Abstract
Animal models are commonly used to determine the efficacy of various antimicrobial agents for treatment of bacterial endocarditis. Previously we have utilized an in vitro infection model, which incorporates simulated endocardial vegetations (SEVs) to evaluate the pharmacodynamics of various antibiotics. In the present study, we compared four experimental rabbit endocarditis protocols to an in vitro infection model in an effort to determine if these models are comparable. We have evaluated the activity of clinafloxacin, trovafloxacin, sparfloxacin, and ciprofloxacin in rabbit models against Staphylococcus aureus and Enterococcus spp. In vitro models were performed simulating the antibiotic pharmacokinetics obtained in the in vivo studies. Models were dosed the same as rabbit models, and SEVs were evaluated at the same time the rabbit vegetations were examined. Clinafloxacin and trovafloxacin were evaluated against methicillin-susceptible (MSSA1199) and -resistant (MRSA494) strains of S. aureus. Ciprofloxacin was studied against MSSA1199 and MSSA487. Sparfloxacin and clinafloxacin were evaluated against Enterococcus faecium SF2149 and Enterococcus faecalis WH245, respectively. We found that reductions in SEV bacterial density obtained in the in vitro model were similar to those obtained in rabbit vegetations, indicating that the SEV model may be a valuable tool for assessing antibiotic potential in the treatment of bacterial endocarditis.
Animal models are commonly used to evaluate the activity of antimicrobial agents in bacterial endocarditis. Although endocarditis is artificially induced, this system represents the best in vivo model to determine the efficacy of antimicrobial agents in the treatment of this problematic infection. Unfortunately, these models are also associated with high cost, extensive labor, and ethical considerations. Alternative methods that are less costly and can be performed in a timely manner may offer certain advantages over the animal models. In recent years, the use of in vitro infection models to evaluate antimicrobial activity has increased significantly. These models have both advantages and disadvantages over in vivo models. For example, in vitro models allow the study of antimicrobial agents under optimal dosing situations since they can simulate human pharmacokinetics. These models can also be utilized to study antibiotic combinations, multiple dosing regimens, and the effect of inoculum on antimicrobial activity. They also can be sampled frequently to determine the bacterial count and antibiotic concentration. On the other hand, the in vitro systems lack host defense mechanisms present in the animal models. Although in vitro models have increased our understanding of antimicrobial pharmacodynamics and the potential for antimicrobial resistance, there have been relatively few attempts to correlate these systems to in vivo data (5). We have developed an in vitro infection model that incorporates simulated vegetations to evaluate the activity of antibiotics in the treatment of bacterial endocarditis. Previously, we have indirectly compared the results of our in vitro model to that of a rabbit model; however, a direct comparison has not been made. If the data between these two systems prove to be comparable, then the in vitro fibrin-clot infection model may be an effective tool in the study of bacterial endocarditis.
MATERIALS AND METHODS
Bacterial strains.
Staphylococcus aureus strains MRSA494 (methicillin resistant) and MSSA1199 and MSSA487 (methicillin susceptible) were obtained from the bloodstreams of patients with bacterial endocarditis. Enterococcus faecalis WH245 (β-lactamase producer) and Enterococcus faecium SF2149 (non-β-lactamase producer) were also isolated from the bloodstreams of patients.
Antibiotics.
Clinafloxacin (lot no. PD127391-0002 Lot J) was supplied by Parke-Davis Pharmaceutical Research, Ann Arbor, Mich. Trovafloxacin (lot no. 25381-086-02) was supplied by Pfizer Inc., Groton, Conn. Sparfloxacin (lot no. 721A) was supplied by Rhone-Poulenc Rorer, Collegeville, Pa. Ciprofloxacin (lot no. 851640; Bayer Corporation, West Haven, Conn.) for injection was obtained commercially.
Susceptibility testing.
MICs were determined by broth microdilution in Mueller-Hinton broth supplemented with calcium and magnesium (SMHB) according to National Committee for Clinical Laboratory Standards guidelines (6). Samples (5 μl) from clear wells were plated onto tryptic soy agar (TSA) plates to determine minimal bactericidal concentrations (MBCs). All samples were incubated at 35°C for 24 h.
Rabbit model of endocarditis.
For S. aureus, all experiments were performed in male New Zealand White rabbits weighing 2 to 3 kg. Left-sided endocarditis was established in rabbits as described previously, and 18 to 24 h after the infection was established, animals were randomized to different treatment arms (6). Control rabbits were sacrificed at the time that treatment was initiated in the study groups. Serum samples were obtained 1 h postdose and just prior to a scheduled dose for the measurement of peak and trough, respectively. Rabbits treated with clinafloxacin received 20 mg/kg of body weight every 8 h and were sacrificed 10 to 12 h following the final dose in order to enumerate residual bacterial counts in the vegetations (4; expressed as log10 CFU/gram). The half-life of clinafloxacin in rabbits was approximately 1.6 h with peak and trough concentrations being 3.5 and 0.1 μg/ml, respectively. In another study, rabbits were treated for 4 days with trovafloxacin at 13.3 mg/kg every 12 h and were sacrificed 14 to 16 h following the last dose of antibiotic (1). This dose resulted in a peak and trough of approximately 4.0 and 0.1 μg/ml, respectively. In the ciprofloxacin treatment study, rabbits received this antimicrobial agent at a dose of 25 mg/kg every 8 h for 6 days. These animals were sacrificed 8 h following the last dose of antibiotic (2). This dosing regimen achieved peak and trough concentrations of 6.0 and 0.5 μg/ml, respectively. The emergence of resistance was evaluated by plating samples of homogenized vegetation onto Mueller-Hinton agar containing the appropriate antibiotic at fivefold the agar dilution MIC for each strain.
For Enterococcus spp., New Zealand White rabbits (1.8 to 3.2 kg) were also used. Endocarditis was produced in the rabbits as previously described (3). Treatment with sparfloxacin and clinafloxacin was initiated 24 h following bacterial challenge, and rabbits were sacrificed 12 h after the last dose of antibiotic. Doses of sparfloxacin and clinafloxacin were given every 12 h (50 mg/kg) to achieve peaks of 15.5 and 5.0 μg/ml, respectively (7). Control rabbits were sacrificed 24 h after inoculation or at the same time as the treatment group. Blood samples were drawn 1 h postdose and just prior to a scheduled dose for the determination of peak and trough antibiotic concentrations, respectively. Emergence of resistance was not evaluated in this study.
Simulated endocardial vegetations (SEVs).
Organism stocks were prepared by inoculating 5-ml test tubes of SMHB with colonies harvested from overnight growth on TSA to achieve a concentration of 1010 CFU/ml. Simulated vegetations were prepared by mixing 0.25 to 1.0 ml of human cryoprecipitate from volunteer donors (American Red Cross, Detroit, Mich.), 0.1 ml of organism suspension (final inoculum, 109 CFU), and 0.025 ml of platelet suspension (platelets mixed with normal saline; 250,000 to 500,000 platelets) in 1.5-ml siliconized Eppendorf tubes. After insertion of a sterile monofilament line into the mixture, bovine thrombin (5,000 U) was added to each tube.
In vitro model.
A 250-ml one-compartment glass apparatus with ports from which SEVs were suspended was used. The apparatus was prefilled with SMHB, and antibiotics were administered into the central compartment via an injection port. A magnetic stir bar was placed in the media for thorough mixing of the drug in the model, and the apparatus was placed in a 37°C water bath. Fresh medium was continuously supplied, and spent media and drug were removed from the compartment via a peristaltic pump set to simulate the half-lives of the antibiotics in the rabbits. SEVs were then removed in triplicate from each model over time. Pharmacokinetic parameters simulated in the model were based on data derived from serum concentrations obtained in the rabbit experiments. For models employing S. aureus, clinafloxacin was administered every 8 h with a simulated half-life of 1.6 h and a corresponding peak of 3.5 μg/ml and trough of 0.1 μg/ml. Trovafloxacin models were dosed twice daily to achieve a peak of 4.0 μg/ml, a trough of 0.1 μg/ml, and a simulated half-life of 2.4 h. Ciprofloxacin was administered every 8 h for 6 days to simulate a peak and trough of 6.0 and 0.5 μg/ml, respectively, and a half-life of 2.2 h. For models employing Enterococcus spp., doses of clinafloxacin and sparfloxacin were given every 12 h, with simulated half-lives of 1.6 and 1.3 h, respectively. The simulated peaks and troughs were 5.0 and 0.3 μg/ml for clinafloxacin, respectively, and 15.5 and 0.1 μg/ml for sparfloxacin, respectively. All experiments were done in duplicate, and models without antibiotics were performed to assure adequate growth of the organisms.
Pharmacodynamic analysis.
Three SEVs were removed from each in vitro model (total of six) at 0, 8, 24, 48, 72, and 96 h to mimic up to 1 to 4 days of therapy. In the ciprofloxacin models, samples were removed at 144 h to be consistent with the time that rabbits were sacrificed in the in vivo trial. The simulated vegetations were homogenized and diluted in normal saline and then plated onto TSA plates. Colony counts were performed after incubation at 35°C for 24 h. The total reduction in bacterial densities was determined by plotting time-kill curves based on the number of remaining organisms at each sampling time.
Pharmacokinetic analysis.
Samples were obtained from in vitro models at 0.5, 1, 2, 4, 8, and 24 h for determination of antibiotic concentrations. All samples were stored at −70°C until ready for analysis. Concentrations of the fluoroquinolones were determined by bioassay utilizing Klebsiella pneumoniae ATCC 10031. Paper disks were spotted with 20 μl of standards or samples. Each standard was tested in triplicate by placing the disk on Mueller-Hinton agar plates preswabbed with a 0.5 McFarland suspension of the test organism. Plates were incubated for 18 to 24 h at 37°C, followed by measurement of zones of inhibition. A correlation coefficient of ≥0.98 was achieved for all plates. Concentrations of 5.0, 1.25, and 0.3125 μg/ml were used as standards, and the coefficient of variation was <10% for each standard. The half-lives and peak concentrations of the antibiotics were determined by trapezoidal methods utilizing RStrip software (MicroMath, Salt Lake City, Utah).
Resistance.
In order to detect the emergence of resistance during therapy, 100 μl of the homogenized SEVs taken at the final time point was plated onto Mueller-Hinton agar plates containing fivefold the agar MIC of the appropriate antibiotic. Plates were examined for growth after 48 h of incubation at 35°C.
Statistical analysis.
The initial and final bacterial inocula (log10 CFU/g) of the two methods were compared by two-way analysis of variance with Tukey's post-hoc test. A P value of ≤0.05 was considered significant.
RESULTS
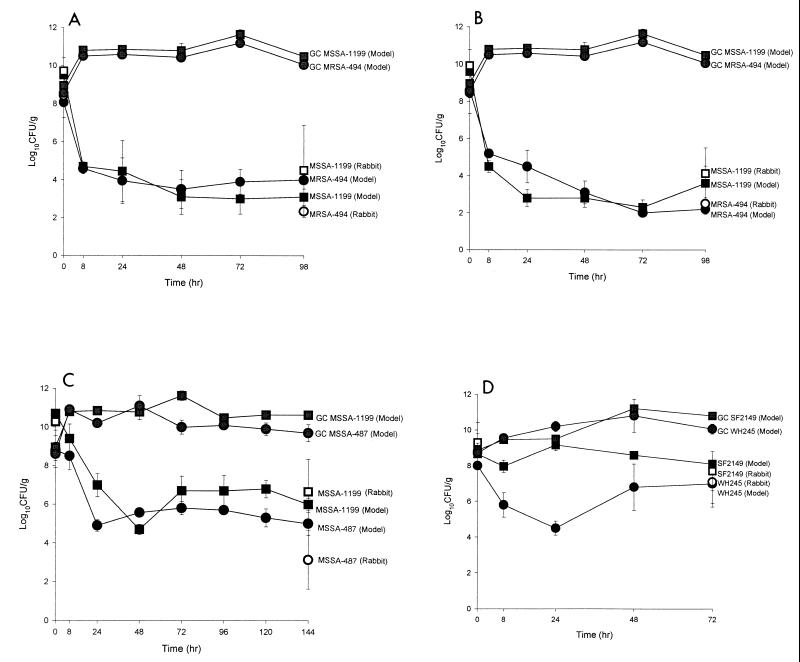
The MICs and MBCs for test organisms are given in Table 1. Table 2 summarizes initial and final bacterial densities found in both models. Figure 1 illustrates changes in bacterial densities observed versus time. No significant differences were found between the in vitro and in vivo models of endocarditis. Resistance to ciprofloxacin at fivefold the MIC emerged in 82% of rabbits infected with MSSA1199. In the in vitro model, resistance to ciprofloxacin developed in 60% of the MSSA1199 SEVs and 87.5% of the MSSA487 SEVs after 6 days of therapy. Resistance was not observed in either model with any of the other regimens evaluated.
TABLE 1.
MICs and MBCs of the drugs
| Drug | MIC/MBC (μg/ml) of drugs for isolatesa
|
||||
|---|---|---|---|---|---|
| MRSA494 | MSSA1199 | MSSA487 | EFWH245 | EFSF2149 | |
| Clinafloxacin | 0.06/0.06 | 0.06/0.06 | ND | 0.06/0.125 | ND |
| Trovafloxacin | 0.031/0.06 | 0.06/0.06 | ND | ND | ND |
| Ciprofloxacin | ND | 0.125/0.125 | 0.5/0.5 | ND | ND |
| Sparfloxacin | ND | ND | ND | ND | 2.0/2.0 |
ND, not done.
TABLE 2.
Initial and final bacterial inocula in the modelsa
| Drug and strain | Initial inoculum (log10 CFU/g)
|
Final inoculum (log10 CFU/g)
|
||
|---|---|---|---|---|
| Rabbit (no. of vegetations) | In vitro (no. of SEVs) | Rabbit (no. of vegetations) | In vitro (no. of SEVs) | |
| Trovafloxacin, MSSA1199 | 9.71 ± 0.71 (11) | 9.5 ± 0.43 (4) | 4.51 ± 2.38 (17) | 3.1 ± 0.85 (8) |
| Trovafloxacin, MRSA494 | 8.43 ± 1.16 (10) | 8.1 ± 0.24 (4) | 2.35 ± 0.32 (17) | 4.0 ± 0.47 (7)* |
| Clinafloxacin, MSSA1199 | 9.92 ± 0.85 (11) | 9.6 ± 0.08 (4) | 4.13 ± 1.38 (16) | 3.6 ± 0.93 (10) |
| Clinafloxacin, MRSA494 | 8.45 ± 1.12 (11) | 8.6 ± 0.42 (4) | 2.5 ± 0.32 (15) | 2.2 ± 0.27 (10) |
| Ciprofloxacin, MSSA1199 | 10.27 ± 0.44 (11) | 10.7 ± 0 (4) | 6.66 ± 1.67 (11) | 6.0 ± 0.33 (10) |
| Ciprofloxacin, MSSA487 | 8.72 ± 0.82 (11) | 8.8 ± 0.41 (4) | 3.13 ± 1.5 (10) | 5.0 ± 0.59 (8) |
| Clinafloxacin, EFWH245 | 8.8 ± 1.0 (5) | 8.0 ± 0.17 (4) | 7.1 ± 1.2 (6) | 7.0 ± 1.3 (20) |
| Sparfloxacin, SF2149 | 9.3 ± 1.1 (9) | 8.7 ± .06 (4) | 7.7 ± 1.1 (9) | 8.1 ± 0.23 (13) |
Values are means ± standard deviations. *, P < 0.05.
FIG. 1.
Activities of trovafloxacin against MRSA494 and MSSA1199 (A), clinafloxacin against MRSA494 and MSSA1199 (B), ciprofloxacin against MSSA487 and MSSA1199 (C), and clinafloxacin against WH245 (D) and sparfloxacin against SF2149 in the in vitro model (● and ■) versus the rabbit model (○ and □) (D). GC, growth control.
Table 3 summarizes the pharmacokinetic parameters observed in the in vitro models. The peak concentrations and half-lives achieved were similar to those achieved in rabbits. Table 4 presents a comparison of the weights of rabbit vegetations and SEVs. Overall, vegetation weights were greater in the in vitro model than in the rabbit model.
TABLE 3.
Pharmacokinetics of the antimicrobial agents in the in vitro modelsa
| Drug | t1/2 (h) | Peak (μg/ml) |
|---|---|---|
| Trovafloxacin | 3.33 ± 0.52 | 4.37 ± 0.43 |
| Clinafloxacin | 1.98 ± 0.22 | 3.16 ± 0.62 |
| Ciprofloxacin | 2.79 ± 0.93 | 5.54 ± 1.20 |
| Sparfloxacin | 1.26 ± 0.08 | 13.85 ± 0.21 |
Values are means ± standard deviations. t1/2, half-life.
TABLE 4.
Vegetation weights of rabbit and in vitro models
| Regimen (drug and strain) | Vegetation wt (mg) ± SD
|
|
|---|---|---|
| Rabbit | In vitro | |
| Growth control, MRSA494 | 50.5 ± 25.7 | 260.0 ± 40.0 |
| Growth control, MSSA1199 | 62.7 ± 41.2 | 270.0 ± 40.0 |
| Growth control, MSSA487 | 43.1 ± 30.6 | 310.0 ± 50.0 |
| Growth control, EFWH245 | 79.18 ± 34.0 | 940.0 ± 70.0 |
| Growth control, SF2149 | 36.7 ± 10.9 | 930.0 ± 30.0 |
| Trovafloxacin, MSSA1199 | 117.1 ± 46.0 | 160.0 ± 20.0 |
| Trovafloxacin, MRSA494 | 59.0 ± 55.2 | 220.0 ± 90.0 |
| Clinafloxacin, MSSA1199 | 63.5 ± 43.5 | 300.0 ± 40.0 |
| Clinafloxacin, MRSA494 | 39.6 ± 16.9 | 220.0 ± 40.0 |
| Ciprofloxacin, MSSA1199 | 125.2 ± 78.6 | 80.0 ± 10.0 |
| Ciprofloxacin, MSSA487 | 54.3 ± 43.1 | 120.0 ± 30.0 |
| Clinafloxacin, EFWH245 | 74.38 ± 63.4 | 950.0 ± 70.0 |
| Sparfloxacin, SF2149 | 44.43 ± 34.98 | 440.0 ± 60.0 |
DISCUSSION
The rabbit model of bacterial endocarditis has served as a valuable tool for evaluating optimal therapy of this disease. The efficacy of new antimicrobial agents in the treatment of serious systemic infections, like endocarditis, compared to those of standard forms of therapy often is evaluated using animal models, such as the rabbit model, prior to being studied in humans. Although these models are quite useful, they also are associated with many obstacles. The rabbit model is technically difficult to conduct in that endocarditis has to be surgically induced and vegetations have to be surgically removed at the end of the experiment. Another disadvantage of this model is the variation in the pharmacokinetics of the antimicrobial agents in the rabbits versus those in humans. Elimination half-lives of most antibiotics are much shorter in rabbits than in humans. Also, the presence of a foreign body in situ across the valve may underestimate the activity of antimicrobial agents. The high cost associated with these models also is a limiting factor. On average, it costs approximately $600 per rabbit, or $6,000 for a standard treatment arm of 10 animals, to conduct a trial employing the rabbit model. This cost includes the rabbit, veterinary care, surgery and necropsy, and all associated supplies. Besides the labor and the high cost, ethical considerations also may restrict the use of animals in the study of endocarditis. Considering these problems, in vitro models incorporating simulated vegetations may offer some advantages in understanding the initial dose-response relationship. This model is not only less labor-intensive and less costly, but it also can reduce animal use and can be performed in a timely manner. The cost associated with the in vitro model is approximately $300/model ($600 per regimen), which includes cryoprecipitate, broth, labor, and other supplies used during the experiments. Another advantage of the in vitro model is that human pharmacokinetics can be used and simulated vegetations can be removed throughout the experiment for analysis. As it is demonstrated in Table 4, the size of the SEVs can be significantly greater than that of rabbit vegetations. The reason for the large size of the SEVs is to assure stability of the clots during handling. Although SEVs are larger, it appears that the final bacterial inoculum is similar to those seen in the rabbit vegetations.
One of the major drawbacks of in vitro systems is that they incorporate artificial media and therefore lack host defense mechanisms. They also are unable to simulate other characteristics of infection, including the spread of the disease to other organs. However, in the case of endocarditis, organisms are present at the core of the vegetation, which decreases the ability of the immune system to contribute to the antibiotic action. In this case, the lack of immunofactors in the in vitro system may not hamper the initial evaluation of antibiotic activity. However, other factors, such as complement, antibody, or serum enhancing proteins, such as interleukin 1, tumor necrosis factor, and immunoglobulin, may be a factor for certain classes of antibiotics.
Although this system has some disadvantages, it may have a role in initial studies of antibiotics in the treatment of bacterial endocarditis. Previously, we have found end results with our in vitro SEV model to be similar to those of the rabbit model (5). In the present study, we were able to demonstrate a decrease in killing activity and the potential for emergence of resistance comparable to that of the rabbit models by closely mimicking the rabbit pharmacokinetics. Due to more frequent sampling, we were also able to better characterize the activity of the antibiotics against the isolates evaluated. Figure 1 illustrates that the greatest kill occurs during the first 24 h, an observation which would be currently missed using standard rabbit model sampling. As Table 2 demonstrates, the initial and final bacterial densities in the SEVs were similar to those found in the rabbit vegetations. The result of this study indicates the in vitro model to be comparable to the rabbit model of endocarditis for study of the activity of fluoroquinolones. However, studies with other pathogens and antimicrobial agents are warranted to further evaluate the application of this system. In view of the advantages of the in vitro model related to cost and use of animals, it might be advantageous in the initial assessment of antimicrobial agents in the treatment of bacterial endocarditis.
REFERENCES
- 1.Kaatz G W, Seo S M, Aeschlimann J R, Houlihan H H, Mercier R C, Rybak M J. Efficacy of trovafloxacin against experimental Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1998;42:254–256. doi: 10.1128/aac.42.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaatz G W, Seo S M, Barriere S L, Albrecht L M, Rybak M J. Ciprofloxacin and rifampin, alone and in combination, for therapy of experimental Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1989;33:1184–1187. doi: 10.1128/aac.33.8.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaatz G W, Seo S M, Barriere S L, Schaberg D R, Fekety R. Ciprofloxacin versus vancomycin in the therapy of experimental methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1987;31:527–530. doi: 10.1128/aac.31.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaatz G W, Seo S M, Lamp K C, Bailey E M, Rybak M J. CI-960, a new fluoroquinolone, for therapy of experimental ciprofloxacin-susceptible and -resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1992;36:1192–1197. doi: 10.1128/aac.36.6.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGrath B J, Kang S L, Kaatz G W, Rybak M J. Bactericidal activities of teicoplanin, vancomycin, and gentamicin alone and in combination against Staphylococcus aureus in an in vitro pharmacodynamic model of endocarditis. Antimicrob Agents Chemother. 1994;38:2034–2040. doi: 10.1128/aac.38.9.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 3rd ed. 1993. Approved standard M7-A3. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 7.Vazquez J, Perri M B, Thal L A, Donabedian S, Zervos M J. Sparfloxacin and clinafloxacin alone or in combination with gentamicin for therapy of experimental ampicillin-resistant enterococcal endocarditis in rabbits. J Antimicrob Chemother. 1993;32:715–721. doi: 10.1093/jac/32.5.715. [DOI] [PubMed] [Google Scholar]