Abstract
To estimate the diversity of extended-spectrum β-lactamases in Brazil, 18 strains from different species of the family Enterobacteriaceae exhibiting a positive double-disk synergy test were collected by a clinical laboratory from several hospitals in Rio de Janeiro, Brazil, in 1996 and 1997. Four strains (Proteus mirabilis, Enterobacter cloacae, Enterobacter aerogenes, and Citrobacter amalonaticus) hybridized with a 550-bp CTX-M probe. The P. mirabilis strain produced a CTX-M-2 enzyme. The E. cloacae, E. aerogenes, and C. amalonaticus isolates harbored a bla gene which was identified by cloning and sequencing as a blaCTX-M gene. E. coli HB101 transconjugants and the E. coli DH5α transformant harboring a recombinant plasmid produced a CTX-M β-lactamase with an isoelectric point of 7.6 conferring a resistance phenotype characterized by a higher level of resistance to cefotaxime than to ceftazidime, as observed with the other CTX-M enzymes. The deduced protein sequence showed a novel Ambler class A CTX-M enzyme, named CTX-M-8, which had 83 to 88% identity with the previously described CTX-M enzymes. The phylogenic study of the CTX-M family including CTX-M-8 revealed four CTX-M types, CTX-M-8 being the first member of a new phylum of CTX-M enzymes. The evolutionary distances between the four types of CTX-M were large, suggesting that the four clusters branched off early from a distant unknown enzyme and that intermediate enzymes probably existed.
Shortly after the introduction of the broad-spectrum cephalosporins such as cefotaxime, aztreonam, and ceftazidime, extended-spectrum β-lactamases (ESBLs) were isolated, first in Europe (24, 40) and then worldwide. According to the structural classification of Ambler et al. (1) and the latest function scheme of Bush et al., these ESBLs are generally class A enzymes of the 2be group, arising subsequently to a few amino acid substitutions, from the common plasmid-mediated TEM and SHV-1 β-lactamases (12, 20).
The CTX-M β-lactamases, a new family in class A ESBLs, were characterized at the beginning of the 1990s in the first reports of the MEN-1 (CTX-M-1) enzyme (4, 6). In contrast to TEM and SHV type cefotaxime-hydrolyzing ESBLs, CTX-Ms are much more active against cefotaxime than against ceftazidime. The amino acid residues critical for their extended-spectrum activity are distinct from those of TEM- and SHV-1-derived ESBLs (4, 14–16, 18, 27).
The growing CTX-M family comprises nine members: CTX-M-1 (MEN-1) (4, 6), CTX-M-2 (5), Toho-1 (19), CTX-M-3 (17), CTX-M-4 (16), CTX-M-5 (11), Toho-2 (27), CTX-M-7 (15) (previously designated CTX-M-5), and CTX-M-6 (15). They have high homology with the class A chromosomally encoded β-lactamases of Proteus vulgaris (31), Serratia fonticola (30), Citrobacter diversus (32), and Klebsiella oxytoca (2, 33) and plasmid-mediated β-lactamase SFO-1 (28). However, there is no clear direct phylogenic connection between CTX-M enzymes and these β-lactamases (7).
First described in Europe, CTX-M-producing strains have now been reported over a wide geographic area including the Near East (7), the Far East (19, 27, 44), South America (5, 7; M. Galas, F. Pasteran, R. Melano, A. Petroni, G. Lopez, A. Corso, A. Rossi and the WHONET Collaborative Group, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-109, p. 201, 1998), and Europe (4, 6, 11, 15–17). CTX-M enzymes have been observed in Escherichia coli (4, 6, 19, 27, 44; Galas et al. 38th ICAAC, abstr. E-109) and Salmonella enterica serovar Typhimurium (5, 11, 15, 16) and also less frequently in Klebsiella pneumoniae (7), Proteus mirabilis (7), Citrobacter freundii (7), and Vibrio cholerae El Tor (M. Galas, A. Petroni, R. Melano, A. Corso, M. Rodriguez, M. L. Cacace, A. Bru, and A. Rossi, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-124, p. 119, 1998).
To estimate the diversity of ESBLs in Brazil, clinical strains that exhibited ESBL phenotypes in different species were collected in hospitals of Rio de Janeiro in 1996 and 1997. In this report, we describe a novel CTX-M type enzyme, designated CTX-M-8, produced by three different strains of the family Enterobacteriaceae.
MATERIALS AND METHODS
Clinical strains.
Table 1 shows the clinical strains and plasmids used in this study. Clinical strains Rio-1, Rio-2, and Rio-3, which produced a novel β-lactamase, were isolated from patients hospitalized in intensive care units of three private hospitals of Rio de Janeiro, Brazil. Enterobacter cloacae Rio-1 was isolated in November 1996 from blood. Citrobacter amalonaticus Rio-2 and Enterobacter aerogenes Rio-3 were isolated in March 1997 from a surgical wound and blood, respectively. CTX-M-1-producing E. coli MEN (4), CTX-M-2-producing P. mirabilis Rio-4, and TEM-1-producing E. coli TR4 (38) were used as reference strains.
TABLE 1.
Strains and plasmids used in the study
| Strain or plasmid | Relevant genotype or phenotype (place and yr of isolation) | Source or reference |
|---|---|---|
| Strains | ||
| E. cloacae Rio-1 | Broad-spectrum cephalosporin resistant (Rio de Janeiro, Brazil, 1996) | This study |
| C. amalonaticus Rio-2 | Broad-spectrum cephalosporin resistant (Rio de Janeiro, Brazil, 1997) | This study |
| E. aerogenes Rio-3 | Broad-spectrum cephalosporin resistant (Rio de Janeiro, Brazil, 1997) | This study |
| P. mirabilis Rio-4 | CTX-M-2-producing P. mirabilis (Rio de Janeiro, Brazil, 1997) | This study |
| E. coli MEN | CTX-M-1-producing E. coli | 4 |
| E. coli TR4 | TEM-1-producing E. coli HB101 | 38 |
| E. coli HB101 | supE44 hsdS20 (rB− mB−) recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 | 35 |
| E. coli DH5α | supE44 ΔlacU169 (Φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 35 |
| Plasmids | ||
| pRio-2 | Natural plasmid from C. amalonaticus Rio-2 containing blaCTX-M-8 and blaTEM-1; resistance phenotype: ESBL, amikacin, gentamicin, kanamycin, netilmicin, tobramycin | This study |
| pRio-3 | Natural plasmid from E. aerogenes Rio-3 containing blaCTX-M-8 and blaTEM-1; resistance phenotype: ESBL, amikacin, gentamicin, kanamycin, netilmicin, tobramycin | This study |
| pC1Rio-2 | Recombinant plasmid containing a 10-kb EcoRI fragment with blaCTX-M-8 | This study |
Mating-out assays.
Direct transfers of plasmids carrying resistance genes were performed by mating donor strains with in vitro-obtained rifampin- or nalidixic acid-resistant mutants of E. coli HB101 (35) as the recipient strain at 37°C in solid and liquid Mueller-Hinton medium. Transconjugants were selected on Mueller-Hinton agar containing rifampin (300 μg/ml) or nalidixic acid (150 μg/ml) and cefotaxime (2 μg/ml). The transconjugants E. coli TrRio-2 and TrRio-3 were obtained from clinical strains of C. amalonaticus Rio-2 and of E. aerogenes Rio-3, respectively.
Susceptibility of β-lactams.
MICs were determined by a dilution method on Mueller-Hinton agar (Sanofi Diagnostics Pasteur, Marnes la Coquette, France) with an inoculum of 104 CFU per spot. Antibiotics were provided as powders by SmithKline Beecham Pharmaceuticals (amoxicillin, ticarcillin, and clavulanate), Lederle Laboratories (piperacillin, tazobactam), Eli Lilly, Paris, France (cephalothin), Roussel-Uclaf (cefotaxime, cefpirome), Glaxo Wellcome Research and Development (ceftazidime), Bristol-Myers Squibb (aztreonam, cefepime), and Merck Sharp & Dohme (imipenem).
Detection of ESBLs was performed with the standard and modified double-disk synergy tests as described previously (21, 41). Antibiotic discs for agar tests were obtained from Sanofi Diagnostics Pasteur.
Isoelectric focusing.
Isoelectric focusing was performed with polyacrylamide gels containing ampholines with a pH range of 3.5 to 10 as previously described (37). The following β-lactamases of known pIs were used as standards: TEM-1 (pI 5.4), SHV-1 (pI 7.6), and CTX-M-1 (pI 8.6).
Determination of β-lactamases kinetic constants.
The Km and Vmax constants of the β-lactamases were obtained by a computerized microacidimetric method as previously described (26) with extracts purified as reported previously (10). The relative Vmax rates of hydrolysis were compared with that for benzylpenicillin, which was taken as 100%. The concentrations of the inhibitors (clavulanate and tazobactam) required to inhibit enzyme activity by 50% (IC50s) were determined as described previously for penicillin G (39).
PCR of CTX-M genes.
The detection of genes encoding CTX-M-1 and CTX-M-2 type enzymes (CTX-M-1 and CTX-M-2 type genes) and the synthesis of probe CTX-M were performed with the primers CTX-MA (5′-CGCTTTGCGATGTGCAG-3′) and CTX-MB (5′-ACCGCGATATCGTTGGT-3′) (temperature of annealing, 52°C). An internal fragment of 550 bp was amplified from positions 264 to 814 (blaCTX-M-1 numbering), which correspond to conserved regions of CTX-M-1 and CTX-M-2 type genes.
Hybridization.
Plasmid DNAs were extracted by the method of Birnboim and Doly (9) and the bromide-CsCl linear gradient method (35). DNA probes used for hybridization were PCR products obtained with primers CTX-MA and CTX-MB. Labeling was performed by random priming with the dinitrophenyl (DNP) DNA-labeling kit purchased from Appligene Oncor (Illkirch, France). Hybridization and revelation were performed with the DNP DNA chemiluminescence detection kit (Appligene Oncor) according to the manufacturer's recommendations on DNA extracts denatured, transferred, and immobilized on Nytran filters.
β-Lactamase gene cloning.
Recombinant DNA manipulation and transformations were performed as described by Sambrook et al. (35). T4 DNA ligase was purchased from Boehringer GmbH, Mannheim, Germany. The CTX-M-encoding gene was cloned as follows: plasmid DNA of strain TrRio-2 was cleaved by EcoRI, and the resultant fragments were ligated in the EcoRI site of pACYC184 (34). E. coli DH5α (35) was transformed by electroporation. The transformant C1Rio-2 harboring the CTX-M-8-encoding plasmid pC1Rio-2 was selected on Mueller-Hinton agar supplemented with 2 μg of cefotaxime per ml.
DNA sequencing.
The sequences were determined by sequencing both strands of recombinant plasmid DNA. The strategies used to establish the nucleotide sequences of the CTX-M type genes are summarized in Fig. 1. The sequence of blaCTX-M-8 was determined from recombinant plasmid pC1Rio-2 with a primer localized on plasmid pACYC184, close to the EcoRI restriction site. It was performed by the dideoxy chain termination procedure of Sanger et al. (36) on an ABI 1377 automatic sequencer using the ABI PRISM dye terminator cycle sequencing ready reaction kit with AmpliTaq DNA polymerase FS (Perkin-Elmer/Applied Biosystems Division, Foster City, Calif.).
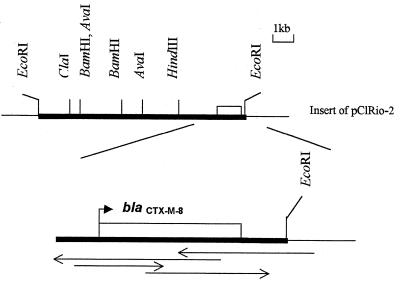
FIG. 1.
Map of the EcoRI CTX-M-8-encoding insert of pC1Rio-2. Arrows indicate the strategy used to establish the nucleotide sequence.
Computer analysis.
The nucleotide sequence and the deduced protein sequence were analyzed with the software available over the Internet at the National Center for Biotechnology Information. A hydrophobic plot was obtained by the method of Nielsen et al. (29). Multiple sequence alignment and pairwise comparisons of sequences were carried out with the help of the software ClustalW, version 1.74 (43). Nine class A CTX-M enzymes were compared to CTX-M-8: CTX-M-1, CTX-M-2, Toho-1, CTX-M-3, CTX-M-4, CTX-M-5, CTX-M-6, CTX-M-7, and Toho-2. A dendrogram was derived from the protein multiple-sequence alignment by the parsimony method using the phylogenetic package PAUP (phylogenetic analysis using parsimony), version 3.0 (42).
Nucleotide sequence accession number.
The blaCTX-M-8 gene nucleotide sequence data appear in the GenBank nucleotide sequence database under accession no. AF189721.
RESULTS
Characterization of clinical isolates.
The clinical isolates Rio-1, Rio-2, and Rio-3 (Table 1) exhibited a resistance to broad-spectrum cephalosporins and a positive double-disk synergy test and produced β-lactamases of pI 7.6 and 5.4. In addition, the Enterobacter strains Rio-1 and Rio-3 harbored β-lactamases with alkaline pI values consistent with cephalosporinase production.
PCR and DNA sequencing identified the β-lactamase of pI 5.4 as TEM-1 penicillinase. The enzyme of pI 7.6 was not of the SHV type, and no PCR products were obtained with the CTX-M-1 and CTX-M-2 type gene-specific primers CTX-MA and CTX-MB. In contrast, CTX-M type genes were detected in the three strains by hybridization with a CTX-M type gene probe, suggesting the presence of new CTX-M type genes in these strains.
Transfer of β-lactam resistance.
Transconjugants TrRio-2 and TrRio-3 were only obtained from C. amalonaticus Rio-2 and E. aerogenes Rio-3 strains. They produced cefotaxime-hydrolyzing β-lactamase of pI 7.6, associated with the TEM-1 penicillinase. The CTX-M gene was carried by large plasmids (≥75 kb), which also harbored aminoglycoside resistance genes (Table 1).
Cloning of the β-lactamase gene.
The genes carried by pRio-2 were cloned in plasmid pACYC184. Transformant C1Rio-2 contained recombinant plasmid pC1Rio-2 of 14 kb, producing only the CTX-M type β-lactamase of pI 7.6. The restriction map of the insert and hybridization with CTX-M type gene probe localized gene blaCTX-M close to the cloning site of pACYC184 (Fig. 1).
β-Lactam susceptibility.
MICs of β-lactams for C. amalonaticus Rio-2, its E. coli HB101 transconjugant TrRio-2 harboring plasmid pRio-2, and E. coli DH5α harboring recombinant plasmid pC1Rio-2 are listed in Table 2. These CTX-M-producing strains exhibited a high level of resistance to amoxicillin (MICs > 2,048 μg/ml), ticarcillin (MICs > 2,048 μg/ml), and cephalothin (MICs ≥ 1,024 μg/ml) and a low level of resistance to cefotaxime (MICs, 8 to 32 μg/ml). Unlike those for the C. amalonaticus Rio-2 isolate, MICs for E. coli transconjugant TrRio-2 and transformant C1Rio-2 of cefotaxime were 8- to 16-fold higher than those of ceftazidime and 2- to 4-fold higher than those of aztreonam. The same results were observed with strains Rio-1, Rio-3, and TrRio-3 (data not shown). MICs of cefepime and cefpirome, even when they were weak, were appreciably higher than those obtained with TEM-1-producing E. coli (2 to 16 μg/ml versus 0.12 μg/ml). In contrast, the activities of imipenem and cefoxitin were not affected.
TABLE 2.
β-Lactam MICs for the CTX-M-8-producing C. amalonaticus and its E. coli derivatives
| β-Lactam(s) | MIC for strain:
|
||||
|---|---|---|---|---|---|
| C. amalonaticus Rio-2 (pRio-2)a | E. coli HB101 TrRio-2 (pRio-2)b | E. coli DH5α C1Rio-2 (pC1Rio-2)c | E. coli HB101 TR4 (pTR4)d | E. coli DH5α | |
| Amoxicillin | >2,048 | >2,048 | >2,048 | >2,048 | 2 |
| Amoxicillin + cae | 64 | 16 | 32 | 16 | 1 |
| Ticarcillin | >2,048 | >2,048 | >2,048 | >2,048 | 2 |
| Ticarcillin + ca | 64 | 16 | 32 | 16 | 2 |
| Cephalothin | >1,024 | 1,024 | >1,024 | 256 | 4 |
| Cefoxitin | 4 | 2 | 4 | 2 | 2 |
| Cefotaxime | 32 | 8 | 16 | 0.12 | 0.06 |
| Cefotaxime + ca | 0.25 | 0.06 | 0.06 | 0.06 | 0.06 |
| Aztreonam | 64 | 4 | 8 | 0.25 | 0.12 |
| Aztreonam + ca | 0.50 | 0.12 | 0.12 | 0.12 | 0.12 |
| Ceftazidime | 64 | 0.50 | 1 | 0.5 | 0.25 |
| Ceftazidime + ca | 1 | 0.12 | 0.12 | 0.5 | 0.25 |
| Cefepime | 8 | 2 | 4 | 0.12 | 0.06 |
| Cefepime + ca | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| Cefpirome | 16 | 4 | 8 | 0.12 | 0.06 |
| Cefpirome + ca | 0.12 | 0.06 | 0.06 | 0.06 | 0.06 |
| Imipenem | 0.12 | 0.25 | 0.25 | 0.12 | 0.06 |
C. amalonaticus Rio-2 producing β-lactamase CTX-M-8 along with a TEM-1 penicillinase.
E. coli HB101 harboring natural plasmid pRio-2, which encodes β-lactamase CTX-M-8 along with a TEM-1 penicillinase.
E. coli DH5α harboring recombinant plasmid pC1Rio-2, which encodes β-lactamase CTX-M-8.
E. coli HB101 harboring plasmid pTR4, which encodes β-lactamase TEM-1.
ca, clavulanate at fixed concentration of 2 μg/ml.
Clavulanate restored partially or totally the activities of the β-lactams. All strains were susceptible to associations of clavulanate and broad-spectrum cephalosporins (MICs, 0.06 to 1 μg/ml).
Kinetic parameters.
The substrate and inhibition profiles of CTX-M-8 are shown in Table 3. The best affinities were observed with penicillins and cefuroxime (Km, 11 to 19 μM) and led to good catalytic efficiency. However, the best substrate of CTX-M-8 was cephalothin. The catalytic activity of the enzyme against cephalothin was 10-fold higher than that against penicillins despite the fact that cephalothin had higher Km values (Km, 87 μM).
TABLE 3.
Substrate profile of CTX-M-8 β-lactamase
| Substrate | Relative Vmaxa | Km (μM) | Relative Vmax/Kmb |
|---|---|---|---|
| Benzylpenicillin | 100 | 11 | 100 |
| Amoxicillin | 37 | 12 | 34 |
| Ticarcillin | 12 | 14 | 10 |
| Piperacillin | 49 | 19 | 29 |
| Cephalothin | 1,070 | 87 | 137 |
| Cefuroxime | 25 | 12 | 23 |
| Cefotaxime | 49 | 74 | 7 |
| Cefpirome | 300 | 1,200 | 3 |
| Cefepime | 96 | 990 | 1 |
| Aztreonam | 9 | 800 | 0.12 |
| Ceftazidime | ≤1 | >500c | ≤0.02 |
| Cefoxitin | NDd | 5c | |
| Imipenem | ND | 1c |
Values are percentages of the Vmax for benzylpenicillin.
Values are percentages of the relative Vmax/Km ratio for benzylpenicillin.
Values were determined as Ki by substrate competition with benzylpenicillin.
ND, no catalytic activity detected.
Likewise, CTX-M-8 had activity against cefotaxime, cefepime, and cefpirome. The greater catalytic efficiency of CTX-M-8 against cefotaxime than against cefepime, and to lesser extent cefpirome, was due to the higher Km values for the last two substrates (cefepime Km, 990 μM; cefpirome Km, 1,200 μM; cefotaxime Km, 74 μM). In contrast, the catalytic activities for ceftazidime (relative Vmax ≤ 1%) and aztreonam (relative Vmax, 9%) were at least 49-fold and 5-fold lower, respectively, than that for cefotaxime (relative Vmax, 49%). Aztreonam and ceftazidime were poor substrates of CTX-M-8. Imipenem and cefoxitin were not affected by CTX-M-8.
CTX-M-8 was susceptible to tazobactam (IC50, 0.010 μM), clavulanate (IC50, 0.036 μM), and sulbactam (IC50, 4.0 μM).
DNA sequencing.
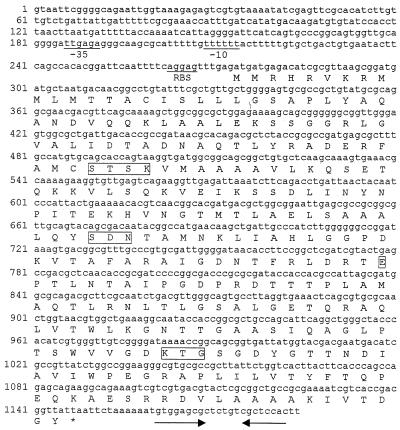
The nucleotide sequence and the deduced amino acid sequence are given in Fig. 2. There was an open reading frame of 873 nucleotides. This coding region had 73 to 75% identity with the previously described CTX-M-type genes. The initiation codon sequence was preceded by putative −35 (TTGAGA) and −10 (TTTTTT) consensus sequences. A terminator hairpin loop was detected 10 nucleotides from the stop codon (Fig. 2). Sequencing of CTX-M type genes of strains Rio-1 and Rio-3 confirmed that these strains harbored the same blaCTX-M gene.
FIG. 2.
Nucleotide sequence of the 1,184-bp fragment of pC1Rio-2 containing blaCTX-M-8. The deduced amino acid sequence is designated in single-letter code below the nucleotide sequence. The potential promoter sequences (−10 and −35 regions) and ribosome-binding site (RBS) are shown. Solid arrows, inverted repeat sequences possibly acting as terminators. Conserved residues in serine β-lactamases (SXXK, SDN, E, KTG) are boxed.
The precursor amino acid sequence deduced from the nucleotide sequence consisted of 291 amino acid residues. On the basis of alignments with the CTX-M peptide sequence previously determined by direct amino acid sequencing (4, 27) (Fig. 3) and hydropathy plots, it is likely that the signal peptide comprised 28 amino acids. Thus, the putative mature CTX-M type enzyme consisted of 263 amino acid residues with a calculated isoelectric point of 7.72 and a calculated molecular weight of 28,039. The four structural elements characteristic of class A β-lactamases were found: S-X-X-K at positions 70 to 73, S-D-N at positions 130 to 132, E at position 166, and K-T-G at positions 234 to 236 (Fig. 2).
FIG. 3.
Alignments of the CTX-M-8 amino acid sequence with those of CTX-M-1 (4, 6), CTX-M-2 (5), CTX-M-3 (19), CTX-M-4 (18), CTX-M-5 (11), CTX-M-6 (15), CTX-M-7 (15) (previously designated CTX-M-5), Toho-1 (19), and Toho-2 (27). Dots, amino acids identical to those of CTX-M-8; boldface letters, specific amino acid residues of CTX-M-8 in the CTX-M family; italic letters, peptide signal of CTX-M-8 as determined by a hydopathy plot; boxes, peptide signals determined by amino acid sequencing; roman numerals, boxes described by Joris et al. (23). Amino acids are numbered according to the standard numbering scheme for the class A β-lactamases of Ambler et al. (1).
Homology with other β-lactamases.
The sequence of the mature form of the CTX-M type enzyme has less than 37% amino acid identity with the sequences of TEM-1 and SHV-1 but 75 to 77% amino acid identity with the sequences of class A β-lactamases of P. vulgaris R0104 (31), S. fonticola CUV (30), C. diversus ULA27 (32), K. oxytoca E23004 (2), and K. oxytoca D488 (33). The previously described CTX-M β-lactamases have 83 to 88% amino acid identity with this novel enzyme, which was designated CTX-M-8.
The peptide sequence alignment shown in Fig. 3 displays the conserved regions and the amino acid substitutions observed in different CTX-M enzymes. Excluding positions 185 to 219 of Toho-2, whose sequences have been discussed elsewhere (25), seven conserved regions were observed. Still excluding positions 186 to 218 of Toho-2, the alignments of CTX-M enzymes showed 72 polymorphic positions, of which 5 were reported solely in CTX-M-8 (positions 92, 103, 109, 158, and 197). Twelve amino acid residues of CTX-M-8 had not previously been observed in the other CTX-Ms (Fig. 3).
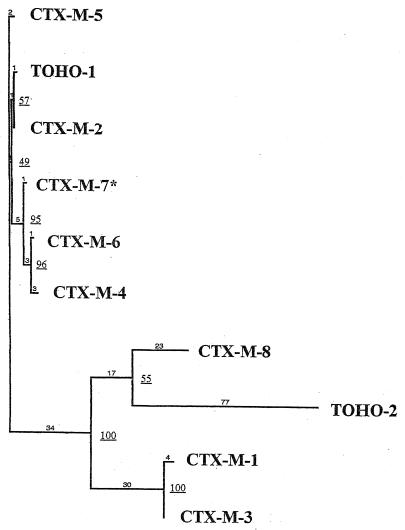
Phylogenic analysis.
A dendrogram (Fig. 4) was constructed on the basis of the peptide alignment shown in Fig. 3. Bootstrapping gave a high degree of resolution for internal nodes (greater than 50% majority consensus confidence) in all but one branch (49%). The dendrogram showed four major branches, whose members were closely related, separated by large evolutionary distances. Four types of CTX-M were obtained: the CTX-M-1 type including CTX-M-1 and CTX-M-3; the CTX-M-2 type including CTX-M-2, Toho-1, CTX-M-5, CTX-M-4, CTX-M-6, and CTX-M-7; and Toho-2 and CTX-M-8, both of which had only one member. Doubt over the length and also the position of the Toho-2 branch persists because of disagreements relating to its sequence (25).
FIG. 4.
Dendrogram of CTX-M family. Branch lengths are to scale and are proportional to the numbers of amino acid changes. The percentages at the branch points refer to the numbers of times particular nodes were found in 100 bootstrap replications (underlined numbers). The distance along the vertical axis has no significance. ∗, previously designated CTX-M-5 (15).
DISCUSSION
The starting point of this work was the observation of three clinical strains that exhibited a positive double-disk synergy test, resistance to broad-spectrum cephalosporins, and a β-lactamase of pI 7.6. A distinctly higher level of resistance to cefotaxime than to ceftazidime was observed with the transconjugants obtained. However, C. amalonaticus Rio-2, unlike transconjugant TrRio-2, exhibited an ESBL phenotype of the ceftazidimase type. These differences of behavior between the wild-type strain Rio-2 and its transconjugant suggest an additional resistance mechanism directed mainly against ceftazidime in C. amalonaticus Rio-2. A chromosomally mediated cephalosporinase, which has mainly cefotaximase activity, in C. amalonaticus has been previously reported (8), but no β-lactamase of corresponding pI (5.5 and 6.05) was detected in our strain. Decreased permeability might also explain the enhanced resistance to ceftazidime. However, such a mechanism in this species has not been reported.
In the course of cloning, a novel CTX-M type gene was characterized. The deduced enzyme, designated CTX-M-8, had 80 to 88% identity with previously described CTX-M enzymes. The conserved regions are known to have a critical role in the catalytic activity of active-site serine penicillin-recognizing enzymes (23). In addition, CTX-M-8 harbors amino acid residues Ser-237 and Arg-276, which have been suspected of playing a part in the cefotaxime-hydrolyzing activity of CTX-M enzymes (4, 14–16, 18, 27). The alignments of CTX-M enzymes showed the high level of polymorphism of the CTX-M family compared to those of the TEM and SHV families. The amino acid substitutions are generally conservative or semiconservative (43). Crystallographic data (18, 22) demonstrate that the majority of the substitutions are localized far from the active site, in weakly conserved zones of class A β-lactamases. This explains the close similarities in the catalytic activities of CTX-M enzymes.
The phylogenic study of the CTX-M family showed four major types of CTX-M: the CTX-M-1 type, the CTX-M-2 type, Toho-2, and CTX-M-8. The evolutionary distances between the four types of CTX-M were large, suggesting that the four clusters of CTX-M branched off early from an unknown protein. Thus, these enzymes could be mutant derivatives from a distant common ancestor. Closely related enzymes of the CTX-M-1 type (M-1, M-3) and of the CTX-M-2 type (M-4, M-5, M-6, M-7) have been observed in a concentrated geographic area where they may have occurred as a result of point mutations, which suggests the existence of many unknown intermediate enzymes. In contrast, CTX-M-2 and Toho-1, classified on the same branch of the CTX-M-2 cluster, have been characterized in geographically distant areas (Japan and South America). The expansion of the CTX-M family may therefore be due to the spread and mutations of the CTX-M-encoding genes, but independent genetic events cannot be excluded.
The catalytic properties of CTX-M-8 are close to those previously reported for CTX-M enzymes. Cephalothin is the best substrate. Catalytic efficiency against cefotaxime is better than that against ceftazidime. CTX-M-8 is slightly more susceptible to the inhibitors than TEM penicillinases (10). Tazobactam is the best inhibitor, as previously described (5, 15, 16, 27).
The strains studied in this report were selected from 18 strains of the family Enterobacteriaceae chosen to characterize the different ESBLs present in Brazil. The majority of these strains (10 of 18) produced SHV type ESBLs. Two TEM type ESBLs were also identified. Four strains produced CTX-M type enzymes: E. cloacae Rio-1, C. amalonaticus Rio-2, and E. aerogenes Rio-3 (CTX-M-8) and P. mirabilis Rio-4 (CTX-M-2). Another CTX-M-like enzyme produced by an E. coli strain and a broad-spectrum cephalosporin-hydrolyzing enzyme not related to the CTX-M, SHV, and TEM type enzymes produced by a Serratia marcescens strain are being studied. These data show the diversity of the ESBLs in Brazil and the spread of CTX-M enzymes.
Like CTX-M-2, which is observed in a large variety of species such as E. coli, P. mirabilis, K. pneumoniae, and V. cholerae (7; Galas et al., 38th ICAAC, abstr. C-174), the blaCTX-M-8 gene was characterized for three different species of the family Enterobacteriaceae. The transferable capacity of plasmids could explain the in vivo transfer of the blaCTX-M-8 gene in different strains and the spread of this enzyme.
CTX-M-2 was first characterized in Argentina (5, 7), and Galas et al. (38th ICAAC, abstr. E-109) report that the predominant ESBL types are first CTX-M-2 and then SHV in that country. We report 5 CTX-M-producing strains out of 18 ESBL-producing strains isolated in Brazil. Thus, like eastern Europe (11, 16, 17), South America could be an important source of CTX-M type β-lactamases. The spread of CTX-M enzymes and the paucity of TEM type mutants seem therefore to be a regional phenomenon in South America.
The first CTX-M-producing strains were sporadic isolates. Recently, outbreaks involving S. enterica serovar Typhimurium (11) and V. cholerae El Tor (Galas et al., 38th ICAAC, abstr. C-174) have been recently described. Nosocomial infections of the urinary tract induced by CTX-M-producing C. freundii have also been reported (17). In this study, E. aerogenes and E. cloacae, two species known to be responsible for nosocomial infections (3, 13) and isolated from patients hospitalized in separate intensive care units of different hospitals, produced CTX-M-8, suggesting that they are involved in nosocomial infections.
Since the first report of the MEN-1 (CTX-M-1) in the 1990s (4, 6), a great variety of CTX-M enzymes have been observed, all of which belong to a new group among the ESBL enzymes. In this study, we report a novel member of the CTX-M family, CTX-M-8, which is not directly related to other CTX-M enzymes. Hence, CTX-M-8 constitutes a novel potential phylum of the CTX-Ms. The intensive use of broad-spectrum cephalosporins such as cefotaxime could account for the emergence and spread of the CTX-M plasmid-mediated enzymes among enteric pathogens. The analysis of novel blaCTX-M could shed light on the origin and the intermediate enzymes of the plasmid-mediated CTX-M β-lactamases.
ACKNOWLEDGMENTS
We thank Rolande Perroux, Marlène Jan, and Dominique Rubio for technical assistance. We are also grateful to Thierry Naas, Service de Bactériologie-Virologie, Faculté de Médecine Paris-Sud, for assistance in the phylogenic study and for use of the phylogenic package PAUP.
This work was supported in part by a grant from Ministère de l'Education Nationale, de la Recherche et de la Technologie.
REFERENCES
- 1.Ambler R P, Coulson A F W, Frere J-M, Ghuysen J M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–272. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa Y, Ohta M, Kido N, Mori M, Ito H, Komatsu T, Fujii Y, Kato N. Chromosomal β-lactamase of Klebsiella oxytoca, a new class A enzyme that hydrolyzes broad-spectrum β-lactam antibiotics. Antimicrob Agents Chemother. 1989;33:63–70. doi: 10.1128/aac.33.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballow C, Schentag J J. Trends in antibiotic utilization and bacterial resistance. Report of the National Nosocomial Resistance Surveillance Group. Diagn Microbiol Infect Dis. 1992;15:37S–42S. [PubMed] [Google Scholar]
- 4.Barthelemy M, Peduzzi J, Bernard H, Tancrede C, Labia R. Close amino acid sequence relationship between the new plasmid-mediated extended-spectrum β-lactamase MEN-1 and chromosomally encoded enzymes of Klebsiella oxytoca. Biochim Biophys Acta. 1992;1122:15–22. doi: 10.1016/0167-4838(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 5.Bauernfeind A, Casellas J M, Goldberg M, Holley M, Jungwirth R, Mangold P, Rohnisch T, Schweighart S, Wilhelm R. A new plasmidic cefotaximase from patients infected with Salmonella typhimurium. Infection. 1992;20:158–163. doi: 10.1007/BF01704610. [DOI] [PubMed] [Google Scholar]
- 6.Bauernfeind A, Grimm H, Schweighart S. A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection. 1990;18:294–298. doi: 10.1007/BF01647010. [DOI] [PubMed] [Google Scholar]
- 7.Bauernfeind A, Stemplinger I, Jungwirth R, Ernst S, Casellas J M. Sequences of β-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other β-lactamases. Antimicrob Agents Chemother. 1996;40:509–513. doi: 10.1128/aac.40.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben Yaghlane Bouslama H, Labia R, Sirot D, Vu Thien H. Chromosomally-mediated β-lactamases produced by Levinea amalonatica with activity against methoxyimino-cephalosporins. J Antimicrob Chemother. 1991;27:191–198. doi: 10.1093/jac/27.2.191. [DOI] [PubMed] [Google Scholar]
- 9.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnet R, De Champs C, Sirot D, Chanal C, Labia R, Sirot J. Diversity of TEM mutants in Proteus mirabilis. Antimicrob Agents Chemother. 1999;43:2671–2677. doi: 10.1128/aac.43.11.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradford P A, Yang Y, Sahm D, Grope I, Gardovska D, Storch G. CTX-M-5, a novel cefotaxime-hydrolyzing β-lactamase from an outbreak of Salmonella typhimurium in Latvia. Antimicrob Agents Chemother. 1998;42:1980–1984. doi: 10.1128/aac.42.8.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bush K, Jacoby J A, Medeiros A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Champs C, Henquell C, Guelon D, Sirot D, Gazuy N, Sirot J. Clinical and bacteriological study of nosocomial infections due to Enterobacter aerogenes resistant to imipenem. J Clin Microbiol. 1993;31:123–127. doi: 10.1128/jcm.31.1.123-127.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazouli M, Legakis N J, Tzouvelekis L S. Effect of substitution of Asn for Arg-276 in the cefotaxime-hydrolyzing class A β-lactamase CTX-M-4. FEMS Microbiol Lett. 1998;169:289–293. doi: 10.1111/j.1574-6968.1998.tb13331.x. [DOI] [PubMed] [Google Scholar]
- 15.Gazouli M, Tzelepi E, Markogiannakis A, Legakis N J, Tzouvelekis L S. Two novel plasmid-mediated cefotaxime-hydrolyzing β-lactamases (CTX-M-5 and CTX-M-6) from Salmonella typhimurium. FEMS Microbiol Lett. 1998;165:289–293. doi: 10.1111/j.1574-6968.1998.tb13159.x. [DOI] [PubMed] [Google Scholar]
- 16.Gazouli M, Tzelepi E, Sidorenko S V, Tzouvelekis L S. Sequence of the gene encoding a plasmid-mediated cefotaxime-hydrolyzing class A β-lactamase (CTX-M-4): involvement of serine 237 in cephalosporin hydrolysis. Antimicrob Agents Chemother. 1998;42:1259–1262. doi: 10.1128/aac.42.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gniadkowski M, Schneider I, Palucha A, Jungwirth R, Mikiewicz B, Bauernfeind A. Cefotaxime-resistant Enterobacteriaceae isolates from a hospital in Warsaw, Poland: identification of a new CTX-M-3 cefotaxime-hydrolyzing β-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob Agents Chemother. 1998;42:827–832. doi: 10.1128/aac.42.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibuka A, Taguchi A, Ishiguro M, Fushinobu S, Ishii Y, Kamitori S, Okuyama K, Yamaguchi K, Konno M, Matsuzawa H. Crystal structure of the E166A mutant of extended-spectrum β-lactamase Toho-1 at 1.8 A resolution. J Mol Biol. 1999;285:2079–2087. doi: 10.1006/jmbi.1998.2432. [DOI] [PubMed] [Google Scholar]
- 19.Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A β-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacoby G A. Genetics of extended-spectrum β-lactamases. Eur J Clin Microbiol Infect Dis. 1994;13:2–11. doi: 10.1007/BF02390679. [DOI] [PubMed] [Google Scholar]
- 21.Jarlier V, Nicolas M H, Fournier G, Philippon A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 22.Jelsch C, Monrey L, Masson J M, Samama J P. Crystal structure of Escherichia coli TEM-1 beta-lactamase at 1.8 A resolution. Proteins. 1993;16:364–383. doi: 10.1002/prot.340160406. [DOI] [PubMed] [Google Scholar]
- 23.Joris B, Ledent P, Dideberg O, Fonze E, Lamotte-Brasseur J, Kelly J A, Ghuysen J M, Frere J M. Comparison of the sequences of class A β-lactamases and of the secondary structure elements of penicillin-recognizing proteins. Antimicrob Agents Chemother. 1991;35:2294–2301. doi: 10.1128/aac.35.11.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kliebe C, Nies B A, Meyer S F, Tolxdorff-Neutzling R M, Wiedeman B. Evolution of plasmid-coded resistance to broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1985;28:302–307. doi: 10.1128/aac.28.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labia R. Analysis of the blatoho gene coding for Toho-2-β-lactamase. Antimicrob Agents Chemother. 1999;43:2576–2577. doi: 10.1128/aac.43.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labia R, Andrillon J, Le Goffic F. Computerized microacidimetric determination of β-lactamase Michaelis-Menten constants. FEBS Lett. 1973;33:42–44. doi: 10.1016/0014-5793(73)80154-1. [DOI] [PubMed] [Google Scholar]
- 27.Ma L, Ishii Y, Ishiguro M, Matsuzawa H, Yamaguchi K. Cloning and sequencing of the gene encoding Toho-2, a class A β-lactamase preferentially inhibited by tazobactam. Antimicrob Agents Chemother. 1998;42:1181–1186. doi: 10.1128/aac.42.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto Y, Inoue M. Characterization of SFO-1, a plasmid-mediated inducible class A beta-lactamase from Enterobacter cloacae. Antimicrob Agents Chemother. 1999;43:307–313. doi: 10.1128/aac.43.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Peduzzi J, Farzaneh S, Reynaud A, Barthelemy M, Labia R. Characterization and amino acid sequence analysis of a new oxyimino cephalosporin-hydrolyzing class A beta-lactamase from Serratia fonticola CUV. Biochim Biophys Acta. 1997;1341:58–70. doi: 10.1016/s0167-4838(97)00020-4. [DOI] [PubMed] [Google Scholar]
- 31.Peduzzi J, Reynaud A, Baron P, Barthelemy M, Labia R. Chromosomally encoded cephalosporin-hydrolyzing β-lactamase of Proteus vulgaris R0104 belongs to Ambler's class A. Biochim Biophys Acta. 1994;1207:31–39. doi: 10.1016/0167-4838(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 32.Perilli M G, Franceschini N, Segatore B, Amicosante G, Oratore A, Duez C, Joris B, Frere J M. Cloning and nucleotide sequencing of the gene encoding the beta-lactamase from Citrobacter diversus. FEMS Microbiol Lett. 1991;67:79–84. doi: 10.1016/0378-1097(91)90448-j. [DOI] [PubMed] [Google Scholar]
- 33.Reynaud A, Peduzzi J, Barthelemy M, Labia R. Cefotaxime-hydrolyzing activity of the β-lactamase of Klebsiella oxytoca D488 could be related to a threonine residue at position 140. FEMS Microbiol Lett. 1991;81:185–192. doi: 10.1016/0378-1097(91)90301-p. [DOI] [PubMed] [Google Scholar]
- 34.Rose R E. The nucleotide sequence of PACYC184. Nucleic Acids Res. 1988;16:355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 36.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sirot D, Chanal C, Henquell C, Labia R, Sirot J, Cluzel R. Clinical isolates of Escherichia coli producing multiple TEM-mutants resistant to β-lactamase inhibitors. J Antimicrob Chemother. 1994;33:1117–1126. doi: 10.1093/jac/33.6.1117. [DOI] [PubMed] [Google Scholar]
- 38.Sirot D, De Champs C, Chanal C, Labia R, Darfeuille-Michaud A, Perroux R, Sirot J. Translocation of antibiotic resistance determinants including an extended-spectrum β-lactamase between conjugative plasmids of Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother. 1991;35:1576–1581. doi: 10.1128/aac.35.8.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sirot D, Recule C, Chaibi E B, Bret L, Croize J, Chanal-Claris C, Labia R, Sirot J. A complex mutant of TEM-1 β-lactamase with mutations encountered in both IRT-4 and extended-spectrum TEM-15, produced by an Escherichia coli clinical isolate. Antimicrob Agents Chemother. 1997;41:1322–1325. doi: 10.1128/aac.41.6.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sirot D, Sirot J, Labia R, Morand A, Courvalin P, Darfeuille-Michaud A, Perroux R, Cluzel R. Transferable resistance to third-generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel β-lactamase. J Antimicrob Chemother. 1987;20:323–334. doi: 10.1093/jac/20.3.323. [DOI] [PubMed] [Google Scholar]
- 41.Sirot J. Detection of extended-spectrum plasmid-mediated β-lactamases by disk diffusion. Clin Microbiol Infect. 1996;2:S35–S39. doi: 10.1111/j.1469-0691.1996.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 42.Swofford D L. PAUP (version 3.0): phylogenetic analysis using parsimony. Champaign, Ill: Illinois Natural History Survey; 1989. [Google Scholar]
- 43.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yagi T, Kurokawa H, Senda K, Ichiyama S, Ito H, Ohsuka S, Shibayama K, Shimokata K, Kato N, Ohta M, Arakawa Y. Nosocomial spread of cephem-resistant Escherichia coli strains carrying multiple Toho-1-like β-lactamase genes. Antimicrob Agents Chemother. 1997;41:2606–2611. doi: 10.1128/aac.41.12.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]