Abstract
Daptomycin, a lipopeptide antibiotic, has broad activity against gram-positive organisms, similar to vancomycin; however, its mechanism of action differs, resulting in interference with cell membrane transport and a more rapid bactericidal activity. In light of increasing need for alternative treatments against intermediate-resistant Staphylococcus aureus, there is revitalized interest in this antibiotic. We, therefore, evaluated the activity of daptomycin alone or in combination in an in vitro infection model against two glycopeptide intermediate-resistant S. aureus (GISA) isolates. Newly designed regimens of daptomycin at 4 and 6 mg/kg of body weight every 24 h (q24h) were compared to the previous regimen of 3 mg/kg q12h. Daptomycin MICs and minimal bactericidal concentrations (MBCs) (MIC/MBC) for Mu-50, HIP5836 (992), and MRSA-67 were 0.5/1.0, 0.5/1.0, and 0.125/0.5 μg/ml, respectively. MICs and MBCs of arbekacin for the three strains were 2.0/8.0, 0.125/0.5, and 0.125/0.25 μg/ml, respectively. Vancomycin and gentamicin MICs and MBCs for the three strains were 8.0/8.0, 8.0/8.0, and 0.5/1.0 μg/ml and 128/128, 0.5/1.0, and 0.25/0.5 μg/ml, respectively. Our experience with daptomycin in an in vitro infection model has shown significant kill against the two GISA strains (Mu-50 and 992) (P < 0.03). We also noted that kill was related to a total dose effect for 992, in which simulated daptomycin in vivo dosages of 6 mg/kg q24h and 3 mg/kg q12h produced similar kill and 4 mg/kg q24h resulted in significant regrowth (P ≤ 0.05). Combination therapy with arbekacin resulted in synergistic activity against Mu-50. Daptomycin area under the concentration-time curve/MIC and Cmax/MIC ranges for GISA isolates were 80 to 116 and 6 to 12, respectively, and ranges for MRSA-67 were 320 to 461 and 24 to 48, respectively, and appeared to have an association with kill (i.e., decreased CFU/milliliter) at 24 and 48 h. Therefore, these experiments suggest that daptomycin alone or in combination could provide an alternative for the treatment of GISA.
Glycopeptide intermediate-resistant Staphylococcus aureus (GISA) has now been isolated in France, Japan, the United States, and Hong Kong. Clinical isolates of GISA containing the vanA or vanB gene have not been reported, and therefore, plasmid-mediated resistance is currently not likely the mechanism for GISA resistance (16). All strains of S. aureus with reduced susceptibilities to vancomycin in vivo have initially been methicillin-resistant S. aureus (MRSA), which developed decreased susceptibility with prolonged exposure to vancomycin (16). The continued inappropriate use of vancomycin will likely lead to further development of resistance by gram-positive organisms (4, 7, 20). As with most drug-resistant organisms, alternative treatment options are extremely limited, often to less-proven or investigational drugs (5). Therefore, the potential for continuing emergence of strains of vancomycin intermediate-resistant S. aureus increases the need to find new therapeutic options.
The mechanism of glycopeptide resistance in S. aureus is unknown (10, 16). However, it is thought that alteration of cell wall structure resulting in thickened cell walls may be a key factor in the development of resistance (15). S. aureus carries the mecA gene, which encodes a penicillin-binding protein (PBP) that confers penicillin resistance (13). However, it has been shown that GISA actually has a three- to fourfold increase in production of PBP2 and that the increase in production is strongly correlated with the increase in vancomycin MIC (10).
Ampicillin-sulbactam or the combination of ampicillin-sulbactam plus arbekacin has been shown to be synergistic and was successfully used in the first reported case of infection caused by GISA (5). Arbekacin is a broad-spectrum aminoglycoside, used in Japan for a number of years, that is active against MRSA organisms that have a variety of aminoglycoside-inactivating enzymes (19). The enzyme AAC-6/APH-2", which can inactivate aminoglycosides, has been reported to have a low modification rate, and the ANT (4′)-1 aminoglycoside-modifying enzyme has no effect on arbekacin (12). Therefore, bactericidal activity of arbekacin against MRSA has been observed at 0.5 to 2 times the MIC, resulting in a 90 to 99.9% kill (18). Another agent with potential use against GISA is the investigational drug daptomycin. It is a lipopeptide antibiotic with a unique mechanism of action that has broad activity against gram-positive organisms. However, its site of activity, differing from those of the glycopeptides and β-lactams, acts by specifically inhibiting the synthesis of the cell wall, and in S. aureus it inhibits the incorporation of [14C]alanine into peptidoglycan. Initially, this drug was investigated as an alternative to vancomycin. However, studies were discontinued in phase II when less-than-optimal effects were observed clinically. Consequently, due to the increasing need for alternative treatments against intermediate-resistant S. aureus, there is a revitalized interest in daptomycin along with other therapeutic options.
With this in mind, we decided to evaluate a number of various antibiotics against two strains of glycopeptide intermediate-resistant S. aureus and a control strain of MRSA.
MATERIALS AND METHODS
Bacterial strains.
The two strains of GISA tested in this evaluation were Mu-50 (Juntendo Hospital, Tokyo, Japan) and HIP5836 (992) (New Jersey strain; Centers for Disease Control and Prevention, Atlanta, Ga.). MRSA-67, a clinical isolate, was tested as a control strain.
Antibiotics.
Daptomycin (lot 444BYO; Cubist Pharmaceuticals, Inc., Cambridge, Mass.) and arbekacin (lot ABKMC-1300; Meiji Seika Kaisha, Ltd., Pharmaceutical Division, Tokyo, Japan) were used. Vancomycin (lot 1NJ03M; Sigma Chemical Company, St. Louis, Mo.), and gentamicin (lot 96HO975; Sigma Chemical Company) were commercially purchased.
Medium.
All in vitro infection models, except for the daptomycin models, used Mueller-Hinton broth (Difco, Detroit, Mich.) supplemented with 25 mg of calcium/liter and 12.5 mg of magnesium/liter (SMHB). All daptomycin models used Mueller-Hinton broth supplemented with 75 mg of calcium/liter and 12.5 mg of magnesium/liter (8). Colony counts were determined using tryptic soy agar (TSA) (Difco) plates.
In vitro susceptibility determination.
MICs and minimal bactericidal concentrations (MBCs) were determined by microdilution technique with an inoculum of 5 × 105 CFU/ml by following National Committee for Clinical Laboratory Standards guidelines (11). Daptomycin MICs and MBCs were determined in supplemented broth as described above.
In vitro pharmacodynamic infection model.
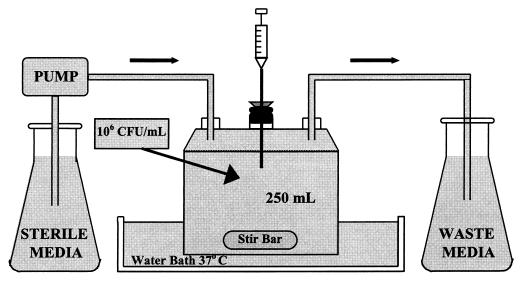
An in vitro infection model consisting of a one-compartment glass chamber with ports for the addition and removal of SMHB, delivery of antibiotics, and collection of bacterial samples and drug concentrations was utilized over 48 h (Fig. 1). Before each experiment, bacterial colonies from an overnight growth on TSA were added to the SMHB to obtain a 108-CFU/ml McFarland suspension. Then, 2.5 ml of this suspension was added to each of the infection models to produce a starting inoculum of 106 CFU/ml. The model was placed in a 37°C water bath during the duration of the experiment with magnetic stir bars in the media to allow for continuous mixing. A peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, Ill.) was used to replace antibiotic-containing media with fresh SMHB and to simulate the half-lives of the study drugs. The pH was monitored throughout all experiments, at 0, 8, 24, and 48 h, with daptomycin due to the possible effects on its activity (8). To ensure reproducibility, each experimental regimen was performed in duplicate. Regimen simulations were as follows. Daptomycin was given at three dosages—6 mg/kg of body weight every 24 h (q24h) (D6), 4 mg/kg q24h (D4), and 3 mg/kg q12h (D3). Since daptomycin is approximately 93% protein bound (9, 14; G. L. Brier, J. D. Wolny, and H. R. Black, Abstr. 29th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1347, 1989), a free simulated maximum concentration of drug in serum (Cmax) and minimum concentration of drug in serum (Cmin) (Cmax/Cmin) of 6/0.07, 4/0.03, and 3/0.02 μg/ml for the respective doses and an average half-life of 8 h was targeted. These regimens simulate human-targeted Cmax/Cmin of 80/10, 60/7.5, and 40/15 μg/ml, respectively. Arbekacin was administered at 100 mg q12h, with an estimated Cmax of 8 μg/ml and Cmin of 0.5 μg/ml and with a half-life of 3 h, which is the target concentration in humans at that dose. Vancomycin was administered at 1 g q12h for an estimated Cmax and Cmin of 30 to 40 and 5 to 10 μg/ml, respectively, with a half-life of 6 h. Gentamicin was given at 1.5 mg/kg q12h for an estimated Cmax of 5 μg/ml and Cmin of 0.3 μg/ml, with a half-life of 3 h. Combinations of the D6, D4, and D3 regimens and vancomycin with arbekacin were performed at the above listed concentrations. In the combination regimen models, the elimination rate was set for the drug with the shorter half-life, and the drug with the longer half-life was supplemented (3).
FIG. 1.
Bacteremia model.
Pharmacokinetic analyses.
Antibiotic concentrations were determined from duplicate samples obtained from each of the models at 0, 0.5, 1, 2, 4, 6, 8, 24, 28, 32, and 48 h and stored at −70°C until analysis. Daptomycin concentrations were determined by microbioassay utilizing Micrococcus luteus ATCC 9341 as the reference organism. Standards and samples were tested in triplicate using blank 1/4-in. disks saturated with 20 μl of the appropriate solution. The disks were then placed on Antibiotic Assay Medium #1 (AAM-1; Difco) agar plates preswabbed with 0.5 McFarland suspension of the reference organism, forming a confluent lawn. The plates were incubated at 37°C for 24 h, at which time the zones of inhibition were measured. All plates achieved a correlation coefficient of ≥0.95. Daptomycin standard antibiotic concentrations used were 10, 2.5, and 1.25 μg/ml, with the last being the lower limit of detection due to the limitation of the blank disk size. The between-day coefficient of variation for the high, medium, and low standards was ≤12% for daptomycin. Arbekacin, vancomycin, and gentamicin concentrations were determined by fluorescence polarization immunoassay (TDx; Abbott Laboratories, Irving, Tex.). Lower limits of detection for the arbekacin, vancomycin, and gentamicin TDx assays were 0.4, 2.0, and 0.27 μg/ml, respectively, and the between-day coefficient of variation for the high, medium, and low standards was ≤10% for each. The antibiotic Cmax/Cmin and half-lives were calculated from plots of the concentration versus time plots. Area under the concentration-time curve from 0 to 24 h (AUC0–24) was determined by trapezoidal methods utilizing the RStrip program, version 3.1 (MicroMath, Salt Lake City, Utah).
Pharmacodynamic analyses.
Samples of approximately 0.5 ml were collected in duplicate from each of the infection models at 0, 1, 2, 4, 6, 8, 24, 28, 32, and 48 h. Samples were then serially diluted in cold 0.9% sodium chloride. Bacterial counts were determined by spiral plating 50-μl samples of the appropriate diluted sample on TSA and then incubating for 24 h at 37°C. The limit of detection was previously determined to be 2.5 log10 CFU/ml. An appropriate number of dilutions (≥5) were made to account for and to minimize antibiotic carryover. Time-kill curves were determined by plotting average colony counts (log10 CFU/milliliter) of the infection models versus time. Bactericidal activity (99.9% kill) was defined as a ≥3 log10 CFU/ml reduction from the starting inoculum. Synergistic activity was defined as a ≥2 log10 CFU/ml reduction from the most active agent. Reductions in the log10 CFU/milliliter were determined over the 48-h period and compared between regimens. Time to achieve 99.9% killing was determined by linear regression, if r2 was greater than or equal to 0.95, or by visual inspection. The following pharmacodynamic parameters were evaluated: Cmax/MIC, AUC0–24/MIC, and time above the MIC for 24 h (T > MIC) versus change in CFU/milliliter. Samples from the 24- and 48-h time points were also plated onto TSA plates containing four to eight times the MIC and incubated for 48 h for determining development of resistance. Any organism growth, noted on the antibiotic-containing resistance plates after 48 h of incubation, would be considered resistant. If resistance occurred, MIC and MBC testing was completed to determine the level of resistance.
Statistical analyses.
Differences in log10 CFU/milliliter at 48 h, time to 99.9% kill, and pharmacodynamic variables (Cmax/MIC and AUC0–24/MIC) between regimens were determined by analysis of variance with Tukey's test for multiple comparisons. A P value of <0.05 was considered significant. All statistical analyses were performed using SPSS Statistical Software (Release 6.1.3; SPSS, Inc., Chicago, Ill.).
RESULTS
Susceptibility testing.
Microdilution MICs and MBCs of daptomycin, arbekacin, vancomycin, and gentamicin for Mu-50, 992, and MRSA-67 are listed in Table 1. Daptomycin's MIC and MBC were the same for both strains of GISA. However, despite similarities in MIC and MBC, kill was significantly less (P < 0.05) over the 48-h period for the daptomycin 4-mg/kg/day regimen compared to the 3 mg/kg q12h or the 6-mg/kg/day regimen with the 992 strain. Arbekacin, on the other hand, produced a kill that was reflective of the differences in MICs for the two GISA strains.
TABLE 1.
Microdilution MICs and MBCs of antibiotics for S. aureus strains
| Antibiotic | MIC/MBC (μg/ml) for strain
|
||
|---|---|---|---|
| Mu-50 | 992 | MRSA-67 | |
| Daptomycin | 0.5/1.0 | 0.5/1.0 | 0.125/0.5 |
| Arbekacin | 2.0/8.0 | 0.125/0.5 | 0.125/0.25 |
| Vancomycin | 8.0/8.0 | 8.0/8.0 | 0.5/1.0 |
| Gentamicin | 128.0/128.0 | 0.5/1.0 | 0.25/0.5 |
Pharmacokinetics.
Daptomycin's Cmaxs were (mean ± standard deviation) 5.84 ± 0.17, 3.92 ± 0.16, and 3.04 ± 0.16 μg/ml for D6, D4, and D3, respectively, with an average half-life of 8.4 ± 0.4 h obtained for all regimens. Arbekacin had an average half-life of 3.11 ± 0.18 h, with Cmax and Cmin being 8.0 ± 0.19 and 0.73 ± 0.14 μg/ml, respectively. Vancomycin's Cmax and Cmin were 32.10 ± 2.03 and 12.15 ± 3.40 μg/ml, respectively, and an average half-life of 6.05 ± 0.37 h was obtained. The Cmax and Cmin for gentamicin obtained in the models were 4.77 ± 0.12 and 0.46 ± 0.25 μg/ml, respectively, with an average half-life of 3.08 ± 0.55 h. Concentrations in combination regimens were individually determined and found to have levels similar to those of monotherapy concentrations (data not shown).
Pharmacodynamics.
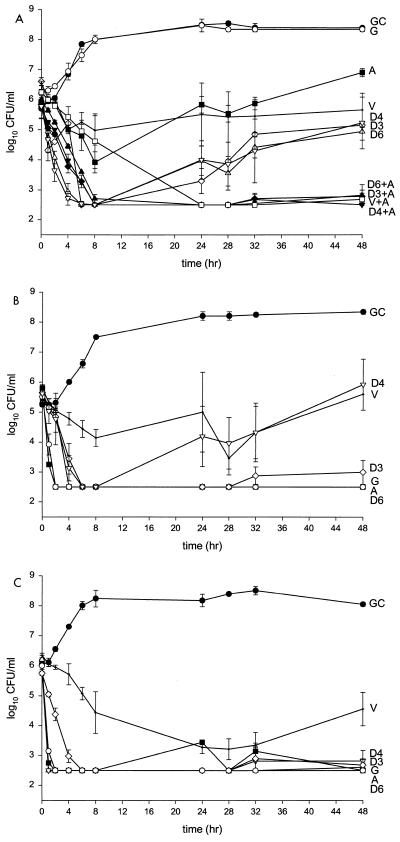
Reductions in bacterial inocula at 48 h and pharmacodynamic parameters for Mu-50, 992, and MRSA-67 are shown in Table 2. Overall, daptomycin's killing activity was greater for 992 and MRSA-67 than for Mu-50 (Fig. 2). Time to 99.9% kill was achieved by 6 h for all daptomycin regimens against Mu-50, but considerable regrowth was observed at 48 h. There was minimal kill, although never resulting in 99.9% kill, observed for arbekacin, with significant regrowth at 48 h. Vancomycin produced only static activity while gentamicin resulted in no kill and was similar to the growth control. Greater and more sustained killing activity at 48 h was noted for each daptomycin regimen when combined with arbekacin against Mu-50, resulting in synergistic activity. Against 992, time to 99.9% kill was achieved by 6, 2, and 4 h for daptomycin, arbekacin, and gentamicin, respectively. However, the D4 regimen was noted to have significant regrowth at 48 h (P ≤ 0.05).
TABLE 2.
Bacterial inocula and pharmacodynamic parameters
| Strain | Regimena | Initial inoculumb (log10 CFU/ml) | Residual inoculumc (log10 CFU/ml) | AUC0–24/MIC | Cmax/MIC |
|---|---|---|---|---|---|
| Mu-50 | D6 | 6.0 ± 0 | 4.94 ± 0.28 | 116.0 ± 7.6 | 11.7 ± 0.6 |
| D4 | 5.78 ± 0.39 | 5.23 ± 0.86 | 80.0 ± 3.7 | 7.7 ± 0.4 | |
| D3 | 6.61 ± 0.14 | 5.15 ± 0.07 | 93.0 ± 4.1 | 6.0 ± 0.3 | |
| A | 5.8 ± 0 | 6.91 ± 0.14 | 36.0 ± 1.8 | 4.0 ± 0.1 | |
| V | 6.55 ± 0.07 | 5.66 ± 0.56 | 53.0 ± 0.4 | 3.7 ± 0.3 | |
| G | 6.21 ± 0.14 | 8.35 ± 0.07 | 0.4 ± 0.01 | 0.04 ± 0.0003 | |
| D6+A | 5.95 ± 0.07 | 2.82 ± 0.35 | |||
| D4+A | 5.72 ± 0.35 | 2.5 ± 0 | |||
| D3+A | 5.85 ± 0.07 | 2.78 ± 0.21 | |||
| V+A | 6.25 ± 0.07 | 2.68 ± 0.21 | |||
| 992 | D6 | 5.35 ± 0.07 | 2.5 ± 0 | 112.0 ± 9.3 | 11.6 ± 0.7 |
| D4 | 5.62 ± 0.19 | 5.92 ± 0.85 | 80.0 ± 3.6 | 7.5 ± 0.4 | |
| D3 | 5.65 ± 0.26 | 2.99 ± 0.4 | 96.0 ± 3.7 | 6.3 ± 0.4 | |
| A | 5.8 ± 0 | 2.5 ± 0 | 521.0 ± 28.0 | 62.4 ± 1.4 | |
| V | 5.8 ± 0 | 5.6 ± 0 | 53.0 ± 0.5 | 4.4 ± 0.4 | |
| G | 5.51 ± 0.14 | 2.5 ± 0 | 96.0 ± 2.8 | 10.7 ± 0.08 | |
| MRSA-67 | D6 | 6.21 ± 0.14 | 2.61 ± 0.14 | 461.0 ± 30.2 | 48.0 ± 2.8 |
| D4 | 5.95 ± 0.07 | 2.82 ± 0.35 | 320.0 ± 14.9 | 30.4 ± 1.6 | |
| D3 | 6.11 ± 0.14 | 3.42 ± 0.35 | 371.0 ± 15.4 | 24.2 ± 1.2 | |
| A | 6.15 ± 0.07 | 2.5 ± 0 | 520.0 ± 28.8 | 65.4 ± 1.6 | |
| V | 6.35 ± 0.07 | 4.56 ± 0.56 | 834.0 ± 7.2 | 68.4 ± 5.4 | |
| G | 6.0 ± 0 | 2.5 ± 0 | 201.0 ± 5.7 | 21.6 ± 0.18 |
D6, daptomycin at 6 mg/kg q24h; D4, daptomycin at 4 mg/kg q24h; D3, daptomycin at 3 mg/kg q12h; A, arbekacin q12h; V, vancomycin q12h; G, gentamicin q12h; D6+A, daptomycin at 6 mg/kg q24h plus arbekacin q12h; D4+A, daptomycin at 4 mg/kg q24h plus arbekacin q12h; D3+A, daptomycin at 3 mg/kg q12h plus arbekacin q12h.
Counts of initial inocula (log10 CFU/milliliter) were taken at 0 h.
Counts of residual inocula (log10 CFU/milliliter) were taken at 48 h.
FIG. 2.
(A) Mu-50. D6, daptomycin at 6 mg/kg q24h (▵); D4, daptomycin at 4 mg/kg q24h (▿); D3, daptomycin at 3 mg/kg q12h (◊); A, arbekacin q12h (■); G, gentamicin q12h (○); V, vancomycin q12h (+); D6+A, daptomycin at 6 mg/kg q24h plus arbekacin q12h (▴); D4+A, daptomycin at 4 mg/kg q24h plus arbekacin q12h (▾); D3+A, daptomycin at 3 mg/kg q12h plus arbekacin q12h (⧫); V+A, vancomycin q12h plus arbekacin q12h (□); GC, growth control (●). (B) 992. D6, daptomycin at 6 mg/kg q24h (▵); D4, daptomycin at 4 mg/kg q24h (▿); D3, daptomycin at 3 mg/kg q12h (◊); A, arbekacin q12h (■); G, gentamicin q12h (○); V, vancomycin q12h (+); GC, growth control (●). (C) MRSA-67. D6, daptomycin at 6 mg/kg q24h (▵); D4, daptomycin at 4 mg/kg q24h (▿); D3, daptomycin at 3 mg/kg q12h (◊); A, arbekacin q12h (■); G, gentamicin q12h (○); V, vancomycin q12h (+); GC, growth control (●).
AUC0–24/MIC and Cmax/MIC of daptomycin were similar for GISA strains (Mu-50 and 992) with D6, D4, and D3 treatments. MRSA-67 daptomycin AUC0–24/MICs and Cmax/MIC were substantially higher than those for the GISA strains. Arbekacin AUC0–24/MICs were comparable for 992 and MRSA-67. However, the AUC0–24/MIC for Mu-50 was significantly lower, which was expected due to the higher MIC. Cmax/MICs of arbekacin were similar for 992 and MRSA-67 but significantly less for Mu-50. Vancomycin AUC0–24/MIC and Cmax/MIC were equivalent for both GISA strains, with higher values for MRSA-67. Gentamicin AUC0–24/MICs and Cmax/MICs were considerably variable between the three strains. No relationships were found for any regimen between AUC0–24/MICs and Cmax/MICs for any of the three organisms tested.
T > MIC of 100% was achieved for all daptomycin regimens. Arbekacin T > MIC was 100% against 992 and MRSA-67, although against Mu-50 T > MIC was only achieved 50% of the time. Vancomycin's T > MIC was 50% with the two GISA strains and 100% for MRSA-67. T > MIC for gentamicin was 100% for 992 and MRSA-67; however, against Mu-50, no time above the MIC was achieved.
The pH had a mean (± standard deviation) of 7.21 ± 0.07, 7.15 ± 0.11, 7.14 ± 0.07, and 7.08 ± 0.1 at 0, 8, 24, and 48 h, respectively, for all the daptomycin experiments.
Resistance.
There was no evidence of resistance developing to any of the regimens at four and eight times the MIC. This includes the daptomycin 4-mg/kg q24h treatment against 992, with which significant regrowth was seen.
DISCUSSION
Daptomycin, derived from Streptomyces roseosporus, is a potent semisynthetic lipopeptide antibiotic with a unique mechanism of action. It has a broad spectrum of activity against a variety of gram-positive organisms, including MRSA, vancomycin-resistant enterococci, penicillin-resistant Streptococcus pneumoniae, and GISA. Early clinical trials did have favorable results; however, less-than-desired outcomes were obtained in infections such as endocarditis when lower dosages of 2 mg/kg q24h and 3 mg/kg q12h were used (14). Daptomycin possesses a concentration-dependent killing and an extended postantibiotic effect of 3 to 6 h, which lends itself to once-daily administration. New dosage regimens of 4 and 6 mg/kg q24h have now been proposed for the treatment of moderate to severe infections. Our objective was to evaluate the bactericidal activity of daptomycin, with the newly proposed regimens, against GISA. In addition, we determined if any synergistic effect could be observed with the addition of an aminoglycoside, arbekacin, which possesses activity against gram-positive organisms that are normally resistant to gentamicin and tobramycin. Arbekacin has protection against the modifying enzymes AAC-6/APH-2" and ANT (4′)-1, which generally inactivate aminoglycosides (12).
Our data support the administration of daptomycin once daily, as there was a pronounced killing rate observed with no to slight regrowth. The development of resistance was not noted in any regimen, regardless of regrowth. The significance of regrowth observed in the in vitro model is unknown, especially since we observed no change in the MICs of vancomycin or daptomycin and the models lack an immune response, which is likely to contribute to this in vivo. The addition of arbekacin did significantly enhance the activity of daptomycin against the Mu-50 strain. However, this was not evident with the 992 strain, as daptomycin or arbekacin alone killed to our limit of detection. The diversity in subpopulations of these two isolates may explain the variation in killing activity since there were no apparent differences noted in the MIC or MBC. Previous work in our laboratory has shown that 992 is a fairly homogeneous strain (similar susceptibilities between subpopulations), while Mu-50 expresses a more hetergeneous susceptibility pattern (1). Interestingly, there was less kill noted when daptomycin was administered as a 4-mg/kg q24h dose compared to dosages of 6 mg/kg q24h or 3 mg/kg q12h against 992. This could be due to a total dose phenomenon since we repeated the experiment three times in duplicate, with similar findings. A mouse thigh infection model demonstrated that similar kill rates were obtained when the same total dose was administered in variable dosing schedules (A. Louie, P. Kaw, W. Liu, N. L. Jumbe, G. Vasudevan, M. H. Miller, and G. L. Drusano, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1770, p. 42, 1999).
The combination of daptomycin and arbekacin offers a potentially unique advantage. Daptomycin has been shown to have a protective effect on renal proximal tubular cells exposed to an aminoglycoside (2). This protective mechanism of action on aminoglycoside nephrotoxicity is still unknown. However, studies have suggested that daptomycin may interfere with the interaction between the aminoglycoside and phospholipids inside the lysosomes of the proximal tubular cells. In an evaluation of staphylococcal abscesses in rats, it was determined that there was less of an increase in serum creatinine and less cortical necrosis when daptomycin plus tobramycin was used than with tobramycin alone (21). Another animal study evaluating the protective effect of daptomycin on gentamicin-induced nephrotoxicity in rats found that daptomycin was detected within the lysosomes of the proximal tubular cells as early as 1 h after a single infusion (17). This combination resulted in less nephrotoxic effects (i.e., lower serum creatinine levels and less histopathologic change) for up to 20 days postinfusion of the drugs.
Although no statistical significance was obtained, there appears to be a trend of daptomycin pharmacodynamic parameters (AUC0–24/MIC and Cmax/MIC) having an association with decreased CFU/milliliter at both 24 and 48 h. However, a clear relationship could not be completely established due to the minimal differences in MICs. A rigorous pharmacodynamic evaluation testing multiple organisms requiring various MICs and variable doses and dosing intervals of daptomycin would be needed to fully characterize the pharmacodynamics of this drug. This relationship was confirmed recently by Louie et al., who compared multiple dosing regimens of daptomycin in mice and found that AUC/MIC is the parameter dynamically linked to outcome (Louie et al., 39th ICAAC).
In conclusion, we found that daptomycin alone or in combination with arbekacin results in significant kill against GISA (P ≤ 0.03). While little to no regrowth was observed with the homogeneous strain 992 for the 3-mg/kg q12h and the 6-mg/kg/day dosage regimens, Mu-50 demonstrated considerable regrowth after treatment with daptomycin. The addition of arbekacin resulted in synergistic activity with little to no Mu-50 regrowth. Therefore, daptomycin alone or in combination with arbekacin represents a viable alternative for treatment against GISA.
ACKNOWLEDGMENTS
This work was supported by a research grant from Cubist Pharmaceuticals, Inc., Cambridge, Mass.
We acknowledge Meiji Seika Kaisha, Ltd., for kindly supplying arbekacin powder. We also acknowledge Abbott Diagnostics for the use of the TDx analyzer for the assay of vancomycin, gentamicin, and arbekacin.
REFERENCES
- 1.Aeschlimann J R, Hershberger E, Rybak M J. Analysis of vancomycin population susceptibility profiles, killing activity, and postantibiotic effect against vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:1914–1918. doi: 10.1128/aac.43.8.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beauchamp D, Pellerin M, Gourde P, Pettigrew M, Bergeron M G. Effects of daptomycin and vancomycin on tobramycin nephrotoxicity in rats. Antimicrob Agents Chemother. 1990;34:139–147. doi: 10.1128/aac.34.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser J. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J Antimicrob Chemother. 1985;15(Suppl. A):125–130. doi: 10.1093/jac/15.suppl_a.125. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Interim guidelines for prevention and control of staphylococcal infection associated with reduced susceptibility to vancomycin. Morb Mortal Wkly Rep. 1997;46:626–628. , 635. [PubMed] [Google Scholar]
- 5.Flores P A, Gordon S M. Vancomycin-resistant Staphylococcus aureus: an emerging public health threat. Clevel Clin J Med. 1997;64:527–532. doi: 10.3949/ccjm.64.10.527. [DOI] [PubMed] [Google Scholar]
- 6.Hiramatsu K. The emergence of Staphylococcus aureus with reduced susceptibility vancomycin in Japan. Am J Med. 1998;104:7S–10S. doi: 10.1016/s0002-9343(98)00149-1. [DOI] [PubMed] [Google Scholar]
- 7.Hospital Infection Control Practices Advisory Committee. Recommendations for preventing the spread of vancomycin resistance. Morb Mortal Wkly Rep. 1995;44:1–13. [PubMed] [Google Scholar]
- 8.Lamp K C, Rybak M J, Bailey E M, Kaatz G W. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob Agents Chemother. 1992;36:2709–2714. doi: 10.1128/aac.36.12.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee B L, Sachdeva M, Chambers H F. Effect of protein binding of daptomycin on MIC and antibacterial activity. Antimicrob Agents Chemother. 1991;35:2505–2508. doi: 10.1128/aac.35.12.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreira B, Bolye-Vavra S, deJonge B L M, Daum R. Increased production of penicillin-binding protein 2, increased detection of other penicillin-binding proteins, and decreased coagulase activity associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:1788–1793. doi: 10.1128/aac.41.8.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. 1997. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 12.Obayashi Y, Fujita J, Ichiyama S, Hojo S, Negayama K, Takashima C, Miyawaki H, Tanabe T, Yamaji Y, Kawanishi K, Takahara J. Investigation of nosocomial infection caused by arbekacin-resistant, methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 1997;28:53–59. doi: 10.1016/s0732-8893(97)00005-9. [DOI] [PubMed] [Google Scholar]
- 13.Palmer S M, Rybak M J. An evaluation of the bactericidal activity of ampicillin/sulbactam, piperacillin/tazobactam, imipenem or nafcillin alone and in combination with vancomycin against methicillin-resistant Staphylococcus aureus (MRSA) in time-kill curves with infected fibrin clots. J Antimicrob Chemother. 1997;39:515–518. doi: 10.1093/jac/39.4.515. [DOI] [PubMed] [Google Scholar]
- 14.Rybak M J, Bailey E M, Lamp K C, Kaatz G W. Pharmacokinetics and bactericidal rates of daptomycin and vancomycin in intravenous drug abusers being treated for gram-positive endocarditis and bacteremia. Antimicrob Agents Chemother. 1992;36:1109–1114. doi: 10.1128/aac.36.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sieradzki K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J Bacteriol. 1997;179:2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenover F C, Lancaster M V, Hill B C, Steward C D, Stocker S A, Hancock G A, O'Hara C M, Clark N C, Hiramatsu K. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J Clin Microbiol. 1998;36:1020–1027. doi: 10.1128/jcm.36.4.1020-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thibault N, Grenier L, Simard M, Bergeron M G, Beauchamp D. Attenuation by daptomycin of gentamicin-induced experimental nephrotoxicity. Antimicrob Agents Chemother. 1994;38:1027–1035. doi: 10.1128/aac.38.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turco T F, Melko G P, Williams J R. Vancomycin intermediate-resistant Staphylococcus aureus. Ann Pharmacother. 1998;32:758–760. doi: 10.1345/aph.18017. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe T, Ohashi K, Matsui K, Kubota T. Comparative studies of the bactericidal, morphological and post-antibiotic effects of arbekacin and vancomycin against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1997;39:471–476. doi: 10.1093/jac/39.4.471. [DOI] [PubMed] [Google Scholar]
- 20.Wenzel R P, Edmond M B. Vancomycin-resistant Staphylococcus aureus: infection control considerations. Clin Infect Dis. 1998;27:245–251. doi: 10.1086/514646. [DOI] [PubMed] [Google Scholar]
- 21.Wood C A, Finkbeiner H C, Kohlhepp S J, Gilbert D N. Influence of daptomycin on staphylococcal abscesses and experimental tobramycin nephrotoxicity. Antimicrob Agents Chemother. 1989;33:1280–1285. doi: 10.1128/aac.33.8.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]