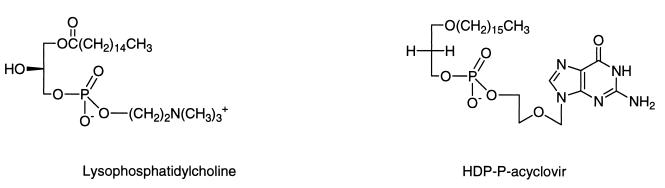Abstract
Acyclovir triphosphate is a potent inhibitor of hepatitis B virus DNA polymerase, but acyclovir treatment provides no benefit in patients with hepatitis B virus infection. This is due in part to the fact that hepatitis B virus, unlike herpes simplex virus, does not code for a viral thymidine kinase which catalyzes the initial phosphorylation of acyclovir. We synthesized 1-O-octadecyl-sn-glycero-3-phospho (3-P)-acyclovir and found that it was highly active in reducing hepatitis B virus replication in 2.2.15 cells, while acyclovir was inactive. The greater antiviral activity of 1-O-octadecyl-sn-glycero-3-P-acyclovir appeared to be due to liver cell metabolism of the compound to acyclovir monophosphate (K. Y. Hostetler et al., Biochem. Pharmacol. 53:1815–1822, 1997). However, a closely related compound without a hydroxyl group at the sn-2 position of glycerol, 1-O-hexadecylpropanediol-3-P-acyclovir, was more active and selective in 2.2.15 cells in vitro. In this study, we treated woodchucks chronically infected with woodchuck hepatitis virus with increasing oral doses of 1-O-hexadecylpropanediol-3-P-acyclovir and assessed the response to therapy versus acyclovir or a placebo. At a dosage of 10 mg/kg of body weight twice a day, the test compound significantly inhibited viral replication in vivo, as indicated by a 95% reduction in serum woodchuck hepatitis virus DNA levels and by a 54% reduction in levels of woodchuck hepatitis virus replicative intermediates in the liver. Higher doses were somewhat less effective. In contrast, 20 mg of acyclovir/kg twice daily, a 5.3-fold-higher molar dosage, had no demonstrable activity against woodchuck hepatitis virus. Oral 1-O-hexadecylpropanediol-3-P-acyclovir appeared to be safe and effective in chronic woodchuck hepatitis virus infection.
Acyclovir [ACV; 9-(2-hydroxyethoxymethyl)guanine] is remarkably effective against herpes simplex virus (HSV) infection (7, 27). ACV is phosphorylated by an HSV-coded thymidine kinase (9) and is subsequently converted to ACV triphosphate by cellular enzymes (22). ACV triphosphate inhibits the DNA polymerase of HSV (6) and is incorporated into viral DNA, causing chain termination because ACV lacks the equivalent of a 3′-hydroxyl group.
ACV triphosphate also inhibits the DNA polymerase of hepatitis B virus (HBV) and the DNA polymerase of woodchuck hepatitis virus (WHV) by 50% at 0.9 and 0.7 μM, respectively (13). Nevertheless, ACV treatment of patients with HBV infection had no additional effect on serum HBeAg levels in patients also treated with alpha interferon (3). ACV given intravenously for 28 days had only a weak effect on viral replication and did not significantly increase the rate of seroconversion to anti-HBe in chronically infected patients (1). Zidovudine (AZT) triphosphate inhibits HBV DNA polymerase by 50% at 0.3 μM (4), but AZT treatment had no effect on serum HBV DNA levels in AIDS patients with concomitant HBV infection (12, 17). Poor phosphorylation of ACV has been noted previously in HepG2 cells (15) and in 2.2.15 cells (29). Thus, the lack of clinical efficacy of AZT and ACV in chronic HBV infection is presumably due to inefficient hepatic phosphorylation of AZT and ACV.
To develop an orally bioavailable form of ACV and other nucleosides which can bypass thymidine kinase phosphorylation, we synthesized several 1-O-alkylglycerol- or 1-O-alkylpropanediol phosphate analogs of ACV and AZT and found them to have substantial antiviral activity in HBV-producing 2.2.15 cells, while AZT and ACV were inactive (15). Metabolic studies with HepG2 cells using radiolabeled compounds indicated that the antiviral activity in 2.2.15 cells was most likely due to a bypass of thymidine kinase. Furthermore, the 1-O-octadecylglycerol-3-phosphate analogs of ACV and AZT were 100% orally bioavailable in mice (15). The low toxicity of ACV in herpes simplex infection is due primarily to the fact that ACV is not phosphorylated to a significant degree in cells which are not infected with HSV. However, there is 30- to 60-fold selectivity of ACV triphosphate for inhibition of HSV DNA polymerase versus human DNA polymerase alpha (16, 25, 26, 30), suggesting that 1-O-hexadecylpropanediol-3-phospho-ACV (HDP-P-ACV) might be reasonably well tolerated in spite of its ability to bypass the initial phosphorylation step.
In this paper, we report the results of oral administration of HDP-P-ACV to woodchucks chronically infected with WHV. The objectives of the studies were to compare the antiviral activity of orally administered HDP-P-ACV with that of ACV and to assess antiviral responses to varying doses of HDP-P-ACV.
MATERIALS AND METHODS
Chemistry.
HDP-P-ACV was synthesized using the phosphotriester approach as described previously (2, 15). The final product was analytically pure by thin-layer chromatography and high-performance liquid chromatography and gave the expected proton nuclear magnetic resonance spectrum. Elemental analysis of the final product (Oneida Research Service, Whitesboro, N.Y.) gave the following results. Calculated for C22H49H5O7PNa · 1.15H2O: C, 51.44; H, 8.20; N, 11.1. Found: C, 51.19; H, 8.15; N, 11.07.
Woodchuck hepatitis studies.
The chronic WHV carrier woodchucks used in these studies were born in laboratory animal facilities at Cornell University and were inoculated at the age of 3 days with WHV (19, 20, 31). Woodchucks selected for use developed acute woodchuck hepatitis surface antigen (WHs) antigenemia and became chronic WHV carriers. The chronic carrier status of all the woodchucks was confirmed prior to initiation of drug treatment.
(i) Experiment 1.
The 16 experimental woodchucks used in the first study were assigned on the basis of age, sex, and serum gamma-glutamyltranspeptidase (GGT) activity to four treatment groups: (i) HDP-P-ACV at 20 mg/kg of body weight twice a day (b.i.d.), (ii) HDP-P-ACV at 10 mg/kg b.i.d., (iii) ACV at 20 mg/kg b.i.d., and (iv) a placebo control. The animals were treated daily for 4 weeks and observed for an additional 12 weeks.
(ii) Experiment 2.
The eight chronic WHV carrier woodchucks used in the second study were similar to those used in study 1 and were assigned on the basis of age, sex, and serum GGT activity to three treatment groups: (i) HDP-P-ACV at 30 mg/kg once a day (q.d.), (ii) HDP-P-ACV at 5 mg/kg b.i.d., and (iii) a placebo control. All other experimental conditions were identical to those in experiment 1.
Each week, sufficient ACV and HDP-P-ACV were weighed to treat the woodchucks of the various groups for 1 week. The drugs were suspended in a semisynthetic liquid diet to ensure their complete consumption. Two separate concentrations of the drug in the diet were made (4 and 8 mg/ml) so that the dosing volume was consistent at 2.5 ml/kg. Volumes of drug sufficient to treat each individual woodchuck were withdrawn from the stock suspensions and administered orally twice daily by dose syringe.
Animals were treated for 4 weeks and were monitored while off treatment for an additional 12 weeks. Blood samples were obtained under general anesthesia (ketamine at 50 mg/kg and xylazine at 5 mg/kg) on the 1st day of treatment and weekly until week 6 (19, 31). Thereafter samples were obtained every 2 weeks until week 16. Body weight was recorded each time the woodchucks were anesthetized and bled. Body weights of drug-treated principals were compared to those of placebo recipient controls to assess possible drug toxicity. Drug dosages for individual woodchucks were adjusted each time the woodchucks were weighed.
Complete blood counts were performed on day 0 and on day 28, the final day of treatment, to evaluate hematological parameters. Biochemical profiles were also performed at day 0 and day 28. Serum GGT, sorbitol dehydrogenase (SDH), aspartate aminotransferase (AST), alanine aminotransferase and alkaline phosphatase (AP) activities, the serum bilirubin concentration, and serum albumin levels were determined in order to assess possible hepatocellular injury and hepatic function. The serum urea nitrogen (BUN) and creatinine levels were determined to assess renal function. Serum Na, K, Cl, and bicarbonate levels were determined to assess electrolyte and acid-base status. Iron status was evaluated by determining total serum iron levels, iron binding capacity, and percent iron saturation (19, 31).
Serum WHV DNA levels were measured during treatment and during the posttreatment follow-up period weekly for 6 weeks, and then every other week until week 16. Other serologic markers of WHV infection, including WHsAg, anti-woodchuck hepatitis virus core antigen (anti-WHc), and anti-WHs, were determined on day 0, after treatment (at 4 weeks), and after 9 weeks off treatment.
Liver biopsy specimens were obtained prior to treatment, at the end of treatment, and at 4 and 12 weeks following drug withdrawal. Biopsies were performed under general anesthesia (ketamine at 50 mg/kg and xylazine at 5 mg/kg) using 16-gauge Bard Biopty-Cut disposable biopsy needles directed by ultrasound imaging (19, 31). These specimens were stored at −70°C until nucleic acid analyses were performed. A second liver sample was fixed in phosphate-buffered formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin for conventional light microscopy, and sectioned and stained for WHsAg and WHc detection using immunohistochemical methods.
The effects of oral ACV and HDP-P-ACV on WHV replication were determined in two ways. First, the serum WHV DNA levels of treated groups were determined, and the concentrations before, during, and following treatment were compared to those of placebo-treated control woodchucks as previously described (19, 31). Second, levels of WHV nucleic acids (WHV DNA, WHV RNA, and WHV DNA monomers) in hepatic biopsy tissue were determined by Southern and Northern blot analysis before treatment, at the end of treatment, and at 4 and 12 weeks posttreatment (19, 31), and the respective values for drug-treated and control woodchucks were compared.
RESULTS
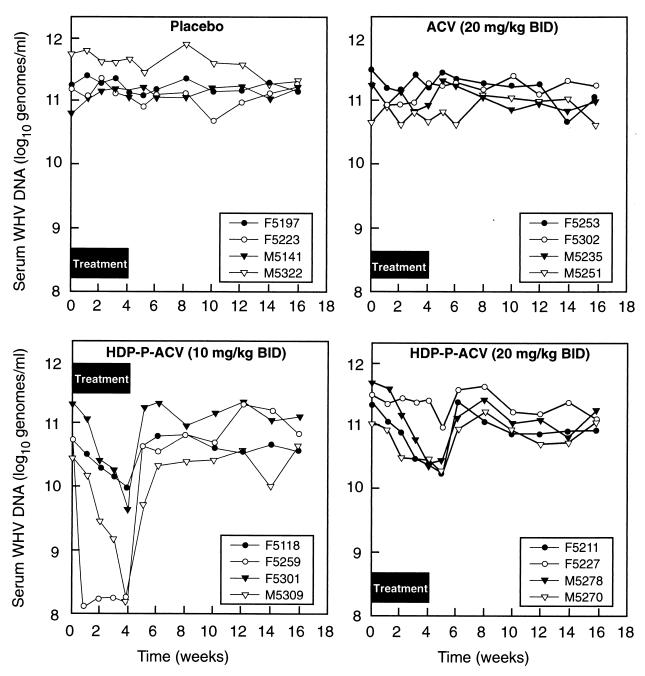
In experiment 1, serum WHV DNA levels did not decline significantly after 4 weeks in woodchucks treated with ACV at 20 mg/kg twice daily (176 μmol/kg/day) or in placebo-treated animals (Fig. 1). However, HDP-P-ACV at a dose of 10 mg/kg twice daily (33.3 μmol/kg/day) had substantial antiviral activity, reducing serum WHV DNA levels by 95% (Fig. 1 and Table 1) and hepatic WHV DNA replicative intermediates (RI) by 50% (Table 2). At 20 mg/kg twice daily, HDP-P-ACV showed somewhat lower antiviral activity, with percentage decreases of 66% at 4 weeks and 84% at the nadir, but the difference between the doses was not statistically significant. Results with both doses of HDP-P-ACV were statistically significant (P < 0.05) versus results with the placebo (Table 1). In the group treated with 10 mg of HDP-P-ACV/kg b.i.d., drug withdrawal after 4 weeks of treatment was associated with a prompt return of serum WHV DNA levels to baseline, but at 20 mg/kg b.i.d., the serum WHV DNA levels continued to decline for a week after the drug was withdrawn before returning to baseline (Fig. 1).
FIG. 1.
Serum WHV DNA levels of woodchucks with chronic WHV infection treated with HDP-P-ACV, ACV, or placebo for 4 weeks, measured during treatment and during posttreatment follow-up (experiment 1).
TABLE 1.
Effects of ACV and HDP-P-ACV on serum WHV DNA levels in WHV infection
| Treatmenta | 109 WHV genomes/ml of serum ± SD at:
|
% Changeb ± SD at:
|
P vs placeboc
|
||||
|---|---|---|---|---|---|---|---|
| Time zero | 4 Wks | Nadir | 4 Wks | Nadir | At 4 wks | Maximum | |
| Expt 1 | |||||||
| Placebo | 234 ± 221 | 212 ± 165 | 166 ± 90 | +14 ± 65 | +2.3 ± 84 | ||
| ACV (20 mg/kg b.i.d.) | 167 ± 96 | 127 ± 69 | 88 ± 40 | −15 ± 33 | −39 ± 22 | NS | NS |
| HDP-P-ACV (10 mg/kg b.i.d.) | 86 ± 77 | 3.7 ± 4.7 | 3.7 ± 4.7 | −95 ± 9 | −95 ± 8.5 | <0.05 | <0.05 |
| HDP-P-ACV (20 mg/kg b.i.d.) | 282 ± 165 | 89 ± 120 | 42 ± 39 | −66 ± 38 | −84 ± 12 | <0.05 | <0.05 |
| Expt 2 | |||||||
| Placebo | 17.0 ± 11.7 | 29.3 ± 19.1 | 29.4 ± 18.8 | +61 ± 43 | +72 ± 28 | ||
| HDP-P-ACV (5 mg/kg b.i.d.) | 34.0 ± 14.5 | 11.3 ± 12.8 | 5.36 ± 4.78 | −72 ± 21 | −86 ± 8.2 | <0.001 | <0.001 |
| HDP-P-ACV (30 mg/kg q.d.) | 47.3 ± 42.0 | 22.0 ± 22.6 | 16.5 ± 20.1 | −58 ± 13 | −72 ± 13 | <0.001 | <0.001 |
The number of animals in each group was four in every instance.
By using each WHV-infected animal as its own control, the percent change from time zero was calculated for each animal at 4 weeks and also for the nadir observed during weeks 1 through 6.
The means ± standard deviations of the percent change at 4 weeks and the maximal percent change (weeks 1 through 6) were calculated and tested for statistical significance versus values for the placebo by using the Student-Newman-Keuls multiple comparisons test (Instat2; Graphpad Software, San Diego, Calif.). NS, not significant.
TABLE 2.
Hepatic WHV replicative intermediates of woodchucks with chronic WHV infection treated with HDP-P-ACVa
| Treatment and time | Hepatic WHV RI (mean pg/μg of cell DNA ± SD)b |
|---|---|
| Expt 1 | |
| Placebo | |
| Time zero | 1,388 ± 485 (4) |
| Wk 4 | 1,420 ± 369 (4) |
| Wk 12 | 1,440 ± 429 (4) |
| ACV (20 mg/kg b.i.d.) | |
| Time zero | 1,488 ± 498 (4) |
| Wk 4 | 1,250 ± 206 (4) |
| Wk 12 | 1,455 ± 497 (4) |
| HDP-P-ACV (10 mg/kg b.i.d.) | |
| Time zero | 1,613 ± 477 (4) |
| Wk 4 | 733 ± 215 (4)* |
| Wk 12 | 1,450 ± 320 (4) |
| HDP-P-ACV (20 mg/kg b.i.d.) | |
| Time zero | 1,775 ± 311 (4) |
| Wk 4 | 1,293 ± 360 (4) |
| Wk 12 | 1,273 ± 329 (4) |
| Expt 2 | |
| Placebo | |
| Time zero | 998 ± 552 (4) |
| Wk 4 | 1,443 ± 412 (4) |
| Wk 12 | 1,175 ± 83 (4) |
| HDP-P-ACV (5 mg/kg b.i.d.) | |
| Time zero | 1,450 ± 320 (4) |
| Wk 4 | 1,208 ± 301 (4) |
| Wk 12 | 1,350 ± 206 (4) |
| HDP-P-ACV (30 mg/kg q.d.) | |
| Time zero | 1,650 ± 320 (4) |
| Wk 4 | 1,525 ± 415 (4) |
| Wk 12 | 1,433 ± 236 (3) |
Woodchucks were treated with HDP-P-ACV for 4 weeks or were left untreated. Measurements were taken at time zero, at the end of treatment, and during posttreatment follow-up.
Number of replicates is given in parentheses. Asterisk, P < 0.05 versus value at time zero.
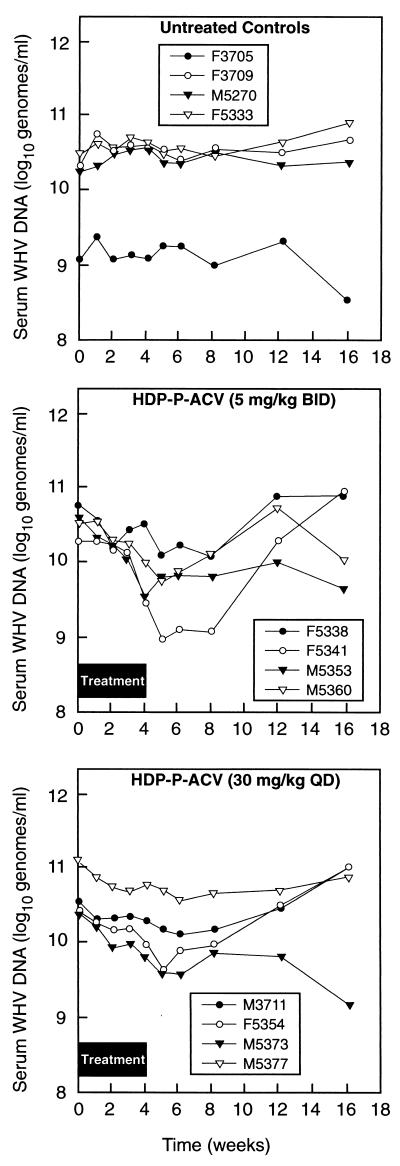
In experiment 2, we examined HDP-P-ACV doses of 5 mg/kg b.i.d. and 30 mg/kg q.d. versus a placebo control (Fig. 2; Table 1). At week 4, serum WHV DNA levels were generally slightly higher in the placebo group but had declined in both the 5-mg/kg b.i.d. and the 30-mg/kg q.d. group, by 72 and 58%, respectively. In experiment 2, serum WHV DNA levels in most HDP-P-ACV-treated animals reached a nadir 1 or 2 weeks after drug withdrawal (Fig. 2). The maximum percent declines in serum WHV DNA levels for animals treated with 5 mg/kg b.i.d. and 30 mg/kg q.d. were 86 and 72%, respectively (Table 1). The percent differences in serum WHV DNA levels after 4 weeks or at the nadir were highly significant versus those for the placebo-treated animals (P < 0.001) (Table 1).
FIG. 2.
Serum WHV DNA levels of woodchucks with chronic WHV infection treated with HDP-P-ACV, ACV, or placebo for 4 weeks, measured during treatment and during posttreatment follow-up (experiment 2).
Significant reductions in mean hepatic WHV DNA RI levels were seen after 4 weeks of treatment at the 10-mg/kg b.i.d. dose level (Table 2). With one exception, no effects of HDP-P-ACV treatment were observed on WHV DNA RI at other dosages (Table 2) or on WHV RNA at any dosage (data not shown). The one exception occurred in hepatic RNA concentrations 12 weeks posttreatment, when the 30-mg/kg q.d. HDP-P-HCV group (56 ± 4 pg of WHV RNA per μg of whole-cell RNA) had a concentration statistically different (P = 0.02) from that of controls (70 ± 5). This is possibly a statistical anomaly, because the woodchuck in the treated group that had consistently had the highest hepatic RNA levels (woodchuck M3711) died prior to the final biopsy and therefore did not contribute data at the +12-week time point.
No physical evidence of toxicity was observed in ACV-treated woodchucks or in HDP-P-ACV-treated woodchucks at any of the dose levels used, and body weights of drug-treated groups were similar to those of controls. No drug- or dose-related changes in hepatic enzymes, in bilirubin, in total serum protein, in serum albumin, or in amylase were apparent in treated groups compared to controls. Similarly, no treatment-related changes were observed in comparison with controls in BUN, creatinine, blood glucose, cholesterol, serum electrolytes, calcium, phosphorus, iron, iron binding capacity, or percent iron saturation. Hematological parameters in all treatment groups were similar to those in controls, including hematocrit, leukocyte count, neutrophil count, and platelet count (data not shown).
No differences between treatment groups were observed histologically in biopsy specimens of the liver obtained prior to treatment. Portal and parenchymal hepatitis in all groups initially was mild to moderate in nature (median scores, 1 to 2 of 4). At the end of 4 weeks of treatment, there were no remarkable differences in the histologic appearance of biopsy specimens from drug-treated and control animals, and no remarkable differences between groups were observed in the biopsy specimens obtained at 4 and 12 weeks posttreatment. Similarly, no differences were observed between treated groups and control woodchucks in the immunohistochemical expression of either WHcAg or WHsAg in hepatic biopsy specimens obtained at the end of the 4-week treatment period or at 4 or 12 weeks posttreatment.
DISCUSSION
In previous experiments with lipid prodrugs in WHV infection, we studied intraperitoneal administration of a liposomal phospholipid analog of 2′,3′-dideoxyguanosine (ddG), 1,2-dipalmitoylphosphatidyl-ddG (DPP-ddG), to achieve liver targeting. Daily intraperitoneal injections of liposomal DPP-ddG at 2.6 mg/kg/day resulted in a decrease in serum WHV DNA levels of 96 to 98%, while an equimolar dose of ddG had little effect (19). While this study clearly demonstrated the utility of liver targeting using a liposomal antiviral agent, parenteral administration of the formulation was required. DPP-ddG and phosphatidyl analogs of other nucleosides are poorly absorbed after oral administration in mice (K. Y. Hostetler, unpublished data, 1999). We next focused on finding a way to develop orally active lipid prodrugs.
To develop an orally bioavailable form of ACV and other poorly absorbed nucleosides which bypass thymidine kinase phosphorylation, we synthesized ACV analogs of lysophosphatidylcholine. Early studies on the absorption of dietary fats using radioisotopically labeled lipids showed that a high percentage of lysophosphatidylcholine, a partially degraded phospholipid, is absorbed intact from the small intestine (23, 28). We prepared a prodrug of lysophosphatidylcholine in which the choline moiety was replaced by ACV (2, 15) or ganciclovir (GCV) (Hostetler et al., submitted), where the sn-1 fatty acid ester was changed to an alkyl ether and the hydroxyl at the sn-2 position of the glycerol backbone was lacking (Fig. 3). The 1-O-alkylglycerol phosphate or alkylpropanediol phosphate analogs of ACV and AZT had substantial antiviral activity in HBV-producing 2.2.15 cells, while AZT and ACV were inactive (15). Metabolic studies with HepG2 cells using radiolabeled compounds suggested that the antiviral activity in 2.2.15 cells was most likely due to direct formation of ACV monophosphate, bypassing the necessity for phosphorylation by a nucleoside kinase. Furthermore, the 1-O-octadecyl-glycerol-3-phosphate analogs of ACV and AZT were 100% orally bioavailable in mice (15), while a GCV analog was 80% orally bioavailable (Hostetler et al., submitted). Although the low toxicity of ACV in herpes simplex infection is due in part to the fact that ACV is not phosphorylated to a significant degree in cells which are not infected with HSV, there is substantial selectivity of ACV triphosphate for inhibition of HSV DNA polymerase, 30- to 60-fold, versus human DNA polymerase alpha (16, 25, 26, 30). This suggests that HDP-P-ACV might be well tolerated despite its ability to bypass the initial phosphorylation by thymidine kinase.
FIG. 3.
Structures of lysophosphatidylcholine and HDP-P-ACV.
WHV and its natural host, the Eastern woodchuck (Marmota monax), represent a useful model of HBV disease, including hepatocellular carcinoma. Previous investigators have used this model system to evaluate potential antiviral therapies (5, 8, 10, 11, 19, 20, 31). The antiviral activity and toxicity profiles of several antiviral agents in chronic WHV infection correlated with their anti-HBV activities in human clinical trials. HDP-P-ACV was well tolerated in WHV-infected woodchucks at oral doses of 5, 10, and 20 mg/kg b.i.d. and 30 mg/kg q.d. for 4 weeks and produced no physical, biochemical, or hematological evidence of toxicity at any dose. At an oral dose of 20 mg/kg b.i.d. (40 mg/kg/day), ACV itself had no demonstrable antiviral effect against WHV, but HDP-P-ACV at 10 mg/kg b.i.d. significantly inhibited WHV replication, as shown by a 95% reduction in serum WHV DNA levels (P < 0.05) and by a 50% reduction in WHV DNA RI levels (P < 0.05). Lower antiviral activity was observed at higher and lower HDP-P-ACV doses. The reason for the lower antiviral activity of the higher doses, 20 mg/kg b.i.d. and 30 mg/kg q.d., is unclear.
Other workers synthesized and evaluated bis(S-acyl-2-thioethyl) phosphotriester analogs of ACV to achieve a bypass of ACV phosphorylation in the liver. Bis(S-acyl-2-thioethyl) analogs of ACV monophosphate were very active in 2.2.15 cells in vitro, with 90% effective concentrations (EC90) of 5.1 to 7.1 μM (24). This degree of activity in 2.2.15 cells compares favorably with that of HDP-P-ACV, which had an EC90 of 3.9 μM, as we reported previously (15). However, although the bis(S-acyl-2-thioethyl)phosphate ACV analogs were active when administered intraperitoneally in ducks with duck HBV infection, the pronucleotides had no statistically significant effect on serum HBV levels versus those of controls when administered orally (14).
In summary, oral HDP-P-ACV was safe and effective in reducing levels of WHV DNA and WHV DNA RI in serum and in the liver in the woodchuck model of HBV infection, while a 5.3-fold-higher molar equivalent dose of ACV had no significant effect. This demonstrates that the antiviral spectrum of ACV can be extended to HBV using this prodrug strategy. Optimal activity was seen at HDP-P-ACV doses of 10 mg/kg twice daily, and higher doses did not provide any further benefit. Additional studies will be required to establish the relationship of HDP-P-ACV dose to antiviral effect in the woodchuck model of HBV infection. This prodrug approach may also be useful for other poorly absorbed nucleosides such as penciclovir (PCV) and GCV. Studies with HDP-P-GCV show 80% oral bioavailability (Hostetler et al., submitted), and preliminary studies with HDP-P-GCV and HDP-P-PCV show good antiviral activity in 2.2.15 cells which constitutively produce HBV (18).
ACKNOWLEDGMENTS
This work was supported in part by NIH grants AI-41928, AI-29614, and EY11832, by the San Diego Veterans Affairs Medical Center Research Center for AIDS and HIV Infections (K.Y.H.) and by NIH grants N01-AI-35164 (B.C.T.) and N01-AI-45179 (J.L.G.).
REFERENCES
- 1.Alexander G J M, Fagan E A, Hegarty J E, Rolando N, Guarner P, Eddleston A L W F, Williams R. A controlled trial of acyclovir in stable chronic HBsAg, HBeAg-positive carriers. J Hepatol. 1986;3(Suppl. 2):S123–S127. doi: 10.1016/s0168-8278(86)80110-6. [DOI] [PubMed] [Google Scholar]
- 2.Beadle, J. R., G. D. Kini, K. A. Aldern, M. F. Gardner, K. N. Wright, R. J. Ryback, and E. R. Kern. Synthesis and antiviral evaluation of 1-O-hexadecylpropanediol-3-P-acyclovir: efficacy against HSV-1 infection in mice. Nucleosides Nucleotides 19:471–479. [DOI] [PubMed]
- 3.Berk L, Schalm S W, de Man R A, Heytink R A, Berthelot P, Brechot C, Boboc B, Degos F, Marcellin P, Benhamou J P, Hess G, Rossol S, zum Büschenfelde K-H M, Chamuleau A F M, Jansen P L M, Reesink H W, Meyer B, Beglinger C, Stalder G A, den Ouden-Muller J W, deJong M J. Failure of acyclovir to enhance the antiviral effect of α-lymphoblastoid interferon on HBe-seroconversion in chronic hepatitis B. A multi-centre randomized controlled trial. J Hepatol. 1992;14:305–309. doi: 10.1016/0168-8278(92)90175-o. [DOI] [PubMed] [Google Scholar]
- 4.Berk L, Schalm S W, Heijtink R A. Zidovudine inhibits hepatitis B virus replication. Antivir Res. 1992;19:111–118. doi: 10.1016/0166-3542(92)90070-l. [DOI] [PubMed] [Google Scholar]
- 5.Cullen J M, Smith S L, Davis M G, Dunn S E, Botteron C, Cecchi A, Linsey D, Linzey D, Frick L, Paff M T, Goulding A, Biron K. In vivo antiviral activity and pharmacokinetics of (−)-cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine in woodchuck hepatitis virus-infected woodchucks. Antimicrob Agents Chemother. 1997;41:2076–2082. doi: 10.1128/aac.41.10.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derse D, Cheng Y-C, Furman P A, St. Clair M H, Elion G B. Inhibition of purified human and herpes simplex virus-induced DNA polymerases by 9-(2-hydroxyethoxymethyl)guanine triphosphate. Effects on primer-template function. J Biol Chem. 1981;256:11447–11451. [PubMed] [Google Scholar]
- 7.Elion G B, Furman P A, Fyfe J A, de Miranda P, Beauchamp L, Schaeffer H J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl)guanine. Proc Natl Acad Sci USA. 1977;74:5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fourel I, Hantz O, Watanabe K A, Jacquet C, Chomel B, Fox J J, Trepo C. Inhibitory effects of 2′-fluorinated arabinosyl-pyrimidine nucleosides on woodchuck hepatitis virus replication in chronically infected woodchucks. Antimicrob Agents Chemother. 1990;34:473–475. doi: 10.1128/aac.34.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fyfe J A, Keller P M, Furman P A, Miller R L, Elion G B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978;253:8721–8727. [PubMed] [Google Scholar]
- 10.Genovesi E V, Lamb L, Medina I, Taylor D, Seifer M, Innalmo S, Colonno R J, Standring D N, Clark J M. Efficacy of the carbocyclic 2′-deoxyguanosine nucleoside BMS-200475 in the woodchuck model of hepatitis B virus infection. Antimicrob Agents Chemother. 1998;42:3209–3217. doi: 10.1128/aac.42.12.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerin J L. Experimental WHV infection of woodchucks: an animal model of hepadnavirus-induced liver cancer. Gastroenterol Jpn. 1990;25(Suppl.):38–42. doi: 10.1007/BF02779926. [DOI] [PubMed] [Google Scholar]
- 12.Gilson R J, Hawkins A E, Kelly G K, Gill S K, Weller I V. No effect of zidovudine on hepatitis B virus replication in homosexual men with symptomatic HIV-1 infection. AIDS. 1991;5:217–220. doi: 10.1097/00002030-199102000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Hantz O, Allaudeen H S, Ooka T, De Clercq E, Trepo C. Inhibition of human and woodchuck hepatitis virus DNA polymerase by the triphosphates of acyclovir, 1-(2′-deoxy-2′-fluoro-β-d-arabinofuranosyl)-5-iodocytosine and E-5-(2-bromovinyl)-2′-deoxyuridine. Antivir Res. 1984;4:187–199. doi: 10.1016/0166-3542(84)90017-2. [DOI] [PubMed] [Google Scholar]
- 14.Hantz O, Perigaud C, Borel C, Jamard C, Zoulim F, Trepo C, Imbach J-L, Gosselin G. The SATE pronucleotide approach applied to acyclovir. Part II. Effects of bis(SATE)phosphotriester derivatives of acyclovir on duck hepatitis B virus replication in vitro and in vivo. Antivir Res. 1999;40:179–187. doi: 10.1016/s0166-3542(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 15.Hostetler K Y, Beadle J R, Kini G D, Gardner M F, Wright K N, Wu T-H, Korba B A. Enhanced oral absorption and antiviral activity of 1-O-octadecyl-sn-glycero-3-phospho-acyclovir in hepatitis B virus infection, in vitro. Biochem Pharmacol. 1997;53:1815–1822. doi: 10.1016/s0006-2952(97)82446-x. [DOI] [PubMed] [Google Scholar]
- 16.Ilsley D D, Lee S-H, Miller W H, Kuchta R D. Acyclic guanosine analogs inhibit DNA polymerases α, δ, ɛ with very different potencies and have unique mechanisms of action. Biochemistry. 1995;34:2504–2510. doi: 10.1021/bi00008a014. [DOI] [PubMed] [Google Scholar]
- 17.Janssen H L, Berk L, Heijtink R A, ten Kate F J, Schalm S W. Interferon-α and zidovudine combination therapy for chronic hepatitis B: results of a randomized, placebo-controlled trial. Hepatology. 1993;17:383–388. [PubMed] [Google Scholar]
- 18.Kini G D, Beadle J R, Aldern K A, Richman D D, Korba B E, Hostetler K Y. Anti-hepatitis B virus activity of 3-hexadecyloxypropane-1-phospho-penciclovir and 3-hexadecyloxypropane-1-phospho-dideoxyguanosine in 2.2.15 cells. Antivir Res. 1998;37:A65. . (Abstract 92.) [Google Scholar]
- 19.Korba B A, Xie H, Wright K N, Hornbuckle W E, Gerin J L, Tennant B C, Hostetler K Y. Liver-targeted antiviral nucleosides: enhanced antiviral activity of phosphatidyl-dideoxyguanosine versus dideoxyguanosine in woodchuck hepatitis virus infection in vivo. Hepatology. 1996;23:958–963. doi: 10.1002/hep.510230503. [DOI] [PubMed] [Google Scholar]
- 20.Korba B E, Cote P J, Hornbuckle W E, Tennant B C, Gerin J L. Treatment of chronic WHV infection in the Eastern woodchuck (M. monax) with nucleoside analogs as a model for therapy of chronic hepatitis virus B infection in man. Hepatology. 2000;31:1165–1175. doi: 10.1053/he.2000.5982. [DOI] [PubMed] [Google Scholar]
- 21.Mason W S, Cullen J, Moraleda G, Saputelli J, Aldrich C E, Miller D S, Tennant B, Firck L, Averett D, Condreay L D, Jilbert A R. Lamuvidine therapy of WHV-infected woodchucks. Virology. 1998;245:18–32. doi: 10.1006/viro.1998.9150. [DOI] [PubMed] [Google Scholar]
- 22.Miller W H, Miller R L. Phosphorylation of acyclovir diphosphate by cellular enzymes. Biochem Pharmacol. 1982;31:3879–3884. doi: 10.1016/0006-2952(82)90305-7. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson A, Borgstrom B. Absorption and metabolism of lecithin and lysolecithin by intestinal slices. Biochim Biophys Acta. 1967;137:240–254. doi: 10.1016/0005-2760(67)90100-2. [DOI] [PubMed] [Google Scholar]
- 24.Perigaud C, Gosselin G, Girardet J-L, Korba B E, Imbach J-L. The S-acyl-2-thioethyl pronucleotide approach applied to acyclovir. Part I. Synthesis and in vitro anti-hepatitis B virus activity of bis(S-acyl-2-thioethyl)phosphotriester derivatives of acyclovir. Antivir Res. 1999;40:167–178. doi: 10.1016/s0166-3542(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 25.Reardon J E. Herpes simplex virus type 1 and human DNA polymerase interactions with 2′-deoxyguanosine 5′-triphosphate analogues. Kinetics of incorporation into DNA and induction of inhibition. J Biol Chem. 1989;264:19039–19044. [PubMed] [Google Scholar]
- 26.Reardon J E, Spector T. Herpes simplex virus type 1 DNA polymerase. Mechanism of inhibition by acyclovir triphosphate. J Biol Chem. 1989;264:7405–7411. [PubMed] [Google Scholar]
- 27.Schaeffer H J, Beauchamp L, de Miranda P, Elion G B, Bauer D J, Collins P. 9-(2-Hydroxyethoxymethyl)guanine activity against viruses of the herpes group. Nature. 1978;272:583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- 28.Scow R O, Stein Y, Stein O. Incorporation of dietary lecithin and lysolecithin into lymph chylomicrons in the rat. J Biol Chem. 1967;242:4919–4924. [PubMed] [Google Scholar]
- 29.Shaw T, Mok S S, Locarnini S A. Inhibition of hepatitis B virus DNA polymerase by enantiomers of penciclovir triphosphate and metabolic basis selective inhibition of HBV replication by penciclovir. Hepatology. 1996;24:996–1002. doi: 10.1002/hep.510240504. [DOI] [PubMed] [Google Scholar]
- 30.St. Clair M H, Miller W H, Miller R L, Lambe C U, Furman P A. Inhibition of cellular α-DNA polymerase and herpes simplex virus-induced DNA polymerases by the triphosphate of BW759U. Antimicrob Agents Chemother. 1984;25:191–194. doi: 10.1128/aac.25.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tennant B C, Baldwin B H, Graham L A, Ascenzi M A, Hornbuckle W E, Rowland P H, Tochkov I A, Yeager A E, Erb H N, Colacio J M, Lopez C, Engelhardt J A, Bowsher R R, Richardson F C, Lewis W, Cote P J, Korba B E, Gerin J L. Antiviral activity and toxicity of fialuridine in the woodchuck model of hepatitis B virus infection. Hepatology. 1998;28:179–191. doi: 10.1002/hep.510280124. [DOI] [PubMed] [Google Scholar]