from Africa on the use of antibiotics among suspected and confirmed COVID-19 patients, and there are no studies on this issue from Sierra Leone. Studies documenting the use of antibiotics in COVID-19 have the potential to inform national policies and can help to design antibiotic stewardship programs in future outbreaks and the choice of antibiotics in routine patient care. Furthermore, findings from this study will add to the global body of evidence on the use of antibiotics in the management of viral infections. The aim of the study, therefore, was to assess antibiotic use among suspected and confirmed COVID-19 patients in Sierra Leone. The specific objectives were the following: (i) to assess the prevalence of antibiotic use, (ii) to classify prescribed antibiotics according to the WHO antibiotic AWaRe classification, and (iii) to determine demographic and clinical factors associated with antibiotic use among suspected and confirmed COVID-19 patients admitted to health facilities and community care centres in Sierra Leone from 31 March 2020 to 31 March 2021.
2. Materials and Methods
2.1. Study Design
This was a cross-sectional study involving secondary analysis of routinely collected patient data.
2.2. Study Setting
Sierra Leone is a country in West Africa bordered by Guinea, Liberia, and the Atlantic Ocean and is divided into 16 districts. The estimated population is 8 million and most (59%) of the people live in rural areas [28]. In 2018, the life expectancy was 53 years for males and 55 years for females, with communicable diseases accounting for 57% of all-cause mortality [25]. The total expenditure on health as a percentage of gross domestic product for Sierra Leone in 2020 was 16% [25].
2.3. Diagnosis and Management of COVID-19 Patients
Individuals suspected of COVID-19 infection (defined as any person presenting with a history of fever ≥ 37.5 °C and at least one sign/symptom of respiratory disease, e.g., cough, shortness of breath, sore throat, loss of smell, fatigue) visiting health facilities are usually admitted to the ‘isolation units’ and investigated for COVID-19 using RT-PCR (reverse transcriptase-polymerase chain reaction) laboratory tests. Asymptomatic patients were admitted during the study period to reduce community transmission. Once confirmed as COVID-19 positive, the patient is referred to the ‘COVID-19 treatment centres’ or ‘community care centres’. Sierra Leone has a total of 21 centres (18 treatment and 3 community care centres) for the management of confirmed COVID-19 patients across the country. The ‘COVID-19 treatment centres’ provide care for moderate, severe, and critical COVID-19 patients whereas ‘community care centres’ are structures (such as schools or university buildings) or healthcare facilities repurposed to provide care to asymptomatic patients and those with mild disease. COVID-19 treatment is offered free of cost to the patients. According to the policy in Sierra Leone, medical doctors and community health officers are responsible for the prescription of antimicrobial agents. The national case management guidelines, including antibiotic use in Sierra Leone, follow WHO guidelines (Table 1).
Table 1.
COVID-19 disease classification, signs and symptoms, and antibiotic use according to the 2020 WHO clinical management guidelines.
| Disease Severity | Signs and Symptoms | Antibiotic Use |
|---|---|---|
| Asymptomatic | No symptoms | No |
| Mild | Fever, cough, fatigue, anorexia, shortness of breath, myalgia | No |
| Moderate | Fever, cough, dyspnoea, fast breathing, SpO2 ≥ 90% on room air | Yes, only if suspicion of bacterial infection |
| Severe | Fever, cough, dyspnoea, fast breathing, respiratory rate > 30 breaths/min, severe respiratory distress, SpO2 < 90% on room air | Yes, only if suspicion of bacterial infection |
| Critical | Lobar or lung collapse, respiratory failure, PaO2/FiO2a ≤ 300 mmHg- PaO2/FiO2 ≤ 100 mmHg, acute life-threatening organ dysfunction, fast heart rate, weak pulse, cold extremities or low blood pressure, skin mottling, coagulopathy, thrombocytopenia, acidosis, high lactate, or hyperbilirubinemia. | Yes, within one hour of admission |
SpO2—Oxygen saturation; PaO2—Partial pressure of oxygen; FiO2—Fraction of inspired oxygen.
2.4. AWaRe Classification
The AWaRe classification of antibiotics is an excellent tool to prevent AMR and strengthen antimicrobial stewardship at local, national, and global levels. AWaRe classifies antibiotics into the following three groups: ACCESS, WATCH, and RESERVE groups [26]. The ‘ACCESS’ group of antibiotics are those which should always be available as they are used in the treatment of common bacterial infections, and they show lower resistance potential [29]. The ‘WATCH’ group of antibiotics includes most of the highest priority agents among the critically important antimicrobials. They have higher resistance potential and are the key targets for antimicrobial stewardship programmes and monitoring [30]. The ‘RESERVE’ group should be treated as ‘last resort’ antibiotics to be used in the management of multidrug-resistant infections, and they are a key target for national and international antimicrobial stewardship programs [31].
2.5. Study Population and Period
We included all the people with suspected and confirmed COVID-19 infection admitted to community care centres, treatment centres, and isolation units between 31st March 2020 and 31 March 2021. For the study of confirmed COVID-19 patients, all the community care centres (n = 3) and treatment centres (n = 18) in the country were included. For the study of suspected COVID-19 patients, we included isolation units (n = 14) in secondary and tertiary healthcare facilities in the country. There was no sampling, and we reviewed all the patient records that were present at the study sites from the day of the commissioning of these designated units to the time of data collection.
2.6. Data Variables, Data Sources, and Data Collection
Data variables included age, sex, location of the centre (rural or urban), COVID-19 case type (suspected or confirmed), the severity of disease at the time of admission (asymptomatic, mild, moderate, severe, and critical), duration of admission, antibiotic use (yes or no), and if ‘yes’, the name of the antibiotic used. A paper-based, structured data collection proforma was used to collect data after pre-testing and validation. The primary source of data was the patient chart (booklet) that contained details of the treatments (including antibiotic use) and other medical care given to the patient at the COVID-19 isolation units, community care, and treatment centres. Data collection was performed by the principal investigator and four data collectors, who were trained before data collection for this purpose from 31 May to 31 August 2021.
2.7. Data Entry and Analysis
To ensure the highest standards in data quality, we performed double entry and validation using EpiData (version 3.1, EpiData Association, Odense, Denmark). Data analysis was performed using EpiData analysis (v2.2.2.187) and Stata (v16, StataCorp, College Station, TX, USA) software.
Data regarding demographic and clinical characteristics were summarized using frequencies and proportions. Duration of hospital stay among confirmed COVID-19 patients was presented as the median number of days with an interquartile range. Analysis was conducted separately for suspected COVID-19 and confirmed COVID-19 patients. Prevalence of antibiotic use was reported as a percentage with 95% confidence intervals (CI). The pattern of antibiotic use was described according to the WHO AWaRe classification.
We assessed associations of demographic and clinical characteristics with antibiotic use using log-binomial regression. We calculated adjusted prevalence ratios (and 95% CI) by including all the variables in the multivariable model in line with our exploratory approach. In all analyses, a p-value ≤ 0.05 was considered statistically significant.
3. Results
3.1. Patients with Confirmed COVID-19
The demographic and clinical characteristics of COVID-19 patients are described in Table 2. There were a total of 700 confirmed COVID-19 patients, of whom 406 (58%) were males and nearly two-thirds (64%) were from rural areas. The median (IQR) age of patients was 35 (25–52) years. About 85% of all admitted patients were classified as asymptomatic or mild, and the median duration of admission was 13 (10–18) days. There were no critical cases of COVID-19.
Table 2.
Demographic and clinical characteristics of people with confirmed COVID-19 infection admitted in community care and treatment centres in Sierra Leone, March 2020–March 2021 (N = 700).
| Variable | N | (%) |
|---|---|---|
| Region | ||
| Urban | 254 | (36.3) |
| Rural | 446 | (63.7) |
| Sex | ||
| Male | 406 | (58.0) |
| Female | 288 | (41.1) |
| Missing | 6 | (0.9) |
| Age (years) | ||
| 0–14 | 62 | (8.9) |
| 15–24 | 103 | (14.7) |
| 25–34 | 165 | (23.6) |
| 35–44 | 118 | (16.9) |
| 45–54 | 95 | (13.6) |
| 55–64 | 74 | (10.6) |
| ≥65 | 80 | (11.4) |
| Missing | 3 | (0.4) |
| Disease classification | ||
| Asymptomatic | 441 | (63.0) |
| Mild | 160 | (22.9) |
| Moderate | 24 | (3.4) |
| Severe | 65 | (9.3) |
| Missing | 10 | (1.4) |
| Duration of admission | ||
| <7 days | 116 | (16.6) |
| 7–14 days | 283 | (40.5) |
| >14 days | 299 | (42.8) |
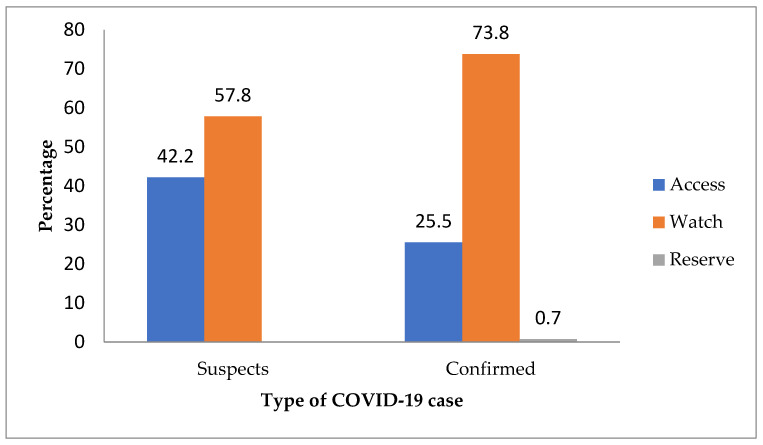
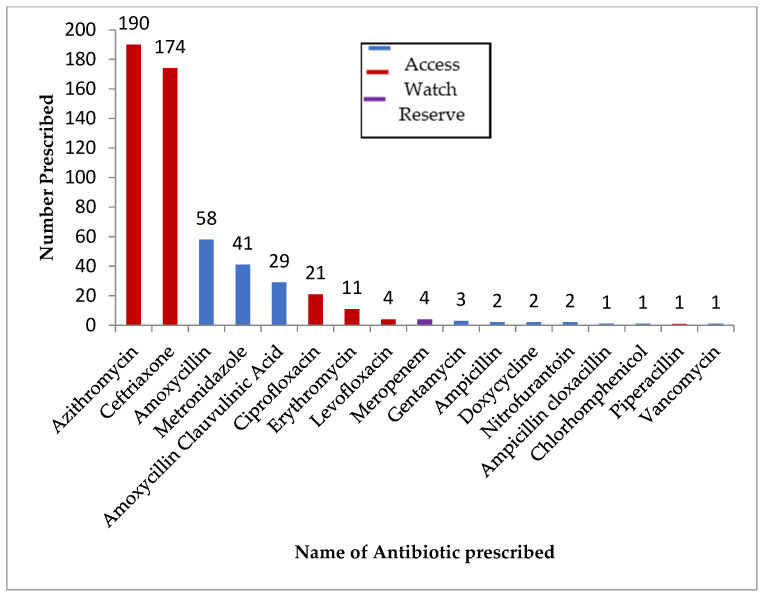
The prevalence of antibiotic use among confirmed COVID-19 patients was 47% (95% CI: 43–51%). The prevalence of antibiotic use ranged from 32% to 100% across the districts among COVID-19 confirmed patients. The majority (73%) of the antibiotics prescribed fell under ‘WATCH’ category of the WHO antibiotic AWaRe classification, and a total of four patients were prescribed ‘RESERVE’ category antibiotics (meropenem) as shown in Figure 1. The median number of antibiotics prescribed to a patient was 2 (IQR: 1–2), and the maximum number was six. The most frequently prescribed antibiotics were azithromycin, ceftriaxone, amoxycillin, metronidazole, and amoxycillin-clavulanic acid (Figure 2).
Figure 1.
Prescription of antibiotics according to the WHO AWaRe classification of antibiotic use in suspected and confirmed COVID-19 patients admitted to isolation units and treatment centres in Sierra Leone (March 2020–March 2021).
Figure 2.
Different antibiotics prescribed to COVID-19 confirmed patients admitted to community care and treatment centres in Sierra Leone, March 2020–March 2021 (N = 545).
The prevalence of antibiotic use was significantly higher in people with mild (PR: 2.0, 95% CI: 1.8–2.7), moderate (PR: 2.1, 95% CI: 1.5–2.8) and severe disease (PR: 2.2, 95% CI: 1.9–2.9) as compared to asymptomatic patients. There was no significant difference in antibiotic use between mild, moderate, and severe patients. In an unadjusted analysis, we found that patients in the 25–34 year age group had a lower prevalence (PR: 0.7, 95% CI: 0.5–1.0) and the older age group had a higher prevalence of antibiotic use (PR: 1.9, 95% CI: 1.4–2.6) compared to children. Patients in urban areas had a higher prevalence (PR: 1.9, 95% CI: 1.6–2.2) of antibiotic use compared to those in rural areas. Duration of admission and sex were not associated with antibiotic use. In adjusted analysis, only age (25–34 years) and disease severity (mild, moderate, and severe) emerged as independent predictors of antibiotic use (Table 3).
Table 3.
Prevalence of antibiotic use and its associated factors among people with confirmed COVID-19 infection admitted in community care centres and treatment centres of Sierra Leone, March 2020–March 2021 (N = 700).
| Variable | Total | Antibiotic Use N | (%) | PR | (95% CI) | aPR | 95% CI |
|---|---|---|---|---|---|---|---|
| Total | |||||||
| Region | |||||||
| Urban | 254 | 172 | (67.7) | 1.91 | (1.6–2.2) | 1.19 | (1.0–1.5) |
| Rural | 446 | 158 | (35.4) | Ref | Ref | Ref | Ref |
| Sex | |||||||
| Male | 406 | 203 | (50.0) | Ref | Ref | Ref | Ref |
| Female | 288 | 126 | (43.8) | 0.88 | (0.7–1.0) | 1.02 | (0.9–1.1) |
| Age (years) | |||||||
| 0–14 | 62 | 26 | (41.9) | Ref | Ref | Ref | Ref |
| 15–24 | 103 | 45 | (43.7) | 1.04 | (0.7–1.5) | 0.97 | (0.7–1.4) |
| 25–34 | 165 | 49 | (29.7) | 0.71 | (0.5–1.0) | 0.64 * | (0.4–0.9) |
| 35–44 | 118 | 45 | (38.1) | 0.91 | (0.6–1.3) | 0.75 | (0.5–1.1) |
| 45–54 | 95 | 52 | (54.7) | 1.31 | (0.9–1.8) | 0.86 | (0.6–1.2) |
| 55–64 | 74 | 49 | (66.2) | 1.58 | (1.1–2.2) | 0.95 | (0.7–1.4) |
| ≥65 | 80 | 64 | (80.0) | 1.91 | (1.4–2.6) | 1.03 | (0.7–1.5) |
| Disease classification | |||||||
| Asymptomatic | 441 | 136 | (30.8) | Ref | Ref | Ref | Ref |
| Mild | 160 | 121 | (75.6) | 2.45 | (2.1–2.9) | 2.00 * | (1.8–2.7) |
| Moderate | 24 | 16 | (66.7) | 2.16 | (1.6–3.0) | 2.05 * | (1.5–2.8) |
| Severe | 65 | 54 | (83.1) | 2.69 | (2.3–3.2) | 2.16 * | (1.9–2.9) |
| Duration of admission | |||||||
| <7 days | 116 | 66 | (56.9) | Ref | Ref | Ref | Ref |
| 7–14 days | 283 | 121 | (42.8) | 0.75 | (0.6–0.9) | 0.91 | (0.7–1.1) |
| >14 days | 299 | 141 | (47.2) | 0.83 | (0.1–0.7) | 0.97 | (0.8–1.2) |
PR–Prevalence ratio; CI–confidence intervals; aPR–adjusted prevalence ratio; * statistically significant (p value < 0.05).
There were a total of 755 suspected COVID-19 patients, with the majority (74%) from the urban area. Of these patients, 369 (49%) were males, and the median (IQR) age was 33 (25–45) years (Table 4).
Table 4.
Demographic characteristics of people with suspected COVID-19 infection admitted to isolation units in Sierra Leone, March 2020–March 2021 (N = 755).
| Variable | N | (%) |
|---|---|---|
| Location | ||
| Urban | 584 | (77.4) |
| Rural | 171 | (22.6) |
| Sex | ||
| Male | 369 | (48.9) |
| Female | 385 | (51) |
| Missing | 1 | (0.1) |
| Age (years) | ||
| 0–14 | 67 | (8.9) |
| 15–24 | 119 | (15.8) |
| 25–34 | 205 | (27.2) |
| 35–44 | 161 | (21.3) |
| 45–54 | 94 | (12.4) |
| 55–64 | 49 | (6.5) |
| ≥65 | 59 | (7.8) |
| Missing | 1 | (0.1) |
3.2. Patients with Suspected COVID-19
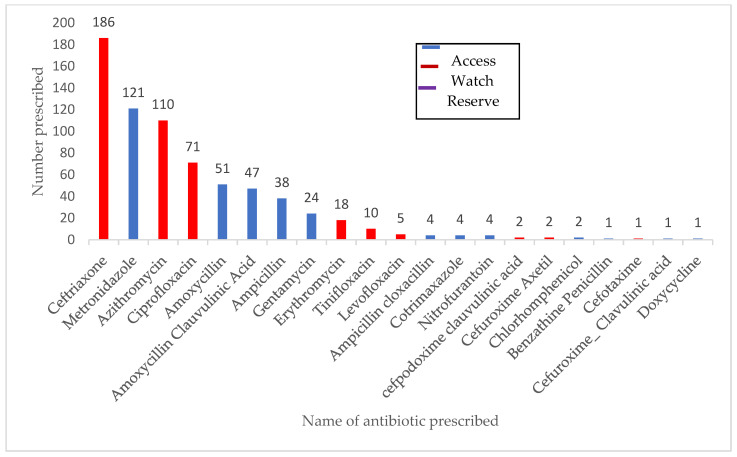
The prevalence of antibiotic use among suspected COVID-19 patients was 61% (95% CI: 58–65%) and the majority (58%) of the antibiotics prescribed fell under the ‘WATCH’ category, as shown in Figure 1 and Figure 3. The prevalence of antibiotic use ranged from 55% to 94% across the districts among COVID-19 suspects. The median number of antibiotics prescribed to a patient was two (one–two); the minimum number prescribed was one, and the maximum number was six. The most frequently prescribed antibiotics were ceftriaxone, metronidazole, azithromycin, ciprofloxacin, and amoxycillin, respectively (Figure 3).
Figure 3.
Different antibiotics prescribed to COVID-19 suspected patients admitted to isolation units in Sierra Leone, March 2020–March 2021 (N = 703).
Adjusted analysis showed that the prevalence of antibiotic use was significantly lower in urban areas compared to rural areas, and older age groups had a higher prevalence of antibiotic use compared to children (Table 5).
Table 5.
Prevalence of antibiotic use and its associated factors among people with suspected COVID-19 infection admitted in isolation units in Freetown, Sierra Leone, March 2020–March 2021 (N = 755).
| Variable | Total | Antibiotic Use N | (%) | PR | 95% CI | aPR | 95% CI |
|---|---|---|---|---|---|---|---|
| Location | |||||||
| Urban | 584 | 319 | (54.6) | 0.65 | (0.6–0.7) | 0.67 * | (0.6–0.7) |
| Rural | 171 | 144 | (84.2) | Ref | Ref | Ref | Ref |
| Sex | |||||||
| Male | 369 | 233 | (63.1) | Ref | Ref | Ref | Ref |
| Female | 385 | 229 | (59.5) | 0.94 | (0.8–1.1) | 0.97 | (0.9–1.1) |
| Age (years) | |||||||
| 0–14 | 67 | 26 | (38.8) | Ref | Ref | Ref | Ref |
| 15–24 | 119 | 75 | (63) | 1.62 | (1.2–2.3) | 1.54 * | (1.1–2.1) |
| 25–34 | 205 | 134 | (65.4) | 1.68 | (1.2–2.3) | 1.56 * | (1.1–2.1) |
| 35–44 | 161 | 93 | (57.8) | 1.49 | (1.1–2.1) | 1.41 * | (1.0–1.9) |
| 45–54 | 94 | 61 | (64.5) | 1.67 | (1.2–2.3) | 1.52 * | (1.1–2.1) |
| 55–64 | 49 | 33 | (67.4) | 1.73 | (1.2–2.5) | 1.63 * | (1.2–2.3) |
| ≥65 | 59 | 41 | (69.5) | 1.79 | (1.3–2.5) | 1.55 * | (1.1–2.2) |
PR—Prevalence ratio; CI—confidence intervals; aPR—adjusted prevalence ratio; * statistically significant (p value < 0.05).
4. Discussion
This is the first study from Sierra Leone reporting on the prevalence of antibiotic use and its associated factors among suspected and confirmed COVID-19 patients admitted to healthcare facilities. This adds to the global evidence on the use of antibiotics in the management of COVID-19. It further contributes to the evidence of inappropriate use of antimicrobial agents globally, which has the potential to increase antimicrobial resistance. There were three key findings, and we will discuss them below.
First, nearly half of all confirmed COVID-19 patients received antibiotics in Sierra Leone. Most (~85%) of the confirmed COVID-19 patients were either ‘asymptomatic’ or had a mild illness and should not have received antibiotics. About six in ten suspected COVID-19 patients received antibiotics when none should have received them as per the WHO and national case management guidelines. In our view, such high levels of antibiotic use were unnecessary and not in line with both the national and WHO clinical case management guidelines. In addition to the misuse of antibiotics among COVID-19 patients, our study provided an insight into the prescribing practises of clinicians which is relevant to the establishment of antimicrobial stewardship programmes in health care facilities. However, despite these worrying results, they are similar to reports from other countries in the African Region (47% in Kenya, 71% in South Africa, and 76% in Uganda) and elsewhere (78% in Spain, 83% in the USA, 100% in Bangladesh, and 67–90% in several studies from China) showing high use of antibiotics in COVID-19 patients [32,33,34,35,36,37,38,39]. In contrast, a study from Singapore reported a very low level (~5%) of antibiotic use [40]. Most of the studies have focused on antibiotic use among confirmed COVID-19 patients and not in suspected patients. Our study is the first from Africa reporting on suspected COVID-19 patients, and we add to the limited evidence for this group of patients. The only other study that looked at suspected COVID-19 patients was from Singapore, which reported a prevalence of 39% antibiotic use [40]. This inappropriate use of antibiotics during the ongoing COVID-19 pandemic will increase multidrug resistance and be associated with longer hospital stays and increased costs to both patients and hospital management.
Second, the majority of patients in our study received at least two antibiotics, with the most predominant antibiotics being azithromycin, ceftriaxone, amoxycillin, metronidazole, and amoxycillin-clavulanic acid. Most of these antibiotics fall under the ‘WATCH’ group of drugs according to the WHO AWaRe categorization. This is in contrast to the national and WHO guidelines which recommend using the ‘ACCESS’ group of antibiotics where warranted and restricting the use of ‘WATCH’ and ‘RESERVE’ groups of antibiotics. There has been a lot of inconsistency in the normative guidance available, adding to the confusion regarding the choice of antibiotics in COVID-19 patients. A rapid review of national treatment guidelines for COVID-19 in 10 African countries showed that various antibiotics, such as azithromycin, doxycycline, clarithromycin, ceftriaxone, erythromycin, amoxicillin, amoxicillin-clavulanic acid, ampicillin, gentamicin, benzylpenicillin, piperacillin/tazobactam, ciprofloxacin, ceftazidime, cefepime, vancomycin, meropenem, and cefuroxime, among others, were recommended for use in the management of COVID-19 [41]. Most of these antibiotics were from the ‘WATCH’ and ‘RESERVE’ categories, in contrast to the WHO guidelines.
Third, severity of disease and age were independent predictors of antibiotic use among confirmed COVID-19 patients. In suspected COVID-19 patients, adults were more likely to receive antibiotics compared to children, and patients admitted in rural areas were more likely to receive antibiotics compared to those in urban areas. These findings are similar to reports from other settings. The study from Bangladesh reported that patients presenting with severe disease (especially those with co-morbidities such as diabetes mellitus) received more antibiotics on average [35]. The study from Spain reported that inappropriate antibiotic use was more likely in patients in the younger age groups and in those without co-morbidities [36]. Similarly, a study from USA reported an association of antibiotic use with increased duration of hospital stay and with patients admitted to ICU and needing mechanical ventilation [37]. In contrast, we did not find any association between the duration of a hospital stay and antibiotic use. The study from Singapore reported that antibiotic use was more appropriate when it was prescribed by infectious disease physicians [40].
Our study had several strengths. First, it was a countrywide study with a large sample size, and, therefore, the findings are representative of all COVID-19 patients and suspects who were admitted to health care facilities in Sierra Leone. Second, data collection was performed by clinicians who were well versed in reading patient clinical files. Third, we used a structured data collection proforma that was pretested and validated before use. This facilitated the implementation of uniform procedures in data collection. Finally, we adhered to ‘STROBE’ (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting the study findings.
There were some limitations. The first relates to the study population. As of 31 March 2021, Sierra Leone had reported a total of 3964 confirmed COVID-19 patients. We did not include COVID-19 patients who received home care or any other treatment options. Thus, we will not be able to extrapolate our findings to these patients. This requires future research. The second limitation relates to the lack of information on the prevalence of bacterial co-infection in our study, which made it difficult for us to make a judgement on the appropriateness of antibiotic use in individual patients. Nevertheless, studies globally have reported a pooled low prevalence of 8.6% of bacterial co-infections in COVID-19 patients, and we have no reason to believe that Sierra Leone will be any different [42]. The third limitation was that we did not look at variation in antibiotic use among suspected and confirmed COVID-19 patients during the different phases of the ongoing COVID-19 pandemic in the country. Despite these limitations, there are some important policy and practice implications arising out of the study.
Inappropriate use of antibiotics has many implications, which include increased costs of health care, increased incidence of adverse drug events, increased mortality, and an increase in antimicrobial resistance in the long run, making antibiotic treatments ineffective when needed. A study from Spain reported that the incidence of adverse drug events was four times higher in COVID-19 patients who received antibiotics inappropriately compared to those with appropriate antibiotic use [36]. Possible reasons for inappropriate use of antibiotics in COVID-19 patients include (i) lack of an effective antibiotic stewardship programme in the health care facilities, (ii) lack of knowledge about the WHO and national treatment guidelines and AWaRe classification, (iii) lack of access to rapid diagnostics leading to empirical antibiotic use, and (iv) lack of supervision, monitoring, and review. It is also possible that clinicians in the early stages of the pandemic used antibiotics as a ‘safety net,’ in the absence of specific and effective antivirals to treat COVID-19.
We make the following recommendations to address the overuse of antibiotics in COVID-19 patients in Sierra Leone. First, all healthcare workers should be trained in national case management guidelines, including when to use antibiotics, what antibiotics to be used, and the AWaRe classification. Although the national guidelines have been developed, they have not been widely disseminated. This is of the utmost priority. Second, antimicrobial stewardship programmes should be established and strengthened at all the hospitals in Sierra Leone. This includes having dedicated focal points and teams reviewing antibiotic use in the respective health care facilities by conducting periodic point prevalence surveys (once every three or six months) to monitor the situation and take appropriate action. Third, access to quality-assured rapid diagnostics, including culture and sensitivity testing and molecular technologies, should be strengthened. This will obviate the need for empirical treatment and promote a more rational use of antibiotics.
5. Conclusions
In this first study from Sierra Leone, we found that there was a high prevalence of antibiotic use among suspected (60%) and confirmed COVID-19 (48%) patients admitted to the health care facilities and community care centres. Since 85% of the confirmed COVID-19 patients were asymptomatic or had mild illnesses, they did not need antibiotics. Similarly, about 60% of suspected COVID-19 patients received antibiotics, when none should have received them as per the guidelines. Such high levels of antibiotic use were unjustified and were not in line with the national and global guidelines. The most frequently prescribed antibiotics were from the ‘Watch’ group of the AWaRe categorisation, which are toxic and prone to resistance. The most frequently prescribed antibiotics were azithromycin, amoxycillin, ceftriaxone, and metronidazole. These issues need to be addressed urgently because inappropriate use of antibiotics leads to increased costs of health care, an increased incidence of adverse drug events, and increased antimicrobial resistance in the long run. We recommend that (i) healthcare workers be trained on national case management guidelines, including the use of antibiotics; (ii) antimicrobial stewardship programmes be strengthened at hospitals; (iii) laboratory capacities be improved to conduct culture and antibiotic sensitivity testing.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership coordinated by TDR, the Special Programme for Research and Training in Tropical Diseases at the World Health Organization. The specific SORT IT program that led to these publication included a partnership of TDR with the WHO Country office of Sierra Leone and was implemented along with The Tuberculosis Research and Prevention Center Non-Governmental Organization, Armenia; The International Union Against Tuberculosis and Lung Diseases, Paris and South East Asia offices; Medicins Sans Frontières—Luxembourg, Luxembourg; ICMR–National Institute of Epidemiology, Chennai, India; Sustainable Health Systems, Freetown, Sierra Leone; Ministry of Health and Sanitation, Freetown, Sierra Leone; Ministry of Livestock, Freetown, Sierra Leone; University of Guinea, Guinea; The Centre for Rural Training and Research, Meferinyah, Guinea; The Madhira institute, Nairobi, Kenya; Institute of Tropical Medicine, Antwerp, Belgium; University of Chester, United Kingdom; University of Liverpool, United Kingdom and; the University of Washington, USA.
Author Contributions
Conceptualization, I.F.K., A.M.V.K., R.Z., M.K., A.M., B.D.F., S.M.T., J.S.K., C.K.N., S.S., F.M. and A.H.D.M.; methodology, I.F.K., A.M.V.K., R.Z., M.K., K.S., A.M., B.D.F., S.M.T., J.S.K. and F.M.; software, I.F.K., A.M.V.K. and K.S.; validation, I.F.K., A.M.V.K., R.Z., M.K., K.S., A.M., B.D.F., S.M.T., J.S.K., F.M., S.S., C.K.N. and S.L.; formal analysis, I.F.K., A.M.V.K., K.S., R.Z. and M.K.; investigation, I.F.K., A.M.V.K., K.S. and S.M.T.; resources, I.F.K., A.M.V.K., R.Z., M.K., K.S. and S.M.T.; data curation, I.F.K., A.M.V.K., R.Z., M.K., A.M., B.D.F. and S.M.T.; writing—original draft preparation, I.F.K., A.M.V.K. and R.Z.; writing—review and editing, I.F.K., A.M.V.K., R.Z., M.K., K.S., S.S., C.K.N., A.M., B.D.F., S.M.T., J.S.K., F.M., S.L. and A.H.D.M.; visualization, I.F.K., A.M.V.K. and K.S.; supervision,; I.F.K. and A.M.V.K.; project administration, I.F.K., A.M.V.K., R.Z. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
The UK Department of Health & Social Care has contributed designated funding for this SORT IT-AMR initiative, which is branded as the NIHR-TD-R partnership. TDR is able to conduct its work thanks to the commitment and support from a variety of funders. A full list of TDR donors is available at: https://tdr.who.int/about-us/our-donors (accessed on 26 January 2022). The funding was for conducting the SORT IT training and not for the conduct of the research per se. The research was conducted in routine operational setting using available resources without any specific funding.
Institutional Review Board Statement
Permission was obtained from the heads of COVID-19 centres and isolation units to access patients’ data. Ethics clearance was obtained from the Sierra Leone Ethics and Scientific Review Committee, Ministry of Health and Sanitation, Freetown (5 July 2021), and the Ethics Advisory Group of International Union Against Tuberculosis and Lung Disease (The Union), Paris, France (10/21; 21 June 2021).
Informed Consent Statement
As this study only used anonymized secondary data, the need for written informed consent was waived by the ethics committees.
Data Availability Statement
The dataset used in this paper has been deposited at https://doi.org/10.6084/m9.figshare.19158734 (accessed on 11 February 2022) and is available under a CC BY 4.0 license.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alsan M., Schoemaker L., Eggleston K., Kammili N., Kolli P., Bhattacharya J. Out-of-pocket health expenditures and antimicrobial resistance in low-income and middle-income countries: An economic analysis. Lancet Infect. Dis. 2015;15:1203–1210. doi: 10.1016/S1473-3099(15)00149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jasovský D., Littmann J., Zorzet A., Cars O. Antimicrobial resistance-a threat to the world’s sustainable development. Ups. J. Med. Sci. 2016;121:159–164. doi: 10.1080/03009734.2016.1195900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talebi Bezmin Abadi A., Rizvanov A.A., Haertlé T., Blatt N.L. World Health Organization Report: Current Crisis of Antibiotic Resistance. BioNanoScience. 2019;9:778–788. doi: 10.1007/s12668-019-00658-4. [DOI] [Google Scholar]
- 4.Murray C.J.L., Ikuta K.S., Sharara F., Swetschinski L., Robles Aguilar G., Gray A., Han C., Bisignano C., Rao P., Wool E., et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edelstein M., Agbebiyi A., Ashiru-Oredope D., Hopkins S. Trends and patterns in antibiotic prescribing among out-of-hours primary care providers in England, 2010–2014. J. Antimicrob. Chemother. 2017;72:3490–3495. doi: 10.1093/jac/dkx323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z., Hu Y., Zou G., Lin M., Zeng J., Deng S., Zachariah R., Walley J., Tucker J.D., Wei X. Antibiotic prescribing for upper respiratory infections among children in rural China: A cross-sectional study of outpatient prescriptions. Glob. Health Action. 2017;10:1287334. doi: 10.1080/16549716.2017.1287334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sartelli M., Weber D.G., Ruppé E., Bassetti M., Wright B.J., Ansaloni L., Catena F., Coccolini F., Abu-Zidan F.M., Coimbra R., et al. Antimicrobials: A global alliance for optimizing their rational use in intra-abdominal infections (AGORA) World J. Emerg. Surg. 2016;11:33. doi: 10.1186/s13017-016-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IACG . Future Global Governance for Antimicrobial Resistance. IACG; Geneva, Switzerland: 2018. [Google Scholar]
- 9.Mhondoro M., Ndlovu N., Bangure D., Juru T., Gombe N.T., Shambira G., Nsubuga P., Tshimanga M. Trends in antimicrobial resistance of bacterial pathogens in Harare, Zimbabwe, 2012–2017: A secondary dataset analysis. BMC Infect. Dis. 2019;19:746. doi: 10.1186/s12879-019-4295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpi C., Shehadeh F., Mylonakis E. Correlation of antimicrobial prescription rate and county income in medicare part D. Medicine. 2019;98:e15914. doi: 10.1097/MD.0000000000015914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abubakar U. Antibiotic use among hospitalized patients in northern Nigeria: A multicenter point-prevalence survey. BMC Infect. Dis. 2020;20:86. doi: 10.1186/s12879-020-4815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chui C.S.L., Cowling B.J., Lim W.W., Hui C.K.M., Chan E.W., Wong I.C.K., Wu P. Patterns of Inpatient Antibiotic Use Among Public Hospitals in Hong Kong from 2000 to 2015. Drug Saf. 2020;43:595–606. doi: 10.1007/s40264-020-00920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee C., Walker S.A., Daneman N., Elligsen M., Palmay L., Coburn B., Simor A. Point prevalence survey of antimicrobial utilization in a Canadian tertiary-care teaching hospital. J. Epidemiol. Glob. Health. 2015;5:143–150. doi: 10.1016/j.jegh.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spivak E.S., Cosgrove S.E., Srinivasan A. Measuring Appropriate Antimicrobial Use: Attempts at Opening the Black Box. Clin. Infect. Dis. 2016;63:1–6. doi: 10.1093/cid/ciw658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabba J.A., James P.B., Li Z., Hanson C., Chang J., Kitchen C., Jiang M., Zhao M., Yang C., Fang Y. Prescribing for Patients Seeking Maternal and Child Healthcare in Sierra Leone: A Multiregional Retrospective Cross-Sectional Assessments of Prescribing Pattern Using WHO Drug Use Indicators. Risk Manag. Healthc. Policy. 2020;13:2525–2534. doi: 10.2147/RMHP.S256648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giraldi G., Montesano M., Napoli C., Frati P., La Russa R., Santurro A., Scopetti M., Orsi G.B. Healthcare-Associated Infections Due to Multidrug-Resistant Organisms: A Surveillance Study on Extra Hospital Stay and Direct Costs. Curr. Pharm. Biotechnol. 2019;20:643–652. doi: 10.2174/1389201020666190408095811. [DOI] [PubMed] [Google Scholar]
- 17.Strathdee S.A., Davies S.C., Marcelin J.R. Confronting antimicrobial resistance beyond the COVID-19 pandemic and the 2020 US election. Lancet. 2020;396:1050–1053. doi: 10.1016/S0140-6736(20)32063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacIntyre C.R., Bui C.M. Pandemics, public health emergencies and antimicrobial resistance-putting the threat in an epidemiologic and risk analysis context. Arch. Public Health. 2017;75:54. doi: 10.1186/s13690-017-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu J. How COVID-19 is accelerating the threat of antimicrobial resistance. BMJ. 2020;369:m1983. doi: 10.1136/bmj.m1983. [DOI] [PubMed] [Google Scholar]
- 20.Douglas M., Katikireddi S.V., Taulbut M., McKee M., McCartney G. Mitigating the wider health effects of COVID-19 pandemic response. BMJ. 2020;369:m1557. doi: 10.1136/bmj.m1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., Agha M., Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X., et al. Compassionate Use of Remdesivir for Patients with Severe COVID-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization . Therapeutics and COVID-19 Living Guideline. WHO; Geneva, Switzerland: 2020. [PubMed] [Google Scholar]
- 24.Seaton R.A., Gibbons C.L., Cooper L., Malcolm W., McKinney R., Dundas S., Griffith D., Jeffreys D., Hamilton K., Choo-Kang B., et al. Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID-19 in Scottish hospitals. J. Infect. 2020;81:952–960. doi: 10.1016/j.jinf.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Bank Current Health Expenditure (% of GDP)-Sierra Leone. [(accessed on 17 September 2020)]. Available online: https://data.worldbank.org/indicator/SH.XPD.CHEX.GD.ZS?locations=SL.
- 26.World Health Organization . The 2019 WHO AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use. World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 27.Langford B.J., So M., Raybardhan S., Leung V., Soucy J.R., Westwood D., Daneman N., MacFadden D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021;27:520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Statistics Sierra Leone . Sierra Leone 2015 Population and Housing Census: National Analytical Report. Statistics Sierra Leone; Freetown, Sierra Leone: 2017. [Google Scholar]
- 29.Gandra S., Kotwani A. Need to improve availability of “access” group antibiotics and reduce the use of "watch" group antibiotics in India for optimum use of antibiotics to contain antimicrobial resistance. J. Pharm. Policy Pract. 2019;12:20. doi: 10.1186/s40545-019-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsia Y., Lee B.R., Versporten A., Yang Y., Bielicki J., Jackson C., Newland J., Goossens H., Magrini N., Sharland M. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): An analysis of paediatric survey data from 56 countries. Lancet Glob. Health. 2019;7:e861–e871. doi: 10.1016/S2214-109X(19)30071-3. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen N.V., Do N.T.T., Nguyen C.T.K., Tran T.K., Ho P.D., Nguyen H.H., Vu H.T.L., Wertheim H.F.L., van Doorn H.R., Lewycka S. Community-level consumption of antibiotics according to the AWaRe (Access, Watch, Reserve) classification in rural Vietnam. JAC-Antimicrob. Resist. 2020;2:dlaa048. doi: 10.1093/jacamr/dlaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mariita K.M., Wanjala A.N., Maina C.K. Characteristics and pharmacological management of COVID-19 patients admitted at a hospital in Nairobi, Kenya. Afr. J. Pharm. Pharmacol. 2021;15:92–100. [Google Scholar]
- 33.Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirenga B., Muttamba W., Kayongo A., Nsereko C., Siddharthan T., Lusiba J., Mugenyi L., Byanyima R.K., Worodria W., Nakwagala F. Characteristics and outcomes of admitted patients infected with SARS-CoV-2 in Uganda. BMJ Open Respir. Res. 2020;7:e000646. doi: 10.1136/bmjresp-2020-000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molla M.M.A., Yeasmin M., Islam M.K., Sharif M.M., Amin M.R., Nafisa T., Ghosh A.K., Parveen M., Arif M.M.H., Alam J.A.J., et al. Antibiotic Prescribing Patterns at COVID-19 Dedicated Wards in Bangladesh: Findings from a Single Center Study. Infect. Prev. Pract. 2021;3:100134. doi: 10.1016/j.infpip.2021.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calderón-Parra J., Muiño-Miguez A., Bendala-Estrada A.D., Ramos-Martínez A., Muñez-Rubio E., Fernández Carracedo E., Tejada Montes J., Rubio-Rivas M., Arnalich-Fernandez F., Beato Pérez J.L., et al. Inappropriate antibiotic use in the COVID-19 era: Factors associated with inappropriate prescribing and secondary complications. Analysis of the registry SEMI-COVID. PLoS ONE. 2021;16:e0251340. doi: 10.1371/journal.pone.0251340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin A.J., Shulder S., Dobrzynski D., Quartuccio K., Pillinger K.E. Antibiotic use and associated risk factors for antibiotic prescribing in COVID-19 hospitalized patients. J. Pharm. Pract. 2021 doi: 10.1177/08971900211030248. [DOI] [PubMed] [Google Scholar]
- 38.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moolla M., Reddy K., Fwemba I., Nyasulu P., Taljaard J., Parker A., Louw E., Nortje A., Parker M., Lalla U. Bacterial infection, antibiotic use and COVID-19: Lessons from the intensive care unit. S. Afr. Med. J. 2021;111:575–581. [PubMed] [Google Scholar]
- 40.Tan S.H., Ng T.M., Tay H.L., Yap M.Y., Heng S.T., Loo A.Y.X., Teng C.B., Lee T.H. A point prevalence survey to assess antibiotic prescribing in patients hospitalized with confirmed and suspected coronavirus disease 2019 (COVID-19) J. Glob. Antimicrob. Resist. 2021;24:45–47. doi: 10.1016/j.jgar.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adebisi Y.A., Jimoh N.D., Ogunkola I.O., Uwizeyimana T., Olayemi A.H., Ukor N.A., Lucero-Prisno D.E. The use of antibiotics in COVID-19 management: A rapid review of national treatment guidelines in 10 African countries. Trop. Med. Health. 2021;49:51. doi: 10.1186/s41182-021-00344-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used in this paper has been deposited at https://doi.org/10.6084/m9.figshare.19158734 (accessed on 11 February 2022) and is available under a CC BY 4.0 license.