Abstract
In a single-center, randomized study, zanamivir (Relenza) concentrations in induced sputum samples and nasal washings of healthy adults following oral inhalation were measured. Concentrations in sputum exceeded the median viral neuraminidase 50% inhibitory concentration at 6, 12, and 24 h, and those in nasal washings did so at 6 and 12 h. There were no zanamivir-related adverse events or laboratory abnormalities.
Influenza virus is highly infectious and can cause worldwide pandemics with significant morbidity and mortality (3). A new strategy in the management of influenza is the use of agents that inhibit the action of neuraminidase, the glycoprotein component of influenza virus that is responsible for liberating new virus particles from infected airway cells (4). The first of these neuraminidase inhibitors approved for influenza treatment was zanamivir (Relenza) (14).
Zanamivir is not metabolized and has a short plasma half-life (∼2 h), low protein binding (<10% of systemically circulating zanamivir), and limited bioavailability (<20% following oral inhalation) (6). Twice-daily oral administration of 10 mg of zanamivir is effective in the treatment of naturally occurring influenza A and B virus infections (8, 10). Recent clinical studies with healthy adults showed that once-daily administration of 10 mg of zanamivir prevents symptomatic, laboratory-confirmed influenza (11).
In order to provide data that further support the treatment and prophylaxis dosing regimens for zanamivir, in the present study we directly measured zanamivir concentrations in induced sputum and nasal wash samples at 6, 12, and 24 h following a single, inhaled 10-mg dose in male and female healthy subjects.
Methods and results.
A single-center, parallel group, uncontrolled, open-label, randomized sampling-time design was used (protocol NAI10902). All subjects provided written informed consent prior to entry. Of 30 volunteers screened, 18 were enrolled to receive treatment. The majority (10 of 12) of screening failures resulted from poor quality or absence of induced sputum, as determined by a sputum induction test. Medical histories were recorded, and physical examinations and clinical laboratory tests were performed during screening.
Descriptive statistics, including the mean, standard deviation, median, and range, were determined relative to sampling time. Statistical analysis was performed to estimate the population mean zanamivir concentration and its 95% confidence interval (CI) at each sampling time by using an analysis-of-variance technique [log(zanamivir) versus sampling time] (SAS Procedure Guide; SAS Institute, Cary, N.C.; 1999).
Subjects were randomized (six to each sampling group) to one of the three postdose sampling time groups: 6, 12, or 24 h following the single zanamivir dose. Treatment groups were similar with respect to gender, age, weight, height, and body mass index (Table 1). Baseline FEV1 measurements ranged from 91 to 133% of predicted values (Table 1). All laboratory values at screening were within normal ranges, and there were no significant changes in FEV1 during or after the sputum induction procedure.
TABLE 1.
Demographics and baseline characteristics of study subjects
| Group (n) | Mean (SD) age (yr) | No. (%)
|
Mean (SD)
|
Mean (SD) baseline FEV1 (liters/min) | ||
|---|---|---|---|---|---|---|
| Female | Male | Height (cm) | Weight (kg) | |||
| Total (18) | 29 (7) | 10 (56) | 8 (44) | 173 (8) | 66 (8) | 4.18 (0.74) |
| 6 h | 31 (6) | 3 (50) | 3 (50) | 177 (5) | 68 (7) | 4.33 (0.84) |
| 12 h | 28 (9) | 4 (67) | 2 (33) | 169 (9) | 65 (12) | 4.11 (0.81) |
| 24 h | 28 (5) | 3 (50) | 3 (50) | 173 (8) | 65 (7) | 4.10 (0.68) |
Subjects spent 1 to 2 days at the research study site for dosing and sample collection. Subjects received one dose of 10 mg (two 5-mg blisters) of zanamivir via the Relenza Diskhaler, and samples were collected one time only at either 6, 12, or 24 h postdosing. Characteristics of the inhaler device and zanamivir formulation have been described previously (5, 12). Safety and tolerability were monitored by using vital signs, clinical laboratory assays, and recorded adverse events during treatment and follow-up.
At the specified sampling times (6, 12, or 24 h postdosing), subjects underwent nasal washings (5 ml of hypertonic saline/nasal opening) and sputum inductions according to the standard study site protocol. For sputum inductions, each subject inhaled hypertonic saline (3.5% [wt/vol]) via an ultrasonic nebulizer until 10 ml of sputum was produced. The quality of all sputum samples was assessed by counting cells in cytospin preparations according to standard procedures. All sputum samples met standards for acceptability (<80% squamous cells and >200 nonsquamous cells). Samples were frozen at −20°C or lower until analysis. Blood samples were collected for determination of urea concentrations immediately after sputum samples were collected.
The primary end point was the measurement of dilution-adjusted zanamivir concentrations in sputum and nasal wash samples at 6, 12, and 24 h postdose in at least six evaluable individuals per sampling time. Liquid chromatography-tandem mass spectrometry was used to detect total zanamivir levels in sputum and nasal wash samples (1). The analytical linear range (determined with spiked sputum and nasal washings) was 0.5 to 1,000 ng/ml, with coefficients of variation of 3.8 to 11.7% over the linear range. Urea concentrations in sputum, nasal washings, and blood were assayed with a high-resolution blood urea nitrogen test (Sigma BUN Reagent and Urea Standard Diluent) (13). Since recovered sputum and nasal wash volumes differ among individuals, all final zanamivir concentrations were adjusted by using a urea dilution method to compare urea concentrations in body fluid with those in blood so that comparisons could be made between subjects: dilution-adjusted zanamivir concentration in sputum or nasal washing = unadjusted zanamivir concentration × (urea level in blood/urea level in sputum or nasal washing).
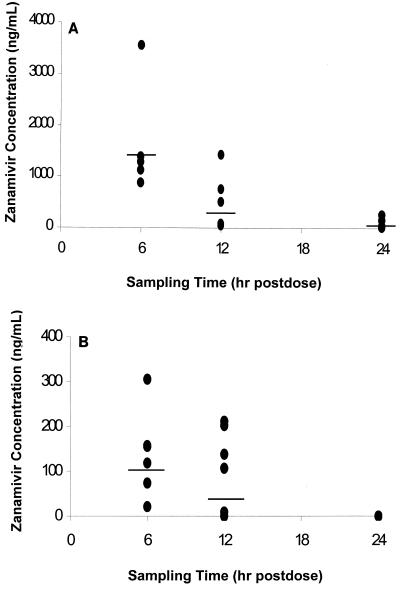
Median (range) dilution-adjusted zanamivir concentrations at 6, 12, and 24 h postdosing were 1,336 (888 to 3,563), 304 (53 to 1,434), and 47 (16 to 258) ng/ml in sputum samples and 137 ng/ml (21 to 305), 122 ng/ml (below quantifiable levels to 212), and below quantifiable levels in nasal wash samples, respectively.
The median (range) fold zanamivir concentrations above the median influenza A and B virus neuraminidase 50% inhibitory concentration (IC50) (0.9 ng/ml [16]) at 6, 12, and 24 h postdosing were 1,483 (985 to 3,958), 337 (58 to 1,593), and 52 (17 to 286) for sputum samples and 151 (23 to 338), 135 (<5 to 234), and <5 for nasal wash samples, respectively. The neuraminidase IC50 assay is the most reproducible assay for predicting the susceptibility of clinical isolates to neuraminidase inhibitors (2).
The concentrations in sputum and nasal washings as a function of sampling time are shown in Fig. 1A and B, respectively. Zanamivir concentrations detected in sputum and nasal washings decreased over time. Using the median urea-adjusted zanamivir concentrations at 6 and 12 h postdose and assuming an essentially first-order elimination of inhaled zanamivir from the lungs up to 12 h postdose, we determined that the elimination half-life would be 2.8 h (elimination rate constant = 0.2467 h−1).
FIG. 1.
(A) Dilution-adjusted zanamivir concentrations in sputum 6, 12, and 24 h after oral inhalation of 10 mg of zanamivir powder. There were six subjects for each time point. Model means (95% Cls) at 6, 12, and 24 h—1,441 (569, 3,650), 235 (93, 594), and 58 (23, 148)—are indicated by horizontal lines. (B) Dilution-adjusted zanamivir concentrations in nasal washings 6, 12, and 24 h after oral inhalation of 10 mg of zanamivir powder. There were six subjects for each time point. Model means (95% Cls) at 6, 12, and 24 h—106 (26, 435), 34 (8, 139), and below the quantification limit—are indicated by horizontal lines.
Within 2 days of zanamivir administration, symptom-directed physical examinations, laboratory tests, and pregnancy tests for female subjects were performed. No drug-related adverse events were reported, and no laboratory tests showed clinically significant abnormalities. Mild to moderate headache occurred in five subjects (one in the 6-h group and two each in the 12- and 24-h groups). No subjects withdrew from the study.
Commentary.
Zanamivir is delivered directly to the primary site of influenza virus infection and replication (the epithelial lining of the respiratory tract) by use of a dry-powder inhaler (Relenza Diskhaler), thereby minimizing systemic exposure. In the present study, we directly measured zanamivir concentrations in sputum and nasal wash samples following a single 10-mg orally inhaled dose in order to further substantiate the dosing regimen in the clinical use of zanamivir. Zanamivir concentrations exceeded the neuraminidase IC50 at all time points examined, except for nasal wash samples at 24 h postdose. Therefore, these data provide support for a twice-daily dosing regimen of zanamivir in order to maintain effective concentrations throughout the respiratory tract during the treatment of subjects with active infection.
The development of viral resistance has been a limitation to therapy with other antiviral agents (15, 17). Resistance to zanamivir has so far not been found in samples collected from clinical trials (2) or in animal models under conditions that readily select for variants resistant to amantadine and rimantadine (9). Achieving supermaximal inhibitory concentrations (above the IC50) at the site of viral replication is an effective strategy for preventing the emergence of viral resistance during therapy, since this substantially limits the percentage of the viral population that can replicate (7). The high local concentrations of zanamivir maintained throughout the respiratory tract following twice-daily dosing may be responsible for the low level of viral resistance observed to date with zanamivir.
This study also provides support for once-daily prophylaxis with zanamivir, as pulmonary concentrations at least 17-fold higher than the viral IC50 were maintained throughout the entire 24-h measurement period, in addition to significant zanamivir concentrations being found in nasal washings at 6 and 12 h postdose. Thus, exposure to the influenza virus in the nasal mucosa during times when the drug concentrations at this site are below the IC50 would not result in pulmonary infection, as concentrations of the drug in the bronchi are maintained well above the IC50 throughout the entire 24-h measurement period.
A recent clinical prophylaxis study randomized subjects to receive once-daily placebo or 10 mg of zanamivir by oral inhalation for a 4-week period (11). Zanamivir was 84% effective in preventing symptomatic laboratory-confirmed influenza with fever present. Interestingly, 79% of those infected in the zanamivir group were asymptomatic, compared to 56% in the placebo group. This finding suggests that zanamivir prevents symptomatic disease as well as infection, possibly due to the sustained pulmonary concentrations after inhalation, which would inhibit the spread of virus from the upper respiratory tract. Importantly, antibodies produced in asymptomatic infected individuals may be protective against subsequent infection with the same strain of influenza virus.
A previous pharmacokinetic study indirectly estimated local concentrations of zanamivir in the lungs within minutes after a single 10-mg orally inhaled dose (5). The zanamivir concentration in the peripheral lung region within minutes postdose was estimated to be approximately 6,900 ng/ml, or 7,676-fold above the median influenza virus neuraminidase IC50 (0.9 ng/ml [16]). Since in the present study we used a direct method for the assessment of respiratory tract zanamivir levels, a comparison of the results with those from the previous study was made. The zanamivir concentration minutes after a 10-mg inhaled dose was calculated in the present study to be 5,870 ng/ml (using C0 = Ct × ekt). Therefore, the indirect estimation of peripheral lung concentration minutes postdose (6,900 ng/ml) determined in the previous scintigraphy study (5) appears to be a reasonable method for the clinical estimation of lung exposure with inhaled medication.
In conclusion, this study demonstrates that zanamivir concentrations significantly higher than the median viral neuraminidase IC50 are retained in the respiratory tract following a single 10-mg zanamivir dose. Therefore, the sustained zanamivir concentrations in the respiratory tract following the recommended dosing regimen are sufficient to achieve effective inhibition of influenza virus replication.
Acknowledgments
We thank Lisa Squassante for statistical analyses, Christine M. Grosse for sample analyses, and Patrice Ferriola for writing and editing assistance.
This study was supported by Glaxo Wellcome Research and Development.
REFERENCES
- 1.Allen G, Brookes S, Barrow A, Dunn J, Grosse C. Liquid chromatographic-tandem mass spectrometric method for the determination of the neuraminidase inhibitor zanamivir (GG167) in human serum. J Chromatogr. 1999;732:383–393. doi: 10.1016/s0378-4347(99)00306-0. [DOI] [PubMed] [Google Scholar]
- 2.Barnett J M, Cadman A, Gor D, Dempsey M, Walters M, Candlin A, Tisdale M, Morley P J, Owens I J, Fenton R J, Lewis A P, Claas E C, Rimmelzwaan G F, DeGroot R, Osterhaus A D. Zanamivir susceptibility monitoring and characterization of influenza virus clinical isolates obtained during phase II clinical efficacy studies. Antimicrob Agents Chemother. 2000;44:78–87. doi: 10.1128/aac.44.1.78-87.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betts R. Influenza virus. In: Mandell G, Bennett J, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 1546–1567. [Google Scholar]
- 4.Calfee D P, Hayden F G. New approaches to influenza chemotherapy. Neuraminidase inhibitors. Drugs. 1998;56:537–553. doi: 10.2165/00003495-199856040-00003. [DOI] [PubMed] [Google Scholar]
- 5.Cass L, Brown J, Pickford M, Fayinka S, Newman S, Johansson C, Bye A. Pharmacoscintigraphic evaluation of lung deposition of inhaled zanamivir in healthy volunteers. Clin Pharmacokinet. 1999;36(Suppl. 1):21–31. doi: 10.2165/00003088-199936001-00003. [DOI] [PubMed] [Google Scholar]
- 6.Cass L, Efthymiopoulos C, Bye A. Review of the pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal dosing in healthy volunteers. Clin Pharmacokinet. 1999;36:1–11. doi: 10.2165/00003088-199936001-00001. [DOI] [PubMed] [Google Scholar]
- 7.Drusano G, Bilello J, Stein D, Nessly M, Meibohm A, Emini E, Deutsch P, Condra J, Chodakewitz J, Holder D. Factors influencing the emergence of resistance to indinavir: role of virologic, immunologic, and pharmacologic variables. J Infect Dis. 1998;178:360–367. doi: 10.1086/515631. [DOI] [PubMed] [Google Scholar]
- 8.Hayden F G, Osterhaus A D, Treanor J J, Fleming D M, Aoki F Y, Nicholson K G, Bohnen A M, Hirst H M, Keene O, Wightman K. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. GG167 Influenza Study Group. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 9.Mendel D, Sidwell R. Influenza virus resistance to neuraminidase inhibitors. Drug Resist Updates. 1998;1:184–189. doi: 10.1016/s1368-7646(98)80038-1. [DOI] [PubMed] [Google Scholar]
- 10.MIST, Campion K, Silagy C, Cooper C, Bolton P, Watts R, Liaw T, Narayan K, Deloze F. Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. The MIST (Management of Influenza in the Southern Hemisphere Trialists) Study Group. Lancet. 1998;352:1877–1881. [PubMed] [Google Scholar]
- 11.Monto A, Robinson D, Herlocher M, Hinston J, Elliott M, Crisp A. Zanamivir in the prevention of influenza among healthy adults. JAMA. 1999;282:31–35. doi: 10.1001/jama.282.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen K, Okamoto L, Shah T. Importance of selected inhaler characteristics and acceptance of a new breath-actuated powder inhalation device. J Asthma. 1997;34:249–253. doi: 10.3109/02770909709068196. [DOI] [PubMed] [Google Scholar]
- 13.Rennard S, Basset G, Lecossier D, O'Donnell K, Pinkston P, Martin P, Crystal R. Estimation of volume of epithelial lining fluid recovered by lavage using urea as a marker of dilution. J Appl Physiol. 1986;60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 14.von Itzstein M, Wu W Y, Kok G B, Pegg M S, Dyason J C, Jin B, Van Phan T, Smythe M L, White H F, Oliver S W, Colman P, Varghese J, Ryan D, Woods J, Bethell R, Hotham V, Cameron J, Penn C. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 15.Wade R C. ‘Flu’ and structure-based drug design. Structure. 1997;5:1139–1145. doi: 10.1016/s0969-2126(97)00265-7. [DOI] [PubMed] [Google Scholar]
- 16.Woods J M, Bethell R C, Coates J A, Healy N, Hiscox S A, Pearson B A, Ryan D M, Ticehurst J, Tilling J, Walcott S M, Penn C. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob Agents Chemother. 1993;37:1473–1479. doi: 10.1128/aac.37.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerman R K, Ruben F L, Ahwesh E R. Influenza, influenza vaccine and amantadine/rimantadine. J Fam Pract. 1997;45:107–122. [PubMed] [Google Scholar]