Abstract
Background and aims:
Several scoring systems predict mortality in alcohol-associated hepatitis (AH), including the Maddrey’s discriminant function (mDF) and MELD score developed in the USA, Glasgow Alcoholic Hepatitis Score (GAHS) in the UK, and ABIC in Spain. To date, no global studies have examined the utility of these scores, nor has the MELD-Na been evaluated for outcome prediction in AH. In this study, we assessed the accuracy of the different scores to predict short-term mortality in AH and investigated additional factors to improve mortality prediction.
Methods:
Patients admitted to hospital with a definite or probable AH were recruited by 85 tertiary centers in 11 countries and across three continents. Baseline demographic and laboratory variables were obtained. The primary outcome was all-cause mortality at 28 and 90 days.
Results:
In total 3,101 patients were eligible for inclusion. After exclusions (n=520), 2,581 patients were enrolled (74.4% male, median age 48, IQR 40.9–55.0 years). The median MELD score was 23.5 (IQR 20.5–27.8). Mortality at 28 and 90 days was 20% and 30.9%, respectively. The AUROC for 28 day mortality ranged from 0.776 for MELD-Na to 0.701 for mDF, for 90 day mortality it ranged from 0.773 for MELD to 0.709 for mDF. The AUROC for mDF to predict death were significantly lower than all other scores. Age added to MELD obtained only a small improvement of AUC.
Conclusions:
These results suggest that mDF score should no longer be used to assess AH’s prognosis. MELD score has the best performance in predicting short-term mortality.
Keywords: alcohol-associated hepatitis, prognostic scores, severity, mortality
Introduction
Alcohol-associated hepatitis (AH) is the most severe acute form of alcohol-related liver disease. AH portends a poor prognosis with high short-term mortality between 20–50% 1. Prognostic scores are used in clinical practice to assess the severity of the disease and identify high-risk patients for consideration of corticosteroid treatment 2. The Maddrey’s discriminant function (mDF) has been traditionally used in guidelines and clinical trials to define severe disease (mDF > 32) 3. However, mDF has some limitations, such as it does not include creatinine, a widely recognized prognostic factor in AH 4, and measurement of the prothrombin time varies significantly between laboratories 5. Several studies have reported the model for end-stage liver disease (MELD) is a better predictor of mortality in AH than the mDF 6, 7. Other validated prognostic scores in AH are the age, bilirubin, international normalized ratio and creatinine (ABIC) score 8 and Glasgow alcoholic hepatitis score (GAHS) 9. The mDF and MELD scores were initially derived and validated in the USA, the ABIC in a cohort from Spain and the GAHS in Glasgow, UK. These scores have not been validated globally despite the expected influence of genetic, socioeconomic, climatic, and local diagnostic and technical laboratory factors 1, 10, 11.
Serum Na is an independent predictor of mortality in patients with cirrhosis12. Both the MELD score and the MELD-Na score which incorporates serum Na into the MELD score are currently used to prioritize allocation of organs for liver transplantation in patients with cirrhosis 13 but MELD-Na has been validated in patients with AH only in a small study14. Moreover, around 30% of patients with AH do not have cirrhosis 15.
Therefore, we aimed to assess the predictive accuracy of MELD-Na in a global cohort of patients with AH as well as other prognostic score such as the mDF, MELD, GAHS, and ABIC scores in determining mortality at 28 and 90 days. We also determined whether there were other parameters that could improve mortality prediction.
Methods
Study design and population
We analyzed individual patient data of well-characterized patients hospitalized with AH in 85 tertiary centers in 11 countries. The diagnosis of AH was determined across all centers using the standardized definition, as described below. All participant centers followed the same inclusion/exclusion criteria. Institutional review boards from each center approved the study.
A diagnosis of AH made following epidemiological, clinical, and biological criteria according to the Standard Definitions of the National Institute on Alcohol Abuse and Alcoholism NIAAA 16. According to clinical criteria, patients with uncertain of AH diagnosis had undergone a liver biopsy, which must confirm the diagnosis of AH. Only patients with biopsy proven AH (definite AH); and patients with history of alcohol use and liver test abnormalities as described below without confounding factors (probable AH) were included.
Specific inclusion criteria were a) a history of alcohol use of >60 g/day in men and >40 g/day in women, b) an aspartate aminotransferase <400 U/l with AST/ALT ratio >1.5, c) Serum γ-glutamyl transpeptidase –GGT– levels >80 mg/dl, d) altered coagulation tests (prolonged prothrombin time and/or INR values), and e) serum bilirubin levels >3 mg/dl. For patients with more than one admission, information was collected only for the index episode. Exclusion criteria were a) other identifiable causes of liver disease: hepatitis B or C virus infection, autoimmune hepatitis, hemochromatosis, Wilson’s disease, suspicion for drug induced liver injury disease, or alternative diagnosis on liver biopsy, b) complete portal vein thrombosis, c) hepatocellular carcinoma and other malignancies, d) human immunodeficiency virus infection, e) other extrahepatic severe illness with low life expectancy according to investigators’ criteria.
Data collection
Age, sex, alcohol consumption (g/day), mortality status (including date of death), and the presence clinical complications at admission (viz. systemic inflammatory response syndrome (SIRS), infection, gastrointestinal (GI) bleeding, acute kidney injury (AKI) and encephalopathy) were collected. AKI was defined as serum creatinine ≥1.5 mg/dL and/or AKIN criteria 17. Baseline laboratory variables were also collected and the MELD, MELD-Na, mDF, GAHS, and ABIC scores derived using these data.
Regarding socioeconomic determinants of health, we obtained data from Human Development Reports of United Nations from 2019 to include surrogate inequality and economic parameters (Addendum, Supplemental Digital Content 9). We focused on the Human Development Index (HDI), a summary measure of a long and healthy life, being knowledgeable and have a decent standard of living, and the Inequality-adjusted HDI (IHDI) that takes into account not only the average achievements of a country in health, education and income, but also how those achievements are distributed among its population by “discounting” each dimension’s average value according to its level of inequality as parameters reflecting inequality and the economy of each country.
Statistical analysis
Baseline characteristics are expressed as percentages and median and interquartile range (IQR). The primary endpoint was all-cause mortality at 28 and 90 days. The prognostic scores’ accuracy was evaluated using receiver operating characteristics (ROC) curves, and the area under the curves (AUROC) was calculated. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and positive and negative likelihood ratio (LR) were calculated for each score. The DeLong method 18 was used to test for statistically significant differences between ROC curves. Cox regression analysis was used to identify risk factors for mortality at 28 and 90 days. The variables demonstrating statistically significant association on both 28 and 90-day mortality univariate analysis (p<0.001) were included in the multivariate analysis. Logistic regression was performed to generate equations.
P<0.05 was considered statistically significant.
We performed all statistical analyses with IBM SPSS software (Version 26) and MedCalc Statistical Software version 16.4.3 (MedCalc Software bv, Ostend, Belgium; https://www.medcalc.org; 2016) for AUROC comparison.
Results
Baseline characteristics of included patients
A total of 3,101 patients with AH were recruited from 85 tertiary centers in 11 countries (Table, Supplemental Digital Content 1). After exclusions (n=520), 2,581 patients (74.4% male, median 48, IQR 40.9–55 years) were finally included (Figure 1). Global and by country baseline characteristics of included patients are shown in Table 1, and baseline laboratory variables are shown in Table, Supplemental Digital Content 2. The majority of patients were white (57.7%), followed by Indians (14.1%), Asians (12%), Latin Americans (9%), and Blacks (1.8%). Regarding sex distribution, Anglo-Saxon countries (USA, UK, and Canada) had a higher percentage of women with a diagnosis of AH (38.6% vs. 10.0%, p<0.001). Globally, women compared with men were younger, with less alcohol consumption, and lower mortality at 28 and 90 days, similar than Anglo-Saxon countries (Table 2).
Figure 1.
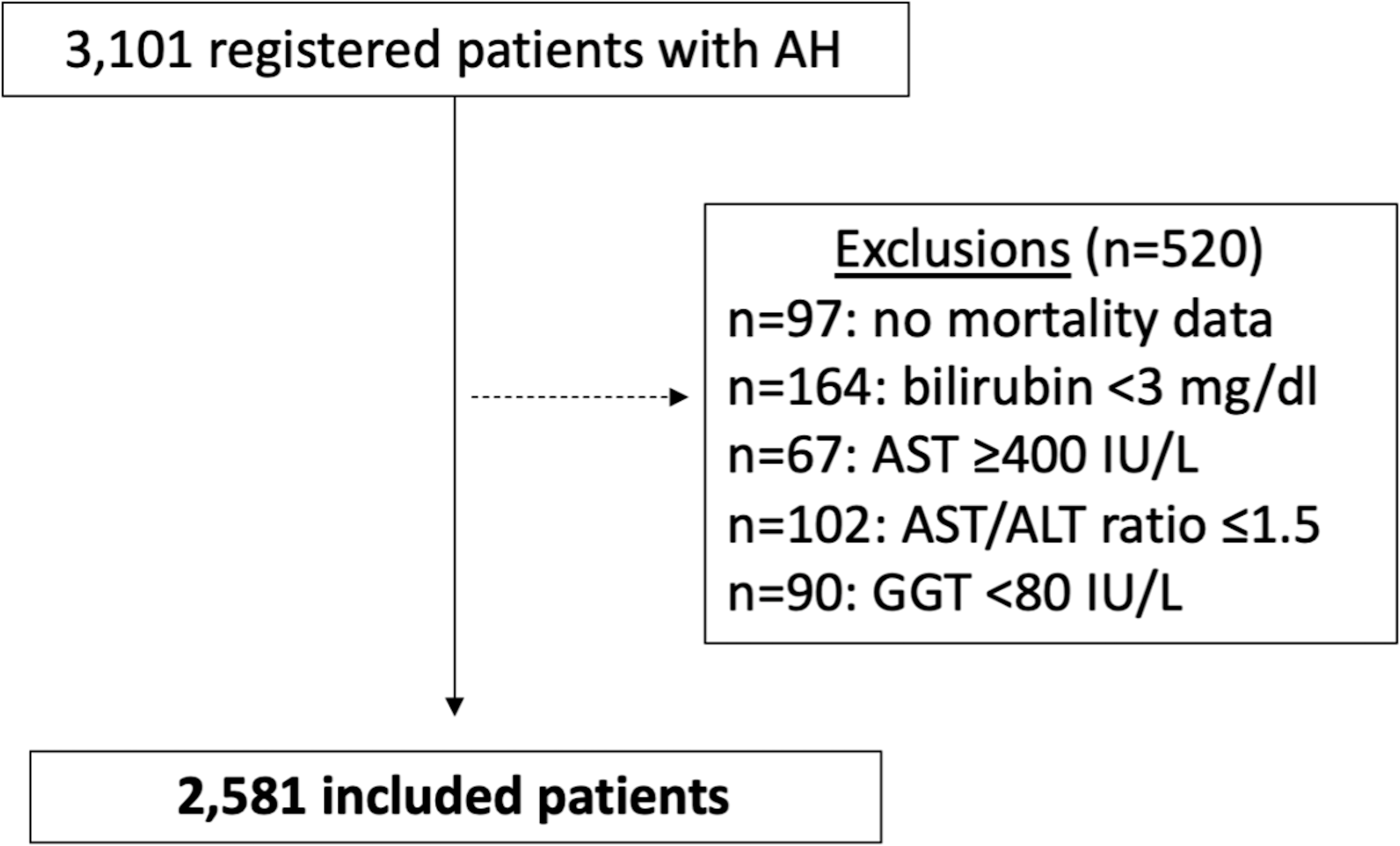
Flow-chart of patient inclusion.
AST: aspartate aminotransferase, ALT: alanine aminotransferase, GGT: gamma glutamyl transferase
Table 1.
Baseline characteristic of included patients.
| Spain n=84 |
Mexico n=222 |
Korea n=274 |
USA n=291 |
Colombia n=40 |
France n=66 |
Brazil n=90 |
India n=366 |
Chile n=28 |
UK n=1,092 |
Canada n=28 |
Total n=2,581 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (male, %) | 76.2 | 87.8 | 87.6 | 57.4 | 82.5 | 92.4 | 90 | 97.5 | 78.6 | 62.6 | 53.6 | 74.4 |
|
| ||||||||||||
| Age (years) | 47 (43–55) | 43 (35–50) | 51 (45–58) | 48 (38–55) | 52 (48–60) | 55 (49–61) | 43.5 (37–50) | 45 (39–51) | 54.5 (47–64) | 48.8 (42–56) | 51.5 (38–58) | 48 (41–55) |
|
| ||||||||||||
| Race | ||||||||||||
| White (%) | 77.4 | 0 | 0 | 79.7 | 0 | 89.4 | 67.8 | 0.3 | 0 | 96.1 | 78.6 | 57.7 |
| Black (%) | 0 | 0 | 0 | 12.7 | 0 | 3 | 2.2 | 0 | 0 | 0.5 | 0 | 1.8 |
| Asian (%) | 2.4 | 0 | 100 | 0 | 0 | 7.6 | 0 | 0 | 0 | 2.5 | 3.6 | 12 |
| Latin American (%) | 8.3 | 70.7 | 0 | 5.8 | 0 | 0 | 28.9 | 0 | 85.7 | 0 | 0 | 9 |
| Indian (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 99.7 | 0 | 0 | 0 | 14.1 |
| No registered (%) | 11.9 | 29.3 | 0 | 1.7 | 100 | 0 | 1.1 | 0 | 14.3 | 0.9 | 17.9 | 5.4 |
|
| ||||||||||||
| Deaths at 28 days (%) | 14.3 | 37.8 | 16.1 | 24.1 | 27.5 | 15.2 | 22.2 | 23.2 | 14.3 | 15.9 | 7.1 | 20 |
|
| ||||||||||||
| Lost to follow-up 28d | 2.4 | 3.2 | 10.9 | 5.5 | 15 | 0 | 17.8 | 16.7 | 0 | 0 | 7.1 | 5.4 |
|
| ||||||||||||
| Deaths at 90 days (%) | 20.2 | 56.8 | 22.3 | 36.8 | 27.5 | 25.8 | 33.3 | 35 | 17.9 | 26.2 | 32.1 | 30.9 |
|
| ||||||||||||
| Lost to follow-up 90d | 11.9 | 6.3 | 27 | 11 | 22.5 | 7.6 | 27.8 | 29.8 | 3.6 | 0 | 28.6 | 11.1 |
|
| ||||||||||||
| MELD | 22.7 (18–27) | 30.9 (25–37) | 20.3 (17–26) | 24.7 (21–31) | 25.5 (19–31) | 22.3 (19–26) | 26.5 (21–36) | 22.6 (19–26) | 21.2 (18–27) | 23.4 (21–26) | 21.8 (20–26) | 23.5 (20–28) |
|
| ||||||||||||
| MELD-Na | 21.2 (19–24) | 32.7 (28–38) | 23.5 (20–30) | 28.4 (25–33) | 27.5 (22–33) | 25.3 (23–29) | 30.7 (25–38) | - | 22.6 (19–32) | 26.3 (24–30) | 25.8 (23–30) | 26.8 (23–31) |
|
| ||||||||||||
| mDF | 56.3 (40–87) | 71.6 (50–103) | 38 (21–61) | 52.5 (37–72) | 51.6 (25–77) | 47.3 (35–68) | 53.9 (46–91) | 66.1 (47–97) | 40.9 (22–52) | 55.4 (43 −74) | - | 55.6 (41–79) |
|
| ||||||||||||
| GAHS | 9 (8–10) | 10 (9–11) | 8 (7–9) | 9 (8–10) | 9 (8–11) | - | 9 (8–10) | - | 8 (7–9) | 8 (7–9) | 8 (7–9.7) | 9 (7–10) |
|
| ||||||||||||
| ABIC | 7.6 (7–9) | 8.5 (7–10) | 7.5 (7–9) | 7.9 (7–9) | 8.8 (7–10) | 8.2 (7–9) | 8.3 (7–11) | 7.5 (7–8) | 8.2 (7–9) | 8 (7–9) | 8.1 (7–9) | 7.9 (7–9) |
|
| ||||||||||||
| Alcohol consumption (g/day) | 100 (80–140) | 180 (118–320) | 113 (60–150) | 120 (45–229) | 100 (100–180) | 100 (75–150) | 227.5 (125–584) | - | 130 (92–181) | 131.5 (84–210) | 160 (85–200) | 126 (84–210) |
|
| ||||||||||||
| Corticosteroid treatment (%) | 67.9 | 52.7 | 2.2 | 41.9 | 12.5 | - | 50 | - | 46.4 | 50.1 | 60.7 | 36.3 |
MELD: model for end-stage liver disease, mDF: Maddrey’s discriminant function, GAHS: Glasgow alcoholic hepatitis score, ABIC: age, bilirubin, international normalized ratio and creatinine score
Table 2.
Baseline characteristics by country according to gender.
| Age (years) | P* | Deaths at 28 days (%) | P* | Deaths at 90 days (%) | P* | Alcohol consumption (g/day) | P* | ||
|---|---|---|---|---|---|---|---|---|---|
|
Spain
n=84 |
Male
n=64 |
48 (43–55) | 0.152 | 14.1 | 0.670 | 18.8 | 0.816 | 100 (80–140) | 0.387 |
|
Female
n=20 |
45 (39–53) | 15 | 25 | 80 (64–90) | |||||
|
Mexico
n=222 |
Male
n=195 |
43 (35–50) | 0.991 | 42.1 | 0.002 | 61.5 | <0.001 | 196 (116–320) | 0.307 |
|
Female
n=27 |
40 (38–52) | 7.4 | 22.2 | 160 (120–240) | |||||
|
Korea
n=274 |
Male
n=240 |
51 (45–59) | 0.030 | 16.3 | 0.966 | 22.9 | 0.782 | 113 (60–150) | 0.029 |
|
Female
n=34 |
45 (40–54) | 14.7 | 17.6 | 60 (50–115) | |||||
|
USA
n=291 |
Male
n=167 |
49 (40–56) | 0.004 | 28.7 | 0.016 | 43.1 | 0.007 | 140 (45–246) | 0.088 |
|
Female
n=124 |
44 (35–53) | 17.7 | 28.2 | 100 (34–186) | |||||
|
Colombia
n=40 |
Male
n=33 |
51 (48–60) | 0.770 | 27.3 | 27.3 | 0.842 | 52 (51–61) | 0.089 | |
|
Female
n=7 |
52 (51–61) | 28.6 | 28.6 | 100 (90–100) | |||||
|
France
n=66 |
Male
n=61 |
55 (49–61) | 1.000 | 16.4 | 1.000 | 26.2 | 0.547 | 55 (40–60) | 0.589 |
|
Female
n=5 |
55 (40–60) | 0 | 20 | 97 (71–123) | |||||
|
Brazil
n=90 |
Male
n=81 |
44 (37–50) | 1.000 | 22.2 | 0.850 | 33.3 | 0.909 | 43 (30–49) | 0.482 |
|
Female
n=9 |
43 (30–49) | 22.2 | 33.3 | 500 (150–723) | |||||
|
India
n=366 |
Male
n=357 |
45 (39–51) | 0.521 | 22.7 | 0.311 | 34.7 | 0.809 | - | - |
|
Female
n=9 |
37 (35–49) | 44.4 | 44.4 | - | |||||
|
Chile
n=28 |
Male
n=22 |
52 (48–64) | 0.648 | 9.1 | 0.191 | 13.6 | 0.488 | 140 (100–200) | 0.317 |
|
Female
n=6 |
62 (42–66) | 33.3 | 33.3 | 100 (85–135) | |||||
|
UK
n=1,092 |
Male
n=684 |
49 (42–57) | 0.012 | 16.7 | 0.442 | 28.7 | 0.019 | 140 (100–218) | <0.001 |
|
Female
n=408 |
47 (41–55) | 14.7 | 22.1 | 105 (70–181) | |||||
|
Canada
n=28 |
Male
n=15 |
53 (46–59) | 0.449 | 6.7 | 0.393 | 33.3 | 0.755 | 160 (96–200) | 0.695 |
|
Female
n=13 |
51 (36–57) | 7.7 | 23.1 | 120 (72–200) | |||||
|
Total
n=2,581 |
Male
n=1,919 |
49 (41–56) | 0.005 | 21.5 | <0.001 | 33.3 | <0.001 | 140 (98–224) | <0.001 |
|
Female
n=662 |
47 (40–54) |
15.6 | 23.9 | 101 (68–180) |
Global and by country gender differences in age, mortality and alcohol consumption
Overall, 28 and 90-day mortality was 20% and 30.9%, respectively. Mexico showed the highest rates of short-term mortality at 28 (37.8%, p<0.001) and 90 days (56.8%, p<0.001) than the entire cohort. Other countries with higher mortality than the entire cohort were Colombia at 28 days (27.5%, p=0.008), Canada at 90 days (32.1%, p=0.009), Brazil at 28 (22.2%, p<0.001) and 90 days (33.3%, p<0.001), and India at 28 (23.2%, p<0.001) and 90 days (35%, p<0.001); whereas UK and Korea showed a lower mortality rate at 28 (15.9%, p<0.001; 16.1%, p<0.001; respectively) and 90 days (26.2%, p<0.001; 22.3%, p<0.001; respectively). The median (IQR) MELD score, MELD-Na, mDF, GAHS, and ABIC scores were 23.5 (20.5–27.8), 26.8 (23.4–31.3), 55.6 (41.4–78.9), 9 (7–10), and 7.9 (6.9–9) respectively. Mexico exhibited substantially higher scores, median MELD 30.9 (24.9–37.4) vs, 23.1 (20.3–27.9), p<0.001. By contrary, Korea obtained lower scores, median MELD 20.3 (17–26) vs, 23.7 (20.8–28), p<0.001. The rest of significant differences between the different countries and the entire cohort are shown in Table, Supplemental Digital Content 3.
Performance of existing prognostic models and MELD-Na
AUROCs for the accuracy to predict mortality at 28 and 90 days for the different scores are shown in Figures 2 and 3. The AUROCs for prediction of mortality at 28 days ranged from 0.776 for MELD-Na and 0.775 for MELD to 0.701 for mDF, whereas for 90 days, mortality predictions ranged from 0.773 for both MELD-Na and MELD to 0.709 for mDF. mDF had the lowest AUROC to predict death, with significant differences between all other scores and mDF (Figure 2 and 3). ABIC score was inferior to MELD and MELD-Na but superior to mDF and without significant difference with GAHS that globally had not significant differences with MELD and MELD-Na but it was also superior than mDF.
Figure 2.
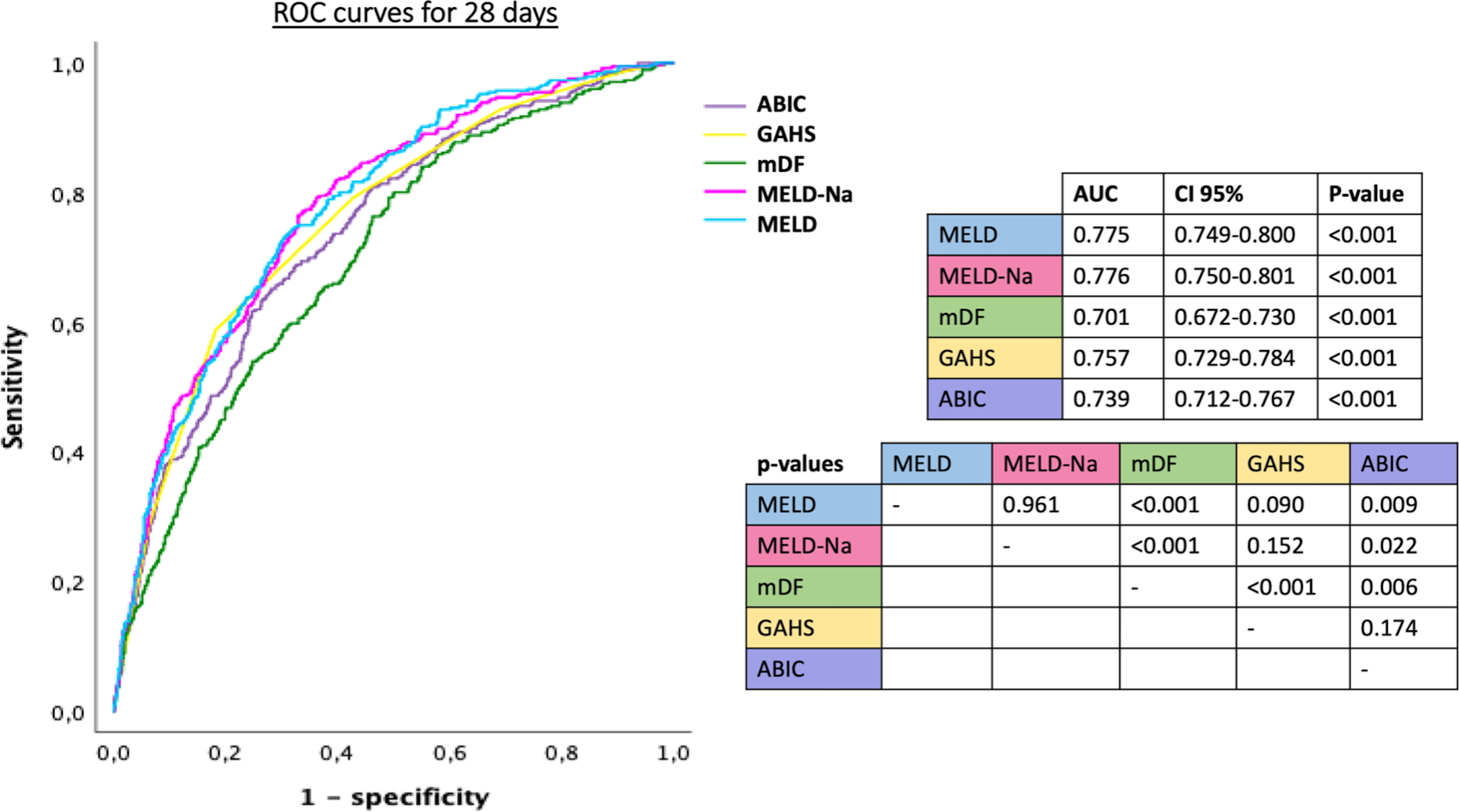
ROC curves of the different prognostic scores for alcohol-associated hepatitis calculated baseline, used to predict mortality at 28 days, and p-values comparing scores.
MELD: model for end-stage liver disease, mDF: Maddrey’s discriminant function, GAHS: Glasgow alcoholic hepatitis score, ABIC: age, bilirubin, international normalized ratio and creatinine score, AUC: area under curve, CI: confidence interval
Figure 3.
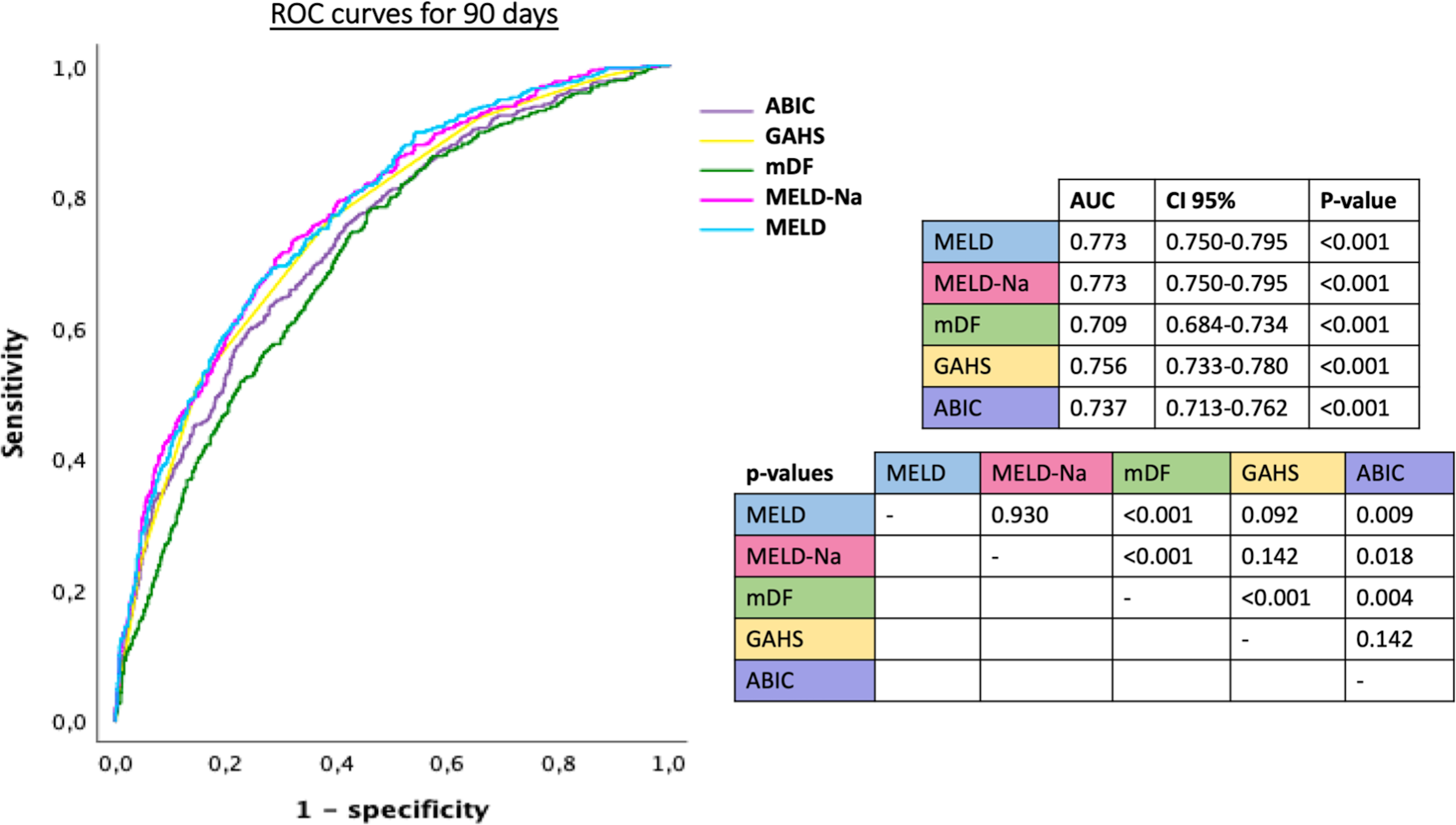
ROC curves of the different prognostic scores for alcohol-associated hepatitis calculated baseline, used to predict mortality at 90 days, and p-values comparing scores.
MELD: model for end-stage liver disease, mDF: Maddrey’s discriminant function, GAHS: Glasgow alcoholic hepatitis score, ABIC: age, bilirubin, international normalized ratio and creatinine score, AUC: area under curve, CI: confidence interval
Since the 42% of included patients were from UK (Addendum, Supplemental Digital Content 10), an analysis was performed excluding UK patients; MELD and MELD-Na scores showed the highest AUROCs predicting mortality (Table, Supplemental Digital Content 4 and 5). Table 3 shows the AUROC of all scores by country. ABIC score significantly predicted mortality at 28 and 90 days in all countries, and in France was the only score that significantly predict mortality. Particularly, Glasgow score was not useful predicting mortality in Colombia and France, and mDF was not useful in Spain and France.
Table 3.
ROC curves to predict mortality at 28 and 90 days per country.
| Mortality at 28 days | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spain | Mexico | Korea | USA | Colombia | France | Brazil | India | Chile | UK | Canada | |
|
MELD
|
0.740 # | 0.748 * | 0.810 * | 0.727 * | 0.759 # | 0.698 | 0.775 * | 0.640 * | 0.967 * | 0.739 * | 0.909 * |
| AUC, 95% CI | (0.550–0.929) | (0.684–0.812) | (0.745–0.875) | (0.650–0.803) | (0.597–0.920) | (0.414–0.981) | (0.648–0.902) | (0.577–0.703) | (0.877–1,057) | (0.699–0.780) | (0.749–1,069) |
| MELD-Na | 0.875 * | 0.749 * | 0.802 * | 0.718 * | 0.721 # | 0.673 | 0.759 * | - | 0.967 * | 0.742 * | 0.932 * |
| AUC, 95% CI | (0.762–0.988) | (0.685–0.813) | (0.735–0.870) | (0.640–0.797) | (0.547–0.895) | (0.431–0.914) | (0.637–0.881) | (0.877–1,057) | (0.701–0.783) | (0.696–1,077) | |
| mDF | 0.656 | 0.677 * | 0.744 * | 0.654 * | 0.702 # | 0.679 | 0.753 * | 0.624 * | 0.767 # | 0.679 * | - |
| AUC, 95% CI | (0.468–0.845) | (0.606–0.748) | (0.661–0.828) | (0.570–0.738) | (0.503–0.901) | (0.399–0.959) | (0.629–0.877) | (0.559–0.688) | (0.553–0.980) | (0.636–0.723) | |
| GAHS | 0.807 # | 0.718 * | 0.760 * | 0.653 * | 0.633 | - | 0.820 * | - | 0.867 * | 0.759 * | 0.830 # |
| AUC, 95% CI | (0.608–1,007) | (0.649–0.786) | (0.685–0.834) | (0.568–0.738) | (0.427–0.839) | (0.696–0.943) | (0.695–1,038) | (0.719–0.799) | (0.584–1,075) | ||
| ABIC | 0.802 * | 0.665 * | 0.737 * | 0.727 * | 0.743 # | 0.802 # | 0.701 # | 0.601 # | 0.967 * | 0.751 * | 0.886 * |
| AUC, 95% CI | (0.644–0.961) | (0.594–0.735) | (0.655–0.819) | (0.646–0.808) | (0.562–0.923) | (0.589–1,016) | (0.569–0.834) | (0.533–0.669) | (0.877–1,057) | (0.711–0.792) | (0.696–1,077) |
| Mortality at 90 days | |||||||||||
| Spain | Mexico | Korea | USA | Colombia | France | Brazil | India | Chile | UK | Canada | |
| MELD | 0.847 * | 0.765 * | 0.836 * | 0.766 * | 0.759 # | 0.694 | 0.748 * | 0.645 * | 0.967 * | 0.709 * | 0.914 * |
| AUC, 95% CI | (0.701–0.992) | (0.700–0.829) | (0.784–0.889) | (0.698–0.834) | (0.597–0.920) | (0.478–0.911) | (0.624–0.871) | (0.587–0.703) | (0.877–1,057) | (0.674–0.745) | (0.803–1,025) |
| MELD-Na | 0.867 * | 0.771 * | 0.831 * | 0.766 * | 0.721 # | 0.708 # | 0.736 * | - | 0.967 * | 0.709 * | 0.898 * |
| AUC, 95% CI | (0.726–1,007) | (0.707–0.835) | (0.775–0.888) | (0.699–0.834) | (0.547–0.895) | (0.516–0.900) | (0.613–0.860) | (0.877–1,057) | (0.673–0.744) | (0.772–1,025) | |
| mDF | 0.693 | 0.686 * | 0.764 * | 0.696 * | 0.702 # | 0.519 | 0.673 # | 0.619 * | 0.767 # | 0.673 * | - |
| AUC, 95% CI | (0.495–0.902) | (0.613–0.759) | (0.693–0.836) | (0.622–0.770) | (0.503–0.901) | (0.265–0.772) | (0.535–0.912) | (0.560–0.678) | (0.553–0.980) | (0.637–0.710) | |
| GAHS | 0.897 * | 0.729 * | 0.802 * | 0.724 * | 0.633 | - | 0.791 * | - | 0.867 * | 0.718 * | 0.941 * |
| AUC, 95% CI | (0.760–1,033) | (0.662–0.797) | (0.743–0.861) | (0.652–0.796) | (0.427–0.839) | (0.675–0.906) | (0.695–1,038) | (0.682–0.753) | (0.852–1,031) | ||
| ABIC | 0.880 * | 0.701 * | 0.739 * | 0.789 * | 0.743 # | 0.736 # | 0.694 # | 0.599 # | 0.967 * | 0.726 * | 0.945 * |
| AUC, 95% CI | (0.760–1,000) | (0.631–0.772) | (0.669–0.809) | (0.724–0.854) | (0.562–0.923) | (0.538–0.934) | (0.561–0.827) | (0.539–0.659) | (0.877–1,057) | (0.691–0.761) | (0.856–1,035) |
p<0.05
p<0.001
MELD: model for end-stage liver disease, mDF: Maddrey’s discriminant function, GAHS: Glasgow alcoholic hepatitis score, ABIC: age, bilirubin, international normalized ratio and creatinine score, AUC: area under curve, CI: confidence interval
The optimal baseline cut-off values for predicting death at 28 and 90 days using this global cohort was 25 for MELD, 28 for MELD-Na, 51 and 52 for mDF, 9 and 8 for GAHS, and 9 for ABIC. The receiver operating characteristic for each cut-off value is shown in Table 4.
Table 4.
Receiver operation characteristic analysis for different cut-off values of the different prognostic scores to predict death at 28 and 90 days.
| Mortality at 28 days | |||||
|---|---|---|---|---|---|
| MELD | MELD-Na | mDF | GAHS | ABIC | |
| Cut-off value | 25 | 28 | 51 | 9 | 9 |
| Sensitivity, 95% CI | 68 (64–72) | 77 (73–81) | 81 (77–84) | 59 (54–64) | 57 (53–61) |
| Specificity, 95% CI | 70 (68–72) | 67 (65–69) | 48 (45–50) | 81 (79–83) | 77 (75–79) |
| Positive LR, 95% CI | 2.3 (2.1–2.5) | 2.3 (2.1–2.5) | 1.5 (1.5–1.6) | 3.1 (2.7–3.5) | 2.5 (2.2–2.8) |
| Negative LR, 95% CI | 0.4 (0.4–0.5) | 0.3 (0.3–0.4) | 0.4 (0.3–0.5) | 0.5 (0.4–0.6) | 0.6 (0.5–0.6) |
| PPV, 95% CI | 36 (34–38) | 36 (34–38) | 29 (27–30) | 43 (40–46) | 38 (36–41) |
| NPV, 95% CI | 90 (89–91) | 92 (91–94) | 90 (89–92) | 89 (88–90) | 88 (87–89) |
| Mortality at 90 days | |||||
| MELD | MELD-Na | mDF | GAHS | ABIC | |
| Cut-off value | 25 | 28 | 52 | 8 | 9 |
| Sensitivity, 95% CI | 63 (60–67) | 71 (67–74) | 76 (72–79) | 77 (73–80) | 55 (51–58) |
| Specificity, 95% CI | 73 (71–76) | 71 (69–73) | 54 (52–57) | 60 (57–63) | 77 (75–79) |
| Positive LR, 95% CI | 2.4 (2.2–2.6) | 2.4 (2.2–2.7) | 1.6 (1.5–1.8) | 1.9 (1.8–2.1) | 2.4 (2.2–2.7) |
| Negative LR, 95% CI | 0.5 (0.5–0.6) | 0.4 (0.4–0.5) | 0.4 (0.4–0.5) | 0.4 (0.3–0.4) | 0.6 (0.5–0.6) |
| PPV, 95% CI | 52 (49–54) | 51 (49–54) | 43 (41–45) | 46 (44–48) | 52 (49–54) |
| NPV, 95% CI | 82 (80–83) | 85 (83–86) | 83 (81–85) | 85 (83–87) | 79 (78–81) |
MELD: model for end-stage liver disease, mDF: Maddrey’s discriminant function, GAHS: Glasgow alcoholic hepatitis score, ABIC: age, bilirubin, international normalized ratio and creatinine score, CI: confidence interval, LR: likelihood ratio, PPV: positive predictive value, NPV: negative predictive value
Figure 4 represents the equivalence between the different values of the scores in relation to the probability of death.
Figure 4.
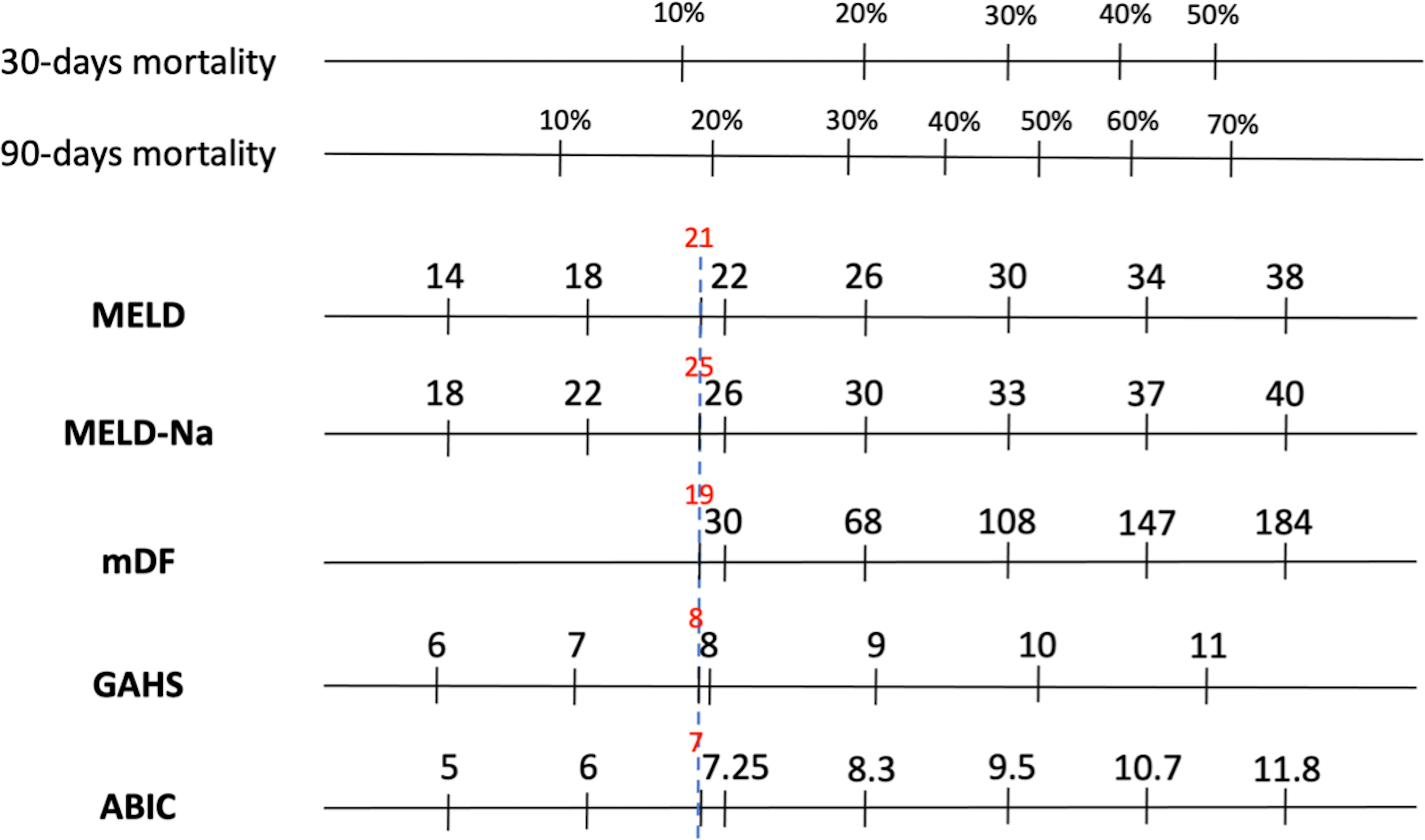
Equivalence between the different prognostic scores according to the probability of death.
MELD: model for end-stage liver disease, mDF: Maddrey’s discriminant function, GAHS: Glasgow alcoholic hepatitis score, ABIC: age, bilirubin, international normalized ratio and creatinine score
Identification of independent predictors of short-term mortality
The variables associated with mortality in the univariate analysis are displayed in Table, Supplemental Digital Content 6.
On multivariate analysis the independent predictors of death were bilirubin (p<0.001), age (p<0.001), leucocytes (p<0.001), international normalized ratio (INR) (p<0.001), creatinine (p<0.001), sodium (p<0.001), and aspartate aminotransferase (AST) (p=0.005 for 28 days and p=0.024 for 90 days) (Table, Supplemental Digital Content 7).
The AUC for 28 and 90 days for age added to the MELD score (Age-MELD score = −6.031+0.033*lnAGE+0.141*lnMELD) compared to MELD alone was 0.761 vs. 0.750, p<0.001, and 0.760 vs. 0.749, p<0.001, respectively. Fifty-five years or over was associated with an increased risk of death (AUC 0.567, p<0.001; sensitivity 34.5%, specificity 77%).
A novel score combining all the independent predictors of mortality [0.445+0.042*ln(bilirubin)+0.041*ln(age)+0.039*ln(leukocytes)+0.460*ln(INR)+0.002*ln(AST)+0.452*ln(Cr)+(−0.041)*ln(Na)] was marginally more accurate than MELD score in predicting 28 mortality (AUC for 28 days 0.795 vs. 0.779, p=0.026), but not 90 day mortality 0.779 vs. 0.775, p=0.570).
Considering socioeconomic factors, IHDI was significantly associated with mortality at 28 (p<0.001) and 90 days (p<0.001) (Figure, Supplemental Digital Content 8); not associated with mortality in a multiple regression analysis considering all independent predictors of mortality analyzed previously (data not shown).
Discussion
Although many studies have assessed the performance of different scoring systems in AH, this is the first global study including patients from 3 continents that addresses this question. Also, this is the first large study that evaluates the performance of MELD-Na in AH, the previous study including only 26 patients 14. Our results showed that the MELD score is the best prognostic score to predict short-term mortality in AH, whereas mDF showed a significantly lower prediction capacity than all previously validated scores. MELD-Na score did not significantly improve the accuracy of the MELD score. Interestingly, age is a relevant independent predictive factor of mortality.
We included well-characterized AH cohorts from different countries in Europe, North America, South America, and Asia. There were no patients from Africa, Oceania, large parts of Asia, and Eastern Europe. In addition to the currently available prognostic scores, different variables that may influence the short-term prognosis in AH were evaluated 19, including those associated with treatment, socioeconomic, cultural, and behavioral factors such as sex and age that may influence the amount of alcohol intake 10, 20.
This study found global interesting demographic differences in patients with AH across different world regions. Almost half of the patients with AH in the USA, UK, and Canada were women, unlike the other countries where AH was more frequent in men. It is believed that globally AH is more common in men 1. In the USA, the reported percentage of hospitalized men with AH is lower than in other countries, but still around 69.7% using inpatient databases from 2010 to 2014 among 56,973 registered patients 21. Similarly, 63% of patients recruited to the STOPAH trial were men 22. This data maybe be explained by an increased alcohol consumption and admissions related to alcohol misuse among young women in these countries 23. Finally, we demonstrate that the reported alcohol intake in patients with AH in countries such as Mexico and Brazil is somewhat greater than in other countries and, furthermore, is associated with higher mortality. This corroborates our previously published data indicating that the quantity of alcohol intake influences survival in Mexican patients with AH 24.
In the current study, MELD was the best score to predict short-term mortality, and mDF had a significantly worse prediction capacity than all the previously validated scores. We also provide evidence that the performance of MELD-Na is not predictively superior to MELD alone in this patient population. Whereas other scoring systems (i.e. ABIC and GAHS), performed similar to MELD but inferior to mDF; this study strongly suggests that mDF should not be used to assess the prognosis in patients with AH. An analysis of the STOPAH trial data by Forrest EH et al. reported a similar conclusion but lacked the global scale of these findings 25. Indeed, mDF is one of the most commonly used scores to determine prognosis and determine need for steroids in clinical trials 26. In recent studies, mDF has shown a lower accuracy for mortality prediction than the others prognostic scores 6, 27, 28. This is probably because mDF does not include renal function, an important prognostic factor in AH 29. Therefore, the MELD score, rather than the mDF score, should be used to identify patient at high risk of mortality in the clinical practice, and should be used to assess prognosis in multicentric international clinical trials.
In addition, the performance of each score was analyzed in individual countries showing that the ABIC score significantly predicted mortality at 28 and 90 days in all countries being the only score that predicted mortality in France. However, the cohort from France was relatively small and had less mortality than the other countries. Therefore, the finding that MELD does not predict mortality in France should be taken cautiously.
We also attempted to improve upon the performance of the MELD score to predict short term survival. First, we assessed the performance of the MELD-Na score, which is widely used in patients with decompensated cirrhosis 30. We provide evidence that the performance of MELD-Na was not superior to MELD alone in this patient population besides its similar AUC.
Moreover, we identified bilirubin, leucocytes, INR, AST, creatinine, sodium, and age as independent predictors of mortality in our series. In several studies age has been shown to be a predictive factor of survival in AH and it is included within the ABIC and GAHS scores calculations 8,9. When we generated a predictor combining MELD and age it resulted in a statistically significant but small improvement in the AUC. No increase of MELD accuracy was observed, neither with the calculation of a global score taking into account all the predictive factors of mortality in our cohort.
Interestingly, we also found that inequality in socioeconomic factors between countries influences AH mortality at 28 and 90 days.
Of note, dynamic factors other than baseline parameters could influence the outcome of patients with AH. Two recent meta-analysis including patients from the STOPAH study, confirmed that prednisolone improves 28-day survival in these patients. However, this effect is not significant at later time points 31,32. Moreover, alcohol relapse negatively impacts medium and long-term survival 33. Finally, a recent report indicates that early referral to an addiction specialist improves short-term survival 34. Therefore, the patients’ outcome is influenced not only by baseline parameters reflecting liver and renal dysfunction but also specific therapy, abstaining from alcohol, and comorbid conditions 35.
The major strength of this study is that a global cohort has been analyzed. However, there are some limitations of this study. First, all included AH were not biopsy-proven. However, current clinical guidelines suggest performing a liver biopsy only when the diagnosis of AH (unclear alcohol use history; atypical laboratory data, or suspicion of another etiology of liver disease) 36. In fact, patients were included in the analysis if they did not meet all restrictive exclusion criteria. Second, data on genetic factors such as PNPLA3 polymorphisms were not available in many of the countries included in the study since PNPLA3 polymorphisms failed to influence mortality in AH 37. In fact, some of the countries with higher mortality (i.e. Mexico) have shown a higher prevalence of the most unfavorable PNPLA3 genotype 38. Third, the utility of these scores in predicting survival beyond 3 months is not clear.
In conclusion, our study demonstrates that in AH, MELD is the best scoring system to globally predict short-term survival and that mDF has the lowest predictive capability of the scores analyzed. Future studies should define optimal MELD cut-offs to determine not only treatment benefit but also when all treatment might be futile and analyze other biomarkers as predictors of severity and mortality in AH.
Supplementary Material
Study highlights
What is Known
The MELD and mDF scores have been assessed to predict mortality in AH in several countries. However, the GAHS and ABIC scores have only been examined in the UK and Spain, respectively. Global validation of these scores is necessary to determine whether regional variables could influence their utility in AH.
Furthermore, the predictive accuracy of the MELD-Na score in AH, previously examined in cirrhosis, and other predictive variables to improve the prediction of existing scores, are unknown.
What is new here
MELD was the best score to predict short-term mortality in AH.
The mDF score had significantly less accurate than all previously validated scores.
MELD-Na did not further improve the accuracy of MELD.
There were no other independent predictors that could be added on to significantly increase the mortality prediction of MELD.
Financial support:
Ramón Bataller is a recipient of NIAAA U01AA021908 and U01AA020821. Dalia Morales Arraez, Meritxell Ventura Cots, Josepmaria Argemi, Carlos Fernández Carillo, and Ana Clemente are recipients of a scholarship grant for study extension abroad, sponsored by the Spanish Association for the Study of the Liver (AEEH). Meritxell Ventura Cots is recipient of the Juan Rodes grant from Instituto de Salud Carlos III. During the conduct of the study AK Singal is funded by the NIAAA 1R21AA023273–01A1 grant. Juan Pablo Arab receives support from the Chilean government through the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1200227) and the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT, AFB170005, CARE Chile UC). Mónica Cruz Lemini is supported by Juan Rodés contract JR19/00047, Instituto de Salud Carlos III - Spanish Ministry of Health”.
Abbreviations:
- AH
alcohol-associated hepatitis
- mDF
Maddrey’s discriminant function
- MELD
model for end-stage liver disease
- ABIC
age, bilirubin, international normalized ratio, and creatinine score
- GAHS
Glasgow alcoholic hepatitis score
- NIAAA
national institute on alcohol abuse and alcoholism
- INR
international normalized ratio
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- GGT
serum γ-glutamyl transpeptidase
- SIRS
systemic inflammatory response syndrome
- GI
gastrointestinal
- AKI
acute kidney injury
- IQR
interquartile range
- ROC
receiver operating characteristics
- AUROC
area under receiver operating characteristics curves
- PPV
positive predictive value
- NPV
negative predictive value
- LR
likelihood ratio
Footnotes
Potential competing interests:
The authors declare that there is no conflict of interest.
References
- 1.Seitz HK, Bataller R, Cortez-Pinto H, et al. Alcoholic liver disease. Nat Rev Dis Primers 2018;4:16. [DOI] [PubMed] [Google Scholar]
- 2.Prado V, Caballería J, Vargas V, et al. Alcoholic hepatitis: How far are we and where are we going? Ann Hepatol 2016;15:463–473. [PubMed] [Google Scholar]
- 3.Maddrey WC, Boitnott JK, Bedine MS, et al. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978;75:193–199. [PubMed] [Google Scholar]
- 4.Asrani SK, Jennings LW, Kim WR, et al. MELD-GRAIL-Na: Glomerular Filtration Rate and Mortality on Liver-Transplant Waiting List. Hepatology 2019. [DOI] [PubMed] [Google Scholar]
- 5.Srikureja W, Kyulo NL, Runyon BA, et al. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol 2005;42:700–706. [DOI] [PubMed] [Google Scholar]
- 6.Louvet A, Labreuche J, Artru F, et al. Combining Data From Liver Disease Scoring Systems Better Predicts Outcomes of Patients With Alcoholic Hepatitis. Gastroenterology 2015;149:398–406.e8; quiz e16–7. [DOI] [PubMed] [Google Scholar]
- 7.Papastergiou V, Tsochatzis EA, Pieri G, et al. Nine scoring models for short-term mortality in alcoholic hepatitis: cross-validation in a biopsy-proven cohort. Aliment Pharmacol Ther 2014;39:721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominguez M, Rincon D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol 2008;103:2747–2756. [DOI] [PubMed] [Google Scholar]
- 9.Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005;54:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ventura-Cots M, Ballester-Ferré MP, Ravi S, et al. Public health policies and alcohol-related liver disease. JHEP Rep 2019;1(5):403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ventura-Cots M, Watts AE, Cruz-Lemini M, et al. Colder Weather and Fewer Sunlight Hours Increase Alcohol Consumption and Alcoholic Cirrhosis Worldwide. Hepatology 2019;69(5):1916–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biggins SW, Kim WR, Terrault NA, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 2006;130:1652–1660. [DOI] [PubMed] [Google Scholar]
- 13.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008;359:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaa BE, Asrani SK, Dunn W, et al. Influence of serum sodium on MELD-based survival prediction in alcoholic hepatitis. Mayo Clin Proc 2011. Jan;86(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009;360:2758–2769. [DOI] [PubMed] [Google Scholar]
- 16.Crabb DW, Bataller R, Chalasani NP, et al. Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology, 150(4), 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut 2015. Apr;64(4):531–7. [DOI] [PubMed] [Google Scholar]
- 18.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44(3):837–845. [PubMed] [Google Scholar]
- 19.Michelena J, Altamirano J, Abraldes JG, et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology 2015;62(3):762–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein E, Cruz-Lemini M, Altamirano J, et al. Heavy daily alcohol intake at the population level predicts the weight of alcohol in cirrhosis burden worldwide. J Hepatol 2016;65(5):998–1005. [DOI] [PubMed] [Google Scholar]
- 21.Doshi SD, Stotts MJ, Hubbard RA, et al. The Changing Burden of Alcoholic Hepatitis: Rising Incidence and Associations with Age, Gender, Race, and Geography. Dig Dis Sci 2020. [DOI] [PubMed] [Google Scholar]
- 22.Thursz MR, Richardson P, Allison M, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;372(17):1619–28. [DOI] [PubMed] [Google Scholar]
- 23.Singal AK, Arora S, Wong RJ, et al. Increasing Burden of Acute-On-Chronic Liver Failure Among Alcohol-Associated Liver Disease in the Young Population in the United States. Am J Gastroenterol 2020;115(1):88–95. [DOI] [PubMed] [Google Scholar]
- 24.Altamirano J, Higuera-de laTijera F, Duarte-Rojo A, et al. The amount of alcohol consumption negatively impacts short-term mortality in Mexican patients with alcoholic hepatitis. Am J Gastroenterol 2011;106(8):1472–80. [DOI] [PubMed] [Google Scholar]
- 25.Forrest EH, Atkinson SR, Richardson P, et al. Application of prognostic scores in the STOPAH trial: Discriminant function is no longer the optimal scoring system in alcoholic hepatitis. J Hepatol 2018;68(3):511–518. [DOI] [PubMed] [Google Scholar]
- 26.Mathurin P, Thursz M. Endpoints and patient stratification in clinical trials for alcoholic hepatitis. J Hepatol 2019;70(2):314–318. [DOI] [PubMed] [Google Scholar]
- 27.Goyal SK, Dixit VK, Jain AK, et al. Assessment of the Model for End-stage Liver Disease (MELD) Score in Predicting Prognosis of Patients with Alcoholic Hepatitis. J Clin Exp Hepatol 2014;4(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadian M, Kakkar R, Dhar M, et al. Model for end-stage liver disease score versus Maddrey discriminant function score in assessing short-term outcome in alcoholic hepatitis. J Gastroenterol Hepatol 2014;29(3):581–8. [DOI] [PubMed] [Google Scholar]
- 29.Altamirano J, Fagundes C, Dominguez M, et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol 2012;10(1):65–71.e3. [DOI] [PubMed] [Google Scholar]
- 30.Leise MD, Kim WR, Kremers WK, et al. A revised model for end-stage liver disease optimizes prediction of mortality among patients awaiting liver transplantation. Gastroenterology 2011;140(7):1952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louvet A, Thursz MR, Kim DJ, et al. Corticosteroids Reduce Risk of Death Within 28 Days for Patients With Severe Alcoholic Hepatitis, Compared With Pentoxifylline or Placebo-a Meta-analysis of Individual Data From Controlled Trials. Gastroenterology 2018;155(2):458–468.e8. [DOI] [PubMed] [Google Scholar]
- 32.Singh S, Murad MH, Chandar AK, Bongiorno CM, Singal AK, Atkinson SR, Thursz MR, Loomba R, Shah VH. Comparative Effectiveness of Pharmacological Interventions for Severe Alcoholic Hepatitis: A Systematic Review and Network Meta-analysis. Gastroenterology 2015. Oct;149(4):958–70.e12. [DOI] [PubMed] [Google Scholar]
- 33.Altamirano J, López-Pelayo H, Michelena J, et al. Alcohol abstinence in patients surviving an episode of alcoholic hepatitis: Prediction and impact on long-term survival. Hepatology 2017;66(6):1842–1853. [DOI] [PubMed] [Google Scholar]
- 34.Peeraphatdit TB, Kamath PS, Karpyak VM, et al. Alcohol Rehabilitation Within 30 Days of Hospital Discharge Is Associated With Reduced Readmission, Relapse, and Death in Patients With Alcoholic Hepatitis. Clin Gastroenterol Hepatol 2020;18(2):477–485.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duseja A, De A, Taneja S, et al. Impact of metabolic risk factors on the severity and outcome of patients with alcohol-associated acute-on-chronic liver failure. Liver Int 2021;41(1):150–157. [DOI] [PubMed] [Google Scholar]
- 36.Bataller R, Cabezas J, Aller R, et al. Alcohol-related liver disease. Clinical practice guidelines. Consensus document sponsored by AEEH. Gastroenterol Hepatol 2019;42(10):657–676. [DOI] [PubMed] [Google Scholar]
- 37.Atkinson SR, Way MJ, McQuillin A, et al. Homozygosity for rs738409:G in PNPLA3 is associated with increased mortality following an episode of severe alcoholic hepatitis. J Hepatol 2017;67(1):120–127. [DOI] [PubMed] [Google Scholar]
- 38.Hernaez R, McLean J, Lazo M, et al. Association between variants in or near PNPLA3, GCKR, and PPP1R3B with ultrasound-defined steatosis based on data from the third National Health and Nutrition Examination Survey. Clin Gastroenterol Hepatol 2013;11(9):1183–1190.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


