Abstract
Background
Quantifying cancer risk in primary care patients with abdominal pain informs diagnostic strategies.
Aim
To quantify oesophagogastric, colorectal, liver, pancreatic, ovarian, uterine, kidney, and bladder cancer risks associated with newly reported abdominal pain with or without other symptoms, signs, or abnormal blood tests (that is, features) indicative of possible cancer.
Design and setting
This was an observational prospective cohort study using Clinical Practice Research Datalink records with English cancer registry linkage.
Method
The authors studied 125 793 patients aged ≥40 years with newly reported abdominal pain in primary care between 1 January 2009 and 31 December 2013. The 1-year cumulative incidence of cancer, and the composite 1-year cumulative incidence of cancers with shared additional features, stratified by age and sex are reported.
Results
With abdominal pain, overall risk was greater in men and increased with age, reaching 3.4% (95% confidence interval [CI] = 3.0 to 3.7, predominantly colorectal cancer 1.9%, 95% CI = 1.6 to 2.1) in men ≥70 years, compared with their expected incidence of 0.88% (95% CI = 0.87 to 0.89). Additional features increased cancer risk; for example, for men, colorectal or pancreatic cancer risk with abdominal pain plus diarrhoea at 60–69 years of age was 3.1% (95% CI = 1.9 to 4.9) predominantly colorectal cancer (2.2%, 95% CI = 1.2 to 3.8).
Conclusion
Abdominal pain increases intra-abdominal cancer risk nearly fourfold in men aged ≥70 years, exceeding the 3% threshold warranting investigation. This threshold is surpassed for the >60 years age group only with additional features. These results will help direct appropriate referral and testing strategies for patients based on their demographic profile and reporting features. The authors suggest non-invasive strategies first, such as faecal immunochemical testing, with safety-netting in a shared decision-making framework.
Keywords: abdominal pain, cancer, diagnosis, general practice, primary health care
INTRODUCTION
Early recognition of cancer symptoms and appropriate referral are key to favourable survival outcomes.1 Historically UK cancer survival rates are relatively poor, with one-fifth of UK cancers diagnosed by the emergency route.2
The NHS Long Term Plan pledges that, by 2028, an extra 55 000 people annually should survive their diagnosis for ≥5 years.3 As part of this strategy, rapid diagnostic centres (RDCs) are being established to assess patients whose potentially serious, but non-specific, symptoms do not warrant an urgent, site-specific referral.4–6 Piloted in Denmark, these multidisciplinary units conduct diagnostic imaging, blood, and urine investigations, with cancer detection rates of 7–12%.5–8 Patients attending RDCs were likely to have multiple signs, symptoms, or abnormal tests indicating possible cancer (termed ‘features’) before diagnosis.5,6 Norway and Sweden implemented diagnostic centres for nonspecific symptoms in 2015/2016. In Sweden, the cancer detection rate was 22%, with weight loss, fatigue and pain commonly reported.9
The NHS Long Term Plan has been jeopardised by COVID-19-related disruption of cancer services, with worsening cancer outcomes predicted, particularly for patients with non-specific features.10,11 Furthermore, National Institute for Health and Care Excellence (NICE) suspected-cancer guideline (NG12)4 recommendations are not followed rigorously, even for patients with ‘red flags’.12
Abdominal pain has many causes, an ambiguity that may delay cancer diagnosis. Approximately 2.5% of the UK population consults primary care with abdominal pain annually,13 with no underlying cause identified for at least one-third of patients.14 Abdominal pain was reported before a cancer diagnosis in 8% of patients in the National Audit of Cancer Diagnosis in Primary Care.15
NG12 guidelines advise GPs to consider pancreatic, colorectal, ovarian, stomach, or oesophageal cancer when abdominal pain is accompanied by another clinical feature.4 Abdominal pain may also present in kidney,16 bladder,17 and uterine18 cancers, and may indicate diagnostic imaging for liver cancer.19 However, the individual positive predictive values (PPVs) of abdominal pain for each cancer site is low. Colorectal cancer was the most common intra-abdominal cancer diagnosed in patients with abdominal pain, with PPVs of 0.6% and 0.3% for men and women, respectively.20
Knowing the cancer risk in patients with abdominal pain plus another cancer feature may improve patient selection for specific diagnostic pathways for those whose individual cancer risks are >3%. The collective risk of a number of cancers may also be >3% in patients with abdominal pain, particularly when they have additional features common to those cancers. Knowing the hierarchy of risk within that group may help inform diagnostic strategies where clinicians consider that investigation is warranted based on overall cancer risk.
How this fits in
| Abdominal pain is a non-specific symptom, which may portend serious disease, including intra-abdominal cancers. There is no unified pathway for investigation. This paper reports the 1-year cumulative incidence risk of intra-abdominal cancer with or without concurrent clinical features for men and women aged 40–59, 60–69 and ≥70 years. Results show that patient demographics and type of concurrent feature effects the cancer risk. These results will inform appropriate testing strategies and specialist referral. |
Finally, it would be useful to know the collective risk of cancers that share a common diagnostic pathway (for example, bladder and kidney). This study aimed to quantify the risk of cancers in the abdominal cavity or cancers that may present with abdominal pain (termed ‘intra-abdominal’) in patients aged ≥40 years in the year after newly reported abdominal pain, with or without other features of intraabdominal cancer.
METHOD
Study design and setting
This prospective cohort study was set in English primary care, and used Clinical Practice Research Datalink (CPRD GOLD) data with partial linkage to data from England’s cancer registry (National Cancer Registration and Analysis Service [NCRAS]). The CPRD contains prospectively collected and anonymised electronic medical records of patient demographics, symptoms, signs, tests, diagnoses, and treatments.21 Participants were followed-up for intra-abdominal cancers in the year after abdominal pain newly reported during 2009–2013.
Sample selection criteria and study size
Study participants were aged ≥40 years, with an abdominal pain code (Supplementary Table S1) recorded between 1 January 2009 and 31 December 2013, but no such code in the previous year. Participants had continuous CPRD records meeting up-to- date standards from at least 1 year before their first abdominal pain code (‘index date’) to the end of the 1-year follow-up. Age was identified from the CPRD year of birth, assigning a birthday of 1 July.
Participants who were <40 years or with a cancer diagnostic code recorded before their first abdominal pain code were excluded.
The risk of intra-abdominal cancer varies with age and sex, and additional features were assumed to occur in 10% of participants <70 years (20% of those ≥70 years). Data over 5 years (2009–2013) ensured sample sizes sufficient to provide ≥95% power to detect the following increases in cancer risk associated with an additional feature (α = 0.05):
40–59 years (n = 29 920 women, n = 29 944 men) increase in risk from cancer: 0.1% to 1.5%;
60–69 years (n = 14 955 women, n = 14 506 men): 0.4% in men and 0.3% in women to 1.5% in either sex;
≥70 years (n = 23 008 women, n = 13 460 men): 0.9% in men and 0.7% in women to 2% in either sex.
Follow-up
NCRAS and CPRD records in the year after the index date were searched for diagnostic codes for common ‘intra-abdominal’ cancers, that is sited within the abdomen or that present with abdominal pain: oesophagogastric (International Classification of Diseases [ICD] C15, C16),22 colorectal (C17–C20),23 liver (C22.8), pancreas (C25),24 ovary (C56),25 uterus (C54, C55),18 kidney (C64),16 or bladder (C67)17 cancers. Intra-abdominal lymphoma was not included, because CPRD codes usually omit the anatomical site. The first cancer diagnostic code determined the participant’s incident diagnosis and its date.
Exposure
‘Additional features’ were the signs, symptoms or abnormal test results listed in NG124 presenting in more than one of the above-listed cancers:
abdominal mass: colorectal, ovarian, oesophagogastric, liver;
change in bowel habit: colorectal, ovarian;
diarrhoea/constipation: colorectal, pancreatic;
nausea/vomiting: pancreatic, oesophagogastric;
weight loss: colorectal, oesophagogastric, pancreatic, ovarian;
haematuria/urinary tract infection: bladder, kidney;
low haemoglobin/raised platelets: uterine, oesophagogastric.
Code lists for each feature were collated.26 Nausea and/or vomiting were combined because of overlapping codes.
Participants with concurrent additional features were identified by searching the CPRD records in a 6-month window centred on the index date.
Outcomes
The outcomes were incident cancers, individually and collectively (composite outcomes consisting of any one of the cancer sites sharing a feature, see above).
Analyses
Analyses were stratified by age group and sex. The 1-year cumulative incidence of individual cancer sites in participants with or without an additional feature is reported. For context, the expected incidence of each cancer was estimated, based on 2011 data for cancer incidence and population size.27,28 For composite outcomes, the 1-year cumulative incidence for participants with abdominal pain plus an additional feature are reported. Estimates are reported with binomial exact 95% confidence intervals (CIs). Data analysis was conducted using Stata (version 16).
Missing data and bias
All code lists are available on request. Code absence was interpreted as non-occurrence of the clinical event.21 Confounding by sex and age were controlled by stratified analyses.
RESULTS
Sample
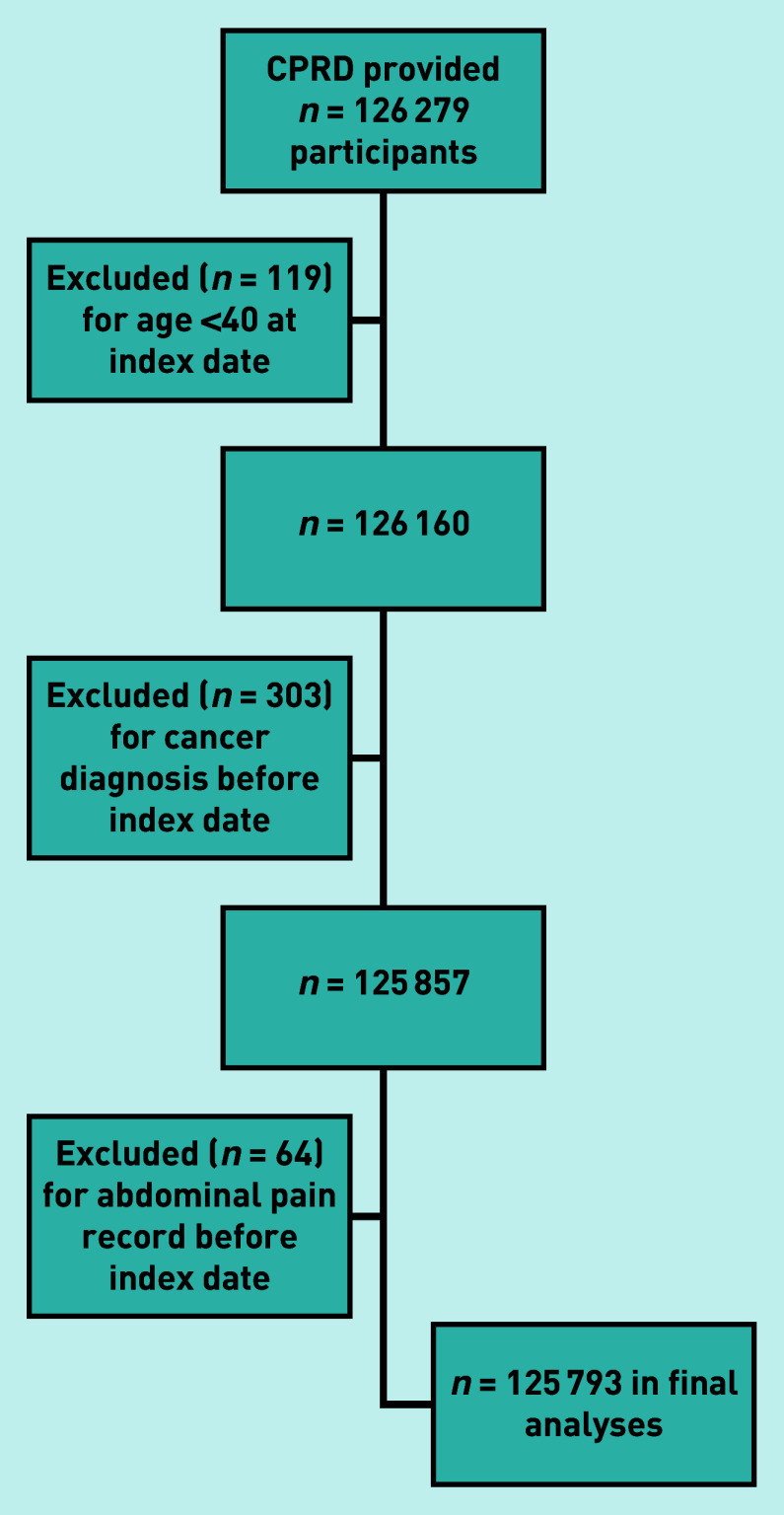
The CPRD provided 126 279 potentially eligible participants, of whom 486 were excluded (Figure 1), leaving 125 793 in the study (Table 1).
Figure 1.
Flow chart of individuals included in the study with application of exclusion criteria. CPRD = Clinical Practice Research Datalink.
Table 1.
Participant characteristics
| Age group, years | Participants, n (% of total) | Men, n (% in age group) | ≥1 additional feature,a n (%) |
|---|---|---|---|
| 40–59 | 59 864 (47.6) | 29 944 (50.0) | 10 132 (16.9) |
| 60–69 | 29 461 (23.4) | 14 506 (49.2) | 6632 (22.5) |
| ≥70 | 36 468 (29.0) | 13 460 (36.9) | 13 790 (37.8) |
| Total | 125 793 (100) | 57 910 (46.0) | 30 554 (24.3) |
Additional feature = the signs, symptoms or abnormal test results, in addition to abdominal pain, that are listed in NG12 as possible presenting features of intra-abdominal cancers.
Numbers of participants with additional features
Additional features were more common in women and with increasing age. At least one additional feature occurred in 12.9%, 19.2%, and 34.6% of men, and in 21.0%, 25.7%, and 39.7% of women aged 40–59, 60–69, and ≥70 years, respectively (see Table 2 for the numbers of participants with additional features, by age group and sex, and by composite cancer grouping), (see Supplementary Table S2 for breakdown by cancer site).
Table 2.
Numbers of participants with additional features, by age group and sex, and by feature–cancer combination
| Cancer and additional feature | Women, n (%) | Men, n (%) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Aged 40–59 years (n= 29 920) | Aged 60–69 years (n = 14 955) | Aged ≥70 years (n= 23 008) | Aged 40–59 years (n= 29 944) | Aged 60–69 years (n = 14 506) | Aged ≥70 years (n= 13 460) | |
| Colorectal, ovary, oesophagogastric, liver | ||||||
| Abdominal mass | 67 (0.2) | 51 (0.3) | 85 (0.4) | 63 (0.2) | 32 (0.2) | 57 (0.4) |
|
| ||||||
| Colorectal, ovary | ||||||
| Change in bowel habit | 281 (0.9) | 201 (1.3) | 330 (1.4) | n/a | n/a | n/a |
|
| ||||||
| Colorectal, pancreas | ||||||
| Constipation | 810 (2.7) | 586 (3.9) | 1765 (7.7) | 645 (2.2) | 574 (4.0) | 1118 (8.3) |
| Diarrhoea | 988 (3.3) | 648 (4.3) | 1474 (6.4) | 1065 (3.6) | 578 (4.0) | 644 (4.8) |
|
| ||||||
| Pancreas, oesophagogastric | ||||||
| Nausea and/or vomiting | 833 (2.8) | 567 (3.8) | 1486 (6.5) | 626 (2.1) | 325 (2.2) | 587 (4.4) |
|
| ||||||
| Colorectal, oesophagogastric, pancreas, ovary | ||||||
| Weight loss | 108 (0.4) | 91 (0.6) | 316 (1.4) | 158 (0.5) | 116 (0.8) | 222 (1.6) |
|
| ||||||
| Bladder, kidney | ||||||
| Haematuria | 100 (0.3) | 88 (0.6) | 129 (0.6) | 182 (0.6) | 119 (0.8) | 199 (1.5) |
| Urinary tract infection | 1514 (5.1) | 1013 (6.8) | 2183 (9.5) | 352 (1.2) | 261 (1.8) | 506 (3.8) |
|
| ||||||
| Uterus, oesophagogastric | ||||||
| Low haemoglobin | 2145 (7.2) | 1209 (8.1) | 4069 (17.7) | n/a | n/a | n/a |
| Raised platelets | 1001 (3.3) | 636 (4.3) | 1348 (5.9) | n/a | n/a | n/a |
n/a = not applicable: included because people born of male sex cannot be diagnosed with uterine cancer or with ovarian cancer.
Cancer incidence with abdominal pain
In this sample with abdominal pain, the 1-year cumulative incidence of intra-abdominal cancer was higher for men than women, and increased with age (Supplementary Table S3), reaching 3.4% (95% CI = 3.0 to 3.7) and 2.3% (95% CI = 2.1 to 2.5) for men and women, respectively, aged ≥70 years. For all age groups, participants were at greatest risk of colorectal cancer, followed by pancreatic and oesophagogastric cancers (and ovarian cancer for women).
Bladder, kidney, and liver cancers had the lowest incidence estimates. The 1-year cumulative incidence values in participants with abdominal pain were consistently higher than the population estimates, apart from liver cancer in women aged 40—59 years (Supplementary Table S3).
Composite cancer risk in participants with additional features
Additional features increased cancer risk over that with abdominal pain alone. At 40–59 years (Supplementary Table S4), having an abdominal mass increased colorectal, ovarian, oesophagogastric, or liver cancer risk in women to 7% (95% CI = 2.0 to 17). Weight loss increased colorectal, ovarian, pancreatic, or oesophagogastric cancer risk to 4% (95% CI = 1 to 9) in women and to 4% (95% CI = 2 to 9) in men. Constipation increased colorectal or pancreatic cancer risk to 2.6% (95% CI = 1.5 to 4.2) in men.
Additional weight loss increased colorectal, ovarian, pancreatic, or oesophagogastric cancer risk to >3% in both sexes (Tables 3 and 4), with pancreas the most likely site at 60–69 years (Table 3). Additional nausea and/or vomiting increased pancreatic or oesophagogastric cancer risk >3% in men aged 60–69 and ≥70 years, with pancreatic more likely.
Table 3.
The 1-year incidence (%, 95% CI) of cancers in participants aged 60–69 years with abdominal pain plus another feature of possible cancer, stratified by sexa
| Additional feature and cancer | Women | Men | ||
|---|---|---|---|---|
|
| ||||
| 1-year incidence (%) | 95% CI | 1-year incidence (%) | 95% CI | |
| Constipation | ||||
| Colorectal or pancreatic | 2.7c | 1.6 to 4.4c | 4.0d | 2.6 to 6.0d |
| Colorectal | 1.7b | 0.8 to 3.1b | 1.2b | 0.5 to 2.5b |
| Pancreatic | 1.0b | 0.4 to 2.2b | 2.8c | 1.6 to 4.5c |
|
| ||||
| Diarrhoea | ||||
| Colorectal or pancreatic | 1.5b | 0.7 to 2.8b | 3.1d | 1.9 to 4.9d |
| Colorectal | 1.1b | 0.4 to 2.2b | 2.2c | 1.2 to 3.8c |
| Pancreatic | 0.5 | 0.1 to 1.3 | 0.9 | 0.3 to 2.0 |
|
| ||||
| Change in bowel habit | ||||
| Colorectal or ovarian | 3c | 1 to 6c | n/ae | n/a |
| Colorectal | 2c | 1 to 6c | ||
| Ovarian | 0.5 | 0.1 to 2.7 | n/a | n/a |
|
| ||||
| Nausea and/or vomiting | ||||
| Pancreatic or oesophagogastric | 1.4b | 0.6 to 2.8b | 4d | 2 to 7d |
| Pancreatic | 1.2b | 0.5 to 2.5b | 3.1d | 1.5 to 5.6d |
| Oesophagogastric | 0.2 | 0.1 to 1.0 | 0.9 | 0.2 to 2.7 |
|
| ||||
| Abdominal mass | ||||
| Colorectal, ovarian, oesophagogastric, or liver | 10d | 3 to 21d | 9d | 2 to 25d |
| Colorectal | 6d | 1 to 16d | 3d | 0 to16d |
| Oesophagogastric | 0 | n/a | 3d | 0 to 16d |
| Ovarian | 2c | 0 to 10c | n/a | |
| Liver | 2c | 0 to 10c | 3d | 0 to 16d |
|
| ||||
| Weight loss | ||||
| Colorectal, ovarian, pancreatic, or oesophagogastric | 5d | 2 to 12d | 9d | 5 to 16d |
| Colorectal | 2c | 0 to 8c | 2c | 0 to 6c |
| Oesophagogastric | 0 | n/a | 2c | 0 to 6c |
| Ovarian | 0 | n/a | n/a | n/a |
| Pancreatic | 3d | 1 to 9d | 6d | 2 to 12d |
For each feature, the total risk and that of contributing cancers is reported. Estimates are reported to the precision afforded by the standard errors, which varies with the cancer–feature combination.
Cancer risk ≥1% and <2%.
Cancer risk ≥2% and <3%.
Cancer risk ≥3%.
There are no data for men for change in bowel habit, as the composite outcome is not meaningful for male sex, who are not diagnosed with ovarian cancer. CI = confidence interval. n/a = not applicable.
Table 4.
The 1-year cumulative incidence of cancers in participants aged ≥70 years with abdominal pain plus another feature of possible cancer, stratified by sexa
| Additional feature and cancer | Women | Men | ||
|---|---|---|---|---|
|
| ||||
| 1-year incidence (%) | 95% CI | 1-year incidence (%) | 95% CI | |
| Constipation | ||||
| Colorectal or pancreatic | 1.9b | 1.3 to 2.7b | 4.9d | 3.7 to 6.4d |
| Colorectal | 1.3b | 0.8 to 1.9b | 3.8d | 2.7 to 5.0d |
| Pancreatic | 0.6 | 0.3 to 1.1 | 1.2b | 0.6 to 2.0b |
|
| ||||
| Diarrhoea | ||||
| Colorectal or pancreatic | 2.0c | 1.4 to 2.9 c | 3.6d | 2.3 to 5.3d |
| Colorectal | 1.7b | 1.1 to 2.5b | 3.3d | 2.0 to 4.9d |
| Pancreatic | 0.3 | 0.1 to 0.8 | 0.3 | 0.0 to 1.1 |
|
| ||||
| Change in bowel habit | ||||
| Colorectal or ovarian | 5d | 3 to 8d | n/a | n/a |
| Colorectal | 4d | 2 to 6d | n/a | n/a |
| Ovarian | 1.5b | 0.5 to 3.5b | n/a | n/a |
|
| ||||
| Nausea and/or vomiting | ||||
| Pancreatic or oesophagogastric | 1.1b | 0.7 to 1.8b | 3.6d | 2.2 to 5.4d |
| Pancreatic | 0.5 | 0.2 to 1.1 | 2.2c | 1.2 to 3.8c |
| Oesophagogastric | 0.6 | 0.3 to 1.1 | 1.4b | 0.6 to 2.7b |
|
| ||||
| Abdominal mass | ||||
| Colorectal, ovarian, oesophagogastric, or liver | 20d | 12 to 30d | 9d | 3 to 19d |
| Colorectal | 12d | 6 to 21d | 5d | 1 to 15d |
| Oesophagogastric | 0 | 4d | 0 to 12d | |
| Ovarian | 7d | 3 to 15d | n/a | |
| Liver | 1b | 0 to 6b | 0 | – |
|
| ||||
| Weight loss | ||||
| Colorectal, ovarian, pancreatic or oesophagogastric | 5d | 3 to 8d | 9d | 6 to 14d |
| Colorectal | 1.6b | 0.5 to 3.7b | 4d | 2 to 8d |
| Ovarian | 0.9 | 0.2 to 2.7 | n/a | |
| Pancreatic | 1.3b | 0.3 to 3.2b | 3d | 1 to 6d |
| Oesophagogastric | 0.9 | 0.2 to 2.7 | 3d | 1 to 6d |
For each feature, the total risk and that of individual cancers is reported.
Cancer risk ≥1% and <2%.
Cancer risk ≥ 2% and <3%.
Cancer risk ≥3%. CI = confidence interval. n/a = not applicable.
Additional constipation or diarrhoea increased colorectal or pancreatic cancer risk >3% in men, with the more likely sites being colorectal at ≥70 years, but pancreatic cancer for constipation and colorectal cancer for diarrhoea at 60–69 years. Additional change in bowel habit increased colorectal or ovarian cancer risk in women >3% at 60–69 and ≥70 years, with colorectal more likely than ovarian (Table 3).
Haematuria, urinary tract infection and abnormal blood test results
Bladder/kidney cancer and uterine/oesophagogastric cancer risks were similarly low in participants with abdominal pain alone, or plus urinary tract infection and abnormal blood tests, respectively (Supplementary Table S5). Bladder or kidney cancer risk in women with abdominal pain and haematuria was 3.0% (95% CI = 0.6 to 8.5) at age 40–59 years and 8% (95% CI = 4 to 14) in those ≥70 years, with bladder the more likely site.
DISCUSSION
Summary
This study examined a common diagnostic problem — abdominal pain — and quantified intra-abdominal cancer risk in the subsequent year. Overall, the risk with abdominal pain per se was lowest in women aged 40–59 (0.49%), and highest in men aged ≥70 (3.39%) (see Supplementary Table S3). The higher risk in men likely reflects sex differences in colorectal, oesophagogastric, pancreatic, bladder, and kidney cancer incidence. Abdominal pain increased intra-abdominal cancer risk over that in the general population.
For example, men aged ≥70 years have a general risk of 0.88% (95% CI = 0.87% to 0.89%) compared with 3.39% with newly reported abdominal pain (see Supplementary Table S3). Having additional features increased cancer risk further, more so with age.
This study has identified which cancers were more likely within the cancers that share features. For example, a man aged 60–69 years with abdominal pain and weight loss has a cumulative intra-abdominal cancer risk of 9%, made up of pancreatic (6%), colorectal, and oesophagogastric (each approximately 2%) cancers.
Strengths and limitations
This is a large study of data in a frequently used primary care database.16–18,22,24 The healthcare setting is important, as most patients with abdominal pain present to primary care. Robust methods were used to identify cancer features and diagnoses.26 CPRD cancer recording is >90%, and was supplemented by cancer registry linkage.29 Some symptom data will be missing: patients may not mention abdominal pain or other cancer features, and doctors may not record them, or only record them in text.30 Text-only abdominal pain records may have reduced the pool of possible patients; nevertheless, the current study was sufficiently powered.30
Stratified analyses ensured that the results would not be skewed by the varying incidences of abdominal pain and cancers by age and sex. The decision in this study to seek additional cancer features within a 6-month window centred on the index date was pragmatic. The authors acknowledge omission of cancer features recorded outside this timeframe.
Comparison with existing literature
Most existing analyses were not stratified, complicating direct comparisons with the results of the current study. Holtedahl et al followed-up 6264 adults attending European primary care with abdominal symptoms.31 The PPVs of upper and lower abdominal pain, respectively, for any abdominal cancer were 1.5% (95% CI = 1.0 to 2.1) and 1.0% (95% CI = 0.6 to 1.5). Their collective value of 2.5% is of similar magnitude to the estimates in the current study for the ≥70 years age group. Additional constipation, diarrhoea, or weight loss increased the hazard of new abdominal cancer,31 consistent with the findings of the current study. Of the 511 cancers diagnosed, 94 were in the colon or rectum (mean age 71 years).31 Lower abdominal pain was a common pre-diagnostic symptom, with a PPV of 0.7% (95% CI = 0.4 to 1.1).31 This is lower than the estimated colorectal cancer risk in the ≥70 years age group in the current study, possibly because of differences in age distribution and abdominal pain location.32 Herbert et al 20 followed-up adults aged ≥30 years with abdominal symptoms in primary care for 1 year. Similar to the findings in the current study, colorectal cancer was the most common intraabdominal cancer diagnosed, with PPVs of 0.6% and 0.3% for men and women, respectively.
Hippisley-Cox and Coupland33,34 followed-up adults aged 25–89 years attending primary care for 2 years, reporting the PPVs of abdominal pain for any incident cancer of 4.0% for men and 2.8% for women. Discrepancies with estimates in the current study probably relate to the restriction of diagnoses to intra-abdominal cancers, differences in age/sex profile, and follow-up period. The increased risks with additional features in the current study are similar to those reported elsewhere.
For example, nausea and/or vomiting has been found to increase the PPVs of abdominal pain from 0.3% for both pancreatic and oesophagogastric cancers to 2.2% (95% CI = 1.1 to 4.6) for pancreatic cancer and 0.7% (95% CI = 0.5 to 0.9) for oesophagogastric cancer.22,24
Implications for clinical practice
It is important to remember that non- malignant abdominal pain causes were not sought. For many of the clinical profiles studied, clinicians may be able to diagnose a non-malignant disease, without considering cancer in the differential. This, supplemented by treatment response, means that patient groups with profiles suggesting a cancer risk ≥3% may be categorised further: a lower-risk group not requiring initial cancer investigation, and a (much) higher-risk group warranting cancer investigation. This selection process is too subtle for observational studies to elucidate entirely. However, managing older patients with cancer features requires assessing the risks and benefits of possibly invasive investigations, such as colonoscopy, and the results of this study aid that. Older patients wish to be involved in decision making, but this is difficult for individuals who are cognitively impaired or frail in standard settings.35 Decisions to investigate are more likely to be deferred in older patients (that is ≥65 years), who tend to have longer diagnostic intervals than younger patients.35 This suggests a real risk of harm to older patients from diagnostic delays, reinforcing the need for rigorous safety-netting in this patient group.36
Not all patients will need referral: where colorectal cancer is the likeliest, faecal immunochemical testing may be used before invasive colonoscopy. In some healthcare systems, primary care clinicians may order computed tomography for possible pancreatic cancer and intra-abdominal lymphomas. Even so, some patients with negative primary care testing will still harbour cancer, and may need specialist referral perhaps to an RDC. This selection is more than totting up estimated risks; intuition and experience may play a part,37 and in the UK, NICE supports GPs using these to make referrals. The results of the current study may guide clinicians in RDCs as to the optimum investigation, or sequence of investigations, to facilitate early diagnosis.
In conclusion, abdominal pain may indicate cancer, the chance being higher in men and with increasing age. Additional features in the history may indicate specific cancers, allowing targeted investigation. This is relevant to primary care, and to facilities for investigating non-specific features of possible cancer.
Abdominal pain alone increases baseline cancer risk nearly fourfold in men aged ≥70 years, to over the threshold warranting investigation. The authors suggest starting with non-invasive testing strategies, such as faecal immunochemical testing, with robust safety-netting in a shared decision- making framework.
The 3% threshold is surpassed for participants >60 years of age only when additional features are present. These results help direct appropriate referral and testing strategies for patients based on their demographic profile and the features they report.
Funding:
This study was supported by Department of Health and Social Care Grant ‘Unexplained abdominal pain in primary care: establishing a clinical profile and diagnostic pathway for identifying cancer’, under the Cancer Awareness, Screening and Early Diagnosis Policy Research Unit. Sarah Price is funded by the National Institute for Health Research (NIHR) Policy Research Programme, conducted through the Policy Research Unit in Cancer Awareness, Screening and Early Diagnosis, PR-PRU-1217-21601. Elizabeth Shephard is funded through the ERICA trial (https://www.theericatrial.co.uk/), which is funded by the Dennis and Mirelle Gillings Foundation, and receives support from CRUK and Macmillan charities. This current research is also linked to the CanTest Collaborative (of which Willie Hamilton is Co-Director), which is funded by CRUK (grant reference number: C8640/A23385). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Ethical Approval
The Independent Scientific Advisory Committee approved the study protocol (18 015) on 5 April 2018.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Abel GA, Mendonca SC, McPhail S, et al. Emergency diagnosis of cancer and previous general practice consultations: insights from linked patient survey data. Br J Gen Pract. 2017. DOI: . [DOI] [PMC free article] [PubMed]
- 2.Elliss-Brookes L, McPhail S, Ives A, et al. Routes to diagnosis for cancer – determining the patient journey using multiple routine data sets. Br J Cancer. 2012;107(8):1220–1226. doi: 10.1038/bjc.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department of Health . NHS Long Term Plan. London: Department of Health; 2019. [Google Scholar]
- 4.National Institute for Health and Care Excellence . Suspected cancer: recognition and referral [NG12] London: NICE; 2015. [PubMed] [Google Scholar]
- 5.Chapman D, Poirier DV, Fitzgerald K, et al. First results from five multidisciplinary diagnostic centre (MDC) projects for non-specific but concerning symptoms, possibly indicative of cancer. Br J Cancer. 2020;123:722–729. doi: 10.1038/s41416-020-0947-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolly SO, Jones G, Allchorne P, et al. The effectiveness of the Guy’s Rapid Diagnostic Clinic (RDC) in detecting cancer and serious conditions in vague symptom patients. Br J Cancer. 2021;124(6):1079–1087. doi: 10.1038/s41416-020-01207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mouseholm E, Lindhardt B. Patient characteristics and cancer prevalence in the Danish cancer patient pathway for patients with serious non-specific symptoms and signs of cancer–a nationwide, population-based cohort study. Cancer Epidemiol. 2017;50:166–172. doi: 10.1016/j.canep.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Næser E, Fredburg U, Møller H, et al. Clinical characteristics and risk of serious disease in patients referred to a diagnostic centre: a cohort study. Cancer Epidemiol. 2017;50:158–165. doi: 10.1016/j.canep.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Stenman E, Palmér K, Rydén S, et al. Diagnostic spectrum and time intervals in Sweden’s first diagnostic centre for patients with nonspecific symptoms of cancer. Acta Oncol. 2019;58(3):296–305. doi: 10.1080/0284186X.2018.1537506. [DOI] [PubMed] [Google Scholar]
- 10.Jones D, Neal RD, Duffy SRG, et al. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol. 2020;21(6):748–750. doi: 10.1016/S1470-2045(20)30242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott LJ, Murphy M, Price S, et al. Changes in presentations with features potentially indicating cancer in primary care during the COVID-19 pandemic: a retrospective cohort study. BMJ Open. 2021;11(5):e050131. doi: 10.1136/bmjopen-2021-050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiering B, Lyratzopoulos G, Hamilton W, et al. Concordance with urgent referral guidelines in patients presenting with any of six ‘alarm’ features of possible cancer: a retrospective cohort study using linked primary care records. BMJ Qual Saf. 2021 doi: 10.1136/bmjqs-2021-013425.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallander MA, Johansson S, Ruigómez A, et al. Unspecified abdominal pain in primary care: the role of gastrointestinal morbidity. Int J Clin Pract. 2007;61(10):1663–1670. doi: 10.1111/j.1742-1241.2007.01529.x. [DOI] [PubMed] [Google Scholar]
- 14.Viniol A, Keunecke C, Biroga T, et al. Studies of the symptom abdominal pain – a systematic review and meta-analysis. Fam Pract. 2014;31(5):517–529. doi: 10.1093/fampra/cmu036. [DOI] [PubMed] [Google Scholar]
- 15.Koo MM, von Wagner C, Abel GA, et al. The nature and frequency of abdominal symptoms in cancer patients and their associations with time to help-seeking: evidence from a national audit of cancer diagnosis. J Public Health (Oxf) 2018;40(3):e388–e395. doi: 10.1093/pubmed/fdx188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shephard E, Neal R, Rose P, et al. Clinical features of kidney cancer in primary care: a case–control study using primary care records. Br J Gen Pract. 2013. DOI: . [DOI] [PMC free article] [PubMed]
- 17.Shephard E, Stapley S, Neal RD, et al. Clinical features of bladder cancer in primary care. Br J Gen Pract. 2012. DOI: . [DOI] [PMC free article] [PubMed]
- 18.Walker S, Hyde C, Hamilton W. Risk of uterine cancer in symptomatic women in primary care: case–control study using electronic records. Br J Gen Pract. 2013. DOI: . [DOI] [PMC free article] [PubMed]
- 19.Volk ML, Marrero JA. Early detection of liver cancer: diagnosis and management. Current Gastroenterol Reports. 2008;10(1):60–66. doi: 10.1007/s11894-008-0010-2. [DOI] [PubMed] [Google Scholar]
- 20.Herbert A, Rafiq M, Pham TM, et al. Predictive values for different cancers and inflammatory bowel disease of 6 common abdominal symptoms among more than 1.9 million primary care patients in the UK: a cohort study. PLoS Med. 2021;18(8):e1003708. doi: 10.1371/journal.pmed.1003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Int J Epidemiol. 2015;44(3):827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stapley S, Peters TJ, Neal RD, et al. The risk of oesophago-gastric cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer. 2013;108(1):25–31. doi: 10.1038/bjc.2012.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton W, Round A, Sharp D, et al. Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer. 2005;93(4):399–405. doi: 10.1038/sj.bjc.6602714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stapley S, Peters TJ, Neal RD, et al. The risk of pancreatic cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer. 2012;106(12):1940–1944. doi: 10.1038/bjc.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton W, Peters TJ, Bankhead C, et al. Risk of ovarian cancer in women with symptoms in primary care: population based case-control study. BMJ. 2009;339:b2998. doi: 10.1136/bmj.b2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson J, Nicholson BD, Hamilton W, et al. Identifying clinical features in primary care electronic health record studies: methods for codelist development. BMJ Open. 2017;7(11):e019637. doi: 10.1136/bmjopen-2017-019637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Office for National Statistics 2011 Census: usual resident population by single year of age, unrounded estimates, local authorities in the United Kingdom. Tables PP02UK and PP03UK. 2013. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/2011censuspopulationestimatesbysingleyearofageandsexforlocalauthoritiesintheunitedkingdom (accessed 20 February 2022).
- 28.Office for National Statistics Cancer Registration Statistics, England: 2011 [Table 1 of the Reference Tables associated with this publication] 2013. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/2013-06-26 (accessed 20 February 2022).
- 29.Dregan A, Moller H, Murray-Thomas T, et al. Validity of cancer diagnosis in a primary care database compared with linked cancer registrations in England. Population-based cohort study. Cancer Epidemiol. 2012;36(5):425–429. doi: 10.1016/j.canep.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Price SJ, Stapley SA, Shephard E, et al. Is omission of free text records a possible source of data loss and bias in Clinical Practice Research Datalink studies? A case–control study. BMJ Open. 2016;6(5):e011664. doi: 10.1136/bmjopen-2016-011664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holtedahl K, Hjertholm P, Borgquist L, et al. Abdominal symptoms and cancer in the abdomen: prospective cohort study in European primary care. Br J Gen Pract. 2018. DOI: . [DOI] [PMC free article] [PubMed]
- 32.Holtedahl K, Borgquist L, Donker GA, et al. Symptoms and signs of colorectal cancer, with differences between proximal and distal colon cancer: a prospective cohort study of diagnostic accuracy in primary care. BMC Fam Pract. 2021;22(1):148. doi: 10.1186/s12875-021-01452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hippisley-Cox J, Coupland C. Symptoms and risk factors to identify women with suspected cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract. 2013. DOI: . [DOI] [PMC free article] [PubMed]
- 34.Hippisley-Cox J, Coupland C. Symptoms and risk factors to identify men with suspected cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract. 2013. DOI: . [DOI] [PMC free article] [PubMed]
- 35.Jones D, Di Martino E, Bradley S, et al. Factors affecting the decision to investigate older adults with potential cancer symptoms: a systematic review. Br J Gen Pract. 2021. DOI: . [DOI] [PMC free article] [PubMed]
- 36.Nicholson B, Mant D, Bankhead C. Can safety-netting improve cancer detection in patients with vague symptoms? BMJ. 2016;355:i5515. doi: 10.1136/bmj.i5515. [DOI] [PubMed] [Google Scholar]
- 37.Smith CF, Drew S, Ziebland S, et al. Understanding the role of GPs’ gut feelings in diagnosing cancer in primary care: a systematic review and meta-analysis of existing evidence. Br J Gen Pract. 2020. DOI: . [DOI] [PMC free article] [PubMed]