Abstract
An analytical method was developed to simultaneously determine pyridate, quizalofop-ethyl, and cyhalofop-butyl in brown rice, soybean, potato, pepper, and mandarin using LC-MS/MS. Purification was optimized using various sorbents: primary–secondary amine, octadecyl (C18) silica gel, graphitized carbon black, zirconium dioxide-modified silica particles, zirconium dioxide-modified silica particles (Z-SEP), and multi-walled carbon nanotubes (MWCNTs). Three versions of QuECHERS methods were then tested using the optimal purification agent. Finally, samples were extracted using acetonitrile and QuEChERS EN salts and purified using the Z-SEP sorbent. A six-point matrix-matched external calibration curve was constructed for the analytes. Good linearity was achieved with a determination coefficient ≥0.999. The limits of detection and quantification were 0.0075 mg/kg and 0.01 mg/kg, respectively. The method was validated after fortifying the target standards to the blank matrices at three concentration levels with five replicates for each concentration. The average recovery was within an acceptable range (70–120%), with a relative standard deviation <20%. The applicability of the developed method was evaluated with real-world market samples, all of which tested negative for these three herbicide residues. Therefore, this method can be used for the routine analysis of pyridate, quizalofop-ethyl, and cyhalofop-butyl in agricultural products.
Keywords: pyridate, quizalofop-ethyl, cyhalofop-butyl, Z-SEP, agricultural products, tandem mass spectrometry analysis
1. Introduction
Various types of pesticides are being increasingly used worldwide to enhance the production and quality of agricultural commodities and to protect them from pests, diseases, and weeds [1]. Herbicides, in particular, have been an essential tool for practical farming for many years, as they can stop unwanted plant growth while leaving the desired crop unharmed [2]. Hence, herbicides have been widely used in row-crop farming and applied before or during planting [3]. They also may be applied to crops in the fall to improve harvesting [4]. Despite these benefits, herbicides inevitably cause serious environmental and health concerns [5]. This is because many agricultural products are left with residues of various herbicides, which naturally poses a potentially hazardous risk to consumers and various ecosystems [6]. Thus, herbicide residues in agricultural commodities should be analyzed to improve public health and food safety [7].
The Positive List System (PLS) was recently implemented to strengthen pesticide acceptance standards for safe agricultural products in the Republic of Korea; as a result, quantitative analysis methods should have detection levels as low as 0.01 mg/kg. Thus, more practical strategies are urgently required to monitor various xenobiotics in different foods (e.g., cereals, root and tuber crops, legumes, fruits, and vegetables). The Ministry of Food and Drug Safety (MFDS) has been developing simultaneous methods to meet the requirement for the residue analysis of pesticides in agricultural foods using liquid chromatography-tandem mass spectrometry (LC-MS/MS) [8]. Notably, pyridate, quizalofop-ethyl, and cyhalofop-butyl residues could not be determined, which necessitated the development of a method that could detect these compounds.
Cyhalofop-butyl and quizalofop-ethyl, which belong to a group of phenoxy compounds, have remarkable crop selectivity, are highly effective in controlling weeds, and demonstrate good systemic abilities [9]. The mechanism of action of these compounds is based on the inhibition of acetyl CoA carboxylase (ACCase); this excludes pyridate, which functions as a photosynthetic electron transport inhibitor [10]. Pyridate controls annual broad-leaved weeds, especially triazine-resistant biotypes and some grass weeds [11]. Quizalofop-ethyl, introduced by Nissan Chemical Industries Ltd. in 1984, was used for the selective post-emergence control of annual and perennial grass weeds in potatoes, soybeans, peanuts, and cotton [12]. Cyhalofop-butyl was manufactured in the mid-1980s by The Dow Chemical Company (now Dow AgroSciences). It is used to control barnyard grass in rice paddies [13]. At present, pyridate is not used domestically. However, the analysis of cyhalofop-butyl residues in agricultural products needs to be further developed because several countries that export agricultural products to Korea use this herbicide.
Currently, there are a few literature methods on the analyses of these herbicides (pyridate, quizalofop-ethyl, and cyhalofop-butyl) residues. For example, a study by Wu et al. [13] identified cyhalofop-butyl and its metabolite in a rice ecosystem using high-performance LC liquid chromatography (HPLC)-MS/MS. This technique was also employed by Lindner et al. [11] to detect pyridate in cereals. Furthermore, quizalofop-ethyl has been detected in soils and plants by gas-liquid chromatography (GLC) and enzyme-linked immunosorbent assays (ELISAs) [14]. However, no existing technology can analyze these herbicide residues simultaneously. Additionally, the methods mentioned above are time-consuming and environmentally harmful. The simultaneous determination of pesticides is a desirable and practical approach because it would provide increased efficiency, lower costs, and reduced labor relative to conventional methods [15,16]. Moreover, it would reduce the amount of materials required for experiments, thus protecting the environment. However, simultaneous analysis requires the removal of interferences (e.g., matrix components) and the consideration of analyte characteristics [17].
Presently, the quick, easy, cheap, rugged, effective, and safe (“QuEChERS”) sample preparation method is earning popularity in the area of green chemistry because it enables multi-residue pesticide analysis for complex matrices and minimizes sample size and the quantity of required materials [18,19,20]. It can be applied to pesticides with diverse polarity ranges and volatility [21]. LC-MS/MS is another powerful tool for analyzing pesticide residues in complex matrices [22]. It is characterized by high precision and sensitivity with fewer extraction procedures than other methodologies, such as gas chromatography-mass spectrometry (GC-MS) [23]. Unlike GC analysis, sample volatilization is not required for LC, which avoids problems associated with chemical degradation and the formation of new products common under high heat conditions. Further, LC–MS/MS specimens typically require no derivatization and minimal sample preparation. In some cases, the specimens can be diluted and directly injected into the LC-MS/MS, significantly increasing throughput. Consequently, the present study aimed to establish an accurate, cost-effective, and environmentally friendly simultaneous detection method for detecting herbicides (pyridate, cyhalofop-butyl, and quizalofop-ethyl) in different agricultural products (brown rice, soybean, potato, pepper, and mandarin.) Because the complex matrices of farming products affect analysis precision, this study employs sample extraction and clean-up (pre-treatment) steps [24]. Specifically, samples were extracted by QuEChERS methods (Original, AOAC, and EN), followed by clean-up and analysis using LC-M/MS.
2. Materials and Methods
2.1. Chemicals and Reagents
Cyhalofop-butyl (99.7%), quizalofop-ethyl (96.4%), and pyridate (96%) were procured from Wako (Richmond, VA, USA), Sigma–Aldrich (Buchs, Switzerland), and Dr. Ehrenstorfer (Augsburg, Germany), respectively. The physicochemical characteristics are shown in Table 1. Acetonitrile and methanol (HPLC grade) were obtained from J.T. Baker (Avantor, Radnor, PA, USA). Formic acid was acquired from Daejung (Siheung-si, Korea), ammonium formate was secured from Sigma–Aldrich and QuEChERS kits (Original, EN, AOAC) were supplied by KRIAT (Daejeon, Korea). Zirconium dioxide-modified silica particles (Z-SEP) and zirconium dioxide-modified silica particles + (Z-SEP+) were obtained from Supelco (Bellefonte, PA, USA). Primary–secondary amine (PSA), octadecyl (C18) silica gel, and graphitized carbon black (GCB) sorbents were acquired from Agilent (Santa Clara, CA, USA). Multi-walled carbon nanotubes (MWCNTs) were supplied by Cheap Tubes (Grafton Vermont, VT, USA). Matrices used for establishing the residual-detection method include cereal (brown rice), legume (soybean), root and tuber crops (potato), vegetables (pepper), and fruits (mandarin); they were homogenized and stored in a sealed container at −70 °C.
Table 1.
Physicochemical characteristics of pyridate, cyhalofop-butyl, and quizalofop-ethyl.
| Analyte | CAS No. | Molecular Formula |
Chemical Structure | Log Pow |
|---|---|---|---|---|
| Cyhalofop-butyl | 122008-85-9 | C20H20FNO4 |

|
3.31 |
| Quizalofop-ethyl | 76578-14-8 | C19H17ClN2O4 |

|
4.28 |
| Pyridate | 55512-33-9 | C19H23ClN2O2S |

|
6.61 |
2.2. Preparation of Standard Stock Solution and Calibration
Solutions of pyridate, quizalofop-ethyl, and cyhalofop-butyl in methanol (1000 ppm) were separately prepared. The stock solution was diluted with the same solvent to 100 ppm and used as a mixed standard solution. To prepare a calibration curve, the intermediate standards solutions of the three pesticides were diluted to 2.5, 5, 7.5, 10, 25, and 50 ng/mL. The standard stock, intermediate, and working solutions were stored at 4 °C until analysis. Brown vials were used throughout the experiment.
2.3. Sample Preparation
A homogenized sample (10 g, 5 g of both cereal and legume) was placed in a 50-mL Teflon centrifuge tube. A 20 mL acetonitrile (for grain and legume, 5 mL of water was added, wet for 30 min, and 10 mL of acetonitrile was added) was then added to the sample in the centrifuge tube, and the mixture was homogenized with a tissue homogenizer for 1 min. A QuEChERS EN extraction kit containing 4 g of MgSO4, 1 g of NaCl, 1 g of trisodium citrate dihydrate, and 0.5 g of disodium hydrogencitrate sesquihydrate was shaken manually for 1 min. The sample tube was centrifuged at 4000 rpm for 5 min, and a 1.5-mL portion of the supernatant was transferred to a microtube that contained 75 mg of Z-SEP. The microtube was then vortexed for 1 min and centrifuged at 4000 rpm for 5 min. The supernatant was diluted with methanol (1:1) and then transferred to a vial for LC-MS/MS analysis.
2.4. Instrumental Conditions
An Alliance 2695 LC separation module (Waters, Milford, MA, USA) coupled with a Micromass Quattro Micro triple quadrupole tandem mass spectrometer (Waters) was used to detect and analyze pyridate, quizalofop-butyl, and cyhalofop-ethyl in five food products (brown rice, soybean, pepper, potato, and mandarin). An Agilent ZORBAX Eclipse Plus C18 column (3.0 mm I.D × 150 mm, 3.5 μm) was used for separation (Table 2).
Table 2.
Analytical conditions for the determination of the tested analytes in various matrices.
| Condition | Content | ||
|---|---|---|---|
| Instrument | LC: Alience 2695 LC separation module, Waters MS/MS: Micromass Quattro Micro triple quadrupole tandem mass, Waters |
||
| Chromatographic separation | |||
| Column | Agilent ZORBAX Eclipse Plus C18 (3.0 mm I.D. × 150 mm L, 3.5 μm) | ||
| Flow rate | 0.3 mL/min | ||
| Injection volume | 5 μL | ||
| Oven temp. | 40 °C | ||
| Mobile phase | A: 5 mM ammonium formate, 0.1% formic acid in methanol B: 5 mM ammonium formate, 0.1% formic acid in water |
||
| Gradient | Time | A (%) | B (%) |
| 0.0 | 5 | 95 | |
| 2.0 | 5 | 95 | |
| 4.0 | 95 | 5 | |
| 10.0 | 95 | 5 | |
| 12.0 | 5 | 95 | |
| 15.0 | 5 | 95 | |
| MS/MS condition | |||
| Capillary voltage | 3.9 kV | ||
| Source temp. | 150 °C | ||
| Desolvation gas temp. | 250 °C | ||
| Desolvation gas flow | 500 L/h | ||
The MS/MS was operated in multiple reaction monitoring (MRM) mode with positive electrospray ionization. MassLynx (version 4.1) software was used for data acquisition.
2.5. Statistical Analyses
The statistics required for interpreting analytical method validation results are the calculation of the mean, standard deviation, relative standard deviation, and regression analysis (IBM SPSS Statistics, v25, Armonk, NY, USA). The acceptance criteria for each validation characteristic are typically around the individual values as well as the mean and relative standard deviation.
3. Results and Discussion
3.1. Optimization of the Extraction Procedure
The extraction conditions were first considered by referring to the physicochemical properties of the three analytes (pyridate, cyhalofop-butyl, and quizalofop-ethyl). When a sample contains moisture, its surface is hydrated with water; this provides better extraction efficiency when using water-soluble organic solvents than non-polar organic solvents with low permeability. Typical water-soluble organic solvents include acetonitrile and acetone, although acetonitrile is preferentially considered because acetone had the disadvantage of extracting large amounts of non-polar or polar interference materials. To simplify the pre-processing of the samples, we employed the QuEChERS method, which has advantages of relatively low solvent usage and rapid analysis time [25]. According to the reagents used, the QuEChERS method is divided into three categories: original, EN, and AOAC. A comparison of these extraction methods showed differences in their average recovery rates. Among the grain, root and tuber crop, legume, fruit, and vegetable matrices, the original method could not recover the three analytes in an average of 70% to 120% from three matrices. In contrast, the AOAC method could not recover 70% to 120% of the analytes from all six matrices. Therefore, with the best average recovery rate for all samples, the EN method was selected and used for the experiments (Table 3).
Table 3.
Comparison of QuEChERS extractions for the determination of three residual herbicides in (A) brown rice, (B) soybean, (C) potato, (D) pepper, and (E) mandarin matrices.
| Analyte | Spiking Level | Recovery ± RSD a (%) | |||
|---|---|---|---|---|---|
| Original | AOAC | EN | |||
| (A) | Cyhalofop-butyl | LOQ | 110.6 ± 8.4 | 71.2 ± 22.5 | 98.3 ± 4.2 |
| 10 × LOQ | 86.3 ± 7.7 | 127.3 ± 12.7 | 101.5 ± 1.5 | ||
| Quizalofop-ethyl | LOQ | 76.8 ± 0.6 | 111.0 ± 7.6 | 96.5 ± 7.2 | |
| 10 × LOQ | 99.0 ± 6.2 | 104.2 ± 0.6 | 94.4 ± 3.6 | ||
| Pyridate | LOQ | 92.2 ± 7.9 | 121.9 ± 8.5 | 82.0 ± 8.7 | |
| 10 × LOQ | 91.0 ± 4.3 | 86.8 ± 9.2 | 81.3 ± 1.1 | ||
| (B) | Cyhalofop-butyl | LOQ | 51.3 ± 19.9 | 72.3 ± 22.0 | 80.0 ± 12.2 |
| 10 × LOQ | 78.6 ± 2.2 | 101.5 ± 17.6 | 95.4 ± 12.5 | ||
| Quizalofop-ethyl | LOQ | 85.5 ± 3.3 | 99.3 ± 2.7 | 86.3 ± 1.7 | |
| 10 × LOQ | 85.2 ± 1.3 | 108.5 ± 7.9 | 102.4 ± 1.9 | ||
| Pyridate | LOQ | 87.9 ± 0.4 | 85.5 ± 3.5 | 78.0 ± 4.5 | |
| 10 × LOQ | 77.0 ± 3.5 | 86.8 ± 11.0 | 83.6 ± 0.7 | ||
| (C) | Cyhalofop-butyl | LOQ | 93.9 ± 1.7 | 115.3 ± 0.3 | 76.5 ± 9.2 |
| 10 × LOQ | 113.5 ± 6.9 | 124.6 ± 9.4 | 102.4 ± 13.2 | ||
| Quizalofop-ethyl | LOQ | 90.5 ± 4.6 | 129.3 ± 1.0 | 104.2 ± 1.7 | |
| 10 × LOQ | 92.4 ± 7.5 | 126.3 ± 8.5 | 106.9 ± 5.5 | ||
| Pyridate | LOQ | 76.9 ± 2.0 | 128.1 ± 3.3 | 86.7 ± 10.4 | |
| 10 × LOQ | 92.8 ± 6.5 | 110.5 ± 0.6 | 99.9 ± 1.7 | ||
| (D) | Cyhalofop-butyl | LOQ | 81.5 ± 37.7 | 27.8 ± 11.4 | 86.5 ± 2.6 |
| 10 × LOQ | 62.7 ± 14.1 | 100.9 ± 7.7 | 85.1 ± 4.9 | ||
| Quizalofop-ethyl | LOQ | 68.4 ± 7.2 | 90.0 ± 6.9 | 93.3 ± 5.0 | |
| 10 × LOQ | 75.9 ± 1.1 | 92.4 ± 3.3 | 91.7 ± 0.7 | ||
| Pyridate | LOQ | 80.2 ± 11.2 | 87.3 ± 11.2 | 100.5 ± 1.9 | |
| 10 × LOQ | 76.9 ± 3.4 | 97.0 ± 2.8 | 90.3 ± 4.8 | ||
| (E) | Cyhalofop-butyl | LOQ | 117.5 ± 14.0 | 95.8 ± 35.0 | 80.4 ± 17.3 |
| 10 × LOQ | 75.4 ± 0.8 | 91.0 ± 6.9 | 88.7 ± 9.0 | ||
| Quizalofop-ethyl | LOQ | 81.1 ± 5.0 | 99.0 ± 4.6 | 82.2 ± 5.7 | |
| 10 × LOQ | 94.0 ± 1.2 | 101.7 ± 7.0 | 94.0 ± 0.4 | ||
| Pyridate | LOQ | 70.1 ± 7.1 | 78.4 ± 7.8 | 79.8 ± 5.2 | |
| 10 × LOQ | 84.1 ± 1.2 | 81.2 ± 1.9 | 73.2 ± 2.1 | ||
a Mean value of three measurements with relative standard deviation.
3.2. Optimization of Clean-Up Procedure
Typical clean-up procedures are not always satisfactory for analysis, especially for pigments, sugars, and organic acids [26]. Thus, we considered various purification agents to develop a new clean-up method for analyzing our target herbicide residues. The optimal sorbent was selected by comparing the recovery rates of the analytes obtained using different quantities of sorbent. First, C18 and PSA, which effectively remove organic acids, sugars, oil, and fat components, were considered. GCB and MWCNTs, which can effectively remove pigments, such as sterol, chlorophyll, and carotenoid, were also tested [27]. When 50, 75, or 100 mg of C18 was used in the clean-up step, there was a decline in the recovery value of pyridate (14.3%, 10.0%, and 1.8%, respectively). Similarly, with 50, 75, or 100 mg of PSA, the recovery value of the three tested analytes was remarkably low (0%). Notably, 50, 75, and 100 mg of GCB were adequate for recovering cyhalofop-butyl (88–108%), but there were slight decreases in the recovery value of quizalofop-ethyl (7–50%) and pyridate (10–32%). Furthermore, the efficiency of the MWCNTs sorbent was excellent for cyhalofop-butyl recovery (58–107%) but not for those of quizalofop-ethyl (0–1.6%) and pyridate (0–5.8%). Based on these results, we employed Z-SEP and Z-SEP+ as alternative absorbents for removing pigments. These are relatively new, commercially available dispersive phases that can effectively remove larger amounts of fat and coloring from samples than conventional sorbents like C18 and PSA [27]. With Z-SEP and Z-SEP+, the recovery value of quizalofop-ethyl and pyridate was 77–220%. However, Z-SEP provided a better recovery value for cyhalofop-butyl (71–99%) than Z-SEP+ (105–139%). Finally, Z-SEP (75 mg) was chosen as the optimal sorbent due to its reasonable recovery rates; this excluded recovery rates of less than 70% and more than 120% (Table 4).
Table 4.
Optimization of sorbent type and amounts in terms of analyte recovery.
| Purification Material | Recovery (%) | |||
|---|---|---|---|---|
| Cyhalofop-butyl | Quizalofop-ethyl | Pyridate | ||
| PSA | 50 mg | 121.4 | 89.8 | 0.0 |
| 75 mg | 70.0 | 96.4 | 0.0 | |
| 100 mg | 104.6 | 82.2 | 0.0 | |
| C18 | 50 mg | 107.4 | 90.2 | 14.3 |
| 75 mg | 98.3 | 87.4 | 10.0 | |
| 100 mg | 94.6 | 74.0 | 1.8 | |
| GCB | 5 mg | 108.0 | 50.2 | 32.6 |
| 10 mg | 95.9 | 20.7 | 14.4 | |
| 15 mg | 88.4 | 6.9 | 10.0 | |
| MWCNTs | 5 mg | 107.2 | 1.6 | 5.8 |
| 10 mg | 58.4 | 0.0 | 2.1 | |
| 15 mg | 57.9 | 0.0 | 0.0 | |
| Z-SEP | 50 mg | 92.2 | 88.3 | 110.3 |
| 75 mg | 99.5 | 101.4 | 93.2 | |
| 100 mg | 71.3 | 91.3 | +95.9 | |
| Z-SEP+ | 50 mg | 105.1 | 86.2 | 77.2 |
| 75 mg | 139.7 | 101.1 | 98.3 | |
| 100 mg | 124.0 | 90.1 | 81.9 | |
Mean value of four measurements.
3.3. Analytical Procedure
The positive electrospray ionization (ESI) mode was used to detect pyridate, cyhalofop-butyl, and quizalofop-butyl in agricultural products. One ppm of each standard solution was injected into the mass detector, the production ion was selected through the production scan mode, and collision energy with high detection intensity was selected. The product ions detected with the best sensitivity were considered quantitative ions, while those detected with poorer sensitivity were considered qualitative ions. The characteristic ions chosen for analysis and optimal collision energy are shown in Table 5. It is worth noting that because LC-MS/MS analysis can suppress ionization or augment target components due to non-target components extracted from the sample, we quantified the analytes based on matrix-matched calibration (MMC).
Table 5.
Characteristic ions observed via LC-MS/MS for three residual herbicides.
| Analyte | Molecular Weight (g/mol) | Exact Mass (g/mol) |
Precursor Ion (m/z) |
Product Ion (m/z) |
CE a (V) |
|
|---|---|---|---|---|---|---|
| 1 | Cyhalofop-butyl | 357.4 | 357.14 | 358.1319 | 120.0795 | 26 |
| 256.1212 b | 10 | |||||
| 2 | Quizalofop-ethyl | 372.8 | 372.09 | 373.0561 | 91.062 | 32 |
| 299.1621 b | 20 | |||||
| 3 | Pyridate | 378.9 | 378.12 | 379.1319 | 207.0993 b | 28 |
| 351.1753 | 10 | |||||
a Collision energy; b quantitative ion.
3.4. Method Validation
3.4.1. Specificity
The selectivities of pyridate, cyhalofop-butyl, and quizalofop-ethyl were evaluated by comparing the blank and spiked samples’ chromatograms to those of the standard solutions. An analysis of the amounts of herbicides recovered from the spiked samples compared to the blank samples showed that this method has high separation and selectivity for analyzing pyridate, cyhalofop-butyl, and quizalofop-ethyl in agricultural products. No interference was detected with the same retention time mass-to-charge ratio (m/z).
3.4.2. Matrix Effects and Linearity
To evaluate and minimize matrix effects on the analyte responses and performances, the comparative data between solvent calibration curves were used following the Kanrar equation [28].
To check the linearity of the matrix-matched standard solution, a mixed stock solution comprising all three analytes was diluted with the blank sample of each extract (brown rice, soybean, green pepper, potato, and mandarin). Then 5 µL of various concentrations of these samples (0.0025, 0.005, 0.0075, 0.01, 0.025, or 0.05 µg/mL) were injected into the LC-MS/MS system and analyzed. The linearity was satisfactory with a coefficient of determination (R2) of ≥0.99.
The ME was tested at a concentration rate of 0.05 ppm, and the value was ranged from +21–+43, −33–+42, and −10–+17 for cyhalofop-butyl, quizalofop-ethyl, and pyridate, respectively. On the other hand, the values were in between −33–+21, +17–+40, +10–+44, +7–+41, and +7–43, in brown rice, soybean, mandarin, pepper, and potato, respectively. As MEs cannot be ruled out, matrix-matched calibration was used throughout the experimental work and quantification.
3.4.3. Limits of Detection and Quantitation
The LODs and LOQs of pyridate, quizalofop-ethyl, and cyhalofop-butyl were calculated using the test solution preparation and device analysis methods. The calculated LOD and LOQ were obtained as signal-to-noise ratios of (S/N) ≥3 and S/N ≥10. The LOD and LOQ were 0.0075 mg/kg and 0.01 mg/kg, respectively, for all matrices. For comparison, a previous work detecting cyhalofop-butyl in a rice paddy field achieved a LOQ of 0.001 mg/kg and a LOD of 0.003 mg/kg [13].
3.4.4. Recovery
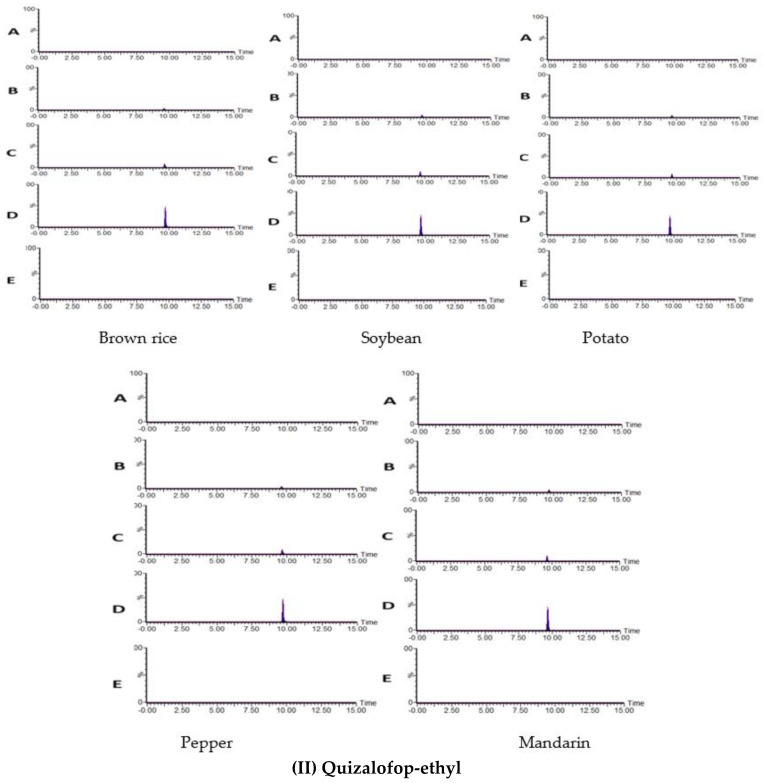
To evaluate the accuracy, reproducibility, and efficiency of our method, experiments for recovery of pyridate, quizalofop-ethyl, and cyhalofop-butyl were conducted five times with fortification levels of 0.01, 0.02, and 0.1 mg/kg, corresponding to the following levels: LOQ, 2 × LOQ, and 10 × LOQ. The average recovery of pyridate, quizalofop-ethyl, and cyhalofop-butyl are as follows: pyridate 85.7–109.6%; quizalofop-ethyl 89.1–112.4%; and cyhalofop-butyl 84.5–106.5%. The respective RSD values were 9.2%, 8.9%, and 15.0%. It was confirmed that these values meet the standards of the codex guidelines (recovery rate = 70–120%) [29] and the Korea Food and Drug Safety Assessment Institute’s “Guidelines on Standard Procedures for Preparation of Test Methods (RSDs < 22%) [30]” regarding the analysis of pesticide residues (Table 6). Cross-validation was carried out for this established test method at two verification institutions, which determined a recovery value of 74.3–117.6% for each concentration of all samples; the standard deviation was within 15% (Table 7). Consequently, compliance with the test method verification criteria was confirmed. Recovery chromatograms of pyridate, cyhalofop-butyl, and quizalofop-ethyl are shown in Figure 1. Moreover, of note is the recovery value (78.9–107.5%, standard deviation = 2.0–6.9%) of cyhalofop-butyl obtained when using HPLC-MS/MS with C18 as an adsorbent [13], which were lower than those achieved by our LC-MS/MS methods using Z-SEP as the adsorbent.
Table 6.
Validation results of the proposed analytical method for the determination of three residual herbicides in food samples.
| Analyte | Spiking Level (mg/kg) |
Recovery ± RSD a (%) | LOQ (mg/kg) |
||||
|---|---|---|---|---|---|---|---|
| Brown Rice | Soybean | Potato | Green Pepper | Mandarin | |||
| Cyhalofop-butyl | 0.01 | 87.0 ± 15.0 | 85.9 ± 14.8 | 102.9 ± 6.8 | 84.5 ± 4.5 | 92.0 ± 12.7 | 0.01 |
| 0.02 | 91.1 ± 9.8 | 95.6 ± 8.2 | 92.8 ± 10.0 | 93.4 ± 7.0 | 105.8 ± 9.3 | ||
| 0.1 | 96.9 ± 8.2 | 106.5 ± 6.0 | 89.8 ± 4.5 | 90.3 ± 7.1 | 94.4 ± 7.9 | ||
| Quizalofop-ethyl | 0.01 | 94.4 ± 7.8 | 101.3 ± 7.4 | 97.5 ± 3.8 | 89.1 ± 8.5 | 98.5 ± 6.9 | 0.01 |
| 0.02 | 97.8 ± 6.6 | 104.4 ± 3.1 | 91.2 ± 5.5 | 99.4 ± 6.7 | 104.7 ± 8.9 | ||
| 0.1 | 91.2 ± 5.2 | 106.0 ± 1.5 | 92.0 ± 1.8 | 103.8 ± 1.8 | 112.4 ± 4.0 | ||
| Pyridate | 0.01 | 106.8 ± 2.9 | 94.6 ± 5.6 | 91.0 ± 3.0 | 89.9 ± 5.4 | 93.6 ± 3.4 | 0.01 |
| 0.02 | 99.6 ± 2.0 | 90.6 ± 4.2 | 94.2 ± 5.5 | 86.8 ± 2.8 | 91.8 ± 9.2 | ||
| 0.1 | 109.6 ± 3.7 | 88.4 ± 3.1 | 94.3 ± 2.6 | 106.4 ± 0.5 | 85.7 ± 3.7 | ||
a Mean value of five measurements with relative standard deviation.
Table 7.
Inter-lab A and B validation results of the proposed analytical method for the determination of three residual herbicides in food samples.
| Analyte | Spiking Level (mg/kg) |
Recovery ± RSD a (%) | |||||
|---|---|---|---|---|---|---|---|
| Brown Rice | Soybean | Potato | Green Pepper | Mandarin | |||
| Cyhalofop-butyl | 0.01 | A | 105.6 ± 8.3 | 117.6 ± 4.6 | 84.9 ± 3.5 | 99.6 ± 4.1 | 75.8 ± 9.9 |
| B | 87.0 ± 2.8 | 95.0 ± 1.8 | 95.3 ± 2.2 | 94.0 ± 4.1 | 95.3 ± 4.7 | ||
| 0.02 | A | 103.4 ± 6.9 | 104.3 ± 4.9 | 91.3 ± 6.2 | 82.0 ± 8.7 | 79.4 ± 8.0 | |
| B | 88.2 ± 3.0 | 93.2 ± 5.1 | 86.2 ± 3.4 | 92.4 ± 1.6 | 98.3 ± 3.7 | ||
| 0.1 | A | 102.5 ± 5.5 | 90.5 ± 6.0 | 90.9 ± 4.5 | 79.8 ± 3.5 | 96.8 ± 7.8 | |
| B | 86.9 ± 3.6 | 103.0 ± 2.3 | 83.7 ± 2.5 | 93.0 ± 3.1 | 99.2 ± 1.7 | ||
| Quizalofop-ethyl | 0.01 | A | 71.8 ± 2.7 | 88.9 ± 2.0 | 72.2 ± 7.5 | 76.7 ± 9.9 | 76.6 ± 4.4 |
| B | 81.3 ± 3.2 | 83.7 ± 5.5 | 89.5 ± 3.2 | 101.0 ± 2.9 | 112.9 ± 6.5 | ||
| 0.02 | A | 81.3 ± 4.6 | 81.6 ± 2.0 | 80.2 ± 3.3 | 89.6 ± 2.4 | 83.7 ± 2.1 | |
| B | 86.9 ± 1.5 | 85.4 ± 1.9 | 91.9 ± 3.4 | 96.5 ± 1.0 | 111.1 ± 4.2 | ||
| 0.1 | A | 78.2 ± 5.4 | 74.9 ± 1.4 | 82.4 ± 1.7 | 97.3 ± 2.2 | 98.8 ± 3.5 | |
| B | 85.6 ± 4.6 | 94.0 ± 8.3 | 99.8 ± 4.1 | 88.7 ± 4.1 | 105.9 ± 1.8 | ||
| Pyridate | 0.01 | A | 89.6 ± 3.2 | 107.1 ± 2.9 | 86.3 ± 6.2 | 74.3 ± 3.3 | 74.5 ± 4.8 |
| B | 85.5 ± 2.2 | 92.7 ± 3.4 | 89.1 ± 3.1 | 103.4 ± 2.9 | 98.3 ± 1.5 | ||
| 0.02 | A | 86.9 ± 5.1 | 105.6 ± 2.9 | 82.2 ± 1.9 | 86.9 ± 5.1 | 105.6 ± 2.9 | |
| B | 86.1 ± 1.8 | 91.4 ± 0.8 | 93.7 ± 5.4 | 99.5 ± 1.5 | 99.0 ± 1.0 | ||
| 0.1 | A | 80.2 ± 3.9 | 97.8 ± 1.6 | 97.5 ± 3.4 | 80.2 ± 3.9 | 97.8 ± 1.6 | |
| B | 87.5 ± 2.2 | 95.0 ± 1.7 | 97.2 ± 3.1 | 93.8 ± 1.5 | 98.9 ± 1.0 | ||
a Mean value of five measurements with relative standard deviation.
Figure 1.
Representative MRM (quantification ion) chromatograms of (I) cyhalofop-butyl, (II) quizalofop-ethyl, and (III) pyridate in five different matrices (brown rice, soybean, potato, pepper, and mandarin: (A) blank sample, (B) sample spiked at 0.005 mg/kg, (C) sample spiked at 0.01 mg/kg, (D) sample spiked at 0.2 mg/kg, and (E) market sample.
3.5. Method Application
The developed method was applied to detect pyridate, cyhalofop-butyl, and quizalofop-ethyl in soybean, mandarin, pepper, potato, and brown rice (10 per each matrix) procured from different local markets in Gwangju, Republic of Korea. The samples were extracted and analyzed according to the above-established procedure. None of the samples showed any residues of pyridate, cyhalofop-butyl, or quizalofop-ethyl (Figure 1). In line with our finding, Wu et al. [13] found that the ultimate residues of cyhalofop-butyl and its metabolite in the rice samples were not detectable or below 0.01 mg/kg at harvest.
4. Conclusions
A simple LC-MS/MS method was developed and optimized for the simultaneous analysis of pyridate, quizalofop-butyl, and cyhalofop-ethyl in five agricultural products (soybean, mandarin, pepper, potato, and brown rice). Among the three types of QuEChERS methods, the EN version most effectively extracted the target analytes. Furthermore, Z-SEP demonstrated a superior analyte recovery rate to those of the other evaluated sorbents (C18, GCB, PSA, MWCNTs, and Z-SEP+). These components provided a system capable of multi-residue detection within complex matrices, with sample sizes and quantities of required materials lower than those of conventional detection methods. The recovery and repeatability of the developed method are within the acceptable range as determined by CODEX guidelines, thus making our LC-MS/MS system suitable for monitoring pyridate, quizalofop-ethyl, and cyhalofop-butyl residues in agricultural products. Shandong.
Author Contributions
J.-H.S.: conceptualization, resources, writing—review and editing, funding acquisition, project administration, supervision. M.M.R. and S.-J.L.: formal analysis, investigation. A.A.Z.: writing—review and editing. A.J. and S.-H.Y.: methodology. J.-B.E.: methodology and visualization. J.-H.K. and J.-W.P.: methodology. E.O.: writing—review and editing. C.P.: writing—review and editing. F.O.: validation, writing—review and editing. A.M.A.E.-A.: data curation, formal analysis, validation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from the Ministry of Food and Drug Safety (MFDS) (Project no.20162 MFDS 607), Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Park K.H., Choi J.H., Abd El-Aty A.M., Cho S.K., Park J.H., Kim B.M., Yang A., Na T.W., Rahman M., Im G.J., et al. Determination of spinetoram and its metabolites in amaranth and parsley using QuEChERS-based extraction and liquid chromatography-tandem mass spectrometry. Food Chem. 2012;134:2552–2559. doi: 10.1016/j.foodchem.2012.04.066. [DOI] [PubMed] [Google Scholar]
- 2.Chemistry Libretexts 20.3, Herbicides and Defoliants. Jun 27, 2021. [(accessed on 18 February 2022)]. Available online: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map%3A_Chemistry_for_Changing_Times_(Hill_and_McCreary)/20%3A_Chemistry_Down_on_the_Farm/20.03%3A_Herbicides_and_Defoliants.
- 3.Saha A., Shabeer T.P.A., Banerjee K., Hingmire S., Bhaduri D., Jain N.K., Utture S. Simultaneous analysis of herbicides pendimethalin, oxyfluorfen, imazethapyr and quizalofop-p-ethyl by LC-MS/MS and safety evaluation of their harvest time residues in peanut (Arachis hypogaea L.) J. Food Sci. Technol. 2015;52:4001–4014. doi: 10.1007/s13197-014-1473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Environmental Protection Agency (EPA) Herbicides, Volume 2. [(accessed on 18 February 2022)]; Available online: https://www.epa.gov/caddis-vol2/caddis-volume-2-sources-stressors-responses-herbicides.
- 5.Wang S., Hou Z., Liang S., Lu Z. Residue Behavior and Risk Assessment of Rimsulfuron and Quizalofop-P-ethyl in Potato under Field Conditions. Bull. Environ. Contam. Toxicol. 2020;105:602–606. doi: 10.1007/s00128-020-03002-9. [DOI] [PubMed] [Google Scholar]
- 6.Ko A.Y., Abd El-Aty A.M., Rahman M.M., Jang J., Kim S.W., Choi J.H., Shim J.H. A modified QuEChERS method for simultaneous determination of flonicamid and its metabolites in paprika using tandem mass spectrometry. Food Chem. 2014;157:413–420. doi: 10.1016/j.foodchem.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 7.Guo W., Bian Z., Zhang D., Tang G., Liu W., Wang J., Li Z., Yang F. Simultaneous determination of herbicide residues in tobacco using ultraperformance convergence chromatography coupled with solid-phase extraction. J. Sep. Sci. 2015;38:858–863. doi: 10.1002/jssc.201401063. [DOI] [PubMed] [Google Scholar]
- 8.Food News Co-Development of 511 Types of Pesticide Rapid Inspection Methods, Unification of the Residual Pesticide Test Method in the Production, Distribution, and Import Stages. 2021. [(accessed on 18 February 2022)]. Available online: http://www.foodnews.co.kr/news/articleView.html?idxno=91194.
- 9.Tsochatzis E.D., Menkissoglu-Spiroudi U., Karpouzas D.G., Tzimou-Tsitouridou R. A multi-residue method for pesticide residue analysis in rice grains using matrix solid-phase dispersion extraction and high-performance liquid chromatography-diode array detection. Anal. Bioanal. Chem. 2010;397:2181–2190. doi: 10.1007/s00216-010-3645-4. [DOI] [PubMed] [Google Scholar]
- 10.Xiang S., Callaghan M.M., Watson K.G., Tong L. A different mechanism for the inhibition of the carboxyltransferase domain of acetyl-coenzyme A carboxylase by tepraloxydim. Proc. Natl. Acad. Sci. USA. 2009;106:20723–20727. doi: 10.1073/pnas.0908431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindner W., Ruckendorfer H. HPLC—Residue analysis of the herbicide pyridate in cereals. Int. J. Environ. Anal. Chem. 1983;16:205–218. doi: 10.1080/03067318308078362. [DOI] [PubMed] [Google Scholar]
- 12.Mandal K., Sahoo S.K., Battu R.S., Singh B. Estimation of quizalofop ethyl residues in black gram (Vigna mungo L.) by gas liquid chromatography. Bull. Environ. Contam. Toxicol. 2014;92:115–118. doi: 10.1007/s00128-013-1159-4. [DOI] [PubMed] [Google Scholar]
- 13.Wu J., Wang K., Zhang Y., Zhang H. Determination and study on dissipation and residue determination of cyhalofop-butyl and its metabolite using HPLC-MS/MS in a rice ecosystem. Environ. Monit. Assess. 2014;186:6959–6967. doi: 10.1007/s10661-014-3902-7. [DOI] [PubMed] [Google Scholar]
- 14.Kim H.K., Kim B.H., Shim J.H., Shu Y.T. Study on analysis method of herbicide quizalofop-ethyl. Korean J. Environ. Agric. 1998;17:22–25. [Google Scholar]
- 15.Mantzos N., Karakitsou A., Nikolaki S., Leneti E., Konstantinou I.J.E.S., Research P. Dissipation and transport of quizalofop-p-ethyl herbicide in sunflower cultivation under field conditions. Environ. Sci. Pollut. Res. 2016;23:3481–3490. doi: 10.1007/s11356-015-5572-6. [DOI] [PubMed] [Google Scholar]
- 16.Park J.-A., Zhang D., Kim S.-K., Cho S.-H., Cho S.-M., Yi H., Shim J.-H., Kim J.-S., El-Aty A.M.A., Shin H.-C. Simultaneous determination of aminopyrine and antipyrine in porcine muscle, milk, and eggs using liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2015;38:4048–4054. doi: 10.1002/jssc.201500920. [DOI] [PubMed] [Google Scholar]
- 17.Kumar B., Sharma R., Singh S.B.J. Evaluation of harvest residues of cyhalofop-butyl in paddy soil. Bull. Environ. Contam. Toxicol. 2012;89:344–347. doi: 10.1007/s00128-012-0701-0. [DOI] [PubMed] [Google Scholar]
- 18.Fedeniuk R.W., Mizuno M., Neiser C., O’Byrne C. Development of LC–MS/MS methodology for the detection/determination and confirmation of chloramphenicol, chloramphenicol 3-O-β-d-glucuronide, florfenicol, florfenicol amine and thiamphenicol residues in bovine, equine and porcine liver. J. Chromatogr. B. 2015;991:68–78. doi: 10.1016/j.jchromb.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Riedel M., Speer K., Stuke S., Schmeer K. Simultaneous analysis of 70 pesticides using HPLC/MS/MS: A comparison of the multiresidue method of Klein and Alder and the QuEChERS method. J. AOAC Int. 2010;93:1972–1986. doi: 10.1093/jaoac/93.6.1972. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava A., Rai S., Kumar Sonker A., Karsauliya K., Pandey C.P., Singh S.P. Simultaneous determination of multiclass pesticide residues in human plasma using a mini QuEChERS method. Anal. Bioanal. Chem. 2017;409:3757–3765. doi: 10.1007/s00216-017-0317-7. [DOI] [PubMed] [Google Scholar]
- 21.Lehotay S.J., Kok A.D., Hiemstra M., Bodegraven P.V. Validation of a fast and easy method for the determination of residues from 229 pesticides in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection. J. AOAC Int. 2005;88:595–614. doi: 10.1093/jaoac/88.2.595. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D., Park J.-A., Kim D.-S., Kim N.-H., Kim S.-K., Cho K.-S., Jeong D., Shim J.-H., Abd El-Aty A.M., Shin H.-C. Simultaneous detection of bacitracin and polymyxin B in livestock products using liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2015;38:2371–2380. doi: 10.1002/jssc.201500282. [DOI] [PubMed] [Google Scholar]
- 23.Rahman M.M., Abd El-Aty A.M., Kabir M.H., Chung H.S., Lee H.S., Hacımüftüoğlu F., Jeong J.H., Chang B.-J., Shin H.-C., Shim J.-H. A quick and effective methodology for analyzing dinotefuran and its highly polar metabolites in plum using liquid chromatography-tandem mass spectrometry. Food Chem. 2018;239:1235–1243. doi: 10.1016/j.foodchem.2017.07.073. [DOI] [PubMed] [Google Scholar]
- 24.Zeng D., Shi H., Li B., Wang M., Song B.J.J. Development of an enzyme-linked immunosorbent assay for quantitative determination of quizalofop-p-ethyl. J. Agric. Food Chem. 2006;54:8682–8687. doi: 10.1021/jf061492n. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y.-J., Rahman M.M., Abd El-Aty A.M., Choi J.-H., Chung H.S., Kim S.-W., Abdel-Aty A.M., Shin H.-C., Shim J.-H. Detection of three herbicide, and one metabolite, residues in brown rice and rice straw using various versions of the QuEChERS method and liquid chromatography-tandem mass spectrometry. Food Chem. 2016;210:442–450. doi: 10.1016/j.foodchem.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Han Y., Song L., Zou N., Chen R., Qin Y., Pan C.J.J.o.C.B. Multi-residue determination of 171 pesticides in cowpea using modified QuEChERS method with multi-walled carbon nanotubes as reversed-dispersive solid-phase extraction materials. J. Chromatogr. B. 2016;1031:99–108. doi: 10.1016/j.jchromb.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 27.Tuzimski T., Szubartowski S. Method development for selected bisphenols analysis in sweetened condensed milk from a can and breast milk samples by HPLC–DAD and HPLC-QqQ-MS: Comparison of sorbents (Z-SEP, Z-SEP Plus, PSA, C18, Chitin and EMR-Lipid) for clean-up of QuEChERS extract. Molecules. 2019;24:2093. doi: 10.3390/molecules24112093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman M.M., Lee H.S., Abd El-Aty A.M., Kabir M.H., Chung H.S., Park J.-H., Kim M.-R., Kim J.-H., Shin H.-C., Shin S.H. Determination of endrin and δ-keto endrin in five food products of animal origin using GC-μECD: A modified QuEChERS approach to traditional detection. Food Chem. 2018;263:59–66. doi: 10.1016/j.foodchem.2018.04.099. [DOI] [PubMed] [Google Scholar]
- 29.Codex Alimentarius Commission (CAC) Guidelines on Good Laboratory Practice in Residue Analysis. Codex Alimentarius Commission (CAC); Rome, Italy: 2003. CAC/GL 40-1993, Rev.1-2003. [Google Scholar]
- 30.Ministry of Food and Drug Safety . Practical Manual on the Analysis of Residual Pesticides in Food. National Institute of Food and Drug Safety Evaluation; Osong, Korea: 2016. Guidelines on Standard Procedures for Preparation of Test Methods. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.