Abstract
The flavonoid apigenin (4′,5,7-trihydroxyflavone), which is one of the most widely distributed phytochemicals in the plant kingdom, is one of the most thoroughly investigated phenolic components. Previous studies have attributed the physiological effects of apigenin to its anti-allergic, antibacterial, antidiabetic, anti-inflammatory, antioxidant, antiviral, and blood-pressure-lowering properties, and its documented anticancer properties have been attributed to the induction of apoptosis and autophagy, the inhibition of inflammation, angiogenesis, and cell proliferation, and the regulation of cellular responses to oxidative stress and DNA damage. The most well-known mechanism for the compound’s anticancer effects in human cancer cell lines is apoptosis, followed by autophagy, and studies have also reported that apigenin induces novel cell death mechanisms, such as necroptosis and ferroptosis. Therefore, the aim of this paper is to review the therapeutic potential of apigenin as a chemopreventive agent, as well as the roles of programmed cell death mechanisms in the compound’s chemopreventive properties.
Keywords: apigenin, apoptosis, autophagy, necroptosis, ferroptosis
1. Introduction
Cancer is a globally important health issue and is the second leading cause of death in the United States, where 1,898,160 new cancer cases and 608,570 cancer deaths were expected in 2021. Cancer mortality increased during the 20th century but decreased by 31% from 1991 to 2018 [1]. However, even though cancer treatment has been advanced significantly over the past two decades (e.g., development of safer, more effective, and more precise drugs) and molecular approaches have been used to treat neoplasms and reduce mortality, the side effects of such treatments remain a major problem [2], and some cancer cells develop resistance or evasion mechanisms [3]. Furthermore, modern cancer treatments are expensive. Therefore, chemoprevention is attracting increasing attention as a cheaper and more effective strategy for reducing cancer-related mortality [2].
Chemopreventive strategies involve the use of natural, synthetic, or biological agents to prevent, inhibit, or reverse the early stages of carcinogenesis or to prevent invasion by premalignant cells. Natural compounds may also reduce side effects [4]. Clinically, these strategies are classified as primary, secondary, or tertiary and are used to reduce the risk of cancer incidence in high-risk populations, to reduce the progression of cancer (via drug treatment) in patients with premalignant lesions, and to prevent cancer recurrence, respectively [5,6]. The definitions of primary and secondary chemoprevention change, and some researchers do not distinguish between primary and secondary chemoprevention. However, typical examples of primary chemoprevention agents include dietary phytochemicals and nonsteroidal anti-inflammatory drugs.
Recently, both herbal and phytochemical-based medicines have attracted attention for their effectiveness against cancer, as well as a wide variety of other diseases [7,8,9,10,11,12,13,14,15]. Indeed, researchers around the world are focusing on the chemopreventive, antioxidant, and anti-inflammatory properties of bioactive compounds [16,17,18], and natural products and their derivatives account for one-third of all new drugs approved by the United States Food and Drug Administration (FDA) [19,20,21]. Plant-based medicines contain multiple bioactive compounds (e.g., alkaloids, carotenoids, diterpenoids, flavonoids, phenolic compounds, and tannins) that impart unique medicinal properties [22,23], and accordingly, plant-derived compounds play important roles in increasing the sensitivity of cells to standard chemotherapy and in reducing cancer risk, invasion, and metastasis [22,24,25,26].
The naturally occurring flavonoid apigenin (4′,5,7-trihydroxyflavone), in particular, which is one of the most widely distributed phytochemicals in the plant kingdom, is one of the most thoroughly researched phenolic compounds [27]. The compound has very low toxicity, is abundant in fruits and vegetables, has many potential biological activities, including anticancer effects, and can simultaneously exert multiple anticancer effects through the modulation of important molecular targets [28,29]. The aim of this paper is to review the therapeutic potential of apigenin as a chemopreventive agent, as well the roles of programmed cell death (PCD) mechanisms in the compound’s chemopreventive properties.
2. Apigenin
The common name of apigenin (i.e., 4′,5,7-trihydroxyflavone; C15H10O5, 270.24 g/mol) is derived from the genus Apium (Apiaceae or Umbelliferae). The yellow crystalline compound possesses hydroxyl groups at the C-5 and C-7 positions of the A-ring and at the C-4′ position of the B-ring and is insoluble in water but soluble in dimethyl sulfoxide and hot ethanol [5,30].
Apigenin is considered an important flavonoid, due to its abundance in a variety of natural sources, including fruits and vegetables, and major sources include parsley, chamomile, celery, spinach, artichoke, and oregano. Dried parsley contains 45,035 μg/g of apigenin, whereas chamomile (dried flowers), celery seed, vine spinach, and Chinese celery contain 3000–5000, 786.5, 622, and 240.2 µg/g, respectively. Glycosylated derivatives (e.g., apiin and apigetrin) and dimers (e.g., amentoflavones, such as 3′,8″-biapigenin) of apigenin have also been isolated from natural sources [5].
3. Physiological Functions of Apigenin
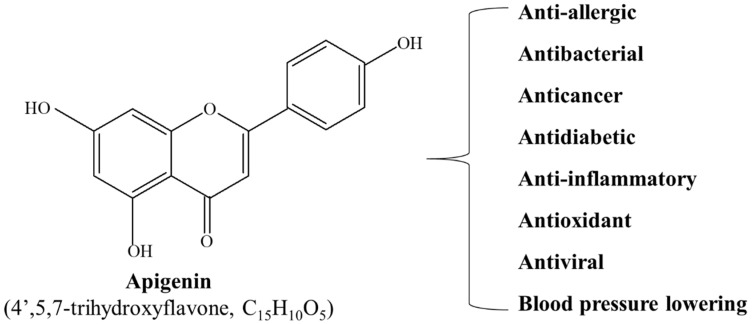
Apigenin has been used in traditional medicines, owing to its anti-inflammatory and antioxidant [29,31], blood-pressure-lowering [32], antibacterial and antiviral [33], antidiabetic [34], and anti-allergic properties [35]. Recently, apigenin has also been demonstrated to possess tumor-suppressive effects, and since Birt et al. [36] first reported the anticancer activity of apigenin in 1986, the compound has been reported to exert anti-tumor effects in a variety of cancer types in both in vitro cell lines and in vivo mouse models (Figure 1).
Figure 1.
Molecular structure and physiological function of apigenin.
4. Apigenin in Cancer Therapy
Carcinogenesis is a multi-step process that involves a series of genetic and epigenetic changes that contribute to the initiation, promotion, and development of cancer [37,38,39]. Cancer treatment strategies include the induction of cell apoptosis to eradicate tumor cells and the induction of cell cycle arrest to prevent cancer cell proliferation, thereby prolonging patient survival [40,41,42], and strategies involving the promotion of apoptosis/autophagy, control of the cell cycle, prevention of tumor cell migration and invasion, and induction of patient immune responses have also been proposed [43,44,45,46].
Apigenin has been demonstrated, in both in vitro and in vivo models, to exert broad anticancer effects in a variety of cancer types, including colorectal cancer, breast cancer, liver cancer, lung cancer, melanoma, prostate cancer, and osteosarcoma [47,48,49,50,51,52]. The compound can prevent cancer cell proliferation by triggering apoptosis, which leads to autophagy and cell cycle regulation, and can also reduce cancer cell motility, thereby preventing cancer cell migration and invasion. It was also recently reported that apigenin can inhibit cancer by stimulating patient immune response [53] and that the compound can regulate several protein kinases and signaling pathways, including PI3K/AKT, MAPK/ERK, JAK/STAT, NF-κB, and Wnt/β-catenin pathways [28].
5. Effect of Apigenin on Apoptosis
5.1. Apoptosis
The term apoptosis was first used by Kerr et al. [54] in 1972 to describe a morphologically distinct type of cell death. Apoptosis, or Type I PCD, is a closely linked cellular process that plays an important role in the development and homeostasis of multicellular organisms [5]. Because tissue homeostasis involves a balance between apoptosis and cell proliferation, disruption of this balance (e.g., uncontrolled apoptosis) may be implicated in a variety of human diseases, including cancer [55,56]. Apoptosis is mainly induced through the intrinsic (mitochondrial) and extrinsic (death receptor) pathway.
5.2. Types of Apoptosis
5.2.1. Intrinsic (Mitochondrial) Pathway
The intrinsic pathway, also known as the mitochondrial pathway of apoptosis, involves various stimuli that act on multiple cellular targets within the cell. This form of apoptosis depends on factors that are released from the mitochondria and begins in either a positive or a negative pathway. Negative signals are caused by the absence of cytokines, hormones, and growth factors in the cell’s immediate environment. In the absence of these survival signals, pro-apoptotic molecules within cells, such as Bax, Noxa, and the p53-upregulated modulator of apoptosis (PUMA), which are normally restrained, are activated to initiate apoptosis. Other factors initiating apoptosis are positive and include exposure to viruses and various toxic substances, radiation, hypoxia, reactive oxygen species (ROS), and toxins [57].
The intrinsic apoptotic pathway is controlled by the mitochondria, including key apoptotic factors, such as cytochrome c [58]. The intrinsic pathway is also controlled by the members of the Bcl-2 family. Pro- and anti-apoptotic Bcl-2 proteins are localized in mitochondria to manage the release of apoptogenic factors [59]. The pro-apoptotic Bcl-2 protein induces permeability of the outer mitochondrial membrane, allowing cytochrome c to be released from the mitochondrial intermembrane space [60]. Consequently, in the presence of ATP, it binds to apoptotic protease activating factor 1 (Apaf-1) and participates in the formation of a multimeric Apaf-1/cytochrome c complex. Subsequently, the Apaf-1/cytochrome c complex binds to procaspase-9 to generate an apoptosome [61]. Consequently, procaspase-9 is cleaved, activated, and dissociated from the apoptosome. Once activated, caspase-9 is activated by cleaving executive caspase-3, -6, and/or -7 [62].
5.2.2. Extrinsic (Death Receptor) Pathway
The extrinsic apoptotic pathway relies on cell surface death receptors, such as tumor necrosis factor (TNF), which are controlled by the expression levels of triggering ligands [63,64,65]. Ligands that stimulate cell surface death receptors contain cytokines, such as transforming growth factor beta 1 (TGF-β1), TNF-α, and interferon gamma [65]. Cell surface death receptors initiate procaspases via ligand binding [66]. Death domains play an important role in the transduction of death signals from the cell surface to intracellular signaling pathways. Therefore, when cell surface death receptor–ligand binding occurs, cytoplasmic adapter proteins are recruited and associated with procaspase-8 via dimerization of the death effector domain [65]. Next, a death-inducing signaling complex (DISC) is formed, which triggers the autocatalytic activation of procaspase-8. Once activated, caspase-8 prompts executioner caspases, such as caspase-3, -6, and -7, which mediate the execution stage of apoptosis [65,67].
5.3. Induction of Apoptosis by Apigenin
The modulation of apoptosis has significant implications for cancer therapy, and thus, the effects of apigenin on molecular targets have attracted extensive investigation (Table 1).
Table 1.
Molecular targets of apigenin-induced apoptosis.
| Cancer/Cell Lines | Up-Regulation | Down-Regulation | Refs. |
|---|---|---|---|
| Bladder | |||
| T24 | PARP cleavage, caspase-3, -7 and -9 cleavage, Bax, Bak, Bad, p–p53, p53, p21, p27, Cyt c (cytosol) | p-Akt, PDK, PI3K, Bcl-2, Bcl-xL, cyclin A, B1, and E, CDK2, Cdc2, Cdc25c, Bcl-xL, Mcl-1, Cyt c (mitochondrial) | [68,69] |
| RT112 | PARP cleavage | [70] | |
| Breast | |||
| SK-BR-3 | p53, p21, Bax, Cyt c, caspase-8 and -3, PARP, DFF45 cleavage, p27 | cyclin A, B, D, and E, CDK1, p-JAK, p-STAT3, VEGF, cyclin D1 and D3, CDK4 | [71,72,73] |
| MDA-MB-231 and MDA-MB-231 xenograft | p-p53 (Ser-15), p21, Bax, PARP cleavage, IκBα, caspase-3 and -7, FOXO3a, p27, Cyt c | Bcl-xL, cyclin B1, Bcl-2, PI3K, PKB/AKT | [74,75,76,77] |
| MCF-7 | p53, p-p53 (Ser-15), p21, caspase-8 and PARP cleavage, ROS, Cyt c, caspase-3, DFF45 cleavage, p27, FOXO3a | p-MDM2, p-JAK1, p-STAT3, NF-κB/p65, p-IκBα, cyclin D1 and D3, CDK4, PI3K, PKB/AKT | [72,77,78,79,80] |
| BT-474 | caspase-8 and -3, PARP, Cyt c, DFF45 cleavage, p27 | p-JAK1, p-JAK2, p-STAT3, VEGF, HIF-1α, cyclin D1 and D3, CDK4 | [72,81] |
| Hs578T | FOXO3a, p21, p27, PARP, Cyt c release | PI3K, PKB/AKT | [77] |
| MDA-MB-453 | caspase-3, -6, -7, -8, and PARP cleavage, Cyt c release, DFF45 cleavage, p27 | procaspase-9, p-JAK2, p-STAT3 | [72,82,83] |
| T47D | caspase-3 and PARP cleavage, Bax | Bcl-2, Bcl-xL | [75] |
| HBL-100 | Cyt c, caspase-3, DFF45 cleavage, p27 | cyclin D1 and D3, CDK4 | [72] |
| Cervical | |||
| HeLa | p53, p21, caspase-2 and -3, Fas, mitochondrial redox impairment, PARP, ROS, AIF, Endo G, Cyt c | Bcl-2, MMP, superoxide dismutase | [84,85,86] |
| SiHa, CaSki, and C-33A | mitochondrial redox impairment, ROS | MMP | [85] |
| Colon | |||
| HCT116 | p21, p53, NAG-1, Bim-EL, Bim-L, PARP cleavage | cyclin B1, Cdc2, Cdc25c, procaspase-3, -8, and -9, Mcl-1, Bcl-xL, STAT3, p-AKT, p-ERK | [87,88,89,90] |
| LoVo | p21, NAG-1 | [88] | |
| DLD-1 | PARP cleavage | Mcl-1, p-AKT, p-ERK, Bcl-xL, Mcl-1, STAT3 | [87,90] |
| SW480 | Cdc2, cyclin B1 | [91] | |
| HT-29 | Bax, PARP cleavage, caspase-3 and -8 | Cdc2, Bcl-2, m-TOR/PI3K/AKT, Bcl-xL, Mcl-1, STAT3, caspase-3 and -8, cyclin D1 | [90,91,92,93] |
| Caco-2 | Cdc2 | [91] | |
| COLO320 | PARP cleavage | Bcl-xL, Mcl-1, STAT3 | [90] |
| Esophageal | |||
| KYSE-510 | p21, PIG3, p63, p73, caspase-3 and -9, Bax | cyclin B1, Bcl-2 | [94] |
| Eca-109 and KYSE-30 | PARP cleavage, caspase-8 | IL-6, VEGF | [95] |
| Gastric | |||
| HGC-27 and SGC-7901 | Bax, Bcl-2, caspase-3 | MMP | [96] |
| Glioblastoma | |||
| U-1242MG | PARP cleavage | MAPK, AKT, mTOR, Bcl-xL | [97] |
| T98G and U-87MG | p-p38 MAPK, c-Jun1, caspase-3, -8, and -9, Bax, tBid, Smac (cytosol), SBDP, CAD (nuclear) | ROS, MMP, Bcl-2, Cyt c (mitochondrial), Smac (mitochondrial), calpastatin, ICAD | [98] |
| Head and Neck | |||
| SCC-25 | TRAIL, TRAIL-R1, and -R2, Fas, TNF-α, TNF-R1 and -R2, Bax, caspase-3 | Bcl-2 | [99] |
| Melanoma | |||
| A375 and C8161 | Cyt c release, Bax, Apaf-1, caspase-3, -9, and PARP cleavage | Bcl-2, Cyt c (mitochondrial), p-ERK1/2, p-AKT, p-mTOR | [50,100,101] |
| Leukemia | |||
| THP-1 | caspase-3 activity, p-p38, p-ERK, PKCδ activity, p-ATM | caspase-9 activity, p-H2AX | [102,103] |
| U937 | caspase-3, -7, -9, and PARP cleavage, p-JNK, Bcl-2 cleavage | hTERT, c-Myc, Mcl-1, p-AKT, AKT, p-Bad, p-mTOR, p-GSK3β, JNK, Mcl-1, Bcl-2 | [104,105] |
| HL60 | p-Cdc2, p-p38, caspase-3, -8, and PARP cleavage | PI3Kp85, p-AKT, p-GSK3β, p-JAK2, p-Src, p-STAT3 | [106,107] |
| TF-1 | LMWPTP | CDK6, p-Src, p-JAK2, p-SHP2, p-STAT3 and 5, p-p70S6K | [106] |
| Liver | |||
| Huh-7 | caspase-3, -8, and -9 cleavage, PARP, Bax/Bcl-2 ratio | [108,109] | |
| HepG2 | caspase-3, -7, -8, -9, and -10, Bid, p21, p16, PARP cleavage, Bax, DR5, ROS, TNF-α, IFN-γ | Bcl-2, PI3K/AKT/mTOR, p-LRP6, Skp2 | [48,110,111,112,113,114] |
| Hep3B | DR5, ROS, caspase activation | [115] | |
| SK-HEP-1 | ROS, caspase 3, PARP | MMP, Bcl-2 | [116] |
| BEL-7402 and BEL-7402 xenograft | ROS, caspase 3, PARP | MMP, Bcl-2, Nrf2 | [116,117] |
| Lung | |||
| A549 | p21, Cyt c release, Bax, p53, p-p53, Wee1, Chk2, Bid, GRP78, caspase-3, -9, and PARP cleavage, GADD153, AIF, MAPK, DR4, DR5 | XIAP, Bcl-2, MMP, cyclin B, Cdc25c, procaspase-8, Bcl-xL, NF-κB, ERK, AKT, Cyt c (mitochondrial) | [81,100,118,119,120] |
| H460 | p21, Bax, FasL, p53, AIF, Cyt c, caspase-3, GRP78, GADD153 | XIAP, Bcl-2, Bid, procaspase-8 | [118,121,122] |
| H1299 | MAPK, DR4, DR5, Bax, Bad | Bcl-xL, Bcl-2, NF-κB, ERK, AKT | [120] |
| Diffuse large B-cell lymphoma | |||
| U2932 and OCI-LY10 | caspase family, PARP cleavage | Bcl-xL, PI3K/mTOR, p-GS3K-β, MCL-X, p38, p-p65, p-AKT | [123] |
| Mesothelioma | |||
| MM-B1, H-Meso-1 and MM-F1 | Bax/Bcl-2 ratio, p53, caspase-8, -9, and PARP-1 cleavage | p-ERK1/2, p-JNK, p-p38 MAPK, p-AKT, c-Jun, p-c-Jun, NF-κB nuclear translocation | [124] |
| Multiple myeloma | |||
| U266 and RPMI 8226 | PARP cleavage | p-STAT3, p-ERK, p-AKT, NF-κB, Mcl-1, Bcl-2, Bcl-xL, XIAP, survivin | [125] |
| Neuroblastoma | |||
| SK-N-DZ, SK-N-BE2, SK-N-DZ and SK-N-BE2 xenograft | caspase-3, -8, and PARP cleavage, Bax, Bid, tBid, calpain, ICAD fragment, p21, Noxa, PUMA, p53, ICAD, SBDP | N-Myc, E-cadherin, Notch-1, hTERT, PCNA, Smac, survivin, SBDP, Bcl-2, Mcl-1 | [126,127,128,129] |
| NUB-7 | PARP cleavage, p53 (NE), p21, Bax, p-ERK | [130] | |
| IMR-32 | Bax, Noxa, PUMA, p53, caspase-3, ICAD | Bcl-2, Mcl-1 | [128] |
| Oral | |||
| SCC-25 | TRAIL, TRAIL-R1 and -R2, Fas, TNF-α, TNF-R1 and -R2, Bax, caspase-3 | cyclin D1 and E, CDK1 | [99] |
| Osteosarcoma | |||
| U-2 OS | Bax, PARP cleavage, p53, AIF | procaspase-3, -8, and -9, GADD153 (NE) | [131] |
| Ovarian | |||
| SKOV-3 | caspase-3 and -9, Bax, Bcl-2, COX-2, ROS | [132,133,134] | |
| A2780 and OVCAR-3 | ROS, MDA, caspase-3 and -9 | [133] | |
| Pancreatic | |||
| BxPC-3 | Ac-p53, p21, PUMA, Cyt c release, caspase-3 cleavage | Bcl-xL/p53 interaction, Bcl-xL/PUMA interaction, cyclin B1, Bcl-2, XIAP, p-GSK3β, NF-κB/p65 (NE) | [135,136,137] |
| MIA PaCa-2 | Ac-p53, p21, PUMA, Cyt c release, PARP cleavage | Bcl-xL/p53 interaction, Bcl-xL/PUMA interaction | [135,138] |
| PANC-1 | Cyt c release, caspase-3 cleavage | cyclin B1, XIAP, p-GSK3β, NF-κB/p65 (NE) | [136] |
| PEL | |||
| BC3, BCBL1, and B | p53 | STAT3, ROS | [139] |
| Prostate | |||
| 22Rv1 and 22Rv1 xenograft | p53, p-p53, p21, p14, Cyt c release, Bax, Apaf-1, caspase-3, -8, -9, and PARP cleavage | MDM2, MMP, Bcl-2, Bcl-xL, p-IKKα, NF-ĸB/p65, PCNA, HDAC1 and 3, Bcl-2 | [140,141,142] |
| PC-3 and PC-3 xenograft | caspase-3, -9, and PARP cleavage, Bax, Bad, Ku70, Cyt c release, p27, p21 | XIAP, cIAP-1, -2, Bcl-2, Bcl-xL, survivin, HDAC1, procaspase-3, -7, and -9, cyclin D1, p-IKKα, NF-ĸB/p65, PCNA, ER-β, PSMA5, PLK-1, HDAC1, and 3, Bcl-2 | [141,142,143,144,145,146,147] |
| LNCaP | p21, p27, Bax, PARP cleavage, Cyt c release | cyclin D1, D2, and E, CDK2, 4, and 6, Bcl-2, procaspase-3, -8, and -9, NF-κB/p65, PLK-1 | [51,145,147] |
| DU145 | caspase-3, -9, and PARP cleavage, DR5, Cyt c release | XIAP, cIAP-1 and -2, survivin, procaspase-3, -7, and -9 | [143,145,148] |
| Renal | |||
| ACHN, 786-O, and Caki-1 | p53, Bax, caspase-3 and -9 | [149] | |
| Thyroid | |||
| FRO | c-Myc, Bid, Fas, p-p53, caspase-3 and PARP cleavage | Bcl-2, p27, p21 | [150] |
AIF, apoptosis-inducing factor; Apaf-1, apoptotic protease activating factor-1; ATM, ataxia telangiectasia mutated; Bad, Bcl-2-associated death promoter; Bax, Bcl-2 associated X protein; Bcl-2, B-cell lymphoma-2; Bcl-xL, B-cell lymphoma extra-large; Bid, BH3-interacting-domain death agonist; Bim-EL, Bcl-2-interacting mediator of cell death (Bim)-extralong; Bim-L, Bim-long; CAD, caspase-activated DNase; Cdc2, cell division control protein 2; Cdc25c, cell division cycle 25c; CDK, cyclin-dependent kinase; Chk2, checkpoint kinase 2; cIAP, cellular inhibitor of apoptosis protein; COX-2, cyclooxygenase-2; Cyt c, cytochrome c; DFF45, DNA fragmentation factor 45; DR4, death receptors 4; DR5, death receptors 5; Endo G, endonuclease G; ER-β, estrogen receptor-beta; ERK, extracellular signal-regulated protein kinases; FasL, apoptosis stimulating fragment (Fas) ligand; FOXO3a, forkhead box O3a; GADD153, growth-arrest- and DNA-damage-inducible gene 153; GRP78, glucose-regulated protein 78; GSK-3β, glycogen synthase kinase-3 beta; H2AX, histone H2A, X; HDAC, histone deacetylase; hTERT, human telomerase reverse transcriptase; HIF-1α, hypoxia-inducible factor 1 alpha subunit; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha; JAK, Janus family of tyrosine kinase; JNK, c-Jun N-terminal kinases; LMWPTP, low-molecular-weight protein tyrosine phosphatase; MAPK, mitogen-activated protein kinase; Mcl-1, myeloid cell leukemia-1; MDA, malondialdehyde; MDM2, mouse double minute 2; mTOR, mammalian target of rapamycin; MMP, mitochondrial membrane potential; NAG-1, nonsteroidal anti-inflammatory drug (NSAID)-activated gene-1; NE, nuclear extract; Nrf2, nuclear factor erythroid 2-related factor 2; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; p70S6K, 70-kDa ribosomal protein S6 kinase; PARP, poly(ADP-ribose) polymerase; PCNA, proliferating cell nuclear antigen; PDK, phosphoinositide-dependent protein kinase; PEL, primary effusion lymphoma; PI3K, phosphoinositide 3-kinase; PIG3, p53 induced gene 3; PKB, protein kinase B; PKC, protein kinase C; PUMA, p53-upregulated modulator of apoptosis; ROS, reactive oxygen species; SBDP, spectrin breakdown product; SMAC, second mitochondria-derived activator of caspases; STAT, signal transducer and activator of transcription; TNFR, TNF receptor; TNF-α, tumor necrosis factor alpha; TRAIL, TNF-related apoptosis-inducing ligand; TRAIL-R, TRAIL receptor; VEGF, vascular endothelial growth factor; XIAP, X-linked inhibitor of apoptosis protein. Adapted in part from Sung, B.; Kim, N.D. Apigenin and Naringenin; Nova Science Publishers, Inc.: New York, NY, USA, 2015; pp. 75–106.
5.3.1. Effect of Apigenin on Caspase-Mediated Apoptosis
Caspases are a family of cysteine proteases that provide important connections in the cellular networks that control inflammation and apoptosis. More than 12 caspases have been reported to date, and caspase-2, -3, -6, -7, -8, -9, and -10 have been implicated in apoptosis. Depending on their mechanism of action, these enzymes are broadly categorized as initiator caspases (caspase-2, -8, -9, and -10) and effector (or executioner) caspases (caspase-3, -6, and -7). Initiator caspases activate effector caspases, which modulate their activity to destroy key structural proteins and to activate other enzymes. In addition, caspase activation is mediated by both intrinsic and extrinsic pathways. Therefore, caspase function and expression are downregulated in tumors, which suggests that caspase activation may be an effective strategy for cancer treatment [151].
The ability of apigenin to induce caspase activation and caspase-dependent apoptosis has been demonstrated in cell lines associated with a variety of cancer types, including bladder cancer, breast cancer, cervical cancer, colon cancer, esophageal cancer, gastric cancer, glioblastoma, head and neck cancer, melanoma, leukemia, liver cancer, lung cancer, mesothelioma, neuroblastoma, osteosarcoma, ovarian cancer, pancreatic cancer, prostate cancer, renal cancer, and thyroid cancer. For example, many studies have demonstrated the apoptotic effect of apigenin, via caspase activation, on breast cancer cells. In MDA-MB-453 breast cancer cells, apigenin activates caspase-8, -9, and -3 and causes the cleavage of poly(ADP-ribose) polymerase (PARP), which results in apoptosis [82,83], and apoptosis is also induced by apigenin-mediated caspase-3 activation in MDA-MB-231, BT-474, SKBR3, T47D, and HBL-100 breast cancer cells [71,72,73,74,75,76,77,80]. Seo et al. [80] reported that extrinsic caspase-dependent apoptosis upregulates levels of cleaved caspase-8 and -3 in apigenin-treated BT-474 breast cancer cells, and consequently, the induction of PARP cleavage was confirmed. In addition, treatment with the caspase-8 inhibitor Z-IETD-FMK and the caspase-9 inhibitor Z-LEHD-FMK, together with apigenin, induced caspase-dependent apoptosis in BT-474 cells, and apigenin has been reported to trigger apoptotic cell death in caspase-3-deficient MCF-7 cells [152]. This can be demonstrated by the activation of caspase-8 by apigenin, which results in proteolytic cleavage of PARP [72,77,78,79]. Furthermore, using the caspase-9-specific inhibitor Z-LEHD-FMK and the general caspase inhibitor Z-VAD-FMK, apigenin was confirmed to induce apoptosis caspase-dependent apoptosis in PC3 and DU145 cells in a dose-dependent manner [153]. A similar effect was demonstrated in 22Rv1 human prostate cancer epithelial cells treated with apigenin, using the general caspase inhibitor Z-VAD-FMK [140]. Das et al. [100] demonstrated that reduced cytochrome c levels, owing to apigenin-induced increases in caspase-3 and -9 levels, induce apoptosis in A375 melanoma cells. Furthermore, apigenin with poly(lactide-co-glycolide)-containing nanoparticles was reported to improve the regulation of cell death and cytochrome c release and the expression of Apaf-1, Bax, Bcl-2, caspase-9, caspase-3, and PARP cleavage in A375 cells [101], and apigenin nanoparticles have been reported to contribute to the inhibition of ultraviolet (UV)-B-induced skin tumor growth by inducing caspase-3-mediated apoptosis [154].
5.3.2. Effect of Apigenin on Tumor Suppressor p53-Dependent Apoptosis
The tumor suppressor protein p53 is a transcription product of the anti-oncogene TP53 and is an important factor in the termination of cellular cancerization and induction of apoptosis in cancer cells. As such, p53 is described as the “guardian of the genome” [155]. The ability of p53 to regulate apoptosis is one of the most widely studied areas, and studies have shown that apoptosis contributes to the tumor-suppressive activity of p53. As a proapoptotic mediator, p53 can activate the transcription of proapoptotic genes, and p53 includes BH-3-specific proteins that encode members of the Bcl-2 family, such as Bax, Noxa, and PUMA. However, p53 may also promote caspase activation by inhibiting anti-apoptotic genes, such as survivin, upregulating apoptosis-inducing gene products, including Fas, TRAIL receptor DR5, Bid, and Apaf-1 [156]. Torkin et al. [130] reported that apigenin induces apoptosis in human neuroblastoma cells but not in untransformed cells. The action of apigenin appears to be mediated by p53, since it increases the levels of p53 and p53 target genes, p21WAF1/CIP1 and Bax. Furthermore, apigenin-mediated apoptotic cell death has been reported to occur in wild-type p53 cells, but not in non-functional mutant p53 cells. Shukla et al. [140] used p53 antisense oligonucleotide experiments to demonstrate that a p53-associated pathway is required for apigenin-mediated apoptosis. In prostate cancer 22Rv1 cells, apigenin treatment increased the expression and transcriptional activation of p53. Therefore, increased p53 protein expression correlated with an increase in the level of the transcriptional target p21WAF1/CIP1. Moreover, consistent with in vitro findings, the uptake of apigenin by 22Rv1-transplanted nude mice was reported to increase wild-type p53, p53-Ser15 phosphorylation, cytochrome c, and cleaved caspase-3 expression in a dose-dependent manner, and the resulting up- and down-regulation of Bax and Bcl-2 levels, respectively, suggest that the inhibited growth of 22Rv1 tumor xenografts is due to the induction of p53 pathway-mediated apoptosis. According to Shendge et al. [152], the apoptosis of apigenin-treated MCF-7 cells involved increased p53 expression, Bax/Bcl-2 ratio, caspase activation, and PARP cleavage. Meanwhile, treatment with both apigenin and the p53-mediated apoptosis inhibitor pifithrin-μ reduced the apoptotic cell population, thereby revealing the important role of p53 in apigenin-induced apoptosis in MCF-7 cells.
5.3.3. Effect of Apigenin on Tumor Suppressor p53-Independent Apoptosis
Mutations in p53 have been identified in more than 50% of human tumor tissues. In certain tumor types, the loss of p53 function is associated with chemoresistance, and cancers with p53 mutations generally respond poorly to therapeutics [157], thereby prompting the investigation of anticancer agents that act independently of p53 status. Zhang et al. [94] reported that apigenin induced apoptosis in p53 mutants of human esophageal squamous cell carcinoma KYSE-510 cells via the mitochondrial apoptosis pathway and induction of p21WAF1/CIP1. Meanwhile, in prostate cancer cells, DU145 (with mutated p53) and PC-3 (with null p53), apigenin treatment increased p21WAF1/CIP1 expression and induced apoptosis. These results demonstrate that apigenin exerts a p53-independent chemopreventive effect [141,142,143,144,145,146,147,148]. King et al. [135] reported that, in human pancreatic cancer cells (BxPC-3 and MIA PaCa-2), the p53 DNA binding-specific inhibitor pipitrin-α blocked transcription-dependent p53 activation and, thus, apigenin’s anti-proliferative and pro-apoptotic effects. Even though there was little reversal of this effect, the p53-regulated apoptosis p21WAF1/CIP1 and PUMA was inhibited by pifithrin-α. Therefore, apigenin can activate p53 through a parallel and transcriptionally independent pathway of PCD.
6. Effect of Apigenin on Autophagy
6.1. Autophagy
Autophagy, or Type 2 PCD [158], is characterized by the sequestration of cytoplasmic material into vacuoles for mass degradation by lysosomal enzymes and is defined as the cellular process through which cytoplasmic macromolecules and organelles are delivered to lysosome for degradation [159]. Much evidence supports the hypothesis that autophagy has a complex and contradictory relationship with cancer. [160] During starvation, autophagy provides recycled metabolic substrates and may promote cell survival by maintaining energy homeostasis. However, autophagy can either cooperate with apoptosis or trigger apoptosis as a backup mechanism [161]. Autophagy involves a variety of proteins that are encoded by autophagy-related genes (ATGs), of which more than 30 have been reported. In general, autophagy is induced by the activation of AMP-activated protein kinase (AMPK), which results from a lack of energy in the form of ATP. However, the process is also negatively regulated by mammalian target of rapamycin (mTOR), and the activation of mTOR complex 1 (mTORC1) has been reported to prevent autophagy, whereas its inhabitation has been reported to trigger autophagy when growth factors and/or amino acids are insufficient [162].
6.2. Types of Autophagy
The main types of autophagy (i.e., microautophagy, macroautophagy, and chaperone-mediated autophagy (CMA)) are characterized by their functions and by the way cargo is delivered to lysosomes [163]. The most well-known type, macroautophagy, involves the formation of double-membrane vesicles (i.e., autophagosomes) that swallow other vesicles (such as proteins, mitochondria, and peroxisomes) and fuse with other lysosomes and lysosomal hydrolases to degrade their contents [164]. Meanwhile, microautophagy is a non-selective lysosomal degradation process by which cytoplasmic cargo are engulfed directly from the boundary membrane via autophagy tubes that mediate endoluminal incorporation and vesicle cleavage [165], and CMA, which only occurs in mammalian cells, differs from other forms of autophagy in both the way transport proteins are perceived for lysosome transfer and the way these proteins reach the lysosomal lumen. In CMA, the internalization of substrate proteins precedes deployment, a step that is not necessary for other types of autophagy [166]. Several recent studies have highlighted the significant role of microautophagy and CMA in tumor growth and progression. However, nearly all studies of the role of autophagy in cancer development, progression, and treatment refer to macroautophagy [5].
6.3. Induction of Autophagy by Apigenin
The diverse molecular targets of apigenin-induced autophagy are summarized in Table 2. The induction of non-apoptotic autophagy by apigenin treatment was first reported by Ruela-de-Sousa et al. [106] in erythroleukemia TF1 cells, in which the autophagy inhibitor mTOR and its downstream 70-kDa ribosomal protein S6 kinase (p70S6K) were inhibited. The treatment failed to affect beclin 1 levels but strongly reduced Atg5, 7, and 12 and induced the production of both non-electron-dense vacuoles and double-membrane vacuoles, which constitute strong evidence of TF-1 cell autophagy. Subsequent studies have confirmed that apigenin can induce autophagy and have reported that apigenin can function as either a tumor suppressor or protector [5,167]. In one study [89], apigenin-induced autophagy was characterized by an increase in the level of LC3-II, which is a processing form of LC3-I, the appearance of autophagosomes, and the accumulation of acid vesicles. In addition, the autophagy inhibitor 3-methyladenine (3-MA) significantly enhanced apigenin-induced apoptosis, with increased levels of PARP cleavage, but reversed apigenin-induced LC3 puncta, which suggested that apigenin induced apoptosis and autophagy simultaneously and that apigenin-induced autophagy plays a cytoprotective role in apigenin-caused apoptosis. Similarly, Yang et al. [111] reported that apigenin increased the expression of LC3-II and the number of GFP-LC3 puncta in HepG2 cells. In addition, it has been reported that the inhibition of autophagy by 3-MA and Atg5 gene silencing enhances the apigenin-induced inhibition of proliferation and apoptosis and that apigenin induces both apoptosis and autophagy by suppressing the PI3K/Akt/mTOR pathway. Most importantly, in vivo data demonstrate that apigenin can reduce tumor growth, and the inhibition of autophagy by 3-MA notably enhances the anticancer effect of apigenin. Chen et al. [92] reported that apigenin induces autophagy and apoptosis in cisplatin-resistant colon cancer cells by inhibiting the m-TOR/PI3K/AKT signaling pathway, increases levels of the autophagy-related proteins Beclin-1 and LC3-II, and inhibits p62 expression. In vivo data have also demonstrated that apigenin can inhibit tumor growth in xenografted mouse models.
Table 2.
Molecular targets of apigenin-induced autophagy.
| Cancer/Cell Lines | Up-Regulation | Down-Regulation | Refs. |
|---|---|---|---|
| Breast | |||
| T47D and MDA-MB-231 | LC3-I, LC3-II | [75] | |
| Cevical | |||
| HeLa | GRP78 | [169] | |
| Colon | |||
| HCT116 | LC3-II | Wnt, c-Myc, Axin2, cyclin D1, β-catenin, p-AKT, p70S6, p-p70, S6, 4EBP1, p-4EBP1 | [89,170] |
| SW480 | LC3-II | Wnt | [170] |
| HT-29 | Beclin-1, LC3-II | p62, p-mTOR, p-PI3K, p-AKT | [92] |
| Gastric | |||
| AGS and SNU-638 | Atg5, Beclin1, LC3-II AMPKα ULK1, GRP78, p-PERK, p-eIF2α ATF4, CHOP, GRP78, CD63 | p62, p-mTOR, Ezh2 | [168] |
| Liver | |||
| HepG2 and HepG2 xenograft | LC3-I, LC3-II, Atg5, Beclin1, LC3-II/I ratio, AMPK | SQSTM1/p62, p-PI3K, p-AKT, p-mTOR, p-mTOR/mTOR ratio, NQO2 | [111,171,172,173] |
| Hep3B | LC3-II, Atg7, ROS | [115] | |
| SMMC-7721 and SK-HEP-1 | LC3B-II, ULK1 | p62 | [174] |
| Leukemia | |||
| TF-1 | LC3-II, Atg5, Atg12, LMWPTP | p-Src, p-JAK2,p-STAT3, p-STAT5, p-SHP2, p-mTOR, p-p70S6K | [106] |
| Lung | |||
| H1975 | LC3-II | p-EGFR, Kras, c-Myc, HIF-1α, p-AMPKα | [175] |
| Multiple myeloma | |||
| NCI-H929 | Beclin1, LC3B-II | [176] | |
| Neuroblastoma | |||
| SH-SY5Y | LC3-II, p-AKT, mTOR | Beclin 1, TLR-4, Myd88 | [177] |
| Pancreatic | |||
| PANC-1 | LC3-I, LC3-II, p-AKT | p62, NRF2, SOD, CATALASE, HSP90, p-4EBP1 | [178] |
| PaCa-44 | LC3-I, LC3-II, p62, NRF2, SOD, catalase, HSP90, 4EBP1, p-AKT | [178] | |
| Renal | |||
| ACHN and OS-RC-2 | Beclin1, LC3-II, p-AMPKα, p-JNK | Ki-67, PCNA, p62, p-PI3K, p-AKT, p-mTOR | [179] |
| Skin | |||
| COLO-16 and HEK | ATM, ATR, UPR, BiP, IRE1α, PERK, Atg, LC3-I, LC3-II | [180] | |
| Thyroid | |||
| BCPAP | Beclin1, LC3-I, LC3-II, Nrf2, HO-1 | p62 | [181] |
AMPK, 5′ adenosine monophosphate-activated protein kinase; ATR, ATF4, activating transcription factor 4; ATR, ataxia telangiectasia and Rad3-related protein; ATM, ataxia-telangiectasia mutated; Atg5, autophagy-related 5; Atg7, autophagy-related 7; Atg12, autophagy-related 12; Axin2, axis inhibition protein 2; CHOP, C/EBP homologous protein; 4EBP1, eukaryotic translation initiation factor 4E binding protein 1; EGFR, epidermal growth factor receptor; Ezh2, enhancer of zeste homolog 2; GRP78, binding immunoglobulin protein; HIF-1α, hypoxia-inducible factor 1-alpha, HO-1, heme oxygenase-1; Hsp90, heat shock protein 90; IRE1α, inositol requiring transmembrane kinase endoribonuclease-1α; JAK2, Janus kinase 2; LMWPTP, low-molecular-weight protein tyrosine phosphatase; mTOR, mammalian target of rapamycin; MYD88, myeloid differentiation primary response 88; Nrf2, nuclear factor erythroid 2-related factor 2; NQO2, NRH-quinone oxidoreductase 2; p70S6K, 70-kDa ribosomal protein S6 kinase; PI3K, phosphoinositide 3-kinase; PCNA, proliferating cell nuclear antigen; PERK, protein kinase RNA-like endoplasmic reticulum kinase; ROS, reactive oxygen species; SHP2, Src homology region 2 domain-containing phosphatase-2; SOD, superoxide dismutase; STAT, signal transducer and activator of transcription; TLR-4, Toll-like receptor 4; ULK1, autophagy-activating kinase 1.
According to Kim et al. [168], apigenin treatment increases the phosphorylation of ATG5, LC3-II, AMPK, and ULK1 and downregulates p62, thereby promoting autophagic cell death, in gastric cancer AGS and SNU-638 cell lines under hypoxic conditions. Apparently, apigenin can also induce autophagic cell death by activating protein kinase R-like endoplasmic reticulum kinase (PERK) signaling, which is indicative of the endoplasmic reticulum (ER) stress response, and induces ER stress and autophagy-related apoptosis by inhibiting hypoxia-inducible factor 1, alpha subunit (HIF-1α), and enhancer of zeste homolog 2 (Ezh2) under both normoxic and hypoxic conditions. Therefore, apigenin clearly activates autophagic cell death by suppressing HIF-1α and Ezh2 in gastric cancer cells under hypoxic conditions.
7. Effect of Apigenin on Necroptosis
7.1. Necroptosis
Necroptosis is a novel form of PCD with morphological features similar to necroptosis, as described by Degterev et al. [182] in 2005. Necroptosis has several features, such as apoptosis and necrosis. For example, morphological signs, such as increased cell size, expanded organelles, translucent cytoplasm, premature plasma membrane destruction, and apoptosis, can be reversed [183]. Even though necroptosis plays an important role in the efficacy of several cancer therapeutics, several signaling pathways have been implicated in the activation of necrosis [184,185]. Necroptosis is a caspase-independent process that is involved in the activation of death receptors [186]. During necroptosis, substrate mixed-lineage kinase domain-like (MLKL)/receptor-interacting serine/threonine kinase 3 (PIRK3) plays an important role in the activation and execution of cell death [187]. After the phosphorylation of MLKL by PIRK3, MLKL is oligomerized and translated into the plasma membrane, where it improves membrane permeability by interacting with phospholipids. Permeability is the main difference between apoptosis and necrosis. To further characterize necroptosis, as well as the difference between apoptosis and necrosis, apoptotic cells are surrounded by adjacent cells or antigen-presenting cells, whereas in necroptosis, permeability increases the release of cytokines and chemokines to induce immune responses and inflammation [188].
7.2. Necroptosis in Cancer
Necroptosis has been described as both a friend and an enemy of cancer and has been reported to exert this dual effect on the growth of tumors associated with various types of cancers. As an unsafe form of cell death that occurs in non-apoptotic cells, necroptosis can stop tumor development. Nevertheless, as a form of necrotic cell death, necroptosis can induce inflammatory responses and has been reported to promote cancer metastasis and immunosuppression [189,190]. Therefore, the manipulating and/or induction of necroptosis in anticancer therapy represent promising therapeutic approaches that could bypass acquired or intrinsic apoptosis resistance and serve as alternatives for eliminating apoptosis-resistant cancer cells. A growing number of compounds and chemotherapeutic agents have been reported to induce necroptosis in cancer cells [191].
7.3. Induction of Necroptosis by Apigenin
Even though few studies have investigated the role of apigenin in necroptosis, the several molecular targets of apigenin-induced necroptosis are summarized in Table 3. Necroptosis involves activation of receptor-interacting protein kinase (RIPK) 1, which binds to RIPK3 to form a necrosome. These events ultimately activate mixed-lineage kinase domain-like protein (MLKL), which causes necroptosis [192]. Lee et al. [193] reported that apigenin treatment can increase p-MLKL and p-RIP3 levels in malignant mesothelioma cell lines (MSTO-211H and H2452) and that apigenin can significantly inhibit cell viability, increase ROS, and induce ATP depletion through mitochondrial dysfunction, thus promoting ROS-dependent necroptosis. Meanwhile, Warkad et al. [194] reported that combined treatment with metformin and apigenin upregulates the necroptosis-related factors p-MLKL and p-RIP3 in AsPC-1 pancreatic cancer cells and that metformin and apigenin together, but not individually, can dramatically increase ROS levels and reduce cell viability in a variety of cancer cells, including AsPC-1 cells. Warkad et al. also reported that metformin differentially regulates cellular ROS levels through the AMPK-FOXO3a, forkhead box O3a (FOXO3a)-MnSOD pathways in AsPC-1 pancreatic cancer cells and that the combination of metformin and apigenin induces DNA damage by AsPC-1 pancreatic cancer cell-specific ROS amplification, which results in apoptosis, autophagy, and necroptosis.
Table 3.
Molecular targets of apigenin-induced necroptosis.
| Cancer/Cell Lines | Up-Regulation | Down-Regulation | Refs. |
|---|---|---|---|
| Mesothelioma | |||
| MSTO-211H and H2452 | ROS, γ-H2AX, p-ATM, p-ATR, p-CHK1, p-CHK2, Bax, caspase-3 and PARP cleavage, p-MLKL, p-RIP3, Bax/Bcl-2 ratio | MMP, ATP, Bcl-2 | [193] |
| Pancreatic | |||
| AsPC-1 | p-ATM, γ-H2AX, p-p53, Bim, Bid, Bax, PARP cleavage, caspasae-3, -8, and -9, Cyt c, AIF1, p62, LC3B, p-MLKL, p-RIP | Bcl-2 | [194] |
AIF1, apoptosis-inducing factor; ATM, ataxia telangiectasia mutated kinase; ATP, adenosine triphosphate; ATR, ataxia telangiectasia and Rad3-related kinase; Bax, Bcl-2-associated X protein; Bid, BH3 interacting-domain death agonist; Bim, Bcl-2 interacting mediator of cell death; Cyt c, cytochrome c; H2AX, H2A histone family member X; MLKL, mixed-lineage kinase domain-like pseudokinase; MMP, mitochondrial membrane potential; PARP, poly(ADP-ribose) polymerase; RIP3, receptor-interacting protein 3; ROS, reactive oxygen species.
8. Effect of Apigenin on Ferroptosis
8.1. Ferroptosis
Ferroptosis, which was first reported by Dixon et al. [195] in 2012, is a form of apoptosis characterized by intracellular iron accumulation and the cellular accumulation of lipid ROS. The process can be stimulated by ROS generation, GSH depletion, and nicotinamide adenine dinucleotide phosphate (NADPH)-dependent lipid peroxidation [196] and involves mitogen-activated protein kinases (MAPKs), including c-Jun NH2-terminal kinase (JNK), ERK, and p38 [162]. The morphological features of ferroptosis include increased mitochondrial membrane density, reduced mitochondrial crista and mitochondrial size, and mitochondrial exoplanet rupture, possibly owing to the dysfunction of voltage-dependent anionic channels and changes in mitochondrial membranes fluidity via lipid peroxidation [197,198].
8.2. Ferroptosis and Cancer
Cell death is important for homeostasis, normal development, and the prevention of hyperproliferative diseases, such as cancer. Despite the success of clinical cancer treatment, genetic resistance to conventional chemotherapeutic agents remains problematic [199]. Ferroptosis has been used to treat a variety of physiological and pathological processes and diseases, including several types of cancer. Many studies have reported that ferroptosis plays an important role in killing tumor cells and preventing tumor growth. For example, ferroptosis has been reported to inhibit tumorigenic cells associated with hepatocellular carcinoma [200], leukemia [201], non-small-cell lung cancer [202], pancreatic cancer [203], and breast cancer [204]. Therefore, ferroptosis could be used as a novel therapeutic strategy for cancer treatment. Because several FDA-approved clinical drugs (e.g., artesunate, sorafenib, and sulfasalazine) are known to induce ferroptosis in certain types of cancer, ferroptosis can be used in preclinical and clinical studies. Moreover, ferroptosis-inducing agents, such as erastin, piperazine erastin, and RSL3, have been reported to inhibit tumor growth in xenograft models of HT-1080 cells in vivo [196]. Therefore, there is a need for clinical studies of ferroptosis-inducing drugs for use in tumor therapy [205].
8.3. Induction of Ferroptosis by Apigenin
Few studies have investigated the effects of apigenin on ferroptosis. Therefore, a limited numbers of molecular targets of apigenin-induced ferroptosis are summarized in Table 4. According to Adham et al. [176], apigenin treatment can induce cell cycle arrest, apoptosis, autophagy, and ferroptosis in the multiple myeloma cell line NCI-H929. Apigenin-induced ferroptosis was confirmed by treating NCI-H929 cells with apigenin and the ferroptosis inhibitor ferrostatin-1, which completely ameliorated apigenin’s cytotoxicity. Meanwhile, another ferroptosis inhibitor, namely, deferoxamine, reduced the cytotoxicity of apigenin by 3.1-fold. In addition to providing the first evidence that apigenin is involved in ferroptosis, Adham et al. also demonstrated that apigenin is an important contributor to the inhibition of the STAT1/COX-2/iNOS signaling pathway to inhibit inflammation and induce apoptosis and that apigenin may be a suitable candidate for treating multiple myeloma. Shao et al. [206] reported that myeloperoxidase (MPO)-mediated oxidative stress plays an important role in pathological dysfunction and also demonstrated that apigenin can relieve MPO-mediated oxidative stress and inhibit neuronal ferroptosis, thereby significantly increasing GPX4, an important marker of ferroptosis. Liu et al. [207], who investigated mesoporous magnetic nanosystems for apigenin (API) delivery, reported that the targeted Fe2O3/Fe3O4@mSiO2-HA nanocomposite delivery system significantly increased ROS levels and cellular lipid peroxidation levels, which is typical of ferroptosis in A549 cells, and upregulated COX2 and p53, an important gene in ferroptosis, while also downregulating GPX4 and FTH1. The downregulation of GPX4, which is also an important component of the ferroptosis signaling pathway, which involves iron ions. The simultaneous administration of apigenin and the ferroptosis inhibitor ferrostatin-1 was reported to yield less pronounced cell inhibitory effects than the administration of apigenin alone. Adham et al. [176] reported that extracts of Thymus vulgaris and Arctium lappa induced apoptosis, autophagy, and ferroptosis in leukemia and multiple myeloma cell lines, and apigenin has been identified in T. vulgaris. In a multiple-myeloma cell line (NCI-H929), T. vulgaris and A. lappa extracts neutralized cytotoxic activity up to the highest concentration of the experiment (100 μg/mL) using the ferroptosis inhibitors ferrostatin-1 and deferoxamine.
Table 4.
Molecular targets of apigenin-induced ferroptosis.
| Cancer/Cell Lines | Up-Regulation | Down-Regulation | Refs. |
|---|---|---|---|
| Lung | |||
| A549 | ROS, COX-2, p53, MDA, Bax, caspase-3 and -8, Cyt c | GPX4, FTH1, SOD, Bcl-2 | [207] |
| Multiple Myeloma | |||
| HEK293 | caspase-3 and -9, p38, JNK, LC3-II, Beclin-1, ROS | AKT, MMP, STAT1, COX-2, iNOS | [176] |
| NCI-H929 | LC3-II, Beclin-1, ROS | MMP | [208] |
| Neuroblastoma | |||
| SH-SY5Y | GPX4 | MMP | [206] |
Bax, Bcl-2-associated X protein; Cyt c, cytochrome c; COX-2, cyclooxygenase-2; FTH1, ferritin heavy chain 1; GPX4, glutathione peroxidase; iNOS, inducible nitric oxide synthase; JNK, c-Jun N-terminal kinases; MDA, malondialdehyde; MMP, mitochondrial membrane potential; ROS, reactive oxygen species; SOD, superoxide dismutase; STAT, signal transducer and activator of transcription.
9. Conclusions
This paper reviews the chemopreventive effects of apigenin and the roles of apoptosis, autophagy, necroptosis, and ferroptosis in the compound’s physiological effects. Evidence from both in vitro and in vivo studies indicates that apigenin exerts significant anticancer activity. However, even though apigenin is bioavailable after oral administration in rats and mice, there are no data regarding the compound’s pharmacodynamic or pharmacokinetic profiles in humans. Therefore, additional data, including the bioavailability and safety of apigenin in humans, are needed to promote further investigation and the development of apigenin as a chemopreventive or therapeutic anticancer agent.
Acknowledgments
The authors are grateful to the Aging Tissue Bank (http://grscicoll.org/institution/aging-tissue-bank, accessed on 21 February 2022) for providing research information.
Author Contributions
Conceptualization, J.Y.J., B.S. and N.D.K.; Investigation, J.Y.J.; Writing—Original Draft Preparation, J.Y.J. and B.S.; Writing—Review and Editing, N.D.K.; Supervision, N.D.K.; Project Administration, N.D.K.; Funding Acquisition, N.D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Research Foundation of Korea (NRF), which is funded by the Korean government (MSIT) (grant No. 2021R1F1A1051265), and by the NRF Basic Research Program, which is funded by the Ministry of Education (grant No. 2018R1D1A1B07044648).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Umezawa S., Higurashi T., Komiya Y., Arimoto J., Horita N., Kaneko T., Iwasaki M., Nakagama H., Nakajima A. Chemoprevention of colorectal cancer: Past, present, and future. Cancer Sci. 2019;110:3018–3026. doi: 10.1111/cas.14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F., Jemal A., Grey N., Ferlay J., Forman D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 4.Ranjan A., Ramachandran S., Gupta N., Kaushik I., Wright S., Srivastava S., Das H., Srivastava S., Prasad S., Srivastava S.K. Role of phytochemicals in cancer prevention. Int. J. Mol. Sci. 2019;20:4981. doi: 10.3390/ijms20204981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung B., Chung H.Y., Kim N.D. Role of apigenin in cancer prevention via the induction of apoptosis and autophagy. J. Cancer Prev. 2016;21:216–226. doi: 10.15430/JCP.2016.21.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Flora S., Ferguson L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. 2005;591:8–15. doi: 10.1016/j.mrfmmm.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Khanna D., Sethi G., Ahn K.S., Pandey M.K., Kunnumakkara A.B., Sung B., Aggarwal A., Aggarwal B.B. Natural products as a gold mine for arthritis treatment. Curr. Opin. Pharmacol. 2007;7:344–351. doi: 10.1016/j.coph.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Liu C., Ho P.C., Wong F.C., Sethi G., Wang L.Z., Goh B.C. Garcinol: Current status of its anti-oxidative, anti-inflammatory and anti-cancer effects. Cancer Lett. 2015;362:8–14. doi: 10.1016/j.canlet.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Ajaikumar K.B., Asheef M., Babu B.H., Padikkala J. The inhibition of gastric mucosal injury by Punicagranatum L. (pomegranate) methanolic extract. J. Ethnopharmacol. 2005;96:171–176. doi: 10.1016/j.jep.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Nair A.S., Shishodia S., Ahn K.S., Kunnumakkara A.B., Sethi G., Aggarwal B.B. Deguelin, an Akt inhibitor, suppresses IkappaBalpha kinase activation leading to suppression of NF-kappaB-regulated gene expression, potentiation of apoptosis, and inhibition of cellular invasion. J. Immunol. 2006;177:5612–5622. doi: 10.4049/jimmunol.177.8.5612. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S.C., Prasad S., Tyagi A.K., Kunnumakkara A.B., Aggarwal B.B. Neem (Azadirachta indica): An indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14–20. doi: 10.1016/j.phymed.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Amalraj A., Varma K., Jacob J., Divya C., Kunnumakkara A.B., Stohs S.J., Gopi S. A novel highly bioavailable curcumin formulation improves symptoms and diagnostic indicators in rheumatoid arthritis patients: A randomized, double-blind, placebo-controlled, two-dose, three-arm, and parallel-group study. J. Med. Food. 2017;20:1022–1030. doi: 10.1089/jmf.2017.3930. [DOI] [PubMed] [Google Scholar]
- 13.Gopi S., Jacob J., Varma K., Jude S., Amalraj A., Arundhathy C.A., George R., Sreeraj T.R., Divya C., Kunnumakkara A.B., et al. Comparative oral absorption of curcumin in a natural turmeric matrix with two other curcumin formulations: An open-label parallel-arm study. Phytother. Res. 2017;31:1883–1891. doi: 10.1002/ptr.5931. [DOI] [PubMed] [Google Scholar]
- 14.Dai X., Zhang J., Arfuso F., Chinnathambi A., Zayed M.E., Alharbi S.A., Kumar A.P., Ahn K.S., Sethi G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp. Biol. Med. 2015;240:760–773. doi: 10.1177/1535370215579167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shanmugam M.K., Nguyen A.H., Kumar A.P., Tan B.K., Sethi G. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: Potential role in prevention and therapy of cancer. Cancer Lett. 2012;320:158–170. doi: 10.1016/j.canlet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 16.Roy N.K., Deka A., Bordoloi D., Mishra S., Kumar A.P., Sethi G., Kunnumakkara A.B. The potential role of boswellic acids in cancer prevention and treatment. Cancer Lett. 2016;377:74–86. doi: 10.1016/j.canlet.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Monisha J., Padmavathi G., Roy N.K., Deka A., Bordoloi D., Anip A., Kunnumakkara A.B. NF-κB blockers gifted by mother nature: Prospectives in cancer cell chemosensitization. Curr. Pharm. Des. 2016;22:4173–4200. doi: 10.2174/1381612822666160609110231. [DOI] [PubMed] [Google Scholar]
- 18.Sailo B.L., Banik K., Girisa S., Bordoloi D., Fan L., Halim C.E., Wang H., Kumar A.P., Zheng D., Mao X., et al. FBXW7 in cancer: What has been unraveled thus far? Cancers. 2019;11:246. doi: 10.3390/cancers11020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias D.A., Urban S., Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2:303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanmugam M.K., Lee J.H., Chai E.Z., Kanchi M.M., Kar S., Arfuso F., Dharmarajan A., Kumar A.P., Ramar P.S., Looi C.Y., et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016;40–41:35–47. doi: 10.1016/j.semcancer.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 22.Kunnumakkara A.B., Harsha C., Banik K., Vikkurthi R., Sailo B.L., Bordoloi D., Gupta S.C., Aggarwal B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 2019;15:705–733. doi: 10.1080/17425255.2019.1650914. [DOI] [PubMed] [Google Scholar]
- 23.Henamayee S., Banik K., Sailo B.L., Shabnam B., Harsha C., Srilakshmi S., Vgm N., Baek S.H., Ahn K.S., Kunnumakkara A.B. Therapeutic emergence of rhein as a potential anticancer drug: A review of its molecular targets and anticancer properties. Molecules. 2020;25:2278. doi: 10.3390/molecules25102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banik K., Ranaware A.M., Deshpande V., Nalawade S.P., Padmavathi G., Bordoloi D., Sailo B.L., Shanmugam M.K., Fan L., Arfuso F., et al. Honokiol for cancer therapeutics: A traditional medicine that can modulate multiple oncogenic targets. Pharmacol. Res. 2019;144:192–209. doi: 10.1016/j.phrs.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Kunnumakkara A.B., Bordoloi D., Sailo B.L., Roy N.K., Thakur K.K., Banik K., Shakibaei M., Gupta S.C., Aggarwal B.B. Cancer drug development: The missing links. Exp. Biol. Med. 2019;244:663–689. doi: 10.1177/1535370219839163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girisa S., Shabnam B., Monisha J., Fan L., Halim C.E., Arfuso F., Ahn K.S., Sethi G., Kunnumakkara A.B. Potential of zerumbone as an anti-cancer agent. Molecules. 2019;24:734. doi: 10.3390/molecules24040734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salehi B., Venditti A., Sharifi-Rad M., Kręgiel D., Sharifi-Rad J., Durazzo A., Lucarini M., Santini A., Souto E.B., Novellino E., et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019;20:1305. doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan X., Qi M., Li P., Zhan Y., Shao H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017;7:50. doi: 10.1186/s13578-017-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pápay Z.E., Kósa A., Böddi B., Merchant Z., Saleem I.Y., Zariwala M.G., Klebovich I., Somavarapu S., Antal I. Study on the pulmonary delivery system of apigenin-loaded albumin nanocarriers with antioxidant activity. J. Aerosol Med. Pulm. Drug Deliv. 2017;30:274–288. doi: 10.1089/jamp.2016.1316. [DOI] [PubMed] [Google Scholar]
- 30.Ashrafizadeh M., Bakhoda M.R., Bahmanpour Z., Ilkhani K., Zarrabi A., Makvandi P., Khan H., Mazaheri S., Darvish M., Mirzaei H. Apigenin as tumor suppressor in cancers: Biotherapeutic activity, nanodelivery, and mechanisms with emphasis on pancreatic cancer. Front. Chem. 2020;8:829. doi: 10.3389/fchem.2020.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y.C., Huang K.M. In vitro anti-inflammatory effect of apigenin in the Helicobacter pylori-infected gastric adenocarcinoma cells. Food Chem. Toxicol. 2013;53:376–383. doi: 10.1016/j.fct.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Z.Y., Gao T., Huang Y., Xue J., Xie M.L. Apigenin ameliorates hypertension-induced cardiac hypertrophy and down-regulates cardiac hypoxia inducible factor-lα in rats. Food Funct. 2016;7:1992–1998. doi: 10.1039/C5FO01464F. [DOI] [PubMed] [Google Scholar]
- 33.Ozçelik B., Kartal M., Orhan I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011;49:396–402. doi: 10.3109/13880209.2010.519390. [DOI] [PubMed] [Google Scholar]
- 34.Pamunuwa G., Karunaratne D.N., Waisundara V.Y. Antidiabetic properties, bioactive constituents, and other therapeutic effects of Scoparia dulcis. Evid.-Based Complement. Alternat. Med. 2016;2016:8243215. doi: 10.1155/2016/8243215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li R.R., Pang L.L., Du Q., Shi Y., Dai W.J., Yin K.S. Apigenin inhibits allergen-induced airway inflammation and switches immune response in a murine model of asthma. Immunopharmacol. Immunotoxicol. 2010;32:364–370. doi: 10.3109/08923970903420566. [DOI] [PubMed] [Google Scholar]
- 36.Birt D.F., Walker B., Tibbels M.G., Bresnick E. Anti-mutagenesis and anti-promotion by apigenin, robinetin and indole-3-carbinol. Carcinogenesis. 1986;7:959–963. doi: 10.1093/carcin/7.6.959. [DOI] [PubMed] [Google Scholar]
- 37.Farhood B., Goradel N.H., Mortezaee K., Khanlarkhani N., Najafi M., Sahebkar A. Melatonin and cancer: From the promotion of genomic stability to use in cancer treatment. J. Cell. Physiol. 2019;234:5613–5627. doi: 10.1002/jcp.27391. [DOI] [PubMed] [Google Scholar]
- 38.Mortezaee K., Najafi M., Farhood B., Ahmadi A., Potes Y., Shabeeb D., Musa A.E. Modulation of apoptosis by melatonin for improving cancer treatment efficiency: An updated review. Life Sci. 2019;228:228–241. doi: 10.1016/j.lfs.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Woo S.M., Seo S.U., Kubatka P., Min K.J., Kwon T.K. Honokiol enhances TRAIL-Mediated Apoptosis through STAMBPL1-induced survivin and c-FLIP degradation. Biomolecules. 2019;9:838. doi: 10.3390/biom9120838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mortezaee K., Najafi M., Farhood B., Ahmadi A., Shabeeb D., Musa A.E. NF-κB targeting for overcoming tumor resistance and normal tissues toxicity. J. Cell. Physiol. 2019;234:17187–17204. doi: 10.1002/jcp.28504. [DOI] [PubMed] [Google Scholar]
- 41.Mortezaee K., Potes Y., Mirtavoos-Mahyari H., Motevaseli E., Shabeeb D., Musa A.E., Najafi M., Farhood B. Boosting immune system against cancer by melatonin: A mechanistic viewpoint. Life Sci. 2019;238:116960. doi: 10.1016/j.lfs.2019.116960. [DOI] [PubMed] [Google Scholar]
- 42.Mortezaee K., Salehi E., Mirtavoos-Mahyari H., Motevaseli E., Najafi M., Farhood B., Rosengren R.J., Sahebkar A. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J. Cell. Physiol. 2019;234:12537–12550. doi: 10.1002/jcp.28122. [DOI] [PubMed] [Google Scholar]
- 43.Najafi M., Cheki M., Rezapoor S., Geraily G., Motevaseli E., Carnovale C., Clementi E., Shirazi A. Metformin: Prevention of genomic instability and cancer: A review. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018;827:1–8. doi: 10.1016/j.mrgentox.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Najafi M., Goradel N.H., Farhood B., Salehi E., Solhjoo S., Toolee H., Kharazinejad E., Mortezaee K. Tumor microenvironment: Interactions and therapy. J. Cell. Physiol. 2019;234:5700–5721. doi: 10.1002/jcp.27425. [DOI] [PubMed] [Google Scholar]
- 45.Najafi M., Hashemi Goradel N., Farhood B., Salehi E., Nashtaei M.S., Khanlarkhani N., Khezri Z., Majidpoor J., Abouzaripour M., Habibi M., et al. Macrophage polarity in cancer: A review. J. Cell. Biochem. 2019;120:2756–2765. doi: 10.1002/jcb.27646. [DOI] [PubMed] [Google Scholar]
- 46.Hashemi Goradel N., Najafi M., Salehi E., Farhood B., Mortezaee K. Cyclooxygenase-2 in cancer: A review. J. Cell. Physiol. 2019;234:5683–5699. doi: 10.1002/jcp.27411. [DOI] [PubMed] [Google Scholar]
- 47.Xu M., Wang S., Song Y.U., Yao J., Huang K., Zhu X. Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/β-catenin signaling pathway. Oncol. Lett. 2016;11:3075–3080. doi: 10.3892/ol.2016.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang C., Wei Y.X., Shen M.C., Tu Y.H., Wang C.C., Huang H.C. Chrysin, Abundant in morinda citrifolia fruit water-EtOAc extracts, combined with apigenin synergistically induced apoptosis and inhibited migration in human breast and liver cancer cells. J. Agric. Food Chem. 2016;64:4235–4245. doi: 10.1021/acs.jafc.6b00766. [DOI] [PubMed] [Google Scholar]
- 49.Lee Y.M., Lee G., Oh T.I., Kim B.M., Shim D.W., Lee K.H., Kim Y.J., Lim B.O., Lim J.H. Inhibition of glutamine utilization sensitizes lung cancer cells to apigenin-induced apoptosis resulting from metabolic and oxidative stress. Int. J. Oncol. 2016;48:399–408. doi: 10.3892/ijo.2015.3243. [DOI] [PubMed] [Google Scholar]
- 50.Zhao G., Han X., Cheng W., Ni J., Zhang Y., Lin J., Song Z. Apigenin inhibits proliferation and invasion, and induces apoptosis and cell cycle arrest in human melanoma cells. Oncol. Rep. 2017;37:2277–2285. doi: 10.3892/or.2017.5450. [DOI] [PubMed] [Google Scholar]
- 51.Gupta S., Afaq F., Mukhtar H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21:3727–3738. doi: 10.1038/sj.onc.1205474. [DOI] [PubMed] [Google Scholar]
- 52.Angulo P., Kaushik G., Subramaniam D., Dandawate P., Neville K., Chastain K., Anant S. Natural compounds targeting major cell signaling pathways: A novel paradigm for osteosarcoma therapy. J. Hematol. Oncol. 2017;10:10. doi: 10.1186/s13045-016-0373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cardenas H., Arango D., Nicholas C., Duarte S., Nuovo G.J., He W., Voss O.H., Gonzalez-Mejia M.E., Guttridge D.C., Grotewold E., et al. Dietary apigenin exerts immune-regulatory activity in vivo by reducing NF-κB activity, halting leukocyte infiltration and restoring normal metabolic function. Int. J. Mol. Sci. 2016;17:323. doi: 10.3390/ijms17030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnstone R.W., Ruefli A.A., Lowe S.W. Apoptosis: A link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/S0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 56.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Brenner D., Mak T.W. Mitochondrial cell death effectors. Curr. Opin. Cell Biol. 2009;21:871–877. doi: 10.1016/j.ceb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Peña-Blanco A., García-Sáez A.J. Bax, Bak and beyond—Mitochondrial performance in apoptosis. FEBS J. 2018;285:416–431. doi: 10.1111/febs.14186. [DOI] [PubMed] [Google Scholar]
- 59.Edlich F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018;500:26–34. doi: 10.1016/j.bbrc.2017.06.190. [DOI] [PubMed] [Google Scholar]
- 60.Suhaili S.H., Karimian H., Stellato M., Lee T.H., Aguilar M.I. Mitochondrial outer membrane permeabilization: A focus on the role of mitochondrial membrane structural organization. Biophys. Rev. 2017;9:443–457. doi: 10.1007/s12551-017-0308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elena-Real C.A., Díaz-Quintana A., González-Arzola K., Velázquez-Campoy A., Orzáez M., López-Rivas A., Gil-Caballero S., De la Rosa M., Díaz-Moreno I. Cytochrome c speeds up caspase cascade activation by blocking 14-3-3ε-dependent Apaf-1 inhibition. Cell Death Dis. 2018;9:365. doi: 10.1038/s41419-018-0408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Opdenbosch N., Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50:1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bertheloot D., Latz E., Franklin B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021;18:1106–1121. doi: 10.1038/s41423-020-00630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leonard B.C., Johnson D.E. Signaling by cell surface death receptors: Alterations in head and neck cancer. Adv. Biol. Regul. 2018;67:170–178. doi: 10.1016/j.jbior.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’ Reilly E., Tirincsi A., Logue S.E., Szegezdi E. The Janus face of death receptor signaling during tumor immunoediting. Front. Immunol. 2016;7:446. doi: 10.3389/fimmu.2016.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erekat N.S. Apoptosis and its therapeutic implications in neurodegenerative diseases. Clin. Anat. 2022;35:65–78. doi: 10.1002/ca.23792. [DOI] [PubMed] [Google Scholar]
- 67.Erekat N.S. Cerebellar upregulation of cell surface death receptor-mediated apoptotic factors in harmaline-induced tremor: An immunohistochemistry study. J. Cell Death. 2018;11:1179066018809091. doi: 10.1177/1179066018809091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu Y., Mao Y., Chen H., Lin Y., Hu Z., Wu J., Xu X., Xu X., Qin J., Xie L. Apigenin promotes apoptosis, inhibits invasion and induces cell cycle arrest of T24 human bladder cancer cells. Cancer Cell Int. 2013;13:54. doi: 10.1186/1475-2867-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi M.D., Shiao C.K., Lee Y.C., Shih Y.W. Apigenin, a dietary flavonoid, inhibits proliferation of human bladder cancer T-24 cells via blocking cell cycle progression and inducing apoptosis. Cancer Cell Int. 2015;15:33. doi: 10.1186/s12935-015-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kilani-Jaziri S., Frachet V., Bhouri W., Ghedira K., Chekir-Ghedira L., Ronot X. Flavones inhibit the proliferation of human tumor cancer cell lines by inducing apoptosis. Drug Chem. Toxicol. 2012;35:1–10. doi: 10.3109/01480545.2011.564180. [DOI] [PubMed] [Google Scholar]
- 71.Choi E.J., Kim G.H. Apigenin causes G(2)/M arrest associated with the modulation of p21(Cip1) and Cdc2 and activates p53-dependent apoptosis pathway in human breast cancer SK-BR-3 cells. J. Nutr. Biochem. 2009;20:285–290. doi: 10.1016/j.jnutbio.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 72.Way T.D., Kao M.C., Lin J.K. Degradation of HER2/neu by apigenin induces apoptosis through cytochrome c release and caspase-3 activation in HER2/neu-overexpressing breast cancer cells. FEBS Lett. 2005;579:145–152. doi: 10.1016/j.febslet.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 73.Seo H.S., Ku J.M., Choi H.S., Woo J.K., Jang B.H., Go H., Shin Y.C., Ko S.G. Apigenin induces caspase-dependent apoptosis by inhibiting signal transducer and activator of transcription 3 signaling in HER2-overexpressing SKBR3 breast cancer cells. Mol. Med. Rep. 2015;12:2977–2984. doi: 10.3892/mmr.2015.3698. [DOI] [PubMed] [Google Scholar]
- 74.Seo H.S., Ju J.H., Jang K., Shin I. Induction of apoptotic cell death by phytoestrogens by up-regulating the levels of phospho-p53 and p21 in normal and malignant estrogen receptor α-negative breast cells. Nutr. Res. 2011;31:139–146. doi: 10.1016/j.nutres.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 75.Cao X., Liu B., Cao W., Zhang W., Zhang F., Zhao H., Meng R., Zhang L., Niu R., Hao X., et al. Autophagy inhibition enhances apigenin-induced apoptosis in human breast cancer cells. Chin. J. Cancer Res. 2013;25:212–222. doi: 10.3978/j.issn.1000-9604.2013.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen D., Landis-Piwowar K.R., Chen M.S., Dou Q.P. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007;9:R80. doi: 10.1186/bcr1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin C.-H., Chang C.-Y., Lee K.-R., Lin H.-J., Chen T.-H., Wan L. Flavones inhibit breast cancer proliferation through the Akt/FOXO3a signaling pathway. BMC Cancer. 2015;15:958. doi: 10.1186/s12885-015-1965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seo H.S., Choi H.S., Kim S.R., Choi Y.K., Woo S.M., Shin I., Woo J.K., Park S.Y., Shin Y.C., Ko S.G. Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFκB signaling in HER2-overexpressing breast cancer cells. Mol. Cell. Biochem. 2012;366:319–334. doi: 10.1007/s11010-012-1310-2. [DOI] [PubMed] [Google Scholar]
- 79.Bai H., Jin H., Yang F., Zhu H., Cai J. Apigenin induced MCF-7 cell apoptosis-associated reactive oxygen species. Scanning. 2014;36:622–631. doi: 10.1002/sca.21170. [DOI] [PubMed] [Google Scholar]
- 80.Seo H.-S., Jo J.K., Ku J.M., Choi H.-S., Choi Y.K., Woo J.-K., Kim H.i., Kang S.-y., Lee K.m., Nam K.W., et al. Induction of caspase-dependent extrinsic apoptosis by apigenin through inhibition of signal transducer and activator of transcription 3 (STAT3) signalling in HER2-overexpressing BT-474 breast cancer cells. Biosci. Rep. 2015;35:e00276. doi: 10.1042/BSR20150165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu R., Ji P., Liu B., Qiao H., Wang X., Zhou L., Deng T., Ba Y. Apigenin enhances the cisplatin cytotoxic effect through p53-modulated apoptosis. Oncol. Lett. 2017;13:1024–1030. doi: 10.3892/ol.2016.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seo H.S., Ku J.M., Choi H.S., Woo J.K., Jang B.H., Shin Y.C., Ko S.G. Induction of caspase-dependent apoptosis by apigenin by inhibiting STAT3 signaling in HER2-overexpressing MDA-MB-453 breast cancer cells. Anticancer Res. 2014;34:2869–2882. [PubMed] [Google Scholar]
- 83.Choi E.J., Kim G.H. Apigenin induces apoptosis through a mitochondria/caspase-pathway in human breast cancer MDA-MB-453 Cells. J. Clin. Biochem. Nutr. 2009;44:260–265. doi: 10.3164/jcbn.08-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng P.W., Chiang L.C., Lin C.C. Apigenin induced apoptosis through p53-dependent pathway in human cervical carcinoma cells. Life Sci. 2005;76:1367–1379. doi: 10.1016/j.lfs.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 85.Souza R.P., Bonfim-Mendonça P.d.S., Gimenes F., Ratti B.A., Kaplum V., Bruschi M.L., Nakamura C.V., Silva S.O., Maria-Engler S.S., Consolaro M.E.L. Oxidative stress triggered by apigenin induces apoptosis in a comprehensive panel of human cervical cancer-derived cell lines. Oxid. Med. Cell. Longev. 2017;2017:1512745. doi: 10.1155/2017/1512745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu Y., Xin Y., Diao Y., Lu C., Fu J., Luo L., Yin Z. Synergistic effects of apigenin and paclitaxel on apoptosis of cancer cells. PLoS ONE. 2011;6:e29169. doi: 10.1371/journal.pone.0029169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shao H., Jing K., Mahmoud E., Huang H., Fang X., Yu C. Apigenin sensitizes colon cancer cells to antitumor activity of ABT-263. Mol. Cancer Ther. 2013;12:2640–2650. doi: 10.1158/1535-7163.MCT-13-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhong Y., Krisanapun C., Lee S.H., Nualsanit T., Sams C., Peungvicha P., Baek S.J. Molecular targets of apigenin in colorectal cancer cells: Involvement of p21, NAG-1 and p53. Eur. J. Cancer. 2010;46:3365–3374. doi: 10.1016/j.ejca.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee Y., Sung B., Kang Y.J., Kim D.H., Jang J.Y., Hwang S.Y., Kim M., Lim H.S., Yoon J.H., Chung H.Y., et al. Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int. J. Oncol. 2014;44:1599–1606. doi: 10.3892/ijo.2014.2339. [DOI] [PubMed] [Google Scholar]
- 90.Maeda Y., Takahashi H., Nakai N., Yanagita T., Ando N., Okubo T., Saito K., Shiga K., Hirokawa T., Hara M., et al. Apigenin induces apoptosis by suppressing Bcl-xl and Mcl-1 simultaneously via signal transducer and activator of transcription 3 signaling in colon cancer. Int. J. Oncol. 2018;52:1661–1673. doi: 10.3892/ijo.2018.4308. [DOI] [PubMed] [Google Scholar]
- 91.Wang W., Heideman L., Chung C.S., Pelling J.C., Koehler K.J., Birt D.F. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol. Carcinog. 2000;28:102–110. doi: 10.1002/1098-2744(200006)28:2<102::AID-MC6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 92.Chen X., Xu H., Yu X., Wang X., Zhu X., Xu X. Apigenin inhibits in vitro and in vivo tumorigenesis in cisplatin-resistant colon cancer cells by inducing autophagy, programmed cell death and targeting m-TOR/PI3K/Akt signalling pathway. J. BUON. 2019;24:488–493. [PubMed] [Google Scholar]
- 93.Turktekin M., Konac E., Onen H.I., Alp E., Yilmaz A., Menevse S. Evaluation of the effects of the flavonoid apigenin on apoptotic pathway gene expression on the colon cancer cell line (HT29) J. Med. Food. 2011;14:1107–1117. doi: 10.1089/jmf.2010.0208. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Q., Zhao X.H., Wang Z.J. Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis. Toxicol. In Vitro. 2009;23:797–807. doi: 10.1016/j.tiv.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 95.Qiu J.-G., Wang L., Liu W.-J., Wang J.-F., Zhao E.-J., Zhou F.-M., Ji X.-B., Wang L.-H., Xia Z.-K., Wang W., et al. Apigenin inhibits IL-6 transcription and suppresses esophageal carcinogenesis. Front. Pharmacol. 2019;10:1002. doi: 10.3389/fphar.2019.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen J., Chen J., Li Z., Liu C., Yin L. The apoptotic effect of apigenin on human gastric carcinoma cells through mitochondrial signal pathway. Tumour Biol. 2014;35:7719–7726. doi: 10.1007/s13277-014-2014-x. [DOI] [PubMed] [Google Scholar]
- 97.Stump T.A., Santee B.N., Williams L.P., Kunze R.A., Heinze C.E., Huseman E.D., Gryka R.J., Simpson D.S., Amos S. The antiproliferative and apoptotic effects of apigenin on glioblastoma cells. J. Pharm. Pharmacol. 2017;69:907–916. doi: 10.1111/jphp.12718. [DOI] [PubMed] [Google Scholar]
- 98.Das A., Banik N.L., Ray S.K. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer. 2010;116:164–176. doi: 10.1002/cncr.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chan L.P., Chou T.H., Ding H.Y., Chen P.R., Chiang F.Y., Kuo P.L., Liang C.H. Apigenin induces apoptosis via tumor necrosis factor receptor- and Bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatin. Biochim. Biophys. Acta. 2012;1820:1081–1091. doi: 10.1016/j.bbagen.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 100.Das S., Das J., Samadder A., Boujedaini N., Khuda-Bukhsh A.R. Apigenin-induced apoptosis in A375 and A549 cells through selective action and dysfunction of mitochondria. Exp. Biol. Med. 2012;237:1433–1448. doi: 10.1258/ebm.2012.012148. [DOI] [PubMed] [Google Scholar]
- 101.Das S., Das J., Samadder A., Paul A., Khuda-Bukhsh A.R. Strategic formulation of apigenin-loaded PLGA nanoparticles for intracellular trafficking, DNA targeting and improved therapeutic effects in skin melanoma in vitro. Toxicol. Lett. 2013;223:124–138. doi: 10.1016/j.toxlet.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 102.Arango D., Parihar A., Villamena F.A., Wang L., Freitas M.A., Grotewold E., Doseff A.I. Apigenin induces DNA damage through the PKCδ-dependent activation of ATM and H2AX causing down-regulation of genes involved in cell cycle control and DNA repair. Biochem. Pharmacol. 2012;84:1571–1580. doi: 10.1016/j.bcp.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vargo M.A., Voss O.H., Poustka F., Cardounel A.J., Grotewold E., Doseff A.I. Apigenin-induced-apoptosis is mediated by the activation of PKCdelta and caspases in leukemia cells. Biochem. Pharmacol. 2006;72:681–692. doi: 10.1016/j.bcp.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 104.Budhraja A., Gao N., Zhang Z., Son Y.O., Cheng S., Wang X., Ding S., Hitron A., Chen G., Luo J., et al. Apigenin induces apoptosis in human leukemia cells and exhibits anti-leukemic activity in vivo. Mol. Cancer Ther. 2012;11:132–142. doi: 10.1158/1535-7163.MCT-11-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jayasooriya R.G., Kang S.H., Kang C.H., Choi Y.H., Moon D.O., Hyun J.W., Chang W.Y., Kim G.Y. Apigenin decreases cell viability and telomerase activity in human leukemia cell lines. Food Chem. Toxicol. 2012;50:2605–2611. doi: 10.1016/j.fct.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 106.Ruela-de-Sousa R.R., Fuhler G.M., Blom N., Ferreira C.V., Aoyama H., Peppelenbosch M.P. Cytotoxicity of apigenin on leukemia cell lines: Implications for prevention and therapy. Cell Death Dis. 2010;1:e19. doi: 10.1038/cddis.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Landis-Piwowar K.R., Milacic V., Dou Q.P. Relationship between the methylation status of dietary flavonoids and their growth-inhibitory and apoptosis-inducing activities in human cancer cells. J. Cell. Biochem. 2008;105:514–523. doi: 10.1002/jcb.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim B.R., Jeon Y.K., Nam M.J. A mechanism of apigenin-induced apoptosis is potentially related to anti-angiogenesis and anti-migration in human hepatocellular carcinoma cells. Food Chem. Toxicol. 2011;49:1626–1632. doi: 10.1016/j.fct.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 109.Kim E.Y., Kim A.K. Apigenin Sensitizes Huh-7 Human Hepatocellular Carcinoma Cells to TRAIL-induced Apoptosis. Biomol. Ther. 2012;20:62–67. doi: 10.4062/biomolther.2012.20.1.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Şirin N., Elmas L., Seçme M., Dodurga Y. Investigation of possible effects of apigenin, sorafenib and combined applications on apoptosis and cell cycle in hepatocellular cancer cells. Gene. 2020;737:144428. doi: 10.1016/j.gene.2020.144428. [DOI] [PubMed] [Google Scholar]
- 111.Yang J., Pi C., Wang G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 2018;103:699–707. doi: 10.1016/j.biopha.2018.04.072. [DOI] [PubMed] [Google Scholar]
- 112.Kim E.Y., Yu J.S., Yang M., Kim A.K. Sub-toxic dose of apigenin sensitizes HepG2 cells to TRAIL through ERK-dependent up-regulation of TRAIL receptor DR5. Mol. Cells. 2013;35:32–40. doi: 10.1007/s10059-013-2175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Valdameri G., Trombetta-Lima M., Worfel P.R., Pires A.R., Martinez G.R., Noleto G.R., Cadena S.M., Sogayar M.C., Winnischofer S.M., Rocha M.E. Involvement of catalase in the apoptotic mechanism induced by apigenin in HepG2 human hepatoma cells. Chem. Biol. Interact. 2011;193:180–189. doi: 10.1016/j.cbi.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 114.Khan T.H., Sultana S. Apigenin induces apoptosis in Hep G2 cells: Possible role of TNF-α and IFN-γ. Toxicology. 2006;217:206–212. doi: 10.1016/j.tox.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 115.Kang C.H., Molagoda I.M.N., Choi Y.H., Park C., Moon D.O., Kim G.Y. Apigenin promotes TRAIL-mediated apoptosis regardless of ROS generation. Food Chem. Toxicol. 2018;111:623–630. doi: 10.1016/j.fct.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 116.Hu X.Y., Liang J.Y., Guo X.J., Liu L., Guo Y.B. 5-Fluorouracil combined with apigenin enhances anticancer activity through mitochondrial membrane potential (ΔΨm)-mediated apoptosis in hepatocellular carcinoma. Clin. Exp. Pharmacol. Physiol. 2015;42:146–153. doi: 10.1111/1440-1681.12333. [DOI] [PubMed] [Google Scholar]
- 117.Gao A.M., Ke Z.P., Wang J.N., Yang J.Y., Chen S.Y., Chen H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis. 2013;34:1806–1814. doi: 10.1093/carcin/bgt108. [DOI] [PubMed] [Google Scholar]
- 118.Kim K.C., Choi E.H., Lee C. Axl receptor tyrosine kinase is a novel target of apigenin for the inhibition of cell proliferation. Int. J. Mol. Med. 2014;34:592–598. doi: 10.3892/ijmm.2014.1804. [DOI] [PubMed] [Google Scholar]
- 119.Lu H.F., Chie Y.J., Yang M.S., Lee C.S., Fu J.J., Yang J.S., Tan T.W., Wu S.H., Ma Y.S., Ip S.W., et al. Apigenin induces caspase-dependent apoptosis in human lung cancer A549 cells through Bax- and Bcl-2-triggered mitochondrial pathway. Int. J. Oncol. 2010;36:1477–1484. doi: 10.3892/ijo_00000634. [DOI] [PubMed] [Google Scholar]
- 120.Chen M., Wang X., Zha D., Cai F., Zhang W., He Y., Huang Q., Zhuang H., Hua Z.C. Apigenin potentiates TRAIL therapy of non-small cell lung cancer via upregulating DR4/DR5 expression in a p53-dependent manner. Sci. Rep. 2016;6:35468. doi: 10.1038/srep35468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pan X., Yang Z., Yang Z., Zhou S., Zhang H., Zang L. Effect of apigenin on proliferation and apoptosis of human lung cancer NCI-H460 cells. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:1137–1140. [PubMed] [Google Scholar]
- 122.Lu H.F., Chie Y.J., Yang M.S., Lu K.W., Fu J.J., Yang J.S., Chen H.Y., Hsia T.C., Ma C.Y., Ip S.W., et al. Apigenin induces apoptosis in human lung cancer H460 cells through caspase- and mitochondria-dependent pathways. Hum. Exp. Toxicol. 2011;30:1053–1061. doi: 10.1177/0960327110386258. [DOI] [PubMed] [Google Scholar]
- 123.Huang S., Yu M., Shi N., Zhou Y., Li F., Li X., Huang X., Jin J. Apigenin and Abivertinib, a novel BTK inhibitor synergize to inhibit diffuse large B-cell lymphoma in vivo and vitro. J. Cancer. 2020;11:2123–2132. doi: 10.7150/jca.34981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Masuelli L., Benvenuto M., Mattera R., Di Stefano E., Zago E., Taffera G., Tresoldi I., Giganti M.G., Frajese G.V., Berardi G., et al. In vitro and in vivo anti-tumoral effects of the flavonoid apigenin in malignant mesothelioma. Front. Pharmacol. 2017;8:373. doi: 10.3389/fphar.2017.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao M., Ma J., Zhu H.Y., Zhang X.H., Du Z.Y., Xu Y.J., Yu X.D. Apigenin inhibits proliferation and induces apoptosis in human multiple myeloma cells through targeting the trinity of CK2, Cdc37 and Hsp90. Mol. Cancer. 2011;10:104. doi: 10.1186/1476-4598-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hossain M.M., Banik N.L., Ray S.K. N-Myc knockdown and apigenin treatment controlled growth of malignant neuroblastoma cells having N-Myc amplification. Gene. 2013;529:27–36. doi: 10.1016/j.gene.2013.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chakrabarti M., Banik N.L., Ray S.K. miR-138 overexpression is more powerful than hTERT knockdown to potentiate apigenin for apoptosis in neuroblastoma in vitro and in vivo. Exp. Cell Res. 2013;319:1575–1585. doi: 10.1016/j.yexcr.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mohan N., Ai W., Chakrabarti M., Banik N.L., Ray S.K. KLF4 overexpression and apigenin treatment down regulated anti-apoptotic Bcl-2 proteins and matrix metalloproteinases to control growth of human malignant neuroblastoma SK-N-DZ and IMR-32 cells. Mol. Oncol. 2013;7:464–474. doi: 10.1016/j.molonc.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karmakar S., Davis K.A., Choudhury S.R., Deeconda A., Banik N.L., Ray S.K. Bcl-2 inhibitor and apigenin worked synergistically in human malignant neuroblastoma cell lines and increased apoptosis with activation of extrinsic and intrinsic pathways. Biochem. Biophys. Res. Commun. 2009;388:705–710. doi: 10.1016/j.bbrc.2009.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Torkin R., Lavoie J.F., Kaplan D.R., Yeger H. Induction of caspase-dependent, p53-mediated apoptosis by apigenin in human neuroblastoma. Mol. Cancer Ther. 2005;4:1–11. doi: 10.1158/1535-7163.1.4.1. [DOI] [PubMed] [Google Scholar]
- 131.Lin C.C., Chuang Y.J., Yu C.C., Yang J.S., Lu C.C., Chiang J.H., Lin J.P., Tang N.Y., Huang A.C., Chung J.G. Apigenin induces apoptosis through mitochondrial dysfunction in U-2 OS human osteosarcoma cells and inhibits osteosarcoma xenograft tumor growth in vivo. J. Agric. Food Chem. 2012;60:11395–11402. doi: 10.1021/jf303446x. [DOI] [PubMed] [Google Scholar]
- 132.Ittiudomrak T., Puthong S., Roytrakul S., Chanchao C. α-Mangostin and apigenin induced cell cycle arrest and programmed cell death in SKOV-3 ovarian cancer cells. Toxicol. Res. 2019;35:167–179. doi: 10.5487/TR.2019.35.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tavsan Z., Kayali H.A. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed. Pharmacother. 2019;116:109004. doi: 10.1016/j.biopha.2019.109004. [DOI] [PubMed] [Google Scholar]
- 134.Pal M.K., Jaiswar S.P., Dwivedi A., Goyal S., Dwivedi V.N., Pathak A.K., Kumar V., Sankhwar P.L., Ray R.S. Synergistic effect of graphene oxide coated nanotised apigenin with paclitaxel (GO-NA/PTX): A ROS dependent mitochondrial mediated apoptosis in ovarian cancer. Anticancer Agents Med. Chem. 2017;17:1721–1732. doi: 10.2174/1871520617666170425094549. [DOI] [PubMed] [Google Scholar]
- 135.King J.C., Lu Q.Y., Li G., Moro A., Takahashi H., Chen M., Go V.L., Reber H.A., Eibl G., Hines O.J. Evidence for activation of mutated p53 by apigenin in human pancreatic cancer. Biochim. Biophys. Acta. 2012;1823:593–604. doi: 10.1016/j.bbamcr.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Johnson J.L., de Mejia E.G. Flavonoid apigenin modified gene expression associated with inflammation and cancer and induced apoptosis in human pancreatic cancer cells through inhibition of GSK-3β/NF-κB signaling cascade. Mol. Nutr. Food Res. 2013;57:2112–2127. doi: 10.1002/mnfr.201300307. [DOI] [PubMed] [Google Scholar]
- 137.Johnson J.L., Gonzalez de Mejia E. Interactions between dietary flavonoids apigenin or luteolin and chemotherapeutic drugs to potentiate anti-proliferative effect on human pancreatic cancer cells, in vitro. Food Chem. Toxicol. 2013;60:83–91. doi: 10.1016/j.fct.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 138.Hamacher R., Saur D., Fritsch R., Reichert M., Schmid R.M., Schneider G. Casein kinase II inhibition induces apoptosis in pancreatic cancer cells. Oncol. Rep. 2007;18:695–701. doi: 10.3892/or.18.3.695. [DOI] [PubMed] [Google Scholar]
- 139.Granato M., Gilardini Montani M.S., Santarelli R., D’Orazi G., Faggioni A., Cirone M. Apigenin, by activating p53 and inhibiting STAT3, modulates the balance between pro-apoptotic and pro-survival pathways to induce PEL cell death. J. Exp. Clin. Cancer Res. 2017;36:167. doi: 10.1186/s13046-017-0632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shukla S., Gupta S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic. Biol. Med. 2008;44:1833–1845. doi: 10.1016/j.freeradbiomed.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shukla S., Kanwal R., Shankar E., Datt M., Chance M.R., Fu P., MacLennan G.T., Gupta S. Apigenin blocks IKKα activation and suppresses prostate cancer progression. Oncotarget. 2015;6:31216–31232. doi: 10.18632/oncotarget.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pandey M., Kaur P., Shukla S., Abbas A., Fu P., Gupta S. Plant flavone apigenin inhibits HDAC and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: In vitro and in vivo study. Mol. Carcinog. 2012;51:952–962. doi: 10.1002/mc.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shukla S., Fu P., Gupta S. Apigenin induces apoptosis by targeting inhibitor of apoptosis proteins and Ku70-Bax interaction in prostate cancer. Apoptosis. 2014;19:883–894. doi: 10.1007/s10495-014-0971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shukla S., Gupta S. Apigenin suppresses insulin-like growth factor I receptor signaling in human prostate cancer: An in vitro and in vivo study. Mol. Carcinog. 2009;48:243–252. doi: 10.1002/mc.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morrissey C., O’Neill A., Spengler B., Christoffel V., Fitzpatrick J.M., Watson R.W. Apigenin drives the production of reactive oxygen species and initiates a mitochondrial mediated cell death pathway in prostate epithelial cells. Prostate. 2005;63:131–142. doi: 10.1002/pros.20167. [DOI] [PubMed] [Google Scholar]
- 146.Singh V., Sharma V., Verma V., Pandey D., Yadav S.K., Maikhuri J.P., Gupta G. Apigenin manipulates the ubiquitin-proteasome system to rescue estrogen receptor-β from degradation and induce apoptosis in prostate cancer cells. Eur. J. Nutr. 2015;54:1255–1267. doi: 10.1007/s00394-014-0803-z. [DOI] [PubMed] [Google Scholar]
- 147.Seo Y.J., Kim B.S., Chun S.Y., Park Y.K., Kang K.S., Kwon T.G. Apoptotic effects of genistein, biochanin-A and apigenin on LNCaP and PC-3 cells by p21 through transcriptional inhibition of polo-like kinase-1. J. Korean Med. Sci. 2011;26:1489–1494. doi: 10.3346/jkms.2011.26.11.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Oishi M., Iizumi Y., Taniguchi T., Goi W., Miki T., Sakai T. Apigenin sensitizes prostate cancer cells to Apo2L/TRAIL by targeting adenine nucleotide translocase-2. PLoS ONE. 2013;8:e55922. doi: 10.1371/journal.pone.0055922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meng S., Zhu Y., Li J.F., Wang X., Liang Z., Li S.Q., Xu X., Chen H., Liu B., Zheng X.Y., et al. Apigenin inhibits renal cell carcinoma cell proliferation. Oncotarget. 2017;8:19834–19842. doi: 10.18632/oncotarget.15771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim S.H., Kang J.G., Kim C.S., Ihm S.H., Choi M.G., Yoo H.J., Lee S.J. Apigenin induces c-Myc-mediated apoptosis in FRO anaplastic thyroid carcinoma cells. Mol. Cell. Endocrinol. 2013;369:130–139. doi: 10.1016/j.mce.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 151.Boice A., Bouchier-Hayes L. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta. 2020;1867:118688. doi: 10.1016/j.bbamcr.2020.118688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shendge A.K., Chaudhuri D., Basu T., Mandal N. A natural flavonoid, apigenin isolated from Clerodendrum viscosum leaves, induces G2/M phase cell cycle arrest and apoptosis in MCF-7 cells through the regulation of p53 and caspase-cascade pathway. Clin. Trans. Oncol. 2021;23:718–730. doi: 10.1007/s12094-020-02461-0. [DOI] [PubMed] [Google Scholar]
- 153.Kaur P., Shukla S., Gupta S. Plant flavonoid apigenin inactivates Akt to trigger apoptosis in human prostate cancer: An in vitro and in vivo study. Carcinogenesis. 2008;29:2210–2217. doi: 10.1093/carcin/bgn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Das S., Das J., Samadder A., Paul A., Khuda-Bukhsh A.R. Efficacy of PLGA-loaded apigenin nanoparticles in Benzo[a]pyrene and ultraviolet-B induced skin cancer of mice: Mitochondria mediated apoptotic signalling cascades. Food Chem. Toxicol. 2013;62:670–680. doi: 10.1016/j.fct.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 155.Hibino E., Hiroaki H. Potential of rescue and reactivation of tumor suppressor p53 for cancer therapy. Biophys. Rev. 2022;14:267–275. doi: 10.1007/s12551-021-00915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ou A., Zhao X., Lu Z. The potential roles of p53 signaling reactivation in pancreatic cancer therapy. Biochim. Biophys. Acta. 2022;1877:188662. doi: 10.1016/j.bbcan.2021.188662. [DOI] [PubMed] [Google Scholar]
- 157.Feng J. The p53 pathway related genes predict the prognosis of colon cancer. Int. J. Gen. Med. 2022;15:169–177. doi: 10.2147/IJGM.S346280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hazari Y., Bravo-San Pedro J.M., Hetz C., Galluzzi L., Kroemer G. Autophagy in hepatic adaptation to stress. J. Hepatol. 2020;72:183–196. doi: 10.1016/j.jhep.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 159.Yang Y., Klionsky D.J. Autophagy and disease: Unanswered questions. Cell Death Differ. 2020;27:858–871. doi: 10.1038/s41418-019-0480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Galluzzi L., Kroemer G. Transient autophagy inhibition precipitates oncogenesis: A red flag for pharmacological autophagy inhibitors? Trends Cell Biol. 2020;30:339–340. doi: 10.1016/j.tcb.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 161.Nazim U.M., Yin H., Park S.Y. Downregulation of c-FLIP and upregulation of DR-5 by cantharidin sensitizes TRAIL-mediated apoptosis in prostate cancer cells via autophagy flux. Int. J. Mol. Med. 2020;46:280–288. doi: 10.3892/ijmm.2020.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ashrafizadeh M., Mohammadinejad R., Tavakol S., Ahmadi Z., Roomiani S., Katebi M. Autophagy, anoikis, ferroptosis, necroptosis, and endoplasmic reticulum stress: Potential applications in melanoma therapy. J. Cell. Physiol. 2019;234:19471–19479. doi: 10.1002/jcp.28740. [DOI] [PubMed] [Google Scholar]
- 163.Shakeri A., Cicero A.F.G., Panahi Y., Mohajeri M., Sahebkar A. Curcumin: A naturally occurring autophagy modulator. J. Cell. Physiol. 2019;234:5643–5654. doi: 10.1002/jcp.27404. [DOI] [PubMed] [Google Scholar]
- 164.Klionsky D.J., Codogno P. The mechanism and physiological function of macroautophagy. J. Innate Immun. 2013;5:427–433. doi: 10.1159/000351979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Matsui C., Yuliandari P., Deng L., Abe T., Shoji I. The role of chaperone-mediated autophagy in hepatitis C virus-induced pathogenesis. Front. Cell. Infect. Microbiol. 2021;11:796664. doi: 10.3389/fcimb.2021.796664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Albornoz A., Sequeida A., Rodríguez C., Budini M. Chapter 27—Chaperone-mediated autophagy—mechanisms and disease role. In: Rothermel B.A., Diwan A., editors. Autophagy in Health and Disease. 2nd ed. Academic Press; Cambridge, MA, USA: 2021. pp. 399–412. [Google Scholar]
- 167.Salmani J.M.M., Zhang X.-P., Jacob J.A., Chen B.-A. Apigenin’s anticancer properties and molecular mechanisms of action: Recent advances and future prospectives. Chin. J. Nat. Med. 2017;15:321–329. doi: 10.1016/S1875-5364(17)30052-3. [DOI] [PubMed] [Google Scholar]
- 168.Kim T.W., Lee H.G. Apigenin induces autophagy and cell death by targeting EZH2 under hypoxia conditions in gastric cancer cells. Int. J. Mol. Sci. 2021;22:13455. doi: 10.3390/ijms222413455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kayacan S., Yilancioglu K., Akdemir A.S., Kaya-Dagistanli F., Melikoglu G., Ozturk M. Synergistic effect of apigenin and curcumin on apoptosis, paraptosis and autophagy-related cell death in HeLa cells. Anticancer Res. 2021;41:1271–1282. doi: 10.21873/anticanres.14884. [DOI] [PubMed] [Google Scholar]
- 170.Lin C.M., Chen H.H., Lin C.A., Wu H.C., Sheu J.J., Chen H.J. Apigenin-induced lysosomal degradation of β-catenin in Wnt/β-catenin signaling. Sci. Rep. 2017;7:372. doi: 10.1038/s41598-017-00409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lu J., Meng Z., Chen Y., Yu L., Gao B., Zheng Y., Guan S. Apigenin induced autophagy and stimulated autophagic lipid degradation. Food Funct. 2020;11:9208–9215. doi: 10.1039/D0FO00949K. [DOI] [PubMed] [Google Scholar]
- 172.Janda E., Martino C., Riillo C., Parafati M., Lascala A., Mollace V., Boutin J.A. Apigenin and luteolin regulate autophagy by targeting NRH-Quinone oxidoreductase 2 in liver cells. Antioxidants. 2021;10:776. doi: 10.3390/antiox10050776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lascala A., Martino C., Parafati M., Salerno R., Oliverio M., Pellegrino D., Mollace V., Janda E. Analysis of proautophagic activities of Citrus flavonoids in liver cells reveals the superiority of a natural polyphenol mixture over pure flavones. J. Nutr. Biochem. 2018;58:119–130. doi: 10.1016/j.jnutbio.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 174.Zeng J., Xie H., Zhang Z.L., Li Z.X., Shi L., Wu K.Y., Zhou Y., Tian Z., Zhang Y., Zhou W., et al. Apigenin regulates the migration, invasion, and autophagy of hepatocellular carcinoma cells by downregulating YAP. Neoplasma. 2022 doi: 10.4149/neo_2021_210615N798. [DOI] [PubMed] [Google Scholar]
- 175.Chen Z., Tian D., Liao X., Zhang Y., Xiao J., Chen W., Liu Q., Chen Y., Li D., Zhu L., et al. Apigenin combined with gefitinib blocks autophagy flux and induces apoptotic cell death through inhibition of HIF-1α, c-Myc, p-EGFR, and glucose metabolism in EGFR L858R+T790M-mutated H1975 Cells. Front. Pharmacol. 2019;10:260. doi: 10.3389/fphar.2019.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Adham A.N., Abdelfatah S., Naqishbandi A.M., Mahmoud N., Efferth T. Cytotoxicity of apigenin toward multiple myeloma cell lines and suppression of iNOS and COX-2 expression in STAT1-transfected HEK293 cells. Phytomedicine. 2021;80:153371. doi: 10.1016/j.phymed.2020.153371. [DOI] [PubMed] [Google Scholar]
- 177.Mohan N., Banik N.L., Ray S.K. Combination of N-(4-hydroxyphenyl) retinamide and apigenin suppressed starvation-induced autophagy and promoted apoptosis in malignant neuroblastoma cells. Neurosci. Lett. 2011;502:24–29. doi: 10.1016/j.neulet.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gilardini Montani M.S., Cecere N., Granato M., Romeo M.A., Falcinelli L., Ciciarelli U., D’Orazi G., Faggioni A., Cirone M. Mutant p53, stabilized by its interplay with HSP90, activates a positive feed-back loop between NRF2 and p62 that induces chemo-resistance to apigenin in pancreatic cancer cells. Cancers. 2019;11:703. doi: 10.3390/cancers11050703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li Y., Sun Q., Li H., Yang B., Wang M. Vitexin suppresses renal cell carcinoma by regulating mTOR pathways. Transl. Androl. Urol. 2020;9:1700–1711. doi: 10.21037/tau-20-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li L., Li M., Xu S., Chen H., Chen X., Gu H. Apigenin restores impairment of autophagy and downregulation of unfolded protein response regulatory proteins in keratinocytes exposed to ultraviolet B radiation. J. Photochem. Photobiol. B. 2019;194:84–95. doi: 10.1016/j.jphotobiol.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 181.Zhang L., Cheng X., Gao Y., Zheng J., Xu Q., Sun Y., Guan H., Yu H., Sun Z. Apigenin induces autophagic cell death in human papillary thyroid carcinoma BCPAP cells. Food Funct. 2015;6:3464–3472. doi: 10.1039/C5FO00671F. [DOI] [PubMed] [Google Scholar]
- 182.Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G.D., Mitchison T.J., Moskowitz M.A., Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 183.Ofengeim D., Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat. Rev. Mol. Cell Biol. 2013;14:727–736. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- 184.Mohammadinejad R., Moosavi M.A., Tavakol S., Vardar D., Hosseini A., Rahmati M., Dini L., Hussain S., Mandegary A., Klionsky D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy. 2019;15:4–33. doi: 10.1080/15548627.2018.1509171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cruz S.A., Qin Z., Stewart A.F.R., Chen H.H. Dabrafenib, an inhibitor of RIP3 kinase-dependent necroptosis, reduces ischemic brain injury. Neural Regen. Res. 2018;13:252–256. doi: 10.4103/1673-5374.226394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Galluzzi L., Kepp O., Chan F.K., Kroemer G. Necroptosis: Mechanisms and relevance to disease. Annu. Rev. Pathol. 2017;12:103–130. doi: 10.1146/annurev-pathol-052016-100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hadifar S., Behrouzi A., Fateh A., Khatami S., Rahimi Jamnani F., Siadat S.D., Vaziri F. Comparative study of interruption of signaling pathways in lung epithelial cell by two different Mycobacterium tuberculosis lineages. J. Cell. Physiol. 2019;234:4739–4753. doi: 10.1002/jcp.27271. [DOI] [PubMed] [Google Scholar]
- 188.Pasparakis M., Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 189.Strilic B., Yang L., Albarrán-Juárez J., Wachsmuth L., Han K., Müller U.C., Pasparakis M., Offermanns S. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature. 2016;536:215–218. doi: 10.1038/nature19076. [DOI] [PubMed] [Google Scholar]
- 190.Seifert L., Werba G., Tiwari S., Giao Ly N.N., Alothman S., Alqunaibit D., Avanzi A., Barilla R., Daley D., Greco S.H., et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature. 2016;532:245–249. doi: 10.1038/nature17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gong Y., Fan Z., Luo G., Yang C., Huang Q., Fan K., Cheng H., Jin K., Ni Q., Yu X., et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer. 2019;18:100. doi: 10.1186/s12943-019-1029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Grootjans S., Vanden Berghe T., Vandenabeele P. Initiation and execution mechanisms of necroptosis: An overview. Cell Death Differ. 2017;24:1184–1195. doi: 10.1038/cdd.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lee Y.J., Park K.S., Nam H.S., Cho M.K., Lee S.H. Apigenin causes necroptosis by inducing ROS accumulation, mitochondrial dysfunction, and ATP depletion in malignant mesothelioma cells. Korean J. Physiol. Pharmacol. 2020;24:493–502. doi: 10.4196/kjpp.2020.24.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Warkad M.S., Kim C.H., Kang B.G., Park S.H., Jung J.S., Feng J.H., Inci G., Kim S.C., Suh H.W., Lim S.S., et al. Metformin-induced ROS upregulation as amplified by apigenin causes profound anticancer activity while sparing normal cells. Sci. Rep. 2021;11:14002. doi: 10.1038/s41598-021-93270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.DeHart D.N., Fang D., Heslop K., Li L., Lemasters J.J., Maldonado E.N. Opening of voltage dependent anion channels promotes reactive oxygen species generation, mitochondrial dysfunction and cell death in cancer cells. Biochem. Pharmacol. 2018;148:155–162. doi: 10.1016/j.bcp.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gao M., Yi J., Zhu J., Minikes A.M., Monian P., Thompson C.B., Jiang X. Role of mitochondria in ferroptosis. Mol. Cell. 2019;73:354–363.e3. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lu B., Chen X.B., Ying M.D., He Q.J., Cao J., Yang B. The role of ferroptosis in cancer development and treatment response. Front. Pharmacol. 2017;8:992. doi: 10.3389/fphar.2017.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sun X., Ou Z., Chen R., Niu X., Chen D., Kang R., Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Trujillo-Alonso V., Pratt E.C., Zong H., Lara-Martinez A., Kaittanis C., Rabie M.O., Longo V., Becker M.W., Roboz G.J., Grimm J., et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat. Nanotechnol. 2019;14:616–622. doi: 10.1038/s41565-019-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Guo J., Xu B., Han Q., Zhou H., Xia Y., Gong C., Dai X., Li Z., Wu G. Ferroptosis: A novel anti-tumor action for cisplatin. Cancer Res. Treat. 2018;50:445–460. doi: 10.4143/crt.2016.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yamaguchi Y., Kasukabe T., Kumakura S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int. J. Oncol. 2018;52:1011–1022. doi: 10.3892/ijo.2018.4259. [DOI] [PubMed] [Google Scholar]
- 204.Ma S., Henson E.S., Chen Y., Gibson S.B. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7:e2307. doi: 10.1038/cddis.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Su Y., Zhao B., Zhou L., Zhang Z., Shen Y., Lv H., AlQudsy L.H.H., Shang P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020;483:127–136. doi: 10.1016/j.canlet.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 206.Shao C., Yuan J., Liu Y., Qin Y., Wang X., Gu J., Chen G., Zhang B., Liu H.K., Zhao J., et al. Epileptic brain fluorescent imaging reveals apigenin can relieve the myeloperoxidase-mediated oxidative stress and inhibit ferroptosis. Proc. Natl. Acad. Sci. USA. 2020;117:10155–10164. doi: 10.1073/pnas.1917946117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu R., Rong G., Liu Y., Huang W., He D., Lu R. Delivery of apigenin-loaded magnetic Fe(2)O(3)/Fe(3)O(4)@mSiO(2) nanocomposites to A549 cells and their antitumor mechanism. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;120:111719. doi: 10.1016/j.msec.2020.111719. [DOI] [PubMed] [Google Scholar]
- 208.Adham N.A., Hegazy M.E.F., Naqishbandi A.M., Efferth T. Induction of apoptosis, autophagy and ferroptosis by Thymus vulgaris and Arctium lappa extract in leukemia and multiple myeloma cell lines. Molecules. 2020;25:5016. doi: 10.3390/molecules25215016. [DOI] [PMC free article] [PubMed] [Google Scholar]