Abstract
A broad-spectrum vaginal microbicide must be effective against a variety of sexually transmitted disease pathogens and be minimally toxic to the cell types found within the vaginal epithelium, including vaginal keratinocytes. We assessed the sensitivity of primary human vaginal keratinocytes to potential topical vaginal microbicides nonoxynol-9 (N-9), C31G, and sodium dodecyl sulfate (SDS). Direct immunofluorescence and fluorescence-activated cell sorting analyses demonstrated that primary vaginal keratinocytes expressed epithelial cell-specific keratin proteins. Experiments that compared vaginal keratinocyte sensitivity to each agent during a continuous, 48-h exposure demonstrated that primary vaginal keratinocytes were almost five times more sensitive to N-9 than to either C31G or SDS. To evaluate the effect of multiple microbicide exposures on cell viability, primary vaginal keratinocytes were exposed to N-9, C31G, or SDS three times during a 78-h period. In these experiments, cells were considerably more sensitive to C31G than to N-9 or SDS at lower concentrations within the range tested. When agent concentrations were chosen to result in an endpoint of 25% viability after three daily exposures, each exposure decreased cell viability at the same constant rate. When time-dependent sensitivity during a continuous 48-h exposure was examined, exposure to C31G for 18 h resulted in losses in cell viability not caused by either N-9 or SDS until at least 24 to 48 h. Cumulatively, these results reveal important variations in time- and concentration-dependent sensitivity to N-9, C31G, or SDS within populations of primary human vaginal keratinocytes cultured in vitro. These investigations represent initial steps toward both in vitro modeling of the vaginal microenvironment and studies of factors that impact the in vivo efficacy of vaginal topical microbicides.
The global spread of the human immunodeficiency virus type 1 (HIV-1) has recently been driven by a dramatic increase in heterosexual transmission, which is the predominant route of transmission in developing countries (8). This disturbing trend in the AIDS epidemic has highlighted the necessity for additional measures to control the transmission of HIV-1, including the development and distribution of broad-spectrum, topical vaginal microbicides for use during heterosexual intercourse (7). An ideal microbicide would be female-controlled, broadly effective against HIV-1 as well as other sexually transmitted disease (STD) pathogens such as human papillomavirus (HPV) and herpes simplex virus types 1 and 2 (HSV-1 and HSV-2, respectively), inexpensive, easy to use and store, and safe during repeated and long-term use.
Products containing nonoxynol-9 (N-9), a widely available, commercially marketed spermicidal agent, have been tested for microbicidal use. N-9 has in vitro activity against several STD pathogens, including HIV-1 (3, 9), but cannot be classified as broadly effective, since it has no activity against nonenveloped viruses such as HPV (4). In vivo effectiveness of N-9 as a microbicide is unclear. Clinical studies have provided conflicting indications of N-9 effectiveness against transmission of HIV-1 and other STD pathogens (2, 10, 11, 15, 20, 27). Results from human and animal studies also indicate a narrow margin between N-9 effectiveness and safety (22), as well as associations between N-9 use and vaginal irritation, inflammation, tissue infiltration by host immune cells, and changes in vaginal flora (10, 16, 21, 22, 24). These adverse effects may increase the risk for HIV-1 transmission during sexual intercourse.
Because of the limitations of N-9 as a microbicidal agent, efforts have been directed toward the development of second-generation microbicidal agents with broader activity and lower toxicity. Our efforts have focused on characterizing the in vitro virucidal potential of and inherent cellular sensitivity to C31G and sodium dodecyl sulfate (SDS), novel microbicidal agents that demonstrate activity against a broad spectrum of STD pathogens, including HIV-1. C31G (in our present studies) is an equimolar mixture of two amphoteric, surface-active molecules: a C14 alkyl amine oxide and a C16 alkyl betaine. C31G is a broad-spectrum antimicrobial and spermicidal agent (1, 5, 9, 25). However, like N-9, C31G has no activity against HPV (5), a sexually transmitted virus that has a direct causative role in the development of human cervical cancer. SDS, an alkyl sulfate commonly used in research applications and in commercially available personal hygiene products, is significantly less cytotoxic than either N-9 or C31G and is effective against HIV-1, HSV-2, and, importantly, papillomaviruses from several species, including humans (5, 9). Other investigators (18; J. Piret, A. Desormeaux, P. Gourde, and M. G. Bergeron, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. H-8, 1998) have subsequently confirmed our original observations regarding the activity of SDS against HIV-1 and HSV-2 infectivity (National Institute of Allergy and Infectious Diseases Preclinical Topical Microbicides Workshop, May 1998) (5, 9, 12).
The present studies were performed to compare the sensitivity of primary human vaginal keratinocytes to N-9, C31G, or SDS exposure. Primary vaginal epithelial cells provide a realistic target for studies of microbicidal cytotoxicity, since, as progenitors of the stratified epithelium that lines the vagina, these cells form part of the physical barrier which impedes the infection of HIV-1-susceptible target cells such as dendritic cells (23), tissue macrophages, and CD4-positive lymphocytes (28). Compromise of this barrier by trauma or cellular damage that may accompany microbicide use could therefore increase the risk of HIV-1 infection. The results reported herein demonstrate that primary vaginal keratinocytes were less sensitive to SDS exposure than to either N-9 or C31G exposure. Time- and concentration-dependent differences in the cytotoxicity of N-9, C31G, and SDS were also observed.
MATERIALS AND METHODS
Tissue isolation and cell culture.
Primary human vaginal keratinocytes were isolated from the vaginal wall of tissues removed during reconstructive surgeries. The average donor age was 63 years (range, 25 to 79 years). Tissue samples were stored (for up to 2 h) at 4°C in minimal essential medium supplemented with penicillin and streptomycin (0.08 mg/ml each), gentamycin (0.4 mg/ml), fungizone (2.5 μg/ml), sodium bicarbonate (0.05%), and HEPES (10 mM) prior to use. Vaginal tissues were cut full-thickness and washed two times in phosphate-buffered saline (PBS). Tissues were digested overnight at 4°C and then at 37°C for 15 to 20 min in 0.25% trypsin–0.01% EDTA in Hanks' balanced salt solution (Clonetics). Following digestion, tissues were transferred to tissue culture dishes containing 2 ml of fetal bovine serum (HyClone) and 2 ml of defined keratinocyte growth medium (KGM; Clonetics). Keratinocytes were then scraped away from the dermis with blunt-tipped forceps, washed, pelleted, and plated in KGM (without serum) at a density of 2,500 cells/cm2 in T-25 tissue culture flasks (for fluorescence-activated cell sorting [FACS] analysis), 35-mm culture dishes (for immunofluorescence), or 12-well tissue culture plates [for 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) analyses]. The medium was changed 24 h after seeding and twice weekly thereafter. Primary, subconfluent keratinocyte cultures were maintained for 10 to 14 days prior to experimental use. To decrease the contributions of variations between donors, each set of experiments was performed using cell populations from 2 to 5 donors.
Flow cytometric analyses.
Primary vaginal keratinocyte cell populations were analyzed using fluorescence-activated cell sorting (FACS). A monoclonal, fluorescein isothiocyanate-conjugated, anti-human pan cytokeratin antibody (mouse immunoglobulin G1 [IgG1] isotype; Sigma) was used to identify cells expressing a broad spectrum of human cytokeratins. This antibody reacts with simple, cornifying and noncornifying squamous epithelia and pseudostratified epithelia but not with nonepithelial human tissues. CD4 expression was assessed using an fluorescein isothiocyanate-conjugated, anti-human CD4 monoclonal antibody (mouse IgG1 isotype; BD PharMingen). Fibronectin expression was quantitated using a monoclonal, mouse anti-human fibronectin antibody (IgG1 isotype; Sigma) and an FITC-conjugated anti-mouse secondary antibody (isotype IgG1; Boehringer Mannheim). Trypsinized cells were fixed in 2% paraformaldehyde for 30 min at 4°C and washed twice with PBS. Following a 1-h incubation period at 4°C with each antibody (diluted 1:7.5), the cells were washed twice with PBS prior to suspension in 2% paraformaldehyde and FACS analysis. For fibronectin detection, the secondary antibody was introduced in a second 1-h incubation period prior to final fixation.
Cell-surface marker identification by immunofluorescence.
Primary vaginal keratinocytes were characterized with respect to keratin and CD4 expression by direct fluorescence using the keratin- and CD4-specific antibodies described above. Cells cultured in 35-mm tissue culture dishes were fixed and stained as described above.
Determination of cellular sensitivity to microbicide exposure.
The effect of each agent on keratinocyte viability was determined by monitoring MTT cleavage by mitochondrial dehydrogenases in viable cells, yielding a measurable, purple product (formazan) (17). Formazan production is proportional to the viable cell number and inversely proportional to the degree of cytotoxicity. N-9 (FW 616), C31G (C14 amine oxide [FW 257] and C16 betaine [FW 327]), and SDS (FW 288) were obtained from Biosyn, Inc., as 1% stock solutions. To eliminate the potential effects of cell confluence and keratinization on cellular sensitivity, each experiment was initiated and concluded before keratinocytes reached confluence. For each experiment, agents were filter sterilized, diluted in sterile water, and added (10 μl per 2 ml of medium) to triplicate wells of subconfluent keratinocytes in KGM at the indicated concentrations. Cells were incubated in the absence or presence of each agent at 37°C in 5% CO2 and 90% humidity. At the conclusion of each experiment, 250 μl of MTT (5 mg/ml) was added to each well and incubated for 3 h at 37°C. Following removal of the medium, intracellular formazan crystals were solubilized for 5 min in 1 ml of 10% Triton X-100 in acidified isopropanol (0.1 N). The resulting solutions were assayed spectrophotometrically at 570 nm and corrected for nonspecific absorption at 690 nm. Statistical analyses were performed using Microsoft Excel.
RESULTS
Primary vaginal keratinocytes express keratin.
To verify the identity of cell populations derived from the vaginal tissues, cells used for the assessment of cellular sensitivity to candidate microbicides were characterized for the expression of cellular markers using FACS analysis. A broad-spectrum anti-cytokeratin antibody was selected to verify the presence of keratinocytes. Keratins comprise a heterogeneous family of proteins whose expression is limited almost exclusively to epithelial cells. Since keratinocytes do not express CD4, an antibody specific for CD4 was used both as a negative control and to detect contaminating immune cell types (i.e., T lymphocytes and cells of monocyte or macrophage lineage). An antibody directed against fibronectin was used as a nonreactive, isotype-matched negative control.
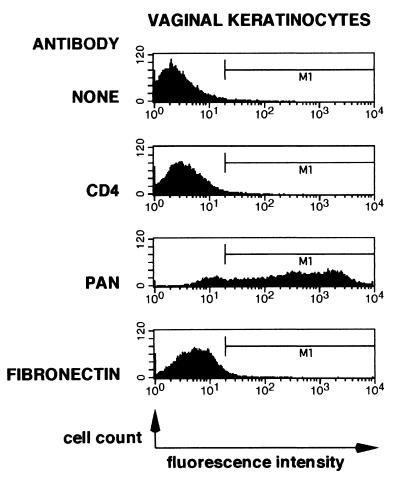
FACS analyses of primary vaginal keratinocytes demonstrated that these cells express members of the keratin protein family (Fig. 1). Approximately 85% of the total cell population expressed keratins above background levels. Keratin expression in primary vaginal keratinocytes was heterogeneous, with levels of expression spanning over two logs of intensity. Similar patterns of keratin expression were observed using the AE3 antibody (ICN Biomedicals, Inc.), which is specific to basic forms of keratin (data not shown). CD4 or fibronectin expression was not detected.
FIG. 1.
Primary human vaginal keratinocytes express members of the keratin protein family. Primary vaginal keratinocytes were examined using flow cytometry for expression of CD4, members of the keratin protein family, or fibronectin. Analyses were conducted as described in Materials and Methods. The horizontal and vertical axes represent fluorescence intensity (log scale) and cell counts, respectively.
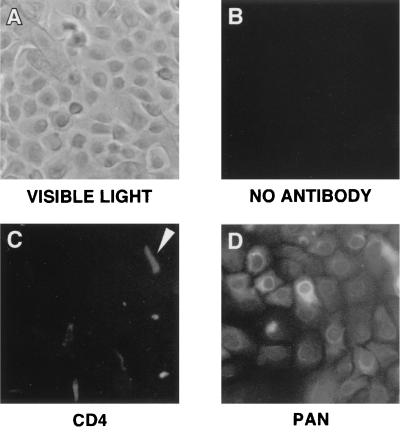
Primary human vaginal keratinocytes were also characterized using visible light microscopy (Fig. 2A) and direct immunofluorescence (Fig. 2B to D). Like the FACS analyses, direct immunofluorescence of primary vaginal keratinocytes revealed expression of the keratin protein family. Keratin expression in vaginal keratinocytes (Fig. 2D) was readily detectable. The apparent cytoplasmic localization was consistent with the role of keratins as intracellular intermediate filaments. The heterogeneous levels of fluorescence observed in the vaginal keratinocytes paralleled the spectrum of keratin expression observed in the FACS analyses. No autofluorescence was observed (Fig. 2B). No CD4 expression was detectable on the majority of the cells examined. However, a small number of cells did appear to be weakly positive for CD4 (Fig. 2C). These cells were distinct in size and shape from the vast majority of cells observed under both visible light (Fig. 2A) and by direct immunofluorescence (Fig. 2D), suggesting that they may have been cells of a different type. Unlike the field illustrated in Fig. 2C, most of the observable fields were devoid of cells positive for CD4 expression, indicating that the number of CD4-positive cells was low (as also suggested by the FACS analyses). Because of their low number, these cells were not characterized further with respect to these studies.
FIG. 2.
Keratin expression in primary human vaginal keratinocytes can be identified by immunofluorescence. Primary vaginal keratinocytes were examined by direct immunofluorescence for expression of CD4 and members of the keratin protein family. Analyses were conducted as described in Materials and Methods. (A) Representative field under visible light; (B, C, and D) direct immunofluorescent micrographs of vaginal keratinocytes in the absence of antibody, labeled with CD4 antibodies, or labeled with pan cytokeratin antibodies, respectively. The arrow in panel C indicates a cell weakly positive for CD4 expression. The field of cells in panel C was selected specifically to show the cells that expressed low levels of CD4 and is not representative of a typical field; cells in most fields were devoid of any detectable CD4 expression.
Primary cultures of human vaginal keratinocytes are much less sensitive to SDS or C31G than to N-9.
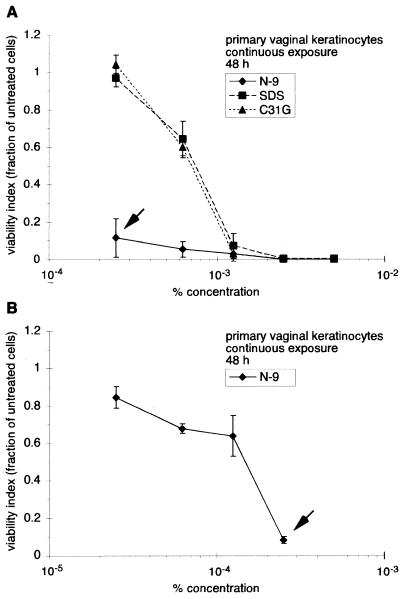
To determine the sensitivity of vaginal keratinocytes to N-9, C31G, or SDS, primary cultures of human vaginal keratinocytes were incubated with each agent and cell viability was evaluated after 48 h of continuous exposure (Fig. 3). Over the concentration range examined (2.5 × 10−4% to 5 × 10−3%), C31G and SDS were equally cytotoxic (Fig. 3A). Exposure to either C31G or SDS at 2.5 × 10−4% resulted in no decreases in cell viability. However, at 1.25 × 10−3%, the presence of either C31G or SDS reduced the number of viable cells to approximately 7 or 3% of the number of control cells, respectively. At the highest concentrations of C31G or SDS (2.5 × 10−3% or 5 × 10−3%), viable cells were not detected after 48 h of exposure.
FIG. 3.
Primary keratinocytes are more sensitive to N-9 than to C31G or SDS during long-term exposure. Primary human vaginal keratinocytes were exposed to N-9, C31G, or SDS for 48 h and subsequently assessed for viability using MTT assays. (A) Cell survival following exposure to concentrations of 2.5 × 10−4 to 5 × 10−3%; (B) Cell survival following exposure to N-9 at concentrations of 2.5 × 10−5 to 2.5 × 10−4%. The arrows in panels A and B both indicate cell viability at 2.5 × 10−4%. Cell viability following microbicide exposure is expressed as the fraction of viable cells relative to the number of mock-exposed cells. The results illustrated are averages from two experiments in which triplicate wells for each concentration were assayed.
Primary human vaginal keratinocytes were particularly sensitive to the presence of N-9. At a concentration of 2.5 × 10−4%, N-9 reduced cell viability to 11% (Fig. 3A). In sharp contrast, exposure to either C31G or SDS at the same concentration resulted in negligible cytotoxicity. To further explore the cytotoxicity of N-9, additional MTT experiments using lower concentrations of N-9 were performed (Fig. 3B). In these analyses, a 50% reduction in concentration, from 2.5 × 10−4% to 1.25 × 10−4%, resulted in an eightfold increase in cell viability (to 64%). Even at the lowest concentration tested (2.5 × 10−5%), cell viability was only 85%. Comparing concentrations which would be expected to result in a 50% reduction in cell viability (50% cytotoxicity concentration [TC50]), vaginal keratinocytes were almost five times more sensitive to N-9 (TC50 = 1.6 × 10−4%) than to either C31G (TC50 = 7.4 × 10−4%) or SDS (TC50 = 7.8 × 10−4%) during long-term (48 h) exposure.
Repetitive exposure to N-9, C31G, or SDS affects the viability of primary keratinocytes.
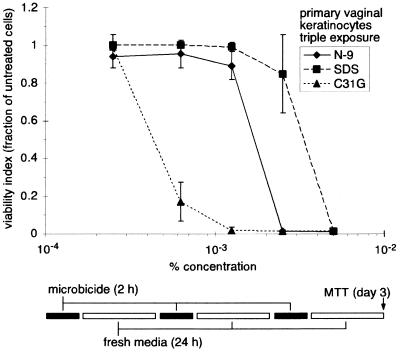
Repeated application of microbicidal products may result in toxic effects not evident after single or infrequent use. Indeed, increased epithelial disruption was evident in women who used N-9 daily for 2 weeks (19). In addition, a recent animal study demonstrated that repeated application of N-9 was detrimental to vaginal epithelial tissues (16). To evaluate the in vitro cytotoxicity of N-9, C31G, or SDS after repeated exposure, primary vaginal keratinocytes were exposed to three daily applications of each agent (Fig. 4). Each application cycle consisted of a 2-h exposure followed by a wash with PBS and a 24-h incubation in new media. Following the third cycle, cell viability was assessed by MTT assay. Results of these experiments indicated that between 2.5 × 10−4 and 1.25 × 10−3%, repeated exposure to either N-9 or SDS resulted in minimal reductions in cell viability. However, above 1.25 × 10−3%, viability in the presence of N-9 decreased sharply to undetectable levels. A similar decrease in viability was observed in the presence of SDS, but the sharp decline occurred at twice the concentration. Cells were less sensitive to N-9 and SDS than to C31G under these in vitro conditions. Although cell mortality following repeated exposure to the lowest concentration of C31G was minimal, viability decreased dramatically at higher concentrations and was undetectable at and above 1.25 × 10−3%.
FIG. 4.
Repetitive microbicide exposure affects the viability of human vaginal keratinocytes. Primary human vaginal keratinocytes were exposed to N-9, C31G, or SDS for 2 h. The cells were then washed once with PBS and cultured under new medium for 24 h. This cycle of exposure and recovery was repeated two more times. Cell survival was assessed using MTT assays following the third exposure/recovery cycle. Cell viability following microbicide exposure is expressed as the fraction of viable cells relative to the number of mock-exposed cells. The results illustrated are averages from two experiments in which triplicate wells for each concentration were assayed.
Repetitive in vitro exposure to N-9, C31G, or SDS results in steadily increasing cytotoxicity.
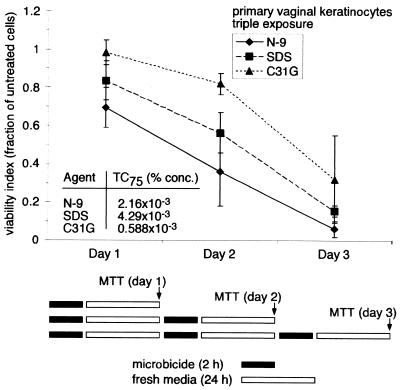
Although the preceding experiment demonstrated concentration-dependent cytotoxicity as a consequence of repeated microbicide exposure, it did not address the contribution of each application to the cumulative loss of cell viability. To examine the contribution of each exposure cycle to the total cytotoxicity, primary vaginal keratinocytes were subjected to the same multiple application protocol as in the preceding experiment and assessed for viability following the 24-h recovery period in each application cycle. The results illustrated in Fig. 4 were used to select concentrations at which multiple exposures to each microbicide resulted in 25% cell viability (TC75). These concentrations were selected to provide a low yet measurable level of cell viability at the endpoints of each experiment as well as detectable losses in cell viability at early time points. The calculated TC75 of N-9 and SDS after the three 2-h microbicide exposures were approximately 3.7- and 7.3-fold higher than the TC75 of C31G, reflecting the higher cytotoxicity of C31G under these conditions. Results indicated that each exposure cycle during the 3-day exposure protocol contributed equally toward the total amount of cytotoxicity (Fig. 5). Daily incubation with N-9, C31G, or SDS resulted in a linear decrease in cell viability.
FIG. 5.
Repetitive microbicide exposure results in a steady reduction in human vaginal keratinocyte viability. TC75 were determined from the results illustrated in Fig. 4. Cell survival was assessed by MTT assay after each exposure and recovery cycle. The cell viability following microbicide exposure is expressed as the fraction of viable cells relative to the number of mock-exposed cells. The results illustrated are averages from two experiments in which triplicate wells for each concentration were assayed.
Decreased cell viability during continuous exposure to C31G occurs earlier than similar decreases after N-9 or SDS exposure.
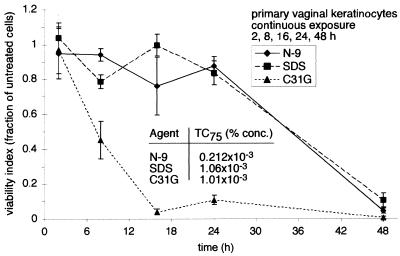
Cytotoxicity during continuous exposure must also be considered in the evaluation of chemical agents as potential microbicides, since significant levels of microbicides are likely retained in the vagina hours after the initial application (14). To determine the time course of microbicide cytotoxicity during continuous exposure, primary vaginal keratinocytes were exposed to a single concentration of N-9, C31G, or SDS and assessed for cell viability 2, 8, 16, 24, and 48 h after the application of each microbicide. The results depicted in Fig. 3A and B were used to select concentrations at which exposure to each microbicide reduced cell viability to 25% (TC75). The TC75 of C31G and SDS were approximately fivefold higher than the TC75 of N-9, reflecting the cytotoxicity of N-9 at low concentrations. Despite the fivefold difference in concentration, N-9 or SDS exposure resulted in similar levels of time-dependent cytotoxicity (Fig. 6). During the first 24 h of exposure to either N-9 or SDS, cell viability was generally constant and did not decrease below 76%. Between 24 and 48 h, cell viability decreased at a much higher rate. By 48 h, the presence of N-9 or SDS reduced cell viability to 5 and 11%, respectively. In contrast, cell viability in the presence of C31G decreased linearly after a 2-h exposure and was reduced to 4% by 16 h. These results indicate that in vitro exposure to C31G leads to a more rapid decline in cell viability than exposure to SDS, despite similar levels of cellular sensitivity following long-term (48 h) exposure.
FIG. 6.
Microbicide toxicity increases with continued exposure to equivalent concentrations of N-9, C31G, or SDS. TC75 were determined from data illustrated in Fig. 3A and B. Primary human vaginal keratinocytes were exposed to N-9, C31G, or SDS for 2, 8, 16, 24, or 48 h. At the end of each exposure interval, cell survival was assessed by an MTT assay. The cell viability following microbicide exposure is expressed as the fraction of viable cells relative to the number of mock-exposed cells. The results illustrated are averages from two experiments in which triplicate wells for each concentration were assayed.
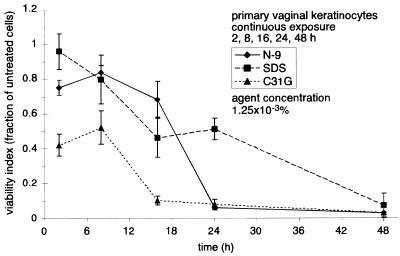
In a similar experiment, primary vaginal keratinocytes were exposed continuously to identical concentrations of N-9, C31G, or SDS (1.25 × 10−3%) for up to 48 h (Fig. 7). Earlier experiments (Fig. 3A) demonstrated that a 48-h exposure to each microbicide at this concentration resulted in less than 10% cell survival. After 16 h of continuous N-9 exposure at this concentration, cell survival remained generally constant between 83 and 69%. However, by 24 h, cell viability in the presence of N-9 fell dramatically to 8%. In contrast, there was no abrupt drop in cell survival during exposure to SDS. The decrease in cell viability associated with SDS exposure was approximately linear over 48 h, decreasing from 96% viability at 2 h to 7% viability at 48 h. At 2 and 8 h, cell survival during exposure to C31G was at 42 and 52%, respectively. Like the results illustrated in Fig. 6, cell survival 16 h after the introduction of C31G decreased abruptly (to approximately 10%).
FIG. 7.
Microbicide toxicity increases with continued exposure to equal concentrations of N-9, C31G, or SDS. Primary human vaginal keratinocytes were exposed to 1.25 × 10−3% N-9, C31G, or SDS for 2, 8, 16, 24, or 48 h. At the end of each exposure interval, cell survival was assessed by an MTT assay. Cell viability following microbicide exposure is expressed as the fraction of viable cells relative to the number of mock-exposed cells. The results illustrated are averages from two experiments in which triplicate wells for each concentration were assayed.
DISCUSSION
The studies presented above represent one of the most extensive uses of primary cell populations of human vaginal keratinocytes in assessments of in vitro microbicide cytotoxicity. The effect of microbicides on primary vaginal keratinocyte viability is highly relevant to clinical microbicide tolerance, since vaginal epithelial cells form part of the physical barrier that may impede the passage of cell-free or cell-associated HIV-1 into subepithelial tissues (23). Microbicidal agents that compromise this barrier may increase the risk of HIV-1 transmission.
One of the principal observations of these studies was that N-9 was 10-fold more toxic than C31G or SDS in long-term experiments using primary vaginal keratinocytes. This observation may be particularly relevant to considerations of long-term toxicity during N-9 use. A clinical study of N-9 retention following vaginal insertion of a contraceptive film containing N-9 demonstrated that levels of N-9 recovered by vaginal lavage remained constant for up to 2 h after product insertion and decreased to under 50% after 4 h (14). A similar report described levels of retention between 19 and 7% after 2 h, detectable levels of N-9 as long as 24 h after insertion, and levels of retention dependent on the contraceptive formulation (26). The toxicity of N-9 at low concentrations illustrated in our long-term (48 h) in vitro experiments (Fig. 3B) and the extended retention of N-9 observed in clinical studies suggest that use of products containing N-9 may result in greater levels of toxicity for longer durations following product insertion compared to products containing either C31G or SDS.
Experiments which examined the time-dependent accumulation of cytotoxicity emphasized differences between microbicides that may be related to each agent's mechanism of activity. In both experiments (Fig. 6 and 7), the time-dependent reductions in cell viability were highly divergent, despite nearly identical levels of cytotoxicity at the 48-h endpoint. These results suggest differences in the mechanisms by which N-9, C31G, and SDS kill cells. The delayed reductions in cell survival during N-9 or SDS exposure (Fig. 6) are also interesting and suggest more complex mechanisms of cell death. Perhaps while a small number of cells are killed quickly, most survive the first 24 h, accumulating damage in their cell membranes. Once cell integrity is compromised beyond a certain threshold, cells may die at a considerably faster rate. Although C31G appears to act similarly, its higher cytotoxicity under these conditions may result from reaching its threshold sooner after exposure. A similar effect may account for the higher sensitivity of primary vaginal keratinocytes to C31G during repeated exposure (Fig. 4). These findings, which will be addressed in future investigations, stress the need for examining not only experimental endpoints but also intermediate exposure times when evaluating microbicide formulations for cytotoxicity.
Examination of time-dependent cytotoxicity also highlighted the relationship between microbicide concentration and duration of exposure. Previous in vitro studies indicated that Escherichia coli (13) and sperm cells (25) were killed by C31G in less than 10 s. These experiments were performed using C31G concentrations of 2 × 10−2 and 5 × 10−2%, respectively. In studies with SupT1 T lymphocytes, complete cell killing was achieved after 10 min at C31G concentrations above 1.25 × 10−2% (9). In contrast, the results illustrated in Fig. 7, which demonstrated that a 16-h exposure was required to reduce cell viability to 10%, were obtained using a C31G concentration that was 16- to 40-fold lower than those used to kill bacteria (13) or sperm (25) and at least 10-fold lower than the concentration that was completely toxic to SupT1 cells (9). These results demonstrate that in vitro cytotoxicity is a function not only of concentration but also of exposure duration.
At low microbicide concentrations, differences in cytotoxicity are revealed that are otherwise masked at higher concentrations. For example, during extended exposure (48 h), C31G and SDS were as cytotoxic as N-9 at 5 × 10−3% but dramatically less cytotoxic than N-9 at a 20-fold-lower concentration (2.5 × 10−4%) (Fig. 3A). Although microbicide formulations intended for in vivo use contain active ingredients exceeding 1%, local concentrations at the vaginal epithelial cell membrane may be far less than in the original product due to dilution in cervical and vaginal secretions and semen, uneven product distribution across the vaginal epithelium, product redistribution by movement during intercourse and normal activities, diffusion across a keratin and cell gradient, and postapplication product loss. At these locally low concentrations, differences in cellular sensitivity to the active ingredient may become important. Because of its persistent cytotoxicity at low concentration, the adverse effects of N-9 on the vaginal epithelium may persist long after the effects of other products with less cytotoxic ingredients, such as C31G or SDS. To determine the relevance of low concentration differences in cytotoxicity to in vivo microbicide use, studies of product distribution and retention will be necessary to complement in vitro examinations of microbicide cytotoxicity and activity.
Studies of sensitivity to microbicide exposure performed with primary vaginal keratinocytes presage ongoing efforts to examine the effect of elements within the vaginal milieu on microbicide efficacy. In vitro assays described in this manuscript can provide assessments of potential activity and cytotoxicity relative to other agents, but they are not suited to accurately reflect the in vivo value of a microbicide, measured in terms of both microbicidal activity and its effect on cell viability. More complete models would take into account additional factors relevant to the vaginal microenvironment, including the presence of vaginal and cervical secretions, changes in pH, osmolarity, and protein content, the influx of semen during intercourse, the architecture of the vaginal epithelium, and time-dependent reductions in product retention (14). These factors may independently affect the in vivo activity and cytotoxicity of a given agent and change the concentrations at which these properties are manifested. We presume that these in vivo factors will decrease cytotoxicity associated with microbicide application and increase the effective antimicrobial activity of the microbicidal agent. Although animal models (16) and clinical trials may offer more complete experimental conditions, in vitro experiments have the advantage of convenience, flexibility, speed, and low cost. Ongoing investigations of vaginal microenvironmental factors are now focused on the influence of protein on microbicide activity. Proteins and mucins within the vaginal and cervical mucus may serve to sequester surfactant microbicides, lessening their effective concentrations and their interactions with STD pathogens and the surrounding tissues. This may be especially true of protein sequestration of SDS, which binds with high affinity to proteins.
These investigations have served to advance our understanding of microbicidal toxicity. First, surface-active agents like N-9, C31G, and SDS may function as microbicides using distinctly different mechanisms, as suggested by the divergence in cellular sensitivity during long- and short-term exposure. Additionally, SDS, which is well-known for its ability to denature proteins, may function as a protein denaturant at low concentrations and as a surfactant at higher concentrations. Understanding the different mechanisms by which these agents act may facilitate the design of more effective microbicides with broader activity and lower cytotoxicity. Future experimentation will explore these and other aspects of microbicide cytotoxicity. Second, these studies have provided the foundation upon which in vitro models of the vaginal microenvironment can be constructed to explore the impact of factors such as pH and the epithelial architecture on microbicide efficacy. Efforts are being directed toward constructing in vitro vaginal microenvironment models that would more closely emulate the conditions within the vagina and allow questions of in vivo efficacy to be better addressed.
In vitro experiments regarding antiviral potential as well as cytotoxicity have implied that C31G and SDS may be attractive alternatives to N-9 as topical vaginal microbicides (9). The present studies, which demonstrate high N-9 cytotoxicity, reinforce the concept that C31G and SDS may be desirable alternatives to N-9. These in vitro experiments will be complemented by ongoing investigations exploring the efficacy of potential vaginal microbicides, including C31G and SDS, using a human vaginal epithelial xenograft system in immunocompromised mice (6). Both approaches will advance the characterization and formulation of these potential vaginal microbicides and provide the foundation for additional human trials.
ACKNOWLEDGMENT
These studies, performed in the laboratories of B.W. and M.K.H., were supported by Public Health Service grant P01 AI37829.
REFERENCES
- 1.Calis S, Yulug N, Sumnu M, Ayhan A, Hincal A A. A non-antibiotic antimicrobial mixture (C31G): evaluation of the antimicrobial efficiency of C31G on vaginal cultures. Boll Chim Farm. 1992;131:335–338. [PubMed] [Google Scholar]
- 2.Cook R L, Rosenberg M J. Do spermicides containing nonoxynol-9 prevent sexually transmitted infections? A meta-analysis. Sex Transm Dis. 1998;25:144–150. doi: 10.1097/00007435-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Harrison C, Chantler E. The effect of nonoxynol-9 and chlorhexidine on HIV and sperm in vitro. Int J STD AIDS. 1998;9:92–97. doi: 10.1258/0956462981921747. [DOI] [PubMed] [Google Scholar]
- 4.Hermonat P L, Daniel R W, Shah K V. The spermicide nonoxynol-9 does not inactivate papillomavirus. Sex Transm Dis. 1992;19:203–205. doi: 10.1097/00007435-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Howett M K, Neely E B, Christensen N D, Wigdahl B, Krebs F C, Malamud D, Patrick S D, Pickel M D, Welsh P A, Reed C A, Ward M G, Budgeon L R, Kreider J W. A broad-spectrum microbicide with virucidal activity against sexually transmitted viruses. Antimicrob Agents Chemother. 1999;43:314–321. doi: 10.1128/aac.43.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howett, M. K., P. A. Welsh, L. R. Budgeon, M. G. Ward, E. B. Neely, S. D. Patrick, J. Weisz, and J. W. Kreider. Transformation of human vaginal xenografts by human papillomavirus type 11: prevention of infection with a microbicide from the alkyl sulfate chemical family. Pathogenesis, in press.
- 7.Irwin K, Scarlett M, Moseley R. Observations from the CDC. The urgent need for new HIV/STD prevention options for women. J Women's Health. 1998;7:1081–1086. doi: 10.1089/jwh.1998.7.1081. [DOI] [PubMed] [Google Scholar]
- 8.Joint United Nations Programme on HIV/AIDS and the World Health Organization. Report on the global HIV/AIDS epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS and the World Health Organization; 1998. [Google Scholar]
- 9.Krebs F C, Miller S R, Malamud D, Howett M K, Wigdahl B. Inactivation of human immunodeficiency virus type 1 by nonoxynol-9, C31G, or an alkyl sulfate, sodium dodecyl sulfate. Antivir Res. 1999;43:147–163. doi: 10.1016/s0166-3542(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 10.Kreiss J, Ngugi E, Holmes K, Ndinya-Achola J, Waiyaki P, Roberts P L, Ruminjo I, Sajabi R, Kimata J, Fleming T R. Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268:477–482. [PubMed] [Google Scholar]
- 11.Louv W C, Austin H, Alexander W J, Stagno S, Cheeks J. A clinical trial of nonoxynol-9 for preventing gonococcal and chlamydial infections. J Infect Dis. 1988;158:518–523. doi: 10.1093/infdis/158.3.518. [DOI] [PubMed] [Google Scholar]
- 12.Malamud D, Howett M K, Wigdahl B, Christensen N D, Krebs F C, Weisz J, Kreider J W. Proceedings of the 12th World AIDS Conference: epidemiology and prevention. Geneva, Switzerland: International Proceedings Division; 1998. Anti-viral activity of microbicides in model systems; pp. 223–227. [Google Scholar]
- 13.Malamud D, Schnaare R L. Formulation of C31G as a vaginal microbicide. In: Rencher W F, editor. Vaginal microbicide formulations workshop. Philadelphia, Pa: Lippincott-Raven; 1998. pp. 79–89. [Google Scholar]
- 14.Mauck C K, Allen S, Baker J M, Barr S P, Abercrombie T, Archer D F. An evaluation of the amount of nonoxynol-9 remaining in the vagina up to 4 h after insertion of a vaginal contraceptive film (VCF) containing 70 mg nonoxynol-9. Contraception. 1997;56:103–110. doi: 10.1016/s0010-7824(97)00100-5. [DOI] [PubMed] [Google Scholar]
- 15.Niruthisard S, Roddy R E, Chutivongse S. Use of nonoxynol-9 and reduction in rate of gonococcal and chlamydial cervical infections. Lancet. 1992;339:1371–1375. doi: 10.1016/0140-6736(92)91195-e. [DOI] [PubMed] [Google Scholar]
- 16.Patton D L, Kidder G G, Sweeney Y C, Rabe L K, Hillier S L. Effects of multiple applications of benzalkonium chloride and nonoxynol 9 on the vaginal epithelium in the pigtailed macaque (Macaca nemestrina) Am J Obstet Gynecol. 1999;180:1080–1087. doi: 10.1016/s0002-9378(99)70598-3. [DOI] [PubMed] [Google Scholar]
- 17.Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, Desmyter J, De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 18.Piret J, Lamontagne J, Bestman-Smith J, Roy S, Gourde P, Desormeaux A, Omar R F, Juhasz J, Bergeron M G. In vitro and in vivo evaluations of sodium lauryl sulfate and dextran sulfate as microbicides against herpes simplex and human immunodeficiency viruses. J Clin Microbiol. 2000;38:110–119. doi: 10.1128/jcm.38.1.110-119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roddy R E, Cordero M, Cordero C, Fortney J A. A dosing study of nonoxynol-9 and genital irritation. Int J STD AIDS. 1993;4:165–170. doi: 10.1177/095646249300400308. [DOI] [PubMed] [Google Scholar]
- 20.Roddy R E, Zekeng L, Ryan K A, Tamoufe U, Weir S S, Wong E L. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998;339:504–510. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 21.Rosenstein I J, Stafford M K, Kitchen V S, Ward H, Weber J N, Taylor-Robinson D. Effect on normal vaginal flora of three intravaginal microbicidal agents potentially active against human immunodeficiency virus type 1. J Infect Dis. 1998;177:1386–1390. doi: 10.1086/517820. [DOI] [PubMed] [Google Scholar]
- 22.Rustomjee R, Abdool Karim Q, Abdool Karim S S, Laga M, Stein Z. Phase 1 trial of nonoxynol-9 film among sex workers in South Africa. AIDS. 1999;13:1511–1515. doi: 10.1097/00002030-199908200-00011. [DOI] [PubMed] [Google Scholar]
- 23.Spira A I, Marx P A, Patterson B K, Mahoney J, Koup R A, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stafford M K, Ward H, Flanagan A, Rosenstein I J, Taylor-Robinson D, Smith J R, Weber J, Kitchen V S. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:327–331. doi: 10.1097/00042560-199804010-00006. [DOI] [PubMed] [Google Scholar]
- 25.Thompson K A, Malamud D, Storey B T. Assessment of the antimicrobial agent C31G as a spermicide: comparison with nonoxynol-9. Contraception. 1996;53:313–318. [PubMed] [Google Scholar]
- 26.Witter F R, Barditch-Crovo P, Rocco L, Trapnell C B. Duration of vaginal retention and potential duration of antiviral activity for five nonoxynol-9 containing intravaginal contraceptives. Int J Gynaecol Obstet. 1999;65:165–170. doi: 10.1016/s0020-7292(99)00018-1. [DOI] [PubMed] [Google Scholar]
- 27.Zekeng L, Feldblum P J, Oliver R M, Kaptue L. Barrier contraceptive use and HIV infection among high-risk women in Cameroon. AIDS. 1993;7:725–731. doi: 10.1097/00002030-199305000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus K A, Reimann K A, Reinhart T A, Rogan M, Cavert W, Miller C J, Veazey R S, Notermans D, Little S, Danner S A, Richman D D, Havlir D, Wong J, Jordan H L, Schacker T W, Racz P, Tenner-Racz K, Letvin N L, Wolinsky S, Haase A T. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]