Abstract
For decades, intensive chemotherapy (IC) has been considered the best therapeutic option for treating acute myeloid leukemia (AML), with no curative option available for patients who are not eligible for IC or who have had failed IC. Over the last few years, several new drugs have enriched the therapeutic arsenal of AML treatment for both fit and unfit patients, raising new opportunities but also new challenges. These include the already approved venetoclax, the IDH1/2 inhibitors enasidenib and ivosidenib, gemtuzumab ozogamicin, the liposomal daunorubicin/cytarabine formulation CPX-351, and oral azacitidine. Venetoclax, an anti BCL2-inhibitor, in combination with hypomethylating agents (HMAs), has markedly improved the management of unfit and elderly patients from the perspective of improved quality of life and better survival. Venetoclax is currently under investigation in combination with other old and new drugs in early phase trials. Recently developed drugs with different mechanisms of action and new technologies that have already been investigated in other settings (BiTE and CAR-T cells) are currently being explored in AML, and ongoing trials should determine promising agents, more synergic combinations, and better treatment strategies. Access to new drugs and inclusion in clinical trials should be strongly encouraged to provide scientific evidence and to define the future standard of treatment in AML.
Keywords: acute myeloid leukemia, immunotherapy, target therapy, venetoclax
1. Introduction
Acute myeloid leukemias (AMLs) comprise a heterogeneous group of conditions that are characterized by the uncontrolled proliferation of a transformed malignant hematopoietic myeloid cell, inevitably leading to bone marrow failure.
Intensive chemotherapy (IC), consisting of anthracycline and cytarabine in induction treatment, high or intermediate doses of cytarabine as consolidation followed by allogeneic stem cell transplantation (HSCT) in high/intermediate-risk patients, has been considered as the gold standard in the treatment of AML for decades.
However, with a median age at diagnosis of 68 years, a high percentage of AML patients are not eligible for such an intensive therapeutic program [1]. Moreover, AML with myelodysplastic-related changes and therapy-related AML are associated with poor prognostic cytogenetic features, such as a complex karyotype and TP53 mutation, and are more frequent in the elderly; they show very unsatisfactory response rates to conventional IC, making it suitable neither for all patients nor for all diseases [2].
In recent decades, the hypomethylating agents (HMAs) azacitidine and decitabine have become the preferred low-intensity options in AML treatment for unfit patients, allowing about one-third of treated patients to reach remission and transfusion independence, improving their overall survival (OS) and quality of life [3,4,5]. In particular, azacitidine has shown better outcomes compared to IC in poor cytogenetic risk patients and less myelotoxicity compared to decitabine [6,7].
Recent advances in the genetic characterization of AML and in understanding the molecular mechanisms at the basis of leukemogenesis has allowed for a better prognostic assessment of the disease and opened the way to the exploration of new tailored therapeutic strategies based on patients’ risk profiles.
In parallel, owing to advances in drug manufacturing, bioengineering, and cellular therapy, more potent and selective agents and more advantageous formulations of old drugs are now available.
This progress is driving AML treatment from a ‘one fits all’ strategy to a precision therapy dimension, providing a real paradigm shift in the treatment of leukemia patients.
With this work, we aimed to write a narrative review of the recent advances in AML treatment and describe the landscape of future possibilities in this setting.
The most relevant papers were chosen after an extensive PubMed query; oral communications were selected from international hematology congresses (ASH and EHA congress). The main keywords used in the search strategy were adult acute myeloid leukemia, new drugs, target therapy, and immunotherapy.
2. Harnessing the Apoptosis Pathway
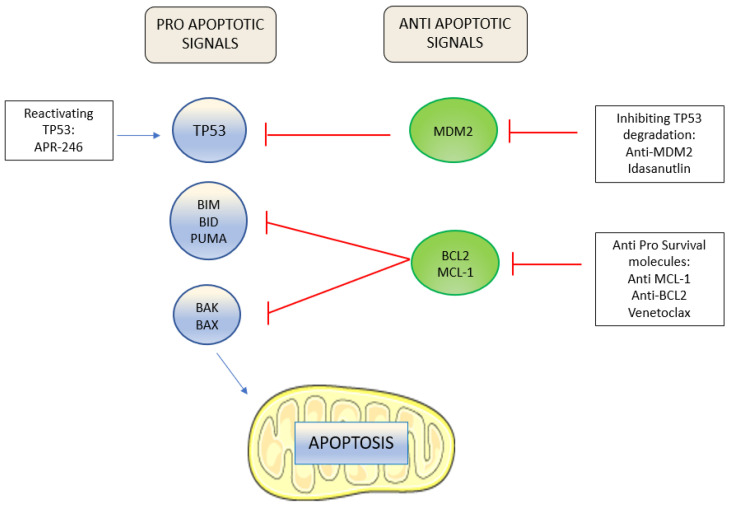
The B-cell lymphoma 2 (BCL-2) family proteins finely regulate the intrinsic cellular apoptosis pathway (Figure 1). In normal conditions, cell death is prevented by the BCL-2 family anti-apoptotic proteins BCL2 and MCL1 through the sequestration of pro-apoptotic molecules.
Figure 1.
Pro and anti-apoptotic signals and drugs acting on the intrinsic apoptosis pathway. Proapoptotic signals are counterbalanced by anti-apoptotic signals to promote cell survival. Drugs can induce apoptosis through the reactivation of mutated TP53, the inhibition of TP53 degradation, and by blocking pro-survival molecules.
In response to cell stress or damage, the pro-apoptotic members of the BCL-2 family, BID and BIM, sensitized by BH3-only proteins, interact with the effector proteins BAX and BAK to provoke mitochondrial membrane permeabilization and caspase release, and, consequently, cell apoptosis [8].
TP53 plays a key role in the intrinsic apoptosis pathway: it is activated in stress conditions, for example, with DNA damage induced by chemotherapy, and triggers apoptosis mainly by promoting the transcription of BH3-only proteins [9].
A better understanding of mechanisms at the basis of the cellular apoptosis pathway prompted the development of different classes of molecules with anti-leukemic activity.
2.1. BCL Inhibitors—Venetoclax
First identified in follicular lymphoma (FL), the anti-apoptotic protein BCL-2 prevents cell death by binding the pro-apoptotic BAX/BAK proteins and protecting the integrity of the outer mitochondrial membrane [10,11,12].
BCL-2 has been found to be overexpressed in AML cells and to confer resistance to conventional chemotherapy [13,14].
While the anti-sense oligonucleotide oblimersen and the first BH3 mimetic obatoclax showed limited efficacy in early clinical trials, the oral BCL-xL and BCL-2 inhibitor navitoclax showed significant anti-tumoral activity in preclinical AML cells and xenograft models [15,16]. Navitoclax was subsequently tested in patients affected by solid tumors and chronic lymphocytic leukemia (CLL), confirming its preclinical efficacy but also significant hematological toxicity, mainly thrombocytopenia [17]. To overcome this effect caused by the inhibition of BCL-xL by navitoclax, the BCL-2 selective inhibitor venetoclax was developed [18,19].
Venetoclax is an oral, highly selective, and potent BCL-2 inhibitor, first employed in CLL treatment [19,20]. By binding the BCL-2 protein, venetoclax allows for the release of pro-apoptotic proteins, restoring the apoptosis pathway [21,22].
After preclinical studies confirmed the pro-apoptotic activity of venetoclax in AML cell lines, it was administered as monotherapy in relapsed or refractory (R/R) AML patients, where it showed a tolerable profile but modest clinical efficacy, suggesting the need to explore its potential combinations with other agents, particularly HMAs [23,24].
Venetoclax is already used in clinical combination with HMAs in AML patients who are not eligible for IC, and many associations with other agents (IC and new drugs) are currently under exploration (Table 1).
Table 1.
List of studies assessing Venetoclax in association with other agents in acute myeloid leukemia. AZA—azacitidine; BETi—BET inhibitor; CLA—cladribine; CCML—chronic myelomonocytic leukemia; COB—cobimetinib; DEC—decitabine; ENA—enasidenib; FLAG—fludarabine; G-CSF—Granulocyte colony-stimulating factor; GILT—gilteritinib; HIDAC—high-dose cytarabine; HMA—hypomethylating agent; IDA—idarubicin; IDASA—idasanutlin; IVO—ivosidenib; LINT-AC225—lintuzumab-Ac225; LDAC—low dose cytarabine; MAGRO—magrolimab; MCL1i—MCL1 inhibitor; MDS—myelodysplastic syndrome; MEKi—MEK inhibitor; MIDO—midostaurin; MIVE—mivebresib; SAB—sabatolimab; SORA—sorafenib; TAGR—tagraxofusp; VEN—venetoclax.
| Agents in Combination with VEN | Population | Phase Study | References |
|---|---|---|---|
| VEN plus HMA/LDAC | |||
| AZA | ND AML ineligible for IC | 3 | 27 |
| LDAC | ND AML ineligible for IC | 3 | 33 |
| Intensive Chemotherapy | |||
| 7 + 3 | ND AML | 1b | NCT03709758 |
| 7 + 3 | ND AML and MDS-EB | 3 | NCT04628026 |
| CLA, HIDAC, IDA | ND AML, HR-MDS, and MPAL | 2 | 34 |
| 5 + 2 | ND AML ≥ 65 years old | 1b | 35 |
| FLAG-IDA | ND and R/R-AML | 1b/2 | 36–37 |
| CPX-351 | ND AML | 1b | NCT04075747 |
| ‘Non-Intensive’ Combinations | |||
| GILT | R/R FLT3 mutated AML | 1 | 42 |
| AZA plus GILT | R/R and ND AML/high risk CMML/MDS FLT3-ITD or -TKD mutated | ½ | 43 |
| DEC and FLT3i (GILT/SORA/MIDO) | ND or R/R FLT3 mutated AML | 2 | 44 |
| IVO and AZA | MDS, ND, and R/R AML IDH1+ | 1b/2 | 49 |
| ENA | R/R AML IDH2+ | 1b/2 | 50 |
| AZA and MAGRO | R/R AML, ND AML IC ineligible | 1/2 | NCT04435691 |
| MIVE (pan-BETi) | R/R AML | 1 | NCT02391480 |
| S64315 (MCL1i) | R/R Hematological malignancies | 1 | NCT03672695 |
| IDASA | R/R AML ≥ 60 years old | 1b | 77 |
| GO | R/R AML | 1b | 94 |
| TAGR plus AZA | ND and R/R AML, MDS, or BPDCN | 1b | 111 |
| LINT-AC225 | R/R AML | 1/2 | 119 |
| SAB | High or very high risk MDS | 2 | NCT04812548 |
| COB (MEKi) | R/R AML ≥ 60 years old, IC ineligible | 1b | 207 |
| CA-4948 | R/R AML and high risk MDS | 1/2a | NCT04278768 |
2.1.1. Venetoclax in Association with Hypomethylating Agents
Preclinical data has shown a synergistic effect of the combination of venetoclax with HMAs and the association of venetoclax with azacitidine/decitabine has been proven to be safe and able to produce a favorable response even in high-risk AML patients in early trials [25,26].
In the phase III study VIALE-A, azacitidine and venetoclax produced significantly better OS (14.7 versus 9.6 months), complete remission (CR) rates (37% vs. 18%), and composite remission rate (CR + CRi; 66% versus 28%) compared to azacytidine alone in AML patients who had not been previously treated and who were ineligible for IC [27].
Based on the VIALE-A study results, venetoclax received full approval from the Food and Drug Administration (FDA) in October 2020 in combination with azacitidine, decitabine, or low-dose cytarabine (LDAC) for the treatment of newly diagnosed (ND) AML in patients 75 years or older or in patients with comorbidities precluding the use of IC. Similarly, the European Medical Agency (EMA) approved the use of venetoclax in combination with an HMA for IC-ineligible adult AML patients.
In the R/R setting, no randomized controlled trials are available; however, retrospective data show interesting results with ORR about three times higher than the conventional salvage treatment with a single HMA, allowing in some cases to proceed to allogeneic stem cell transplantation (ASCT) [28,29].
In one study, a ten-day decitabine schedule was associated with venetoclax and compared to standard IC in 65 patients with R/R AML; the experimental arm showed a better ORR (60% vs. 36%, respectively), minimal residual disease (MRD) negativity by flow cytometry (28% vs. 13%), longer event-free survival (EFS; 5.7 vs. 1.5 months), and OS (6.8 vs. 4.7 months) [30].
The combination of azacitidine or decitabine with venetoclax can also be effective in AML relapse following ASCT, as shown in a retrospective analysis of 32 R/R patients conducted by the German Cooperative Transplant Study Group that reported an ORR of 47%, of which 50% were CR [31].
2.1.2. Venetoclax in Combination with Low-Dose Ara-C
The phase III study VIALE-C compared venetoclax plus low dose Ara-C (LDAC) with placebo plus LDAC in ND AML patients who were ineligible for IC [32].
Although no statistically significant differences were observed in OS (7.2 for the combination of LDAC and venetoclax versus 4.1 months in the control arm), and thus the primary endpoint of the study was not met, venetoclax provided a 25% reduction in the risk of death over the LDAC alone arm. A higher rate of CR/CRi was observed with venetoclax plus LDAC vs. LDAC alone: 48% vs. 13% with CR achieved in 27% vs. 7% of the patients, respectively. A post-hoc analysis with an additional 6 months of follow-up reported a median OS for the venetoclax and placebo arm of 8.4 and 4.1 months, respectively [33].
2.1.3. Venetoclax in Association with Intensive Chemotherapy
Considering the improved outcome when added to the treatment of elderly or unfit AML patients, venetoclax is under evaluation for younger and fit patients in addition to different IC regimens.
Thus far, no published data is available concerning the association of the standard IC backbone (7 + 3) to venetoclax. A phase Ib trial (NCT03709758) enrolling ND AML is ongoing and a phase III randomized placebo-controlled 7 + 3 and venetoclax in ND AML or MDS-EB will be recruiting soon (NCT04628026).
In a single arm, phase II trial at the MD Anderson Cancer Center, CLIA (cladribine plus high-dose aracytin and idarubicin) was associated with venetoclax in ND AML, higher risk MDS, and mixed phenotype acute leukemia patients. Composite complete response (CCR) was achieved by 94% of patients, with 82% having undetectable MRD. At a median follow-up of 13.5 months, the median duration of response (DOR), EFS, and OS were not reached. The toxicity profile was excellent, with only two deaths in CR occurring in patients receiving a concomitant FLT3 inhibitor (FLT3i) [34].
In a phase Ib dose escalation study, venetoclax was associated with a 5 + 2 regimen in 51 elderly AML patients who were ineligible for IC; the authors reported noteworthy CR/CRi rates (72% in the whole population with 94% in de novo and 43% in secondary AML) and a median OS of 11 months. An impressive marrow blast reduction ≥ 50% was observed in NPM1-, IDH2-, and SRSF2-mutant AML during the venetoclax monotherapy pre-phase [35].
Di Nardo and colleagues published very promising results of a phase Ib/II trial of venetoclax in association with FLAG-Ida in ND and R/R AML [36]. These results were recently updated at ASH 2021: the CRc rate was 88%, with 92% of this group achieving an MRD-negative status, and 66% of patients bridged to ASCT. The toxicity profile was acceptable, with febrile neutropenia (39%) and pneumonia (24%) being the most frequent adverse events (AEs). With a median follow-up of 16 months, median OS and EFS were both not reached, with 1-year OS and EFS rates of 96% and 77%, respectively. The presence of a TP53 mutation at diagnosis conferred a significantly inferior outcome compared to wild type (WT) status, with an OS of 24 months compared to not reached (p = 0.03) and an EFS of 8 months compared to not reached in the two groups (p = < 0.001), respectively [37].
All patients with a TP53 mutated at diagnosis inevitably relapsed, and two patients who were initially TP53 WT relapsed as TP53 mutated [38].
Novel associations are still under evaluation, such as CPX 351 plus venetoclax in ND AML without FLT3 or IDH mutations (NCT04075747) or a low-intensity dose of CPX 351 plus venetoclax in ND AML patients who are ineligible for IC. These studies are currently recruiting and thus have no available data concerning efficacy and safety.
2.1.4. Venetoclax—Future Non-Intensive Combinations
Considering the efficacy and tolerability of combinations based on venetoclax and the existence of driver mutations for which several drugs were recently approved, venetoclax is being actively studied in combination trials.
The use of multiple drug combinations could likely offer deeper and prolonged responses, owing to synergism and the simultaneous activity on different subclones of AML. The non-overlapping toxicities of different agents could also account for safe and well-tolerated combinations in the elderly and in frail patients [39].
2.1.5. Venetoclax in Association with FLT3 Inhibitors
FLT3 and ITD mutations in AML seem to confer resistance to venetoclax treatment by enhancing the expression of anti-apoptotic proteins (BCL-xL and MCL1), explaining the relatively weak response to venetoclax-based regimens noted in patients harboring these alterations [26,40,41].
The development of FLT3is prompted an assessment of their efficacy in combination with venetoclax and as part of triple combinations in this unfavorable subgroup of AML patients.
In a phase I study, the association of venetoclax and gilteritinib showed a desirable CR rate (85.4%) in 41 R/R AML patients with an FLT3 mutation, persisting also in patients already exposed to FLT3is [42].
In a phase I/II trial, the combination of azacitidine, venetoclax, and gilteritinib was evaluated in R/R AML, ND AML unfit for IC, or high risk CMML/MDS FLT3-ITD or TKD-mutated AML. This triplet resulted in a CRc rate of 100% and 69% for de novo and R/R patients, respectively [43].
A phase II trial explored the use of a combination of decitabine, venetoclax, and FLT3is (gilterinib, sorafenib, and midostaurin) in 25 ND or R/R FLT3-mutated AML patients. It should be noted that eight R/R patients were previously exposed to FLT3is and four patients previously underwent ASCT [39].
In ND and R/R patients, the CCRs were 92% and 62%, respectively, with more than 50% of responders achieving MRD negativity in both groups. The median OS was 14.5 and 6.8 months in ND and R/R patients, respectively.
The median DOR was not reached in either ND or R/R patients. Nine patients were able to proceed to ASCT: four patients in the ND cohort and five in the R/R cohort.
A triplet combination of decitabine, venetoclax, and quizartinib was evaluated in 17 FLT3-mutated AML patients (13 R/R and four ND) who were ineligible for IC. Significant responses were achieved in both the ND (CR 100%, with 4/4 FLT3-PCR MRD negativity and 2/3 flow cytometry MRD negativity) and R/R group (CR 69% with 4/9 FLT3-PCR MRD negativity and 5/9 flow cytometry MRD negativity). The sixty-day mortality rate was 8% in the R/R setting and 0% in the ND group [44].
2.1.6. Venetoclax in Association with IDH Inhibitors
In accordance with preclinical observations reporting an increased susceptibility to venetoclax in IDH1/2-mutated AML cells, the subgroup of AML patients harboring IDH1/2 mutations has shown high rates of durable remissions in clinical studies associating HMAs with venetoclax. Recently, Pollyea et al. reported a CCR of 79%, a median duration of remission of 29.5 months, and an OS of 24.5 months in ND IDH1/2-mutated AML [27,45,46,47].
A triplet strategy combining ivosidenib and venetoclax with or without azacitidine in 25 patients with IDH1-mutated myeloid malignancies was evaluated in a phase Ib/II study; it showed an acceptable toxicity profile and high rates of MRD-negativity in AML patients [48].
The combination of venetoclax and enasidenib is under investigation in patients with IDH2-mutated myeloid malignancies in a phase Ib/II study (NCT04092179); preliminary results from 11 patients, mainly R/R AML, reported a CR + CRi rate of 55% with a tolerable toxicity profile [49].
2.1.7. Other Associations
Other associations with venetoclax are being explored in phase I–II trials, for example, with anti-CD47 antibodies, such as magrolimab, BET-inhibitors, and antibody–drug-conjugated anti CD-123 (NCT03113643, NCT04086264) [50,51].
2.2. Anti-Myeloid Leukemia Cell Differentiation Protein-1 (MCL-1)
MCL-1 (myeloid leukemia cell differentiation protein-1) is a pro-survival protein that is implicated in the intrinsic apoptosis pathway, acting by neutralizing BH3-only proteins and preventing mitochondrial membrane permeabilization and consequent cell death [52].
High levels of MCL-1 expression are induced by FLT3 mutations in AML and are correlated with venetoclax resistance, leukemia relapse, and poor outcomes, suggesting a prominent role of MCL-1 as an anti-apoptotic protein in AML [53,54,55].
Preclinical studies have shown that anti-MCL1 treatments have a synergistic activity with venetoclax in AML cells. Moreover, venetoclax-resistant cells keep their susceptibility to MCL-1 inhibition, allowing for the restoration of venetoclax sensitivity [56,57].
MCL1 inhibitors, such as AMG-176 (NCT03797261), AZD5991 (NCT03218683), and S64315 in combination with venetoclax (NCT03672695), are currently under exploration in patients with R/R hematological malignancies.
3. Reactivating TP53
TP53 mutations occur in about 10% of AML patients and confer a poor prognosis and refractoriness to conventional IC. The response to HMAs in TP53-mutated patients is less impaired; hence, this class of agents is preferable as a first-line treatment option in this setting. However, the durable response is dismal and long-term survival after ASCT is uncommon, making TP53-mutated AML a disease with an unmet therapeutic need [30,58,59,60].
3.1. Eprenetapopt (APR-246)
Eprenetapopt (APR-246) induces a conformational change in mutated TP53 that confers thermostability and restores the protein’s native onco-suppressive function [61].
After demonstrating its safety and good tolerability in monotherapy, a phase Ib/II study of a combination with azacitidine provided encouraging remission rates (CR of 44%) in refractory AML patients [62,63,64].
A phase III trial comparing azacitidine plus eprenetapopt to azacitidine alone in patients with TP53-mutated MDS is currently ongoing. (NCT03745716).
3.2. Murine Double Minute 2 (MDM2) Inhibitors
Murine double minute 2 (MDM2) is an E3 ubiquitin ligase that degrades TP53. MDMX is an inhibitor of TP53 transactivation [65,66].
In WT TP53 AML, the overexpression of MDM2 and MDMX causes the inactivation of TP53, providing a very strong rationale for the use of MDM2/MDMX inhibitors in synergistic strategies and as venetoclax resensitizing agents [67].
The development of inhibitors acting on both MDM2 and MDMX has encountered some difficulties related to the inadequate size of the molecules and their limited bioavailability. Nevertheless, some MDM2 inhibitors have been developed [68].
RG7112 was the first selective MDM2 inhibitor that showed the capacity to restore TP53 activity and clinical efficacy in R/R AML patients in phase I studies, as monotherapy and in combination with LDAC [69,70,71].
Idasanutlin (RG7388) is a second-generation MDM2 inhibitor that is more potent, more selective, and has a more predictable pharmacokinetic profile than RG7112 [72,73].
After some encouraging results from idasanutlin in a phase I study, the phase III study MIRROR failed to meet the primary endpoint of survival benefit for the combination of idasanutlin and cytarabine compared to cytarabine alone [74,75].
Idasanutlin was studied in combination with venetoclax in a phase I study in the upfront treatment of R/R AML patients unfit for IC. This combination was associated with an ORR of 41%, a median time to response of 1.4 months, and a median DOR of 4.9 months [76].
Other small MDM2 inhibitors are currently undergoing clinical investigation, including MK8242 in monotherapy, AMG323 in combination with trametinib and decitabine, DS3032s in monotherapy and in association with azacitidine (NCT02319369), quizartinib, and cytarabine (NCT03552029) [77,78,79].
ALRN-6924, an MDM2-MDMX inhibitor, is under investigation in monotherapy and in combination with cytarabine in a phase I study [80].
Preclinical and clinical research on targeting MDM2 and MDMX is a current field of great interest in the treatment of AML. Challenges for MDM2 inhibitors and anti-MDM2-MDMX development include defining the optimal dose, finding the optimal combination, managing hematological and gastrointestinal toxicities, and defining which type of patients could potentially reap the greatest benefits from them [81].
4. Harnessing Immunity
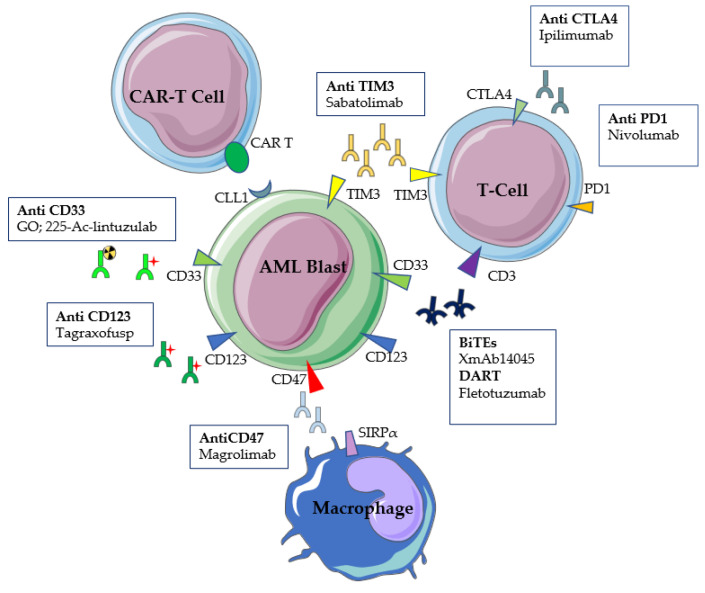
A more detailed knowledge of the evasion mechanisms carried out by cancer cells to escape antitumor immunity, together with technical advances in the production of monoclonal antibodies (mAbs), led to the conception of a plethora of drugs specifically directed to harness immunity against AML cells (Figure 2).
Figure 2.
Harnessing immunity against AML blasts. Antibodies, checkpoint inhibitors, bispecific antibodies, and CAR-T cells are possible strategies in leukemia treatment.
These comprise gemtuzumab ozogamycin, the first antibody–drug conjugate (ADC) targeting CD33, new anti-CD33s mAbs, mAbs targeting CD123, and radiolabeled mAbs.
Checkpoint inhibitors (anti-CTLA4 and anti-PD1-PDL1), which are already largely employed in solid oncology, have also been tested in the AML setting and may represent an interesting treatment in combination with other agents. Alternative molecules that are able to restore immune cytotoxic effect, such as sabatolimab, an anti-T cell immunoglobulin and mucin domain 3 (TIM3), and magrolimab, an anti-CD47 macrophage checkpoint inhibitor, have recently made their appearance in early phase clinical trials.
Finally, some bispecific T-cell engager (BiTE) antibodies and chimeric antigen receptor T (CAR-T) cells adapted to myeloid leukemia targets are currently being developed.
4.1. Monoclonal Antibodies (mAb) Anti-CD33
CD33 (or siglec 3) is a transmembrane receptor expressed by myeloid cells that is present on the surface of AML blasts in 85–90% of adult and pediatric AML patients [82].
Gemtuzumab ozogamicin (GO) is an ADC-targeting CD33 associated with calicheamicin, a cytotoxic component that is released into target cells [83,84,85].
After an accelerated approval by the FDA for R/R AML in 2000, GO was withdrawn in 2010 due to the absence of benefit and excessive toxicity in addition to standard induction chemotherapy in the phase III study SWOG-S0106 [86,87,88,89,90].
More recently, a fractionated administration schedule allowed the relocation of GO as a therapeutic option in AML [91]. In the ALFA-0701 trial (NCT00927498), fractionated GO (3 mg/m2 on day 1-4-7 of induction and day 1 of consolidation treatment) in association with standard chemotherapy in adult ND (50–70 years old) AML patients resulted in an EFS gain (13.6 versus 8.8 months), despite a slightly higher early mortality (4% vs. 2%) due to hemorrhage and VOD (5%) in the GO arm compared to the control arm.
An advantage in EFS, OS, and relapse-free survival (RFS) for low and intermediate-risk cytogenetic AML groups was confirmed by a meta-analysis including 3300 patients from five randomized controlled trials that corroborated the clinical benefit of GO in these categories of patients [91].
Potential GO toxicity was considered to be outweighed by the gain in EFS, leading to the reapproval of the drug by the FDA in 2017 for newly-diagnosed CD33-positive AML in adults and relapsed or refractory CD33-positive AML in adults and pediatric patients aged 2 years and older.
The EMA approved GO for adults (>15 years old) with de novo CD33+ AML in combination with daunorubicin and cytarabine.
4.1.1. New Perspectives on Gemtuzumab Ozogamycin
Several phase I and II studies are currently exploring the benefit of associating GO with other drugs, such as azacitidine, CPX-351, venetoclax, and other conventional drugs, in the induction phase of treatment.
In a retrospective study on 17 R/R AML patients, the combination of GO and azacitidine provided an overall response rate of 76.9%, emerging as a possible bridge to transplant treatment [92].
GO plus venetoclax was revealed to be safe in a phase Ib trial and the effectiveness of this combination is currently under investigation [93].
The combination of GO with the poly (ADP-ribose) polymerase inhibitors olaparib and talazoparib showed an intense antileukemic effect in preclinical models and could be included in novel combinations suitable for clinical trials in the near future [94,95,96].
In the phase I MOSAIC trial, GO was associated with midostaurin and standard 3 + 7 chemotherapy in upfront AML with a cCR of 91% [97].
Finally, advances in cellular therapy may open new opportunities for GO. Currently, an ongoing trial is evaluating post-transplant GO administration after an allogeneic engineered hematopoietic stem cell transplant lacking CD33 (NCT04849910).
Next generation anti-CD33s are under development, including vadastuximab talirine (SNG33A), a humanized murine anti-CD33 IgG1 mAb coupled with two molecules of pyrrolobenzodiazepine, which showed good tolerability in monotherapy and an encouraging CCR (CR + CRi 73%) in a study on 53 elderly AML patients who were ineligible for IC [98,99].
4.1.2. Anti-CD123—Tagraxofusp
CD123 is a component of the interleukin 3 receptor (IL-3Rα) expressed by hematopoietic stem cells that transmit, through the JAK2/STAT pathway, a signal for survival and proliferation [100].
CD123 has been found to be extensively overexpressed not only in AML but also in other hematological malignancies, such as blastic plasmacytoid dendritic cell neoplasm (BPDCN), MDS, systemic mastocytosis, chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), and hairy cell leukemia (HCL) [101,102,103,104,105,106,107].
Tagraxofusp (SL-401) is an anti-CD123 antibody conjugated to diphtheria toxin that has shown efficacy in the treatment of BPDCN [108].
In a phase I study, SL-401 was employed in 45 AML patients and showed a good safety profile but limited efficacy [109].
The combination of tagraxofusp with azacitidine or with azacitidine and venetoclax was tested in a phase Ib trial in AML, MDS, and BPDCN patients. Twelve AML patients received doublet and 14 AML patients triplet therapy, with CR or CRi in eight ND AML patients. No R/R AML patients responded in either of the two groups [110].
4.1.3. Radiolabeled Monoclonal Antibodies
Radiolabeled mAbs can deliver ionizing radiation to target cells in a far more precise manner than the classical irradiation technique, leading to the development of different mAbs labeled with radioisotopes that decay via the emission of alpha or beta particles [111].
While humanized, the unconjugated anti-CD33 mAb lintuzumab did not confer a survival benefit in AML treatment as monotherapy and in association with IC; it has been employed for the delivery of radionuclides [112,113,114].
The radioconjugate α-emitters bismuth-213 (213Bi-) and actinium-225 (225Ac-) conjugated with lintuzumab have shown antileukemic efficacy as single agents in R/R AML and in association with cytarabine [115,116,117]. Moreover, 225Ac–lintuzumab has been shown to reverse resistance to venetoclax in AML models. A phase I/II study of venetoclax and lintuzumab–225Ac in R/R AML patients is currently ongoing (NCT03867682) [118].
4.2. Checkpoint Inhibitors
Regulatory T cells expressing PD1/TIM3 are increased in leukemic bone marrow and AML blasts have been found to express PDL1, making T cell harnessing through checkpoint inhibitors (CPI) an attractive therapeutic strategy in AML [119].
4.2.1. Anti-CTLA4 and Anti-PD1
The anti-CTLA4 agent ipilimumab was explored in a phase I study on 12 R/R AML patients post ASCT; it allowed a complete response lasting greater than one year in five cases, despite reported immune-mediated toxic effects and graft versus host disease (GVHD) [120].
While the antileukemic activity of PD1 inhibition alone seems to be low, the observation that HMAs, among their immunomodulatory effects, induce an increased expression of inhibitory molecules (PD1 and CTLA4), provided the rational to obtain a synergistic effect by combining HMAs and CPI [121,122].
The combination of nivolumab and azacitidine seems to be more effective for R/R AML patients in early salvage conditions (patients with less than two prior lines of therapy) compared to HMA alone. In this setting, in a non-randomized, open-label, phase II study, Daver and colleagues reported a median OS of 10.5 months and a 12-month OS of 50% [123].
Encouraging results have also been reported for the combination of azacitidine and pembrolizumab, which was tested in a phase II trial in R/R and ND AML patients greater than 65 years old, with a particularly notable efficacy in the ND setting (CCR = 47%, 8/22 pts) [124].
The triplet consisting of azacitidine, nivolumab, and ipilimumab was shown to slightly improve OS in R/R AML patients. Single-cell immunophenotype profiling could help in selecting and predicting the response to CPI [125,126].
4.2.2. Anti-T Cell Immunoglobulin and Mucin Domain 3 (TIM3)
T-cell immunoglobulin and mucin domain 3 (TIM3) is a transmembrane co-inhibitor receptor expressed by T cells that leads to T-cell exhaustion [127].
The blockage of TIM3 and PD1 with antibodies improves the anti-cancer T cell response in vitro and in murine models [128,129].
Galectin-9, a TIM3 ligand, is produced by leukemia stem cells (LSCs) and protects them from T cell targeting. Moreover, LSCs—but not hematopoietic stem cells (HSCs)—have been found to express TIM3, which seems to be activated in an autocrine manner, leading to the accumulation of beta catenin in the intracellular compartment and the promotion of a self-renewal signal [130].
In fact, sabatolimab, a humanized IgG4 anti TIM3 mAb, is the only TIM3 inhibitor currently being studied in AML and MDS clinical trials in monotherapy or in combination with other drugs (e.g., venetoclax, HMAs); it has shown promising response rates and manageable toxicities [131].
4.3. Anti-CD47 Antibodies—Magrolimab
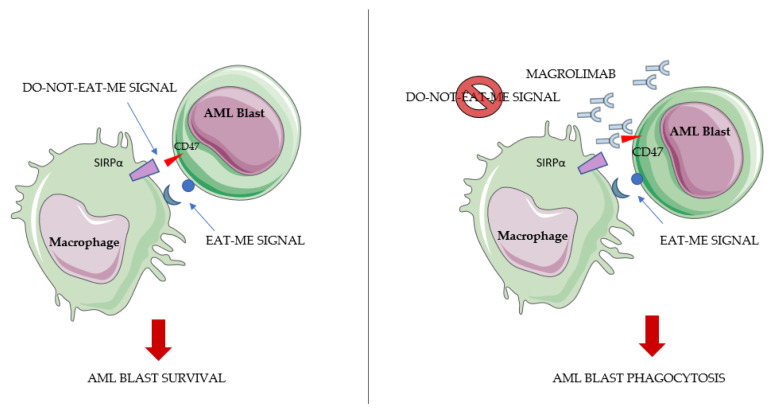
CD47 is a transmembrane protein that transmits a “do not eat me” signal. CD47 expression counterbalances pro-phagocytosis signals in malignant cells, allowing for the evasion of macrophage phagocytosis [132].
CD47 is expressed in AML cells and its inhibition has shown a capacity for eliminating LSCs in preclinical models (Figure 3) [133].
Figure 3.
AML blasts avoid phagocytosis through CD47–SIRPα interaction, transmitting a ‘do-not-eat-me’ signal that cancels the malignant cell’s ‘eat-me’ signal expression. Magrolimab (anti-CD47) interferes with the CD47 and SIRPα interaction, abolishing this escape mechanism and inducing blast phagocytosis.
The IgG4 anti-CD47 magrolimab showed a satisfactory tolerability profile with limited antileukemic activity as monotherapy in R/R AML patients in a phase I trial [134].
The observation that azacitidine upregulates the ‘eat me’ signal on leukemic cells, increasing magrolimab efficacy, provided the rationale for testing their association [135].
In a recent phase Ib trial, the combination of magrolimab with azacitidine showed a 64% and 91% CCR in untreated AML and high risk MDS patients non eligible for IC, respectively. Interestingly, 74% of CCR was found in the TP53-mutated AML subgroup [136].
A phase III study evaluating the combination of magrolimab with azacitidine (NCT04778397) and a phase I study evaluating the combination magrolimab, azacytidine, and venetoclax are currently ongoing. (NCT04435691).
4.4. Bi-Specific T-Cell Engagers (BiTEs)
Bispecific antibodies are compounds capable of binding two different targets, usually expressed by different cells. Particularly, bispecific T-cell engagers (BiTEs) are agents whose action is triggered by the engagement of both antigen sites, determining T-cell activation with subsequent killing of the target cell and increased pro-inflammatory cytokine release, independent of the costimulatory signal [137,138].
This technology started to be investigated more than 50 years ago; however, its clinical development and application in a hematological setting is much more recent and is mainly related to lymphoproliferative disorders [139,140,141].
The first bispecific antibody approved for use in a hematological malignancy was blinatumomab, which granted the indication for treatment of relapsed or refractory (R/R) acute lymphoblastic leukemia (ALL) in 2014.
The first BiTE designed and employed for AML treatment was AMG330, which targets the antigens CD33 on tumor cells and CD3 on T cells; preliminary data showed important in vitro antileukemic activity and particularly the capacity of avoiding key mechanisms for tumor resistance, such as CD33 downregulation [142].
The preliminary results of a phase I trial with AMG330 for R/R AML showed an ORR of 19% with an acceptable toxicity profile characterized mostly by cytokine release syndrome (CRS; 67% all grades, 13% grade 3–4) and an AE correlating with blast count at the baseline and dose level. This drug presents a short half-life of approximately two hours, needing continuous intravenous administration [143].
A modified CD33/CD3 BiTE is AMG673, which presents a longer half-life than AMG330, permitting an intermittent schedule of intravenous administration. Efficacy data reported in the phase I trial showed a decrease in blast count in 44% of evaluable patients; however, 50% of patients experienced CRS (27% of grade 3, no grade 4 observed) [144]. Other CD33/CD3 BiTEs currently under investigation are AMV564, which seems to be correlated with less CRS in the preliminary data of a phase I trial, and JNJ-67371244, for which no data are available yet [145].
Vibecotamab (XmAb14045) has been conceived to target CD3 and CD123 and was tested in a phase I study in 63 heavily pre-treated R/R AML patients.
Efficacy was reported only for the two highest dose level studies in the trial (1.3 and 2.3 µg/kg weekly) with CR/CRi in three of thirteen AML patients, of which two could proceed to transplant.
The main toxicities were CRS (77%, with 11% of grade ≥ 3), fatigue, neutropenic fever, and peripheral edema [146].
A different type of molecule called DART (dual-affinity re-targeting) was recently developed; it differs from BiTE due to the presence of variable domains of heavy and light chains which engage the target antigens on two separate polypeptides [147]. This configuration provides a more stable molecule due to the presence of an additional disulfide bridge and seems to also give an advantage in terms of efficacy when compared to the BiTE format [148].
The first DART investigated in AML was flotetuzumab (MGD006 or S80880), which binds to CD3 and CD123; the phase I/II trial showed a CR/CRi rate of 27% and a median OS of 10.2 months, independent from cytogenetic risk category. Grade 3 CRS was observed in 8% of patients and mostly reversible and frequently treated with the early employment of tocilizumab. The recommended dose was 500 ng/kg/day by continuous intravenous administration [149].
4.5. Chimeric Antigen Receptor T Cells (CAR-Ts)
One of the most innovative approaches in modern oncohematologic immunotherapy is represented by chimeric antigen receptor T cells (CAR-Ts). This technology is based on engineered synthetic receptors permitting the regulation of T cell activity to recognize and eliminate neoplastic cells expressing a specific target antigen. The induced activity of CARs is independent from the presence of co-stimulatory proteins or MHC receptors [150].
Despite its great impact in lymphoproliferative disorders, leading to the approval of several CAR-T compounds by EMA and FDA in the last years, the development of this immunotherapy for AML treatment has been slower and less successful, due to adverse microenvironmental conditions and the reduced number of target antigens selectively expressed by AML cells, determining suboptimal efficacy and increased toxicity [151].
Two promising target antigens are CD33, which is highly expressed on both AML and healthy stem cells, and CLL-1 (or CLEC12A), a more selective agent for leukemic blasts. In the first phase I trial using dual CD33-CLL-1 CAR-T cells on 9 R/R AML patients, a 4-week evaluation reported an interesting MRD negative rate of 78%, using flow cytometry, with six patients proceeding to ASCT. CRS was common, but was mostly of grade 1–2 (75% of cases) and efficaciously managed with corticosteroid treatment [152].
Another widely studied AML antigen is CD123; in a phase I trial using a CD123 CAR-T cell treatment, the data showed significant antileukemic activity with manageable CRS [153]. CD38 CAR-T cells are currently under investigation in a phase I/II trial: this antigen, well known for its implication in plasma cell dyscrasias, has also been found to be highly expressed in AML blasts [154].
In a phase I/II trial with six patients, four of them achieved CR or CRi, with a median PFS of 6.4 months. CRS was highly manageable, mostly of grade 1–2 (83% of patients) [155].
A further improvement of CAR-T technology is the universal CAR-T platform (UniCAR): this CAR is designed to recognize a peptide motif included in the second component of the molecule, called the targeting module, which confers specificity against the antigen of choice. Three AML patients were treated with UniCAR-T-CD123 with encouraging efficacy (one PR, two CRi) and toxicity (two cases of grade 1 CRS) results [156].
5. New Formulations, Old Drugs
5.1. CPX-351 (Vyxeos)
CPX-351 is a liposomal formulation with a 1:5 molecular ratio of daunorubicin and cytarabine that optimizes drug delivery, with preferential uptake by LSCs compared to normal HSCs [157,158,159].
In a phase III trial including 309 patients from 60 to 75 years old with ND high-risk AML, CPX-351 showed a significantly improved median OS and higher ORR compared to the classic 7 + 3 approach, despite a longer time to neutrophil and platelet count recovery [160]. CPX-351 was approved by the FDA and EMA for ND and therapy-related (TR) AML, and real-world data confirmed the efficacy of CPX-351 in monotherapy, with promising outcomes after HSCT [161].
Ongoing trials are exploring the role of CPX-351 beyond TR-AML (MDS, LMMC, myelofibrosis, and myeloproliferative neoplasms) and its associations with other drugs (venetoclax, gilteritinib, palbociclib, glasdegib, and GO).
5.2. Oral Azacitidine (Oral-Aza)
The well-known HMA azacitidine has been recently developed in an oral form, which is administered in an extended doses schedule of 14 or 21 days, that exercises anti tumoral activity through hypomethylating and immunomodulatory effects which are not yet fully understood [162,163].
The oral formulation of azacitidine allows a prolonged schedule of administration, which consequently extends the exposure of AML cells to the drug, sparing inconveniences related to injections and promoting a particular hypomethylating profile and weaker cytotoxic effects [164,165,166,167].
A phase I study showed that oral-aza, administered daily for 14- and 21-day affords 38% and 57% greater azacitidine exposure, respectively, in comparison with the classical injectable schedule (75 mg/m2 for 7 consecutive days) [168,169].
An ORR of 22% in AML patients was reported by phase II AZA-MDS-004 study, in which oral-aza was administered for 21 days [170].
Nowadays, the approval of oral azacitidine in the EU, USA, and Canada for AML patients in RC1 or RCi1 after IC who are not eligible for ASCT is based on the results provided by the QUAZAR AML-001 study. In this trial, oral-aza maintenance after IC significantly prolonged OS (24.7 versus 14.8 months) and RFS (10.2 versus 4.8 months) in AML patients older than 55 years, compared to a placebo [171].
6. Tyrosine Kinase Inhibitors (TKIs) and the RAS Pathway Inhibitors
6.1. FLT3 Inhibitors
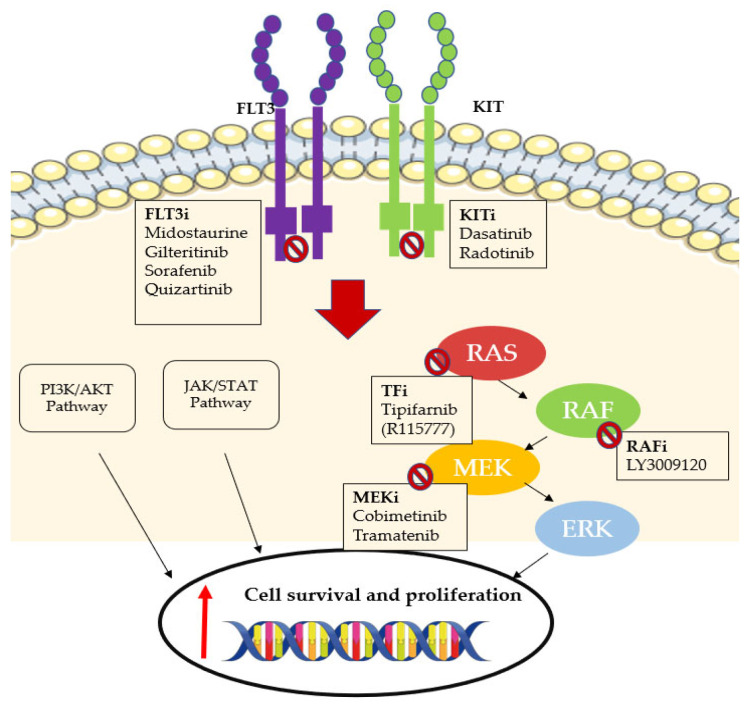
Over the last two decades, several TKIs have been developed to treat patients with FLT3 gene mutations (Figure 4). Approximately 30% of AML patients carry a mutation at diagnosis, whether an internal tandem duplication (FLT3-ITD) or a point mutation in the tyrosine kinase domain (FLT3-TKD). These patients are historically associated with a bad prognosis, a high risk of relapse, and low cure rates. FLT3is have been evaluated in frontline treatment, salvage settings, or as maintenance treatment after ASCT [172].
Figure 4.
FLT3 and KIT inhibitors and the RAS-RAF-MEK-ERK signaling pathway and its inhibitors.
New clinical trials are now exploring the use of first and second-generation FLT3is in association with non-intensive treatments in frontline or R/R settings and the use of second-generation FLT3is in association with IC in a frontline setting.
6.1.1. Midostaurin
Midostaurin is a first-generation FLT3i now approved by the FDA and EMA in association with standard IC for ND AML based on the results from the RATIFY Trial [173].
The trial randomized 717 patients to receive classic IC and midostaurin or classic IC and a placebo. The CR rate was 58.9% and 53.6% while the median 4-year OS was 51.4% and 44.3% in the midostaurin and placebo groups, respectively. The safety profile was similar in both groups, and this has recently been confirmed in the RADIUS-X expanded access program [174].
Midostaurin in association with azacitidine was evaluated in 54 patients in a phase I/II study of AML and MDS unfit for IC or R/R after previous treatments: the ORR was 26%, with a CR + CRi rate of 13%. The median OS was 22 weeks [175].
6.1.2. Sorafenib
Sorafenib is another first-generation FLT3i. It has been tested in phase II and III trials in ND AML in association with IC with discordant outcomes [176,177].
Very interesting results have been published in the post-transplant setting. The SORMAIN study is the only prospective RCT in this setting, randomizing 83 adult patients with FLT3-ITD AML in CR post ASCT to receive sorafenib or a placebo. The 2-year RFS was 85% and 53.3% in the sorafenib and placebo groups, respectively (p = 0.013), and the OS was significantly longer in the sorafenib arm (p = 0.03) [178].
6.1.3. Gilteritinib
Gilterinib is a highly selective second-generation FLT3i with activity against AML cells harboring FLT3-ITD and TKD mutations. Significant results in R/R patients lead to its experimental use in the frontline setting.
The phase III ADMIRAL trial led to the approval of gilteritinib in monotherapy in R/R FLT3-mutated patients. Gilteritinib was compared to salvage chemotherapy in 371 patients. The median OS was improved in the gilteritinib arm (9.3 vs. 5.6 months; p < 0.001), as was the CR rate (34% vs. 15.3%). It should be noted that 88% of patients were not pre-exposed to other FLT3is [179].
The phase III trial LACEWING study (NCT02752035) evaluated gilteritinib with azacitidine compared to azacitidine alone in ND FLT3-mutated AML patients who were ineligible for IC. Higher CRc rates were observed in the experimental arm, but a similar OS was reported in both arms [180].
As already discussed, gilterinib has been tested in association with venetoclax and in triplet with azacitidine and venetoclax for R/R and ND AML patients with interesting results [42,43].
6.1.4. Quizartinib
In the QUANTUM-R phase III study, which compared the second-generation FLT3 inhibitor quizartinib to salvage chemotherapy in R/R FLT3-ITD AML, quizartinib showed a survival benefit with an OS of 6.2 months compared to 4.7 in the control arm [181].
Quizartinib is currently approved in Japan for the treatment of R/R FLT3-ITD-mutated AML.
In a phase I/II trial, the combinations of quizartinib with azacitidine or LDAC were evaluated in ND or RR FLT3-ITD MDS-AML patients, producing a median OS of 19.2 months (quizartinib/azacitidine) and 8.5 months (quizartinib/LDAC) in the frontline setting and 10.5 months (quizartinib/azacitidine) and 6.4 months (quizartinib/LDAC) in the R/R setting [182].
Quizartinib was also evaluated in a triplet therapy in association with azacitidine and venetoclax in ND and R/R AML FLT3-ITD-mutated patients who were ineligible for IC. All five patients in the ND cohort achieved CRc. The CRc rate among R/R patients was 65%, with an encouraging OS of 7.5 months and a 1-year OS of 34%. It should be noted that 68% of these patients received gilteritinib and that RAS/MAPK mutations were associated with primary and secondary resistance [183].
6.2. KIT Inhibitors
The KIT gene encodes for a membrane receptor tyrosine kinase (CD117) that is frequently mutated in core-binding factor AML, suggesting a possible role of KIT inhibition in AML treatment (Figure 4) [184,185].
The BCR/ABL inhibitors dasatinib and radotinib have been shown to promote AML cell death by targeting c-KIT in AML cell lines and murine models, suggesting a potential role in clinical application [186,187].
6.3. RAS Pathway Inhibitors
Rat sarcoma (RAS) proto-oncogenic proteins have been largely studied in recent decades, and RAS mutations have been found to play a role in about 30% of human cancers [188].
The three highly homologous RAS proteins (KRAS, NRAS, and HRAS) are activated through a GTP phosphorylation process and promote cell survival and proliferation through the RAS/RAF/MEK/ERK pathway (Figure 4) [189].
RAS mutations are found in approximately 15–40% of AML diagnoses, and the most frequent mutated RAS protein in myeloid malignancies is NRAS [190,191,192,193,194,195].
Once synthesized, RAS proteins undergo cytosolic modifications before being collocated to the membrane surface where they are effective. One of these modifications is prenylation, which requires an enzyme called farnesyltransferase (FT) [196].
Tipifarnib (R115777) is an inhibitor of FT that has been tested in R/R AML, with reported clinical responses in 29% of patients (10 out on 34) with two CR [197,198].
In phase I studies, tipifarnib was shown to be safe in combination with IC and with bortezomib in patients with ND AML [199].
To target the RAS pathway, a RAF inhibitor has also been developed (LY3009120) and has shown synergistic proprieties with cytarabine in RAS-mutated AML cell lines [200].
Acting on the same signal transmission pathway, some MEK inhibitors have been developed and studied in early phase trials. Their clinical development is still at an immature stage and their role will likely be in combination with other agents.
The MEK inhibitor binimetinib has been shown to be safe in RAS-mutated AML, with very limited activity as monotherapy and in combination with the ATP-competitive pan-AKT inhibitor GSK2141795 (targeting the PI3K/PTEN/AKT/mTOR pathway) [201,202].
Trametinib is another MEK inhibitor that showed activity in RAS-mutated AML in a phase I/II study [203].
Cobimetinib, a third MEK inhibitor, is currently being studied in combination with venetoclax [204].
Downstream in the same pathway, ERK activation was shown to confer resistance to TKI; the association of trametinib with midostaurin showed a synergistic effect and could emerge as a new exploitable strategy in FLT3-mutated AML patients [205,206].
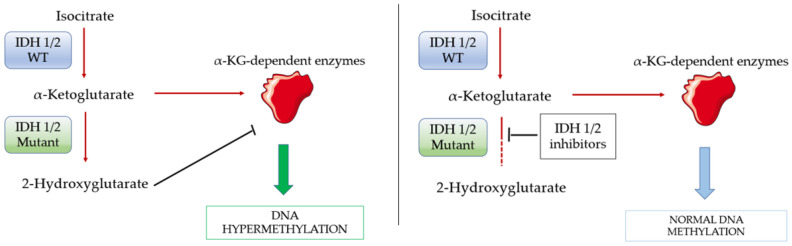
7. Isocitrate Dehydrogenase Inhibitors
IDHs belong to a group of enzymes that catalyze the oxidative decarboxylation of isocitrate to alfa-ketoglutarate (α-KG), generating NADPH.
Mutations involving arginine in the active site of IDH1/2 (R132 in IDH1 and R140 or R172 in IDH2) result in mutant IDH1/2 proteins that have acquired the capability of converting α-ketoglutarate in 2-hydroxyglutarate (2-HG) [207,208].
2-HG acts as a competitive inhibitor of α-KG, interfering with α-KG-dependent enzymes, which play a role in several metabolic processes and DNA methylation, and produces a characteristic hypermethylated DNA phenotype (Figure 5) [209,210].
Figure 5.
IDH1/2 mutant converts α-ketoglutarate into 2-hydroxyglutarate, which interacts with α-KG-dependent enzymes and leads to DNA hypermethylation. IDH1/2 inhibitors prevent α-KG production and restore a normal DNA methylation profile.
IDH1 and IDH2 mutations are found in approximately 8% and 12% of AML cases, respectively, frequently co-occurring with FLT3/NPM1 mutations [211,212].
It should be noted that IDH mutations have been also described in other malignancies (glioblastoma, chondrosarcoma, cholangiocarcinoma, and angioimmunoblastic T cell lymphoma) [209,213,214,215].
The prognostic impact of IDH1/2 mutations in AML is still a matter of debate [216].
Enasidenib (AG-221) and ivosidenib (AG-120), which are IDH2 and IDH1 inhibitors, respectively, target IDH-mutated proteins.
Enasidenib was proven to be well tolerated and safe as a single agent and capable of inducing responses in R/R IDH2-mutated AML patients in a phase I/II study, with an ORR of 40.3% and a median DOR of 5.8 months [217]. These results led to enasidenib approval for R/R IDH2-mutated AML in the USA.
Unfortunately, the phase III trial IDHENTIFY (ClinicalTrials.gov (accessed on 7 February 2022), NCT02577406), which compared enasidenib to conventional treatments (best supportive care alone or in combination with azacytidine or low-dose or intermediate dose cytarabine) in R/R IDH2-mutated AML patients was announced to have missed the primary endpoint of OS in the enasidenib arm [218].
In an ND setting, a phase I/II clinical trial investigating enasidenib monotherapy in 39 ND IDH2-mutated AML patients reported an ORR of 30.8% with a CCR of 21% and a median OS of 11.3 months [219].
Ivosidenib monotherapy was tested in a phase I study in 268 IDH1-mutated AML patients, with 179 patients in the R/R setting. CR + CRi was obtained in 30.4% of treated patients, with a global DOR of 6.5 months that reached 9.3 months in CR patients and a low rate of AEs of grade 3–4 (mainly QT prolongation in 7.8%, IDH differentiation syndrome in 3.9%, and thrombocytopenia in 3.4% of cases) [220].
Resistance to ivosidenib has been found to be correlated with receptor tyrosine kinase pathway mutations in this population of R/R AML patients [221].
Both in the ND and R/R settings, enasidenib and ivosidenib are under investigation in association with other agents.
Compared with azacitidine alone, Dinardo et al. showed that the combination of enasidenib with azacitidine resulted in a better and greater-than-additive effect on ORR (74% versus 36%) and a better CRR (54% versus 12%) [222].
The safety and clinical activity of the combination of ivosidenib with azacitidine was evaluated in a phase b study in patients with ND IDH1-mutated AML who were ineligible for IC.
This association, beside good tolerance without dose-limiting toxicities, reported interesting and encouraging results concerning clinical efficacy, with an ORR of 78.3%, a CR rate of 61%, and a median time to first response of 1.8 months (range, 0.7–3.8 months); the median duration of CR, CR/CRh, and overall response were not reached [223].
The association of both agents with standard IC in ND AML in a phase I study did not delay hematological recovery compared with historical data. Ivosidenib-treated and enasidenib-treated patients had encouraging 12-month survival probabilities of >75%, considering the high portion of secondary AML (~30%) and a majority of elderly patients (more than 60 years old) in the study population. Phase III studies are currently ongoing to further evaluate the association of IDH inhibitors with IC [224].
In the phase Ib/II enaven-AML trial, enasidenib was associated with venetoclax in 11 RR/AML very high-risk (VHR) MDS patients with an encouraging ORR (55%) [49].
Multiple combinations of enasidenib/ivosidenib with venetoclax (NCT04092179), decitabine (NCT05010772), decitabine and venetoclax (NCT04774393), and classical induction/consolidation therapy (NCT03839771) are currently under investigation along with the role of IDH inhibitors in maintenance therapy following ASCT in AML (NCT03728335, NCT04522895); in IDH2-mutated MDS (NCT03744390); in association with CPX-351, fedratinib (NCT04955938), and ruxolitinib (NCT04281498); and in IDH1/2-mutated high risk myeloproliferative syndromes.
8. Others
8.1. Hedgehog Inhibitors—Glasdegib
The hedgehog signaling pathway plays an essential role in embryonal and adult hematopoiesis and its aberrant activation has been found to drive hematological malignancies, including AML [225,226,227].
In the phase II BRIGHT AML 1003 trial, the combination of glasdegib and LDAC improved CR rate (17 versus 2.3%) and median OS (8.8 versus 4.9 months) compared to LDAC alone in a population of AML and high-risk MDS patients [228].
These results led to the FDA and EMA approval of glasdegib in combination with LDAC in AML patients aged more than 75 years who are ineligible for IC [229].
Moreover, the long-term BRIGHT AML 1003 analysis showed a survival advantage of the association of glasdegib with LDAC versus LDAC alone, with a more pronounced benefit in secondary AML [230].
Ongoing trials are currently investigating new associations of glasdegib with azacitidine and IC with cytarabine/daunorubicin (NCT03416179, NCT02367456, and NCT01546038).
8.2. IRAK4 Inhibitors
IRAK4 (interleukin-1 receptor-associated kinase 4) in association with IRAK1 can activate NF-κB pathway signaling, triggering cell survival. IRAK4-L RNA isoform expression, generated by alternating splicing promoted by mutant U2AF1 splicing factor, has been found to be associated with AML and is required for leukemic cell function [231].
The inhibition of IRAK-4 through the IRAK-kinase inhibitor CA-4948 was shown to arrest leukemic growth in AML cells expressing IRAK4-L and to limit the spread of THP1 leukemic cells in a xenograft murine model [231].
Currently, the oral IRAK-4 inhibitor CA-4948 is under investigation in monotherapy and in combination with azacitidine or venetoclax in R/R AML and R/R MDS settings (NCT04278768).
8.3. Menin-KMT2A (MLL) Inhibitor
The histone-lysine-N-methyltransferase 2A (KMT2A) gene and menin-1 gene are part of a complex that plays an essential role in regulating the expression of homeobox genes. Mutations in KMT2A are responsible for aberrant Hox gene expression leading to leukemogenesis [232,233].
Accounting for the 5–10% of AML diagnoses, DNMT3A-mutated cases constitute a very poor prognostic group of disease [234].
The menin inhibitor MI-3454 was revealed to block proliferation and induce differentiation in AML cells and remission in AML KMT3A/NPM1 mutated murine models [235].
Indeed, the menin inhibitor KO-539 is under investigation in the KOMET-001 phase I/II study in R/R AML patients [236].
9. Discussion and Conclusions
Until recent years, AML has been treated with IC followed by ASCT for fit patients with the intention to cure, and with HMA for patients ineligible for IC, mainly with an intent to improve quality of life. A significant portion of more fragile patients could also be oriented towards pure palliative care.
The absence of valid alternative treatments outside clinical trials leaves little room for therapeutical strategy in frontline treatment and, in the R/R setting, if remission cannot be obtained with salvage chemotherapy to proceed to transplant, supportive care should be encouraged.
Recent advances in the understanding of the molecular basis of leukemogenesis and the mechanisms sustaining LSC survival has allowed a better characterization of AML disease and a more precise prognostic stratification.
With the therapeutical plan, these advances led to the development of several classes of agents with different mechanisms of action, opening opportunities for new hopes for patients and prompting new management possibilities for a disease treated for many years in a single way.
Nowadays, IDH1-2 inhibitors, FLT3is, and GO are frequently incorporated in AML treatment according to a tailored approach; future challenges include assessing their role in new combinations and the development of new, more potent, and better tolerated next-generation molecules. New formulations of drugs (CPX-351 and oral azacitidine) with particularly interesting pharmacokinetic and tolerability profiles are also currently available and widely used.
Among recently approved drugs, the BCL-2 inhibitor venetoclax is one of the most interesting agents, even if its role in treating AML is still largely unexplored. We know that in combination with HMAs and other agents, is a valuable upfront treatment component for unfit and fit patients; however, new combinations carrying promising results in early phase trials are currently under investigation.
Beyond induction, venetoclax may keep an interesting role in consolidation and maintenance; however, again, appropriate schedules and more advantageous combinations need to be established.
Following the path that has been traced by venetoclax, new classes of drugs will likely enrich the therapeutical landscape for AML, from which every population of patients could benefit, opening the pathway for several different treatment strategies.
In this race, agents harnessing the intrinsic apoptotic pathway and their associations, target therapies, and mAbs are progressing more rapidly; however, owing to the acquired knowledge in lymphoproliferative disease, BiTEs and CAR-T cells could also gain ground in the next years and may turn out to be key weapons against AML.
Frail patients could particularly benefit from the synergic association of new molecules without excessive side effects, owing to different and non-overlapping toxicity profiles of new single agents, allowing for globally better outcomes in terms of treatment efficacy and quality of life. Moreover, a larger portion of patients with preserved performance status could proceed to transplant, with expected advantages in long-term survival, questioning the optimal employment of new drugs in the maintenance post-transplant setting.
On the other hand, the incorporation of new agents in IC schedules represents a hope for deeper and long-lasting responses in fit patients, maybe questioning the role of transplant for some AML patients in the future.
Moreover, several articulated sequential strategies combining different agents at different moments (induction, consolidation, and maintenance) of treatment could be imagined. Defining which is the best place for every given agent is another challenge to deal with.
No less important, on the bases of molecular and cytogenetic disease characteristics together with a better understanding in molecular biology and the immunology of LSCs, specific disease-tailored treatment could emerge.
Complex karyotype disease and very bad prognosis mutations (such as EVI1 and TP53) characterize a population that is still difficult to treat with very poor outcomes, constituting an unmet therapeutical need. More effective drugs for such poor-prognosis disease should be determined and new agents will perhaps represent interesting therapeutic options.
Undoubtedly, there is an unmet necessity for curative therapy in AML; access to new drugs should be encouraged, and well-designed, large-scale trials are needed to assess the efficacy of new molecules and to trace patient-tailored strategy. Observational data will be essential to support and to confirm clinical trial results in the real world AML population and, moreover, to provide survival outcomes on a longer-term follow-up.
In conclusion, important and exciting progress in recent years is offering new therapeutical possibilities, projecting AML treatment into a precision medicine dimension, which is disease and patient tailored and capable of improving life quality and life expectancy for most patients.
Author Contributions
Writing and editing, F.A., F.M. and A.S.; review and editing, S.W., P.L. and C.S.; F.A. created the figures (smart.servier.com accessed on 25 February 2022) and tables. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Song X., Peng Y., Wang X., Chen Y., Jin L., Yang T., Qian M., Ni W., Tong X., Lan J. Incidence, Survival, and Risk Factors for Adults with Acute Myeloid Leukemia Not Otherwise Specified and Acute Myeloid Leukemia with Recurrent Genetic Abnormalities: Analysis of the Surveillance, Epidemiology, and End Results (SEER) Database, 2001–2013. Acta Haematol. 2018;139:115–127. doi: 10.1159/000486228. [DOI] [PubMed] [Google Scholar]
- 2.Daneshbod Y., Kohan L., Taghadosi V., Weinberg O.K., Arber D.A. Prognostic Significance of Complex Karyotypes in Acute Myeloid Leukemia. Curr. Treat. Options Oncol. 2019;20:15. doi: 10.1007/s11864-019-0612-y. [DOI] [PubMed] [Google Scholar]
- 3.Kaminskas E., Farrell A.T., Wang Y.-C., Sridhara R., Pazdur R. FDA Drug Approval Summary: Azacitidine (5-Azacytidine, VidazaTM) for Injectable Suspension. Oncologist. 2005;10:176–182. doi: 10.1634/theoncologist.10-3-176. [DOI] [PubMed] [Google Scholar]
- 4.Zeidan A.M., Wang R., Wang X., Shallis R.M., Podoltsev N.A., Bewersdorf J.P., Huntington S.F., Neparidze N., Giri S., Gore S.D., et al. Clinical Outcomes of Older Patients with AML Receiving Hypomethylating Agents: A Large Population-Based Study in the United States. Blood Adv. 2020;4:2192–2201. doi: 10.1182/bloodadvances.2020001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steensma D.P., Baer M.R., Slack J.L., Buckstein R., Godley L.A., Garcia-Manero G., Albitar M., Larsen J.S., Arora S., Cullen M.T., et al. Multicenter Study of Decitabine Administered Daily for 5 Days Every 4 Weeks to Adults with Myelodysplastic Syndromes: The Alternative Dosing for Outpatient Treatment (ADOPT) Trial. J. Clin. Oncol. 2009;27:3842–3848. doi: 10.1200/JCO.2008.19.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dombret H., Seymour J.F., Butrym A., Wierzbowska A., Selleslag D., Jang J.H., Kumar R., Cavenagh J., Schuh A.C., Candoni A., et al. International Phase 3 Study of Azacitidine vs Conventional Care Regimens in Older Patients with Newly Diagnosed AML with >30% Blasts. Blood. 2015;126:291–299. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma J., Ge Z. Comparison between Decitabine and Azacitidine for Patients with Acute Myeloid Leukemia and Higher-Risk Myelodysplastic Syndrome: A Systematic Review and Network Meta-Analysis. Front. Pharmacol. 2021;12:701690. doi: 10.3389/fphar.2021.701690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czabotar P.E., Lessene G., Strasser A., Adams J.M. Control of Apoptosis by the BCL-2 Protein Family: Implications for Physiology and Therapy. Nat. Rev. Mol. Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 9.Aubrey B.J., Kelly G.L., Janic A., Herold M.J., Strasser A. How Does P53 Induce Apoptosis and How Does This Relate to P53-Mediated Tumour Suppression? Cell Death Differ. 2018;25:104–113. doi: 10.1038/cdd.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsujimoto Y., Finger L.R., Yunis J., Nowell P.C., Croce C.M. Cloning of the Chromosome Breakpoint of Neoplastic B Cells with the t(14;18) Chromosome Translocation. Science. 1984;226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 11.Singh R., Letai A., Sarosiek K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat. Rev. Mol. Cell Biol. 2019;20:175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delbridge A.R.D., Strasser A. The BCL-2 Protein Family, BH3-Mimetics and Cancer Therapy. Cell Death Differ. 2015;22:1071–1080. doi: 10.1038/cdd.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delia D., Aiello A., Soligo D., Fontanella E., Melani C., Pezzella F., Pierotti M., Della Porta G. Bcl-2 Proto-Oncogene Expression in Normal and Neoplastic Human Myeloid Cells. Blood. 1992;79:1291–1298. doi: 10.1182/blood.V79.5.1291.1291. [DOI] [PubMed] [Google Scholar]
- 14.Campos L., Rouault J., Sabido O., Oriol P., Roubi N., Vasselon C., Archimbaud E., Magaud J., Guyotat D. High Expression of Bcl-2 Protein in Acute Myeloid Leukemia Cells Is Associated with Poor Response to Chemotherapy. Blood. 1993;81:3091–3096. doi: 10.1182/blood.V81.11.3091.3091. [DOI] [PubMed] [Google Scholar]
- 15.Marcucci G., Stock W., Dai G., Klisovic R.B., Liu S., Klisovic M.I., Blum W., Kefauver C., Sher D.A., Green M., et al. Phase I Study of Oblimersen Sodium, an Antisense to Bcl-2, in Untreated Older Patients with Acute Myeloid Leukemia: Pharmacokinetics, Pharmacodynamics, and Clinical Activity. J. Clin. Oncol. 2005;23:3404–3411. doi: 10.1200/JCO.2005.09.118. [DOI] [PubMed] [Google Scholar]
- 16.Schimmer A.D., Raza A., Carter T.H., Claxton D., Erba H., DeAngelo D.J., Tallman M.S., Goard C., Borthakur G. A Multicenter Phase I/II Study of Obatoclax Mesylate Administered as a 3- or 24-Hour Infusion in Older Patients with Previously Untreated Acute Myeloid Leukemia. PLoS ONE. 2014;9:e108694. doi: 10.1371/journal.pone.0108694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason K.D., Carpinelli M.R., Fletcher J.I., Collinge J.E., Hilton A.A., Ellis S., Kelly P.N., Ekert P.G., Metcalf D., Roberts A.W., et al. Programmed Anuclear Cell Death Delimits Platelet Life Span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 18.Vogler M., Hamali H.A., Sun X.-M., Bampton E.T.W., Dinsdale D., Snowden R.T., Dyer M.J.S., Goodall A.H., Cohen G.M. BCL2/BCL-XL Inhibition Induces Apoptosis, Disrupts Cellular Calcium Homeostasis, and Prevents Platelet Activation. Blood. 2011;117:7145–7154. doi: 10.1182/blood-2011-03-344812. [DOI] [PubMed] [Google Scholar]
- 19.Souers A.J., Leverson J.D., Boghaert E.R., Ackler S.L., Catron N.D., Chen J., Dayton B.D., Ding H., Enschede S.H., Fairbrother W.J., et al. ABT-199, a Potent and Selective BCL-2 Inhibitor, Achieves Antitumor Activity While Sparing Platelets. Nat. Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 20.Stilgenbauer S., Eichhorst B., Schetelig J., Coutre S., Seymour J.F., Munir T., Puvvada S.D., Wendtner C.-M., Roberts A.W., Jurczak W., et al. Venetoclax in Relapsed or Refractory Chronic Lymphocytic Leukaemia with 17p Deletion: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2016;17:768–778. doi: 10.1016/S1470-2045(16)30019-5. [DOI] [PubMed] [Google Scholar]
- 21.Pollyea D.A., Pratz K.W., Jonas B.A., Letai A., Pullarkat V.A., Wei A., Konopleva M.Y., Recher C., Frankfurt O., Rizzieri D., et al. Venetoclax in Combination with Hypomethylating Agents Induces Rapid, Deep, and Durable Responses in Patients with AML Ineligible for Intensive Therapy. Blood. 2018;132:285. doi: 10.1182/blood-2018-99-117179. [DOI] [Google Scholar]
- 22.Guerra V.A., DiNardo C., Konopleva M. Venetoclax-Based Therapies for Acute Myeloid Leukemia. Best Pract. Res. Clin. Haematol. 2019;32:145–153. doi: 10.1016/j.beha.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan R., Hogdal L.J., Benito J.M., Bucci D., Han L., Borthakur G., Cortes J., DeAngelo D.J., Debose L., Mu H., et al. Selective BCL-2 Inhibition by ABT-199 Causes On-Target Cell Death in Acute Myeloid Leukemia. Cancer Discov. 2014;4:362–375. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konopleva M., Pollyea D.A., Potluri J., Chyla B., Hogdal L., Busman T., McKeegan E., Salem A.H., Zhu M., Ricker J.L., et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016;6:1106–1117. doi: 10.1158/2159-8290.CD-16-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsao T., Shi Y., Kornblau S., Lu H., Konoplev S., Antony A., Ruvolo V., Qiu Y.H., Zhang N., Coombes K.R., et al. Concomitant Inhibition of DNA Methyltransferase and BCL-2 Protein Function Synergistically Induce Mitochondrial Apoptosis in Acute Myelogenous Leukemia Cells. Ann. Hematol. 2012;91:1861–1870. doi: 10.1007/s00277-012-1537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiNardo C.D., Pratz K., Pullarkat V., Jonas B.A., Arellano M., Becker P.S., Frankfurt O., Konopleva M., Wei A.H., Kantarjian H.M., et al. Venetoclax Combined with Decitabine or Azacitidine in Treatment-Naive, Elderly Patients with Acute Myeloid Leukemia. Blood. 2019;133:7–17. doi: 10.1182/blood-2018-08-868752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiNardo C.D., Jonas B.A., Pullarkat V., Thirman M.J., Garcia J.S., Wei A.H., Konopleva M., Döhner H., Letai A., Fenaux P., et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020;383:617–629. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 28.Aldoss I., Yang D., Pillai R., Sanchez J.F., Mei M., Aribi A., Ali H., Sandhu K., Al Malki M.M., Salhotra A., et al. Association of Leukemia Genetics with Response to Venetoclax and Hypomethylating Agents in Relapsed/Refractory Acute Myeloid Leukemia. Am. J. Hematol. 2019;94:E253–E255. doi: 10.1002/ajh.25567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandhu K.S., Dadwal S., Yang D., Mei M., Palmer J., Salhotra A., Al Malki M., Aribi A., Ali H., Khaled S., et al. Outcome of Allogeneic Hematopoietic Cell Transplantation after Venetoclax and Hypomethylating Agent Therapy for Acute Myelogenous Leukemia. Biol. Blood Marrow Transplant. 2020;26:e322–e327. doi: 10.1016/j.bbmt.2020.08.027. [DOI] [PubMed] [Google Scholar]
- 30.Maiti A., DiNardo C.D., Qiao W., Kadia T.M., Jabbour E.J., Rausch C.R., Daver N.G., Short N.J., Borthakur G., Pemmaraju N., et al. Ten-day Decitabine with Venetoclax versus Intensive Chemotherapy in Relapsed or Refractory Acute Myeloid Leukemia: A Propensity Score-matched Analysis. Cancer. 2021;127:4213–4220. doi: 10.1002/cncr.33814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuler E., Wagner-Drouet E.-M., Ajib S., Bug G., Crysandt M., Dressler S., Hausmann A., Heidenreich D., Hirschbühl K., Hoepting M., et al. Treatment of Myeloid Malignancies Relapsing after Allogeneic Hematopoietic Stem Cell Transplantation with Venetoclax and Hypomethylating Agents—A Retrospective Multicenter Analysis on Behalf of the German Cooperative Transplant Study Group. Ann. Hematol. 2021;100:959–968. doi: 10.1007/s00277-020-04321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei A.H., Strickland S.A., Hou J.-Z., Fiedler W., Lin T.L., Walter R.B., Enjeti A., Tiong I.S., Savona M., Lee S., et al. Venetoclax Combined with Low-Dose Cytarabine for Previously Untreated Patients with Acute Myeloid Leukemia: Results from a Phase Ib/II Study. J. Clin. Oncol. 2019;37:1277–1284. doi: 10.1200/JCO.18.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei A.H., Montesinos P., Ivanov V., DiNardo C.D., Novak J., Laribi K., Kim I., Stevens D.A., Fiedler W., Pagoni M., et al. Venetoclax plus LDAC for Newly Diagnosed AML Ineligible for Intensive Chemotherapy: A Phase 3 Randomized Placebo-Controlled Trial. Blood. 2020;135:2137–2145. doi: 10.1182/blood.2020004856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadia T.M., Reville P.K., Borthakur G., Yilmaz M., Kornblau S., Alvarado Y., Dinardo C.D., Daver N., Jain N., Pemmaraju N., et al. Venetoclax plus Intensive Chemotherapy with Cladribine, Idarubicin, and Cytarabine in Patients with Newly Diagnosed Acute Myeloid Leukaemia or High-Risk Myelodysplastic Syndrome: A Cohort from a Single-Centre, Single-Arm, Phase 2 Trial. Lancet Haematol. 2021;8:e552–e561. doi: 10.1016/S2352-3026(21)00192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chua C.C., Roberts A.W., Reynolds J., Fong C.Y., Ting S.B., Salmon J.M., MacRaild S., Ivey A., Tiong I.S., Fleming S., et al. Chemotherapy and Venetoclax in Elderly Acute Myeloid Leukemia Trial (CAVEAT): A Phase Ib Dose-Escalation Study of Venetoclax Combined with Modified Intensive Chemotherapy. J. Clin. Oncol. 2020;38:3506–3517. doi: 10.1200/JCO.20.00572. [DOI] [PubMed] [Google Scholar]
- 36.DiNardo C.D., Lachowiez C.A., Takahashi K., Loghavi S., Xiao L., Kadia T., Daver N., Adeoti M., Short N.J., Sasaki K., et al. Venetoclax Combined With FLAG-IDA Induction and Consolidation in Newly Diagnosed and Relapsed or Refractory Acute Myeloid Leukemia. J. Clin. Oncol. 2021;39:2768–2778. doi: 10.1200/JCO.20.03736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curtis Lachowiez, MD1, Courtney D. DiNardo, MD, MSCE2, Koichi Takahashi, MD, PhD3, Sanam Loghavi, MD4, Lian-Chun Xiao5*, Tapan M. Kadia, MD6, Naval Daver, MD3, Maria Adeoti, R.N.3*, Nicholas J. Short, MD2, Koji Sasaki, MD7, Sa A Wang, MD8*, Gautam Borthakur, MD3, Ghayas C. Issa, MD3, Abhishek Maiti, MBBS2, Yesid Alvarado, MD2, Naveen Pemmaraju, MD3, Guillermo Montalban-Bravo, MD2, Lucia Masarova, MD2*, Musa Yilmaz, MD3, Nitin Jain, MD7, Michael Andreeff, MD, PhD3, Guillermo Garcia-Manero, MD3, Steven Kornblau, MD3, Farhad Ravandi, MBBS3, Elias J. Jabbour, MD3, Marina Konopleva, MD, PhD2 and Hagop Kantarjian, MD2 701 Venetoclax Combined with FLAG-IDA Induction and Consolidation in Newly Diagnosed Acute Myeloid Leukemia–Oral communication ASH. 2021. [(accessed on 13 December 2021)]. Available online: https://ash.confex.com/ash/2021/webprogram/Paper150457.html.
- 38.Lachowiez C., DiNardo C.D., Takahashi K., Loghavi S., Xiao L.-C., Kadia T.M., Daver N., Adeoti M., Short N.J., Sasaki K., et al. Venetoclax Combined with FLAG-IDA Induction and Consolidation in Newly Diagnosed Acute Myeloid Leukemia. Blood. 2021;138:701. doi: 10.1182/blood-2021-150457. [DOI] [PubMed] [Google Scholar]
- 39.Maiti A., DiNardo C.D., Daver N.G., Rausch C.R., Ravandi F., Kadia T.M., Pemmaraju N., Borthakur G., Bose P., Issa G.C., et al. Triplet Therapy with Venetoclax, FLT3 Inhibitor and Decitabine for FLT3-Mutated Acute Myeloid Leukemia. Blood Cancer J. 2021;11:25. doi: 10.1038/s41408-021-00410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chyla B., Daver N., Doyle K., McKeegan E., Huang X., Ruvolo V., Wang Z., Chen K., Souers A., Leverson J., et al. Genetic Biomarkers of Sensitivity and Resistance to Venetoclax Monotherapy in Patients with Relapsed Acute Myeloid Leukemia. Am. J. Hematol. 2018;93:E202. doi: 10.1002/ajh.25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasper S., Breitenbuecher F., Heidel F., Hoffarth S., Markova B., Schuler M., Fischer T. Targeting MCL-1 Sensitizes FLT3-ITD-Positive Leukemias to Cytotoxic Therapies. Blood Cancer J. 2012;2:e60. doi: 10.1038/bcj.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daver N., Perl A.E., Maly J., Levis M., Ritchie E., Litzow M.R., McCloskey J., Smith C.C., Schiller G.J., Bradley T., et al. Venetoclax in Combination with Gilteritinib Demonstrates Molecular Clearance of FLT3 Mutation in Relapsed/Refractory FLT3-Mutated Acute Myeloid Leukemia. Blood. 2021;138:691. doi: 10.1182/blood-2021-150743. [DOI] [Google Scholar]
- 43.Short N.J., DiNardo C.D., Daver N., Nguyen D., Yilmaz M., Kadia T.M., Garcia-Manero G., Issa G.C., Huang X., Qiao W., et al. A Triplet Combination of Azacitidine, Venetoclax and Gilteritinib for Patients with FLT3-Mutated Acute Myeloid Leukemia: Results from a Phase I/II Study. Blood. 2021;138:696. doi: 10.1182/blood-2021-153571. [DOI] [Google Scholar]
- 44.Musa Yilmaz M., Hagop Kantarjian H., Short N.J., Konopleva M., Kadia T.M., DiNardo C.D., Borthakur G., Naveen Pemmaraju N., Maiti A. 798 Hypomethylating Agent (HMA) Therapy and Venetoclax (VEN) with FLT3 Inhibitor “Triplet” Therapy Is Highly Active in Older/Unfit Patients with FLT3 Mutated AML. Oral Communication ASH. 2021. [(accessed on 13 December 2021)]. Available online: https://ashpublications.org/blood/article/138/Supplement%201/798/480052/Hypomethylating-Agent-HMA-Therapy-and-Venetoclax.
- 45.Chan S.M., Thomas D., Corces-Zimmerman M.R., Xavy S., Rastogi S., Hong W.-J., Zhao F., Medeiros B.C., Tyvoll D.A., Majeti R. Isocitrate Dehydrogenase 1 and 2 Mutations Induce BCL-2 Dependence in Acute Myeloid Leukemia. Nat. Med. 2015;21:178–184. doi: 10.1038/nm.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollyea D.A., Dinardo C.D., Arellano M.L., Pigneux A., Fiedler W., Konopleva M., Rizzieri D.A., Smith B.D., Shinagawa A., Lemoli R.M., et al. Results of Venetoclax and Azacitidine Combination in Chemotherapy Ineligible Untreated Patients with Acute Myeloid Leukemia with IDH 1/2 Mutations. Blood. 2020;136:5–7. doi: 10.1182/blood-2020-134736. [DOI] [Google Scholar]
- 47.Pollyea D.A., DiNardo C.D., Arellano M.L., Pigneux A., Fiedler W., Konopleva M., Rizzieri D.A., Smith B.D., Shinagawa A., Lemoli R.M., et al. Impact of Venetoclax and Azacitidine in Treatment-Naïve Patients with Acute Myeloid Leukemia and IDH1/2 Mutations. Clin. Cancer Res. 2022 doi: 10.1158/1078-0432.CCR-21-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lachowiez C.A., Borthakur G., Loghavi S., Zeng Z., Kadia T.M., Masarova L., Takahashi K., Tippett G.D., Smith S., Garcia J.S., et al. A Phase Ib/II Study of Ivosidenib with Venetoclax +/− Azacitidine in IDH1-Mutated Myeloid Malignancies. J. Clin. Oncol. 2021;39:7012. doi: 10.1200/JCO.2021.39.15_suppl.7012. [DOI] [Google Scholar]
- 49.Chan S.M., Cameron C., Cathelin S., Gupta V., Maze D., Minden M.D., Murphy T., Schimmer A.D., Schuh A.C., Sibai H., et al. Enasidenib in Combination with Venetoclax in IDH2-Mutated Myeloid Malignancies: Preliminary Results of the Phase Ib/II Enaven-AML Trial. Blood. 2021;138:1263. doi: 10.1182/blood-2021-153660. [DOI] [Google Scholar]
- 50.Daver N., Konopleva M., Maiti A., Kadia T.M., DiNardo C.D., Loghavi S., Pemmaraju N., Jabbour E.J., Montalban-Bravo G., Tang G., et al. Phase I/II Study of Azacitidine (AZA) with Venetoclax (VEN) and Magrolimab (Magro) in Patients (Pts) with Newly Diagnosed Older/Unfit or High-Risk Acute Myeloid Leukemia (AML) and Relapsed/Refractory (R/R) AML. Blood. 2021;138:371. doi: 10.1182/blood-2021-153638. [DOI] [Google Scholar]
- 51.Borthakur G., Odenike O., Aldoss I., Rizzieri D.A., Prebet T., Chen C., Popovic R., Modi D.A., Joshi R.H., Wolff J.E., et al. A Phase 1 Study of the Pan-bromodomain and Extraterminal Inhibitor Mivebresib (ABBV-075) Alone or in Combination with Venetoclax in Patients with Relapsed/Refractory Acute Myeloid Leukemia. Cancer. 2021;127:2943–2953. doi: 10.1002/cncr.33590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolomsky A., Vogler M., Köse M.C., Heckman C.A., Ehx G., Ludwig H., Caers J. MCL-1 Inhibitors, Fast-Lane Development of a New Class of Anti-Cancer Agents. J. Hematol. Oncol. 2020;13:173. doi: 10.1186/s13045-020-01007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshimoto G., Miyamoto T., Jabbarzadeh-Tabrizi S., Iino T., Rocnik J.L., Kikushige Y., Mori Y., Shima T., Iwasaki H., Takenaka K., et al. FLT3-ITD up-Regulates MCL-1 to Promote Survival of Stem Cells in Acute Myeloid Leukemia via FLT3-ITD–Specific STAT5 Activation. Blood. 2009;114:5034–5043. doi: 10.1182/blood-2008-12-196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaufmann S.H., Karp J.E., Svingen P.A., Krajewski S., Burke P.J., Gore S.D., Reed J.C. Elevated Expression of the Apoptotic Regulator Mcl-1 at the Time of Leukemic Relapse. Blood. 1998;91:991–1000. doi: 10.1182/blood.V91.3.991.991_991_1000. [DOI] [PubMed] [Google Scholar]
- 55.Li X., Zhou J., Wen X., Zhang T., Wu D., Deng Z., Zhang Z., Lian X., He P., Yao X., et al. Increased MCL-1 Expression Predicts Poor Prognosis and Disease Recurrence in Acute Myeloid Leukemia. OncoTargets Ther. 2019;12:3295–3304. doi: 10.2147/OTT.S194549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramsey H.E., Fischer M.A., Lee T., Gorska A.E., Arrate M.P., Fuller L., Boyd K.L., Strickland S.A., Sensintaffar J., Hogdal L.J., et al. A Novel MCL1 Inhibitor Combined with Venetoclax Rescues Venetoclax-Resistant Acute Myelogenous Leukemia. Cancer Discov. 2018;8:1566–1581. doi: 10.1158/2159-8290.CD-18-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hormi M., Birsen R., Belhadj M., Huynh T., Cantero Aguilar L., Grignano E., Haddaoui L., Guillonneau F., Mayeux P., Hunault M., et al. Pairing MCL-1 Inhibition with Venetoclax Improves Therapeutic Efficiency of BH3-mimetics in AML. Eur. J. Haematol. 2020;105:588–596. doi: 10.1111/ejh.13492. [DOI] [PubMed] [Google Scholar]
- 58.Papaemmanuil E., Gerstung M., Bullinger L., Gaidzik V.I., Paschka P., Roberts N.D., Potter N.E., Heuser M., Thol F., Bolli N., et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016;374:2209–2221. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hunter A.M., Sallman D.A. Current Status and New Treatment Approaches in TP53 Mutated AML. Best Pract. Res. Clin. Haematol. 2019;32:134–144. doi: 10.1016/j.beha.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Döhner H., Dolnik A., Tang L., Seymour J.F., Minden M.D., Stone R.M., del Castillo T.B., Al-Ali H.K., Santini V., Vyas P., et al. Cytogenetics and Gene Mutations Influence Survival in Older Patients with Acute Myeloid Leukemia Treated with Azacitidine or Conventional Care. Leukemia. 2018;32:2546–2557. doi: 10.1038/s41375-018-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lambert J.M.R., Gorzov P., Veprintsev D.B., Söderqvist M., Segerbäck D., Bergman J., Fersht A.R., Hainaut P., Wiman K.G., Bykov V.J.N. PRIMA-1 Reactivates Mutant P53 by Covalent Binding to the Core Domain. Cancer Cell. 2009;15:376–388. doi: 10.1016/j.ccr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Deneberg S., Cherif H., Lazarevic V., Andersson P.-O., von Euler M., Juliusson G., Lehmann S. An Open-Label Phase I Dose-Finding Study of APR-246 in Hematological Malignancies. Blood Cancer J. 2016;6:e447. doi: 10.1038/bcj.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lehmann S., Bykov V.J.N., Ali D., Andrén O., Cherif H., Tidefelt U., Uggla B., Yachnin J., Juliusson G., Moshfegh A., et al. Targeting P53 in Vivo: A First-in-Human Study with P53-Targeting Compound APR-246 in Refractory Hematologic Malignancies and Prostate Cancer. J. Clin. Oncol. 2012;30:3633–3639. doi: 10.1200/JCO.2011.40.7783. [DOI] [PubMed] [Google Scholar]
- 64.Sallman D.A., DeZern A.E., Garcia-Manero G., Steensma D.P., Roboz G.J., Sekeres M.A., Cluzeau T., Sweet K.L., McLemore A., McGraw K.L., et al. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes. J. Clin. Oncol. 2021;39:1584–1594. doi: 10.1200/JCO.20.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iwakuma T., Lozano G. MDM2, an Introduction. Mol. Cancer Res. 2003;1:993–1000. [PubMed] [Google Scholar]
- 66.Shvarts A., Steegenga W.T., Riteco N., van Laar T., Dekker P., Bazuine M., van Ham R.C., van der Houven van Oordt W., Hateboer G., van der Eb A.J., et al. MDMX: A Novel P53-Binding Protein with Some Functional Properties of MDM2. EMBO J. 1996;15:5349–5357. doi: 10.1002/j.1460-2075.1996.tb00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lehmann C., Friess T., Birzele F., Kiialainen A., Dangl M. Superior Anti-Tumor Activity of the MDM2 Antagonist Idasanutlin and the Bcl-2 Inhibitor Venetoclax in P53 Wild-Type Acute Myeloid Leukemia Models. J. Hematol. Oncol. 2016;9:50. doi: 10.1186/s13045-016-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carvajal L.A., Neriah D.B., Senecal A., Benard L., Thiruthuvanathan V., Yatsenko T., Narayanagari S.-R., Wheat J.C., Todorova T.I., Mitchell K., et al. Dual Inhibition of MDMX and MDM2 as a Therapeutic Strategy in Leukemia. Sci. Transl. Med. 2018;10:eaao3003. doi: 10.1126/scitranslmed.aao3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andreeff M., Kelly K.R., Yee K., Assouline S., Strair R., Popplewell L., Bowen D., Martinelli G., Drummond M.W., Vyas P., et al. Results of the Phase I Trial of RG7112, a Small-Molecule MDM2 Antagonist in Leukemia. Clin. Cancer Res. 2016;22:868–876. doi: 10.1158/1078-0432.CCR-15-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vu B., Wovkulich P., Pizzolato G., Lovey A., Ding Q., Jiang N., Liu J.-J., Zhao C., Glenn K., Wen Y., et al. Discovery of RG7112: A Small-Molecule MDM2 Inhibitor in Clinical Development. ACS Med. Chem. Lett. 2013;4:466–469. doi: 10.1021/ml4000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yee K., Martinelli G., Assouline S., Kasner M., Vey N., Kelly K.R., Drummond M.W., Dennis M., Seiter K., Blotner S., et al. Phase 1b Study of the MDM2 Antagonist RG7112 in Combination with 2 Doses/Schedules of Cytarabine. Blood. 2013;122:498. doi: 10.1182/blood.V122.21.498.498. [DOI] [Google Scholar]
- 72.Zhang B., Golding B.T., Hardcastle I.R. Small-Molecule MDM2-P53 Inhibitors: Recent Advances. Future Med. Chem. 2015;7:631–645. doi: 10.4155/fmc.15.13. [DOI] [PubMed] [Google Scholar]
- 73.Ding Q., Zhang Z., Liu J.-J., Jiang N., Zhang J., Ross T.M., Chu X.-J., Bartkovitz D., Podlaski F., Janson C., et al. Discovery of RG7388, a Potent and Selective P53–MDM2 Inhibitor in Clinical Development. J. Med. Chem. 2013;56:5979–5983. doi: 10.1021/jm400487c. [DOI] [PubMed] [Google Scholar]
- 74.Lanza B., Martinelli G., Yee K.W.L., Jukofsky L., Reis B., Blotner S., Drummond M.W., Vey N., Seiter K., Dickinson M.J., et al. Minimal Residual Disease (MRD) Assessment by Multiparametric Flow Cytometry Is Prognostic for Progression-Free Survival in Phase 1/1b Relapsed/Refractory Acute Myeloid Leukemia (AML) Patients Treated with Idasanutlin MDM2 Antagonist. Blood. 2016;128:2843. doi: 10.1182/blood.V128.22.2843.2843. [DOI] [Google Scholar]
- 75.Montesinos P., Beckermann B.M., Catalani O., Esteve J., Gamel K., Konopleva M.Y., Martinelli G., Monnet A., Papayannidis C., Park A., et al. MIRROS: A Randomized, Placebo-Controlled, Phase III Trial of Cytarabine ± Idasanutlin in Relapsed or Refractory Acute Myeloid Leukemia. Future Oncol. 2020;16:807–815. doi: 10.2217/fon-2020-0044. [DOI] [PubMed] [Google Scholar]
- 76.Daver N.G., Garcia J.S., Jonas B.A., Kelly K.R., Assouline S., Brandwein J.M., Fenaux P., Olin R.L., Martinelli G., Paolini S., et al. Updated Results from the Venetoclax (Ven) in Combination with Idasanutlin (Idasa) Arm of a Phase 1b Trial in Elderly Patients (Pts) with Relapsed or Refractory (R/R) AML Ineligible for Cytotoxic Chemotherapy. Blood. 2019;134:229. doi: 10.1182/blood-2019-123711. [DOI] [Google Scholar]
- 77.Erba H.P., Becker P.S., Shami P.J., Grunwald M.R., Flesher D.L., Zhang Y., Rasmussen E., Henary H.A., Wang E.S. Dose Escalation Results of a Phase 1b Study of the MDM2 Inhibitor AMG 232 with or without Trametinib in Patients (Pts) with Relapsed/Refractory (r/r) Acute Myeloid Leukemia (AML) J. Clin. Oncol. 2017;35:7027. doi: 10.1200/JCO.2017.35.15_suppl.7027. [DOI] [Google Scholar]
- 78.DiNardo C.D., Rosenthal J., Andreeff M., Zernovak O., Kumar P., Gajee R., Chen S., Rosen M., Song S., Kochan J., et al. Phase 1 Dose Escalation Study of MDM2 Inhibitor DS-3032b in Patients with Hematological Malignancies—Preliminary Results. Blood. 2016;128:593. doi: 10.1182/blood.V128.22.593.593. [DOI] [Google Scholar]
- 79.Daver N.G., Zhang W., Graydon R., Dawra V., Xie J., Kumar P., Andreeff M. A Phase I Study of Milademetan in Combination with Quizartinib in Patients (Pts) with Newly Diagnosed (ND) or Relapsed/Refractory (R/R) FLT3-ITD Acute Myeloid Leukemia (AML) J. Clin. Oncol. 2019;37:TPS7067. doi: 10.1200/JCO.2019.37.15_suppl.TPS7067. [DOI] [Google Scholar]
- 80.Sallman D.A., Borate U., Cull E.H., Donnellan W.B., Komrokji R.S., Steidl U.G., Corvez M.M., Payton M., Annis D.A., Pinchasik D., et al. Phase 1/1b Study of the Stapled Peptide ALRN-6924, a Dual Inhibitor of MDMX and MDM2, As Monotherapy or in Combination with Cytarabine for the Treatment of Relapsed/Refractory AML and Advanced MDS with TP53 Wild-Type. Blood. 2018;132:4066. doi: 10.1182/blood-2018-99-118780. [DOI] [Google Scholar]
- 81.Khurana A., Shafer D.A. MDM2 Antagonists as a Novel Treatment Option for Acute Myeloid Leukemia: Perspectives on the Therapeutic Potential of Idasanutlin (RG7388) OncoTargets Ther. 2019;12:2903–2910. doi: 10.2147/OTT.S172315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.on behalf of the Study Alliance Leukemia. Ehninger A., Kramer M., Röllig C., Thiede C., Bornhäuser M., von Bonin M., Wermke M., Feldmann A., Bachmann M., et al. Distribution and Levels of Cell Surface Expression of CD33 and CD123 in Acute Myeloid Leukemia. Blood Cancer J. 2014;4:e218. doi: 10.1038/bcj.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Appelbaum F.R., Matthews D.C., Eary J.F., Badger C.C., Kellogg M., Press O.W., Martin P.J., Fisher D.R., Nelp W.B., Thomas E.D., et al. The Use of Radiolabeled Anti-Cd33 Antibody to Augment Marrow Irradiation Prior to Marrow Transplantation for Acute Myelogenous Leukemia. Transplantation. 1992;54:829–833. doi: 10.1097/00007890-199211000-00012. [DOI] [PubMed] [Google Scholar]
- 84.Hamann P.R., Hinman L.M., Hollander I., Beyer C.F., Lindh D., Holcomb R., Hallett W., Tsou H.-R., Upeslacis J., Shochat D., et al. Gemtuzumab Ozogamicin, A Potent and Selective Anti-CD33 Antibody−Calicheamicin Conjugate for Treatment of Acute Myeloid Leukemia. Bioconjug. Chem. 2002;13:47–58. doi: 10.1021/bc010021y. [DOI] [PubMed] [Google Scholar]
- 85.Ricart A.D. Antibody-Drug Conjugates of Calicheamicin Derivative: Gemtuzumab Ozogamicin and Inotuzumab Ozogamicin. Clin. Cancer Res. 2011;17:6417–6427. doi: 10.1158/1078-0432.CCR-11-0486. [DOI] [PubMed] [Google Scholar]
- 86.Sievers E.L., Larson R.A., Stadtmauer E.A., Estey E., Löwenberg B., Dombret H., Karanes C., Theobald M., Bennett J.M., Sherman M.L., et al. Efficacy and Safety of Gemtuzumab Ozogamicin in Patients with CD33-Positive Acute Myeloid Leukemia in First Relapse. J. Clin. Oncol. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 87.Larson R.A., Sievers E.L., Stadtmauer E.A., Löwenberg B., Estey E.H., Dombret H., Theobald M., Voliotis D., Bennett J.M., Richie M., et al. Final Report of the Efficacy and Safety of Gemtuzumab Ozogamicin (Mylotarg) in Patients with CD33-Positive Acute Myeloid Leukemia in First Recurrence. Cancer. 2005;104:1442–1452. doi: 10.1002/cncr.21326. [DOI] [PubMed] [Google Scholar]
- 88.Petersdorf S.H., Kopecky K.J., Slovak M., Willman C., Nevill T., Brandwein J., Larson R.A., Erba H.P., Stiff P.J., Stuart R.K., et al. A Phase 3 Study of Gemtuzumab Ozogamicin during Induction and Postconsolidation Therapy in Younger Patients with Acute Myeloid Leukemia. Blood. 2013;121:4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bross P.F., Beitz J., Chen G., Chen X.H., Duffy E., Kieffer L., Roy S., Sridhara R., Rahman A., Williams G., et al. Approval Summary. Clin. Cancer Res. 2001;7:1490. [PubMed] [Google Scholar]
- 90.Giles F.J., Kantarjian H.M., Kornblau S.M., Thomas D.A., Garcia-Manero G., Waddelow T.A., David C.L., Phan A.T., Colburn D.E., Rashid A., et al. Mylotarg? (Gemtuzumab Ozogamicin) Therapy Is Associated with Hepatic Venoocclusive Disease in Patients Who Have Not Received Stem Cell Transplantation. Cancer. 2001;92:406–413. doi: 10.1002/1097-0142(20010715)92:2<406::AID-CNCR1336>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 91.Hills R.K., Castaigne S., Appelbaum F.R., Delaunay J., Petersdorf S., Othus M., Estey E.H., Dombret H., Chevret S., Ifrah N., et al. Addition of Gemtuzumab Ozogamicin to Induction Chemotherapy in Adult Patients with Acute Myeloid Leukaemia: A Meta-Analysis of Individual Patient Data from Randomised Controlled Trials. Lancet Oncol. 2014;15:986–996. doi: 10.1016/S1470-2045(14)70281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Budaeva I., Zaytsev D., Shatilova A., Tochenaya E., Petrov A., Vabishchevich R., Motorin D., Badaev R., Ivanov V., Bogdanov K., et al. AML-288: The Combination of Gemtuzumab Ozogamicin and Azacitidine in the Treatment of Relapsed and Refractory AML. Clin. Lymphoma Myeloma Leuk. 2021;21:S301. doi: 10.1016/S2152-2650(21)01716-X. [DOI] [Google Scholar]
- 93.Arain S., Patel P., Sweiss K., Parkin B., Konig H., Calip G., Yavuz B.G., Quigley J. Proceedings of the Clinical Trials. American Association for Cancer Research; Philadelphi, PA, USA: 2021. Abstract CT224: Phase Ib Study of the Safety and Efficacy of Gemtuzumab Ozogamicin (GO) and Venetoclax in Patients with Relapsed or Refractory CD33+ Acute Myeloid Leukemia: Big Ten Cancer Research Consortium BTCRC-AML17-113; p. CT224. [Google Scholar]
- 94.Yamauchi T., Uzui K., Nishi R., Shigemi H., Ueda T. Gemtuzumab Ozogamicin and Olaparib Exert Synergistic Cytotoxicity in CD33-Positive HL-60 Myeloid Leukemia Cells. Anticancer Res. 2014;34:5487. [PubMed] [Google Scholar]
- 95.Portwood S.M., Cantella M.C., Cronin T.L., Wang E.S. Addition of the PARP Inhibitor, Talazoparib, to Gemtuzumab Ozogamicin Significantly Enhances Anti-Leukemic Activity in Human CD33+ Acute Myeloid Leukemia. Blood. 2019;134:1371. doi: 10.1182/blood-2019-130427. [DOI] [Google Scholar]
- 96.Mizutani Y., Inase A., Maimaitili Y., Miyata Y., Kitao A., Matsumoto H., Kawaguchi K., Higashime A., Goto H., Kurata K., et al. An MTORC1/2 Dual Inhibitor, AZD2014, Acts as a Lysosomal Function Activator and Enhances Gemtuzumab Ozogamicin-Induced Apoptosis in Primary Human Leukemia Cells. Int. J. Hematol. 2019;110:490–499. doi: 10.1007/s12185-019-02701-2. [DOI] [PubMed] [Google Scholar]
- 97.Röllig C., Schliemann C., Mikesch J.-H., Fransecky L., Baldus C.D., Heydrich B.-N., Hanoun M., Noppeney R., Scholl S., Schnetzke U., et al. Gemtuzumab Ozogamicin Plus Midostaurin in Combination with Standard Intensive Induction Therapy in Newly Diagnosed AML: Results from a Phase-I Study. Blood. 2021;138:2324. doi: 10.1182/blood-2021-150069. [DOI] [PubMed] [Google Scholar]
- 98.Bixby D.L., Stein A.S., Fathi A.T., Kovacsovics T.J., Levy M.Y., Erba H.P., Lancet J.E., Jillella A.P., Ravandi F., Walter R.B., et al. Vadastuximab Talirine Monotherapy in Older Patients with Treatment Naive CD33-Positive Acute Myeloid Leukemia (AML) Blood. 2016;128:590. doi: 10.1182/blood.V128.22.590.590. [DOI] [Google Scholar]
- 99.Fathi A.T., Erba H.P., Lancet J.E., Stein E.M., Ravandi F., Faderl S., Walter R.B., Advani A., DeAngelo D.J., Kovacsovics T.J., et al. Vadastuximab Talirine Plus Hypomethylating Agents: A Well-Tolerated Regimen with High Remission Rate in Frontline Older Patients with Acute Myeloid Leukemia (AML) Blood. 2016;128:591. doi: 10.1182/blood.V128.22.591.591. [DOI] [Google Scholar]
- 100.Liu K., Zhu M., Huang Y., Wei S., Xie J., Xiao Y. CD123 and Its Potential Clinical Application in Leukemias. Life Sci. 2015;122:59–64. doi: 10.1016/j.lfs.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 101.Bras A.E., Haas V., Stigt A., Jongen-Lavrencic M., Beverloo H.B., Marvelde J.G., Zwaan C.M., Dongen J.J.M., Leusen J.H.W., Velden V.H.J. CD123 Expression Levels in 846 Acute Leukemia Patients Based on Standardized Immunophenotyping. Cytometry. 2019;96:134–142. doi: 10.1002/cyto.b.21745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xue T., Budde L.E. Immunotherapies Targeting CD123 for Blastic Plasmacytoid Dendritic Cell Neoplasm. Hematol. Oncol. Clin. N. Am. 2020;34:575–587. doi: 10.1016/j.hoc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 103.Stevens B.M., Khan N., D’Alessandro A., Nemkov T., Winters A., Jones C.L., Zhang W., Pollyea D.A., Jordan C.T. Characterization and Targeting of Malignant Stem Cells in Patients with Advanced Myelodysplastic Syndromes. Nat. Commun. 2018;9:3694. doi: 10.1038/s41467-018-05984-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Teodosio C., Mayado A., Sa´nchez-Mun~oz L., Morgado J.M., Jara-Acevedo M., A´lvarez-Twose I., Garci´a-Montero A.C., Matito A., Caldas C., Escribano L., et al. The Immunophenotype of Mast Cells and Its Utility in the Diagnostic Work-up of Systemic Mastocytosis. J. Leukoc. Biol. 2015;97:49–59. doi: 10.1189/jlb.5RU0614-296R. [DOI] [PubMed] [Google Scholar]
- 105.Frolova O., Benito J., Brooks C., Wang R.-Y., Korchin B., Rowinsky E.K., Cortes J., Kantarjian H., Andreeff M., Frankel A.E., et al. SL-401 and SL-501, Targeted Therapeutics Directed at the Interleukin-3 Receptor, Inhibit the Growth of Leukaemic Cells and Stem Cells in Advanced Phase Chronic Myeloid Leukaemia. Br. J. Haematol. 2014;166:862–874. doi: 10.1111/bjh.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Angelova E., Audette C., Kovtun Y., Daver N., Wang S.A., Pierce S., Konoplev S.N., Khogeer H., Jorgensen J.L., Konopleva M., et al. CD123 Expression Patterns and Selective Targeting with a CD123-Targeted Antibody-Drug Conjugate (IMGN632) in Acute Lymphoblastic Leukemia. Haematologica. 2019;104:749–755. doi: 10.3324/haematol.2018.205252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Del Giudice I., Matutes E., Morilla R., Morilla A., Owusu-Ankomah K., Rafiq F., A’Hern R., Delgado J., Bazerbashi M.B., Catovsky D. The Diagnostic Value of CD123 in B-Cell Disorders with Hairy or Villous Lymphocytes. Haematologica. 2004;89:303–308. [PubMed] [Google Scholar]
- 108.Pemmaraju N., Lane A.A., Sweet K.L., Stein A.S., Vasu S., Blum W., Rizzieri D.A., Wang E.S., Duvic M., Sloan J.M., et al. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. N. Engl. J. Med. 2019;380:1628–1637. doi: 10.1056/NEJMoa1815105. [DOI] [PubMed] [Google Scholar]
- 109.Frankel A., Liu J.-S., Rizzieri D., Hogge D. Phase I Clinical Study of Diphtheria Toxin-Interleukin 3 Fusion Protein in Patients with Acute Myeloid Leukemia and Myelodysplasia. Leuk. Lymphoma. 2008;49:543–553. doi: 10.1080/10428190701799035. [DOI] [PubMed] [Google Scholar]
- 110.Lane A.A., Stein A.S., Garcia J.S., Garzon J.L., Galinsky I., Luskin M.R., Stone R.M., Winer E.S., Leonard R., Mughal T.I., et al. Safety and Efficacy of Combining Tagraxofusp (SL-401) with Azacitidine or Azacitidine and Venetoclax in a Phase 1b Study for CD123 Positive AML, MDS, or BPDCN. Blood. 2021;138:2346. doi: 10.1182/blood-2021-147486. [DOI] [Google Scholar]
- 111.Williams B.A., Law A., Hunyadkurti J., Desilets S., Leyton J.V., Keating A. Antibody Therapies for Acute Myeloid Leukemia: Unconjugated, Toxin-Conjugated, Radio-Conjugated and Multivalent Formats. J. Clin. Med. 2019;8:E1261. doi: 10.3390/jcm8081261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feldman E.J., Brandwein J., Stone R., Kalaycio M., Moore J., O’Connor J., Wedel N., Roboz G.J., Miller C., Chopra R., et al. Phase III Randomized Multicenter Study of a Humanized Anti-CD33 Monoclonal Antibody, Lintuzumab, in Combination with Chemotherapy, versus Chemotherapy Alone in Patients with Refractory or First-Relapsed Acute Myeloid Leukemia. J. Clin. Oncol. 2005;23:4110–4116. doi: 10.1200/JCO.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 113.Sekeres M.A., Lancet J.E., Wood B.L., Grove L.E., Sandalic L., Sievers E.L., Jurcic J.G. Randomized Phase IIb Study of Low-Dose Cytarabine and Lintuzumab versus Low-Dose Cytarabine and Placebo in Older Adults with Untreated Acute Myeloid Leukemia. Haematologica. 2013;98:119–128. doi: 10.3324/haematol.2012.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maakaron J.E., Rogosheske J., Long M., Bachanova V., Mims A.S. CD33-Targeted Therapies: Beating the Disease or Beaten to Death? J. Clin. Pharmacol. 2021;61:7–17. doi: 10.1002/jcph.1730. [DOI] [PubMed] [Google Scholar]
- 115.Finn L.E., Levy M., Orozco J.J., Park J.H., Atallah E., Craig M., Perl A.E., Scheinberg D.A., Cicic D., Bergonio G.R., et al. A Phase 2 Study of Actinium-225 (225Ac)-Lintuzumab in Older Patients with Previously Untreated Acute Myeloid Leukemia (AML) Unfit for Intensive Chemotherapy. Blood. 2017;130:2638. doi: 10.1182/blood.V130.Suppl_1.2638.2638. [DOI] [Google Scholar]
- 116.Rosenblat T.L., McDevitt M.R., Mulford D.A., Pandit-Taskar N., Divgi C.R., Panageas K.S., Heaney M.L., Chanel S., Morgenstern A., Sgouros G., et al. Sequential Cytarabine and Alpha-Particle Immunotherapy with Bismuth-213-Lintuzumab (HuM195) for Acute Myeloid Leukemia. Clin. Cancer Res. 2010;16:5303–5311. doi: 10.1158/1078-0432.CCR-10-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jurcic J.G., Levy M.Y., Park J.H., Ravandi F., Perl A.E., Pagel J.M., Smith B.D., Estey E.H., Kantarjian H., Cicic D., et al. Phase I Trial of Targeted Alpha-Particle Therapy with Actinium-225 (225Ac)-Lintuzumab and Low-Dose Cytarabine (LDAC) in Patients Age 60 or Older with Untreated Acute Myeloid Leukemia (AML) Blood. 2016;128:4050. doi: 10.1182/blood.V128.22.4050.4050. [DOI] [Google Scholar]
- 118.Garg R., Allen K.J.H., Dawicki W., Geoghegan E.M., Ludwig D.L., Dadachova E. 225Ac-Labeled CD33-Targeting Antibody Reverses Resistance to Bcl-2 Inhibitor Venetoclax in Acute Myeloid Leukemia Models. Cancer Med. 2021;10:1128–1140. doi: 10.1002/cam4.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Williams P., Basu S., Garcia-Manero G., Hourigan C.S., Oetjen K.A., Cortes J.E., Ravandi F., Jabbour E.J., Al-Hamal Z., Konopleva M., et al. The Distribution of T-cell Subsets and the Expression of Immune Checkpoint Receptors and Ligands in Patients with Newly Diagnosed and Relapsed Acute Myeloid Leukemia. Cancer. 2019;125:1470–1481. doi: 10.1002/cncr.31896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Davids M.S., Kim H.T., Bachireddy P., Costello C., Liguori R., Savell A., Lukez A.P., Avigan D., Chen Y.-B., McSweeney P., et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N. Engl. J. Med. 2016;375:143–153. doi: 10.1056/NEJMoa1601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berger R., Rotem-Yehudar R., Slama G., Landes S., Kneller A., Leiba M., Koren-Michowitz M., Shimoni A., Nagler A. Phase I Safety and Pharmacokinetic Study of CT-011, a Humanized Antibody Interacting with PD-1, in Patients with Advanced Hematologic Malignancies. Clin. Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 122.Daver N., Boddu P., Garcia-Manero G., Yadav S.S., Sharma P., Allison J., Kantarjian H. Hypomethylating Agents in Combination with Immune Checkpoint Inhibitors in Acute Myeloid Leukemia and Myelodysplastic Syndromes. Leukemia. 2018;32:1094–1105. doi: 10.1038/s41375-018-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Daver N., Garcia-Manero G., Basu S., Boddu P.C., Alfayez M., Cortes J.E., Konopleva M., Ravandi-Kashani F., Jabbour E., Kadia T., et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019;9:370–383. doi: 10.1158/2159-8290.CD-18-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gojo I., Stuart R.K., Webster J., Blackford A., Varela J.C., Morrow J., DeZern A.E., Foster M.C., Levis M.J., Coombs C.C., et al. Multi-Center Phase 2 Study of Pembroluzimab (Pembro) and Azacitidine (AZA) in Patients with Relapsed/Refractory Acute Myeloid Leukemia (AML) and in Newly Diagnosed (≥65 Years) AML Patients. Blood. 2019;134:832. doi: 10.1182/blood-2019-127345. [DOI] [Google Scholar]
- 125.Daver N.G., Garcia-Manero G., Konopleva M.Y., Alfayez M., Pemmaraju N., Kadia T.M., DiNardo C.D., Cortes J.E., Ravandi F., Abbas H., et al. Azacitidine (AZA) with Nivolumab (Nivo), and AZA with Nivo + Ipilimumab (Ipi) in Relapsed/Refractory Acute Myeloid Leukemia: A Non-Randomized, Prospective, Phase 2 Study. Blood. 2019;134:830. doi: 10.1182/blood-2019-131494. [DOI] [Google Scholar]
- 126.Guo R., Lü M., Cao F., Wu G., Gao F., Pang H., Li Y., Zhang Y., Xing H., Liang C., et al. Single-Cell Map of Diverse Immune Phenotypes in the Acute Myeloid Leukemia Microenvironment. Biomark Res. 2021;9:15. doi: 10.1186/s40364-021-00265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Acharya N., Sabatos-Peyton C., Anderson A.C. Tim-3 Finds Its Place in the Cancer Immunotherapy Landscape. J. ImmunoTher. Cancer. 2020;8:e000911. doi: 10.1136/jitc-2020-000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sakuishi K., Apetoh L., Sullivan J.M., Blazar B.R., Kuchroo V.K., Anderson A.C. Targeting Tim-3 and PD-1 Pathways to Reverse T Cell Exhaustion and Restore Anti-Tumor Immunity. J. Exp. Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fourcade J., Sun Z., Pagliano O., Chauvin J.-M., Sander C., Janjic B., Tarhini A.A., Tawbi H.A., Kirkwood J.M., Moschos S., et al. PD-1 and Tim-3 Regulate the Expansion of Tumor Antigen-Specific CD8+ T Cells Induced by Melanoma Vaccines. Cancer Res. 2014;74:1045–1055. doi: 10.1158/0008-5472.CAN-13-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kikushige Y., Shima T., Takayanagi S., Urata S., Miyamoto T., Iwasaki H., Takenaka K., Teshima T., Tanaka T., Inagaki Y., et al. TIM-3 Is a Promising Target to Selectively Kill Acute Myeloid Leukemia Stem Cells. Cell Stem Cell. 2010;7:708–717. doi: 10.1016/j.stem.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 131.Zeidan A.M., Komrokji R.S., Brunner A.M. TIM-3 Pathway Dysregulation and Targeting in Cancer. Expert Rev. Anticancer Ther. 2021;21:523–534. doi: 10.1080/14737140.2021.1865814. [DOI] [PubMed] [Google Scholar]
- 132.Chao M.P., Weissman I.L., Majeti R. The CD47–SIRPα Pathway in Cancer Immune Evasion and Potential Therapeutic Implications. Curr. Opin. Immunol. 2012;24:225–232. doi: 10.1016/j.coi.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chao M.P., Weissman I.L., Park C.Y. Cancer Stem Cells: On the Verge of Clinical Translation. Lab. Med. 2008;39:679–686. doi: 10.1309/LMTDDRGLY374WSCQ. [DOI] [Google Scholar]
- 134.Vyas P., Knapper S., Kelly R., Salim R., Lubowiecki M., Royston D., Johnson H., Roberts C., Chen J., Agoram B., et al. Initial Phase 1 Results of The First-In-Class Anti-Cd47 Antibody Hu5f9-G4 in Relapsed/Refractory Acute Myeloid Leukemia Patients. [(accessed on 8 February 2022)]. Available online: https://library.ehaweb.org/eha/2018/stockholm/214718/paresh.vyas.initial.phase.1.results.of.the.first-in-class.anti-cd47.antibody.html.
- 135.Feng D., Gip P., McKenna K.M., Zhao F., Mata O., Choi T.S., Duan J., Sompalli K., Majeti R., Weissman I.L., et al. Combination Treatment with 5F9 and Azacitidine Enhances Phagocytic Elimination of Acute Myeloid Leukemia. Blood. 2018;132:2729. doi: 10.1182/blood-2018-99-120170. [DOI] [Google Scholar]
- 136.Sallman D.A., Al Malki M., Asch A.S., Lee D.J., Kambhampati S., Donnellan W.B., Bradley T.J., Vyas P., Jeyakumar D., Marcucci G., et al. Tolerability and Efficacy of the First-in-Class Anti-CD47 Antibody Magrolimab Combined with Azacitidine in MDS and AML Patients: Phase Ib Results. J. Clin. Oncol. 2020;38:7507. doi: 10.1200/JCO.2020.38.15_suppl.7507. [DOI] [Google Scholar]
- 137.Perez P., Hoffman R.W., Shaw S., Bluestone J.A., Segal D.M. Specific Targeting of Cytotoxic T Cells by Anti-T3 Linked to Anti-Target Cell Antibody. Nature. 1985;316:354–356. doi: 10.1038/316354a0. [DOI] [PubMed] [Google Scholar]
- 138.Offner S., Hofmeister R., Romaniuk A., Kufer P., Baeuerle P.A. Induction of Regular Cytolytic T Cell Synapses by Bispecific Single-Chain Antibody Constructs on MHC Class I-Negative Tumor Cells. Mol. Immunol. 2006;43:763–771. doi: 10.1016/j.molimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 139.Nisonoff A., Rivers M.M. Recombination of a Mixture of Univalent Antibody Fragments of Different Specificity. Arch. Biochem. Biophys. 1961;93:460–462. doi: 10.1016/0003-9861(61)90296-X. [DOI] [PubMed] [Google Scholar]
- 140.de Gast G.C., Haagen I.A., van Houten A.A., Klein S.C., Duits A.J., de Weger R.A., Vroom T.M., Clark M.R., Phillips J., van Dijk A.J. CD8 T Cell Activation after Intravenous Administration of CD3×CD19 Bispecific Antibody in Patients with Non-Hodgkin Lymphoma. Cancer Immunol. ImmunoTher. 1995;40:390–396. doi: 10.1007/BF01525390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hartmann F., Renner C., Jung W., Deisting C., Juwana M., Eichentopf B., Kloft M., Pfreundschuh M. Treatment of Refractory Hodgkin’s Disease with an Anti-CD16/CD30 Bispecific Antibody. Blood. 1997;89:2042–2047. doi: 10.1182/blood.V89.6.2042. [DOI] [PubMed] [Google Scholar]
- 142.Laszlo G.S., Gudgeon C.J., Harrington K.H., Dell’Aringa J., Newhall K.J., Means G.D., Sinclair A.M., Kischel R., Frankel S.R., Walter R.B. Cellular Determinants for Preclinical Activity of a Novel CD33/CD3 Bispecific T-Cell Engager (BiTE) Antibody, AMG 330, against Human AML. Blood. 2014;123:554–561. doi: 10.1182/blood-2013-09-527044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ravandi F., Walter R.B., Subklewe M., Buecklein V., Jongen-Lavrencic M., Paschka P., Ossenkoppele G.J., Kantarjian H.M., Hindoyan A., Agarwal S.K., et al. Updated Results from Phase I Dose-Escalation Study of AMG 330, a Bispecific T-Cell Engager Molecule, in Patients with Relapsed/Refractory Acute Myeloid Leukemia (R/R AML) J. Clin. Oncol. 2020;38:7508. doi: 10.1200/JCO.2020.38.15_suppl.7508. [DOI] [Google Scholar]
- 144.Subklewe M., Stein A., Walter R.B., Bhatia R., Wei A.H., Ritchie D., Bücklein V., Vachhani P., Dai T., Hindoyan A., et al. Preliminary Results from a Phase 1 First-in-Human Study of AMG 673, a Novel Half-Life Extended (HLE) Anti-CD33/CD3 BiTE® (Bispecific T-Cell Engager) in Patients with Relapsed/Refractory (R/R) Acute Myeloid Leukemia (AML) Blood. 2019;134:833. doi: 10.1182/blood-2019-127977. [DOI] [Google Scholar]
- 145.Westervelt P., Cortes J.E., Altman J.K., Long M., Oehler V.G., Gojo I., Guenot J., Chun P., Roboz G.J. Phase 1 First-in-Human Trial of AMV564, a Bivalent Bispecific (2:2) CD33/CD3 T-Cell Engager, in Patients with Relapsed/Refractory Acute Myeloid Leukemia (AML) Blood. 2019;134:834. doi: 10.1182/blood-2019-129042. [DOI] [Google Scholar]
- 146.Ravandi F., Bashey A., Foran J.M., Stock W., Mawad R., Blum W., Saville M.W., Johnson C.M., Vanasse K.G.J., Ly T., et al. Complete Responses in Relapsed/Refractory Acute Myeloid Leukemia (AML) Patients on a Weekly Dosing Schedule of XmAb14045, a CD123 x CD3 T Cell-Engaging Bispecific Antibody: Initial Results of a Phase 1 Study. Blood. 2018;132:763. doi: 10.1182/blood-2018-99-119786. [DOI] [Google Scholar]
- 147.Johnson S., Burke S., Huang L., Gorlatov S., Li H., Wang W., Zhang W., Tuaillon N., Rainey J., Barat B., et al. Effector Cell Recruitment with Novel Fv-Based Dual-Affinity Re-Targeting Protein Leads to Potent Tumor Cytolysis and in Vivo B-Cell Depletion. J. Mol. Biol. 2010;399:436–449. doi: 10.1016/j.jmb.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 148.Moore P.A., Zhang W., Rainey G.J., Burke S., Li H., Huang L., Gorlatov S., Veri M.C., Aggarwal S., Yang Y., et al. Application of Dual Affinity Retargeting Molecules to Achieve Optimal Redirected T-Cell Killing of B-Cell Lymphoma. Blood. 2011;117:4542–4551. doi: 10.1182/blood-2010-09-306449. [DOI] [PubMed] [Google Scholar]
- 149.Uy G.L., Aldoss I., Foster M.C., Sayre P.H., Wieduwilt M.J., Advani A.S., Godwin J.E., Arellano M.L., Sweet K.L., Emadi A., et al. Flotetuzumab as Salvage Immunotherapy for Refractory Acute Myeloid Leukemia. Blood. 2021;137:751–762. doi: 10.1182/blood.2020007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sadelain M., Brentjens R., Rivière I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fiorenza S., Turtle C.J. CAR-T Cell Therapy for Acute Myeloid Leukemia: Preclinical Rationale, Current Clinical Progress, and Barriers to Success. BioDrugs. 2021;35:281–302. doi: 10.1007/s40259-021-00477-8. [DOI] [PubMed] [Google Scholar]
- 152.Liu F., Zhang H., Sun L., Li Y., Zhang S., He G., Yi H., Wada M., Pinz K.G., Chen K.H., et al. First-In-Human Cll1-Cd33 Compound Car (Ccar) T Cell Therapy in Relapsed and Refractory Acute Myeloid Leukemia. [(accessed on 7 February 2022)]. Available online: https://library.ehaweb.org/eha/2020/eha25th/294969/fang.liu.first-in-human.cll1-cd33.compound.car.28ccar29.t.cell.therapy.in.html.
- 153.Budde L., Song J.Y., Kim Y., Blanchard S., Wagner J., Stein A.S., Weng L., Del Real M., Hernandez R., Marcucci E., et al. Remissions of Acute Myeloid Leukemia and Blastic Plasmacytoid Dendritic Cell Neoplasm Following Treatment with CD123-Specific CAR T Cells: A First-in-Human Clinical Trial. Blood. 2017;130:811. doi: 10.1182/blood.V130.Suppl_1.811.811. [DOI] [Google Scholar]
- 154.Naik J., Themeli M., de Jong-Korlaar R., Ruiter R.W.J., Poddighe P.J., Yuan H., de Bruijn J.D., Ossenkoppele G.J., Zweegman S., Smit L., et al. CD38 as a Therapeutic Target for Adult Acute Myeloid Leukemia and T-Cell Acute Lymphoblastic Leukemia. Haematologica. 2019;104:e100–e103. doi: 10.3324/haematol.2018.192757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cui Q., Qian C., Xu N., Kang L., Dai H., Cui W., Song B., Yin J., Li Z., Zhu X., et al. CD38-Directed CAR-T Cell Therapy: A Novel Immunotherapy Strategy for Relapsed Acute Myeloid Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation. J. Hematol. Oncol. 2021;14:82. doi: 10.1186/s13045-021-01092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wermke M., Kraus S., Ehninger A., Bargou R.C., Goebeler M.-E., Middeke J.M., Kreissig C., von Bonin M., Koedam J., Pehl M., et al. Proof of Concept for a Rapidly Switchable Universal CAR-T Platform with UniCAR-T-CD123 in Relapsed/Refractory AML. Blood. 2021;137:3145–3148. doi: 10.1182/blood.2020009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Feldman E.J., Lancet J.E., Kolitz J.E., Ritchie E.K., Roboz G.J., List A.F., Allen S.L., Asatiani E., Mayer L.D., Swenson C., et al. First-In-Man Study of CPX-351: A Liposomal Carrier Containing Cytarabine and Daunorubicin in a Fixed 5:1 Molar Ratio for the Treatment of Relapsed and Refractory Acute Myeloid Leukemia. J. Clin. Oncol. 2011;29:979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lim W.-S., Tardi P.G., Dos Santos N., Xie X., Fan M., Liboiron B.D., Huang X., Harasym T.O., Bermudes D., Mayer L.D. Leukemia-Selective Uptake and Cytotoxicity of CPX-351, a Synergistic Fixed-Ratio Cytarabine:Daunorubicin Formulation, in Bone Marrow Xenografts. Leuk. Res. 2010;34:1214–1223. doi: 10.1016/j.leukres.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 159.Cafaro A., Giannini M.B., Silimbani P., Cangini D., Masini C., Ghelli Luserna Di Rorà A., Simonetti G., Martinelli G., Cerchione C. CPX-351 Daunorubicin-Cytarabine Liposome: A Novel Formulation to Treat Patients with Newly Diagnosed Secondary Acute Myeloid Leukemia. Minerva Med. 2020;111:455–466. doi: 10.23736/S0026-4806.20.07017-2. [DOI] [PubMed] [Google Scholar]
- 160.Lancet J.E., Uy G.L., Cortes J.E., Newell L.F., Lin T.L., Ritchie E.K., Stuart R.K., Strickland S.A., Hogge D., Solomon S.R., et al. CPX-351 (Cytarabine and Daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients with Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018;36:2684–2692. doi: 10.1200/JCO.2017.77.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rautenberg C., Stölzel F., Röllig C., Stelljes M., Gaidzik V., Lauseker M., Kriege O., Verbeek M., Unglaub J.M., Thol F., et al. Real-world experience of CPX-351 as first-line treatment for patients with acute myeloid leukemia. Blood Cancer J. 2021;11:164. doi: 10.1038/s41408-021-00558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Laille E., Shi T., Garcia-Manero G., Cogle C.R., Gore S.D., Hetzer J., Kumar K., Skikne B., MacBeth K.J. Pharmacokinetics and Pharmacodynamics with Extended Dosing of CC-486 in Patients with Hematologic Malignancies. PLoS ONE. 2015;10:e0135520. doi: 10.1371/journal.pone.0135520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stresemann C., Lyko F. Modes of Action of the DNA Methyltransferase Inhibitors Azacytidine and Decitabine. Int. J. Cancer. 2008;123:8–13. doi: 10.1002/ijc.23607. [DOI] [PubMed] [Google Scholar]
- 164.Santini V., Kantarjian H.M., Issa J.-P. Changes in DNA Methylation in Neoplasia: Pathophysiology and Therapeutic Implications. Ann. Intern. Med. 2001;134:573. doi: 10.7326/0003-4819-134-7-200104030-00011. [DOI] [PubMed] [Google Scholar]
- 165.Garcia-Manero G., Almeida A., Giagounidis A., Platzbecker U., Garcia R., Voso M.T., Larsen S.R., Valcarcel D., Silverman L.R., Skikne B., et al. Design and Rationale of the QUAZAR Lower-Risk MDS (AZA-MDS-003) Trial: A Randomized Phase 3 Study of CC-486 (Oral Azacitidine) plus Best Supportive Care vs Placebo plus Best Supportive Care in Patients with IPSS Lower-Risk Myelodysplastic Syndromes and Poor Prognosis Due to Red Blood Cell Transfusion–Dependent Anemia and Thrombocytopenia. BMC Hematol. 2016;16:12. doi: 10.1186/s12878-016-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Saunthararajah Y. Key Clinical Observations after 5-Azacytidine and Decitabine Treatment of Myelodysplastic Syndromes Suggest Practical Solutions for Better Outcomes. Hematology. 2013;2013:511–521. doi: 10.1182/asheducation-2013.1.511. [DOI] [PubMed] [Google Scholar]
- 167.Garcia-Manero G., Döhner H., Wei A.H., La Torre I., Skikne B., Beach C., Santini V. Oral Azacitidine (CC-486) for the Treatment of Myeloid Malignancies. Clin. Lymphoma Myeloma Leuk. 2021 doi: 10.1016/j.clml.2021.09.021. in press. [DOI] [PubMed] [Google Scholar]
- 168.Garcia-Manero G., Gore S.D., Cogle C., Ward R., Shi T., MacBeth K.J., Laille E., Giordano H., Sakoian S., Jabbour E., et al. Phase I Study of Oral Azacitidine in Myelodysplastic Syndromes, Chronic Myelomonocytic Leukemia, and Acute Myeloid Leukemia. J. Clin. Oncol. 2011;29:2521–2527. doi: 10.1200/JCO.2010.34.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Garcia-Manero G., Gore S.D., Kambhampati S., Scott B., Tefferi A., Cogle C.R., Edenfield W.J., Hetzer J., Kumar K., Laille E., et al. Efficacy and Safety of Extended Dosing Schedules of CC-486 (Oral Azacitidine) in Patients with Lower-Risk Myelodysplastic Syndromes. Leukemia. 2016;30:889–896. doi: 10.1038/leu.2015.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Savona M.R., Kolibaba K., Conkling P., Kingsley E.C., Becerra C., Morris J.C., Rifkin R.M., Laille E., Kellerman A., Ukrainskyj S.M., et al. Extended Dosing with CC-486 (Oral Azacitidine) in Patients with Myeloid Malignancies. Am. J. Hematol. 2018;93:1199–1206. doi: 10.1002/ajh.25216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wei A.H., Döhner H., Pocock C., Montesinos P., Afanasyev B., Dombret H., Ravandi F., Sayar H., Jang J.-H., Porkka K., et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020;383:2526–2537. doi: 10.1056/NEJMoa2004444. [DOI] [PubMed] [Google Scholar]
- 172.Thol F. What to Use to Treat AML: The Role of Emerging Therapies. Hematology. 2021;2021:16–23. doi: 10.1182/hematology.2021000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stone R.M., Mandrekar S.J., Sanford B.L., Laumann K., Geyer S., Bloomfield C.D., Thiede C., Prior T.W., Döhner K., Marcucci G., et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017;377:454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Roboz G.J., Strickland S.A., Litzow M.R., Dalovisio A., Perl A.E., Bonifacio G., Haines K., Barbera A., Purkayastha D., Sweet K. Updated Safety of Midostaurin plus Chemotherapy in Newly Diagnosed FLT3 Mutation–Positive Acute Myeloid Leukemia: The RADIUS-X Expanded Access Program. Leuk. Lymphoma. 2020;61:3146–3153. doi: 10.1080/10428194.2020.1805109. [DOI] [PubMed] [Google Scholar]
- 175.Strati P., Kantarjian H., Ravandi F., Nazha A., Borthakur G., Daver N., Kadia T., Estrov Z., Garcia-Manero G., Konopleva M., et al. Phase I/II Trial of the Combination of Midostaurin (PKC412) and 5-Azacytidine for Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome: Azacitidine and Midostaurin for AML/MDS. Am. J. Hematol. 2015;90:276–281. doi: 10.1002/ajh.23924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ravandi F., Arana Yi C., Cortes J.E., Levis M., Faderl S., Garcia-Manero G., Jabbour E., Konopleva M., O’Brien S., Estrov Z., et al. Final Report of Phase II Study of Sorafenib, Cytarabine and Idarubicin for Initial Therapy in Younger Patients with Acute Myeloid Leukemia. Leukemia. 2014;28:1543–1545. doi: 10.1038/leu.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Serve H., Krug U., Wagner R., Sauerland M.C., Heinecke A., Brunnberg U., Schaich M., Ottmann O., Duyster J., Wandt H., et al. Sorafenib in Combination with Intensive Chemotherapy in Elderly Patients with Acute Myeloid Leukemia: Results from a Randomized, Placebo-Controlled Trial. J. Clin. Oncol. 2013;31:3110–3118. doi: 10.1200/JCO.2012.46.4990. [DOI] [PubMed] [Google Scholar]
- 178.Burchert A., Bug G., Fritz L.V., Finke J., Stelljes M., Röllig C., Wollmer E., Wäsch R., Bornhäuser M., Berg T., et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia with FLT3 –Internal Tandem Duplication Mutation (SORMAIN) J. Clin. Oncol. 2020;38:2993–3002. doi: 10.1200/JCO.19.03345. [DOI] [PubMed] [Google Scholar]
- 179.Perl A.E., Martinelli G., Cortes J.E., Neubauer A., Berman E., Paolini S., Montesinos P., Baer M.R., Larson R.A., Ustun C., et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019;381:1728–1740. doi: 10.1056/NEJMoa1902688. [DOI] [PubMed] [Google Scholar]
- 180.Wang E.S., Montesinos P., Minden M.D., Lee J.-H., Heuser M., Naoe T., Chou W.-C., Laribi K., Esteve J., Altman J.K., et al. Phase 3, Open-Label, Randomized Study of Gilteritinib and Azacitidine Vs Azacitidine for Newly Diagnosed FLT3-Mutated Acute Myeloid Leukemia in Patients Ineligible for Intensive Induction Chemotherapy. Blood. 2021;138:700. doi: 10.1182/blood-2021-145379. [DOI] [Google Scholar]
- 181.Cortes J.E., Khaled S., Martinelli G., Perl A.E., Ganguly S., Russell N., Krämer A., Dombret H., Hogge D., Jonas B.A., et al. Quizartinib versus Salvage Chemotherapy in Relapsed or Refractory FLT3-ITD Acute Myeloid Leukaemia (QuANTUM-R): A Multicentre, Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 2019;20:984–997. doi: 10.1016/S1470-2045(19)30150-0. [DOI] [PubMed] [Google Scholar]
- 182.Swaminathan M., Kantarjian H.M., Levis M., Guerra V., Borthakur G., Alvarado Y., DiNardo C.D., Kadia T., Garcia-Manero G., Ohanian M., et al. A Phase I/II Study of the Combination of Quizartinib with Azacitidine or Low-Dose Cytarabine for the Treatment of Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome. Haematologica. 2021;106:2121. doi: 10.3324/haematol.2020.263392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yilmaz M., Muftuoglu M., Kantarjian H., DiNardo C.D., Kadia T.M., Konopleva M., Borthakur G., Pemmaraju N., Short N.J., Alvarado Y., et al. Quizartinib (Quiz) with Decitabine (DAC) and Venetoclax (VEN) Is Highly Active in Patients (Pts) with FLT3-ITD Mutated Acute Myeloid Leukemia (AML)—RAS/MAPK Mutations Continue to Drive Primary and Secondary Resistance. Blood. 2021;138:370. doi: 10.1182/blood-2021-153426. [DOI] [Google Scholar]
- 184.Andre C., Hampe A., Lachaume P., Martin E., Wang X.-P., Manus V., Hu W.-X., Galibert F. Sequence Analysis of Two Genomic Regions Containing the KIT and the FMS Receptor Tyrosine Kinase Genes. Genomics. 1997;39:216–226. doi: 10.1006/geno.1996.4482. [DOI] [PubMed] [Google Scholar]
- 185.Badr P., Elsayed G.M., Eldin D.N., Riad B.Y., Hamdy N. Detection of KIT Mutations in Core Binding Factor Acute Myeloid Leukemia. Leuk. Res. Rep. 2018;10:20–25. doi: 10.1016/j.lrr.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Heo S.-K., Noh E.-K., Kim J.Y., Jeong Y.K., Jo J.-C., Choi Y., Koh S., Baek J.H., Min Y.J., Kim H. Targeting C-KIT (CD117) by Dasatinib and Radotinib Promotes Acute Myeloid Leukemia Cell Death. Sci. Rep. 2017;7:15278. doi: 10.1038/s41598-017-15492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fernandez S., Desplat V., Villacreces A., Guitart A.V., Milpied N., Pigneux A., Vigon I., Pasquet J.-M., Dumas P.-Y. Targeting Tyrosine Kinases in Acute Myeloid Leukemia: Why, Who and How? Int. J. Mol. Sci. 2019;20:3429. doi: 10.3390/ijms20143429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bos J.L. Ras Oncogenes in Human Cancer: A Review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 189.Liu P., Wang Y., Li X. Targeting the Untargetable KRAS in Cancer Therapy. Acta Pharm. Sin. B. 2019;9:871–879. doi: 10.1016/j.apsb.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu X., Ye Q., Zhao X.-P., Zhang P.-B., Li S., Li R.-Q., Zhao X.-L. RAS Mutations in Acute Myeloid Leukaemia Patients: A Review and Meta-Analysis. Clin. Chim. Acta. 2019;489:254–260. doi: 10.1016/j.cca.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 191.Coghlan D.W., Morley A.A., Matthews J.P., Bishop J.F. The Incidence and Prognostic Significance of Mutations in Codon 13 of the N-Ras Gene in Acute Myeloid Leukemia. Leukemia. 1994;8:1682–1687. [PubMed] [Google Scholar]
- 192.Illmer T., Thiede C., Fredersdorf A., Stadler S., Neubauer A., Ehninger G., Schaich M. Activation of the RAS Pathway Is Predictive for a Chemosensitive Phenotype of Acute Myelogenous Leukemia Blasts. Clin. Cancer Res. 2005;11:3217–3224. doi: 10.1158/1078-0432.CCR-04-2232. [DOI] [PubMed] [Google Scholar]
- 193.Kiyoi H., Naoe T., Nakano Y., Yokota S., Minami S., Miyawaki S., Asou N., Kuriyama K., Jinnai I., Shimazaki C., et al. Prognostic Implication of FLT3 and N-RAS Gene Mutations in Acute Myeloid Leukemia. Blood. 1999;93:3074–3080. [PubMed] [Google Scholar]
- 194.Radich J.P., Kopecky K.J., Willman C.L., Weick J., Head D., Appelbaum F., Collins S.J. N-Ras Mutations in Adult de Novo Acute Myelogenous Leukemia: Prevalence and Clinical Significance. Blood. 1990;76:801–807. doi: 10.1182/blood.V76.4.801.801. [DOI] [PubMed] [Google Scholar]
- 195.Parikh C., Subrahmanyam R., Ren R. Oncogenic NRAS, KRAS, and HRAS Exhibit Different Leukemogenic Potentials in Mice. Cancer Res. 2007;67:7139–7146. doi: 10.1158/0008-5472.CAN-07-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dai X., Sun Y., Zhang T., Ming Y., Hongwei G. An Overview on Natural Farnesyltransferase Inhibitors for Efficient Cancer Therapy. J. Enzym. Inhib. Med. Chem. 2020;35:1027–1044. doi: 10.1080/14756366.2020.1732366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kirschbaum M.H., Synold T., Stein A.S., Tuscano J., Zain J.M., Popplewell L., Karanes C., O’Donnell M.R., Pulone B., Rincon A., et al. A Phase 1 Trial Dose-Escalation Study of Tipifarnib on a Week-on, Week-off Schedule in Relapsed, Refractory or High-Risk Myeloid Leukemia. Leukemia. 2011;25:1543–1547. doi: 10.1038/leu.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Karp J.E., Lancet J.E., Kaufmann S.H., End D.W., Wright J.J., Bol K., Horak I., Tidwell M.L., Liesveld J., Kottke T.J., et al. Clinical and Biologic Activity of the Farnesyltransferase Inhibitor R115777 in Adults with Refractory and Relapsed Acute Leukemias: A Phase 1 Clinical-Laboratory Correlative Trial. Blood. 2001;97:3361–3369. doi: 10.1182/blood.V97.11.3361. [DOI] [PubMed] [Google Scholar]
- 199.Muus P., Langemeijer S., van Bijnen S., Blijlevens N., de Witte T. A Phase I Clinical Trial to Study the Safety of Treatment with Tipifarnib Combined with Bortezomib in Patients with Advanced Stages of Myelodysplastic Syndrome and Oligoblastic Acute Myeloid Leukemia. Leuk. Res. 2021;105:106573. doi: 10.1016/j.leukres.2021.106573. [DOI] [PubMed] [Google Scholar]
- 200.Park J., Yoon S.-S., Koh Y. Proceedings of the Molecular and Cellular Biology/Genetics. American Association for Cancer Research; Philadelphia, PA, USA: 2019. Abstract 854: Combination Effect of Low Dose Cytarabine and Pan-RAF Inhibitor LY3009120 in AML Cells with RAS Mutations; p. 854. [Google Scholar]
- 201.Maiti A., Naqvi K., Kadia T.M., Borthakur G., Takahashi K., Bose P., Daver N.G., Patel A., Alvarado Y., Ohanian M., et al. Phase II Trial of MEK Inhibitor Binimetinib (MEK162) in RAS-Mutant Acute Myeloid Leukemia. Clin. Lymphoma Myeloma Leuk. 2019;19:142–148.e1. doi: 10.1016/j.clml.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 202.Ragon B.K., Odenike O., Baer M.R., Stock W., Borthakur G., Patel K., Han L., Chen H., Ma H., Joseph L., et al. Oral MEK 1/2 Inhibitor Trametinib in Combination with AKT Inhibitor GSK2141795 in Patients with Acute Myeloid Leukemia with RAS Mutations: A Phase II Study. Clin. Lymphoma Myeloma Leuk. 2019;19:431–440.e13. doi: 10.1016/j.clml.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Borthakur G., Popplewell L., Boyiadzis M., Foran J., Platzbecker U., Vey N., Walter R.B., Olin R., Raza A., Giagounidis A., et al. Activity of the Oral Mitogen-Activated Protein Kinase Kinase Inhibitor Trametinib in RAS-Mutant Relapsed or Refractory Myeloid Malignancies: Trametinib in RAS-Mutant Malignancies. Cancer. 2016;122:1871–1879. doi: 10.1002/cncr.29986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Daver N., Pollyea D.A., Yee K.W.L., Fenaux P., Brandwein J.M., Vey N., Martinelli G., Kelly K.R., Roboz G.J., Garcia J.S., et al. Preliminary Results from a Phase Ib Study Evaluating BCL-2 Inhibitor Venetoclax in Combination with MEK Inhibitor Cobimetinib or MDM2 Inhibitor Idasanutlin in Patients with Relapsed or Refractory (R/R) AML. Blood. 2017;130:813. doi: 10.1182/blood.V130.Suppl_1.813.813. [DOI] [Google Scholar]
- 205.Morales M.L., Arenas A., Ortiz-Ruiz A., Leivas A., Rapado I., Rodríguez-García A., Castro N., Zagorac I., Quintela-Fandino M., Gómez-López G., et al. MEK Inhibition Enhances the Response to Tyrosine Kinase Inhibitors in Acute Myeloid Leukemia. Sci. Rep. 2019;9:18630. doi: 10.1038/s41598-019-54901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang X., Sexauer A., Levis M. Bone Marrow Stroma-Mediated Resistance to FLT3 Inhibitors in FLT3-ITD AML Is Mediated by Persistent Activation of Extracellular Regulated Kinase. Br. J. Haematol. 2014;164:61–72. doi: 10.1111/bjh.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Stoddard B.L., Koshland D.E. Structure of Isocitrate Dehydrogenase with Alpha-Ketoglutarate at 2.7-A Resolution: Conformational Changes Induced by Decarboxylation of Isocitrate. Biochemistry. 1993;32:9317–9322. doi: 10.1021/bi00087a009. [DOI] [PubMed] [Google Scholar]
- 208.Martelli M.P., Martino G., Cardinali V., Falini B., Martinelli G., Cerchione C. Enasidenib and Ivosidenib in AML. Minerva Med. 2020;111:411–426. doi: 10.23736/S0026-4806.20.07024-X. [DOI] [PubMed] [Google Scholar]
- 209.Cairns R.A., Iqbal J., Lemonnier F., Kucuk C., de Leval L., Jais J.-P., Parrens M., Martin A., Xerri L., Brousset P., et al. IDH2 Mutations Are Frequent in Angioimmunoblastic T-Cell Lymphoma. Blood. 2012;119:1901–1903. doi: 10.1182/blood-2011-11-391748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Figueroa M.E., Lugthart S., Li Y., Erpelinck-Verschueren C., Deng X., Christos P.J., Schifano E., Booth J., van Putten W., Skrabanek L., et al. DNA Methylation Signatures Identify Biologically Distinct Subtypes in Acute Myeloid Leukemia. Cancer Cell. 2010;17:13–27. doi: 10.1016/j.ccr.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.DiNardo C.D., Ravandi F., Agresta S., Konopleva M., Takahashi K., Kadia T., Routbort M., Patel K.P., Brandt M., Pierce S., et al. Characteristics, Clinical Outcome, and Prognostic Significance of IDH Mutations in AML: IDH Mutations in AML. Am. J. Hematol. 2015;90:732–736. doi: 10.1002/ajh.24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.DiNardo C.D., Propert K.J., Loren A.W., Paietta E., Sun Z., Levine R.L., Straley K.S., Yen K., Patel J.P., Agresta S., et al. Serum 2-Hydroxyglutarate Levels Predict Isocitrate Dehydrogenase Mutations and Clinical Outcome in Acute Myeloid Leukemia. Blood. 2013;121:4917–4924. doi: 10.1182/blood-2013-03-493197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Parsons D.W., Jones S., Zhang X., Lin J.C.-H., Leary R.J., Angenendt P., Mankoo P., Carter H., Siu I.-M., Gallia G.L., et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Amary M.F., Bacsi K., Maggiani F., Damato S., Halai D., Berisha F., Pollock R., O’Donnell P., Grigoriadis A., Diss T., et al. IDH1 and IDH2 Mutations Are Frequent Events in Central Chondrosarcoma and Central and Periosteal Chondromas but Not in Other Mesenchymal Tumours: IDH1 and IDH2 Mutations Frequency in Mesenchymal Tumours. J. Pathol. 2011;224:334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 215.Wang P., Dong Q., Zhang C., Kuan P.-F., Liu Y., Jeck W.R., Andersen J.B., Jiang W., Savich G.L., Tan T.-X., et al. Mutations in Isocitrate Dehydrogenase 1 and 2 Occur Frequently in Intrahepatic Cholangiocarcinomas and Share Hypermethylation Targets with Glioblastomas. Oncogene. 2013;32:3091–3100. doi: 10.1038/onc.2012.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Issa G.C., DiNardo C.D. Acute Myeloid Leukemia with IDH1 and IDH2 Mutations: 2021 Treatment Algorithm. Blood Cancer J. 2021;11:107. doi: 10.1038/s41408-021-00497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stein E.M., DiNardo C.D., Pollyea D.A., Fathi A.T., Roboz G.J., Altman J.K., Stone R.M., DeAngelo D.J., Levine R.L., Flinn I.W., et al. Enasidenib in Mutant IDH2 Relapsed or Refractory Acute Myeloid Leukemia. Blood. 2017;130:722–731. doi: 10.1182/blood-2017-04-779405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bristol Myers Squibb Provides Update on Phase 3 IDHENTIFY Trial in Patients with Relapsed or Refractory Acute Myeloid Leukemia. [(accessed on 7 February 2022)]. Available online: https://news.bms.com/news/details/2020/Bristol-Myers-Squibb-Provides-Update-on-Phase-3-IDHENTIFY-Trial-in-Patients-with-Relapsed-or-Refractory-Acute-Myeloid-Leukemia/default.aspx.
- 219.Pollyea D.A., Tallman M.S., de Botton S., Kantarjian H.M., Collins R., Stein A.S., Frattini M.G., Xu Q., Tosolini A., See W.L., et al. Enasidenib, an Inhibitor of Mutant IDH2 Proteins, Induces Durable Remissions in Older Patients with Newly Diagnosed Acute Myeloid Leukemia. Leukemia. 2019;33:2575–2584. doi: 10.1038/s41375-019-0472-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.DiNardo C.D., Stein E.M., de Botton S., Roboz G.J., Altman J.K., Mims A.S., Swords R., Collins R.H., Mannis G.N., Pollyea D.A., et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N. Engl. J. Med. 2018;378:2386–2398. doi: 10.1056/NEJMoa1716984. [DOI] [PubMed] [Google Scholar]
- 221.Choe S., Wang H., DiNardo C.D., Stein E.M., de Botton S., Roboz G.J., Altman J.K., Mims A.S., Watts J.M., Pollyea D.A., et al. Molecular Mechanisms Mediating Relapse Following Ivosidenib Monotherapy in IDH1-Mutant Relapsed or Refractory AML. Blood Adv. 2020;4:1894–1905. doi: 10.1182/bloodadvances.2020001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.DiNardo C.D., Schuh A.C., Stein E.M., Montesinos P., Wei A.H., de Botton S., Zeidan A.M., Fathi A.T., Kantarjian H.M., Bennett J.M., et al. Enasidenib plus Azacitidine versus Azacitidine Alone in Patients with Newly Diagnosed, Mutant-IDH2 Acute Myeloid Leukaemia (AG221-AML-005): A Single-Arm, Phase 1b and Randomised, Phase 2 Trial. Lancet Oncol. 2021;22:1597–1608. doi: 10.1016/S1470-2045(21)00494-0. [DOI] [PubMed] [Google Scholar]
- 223.DiNardo C.D., Stein A.S., Stein E.M., Fathi A.T., Frankfurt O., Schuh A.C., Döhner H., Martinelli G., Patel P.A., Raffoux E., et al. Mutant Isocitrate Dehydrogenase 1 Inhibitor Ivosidenib in Combination with Azacitidine for Newly Diagnosed Acute Myeloid Leukemia. J. Clin. Oncol. 2021;39:57–65. doi: 10.1200/JCO.20.01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Stein E.M., DiNardo C.D., Fathi A.T., Mims A.S., Pratz K.W., Savona M.R., Stein A.S., Stone R.M., Winer E.S., Seet C.S., et al. Ivosidenib or Enasidenib Combined with Intensive Chemotherapy in Patients with Newly Diagnosed AML: A Phase 1 Study. Blood. 2021;137:1792–1803. doi: 10.1182/blood.2020007233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Abraham A., Matsui W. Hedgehog Signaling in Myeloid Malignancies. Cancers. 2021;13:4888. doi: 10.3390/cancers13194888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Freisleben F., Behrmann L., Thaden V., Muschhammer J., Bokemeyer C., Fiedler W., Wellbrock J. Downregulation of GLI3 Expression Mediates Chemotherapy Resistance in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2020;21:5084. doi: 10.3390/ijms21145084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Munchhof M.J., Li Q., Shavnya A., Borzillo G.V., Boyden T.L., Jones C.S., LaGreca S.D., Martinez-Alsina L., Patel N., Pelletier K., et al. Discovery of PF-04449913, a Potent and Orally Bioavailable Inhibitor of Smoothened. ACS Med. Chem. Lett. 2012;3:106–111. doi: 10.1021/ml2002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cortes J.E., Dombret H., Merchant A., Tauchi T., DiRienzo C.G., Sleight B., Zhang X., Leip E.P., Shaik N., Bell T., et al. Glasdegib plus Intensive/Nonintensive Chemotherapy in Untreated Acute Myeloid Leukemia: BRIGHT AML 1019 Phase III Trials. Future Oncol. 2019;15:3531–3545. doi: 10.2217/fon-2019-0373. [DOI] [PubMed] [Google Scholar]
- 229.Norsworthy K.J., By K., Subramaniam S., Zhuang L., Del Valle P.L., Przepiorka D., Shen Y.-L., Sheth C.M., Liu C., Leong R., et al. FDA Approval Summary: Glasdegib for Newly Diagnosed Acute Myeloid Leukemia. Clin. Cancer Res. 2019;25:6021–6025. doi: 10.1158/1078-0432.CCR-19-0365. [DOI] [PubMed] [Google Scholar]
- 230.Heuser M., Smith B.D., Fiedler W., Sekeres M.A., Montesinos P., Leber B., Merchant A., Papayannidis C., Pérez-Simón J.A., Hoang C.J., et al. Clinical Benefit of Glasdegib plus Low-Dose Cytarabine in Patients with de Novo and Secondary Acute Myeloid Leukemia: Long-Term Analysis of a Phase II Randomized Trial. Ann. Hematol. 2021;100:1181–1194. doi: 10.1007/s00277-021-04465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Smith M.A., Choudhary G.S., Pellagatti A., Choi K., Bolanos L.C., Bhagat T.D., Gordon-Mitchell S., Von Ahrens D., Pradhan K., Steeples V., et al. U2AF1 Mutations Induce Oncogenic IRAK4 Isoforms and Activate Innate Immune Pathways in Myeloid Malignancies. Nat. Cell Biol. 2019;21:640–650. doi: 10.1038/s41556-019-0314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Aryal S., Zhang Y., Wren S., Li C., Lu R. Molecular Regulators of HOXA9 in Acute Myeloid Leukemia. FEBS J. 2021 doi: 10.1111/febs.16268. [DOI] [PubMed] [Google Scholar]
- 233.Yokoyama A., Somervaille T.C.P., Smith K.S., Rozenblatt-Rosen O., Meyerson M., Cleary M.L. The Menin Tumor Suppressor Protein Is an Essential Oncogenic Cofactor for MLL-Associated Leukemogenesis. Cell. 2005;123:207–218. doi: 10.1016/j.cell.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 234.Issa G.C., Zarka J., Sasaki K., Qiao W., Pak D., Ning J., Short N.J., Haddad F., Tang Z., Patel K.P., et al. Predictors of Outcomes in Adults with Acute Myeloid Leukemia and KMT2A Rearrangements. Blood Cancer J. 2021;11:162. doi: 10.1038/s41408-021-00557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Klossowski S., Miao H., Kempinska K., Wu T., Purohit T., Kim E., Linhares B.M., Chen D., Jih G., Perkey E., et al. Menin Inhibitor MI-3454 Induces Remission in MLL1-Rearranged and NPM1-Mutated Models of Leukemia. J. Clin. Investig. 2020;130:981–997. doi: 10.1172/JCI129126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wang E. Phase 1/2 First in Human Study of The Menin-Kmt2a (Mll) Inhibitor Ko-539 in Patients with Relapsed or Refractory Acute Myeloid Leukemia. [(accessed on 7 February 2022)]. Available online: https://library.ehaweb.org/eha/2021/eha2021-virtual-congress/324089/eunice.wang.phase.1.2.first.in.human.study.of.the.menin-kmt2a.28mll29.inhibitor.html?f=listing%3D3%2Abrowseby%3D8%2Asortby%3D1%2Amedia%3D1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.