Abstract
Voriconazole is an investigational azole antifungal agent with activity against a variety of fungal species, including fluconazole-susceptible and -resistant Candida species and Cryptococcus neoformans. In this study, we employed in vitro time-kill methods to characterize the relationship between concentrations of voriconazole and its fungistatic activity against Candida albicans, Candida glabrata, Candida tropicalis, and C. neoformans. Isolates were exposed to voriconazole concentrations ranging from 0.0625 to 16 times the MIC, and the viable colony counts were determined over time. The 50 and 90% effective concentrations (EC50 and EC90, respectively) were determined at 8, 12, and 24 h following the addition of voriconazole. At each time point, near-maximal fungistatic activity, as indicated by the EC90, was noted at a drug concentration of approximately three times the MIC. Additionally, EC50 and EC90 did not change over time, thus suggesting that the rate of activity was not improved by increasing concentrations. Voriconazole exhibits non-concentration-dependent pharmacodynamic characteristics in vitro.
Voriconazole is an investigational triazole antifungal, related to fluconazole, which exhibits an enhanced spectrum of activity encompassing a variety of fluconazole-susceptible and -resistant Candida species and filamentous fungi, including Aspergillus (2, 8). Because of its extended spectrum of activity, its favorable pharmacokinetic profile compared to itraconazole, and the pending availability of intravenous and oral formulations, voriconazole is poised to have a significant impact on the management of fungal infections.
The importance of knowing the pharmacodynamic characteristics of antibacterials has been well established. However, despite the contribution that clinical application of pharmacodynamic principles has made regarding appropriate use of antibacterials, data regarding the pharmacodynamic characteristics of antifungal agents are relatively scarce. Through an understanding of antifungal pharmacodynamic properties and clinical application of these principles, practitioners may identify dosing strategies that will result in maximization of the therapeutic effect and minimization of drug-related adverse events. Currently, no data regarding the pharmacodynamic properties of voriconazole have been published. Therefore, we sought to characterize the relationships between concentrations of voriconazole and the rate and extent of its antifungal activity against isolates of Candida albicans, Candida glabrata, Candida tropicalis, and Crytococcus neoformans.
MATERIALS AND METHODS
Antifungal agents.
Stock solutions of voriconazole (Pfizer, New York, N.Y.) and fluconazole (Pfizer) were prepared using RPMI 1640 medium (Sigma Chemical Co., St. Louis, Mo.) buffered to a pH of 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) buffer (Sigma Chemical Co.) as solvent. Dimethyl sulfoxide was used to aid the solubilization of voriconazole. The final concentration of dimethyl sulfoxide in the time-kill test solutions was ≤1% (vol/vol) of the solution composition. Stock solutions were separated into unit-of-use portions and stored at −80°C until used.
Test isolates.
Two clinical isolates of C. glabrata (strains 350 and 582), C. albicans (strains ATCC 90028 and OY31.5), and C. neoformans (strains 887.002 and 1041.007) and one C. tropicalis (strain 3829) were selected for testing. Isolates were obtained from the Department of Pathology, University of Iowa College of Medicine.
Antifungal susceptibility testing.
The MICs of voriconazole and fluconazole were determined against test isolates using broth microdilution techniques as described by the National Committee for Clinical Laboratory Standards (6). The MICs were determined in RPMI 1640 buffered to a pH of 7.0 with MOPS. The starting inoculum was approximately 0.5 × 103 to 2.5 × 103 CFU/ml. Microtiter trays were incubated at 35°C in a moist, dark chamber, and the MICs were recorded after 48 h of incubation for the Candida species and after 72 h of incubation for C. neoformans. The susceptibility endpoints for voriconazole and fluconazole were defined as the lowest concentration of antifungal which resulted in visual growth that was reduced by 80% compared with growth of the control (6). The determinations of the MICs for the isolates were performed in duplicate prior to the use of the isolates in time-kill studies.
Antifungal carryover.
Before the time-kill curve studies were initiated, antifungal carryover was evaluated using previously described methods (2). Briefly, a fungal suspension was prepared with each test isolate to yield an inoculum of approximately 5 × 103 CFU/ml. One hundred-microliter volumes of these suspensions were added to 900-μl volumes of sterile water or sterile water plus voriconazole at concentrations ranging from 0.0625 to 16 times the MIC. This dilution resulted in a starting inoculum of approximately 5 × 102 CFU/ml. Immediately following addition of the fungal inoculum to a test tube, the tube was vortexed and a 30-μl sample was removed and plated without dilution on potato dextrose agar plates (Remel, Lenexa, Kans.) for determination of viable colony counts. Following 48 h of incubation at 35°C, the number of CFU was determined. Tests were conducted in quintuplicate. The mean colony count data for each agent at each multiple of the MIC tested were compared with the data for the control. Significant antifungal carryover was defined as a reduction in the mean number of CFU per milliliter of >25% compared with the colony count for the control (6, 9).
Time-kill curve procedures.
Time-kill studies were performed as described previously (4). Before testing, isolates were subcultured twice on potato dextrose agar plates. Colonies from a 24- to 48-h culture were suspended in 9 ml of sterile water and adjusted to a 0.5 McFarland turbidity standard. One milliliter of the adjusted fungal suspension was then added to either growth medium alone (control) or a solution of RPMI plus an appropriate amount of voriconazole stock solution. These procedures resulted in a starting inoculum of approximately 5 × 104 to 1 × 106 CFU/ml and a voriconazole concentration of 0.0625, 0.25, 1, 4, or 16 times the MIC. Test solutions were placed on an orbital shaker and incubated with agitation at 35°C. At predetermined time points, 100-μl samples were obtained from each solution, serially diluted in sterile water, and plated (30 μl) on potato dextrose agar plates for determination of viable colony counts. The lower limit of reproducibly quantifiable CFU according to these methods was 50 CFU/ml (5). All time-kill experiments were performed in duplicate.
Analysis.
Colony count data (in log10 CFU per milliliter) from duplicate time-kill studies were averaged and plotted as a function of time for each isolate. The rate and extent of antifungal activity at the various voriconazole concentrations were then compared among concentrations. Fungicidal activity was defined as a ≥99.9% reduction in the number of CFU per milliliter from the starting inoculum count, and fungistatic activity was defined as <99.9% reduction in the number of CFU per milliliter from the starting inoculum count. Additionally, the net changes (in log10 CFU per milliliter) in fungal density at 8, 12, and 24 h were determined for each isolate at each multiple of the MIC and plotted. The data were then fitted to a sigmoidal maximal effect (Emax) model using median time-kill results compiled from all isolates. The concentrations producing 50 and 90% of the maximal effect (50 and 90% effective concentrations [EC50 and EC90, respectively) were determined at each time point.
(This work was presented at the International Congress on Clinical Pharmacy, Orlando, Fla., April 1999.)
RESULTS
Antifungal susceptibility.
Susceptibility data for each isolate are presented in Table 1. Voriconazole MICs ranged from 0.007 to 4.0 μg/ml. All isolates were susceptible to fluconazole, with the exception of C. glabrata 350 (fluconazole MIC, ≥128 μg/ml) (10).
TABLE 1.
MICs of voriconazole and fluconazole for test isolatesa
| Microorganism | MIC (μg/ml)
|
|
|---|---|---|
| Voriconazole | Fluconazole | |
| C. albicans | ||
| 90028 | 0.007 | 0.25 |
| OY31.5 | 0.007 | 0.25 |
| C. glabrata | ||
| 350 | 4.0 | >128 |
| 582 | 0.06 | 8 |
| C. tropicalis 3829 | 0.03 | 0.5 |
| C. neoformans | ||
| 887.002 | 0.03 | 4 |
| 1041.007 | 0.12 | 1 |
Voriconazole and fluconazole MICs were determined a total of four times for each isolate, and values are medians.
Antifungal carryover.
Antifungal carryover was not observed with any of the isolates at the concentrations tested by the sampling methodology described above.
Time-kill curves.
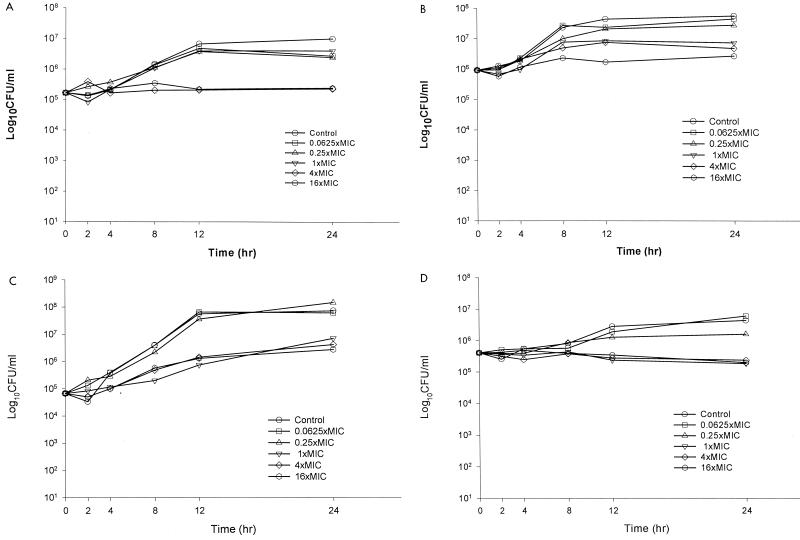
A time-kill plot of the activity of voriconazole against a representative isolate from each of the fungal species tested is presented in Figure 1A to D. Fungistatic activity was observed with voriconazole against all seven isolates. For the isolates of C. neoformans and C. glabrata and the isolate of C. tropicalis, voriconazole concentrations greater than the MIC did not appreciably increase the rate or extent of fungistatic activity. Against both isolates of C. albicans, however, the maximal fungistatic activity was observed with voriconazole concentrations greater than or equal to four times the MIC.
FIG. 1.
Representative time-kill plots with voriconazole against C. albicans 90028 (A), C. glabrata 350 (B), C. tropicalis 3829 (C), and C. neoformans 1041.007 (D).
The antifungal activity (i.e., net change in CFU per milliliter) of voriconazole following 8, 12, and 24 h of exposure against all pathogens was plotted as a function of the multiple of the MIC (Fig. 2). EC50 and EC90 data are summarized in Table 2. The EC50 and EC90 did not change appreciably from 8 to 24 h, thus suggesting that the rate of fungistatic activity does not improve with increasing concentrations of voriconazole. Additionally, the EC50 and EC90 occur within a relatively narrow range of multiples of the MIC. The EC90 at each of the three time points ranged between 2.3 and 3.3 times the MIC.
FIG. 2.
Composite response curves for test isolates of change in colony counts from the starting inoculum count plotted as a function of the multiple of the voriconazole MIC following exposure for 8 h (A), 12 h (B), and 24 h (C).
TABLE 2.
Composite Emax model parameters
Values are multiples of MIC.
DISCUSSION
In this study, we described the pharmacodynamics of voriconazole against a variety of Candida species and C. neoformans using in-vitro time-kill curve methods. We noted that voriconazole exhibited fungistatic activity against test isolates at concentrations greater than or equal to the MIC and near-maximal fungistatic activity at concentrations equal to approximately two to three times the MIC (EC90). Furthermore, the extent of activity afforded by voriconazole did not appear to be dependent on the susceptibility of the isolate to fluconazole, as was evident by similar activity against fluconazole-susceptible and -resistant isolates of C. glabrata.
When evaluating the pharmacodynamic characteristics of an antimicrobial, it is valuable to determine the effect of concentration on both the rate and extent of activity. Using an Emax model, one can generate parameters such as the EC50 and EC90 which provide insight into the relationship between concentration and extent of activity. If the EC50 and EC90 occur over a narrow range of concentrations, then transition from minimal to maximal activity also occurs over a narrow concentration range. This rapid transition is consistent with compounds that exhibit predominantly non-concentration-dependent activity. In contrast, if the EC50 and EC90 occur over a greater concentration range, then concentration-dependent properties are exhibited. To assess the relationship between concentration and the rate of activity one can compare EC50 and EC90 over time. If the rate of activity is not dependent on concentration, the EC50 and EC90 should remain relatively stable at each time point. If the EC90 and/or the EC50 declines with time, however, this suggests that higher concentrations result in more rapid expression of activity. In the case of voriconazole, the EC50 and EC90 occur over a narrow range of multiples of the MIC, 0.8 to 3 times the MIC, and remain constant between 8 and 24 h. These data are consistent with an agent exhibiting non-concentration-dependent activity.
Whether a compound adheres to the pharmacodynamics described in vitro once it is tested in vivo depends on several factors; however, the pharmacokinetic profile is perhaps the most important. The in vivo pharmacodynamic properties of a drug will be determined by where the transition portion of the concentration-effect curve falls with respect to achievable concentrations. If the range of multiples of the MIC which drive the transition from minimal to maximal effect is far surpassed by the concentrations resulting from clinically employed doses, then this agent would exhibit non-concentration-dependent activity in vivo regardless of the slope of the concentration-effect curve. Likewise, if the concentrations observed in vivo fall on the transition portion of the curve, then this agent would exhibit concentration-dependent activity, even if the slope of the transition portion of the curve is steep. In this study, composite dose-effect curves were created and examined at various time points to assess the relation between the multiple of the MIC and the extent of fungistatic activity. It was subsequently determined that the transition from minimal to maximal activity occurred over a relatively small range of concentrations. The calculated composite EC90 for the test isolates was approximately three times the MIC. The MIC90s of voriconazole for a variety of Candida species have been reported to range from 0.06 to 0.12 μg/ml for C. albicans to 1.0 to 2.0 μg/ml for C. glabrata (3, 8). Therefore, we predict that maximal fungistatic activity would be observed against a variety of Candida species in vivo if voriconazole concentrations of 3 to 6 μg/ml were achieved.
The concentration-effect relationships noted with voriconazole are similar to those reported previously for fluconazole against C. albicans and C. neoformans (5, 6). In these in vitro time-kill studies, fluconazole exhibited fungistatic activity that was maximized at concentrations between the MIC and four times the MIC for test isolates. These data suggest that the azoles exhibit non-concentration-dependent activity over a range of clinically achievable concentrations. Animal data have subsequently supported these in vitro observations (1, 7). Furthermore, clinical data also suggest that the fungistatic activity of fluconazole is maximized once concentrations at the site of infection exceed roughly the MIC for the infecting pathogen (10, 11). Noting the similar pharmacodynamics of fluconazole and voriconazole, it would appear reasonable to assume that maximizing the duration of exposure of a fungus to voriconazole would optimize the fungistatic activity of voriconazole against yeasts.
ACKNOWLEDGMENT
We thank Donald Klepser for his assistance in the preparation of the manuscript.
REFERENCES
- 1.Andes D, van Ogtrop M. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob Agents Chemother. 1999;43:2116–2120. doi: 10.1128/aac.43.9.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belanger P, Nast C C, Fratti R, Sanati H, Ghannoum M. Voriconazole (UK-109,496) inhibits the growth and alters the morphology of fluconazole-susceptible and -resistant Candida species. Antimicrob Agents Chemother. 1997;41:1840–1842. doi: 10.1128/aac.41.8.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kauffman C A, Zarins L T. In vitro activity of voriconazole against Candida species. Diagn Microbiol Infect Dis. 1998;31:297–300. doi: 10.1016/s0732-8893(98)00005-4. [DOI] [PubMed] [Google Scholar]
- 4.Klepser M E, Ernst E J, Lewis R E, Ernst M E, Pfaller M A. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob Agents Chemother. 1998;42:1207–1212. doi: 10.1128/aac.42.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klepser M E, Wolfe E J, Jones R N, Nightingale C H, Pfaller M A. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob Agents Chemother. 1997;41:1392–1395. doi: 10.1128/aac.41.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klepser M E, Wolfe E J, Pfaller M A. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B against Cryptococcus neoformans. J Antimicrob Chemother. 1998;41:397–401. doi: 10.1093/jac/41.3.397. [DOI] [PubMed] [Google Scholar]
- 7.Louie A, Drusano G L, Banerjee P, Liu Q F, Liu W, Kaw P, Shayegani M, Taber H, Miller M H. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob Agents Chemother. 1998;42:1105–1109. doi: 10.1128/aac.42.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marco F, Pfaller M A, Messer S, Jones R N. In vitro activities of voriconazole (UK-109,496) and four other antifungal agents against 394 clinical isolates of Candida spp. Antimicrob Agents Chemother. 1998;42:161–163. doi: 10.1128/aac.42.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearson R D, Steigbigel R T, Davis H T, Chapman S W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980;18:699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 11.Witt M D, Lewis R J, Larsen R A, Milefchik E N, Leal M A, Haubrich R H, Richie J A, Edwards J E, Jr, Ghannoum M A. Identification of patients with acute AIDS-associated cryptococcal meningitis who can be effectively treated with fluconazole: the role of antifungal susceptibility testing. Clin Infect Dis. 1996;22:322–328. doi: 10.1093/clinids/22.2.322. [DOI] [PubMed] [Google Scholar]