Abstract
EGFR is a prototypical receptor tyrosine kinase that is overexpressed in multiple cancers including head and neck squamous cell carcinoma (HNSCC). The standard of care for HNSCC remains largely unchanged despite decades of research. While EGFR blockade is an attractive target in HNSCC patients and anti-EGFR strategies including monoclonal antibodies and kinase inhibitors have shown some clinical benefit, efficacy is often due to the eventual development of resistance. In this review, we discuss how the acquisition of mutations in various domains of the EGFR gene not only alter drug binding dynamics giving rise to resistance, but also how mutations can impact radiation response and overall survival in HNSCC patients. A better understanding of the EGFR mutational landscape and its dynamic effects on treatment resistance hold the potential to better stratify patients for targeted therapies in order to maximize therapeutic benefits.
Keywords: EGFR, head and neck squamous cell carcinoma, kinase inhibitors, resistance
1. Introduction
The epidermal growth factor receptor (EGFR) is a member of the ErbB/HER family of receptor tyrosine kinases (RTKs) and is essential for several cellular survival processes [1,2]. An extensively studied biomarker, EGFR is one of the most implicated genes in carcinogenesis due to its frequent overexpression and mutations in multiple cancers [3,4]. Head and neck squamous cell carcinomas (HNSCC) are tumors that arise predominantly in the mucosa of the oral cavity, sinuses, oropharynx, hypopharynx, and larynx [5]. Up to 80–90% of HNSCCs overexpress or harbor mutations in EGFR, and these alterations directly impact overall and progression-free survival [6,7,8]. Targeting EGFR therapeutically using anti-EGFR monoclonal antibodies or kinase domain inhibitors with concomitant radiation remains one therapeutic option for patients with HNSCC [7,9,10]. However, the efficacy of these therapies can be compromised, on the one hand, due to the presence of pre-existing genetic alterations in EGFR that render them resistant to EGFR blockade or, on the other hand, the acquisition of secondary mutations under therapeutic pressure, which helps evade targeting [11,12]. EGFR status is increasingly being recognized as a predictor of survival as well as chemoradiation response in HNSCC. In this review, we provide an overview of various genetic alterations in EGFR and how these mutations impact chemoradiation response as well as survival in HNSCC.
2. The EGFR Structure
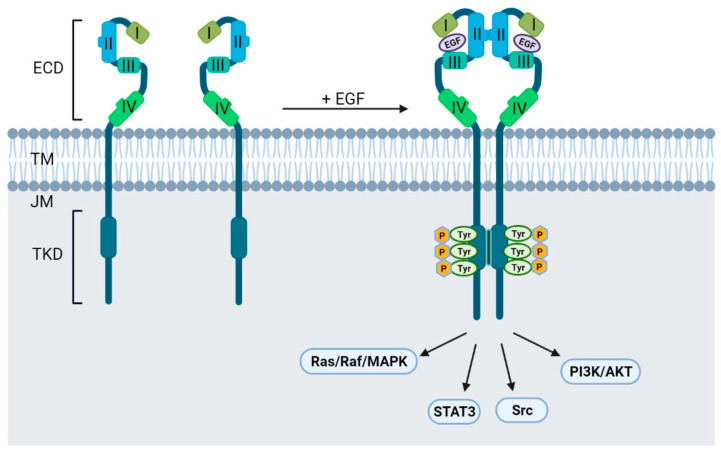
EGFR is a transmembrane protein comprised of an extracellular ligand-binding domain (ECD), a transmembrane domain (TD), a juxtamembrane (JM) segment, a tyrosine kinase domain (TKD), and a C-terminal regulatory tail [13,14]. The structure of EGFR with relevant domains is depicted in Figure 1. The ECD of EGFR is composed of four domains necessary for ligand binding. In an inactive state, domains I, II and III adopt a ‘closed’ conformation, while in an active state, EGF binds to the pocket between domains I and III and favors a conformational change to an ‘open’ untethered state [15]. This rearrangement allows domains II and IV to bind to the corresponding domains on the adjacent receptor leading to homo- or hetero-dimerization. The conformational change caused by ligand binding leads to auto- and trans-phosphorylation of the TKD with subsequent recruitment of adaptor proteins such as Src homology 2 (SH2) or phosphotyrosine-binding (PTB) domains. These domains then activate downstream pathways essential for cell survival and proliferation [15,16,17,18]. Since EGFR plays a critical role in regulating various cellular processes, oncogenic mutations in any of these domains result in aberrant expression and/or dysregulated signaling while also altering responses to EGFR targeting agents.
Figure 1.
EGFR structure with relevant domains and signaling activation.
3. EGFR Mutations and Drug Resistance
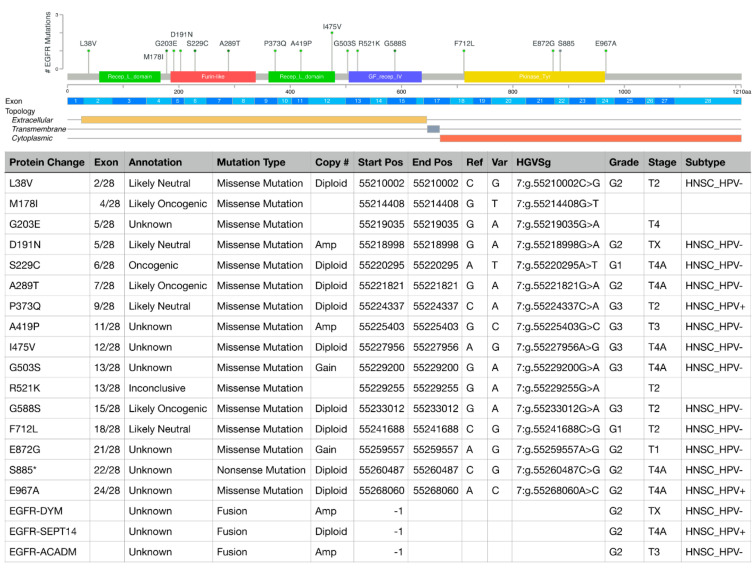
Acquisition of treatment resistance can be due to diverse mechanisms including (a) pre-existing mutations in EGFR or those acquired during therapy, (b) aberrant expression of EGFR ligands, (c) defective endocytosis of EGFR, (d) alterations in downstream signaling cascades, (e) receptor switching or crosstalk with other RTKs, and (f) epigenetic modifications [19,20,21]. Additionally, tumors develop resistance by acquiring mutations in domains where therapeutic antibodies bind. Multiple mutations have been reported in the TKD and the ECD, regions that are targeted for EGFR blockade (Figure 2); mutations in the TM domains have not been reported in HNSCC so far. Here, we discuss how mutations in EGFR domains initiate and drive resistance in HNSCC.
Figure 2.
EGFR mutations and gene fusions in HNSCC patients curated in the cBioPortal for Cancer Genomics in relation to EGFR exons and EGFR protein domains. The table shows details of the specific mutations and corresponding patients, including protein change, exon location, functional annotation according to OncoKB precision oncology knowledge base, type of mutation, EGFR gene copy number, mutation site, nucleotide change (reference [Ref] vs. variant [V]), Human Genome Variation Society genomic nomenclature (HGVSg), tumor grade, tumor, stage, HPV subtype (+, positive; −, negative). EGFR mutational and clinical data were visualized, analyzed, and downloaded from the cBioPortal for Cancer Genomics (https://www.cbioportal.org; accessed on 19 March 2022 [22,23]).
3.1. Extracellular Domain Mutations
Ectodomain targeting monoclonal antibodies against EGFR competitively bind and terminate signaling by binding to domain III of the ECD. Commonly used monoclonal antibodies include the FDA approved Cetuximab (CTX) and Panitumumab (PAN) with the former exhibiting higher efficacy in combination with radiotherapy or other biologic and chemotherapeutic agents such as pembrolizumab or cisplatin [9,24,25]. Until now, mutations reported in the ECD of EGFR have exclusively been associated with CTX resistance in HNSCC while mutational resistance to PAN has been less explored with scant reports [20,26].
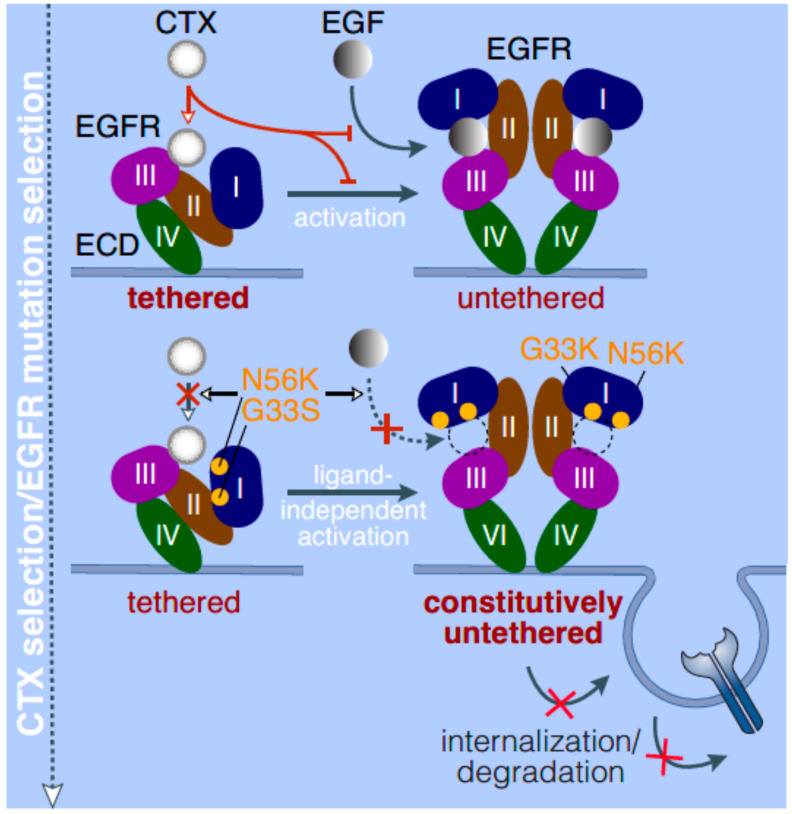
A clinical study investigating the development of resistance in a patient initially sensitive to CTX analyzed both pre- and post-CTX tumor biopsies and found a missense mutation in the EGFR-ECD at the 465th position with a glycine to arginine substitution (G465R) [26]. Further analysis found that the mutation altered the binding of CTX to domain III of the ECD sustaining resistance. Additional non-synonymous mutations (alterations in amino acid sequence) have been reported in the ECD of EGFR (G33S, N56K) in an HNSCC cell line selected for resistance to CTX (20). These acquired mutations in domain I of the ECD resulted in a lower affinity for EGF binding; however, constitutive activation of EGFR was observed along with diminished internalization of EGFR for degradation. Additionally, these mutations trapped EGFR in an open extended conformation that prevented CTX from accessing its binding site on domain III leading to resistance [20] (Figure 3). Interestingly, a similar mechanism has been observed with EGFR ECD mutations in glioblastoma, where missense mutations located at the domain I-to-II interface led to spontaneous untethering of the self-inhibitory tether driving oncogenicity [27]. Additionally, the A289T mutation (Figure 2) has been reported in glioblastoma multiforme, anaplastic astrocytoma, and lung adenocarcinoma [28] and appears to favor a ligand-independent formation of the active state [29]. Therefore, ECD mutations in EGFR across tumors seem to share a common mechanism of bypassing steric inhibition leading to unchecked signaling as well as the acquisition of a resistant phenotype by preventing monoclonal antibody binding to EGFR. CTX resistance has also been reported in patients with an EGFR-K521 (K-allele) polymorphism, in which CTX had lower binding affinity along with an inability to inhibit downstream signaling [30]. The authors suggested this polymorphism could be used as a prognostic predictor of therapeutic resistance, which is discussed in subsequent sections of this review. While most mutations are acquired in response to therapy, one report showed that the presence of a R521K substitution in both in vitro and in vivo HNSCC models rendered tumors resistant to CTX but sensitive to a c-MET TKI (SU11274) [31]. Interestingly, a discrepancy in EGFR expression between the primary tumor and metastatic brain lesions was observed in a patient under long-term CTX treatment who eventually acquired resistance [32]. The metastatic lesions had lower EGFR expression and higher expression of N-cadherin with an upregulation of epithelial-to-mesenchymal (EMT) transition genes indicating a mechanism of therapeutic evasion. In general, overcoming resistance to CTX entails switching to a different monoclonal antibody or targeting alternative domains in EGFR such as the tyrosine kinase domain as discussed below.
Figure 3.
CTX and EGF binding dynamics in normal cells and cells with ECD mutations in EGFR.
3.2. Tyrosine Kinase Domain Mutations
Structurally, the TKD of EGFR is a vital component and mediator of downstream signaling cascades that regulate diverse biological processes [33]. Targeting the TKD is an attractive approach as cancer cells are dependent on RTK signaling for sustenance. TKIs bind to the TKD and inhibit phosphorylation of the kinases preventing downstream signal transduction [19]. Several kinase targeting therapies called tyrosine kinase inhibitors (TKIs) such as Gefitinib, Erlotinib, and Afatinib, have been used to treat HNSCC in the past few decades [9,34,35,36]. As with ECD targeting therapies, the eventual development of resistance to TKIs is a major factor in limiting their efficacy; aberrant activation of the TKD mediated by mutations is a mechanism exploited by cancers to not only propagate signaling but also evade TKI targeting.
A retrospective study of 47 diagnosed HNSCC cases in a Saudi cohort showed that 57% of tumors had mutations in the TKD spanning exons 18 to 21. Specifically, a T790M mutation was observed in 4 patients resistant to tyrosine kinase inhibitor (TKI) therapy. Mutational status also correlated with higher grade and advanced stage of the tumor [37]. Conversely, mutational screening of 52 specimens in a Belgian cohort did not find any missense mutations in the TKD indicating that the prevalence of TKD mutations could possibly vary between different ethnic populations [38]. A systematic review of 53 studies found the overall pooled prevalence of TKD mutations in head and neck cancer patients was 2.8% in 4122 patients studied. It was discovered that 41.5% of these mutations were observed in exon 19, 32.1% in exon 20, 17% in exon 21, and 9.4% in exon 18. The predominant mutation was of the missense type with T790M and L861Q substitutions observed in multiple cases [39]. The T790M mutation has also been reported as the most prevalent resistance alteration in TKI resistant lung carcinoma patients suggesting that the mutational profile with respect to TKI resistance could be shared across tumors [12,40,41]. Kinase domain duplication (KDD), which entails duplication of the exons encoding the TKD, has been reported in HNSCC [42]. These KDD mutants exhibit higher phosphorylation as well as EGF- independent activation states leading to aberrant EGFR signaling [42]. Overall, somatic EGFR mutations and gene copy gain mediate therapeutic resistance in HNSCC underscoring the need for newer strategies such as multitargeted kinase inhibitors or dual targeting of both the ECD and the TKD [36,43,44,45].
4. EGFR Alterations and Radiation Response
For many HNSCCs, the response to a single-agent chemotherapy or monoclonal antibody alone remains dismally low mandating a multi-modal approach combining surgery, chemotherapy and/or radiation [46,47]. Early in vitro studies demonstrated that high concentrations of EGF slowed tumor growth, presumably through negative feedback and resulted in radiosensitization of human HNSCC cells [48,49]. Next, anti-EGFR blocking antibodies, which inhibited EGFR-induced signaling, showed prominent radiosensitization for squamous cell carcinomas in vitro [50,51,52]. Subsequently, patients with locoregionally advanced HNSCC treated with concomitant radiotherapy (RT) plus CTX exhibited a significantly improved 5-year survival and locoregional control without major toxic effects [24,53]. Multiple studies examining the role of EGFR in radiation resistance of HNSCC found that patients with high expression of EGFR had significantly lower overall survival and higher relapse rates [54,55]. At a cellular level, EGFR also modulates the repair of radiation-induced double stranded DNA breaks (DSB) by forming an EGFR–DNA-PK complex [56]. Inhibition of EGFR by CTX in HNSCC increased radiation-induced apoptosis and dysregulation of repair mechanisms mediated by downstream effectors such as JAK-STAT3 and PI3K-AKT pathways confirming the role of EGFR in mediating radioresistance [57]. One study reported that prolonged treatment with CTX promoted p27Kip1-mediated G1 arrest with the induction of autophagy [58]. The activation of autophagy has been shown to have opposing roles with respect to radiation response with some reports showing autophagy to be a radiosensitizer [59,60], while others show it plays a role in radioresistance [61]. Interestingly, a recent study found that EGFR expression and RT response depended on the human papilloma virus (HPV) status of the tumors [62]. In HPV-negative HNSCC cells, EGFR overexpression conferred increased survival, epithelial-to-mesenchymal transition and radioresistance via activation of vital DSB repair proteins post-irradiation. Conversely, in HPV-positive HNSCC cells, EGFR overexpression increased radiosensitization by abrogating the expression of DSB repair proteins post-irradiation. Additionally, HPV E16 levels were significantly suppressed in EGFR overexpressing HPV-positive cells resulting in restoration of p53 activity leading to RT sensitivity. When EGFR was inhibited using the TKI Gefitinib, it led to radiosensitization, further confirming that EGFR expression has a vital role in RT response [62].
EGFR also plays indirect roles in radioresistance by exploiting various oncogenic survival mechanisms. For example, EGFR is known to regulate and maintain cancer stem cells (CSC), which are distinct subpopulations in tumors with the ability to self-renew and differentiate, and which are known to be inherently radioresistant due to their enhanced DNA damage repair abilities [63,64,65]. As a result, discrepancies in RT sensitivity exist within a tumor leading to radioresistance and relapse. Two separate studies found that combining the EGFR inhibitor afatinib with ionizing radiation led to a significant decrease in CSC populations as well as colony forming abilities of these CSCs with an overall decrease in tumor size [66,67]. An increase in phosphorylated γH2AX, indicative of DNA damage, was also observed in afatinib treated cells [66,67]. Another study explored the role of HIF-1α, a marker of hypoxia, which is known to upregulate EGFR expression in tumors [68]. When HIF-1α was inhibited using rapamycin and used in combination with CTX and concomitant ionizing radiation in HNSCC in vivo models, a significant reduction in tumor sizes were observed initially followed by a rapid relapse. The authors found that this treatment regimen induced the expression of another hypoxia gene known as HIF-2α, which mediates resistance to EGFR inhibition as well as radioresistance. Subsequent HIF-2α inhibition with concomitant EGFR inhibition and ionizing radiation led to radiosensitization and a significant decrease in tumor growth [68].
Taken together, EGFR mediates radiation response in HNSCC either directly, by regulating its own downstream activation partners or DNA damage response proteins, or indirectly, by initiating and regulating tumor-specific survival mechanisms.
5. EGFR Alterations as Prognostic Indicators for Disease and Therapeutic Response
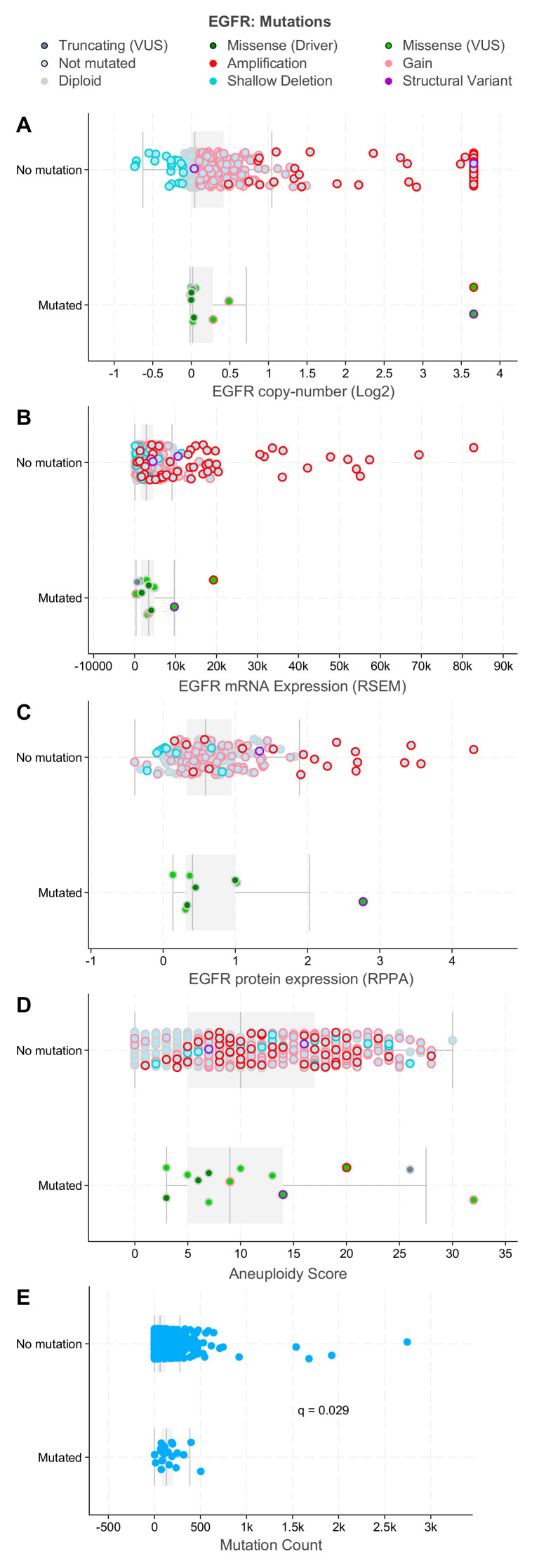
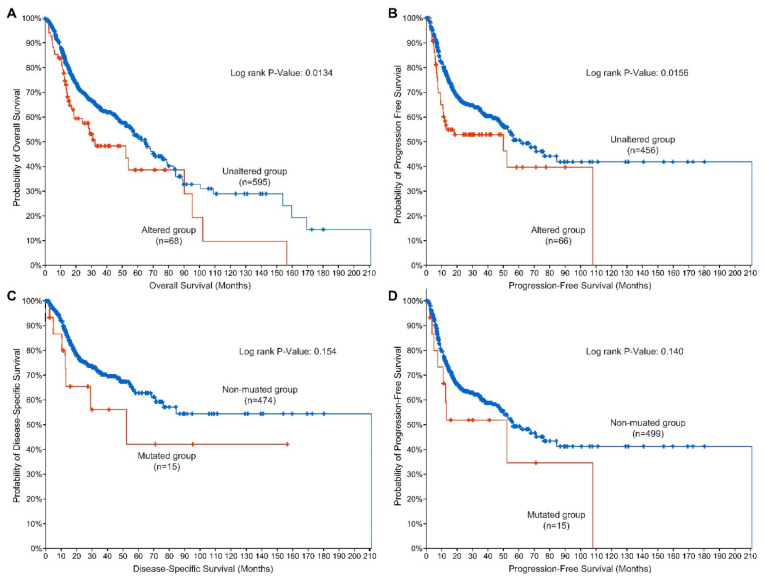
With the importance of EGFR in mediating chemoradiation response discussed in the previous sections, EGFR has been shown to be of prognostic significance in HNSCC. The predictive value of EGFR has been very well established using various methodologies such as immunohistochemical analysis of tumor samples, fluorescent in situ hybridization, tissue microarray, and gene sequencing [69,70,71]. Multiple studies have shown that the overexpression of EGFR in HNSCC directly correlates with worse outcomes [47,54,72,73,74]. Meta-analyses have shown that EGFR overexpression is associated with reduced overall survival (OS), and progression-free survival (PFS), and disease-free survival (DFS) with the magnitude of these effects varying from study to study [75,76,77,78]. EGFR mutations appear to rarely co-segregate with amplifications of the EGFR gene (Figure 4) in H&N cancers as is often the case in glioblastoma. EGFR mutated HNSCCs show a ranges of EGFR mRNA and protein expression comparable to their non-amplified counterparts. EGFR mutated tumors show a range of chromosomal aneuploidy scores similar to non-mutated tumors but demonstrate a significantly lower overall mutational burden (Figure 4). Patients with either EGFR mutation or amplification demonstrate comparatively briefer overall survival and progression-free survival than patients with tumors that do not carry these genetic events, indicating that these alterations could have potential prognostic value. Moreover, EGFR mutations are suggestively associated with disease-specific and progression-free survival in H&N cancers (Figure 5). While these observations are based on univariate analyses and thus have obvious limitations, they are hypothesis-generating for future work. The low variant frequency for the EGFR mutation—a situation in which variance estimates tend to inflate—might make it difficult to model this survival relationship in an adjusted model unless a very large sample size is studied.
Figure 4.
EGFR mutations in relation to EGFR copy number (A), mRNA (B), protein (C), and genome-wide aneuploidy (D) levels and overall mutational burden (E) in HNSCC patients curated in the cBioPortal for Cancer Genomics. EGFR mutations rarely co-segregate with EGFR amplifications (A) and show ranges of EGFR mRNA (B) and protein (C) expression comparable to their non-amplified counterparts. EGFR mutated tumors show a range of chromosomal aneuploidy scores (D) similar to non-mutated tumors, but demonstrate a significantly lower overall mutational burden (E). EGFR genomic, and aneuploidy, and mutational count data were visualized, analyzed, and downloaded from the cBioPortal for Cancer Genomics (https://www.cbioportal.org; accessed on 19 March 2022 [22,23]).
Figure 5.
EGFR alterations and survival in H&N cancer patients curated in the cBioPortal for Cancer Genomics. Patients who carry EGFR mutations and/or amplifications demonstrate a significantly briefer overall (A) and progression-free survival (B) than patients with unaltered EGFR. EGFR mutations are suggestively associated with disease-specific (C) and progression-free survival (D). EGFR genomic and clinical data were visualized, analyzed, and downloaded from the cBioPortal for Cancer Genomics (https://www.cbioportal.org; accessed on 19 March 2022 [22,23]).
A phase III EXTREME study that evaluated EGFR copy number as a predictive marker in patients treated with a combination of CTX and platinum/5-fluorouracil (5-FU) found no association between copy number and OS or PFS [79]. Therefore, in certain situations, EGFR expression may be helpful in discussing outcomes for patients, but this line of investigation needs further discernment.
From a mutational perspective, EGFR status has had diverse effects based on the treatment regimens used. Two separate studies examined the presence of a single nucleotide polymorphism (SNP) EGFR-K521 in different HNSCC clinical cohorts and found that while the SNP was not associated with the risk of cancer, it correlated with response to CTX treatment [30,80]. In vitro studies showed that HNSCC cells with a R521K substitution in the ECD did not respond to CTX treatment; cell proliferation and apoptosis remained unimpacted in both in vitro and in vivo models indicative of intrinsic resistance to CTX [31].
Several EGFR gene polymorphisms have been associated with an elevated HNSCC risk. Genotyping of 578 HNSCC patients and 588 cancer-free controls for 60 EGFR SNPs revealed intronic SNPs rs12535536, rs2075110, rs1253871, rs845561, and rs6970262, and synonymous SNP rs2072454 were associated with HNSCC risk among all HNSCC patients [81]. The EGFR-R497K substitution has been reported to be an independent predictor for both OS and therapeutic response; patients with this SNP exhibited lower response to CTX treatment [82] but had a decreased risk of disease-specificity mortality [83]. On the contrary, an in vitro study found that HNSCCs carrying the EGFR-R497K mutation were more likely to be susceptible to CTX treatment [84]. These discrepant findings may be attributed to a differing experimental approach, namely, an in vitro versus an in vivo setting.
The EGFR mutational spectrum in non-small cell lung cancers (NSCLC) is very well defined with respect to therapeutic response. Systematic reviews in NSCLC show how patients with certain mutations exhibit differential sensitivities and clinical outcomes to TKIs and immunotherapy [85,86,87,88]. In general, most mutations involving exons 18, 19 and 21 are predictive of sensitivity to TKIs while exon 20 mutations are considered predictors of resistance in NSCLC [89,90,91,92,93]. For example, several case studies have shown that exon 19 deletions and exon 21 substitutions such as L858R, which account for approximately 50% of EGFR mutations in NSCLC, are known sensitizers to first and second generation TKIs [94,95,96,97]. Exon 20 substitutions such as T790M and C797S, most implicated in resistance to gefitinib and erlotinib, have been shown to be susceptible to third generation TKIs such as alflutinib and osimertinib with varied efficacies in different patient cohorts [98,99,100,101,102]. Since mutational patterns could be shared between tumors, some studies have attempted to screen for these specific mutations in HNSCC cohorts in order to assess chemoradiation response. A systematic review of a 113-patient HNSCC cohort found that the L858R substitution, associated with sensitivity to EGFR TKIs in NSCLC, was found in only 2.5% of the patients; the T790M substitution in exon 20, was found in 7.5% of patients; and in-frame deletions in exon 19, making up 45% of all EGFR mutations in NSCLC and linked to responsiveness to EGFR TKIs, were observed in 22% of all EGFR-mutated HNSCCs (37). This indicates that some of the predictive value of EGFR mutations, which are known predictors of therapeutic response in NSCLC, could be extrapolated to HNSCC. However, large-scale screening in multiple treatment cohorts as well as detailed analysis is required to demonstrate the prognostic capabilities of EGFR mutations in HNSCC.
Taken together, EGFR genotyping could prove to be useful in predicting response to EGFR-targeted therapies in HNSCC and help stratify patients to escalated or deescalated treatment regimens.
6. Conclusions
Despite the latest advancements in EGFR-targeted therapies, the presence or absence of EGFR mutations plays a role in the response of tumors to various treatments. More research is needed to better define the mutational landscape of EGFR and its dynamic biologic effects in HNSCC as a means to tailor EGFR-targeted therapies and thus enhance the clinical benefit derived from these therapies.
Author Contributions
All authors have jointly conceived this work and jointly written the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
J.A.B. reports occasional consulting for Merck Serono.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Herbst R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004;59((Suppl. 2)):21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 2.Roskoski R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharm. Res. 2014;79:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Liu H., Zhang B., Sun Z. Spectrum of EGFR aberrations and potential clinical implications: Insights from integrative pan-cancer analysis. Cancer Commun. 2020;40:43–59. doi: 10.1002/cac2.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigismund S., Avanzato D., Lanzetti L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018;12:3–20. doi: 10.1002/1878-0261.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leemans C.R., Braakhuis B.J., Brakenhoff R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson D.E., Burtness B., Leemans C.R., Lui V.W.Y., Bauman J.E., Grandis J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalyankrishna S., Grandis J.R. Epidermal growth factor receptor biology in head and neck cancer. J. Clin. Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 9.Fasano M., Della Corte C.M., Viscardi G., Di Liello R., Paragliola F., Sparano F., Iacovino M.L., Castrichino A., Doria F., Sica A., et al. Head and neck cancer: The role of anti-EGFR agents in the era of immunotherapy. Ther. Adv. Med. Oncol. 2021;13:1758835920949418. doi: 10.1177/1758835920949418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundvall M., Karrila A., Nordberg J., Grenman R., Elenius K. EGFR targeting drugs in the treatment of head and neck squamous cell carcinoma. Expert Opin. Emerg. Drugs. 2010;15:185–201. doi: 10.1517/14728211003716442. [DOI] [PubMed] [Google Scholar]
- 11.Cooper J.B., Cohen E.E. Mechanisms of resistance to EGFR inhibitors in head and neck cancer. Head Neck. 2009;31:1086–1094. doi: 10.1002/hed.21109. [DOI] [PubMed] [Google Scholar]
- 12.Byeon H.K., Ku M., Yang J. Beyond EGFR inhibition: Multilateral combat strategies to stop the progression of head and neck cancer. Exp. Mol. Med. 2019;51:1–14. doi: 10.1038/s12276-018-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purba E.R., Saita E.I., Maruyama I.N. Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: The “Rotation Model”. Cells. 2017;6:13. doi: 10.3390/cells6020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bessman N.J., Bagchi A., Ferguson K.M., Lemmon M.A. Complex relationship between ligand binding and dimerization in the epidermal growth factor receptor. Cell Rep. 2014;9:1306–1317. doi: 10.1016/j.celrep.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson K.M. Structure-based view of epidermal growth factor receptor regulation. Annu. Rev. Biophys. 2008;37:53–73. doi: 10.1146/annurev.biophys.37.032807.125829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Z., Lovly C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer. 2018;17:58. doi: 10.1186/s12943-018-0782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemmon M.A., Schlessinger J., Ferguson K.M. The EGFR family: Not so prototypical receptor tyrosine kinases. Cold Spring Harb. Perspect Biol. 2014;6:a020768. doi: 10.1101/cshperspect.a020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deric L., Wheeler Y.Y. Receptor Tyrosine Kinases: Structure, Functions and Role in Human Disease. Humana Press; New York, NY, USA: 2015. [Google Scholar]
- 19.Ortiz-Cuaran S., Bouaoud J., Karabajakian A., Fayette J., Saintigny P. Precision Medicine Approaches to Overcome Resistance to Therapy in Head and Neck Cancers. Front. Oncol. 2021;11:14332. doi: 10.3389/fonc.2021.614332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair S., Trummell H.Q., Rajbhandari R., Thudi N.K., Nozell S.E., Warram J.M., Willey C.D., Yang E.S., Placzek W.J., Bonner J.A., et al. Novel EGFR ectodomain mutations associated with ligand-independent activation and cetuximab resistance in head and neck cancer. PLoS ONE. 2020;15:e0229077. doi: 10.1371/journal.pone.0229077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elferink L.A., Resto V.A. Receptor-tyrosine-kinase-targeted therapies for head and neck cancer. J. Signal Transduct. 2011;2011:982879. doi: 10.1155/2011/982879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Dis. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonner J.A., Harari P.M., Giralt J., Azarnia N., Shin D.M., Cohen R.B., Jones C.U., Sur R., Raben D., Jassem J., et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 25.Sacco A.G., Chen R., Worden F.P., Wong D.J.L., Adkins D., Swiecicki P., Chai-Ho W., Oppelt P., Ghosh D., Bykowski J., et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: An open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021;22:883–892. doi: 10.1016/S1470-2045(21)00136-4. [DOI] [PubMed] [Google Scholar]
- 26.Khattri A., Sheikh N., Acharya R., Tan Y.-H.C., Kochanny S., Lingen M.W., Vokes E.E., Seiwert T.Y. Mechanism of acquired resistance to cetuximab in head and neck cancer. J. Clin. Oncol. 2018;36((Suppl. 15)):e18061. doi: 10.1200/JCO.2018.36.15_suppl.e18061. [DOI] [Google Scholar]
- 27.Orellana L., Thorne A.H., Lema R., Gustavsson J., Parisian A.D., Hospital A., Cordeiro T.N., Bernado P., Scott A.M., Brun-Heath I., et al. Oncogenic mutations at the EGFR ectodomain structurally converge to remove a steric hindrance on a kinase-coupled cryptic epitope. Proc. Natl. Acad. Sci. USA. 2019;116:10009–10018. doi: 10.1073/pnas.1821442116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Consortium A.P.G. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Dis. 2017;7:818–831. doi: 10.1158/2159-8290.CD-17-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGovern S.L., Rojas M.L., Gururaj A., Chiu W., Bogler O., Weinstein J.N. Extracellular Domain Mutations in EGFR Occur Uniquely in Glioblastoma and Favor Ligand-independent Formation of the Active State. Int. J. Radiat. Oncol. Biol. Phys. 2012;84:S177. doi: 10.1016/j.ijrobp.2012.07.459. [DOI] [Google Scholar]
- 30.Braig F., Kriegs M., Voigtlaender M., Habel B., Grob T., Biskup K., Blanchard V., Sack M., Thalhammer A., Ben Batalla I., et al. Cetuximab Resistance in Head and Neck Cancer Is Mediated by EGFR-K521 Polymorphism. Cancer Res. 2017;77:1188–1199. doi: 10.1158/0008-5472.CAN-16-0754. [DOI] [PubMed] [Google Scholar]
- 31.Nelhubel G.A., Cserepes M., Szabo B., Turk D., Karpati A., Kenessey I., Raso E., Barbai T., Hegedus Z., Laszlo V., et al. EGFR Alterations Influence the Cetuximab Treatment Response and c-MET Tyrosine-Kinase Inhibitor Sensitivity in Experimental Head and Neck Squamous Cell Carcinomas. Pathol. Oncol. Res. 2021;27:20256. doi: 10.3389/pore.2021.620256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naruse T., Tokuhisa M., Yanamoto S., Sakamoto Y., Okuyama K., Tsuchihashi H., Umeda M. Lower gingival squamous cell carcinoma with brain metastasis during long-term cetuximab treatment: A case report. Oncol. Lett. 2018;15:7158–7162. doi: 10.3892/ol.2018.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paul M.K., Mukhopadhyay A.K. Tyrosine kinase—Role and significance in Cancer. Int. J. Med. Sci. 2004;1:101–115. doi: 10.7150/ijms.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson F.M., Gray N.S. Kinase inhibitors: The road ahead. Nat. Rev. Drug. Dis. 2018;17:353–377. doi: 10.1038/nrd.2018.21. [DOI] [PubMed] [Google Scholar]
- 35.Muller S., Chaikuad A., Gray N.S., Knapp S. The ins and outs of selective kinase inhibitor development. Nat. Chem. Biol. 2015;11:818–821. doi: 10.1038/nchembio.1938. [DOI] [PubMed] [Google Scholar]
- 36.Sola A.M., Johnson D.E., Grandis J.R. Investigational multitargeted kinase inhibitors in development for head and neck neoplasms. Expert Opin. Investig. Drugs. 2019;28:351–363. doi: 10.1080/13543784.2019.1581172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vatte C., Al Amri A.M., Cyrus C., Chathoth S., Acharya S., Hashim T.M., Ali Z.L., Alshreadah S.T., Alsayyah A., Al-Ali A.K., et al. Tyrosine kinase domain mutations of EGFR gene in head and neck squamous cell carcinoma. Oncol. Targets Ther. 2017;10:527–533. doi: 10.2147/OTT.S132187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boeckx C., Weyn C., Vanden Bempt I., Deschoolmeester V., Wouters A., Specenier P., Laer C.V., Vanden Weyngaert D., Kockx M., Vermorken J.B., et al. Mutation analysis of genes in the EGFR pathway in Head and Neck cancer patients: Implications for anti-EGFR treatment response. BMC Res. Notes. 2014;7:337. doi: 10.1186/1756-0500-7-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perisanidis C. Prevalence of EGFR Tyrosine Kinase Domain Mutations in Head and Neck Squamous Cell Carcinoma: Cohort Study and Systematic Review. In Vivo. 2017;31:23–34. doi: 10.21873/invivo.11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagener-Ryczek S., Heydt C., Suptitz J., Michels S., Falk M., Alidousty C., Fassunke J., Ihle M.A., Tiemann M., Heukamp L., et al. Mutational spectrum of acquired resistance to reversible versus irreversible EGFR tyrosine kinase inhibitors. BMC Cancer. 2020;20:408. doi: 10.1186/s12885-020-06920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaclova T., Grazini U., Ward L., O’Neill D., Markovets A., Huang X., Chmielecki J., Hartmaier R., Thress K.S., Smith P.D., et al. Clinical impact of subclonal EGFR T790M mutations in advanced-stage EGFR-mutant non-small-cell lung cancers. Nat. Commun. 2021;12:1780. doi: 10.1038/s41467-021-22057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du Z., Brown B.P., Kim S., Ferguson D., Pavlick D.C., Jayakumaran G., Benayed R., Gallant J.N., Zhang Y.K., Yan Y., et al. Structure-function analysis of oncogenic EGFR Kinase Domain Duplication reveals insights into activation and a potential approach for therapeutic targeting. Nat. Commun. 2021;12:1382. doi: 10.1038/s41467-021-21613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matar P., Rojo F., Cassia R., Moreno-Bueno G., Di Cosimo S., Tabernero J., Guzman M., Rodriguez S., Arribas J., Palacios J., et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): Superiority over single-agent receptor targeting. Clin. Cancer Res. 2004;10:6487–6501. doi: 10.1158/1078-0432.CCR-04-0870. [DOI] [PubMed] [Google Scholar]
- 44.Subbiah V., Dumbrava E.I., Jiang Y., Thein K.Z., Naing A., Hong D.S., Fu S., Piha-Paul S.A., Tsimberidou A.M., Janku F., et al. Dual EGFR blockade with cetuximab and erlotinib combined with anti-VEGF antibody bevacizumab in advanced solid tumors: A phase 1 dose escalation triplet combination trial. Exp. Hematol. Oncol. 2020;9:1–8. doi: 10.1186/s40164-020-00159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang G., Xia P., Zhao S., Yuan L., Wang X., Li X. Gefitinib Combined with Cetuximab for the Treatment of Lung Adenocarcinoma Harboring the EGFR-Intergenic Region (SEC61G) Fusion and EGFR Amplification. Oncologist. 2021;26:e1898–e1902. doi: 10.1002/onco.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson G., Ebadi M., Vo K., Novak J., Govindarajan A., Amini A. An Updated Review on Head and Neck Cancer Treatment with Radiation Therapy. Cancers. 2021;13:4912. doi: 10.3390/cancers13194912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bossi P., Platini F. Radiotherapy plus EGFR inhibitors: Synergistic modalities. Cancers Head Neck. 2017;2:1–9. doi: 10.1186/s41199-016-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwok T.T., Sutherland R.M. Differences in EGF related radiosensitisation of human squamous carcinoma cells with high and low numbers of EGF receptors. Br. J. Cancer. 1991;64:251–254. doi: 10.1038/bjc.1991.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonner J.A., Maihle N.J., Folven B.R., Christianson T.J., Spain K. The interaction of epidermal growth factor and radiation in human head and neck squamous cell carcinoma cell lines with vastly different radiosensitivities. Int. J. Radiat. Oncol. Biol. Phys. 1994;29:243–247. doi: 10.1016/0360-3016(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 50.Bonner J.A., Raisch K.P., Trummell H.Q., Robert F., Meredith R.F., Spencer S.A., Buchsbaum D.J., Saleh M.N., Stackhouse M.A., LoBuglio A.F., et al. Enhanced apoptosis with combination C225/radiation treatment serves as the impetus for clinical investigation in head and neck cancers. J. Clin. Oncol. 2000;18((Suppl. 21)):47S–53S. [PubMed] [Google Scholar]
- 51.Huang S.M., Bock J.M., Harari P.M. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–1940. [PubMed] [Google Scholar]
- 52.Saleh M.N., Raisch K.P., Stackhouse M.A., Grizzle W.E., Bonner J.A., Mayo M.S., Kim H.G., Meredith R.F., Wheeler R.H., Buchsbaum D.J. Combined modality therapy of A431 human epidermoid cancer using anti-EGFr antibody C225 and radiation. Cancer Biother. Radiopharm. 1999;14:451–463. doi: 10.1089/cbr.1999.14.451. [DOI] [PubMed] [Google Scholar]
- 53.Bonner J.A., Harari P.M., Giralt J., Cohen R.B., Jones C.U., Sur R.K., Raben D., Baselga J., Spencer S.A., Zhu J., et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 54.Ang K.K., Berkey B.A., Tu X., Zhang H.Z., Katz R., Hammond E.H., Fu K.K., Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 55.Hutchinson M.N.D., Mierzwa M., D’Silva N.J. Radiation resistance in head and neck squamous cell carcinoma: Dire need for an appropriate sensitizer. Oncogene. 2020;39:3638–3649. doi: 10.1038/s41388-020-1250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dittmann K., Mayer C., Fehrenbacher B., Schaller M., Raju U., Milas L., Chen D.J., Kehlback R., Rodemann H.R. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J. Biol. Chem. 2005;280:31182–31189. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- 57.Taberna M., Oliva M., Mesia R. Cetuximab-Containing Combinations in Locally Advanced and Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019;9:83. doi: 10.3389/fonc.2019.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okuyama K., Suzuki K., Naruse T., Tsuchihashi H., Yanamoto S., Kaida A., Miura M., Umeda M., Yamashita S. Prolonged cetuximab treatment promotes p27(Kip1)-mediated G1 arrest and autophagy in head and neck squamous cell carcinoma. Sci. Rep. 2021;11:5259. doi: 10.1038/s41598-021-84877-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bos T., Ratti J.A., Harada H. Targeting Stress-Response Pathways and Therapeutic Resistance in Head and Neck Cancer. Front. Oral Health. 2021;26:76643. doi: 10.3389/froh.2021.676643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee M., Nam H.Y., Kang H.B., Lee W.H., Lee G.H., Sung G.J., Han M.W., Cho K.J., Chang E.J., Choi K.C., et al. Epigenetic regulation of p62/SQSTM1 overcomes the radioresistance of head and neck cancer cells via autophagy-dependent senescence induction. Cell Death Dis. 2021;12:250. doi: 10.1038/s41419-021-03539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang M., Kern A.M., Hulskotter M., Greninger P., Singh A., Pan Y., Chowdhuy D., Krause M., Baumann M., Benes C.H., et al. EGFR-mediated chromatin condensation protects KRAS-mutant cancer cells against ionizing radiation. Cancer Res. 2014;74:2825–2834. doi: 10.1158/0008-5472.CAN-13-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alsahafi E.N., Thavaraj S., Sarvestani N., Novoplansky O., Elkabets M., Ayaz B., Tavassoli M. EGFR overexpression increases radiotherapy response in HPV-positive head and neck cancer through inhibition of DNA damage repair and HPV E6 downregulation. Cancer Lett. 2021;4988:80–97. doi: 10.1016/j.canlet.2020.10.035. [DOI] [PubMed] [Google Scholar]
- 63.Arnold C.R., Mangesius J., Skvortsova I.I., Ganswindt U. The Role of Cancer Stem Cells in Radiation Resistance. Front. Oncol. 2020;10:64. doi: 10.3389/fonc.2020.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rycaj K., Tang D.G. Cancer stem cells and radioresistance. Int. J. Radiat. Biol. 2014;90:615–621. doi: 10.3109/09553002.2014.892227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma L., Zhang G., Miao X.B., Deng X.B., Wu Y., Liu Y., Jin Z.R., Li X.Q., Liu Q.Z., Sun D.X., et al. Cancer stem-like cell properties are regulated by EGFR/AKT/beta-catenin signaling and preferentially inhibited by gefitinib in nasopharyngeal carcinoma. FEBS J. 2013;280:2027–2041. doi: 10.1111/febs.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Macha M.A., Rachagani S., Qazi A.K., Jahan R., Gupta S., Patel A., Seshacharyulu P., Lin C., Li S., Wang S., et al. Afatinib radiosensitizes head and neck squamous cell carcinoma cells by targeting cancer stem cells. Oncotarget. 2017;8:20961–20973. doi: 10.18632/oncotarget.15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X.K., He J.H., Xu J.H., Ye S., Wang F., Zhang H., Huang Z.C., To K.K.W., Fu L.W. Afatinib enhances the efficacy of conventional chemotherapeutic agents by eradicating cancer stem-like cells. Cancer Res. 2014;74:4431–4445. doi: 10.1158/0008-5472.CAN-13-3553. [DOI] [PubMed] [Google Scholar]
- 68.Coliat P., Ramolu L., Jegu J., Gaiddon C., Jung A.C., Pencreach E. Constitutive or Induced HIF-2 Addiction is Involved in Resistance to Anti-EGFR Treatment and Radiation Therapy in HNSCC. Cancers. 2019;11:1607. doi: 10.3390/cancers11101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cappuzzo F. Guide to Targeted Therapies: EGFR Mutations in NSCLC. Springer; Berlin/Heidelberg, Germany: 2014. [Google Scholar]
- 70.Angulo B., Conde E., Suarez-Gauthier A., Plaza C., Martinez R., Redondo P., Izquierdo E., Rubio-Viqueira B., Paz-Ares L., Hidalgo M., et al. A comparison of EGFR mutation testing methods in lung carcinoma: Direct sequencing, real-time PCR and immunohistochemistry. PLoS ONE. 2012;7:e43842. doi: 10.1371/journal.pone.0043842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Normanno N., Denis M.G., Thress K.S., Ratcliffe M., Reck M. Guide to detecting epidermal growth factor receptor (EGFR) mutations in ctDNA of patients with advanced non-small-cell lung cancer. Oncotarget. 2017;8:12501–12516. doi: 10.18632/oncotarget.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chung C.H., Ely K., McGavran L., Varella-Garcia M., Parker J., Parker N., Jarrett C., Carter J., Murphy B.A., Netterville J., et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J. Clin. Oncol. 2006;24:4170–4176. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 73.Chung C.H., Zhang Q., Hammond E.M., Trotti A.M., III, Wang H., Spencer S., Zhang H.Z., Cooper J., Jordan R., Rotman M.H., et al. Integrating epidermal growth factor receptor assay with clinical parameters improves risk classification for relapse and survival in head-and-neck squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:331–338. doi: 10.1016/j.ijrobp.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Temam S., Kawaguchi H., El-Naggar A.K., Jelinek J., Tang H., Liu D.D., Lang W., Issa J.P., Lee J.J., Mao L. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J. Clin. Oncol. 2007;25:2164–2170. doi: 10.1200/JCO.2006.06.6605. [DOI] [PubMed] [Google Scholar]
- 75.Alterio D., Marvaso G., Maffini F., Gandini S., Chiocca S., Ferrari A., Preda L., Rocca M.C., Lepanto D., Fodor C., et al. Role of EGFR as prognostic factor in head and neck cancer patients treated with surgery and postoperative radiotherapy: Proposal of a new approach behind the EGFR overexpression. Med. Oncol. 2017;34:107. doi: 10.1007/s12032-017-0965-7. [DOI] [PubMed] [Google Scholar]
- 76.Chen X., Liang R., Lai L., Chen K., Zhu X. Prognostic Role of EGFR/p-EGFR in Patients With Nasopharyngeal Carcinoma: A Meta-Analysis. Front. Oncol. 2021;11:97369. doi: 10.3389/fonc.2021.697369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keren S., Shoude Z., Lu Z., Beibei Y. Role of EGFR as a prognostic factor for survival in head and neck cancer: A meta-analysis. Tumour Biol. 2014;35:2285–2295. doi: 10.1007/s13277-013-1303-0. [DOI] [PubMed] [Google Scholar]
- 78.Zhu X., Zhang F., Zhang W., He J., Zhao Y., Chen X. Prognostic role of epidermal growth factor receptor in head and neck cancer: A meta-analysis. J. Surg. Oncol. 2013;108:387–397. doi: 10.1002/jso.23406. [DOI] [PubMed] [Google Scholar]
- 79.Licitra L., Mesia R., Rivera F., Remenar E., Hitt R., Erfan J., Rottey S., Kawecki A., Zabolotnny D., Benasso M., et al. Evaluation of EGFR gene copy number as a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy in the first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: EXTREME study. Ann. Oncol. 2011;22:1078–1087. doi: 10.1093/annonc/mdq588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y., Zha L., Liao D., Li X. A Meta-Analysis on the Relations between EGFR R521K Polymorphism and Risk of Cancer. Int. J. Genom. 2014;2014:312102. doi: 10.1155/2014/312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fung C., Zhou P., Joyce S., Trent K., Yuan J.M., Grandis J.R., Weissfeld J.L., Romkes M., Weeks D.E., Egloff A.M. Identification of epidermal growth factor receptor (EGFR) genetic variants that modify risk for head and neck squamous cell carcinoma. Cancer Lett. 2015;357:549–556. doi: 10.1016/j.canlet.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stoehlmacher-Williams J., Obermann L., Ehninger G., Goekkurt E. Polymorphisms of the epidermal growth factor receptor (EGFR) and survival in patients with advanced cancer of the head and neck (HNSCC) Anticancer Res. 2012;32:421–425. [PubMed] [Google Scholar]
- 83.Bandres E., Barricarte R., Cantero C., Honorato B., Malumbres R., Zarate R., Alcade J., Garcia-Foncillas J. Epidermal growth factor receptor (EGFR) polymorphisms and survival in head and neck cancer patients. Oral Oncol. 2007;43:713–719. doi: 10.1016/j.oraloncology.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 84.Krohn V., Wiegand S., Werner J.A., Mandic R. EGFR codon 497 polymorphism—Implications for receptor sensitivity to inhibitors in HNSCC cell lines. Anticancer Res. 2011;31:59–65. [PubMed] [Google Scholar]
- 85.Robichaux J.P., Le X., Vijayan R.S.K., Hicks J.K., Heeke S., Elamin Y.Y., Lin H.Y., Udagawa H., Skoulidis F., Tran H., et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature. 2021;597:732–737. doi: 10.1038/s41586-021-03898-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Russo A., Franchina T., Ricciardi G., Battaglia A., Picciotto M., Adamo V. Heterogeneous Responses to Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors (TKIs) in Patients with Uncommon EGFR Mutations: New Insights and Future Perspectives in this Complex Clinical Scenario. Int. J. Mol. Sci. 2019;20:1431. doi: 10.3390/ijms20061431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoshikawa S., Kukimoto-Niino M., Parker L., Handa N., Terada T., Fujimoto T., Terazawa Y., Wakiyama M., Sato M., Sano S., et al. Structural basis for the altered drug sensitivities of non-small cell lung cancer-associated mutants of human epidermal growth factor receptor. Oncogene. 2013;32:27–38. doi: 10.1038/onc.2012.21. [DOI] [PubMed] [Google Scholar]
- 88.Zhang T., Wan B., Zhao Y., Li C., Liu H., Lv T., Zhan P., Song Y. Treatment of uncommon EGFR mutations in non-small cell lung cancer: New evidence and treatment. Transl. Lung Cancer Res. 2019;8:302–316. doi: 10.21037/tlcr.2019.04.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirose T., Ikegami M., Endo M., Matsumoto Y., Nakashima Y., Mano H., Kohsaka S. Extensive functional evaluation of exon 20 insertion mutations of EGFR. Lung Cancer. 2021;152:135–142. doi: 10.1016/j.lungcan.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 90.Passaro A., Mok T., Peters S., Popat S., Ahn M.J., de Marinis F. Recent Advances on the Role of EGFR Tyrosine Kinase Inhibitors in the Management of NSCLC With Uncommon, Non Exon 20 Insertions, EGFR Mutations. J. Thorac. Oncol. 2021;16:764–773. doi: 10.1016/j.jtho.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 91.Remon J., Hendriks L.E.L., Cardona A.F., Besse B. EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat Rev. 2020;90:102105. doi: 10.1016/j.ctrv.2020.102105. [DOI] [PubMed] [Google Scholar]
- 92.Soria J.C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., Dechaphunkul A., Imamura F., Nogami N., Kurata T., et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 93.Vyse S., Huang P.H. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther. 2019;4:5. doi: 10.1038/s41392-019-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hong W., Wu Q., Zhang J., Zhou Y. Prognostic value of EGFR 19-del and 21-L858R mutations in patients with non-small cell lung cancer. Oncol. Lett. 2019;18:3887–3895. doi: 10.3892/ol.2019.10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsu W.H., Yang J.C., Mok T.S., Loong H.H. Overview of current systemic management of EGFR-mutant NSCLC. Ann. Oncol. 2018;29((Suppl. 1)):i3–i9. doi: 10.1093/annonc/mdx702. [DOI] [PubMed] [Google Scholar]
- 96.Stewart E.L., Tan S.Z., Liu G., Tsao M.S. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations—A review. Transl. Lung Cancer Res. 2015;4:67–81. doi: 10.3978/j.issn.2218-6751.2014.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van Assche K., Ferdinande L., Lievens Y., Vandecasteele K., Surmont V. EGFR Mutation Positive Stage IV Non-Small-Cell Lung Cancer: Treatment Beyond Progression. Front. Oncol. 2014;4:50. doi: 10.3389/fonc.2014.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahn M.J., Tsai C.M., Shepherd F.A., Bazhenova L., Sequist L.V., Hida T., Yang J.C.H., Ramalingam S.S., Mitsudomi T., Janne P.A., et al. Osimertinib in patients with T790M mutation-positive, advanced non-small cell lung cancer: Long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer. 2019;125:892–901. doi: 10.1002/cncr.31891. [DOI] [PubMed] [Google Scholar]
- 99.Dong R.F., Zhu M.L., Liu M.M., Xu Y.T., Yuan L.L., Bian J., Xia Y.Z., Kong L.Y. EGFR mutation mediates resistance to EGFR tyrosine kinase inhibitors in NSCLC: From molecular mechanisms to clinical research. Pharmacol. Res. 2021;1671:05583. doi: 10.1016/j.phrs.2021.105583. [DOI] [PubMed] [Google Scholar]
- 100.Nagasaka M., Zhu V.W., Lim S.M., Greco M., Wu F., Ou S.I. Beyond Osimertinib: The Development of Third-Generation EGFR Tyrosine Kinase Inhibitors for Advanced EGFR+ NSCLC. J. Thorac. Oncol. 2021;16:740–763. doi: 10.1016/j.jtho.2020.11.028. [DOI] [PubMed] [Google Scholar]
- 101.Shi Y., Zhang S., Hu X., Feng J., Ma Z., Zhou J., Yang N., Wu L., Liao W., Zhong D., et al. Safety, Clinical Activity, and Pharmacokinetics of Alflutinib (AST2818) in Patients with Advanced NSCLC with EGFR T790M Mutation. J. Thorac. Oncol. 2020;15:1015–1026. doi: 10.1016/j.jtho.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 102.Zhao Z., Li L., Wang Z., Duan J., Bai H., Wang J. The Status of the EGFR T790M Mutation is associated with the Clinical Benefits of Osimertinib Treatment in Non-small Cell Lung Cancer Patients: A Meta-Analysis. J. Cancer. 2020;11:3106–3113. doi: 10.7150/jca.38411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.