Abstract
The endoplasmic reticulum (ER) is a key organelle responsible for the synthesis, modification, folding and assembly of proteins; calcium storage; and lipid synthesis. When ER homeostatic balance is disrupted by a variety of physiological and pathological factors—such as glucose deficiency, environmental toxins, Ca2+ level changes, etc.—ER stress can be induced. Abnormal ER stress can be involved in many diseases. NOD-like receptor family pyrin domain-containing 3 (NLRP3), an intracellular receptor, can perceive internal and external stimuli. It binds to apoptosis-associated speck-like protein containing a CARD (ASC) and caspase-1 to assemble into a protein complex called the NLRP3 inflammasome. Evidence indicates that ER stress and the NLRP3 inflammasome participate in many pathological processes; however, the exact mechanism remains to be understood. In this review, we summarized the role of ER stress and the NLRP3 inflammasome in liver disorders and analyzed the mechanisms, to provide references for future related research.
Keywords: endoplasmic reticulum stress, NLRP3 inflammasome, nonalcoholic fatty liver disease, hepatic ischemia–reperfusion, hepatotoxicity, liver injury
1. Introduction
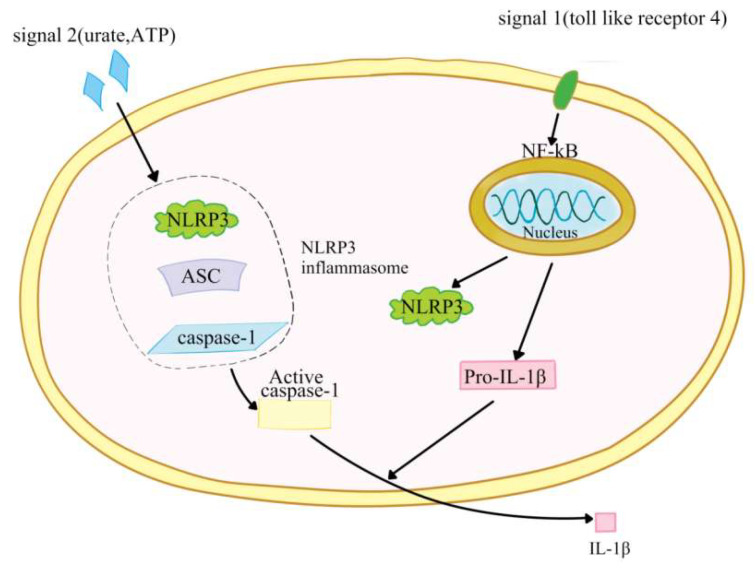
The definition of inflammasomes was first proposed by Tschopp et al. in 2002 [1]. Inflammasomes are a group of cellular protein complexes, which can recognize exogenous microorganisms, endogenous danger signals and different stressors; as a response, they activate caspase-1 to produce IL-1β and IL-18 to initiate inflammation [2,3]. So far, inflammasomes have been found to include nucleotide-binding domain leucine-rich repeat (NLR) and pyran domain-containing receptor 1 (NLRP1); NLRP3; RIG-I; and caspase recruitment domain containing receptor 4 (NLRC4); and they have been found to be absent in melanoma 2 (AIM2) [3,4]. NLRP3 inflammasome is the most thoroughly studied one at present, and is composed of NLRP3, apoptosis-associated speck-like protein (ASC) and pro-caspase-1 precursor [5,6,7,8,9]. NLRP3, a 115 kDa cytoplasmic protein, contains three domains: one is a leucine-rich repeat (LRR) at the C-end; the second is a central nucleotide-binding and oligomeric domain NACHT with ATPase activity, and the third is a pyran domain (PYD) at the N-end, which is used to recruit ASCs [10]. NLRP3 is expressed in monocytes, dendritic cells, neutrophils, epithelial cells, osteoblasts and lymphocytes [11]. ASC contains an amino terminal PYD and a carboxyl terminal CARD. Under specific stimulation, ASC interacts with NLRP3 through the PYD–PYD domain [3]. ASC recruits pro-caspase-1 through CARD–CARD domain interactions [12,13]. Under exogenous or endogenous stimulation, NLRP3 in the cell is activated, interacts with pre-caspase-1 and ASC to form a large protein complex, and activates caspase-1. Activated caspase-1 converts pre-IL-1β and pre-IL-18 into IL-1β and IL-18, which promotes inflammation by inducing the production of pro-inflammatory cytokines, chemokines and growth factors [14]. The activation of NRLP3 inflammasome requires two steps. The first step is induced by the first signal (signal 1) including toll-like receptor 4 and many endogenous risk signals, which activates NF-ĸB to upregulate the expression of NLRP3, pro-IL-1β, and pro-IL-18 [15,16]. The second step is the activation step and is induced by a signal (signal 2) including urate, extracellular adenosine triphosphate (ATP) and cholesterol crystals. Signal 2 promotes NLRP3 inflammasome assembly and activates caspase-1 to convert pre-IL-18 and IL-1β into their active forms [17] (Figure 1). It will lead to host inflammatory injury when NLRP3 inflammasome is overactivated [18]. Therefore, abnormal NLRP3 inflammasome can be involved in a variety of diseases, including liver diseases [19,20,21,22,23].
Figure 1.
The activation of NRLP3 inflammasome.
The endoplasmic reticulum (ER) is an organelle responsible for the synthesis, folding and modification of the secretion/transmembrane protein. It also plays a key role in lipid calcium storage, detoxification and biosynthesis [17,24,25]. When many physiological and pathological factors—including glucose deficiency, environmental toxins, Ca2+ level changes, viral infection, oxidative stress, inflammation and hypoxia–disrupt ER homeostatic balance, ER stress can be induced, forming a large number of unfolded and misfolded proteins, calcium depletion and lipid synthesis disorders [26,27]. Cells can reduce the damage of misfolded protein and alleviate the stress state in two ways. One is that ER stress triggers the unfolded protein reaction (UPR) to reduce the synthesis of new proteins and increase the expression of molecular chaperones that can promote protein folding. The second is to increase the degradation of misfolded proteins [28]. ER stress-induced UPR is mediated by three parallel signaling pathways: the activated transcription factor 6 (ATF6)-mediated pathway; the inositol dependent enzyme 1 (IRE1)-mediated pathway; and the pancreatic endoplasmic reticulum kinase (PERK)-mediated pathway [29]. Moderate ER stress can promote the recovery of ER homeostasis to help cells adapt to environmental changes. Excessive ER stress can induce caspase-12-dependent apoptosis, thus leading to many diseases [30]. Previous studies have shown that ER stress is involved in the occurrence and development of many disorders including diabetes, obesity, cancer, inflammation, neurodegenerative diseases and autoimmune diseases [31,32,33,34,35]. It has also been reported that ER stress and the NLRP3 inflammasome participate in many physiological and pathological processes; however, the exact mechanisms are unclear [36]. In this review, we summarized the role of ER stress and NLRP3 inflammasome in liver disorders and analyzed the mechanisms, to provide references for future related research.
2. The Role of Endoplasmic Reticulum Stress and the NLRP3 Inflammasome in Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease (NAFLD) is a clinicopathological syndrome characterized by the accumulation of liver fat, excluding viral infection and excessive drinking, and includes fatty liver, nonalcoholic steatohepatitis, and liver cirrhosis. It is a common chronic liver disease in the world. Due to its high incidence rate (about 20–30%) and long-term clinical treatment, NAFLD has become a serious public health problem [37,38,39]. Many risk factors are related to the progress of NAFLD, such as type 2 diabetes, visceral obesity, hyperlipidemia, and insulin resistance, but the exact pathogenesis is not yet fully understood [40]. At present, insulin sensitizers, antioxidants, lipid-lowering drugs and liver-protecting drugs are mainly used to treat NAFLD; however, the therapeutic effects are poor, and some drugs are found to have obvious toxic side effects, and can cause great harm to patients [41]. So far, no effective method for the prevention and treatment of NAFLD has been found. Therefore, it is particularly important to deeply study the pathogenesis of NAFLD and explore its effective treatment.
2.1. The Role of Endoplasmic Reticulum Stress and NLRP3 Inflammasome in Nonalcoholic Steatohepatitis
Nonalcoholic steatohepatitis (NASH) is the combination of lipid accumulation, hepatocyte death, inflammation, and fibrosis, which can develop into advanced fibrosis and hepatocellular carcinoma [42,43]. It is unclear how hepatic steatosis transforms into NASH [43]. It has been reported that the ER stress in hepatocytes may be related to the development of steatosis to NASH [44,45]. The results of C Lebeaupin et al. showed that tauroursodeoxycholic acid (TUDCA), an ER stress inhibitor, could inhibit balloon degeneration, apoptosis, and inflammasome activation of hepatocytes in obese mice that have severe steatosis and are stimulated by LPS. In the liver of obese mice, treatment with LPS or tunicamycin (an ER stress inducer) resulted in the activation of IRE1α, PERK and CHOP overexpression, which activated the NLRP3 inflammasome, subsequently triggering caspase-1, caspase-11, interleukin-1β-mediated hepatocyte pyroptosis, and caspase-3-dependent apoptosis. Meanwhile, TUDCA could abolish the above changes caused by LPS, indicating that TUDCA suppressed NLRP3 inflammasome-induced pyroptotic death by inhibiting LPS-induced ER stress to improve the NASH model, and ER stress promoted NLRP3 inflammasome-induced pyroptosis. Knocking-down Chop using siRNA inhibited the activity of caspase-11, caspase-1 and IL-1β, but not of active caspase-3, in tunicamycin or tunicamycin + LPS-induced primary hepatocytes; this indicates that ER stress induced NLRP3 inflammasome pyroptosis to cause injury of the LPS-induced NASH model via CHOP. In conclusion, ER stress could activate the NLRP3 inflammasome and subsequent pyroptosis and apoptosis, which promoted NASH progression. Therefore, the inhibition of ER stress-dependent NLRP3 inflammasome activation and subsequent cell death might be a potential treatment for nonalcoholic hepatitis. The ER stress effectors PERK and IRE1α could both activate CHOP, thereby activating NLRP3 inflammasome, which further proved that ER stress promoted the NLRP3 inflammasome via the CHOP pathway [46]. Reactive oxygen species (ROS) can activate the NLRP3 inflammasome [47]. In the above model, LPS and tunicamycin co-treatment only caused a slight increase in ROS; therefore, further studies are needed to exclude the effect of ER stress on NLRP3 through ROS. Moreover, whether ROS can further enhance the effects of the IRE1α-PERK–HOP axes, thus exacerbating NLRP3 inflammasome-induced pyroptosis in LPS-induced hepatocyte, needs to be studied.
2.2. Bax Inhibitor-1 Improves NAFLD through Endoplasmic Reticulum Stress and NLRP3 Inflammasome
Bax Inhibitor-1 (BI-1) is a negative regulator of ER stress and can improve NAFLD [48,49]. Cynthia Lebeaupin and colleagues found that BI-1 gene ablation in tunicamycin-treated BI-1−/− mice made the liver vulnerable to NAFLD, which led to hepatic steatosis and metabolic collapse; this was evidenced by an increase in fatty acid uptake, inhibition of β-oxidation, and a reduction in fatty acid release. Moreover, the enhanced ER stress promoted NLRP3 inflammasome activation, hepatocyte death, fibrosis, and the dysregulation of lipid homeostasis, leading to liver injury in the livers of tunicamycin-treated BI-1−/− mice. In liver biopsies obtained from NAFLD patients, the activation of the IRE1α signaling pathway was accompanied by BI-1 downregulation, suggesting that the IRE1α signaling pathway contributed to NAFLD. Moreover, the enhanced ER stress evidenced by the increasing expression of liver X-box binding protein 1 (XBP1), IRE1a, and the C/EBP homologous protein (CHOP) in HFD-fed BI-1−/− mice with NASH could also activate the NLRP3 inflammasome. Similarily, in primary mouse hepatocytes lacking BI-1, the IRE1α signaling pathway was shown to mediate NLRP3 inflammasome activation and cell death. Additionly, the inhibition of IRE1α signaling with STF-083010 counteracted the BI-1 deficiency promotion of NAFLD, indicating that BI-1 could improve NAFLD by suppressing ER stress-induced IRE1α-dependent NLRP3 inflammasome activation [50]. It can be seen from the above that ER stress-induced IRE1α-dependent NLRP3 inflammasome activation is involved in lipid metabolism, so it can be speculated that it may be beneficial in treating diabetes, which is worth studing.
2.3. Ginsenoside Rg1 Improves NAFLD through Endoplasmic Reticulum Stress and NLRP3 Inflammasome
Ginsenoside Rg1 (Rg1) is an active ingredient of natural medicine and has a variety of physiological functions, including anti-inflammatory, anti-apoptotic, anti-fibrosis, antioxidant and neuroprotective effects. It has been reported that Rg1 is involved in NAFLD [51,52,53]. Yashu Xu et al. constructed a mouse model of NAFLD by feeding mice a high-fat diet (HFD), and committed a series of experiments. The results showed that Rg1 improved NAFLD by notably decreasing liver weight, serum aspartate aminotransferase (AST), triglyceride (TG), alanine aminotransferase (ALT), and free fatty acids (FFAs), as well as alleviating liver inflammation. The above results were confirmed using a liver tissue staining experiment. Rg1 also decreased the serum level of malondialdehyde (MDA) and upregulated the expression of superoxide dismutase (SOD) and peroxisome proliferator-activated receptor-alpha (PPARα); this promoted fatty acid beta oxidation and the metabolism of FFAs and TG, indicating that Rg1 improved NAFLD by regulating lipid peroxidation. ER stress was promoted in NAFLD, which resulted in apoptosis and inflammation and lead to hepatocyte injury. On the other hand, Rg1 inhibited ER stress by downregulating the expression of CCAAT/enhancer binding protein (C/EBP) homologous protein (CHOP), caspase 12, and glucose-regulated protein 78 (GRP78). Moreover, the NLRP3 inflammasome level and the subsequent production of interleukin 1-beta (IL-1β) and interleukin 18 (IL-18) were increased in the NAFLD model of mice, while the changes decreased with Rg1. In summary, Rg1 ameliorated NAFLD by inhibiting ER stress and inflammasome activation, which needs to be further confirmed [54]. In the above study, the relationship between ER stress and NLRP3 inflammasome in the improvement of NAFLD with Rg1 remains to be further studied.
2.4. Acetylantroquinonol B Improves NAFLD through Endoplasmic Reticulum Stress and the NLRP3 Inflammasome
Acetylantroquinonol B (4-AAQB) is a ubiquinone from Antrodia cinnamomea and has antioxidant, anti-inflammatory and anti-hepatoma properties [55]. The results of I-Chuan Yen, et al. showed that 4-AAQB improved methionine/choline-deficient (MCD) diet-induced NASH by attenuating steatosis, immune cell filtration and hepatic ballooning, and by reducing the plasma levels of AST and ALT. Additionly, inflammation, ER stress, and NLRP3 inflammasome were all upregulated in in vitro and in vivo models, while 4-AAQB decreased these changes. 4-AAQB also activated the nuclear factor erythroid 2-related factor 2 (Nrf2) and Sirtuin 1 signaling pathways in vitro and in vivo [56]. SIRT1 inhibited hepatic inflammation, ER stress, and lipogenesis, indicating that SIRT1 could improve NAFLD [57,58,59,60] and possessed a beneficial metabolic function [61]. An SIRT1 gene knockout exacerbated palmitic acid (PA)-induced NLRP3 inflammasome activation and subsequent inflammation in AML-12 cells, indicating that 4-AAQB suppressed ERS/NLRP3 inflammasome by activating SIRT1 [62]. Studies have shown that Nrf2-deficient mice are prone to NASH [63], showing that Nrf2 is a promising therapeutic target for NAFLD [64,65]. In addition, SIRT1 is related to the activation of the Nrf2 antioxidant pathway in vivo [66]. The activation of the Nrf2 pathway improves NASH progression by inhibiting ER stress [67]. Therefore, it can be deduced that 4-AAQB ameliorated NAFLD through the inhibition of ERS/NLRP3 inflammasome by activating SIRT1-the Nrf2 pathway, which needs to be further verified. In the above study, 4-AAQB could scavenge ROS to inhibit oxidative stress [56]; therefore, in the improvement of NAFLD using 4-AAQB, ER stress may inhibit the NLRP3 inflammasome through ROS, which needs to be further studied.
In summary, at present, there are few studies on the role of ER stress/NLRP3 inflammasome in NAFLD; moreover, some studies are very superficial and can only draw preliminary conclusions, which need further research to be verified. In particular, the mechanism of ER stress/the NLRP3 inflammasome involved in liver lipid metabolism remains to be further explored. In addition, in the above studies, ER stress/the NLRP3 inflammasome were inhibited to improve NAFLD. However, whether enhanced endoplasmic reticulum stress can improve NAFLD by inhibiting NLRP3 inflammatory bodies remains to be clarified. ER stress/the NLRP3 inflammasome may become a new strategy for the treatment of NAFLD.
3. The Role of Endoplasmic Reticulum Stress and the NLRP3 Inflammasome in Hepatic Ischemia–Reperfusion
Hepatic ischemia–reperfusion (HIR) is a physiological and pathological phenomenon, which is difficult to avoid in some types of surgery and is related to liver transplantation, liver injury and hepatectomy [68,69]. When the blood flow is restored, the liver is subjected to new attacks due to the initial metabolic imbalance caused by the sudden supply of nutrients (especially oxygen), resulting in the deterioration of the injury. This phenomenon is called hepatic ischemia–reperfusion injury (HIRI) [70,71]. γ-Oryzanol (ORY) is one of the rice bran oil (RBO) compounds, which is known as the main food source in the world [72]. ORY has been reported to have anti-diabetic, anti-hyperlipidemic, anti-carcinogenic, anti-inflammatory, anti-ulcerogenic, and antioxidant effects [73,74]. Yichao Du and colleagues orally administered ORY to mice for 7 days, followed by liver ischemia for 60 min and reperfusion for 6 h. The results showed that ORY mitigated HIRI in mice by decreasing the serum AST and ALT levels; it also improved hemorrhagic focus, the collapse of the hepatic lobule structure, and hepatocyte necrosis in a HIRI model of rats. The in-depth research revealed that ORY upregulated the levels of GSH and SOD, and downregulated the levels of MDA and MPO in a HIRI model of rats, indicating that ORY inhibited I/R-induced lipid peroxidation, oxidative stress and neutrophil infiltration. ORY also inhibited ER stress during HIRI by reducing the expression levels of CHOP, p-PERK and GRP78. Moreover, ORY significantly decreased the protein expressions of NLRP3, caspase-1, IL-1β, and Bax, and increased Bcl-2 protein expression to protect the liver from inflammation and apoptosis induced by I/R in the rat model. Similar results were obtained in AML12 cells (mouse normal hepatocytes) in vitro. Collectively, ORY improved HIRI by suppressing the NLRP3 inflammasome and ER stress [75]. Studies have revealed that the massive production of ROS is closely related to HIRI [76]. In the above study, ORY notably reduced ROS levels in CoCl2-Induced AML12 cells; therefore, it can be deduced that ER stress inhibits NLRP3 inflammasome by clearing ROS. More and more evidence has proven that ER stress and NLRP3 inflammasome-mediated injury have a great impact on ischemia–reperfusion injury [77,78], which needs further study. ER stress and NLRP3 inflammasome may become a target for the treatment of hepatic ischemia–reperfusion injury in the future.
4. The Role of Endoplasmic Reticulum Stress and the NLRP3 Inflammasome in Hepatotoxicity
4.1. Allicin Improves Hepatotoxicity through Endoplasmic Reticulum Stress and the NLRP3 Inflammasome
Acrylamide (AA) is produced by a Maillard reaction during thermal processing and a well-known potential carcinogenic compound [79]. AA has been reported to have hepatotoxic effects [80]. Allicin is one of the active components in garlic bulbs [81], and has many biological benefits, including anticancer, hypolipidemic, blood-pressure-lowering, diabetes improvement, anti-hepatic-steatosis and anti-inflammation [82,83]. Allicin can inhibit AA-induced hepatocyte injury and toxicity by inhibiting intracellular ROS release and oxidative stress (OS) [84,85]. Bo Nan et al. found that allicin downregulated CYP2E1 protein expression and ROS release to finally reduce OS-induced liver injury in Kupffer cells and SD rat livers treated with AA. Meanwhile, allicin significantly decreased the expression of the ER stress marker proteins CHOP and GRP78, and the expression of IRE1α pathway key proteins TRAF2, p-ASK, p-IRE, and XBP-1s induced by AA; this indicates that allicin inhibited AA-induced ER stress. Furthermore, allicin suppressed AA-induced the MAPK and NF-κB pathways by downregulating p65, JNK, p38, ERK, and IκBα phosphorylation in Kupffer cells and SD rat livers. Additionally, allicin also decreased cleaved caspase-1 expression and the release of IL-1β, IL-6, IL-18, and TNF-α to inhibit AA-induced-NLRP3 inflammasome activation, thus mitigating AA-induced liver inflammation. Collectively, allicin can reduce AA-induced NLRP3 inflammasome activation by inhibiting ER stress and OS, thus improving AA-induced hepatotoxicity; this needs to be further verified using inhibitors, such as ER stress inhibitors and NLRP3 inhibitors [86]. It has been reported that high glucose can produce ROS and activate the MAPK and NF-κB signaling pathways to induce inflammation in HepG2 cells [87]. Additionally, ER stress can regulate the MAPK and NF-κB signaling pathways [88,89]. Therefore, it can be deduced that ER stress inhibits the NLRP3 inflammasome via the MAPK and NF-κB signaling pathways in the improvement of AA-induced hepatotoxicity using allicin, which needs to be further confirmed.
4.2. Baicalin Improves Hepatotoxicity through Endoplasmic Reticulum Stress and NLRP3 Inflammasome 13
Baicalin (BA) is one of the main bioactive components of the Chinese herbal medicine Scutellaria baicalensis. It has many pharmacological activities, such as antitumor, antibacterial, and antioxidant [90,91,92]. BA has been reported to be closely related to lipid metabolism; however, the exact mechanism is unclear [93,94]. The results of Junli Zhang et al. showed that 400 μM PA induced ER stress, evidenced by the elevated expression of the ER stress marker IRE1α and hyperphosphorylation in AML-12 cells. BA (12.5 μM and 25 μM) and TUDCA significantly inhibited p-IRE1α expression to suppress PA-induced ER stress. BA and TUDCA also inhibited intracellular PA-induced ROS generation and apoptosis of AML-12 cells, indicating that BA could suppress oxidative stress and apoptosis induced by ER stress. Moreover, BA and TUDCA significantly inhibited the expression of TXNIP and NLRP3 induced by PA, which was reversed by compound C (an AMPK inhibitor), indicating that BA inhibited ER stress via the TXNIP/NLRP3 pathway through the AMPK pathway. Collectively, BA improved cytotoxicity of AML-12 cells induced by PA, through the inhibition of ER stress via the TXNIP/NLRP3 pathway, through the AMPK pathway [95]. In contrast to the inhibition of ER stress by BA in the above study, Wang et al. found that BA induced apoptosis by promoting ER stress by activating the ATF6 signaling pathway in human hepatoma cells [96]. The reason may be that the basic level of ER stress differs between different types of cells. Studies have shown that ROS/TXNIP-induced activation of the NLRP3 inflammasome plays an important role in NAFLD [97,98,99]. Similarly, in the above study, it can be seen that ER stress regulates the NLRP3 inflammasome through ROS/TXNIP in BA improvement of hepatotoxicity. Targeting the TXNIP/NLRP3 pathway may be promising in liver diseases.
5. Farnesoid X Receptor Improves Liver Injury through Endoplasmic Reticulum Stress and NLRP3 Inflammasome
The farnesoid X receptor (FXR) is a member of the nuclear receptor family and exists in the intestine and liver. It helps to maintain systemic metabolic homeostasis by regulating glucose, bile acid, lipid metabolism, and energy homeostasis. Furthermore, the FXR also plays an important role in many organs, including the liver, cardiovascular system, kidney, intestine, breast, pancreas and brain [100,101,102,103]. Liver FXR activation has beneficial effects on metabolic disorders, such as NAFLD, diabetes mellitus and cholestasis [104,105,106]. The results of Chang Yeob Han et al. showed that, in patients with NAFLD and mice with liver injury, the level of FXR in the liver was negatively correlated with the activation of NLRP3 inflammasome, suggesting an inhibitory role of FXR on the NLRP3 inflammasome. In hepatocytes treated with tunicamycin, FXR levels significantly decreased, the levels of NLRP3, TXNIP, and cleaved caspase-1 and IL-1β increased; this indicates that ER stress-induced NLRP3 inflammasome in rat hepatocytes was related to FXR inhibition. FXR deficiency in mice cooperated with ER stress-induced NLRP3 and the thioredoxin-interacting protein (TXNIP), which aggravated liver injury. Meanwhile the treatment of wild-type mice with GW4064 (an FXR agonist) had the opposite effect, indicating that FXR inhibited ER stress-induced NLRP3 and TXNIP. Moreover, FXR suppressed NLRP3 and TXNIP via the PERK–CHOP pathway. In summary, FXR suppresses ER stress-induced NLRP3 inflammasome via the PERK–CHOP signaling pathway in hepatocytes to improve liver injury. ER stress promoted NLRP3 inflammasome via the PERK–CHOP signaling pathway [107]. TXNIP interacts with and activates NLRP3 [108,109,110]. However, in the above study, TXNIP knockdown had no influence on NLRP3, indicating that ER stress-mediated NLRP3 induction may not be related to TXNIP [107].
6. Conclusions
Accumulating evidence indicates that ER stress and the NLRP3 inflammasome play an important role in liver disorders. In this review, we summarized the following: (1) ER stress could activate NLRP3 inflammasome and subsequent pyroptosis and apoptosis to lead to nonalcoholic hepatitis; (2) BI-1 could improve NAFLD by suppressing ER stress-induced IRE1a-dependent NLRP3 inflammasome activation; (3) Rg1 improved NAFLD through the inhibition of ER stress and NLRP3 inflammasome activation, which needs to be further confirmed; (4) 4-AAQB ameliorated NAFLD through the inhibition of ERS/NLRP3 inflammasome by activating the SIRT1-Nrf2 pathway, which needs to be further confirmed; (5) ORY ameliorated HIRI through the inhibition of the NLRP3 inflammasome and ER stress; (6) ER stress inhibits the NLRP3 inflammasome via the MAPK and NF-κB signaling pathways in the improvement of AA-induced hepatotoxicity using allicin, which needs to be further confirmed; (7) BA improved the PA-induced cytotoxicity of AML-12 cells through the inhibition of ER stress via the TXNIP/NLRP3 pathway, through the AMPK pathway; (8) FXR suppressed the ER stress-induced NLRP3 inflammasome via the PERK–CHOP signaling pathway in hepatocytes to improve liver injury (Table 1).
Table 1.
Summary of the roles of endoplasmic reticulum stress and NLRP3 inflammasome in liver disorders.
| The Type of Pathological Processes | The Role of ER Stress and the NLRP3 Inflammasome | Experimental Model | Reference |
|---|---|---|---|
| Nonalcoholic hepatitis | ER stress promoted the NLRP3 inflammasome and subsequent pyroptosis and apoptosis to promote nonalcoholic hepatitis | Mouse/mouse primary hepatocyte model of nonalcoholic hepatitis | [46] |
| Nonalcoholic fatty liver disease (NAFLD) | BI-1 improved NAFLD through inhibition of ER stress-induced and IRE1a-dependent NLRP3 inflammasome activation | Mouse/mouse hepatocyte model of NAFLD | [50] |
| NAFLD | Rg1 improved NAFLD through the inhibition of ER stress and NLRP3 inflammasome activation | Mouse model of NAFLD | [54] |
| NAFLD | 4-AAQB ameliorated NAFLD through the inhibition of ERS/NLRP3 inflammasome by activating the SIRT1-Nrf2 pathway | Male C57BL/6J mouse model of NAFLD | [56] |
| Hepatic ischemia–reperfusion(HIRI) | ORY ameliorated HIRI through the inhibition of the NLRP3 inflammasome and ER stress | C57BL/6 mouse model of HIRI | [75] |
| Hepatotoxicity | Allicin improved AA-induced hepatotoxicity through ER stress inhibition of NLRP3 inflammasome via the MAPK and NF-κB signaling pathways | Sprague Dawley rats/Kupffer cell model of hepatotoxicity | [86] |
| Hepatotoxicity | BA improved PA-induced cytotoxicity of AML-12 cells through the inhibition of ER stress via the TXNIP/NLRP3 pathway, through the AMPK pathway | AML-12 cell model of hepatotoxicity | [95] |
| Liver injury | FXR improved liver injury through inhibition of ER stress-induced NLRP3 inflammasome via the PERK–CHOP signaling pathway | C57BL/6J mouse/AML-12 cell model of liver injury | [107] |
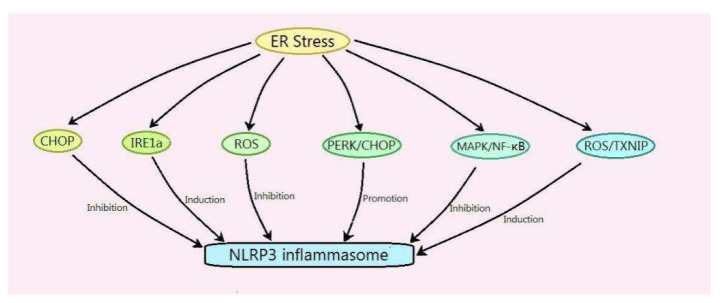
It can be seen from the above summarized research that the mechanism of ER stress-regulating NLRP3 is as follows: (1) ER stress promotes the NLRP3 inflammasome via the CHOP pathway; (2) ER stress induces the NLRP3 inflammasome via IRE1a; (3) ER stress inhibits the NLRP3 inflammasome by clearing ROS; (4) ER stress suppresses the NLRP3 inflammasome via the MAPK and NF-κB signaling pathways; (5) ER stress induces the NLRP3 inflammasome through ROS/TXNIP; and (6) ER stress promotes the NLRP3 inflammasome via the PERK–CHOP signaling pathway (Figure 2). In the regulation of the NLRP3 inflammasome by ER stress in liver disorders, sometimes, ER stress inhibits NLRP3, and sometimes, the opposite is true. The reason may be different physiological and pathological processes, which need further study. Most of the existing studies state that ER stress regulates the NLRP3 inflammasome in the liver. Conversely, whether the NLRP3 inflammasome can regulate ER stress in the liver, and the mechanism, need to be further studied. Evidence indicates that ER stress and the NLRP3 inflammasome are both the regulative target of hydrogen sulfide (H2S) [111,112]. Whether H2S can regulate ER stress and the NLRP3 inflammasome in liver disorders is worth studying. ER stress and the NLRP3 inflammasome will become an important target for the treatment of liver disorders.
Figure 2.
Mechanism of endoplasmic reticulum stress regulating the NLRP3 inflammasome in liver disorders.
Author Contributions
H.W. devised, wrote, and funded the manuscript; X.L., H.H., X.F., C.C., D.W. and H.L. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from key scientific and technological projects in Henan Province, China (Grant No. 202102310153); the construction of first-class medical disciplines at the Medical College of Henan University from 2020 to 2021 for Honggang Wang; the Innovation and Entrepreneurship Training Program for Henan University Students in 2021 (Grant number: 20211021002 and 20217003002); the Henan Provincial Social Science Planning Decision Consulting Project (Grant No. 2018JC38); and the Graduate Education Reform and Quality Improvement Project of Henan Province (Grant No. YJS2021AL074).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martinon F., Burns K., Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 2.Strowig T., Henao-Mejia J., Elinav E., Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 3.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Minkiewicz J., de Rivero Vaccari J.P., Keane R.W. Human astrocytes express a novel NLRP2 inflammasome. Glia. 2013;61:1113–1121. doi: 10.1002/glia.22499. [DOI] [PubMed] [Google Scholar]
- 5.Volt H., Garcia J.A., Doerrier C., Diaz-Casado M.E., Guerra-Librero A., Lopez L.C., Escames G., Tresguerres J.A., Acuna-Castroviejo D. Same molecule but different expression: Aging and sepsis trigger NLRP3 inflammasome activation, a target of melatonin. J. Pineal Res. 2016;60:193–205. doi: 10.1111/jpi.12303. [DOI] [PubMed] [Google Scholar]
- 6.Sharif H., Wang L., Wang W.L., Magupalli V.G., Andreeva L., Qiao Q., Hauenstein A.V., Wu Z., Nunez G., Mao Y., et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570:338–343. doi: 10.1038/s41586-019-1295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agostini L., Martinon F., Burns K., McDermott M.F., Hawkins P.N., Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/S1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 8.Zahid A., Li B., Kombe A.J.K., Jin T., Tao J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front. Immunol. 2019;10:2538. doi: 10.3389/fimmu.2019.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao Y., Wang N., Qiu T., Sun X. The Role of Autophagy and NLRP3 Inflammasome in Liver Fibrosis. Biomed Res. Int. 2020;2020:7269150. doi: 10.1155/2020/7269150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halle A., Hornung V., Petzold G.C., Stewart C.R., Monks B.G., Reinheckel T., Fitzgerald K.A., Latz E., Moore K.J., Golenbock D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong Y., Kinio A., Saleh M. Functions of NOD-Like Receptors in Human Diseases. Front. Immunol. 2013;4:333. doi: 10.3389/fimmu.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai X., Chen J., Xu H., Liu S., Jiang Q.X., Halfmann R., Chen Z.J. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156:1207–1222. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu A., Magupalli V.G., Ruan J., Yin Q., Atianand M.K., Vos M.R., Schroder G.F., Fitzgerald K.A., Wu H., Egelman E.H. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv S., Li X., Wang H. The Role of the Effects of Endoplasmic Reticulum Stress on NLRP3 Inflammasome in Diabetes. Front. Cell Dev. Biol. 2021;9:663528. doi: 10.3389/fcell.2021.663528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swanson K.V., Junkins R.D., Kurkjian C.J., Holley-Guthrie E., Pendse A.A., El Morabiti R., Petrucelli A., Barber G.N., Benedict C.A., Ting J.P. A noncanonical function of cGAMP in inflammasome priming and activation. J. Exp. Med. 2017;214:3611–3626. doi: 10.1084/jem.20171749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019;20:3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokolova M., Ranheim T., Louwe M.C., Halvorsen B., Yndestad A., Aukrust P. NLRP3 Inflammasome: A Novel Player in Metabolically Induced Inflammation-Potential Influence on the Myocardium. J. Cardiovasc. Pharmacol. 2019;74:276–284. doi: 10.1097/FJC.0000000000000704. [DOI] [PubMed] [Google Scholar]
- 18.He Y., Hara H., Nunez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wree A., Eguchi A., McGeough M.D., Pena C.A., Johnson C.D., Canbay A., Hoffman H.M., Feldstein A.E. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59:898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wree A., McGeough M.D., Inzaugarat M.E., Eguchi A., Schuster S., Johnson C.D., Pena C.A., Geisler L.J., Papouchado B.G., Hoffman H.M., et al. NLRP3 inflammasome driven liver injury and fibrosis: Roles of IL-17 and TNF in mice. Hepatology. 2018;67:736–749. doi: 10.1002/hep.29523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mridha A.R., Wree A., Robertson A.A.B., Yeh M.M., Johnson C.D., Van Rooyen D.M., Haczeyni F., Teoh N.C., Savard C., Ioannou G.N., et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatol. 2017;66:1037–1046. doi: 10.1016/j.jhep.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Mamun A., Akter A., Hossain S., Sarker T., Safa S.A., Mustafa Q.G., Muhammad S.A., Munir F. Role of NLRP3 inflammasome in liver disease. J. Dig. Dis. 2020;21:430–436. doi: 10.1111/1751-2980.12918. [DOI] [PubMed] [Google Scholar]
- 23.Knorr J., Wree A., Tacke F., Feldstein A.E. The NLRP3 Inflammasome in Alcoholic and Nonalcoholic Steatohepatitis. Semin. Liver Dis. 2020;40:298–306. doi: 10.1055/s-0040-1708540. [DOI] [PubMed] [Google Scholar]
- 24.Braakman I., Bulleid N.J. Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 2011;80:71–99. doi: 10.1146/annurev-biochem-062209-093836. [DOI] [PubMed] [Google Scholar]
- 25.Chen X., Karnovsky A., Sans M.D., Andrews P.C., Williams J.A. Molecular characterization of the endoplasmic reticulum: Insights from proteomic studies. Proteomics. 2010;10:4040–4052. doi: 10.1002/pmic.201000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandl J., Meszaros T., Banhegyi G., Csala M. Minireview: Endoplasmic reticulum stress: Control in protein, lipid, and signal homeostasis. Mol. Endocrinol. 2013;27:384–393. doi: 10.1210/me.2012-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rangel-Aldao R. The unfolded protein response, inflammation, oscillators, and disease: A systems biology approach. Endoplasmic Reticulum Stress Dis. 2015;2:30–52. doi: 10.1515/ersc-2015-0003. [DOI] [Google Scholar]
- 28.McCaffrey K., Braakman I. Protein quality control at the endoplasmic reticulum. Essays Biochem. 2016;60:227–235. doi: 10.1042/EBC20160003. [DOI] [PubMed] [Google Scholar]
- 29.So J.S. Roles of Endoplasmic Reticulum Stress in Immune Responses. Mol. Cells. 2018;41:705–716. doi: 10.14348/molcells.2018.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji T., Han Y., Yang W., Xu B., Sun M., Jiang S., Yu Y., Jin Z., Ma Z., Yang Y., et al. Endoplasmic reticulum stress and NLRP3 inflammasome: Crosstalk in cardiovascular and metabolic disorders. J. Cell. Physiol. 2019;234:14773–1478821. doi: 10.1002/jcp.28275. [DOI] [PubMed] [Google Scholar]
- 31.Hetz C., Chevet E., Harding H.P. Targeting the unfolded protein response in disease. Nat. Rev. Drug Discov. 2013;12:703–719. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 32.Marciniak S.J., Ron D. Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 33.Oyadomari S., Harding H.P., Zhang Y., Oyadomari M., Ron D. Dephosphorylation of translation initiation factor 2α enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozcan U., Cao Q., Yilmaz E., Lee A.H., Iwakoshi N.N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L.H., Hotamisligil G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 35.Wang S., Kaufman R.J. The impact of the unfolded protein response on human disease. J. Cell Biol. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W., Cao T., Luo C., Cai J., Zhou X., Xiao X., Liu S. Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl. Microbiol. Biotechnol. 2020;104:6129–6140. doi: 10.1007/s00253-020-10614-y. [DOI] [PubMed] [Google Scholar]
- 37.Santhekadur P.K., Kumar D.P., Sanyal A.J. Preclinical models of non-alcoholic fatty liver disease. J. Hepatol. 2018;68:230–237. doi: 10.1016/j.jhep.2017.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen X., Jin C., Wu Y., Zhang Y., Wang X., Huang W., Li J., Wu S., Gao X. Prospective study of perceived dietary salt intake and the risk of non-alcoholic fatty liver disease. J. Hum. Nutr. Diet. 2019;32:802–809. doi: 10.1111/jhn.12674. [DOI] [PubMed] [Google Scholar]
- 39.DeWeerdt S. Disease progression: Divergent paths. Nature. 2017;551:S92–S93. doi: 10.1038/d41586-017-06925-2. [DOI] [PubMed] [Google Scholar]
- 40.Li M., Xu C., Shi J., Ding J., Wan X., Chen D., Gao J., Li C., Zhang J., Lin Y., et al. Fatty acids promote fatty liver disease via the dysregulation of 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Gut. 2018;67:2169–2180. doi: 10.1136/gutjnl-2017-313778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Issa D., Patel V., Sanyal A.J. Future therapy for non-alcoholic fatty liver disease. Liver Int. 2018;38((Suppl. S1)):56–63. doi: 10.1111/liv.13676. [DOI] [PubMed] [Google Scholar]
- 42.Machado M.V., Diehl A.M. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology. 2016;150:1769–1777. doi: 10.1053/j.gastro.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki A., Diehl A.M. Nonalcoholic Steatohepatitis. Annu. Rev. Med. 2017;68:85–98. doi: 10.1146/annurev-med-051215-031109. [DOI] [PubMed] [Google Scholar]
- 44.Xia S.W., Wang Z.M., Sun S.M., Su Y., Li Z.H., Shao J.J., Tan S.Z., Chen A.P., Wang S.J., Zhang Z.L., et al. Endoplasmic reticulum stress and protein degradation in chronic liver disease. Pharmacol. Res. 2020;161:105218. doi: 10.1016/j.phrs.2020.105218. [DOI] [PubMed] [Google Scholar]
- 45.Kim J.Y., Garcia-Carbonell R., Yamachika S., Zhao P., Dhar D., Loomba R., Kaufman R.J., Saltiel A.R., Karin M. ER Stress Drives Lipogenesis and Steatohepatitis via Caspase-2 Activation of S1P. Cell. 2018;175:133–145.e15. doi: 10.1016/j.cell.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lebeaupin C., Proics E., de Bieville C.H., Rousseau D., Bonnafous S., Patouraux S., Adam G., Lavallard V.J., Rovere C., Le Thuc O., et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015;6:e1879. doi: 10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu Z., He Y., Ming H., Lei S., Leng Y., Xia Z.Y. Lipopolysaccharide (LPS) Aggravates High Glucose- and Hypoxia/Reoxygenation-Induced Injury through Activating ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis in H9C2 Cardiomyocytes. J. Diabetes Res. 2019;2019:8151836. doi: 10.1155/2019/8151836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bailly-Maitre B., Belgardt B.F., Jordan S.D., Coornaert B., von Freyend M.J., Kleinridders A., Mauer J., Cuddy M., Kress C.L., Willmes D., et al. Hepatic Bax inhibitor-1 inhibits IRE1alpha and protects from obesity-associated insulin resistance and glucose intolerance. J. Biol. Chem. 2010;285:6198–6207. doi: 10.1074/jbc.M109.056648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lisbona F., Rojas-Rivera D., Thielen P., Zamorano S., Todd D., Martinon F., Glavic A., Kress C., Lin J.H., Walter P., et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1α. Mol. Cell. 2009;33:679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lebeaupin C., Vallee D., Rousseau D., Patouraux S., Bonnafous S., Adam G., Luciano F., Luci C., Anty R., Iannelli A., et al. Bax inhibitor-1 protects from nonalcoholic steatohepatitis by limiting inositol-requiring enzyme 1 alpha signaling in mice. Hepatology. 2018;68:515–532. doi: 10.1002/hep.29847. [DOI] [PubMed] [Google Scholar]
- 51.Hou Y., Gu D., Peng J., Jiang K., Li Z., Shi J., Yang S., Li S., Fan X. Ginsenoside Rg1 Regulates Liver Lipid Factor Metabolism in NAFLD Model Rats. ACS Omega. 2020;5:10878–10890. doi: 10.1021/acsomega.0c00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J.C., Jeon J.Y., Yang W.S., Kim C.H., Eom D.W. Combined Amelioration of Ginsenoside (Rg1, Rb1, and Rg3)-enriched Korean Red Ginseng and Probiotic Lactobacillus on Non-alcoholic Fatty Liver Disease. Curr. Pharm. Biotechnol. 2019;20:222–231. doi: 10.2174/1389201020666190311143554. [DOI] [PubMed] [Google Scholar]
- 53.Gu D., Yi H., Jiang K., Fakhar S.H., Shi J., He Y., Liu B., Guo Y., Fan X., Li S. Transcriptome analysis reveals the efficacy of ginsenoside-Rg1 in the treatment of nonalcoholic fatty liver disease. Life Sci. 2021;267:118986. doi: 10.1016/j.lfs.2020.118986. [DOI] [PubMed] [Google Scholar]
- 54.Xu Y., Yang C., Zhang S., Li J., Xiao Q., Huang W. Ginsenoside Rg1 Protects against Non-alcoholic Fatty Liver Disease by Ameliorating Lipid Peroxidation, Endoplasmic Reticulum Stress, and Inflammasome Activation. Biol. Pharm. Bull. 2018;41:1638–1644. doi: 10.1248/bpb.b18-00132. [DOI] [PubMed] [Google Scholar]
- 55.Wu C.H., Ou C.H., Yen I.C., Lee S.Y. 4-Acetylantroquinonol B Inhibits Osteoclastogenesis by Inhibiting the Autophagy Pathway in a Simulated Microgravity Model. Int. J. Mol. Sci. 2020;21:6971. doi: 10.3390/ijms21186971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yen I.C., Tu Q.W., Chang T.C., Lin P.H., Li Y.F., Lee S.Y. 4-Acetylantroquinonol B ameliorates nonalcoholic steatohepatitis by suppression of ER stress and NLRP3 inflammasome activation. Biomed. Pharmacother. 2021;138:111504. doi: 10.1016/j.biopha.2021.111504. [DOI] [PubMed] [Google Scholar]
- 57.Yoshizaki T., Schenk S., Imamura T., Babendure J.L., Sonoda N., Bae E.J., Oh D.Y., Lu M., Milne J.C., Westphal C., et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010;298:E419–E428. doi: 10.1152/ajpendo.00417.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding R.B., Bao J., Deng C.X. Emerging roles of SIRT1 in fatty liver diseases. Int. J. Biol. Sci. 2017;13:852–867. doi: 10.7150/ijbs.19370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y., Xu S., Giles A., Nakamura K., Lee J.W., Hou X., Donmez G., Li J., Luo Z., Walsh K., et al. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 2011;25:1664–1679. doi: 10.1096/fj.10-173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deng X.Q., Chen L.L., Li N.X. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007;27:708–715. doi: 10.1111/j.1478-3231.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 61.Rolo A.P., Teodoro J.S., Palmeira C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2012;52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Peng Z., Li X., Xing D., Du X., Wang Z., Liu G., Li X. Nobiletin alleviates palmitic acidinduced NLRP3 inflammasome activation in a sirtuin 1dependent manner in AML12 cells. Mol. Med. Rep. 2018;18:5815–5822. doi: 10.3892/mmr.2018.9615. [DOI] [PubMed] [Google Scholar]
- 63.Chowdhry S., Nazmy M.H., Meakin P.J., Dinkova-Kostova A.T., Walsh S.V., Tsujita T., Dillon J.F., Ashford M.L., Hayes J.D. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2010;48:357–371. doi: 10.1016/j.freeradbiomed.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 64.Du J., Zhang M., Lu J., Zhang X., Xiong Q., Xu Y., Bao Y., Jia W. Osteocalcin improves nonalcoholic fatty liver disease in mice through activation of Nrf2 and inhibition of JNK. Endocrine. 2016;53:701–709. doi: 10.1007/s12020-016-0926-5. [DOI] [PubMed] [Google Scholar]
- 65.Chambel S.S., Santos-Goncalves A., Duarte T.L. The Dual Role of Nrf2 in Nonalcoholic Fatty Liver Disease: Regulation of Antioxidant Defenses and Hepatic Lipid Metabolism. Biomed. Res. Int. 2015;2015:597134. doi: 10.1155/2015/597134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding Y.W., Zhao G.J., Li X.L., Hong G.L., Li M.F., Qiu Q.M., Wu B., Lu Z.Q. SIRT1 exerts protective effects against paraquat-induced injury in mouse type II alveolar epithelial cells by deacetylating NRF2 in vitro. Int. J. Mol. Med. 2016;37:1049–1058. doi: 10.3892/ijmm.2016.2503. [DOI] [PubMed] [Google Scholar]
- 67.Sharma R.S., Harrison D.J., Kisielewski D., Cassidy D.M., McNeilly A.D., Gallagher J.R., Walsh S.V., Honda T., McCrimmon R.J., Dinkova-Kostova A.T., et al. Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2) Cell. Mol. Gastroenterol. Hepatol. 2018;5:367–398. doi: 10.1016/j.jcmgh.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu S., Yao W., Chen C., Chen H., Huang F., Liu Y., Cai J., Yuan D., Hei Z. Connexin 32 deficiency protects the liver against ischemia/reperfusion injury. Eur. J. Pharmacol. 2020;876:173056. doi: 10.1016/j.ejphar.2020.173056. [DOI] [PubMed] [Google Scholar]
- 69.de Almeida T.N., Victorino J.P., Bistafa Liu J., Tofoli Queiroz Campos D., Graf C., Jordani M.C., Carneiro d’Albuquerque L.A., Mendes K.D.S., Castro E.S.O. Effect of Hepatic Preconditioning with the Use of Methylene Blue on the Liver of Wistar Rats Submitted to Ischemia and Reperfusion. Transplant. Proc. 2018;50:841–847. doi: 10.1016/j.transproceed.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 70.Zhai Y., Petrowsky H., Hong J.C., Busuttil R.W., Kupiec-Weglinski J.W. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gan X., Zhang R., Gu J., Ju Z., Wu X., Wang Q., Peng H., Qiu J., Zhou J., Cheng F., et al. Acidic Microenvironment Regulates the Severity of Hepatic Ischemia/Reperfusion Injury by Modulating the Generation and Function of Tregs via the PI3K-mTOR Pathway. Front. Immunol. 2019;10:2945. doi: 10.3389/fimmu.2019.02945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramazani E., Akaberi M., Emami S.A., Tayarani-Najaran Z. Biological and Pharmacological Effects of Gamma-oryzanol: An Updated Review of the Molecular Mechanisms. Curr. Pharm. Des. 2021;27:2299–2316. doi: 10.2174/1381612826666201102101428. [DOI] [PubMed] [Google Scholar]
- 73.Minatel I.O., Francisqueti F.V., Correa C.R., Lima G.P. Antioxidant Activity of gamma-Oryzanol: A Complex Network of Interactions. Int. J. Mol. Sci. 2016;17:1107. doi: 10.3390/ijms17081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szczesniak K.A., Ostaszewski P., Ciecierska A., Sadkowski T. Investigation of nutriactive phytochemical-gamma-oryzanol in experimental animal models. J. Anim. Physiol. Anim. Nutr. 2016;100:601–617. doi: 10.1111/jpn.12428. [DOI] [PubMed] [Google Scholar]
- 75.Du Y., Zhong F., Cheng H., Li T., Chen Y., Tan P., Huang M., Liang T., Liu Y., Xia X., et al. The Dietary Supplement gamma-Oryzanol Attenuates Hepatic Ischemia Reperfusion Injury via Inhibiting Endoplasmic Reticulum Stress and HMGB1/NLRP3 Inflammasome. Oxid. Med. Cell. Longev. 2021;2021:4628050. doi: 10.1155/2021/4628050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katwal G., Baral D., Fan X., Weiyang H., Zhang X., Ling L., Xiong Y., Ye Q., Wang Y. SIRT3 a Major Player in Attenuation of Hepatic Ischemia-Reperfusion Injury by Reducing ROS via Its Downstream Mediators: SOD2, CYP-D, and HIF-1alpha. Oxid. Med. Cell. Longev. 2018;2018:2976957. doi: 10.1155/2018/2976957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yue R.C., Lu S.Z., Luo Y., Wang T., Liang H., Zeng J., Liu J., Hu H.X. Calpain silencing alleviates myocardial ischemia-reperfusion injury through the NLRP3/ASC/Caspase-1 axis in mice. Life Sci. 2019;233:116631. doi: 10.1016/j.lfs.2019.116631. [DOI] [PubMed] [Google Scholar]
- 78.Cai J., Zhang X., Chen P., Li Y., Liu S., Liu Q., Zhang H., Wu Z., Song K., Liu J., et al. The ER stress sensor inositol-requiring enzyme 1alpha in Kupffer cells promotes hepatic ischemia-reperfusion injury. J. Biol. Chem. 2022;298:101532. doi: 10.1016/j.jbc.2021.101532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sansano M., Heredia A., Peinado I., Andres A. Dietary acrylamide: What happens during digestion. Food Chem. 2017;237:58–64. doi: 10.1016/j.foodchem.2017.05.104. [DOI] [PubMed] [Google Scholar]
- 80.Rifai L., Saleh F.A. A Review on Acrylamide in Food: Occurrence, Toxicity, and Mitigation Strategies. Int. J. Toxicol. 2020;39:93–102. doi: 10.1177/1091581820902405. [DOI] [PubMed] [Google Scholar]
- 81.Salehi B., Zucca P., Orhan I.E., Azzini E., Adetunji C.O., Mohammed S.A., Banerjee S.K., Sharopov F., Rigano D., Sharifi-Rad J., et al. Allicin and health: A comprehensive review. Trends Food Sci. Technol. 2019;86:502–516. doi: 10.1016/j.tifs.2019.03.003. [DOI] [Google Scholar]
- 82.Shi X., Zhou X., Chu X., Wang J., Xie B., Ge J., Guo Y., Li X., Yang G. Allicin Improves Metabolism in High-Fat Diet-Induced Obese Mice by Modulating the Gut Microbiota. Nutrients. 2019;11:2909. doi: 10.3390/nu11122909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang C., He X., Sheng Y., Yang C., Xu J., Zheng S., Liu J., Xu W., Luo Y., Huang K. Allicin-induced host-gut microbe interactions improves energy homeostasis. FASEB J. 2020;34:10682–10698. doi: 10.1096/fj.202001007R. [DOI] [PubMed] [Google Scholar]
- 84.Hong Y., Nan B., Wu X., Yan H., Yuan Y. Allicin alleviates acrylamide-induced oxidative stress in BRL-3A cells. Life Sci. 2019;231:116550. doi: 10.1016/j.lfs.2019.116550. [DOI] [PubMed] [Google Scholar]
- 85.Wang E.T., Chen D.Y., Liu H.Y., Yan H.Y., Yuan Y. Protective effect of allicin against glycidamide-induced toxicity in male and female mice. Gen. Physiol. Biophys. 2015;34:177–187. doi: 10.4149/gpb_2014038. [DOI] [PubMed] [Google Scholar]
- 86.Nan B., Yang C., Li L., Ye H., Yan H., Wang M., Yuan Y. Allicin alleviated acrylamide-induced NLRP3 inflammasome activation via oxidative stress and endoplasmic reticulum stress in Kupffer cells and SD rats liver. Food Chem. Toxicol. 2021;148:111937. doi: 10.1016/j.fct.2020.111937. [DOI] [PubMed] [Google Scholar]
- 87.Panahi G., Pasalar P., Zare M., Rizzuto R., Meshkani R. High glucose induces inflammatory responses in HepG2 cells via the oxidative stress-mediated activation of NF-kappaB, and MAPK pathways in HepG2 cells. Arch. Physiol. Biochem. 2018;124:468–474. doi: 10.1080/13813455.2018.1427764. [DOI] [PubMed] [Google Scholar]
- 88.Jin J., Ma Y., Tong X., Yang W., Dai Y., Pan Y., Ren P., Liu L., Fan H.Y., Zhang Y., et al. Metformin inhibits testosterone-induced endoplasmic reticulum stress in ovarian granulosa cells via inactivation of p38 MAPK. Hum. Reprod. 2020;35:1145–1158. doi: 10.1093/humrep/deaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qiao Q., Sun C., Han C., Han N., Zhang M., Li G. Endoplasmic reticulum stress pathway PERK-eIF2α confers radioresistance in oropharyngeal carcinoma by activating NF-kB. Cancer Sci. 2017;108:1421–1431. doi: 10.1111/cas.13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang T., Liu Y., Zhang C. Pharmacokinetics and Bioavailability Enhancement of Baicalin: A Review. Eur. J. Drug Metab. Pharmacokinet. 2019;44:159–168. doi: 10.1007/s13318-018-0509-3. [DOI] [PubMed] [Google Scholar]
- 91.Yu H., Chen B., Ren Q. Baicalin relieves hypoxia-aroused H9c2 cell apoptosis by activating Nrf2/HO-1-mediated HIF1alpha/BNIP3 pathway. Artif. Cells Nanomed. Biotechnol. 2019;47:3657–3663. doi: 10.1080/21691401.2019.1657879. [DOI] [PubMed] [Google Scholar]
- 92.Guo L.T., Wang S.Q., Su J., Xu L.X., Ji Z.Y., Zhang R.Y., Zhao Q.W., Ma Z.Q., Deng X.Y., Ma S.P. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflamm. 2019;16:95. doi: 10.1186/s12974-019-1474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu C., Wang Y., Fan Y., Li H., Wang C., Zhang J., Zhang S., Han X., Wen C. Lipidomics revealed idiopathic pulmonary fibrosis-induced hepatic lipid disorders corrected with treatment of baicalin in a murine model. AAPS J. 2015;17:711–722. doi: 10.1208/s12248-014-9714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Q., Liu M., Yu H., Li J., Wang S., Zhang Y., Qiu F., Wang T. Scutellaria baicalensis regulates FFA metabolism to ameliorate NAFLD through the AMPK-mediated SREBP signaling pathway. J. Nat. Med. 2018;72:655–666. doi: 10.1007/s11418-018-1199-5. [DOI] [PubMed] [Google Scholar]
- 95.Zhang J., Zhang H., Deng X., Zhang Y., Xu K. Baicalin protects AML-12 cells from lipotoxicity via the suppression of ER stress and TXNIP/NLRP3 inflammasome activation. Chem. Biol. Interact. 2017;278:189–196. doi: 10.1016/j.cbi.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y.F., Li T., Tang Z.H., Chang L.L., Zhu H., Chen X.P., Wang Y.T., Lu J.J. Baicalein Triggers Autophagy and Inhibits the Protein Kinase B/Mammalian Target of Rapamycin Pathway in Hepatocellular Carcinoma HepG2 Cells. Phytother. Res. 2015;29:674–679. doi: 10.1002/ptr.5298. [DOI] [PubMed] [Google Scholar]
- 97.Mai W., Xu Y., Xu J., Zhao D., Ye L., Yu G., Wang Z., Lu Q., Lin J., Yang T., et al. Berberine Inhibits Nod-Like Receptor Family Pyrin Domain Containing 3 Inflammasome Activation and Pyroptosis in Nonalcoholic Steatohepatitis via the ROS/TXNIP Axis. Front. Pharmacol. 2020;11:185. doi: 10.3389/fphar.2020.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng T., Yang X., Li W., Wang Q., Chen L., Wu D., Bian F., Xing S., Jin S. Salidroside Attenuates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease via AMPK-Dependent TXNIP/NLRP3 Pathway. Oxid. Med. Cell. Longev. 2018;2018:8597897. doi: 10.1155/2018/8597897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang X., Zhang J.H., Chen X.Y., Hu Q.H., Wang M.X., Jin R., Zhang Q.Y., Wang W., Wang R., Kang L.L., et al. Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid. Redox Signal. 2015;22:848–870. doi: 10.1089/ars.2014.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang C., Wang Z., Feng Q., Chen W.D., Wang Y.D. Farnesoid X receptor: A potential therapeutic target in multiple organs. Histol. Histopathol. 2020;35:1403–1414. doi: 10.14670/HH-18-301. [DOI] [PubMed] [Google Scholar]
- 101.Forman B.M., Goode E., Chen J., Oro A.E., Bradley D.J., Perlmann T., Noonan D.J., Burka L.T., McMorris T., Lamph W.W., et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 102.Makishima M., Okamoto A.Y., Repa J.J., Tu H., Learned R.M., Luk A., Hull M.V., Lustig K.D., Mangelsdorf D.J., Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 103.Lambert G., Amar M.J., Guo G., Brewer H.B., Jr., Gonzalez F.J., Sinal C.J. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J. Biol. Chem. 2003;278:2563–2570. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- 104.Adorini L., Pruzanski M., Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov. Today. 2012;17:988–997. doi: 10.1016/j.drudis.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 105.Beuers U., Trauner M., Jansen P., Poupon R. New paradigms in the treatment of hepatic cholestasis: From UDCA to FXR, PXR and beyond. J. Hepatol. 2015;62:S25–S37. doi: 10.1016/j.jhep.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 106.Carr R.M., Reid A.E. FXR agonists as therapeutic agents for non-alcoholic fatty liver disease. Curr. Atheroscler. Rep. 2015;17:500. doi: 10.1007/s11883-015-0500-2. [DOI] [PubMed] [Google Scholar]
- 107.Han C.Y., Rho H.S., Kim A., Kim T.H., Jang K., Jun D.W., Kim J.W., Kim B., Kim S.G. FXR Inhibits Endoplasmic Reticulum Stress-Induced NLRP3 Inflammasome in Hepatocytes and Ameliorates Liver Injury. Cell Rep. 2018;24:2985–2999. doi: 10.1016/j.celrep.2018.07.068. [DOI] [PubMed] [Google Scholar]
- 108.Yoshihara E., Masaki S., Matsuo Y., Chen Z., Tian H., Yodoi J. Thioredoxin/Txnip: Redoxisome, as a redox switch for the pathogenesis of diseases. Front. Immunol. 2014;4:514. doi: 10.3389/fimmu.2013.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 110.Abderrazak A., Syrovets T., Couchie D., El Hadri K., Friguet B., Simmet T., Rouis M. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015;4:296–307. doi: 10.1016/j.redox.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang H., Shi X., Qiu M., Lv S., Liu H. Hydrogen Sulfide Plays an Important Protective Role through Influencing Endoplasmic Reticulum Stress in Diseases. Int. J. Biol. Sci. 2020;16:264–271. doi: 10.7150/ijbs.38143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang H., Shi X., Qiu M., Lv S., Zheng H., Niu B., Liu H. Hydrogen Sulfide Plays an Important Role by Influencing NLRP3 inflammasome. Int. J. Biol. Sci. 2020;16:2752–2760. doi: 10.7150/ijbs.47595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.