Abstract
Cancer is a leading cause of death worldwide. In many cases, the treatment of the disease is limited due to the metastasis of cells to distant locations of the body through the blood and lymphatic drainage. Most of the anticancer therapeutic options focus mainly on the inhibition of tumor cell growth or the induction of cell death, and do not consider the molecular basis of metastasis. The aim of this work is to provide a comprehensive review focusing on cancer metastasis and the mitogen-activated protein kinase (MAPK) pathway (ERK/JNK/P38 signaling) as a crucial modulator of this process.
Keywords: cancer, c-JUN N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), metastasis, mitogen-activated kinases (MAPKs)
1. Introduction
The overgrowth of cells in one specific site usually leads to the formation of primary tumors restricted to one location in the body. However, most patients die because of secondary tumors distant from the original tumor formation sites. The process of tumor metastasis is a complex interplay between the molecular characteristics of cancer cells that acquire certain properties (such as the ability to grow in unfavorable conditions, the capacity to spread across the body, or to produce the enzymes that digest the intracellular matrix) and the tumor microenvironment, which is subject to modifications that facilitate the journey of cells through the blood and lymphatic vessels [1]. All of these new capabilities arise from either genetic or epigenetic changes in the DNA of the transformed cells. Only the minority of tumor cells (subclones) acquire those abilities that enable their spread throughout the organism [2,3].
2. Cancer Metastasis Overview
The basement membrane (basal lamina, BM) is a 100–300 nm highly dynamic, ultrathin, and dense form of the extracellular matrix (ECM) that separates epithelia or endothelia from the connective tissues located beneath [4]. It consists of large glycoproteins and works as an adhesive scaffold providing strong structural support for other tissues. It also plays important role in cell signaling thanks to the presence of binding sites for cell adhesion molecules and ligands for cell surface receptors, including integrins and growth factor receptors, thus regulating cell functions including proliferation, differentiation, angiogenesis, and migration. BM sets apart tissues via adhesions mediated by hemidesmosomes consisting mainly of laminin that allow cell–matrix adhesion through the interaction with cell-surface integrins [5]. Integrins have a broad extracellular domain that interacts with ECM molecules, and an intracellular region that is connected to the cytoskeleton by intracellular focal adhesions (FAs). More than 150 proteins form FAs, including kinases, scaffold, and adaptor proteins. FAs are dynamic structures that assemble, scatter, and recycle during cell migration. The binding of integrins to FAs and ECM molecules not only allows for cell adhesion to the ECM, but also transfers cytoskeletal forces onto the ECM, allowing for cell migration as well as the transmission of signals from the extracellular environment to the intracellular pathways. The transmission is mediated by integrin-activated signaling molecules, such as focal adhesion kinase (FAK), phosphatidylinositol 3-kinase (PI3K), and extracellular signal-regulated kinase 1 (ERK1). Therefore, integrins are involved in adhesion, migration, and invasion. Integrins also aid in the progression of the metastatic process by causing the basement membrane to be proteolytically degraded by the activation of matrix metalloproteinases (MMPs). Furthermore, integrins are involved in the regulation of tumor cell motility through the transforming protein RhoA (RHOA) signaling pathway [6].
BMs encompass 60–200 different proteins and the composition of different BMs is greatly varied. However, four major components with different roles can be distinguished: type IV collagen, laminin, nidogen/entactin, and perlecan. Among others, agrin, bamacan, fibulin, fibronectin, type XV collagen, type XVIII collagen, nephronectin, netrins, SPARC/osteonectin/BM40, BM90, papilin, and usherin can be enumerated [4,7]. The detailed information on the composition of the BM can be found elsewhere: [8,9,10,11]. The composition of tumor BM is different than that of physiological BMs and its function is therefore altered. During the development of neoplasia, multistep spatial and temporal BM remodeling occurs [8]. This process is dynamic and involves de novo deposition and the integration of ECM proteins, their self-assembly, or their degradation [7].
Benign tumors do not cross the basement membrane, and therefore are unable to spread further. In contrast, metastatic tumor cells overexpress certain proteases, such as MMPs, which cleave the basement membrane components and allow the movement of tumor cells across the basement membrane and ECM. After the dissolution of the BM, cancer cells may enter the stroma. Moreover, local stromal cells, such as tumor-associated macrophages (TAM), may also produce MMPs and facilitate the movement of tumor cells [12]. Elevated MMP activity was observed in many tumor types, including osteosarcoma, in humans. Research indicates the key roles of MMP2 and MMP9 in the process of metastasis [13]. Moreover, the decreased activity of tissue MMP inhibitors is also often observed in metastatic cancers, allowing the enzymes to freely act on the ECM [14,15]. Cathepsins constitute another class of degrading enzymes with elevated activity observed in many cancers. Cathepsins act on many ECM components, including fibronectin, laminoprotein, and proteoglycans [16]. Moreover, the role of urokinase plasminogen activator (uPA) in metastasis was shown. uPA exhibits serine protease activity and is responsible for the conversion of inactive plasminogen to active plasmin. However, its activity is not restricted to one type of substrate as its proteolytic activity towards ECM and BM was found. The role of uPA in the process of metastasis is not only direct, as it activates other proteases including MMPs [17]. Furthermore, during remodeling, growth factors may be released. This includes vascular endothelial growth factor (VEGF), which stimulates the formation of new blood vessels and contributes to the improved access of cancer cells to oxygen and nutrients [7,18,19]. In the process called the invasion–metastasis cascade, tumor cells eventually violate the barrier, cross the blood or lymphatic microvessels, and spread through general circulation to other sites of the body. The invasion of cancer cells into the vessels is termed intravasation. The thickness of the blood vessel is the crucial factor influencing the ease of intravasation. Thus, small blood and lymph vessels are the major targets of cancer cells [20]. After the cells get into the bloodstream, circulating tumor cells (CTCs) encounter unfavorable conditions such as attacks from the immune system. CTCs often connect and travel as aggregates. Cells adhere either by homotypic adhesion (among cancer cells) or heterotypic adhesion (with other host cells, usually platelets). This prevents the CTCs from elimination as a result of the immune system’s activity (especially in the case of CTCs covered with platelets). Blood platelets are also usually a great source of growth factors that stimulate the growth of cancer cells, further enhancing their chance of survival in the bloodstream [21,22]. The loss of interaction with the ECM may however lead to anoikis, a form of cell death in detached anchorage-dependent cells. In addition, tumor cells become exposed to various environmental stresses, including the lack of oxygen or nutrients, the encounter with reactive oxygen species (ROS), and aforementioned insults from the immune response components [21,23]. However, in favorable conditions, some cells may leave the vessels (extravasate) and form micrometastases, which, over time, may colonize foreign tissues leading to the formation of macroscopic metastases. Cells are disseminated by the vascular and lymphatic drainage [24]. The lungs and the liver work as sophisticated filters of CTCs owing to their dense coverage in small vessels [25]. The colonization of cells may also result from the interactions of ligands (or receptors) on the surface of cancer cells and receptors (or ligands) of endothelial cells (this is also called the seed-and-soil hypothesis). According to the hypothesis, distinct cancer cells may exhibit different sets of ligands or receptors that are compatible with the ligands or receptors on the cells of different tissues [26,27,28]. In addition, some tissues may further attract cancer cells owing to the release of individual chemotactic factors [29]. The other mechanism for the colonization of cells in distinct locations assumes that CTCs may locate in a multitude of tissues wherein the growth of clinically significant metastatic cells is probably only in tissues that secrete factors that facilitate proliferation and colonization by cancer cells [30,31]. Many micrometastatic cells may stay dormant yet viable for an extended time [32]. However, during evolution, cancer cells may adapt to the new environment, colonize foreign tissues, and form a tumor. After achieving a critical size of the tumor mass, the cancer cells need to form a network of new blood vessels to further supply the cells with nutrients, oxygen, and growth factors [33,34].
3. Molecular Basis of Metastasis
In all cases, tumor formation starts with the initiating mutation that confers the unlimited proliferative potential of cells, followed by the development of genetic instability that leads to autonomic transformed cells. However, the oncogenic transformation is not sufficient for cells to acquire the ability to enter systemic circulation and infiltrate distant tissues. Moreover, the cells need to survive in the new environment and colonize the foreign tissue. The genes that contribute to all these events can be classified into several classes: (a) metastasis initiation genes, (b) metastasis progression genes, and (c) metastasis virulence genes. The first class of genes (initiation genes) allows tissue invasion and their dispersion. This class includes various factors that contribute to angiogenesis, cell motility, or invasion. Progression genes, on the other hand, facilitate tumor development at the site of metastasis. Similar to oncogenes, progression genes contribute to tumorigenesis; however, during the metastatic process they play additional, advantageous functions that enable the tissue-specific spread. The classical examples of these include MMPs. Metastasis virulence genes are responsible for the aggressive potential of tumor cells not in the primary site, but in the secondary site. It is, however, often difficult to classify a gene to a specific class of metastasis genes as their functions usually overlap [35].
So far, hundreds of genes have been shown to contribute to the invasive potential of cells. Mutations in genes encoding the cellular tumor antigen p53 (TP53), cyclin-dependent kinase inhibitor 2A (CDKN2A), phosphatase and tensin homolog (PTEN), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α (PIK3CA), retinoblastoma (RB1), GTPase KRas (KRAS), estrogen receptor (ESR1), MYC proto-oncogene protein (MYC), and serine/threonine-protein kinase B-raf (BRAF) were linked with the tumor metastasis process. However, metastatic research focuses mainly on gene expression changes during the metastatic process, rather than the exploration of specific gene mutations [36].
To acquire the abilities needed to form metastases, epithelial stem cells or differentiated epithelial cells must undergo the epithelial–mesenchymal transition (EMT). The whole EMT process is mediated by various transcription factors involved in developmental programs such as twist-related protein 1 (TWIST), zinc finger proteins SNAI1/2 and SLUG, zinc finger E-box-binding homeobox 1/2 (ZEB1/2), forkhead box protein C2 (FOXC2), or paired mesoderm homeobox protein 1 (PRRX1) [37,38]. The expression levels of noncoding RNAs such as microRNAs and long non-coding RNAs have also been found to change during the EMT process, suggesting that they may play a role in the process [39,40,41,42,43]. The epigenetic regulation, chromatin remodeling, alternative splicing, posttranslational modifications, stabilization, and altered subcellular localization of proteins all contribute to the EMT [44].
Cadherins are a transmembrane glycoprotein superfamily that mediates the adhesion between homophilic (same type of cells) cell to cell adhesions. More than 20 members of the cadherin molecule family have been identified, each of which is expressed differently depending on the cell type. A non-covalent interaction occurs between two cadherins of the same kind belonging to nearby cells results in their holding together [6]. The loss of E-cadherin is mainly due to genetic mutations in the CDH1 gene that promote the synthesis of altered protein, CDH1 epigenetic silencing through promoter methylation, and SRC family kinase-mediated downregulation of the E-cadherin gene, or the inhibition of CDH1 expression by transcriptional repressors [45,46]. A functional E-cadherin–catenin complex not only stabilizes cell–cell adhesion, but also activates downstream signaling pathways such as RHO GTPases, PI3K, and MAPK [6].
During the EMT, cells change polarity and cytoskeleton organization is altered. The downregulation of an epithelial gene expression signature is one of the main hallmarks of the EMT. This includes the diminished expression of cell junction proteins such as vascular endothelial cluster of differentiation 31 (CD31; PECAM1), claudins, occludins, desmoplakin, and plakophylins [47]. While the loss of E-cadherin and cytokeratins is observed in the EMT, the expression of vimentin and neural cell adhesion molecule (NCAM) becomes upregulated. NCAM interacts with the SRC family tyrosine kinase FYN and contributes to cell invasion and migration [47,48]. Furthermore, cells begin to produce fibronectin, an extracellular matrix protein, normally secreted only by mesenchymal cells [49]. Additionally, the expression of N-cadherin may increase the affinity of cancer cells to stromal cells [50,51]. Through connection with α-catenin and β-catenin, N-cadherin attaches to the cytoskeleton and, via p120 catenin, influences receptor tyrosine kinases (RTKs) activity [47]. Reorganization of the cytoskeleton, gain of front–rear polarity, and the dissolution of the epithelial cell-to-cell junctions are therefore prominent hallmarks of the EMT. During the EMT, reorganization of the cortical actin cytoskeleton occurs enabling dynamic cell elongation and motility [52].
Two main mechanisms of cell movement can be distinguished: amoeboid and mesenchymal. The mesenchymal type of movement is dependent on protease (MMPs) activities that enable the movement through the degradation on ECM, while the amoeboid type is a protease-independent process and relies on mechanical forces exerted by the cells on the ECM. Both types of movements are controlled by the signaling pathways of the RHO family of small GTPases that control actin dynamics and their rearrangement during cell migration in response to environmental stimuli. In the mesenchymal type of movement, cells attain a specific elongated spindle-like shape and resemble fibroblasts in shape. RHOA facilitates actin stress fiber formation, whereas Ras-related protein (RAC1) and cell division control protein 42 homolog (CDC42) promote the formation of lamellipodia (network of the actin filaments) and filopodia (rod-like projections of actin fibers) [52]. The activity of Rho GTPases is regulated by guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (GDIs). RHO-associated kinase (ROCK) interacts with formin diaphanous 1 (DIA1) and facilitates actin polymerization. Furthermore, ROCK inactivates myosin-light-chain phosphatase (MLCP), which normally dephosphorylates myosin II light chain (MLC2). The phosphorylation of MLC2 leads to the augmented activity of myosin II ATPase, facilitating its interaction with actin filaments to confer cell contraction. Phosphorylated MLC2 is negatively regulated by myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK). Furthermore, ROCK1 is negatively regulated by RHO-related GTP-binding protein (RHOE). In contrast, 3-phosphoinositide-dependent protein kinase 1 (PDK1) promotes ROCK1-dependent actomyosin filament formation. PI3K is a key regulator of front–rear polarity and is involved in the recruitment of CDC42 and RAC GEFs to the leading edges of the moving cells. RAC and CDC42 regulate actin polymerization via the regulation of Wiskott–Aldrich syndrome protein (WASP) and its interaction with Arp2/3 complex 34 kDa subunit (ARP2/3) [47,52].
In the amoeboid type of migration, rounded cells move via constant cycles of expansion and contraction of the cell body. This is allowed by actin and myosin, which localize cortically and contribute to membrane blebbing. Blebbing results from the inflow of cytoplasm. In contrast, in lamellipodia, actin polymerization is the underlying cause of changes and movement. Again, the RHO/ROCK signaling pathway regulates the process of migration [6,52]. In summary, invadopodia, lamellipodia, filopodia, podosomes, and are actin-rich membrane protrusions formed by metastatic cells. To migrate and invade, protrusions use mechanical forces and proteases. They are either the cell’s sensory organelles (filopodia) for signals such as chemoattractants, or the major organelles for cell motility (lamellipodia), or both allowing motility and ECM degradation (invadopodia). Unlike single-cell migration, collectively migrating cells maintain cell–cell connections by continuously expressing adhesion molecules. Cells may travel as sheets, strands, tubes (coordinated invasion), or clusters (cohort migration). Collective cell migration involves force creation to pull or push cells forward or backward. Substrate-binding integrins generate the energy required for motility. Integrins are expressed on the leading edges of cells to form adhesion complexes with ECM components such as fibronectin. ECM attachment stimulates cytoskeletal adaptor proteins, such as cortactin, vinculin, paxillin, and talin. Migrating cells develop membrane protrusions and integrin-mediated focal adhesions connected to the actin cytoskeleton. Cells also express MMPs at their leading edges to split collagen fibers and arrange them in tube-like structures to travel in the cell mass [6].
MMPs constitute a family of 24 endopeptidases that regulate the ECM composition through proteolytic activity. Based on their structure and function, MMPs can be classified into eight groups encompassing MMPs either secreted or membrane-bound. Due to their proteolytic function, the activity of MMPs must be tightly controlled. This is achieved through strictly controlled transcriptional and translational events. Moreover, MMPs are expressed as proenzymes or zymogens that need to be activated by “cysteine switch” mechanisms and autocatalysis reactions in which the enzyme cleaves its prodomain and gains catalytic activity. Furthermore, as previously mentioned, the activity of enzymes is controlled by four tissue inhibitors of metalloproteinases (TIMPs) that impede their enzymatic function [53]. Due to their proteolytic activity, MMPs have been suggested to play a critical role in all of the metastatic steps from the invasion through ECM degradation [54,55,56], angiogenesis [54,57,58], immune evasion [59,60,61,62], the establishment of premetastatic niche [63,64], extravasation [65], to proliferation and survival in the new environment [66,67,68]. Thus, the inhibition of MMPs may represent a new strategy in targeting metastasis. Despite the obvious roles of MMPs in tumor metastasis, continuous efforts fail to implement MMPs inhibitors into the clinic. This is attributed to the difference between murine and human biology, e.g., differences in lifespan, differences in tumor growth and size without metastatic spread, or the use of genetically homogenous cancer cells in the bolus injection compared with the greater heterogenicity of tumor cells in humans. These factors, together with the lack of the specificity of inhibitors and the potential side-effects, have restricted the use of MMP inhibitors in cancer treatment. Moreover, MMPs act early in the development of metastasis; thus, certain actions, such as MMP expression profiling, should be performed in the pre-and peri-metastatic stages. Both antibodies and small-molecule inhibitors targeting MMPs with varied affinity, specificity, and selectivity have been developed [53].
4. Tumor-Associated Macrophages (TAMs) and Cancer-Associated Fibroblasts (CAFs)
It is now well established that cancer cell proliferation and metastasis require interaction with the tumor milieu, which is also referred to as the tumor microenvironment (TME). Tumor interactions with ECM components are critical for the EMT and tumor invasion. TME is engaged in all four steps of the metastatic process: adhesion, detachment, migration, and invasion. TME contains tumor stroma cells, immune system effectors, platelets, fibroblasts, endothelial cells, proteases, cytokines, hormones, and other humoral components [6].
Cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs) and are the primary components of invading stromal cells. CAFs are a heterogeneous cell population. Every tumor type comprises subpopulations of CAFs that are distinct to the tumor and the surrounding tissue. The activation of CAFs is related to the development of a tumor with invasive characteristics. Cancer progression has been linked to TAMs and CAFs, which have been implicated in the RAS and MAPK signaling cascades. Moreover, CAFs promote primary tumor development via the secretion of cytokines such as interleukin 1 (IL-1) [6,69].
In contrast, TAMs may promote the secretion of various factors associated with tumor progression and metastasis, including tumor necrosis factor α (TNF-α), IL-6, transforming growth factor α (TGF-α), platelet-derived growth factor receptor (PDGFR), and vascular endothelial growth factor receptor (VEGFR). These factors can typically affect the EMT, encouraging tumor growth and metastasis. For example, cholangiocarcinoma cells recruit CAFs by secreting platelet-derived growth factor D (PDGF-D), boosting cell migration through PDGFR-β, RHO-GTPase, and c-JUN N-terminal kinase (JNK) pathway activation [70]. TAMs can release a wide range of growth factors, proteolytic enzymes, cytokines, and inflammatory mediators when activated by cancer cells. TAMs can promote cancer metastasis via the stimulation of angiogenesis, proliferation, migration, and invasion [71].
Gene expression profiles in metastatic cancers resemble those that allow the physiological function of macrophages or stem cells. Many changes that are thought to account for the EMT may also occur in non-metastatic tumors. Accumulating evidence suggests that other mechanisms, such as the alteration of stem cells, may lead to the development of metastatic cancer cells. Particularly, hematopoietic stem cells or yolk sac-derived macrophages comprise a valid source of metastatic cells (myeloid cell hypothesis). There are many similarities between immune cells and metastatic cells, including the ability to intravasate and extravasate from vasculature, the release of pro-angiogenic factors such as EGF, FGF or PDGF, the capability to survive in hypoxic or necrotic conditions, the expression of cathepsins and the downregulation of E-cadherin. Moreover, when inflammation occurs, macrophages can fuse with epithelial cells and exhibit both epithelial and mesenchymal properties further supporting this hypothesis [33].
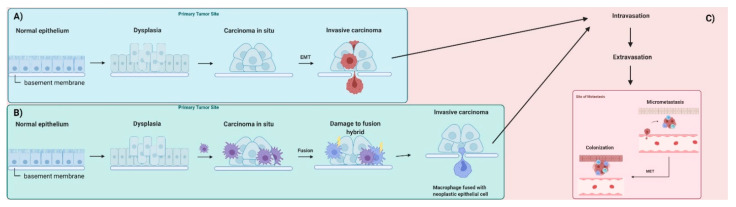
In this hypothesis, macrophage infiltration of carcinoma in situ generates a chronic inflammatory environment that enhances fusion between immune cells and neoplastic epithelial cells. Inflammation leads to the impairment of mitochondrial function and mitochondrial damage, facilitating the use of fermentation as a primary energy source. The emerging hybrids exhibit the properties of mesenchymal cells, similar to those observed for macrophages, such as the ability to intravasate and extravasate. Conventional EMT and the fusion hybrid hypothesis of tumor metastasis are shown in Figure 1 [33].
Figure 1.
Overview of tumor metastasis. (A) Conventional model of metastasis. Normal epithelia are lined with a basement membrane that separates epithelia or endothelia from the connective tissues located beneath. Epithelial cells proliferate and become dysplastic. Accumulation of genetic and epigenetic changes leads to the formation of carcinoma in situ also lined with the basement membrane. During epithelial–mesenchymal transition (EMT), cells acquire abilities that allow them to move through the basement membrane. (B) The metastatic process according to the fusion hybrid hypothesis. Metastatic cancer cells are formed as a result of direct transformation or as a result of fusion of neoplastic epithelial cells and myeloid cells such as macrophages. (C) Metastatic cancer cells intravasate into small blood (shown here) and lymph vessels, where they travel with circulation to distant sites. At secondary sites, carcinoma cells extravasate, form micrometastases, and in the process of mesenchymal–epithelial transition (MET) colonize the foreign tissue (macrometastasis). Based on [33].
5. Cancer Stem Cells in Metastasis
Despite the fact that the presence of cancer stem cells (CSCs) is still widely debated, the CSC hypothesis is of significant clinical significance, because it has the potential to explain tumor resistance to chemotherapy, cancer progression, and recurrence in cancer patients [72,73]. CSCs or tumor-initiating cells (TICs) represent a sub-population of cells that exhibit self-renewal ability and the capacity to differentiate. The undifferentiated state is maintained through the transcriptional regulation of four major transcription factors: SOX2, OCT4, and NANOG [74,75,76,77]. Moreover, compared with normal stem cells, CSCs are highly clonogenic. When a self-renewing stem cell divides, it can choose one of two important cell division fates: symmetric division, which results in the formation of two daughter stem cells; or asymmetric division, which results in the formation of a stem cell and a differentiated daughter cell. Mutations in tumor suppressors that are involved in the restriction of asymmetric stem cell division equip cancer stem cells with mechanisms to hyperproliferate [78,79,80]. Moreover, it was found that cancer cells can undergo the EMT following stimuli from the cells in the tumor environment, which subsequently leads to the formation of cells with features similar to CSCs [81]. Moreover, the conversion of non-stem cancer cells to CSCs, such as in the case of trastuzumab resistance resulting in CSC proliferation, occurs predominantly as a result of IL-6 release by CSCs in the tumor microenvironment. IL-6 is also capable of inducing the expression of other cytokines that are beneficial to CSCs development [82,83,84,85]. Furthermore, through juxtacrine signaling or contact-dependent signaling, TAMs may aid in the formation of a CSC niche [86]. Research indicates that CAFs produce 100 times more interleukin-6 (IL-6) than nonmalignant tissue associated fibroblasts, which can actually enhance the invasive characteristics of the cells [87]. The acquisition of mesenchymal features may help cells to disseminate from primary tumor mass. Likewise, cells may undergo the mesenchymal–epithelial transition (MET) to the epithelial state at the site of metastasis [88,89,90].
CSCs emergence is strongly associated with the development of resistance mechanisms to anticancer agents. Chemoresistance of CSCs and their continuous persistence following treatment with anticancer drugs is associated with a high risk of metastasis and the lower survival rates of patients. There are numerous factors that influence the incidence of CSCs resistance. These include interactions of CSCs with microenvironment components, epigenetic alterations occurring in CSCs (disruptions in DNA methylation, nucleosome remodeling, histone modifications, and non-coding RNAs), enhanced drug efflux due to the upregulation of efflux proteins (such as glycoprotein P) and drug inactivating enzymes, enhanced DNA repair and the development of mechanisms that prevent apoptosis and induce quiescence or dormancy [91].
The deregulation of self-renewal pathways implicated in the development of stemness features is considered a hallmark of CSCs. Recent studies have discovered that targeting of these signaling pathways in CSCs is of particular relevance [92]. Despite their importance in embryonic organogenesis and adult homeostasis, the aberrant activation of these pathways promotes a number of features associated with tumorigenesis, including the growth of tumors. These have been extensively reviewed by others, as in the case of Hedgehog [72,93], NOTCH [94,95], and WNT signaling [96,97].
Many studies have revealed important connections between CSCs, tumor development, and anticancer therapy resistance, highlighting the need for novel therapeutic options that specifically target this aggressive cancer subpopulation. A variety of surface markers are available today that can be used to recognize CSCs by directly targeting the antigens present in these cells. When compared to non-neoplastic stem cells and somatic cells, the expression profiles and sub-localizations of these markers in cancer stem cells are significantly different. Apart from molecular markers, various of analytical procedures and techniques are utilized to identify cancer stem cells. These methods and techniques range from functional tests to cell sorting and filtration strategies or xenotransplantation methods [98]. Several approaches based on CSC targeting may be effective in improving clinical responses to systemic therapy. These rely on CSC ablation with monoclonal antibodies, the inhibition of CSC function, the reversal of CSC-associated resistance mechanisms, and the induction of CSC differentiation with epigenetic differentiation therapy [99]. Small molecule inhibitors, vaccines, antibodies, and chimeric antigen receptor T cell cells (CAR-Ts), are currently being tested in clinical trials to assess their effectiveness and safety [81,100].
6. Major Metabolic Adaptations of Metastatic Cells
As previously mentioned, wandering cells may experience unfavorable conditions such as hypoxia. Thus, cells must undergo metabolic adaptations to survive in low-oxygen environments. One of the crucial factors that determine survival in hypoxic conditions is hypoxia-inducible transcription factor α (HIF1α), which works as a transcription factor for a number of genes involved in anaerobic metabolisms, angiogenesis, and metastasis [35,46]. While the HIFα subunit is targeted for degradation by the von Hippel–Lindau tumor suppressor (VHL) under normoxic conditions, the occurrence of hypoxia results in the translocation of the HIFα subunit to the nucleus and its association with constitutively expressed HIFβ subunit, which results in the activation of expression of various genes containing a HIF-responsive element (HRE). These include carbonic anhydrases, glucose transporters, and VEGF [35,46,101,102]. Additional studies have shown that VEGF promotes endothelial cell survival by activating the phosphotyrosine 3-kinase/RAC-alpha serine/threonine-protein kinase (PI3Ks/AKT) signal transduction pathway and increasing the expression of the anti-apoptotic protein BCL-2 [103].
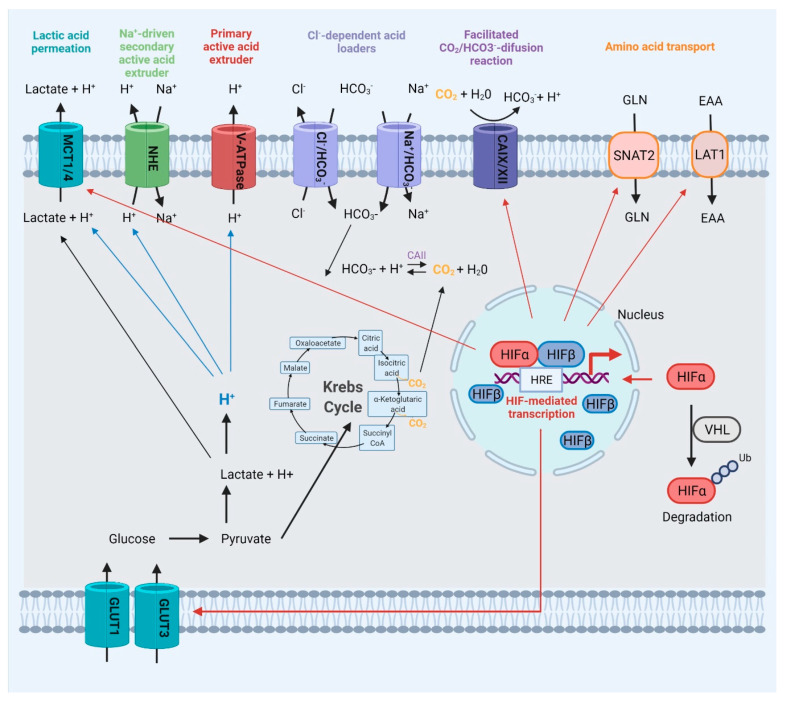
Restricted access to oxygen supply implies heavy energetic reliance of cancer cells on lactate produced in anaerobic respiration [104,105]. Cancer cells have been found to overexpress the monocarboxylate transporters 1 and 4 (MCT1 and MCT4), as well as the glucose transporters 1 to 3 (GLUT1–GLUT3), all of which contribute to cell survival under stress conditions. MCTs are responsible for the transport of monocarboxylic acids (such as lactate, pyruvate, and ketone bodies) into and out of cells through the plasma and mitochondrial membranes. These proteins have a fundamental function in regulating the efflux of lactate and protons produced as byproducts of glycolysis from the intracellular to extracellular space. As a result, they contribute to extracellular acidosis. GLUT1–3, on the other hand, control the uptake of glucose by the cells, which is then transformed to pyruvate, resulting in the production of two ATP molecules. The cytoplasmic pH is regulated by five primary protein families. H+ transporters, such as MCT transporters, sodium hydrogen ion exchangers (NHE), as well as vacuolar-type H+-adenosine triphosphatases (V-ATPases) that transport H+ across membranes, and chloride–bicarbonate exchangers. The sodium-coupled bicarbonate transporters (SLC4 and SLC6) control the intake of bicarbonate used to titrate intracellular H+. Furthermore, carbonic anhydrases are responsible for pH regulation by catalyzing the reversible hydration of CO2 [106,107,108,109,110] (Figure 2).
Figure 2.
Major metabolic adaptations of cancer cells during metastasis with special focus on ways by which cancer cells avoid repercussions resulting from hypoxia and lack of nutrients. Hypoxia-inducible transcription factor α (HIF1α) is a transcription factor for many genes involved in anaerobic metabolisms, angiogenesis, and metastasis. HIFα subunit is targeted for degradation by the von Hippel–Lindau tumor suppressor (VHL) under normoxic conditions; however, the occurrence of hypoxia allows the translocation of the HIFα subunit to the nucleus and formation of transcription factor complexes with constitutively expressed HIFβ subunit. The transcription factor regulates the expression of various genes containing HIF-responsive elements (HRE) such as carbonic anhydrases, glucose transporters, and vascular endothelial growth factor (VEGF). MCT1–MCT4 are proton-coupled monocarboxylate (lactate, pyruvate, and ketone) transporters. Sodium hydrogen ion exchangers (NHE) regulate the intracellular pH regulation via electroneutral, 1:1, exchange of Na+ and H+ along their gradients. Vacuolar-type H+ adenosine triphosphatases (V-ATPases) transport H+ across membranes in an active process. Carbonic anhydrases (CA) are zinc metalloproteins that catalyze the reversible hydration of CO2 to form HCO3− and H+. CO2 is also the main byproduct of the Krebs cycle and may be generated from HCO3− in reactions catalyzed by CAII. HIF transcription factor governs the expression of MCT1/4, glucose transporters (GLUT1/3), amino acid transporters—sodium-coupled neutral amino acid transporter 2 (SNAT2), large neutral amino acid transporter 1 (LAT1), and CAIX/CAXII. Cl−/HCO3−—chloride bicarbonate anion exchangers; Na+/HCO3−—sodium-dependent bicarbonate cotransporters.
Furthermore, glutamine absorption and glutaminolysis are critical for the survival of some tumor cells. Carbon used for the tricarboxylic acid cycle, glutathione production, and nucleotide and lipid synthesis is provided from glutamine via reductive carboxylation. Moreover, glutamine is essential for the supply of reduced nitrogen for biosynthetic pathways. Even though mammalian cells are capable of glutamine production due to the activity of glutamine synthetase (GLUL), some cancer cells require exogenous glutamine, which is catabolized in the mitochondria by another enzyme—glutaminase (GLS) [111]. HIF transcription factor regulates the expression of amino acid transporters—sodium-coupled neutral amino acid transporter 2 (SNAT2) and large neutral amino acid transporter 1 (LAT1) engaged in the transport of glutamine for enhanced growth [112] (Figure 2).
Additionally, CSCs were shown to rewire the energy metabolism to meet the demands of self-renewal and stemness maintenance. Growing data support the fact that changes in lipid metabolism, such as an increase in fatty acid (FA) consumption, de novo lipogenesis, and the stimulation of mitochondrial FA oxidation, are involved in the control of CSC function. The role of lipid metabolism in CSCs was recently reviewed by other authors [113,114].
Cell detachment triggers anoikis, characterized by the simultaneous downregulation of anti-apoptotic protein BCL-xL and the upregulation of FAS ligand that works as a death receptor pathway activator. Therefore, CTCs incorporate mechanisms to avoid anoikis and apoptotic cell death during metastasis. This includes the downregulation of caspase 8 (CASP8) that promotes cell dissemination and survival, and alterations in the signaling pathways that regulate anoikis including small GTPases, various protein kinases, and EMT factors [35,115].
7. The Signaling Pathways in Metastasis
RTKs play a pivotal role in the regulation of various cellular activities including differentiation proliferation, and migration. The ErbB family of receptors includes four receptors, ErbB-1 (EGFR), ErbB-2 (HER2 or NEU), ErbB-3, and ErbB-4. Similar to other receptor tyrosine kinases, epidermal growth factor receptor (EGFR) is composed of extracellular, transmembrane, and intracellular regions. The extracellular region contains domains responsible for ligand binding. Many EGFR ligands have been identified so far. These include amphiregulin, betacellulin, epidermal growth factor (EGF), epiregulin, and transforming growth factor α (TGF-α). Some of them bind directly with the receptor, while others need to be processed in proteolytic cleavage reactions that allow their effective binding [116,117]. On the other hand, the transmembrane domain anchors the receptor in the phospholipid bilayer of the cell, while the intracellular domain possesses catalytic activity. The binding of the ligand triggers homo- or hetero-dimerization with other ErbB family members and leads to subsequent transphosphorylation of intracellular domains, which generates the binding sites for many adaptor proteins involved in signaling events. EGFR receptors form complicated signaling networks with other signaling pathway components including proteins involved in the mitogen-activated kinase MAPK (RAS/RAF/MAPK), phosphatidylinositol 3-kinase (PI3K/AKT), protein kinase C (PKC), and the JAK/STAT pathways. Moreover, EGFR has been shown to translocate to the nucleus, where it controls the expression of various genes including CCND1, coding for cyclin D1 involved in cell cycle progression. For example, EGFR is overexpressed in 80–90% of aggressive head and neck squamous cell carcinomas and is associated with poor survival [118,119].
Several studies have demonstrated that the expression levels of EGF and EGFR are associated with tumor progression, metastasis formation, and resistance to treatment [120]. EGF may play an important role in the acquisition of metastatic properties in gallbladder cancer (GBC). Sasaki et al. reported that EGF may promote the EMT and the development of stemness in GBC cells with a scattering phenotype by increasing the activity of β-catenin in these cells [121]. Experiments on human colon cancer cells in vitro have revealed that metastatic cells may express up to five times more EGFR than nonmetastatic cells. This influences the aggressiveness of the tumor cells [122]. Moreover, research indicates the intrinsic relationship between transforming growth factor α (TGFα) and EGFR [123]. The findings provide evidence for the notion that some cells may rely on TGF-induced EGFR activation and promote IL-8 production and, thus, together with VEGF, stimulate the formation of new blood vessels to enhance the survival of cancer cells and promote their metastatic potential [124]. Since Virchow’s hypothesis, the complex link between cancer and chronic inflammation has been carefully studied. Through the years it was established that inflammation regulates cancer progression [125]. IL-8 and VEGF are both expressed at high levels in a variety of tumors, and they have been shown to enhance tumor angiogenesis, growth, and metastasis. To promote the creation of new blood vessels, ERK1/2 can be employed as an alternate mechanism to stimulate the expression of IL-8 and VEGF. Moreover, it has been shown that the activation of the ERK/MAPK signaling pathway is involved in the promotion of VEGF production in colorectal cancer [126]. VEGF-C contributes to enhanced skin cancer migration, invasion, and stemness via upregulation of SLUG transcription factor in KRAS/MAPK-dependent signaling events [127]. Several members of the MMP family, especially MMP2 and MMP9, were shown to be highly expressed in tumors that were positive for TGFα [124]. Moreover, the expression of TGFα may be the major determinant of response to treatment with EGFR inhibitors [128,129]. Many studies were performed to determine the efficiency of dual inhibition of EGFR and VEGFR. Experiments in murine models have shown that the treatment is effective and reduce both the growth and metastasis in nude mice model of orthotopic human colon cancer [130,131]. Moreover, the use of combination therapy with EGFR, VEGFR [132], and PDGFR inhibitors together with gemcitabine constitutes promising therapy for pancreatic cancer with strong (80–95%) inhibition of tumor growth and protracted survival in the orthotopic nude mouse model [132,133]. Dual inhibition of EGFR and VEGFR was also tested in colon cancer [134], cutaneous squamous cell carcinoma [135], follicular thyroid cancer [136], oral cancer [137], orthotopic ovarian carcinoma [138], and prostate cancer [139,140]. EGFR is currently a key target in anticancer drug discovery. As a result, monoclonal antibodies such cetuximab, panitumumab, nimotuzumab, necitumumab, and others have been introduced into the clinic. There are two basic techniques to combine chemotherapeutic agent toxicity and the precise targeting of EGFR overexpressing tumor tissues: antibody–drug conjugates (ADCs) and antibody–nanoparticle conjugates (ANCs). ADCs are antibodies covalently attached to a cytotoxic agent via a spacer. ADCs undergo chemical and enzymatic processing, resulting in the release of the drug upon binding to EGFR, and endocytosis. ANCs are nanotechnology-based formulations and include lipid, polymeric, and inorganic nanoparticles that preserve drugs against inactivation, allowing regulated release and passive accumulation in tumor tissues through increased permeability and retention. EGFR receptor-mediated endocytosis and the formation of lysosomes results in the release of drug into the cytosol. This topic was reviewed previously in [141]. EGFR is the most well-known growth factor receptor that interacts with G protein-coupled receptors (GPCRs) and is involved in the regulation of tumor development, invasion, and progression in a variety of malignancies [142].
The class of surface receptor proteins known as GPCRs regulates a wide range of cellular functions and has been identified as a potential treatment target for several diseases including cancer. GPCRs are activated by hormones, neurotransmitters, growth factors, or light. Additionally, GPCR ligands such as prostaglandins, bradykinin, and gastrin-releasing peptide can also transactivate EGFR to promote cancer cell proliferation, survival, and invasion. PI3K/AKT, PDK1, and MMPs are some of the essential signaling intermediates involved in GPCR–EGFR crosstalk [142,143,144].
Instead of a single G protein component such as RAS, G protein-coupled GPCRs have three G protein subunits that attach to the plasma membrane via Gα and Gγ subunits. The Gα subunit also binds to GTP (active protein) or GDP (inactive protein) upon activation of GPCR. Heterotrimeric G proteins dissociate into Gα monomers and Gβ–Gγ dimers, relaying the message to downstream signaling partners. Through the modulation of several biological pathways, they have the potential to either suppress or accelerate tumor development, survival, dissemination, and metastasis. GPCRs may also upregulate mesenchymal transcription factors such as the SNAI, ZEB, and TWIST superfamilies, which regulate cell polarity, cytoskeleton remodeling, migration, and invasion, through modulation of downstream signaling pathways such as the nuclear factor kappa B subunit (NF-κB), MAPK/ERK, PI3K/AKT, and TGF-β pathways [142].
The transforming growth factor-β (TGFβ) family constitutes a group of homo- and heterodimeric secreted cytokines that control the multitude of cellular functions including proliferation and differentiation of cells. All cell types express TGF-β receptors responsible for signal transduction upon ligand binding. Type I and II receptors transduce signals through their intrinsic ATP-dependent protein kinase activity. Upon ligand binding, constitutively active type II receptor (TβRII) recruits TGF-β type I receptor (TβRI). TβRII activates the TβR1 via phosphorylation of its regulatory segment called the GS region. The binding of the ligand leads to the recruitment and phosphorylation of mothers against decapentaplegic (SMAD) family proteins: receptor-activated SMADs (R-SMADs) and receptor-regulated SMADs—SMAD2 and SMAD3. R-SMADs associate with SMAD4 and SMAD2/3-SMAD4 complexes, translocate to the nucleus, and act as transcription factors. The whole process is tightly controlled by SMAD6 and SMAD7, which are also called inhibitory SMADs (I-SMADs). However, as with the other signaling pathways discussed in this review, the complexity of the regulation of signal transduction is beyond the scope of this work. Thus, we refer the reader to other reviews on this topic [145].
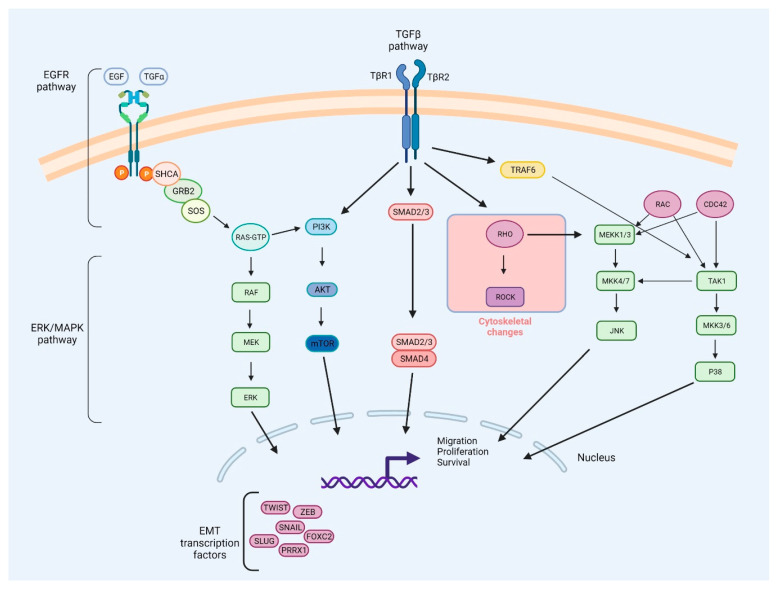
TGFβ signaling plays a dualistic role in cancer. This is due to a versatility of function of TGFβ signaling, such as the influence on ECM remodeling, control of tumor cell interaction with the microenvironment, transcriptional regulation, or angiogenesis. On the one hand, due to the SMAD-dependent downregulation of c-MYC, cyclin-dependent kinases (CDKs), and M-phase inducer phosphatase 1 CDC25A (CDC25A), TGFβ may restrict the proliferation of cells [146]. On the other hand, it may act as a tumor growth and metastasis promoter. This turnover is complex, but may be attributed to the phosphoserine/phosphothreonine-binding protein 14-3-3ζ that induces the tumorigenic activity of TGF-β. It emerged that 14-3-3ζ may stabilize glioma-associated oncogene homolog 2 (GLI2), a partner of SMAD complexes, and induce the expression of the parathyroid hormone-related protein (PTHrP) which, together with TNF-α and cytokines, stimulates osteoblasts to release tumor necrosis factor ligand superfamily member 11 (RANKL). RANKL works as a ligand for the tumor necrosis factor superfamily member 11 (RANK), which enhances osteoclast differentiation, further supporting bone demineralization and release of factors that enhance cell proliferation [46,147]. Moreover, the activation of TGFβ stimulates the expression of SNAI and downregulates the expression of VE-cadherin, CD31, and claudin 5, which facilitates the metastasis process [44]. TGFβ may also stimulate AKT activity through PI3K in epithelial cells undergoing the EMT, which leads to the activation of the mammalian serine/threonine-protein kinase TOR complexes 1/2 (mTORC1 and mTORC2) [148,149,150]. TGFβ increases mTORC2 kinase activity in cells undergoing the EMT, regulating the advancement of epithelial cells through the EMT process. mTORC2 is necessary for cell migration and invasion because it regulates the cytoskeletal alterations and gene expression that occur in the course of EMT [150]. MAPK pathways, such as ERK, P38, and JNK, are also activated by TGFβ signaling [151]. Furthermore, TGFβ-induced transcription is enhanced by ERK/MAPK signaling, which results in a greater reduction in E-cadherin expression and increased expression of N-cadherin and MMPs [47,152]. The integrated network of the EGFR, ERK/MAPK, and TGFβ signaling pathways involved in the metastasis process is presented in Figure 3.
Figure 3.
Integration network of epidermal growth factor receptor (EGFR), mitogen-activated protein kinase (ERK/MAPK), and transforming growth factor-β (TGFβ) signaling pathways involved in metastasis processes. Several signaling pathways are involved in the progress of the epithelial–mesenchymal transition (EMT), and these pathways can work together to elicit complete EMT responses. Apart from its ability to promote EMT through the expression of mothers against decapentaplegic (SMAD) proteins, transforming growth factor β (TGF-β) can also activate the phosphotyrosine 3-kinase/RAC-alpha serine/threonine-protein kinase (PI3K–AKT), ERK–MAPK, P38–MAPK, and c-JUN N-terminal kinase (JNK) signaling pathways. After TβRI phosphorylates the adaptor protein SRC homology 2 domain-containing-transforming A (SHCA), it provides a docking site for the growth factor receptor-bound protein 2 (GRB2) and the son of sevenless (SOS), an event that signals the initiation of the MAPK cascade involving RAS, RAF, MEK, and ERK. Interaction of TNF receptor-associated factor 6 (TRAF6) with the TGF receptor complex stimulates TGF-activated kinase 1 (TAK1), which contributes to activation of P38 and JNK. The EMT is facilitated by the activation of ERK1 and ERK2 MAPK, which increase the expression of EMT transcription factors and proteins involved in cell motility or invasion, such as RHO GTPases that activate the RHO-associated kinase (ROCK) protein to confer cell contraction. Hypoxia-inducible factor 1 (HIF1) (not shown here) can promote EMT by activating the expression of the TWIST protein, which acts as a transcription factor. TGFβ signaling involves activation of SMAD proteins that stimulates expression SNAI/L and down-regulates the expression of VE-cadherin, CD31, and claudin 5 to facilitate the metastasis process. Other EMT-inducing transcription factors include forkhead box protein C2 (FOXC2), paired mesoderm homeobox protein 1 (PRRX1), SLUG, and zinc finger E-box-binding homeobox (ZEB). Furthermore, the PI3K–AKT signaling pathway may be activated both by EGFR and TGFβ signaling.
8. The MAPK Pathway in Metastasis
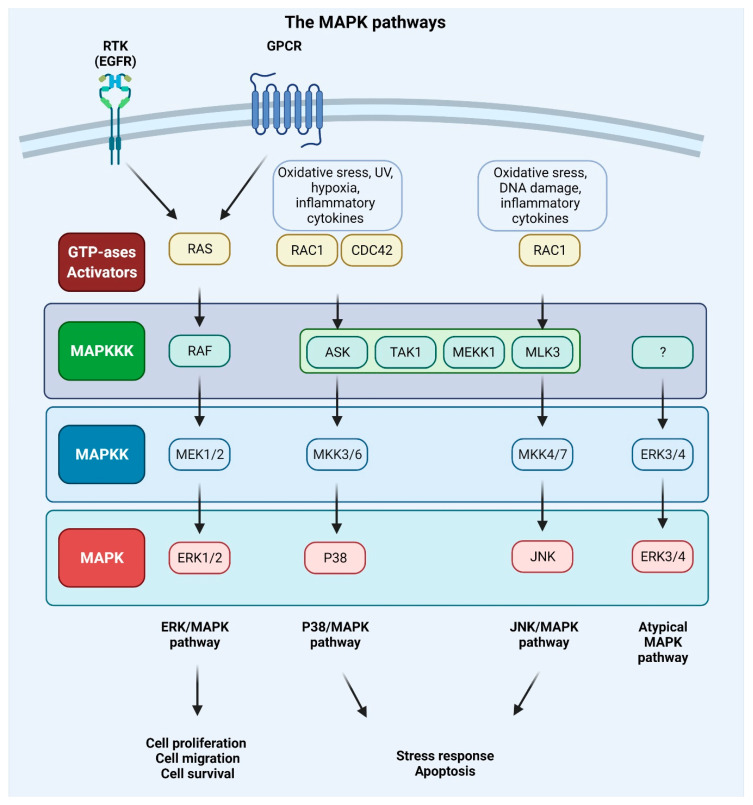
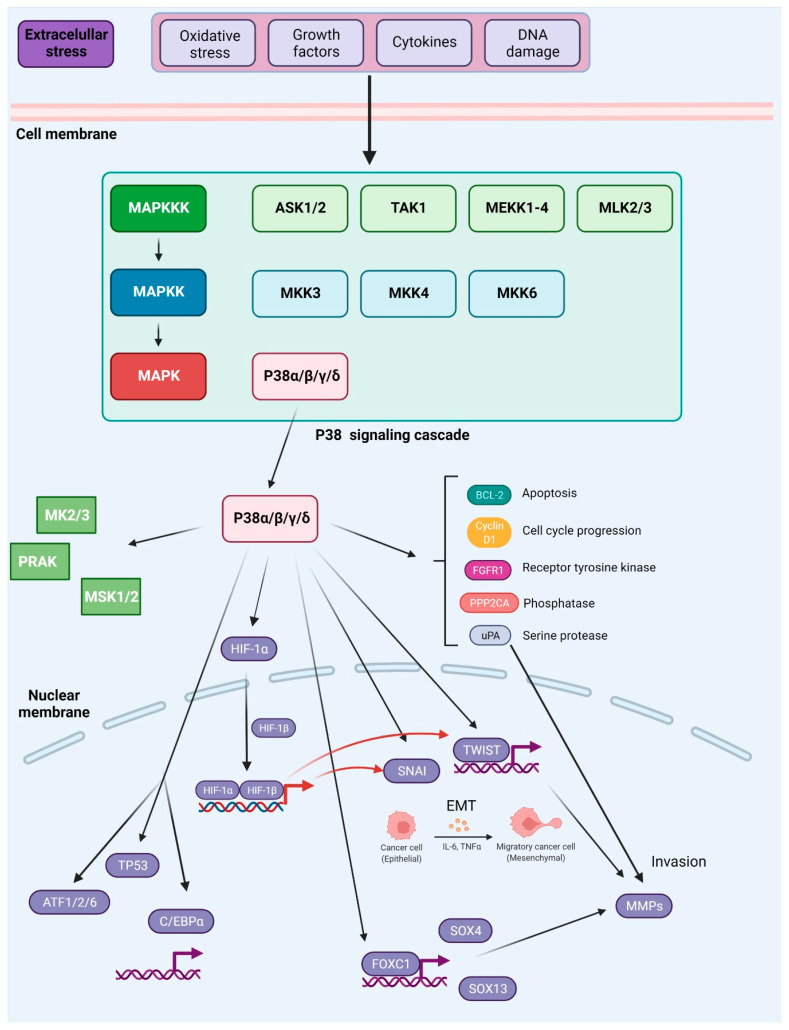
MAPKs are serine/threonine-protein kinases that can be activated by a variety of extracellular stimuli including growth factors, cytokines, insulin, environmental factors, and oxidative and genotoxic stress. Through the use of genetically engineered mouse models and chemically induced tumorigenesis models it has been observed that components of the MAPK pathway not only regulate the behavior of tumor cells, but also the behavior of surrounding normal stromal cells in the TME during cancer pathogenesis. The unique activities of MAPK pathway components in tumor initiation and development vary based on the stimuli and stromal cells involved in tumor growth, as well as the molecular isoforms of the pathway components, as reviewed in [153,154]. The conventional MAPKs in mammals include c-Jun NH2-terminal kinase (JNK), P38 MAPK, and extracellular signal-regulated kinase (ERK), which, in turn, exist in several isoforms. JNK1 and JNK2 are found in nearly all tissues in contrast to JNK3, which is found primarily in neuronal cells. P38 MAPK exists in several isoforms encompassing P38α (also known as MAPK14 or SAPK2a), P38β (MAPK11, SAPK2b), P38γ (MAPK12, SAPK3, ERK6), and P38δ (MAPK13, SAPK4). ERK1 and ERK2 are the subtypes of the eight isoforms of ERK that are activated by MAPK/ERK kinase 1 (MEK1/2) [155,156]. In contrast, ERK3/4 and ERK7/8 are considered atypical MAPKs [157]. The MAPK pathway components are shown in Figure 4.
Figure 4.
Mitogen-activated protein kinase (MAPK) signaling pathways. Extracellular signals such as growth factors and cytokines, as well as intracellular signals such as oxidative and DNA damage, activate MAPK pathways. GTPases (activators) such as RAS, RAS-related protein (RAC), and cell division control protein 42 homolog (CDC42) constitute the first layer of MAPK signaling cascade, which conveys the signal to downstream protein kinases. The MAPK signaling cascades consist of three kinases: mitogen-activated protein kinase kinase kinase (MAPKKK), a mitogen-activated protein kinase kinase (MAPKK), and mitogen-activated protein kinase (MAPK) and results in proliferation, migration, differentiation, survival, or apoptosis. Mammalian MAPK pathways include ERK MAPK, P38 MAPK, and JNK MAPK signaling events. ERK/MAPK pathway is activated by RAS, which is attracted to the plasma membrane through receptor tyrosine kinases (RTKs) and G protein-coupled receptor (GPCRs) activation. In this cascade, MAPK/ERK kinase 1/2 (MEK1/2) activates extracellular signal-regulated kinase 1/2 (ERK1/2). P38/MAPK and JNK/MAPK pathways are triggered by various insults that activate signaling through MKK3/6 or MKK4/7 (MAPKKs), respectively, that are activated upon MAPKKK s such apoptosis signal-regulating kinase 1 (ASK1), transforming growth factor-β-activated kinase 1 (TAK1), MEKK1 (MAPKKK), and MLK3 (MAPKKK). ERK3/4 are considered atypical MAPK kinases [156].
Every MAPK signaling cascade involves at least three core kinases: MAPKKK (mitogen-activated protein kinase kinase kinase), MAPKK (mitogen-activated protein kinase kinase), and MAPK (mitogen-activated protein kinase) [158]. MAPK pathways are present in nearly all eukaryotes and play an important role in numerous cellular activities, including gene expression, metabolism, proliferation, apoptosis, invasion, and metastasis [156]. The RAS–RAF–MEK–ERK pathway is disrupted in approximately 40% of all human malignancies, with mutations in BRAF (10%) and its upstream activator RAS (30%) being the most frequently observed. Inhibitors of MEK were among the first anti-MAPK pathway therapeutics to be created, but despite their high potency and selectivity, they failed in clinical trials. This was due to the negative feedback mechanisms of the pathway components and systemic toxicity of the drugs. Treating metastatic malignant melanoma and other cancers with the use of a combination of RAF and MEK inhibitors has become standard practice. The majority of the pioneering work has been performed in metastatic malignant melanoma, which is characterized by a high prevalence of BRAF (50–60%) and NRAS (15–20%) mutations [159,160,161,162,163].
The MAPK pathway is initiated by the binding of growth factor to the RTK or GPCR and the subsequent phosphorylation of RAS protein and following activation of BRAF or RAF (also known as MAPKKK) kinase. The activation signal is conveyed to the MAPKKs (phosphorylation of two serine residues of the MEK1 or MEK2 protein). The downstream phosphorylation of tyrosine and threonine residues of ERK kinase results in the phosphorylation of a multitude of protein substrates involved in differentiation, apoptosis, and migration [41,156]. Upon stimulation of MAPK signaling, ERK1/2 shuttles from cytoplasm to the nucleus, where it regulates gene expression by phosphorylating numerous transcription factors. In the cytoplasm, cytoskeletal components such as microtubule-associated protein (MAP1, MAP2, MAP4) are the targets of the ERK1/2 kinase. These phosphorylation events control the cell morphology and cytoskeletal redistribution. Moreover, ERK1/2 may phosphorylate other cytoplasmic components, including son of sevenless (SOS), RAF1, and MEK, providing a negative feedback regulation of the pathway. In the nucleus, proto-oncogenes including c-FOS, c-JUN, ETS domain-containing protein (ELK1), c-MYC, and cyclic AMP-dependent transcription factor (ATF2) are all phosphorylated in an ERK1/2-dependent manner [126]. The activation of the ERK/MAPK signaling pathway can promote tumor invasion and metastasis by upregulating MMP expression, whereas the inhibition of this signaling can impede the aforementioned processes [164,165]. It was also discovered that mesothelin regulates the expression of MMP7 through the MAPK/ERK signal transduction pathway, as well as the ERK1/2, AKT, and JNK-mediated pathways, contributing to the invasiveness of ovarian cancer cells [166]. Moreover, RAS-associated protein RAP1A was identified as a significant promoter of ovarian cancer cell metastasis via activation of ERK and P38 signaling and the induction of EMT through enhanced expression of SLUG, ZEB1, vimentin, fibronectin, and MMP9 [167].
The modification of cellular adhesiveness has a direct impact on the mobility of cells. The activation of the ERK/MAPK pathway has been demonstrated to regulate the disassembly of focal adhesions [158]. Moreover, fibroblast de-adhesion triggered by EGFR necessitates the activation of M-calpain, which is downstream of the ERK/MAPK kinase signaling pathway [168,169,170,171]. Previous studies indicate that activation of the MAPK pathway may not be sufficient for the induction of cell mobility and may require phospholipase C activity (PLC) [172,173]. Moreover, the RAS/RAF/MEK/ERK kinase cascade can have a profound impact on HIF-1α protein translation. Activated ERK phosphorylates eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), ribosomal protein S6 kinase (S6K), and MAP kinase interacting kinase (MNK) (which can, in turn, directly phosphorylate eukaryotic translation initiation factor 4E (eIF-4E)) and enhances mRNA translation of HIF-1α protein involved in the response of cells to hypoxia [174]. Moreover, ERK may alter MMP activity, which affects gastric cancer (GC) cell migration or invasion, and many proteins upstream of the ERK/MAPK pathway, such as IL-22, RasGAP-activating-like protein 1 (RASAL1), protein tyrosine phosphatase type IVA 3 (PRL3), nuclear apoptosis-inducing factor 1 (NAIF1), coiled-coil domain-containing protein 134 (CCDC134), and zinc finger protein (ZIC1) that potentially affect invasion and migration in GC cell lines [158]. The RAS/RAF/ERK cell signaling pathway and the P38 MAPK pathway are both responsible for the activation of MNKs that are engaged in oncogenic transformation and can promote metastasis. Alternative splicing results in the production of four MNKs isoforms in human cells (MNK1a/b and MNK2a/b), which are derived from two genes. Through the regulation of eukaryotic translation initiation factor 4E (eIF4E), these kinases play a critical role in the control of the expression of specific proteins involved in the cell cycle, cell survival, and cell motility. However, they also regulate the expression of genes through the modulation of other substrates such as heterogeneous nuclear ribonucleoprotein A1 (HNRNPA1), polypyridine tract-binding protein-associated splicing factor (SFPQ), and sprouty 2 (SPRY2). This topic was recently reviewed in [175].
ROS play a critical role in the regulation of various biological processes. ROS are an integral part of the tumor microenvironment and may promote cancer angiogenesis, metastasis, and survival. Several studies have demonstrated that ROS accumulation is a significant contributor to the EMT process and this topic has been previously reviewed [176,177,178]. For example, ROS causes epigenetic alterations in the promoter region of E-cadherin and several other tumor suppressor genes, resulting in tumor development and metastasis. It may cause gene promotor hypermethylation via SNAI-mediated induction of histone deacetylase 1 (HDAC1) and DNA methyltransferase 1 (DNMT1). Moreover, it was found that TGF-β1 controls the expression of uPA and MMP9, which aids in cell motility and invasion through ROS-mediated events. ROS present in moderate concentrations, stimulate the activation of the cancer cell survival signaling cascade, which includes the MAPK/ERK, P38, JNK, and PI3K/AKT signaling. As a result of the pathway activation ROS contribute to the activation of NF-κB, MMPs, and VEGF. However, cells have to maintain a balance between ROS generation and elimination, as excess ROS production may lead to DNA damage and apoptosis [125]. Moreover, EGFR/RAS/MAPK signaling pathway is involved in NFκB activation, cyclooxygenase-2 (COX2) upregulation, and GC cell proliferation. COX2 upregulation promotes cancer growth and decreases apoptosis. Many studies on GC have linked the MAPK pathway activation to apoptosis and autophagy. The role of the MAPK pathway in GC was extensively reviewed in [42].
The P38 pathway includes the MAPKKKs such as apoptosis signal-regulating kinase 1 (ASK1), transforming growth factor-β-activated kinase 1 (TAK1), mitogen-activated protein kinase kinase kinase 1 (MEKK1), and mixed-lineage kinase 3 (MLK3), and MAPKKs, such as MKK3/6, which in turn activate P38 [70]. In various malignancies, P38 promotes EMT and metastasis via the upregulation of pro-metastatic genes [179,180]. P38 MAPKs perform a wide range of functions through binding to and activating a diverse array of substrates. More than 100 proteins have been demonstrated to be susceptible to direct phosphorylation by P38 MAPKs in vitro and in vivo, with approximately half of these being transcription factors, including ATF-1, -2, and -6, TP53, and CCAAT/enhancer-binding protein α (C/EBPα). Other substrates include protein kinases (e.g., MAP kinase-activated protein kinase 2/3 (MK2/3), ribosomal protein S6 kinase α 5 (MSK1) and phosphatases (e.g., serine/threonine-protein phosphatase 2A catalytic subunit α isoform (PPP2CA)), cell-cycle proteins (e.g., cyclin D1), apoptosis proteins (e.g., BCL-2 family proteins), growth factor receptors (e.g., fibroblast growth factor receptor 1 (FGFR1)), and cytoskeletal proteins (e.g., tau, keratin 8) [181]. For more information on substrates of P38 see [182,183]; moreover the table, a companion to the SnapShot “p38 MAPK Signaling” in the January 31 issue of Cell, describes 66 P38α substrates grouped into eight different categories based on biochemical function [184]. In this review, we will focus on P38 targets with an established role in cancer metastasis.
It has been demonstrated that P38 may be involved in the phosphorylation of the Ser68 residue on TWIST1, which leads to increased protein stability and promotes its capacity to induce EMT and invasiveness in breast cancer [185]. It has been found that elevated TWIST1 levels are also dependent on activation of the ERK signaling [186]. TWIST1 may in turn act as a transcriptional factor for MMPs [187]. Additionally, P38-mediated signaling was shown to regulate the expression of MMP1, MMP2, MMP9, and MMP13 in multiple cancer cell lines [188,189,190,191,192]. In contrast, high expression levels of SNAI together with high expression levels of the phosphorylated P38 MAPK (Thr180/Tyr182) were found in primary tumors. High expression of SNAI in metastatic cells is correlated with an increased risk of death in ovarian cancer patients [193]. A strong link between inflammation and EMT has been established [194]. SNAI was found to trigger IL-6 production, which may in turn act as an EMT trigger. IL-6 also contributes to signal transducer and activator of transcription 3 (STAT3) activation, which affects both tumorigenesis and metastasis [195,196,197]. IL-1β is another cytokine linked to the advancement of cancer including gastric adenocarcinoma, but its molecular causes remain unknown. Both P38 and JNK regulate the IL-1β signaling pathway and the activation of P38 by IL-1β enhances GC cell motility, invasion, and metastatic potential in vitro and in vivo. It was shown that IL-1β induces the IL-1β/P38/AP-1(c-FOS)/MMP2/MMP9 pathway [198].
The Forkhead box (FOX) family of transcription factors, which are distinguished by a conserved DNA-binding domain known as the ‘forkhead’ or ‘winged-helix’, regulate a wide range of biological functions, including cell proliferation, differentiation, apoptosis, and metabolism. FOXC1 and FOXC2, play a critical role in the regulation of embryonic, ocular, and cardiac development. A wide variety of cancers are including breast carcinomas, hepatocellular carcinomas, lymphomas exhibit elevated expression of FOXC1 and FOXC2. FOXC transcription factors aid in the progression of cancer through regulation of cell proliferation, metastasis, EMT, and angiogenesis [199]. FOXC1 promotes tumor metastasis in numerous human malignant cancers. However, the upstream and downstream molecular mechanisms of FOXC1 in metastasis remain unknown. FOXC1 upregulation was related to poor prognosis in colorectal cancer (CRC). In vitro and in vivo, FOXC1 knockdown reduced CRC cell migration and invasion while FOXC1 overexpression increased the metastatic potential of the tested cells. Moreover, it was found that in metastatic CRC cells, FOXC1 regulates MMP10 and the expression of transcription factors SOX4 and SOX13. FOXC1’s Ser241 and Ser272 were found to be important sites for the interaction with P38, phosphorylation of which contribute to its stability [200]. Moreover, the P38-mediated phosphorylation of Ser367 of FOXC2 serves as a regulatory mechanism of ZEB1 in metastatic breast cancer cells. The inhibition of P38–FOXC2 signaling selectively reduces cell metastasis without an effect on primary tumor growth. The genetic or pharmacological suppression of P38 reverses the EMT in a FOXC2-dependent process [201].
As described above, ZEB1 was identified as a downstream target of FOXC2 [201]. ZEB1 is a transcription factor that belongs to the ZEB family of transcription factors. It is distinguished by the presence of two zinc finger clusters, which are important for DNA binding, as well as a homeodomain that is centrally positioned. Other protein binding domains found in ZEB1 include the Smad interaction domain (SID), the CtBP interaction domain (CID), and the p300-P/CAF binding domain, among others (CBD). ZEB1 can bind to certain DNA sequences known as E-boxes and either downregulate or upregulate the expression of its target gene by recruiting co-suppressors or co-activators through the CID, SID, or CBD signaling pathways. The suppression of ZEB1 in MDA-MB-231 human breast cancer cells results in the overexpression of around 200 genes and the downregulation of approximately 30 genes, the majority of which are determinants of epithelial differentiation and cell–cell adhesion. Because of the critical function of ZEB1 in the downregulation of E-cadherin, it is thought to operate as a driver of the EMT and cancer progression. In addition to suppressing the expression of E-cadherin, ZEB1 regulates the expression of several additional target genes that are implicated in tumor growth. For instance, ZEB1 binds to the promoters of epithelial polarity genes and suppresses their transcription, causing breast cancer cells to lose adherence and thus conferring invasive potential [202].
P38 can activate HIF-1 by stabilizing its α subunit (HIF-1α). HIF-1 is also a transcriptional regulator of growth factors and cytokines such as VEGF and TGF-β that are involved in EMT. In addition, HIF-1 can directly stimulate the production of SNAI and TWIST affecting cell migration and EMT [203,204]. P38α also may trigger cell migration or cytoskeletal remodeling via the phosphorylation of heat-shock protein 27 (HSP27), the activation of LIM domain kinase 1 (LIMK1), and the inactivation of cofilin [205,206]. Cofilin is a small abundant protein that binds both G-actin (monomeric) and F-actin (filamentous actin) and thus confers cell migration. Several studies have found that the expression of specific genes in the cofilin pathway is altered in invasive tumor cells, suggesting that cofilin is involved in the initiation of the early phases of the motility cycle. Moreover, the cofilin pathway responds to the TME stimuli that are implicated in cell migration through the activation of other pathways (see Section 5) involved in metastasis. These include cytokines and growth factors such as EGF and TGFα [207].
P38 responds to ROS buildup by encouraging growth stagnation and death, hence preventing carcinogenesis. TNF-α can be stimulated by ROS, resulting in the activation of the JNK signaling pathway and the induction of apoptosis. On the other hand, TNF-α may activate NF-κB and decrease ROS production through the induction of associated genes such as manganese superoxide dismutase (MnSOD) and ferritin heavy chain (FHC), blocking JNK activation and apoptosis. The activation of P38 stimulates the activity of ribosomal protein S6 kinase α 5/4 (RPS6KA5/4 or MSK1/2) and, in turn, promotes the activity of NF-κB [70]. More recently, plasma membrane Ca2+ pump isoform 4b (PMCA4b or ATP2B4) has been established as a metastasis suppressor in BRAF mutant melanoma cells. The activation of P38 triggers the endo/lysosomal internalization and degradation of the ion pump in melanoma cells. Moreover, the inhibition of the P38 MAPK pathway reduces both migration, and metastasis of BRAF mutant cells via the increase in PMCA4b expression and a reduction in β4 integrin yields [208].
While other isoforms of P38 were shown to have a profound influence on cancer metastasis, for many years P38δ was a poorly investigated member of the MAPK family. However, it was found that this isoform is highly expressed in particularly all types of human breast cancers, and the inhibition of P38δ in MCF-7 and MDA-MB-231 breast cancer cell lines results in diminished cell proliferation. Moreover, cells without P38δ seem to exhibit enhanced cell–matrix adhesion. This is attributed to the regulatory role of P38δ on FAK kinase [209]. Moreover, P38δ was shown to enhance the development of CSCs in breast cancer [210]. In contrast, P38γ and P38δ activation may suppress CSCs development in non-small-cell lung cancer (NSCLC) through promotion of the ubiquitin-mediated degradation of SOX2, OCT4, NANOG, KLF4 and MYC transcription factors that normally contribute to the acquisition of cancer stem cell characteristics [211]. The role of P38 signaling in metastasis was also previously summarized in [180] and is shown in Figure 5.
Figure 5.
Activation and downstream targets of P38 MAPK. P38 MAPK is activated through several mechanisms. The canonical MAPK signaling module involves sequential phosphorylation and activation events that pass down from MAP3Ks to MAP2Ks, and from MAP2Ks to P38 MAPK. In response to various external stresses and signals (e.g., oxidative stress, UV irradiation, DNA-damage chemotherapeutic agents, and cytokines), several MAP3Ks can trigger activation of P38 signaling, such as TAK1, MEKK1-4, MLK2/3, and ASK1/2. Three MAP2Ks, namely MKK3, MKK6, and MKK4, are direct upstream activators of P38 MAPK. In addition to canonical activation, P38a, the best-characterized member of the P38 kinase family, can also be activated through autophosphorylation. P38 MAPK has been reported to phosphorylate more than 100 proteins, highlighting the versatility of this signaling pathway. Prominent downstream targets include transcription factors, protein kinases, and phosphatases, growth factor receptors, as well as key regulators of cell cycle and apoptosis (depicted in the main text of the article). Based on [181].
JNK1, JNK2, and JNK3 are the kinases encoded by genes belonging to the JNK family. JNK1 and JNK2 are expressed throughout the body, whereas JNK3 expression is restricted to certain tissues, with the highest levels found in the brain, heart, and testes. For each of the genes, several different splice variants result in a total of 10 isoforms of the protein with molecular weights ranging from 46 to 54 kDa [212]. MKK4 and MKK7 are two representatives of the MAPKK kinases belonging to the JNK sub-pathway activated when MAPKKKs are triggered. These components then phosphorylate and activate JNK, which in turn phosphorylates a multitude of substrates of the AP-1 transcription factor, with c-JUN, FOS, and FOS-related antigen 1/2 (FRA1/2) being the most relevant. Other JNK’s downstream targets include members of the mitochondrial apoptosis regulator BCL-2 family (BCL-2, BCL-xL, BAD, BIM, and BAX), as well as ATF2, ELK-1, TP53, and c-MYC [213,214].
JNKs have a dualistic role in cancer [215]. For example, in mouse embryonic fibroblasts (MEFs), the deletion of JNK2 results in increased cell proliferation, whereas the loss of JNK1 has the opposing effect [216]. The differential regulation of c-JUN is thought to be responsible for these effects [217]. In MEFs, JNK loss in combination with the double knockout of TP53 (TP53–/–) results in MET, as demonstrated by increased E-cadherin expression, decreased N-cadherin expression, and lower colony-forming ability [218]. TGFβ activates JNKs in a cascade that necessitates the involvement of TAK1. This pathway is critical for TGFβ signaling because it is required for the phosphorylation of SMAD3 by JNK and is required for the subsequent transcriptional activation of SMAD3. Not only does the phosphorylation of SMAD3 by JNK increase the efficacy of SMAD-dependent gene expression, but it also increases SMAD3 translocation to the nucleus. Because SMAD3 directly transactivates SNAI1 and SNAI2, JNKs may promote the EMT [212].
Furthermore, the role of JNKs in inflammation is well established [219,220,221]. Activated JNK1 promotes the recruitment of inflammatory macrophages, which release VEGF to stimulate angiogenesis and MMPs to aid in tissue remodeling. Moreover, monocytes release TGF-β, which in turn causes tumor cells to undergo the EMT [212]. Studies suggest that the double knockout JNK1−/− results in reduction in tumor burden, tumor proliferation, and cytokine production, including TNFα and IL-6. Several studies have suggested that the JNK-dependent inflammatory response promotes tumor progression through induction of the EMT in cells [222]. Moreover, JNK-stimulated binding of c-JUN to the VEGF promoter may increase the expression of angiogenic factors facilitating the access of tumor cells to oxygen and nutrients [223,224]. Phosphorylated JNK activates c-JUN, which results in an increase in the expression of MMP2 as a result of the upregulation of astrocyte elevated gene-1 (AEG-1) in cells. The upregulation of AEG-1 dramatically increases the aggressiveness of osteosarcoma cells via the JNK/c-JUN/MMP2 pathway. In addition, it has been shown that the JNK pathway can promote cancer invasion and metastasis by boosting the expression of other MMP family members such as MMP7 and MMP9, which are induced by the activation of the downstream signaling cascade [213].
In human cancer, it is common to observe apparent defects in cell polarity. The fundamental processes through which cell polarity disturbance contributes to tumor growth and metastasis are uncertain. When different apicobasal polarity genes in Drosophila are mutated, JNK signaling is activated and the E-cadherin/β-catenin adhesion complex is downregulated. Both of these events are required and sufficient to cause oncogenic RAS(V12)-induced benign tumors in the developing eye to exhibit metastatic behavior. Furthermore, when oncogenic RAS is present, active JNK and RAS signaling work together to promote tumor development, with JNK signaling switching from a proapoptotic to a pro-growth function depending on the context [225].
The overexpression of glucose-regulated protein 94 (GRP94) has been observed in a variety of malignancies, including breast, liver, lung, colorectal, gastric, pancreatic, and head and neck cancers. GRP94 is a key protein involved in mediating cancer progression, and it is highly expressed in hepatocellular carcinoma (HCC). On the other hand, chaperonin-containing TCP1 complex (CCT1-8) proteins are highly conserved molecular chaperones that are involved in promoting the correct folding of newly synthesized proteins or the refolding of misfolded proteins. Furthermore, it has been proposed that CCT proteins are implicated in the progression of a variety of cancers, including breast cancer, colorectal cancer, uterine sarcoma, and lung cancer, among others. CCT8 overexpression has been discovered in a variety of cancers, including colon cancer, breast cancer, glioma, and HCC. It has been reported that the silencing of GRP94 hindered the wound healing, migration, and invasion of HCC cells. These findings suggested that GRP94 knockdown may have a suppressive impact on HCC cell metastasis via a reduction in CCT8/c-JUN/EMT signaling in HCC cells. The silencing of GRP94 greatly reduced the migration and invasion of cells [226].
JNK is a multifunctional protein that can mediate both cell transformation and apoptosis through a variety of mechanisms that partially overlap with those of the ERK signaling pathway. JNK has been shown to increase resistance to ERK pathway inhibitors as well as chemotherapeutic agents. Moreover, JNK is unquestionably significant in the development of resistance to RAF inhibitors [159]. Evidence suggests that nuclear apoptosis-inducing factor 1 (NAIF1), a protein often downregulated or lost in cancer regulates cellular migration and invasion through the MAPK pathway. The human NAIF1 gene encodes a 327-amino acid protein with a homeodomain-like region and two nuclear localization signals at its N-terminus. The overexpression of NAIF leads to cell growth inhibition and apoptosis. GC cell growth, migration, and invasiveness can be suppressed by NAIF1. NAIF1 can decrease the expression MMP2 and MMP9, and reduce the activity of FAK. Additionally, NAIF1 restrains MAPK1 and MAPK8 activity via the inhibition of their mRNA expression with accompanied ERK and JNK degradation. Thus, the therapeutic targeting of NAIF1 seems to be a new potential strategy in GC treatment [227,228]. Moreover, JNK1 contributes to the survival of circulating cancer cells via inhibition of the transcription of apoptosis-inhibiting genes. As a result, JNK1 and JNK2 may work in concert to improve CTC survival by boosting survival signals and inhibiting apoptosis [212].
Transgelin is an actin-binding protein that is involved in the promotion of cell motility in healthy cells. Although there is debate over whether or not transgelin plays a role in cancer development, many studies have demonstrated that elevated transgelin levels are associated with aggressive tumor behavior, advanced stage of the disease, and poor prognosis [229,230]. Changes in the expression of the transgelin protein mediated by the AKT and JNK signaling pathways increase the metastatic potential of CRC cells. The suppression of transgelin, AKT, or JNK signaling results in a significant reduction in cell migration and invasion in SW620 cells with the concurrent inhibition of actin cytoskeleton dynamics [231].
It has been observed that tenascin-C (TNC), an extracellular matrix glycoprotein, may influence metastases and contribute to the poor prognosis of patients with pancreatic cancer. TNC was shown to induce the migration and invasion of pancreatic cancer cells. This was associated with the upregulation of EMT-associated markers, including MMP9, in a JNK/c-JUN-dependent manner. Moreover, because TNC can activate JNK, it can enhance the association of paxillin with FAK, which promotes pancreatic cancer cell motility and adhesion [232]. The role of JNKs in metastasis was summarized elsewhere [212].
Several investigations have demonstrated that JNK is involved in the migration and invasion of prostate cancer cells. In PC3 and DU145 cells, the inhibition of JNK pathways by the JNK inhibitor SP600125 or JNK siRNA prevented thrombospondin-2-induced migration and invasion [233]. It has been also demonstrated that the CC chemokine receptor 7 (CCR7) may significantly boost the expression of phosphorylated JNK in PC3 cells by activating NOTCH signaling. This results in increased migration and enhanced metastatic activity in PC3 cells [234].
ERK signaling events are tightly controlled cascades. These regulatory components include bispecific phosphatases, scaffold proteins, control of signal duration, and intensity, as well as the dynamic subcellular localization of cascade components in response to environmental stimuli [126]. More recently, MLK3 has been identified as a crucial player in MAPK signaling with an impact on cell invasion and metastasis. MLK3 belongs to the class of MAPKKK that transduce signals from cell surface receptors to JNK, ERK, and P38 kinases. In mammals, MLK comprises four members: MLK1 (MAP3K9), MLK2 (MAP3K10), MLK3 (MAP3K11), and MLK4 with two isoforms (MLK4α and MLK4β). It is essential for migrating cells to undergo cytoskeletal rearrangement and FA changes, which are controlled both spatially and temporally by the activities of the GTPases CDC42, RAC1, and RHOA. MLK3 works as a scaffold protein for RAF1 and allows subsequent BRAF phosphorylation and activation of MEK1/2 and ERK. MLK3 acts as a negative regulator of RHOA GTPase via direct binding to RHOA-specific guanine exchange factor P63RHO-GEF [235]. In breast cancer cells, the catalytic activity of MLK3 is essential for the activation of JNK, which in turn phosphorylates Ser178 of paxillin, resulting in the proliferation of the cancer cells. This phosphorylation event on paxillin engages FAKs, which in turn stimulates further phosphorylation of paxillin on Tyr31 and Tyr118 [236]. Phosphorylated paxillin is capable of competing with the RHOA-specific GAP protein, P190RHO-GAP, for binding to the P120RAS-GAP. In this way, when paxillin attaches to P120RAS-GAP, it releases P190RHO-GAP from the binding site of P120RAS-GAP, allowing P190RHO-GAP to decrease the activity of RHOA [237]. In addition to being essential for optimal JNK activation. MLK3 distribution in the centrosome and on microtubules during mitosis appears to govern microtubule structure in a JNK-independent manner [238]. Several members of the JNK-interacting proteins (JIPs), including JIP-1, -2, and 3, have been shown to function as scaffold proteins for the MLK3-MKK7-JNK signaling subsystem. It has been demonstrated that the JIP-2 protein serves as a docking site for the recruitment of MLK3, MKK3, and either the P38α or P38δ isoforms of MAPK, allowing for MLK3-dependent P38 MAPK activation to occur more efficiently. MLK3 has been demonstrated to signal through a variety of receptors, including EGFR [45], and the discoidin domain receptor 1 (DDR1) [83]. As a result, downstream JNK, ERK, or P38 signaling is triggered. MLK3 has been also shown to be involved in the invasion of triple-negative breast cancer (TNBC) cells triggered by C-X-C chemokine receptor type 4 (CXCR4)/stromal cell-derived factor 1 (CXCL12). Highly metastatic TNBC cells can be prevented from migrating by inhibiting either the MLK3 or JNK pathways, or by silencing the MLK3 gene. In highly invasive breast cancer cells, the depletion of MLK3 or suppression of its activity leads to increased RHOA activity, excessive FA and stress fiber production, and as a result, reduced cell motility. One possible mechanism by which MLK3 may govern cancer cell invasion is through the regulation of the expression of MMPs. For example, the expression of MMP2 and MMP9 is dependent on the MLK3–ERK–AP1 axis. This suggests that MLK3 may promote cancer invasion in part by upregulating MMPs. Furthermore, MLK3 promotes the EMT switch triggered by collagen type I in prostate cancer. In this model, MLK3 transduces signaling from two collagen receptors, the integrin 2 and the DDR1 receptors, increasing the production of the EMT marker N-cadherin in a process mediated through the MKK7-JNK pathway [239]. The function of MLK3 in proliferation, invasion, and metastasis was reviewed in [239].
9. Conclusions
Metastasis is perhaps the most common reason for treatment failure in cancer patients, as well as the leading cause of cancer-related death. Even though the emergence of technological breakthroughs in imaging and cancer cell identification that have significantly advanced our understanding of cancer metastasis, the biological mechanisms driving cancer metastasis and chemoresistance remain largely unclear [240]. The understanding of the complicated biology underpinning the survival strategy of cancer cells, especially CSCs following treatment with anticancer agents, is of particular importance. The understanding of signaling pathways involved in the acquisition of metastatic phenotype such as Hedgehog [71,91], NOTCH [92,93], WNT signaling [94,95], and pathways engaged in the proliferation and survival of cancer cells such as those exerted via stimulation of RTKs offers an opportunity to target cancer cells. Furthermore, cancer cells reprogram their metabolism to survive in the harsh conditions that they experience during metastatic processes. The understanding of the mechanisms that underlie metabolic reprogramming in cancer cells may aid the identification of anticancer targets [241,242]. Despite the emergence of novel small-molecule inhibitors that target critical components of the aforementioned signaling pathways, only a small fraction has been tested in clinical settings due to their non-specific toxicity and solubility difficulties that limit their practical application. This could be resolved by nanoformulating these substances, which will help to overcome these barriers and transport molecules to the designated areas in a more targeted manner [243].
As evidenced by an increase in the number of publications released each year, it is becoming increasingly clear that MAPKs are involved in all the steps required for hyperproliferating cells to develop into metastatic tumors. However, we are currently lacking in vivo data to fully understand how MAPK signaling pathways can affect the progression of metastatic disease. The understanding of interplay between distinct members of the MAPK family, as well as their crosstalk with other signaling pathways, miRNAs, and long non-coding RNAs is crucial. It is hoped that this research will result in a detailed characterization of the function of MAPK pathway members in the tumor microenvironment and their influence on its components that affect the metastatic process, such as tumor-associated macrophages (TAMs) and cancer-associated fibroblasts (CAFs). The generation of genetically engineered animals will allow the manipulation and, therefore, understanding of individual functions of pathway components in the invasion and metastasis of cancer cells. Most significantly, the knowledge that is collected may be applied to the development of novel therapeutic strategies with fewer side effects and higher therapeutic efficiency.
Abbreviations
| 4E-BP1 | Eukaryotic translation initiation factor 4E-binding protein 1 |
| ADCs | Antibody–drug conjugates |
| AEG-1 | Astrocyte elevated gene-1 |
| AKT | RAC-alpha serine/threonine-protein kinase |
| ANCs | Antibody–nanoparticle conjugates |
| ARP2/3 | Arp2/3 complex 34 kDa subunit |
| ASK1 | Apoptosis signal-regulating kinase 1 |
| ATF2 | Cyclic AMP-dependent transcription factor 2 |
| BCL-2 | Apoptosis regulator Bcl-2 |
| BCL-xL | Antiapoptotic protein BCL-xL |
| BM | Basement membrane |
| BRAF | Serine/threonine-protein kinase B-raf |
| CAF | Cancer-associated fibroblasts |
| CBD | p300-P/CAF binding domain |
| CCT | Chaperonin-containing TCP1 complex |
| C/EBPα | CCAAT/enhancer-binding protein alpha |
| CASP8 | Caspase 8 |
| CCDC134 | Coiled-coil domain-containing protein 134 |
| CD31 | Vascular endothelial cluster of differentiation 31 |
| CDC25A | M-phase inducer phosphatase 1 |
| CDC42 | Cell division control protein 42 homolog |
| CDK | Cyclin-dependent kinases |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A |
| CID | CtBP interaction domain |
| COX2 | Cyclooxygenase-2 |
| CRC | Colorectal cancer |
| CSCs | Cancer-stem cells |
| CTCs | Circulating tumor cells |
| CXCL12 | Stromal cell-derived factor 1 |
| CXCR4 | C-X-C chemokine receptor type 4 |
| DDR1 | Discoidin domain receptor 1 |
| DIA1 | Formin diaphanous 1 |
| DNMT1 | DNA methyltransferase 1 |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| eIF-4E | Eukaryotic translation initiation factor 4E |
| ELK-1 | ETS domain-containing protein |
| EMT | Epithelial–mesenchymal transition |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| ESR | Estrogen receptor |
| FA | Focal adhesions |
| FAK | Focal adhesion kinase |
| FGFR1 | Fibroblast growth factor receptor 1 |
| FHC | Ferritin heavy chain |
| FOX | Forkhead box |
| FOXC1/2 | Forkhead box protein C1/2 |
| FRA1/2 | FOS-related antigen 1/2 |
| FYN | SRC family tyrosine kinase FYN |
| GAPs | GTPase-activating proteins |
| GBC | Gallbladder cancer |
| GC | Gastric cancer |
| GDIs | Guanine nucleotide dissociation inhibitors |
| GEFs | Guanine nucleotide exchange factors |
| GLI2 | Glioma-associated oncogene homolog 2 |
| GLS | Glutaminase |
| GLUL | Glutamine synthetase |
| GLUT | Glucose transporters |
| GPCRs | G protein-coupled receptors |
| GRB2 | Growth factor receptor-bound protein 2 |
| GRP94 | Glucose-regulated protein 94 |
| HCC | Hepatocellular carcinoma |
| HDAC | Histone deacetylase 1 |
| HER2 | Human epidermal growth factor receptor 2 |
| HIF1α | Hypoxic-responsive transcription factor α |
| HNRNPA1 | Heterogeneous nuclear ribonucleoprotein A1 |
| HRE | HIF-responsive element |
| HSP27 | Heat-shock protein 27 |
| IL | Interleukin |
| I-SMAD | Inhibitory SMADs |
| JIPs | JNK-interacting proteins |
| JNK | c-Jun N-terminal kinase |
| KRAS | GTPase KRas |
| LAT1 | Large neutral amino acid transporter 1 |
| LIMK1 | LIM domain kinase 1 |
| MAP | Microtubule-associated protein |
| MAPK | Mitogen-activated protein kinase |
| MAPKK | Mitogen-activated protein kinase kinase |
| MAPKKK | Mitogen-activated protein kinase kinase kinase |
| MCT | Monocarboxylate transporters |
| MEF | Mouse embryonic fibroblasts |
| MEK1 | MAPK/ERK kinase 1 |
| MEKK1 | Mitogen-activated protein kinase kinase kinase 1 |
| MET | Mesenchymal–epithelial transition |
| MK2/3 | MAP kinase-activated protein kinase 2/3 |
| MLC2 | Myosin II light chain |
| MLCP | Myosin-light-chain phosphatase |
| MLK3 | Mitogen-activated protein kinase kinase kinase 11 |
| MMPs | Matrix metalloproteinases |
| MNK | MAP kinase interacting kinase |
| MnSOD | Manganese superoxide dismutase |
| MRCK | Myotonic dystrophy kinase-related Cdc42-binding kinase |
| MSK1 | Ribosomal protein S6 kinase alpha-5 |
| mTOR | Serine/threonine-protein kinase mTOR |
| MYC | MYC proto-oncogene protein |
| NAIF1 | Nuclear apoptosis-inducing factor 1 |
| NCAM | Neural cell adhesion molecule |
| NFκB | Nuclear factor kappa B subunit |
| NHE | Sodium hydrogen ion exchangers |
| PDGF-D | Platelet-derived growth factor D |
| PDGFR | Platelet-derived growth factor receptor |
| PDK1 | 3-Phosphoinositide-dependent protein kinase 1 |
| PECAM | Platelet and endothelial cell adhesion molecule 1 |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| PKC | Protein kinase C |
| PLC | Phospholipase C |
| PPP2CA | Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform |
| PRL3 | Protein tyrosine phosphatase type IVA 3 |
| PRRX1 | Paired mesoderm homeobox protein 1 |
| PTEN | Phosphatase and tensin homolog |
| PMCA4b | Plasma membrane Ca2+ pump isoform 4b |
| PTHrP | Parathyroid hormone-related protein |
| R-SMAD | Receptor-activated SMADs |
| RAP1A | Ras-related protein Rap-1A |
| RAC1 | Ras-related protein RAC1 |
| RAF | Proto-oncogene serine/threonine-protein kinase RAF |
| RANK | Tumor necrosis factor superfamily member 11 |
| RANKL | Tumor necrosis factor ligand superfamily member 11 |
| RASAL1 | RasGAP-activating-like protein 1 |
| RB | Retinoblastoma protein |
| RHOA | Transforming protein RhoA |
| RHOE | RHO-related GTP-binding protein E |
| ROCK | RHO-associated kinase |
| ROS | Reactive oxygen species |
| RPS6KA5/4 | Ribosomal protein S6 kinase alpha-5/4 |
| RTK | Receptor tyrosine kinase |
| S6K | Ribosomal protein S6 kinase |
| SID | Smad interaction domain |
| SHCA | SRC homology 2 domain-containing-transforming A |
| SFPQ | Polypyridine tract-binding protein-associated splicing factor |
| SLC4/6 | Sodium-coupled bicarbonate transporters |
| SMAD | Mothers against decapentaplegic |
| SNAT2 | Sodium-coupled neutral amino acid transporter 2 |
| SOS | Son of sevenless |
| SPRY2 | Sprouty 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TAK1 | Transforming growth factor-β-activated kinase 1 |
| TAM | Tumor-associated macrophage |
| TCA | Tricarboxylic acid |
| TGFα/β | Transforming growth factor alpha/beta |
| TICs | Tumor-initiating cells |
| TIMPs | Tissue inhibitors of metalloproteinases |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
| TNC | Tenascin-C |
| TNFα | Tumor necrosis factor alpha |
| TP53 | Cellular tumor antigen p53 |
| TRAF6 | TNF receptor-associated factor 6 |
| TWIST1 | Twist-related protein 1 |
| TβR1/2 | TGF-β type receptor 1/2 |
| uPA | Urokinase plasminogen activator |
| V-ATPase | Vacuolar-type H+-adenosine triphosphatases |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| VHL | Von Hippel–Lindau tumor suppressor |
| WASP | Wiskott–Aldrich syndrome protein |
| ZEB1/2 | Zinc finger E-box-binding homeobox 1/2 |
| ZIC1 | Zinc finger protein ZIC1 |
Author Contributions
Conceptualization, M.K. and R.K.; collected literature, M.K., A.G. and A.B.; wrote the manuscript, M.K. and A.G.; supervised project and proofread the paper, M.K., A.B., M.M. and R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Welch D.R., Hurst D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019;79:3011–3027. doi: 10.1158/0008-5472.CAN-19-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel S.A., Vanharanta S. Epigenetic determinants of metastasis. Mol. Oncol. 2017;11:79–96. doi: 10.1016/j.molonc.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eyles J., Puaux A.-L., Wang X., Toh B., Prakash C., Hong M., Tan T.G., Zheng L., Ong L.C., Jin Y., et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Investig. 2010;120:2030–2039. doi: 10.1172/JCI42002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yurchenco P.D. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011;3:a004911. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hohenester E., Yurchenco P.D. Laminins in basement membrane assembly. Cell Adhes. Migr. 2013;7:56–63. doi: 10.4161/cam.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B. 2015;5:402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeBleu V.S., Macdonald B., Kalluri R. Structure and function of basement membranes. Exp. Biol. Med. Maywood. 2007;232:1121–1129. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- 8.Sekiguchi R., Yamada K.M. Basement Membranes in Development and Disease. Curr. Top. Dev. Biol. 2018;130:143–191. doi: 10.1016/bs.ctdb.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatseva A., Sin Y.Y., Brezzo G., Van Agtmael T. Basement membrane collagens and disease mechanisms. Essays Biochem. 2019;63:297–312. doi: 10.1042/EBC20180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruegel J., Miosge N. Basement membrane components are key players in specialized extracellular matrices. Cell. Mol. Life Sci. 2010;67:2879–2895. doi: 10.1007/s00018-010-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mutgan A.C., Jandl K., Kwapiszewska G. Endothelial Basement Membrane Components and Their Products, Matrikines: Active Drivers of Pulmonary Hypertension? Cells. 2020;9:2029. doi: 10.3390/cells9092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanner K. Regulation of the basement membrane by epithelia generated forces. Phys. Biol. 2012;9:065003. doi: 10.1088/1478-3975/9/6/065003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunz P., Sähr H., Lehner B., Fischer C., Seebach E., Fellenberg J. Elevated ratio of MMP2/MMP9 activity is associated with poor response to chemotherapy in osteosarcoma. BMC Cancer. 2016;16:223. doi: 10.1186/s12885-016-2266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchio-Yamada K., Manabe N., Goto Y., Anann S., Yamamoto Y., Takano K., Ogura A., Matsuda J. Decreased expression of matrix metalloproteinases and tissue inhibitors of metalloproteinase in the kidneys of hereditary nephrotic (ICGN) mice. J. Vet. Med. Sci. 2005;67:35–41. doi: 10.1292/jvms.67.35. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y., Goldberg I.D., Shi Y.E. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. 2002;21:2245–2252. doi: 10.1038/sj.onc.1205291. [DOI] [PubMed] [Google Scholar]
- 16.Vidak E., Javoršek U., Vizovišek M., Turk B. Cysteine Cathepsins and Their Extracellular Roles: Shaping the Microenvironment. Cells. 2019;8:264. doi: 10.3390/cells8030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang L., Han X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed. Pharmacother. 2013;67:179–182. doi: 10.1016/j.biopha.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Vempati P., Mac Gabhann F., Popel A.S. Quantifying the Proteolytic Release of Extracellular Matrix-Sequestered VEGF with a Computational Model. PLoS ONE. 2010;5:e11860. doi: 10.1371/journal.pone.0011860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69:4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 20.Zavyalova M.V., Denisov E.V., Tashireva L.A., Savelieva O.E., Kaigorodova E.V., Krakhmal N.V., Perelmuter V.M. Intravasation as a Key Step in Cancer Metastasis. Biochemistry. 2019;84:762–772. doi: 10.1134/S0006297919070071. [DOI] [PubMed] [Google Scholar]
- 21.García S.A., Weitz J., Schölch S. Circulating Tumor Cells. Methods Mol. Biol. 2018;1692:213–219. doi: 10.1007/978-1-4939-7401-6_18. [DOI] [PubMed] [Google Scholar]
- 22.Balzer E.M., Konstantopoulos K. Intercellular adhesion: Mechanisms for growth and metastasis of epithelial cancers. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012;4:171–181. doi: 10.1002/wsbm.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen K.-Y., Lin J.-A., Yao H.-Y., Hsu A.-C., Tai Y.-T., Ho B.-Y. Monascin accelerates anoikis in circulating tumor cells and prevents breast cancer metastasis. Oncol. Lett. 2020;20:166. doi: 10.3892/ol.2020.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong S.Y., Hynes R.O. Lymphatic or Hematogenous Dissemination: How Does a Metastatic Tumor Cell Decide? Cell Cycle Georget. Tex. 2006;5:812–817. doi: 10.4161/cc.5.8.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaifi J.T., Kunkel M., Das A., Harouaka R.A., Dicker D.T., Li G., Zhu J., Clawson G.A., Yang Z., Reed M.F., et al. Circulating tumor cell isolation during resection of colorectal cancer lung and liver metastases: A prospective trial with different detection techniques. Cancer Biol. Ther. 2015;16:699–708. doi: 10.1080/15384047.2015.1030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langley R.R., Fidler I.J. The seed and soil hypothesis revisited—The role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer. 2011;128:2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fidler I.J. The pathogenesis of cancer metastasis: The “seed and soil” hypothesis revisited. Nat. Rev. Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 28.Fidler I.J., Poste G. The “seed and soil” hypothesis revisited. Lancet Oncol. 2008;9:808. doi: 10.1016/S1470-2045(08)70201-8. [DOI] [PubMed] [Google Scholar]
- 29.Roussos E.T., Condeelis J.S., Patsialou A. Chemotaxis in cancer. Nat. Rev. Cancer. 2011;11:573–587. doi: 10.1038/nrc3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Micalizzi D.S., Maheswaran S., Haber D.A. A conduit to metastasis: Circulating tumor cell biology. Genes Dev. 2017;31:1827–1840. doi: 10.1101/gad.305805.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massagué J., Obenauf A.C. Metastatic Colonization. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurst R.E., Bastian A., Bailey-Downs L., Ihnat M.A. Targeting dormant micrometastases: Rationale, evidence to date and clinical implications. Ther. Adv. Med. Oncol. 2016;8:126–137. doi: 10.1177/1758834015624277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seyfried T.N., Huysentruyt L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013;18:43–73. doi: 10.1615/CritRevOncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valastyan S., Weinberg R.A. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen D.X., Massagué J. Genetic determinants of cancer metastasis. Nat. Rev. Genet. 2007;8:341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 36.Fares J., Fares M.Y., Khachfe H.H., Salhab H.A., Fares Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stemmler M.P., Eccles R.L., Brabletz S., Brabletz T. Non-redundant functions of EMT transcription factors. Nat. Cell Biol. 2019;21:102–112. doi: 10.1038/s41556-018-0196-y. [DOI] [PubMed] [Google Scholar]
- 38.Thiery J.P., Acloque H., Huang R.Y.J., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J., Ma L. MicroRNA control of epithelial-mesenchymal transition and metastasis. Cancer Metastasis Rev. 2012;31:653–662. doi: 10.1007/s10555-012-9368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Q., Deng F., Qin Y., Zhao Z., Wu Z., Xing Z., Ji A., Wang Q.J. Long non-coding RNA regulation of epithelial-mesenchymal transition in cancer metastasis. Cell Death Dis. 2016;7:e2254. doi: 10.1038/cddis.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asl E.R., Amini M., Najafi S., Mansoori B., Mokhtarzadeh A., Mohammadi A., Lotfinejad P., Bagheri M., Shirjang S., Lotfi Z., et al. Interplay between MAPK/ERK signaling pathway and MicroRNAs: A crucial mechanism regulating cancer cell metabolism and tumor progression. Life Sci. 2021;278:119499. doi: 10.1016/j.lfs.2021.119499. [DOI] [PubMed] [Google Scholar]
- 42.Magnelli L., Schiavone N., Staderini F., Biagioni A., Papucci L. MAP Kinases Pathways in Gastric Cancer. Int. J. Mol. Sci. 2020;21:2893. doi: 10.3390/ijms21082893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soleimani A., Rahmani F., Saeedi N., Ghaffarian R., Khazaei M., Ferns G.A., Avan A., Hassanian S.M. The potential role of regulatory microRNAs of RAS/MAPK signaling pathway in the pathogenesis of colorectal cancer. J. Cell. Biochem. 2019;120:19245–19253. doi: 10.1002/jcb.29268. [DOI] [PubMed] [Google Scholar]
- 44.Serrano-Gomez S.J., Maziveyi M., Alahari S.K. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol. Cancer. 2016;15:18. doi: 10.1186/s12943-016-0502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen D.X., Bos P.D., Massagué J. Metastasis: From dissemination to organ-specific colonization. Nat. Rev. Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 46.Chiang A.C., Massagué J. Molecular basis of metastasis. N. Engl. J. Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson A.M., Havel L.S., Koyen A.E., Konen J.M., Shupe J., Wiles W.G., Martin W.D., Grossniklaus H.E., Sica G., Gilbert-Ross M., et al. Vimentin is required for lung adenocarcinoma metastasis via heterotypic tumor cell-cancer-associated fibroblast interactions during collective invasion. Clin. Cancer Res. 2018;24:420–432. doi: 10.1158/1078-0432.CCR-17-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akiyama S.K., Olden K., Yamada K.M. Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev. 1995;14:173–189. doi: 10.1007/BF00690290. [DOI] [PubMed] [Google Scholar]
- 50.Wheelock M.J., Shintani Y., Maeda M., Fukumoto Y., Johnson K.R. Cadherin switching. J. Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 51.Eastham A.M., Spencer H., Soncin F., Ritson S., Merry C.L.R., Stern P.L., Ward C.M. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67:11254–11262. doi: 10.1158/0008-5472.CAN-07-2253. [DOI] [PubMed] [Google Scholar]
- 52.Paňková K., Rösel D., Novotný M., Brábek J. The molecular mechanisms of transition between mesenchymal and amoeboid invasiveness in tumor cells. Cell. Mol. Life Sci. 2010;67:63–71. doi: 10.1007/s00018-009-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winer A., Adams S., Mignatti P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures into Future Successes. Mol. Cancer Ther. 2018;17:1147–1155. doi: 10.1158/1535-7163.MCT-17-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deryugina E.I., Quigley J.P. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015;44:94–112. doi: 10.1016/j.matbio.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jabłońska-Trypuć A., Matejczyk M., Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016;31:177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 56.Amano S., Akutsu N., Matsunaga Y., Nishiyama T., Champliaud M.F., Burgeson R.E., Adachi E. Importance of balance between extracellular matrix synthesis and degradation in basement membrane formation. Exp. Cell Res. 2001;271:249–262. doi: 10.1006/excr.2001.5387. [DOI] [PubMed] [Google Scholar]
- 57.Rundhaug J.E. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quintero-Fabián S., Arreola R., Becerril-Villanueva E., Torres-Romero J.C., Arana-Argáez V., Lara-Riegos J., Ramírez-Camacho M.A., Alvarez-Sánchez M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019;9:1370. doi: 10.3389/fonc.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McQuibban G.A., Gong J.H., Tam E.M., McCulloch C.A., Clark-Lewis I., Overall C.M. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 60.Godefroy E., Manches O., Dréno B., Hochman T., Rolnitzky L., Labarrière N., Guilloux Y., Goldberg J., Jotereau F., Bhardwaj N. Matrix metalloproteinase-2 conditions human dendritic cells to prime inflammatory T(H)2 cells via an IL-12- and OX40L-dependent pathway. Cancer Cell. 2011;19:333–346. doi: 10.1016/j.ccr.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheu B.C., Hsu S.M., Ho H.N., Lien H.C., Huang S.C., Lin R.H. A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res. 2001;61:237–242. [PubMed] [Google Scholar]
- 62.Guedez L., Jensen-Taubman S., Bourboulia D., Kwityn C.J., Wei B., Caterina J., Stetler-Stevenson W.G. TIMP-2 targets tumor-associated myeloid suppressor cells with effects in cancer immune dysfunction and angiogenesis. J. Immunother. 2012;35:502–512. doi: 10.1097/CJI.0b013e3182619c8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shay G., Lynch C.C., Fingleton B. Moving targets: Emerging roles for MMPs in Cancer Progression and Metastasis. Matrix Biol. 2015;44:200–206. doi: 10.1016/j.matbio.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rucci N., Sanità P., Angelucci A. Roles of metalloproteases in metastatic niche. Curr. Mol. Med. 2011;11:609–622. doi: 10.2174/156652411797536705. [DOI] [PubMed] [Google Scholar]
- 65.Voura E.B., English J.L., Yu H.-Y.E., Ho A.T., Subarsky P., Hill R.P., Hojilla C.V., Khokha R. Proteolysis during Tumor Cell Extravasation In Vitro: Metalloproteinase Involvement across Tumor Cell Types. PLoS ONE. 2013;8:e78413. doi: 10.1371/journal.pone.0078413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strand S., Vollmer P., van den Abeelen L., Gottfried D., Alla V., Heid H., Kuball J., Theobald M., Galle P.R., Strand D. Cleavage of CD95 by matrix metalloproteinase-7 induces apoptosis resistance in tumour cells. Oncogene. 2004;23:3732–3736. doi: 10.1038/sj.onc.1207387. [DOI] [PubMed] [Google Scholar]
- 67.Newby A.C. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc. Res. 2006;69:614–624. doi: 10.1016/j.cardiores.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 68.George S.J., Dwivedi A. MMPs, cadherins, and cell proliferation. Trends Cardiovasc. Med. 2004;14:100–105. doi: 10.1016/j.tcm.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Czekay R.-P., Cheon D.-J., Samarakoon R., Kutz S.M., Higgins P.J. Cancer-Associated Fibroblasts: Mechanisms of Tumor Progression and Novel Therapeutic Targets. Cancers. 2022;14:1231. doi: 10.3390/cancers14051231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen C., Nelson L.J., Ávila M.A., Cubero F.J. Mitogen-Activated Protein Kinases (MAPKs) and Cholangiocarcinoma: The Missing Link. Cells. 2019;8:1172. doi: 10.3390/cells8101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alizadeh A.M., Shiri S., Farsinejad S. Metastasis review: From bench to bedside. Tumour Biol. 2014;35:8483–8523. doi: 10.1007/s13277-014-2421-z. [DOI] [PubMed] [Google Scholar]
- 72.Cochrane C.R., Szczepny A., Watkins D.N., Cain J.E. Hedgehog Signaling in the Maintenance of Cancer Stem Cells. Cancers. 2015;7:1554–1585. doi: 10.3390/cancers7030851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aramini B., Masciale V., Grisendi G., Bertolini F., Maur M., Guaitoli G., Chrystel I., Morandi U., Stella F., Dominici M., et al. Dissecting Tumor Growth: The Role of Cancer Stem Cells in Drug Resistance and Recurrence. Cancers. 2022;14:976. doi: 10.3390/cancers14040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swain N., Thakur M., Pathak J., Swain B. SOX2, OCT4 and NANOG: The core embryonic stem cell pluripotency regulators in oral carcinogenesis. J. Oral Maxillofac. Pathol. 2020;24:368–373. doi: 10.4103/jomfp.JOMFP_22_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohiuddin I.S., Wei S.-J., Kang M.H. Role of OCT4 in cancer stem-like cells and chemotherapy resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866:165432. doi: 10.1016/j.bbadis.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiang R., Liao D., Cheng T., Zhou H., Shi Q., Chuang T.S., Markowitz D., Reisfeld R.A., Luo Y. Downregulation of transcription factor SOX2 in cancer stem cells suppresses growth and metastasis of lung cancer. Br. J. Cancer. 2011;104:1410–1417. doi: 10.1038/bjc.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jeter C.R., Yang T., Wang J., Chao H.-P., Tang D.G. NANOG in cancer stem cells and tumor development: An update and outstanding questions. Stem Cells. 2015;33:2381–2390. doi: 10.1002/stem.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Visvader J.E., Lindeman G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 79.Powell A.E., Shung C.-Y., Saylor K.W., Müllendorff K.A., Müllendorf K.A., Weiss J.B., Wong M.H. Lessons from development: A role for asymmetric stem cell division in cancer. Stem Cell Res. 2010;4:3–9. doi: 10.1016/j.scr.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shahriyari L., Komarova N.L. Symmetric vs. Asymmetric Stem Cell Divisions: An Adaptation against Cancer? PLoS ONE. 2013;8:e76195. doi: 10.1371/journal.pone.0076195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang L., Shi P., Zhao G., Xu J., Peng W., Zhang J., Zhang G., Wang X., Dong Z., Chen F., et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020;5:8. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsui W.H. Cancer stem cell signaling pathways. Medicine. 2016;95:S8–S19. doi: 10.1097/MD.0000000000004765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koury J., Zhong L., Hao J. Targeting Signaling Pathways in Cancer Stem Cells for Cancer Treatment. Stem Cells Int. 2017;2017:2925869. doi: 10.1155/2017/2925869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang Y., Li X., Wang T., Guo Q., Xi T., Zheng L. Emerging agents that target signaling pathways in cancer stem cells. J. Hematol. Oncol. 2020;13:60. doi: 10.1186/s13045-020-00901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Korkaya H., Kim G.-I., Davis A., Malik F., Henry N.L., Ithimakin S., Quraishi A.A., Tawakkol N., D’Angelo R., Paulson A.K., et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol. Cell. 2012;47:570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu H., Clauser K.R., Tam W.L., Fröse J., Ye X., Eaton E.N., Reinhardt F., Donnenberg V.S., Bhargava R., Carr S.A., et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 2014;16:1105–1117. doi: 10.1038/ncb3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hugo H.J., Lebret S., Tomaskovic-Crook E., Ahmed N., Blick T., Newgreen D.F., Thompson E.W., Ackland M.L. Contribution of Fibroblast and Mast Cell (Afferent) and Tumor (Efferent) IL-6 Effects within the Tumor Microenvironment. Cancer Microenviron. 2012;5:83–93. doi: 10.1007/s12307-012-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mani S.A., Guo W., Liao M.-J., Eaton E.N., Ayyanan A., Zhou A.Y., Brooks M., Reinhard F., Zhang C.C., Shipitsin M., et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Le N.H., Franken P., Fodde R. Tumour–stroma interactions in colorectal cancer: Converging on β-catenin activation and cancer stemness. Br. J. Cancer. 2008;98:1886–1893. doi: 10.1038/sj.bjc.6604401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fodde R., Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr. Opin. Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 91.Arjmand B., Hamidpour S.K., Alavi-Moghadam S., Yavari H., Shahbazbadr A., Tavirani M.R., Gilany K., Larijani B. Molecular Docking as a Therapeutic Approach for Targeting Cancer Stem Cell Metabolic Processes. Front. Pharmacol. 2022;13:768556. doi: 10.3389/fphar.2022.768556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Borah A., Raveendran S., Rochani A., Maekawa T., Kumar D.S. Targeting self-renewal pathways in cancer stem cells: Clinical implications for cancer therapy. Oncogenesis. 2015;4:e177. doi: 10.1038/oncsis.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sari I.N., Phi L.T.H., Jun N., Wijaya Y.T., Lee S., Kwon H.Y. Hedgehog Signaling in Cancer: A Prospective Therapeutic Target for Eradicating Cancer Stem Cells. Cells. 2018;7:208. doi: 10.3390/cells7110208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Venkatesh V., Nataraj R., Thangaraj G.S., Karthikeyan M., Gnanasekaran A., Kaginelli S.B., Kuppanna G., Kallappa C.G., Basalingappa K.M. Targeting Notch signalling pathway of cancer stem cells. Stem Cell Investig. 2018;5:5. doi: 10.21037/sci.2018.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang J., Sullenger B.A., Rich J.N. Notch signaling in cancer stem cells. Adv. Exp. Med. Biol. 2012;727:174–185. doi: 10.1007/978-1-4614-0899-4_13. [DOI] [PubMed] [Google Scholar]
- 96.Reya T., Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 97.de Sousa e Melo F., Vermeulen L. Wnt Signaling in Cancer Stem Cell Biology. Cancers. 2016;8:60. doi: 10.3390/cancers8070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gopalan V., Islam F., Lam A.K.-Y. Surface Markers for the Identification of Cancer Stem Cells. Methods Mol. Biol. 2018;1692:17–29. doi: 10.1007/978-1-4939-7401-6_2. [DOI] [PubMed] [Google Scholar]
- 99.Schatton T., Frank N.Y., Frank M.H. Identification and targeting of cancer stem cells. BioEssays. 2009;31:1038–1049. doi: 10.1002/bies.200900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pan Y., Ma S., Cao K., Zhou S., Zhao A., Li M., Qian F., Zhu C. Therapeutic approaches targeting cancer stem cells. J. Cancer Res. Ther. 2018;14:1469–1475. doi: 10.4103/jcrt.JCRT_976_17. [DOI] [PubMed] [Google Scholar]
- 101.Kaelin W.G., Ratcliffe P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 102.Lendahl U., Lee K.L., Yang H., Poellinger L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat. Rev. Genet. 2009;10:821–832. doi: 10.1038/nrg2665. [DOI] [PubMed] [Google Scholar]
- 103.Hicklin D.J., Ellis L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 104.Liberti M.V., Locasale J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Potter M., Newport E., Morten K.J. The Warburg effect: 80 years on. Biochem. Soc. Trans. 2016;44:1499–1505. doi: 10.1042/BST20160094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McIntyre A., Harris A.L. The Role of pH Regulation in Cancer Progression. Recent Results Cancer Res. 2016;207:93–134. doi: 10.1007/978-3-319-42118-6_5. [DOI] [PubMed] [Google Scholar]
- 107.Damaghi M., Wojtkowiak J.W., Gillies R.J. pH sensing and regulation in cancer. Front. Physiol. 2013;4:370. doi: 10.3389/fphys.2013.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koltai T. Cancer: Fundamentals behind pH targeting and the double-edged approach. Onco Targets Ther. 2016;9:6343–6360. doi: 10.2147/OTT.S115438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parks S.K., Pouysségur J. Targeting pH regulating proteins for cancer therapy-Progress and limitations. Semin. Cancer Biol. 2017;43:66–73. doi: 10.1016/j.semcancer.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 110.Asgharzadeh M.R., Barar J., Pourseif M.M., Eskandani M., Jafari Niya M., Mashayekhi M.R., Omidi Y. Molecular machineries of pH dysregulation in tumor microenvironment: Potential targets for cancer therapy. BioImpacts. 2017;7:115–133. doi: 10.15171/bi.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cluntun A.A., Lukey M.J., Cerione R.A., Locasale J.W. Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer. 2017;3:169–180. doi: 10.1016/j.trecan.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McDonald P.C., Swayampakula M., Dedhar S. Coordinated Regulation of Metabolic Transporters and Migration/Invasion by Carbonic Anhydrase IX. Metabolites. 2018;8:E20. doi: 10.3390/metabo8010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu H., Zhang Z., Song L., Gao J., Liu Y. Lipid metabolism of cancer stem cells. Oncol. Lett. 2022;23:119. doi: 10.3892/ol.2022.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deng C.-F., Zhu N., Zhao T.-J., Li H.-F., Gu J., Liao D.-F., Qin L. Involvement of LDL and ox-LDL in Cancer Development and Its Therapeutical Potential. Front. Oncol. 2022;12:803473. doi: 10.3389/fonc.2022.803473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buchheit C.L., Weigel K.J., Schafer Z.T. Cancer cell survival during detachment from the ECM: Multiple barriers to tumour progression. Nat. Rev. Cancer. 2014;14:632–641. doi: 10.1038/nrc3789. [DOI] [PubMed] [Google Scholar]
- 116.Dong J., Opresko L.K., Chrisler W., Orr G., Quesenberry R.D., Lauffenburger D.A., Wiley H.S. The Membrane-anchoring Domain of Epidermal Growth Factor Receptor Ligands Dictates Their Ability to Operate in Juxtacrine Mode. Mol. Biol. Cell. 2005;16:2984–2998. doi: 10.1091/mbc.e04-11-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu M.J., Johnson D.E., Grandis J.R. EGFR-Targeted Therapies in the Post-Genomic Era. Cancer Metastasis Rev. 2017;36:463–473. doi: 10.1007/s10555-017-9687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alsahafi E., Begg K., Amelio I., Raulf N., Lucarelli P., Sauter T., Tavassoli M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019;10:540. doi: 10.1038/s41419-019-1769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stadler M.E., Patel M.R., Couch M.E., Hayes D.N. Molecular biology of head and neck cancer: Risks and pathways. Hematol. Oncol. Clin. N. Am. 2008;22:1099–1124.vii. doi: 10.1016/j.hoc.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mendelsohn J. The epidermal growth factor receptor as a target for cancer therapy. Endocr. Relat. Cancer. 2001;8:3–9. doi: 10.1677/erc.0.0080003. [DOI] [PubMed] [Google Scholar]
- 121.Sasaki T., Kuniyasu H., Luo Y., Kato D., Shinya S., Fujii K., Ohmori H., Yamashita Y. Significance of epithelial growth factor in the epithelial-mesenchymal transition of human gallbladder cancer cells. Cancer Sci. 2012;103:1165–1171. doi: 10.1111/j.1349-7006.2012.02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Radinsky R., Risin S., Fan D., Dong Z., Bielenberg D., Bucana C.D., Fidler I.J. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin. Cancer Res. 1995;1:19–31. [PubMed] [Google Scholar]
- 123.Kidd M., Schimmack S., Lawrence B., Alaimo D., Modlin I.M. EGFR/TGFα and TGFβ/CTGF Signaling in Neuroendocrine Neoplasia: Theoretical Therapeutic Targets. Neuroendocrinology. 2013;97:35–44. doi: 10.1159/000334891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sasaki T., Nakamura T., Rebhun R.B., Cheng H., Hale K.S., Tsan R.Z., Fidler I.J., Langley R.R. Modification of the Primary Tumor Microenvironment by Transforming Growth Factor α-Epidermal Growth Factor Receptor Signaling Promotes Metastasis in an Orthotopic Colon Cancer Model. Am. J. Pathol. 2008;173:205–216. doi: 10.2353/ajpath.2008.071147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aggarwal V., Tuli H.S., Varol A., Thakral F., Yerer M.B., Sak K., Varol M., Jain A., Khan M.A., Sethi G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules. 2019;9:735. doi: 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo Y.-J., Pan W.-W., Liu S.-B., Shen Z.-F., Xu Y., Hu L.-L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020;19:1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yeh Y.-W., Cheng C.-C., Yang S.-T., Tseng C.-F., Chang T.-Y., Tsai S.-Y., Fu E., Chiang C.-P., Liao L.-C., Tsai P.-W., et al. Targeting the VEGF-C/VEGFR3 axis suppresses Slug-mediated cancer metastasis and stemness via inhibition of KRAS/YAP1 signaling. Oncotarget. 2017;8:5603–5618. doi: 10.18632/oncotarget.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kuwai T., Nakamura T., Sasaki T., Kim S.-J., Fan D., Villares G.J., Zigler M., Wang H., Bar-Eli M., Kerbel R.S., et al. Phosphorylated epidermal growth factor receptor on tumor-associated endothelial cells is a primary target for therapy with tyrosine kinase inhibitors. Neoplasia. 2008;10:489–500. doi: 10.1593/neo.08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu W., O′Reilly M.S., Langley R.R., Tsan R.Z., Baker C.H., Bekele N., Tang X.M., Onn A., Fidler I.J., Herbst R.S. Expression of epidermal growth factor (EGF)/transforming growth factor-alpha by human lung cancer cells determines their response to EGF receptor tyrosine kinase inhibition in the lungs of mice. Mol. Cancer Ther. 2007;6:2652–2663. doi: 10.1158/1535-7163.MCT-06-0759. [DOI] [PubMed] [Google Scholar]
- 130.Yokoi K., Thaker P.H., Yazici S., Rebhun R.R., Nam D.-H., He J., Kim S.-J., Abbruzzese J.L., Hamilton S.R., Fidler I.J. Dual inhibition of epidermal growth factor receptor and vascular endothelial growth factor receptor phosphorylation by AEE788 reduces growth and metastasis of human colon carcinoma in an orthotopic nude mouse model. Cancer Res. 2005;65:3716–3725. doi: 10.1158/0008-5472.CAN-04-3700. [DOI] [PubMed] [Google Scholar]
- 131.Sasaki T., Kitadai Y., Nakamura T., Kim J.-S., Tsan R.Z., Kuwai T., Langley R.R., Fan D., Kim S.-J., Fidler I.J. Inhibition of epidermal growth factor receptor and vascular endothelial growth factor receptor phosphorylation on tumor-associated endothelial cells leads to treatment of orthotopic human colon cancer in nude mice. Neoplasia. 2007;9:1066–1077. doi: 10.1593/neo.07667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yokoi K., Kim S.-J., Thaker P., Yazici S., Nam D.-H., He J., Sasaki T., Chiao P.J., Sclabas G.M., Abbruzzese J.L., et al. Induction of apoptosis in tumor-associated endothelial cells and therapy of orthotopic human pancreatic carcinoma in nude mice. Neoplasia. 2005;7:696–704. doi: 10.1593/neo.05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yokoi K., Sasaki T., Bucana C.D., Fan D., Baker C.H., Kitadai Y., Kuwai T., Abbruzzese J.L., Fidler I.J. Simultaneous inhibition of EGFR, VEGFR, and platelet-derived growth factor receptor signaling combined with gemcitabine produces therapy of human pancreatic carcinoma and prolongs survival in an orthotopic nude mouse model. Cancer Res. 2005;65:10371–10380. doi: 10.1158/0008-5472.CAN-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rebhun R.B., Langley R.R., Yokoi K., Fan D., Gershenwald J.E., Fidler I.J. Targeting receptor tyrosine kinase on lymphatic endothelial cells for the therapy of colon cancer lymph node metastasis. Neoplasia. 2006;8:747–757. doi: 10.1593/neo.06322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Park Y.W., Younes M.N., Jasser S.A., Yigitbasi O.G., Zhou G., Bucana C.D., Bekele B.N., Myers J.N. AEE788, a dual tyrosine kinase receptor inhibitor, induces endothelial cell apoptosis in human cutaneous squamous cell carcinoma xenografts in nude mice. Clin. Cancer Res. 2005;11:1963–1973. doi: 10.1158/1078-0432.CCR-04-1665. [DOI] [PubMed] [Google Scholar]
- 136.Younes M.N., Yigitbasi O.G., Park Y.W., Kim S.-J., Jasser S.A., Hawthorne V.S., Yazici Y.D., Mandal M., Bekele B.N., Bucana C.D., et al. Antivascular therapy of human follicular thyroid cancer experimental bone metastasis by blockade of epidermal growth factor receptor and vascular growth factor receptor phosphorylation. Cancer Res. 2005;65:4716–4727. doi: 10.1158/0008-5472.CAN-04-4196. [DOI] [PubMed] [Google Scholar]
- 137.Yigitbasi O.G., Younes M.N., Doan D., Jasser S.A., Schiff B.A., Bucana C.D., Bekele B.N., Fidler I.J., Myers J.N. Tumor cell and endothelial cell therapy of oral cancer by dual tyrosine kinase receptor blockade. Cancer Res. 2004;64:7977–7984. doi: 10.1158/0008-5472.CAN-04-1477. [DOI] [PubMed] [Google Scholar]
- 138.Thaker P.H., Yazici S., Nilsson M.B., Yokoi K., Tsan R.Z., He J., Kim S.-J., Fidler I.J., Sood A.K. Antivascular therapy for orthotopic human ovarian carcinoma through blockade of the vascular endothelial growth factor and epidermal growth factor receptors. Clin. Cancer Res. 2005;11:4923–4933. doi: 10.1158/1078-0432.CCR-04-2060. [DOI] [PubMed] [Google Scholar]
- 139.Yazici S., Kim S.J., Busby J.E., He J., Thaker P., Yokoi K., Fan D., Fidler I.J. Dual inhibition of the epidermal growth factor and vascular endothelial growth factor phosphorylation for antivascular therapy of human prostate cancer in the prostate of nude mice. Prostate. 2005;65:203–215. doi: 10.1002/pros.20283. [DOI] [PubMed] [Google Scholar]
- 140.Busby J.E., Kim S.-J., Yazici S., Nakamura T., Kim J.-S., He J., Maya M., Wang X., Do K.-A., Fan D., et al. Therapy of multidrug resistant human prostate tumors in the prostate of nude mice by simultaneous targeting of the epidermal growth factor receptor and vascular endothelial growth factor receptor on tumor-associated endothelial cells. Prostate. 2006;66:1788–1798. doi: 10.1002/pros.20519. [DOI] [PubMed] [Google Scholar]
- 141.da Silva Santos E., Nogueira K.A.B., Fernandes L.C.C., Martins J.R.P., Reis A.V.F., Neto J.D.B.V., da Silva Júnior I.J., Pessoa C., Petrilli R., Eloy J.O. EGFR targeting for cancer therapy: Pharmacology and immunoconjugates with drugs and nanoparticles. Int. J. Pharm. 2021;592:120082. doi: 10.1016/j.ijpharm.2020.120082. [DOI] [PubMed] [Google Scholar]
- 142.Kumari N., Reabroi S., North B.J. Unraveling the Molecular Nexus between GPCRs, ERS, and EMT. Mediat. Inflamm. 2021;2021:6655417. doi: 10.1155/2021/6655417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bhola N.E., Grandis J.R. Crosstalk between G-protein-coupled receptors and epidermal growth factor receptor in cancer. Front. Biosci. 2008;13:1857–1865. doi: 10.2741/2805. [DOI] [PubMed] [Google Scholar]
- 144.Köse M. GPCRs and EGFR—Cross-talk of membrane receptors in cancer. Bioorg. Med. Chem. Lett. 2017;27:3611–3620. doi: 10.1016/j.bmcl.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 145.Tzavlaki K., Moustakas A. TGF-β Signaling. Biomolecules. 2020;10:487. doi: 10.3390/biom10030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Steeg P.S. Targeting metastasis. Nat. Rev. Cancer. 2016;16:201–218. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xie F., Ling L., van Dam H., Zhou F., Zhang L. TGF-β signaling in cancer metastasis. Acta Biochim. Biophys. Sin. 2018;50:121–132. doi: 10.1093/abbs/gmx123. [DOI] [PubMed] [Google Scholar]
- 148.Hamidi A., Song J., Thakur N., Itoh S., Marcusson A., Bergh A., Heldin C.-H., Landström M. TGF-β promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85α. Sci. Signal. 2017;10:eaal4186. doi: 10.1126/scisignal.aal4186. [DOI] [PubMed] [Google Scholar]
- 149.Lamouille S., Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J. Cell Biol. 2007;178:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lamouille S., Connolly E., Smyth J.W., Akhurst R.J., Derynck R. TGF-β-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J. Cell Sci. 2012;125:1259–1273. doi: 10.1242/jcs.095299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 152.Grände M., Franzen A., Karlsson J.-O., Ericson L.E., Heldin N.-E., Nilsson M. Transforming growth factor-beta and epidermal growth factor synergistically stimulate epithelial to mesenchymal transition (EMT) through a MEK-dependent mechanism in primary cultured pig thyrocytes. J. Cell Sci. 2002;115:4227–4236. doi: 10.1242/jcs.00091. [DOI] [PubMed] [Google Scholar]
- 153.Kamiyama M., Naguro I., Ichijo H. In vivo gene manipulation reveals the impact of stress-responsive MAPK pathways on tumor progression. Cancer Sci. 2015;106:785–796. doi: 10.1111/cas.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wortzel I., Seger R. The ERK Cascade. Genes Cancer. 2011;2:195–209. doi: 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim E.K., Choi E.-J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015;89:867–882. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- 156.Cargnello M., Roux P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Olea-Flores M., Zuñiga-Eulogio M.D., Mendoza-Catalán M.A., Rodríguez-Ruiz H.A., Castañeda-Saucedo E., Ortuño-Pineda C., Padilla-Benavides T., Navarro-Tito N. Extracellular-Signal Regulated Kinase: A Central Molecule Driving Epithelial–Mesenchymal Transition in Cancer. Int. J. Mol. Sci. 2019;20:2885. doi: 10.3390/ijms20122885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang M., Huang C.-Z. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J. Gastroenterol. 2015;21:11673–11679. doi: 10.3748/wjg.v21.i41.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee S., Rauch J., Kolch W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020;21:1102. doi: 10.3390/ijms21031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fiore M., Forli S., Manetti F. Targeting Mitogen-activated Protein Kinase-activated Protein Kinase 2 (MAPKAPK2, MK2): Medicinal Chemistry Efforts to Lead Small Molecule Inhibitors to Clinical Trials. J. Med. Chem. 2016;59:3609–3634. doi: 10.1021/acs.jmedchem.5b01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kiniwa Y., Okuyama R. Recent advances in molecular targeted therapy for unresectable and metastatic BRAF-mutated melanoma. Jpn. J. Clin. Oncol. 2021;51:315–320. doi: 10.1093/jjco/hyaa222. [DOI] [PubMed] [Google Scholar]
- 162.Djanani A., Eller S., Öfner D., Troppmair J., Maglione M. The Role of BRAF in Metastatic Colorectal Carcinoma–Past, Present, and Future. Int. J. Mol. Sci. 2020;21:9001. doi: 10.3390/ijms21239001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Proietti I., Skroza N., Bernardini N., Tolino E., Balduzzi V., Marchesiello A., Michelini S., Volpe S., Mambrin A., Mangino G., et al. Mechanisms of Acquired BRAF Inhibitor Resistance in Melanoma: A Systematic Review. Cancers. 2020;12:E2801. doi: 10.3390/cancers12102801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Braicu C., Buse M., Busuioc C., Drula R., Gulei D., Raduly L., Rusu A., Irimie A., Atanasov A.G., Slaby O., et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers. 2019;11:E1618. doi: 10.3390/cancers11101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gao J., Wang Y., Yang J., Zhang W., Meng K., Sun Y., Li Y., He Q.-Y. RNF128 Promotes Invasion and Metastasis Via the EGFR/MAPK/MMP-2 Pathway in Esophageal Squamous Cell Carcinoma. Cancers. 2019;11:E840. doi: 10.3390/cancers11060840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chang M.-C., Chen C.-A., Chen P.-J., Chiang Y.-C., Chen Y.-L., Mao T.-L., Lin H.-W., Lin Chiang W.-H., Cheng W.-F. Mesothelin enhances invasion of ovarian cancer by inducing MMP-7 through MAPK/ERK and JNK pathways. Biochem. J. 2012;442:293–302. doi: 10.1042/BJ20110282. [DOI] [PubMed] [Google Scholar]
- 167.Lu L., Wang J., Wu Y., Wan P., Yang G. Rap1A promotes ovarian cancer metastasis via activation of ERK/p38 and notch signaling. Cancer Med. 2016;5:3544–3554. doi: 10.1002/cam4.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Glading A., Chang P., Lauffenburger D.A., Wells A. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J. Biol. Chem. 2000;275:2390–2398. doi: 10.1074/jbc.275.4.2390. [DOI] [PubMed] [Google Scholar]
- 169.Glading A., Uberall F., Keyse S.M., Lauffenburger D.A., Wells A. Membrane proximal ERK signaling is required for M-calpain activation downstream of epidermal growth factor receptor signaling. J. Biol. Chem. 2001;276:23341–23348. doi: 10.1074/jbc.M008847200. [DOI] [PubMed] [Google Scholar]
- 170.Glading A., Lauffenburger D.A., Wells A. Cutting to the chase: Calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/S0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- 171.Glading A., Bodnar R.J., Reynolds I.J., Shiraha H., Satish L., Potter D.A., Blair H.C., Wells A. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol. Cell. Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen P., Xie H., Sekar M.C., Gupta K., Wells A. Epidermal growth factor receptor-mediated cell motility: Phospholipase C activity is required, but mitogen-activated protein kinase activity is not sufficient for induced cell movement. J. Cell Biol. 1994;127:847–857. doi: 10.1083/jcb.127.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen P., Murphy-Ullrich J.E., Wells A. A role for gelsolin in actuating epidermal growth factor receptor-mediated cell motility. J. Cell Biol. 1996;134:689–698. doi: 10.1083/jcb.134.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Masoud G.N., Li W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B. 2015;5:378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pinto-Díez C., Ferreras-Martín R., Carrión-Marchante R., González V.M., Martín M.E. Deeping in the Role of the MAP-Kinases Interacting Kinases (MNKs) in Cancer. Int. J. Mol. Sci. 2020;21:2967. doi: 10.3390/ijms21082967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jiang J., Wang K., Chen Y., Chen H., Nice E.C., Huang C. Redox regulation in tumor cell epithelial-mesenchymal transition: Molecular basis and therapeutic strategy. Signal Transduct. Target. Ther. 2017;2:17036. doi: 10.1038/sigtrans.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang Z., Li Y., Sarkar F.H. Signaling Mechanism(S) of Reactive Oxygen Species in Epithelial-Mesenchymal Transition Reminiscent of Cancer Stem Cells in Tumor Progression. Curr. Stem Cell Res. Ther. 2010;5:74–80. doi: 10.2174/157488810790442813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee S.Y., Ju M.K., Jeon H.M., Lee Y.J., Kim C.H., Park H.G., Han S.I., Kang H.S. Reactive oxygen species induce epithelial-mesenchymal transition, glycolytic switch, and mitochondrial repression through the Dlx-2/Snail signaling pathways in MCF-7 cells. Mol. Med. Rep. 2019;20:2339–2346. doi: 10.3892/mmr.2019.10466. [DOI] [PubMed] [Google Scholar]
- 179.Wu X., Zhang W., Font-Burgada J., Palmer T., Hamil A.S., Biswas S.K., Poidinger M., Borcherding N., Xie Q., Ellies L.G., et al. Ubiquitin-conjugating enzyme Ubc13 controls breast cancer metastasis through a TAK1-p38 MAP kinase cascade. Proc. Natl. Acad. Sci. USA. 2014;111:13870–13875. doi: 10.1073/pnas.1414358111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.del Barco Barrantes I., Nebreda A.R. Roles of p38 MAPKs in invasion and metastasis. Biochem. Soc. Trans. 2012;40:79–84. doi: 10.1042/BST20110676. [DOI] [PubMed] [Google Scholar]
- 181.Zou X., Blank M. Targeting p38 MAP kinase signaling in cancer through post-translational modifications. Cancer Lett. 2017;384:19–26. doi: 10.1016/j.canlet.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 182.Whitmarsh A.J. A central role for p38 MAPK in the early transcriptional response to stress. BMC Biol. 2010;8:47. doi: 10.1186/1741-7007-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cuadrado A., Nebreda A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 184.Trempolec N., Dave-Coll N., Nebreda A.R. SnapShot: p38 MAPK substrates. Cell. 2013;152:924. doi: 10.1016/j.cell.2013.01.047. [DOI] [PubMed] [Google Scholar]
- 185.Hong J., Zhou J., Fu J., He T., Qin J., Wang L., Liao L., Xu J. Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1 protein and promotes breast cancer cell invasiveness. Cancer Res. 2011;71:3980–3990. doi: 10.1158/0008-5472.CAN-10-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ansieau S., Bastid J., Doreau A., Morel A.-P., Bouchet B.P., Thomas C., Fauvet F., Puisieux I., Doglioni C., Piccinin S., et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 187.Weiss M.B., Abel E.V., Mayberry M.M., Basile K.J., Berger A.C., Aplin A.E. TWIST1 is an ERK1/2 effector that promotes invasion and regulates MMP-1 expression in human melanoma cells. Cancer Res. 2012;72:6382–6392. doi: 10.1158/0008-5472.CAN-12-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hsieh M.-J., Chen K.-S., Chiou H.-L., Hsieh Y.-S. Carbonic anhydrase XII promotes invasion and migration ability of MDA-MB-231 breast cancer cells through the p38 MAPK signaling pathway. Eur. J. Cell Biol. 2010;89:598–606. doi: 10.1016/j.ejcb.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 189.Johansson N., Ala-aho R., Uitto V., Grénman R., Fusenig N.E., López-Otín C., Kähäri V.M. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J. Cell Sci. 2000;113:227–235. doi: 10.1242/jcs.113.2.227. [DOI] [PubMed] [Google Scholar]
- 190.Kumar P., Yadav A., Patel S.N., Islam M., Pan Q., Merajver S.D., Teknos T.N. Tetrathiomolybdate inhibits head and neck cancer metastasis by decreasing tumor cell motility, invasiveness and by promoting tumor cell anoikis. Mol. Cancer. 2010;9:206. doi: 10.1186/1476-4598-9-206. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 191.Park S.Y., Jeong K.J., Panupinthu N., Yu S., Lee J., Han J.W., Kim J.M., Lee J.-S., Kang J., Park C.G., et al. Lysophosphatidic acid augments human hepatocellular carcinoma cell invasion through LPA1 receptor and MMP-9 expression. Oncogene. 2011;30:1351–1359. doi: 10.1038/onc.2010.517. [DOI] [PubMed] [Google Scholar]
- 192.Xu L., Chen S., Bergan R.C. MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene. 2006;25:2987–2998. doi: 10.1038/sj.onc.1209337. [DOI] [PubMed] [Google Scholar]
- 193.Hipp S., Berg D., Ergin B., Schuster T., Hapfelmeier A., Walch A., Avril S., Schmalfeldt B., Höfler H., Becker K.-F. Interaction of Snail and p38 mitogen-activated protein kinase results in shorter overall survival of ovarian cancer patients. Virchows Arch. 2010;457:705–713. doi: 10.1007/s00428-010-0986-5. [DOI] [PubMed] [Google Scholar]
- 194.Ricciardi M., Zanotto M., Malpeli G., Bassi G., Perbellini O., Chilosi M., Bifari F., Krampera M. Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells. Br. J. Cancer. 2015;112:1067–1075. doi: 10.1038/bjc.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhou J., Zhang C., Pan J., Chen L., Qi S.-T. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human adamantinomatous craniopharyngioma cells and promotes tumor cell migration. Mol. Med. Rep. 2017;15:4123–4131. doi: 10.3892/mmr.2017.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sun Q., Shang Y., Sun F., Dong X., Niu J., Li F. Interleukin-6 Promotes Epithelial-Mesenchymal Transition and Cell Invasion through Integrin β6 Upregulation in Colorectal Cancer. Oxid. Med. Cell. Longev. 2020;2020:8032187. doi: 10.1155/2020/8032187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lyons J.G., Patel V., Roue N.C., Fok S.Y., Soon L.L., Halliday G.M., Gutkind J.S. Snail up-regulates pro-inflammatory mediators and inhibits differentiation in oral keratinocytes. Cancer Res. 2008;68:4525–4530. doi: 10.1158/1078-0432.CCR-07-6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Huang Q., Lan F., Wang X., Yu Y., Ouyang X., Zheng F., Han J., Lin Y., Xie Y., Xie F., et al. IL-1β-induced activation of p38 promotes metastasis in gastric adenocarcinoma via upregulation of AP-1/c-fos, MMP2 and MMP9. Mol. Cancer. 2014;13:18. doi: 10.1186/1476-4598-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen X., Wei H., Li J., Liang X., Dai S., Jiang L., Guo M., Qu L., Chen Z., Chen L., et al. Structural basis for DNA recognition by FOXC2. Nucleic Acids Res. 2019;47:3752–3764. doi: 10.1093/nar/gkz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang Y., Liao Y., Chen C., Sun W., Sun X., Liu Y., Xu E., Lai M., Zhang H. p38-regulated FOXC1 stability is required for colorectal cancer metastasis. J. Pathol. 2020;250:217–230. doi: 10.1002/path.5362. [DOI] [PubMed] [Google Scholar]
- 201.Werden S.J., Sphyris N., Sarkar T.R., Paranjape A.N., LaBaff A.M., Taube J.H., Hollier B.G., Ramirez-Peña E.Q., Soundararajan R., den Hollander P., et al. Phosphorylation of serine 367 of FOXC2 by p38 regulates ZEB1 and breast cancer metastasis, without impacting primary tumor growth. Oncogene. 2016;35:5977–5988. doi: 10.1038/onc.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang P., Sun Y., Ma L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14:481–487. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Emerling B.M., Platanias L.C., Black E., Nebreda A.R., Davis R.J., Chandel N.S. Mitochondrial reactive oxygen species activation of p38 mitogen-activated protein kinase is required for hypoxia signaling. Mol. Cell. Biol. 2005;25:4853–4862. doi: 10.1128/MCB.25.12.4853-4862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.López-Novoa J.M., Nieto M.A. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Laferriere J., Houle F., Taher M.M., Valerie K., Huot J. Transendothelial migration of colon carcinoma cells requires expression of E-selectin by endothelial cells and activation of stress-activated protein kinase-2 (SAPK2/p38) in the tumor cells. J. Biol. Chem. 2001;276:33762–33772. doi: 10.1074/jbc.M008564200. [DOI] [PubMed] [Google Scholar]
- 206.Kobayashi M., Nishita M., Mishima T., Ohashi K., Mizuno K. MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J. 2006;25:713–726. doi: 10.1038/sj.emboj.7600973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang W., Eddy R., Condeelis J. The cofilin pathway in breast cancer invasion and metastasis. Nat. Rev. Cancer. 2007;7:429–440. doi: 10.1038/nrc2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Naffa R., Vogel L., Hegedűs L., Pászty K., Tóth S., Kelemen K., Singh N., Reményi A., Kállay E., Cserepes M., et al. P38 MAPK Promotes Migration and Metastatic Activity of BRAF Mutant Melanoma Cells by Inducing Degradation of PMCA4b. Cells. 2020;9:E1209. doi: 10.3390/cells9051209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wada M., Canals D., Adada M., Coant N., Salama M.F., Helke K.L., Arthur J.S., Shroyer K.R., Kitatani K., Obeid L.M., et al. P38 delta MAPK promotes breast cancer progression and lung metastasis by enhancing cell proliferation and cell detachment. Oncogene. 2017;36:6649–6657. doi: 10.1038/onc.2017.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Xu M., Wang S., Wang Y., Wu H., Frank J.A., Zhang Z., Luo J. Role of p38γ MAPK in regulation of EMT and cancer stem cells. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:3605–3617. doi: 10.1016/j.bbadis.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fang Y., Wang J., Wang G., Zhou C., Wang P., Zhao S., Zhao S., Huang S., Su W., Jiang P., et al. Inactivation of p38 MAPK contributes to stem cell-like properties of non-small cell lung cancer. Oncotarget. 2017;8:26702–26717. doi: 10.18632/oncotarget.15804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ebelt N.D., Cantrell M.A., Van Den Berg C.L. c-Jun N-Terminal Kinases Mediate a Wide Range of Targets in the Metastatic Cascade. Genes Cancer. 2013;4:378–387. doi: 10.1177/1947601913485413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li Y.-S., Deng Z.-H., Zeng C., Lei G.-H. JNK pathway in osteosarcoma: Pathogenesis and therapeutics. J. Recept. Signal Transduct. Res. 2016;36:465–470. doi: 10.3109/10799893.2015.1122045. [DOI] [PubMed] [Google Scholar]
- 214.Tam S.Y., Law H.K.-W. JNK in Tumor Microenvironment: Present Findings and Challenges in Clinical Translation. Cancers. 2021;13:2196. doi: 10.3390/cancers13092196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tournier C. The 2 Faces of JNK Signaling in Cancer. Genes Cancer. 2013;4:397–400. doi: 10.1177/1947601913486349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sabapathy K., Hochedlinger K., Nam S.Y., Bauer A., Karin M., Wagner E.F. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol. Cell. 2004;15:713–725. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 217.Shaulian E., Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 218.Cellurale C., Sabio G., Kennedy N.J., Das M., Barlow M., Sandy P., Jacks T., Davis R.J. Requirement of c-Jun NH(2)-terminal kinase for Ras-initiated tumor formation. Mol. Cell. Biol. 2011;31:1565–1576. doi: 10.1128/MCB.01122-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hammouda M.B., Ford A.E., Liu Y., Zhang J.Y. The JNK Signaling Pathway in Inflammatory Skin Disorders and Cancer. Cells. 2020;9:E857. doi: 10.3390/cells9040857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Guma M., Firestein G.S. c-Jun N-Terminal Kinase in Inflammation and Rheumatic Diseases. Open Rheumatol. J. 2012;6:220–231. doi: 10.2174/1874312901206010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kaminska B. Molecular characterization of inflammation-induced JNK/c-Jun signaling pathway in connection with tumorigenesis. Methods Mol. Biol. 2009;512:249–264. doi: 10.1007/978-1-60327-530-9_13. [DOI] [PubMed] [Google Scholar]
- 222.Takahashi H., Ogata H., Nishigaki R., Broide D.H., Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Krejsgaard T., Vetter-Kauczok C.S., Woetmann A., Lovato P., Labuda T., Eriksen K.W., Zhang Q., Becker J.C., Ødum N. Jak3- and JNK-dependent vascular endothelial growth factor expression in cutaneous T-cell lymphoma. Leukemia. 2006;20:1759–1766. doi: 10.1038/sj.leu.2404350. [DOI] [PubMed] [Google Scholar]
- 224.Saníger M.L., Oya R., Macías D., Domínguez J.N., Aránega A., Luque F. c-Jun kinase mediates expression of VEGF induced at transcriptional level by Rac1 and Cdc42Hs but not by RhoA. J. Cell. Biochem. 2006;98:650–660. doi: 10.1002/jcb.20801. [DOI] [PubMed] [Google Scholar]
- 225.Igaki T., Pagliarini R.A., Xu T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol. 2006;16:1139–1146. doi: 10.1016/j.cub.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 226.Wei P.-L., Huang C.-Y., Tai C.-J., Batzorig U., Cheng W.-L., Hunag M.-T., Chang Y.-J. Glucose-regulated protein 94 mediates metastasis by CCT8 and the JNK pathway in hepatocellular carcinoma. Tumour Biol. 2016;37:8219–8227. doi: 10.1007/s13277-015-4669-3. [DOI] [PubMed] [Google Scholar]
- 227.Yang M., Gu Y.-Y., Peng H., Zhao M., Wang J., Huang S.-K., Yuan X.-H., Li J., Sang J.-L., Luo Q., et al. NAIF1 inhibits gastric cancer cells migration and invasion via the MAPK pathways. J. Cancer Res. Clin. Oncol. 2015;141:1037–1047. doi: 10.1007/s00432-014-1865-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lv B., Shi T., Wang X., Song Q., Zhang Y., Shen Y., Ma D., Lou Y. Overexpression of the novel human gene, nuclear apoptosis-inducing factor 1, induces apoptosis. Int. J. Biochem. Cell Biol. 2006;38:671–683. doi: 10.1016/j.biocel.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 229.Zhou H.-M., Fang Y.-Y., Weinberger P.M., Ding L.-L., Cowell J.K., Hudson F.Z., Ren M., Lee J.R., Chen Q.-K., Su H., et al. Transgelin increases metastatic potential of colorectal cancer cells in vivo and alters expression of genes involved in cell motility. BMC Cancer. 2016;16:55. doi: 10.1186/s12885-016-2105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lin Y., Buckhaults P.J., Lee J.R., Xiong H., Farrell C., Podolsky R.H., Schade R.R., Dynan W.S. Association of the actin-binding protein transgelin with lymph node metastasis in human colorectal cancer. Neoplasia. 2009;11:864–873. doi: 10.1593/neo.09542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhou H., Zhang Y., Chen Q., Lin Y. AKT and JNK Signaling Pathways Increase the Metastatic Potential of Colorectal Cancer Cells by Altering Transgelin Expression. Dig. Dis. Sci. 2016;61:1091–1097. doi: 10.1007/s10620-015-3985-1. [DOI] [PubMed] [Google Scholar]
- 232.Cai J., Du S., Wang H., Xin B., Wang J., Shen W., Wei W., Guo Z., Shen X. Tenascin-C induces migration and invasion through JNK/c-Jun signalling in pancreatic cancer. Oncotarget. 2017;8:74406–74422. doi: 10.18632/oncotarget.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chen P.-C., Tang C.-H., Lin L.-W., Tsai C.-H., Chu C.-Y., Lin T.-H., Huang Y.-L. Thrombospondin-2 promotes prostate cancer bone metastasis by the up-regulation of matrix metalloproteinase-2 through down-regulating miR-376c expression. J. Hematol. Oncol. 2017;10:33. doi: 10.1186/s13045-017-0390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Du R., Tang G., Tang Z., Kuang Y. Ectopic expression of CC chemokine receptor 7 promotes prostate cancer cells metastasis via Notch1 signaling. J. Cell. Biochem. 2019;120:9639–9647. doi: 10.1002/jcb.28242. [DOI] [PubMed] [Google Scholar]
- 235.Swenson-Fields K.I., Sandquist J.C., Rossol-Allison J., Blat I.C., Wennerberg K., Burridge K., Means A.R. MLK3 limits activated Galphaq signaling to Rho by binding to p63RhoGEF. Mol. Cell. 2008;32:43–56. doi: 10.1016/j.molcel.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chen J., Gallo K.A. MLK3 regulates paxillin phosphorylation in chemokine-mediated breast cancer cell migration and invasion to drive metastasis. Cancer Res. 2012;72:4130–4140. doi: 10.1158/0008-5472.CAN-12-0655. [DOI] [PubMed] [Google Scholar]
- 237.Tsubouchi A., Sakakura J., Yagi R., Mazaki Y., Schaefer E., Yano H., Sabe H. Localized suppression of RhoA activity by Tyr31/118-phosphorylated paxillin in cell adhesion and migration. J. Cell Biol. 2002;159:673–683. doi: 10.1083/jcb.200202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Swenson K.I., Winkler K.E., Means A.R. A new identity for MLK3 as an NIMA-related, cell cycle-regulated kinase that is localized near centrosomes and influences microtubule organization. Mol. Biol. Cell. 2003;14:156–172. doi: 10.1091/mbc.e02-02-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rattanasinchai C., Gallo K.A. MLK3 Signaling in Cancer Invasion. Cancers. 2016;8:51. doi: 10.3390/cancers8050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Qian C.-N., Mei Y., Zhang J. Cancer metastasis: Issues and challenges. Chin. J. Cancer. 2017;36:38. doi: 10.1186/s40880-017-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ohshima K., Morii E. Metabolic Reprogramming of Cancer Cells during Tumor Progression and Metastasis. Metabolites. 2021;11:28. doi: 10.3390/metabo11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Herreros-Pomares A. Identification, Culture and Targeting of Cancer Stem Cells. Life. 2022;12:184. doi: 10.3390/life12020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jafri N.F., Ma P.C., Maulik G., Salgia R. Mechanisms of metastasis as related to receptor tyrosine kinases in small-cell lung cancer. J. Environ. Pathol. Toxicol. Oncol. 2003;22:147–165. doi: 10.1615/JEnvPathToxOncol.v22.i3.10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.