Abstract
Investigating vaccine effectiveness (VE) in real-world conditions is crucial, especially its variation across different settings and populations. We undertook a test-negative control study in Galicia (Northwest Spain) to assess BNT162b2 effectiveness against acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection as well as COVID-19 associated hospitalization, intensive care unit (ICU) admission and mortality. A total of 44,401 positive and 817,025 negative SARS-CoV-2 test results belonging to adults were included. Adjusted odds ratios of vaccination and their 95% confidence interval (CI) were estimated using multivariate logistic-regression models. BNT162b2 showed high effectiveness in reducing SARS-CoV-2 infections in all age categories, reaching maximum VE ≥ 14 days after administering the second dose [18–64 years: VE = 92.9% (95%CI: 90.2–95.1); 65–79 years: VE = 85.8% (95%CI: 77.3–91.9), and ≥80 years: VE = 91.4% (95%CI: 87.9–94.1)]. BNT162b2 also demonstrated effectiveness in preventing COVID-19 hospitalization for all age categories, with VE more pronounced for those aged ≥80 years [VE = 60.0% (95%CI: 49.4–68.3)]. Moreover, there was a considerable reduction in ICU admission [VE = 88.0% (95%CI: 74.6–95.8)] and mortality [VE = 38.0% (95%CI: 15.9–55.4)] in the overall population. BNT162b2 showed substantial protection against SARS-CoV-2 infections and COVID-19 severity. Our findings would prove useful for systematic reviews and meta-analysis on COVID-19 VE.
Keywords: SARS-CoV-2, COVID-19, vaccine effectiveness, population study
1. Introduction
In Spain, the vaccination program against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was launched on 27 December 2020 right after the approval of BNT162b2 vaccine by the European Medicines Agency (EMA) on 21 December 2020 [1]. As of 18 March 2021, three vaccines have been approved by EMA: BNT162b2 (21 December 2020) mRNA-1273 (6 January 2021) and ChAdOx1 (29 January 2021). BNT162b2 and mRNA-1273 contain a messenger RNA (mRNA) molecule that encodes a protein from SARS-CoV-2, while ChAdOx1 contains the SARS-CoV-2 spike protein gene, which instructs the host cells to produce the protein of the S-antigen unique to SARS-CoV-2 [1]. A full vaccine course of BNT162b2, mRNA-1273, or ChAdOx1 nCoV-19 consists of two injections with a predefined time separation between the first and the second dose.
When COVID-19 vaccine supplies were limited, vaccination in Spain against COVID-19 followed a priority scale to reduce the case fatality rate as well as the burden on essential services. The highest priority was given to staff and residents of nursing homes (vaccinated with BNT162b2), followed by: (i) frontline healthcare workers (vaccinated with BNT162b2 or mRNA-1273); (ii) elderly individuals (vaccinated with BNT162b2); (iii) non-essential healthcare workers, education staff, security law corps (vaccinated with ChAdOx1); and finally (iv) other groups by decreasing age [2].
According to the established priority order and the vaccine type assigned to each age group, the vast majority of the Spanish vaccinated population until 18 March 2021 had received one or two doses of BNT162b2. Though clinical trials had been already carried out to measure the short-term efficacy of this vaccine prior to its approval [3], observational studies are required to determine vaccine effectiveness (VE) in diverse populations and real-world settings [4]. Several studies from different countries evaluated BNT162b2 effectiveness, and a related meta-analysis reported a substantial protective effect of BNT162b2 against COVID-19 with VE of 53% ≥ 14 days following the first dose and 95% ≥ 7 days after the second dose [5].
It has been shown that variations in adherence to vaccine doses and time intervals for dose administration, as well as differences in access to healthcare, SARS-CoV-2 testing, hospitalization threshold and COVID-19 management across countries and settings may influence the external validity of reported VE [6]. An evaluation of VE in different settings and population would therefore prove useful for future systematic reviews and meta-analysis.
Spain is divided into 17 autonomous communities and two autonomous cities, with diverse sociodemographic characteristics. Though the same vaccines have been administered in all Spanish territory, the epidemiological situation with respect to the COVID-19 pandemic and the applied nonpharmaceutical measures have widely varied across the different Spanish regions.
In the present study, we aim to evaluate, for the first time, BNT162b2 VE in Galicia (Northwest Spain) through a test-negative case-control design. We examined BNT162b2 VE against SARS-CoV-2 infection, and COVID-19 severity in a population consisting of 2,280,288 vaccinated and unvaccinated adults. We also undertook stratified analyses by age, number of administered vaccine doses and time since vaccine administration.
2. Materials and Methods
2.1. Study Design
We carried out a retrospective test-negative control study in Galicia, Northwest Spain [7] to study BNT162b2 VE related to SARS-CoV-2 infection. Test-negative case-control designs are widely used to estimate VE against respiratory viruses [8].
2.2. Data Collection
The study encompassed all adults (≥18 years old) who were vaccinated between 27 December 2020 and 18 March 2021. The vaccination program was introduced in Galicia on 27 December 2020, prioritizing first-line health personnel, elderly people (80 years or older) and residents of care homes [9]. COVID-19 vaccination data is available in the Galician public health system (SERGAS). Data were extracted from SERGAS using randomly assigned pseudo-anonymized codes. The authors were blind to the code assignment. Extracted data included age, gender, SARS-CoV-2 test history (date, type of test and result), history of patients’ hospitalization, admission to intensive care unit (ICU) or death by SARS-CoV-2 since the start of the study. Vaccination information was also obtained (vaccine name, number of administered doses and date of vaccine administration).
2.3. Exposure and Outcome Ascertainment
The start date of the vaccination campaign in Spain, 27 December 2020, was established as an index date. Individuals who had a positive laboratory-confirmed SARS-CoV-2 test result before the index date were excluded from the analysis. Only a positive SARS-CoV-2 test result was considered per individual, provided that the test took place after the index date. When several positive tests existed for the same individual, only the first positive test was considered. The same individual could serve as one or more non-cases depending on the number of corresponding negative tests. For instance, an individual who had three negative tests after the index date could contribute by three non-case observations to the study.
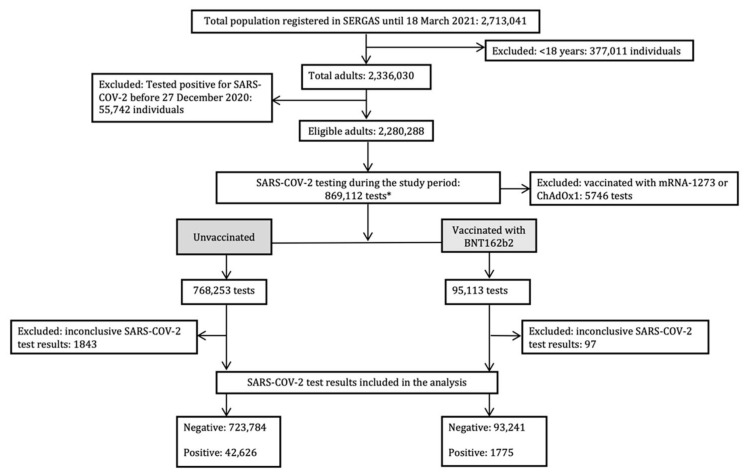
The vaccination status was assigned depending on the number of vaccine doses administrated before test date. As BNT162b2 was the first administered vaccine in Spain, most of the participants had received this vaccine during the study period. Therefore, the number of individuals who had received mRNA-1273 and ChAdOx1 vaccines and subsequently underwent SARS-CoV-2 test during the study period was not sufficient to be considered in the analysis (Figure 1). The vaccination status (unvaccinated, partially vaccinated, or fully vaccinated) was assigned according to the vaccination status of the individual on the test date. Subjects who did not receive any dose of the vaccine before the test date were classified as “unvaccinated”. Subjects who had received only one vaccine dose before the test date were considered “partially vaccinated”. Individuals who received the two doses of the vaccine before the test date were deemed “fully vaccinated”.
Figure 1.
Flow diagram of participants’ selection and SARS-CoV-2 test inclusion in the study. 27 December 2020 was established as the index date for case and non-case selection. The study took place between 27 December 2020 and 18 March 2021. *: the same individual could contribute with more than one test to the study.
2.4. Statistical Analysis
VE was measured by comparing the odds of vaccination between cases and non-cases. Adjusted odds ratios (ORs) of testing positive for SARS-CoV-2 among vaccinated patients was compared to that of unvaccinated patients using a logistic regression model. The time since vaccine administration was introduced in the model as an independent variable. This time was represented by zero days for unvaccinated individuals, and the following vaccination periods were considered for vaccinated individuals: 1–6 days after the first dose, 7–13 days after the first dose, 14–20 days after the first dose, 1–6 days after the second dose, 7–13 days after the second dose, and 14 days or more after the second dose. The models were adjusted for the date of SARS-CoV-2 test (in weekly units since the start of study: December 27th, 2020), age and sex. VE was then calculated and expressed in percentages as follows: VE = (1 − OR) × 100.
To evaluate COVID-19 severity in the vaccinated population, we compared the odds of hospitalization, ICU admission and hospital mortality between SARS-CoV-2 positive-tested individuals who received BNT162b2 to those who tested positive but who had not been vaccinated. Patients with nosocomial infections were excluded from this analysis, since SARS-CoV-2 infection could not be ascertained as the cause of hospitalization. Again, adjusted ORs and their 95% CI were computed using logistic-regression models. The models were adjusted for age, date of positive SARS-CoV-2 test and sex. Upon stratifying the participants according to vaccination status and outcome, only those groups with at least five observations in each arm were considered.
The analyses were stratified by age group (18–64, 65–79 and ≥80 years) when it was possible.
All analyses were carried out using the statistical software R (v. 4.0.3) (University of Auckland, Auckland, New Zealand) [10].
3. Results
3.1. Study Population
At the start of the study (27 December 2020), the total number of participants recorded in SERGAS was 2,713,041, out of whom 2,336,030 were aged 18 years and above. A total of 55,742 individuals had a positive laboratory-confirmed SARS-CoV-2 test result before the index date and were excluded from the analysis. This decision was taking to account the variation in criteria applied for individuals’ vaccination throughout the vaccination campaign, and was in line with procedures carried out in previous studies [11,12]. A total of 2,280,288 individuals were assessed for eligibility during the study period (27 December 2020–18 March 2021). From these, 250,226 received at least one of the approved vaccines. Most of those individuals (n = 169,104; 67.6%) were recipients of BNT162b2 (two doses: n = 95,890; one dose: n = 73,214), almost one-third (n = 73,658; 29.4%) received the first dose of ChAdOx1 and only 3.0% (n = 7464) were vaccinated with mRNA-1273 (two doses: n = 3729; one dose: n = 3735). Therefore, only individuals vaccinated with BNT162b2 were included in the analysis.
A total of 596,850 SARS-CoV-2 test results (44,401 positive and 552,449 negative) fulfilled the inclusion criteria (Figure 1). A total of 78.5% of SARS-CoV-2 tests were performed in the population aged between 18 and 64 years; 12.0% corresponded to individuals in the age range of 65–79; and 9.5% belonged to individuals ≥80 (Table 1). More than half of the eligible test results (57.7%) belonged to women (Table 1).
Table 1.
Description of included SARS-CoV-2 tests stratifying by age, sex, vaccination status and test result.
| No. of Participants | Vaccination Status | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unvaccinated (n = 766,410) | Dose 1 (n = 42,999) | Dose 2 (n = 52,017) | Dose 2 (n = 52,017) | |||||||||||
| Negative Test | Positive Test | Negative Test | Positive Test | Negative Test | Positive Test | |||||||||
| n | % | n | % | n | % | n | % | n | % | % | n | % | ||
| Whole population | 861,426 | - | 723,784 | 94.4% | 42,626 | 5.6% | 41,497 | 96.5% | 1502 | 3.5% | 51,744 | 99.5% | 273 | 0.5% |
| Age category (Years) | ||||||||||||||
| 18–64 | 680,312 | 78.5% | 583,526 | 94.8% | 32,285 | 5.2% | 26,499 | 97.0% | 809 | 3.0% | 31,381 | 99.6% | 122 | 0.4% |
| 65–79 | 104,198 | 12.0% | 88,491 | 93.1% | 6598 | 6.9% | 3735 | 95.3% | 183 | 4.7% | 5108 | 99.2% | 41 | 0.8% |
| ≥80 | 82,650 | 9.5% | 51,767 | 93.3% | 3743 | 6.7% | 11,263 | 95.7% | 510 | 4.3% | 15,255 | 99.3% | 110 | 0.7% |
| Sex | ||||||||||||||
| Male | 366,578 | 42.3% | 322,178 | 94.7% | 20,116 | 5.3% | 10,220 | 96.5% | 366 | 3.5% | 12,356 | 99.5% | 82 | 0.5% |
| Female | 500,567 | 57.7% | 401,591 | 94.1% | 22,510 | 5.9% | 31,277 | 96.5% | 1502 | 3.5% | 39,388 | 99.3% | 191 | 0.7% |
| Missing | 15 | 0.0% | 15 | 100% | - | - | - | - | - | - | - | - | - | - |
3.2. BNT162b2 Vaccine Effectiveness against SARS-CoV-2 Infection
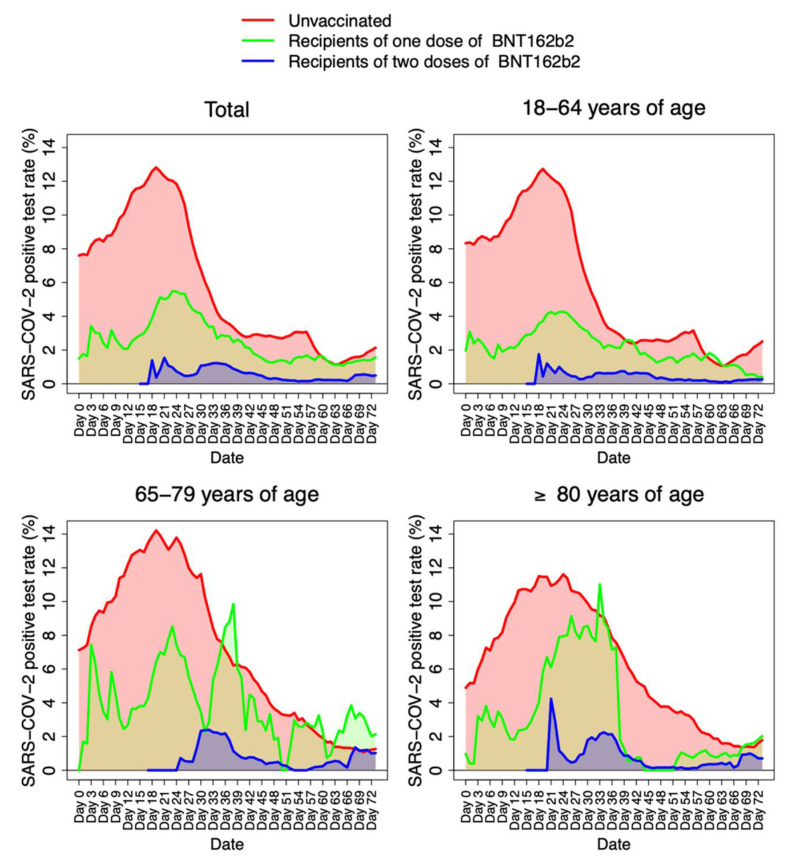
As shown in Figure 2, SARS-CoV-2 positivity rate among the vaccinated population declined with time. A substantial decrease in the odds of SARS-CoV-2 infection was observed in all age categories since the first week (1–6 days) after receiving the first dose of BNT162b2 (Table 2).
Figure 2.
Frequency of weekly positive SARS-CoV-2 tests computed by age groups, and number of administered BNT162b2 vaccine doses. The red line represents the unvaccinated population. The green line represents those individuals who received one dose of BNT162b2. The blue line represents those individuals who received two doses of BNT162b2.
Table 2.
BNT162b2 vaccine effectiveness in preventing SARS-CoV-2 infection stratified by age, time since vaccine administration and number of received doses.
| Whole Population | 18–64 Years | 65–79 Years | ≥80 Years | |||||
|---|---|---|---|---|---|---|---|---|
| Vaccination Status | OR | VE | OR | VE | OR | VE | OR | VE |
| (95% CI) 1 | (95% CI) | (95% CI) 1 | (95% CI) | (95% CI) 2 | (95% CI) | (95% CI) 2 | (95% CI) | |
| Unvaccinated | 1 | - | 1 | - | 1 | - | 1 | - |
| 1–6 days dose 1 | 0.3 | 70.50% | 0.37 | 63.10% | 0.2 | 80.50% | 0.19 | 81.30% |
| (0.26–0.33) | (66.5–74.1) | (0.32–0.42) | (57.5–68.1) | (0.12–0.31) | (69–88.6) | (0.13–0.26) | (74.3–86.8) | |
| 7–13 days dose 1 | 0.36 | 64.30% | 0.36 | 63.80% | 0.38 | 62.90% | 0.43 | 57.90% |
| (0.33–0.39) | (61.2–67.2) | (0.32–0.40) | (59.7–67.7) | (0.29–0.47) | (53.2–71.2) | (0.36–0.50) | (50.8–64.1) | |
| 14–20 days dose 1 | 0.32 | 67.70% | 0.25 | 74.70% | 0.43 | 57.40% | 0.5 | 51.40% |
| (0.29–0.35) | (64.6–70.6) | (0.22–0.29) | (71.0–78.0) | (0.34–0.55) | (46.1–66.9) | (0.42–0.58) | (43.5–58.4) | |
| 1–6 days dose 2 | 0.18 | 81.60% | 0.17 | 83.40% | 0.22 | 78.70% | 0.16 | 84.70% |
| (0.15–0.22) | (77.7–85.0) | (0.12–0.22) | (78.3–87.6) | (0.12–0.36) | (65.1–88.1) | (0.11–0.22) | (78.9–89.3) | |
| 7–13 days dose 2 | 0.25 | 75.40% | 0.21 | 79.00% | 0.17 | 83% | 0.22 | 78.80% |
| (0.20–0.30) | (70.1–80.1) | (0.15–0.28) | (71.7–84.9) | (0.09–0.29) | (71.2–90.9) | (0.16–0.29) | (71.7–84.6) | |
| ≥14 days dose 2 | 0.09 | 90.80% | 0.07 | 92.90% | 0.15 | 85.80% | 0.09 | 91.40% |
| (0.07–0.11) | (88.6–92.7) | (0.05–0.10) | (90.2–95.1) | (0.08–0.23) | (77.3–91.9) | (0.06–0.12) | (87.9–94.1) | |
CI: confidence interval; OR: Adjusted odds ratio; VE: vaccine effectiveness. 1 odds ratio adjusted for sex, age and time period between SARS-CoV-2 test and the start of study (in weeks). 2 odds ratio adjusted for sex and time period between SARS-CoV-2 test and the start of study (in weeks).
The greatest VE against SARS-CoV-2 infection was observed ≥14 days after receiving the second dose of BNT162b2 [VE = 91.0% (95% CI: 89.0–93.0)] (Table 2). Stratifying this result by age, revealed that the VE is substantial for all age categories: VE = 93.0% (95% CI: 90.0–95.0) for 18–64 years; VE = 85.0% (95% CI: 75.0–91.0) for 65–79 years; and VE = 91.0% (95% CI: 87.0–94.0) for ≥80 years (Table 2).
3.3. BNT162b2 Vaccine Effectiveness against COVID-19 Severity
Among 44,401 SARS-CoV-2 positive-tested individuals (Table 1), 606 were excluded due to nosocomial infections.
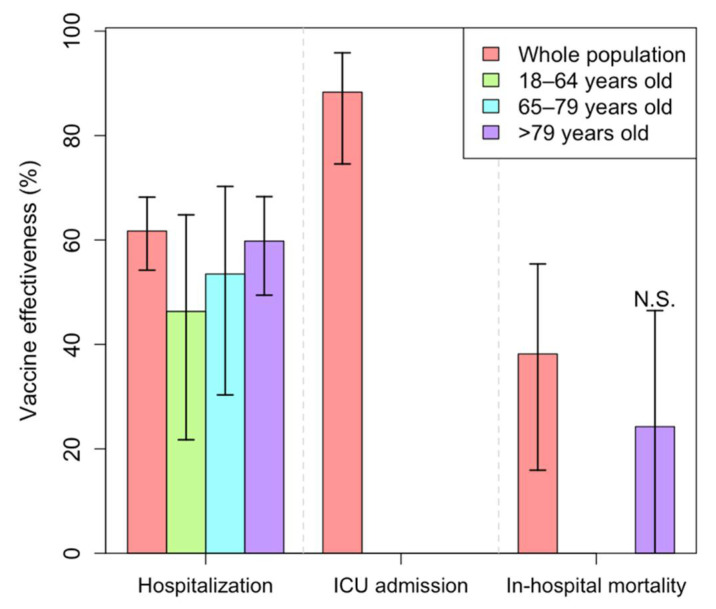
COVID-19 was deemed as the cause of hospitalization in 4254 of the remaining 43,795 adults. A total of 4103 of the 4254 hospitalized adults were unvaccinated and the remaining 151 were vaccinated with BNT162b2 (one dose: 127; two doses: 24) (Table S1). A considerable reduction in hospital admissions for COVID-19 was detected in individuals vaccinated with any dose (one or two) of BNT162b2 as compared to unvaccinated individuals [VE = 62.0% (95% CI: 54.2–68.2)] (Figure 3, Table S2). BNT162b2 administration showed protection against COVID-19 hospitalization for all age categories, with the VE more pronounced for those aged 80 years and older [18–64 years: VE = 46.0% (95% CI: 21.7–64.8); 65–79 years: VE = 53.0% (95% CI: 30.3–70.3), and ≥80 years: VE = 60.0% (95% CI: 49.4–68.3)] (Figure 3, Table S2).
Figure 3.
Vaccine’s effectiveness results ogBNT162b2 vaccine. 95% CI are displayed in black lines. Extended results in Table S2. N.S.: not significant.
Due to the limited number of positive-tested individuals who had received two doses of BNT162b2 during the study period, we could not stratify the COVID-19 severity analysis by the number of received doses.
Among 1762 BNT162b2 vaccinated and SARS-CoV-2 positive individuals, only five incompletely vaccinated (one dose) adults younger than 80 years old were admitted to ICU (Figure 3, Table S2). For this reason, BNT162b2 VE against ICU admission could not be stratified by age, but globally, the vaccine showed high effectiveness against ICU admission for COVID-19 [VE = 88.0% (95% CI: 74.6–95.8)] (Figure 3, Table S2).
Overall, the BNT162b2 vaccine was associated with a decrease in death for COVID-19 [VE = 38.0% (95% CI: 15.9–55.4)] (Figure 3, Table S2). Due to the limited number of registered COVID-19 deaths, the analysis could be only stratified for the age group ≥ 80 years. The BNT162b2 vaccine was suggested to be associated with 24% lower odds of COVID-19 mortality in vaccinated individuals ≥80 years as compared to unvaccinated individuals from the same age category, yet the confidence interval was large, probably due to the insufficient number of observations [OR = 0.76 (95% CI: 0.54–1.05)] (Figure 3, Table S2).
4. Discussion
We provided early real-world evidence of BNT162b2 effectiveness in reducing SARS-CoV-2 infections as well as COVID-19 disease severity in the general adult population in Galicia, Spain. Our findings reveal a substantial protection against SARS-CoV-2 infections in all age categories, a few days after receiving the first dose (one to six days). BNT162b2 VE against SARS-CoV-2 reached its peak ≥14 days after the second dose; exceeding 90% in the elderly population (≥80 years) and in those aged 18–64 years. Remarkable oscillations of SARS-CoV-2 positive tests were observed for partially vaccinated individuals of the age groups 65–79 and ≥80 years. These findings can be related to COVID-19 outbreak events at nursing homes at the start of the vaccination campaign. These events mainly involved residents, being most of them asymptomatic (according to media news). However, it is also remarkable that these outbreaks did not affect fully vaccinated individuals.
A reduction in SARS-CoV-2 infections implies a decrease in hospital and ICU admissions, and consequently a protection against death caused by COVID-19. This is in line with our observation that the odds of hospital admission for COVID-19 are 62% lower in individuals vaccinated with one or two doses of BNT162b2 than in nonvaccinated individuals. Our findings also point to overall reduced odds of ICU admissions and mortality for COVID-19. Nonetheless, upon categorizing the study participants who were admitted to ICU or died from COVID-19 according to their age, a limited number of observations were left in each age category, restraining therefore the stratification of this analysis by age. Replicating the study in a larger population would allow for a comprehensive evaluation of BNT162b2 effectiveness against ICU admissions and mortality caused by COVID-19.
Our findings coincide with those of previous studies that measured BNT162b2 VE. In the United Kingdom, Pritchard et al. demonstrated 80% VE of BNT162b2 against SARS-CoV-2 infections among individuals fully vaccinated as compared to non-vaccinees [13]. Another study in Israel involving healthcare workers, reported a 75% reduction in SARS-CoV-2 infections in the 15 to 28 days after receiving the first dose of BNT162b2 [12]. In England, SARS-CoV-2 infections declined by 89% in elder adults after ≥14 days of vaccinating with the second dose of BNT162b2 [14]. In Scotland, the hospitalization rate for COVID-19 also decreased by 91% in the 28 to 34 days after the administration of the first dose of BNT162b2 [11]. In a study undertaken in nursing homes, Shrotri et al. estimated a 65% decrease in SARS-CoV-2 infections among residents of English long-term care facilities, 35 to 48 days after receiving the first doses of BNT162b2 [15].
In Spain, since the start of COVID-19 vaccination program on 27 December 2020, some studies have evaluated VE. Nonetheless, the studies targeted various populations and/or socioeconomically different regions where the COVID-19 epidemiological situation and the related preventive measures were dissimilar. Cabezas et al. evaluated SARS-CoV-2 infections in a group of residents and healthcare workers of nursing homes in Catalonia (Spain) until 5 March 2021 and reported a 91% and 80% reduction in the infection rate after receiving two doses of BNT162b2 for nursing home residents and staff, respectively [16]. Two other studies in the elderly population were conducted in Spain using national vaccination registers [17,18]. Both studies also provided an early evaluation of VE and were concluded by 10 March 2021 [17] and 4 April 2021 [18]. The study of Mazagatos et al. showed high effectiveness of mRNA vaccines in preventing COVID-19 hospitalizations (88%) and deaths (97%) [18]. Likewise, Monge et al. showed substantial VE against SARS-CoV-2 infections in individuals with and without previous infection (57% and 82%, respectively) [17]. Almost all administered vaccines (99.8%) in that study were BNT162b2 [17]. In Navarre (Spain), Martínez-Baz et al. estimated VE in a population of adults who were in close contact with COVID-19 positive cases between January and April 2021. The study excluded residents of nursing homes and the majority of the participants had received BNT162b2 vaccine [19]. Martínez-Baz et al. showed that full vaccination reduces SARS-CoV-2 infections by 66%, symptomatic COVID-19 by 42% and hospitalization for COVID-19 by 72% [19].
Our findings are unlikely to have been biased. First, since our study is population-based, selection bias is unlikely to have occurred as we had access to the whole population registered in SERGAS. In Galicia, vaccination took place through SERGAS and was made freely available to everyone. All data were electronically registered through sanitary cards that are unique to each person registered in SERGAS. Therefore, exposure misclassification is also a remote possibility. As for outcome ascertainment, misclassification is also improbable, the infection with SARS-CoV-2 and the disease severity were determined through laboratory-based tests and related medical records. Furthermore, confounding bias is unlikely to have occurred, as we adjusted the estimated VE for potentially confounding factors including sex, age and duration of pandemic.
Our study suffers from the following limitations. We did not have data about patients’ comorbidities and COVID-19 symptoms (symptomatic/asymptomatic, and time lapse between symptoms’ appearances and COVID-19 testing). In addition, different nonpharmaceutical measures were applied by the government during the study period, which might have influenced the incidence of SARS-CoV-2 infections. During the COVID-19 pandemic, the Spanish government implemented various nonpharmaceutical interventions based on a four-level pandemic-alert scale that uses the cumulative incidence level proposed by the World Health Organization (WHO) and by the European Center for Disease Prevention and Control (ECDC). The measures included, among others: total lockdown, border closure of autonomous communities, provinces or municipalities, personal protective measures, night-time curfew, mass-gathering cancellations, control of numbers of individuals (capacity limitations) and limited opening hours in specific facilities such as restaurants. Our study also lacks data about the identity of detected SARS-CoV-2 variants in the positively tested individuals [20,21,22]. Individuals in different settings could be exposed at a greater or lesser extent to SARS-CoV-2, hence stratifying the analysis according to patients’ settings such as homecare centers, frontline healthcare workers and administrative occupation would provide a deeper insight on VE. We did not have information on SARS-CoV-2 viral load. SARS-CoV-2 virus quantitation can help understand disease heterogeneity and aid in identifying patients who may benefit from new COVID-19 therapies [23]. Although our study was population-based and encompassed a large sample size, the number of positive SARS-CoV-2 tests was not sufficient to stratify the analysis of VE against ICU admission and mortality for COVID-19 by different age subgroups, hence replicating the study in a larger population would allow to comprehensively measure VE against severe COVID-19 outcomes. Our findings cannot be generalized to disadvantaged populations who have limited access to healthcare services, as in Spain access to healthcare is universal. Nonetheless, confounding bias due to healthcare-seeking behavior is unlikely to have occurred in our study [6].
Evaluating BNT162b2 safety was beyond the scope of the present study, yet the authors recommend to investigate the adverse effects related to this vaccine in the Spanish population in future studies. BNT162b2 was previously associated with increased risk of serious adverse events such as myocarditis and lymphadenopathy [24]. Individuals vaccinated with BNT162b2 also reported side effects such as soreness, fatigue, myalgia, headache, fever and muscle pain [25].
5. Conclusions
Our study shows that BNT162b2 offers substantial protection against SARS-CoV-2 infection from the moment of vaccination, leading to an important reduction in COVID-19 associated hospitalization, ICU admission and mortality. The findings of the present study would prove useful for systematic reviews and meta-analysis on the topic. However, studies on VE should be routinely updated until the end of the pandemic to provide a more comprehensive analysis on protection against COVID-19 severity, new emerging SARS-CoV-2 variants and duration of acquired immunity. Further studies are also required to study the overall vaccine performance and evaluate vaccine safety in different settings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19074039/s1, Table S1: Distribution of COVID-19 attributed hospitalization, intensive care unit (ICU) admission and mortality among SARS-CoV-2 positive cases stratified by vaccination status, age and sex.; Table S2: BNT162b2 VE against COVID-19 associated hospitalization, intensive care unit (ICU) admission and mortality stratified by age.
Author Contributions
Conceived the research idea and designed the study, F.M.-T.; analyzed the data and wrote the original draft of the manuscript, J.P.-S.; critically reviewed, revised and edited the manuscript, N.M.; interpreted the data, J.P.-S. and N.M.; reviewed and revised the manuscript, A.S., A.G.-C., C.R.-T. and I.R.-C.; extracted and processed the data, L.R.L.-P., J.M.G.-P., B.R., M.T.O.-B. and C.D.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Framework Partnership Agreement between the Consellería de Sanidad de la XUNTA de Galicia and GENVIP-IDIS-2021–2024 (SERGAS-IDIS march 2021); and consorcio Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CB21/06/00103; F.M.T.), DIAVIR (Instituto de Salud Carlos III (ISCIII)/DTS19/00049/Cofinanciado FEDER; Proyecto de Desarrollo Tecnológico en Salud), Resvi-Omics (Instituto de Salud Carlos III (ISCIII)/PI19/01039/Cofinanciado FEDER), BI-BACVIR (PRIS-3; Agencia de Conocimiento en Salud (ACIS)—Servicio Gallego de Salud (SERGAS)—Xunta de Galicia; Spain), Programa Traslacional COVID-19 (ACIS—Servicio Gallego de Salud (SERGAS)—XUNTA de Galicia; Spain) and Axencia Galega de Innovación (GAIN; IN607B 2020/08—XUNTA de Galicia; Spain) [A.S.]; and ReSVinext (Instituto de Salud Carlos III (ISCIII)/PI16/01569/Cofinanciado FEDER), Enterogen (Instituto de Salud Carlos III (ISCIII)/PI19/01090/Cofinanciado FEDER), and Axencia Galega de Innovación (GAIN; IN845D 2020/23—Xunta de Galicia; Spain) [F.M.-T.].
Institutional Review Board Statement
This research study is carried out within the framework of the care protocol for the study of adverse effects and failures of vaccines against COVID-19 within the SERGAS pharmacovigilance program and under the Framework Collaboration Agreement between the Consellería de Sanidade of the Xunta de Galicia and the Vaccines and Infections Research Group (GENVIP) of Instituto de Investigación Sanitaria de Santiago de Compostela for applied research on infections and vaccines in Galicia, with pseudonymization being the basis for processing the study data.
Informed Consent Statement
Patient consent was waived due to this study neither involved an intervention, nor used biological samples. The public health agency (SERGAS) provided the authors with pseudo-anonymized data, and SERGAS did not take part of the data analysis.
Data Availability Statement
Not applicable.
Conflicts of Interest
F.M.-T. received honoraria from GSK group of companies, Pfizer Inc., Sanofi Pasteur, MSD, Seqirus, and Janssen for taking part in advisory boards and expert meetings and for acting as a speaker in congresses outside the scope of the submitted work. F.M.-T. has also acted as principal investigator in randomized controlled trials of the above-mentioned companies as well as Ablynx, Gilead, Regeneron, Roche, Abbott, Novavax, and MedImmune, with honoraria paid to his institution. The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.European Medicines Agency . COVID-19 Vaccines: Authorised. European Medicines Agency; Amsterdam, The Netherlands: 2020. [Google Scholar]
- 2.Ministerio de Sanidad . Estrategia de Vacunación Frente al COVD-19 en España. Ministerio de Sanidad; Madrid, Spain: 2020. [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poland G.A., Ovsyannikova I.G., Kennedy R.B., Haralambieva I.H., Jacobson R.M. Vaccinomics and a new paradigm for the development of preventive vaccines against viral infections. OMICS. 2011;15:625–636. doi: 10.1089/omi.2011.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kow C.S., Hasan S.S. Real-world effectiveness of BNT162b2 mRNA vaccine: A meta-analysis of large observational studies. Inflammopharmacology. 2021;29:1075–1090. doi: 10.1007/s10787-021-00839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean N.E., Hogan J.W., Schnitzer M.E. COVID-19 Vaccine Effectiveness and the Test-Negative Design. N. Engl. J. Med. 2021;385:1431–1433. doi: 10.1056/NEJMe2113151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukushima W., Hirota Y. Basic principles of test-negative design in evaluating influenza vaccine effectiveness. Vaccine. 2017;35:4796–4800. doi: 10.1016/j.vaccine.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Chua H., Feng S., Lewnard J.A., Sullivan S.G., Blyth C.C., Lipsitch M., Cowling B.J. The Use of Test-negative Controls to Monitor Vaccine Effectiveness: A Systematic Review of Methodology. Epidemiology. 2020;31:43–64. doi: 10.1097/EDE.0000000000001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SERGAS . Plan Galego de Vacinación Fronte Ao SARS-CoV-2. Sergas; Galicia, Spain: 2021. [(accessed on 15 February 2022)]. Available online: https://coronavirus.sergas.gal/Contidos/Plan-galego-vacinacion-COVID. [Google Scholar]
- 10.R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2021. [Google Scholar]
- 11.Vasileiou E., Simpson C.R., Shi T., Kerr S., Agrawal U., Akbari A., Bedston S., Beggs J., Bradley D., Chuter A., et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A national prospective cohort study. Lancet. 2021;397:1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amit S., Regev-Yochay G., Afek A., Kreiss Y., Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397:875–877. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pritchard E., Matthews P.C., Stoesser N., Eyre D.W., Gethings O., Vihta K.D., Jones J., House T., VanSteenHouse H., Bell I., et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat. Med. 2021;27:1370–1378. doi: 10.1038/s41591-021-01410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harel T., Hacohen N., Shaag A., Gomori M., Singer A., Elpeleg O., Meiner V. Homozygous null variant in CRADD, encoding an adaptor protein that mediates apoptosis, is associated with lissencephaly. Am. J. Med. Genet. A. 2017;173:2539–2544. doi: 10.1002/ajmg.a.38347. [DOI] [PubMed] [Google Scholar]
- 15.Shrotri M., Krutikov M., Palmer T., Giddings R., Azmi B., Subbarao S., Fuller C., Irwin-Singer A., Davies D., Tut G., et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): A prospective cohort study. Lancet Infect. Dis. 2021;21:1529–1538. doi: 10.1016/S1473-3099(21)00289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabezas C., Coma E., Mora-Fernandez N., Li X., Martinez-Marcos M., Fina F., Fabregas M., Hermosilla E., Jover A., Contel J.C., et al. Associations of BNT162b2 vaccination with SARS-CoV-2 infection and hospital admission and death with COVID-19 in nursing homes and healthcare workers in Catalonia: Prospective cohort study. BMJ. 2021;374:n1868. doi: 10.1136/bmj.n1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monge S., Olmedo C., Alejos B., Lapeña M.F., Sierra M.J., Limia A., COVID-19 Registries Study Group Direct and Indirect Effectiveness of mRNA Vaccination against Severe Acute Respiratory Syndrome Coronavirus 2 in Long-Term Care Facilities, Spain. Emerg. Infect. Dis. 2021;27:2595–2603. doi: 10.3201/eid2710.211184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazagatos C., Monge S., Olmedo C., Vega L., Gallego P., Martín-Merino E., Sierra M.J., Limia A., Larrauri A., Working Group for the Surveillance and Control of COVID-19 in Spain Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, Spain, weeks 53 2020 to 13 2021. Euro Surveill. 2021;26:2100452. doi: 10.2807/1560-7917.ES.2021.26.24.2100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez-Baz I., Miqueleiz A., Casado I., Navascués A., Trobajo-Sanmartín C., Burgui C., Guevara M., Ezpeleta C., Castilla J., Working Group for the Study of COVID-19 in Navarra Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Euro Surveill. 2021;26:2100438. doi: 10.2807/1560-7917.ES.2021.26.21.2100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gómez-Carballa A., Pardo-Seco J., Bello X., Martinón-Torres F., Salas A. Superspreading in the emergence of COVID-19 variants. Trends Genet. 2021;37:1069–1080. doi: 10.1016/j.tig.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gómez-Carballa A., Bello X., Pardo-Seco J., Pérez Del Molino M.L., Martinón-Torres F., Salas A. Phylogeography of SARS-CoV-2 pandemic in Spain: A story of multiple introductions, micro-geographic stratification, founder effects, and super-spreaders. Zool. Res. 2020;41:605–620. doi: 10.24272/j.issn.2095-8137.2020.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez-Carballa A., Bello X., Pardo-Seco J., Martinón-Torres F., Salas A. Mapping genome variation of SARS-CoV-2 worldwide highlights the impact of COVID-19 super-spreaders. Genome Res. 2020;30:1434–1448. doi: 10.1101/gr.266221.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunet-Ratnasingham E., Anand S.P., Gantner P., Dyachenko A., Moquin-Beaudry G., Brassard N., Beaudoin-Bussières G., Pagliuzza A., Gasser R., Benlarbi M., et al. Integrated immunovirological profiling validates plasma SARS-CoV-2 RNA as an early predictor of COVID-19 mortality. Sci. Adv. 2021;7:eabj5629. doi: 10.1126/sciadv.abj5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., Hernán M.A., Lipsitch M., Kohane I., Netzer D., et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadali R.A.K., Janagama R., Peruru S., Malayala S.V. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int. J. Infect. Dis. 2021;106:376–381. doi: 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.