Abstract
A new CTX-M-type β-lactamase (CTX-M-9) has been cloned from a clinical cefotaxime-resistant Escherichia coli strain. Despite the close identity that exists between the CTX-M-9 and Toho-2 β-lactamases (88%), the 35 amino acids located between residues Ala-185 and Ala-219 are totally different in both enzymes. Outside of this region there are only six amino acids substitutions between both proteins.
Not long after the beginning of the use of the extended-spectrum β-lactam antibiotics, extended-spectrum β-lactamases (ESBLs) were detected in Europe and the United States and have now become a serious problem around the world. ESBLs are most often derivatives of TEM or SHV enzymes. However, there is a small growing family of plasmid-mediated ESBLs of Ambler class A that are not closely related to TEM or SHV β-lactamases but that show homology to chromosomal β-lactamases of Klebsiella oxytoca, including CTX-M-1 (MEN-1) (3, 5–7), CTX-M-2 (4, 6), CTX-M-3 (14), CTX-M-4 (12, 13), CTX-M-6 (11, 20), Toho-1 (15), Toho-2 (17), and two different enzymes, both designated CTX-M-5, which will be referred to here as CTX-M-5 (8) and CTX-M-7 (11, 20). In this report we present a new β-lactamase closely related to the β-lactamases in this family.
In 1996, a cefotaxime-resistant Escherichia coli strain (785-D) against which synergy of cefotaxime with clavulanic acid was found was detected by a conventional disk diffusion susceptibility test. The strain was isolated from the urine of a 65-year-old woman who had a urinary tract infection and diabetes mellitus type 2. Two months before the isolation, the patient underwent a nephroureterectomy because of a renal carcinoma.
The MICs of the β-lactam antibiotics were determined by the Etest (Biodisk, Solna, Sweden). The β-lactamase crude cell extracts were prepared from 250-ml cultures in Luria-Bertani (LB) broth (Oxoid, Basingstoke, United Kingdom). Washed, centrifuged cell pellets were subjected to three cycles of 15-s sonication treatments at 4°C, and the supernatants of the sonic extracts were frozen at −20°C until they were tested. β-Lactamases were characterized initially by isoelectric focusing as described previously (2) in polyacrylamide gels with a pH gradient from 4 to 11 (SERVALYT 4-9 T, 9-11 T; Serva, Heidelberg, Germany). Enzyme activities in the gel were detected by the iodometric method (2).
Substrate hydrolysis in sonic extracts was monitored spectrophotometrically with a Biochrom 4060 spectrophotometer (Pharmacia, Uppsala, Sweden) as described previously (19).
Conjugation studies were performed in a solid medium as described previously (19) by using E. coli 785-D (susceptible to kanamycin) and E. coli HB101 (Nalr Kanr) as the donor and the recipient, respectively. Kanamycin (50 μg/ml) and cefotaxime (4 μg/ml) were used for transconjugant selection. One of the transconjugants (E. coli MSP492) was used for further experiments.
Extraction of plasmid DNA was by the alkaline lysis procedure reported previously (18). Cloning of the cefotaxime resistance gene was as follows: plasmid DNA from E. coli MSP492 was partially digested with Sau3AI, and the resultant fragments (size range, 2 to 4 kb) were ligated into the BamHI site of the pK184 vector (16). Afterward, E. coli DH5α was transformed with the ligation mixture, and cefotaxime-resistant colonies were selected on LB agar plates supplemented with 4 μg of cefotaxime per ml. One of these E. coli DH5α transformants (MSP493) that contained plasmid pK184, which carries a 2-kb Sau3AI fragment (pMSP072), was selected for further studies. DNA sequencing was carried out by the dideoxy method (9) with fluorescent primers and the Automatic Laser Fluorescent DNA Sequencer (ALF; Pharmacia). The entire nucleotide sequence was determined on both DNA strands.
Isoelectric focusing analysis showed that the wild-type E. coli strain (strain 785-D) expressed two β-lactamases with pIs of 5.4 and about 8.0, respectively. Moreover, E. coli MSP492 and E. coli MSP493 strains expressed only a β-lactamase with a pI of about 8.0.
The β-lactam susceptibility profiles of the parental E. coli strain (strain 785-D), the two reference strains (E. coli DH5α and E. coli HB101), and strains E. coli MSP492 and E. coli MSP493 are presented in Table 1. All strains that contained the β-lactamase with a pI of about 8.0 (strains 785-D, MSP492, and MSP493) have similar susceptibility patterns. The level of resistance to β-lactams of all of the strains was similar to that observed for other strains of the family Enterobacteriaceae that produce CTX-M-type β-lactamases (3–8, 11–15, 17, 20, 21). Thus, these three strains were resistant to all penicillins and cephalosporins tested except cefoxitin, ceftazidime, and aztreonam (Table 1). Furthermore, the activities of penicillins, cefotaxime, and ceftazidime were restored in the presence of inhibitors.
TABLE 1.
MICs of β-lactams for reference and CTX-M-9-producing strains
| Antibiotic(s) | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| E. coli 785-Da | E. coli MSP492b | E. coli MSP493c | E. coli HB101d | E. coli DH5αd | |
| Ampicillin | >256 | >256 | >256 | 1.5 | 1.5 |
| Ampicillin-Sule | 16 | 16 | 16 | 1.5 | 1.5 |
| Amoxicillin-Claf | 4 | 3 | 4 | 1 | 1 |
| Ticarcillin | >256 | >256 | >256 | 1.5 | 1 |
| Piperacillin | 256 | >256 | >256 | 0.5 | 0.38 |
| Piperacillin-Tazg | 3 | 0.5 | 0.5 | 0.75 | 0.38 |
| Cefoxitin | 12 | 1.5 | 2 | 1.5 | 1.5 |
| Cefotaxime | 24 | >32 | >32 | 0.032 | 0.032 |
| Cefotaxime-Claf | 0.19 | 0.032 | 0.047 | ≤0.016 | ≤0.016 |
| Ceftazidime | 1 | 1.5 | 2 | 0.047 | 0.047 |
| Ceftazidime-Claf | 0.38 | ≤0.064 | ≤0.064 | ≤0.064 | ≤0.064 |
| Aztreonam | 2 | 6 | 4 | ≤0.016 | ≤0.016 |
Clinical isolate E. coli 785-D produces CTX-M-9 along with the TEM-1 penicillinase.
E. coli transconjugant (MSP492).
E. coli DH5α harboring recombinant plasmid pMSP072.
Reference strains.
Sul, sulbactam; the ratio of the penicillin/inhibitor was 2:1.
Cla, clavulanic acid; the ratio of the penicillin/inhibitor was 2:1.
Taz, tazobactam at a fixed concentration of 4 μg/ml.
Kinetic parameters for the β-lactamase enzyme with a pI of about 8.0 obtained from the transconjugant strain (strain MSP492) are presented in Table 2. The antibiotic against which it had the greatest activity (relative Vmax), cefotaxime, as well as the inhibitory activities (50% inhibitory concentrations [IC50s]) of β-lactamase inhibitors are similar to those described for the CTX-M-type β-lactamases (3–8, 11–15, 17, 20, 21). Likewise, this enzyme was inhibited by low concentrations of tazobactam (IC50, 1 nM), while inhibition by clavulanate and sulbactam required higher concentrations (IC50s, 10 and 700 nM, respectively).
TABLE 2.
Kinetic parameters of CTX-M-9 β-lactamase
| Substrate or inhibitor | Vmaxa | IC50 (nM)b |
|---|---|---|
| Substrates | ||
| Cephaloridine | 100 | |
| Penicillin | 48 | |
| Cefotaxime | 16 | |
| Ceftazidime | NCc | |
| Aztreonam | 0.1 | |
| Inhibitors | ||
| Clavulanic acid | 10 | |
| Sulbactam | 700 | |
| Tazobactam | 1 |
Relative to that for cephaloridine (which was taken as 100%).
IC50, concentration of β-lactamase inhibitor that inhibits 50% of the activity after 5 min of incubation at 25°C; cephaloridine (0.1 mM) was used as the substrate.
NC, not calculated (rates were to low to obtain reliable values).
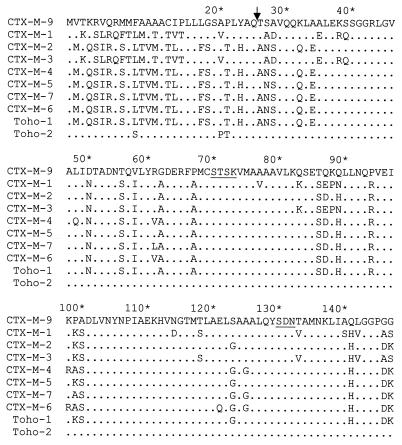
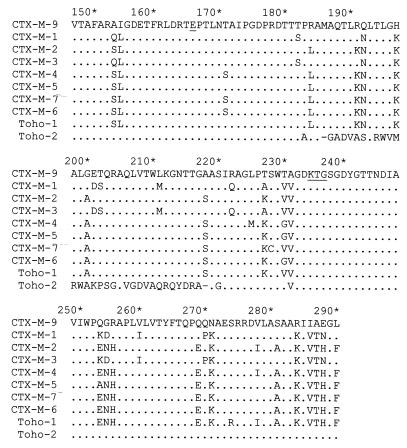
After confirming by isoelectric focusing that E. coli cells that carry the pMSP072 plasmid harbor the same β-lactamase harbored by the E. coli 785-D and MSP492 strains, the nucleotide sequence of the 2-kb fragment cloned in this plasmid was determined. Analysis of this sequence shows an 873-nucleotide open reading frame that presents a close homology with those of CTX-M-type enzymes. The alignment of the deduced 291-amino-acid sequence of this open reading frame with other β-lactamases is shown in Fig. 1. On the basis of its similarity with previously characterized CTX-M-type β-lactamases, the most likely signal peptide would consist of 29 amino acids residues (11, 12), whereas the mature β-lactamase could comprise 262 amino acids. The consensus sequences 70SXXK73, 130SDN132, E166, and 234KTG236 (numbering of Ambler et al. [1]) typical of class A serine β-lactamases were also found in the amino acid sequence of this new β-lactamase (Fig. 1). The degree of amino acid sequence homology of the mature protein with other plasmid-mediated CTX-M-type β-lactamases was higher than 80% (Table 3). In agreement with all of these data, we have designated this β-lactamase CTX-M-9. However, the N- and C-terminal sequences of the CTX-M-9 protein are dramatically different from the sequences of the same regions of other CTX-M-type β-lactamases except Toho-2 (Fig. 1).
FIG. 1.
Alignment of amino acid sequences of the CTX-M-9 β-lactamase with other known cefotaxime-hydrolyzing β-lactamases. Dots indicate identical amino acids; underlining indicates the 70SXXK73, 130SDN132, E166, and 234KTG236 conserved sequences that are typical of class A serine β-lactamases. The arrow indicates the deduced cleavage site of the signal peptide. Amino acid numbering is according to the scheme outlined by Ambler et al. (1). Enzymes CTX-M-1 (3, 5–7), CTX-M-2 (4, 6), CTX-M-3 (14), CTX-M-4 (12, 13), CTX-M-5 (8), CTX-M-7 (11, 20), CTX-M-6 (11, 20), Toho-1 (15), and Toho-2 (17) have been described previously.
TABLE 3.
Homologies of amino acid sequences of various mature class A β-lactamases
| β-Lactamase | % Homology with:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CTX-M-9 | CTX-M-6 | CTX-M-7 | CTX-M-5 | CTX-M-4 | CTX-M-3 | CTX-M-2 | CTX-M-1 | Toho-1 | |
| CTX-M-6 | 82 | ||||||||
| CTX-M-7 | 82 | 98 | |||||||
| CTX-M-5 | 84 | 96 | 96 | ||||||
| CTX-M-4 | 81 | 98 | 97 | 96 | |||||
| CTX-M-3 | 82 | 82 | 83 | 85 | 82 | ||||
| CTX-M-2 | 83 | 96 | 97 | 98 | 96 | 85 | |||
| CTX-M-1 | 81 | 81 | 82 | 84 | 80 | 98 | 83 | ||
| Toho-1 | 83 | 96 | 97 | 98 | 96 | 84 | 99 | 83 | |
| Toho-2 | 88 | 71 | 71 | 73 | 70 | 74 | 72 | 73 | 72 |
The CTX-M-9 mature protein presents an 88% sequence homology with Toho-2, although the 35 amino acids located between Ala-185 and Ala-219 are completely different (Fig. 1). Compared with the other CTX-M enzymes, there are only four or five amino acid substitutions within this 35-amino-acid region. This difference between Toho-2 and the CTX-M-type enzymes, including CTX-M-9, is due to three sets of nucleotide deletions in Toho-2 at positions 564 and 565 (GA), 585 and 586 (GC), and 622 (G) with respect to the CTX-M-9 translational starting point, which change the reading frame. However, a fourth deletion (a G at position 659) recovers the initial reading frame. Outside of this region there are only six amino acid substitutions between CTX-M-9 and Toho-2 proteins. Moreover, all characteristic substitutions assumed to be implicated in cephalosporin hydrolysis (Cys-69, Ala-205, Ser-237, Thr-244, and Arg-276) (11, 12, 17) in the CTX-M-type β-lactamases are also present in CTX-M-9.
Regardless of the comparison of the amino acid sequence that suggests that CTX-M-9 and the other CTX-M-type β-lactamases are closely related, the amino acid sequence differences between them (about 15%) are too many for CTX-M-9 to be considered a direct descendant of any of the others. Otherwise, the analysis of amino acid sequences of several β-lactamases suggests a close relationship of plasmid-mediated CTX-M-type enzymes with the chromosomal β-lactamases of Klebsiella oxytoca, Citrobacter diversus, and Proteus vulgaris (6, 10), but there is no evident phylogenetic connection between the CTX-M-type β-lactamases and other bla genes.
From 1996 to 1998, 23 strains (22 E. coli strains and 1 Salmonella enterica serotype Virchow strain) that presented the same resistance phenotype and that carried a β-lactamase with a pI of about 8.0 have been sporadically detected in our laboratory. All of these isolated strains were PCR positive when two primers selected from the blaCTX-M-9 nucleotide sequence (5′-GTG ACA AAG AGA GTG CAA CGG-3′ and 5′-ATG ATT CTC GCC GCT GAA GCC-3′, which comprise positions 4 to 24 and 860 to 840, respectively, with respect to its translational starting point) were used (data not shown). These results suggest that the CTX-M-9 β-lactamase is synthesized in all of them.
CTX-M-type β-lactamase-producing strains have been incidentally identified for 10 years as single or epidemic clinical isolates in very distant geographic regions: CTX-M-1 in Germany in 1989 (5), MEN-1 in France from a patient from Italy in 1989 (3, 7), CTX-M-2 in Argentina in 1990 (4), CTX-M-3 in Poland in 1996 (14), CTX-M-4 in Russia in 1996 (12, 13), CTX-M-5 in Latvia in 1991 (8), CTX-M-7 in Greece in 1996 (11, 20), CTX-M-6 in Greece in 1997 (11, 20), and Toho-1 in 1993 and Toho-2 in 1995 in Japan (15, 17). In addition to these initial findings, the presence of the CTX-M-type β-lactamases has been described in various species of the family Enterobacteriaceae in widely distant geographic areas, with all them being plasmid encoded. The present work is the first report of a CTX-M-type β-lactamase in Spain. In our laboratory CTX-M-9 is, although rare, the most frequent ESBL detected. The divergence of the amino acid sequences as well as the temporal and geographic dispersion of strains that carry this β-lactamase makes any assumption about the origin of this plasmid-mediated β-lactamase difficult. These facts raise important questions about whether such enzymes arise de novo in multiple geographic locations or, alternatively, are disseminated around the world.
Nucleotide sequence accession number.
The accession number of the nucleotide sequence of the blaCTX-M-9 gene in the GenBank database is AF174129.
Acknowledgments
We are grateful to B. Mirelis for helpful advice and comments. We thank the “Fundación Ma Francisca de Roviralta” for financial support. We are deeply indebted to Joan Ruiz and M. Mar López for excellent technical assistance.
This work was supported partially by grants FIS 97/0623 and FIS 98/1522 to G.P.; J.B. was supported by grants PB97-0194 and BIO99-0779 from the Ministerio de Educación y Ciencia of Spain and 1999SGR-00106 from the Comissionat per Universitats i Recerca de la Generalitat de Catalunya.
REFERENCES
- 1.Ambler R P, Coulson A F W, Frere J M, Ghuysen J M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barthélémy M, Guionie M, Labia R. β-Lactamases: determination of their isoelectric points. Antimicrob Agents Chemother. 1979;13:695–698. doi: 10.1128/aac.13.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthélémy M, Peduzzi J, Bernard H, Tancrède C, Labia R. Close amino acid sequence relationship between the new plasmid-mediated extended-spectrum β-lactamase MEN-1 and chromosomally encoded enzymes of Klebsiella oxytoca. Biochim Biophys Acta. 1992;1122:15–22. doi: 10.1016/0167-4838(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 4.Bauernfeind A, Casellas J M, Goldberg M, Holley M, Jungwirth R, Mangold P, Röhnisch T, Schweighart S, Wilhelm R. A new plasmidic cefotaximase from patients infected with Salmonella typhimurium. Infection. 1992;20:158–163. doi: 10.1007/BF01704610. [DOI] [PubMed] [Google Scholar]
- 5.Bauernfeind A, Grimm H, Schweighart S. A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection. 1990;18:294–298. doi: 10.1007/BF01647010. [DOI] [PubMed] [Google Scholar]
- 6.Bauernfeind A, Stemplinger I, Jungwirth R, Ernst S, Casellas J M. Sequence of β-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other β-lactamases. Antimicrob Agents Chemother. 1996;40:509–513. doi: 10.1128/aac.40.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernard H, Tancrede C, Livrelli V, Morand A, Barthélémy M, Labia R. A novel plasmid-mediated extended-spectrum β-lactamase not derived from TEM- or SHV-type enzymes. J Antimicrob Chemother. 1992;29:590–592. doi: 10.1093/jac/29.5.590. [DOI] [PubMed] [Google Scholar]
- 8.Bradford P A, Yang Y, Sahm D, Grope I, Gardovska D, Storch G. CTX-M-5, a novel cefotaxime-hydrolyzing β-lactamase from an outbreak of Salmonella typhimurium in Latvia. Antimicrob Agents Chemother. 1998;42:1980–1984. doi: 10.1128/aac.42.8.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez de Henestrosa A R, Rivera E, Tapias A, Barbé J. Identification of the Rhodobacter sphaeroides SOS box. Mol Microbiol. 1998;28:991–1003. doi: 10.1046/j.1365-2958.1998.00860.x. [DOI] [PubMed] [Google Scholar]
- 10.Fournier B, Roy P H, Lagrange P H, Philippon A. Chromosomal β-lactamase genes of Klebsiella oxytoca are divided into two main groups, blaOXY-1 and blaOXY-2. Antimicrob Agents Chemother. 1996;40:454–459. doi: 10.1128/aac.40.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gazouli M, Tzelepi E, Markogiannakis A, Legakis N J, Tzouvelekis L S. Two novel plasmid-mediated cefotaxime-hydrolyzing β-lactamases (CTX-M-5 and CTX-M-6) from Salmonella typhimurium. FEMS Microbiol Lett. 1998;165:289–293. doi: 10.1111/j.1574-6968.1998.tb13159.x. [DOI] [PubMed] [Google Scholar]
- 12.Gazouli M, Tzelepi E, Sidorenko S V, Tzouvelekis L S. Sequence of the gene encoding a plasmid-mediated cefotaxime-hydrolyzing class A β-lactamase (CTX-M-4): involvement of serine 237 in cephalosporin hydrolysis. Antimicrob Agents Chemother. 1998;42:1259–1262. doi: 10.1128/aac.42.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazouli M, Sidorenko S V, Tzelepi E, Kozlova N S, Gladin D P, Tzouvelekis L S. A plasmid-mediated β-lactamase conferring resistance to cefotaxime in a Salmonella typhimurium clone found in St Petersburg, Russia. J Antimicrob Chemother. 1998;41:119–121. doi: 10.1093/jac/41.1.119. [DOI] [PubMed] [Google Scholar]
- 14.Gniadkowski M, Schneider I, Palucha A, Jungwirth R, Mikiewicz B, Bauernfeind A. Cefotaxime-resistant Enterobacteriaceae isolates from a hospital in Warsaw, Poland: identification of a new CTX-M-3 cefotaxime-hydrolyzing β-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob Agents Chemother. 1998;42:827–832. doi: 10.1128/aac.42.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A β-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jobbling M G, Holmes R K. Construction of vectors with the p15a replicon, kanamycin resistance, inducible lacZα and pUC18 or pUC19 multiple cloning sites. Nucleic Acids Res. 1990;18:5315–5316. doi: 10.1093/nar/18.17.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma L, Ishii Y, Ishiguro M, Matsuzawa H, Yamaguchi K. Cloning and sequencing of the gene encoding Toho-2, a class A β-lactamase preferentially inhibited by tazobactam. Antimicrob Agents Chemother. 1998;42:1181–1186. doi: 10.1128/aac.42.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller J M. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 19.Miró E, del Cuerpo M, Navarro F, Sabaté M, Mirelis B, Prats G. Emergence of clinical Escherichia coli isolates with decreased susceptibility to ceftazidime and synergic effect with co-amoxiclav due to SHV-1 hyperproduction. J Antimicrob Chemother. 1998;42:535–538. doi: 10.1093/jac/42.4.535. [DOI] [PubMed] [Google Scholar]
- 20.Tzouvelekis L S, Gazouli M, Markogiannakis A, Paraskaki E, Legakis N J, Tzelepi E. Emergence of resistance to third-generation cephalosporins amongst Salmonella typhimurium isolates in Greece: report of the first three cases. J Antimicrob Chemother. 1998;42:273–275. doi: 10.1093/jac/42.2.273. [DOI] [PubMed] [Google Scholar]
- 21.Yagi T, Kurokawa H, Senda K, Ichiyama S, Ito H, Ohsuka S, Shibayama K, Shimokata K, Kato N, Ohta M, Arakawa Y. Nosocomial spread of cephem-resistant Escherichia coli strains carrying multiple Toho-1-like β-lactamase genes. Antimicrob Agents Chemother. 1997;41:2606–2611. doi: 10.1128/aac.41.12.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]