Abstract
Ischemia-reperfusion injury (IRI) can be defined as changes in the functions and structures of the tissues resulting from the restoration of blood after a period of ischemia. This study aimed to assess the potential protective effect of Fimasartan (angiotensin receptor antagonist) in the bilateral renal IRI in male rats through its potential effect on renal functions, modulation of the inflammatory cascade, oxidative stress, and apoptotic effect. The animals were equally assigned into four groups. The sham (negative control) group was exposed to surgical conditions without induction of IRI. The control group was exposed to ischemia by occluding the renal pedicles by clamps for 30 min, followed by restoration of blood for 2h. The vehicle-treated group received dimethyl sulfoxide (DMSO) by intraperitoneal injection (IP) 30 minutes before clamping. Fimasartan-treated group: rats pretreated with Fimasartan a dose of 3 mg/kg IP; this was half hour before occluding the renal pedicles. Animals were then exposed to 30 min ischemia (clamping the renal pedicles) followed by 2h reperfusion by releasing the clamps. Blood samples were collected to examine the levels of serum urea and creatinine. Renal tissue was used to measure the levels of cytokines (TNFα, IL-6) and total antioxidant capacity (TAC). Immunohistochemistry was used to assess the levels of Bax, caspase 3, and Bcl-2. Histopathological analyses were performed to detect the parenchymal injury. The present study shows that pretreatment with Fimasartan improves kidney function through its effects on oxidative stress, cytokines, and apoptotic markers.
Keywords: Fimasartan, renal ischemia, Bcl-2, caspase 3, oxidative stress
Keywords: IRI – ischemia-reperfusion injury, IP – intraperitoneal, TAC – total antioxidant capacity, ROS – reactive oxygen species, AKI – acute kidney injury, ARB – angiotensin II receptor blocker
Introduction
Ischemia-reperfusion injury (IRI) is a pathological condition in the ischemic tissues that suffer from a low blood supply followed by reestablishment of blood flow resulting in further organ damage [1]. Blood flow restoration following ischemia causes a serious cell injury, including production of the reactive oxygen species (ROS), inflammatory responses, oxidative stress, and apoptosis [2, 3]. IRI deteriorates many organs, particularly the kidney, resulting in increased mortality rate injury. IRI is considered a major contributor to chronic renal failure and end-stage renal failure. Different conditions in which the kidneys are exposed to IRI include vascular and cardiac surgery, chronic renal artery stenosis, embolism trauma, atherosclerosis, and kidney transplantation [4–6]. More than 60% of patients with acute kidney injury (AKI) are due to IRI or acute tubular necrosis [7]. Annually, more than 1.5 million individuals die because of AKI, thereby discovering new therapeutic agents with low adverse effects can save the life of these patients [8]. Furthermore, AKI causes a long stay in hospitals, resulting in pressure on the health care providers and an economic burden on health services [9]. Multiple molecular mechanisms underpin the renal IRI, including the inflammatory responses initiated by various inflammatory molecules such as cytokines, chemokines, and increased expression of adhesion molecules leading to tissue necrosis [10]. Immune cells play a critical role in the inflammation response by secreting the TNF-α responsible for regulating a variety of immune, inflammatory and hematopoietic responses [11]. In AKI, the inflammatory response is critical, resulting in an induction of IL-6; therefore, IL-6 can be a useful target in AKI through modulating its effect [12–15]. ROS is another factor playing a critical role in deteriorating the case scenario of IRI as they are released in both phases resulting in deterioration of the affected tissues. Accumulation of ROS causes a state of imbalance, leading to tissue hypoxia and intracellular acidosis due to the generation of lactate.
Furthermore, excessive increase of ROS is also because of the decreased activity of a variety of antioxidant molecules such as superoxide dismutase, catalase, and glutathione peroxidase. These events influence the mitochondrial respiratory chain activity and burst ROS, resulting in oxidative damage to the bimolecular, including proteins, lipids, and DNA, ending in apoptosis and cell death [16]. Apoptosis occurs in response to hypoxic stress, resulting from ischemia and ROS production from reperfusion [17]. This stress can initiate both apoptosis pathways, including the intrinsic and extrinsic mechanisms [6]. Bax and Bcl2 are apoptotic regulators that are key players of IRI apoptosis. Bcl2 has an anti-apoptotic effect resulting in inhibition of the cell apoptosis via antagonizing the mitochondrial membrane permeability. The apoptotic impact of Bax releases a variety of cytokines, thereby inducing cell apoptosis [18, 19]. Caspase 3 is an executioner caspase-activated by initiator caspase to cleave a wide spectrum of cellular proteins [20]. Fimasartan is an angiotensin II receptor blocker (ARB) representing the latest drug from the ARB group [21, 22]. It is licensed in some countries, for example, in Korea by the Korea Food and Drug Administration, to treat hypertension [21].
Fimasartan was developed by Boryung Pharmaceutical, a Korean company, and received approval in many other countries such as China, Singapore, Russia, and India. It has a safety profile, efficacy, and tolerability; it has good properties compared to other ARBs, in addition to pleiotropic effects [22]. In general, ARBs represent the highly used antihypertensive drugs and the first recommended class according to American and European guidelines [23]. In recent studies, Fimasartan has been found to provide therapeutic action in patients suffering from acute coronary syndrome by decreasing inflammation of the carotid atherosclerotic plaque [24]. Particularly the Renin angiotensin aldosterone system (RAAS) modulates the inflammatory process and plays a significant role in causing renal IRI by different effecting mechanisms [25–26]. Especially, Ang II mediates the oxidative condition, inflammatory cascade, and apoptosis of renal tissue [27–29].
Materials and Methods
Animal maintenance, preparation, treatment, and sacrifice
Animals were fed in a standard laboratory in the animal house of the Faculty of Science, University of Kufa. All experiments were performed in the laboratory of the Department of Pharmacology and Therapeutics and Middle Euphrates Unit for Cancer Research, Faculty of Medicine, University of Kufa, Najaf, Iraq. All procedures were reviewed by the Institutional Animal Care and Use Committee (IACUC), University of Kufa, Najaf, Iraq.
Experimental design
Wistar albino rats were between eight and twelve weeks and weighted 220–260 gm. Animals were assigned to four groups (5 animals in each group). Sham (negative control group) was subjected to the same operation without ischemia and reperfusion. The control group was subjected to ischemia for 30 min and 2h reperfusion [30–31]. The vehicle-treated group was pretreated with dimethyl sulfoxide (DMSO) 30 min prior to the induction of ischemia and reperfusion [31]. Fimasartan group was treated with Fimasartan (3 mg/kg) 30 min before the induction of ischemia and reperfusion [32].
Induction of renal IRI
Animals were anesthetized by intraperitoneal injection with ketamine (100 mg/kg) and xylazine (10 mg/kg). To ensure that animals were anesthetized, reflex monitoring, including tail and leg pinching, was performed [33]. A midline incision was performed, and two renal pedicles were occluded for 30 min using clamps. At the time of ischemia, animals were maintained at 37°C using a heating pad. After 30 min of ischemia, the clamps were released, allowing the blood to resort for 2h and closing the abdomen. Normal saline (1 ml) was immediately injected into the animals to maintain fluid balance [34]. When the experiment ended, animals were sacrificed, and the left kidney was removed. The renal tissues were excised into two parts; the frozen one was used for the tissue assessment of cytokines and antioxidant readouts. The second part was put in 10% formalin for histopathological and immunohistochemistry analysis.
Fimasartan preparation
Fimasartan was from Med Chem Express, USA Company. Molecular Formula: C27H31N7OS, Chemical Names: Fimasartan (BR-A-657), CAS.NO 247257-48-3. To prepare the drug, the powder was dissolved in DMSO and immediately used.
Assessment of the renal function
The blood was collected from the heart and put in test tubes for 30 min to clot at room temperature. The blood samples were centrifuged at 3000 rpm for 15 min. The supernatant was collected and used to assess the levels of urea and creatinine using commercial kits.
IL-6, TNF-α and total antioxidant capacity (TAC) measurement in kidney using ELISA
To measure the cytokines and antioxidant markers in the renal tissues, the frozen part of the kidney was washed with cold PBS to remove the blood. The renal tissue was weighted, and PBS containing 1% Triton X-100 and 1% protease cocktail inhibitor was added to the tissue in a ratio of 1:9 W/V, using an appropriate test tube. The samples were homogenized using an ultrasonic liquid processor [35]. The samples were spun down at 3000 rpm for 20 min at 4°C. The supernatant was used to measure the levels of TNFα, IL-6, and TAC using ELISA (Bioassay Technology Laboratory, China).
Tissue preparation for histopathology
The renal tissue in the formalin was washed with cold normal saline to remove the clots. The tissue was processed in paraffin blocks. The tissue sections included the renal cortex and pelvis. Slices of 5 μM thickness were cut and stained with hematoxylin and eosin stain. The changes in the renal tissues were investigated and included the following: cellular swelling, eosinophilic cast, tubular dilation, development of proteinaceous cast, desquamation of epithelial cells, inflammatory cells infiltration, and necrosis [36]. The renal tissue was investigated using a microscope with magnification lenses from 100 to 400X. The investigation was performed by an independent pathologist unaware of the experimental groups. Histopathological changes were scored from 0 to 4 according to the percentages of affected tubules as follow: the normal tissue was assigned 0, score 1: less than 25%, score 2: 25–50%, score 3: 50–75% and score 4: 75–100% [37].
Immunohistochemistry (IHC) study
To measure the levels of BAX, Bcl-2, and caspase 3 in the renal tissue, immunohistochemistry analysis was used. The immunostaining was performed in the paraffin-embedded tissue sections (5 μM thickness). Briefly, the sections were deparaffinized, and the endogenous peroxidase activity was blocked using 3% (v/v). Non-specific binding sites can be reduced by incubating the tissue sections in serum-free proteins. The tissue sections were incubated overnight at 4°C with primary antibodies purchased from the Bioassay Technology Laboratory against BAX (1:100), Bcl-2 (1:100), or caspase 3 (1:100). The sections were then washed for 1h and incubated with a biotinylated secondary antibody for 30 min at 37°C. The tissue sections were then washed and incubated with horseradish peroxidase for 30 min followed by incubation with chromogen for 15 min (100 μL per slide). The tissue sections were then counterstained with hematoxylin [38]. The immunostaining of the BAX, Bcl-2, and caspase 3 was quantified using a Q-score system in which the scores were calculated by multiplying the immunostaining intensity and positive stain area. The labeling intensity was graded as follows: score 0: no staining, score 1: weak staining, score 2: moderate staining, and score 3: strong staining. The stained cells were represented as percentages ranging from 0–100% [39]. An independent pathologist unaware of the study design investigated the immunostained tissue sections.
Statistical analysis
SPSS software version 26.0 was used to analyze the data. The results were represented as mean±SEM unless otherwise stated. Analysis of variance (ANOVA) was used to compare the groups, followed by a post hoc test [40]. Finally, the Kruskal-Wallis test was used to assess the mean differences among the study groups in terms of morphological changes and immunohistochemistry. In this study, the P≤0.05 is considered statistically significant.
Results
Influence of Fimasartan on renal function
The results showed that serum urea and creatinine levels were higher in control and vehicle-treated groups than in sham groups. In contrast, pretreatment with Fimasartan resulted in a marked decrease in serum urea and creatinine levels compared to the control and vehicle-treated, Figures 1 and 2.
Figure 1.
Mean level of serum urea among the groups. Data are represented as mean±SEM, n=5. Statistical analysis was performed using a one-way ANOVA followed by a post hoc test. * – P<0.05 compared to the sham group; #– P<0.05 compared to control and vehicle-treated groups.
Figure 2.
Mean level of serum creatinine among the groups. Data are expressed as mean±SEM, n=5. Statistical analysis was performed using a one-way ANOVA followed by a post hoc test. * – P<0.05 compared to the sham group; #– P<0.05 compared to control and vehicle-treated groups.
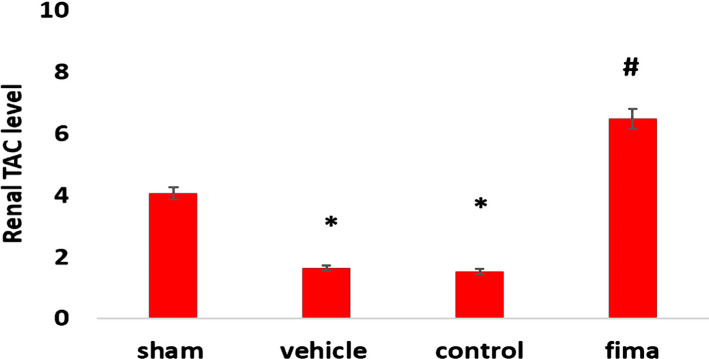
Influence of Fimasartan on oxidative stress in the kidney (TAC)
The data in control and vehicle-treated groups showed a dramatic decrease in the levels of renal (TAC) compared to the sham group (Figure 3). On the other hand, pretreatment with Fimasartan resulted in a marked elevation in TAC levels compared to the control and vehicle-treated groups (Figure 3).
Figure 3.
Tissue levels of TACin the kidney (U/ml) among the group. Data are represented as mean±SEM, n=5. Statistical analysis was performed using a one-way ANOVA followed by a post hoc test. * – P<0.05 compared to the sham group, # – P<0.05 compared to control and vehicle-treated groups.
Influence of Fimasartan on renal cytokines (TNFα, IL-6)
The levels of TNFα and IL-6 in the kidney for the control and vehicle-treated groups increased significantly compared to the sham group. By contrast, these levels significantly dropped in the Fimasartan-treated group versus the control and vehicle-treated groups (Figure 4).
Figure 4.
Mean level of serum creatinine among the groups. Data are expressed as mean±SEM, n=5. Statistical analysis was performed using a one-way ANOVA followed by a post hoc test. * – P<0.05 compared to the sham group; #– P<0.05 compared to control and vehicle-treated groups.
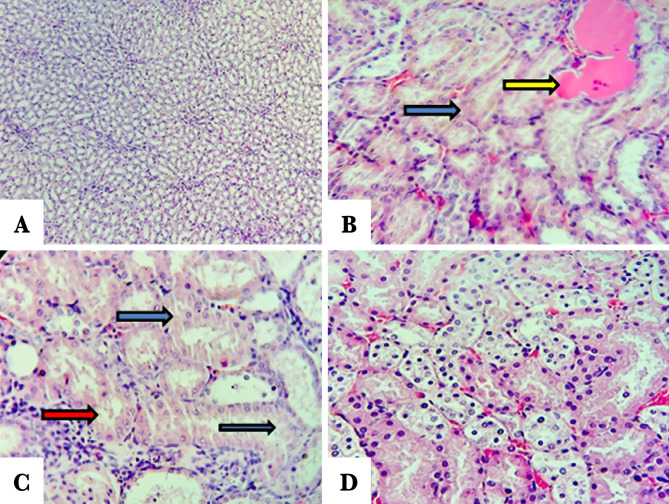
Histopathological examination
The score of damage and morphological changes of kidneys among the groups were shown in Figures 5 and 6. The renal morphology was normal in the sham negative group (Figure 6A). In contrast, the histopathological investigation in the control and vehicle-treated groups showed tubular cell swelling, tubular dilation, and damage and degeneration of tubular structure. Furthermore, there is a cast formation and congestion of the lumen (Figure 6 B and C). Pretreatment with Fimasartan maintained the normal morphology of renal tissue. The treatment effect is characterized by a slight swelling of the renal tubules with mild interstitial congestion (Figure 6D).
Figure 5.
Histopathology scores of the study groups. Statistical analysis was performed using a Kruskal-Wallis test, n=5. * – P<0.05 compared to the sham group; # – P<0.05 compared to control and vehicle-treated groups.
Figure 6.
Representative pictures of the renal tissue sections stained with hematoxylin and eosin. A – Sham negative group reveals normal morphology; B – Control group reveals cellular swelling, increased cytoplasmic eosinophilic (blue arrow), cast formation (yellow arrow); C – Vehicle-treated group shows a cellular injury including swelling, cytoplasmic eosinophilic (blue arrows), and interstitial inflammation (red arrow); D – Fimasartan-treated group showing a slight change in most areas of renal tissue.
Immunohistochemistry finding
Influence of Fimasartan on the anti-apoptotic Bcl-2, proapoptotic Bax, and caspase 3
To investigate Bcl-2 expression, Bax, and caspase, immunohistochemistry was used. Q score was used to calculate the intensity of labeling of these molecules. The results showed that pretreatment with Fimasartan caused a marked increase in Q score of Bcl2 and decrease in Q score of Bax and caspase 3, compared to the control and vehicle-treated groups (Figure 7).
Figure 7.
Q score of BAX, BCL-2, and Caspase 3 staining of the renal tissue among the four groups (N=5). Data are represented as mean±SEM, n=5. * – P<0.05 compared to the sham group; # – P<0.05 compared to control and vehicle-treated groups.
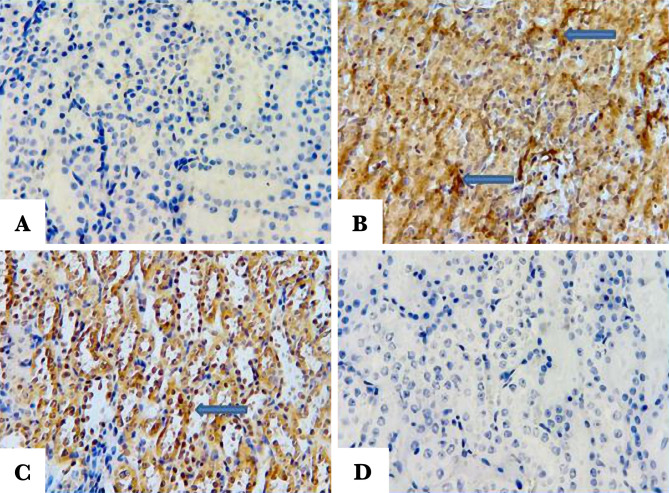
Influence of Fimasartanon the anti-apoptotic Bcl-2
Renal tissue was probed with anti Bcl2 antibody to investigate the Bcl2 expression. Pretreatment with Fimasartan revealed strong labeling intensity (brown color) compared to the control and vehicle-treated groups (Figure 8 A, B, C, and D).
Figure 8.
Representative pictures of renal tissue sections. A – Sham negative group show positive BCL-2 labeling intensity (indicated by arrow); B – Control group showing a negative stain; C – Vehicle-treated group shows negative stain; D – Fimasartan-treated group reveals positive labeling intensity BCL-2 (indicated by arrow).
Influence of Fimasartan on Bax
To investigate the expression level of Bax, the renal tissue was probed with anti-Bax antibody. The tissue sections of the sham negative group and Fimasartan-treated group revealed no staining of the Bax, (Figure 9 A and D). By contrast, the tissue sections of the control and vehicle-treated groups were stained positively (darkly brown color), Figure 9 B and C.
Figure 9.
Representative pictures showing the renal tissue sections of the study groups. A – Sham negative group shows negative Bax labeling intensity; B – Control group reveals positive labeling intensity (indicated by arrow); C – Vehicle-treated group shows positive labeling intensity (indicated by arrow); D – Fimasartan-treated group showing negative Bax stain.
Influence of Fimasartan on the proapoptotic caspase3
To investigate the expression level of caspase 3, the renal tissue was probed with an anti-caspase 3 antibody. The tissue sections of the sham negative group and Fimasartan-treatment group revealed no staining of caspase 3 (Figure 10 A and D). On the other hand, the control and vehicle-treated groups were positively stained (brown stain), Figure 10 B and C.
Figure 10.
Representative pictures of the renal tissue sections. A – Sham negative group reveals negative caspase 3 labeling intensity; B – Control group reveals positive caspase 3 labeling intensity (brown color); C – Vehicle-treated group shows positive caspase 3 labeling intensity; D – Fimasartan-treatment group showing negative caspase 3 labeling intensity.
Discussion
The present study demonstrated significantly high urea and creatinine levels in the control and vehicle-treated groups compared to the sham group. The observed increase in these markers could be attributed to renal tissue damage and decreased glomerular capacity [33]. Pretreatment with Fimasartan reduced the urea and creatinine Fimasartan levels compared to the control and vehicle-treated groups highlighting the potential protective effect of Fimasartan. This finding was also reported by Cho et al. (2018). This result may be explained by the fact that Fimasartan antagonizes AT1 receptors, thereby reducing the deterioration of the renal tissue [40, 41]. The histopathological findings of the current study revealed a significantly lower degree of tissue injury in the Fimasartan-treated group compared to control and vehicle-treated groups. This finding was reported by a previous study [41]. This low degree of injury by Fimasartan treatment is likely to be related to its ability to decrease oxidative stress and inhibit most pro-inflammatory responses [42–45], resulting in a decrease in the movement of the inflammatory cell to ischemic tissue [46]. Prior studies have noted the importance of TNF-α and IL-6 in the molecular pathogenesis of IRI. The results of this study indicate that the TNF-α and IL-6 levels were significantly lower in the Fimasartan-treatment group than in the control and vehicle-treated group. These results agree with those obtained by Cho et al. (2018) [41]. A possible explanation for this might be that Fimasartan could act as an IKK inhibitor, thereby inhibiting the NF-κB. This effect could reduce the accumulation and recruitment of the inflammatory cells in the ischemic tissues [47]. These results reflect those of Lee et al. [48], who found that Fimasartan decreases macrophage number, decreases plague breaking, and enhances plaque stabilization. Shigeoka et al. showed that NLRP3 could signal the injury responses in the renal epithelium [49]. It can thus be suggested that pretreatment with Fimasartan inhibits NLRP3 inflammasome and protects the kidney through modulation of the immune response and cytokine response. The present study demonstrated a significantly high level of TAC in the Fimasartan-treated group in comparison to control and vehicle groups. This result agrees with Kim et al. that revealed that Fimasartan significantly increased the antioxidant enzyme in unilateral ureteral obstruction in mice by up regulating expression of the mRNA of NQO1 and HO-1, the protein expression of those genes, as well as of CuSOD, MnSOD, and catalase [50].
Furthermore, Nezu and Suzuki [51] established the activation of anti-oxidative transcription factor Nrf2 in the renal tubule, diminishing the reactive oxygen species protecting them from damage and fibrosis, this mechanism being involved via Fimasartan action [52]. The current study showed a marked decrease in apoptotic regulators (Bax and caspase 3) and an increase in levels of Bcl2 in the Fimasartan-treated group compared to the control and vehicle groups. These results are in line with previous studies [53] that established ARBs lead to upregulation of Bcl2 levels and decreasing Bax and caspase 3 expression. These outcomes can be explained via the impact of Fimasartan, which decreases apoptosis cells by blocking the AT1 receptor, inhibiting proapoptotic (p53-Bax), and increasing anti-apoptotic (Bcl2) [54].
Conclusion
This study showed that Fimasartan reduces the levels of the inflammatory molecules TNF-α and IL-6, increases the expression levels of anti-apoptotic Bcl2, and decreases the expression levels of proapoptotic Bax and caspase 3. Furthermore, this study also showed that Fimasartan ameliorates the histopathological changes that occur in response to renal IRI. Taken together, these results suggest that Fimasartan could be a protective agent in renal IRI through its effect on inflammation, oxidative stress, and apoptosis regulators.
Acknowledgements
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), University of Kufa, Najaf, Iraq (ID: EC230).
Authorship
WA, MA, AAH, LA contributed to the methodology and writing the original draft. NH contributed to conceptualizing, study design, and editing the manuscript. HQ contributed to editing the manuscript,and QZ, MJ contributed to data curation and analysis.
References
- 1.Pefanis A, Ierino FL, Murphy JM, Cowan PJ. Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int. 2019 Aug;96(2):291–301. doi: 10.1016/j.kint.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Abbas LM, Al-Mudhafar RH, Al-Mudhafar DH, Hadi NR. Ranolazine Protects the Kidney from Ischemia/Reperfusion Injury in Adult Male Rats by Modulation of Inflammatory and Oxidative Pathways and Suppression of Notch2/Hes1 signaling pathway. Systematic Reviews in Pharmacy. 2021;12(1):12. [Google Scholar]
- 3.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev. 2015 Jun 1;4(2):20–7. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2007 Oct;376(1-2):1–43. doi: 10.1007/s00210-007-0183-5. [DOI] [PubMed] [Google Scholar]
- 5.Kezić A, Stajic N, Thaiss F. Innate Immune Response in Kidney Ischemia/Reperfusion Injury: Potential Target for Therapy. J Immunol Res. 2017;2017:6305439. doi: 10.1155/2017/6305439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu MY, Yiang GT, Liao WT, Tsai AP, et al. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol Biochem. 2018;46(4):1650–1667. doi: 10.1159/000489241. [DOI] [PubMed] [Google Scholar]
- 7.Park M, Kwon CH, Ha HK, Han M, Song SH. RNA-Seq identifies condition-specific biological signatures of ischemia-reperfusion injury in the human kidney. BMC Nephrology. 2020;21(1):398. doi: 10.1186/s12882-020-02025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luyckx VA, Tonelli M, Stanifer JW. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. 2018 Jun 1;96(6):414–422D. doi: 10.2471/BLT.17.206441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Legrand M, Rossignol P. Cardiovascular Consequences of Acute Kidney Injury. N Engl J Med. 2020 Jun 4;382(23):2238–2247. doi: 10.1056/NEJMra1916393. [DOI] [PubMed] [Google Scholar]
- 10.Vallés PG, Lorenzo AG, Bocanegra V, Vallés R. Acute kidney injury: what part do toll-like receptors play? Int J Nephrol Renovasc Dis. 2014 Jun 19;7:241–51. doi: 10.2147/IJNRD.S37891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong DH, Kim YK, Kim MR, Jang JH, Lee S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int J Mol Sci. 2018 Apr 2;19(4):1057. doi: 10.3390/ijms19041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vries DK, Lindeman JH, Tsikas D, de Heer E, et al. Early renal ischemia-reperfusion injury in humans is dominated by IL-6 release from the allograft. Am J Transplant. 2009 Jul;9(7):1574–84. doi: 10.1111/j.1600-6143.2009.02675.x. [DOI] [PubMed] [Google Scholar]
- 13.Grigoryev DN, Liu M, Hassoun HT, Cheadle C, et al. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008 Mar;19(3):547–58. doi: 10.1681/ASN.2007040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su H, Lei CT, Zhang C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front Immunol. 2017 Apr 21;8:405. doi: 10.3389/fimmu.2017.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atreya R, Mudter J, Finotto S, Müllberg J, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000 May;6(5):583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 16.Ávila C, Líbano L, Rojas I, Rodrigo R. Role of ischemia-reperfusion in oxidative stress-mediated injury during kidney transplantation. Clin Res Trial. 2019;5(3) doi: 10.15761/CRT.1000260. [DOI] [Google Scholar]
- 17.Liu H, Jing X, Dong A, Bai B, Wang H. Overexpression of TIMP3 Protects Against Cardiac Ischemia/Reperfusion Injury by Inhibiting Myocardial Apoptosis Through ROS/Mapks Pathway. Cell Physiol Biochem. 2017;44(3):1011–1023. doi: 10.1159/000485401. [DOI] [PubMed] [Google Scholar]
- 18.Weng XF, Li ST, Song Q, Zhu Q, et al. Protective Effect of Nicotinamide Adenine Dinucleotide Phosphate on Renal Ischemia-Reperfusion Injury. Kidney Blood Press Res. 2018;43(3):651–663. doi: 10.1159/000489620. [DOI] [PubMed] [Google Scholar]
- 19.Wei Q, Dong G, Chen JK, Ramesh G, Dong Z. Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int. 2013 Jul;84(1):138–48. doi: 10.1038/ki.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galluzzi L, López-Soto A, Kumar S, Kroemer G. Caspases Connect Cell-Death Signaling to Organismal Homeostasis. Immunity. 2016 Feb 16;44(2):221–31. doi: 10.1016/j.immuni.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Lee HY, Oh BH. Fimasartan: A New Angiotensin Receptor Blocker. Drugs. 2016 Jul;76(10):1015–22. doi: 10.1007/s40265-016-0592-1. [DOI] [PubMed] [Google Scholar]
- 22.Pradhan A, Gupta V, Sethi R. Fimasartan: A new armament to fight hypertension. J Family Med Prim Care. 2019 Jul;8(7):2184–2188. doi: 10.4103/jfmpc.jfmpc_300_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018 Jun;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 24.Oh M, Lee CW, Ahn JM, Park DW, et al. Comparison of fimasartan and amlodipine therapy on carotid atherosclerotic plaque inflammation. Clin Cardiol. 2019 Feb;42(2):241–246. doi: 10.1002/clc.23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kontogiannis J, Burns KD. Role of AT1 angiotensin II receptors in renal ischemic injury. Am J Physiol. 1998 Jan;274(1):F79–90. doi: 10.1152/ajprenal.1998.274.1.F79. [DOI] [PubMed] [Google Scholar]
- 26.Hammad FT, Wheatley AM, Davis G. Role of endothelin ET(A) receptor antagonism in the post-transplant renal response to angiotensin II in the rat. Exp Physiol. 2001 May;86(3):365–72. doi: 10.1113/eph8602137. [DOI] [PubMed] [Google Scholar]
- 27.Ranjbar R, Shafiee M, Hesari A, Ferns GA, et al. The potential therapeutic use of renin-angiotensin system inhibitors in the treatment of inflammatory diseases. J Cell Physiol. 2019 Mar;234(3):2277–2295. doi: 10.1002/jcp.27205. [DOI] [PubMed] [Google Scholar]
- 28.Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, el-Dahr SS. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol. 1997 Jul;273(1 Pt 2):F170–7. doi: 10.1152/ajprenal.1997.273.1.F170. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Ortega M, Rupérez M, Esteban V, Rodríguez-Vita J, et al. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant. 2006 Jan;21(1):16–20. doi: 10.1093/ndt/gfi265. [DOI] [PubMed] [Google Scholar]
- 30.Hassoon MF, Hadi NR, Mahboba W, Jawad D, et al. Nephroprotective potential effect of Canagliflozin in renal ischemia reperfusion injury in rat model: Role of Nrf2 pathway. Systematic Reviews in Pharmacy. 2019;10(2):60–69. doi: 10.5530/srp.2019.2.11. [DOI] [Google Scholar]
- 31.Karsh EH, Kadhim RJ, Jabir Maji S. Effect of graphene oxide and gold nanoparticles on kidney parameters of male mice. AIP Conference Proceedings. 2020;2213(1):020145. doi: 10.1063/5.0000167. [DOI] [Google Scholar]
- 32.Choi DJ, Kim EJ, Park MJ, Oh IY, et al. A new angiotensin ii type i receptor blocker, br-a-657, reduces reperfusion injury via anti-apoptotic/anti-inflammatory effect. Journal of the American College of Cardiology. 2011;57(14_Supplement):E967–E967. doi: 10.1016/S0735-1097(11)60967-4. [DOI] [Google Scholar]
- 33.Ali AN, Altimimi ML, Al-Ardi HM, Hadi NR. Nephroprotective Potential Effect of Azilsartan in Renal Ischemia Reperfusion Injury/role VEGF Pathway. Systematic Reviews in Pharmacy. 2019;10(2):90–99. doi: 10.5530/srp.2019.2.15. [DOI] [Google Scholar]
- 34.Hadi NR, Al-Amran F, Tweij TAR, Mansur ME. CDDO Me Provides Kidney Protective Impacts against Ischemia/Reperfusion Injury via Inhibition of Oxidative Stress and Inflammation by Targeting Nrf2 and NF-kB Signaling Pathways. Systematic Reviews in Pharmacy. 2020;11(1):108–118. doi: 10.5530/srp.2020.1.16. [DOI] [Google Scholar]
- 35.Al-Jabbar WA, Hadi NR, Ghafil FAA, Abdulkadhim H, et al. Nephroprotective effects of quercetin in renal ischemia reperfusion injury in Mice. Syst. Rev. Pharm. 2019;10:184–93. [Google Scholar]
- 36.Shimokawa T, Tsutsui H, Miura T, Takama M, et al. Post-treatment with JP-1302 protects against renal ischemia/reperfusion-induced acute kidney injury in rats. J Pharmacol Sci. 2019 Mar;139(3):137–142. doi: 10.1016/j.jphs.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Ali IH, Jabir MS, Al-Shmgani HSA, Sulaiman GM, Sadoon AH. Pathological And Immunological Study On Infection With Escherichia Coli In ale BALB/c mice. J Phys: Conf Ser. 2018;1003:012009. doi: 10.1088/1742-6596/1003/1/012009. [DOI] [Google Scholar]
- 38.Abdelzaher WY, Ahmed SM, Welson NN, Marraiki N, et al. Vinpocetine ameliorates L-arginine induced acute pancreatitis via Sirt1/Nrf2/TNF pathway and inhibition of oxidative stress, inflammation, and apoptosis. Biomedicine & Pharmacotherapy. 2021;133:110976. doi: 10.1016/j.biopha.2020.110976. [DOI] [PubMed] [Google Scholar]
- 39.Meyerholz DK, Beck AP. Principles and approaches for reproducible scoring of tissue stains in research. Lab Invest. 2018 Jul;98(7):844–855. doi: 10.1038/s41374-018-0057-0. [DOI] [PubMed] [Google Scholar]
- 40.Kareem SH, Naji AM, Taqi ZJ, Jabir MS. Polyvinylpyrrolidone Loaded-MnZnFe2O4 Magnetic Nanocomposites Induce Apoptosis in Cancer Cells Through Mitochondrial Damage and P53 Pathway. J Inorg Organomet Polym. 2020;30(12):5009–5023. doi: 10.1007/s10904-020-01651-1. [DOI] [Google Scholar]
- 41.Cho JH, Choi SY, Ryu HM, Oh EJ, et al. Fimasartan attenuates renal ischemia-reperfusion injury by modulating inflammation-related apoptosis. Korean J Physiol Pharmacol. 2018;22(6):661–670. doi: 10.4196/kjpp.2018.22.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hameed AMA, Altemimi ML, Al-Mudhafar RH, Al-Mudhafar DH, Hadi NR. The Anti-Apoptotic, Anti-Inflammatory And Anti-Oxidant Effects Of Olmesartan On Renal I/R Injury In Male Rat Model. Systematic Reviews in Pharmacy. 2021;12(2):404–418. [Google Scholar]
- 43.Molinas SM, Cortés-González C, González-Bobadilla Y, Monasterolo LA, et al. Effects of losartan pretreatment in an experimental model of ischemic acute kidney injury. Nephron Exp Nephrol. 2009;112(1):e10–9. doi: 10.1159/000210574. [DOI] [PubMed] [Google Scholar]
- 44.Blessing E, Preusch M, Kranzhöfer R, Kinscherf R, et al. Anti-atherosclerotic properties of telmisartan in advanced atherosclerotic lesions in apolipoprotein E deficient mice. Atherosclerosis. 2008 Aug;199(2):295–303. doi: 10.1016/j.atherosclerosis. [DOI] [PubMed] [Google Scholar]
- 45.Imayama I, Ichiki T, Inanaga K, Ohtsubo H, et al. Telmisartan downregulates angiotensin II type 1 receptor through activation of peroxisome proliferator-activated receptor γ. Cardiovasc. Res. 2006;72:184–90. doi: 10.1016/j.cardiores.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Benoit SW, Devarajan P. Acute kidney injury: emerging pharmacotherapies in current clinical trials. Pediatr Nephrol. 2018 May;33(5):779–787. doi: 10.1007/s00467-017-3695-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim CK, Yang XL, Kim YJ, Choi IY, et al. Effect of Long-Term Treatment with Fimasartan on Transient Focal Ischemia in Rat Brain. BioMed Research International. 2015;2015:e295925. doi: 10.1155/2015/295925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JY, Lee CW, Kim WJ, Ahn JM, et al. Antiatherosclerotic effects of the novel angiotensin receptor antagonist Fimasartan on plaque progression and stability in a rabbit model: a double-blind placebo-controlled trial. J Cardiovasc Pharmacol. 2013 Aug;62(2):229–36. doi: 10.1097/FJC.0b013e318297458b. [DOI] [PubMed] [Google Scholar]
- 49.Shigeoka AA, Mueller JL, Kambo A, Mathison JC, et al. An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury. J Immunol. 2010 Nov 15;185(10):6277–85. doi: 10.4049/jimmunol.1002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S, Kim SJ, Yoon HE, Chung S, et al. Fimasartan, a Novel Angiotensin-Receptor Blocker, Protects against Renal Inflammation and Fibrosis in Mice with Unilateral Ureteral Obstruction: the Possible Role of Nrf2. Int J Med Sci. 2015 Oct 21;12(11):891–904. doi: 10.7150/ijms.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nezu M, Suzuki N. Roles of Nrf2 in Protecting the Kidney from Oxidative Damage. Int J Mol Sci. 2020 Apr 22;21(8):2951. doi: 10.3390/ijms21082951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang X, Sun J, Kim TJ, Kim YJ, et al. Pretreatment with low-dose fimasartan ameliorates NLRP3 inflammasome-mediated neuroinflammation and brain injury after intracerebral hemorrhage. Exp Neurol. 2018 Dec;310:22–32. doi: 10.1016/j.expneurol.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han J, Park SJ, Thu VT, Lee SR, et al. Effects of the novel angiotensin II receptor type I antagonist, fimasartan on myocardial ischemia/reperfusion injury. Int J Cardiol. 2013 Oct 3;168(3):2851–9. doi: 10.1016/j.ijcard.2013.03.151. [DOI] [PubMed] [Google Scholar]
- 54.Quan H, Oh GC, Seok SH, Lee HY. Fimasartan, an angiotensin II receptor antagonist, ameliorates an in vivo zebrafish model of heart failure. Korean J Intern Med. 2020 Nov;35(6):1400–1410. doi: 10.3904/kjim.2019.038. [DOI] [PMC free article] [PubMed] [Google Scholar]