Abstract
As of 27 March 2022, the β-coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 487 million individuals worldwide, causing more than 6.14 million deaths. SARS-CoV-2 spreads through close contact, causing the coronavirus disease 2019 (COVID-19); thus, emergency lockdowns have been implemented worldwide to avoid its spread. COVID-19 is not the first infectious disease that humankind has had to face during its history. Indeed, humans have recurrently been threatened by several emerging pathogens that killed a substantial fraction of the population. Historical sources document that as early as between the 10th and the 6th centuries BCE, the authorities prescribed physical–social isolation, physical distancing, and quarantine of the infected subjects until the end of the disease, measures that strongly resemble containment measures taken nowadays. In this review, we show a historical and literary overview of different epidemic diseases and how the recommendations in the pre-vaccine era were, and still are, effective in containing the contagion.
Keywords: history of plague, preventive measures, COVID-19, SARS-CoV-2, vaccines
1. Introduction
A novel single-stranded RNA virus from the β-Coronaviridae family named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), initially recognized as the viral cause of a cluster of pneumonia “originally unknown”, emerged firstly in China, spread rapidly worldwide, and led to a global pandemic called “coronavirus disease 2019” (COVID-19). β-Coronaviruses are members of a large family of RNA viruses. One-third of the RNA sequence encodes four core structural proteins: spike (S), membrane-associated envelope (E), membrane (M), and nucleocapsid (N) proteins [1].
COVID-19 is characterized by severe upper respiratory tract infections, respiratory distress, and frequent need for hospitalization. Since the outbreak, COVID-19 has killed more than 6.12 million patients worldwide as of March 27th 2022. The clinical spectrum of COVID-19 ranges from asymptomatic or pauci-symptomatic to severe symptomatic with a large range of clinical manifestations such as cough, fever, myalgia, gastrointestinal symptoms, and anosmia. Admission to intensive care units (ICUs), mechanical ventilation, and mortality are common, especially in subjects with compromised immune systems, in subjects affected by underlying chronic diseases, or in the elderly [2]. The rapid deterioration of clinical conditions has been described as a typical feature of SARS-CoV-2 infection, driven by the pathogen–host interaction. The angiotensin-converting enzyme 2 (ACE2) is the only confirmed SARS-CoV-2 entry receptor [3]. SARS-CoV-2 and ACE2 binding allows the virus to enter a cell through the spike (S) proteins that work in concert with the host cell serine protease transmembrane protease serine 2 (TMPRSS2). TMPRSS2 cleaves the spike protein of SARS-CoV-2, facilitating membrane fusion. ACE2 relies on the renin–angiotensin system (RAS) molecular pathway; ACE2 is a crucial counter-regulatory enzyme to ACE by the breakdown of angiotensin II that is involved in blood pressure regulation and electrolyte homeostasis [4]. ACE2 is expressed in multiple tissues, and thus the existence of multiorgan complications/failure is not surprising [5]. Recently, clinicians started to report prolonged sequelae of symptoms in the post-acute phase of COVID-19, which may be represented in three categories:
-
(1)
Residual symptoms continuing after acute infection recovery;
-
(2)
Organ dysfunction continuing after initial recovery;
-
(3)
New symptoms/syndromes developing after initial asymptomatic or mild infection [6].
As a consequence of the long duration of pandemics and the large number of patients that survived the disease, the priority for National Health Systems is progressively shifting towards mid- and long-term effects of COVID-19. Frail and prefrail subjects who recovered after hospital admission represent an excellent model for studying this new scenario. With the COVID-19 pandemic, consideration of frailty hallmarks in dismissing infected older patients is critical to identify those subjects at higher risk of functional decline that may fall into accelerated aging processes. In this contest, there is the vital role of rehabilitation programs for the coming years and the urgent need to develop strategies to assist COVID-19 survivors.
Communicable Diseases
Communicable diseases, also known as transmissible diseases or infectious diseases, are illnesses that spread, directly or indirectly, from one organism to another through the transfer of a pathogen such as viruses, bacteria, fungi, protozoa, multicellular parasites, and aberrant proteins known as prions. They have been present for all of human history but became important with the rising of agrarian life 10,000 years ago when the demographic expansion and technological innovations made the transmission of infectious diseases more possible. The zoonotic transmission of pathogens from animals to humans is the key mechanism in the process of emerging infections, and the probability of cross-species transmission is enhanced by increasing interactions between humans and animals [7]. According to Wolfe et al. [7], the passage of a pathogen from a different species to humans is characterized by five steps:
-
(1)
The pathogen infects only animals under natural conditions;
-
(2)
The pathogen evolves to be transmitted to humans without continuous human-to-human transmission;
-
(3)
The pathogen undergoes secondary transmission to humans;
-
(4)
The disease exists in animals but different secondary human-to-human transmission occurs without the involvement of animal hosts;
-
(5)
The disease occurs exclusively in humans.
The risk of zoonotic transmission is related to the animal species harboring the pathogen, the nature of human–animal interaction, and the frequency of these interactions.
One of the first pieces of evidence of the insurgence of an epidemic is derived from molecular studies that unambiguously identified the presence of DNA from Yersinia pestis (Y. pestis), the causative agent of plague, in different individuals at Fralsegarden in Gokhem parish, Falbygden, western Sweden, dated to 4900 BCE [8]. Interestingly, the analysis of both the archaeological context and the human genomes supports the notion that the rise and the spread of the above plague were related to lifestyle (high population densities in close contact with animals), population growth, and expanding trade networks. It has been proposed that the Y. pestis plague may have contributed to the Neolithic decline [8].
2. History of “Plagues”
The written texts and artworks of past times are useful as they provide information about the cultural contexts and enhance the understanding of the spread of communicable diseases in the past, centuries before the modern scientific literature. Literary plagues involve different forms, from true-to-life to completely imaginary. The term “plague” derives from the Latin plaga, meaning “blow or wound” [9]. Although plague refers to infection by an extremely contagious Gram-negative bacterium, Y. pestis (formerly called Pasteurella pestis), the word “plague” is used not only as a generic term for an epidemic or pandemic but also as a metaphor for a wide range of calamities. Interestingly, the first book of Western literature, Homer’s Iliad, starts with the story of a plague that strikes the Greek army at Troy (Table 1) [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28].
Table 1.
Plague in the human literary documents.
| Years | Infectious Disease | Literary Documents | Origin | Possible Pathogen | Peer-Reviewed Source |
|---|---|---|---|---|---|
| 1500 BCE | Different diseases | Ebers Papyrus | Egipt | Unknown | [10] |
| 1335 BCE | Hittite plague | The Amarna Letter EA 96; The Amarna Letter EA 137; The Amarna Letter EA 224. | Mesopotamia via Canaanite harbors | Francisella tularensis | [11] |
| The story of the plague is handed down in literature in the Hittite plague prayers (KUB XIV 10 and KUB XXVI 86) | |||||
| 1190 or 1141 BCE | Plague of the Philistines, also known as the Plague of Ashdod | Bible, Book of Samuel according to 1 Samuel 5:9 |
Ashdod (now Israel) | Y. pestis, Francisella tularensis, smallpox, or variola | [12] |
| 800–781 BCE | Plague of the Achaean soldiers’ camp | Homer’s Iliad Book 1:50–56 | Troy or Ilion located at Hisarlik in present-day Turkey | Equine encephalomyelitis? | [13] |
| 463 to 462 BCE | Rome when Lucius Aebutius and Publius Servilius were elected consuls | Quintus Livius Titus, popularly known as Livy, in his Early History of Rome | Rome, Italy | Unknown | [14] |
| 430–420 BCE | Plague of Thebes | Oedipus Rex by Sophocles (original Greek title Oιδίπους τύραννος) |
Thebes, Greece | Leishmania spp., Leptospira spp., Brucella abortus, Orthopoxviridae, and Francisella tularensis in cattle. | [15] |
| Humans could have been affected by a different pathogen such as Salmonella enterica serovar Typhi | |||||
| 430–429 BCE | Plague of Athens | History of the Peloponnesian War by Thucydides | Ethiopia, then all the Mediterranean | Salmonella enterica serovar Typhi | [16,17] |
| 430–429 BCE | Plague of Athens | Titus Lucretius Carus 99–55 BCE De Rerum Natura with the Plague of Athens |
Athens, Greece | Unknown | [18] |
| 433–432 BCE | Rome when Gaius Julius and Lucius Verginius were consuls | Quintus Livius Titus, popularly known as Livy, in his Early History of Rome Ab Urbe Condita 4.20–21, 4.25.3–4, and 4.30.8–10) | Rome, Italy | Anthrax? | [14] |
| 396 BCE | Plague at Syracuse | Punica by Tiberius Catius Asconius Silius Italicus | Syracuse, Italy | Typhoid or smallpox | [19] |
| Mythological plague | 70–19 BCE | Publius Vergilius Maro Third book of Georgics |
Unknown | Unknown | [18] |
| Mythological plague | Plague at Aegina | Publius Ovidius Naso 43 BCE–18 CE Metamorphoses, Book 7 523–613 |
Unknown | Unknown | [18] |
| Mythological plague | Lucius Annaeus Seneca Between 8 and 1 BCE–65 CE |
Oedipus is a fabula crepidata (Roman tragic play with Greek subject) | Unknown | Unknown | [18] |
| 293 BCE | Plague of Rome | The construction of a hospital on Tiber Island in honor of Asclepius, the God of Medicine | Rome, Italy | Unknown | [14] |
| 165 CE, first wave | The Antonine Plague, Marcus Aurelius Antoninus (161–180 CE) | Plague of Galen, Methodus Medendi |
Rome, Italy | Smallpox (Variola major and Variola minor) | [20,21] |
| 251–266 CE second wave | Cyprian Plague | Documented by Saint Cyprian, the Assyrian | Ethiopia | Small-pox | [21] |
| 541–543 CE and subsequent outbreaks 750–1000 CE |
Justinian Plague Justinian I (r. 527–565 CE) | Symptoms described by Procopius of Cesarea (500–565 CE) in his History of the Wars, Book II, are very similar to those later reported during the second pandemic of the 14th to 17th centuries (the Black Death) | Pelusium, near Suez in Egypt | Y. pestis, distinct from the lineage that caused the second pandemic (including the Black Death) 800 years later in human populations | [22] |
| 590 CE | Roman Plague | The plague was described by the bishop and chronicler Gregory of Tours and later chronicler Paul the Deacon | Rome, Italy | Y. pestis | [14] |
| 1347–1351 | Black Death | China and Inner Asia | Y. pestis | ||
| Thomas Walsingham—Norfolk, 1422 | Thomas Walsingham “Towns once packed with people were emptied of their inhabitants, and the plague spread so thickly that the living were hardly able to bury the dead” | Y. pestis | [23] | ||
| Gabriele de Mussi (1280–1356) | Gabriele de Mussi Historia de Morbo | Y. pestis | [24] | ||
| Francesco Petrarch (1304–1374) | Francesco Petrarch chronicles the plague at Parma and bewails the magnitude of the destruction that seems to threaten the very existence of the human race | Y. pestis | [23] | ||
| Agnolo di Tura (14th century) |
Agnolo di Tura, in his Crunica Senese, gives a shocking account of the raging plague in Siena | Y. pestis | [25] | ||
| Simon de Covino (1320–1367) |
At Marseilles, the Black Death entered France, and Simon de Covino, the French poet–physician, describes the symptoms of the disease in Latin hexameter verse | Y. pestis | [25] | ||
| Giovanni Boccaccio 1313–1375 |
Boccaccio acknowledges the Florentine plague of 1348 to be the prime mover of The Decameron, and in the introduction to that work he says “between March and the following July” | Y. pestis | [26] | ||
| Matteo Villani 1275–1363 |
Matteo Villani attributes to the Black Death the social, moral, and political anarchy that were rampant in Florence in succeeding years | Y. pestis | [27] | ||
| The second plague pandemic, caused by Yersinia pestis, devastated Europe and the nearby regions between the 14th and 18th centuries 1360–1363; 1374; 1400; 1438–1439; 1456–1457; 1464–1466; 1481–1485; 1500–1503; 1518–1531; 1544–1548; 1563–1566; 1573–1588; 1596–1599; 1602–1611; 1623–1640; 1644–1654; 1664–1667; 1679 |
Second outbreak of Black Death | Giovanni Baldinucci’s diary | Y. pestis | [28] | |
| Italian Plague of 1629–1631 or the Great Plague of Milan and the Great Plague of Seville (1647–1652), | Francesco Rondinelli | ||||
| the Great Plague of London (1665–1666), | Plague of Florence 1632–1633 |
||||
| and the Great Plague of Vienna (1679) | Alessandro Manzoni’s The Betrothed | ||||
| 1720–today | Third outbreak of Black Death. There is some controversy over the identity of the disease, but in its virulent form, it was responsible for the Great Plague of Marseille in 1720–1722, the Great Plague of 1738 (which hit Eastern Europe), and the Russian plague of 1770–1772 |
Yunnan Province, Southwest China | Y. pestis | [23] |
It is important to remember that no medicine or doctor had been able to cure or prevent infectious diseases for millennia, and the only way to stay safe was to avoid contact with infected people and contaminated objects. The great terror of epidemics was also fueled by the belief in their supernatural origin, as they were often believed to be caused by irate gods. In the Bible (i.e., Exodus (Hebrew: יציאת מצרים) 9:14, Numbers (Hebrew: בְּמִדְבַּר) 11:33, Former Prophets 1 Samuel (Hebrew: נביאים ראשונים, שְׁמוּאֵל]) 4:8, Psalms (Hebrew: תְּהִלִּים) 89:23, the Latter Prophets Isaiah (Hebrew: נביאים אחרונים; ספר ישעיהו) 9:13), the plague was regarded as a divine punishment for sins, and hence the frightening description of its diffusion was a warning to behave morally (reviewed also by Freemon [29]). This supposed causal relationship between plague and sin is also present in Greek literary texts, such as Homer’s Iliad and Sophocles’ King Oedipus (429 BCE) (Table 1). This attitude changed completely with Thucydides (460–395 BCE), a Greek historian, who in his History of the Peloponnesian War, refused a supernatural origin of the disease (the Plague of Athens), describing it with a scientific approach, reporting the origin of the plague, the symptoms, the incapacity of doctors to cure it, and the uncontrolled fear of contagion among the public (Table 1). The plague originated in early May 430 BCE, with a second wave in the summer of 428 BCE and a third in the winter of 427–426 BCE, and spared no segment of the population, including the statesman Pericles and Thucydides himself. The Plague of Athens killed between 75,000 and 100,000 people, ~25% of the population of Athens (429 BCE) (Table 1 and Table 2 and Figure 1) [16,30].
Table 2.
Scientific milestones in infectious disease discovery.
| Years | Discovery | References |
|---|---|---|
| 1530 | Girolamo Fracastoro expresses his ideas on the origin of syphilis, explaining that this disease is spread by “seeds” distributed by intimate contact | [43] |
| 1683 | Anton van Leeuwenhoek observes bacteria under the first microscope | [44] |
| 1701–1714 | Giacomo Pilarino and Emmanuel Timoni give the first smallpox inoculations | [45] |
| 1757 | Francis Home demonstrates that measles is caused by an infectious agent in the blood of patients | [46] |
| 1796 | Edward Jenner develops the process of vaccination for smallpox, the first vaccine for any disease | [45] |
| 1842–1847 | Oliver Wendel Holmes describes puerperal fever and Ignaz Semmelweis discovers how to prevent the transmission of puerperal fever | [44] |
| 1857 | Louis Pasteur identifies germs as a cause of disease | [47] |
| 1870 | Robert Koch and Louis Pasteur establish the germ theory of disease | [48] |
| 1879 | First vaccine developed for chicken cholera by Louis Pasteur | [49] |
| 1881 | First vaccine developed for anthrax by Louis Pasteur | [49] |
| 1882 | First vaccine developed for rabies by Louis Pasteur | [49] |
| 1882 | Koch discovers the Tuberculosis bacillus: Mycobacterium tuberculosis | [50] |
| 1890 | Emil von Behring discovers antitoxins and develops tetanus and diphtheria vaccines | [49] |
| 1892 | Dmitri Ivanovsky shows that sap from a diseased tobacco plant remained infectious to healthy tobacco plants despite having been filtered | [51] |
| 1894 | Isolation in culture and microscopic description of causative bacteria | [52,53] |
| 1896/1897 | Almroth Wright and Richard Pfeiffer develop the first vaccine for typhoid fever | [54] |
| 1897 | Waldemar Haffkine tests on himself the first vaccine developed for bubonic plague | [55] |
| 1897 | Paul Ehrlich develops a standardized unit of measure for diphtheria antitoxin that would play an important role in future developmental work on sera and vaccines | [56] |
| 1898 | Martinus Beijerinck is convinced that filtrate contains a new form of infectious agent called a virus | [57] |
| 1913 | Paul Ehrlich develops the first antimicrobial drug, “Salvarsan”, against the bacterium Treponema pallidum, the etiological agent of syphilis | [58] |
| 1918 | Charles Nicolle and Charles Lebailly advance the hypothesis that the causative agent of the “Spanish” flu is a nonfilterable agent of infinitesimal dimensions: possibly a virus | [59,60] |
| 1923 | First vaccine developed for diphtheria by Alexander Thomas Glenny | [49] |
| 1924 | First vaccine developed for tetanus (tetanus toxoid) by Alexander Thomas Glenny | [49] |
| 1914/1926 | First vaccine developed for whooping cough (pertussis) by Leila Denmark | [49] |
| 1927 | First vaccine developed for tuberculosis by Albert Calmette and Camille Guérin | [49] |
| 1928 | Sir Alexander Fleming discovers penicillin | [61] |
| 1931 | German engineers Ernst Ruska and Max Knoll project the electron microscope | [62] |
| 1931 | Live attenuated bacterial vaccine developed and tested | [63,64] |
| 1932 | Gerhard Domagk announces that the red dye prontosil is active against streptococcal infections in humans; afterward, Ernest Fourneau, Jacques and Thérèse Tréfouël, Daniel Bovet, and Fedrico Nitti show that the active antibacterial agent is sulfanilamide | [65] |
| 1935 | First vaccine developed for yellow fever by Max Theiler | [49] |
| 1937 | First vaccine developed for typhus by Rudolf Weigl | [49] |
| 1938 | Jonas Salk and Thomas Francis develop the first vaccine against flu viruses | [66] |
| 1940–1947 | Large concentrations of blood bacteria correlated with mortality | [67] |
| 1944 | Oswald Avery, Colin MacLeod, and Maclyn McCarty report that DNA is the transforming factor in the experiments of Frederick Griffith where an extract of the pathogenic strain of pneumococcus could transform a harmless strain into a pathogenic one | [68] |
| 1944 | Selman Waksman, Albert Schatz, and Elizabeth Bugie announce the discovery of streptomycin and state that it is active against Mycobacterium tuberculosis | [69] |
| 1949 | John Franklin Enders, Thomas Weller, and Frederick Robbins grow poliovirus for the first time in cultured human embryo cells | [70] |
| 1953 | James Watson and Francis Crick describe the structure of DNA | [71] |
| 1955 | Jonas Salk develops the first polio vaccine | [49] |
| 1964 | First vaccine developed for measles by John Franklin Enders | [49] |
| 1967 | Maurice Hilleman develops the first vaccine for mumps virus | [49] |
| 1970 | Maurice Hilleman develops the first vaccine for rubella | [49] |
| 1977 | First approved vaccine developed for pneumonia | [49] |
| 1978 | First approved vaccine developed for meningitis | [49] |
| 1980 | Genetic relatedness of Yersinia pestis and Yersinia pseudotuberculosis | [72] |
| 1981 | First approved vaccine developed for hepatitis B | [49] |
| 1983 | HIV, the virus that causes AIDS, is identified | [73] |
| 1987 | First approval of a drug against AIDS: zidovudine (AZT, ZDV) | [74] |
| 1992 | First approved vaccine developed for hepatitis A | [49] |
| 1995 | First vaccine developed for varicella (chickenpox) and hepatitis A | [49] |
| 1998 | First approved vaccine developed for rotavirus | [49] |
| 2001–2011 | DNA sequence of Yersinia pestis samples | [75] |
| 2006 | First vaccine developed for human papillomavirus and for herpes zoster (shingles) | [49] |
| 2019 | Food and Drug Administration (FDA) approves rVSVΔG-ZEBOV-GP Ebola vaccine | [76] |
| 2020 | First vaccine against COVID-19 | [77] |
| 2022 | First drugs for COVID-19 (molnupiravir and Paxlovid or nirmatrelvir + Paxlovid) | [78,79,80] |
In bold the most important milestones in science discovery.
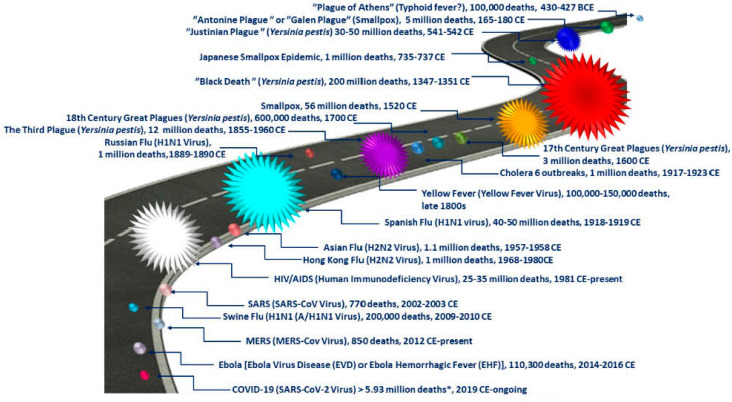
Figure 1.
Emergence of the most important pandemics in the world until today with a possible estimation of the number of casualties. * the number is not definitive since the pandemic is ongoing. Adapted from [31].
The Roman historian Titus Livius, known as Livy in English (Table 1), writing many centuries later, reported that epidemics occurred also in Rome in 433 and 438 BCE, suggesting the same origin of the disease. DNA analysis of skeletal remains recovered in 2001 in a mass burial pit in Greece and dating back to the plague years revealed ancient microbial typhoid (Salmonella enterica serovar Typhi), supporting the hypothesis that the Plague of Athens was a typhoid disease [32].
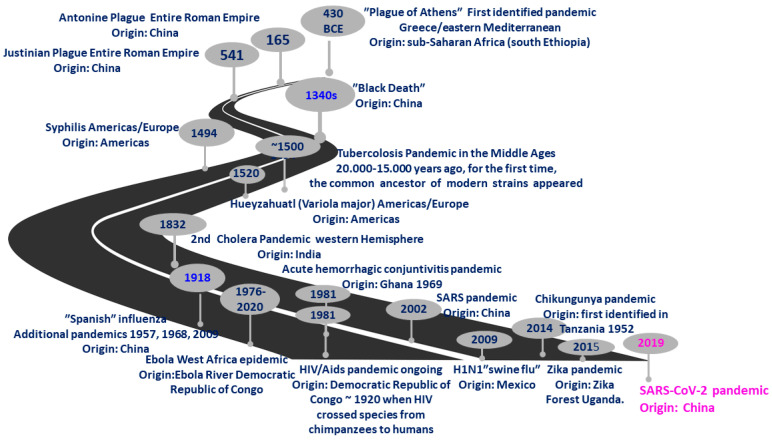
Figure 1 reports the emergence of the most important pandemics in the world until today with a possible estimation of the number of casualties, whereas Figure 2 reports the origin of the pandemics, considering their onset for the first time.
Figure 2.
First place of appearance of pandemics.
Centuries later, between 165 and 180 CE, the Roman empire was challenged by the Antonine Plague. It is supposed that the disease was brought by armies from what is currently Iraq and eventually spread into the entire Roman empire, which comprised Central and Southern Europe. It is estimated that the diseases killed about 5 million people [20]. The symptoms were described by Galen and suggested that it was probably smallpox, as its presentation was characterized by rashes, hemorrhagic pustules, bloody diarrhea, fever, and sometimes hemoptysis [33].
The Justinian Plague (starting in 541 CE with subsequent outbreaks until 750–1000 CE) is the first confirmed pandemic plague caused by Y. pestis based on the analysis of the teeth of people buried at that time [22]. Accordingly, the reported symptoms were those distinctive of Y. pestis infection: fever, cough, and dyspnea in pneumonic plague and groin or axillary buboes in bubonic plague. The transmission was mediated from infected rats to humans through flea bites, but there was also human–human transmission. It has been estimated that the Plague of Justinian killed 60% of people in the Mediterranean area [34].
The Justinian Plague may have contributed to the end of the Roman empire, characterizing the transition from the Classical to the Medieval era [22]. It has been shown that the strain of Y. pestis linked with the Plague of Justinian is different from those associated with later human plague pandemics, and it seems that this strain is extinct or poorly present in wild rodent reservoirs [22].
The first scientific description of the etiology of plague and of the possible way of spreading of the disease did not become available until the 19th century. In 1894, in Hong Kong, Alexander Yersin (1863–1943) isolated in culture and identified the causative bacterial agent (i.e., Yersinia pestis) [35]. After examination of the lymph glands of dead rats, he found the same bacteria described previously in human tissues; consequently, he made the causal connection between rat mortality and human epidemics. Surprisingly, the earliest recorded role of mice in plague is in the Bible that ascribed the pestilence among the Philistines to “the mice that marred the land” (1 שְׁמוּאֵל Samuel. 6:4–18).
For the next centuries, after the different outbreaks of Justinian’s plague, different epidemics occurred frequently. The next great pandemic plague was the dreaded “Black Death” of Europe in the 14th century (Figure 1 and Figure 2 and Table 1).
The Black Death, also known as the Black Plague, spread from the Caspian Sea by the town of Caffa, now Feodosia, a Genoese colony, to almost all European countries, causing the death of one-third of the European population [36] over the next few years, and persisted in Europe until 1750. Following traditional beliefs promoted by Hippocrates and Galen, the poisoned air (“miasma”) was believed to be the causative agent of the epidemics; as already mentioned, an association between the plague and dead rats was not observed until the beginning of the 20th century [30]. The name Black Death derives from a typical symptom of the disease, called acral necrosis, of black color due to subdermal hemorrhages. Although there was a great debate on the etiology of this disease, DNA and protein signatures specific to Y. pestis were found in human skeletons from mass graves associated archaeologically with the Black Death throughout Europe, confirming it as the causative agent. Furthermore, the analysis of 17 single nucleotide polymorphisms (SNPs) and the absence of a deletion in the glpD gene (aerobic glycerol-3-phosphate dehydrogenase) identified two previously unknown but related clades of Y. pestis associated with distinct medieval mass graves, suggesting that the plague arrived in Europe at different times, through distinct routes [37]. The third wave of Y. pestis started in 1772 in Yunnan Province, Southwest China. In this case, Europe was not significantly affected, but some cases were reported in Malta in June 1945 [35,38].
A great nightmare and terror worldwide was represented by smallpox, which was a highly contagious and lethal disease with remarkably high death rates until its global eradication, declared by the WHO on 8 May 1980. The causative agent of smallpox is the variola virus (VARV), a member of the genus Orthopoxvirus, family Poxviridae, and subfamily Chordopoxvirina. Analysis of viruses’ DNA collected from different individuals living both in Eurasia and in the Americas between 200 and 150 years ago revealed that their DNA resembled modern variola. The young pharaoh Rameses V in the 12th century BCE was probably killed by VARV [39]. The etiological cause of the Antonine Plague, which killed the emperor himself, was probably VARV. Queen Elizabeth I of England, at the age of 29 years in October 1562, survived smallpox, which left her without hair and with permanent disfiguring facial scars [40]. In 1694, VARV killed Queen Mary of England; six years later, it killed her son, the Duke of Gloucestershire, and then it went on to kill Emperor Joseph I of Austria (17 April 1711), King Luis I of Spain (31 August 1724), Tsar Peter II of Russia (30 January 1730), Queen Ulrika Eleonora of Sweden (24 November 1741), and the French King Louis XV (10 May 1774) [41]. During the American Revolutionary War (1775–1783), one of the greatest threats to the army was smallpox. In 1775, General George Washington recognized smallpox as a very serious problem for his army, especially after the smallpox outbreak in the city of Boston in the winter of 1775 [42].
Table 2 shows the scientific aspect of the history of infectious diseases [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80], and Table 3 shows infectious diseases studied using molecular techniques, including metagenomics. Different reviews as well as two important books explore this field [81,82,83,84,85,86]. Moreover, Spyrou et al. [87] reviewed the ancient pathogen genomic data recovered from archaeological or historical specimens, including the method of retrieval. DNA analysis from archaeological sites has been a powerful tool for deciphering the steps of the evolution of pathogens [81]. Combining the data obtained from molecular analysis with disease modeling and the genetic history of the human population, together with the information offered by the archaeological, historical, and palaeopathological records, is helpful to define a comprehensive picture of host–pathogen interactions during centuries. Artistic and literary works, on the other hand, are helpful in understanding what kind of measures were taken to deal with pandemic emergencies in ancient times.
Table 3.
Infectious diseases studied using molecular techniques including metagenomics.
| Time Frame of Disease Appearance | Archaeological Discovery and Pathogen Identification | Method of Retrieval | References |
|---|---|---|---|
| 4900 BCE | Different individuals at Fralsegarden in Gokhem parish, Falbygden, western Sweden. Identification of the presence of Y. pestis DNA | Genotyping of Y. pestis strain, phylogenetic and molecular clock analyses, heatmaps and functional classification of variants, and admixture analyses of human genomes | [8] |
| 4800 to 3700 | 563 tooth and bone samples from Russia, Hungary, Croatia, Lithuania, Estonia | Shotgun screening sequencing, in silico screening, deep shotgun sequencing, Y. pestis in-solution capture, genome reconstruction and authentication, individual sample treatment due to laboratory preparation and sequencing strategies, SNP calling, heterozygosity and phylogenetic analysis, dating analysis, SNP effect analysis, virulence factor analysis, and indel analysis | [88] |
| Latvia, and Germany. | |||
| Identification of the presence of extinct clade in the Y. pestis phylogeny | |||
| 3250 BCE to 700 CE | Different individuals from Abusir el-Meleq, located in Middle Egypt. | Metagenomic screening and SNP typing | [89] |
| Identification of Mycobacterium leprae strain and a 2000-year-old human hepatitis B virus | |||
| 3500 BCE to 300 CE | Mummies from Upper and Lower Egypt. | PCR amplification and sequencing | [90,91,92] |
| Identification of Falciparum malaria (Plasmodium Falciparum) and human tuberculosis (Mycobacterium tuberculosis) | |||
| 430 BCE | Dental pulp in Kerameikos mass burial of putative victims of the plague. | PCR amplification and sequencing | [32] |
| Identification of Salmonella enterica serovar Typhi | |||
| 541–543 CE | Radiocarbon dating of individuals to 533 AD (plus or minus 98 years) from the Aschheim-Bajuwarenring, Bavaria, Germany. | PCR amplification and sequencing reconstructed draft genomes of the infectious Y. pestis strains, comparing them with a database of genomes from 131 Y. pestis strains from the second and third pandemics, and constructing a maximum likelihood phylogenetic tree. | [22] |
| Identification of Y. pestis | |||
| 13th and beginning of the 14th century to 19th century | Different subjects from different places in Europe. Identification of Y. pestis |
PCR and sequencing SNP calling and evaluation | [93,94,95,96] |
| 1630–1632 | Different subjects from Imola Northern Italy. Identification of Y. pestis | Genomic and metagenomic analysis of sequencing data | [97] |
| 16th century | Italian mummy from the 16th century. | Genomic and metagenomic analysis of sequencing data | [98] |
| Identification of intact smallpox virus particles | |||
| 1580–1630 VARV | Different subjects from Vilnius, Lithuania. | PCR and sequencing | [99,100] |
| Identification of smallpox |
In modern history, the most devastating pandemic was the 1918–1919 influenza called the “Spanish” flu (or the “Spanish Lady”) (Table 1). The flu was named “Spanish” since the infection spread in 1918 when the majority of nations were involved in World War I and the newspapers were under censorship rules and only the Spanish reported the pandemic. The first wave of flu appeared in March 1918, at Camp Funston in Kansas (USA), a military training camp. The second, more virulent wave appeared in August. In Europe, it appeared firstly at the seaport of Brest, where the American troops arrived. The confirmed number of casualties is unknown, with an estimated number ranging from 20 to 150 million [101]. The etiological agent of Spanish flu is named H1N1 for the hemagglutinin (H) and neuraminidase (N) proteins. The H1N1 influenza virus is an orthomyxovirus. The pandemic was characterized by an uncommonly high mortality rate among healthy young adults (from 15 to 34 years), not observed in either prior or subsequent influenza A epidemics. The case fatality rate was >2.5%, compared to <0.1% in other influenza pandemics [102]. The histopathological analysis of lung tissues of subjects who died in 1918 due to influenza showed acute pulmonary edema and/or hemorrhage with acute bronchiolitis, alveolitis, and bronchopneumonia. Data from DNA sequencing suggest that the 1918 virus consisted of a new virus, and that H1N1 is not a result of recombination of previous virus strains acquiring one or more new genes, as was the case of those causing the 1957 (Asian Flu, H2N2) and 1968 (H3N2) pandemics [103]. In 1918, the biomedical idea of influenza was dictated by Richard Pfeiffer’s 1892 statement that Bacillus influenzae was the only etiological cause. Nevertheless, in October 1918, for the first time, two scientists of the Pasteur Institute, Charles Nicolle and Charles Lebailly, advanced the hypothesis that the causative agent of the “Spanish” flu was a nonfilterable agent of infinitesimal dimensions: possibly a virus [60]. Interestingly, in novels and books written during the Spanish flu pandemic period, there is little or no mention of the flu. The majority of deaths likely resulted directly from secondary bacterial pneumonia caused by common upper respiratory tract bacteria [104] of different species that took advantage of the underlying viral infection. As suggested by a study by Anthony Fauci [104], the majority of deaths likely resulted directly from secondary bacterial pneumonia caused by common upper respiratory tract bacteria. According to the above study, the causes of death involved several diverse bacteria, alone or in complex combinations, working with the co-pathogenic properties of the virus itself, possibly linked to viral growth, facility of cell-to-cell spread, cell tropism, or interference with or induction of immune responses. Thus, the large majority of infected subjects in 1918 (>97%) showed a typical, self-limited course of influenza, without any antivirals, antibiotics, or vaccines.
Public health officials imposed multiple interventions as disease containment measures, including the closure of schools, churches, and dancing halls; banning of mass gatherings; mandatory mask wearing; case isolation; and disinfection/hygiene. The primary lesson of the 1918 influenza pandemic was that it is critical to intervene early and that viral spread will be renewed upon relaxation of such measures [105].
Starting in the early 1980s, a new and unusual disease began to emerge worldwide. The disease was recognized as pandemic when on June 5, 1981, the US Centers for Disease Control (CDC) of Atlanta received information of unusually high rates of the uncommon diseases Pneumocystis jirovecii (then called Pneumocystis carinii) pneumonia (PCP) and Kaposi’s sarcoma in young white healthy homosexual men living in Los Angeles. Afterward, in 1982, cases were reported in injection drug users and then in women possibly infected through heterosexual sex. The disease was named acquired immune deficiency syndrome (AIDS) [106]. The first appearance of the disease was in Kinshasa, in the Democratic Republic of Congo, in around 1920, when HIV, the causal agent, crossed species from chimpanzees to humans. In early 1983, a new human retrovirus, initially named lymphadenopathy-associated virus (LAV), was isolated at the Pasteur Institute, Paris, France, from a culture obtained by a lymph node biopsy from a patient with generalized lymphadenopathy. Then, a similar virus was isolated from patients with AIDS, and in 1985, the virus RNA was sequenced. The main receptor for HIV was identified in the CD4 cell surface molecule [73], confirming that HIV causes AIDS. In November 2020, the WHO reported that at the end of 2019, 38.0 million people, 25.7 million of which were in the African Region, were living with HIV [107].
Table 4 summarizes the interventions adopted to prevent the spread of the modern infectious diseases [108,109,110,111,112,113,114,115,116].
Table 4.
Modern infectious disease prevention and intervention.
| Pandemic | Years | Source | Main Actions | Current Prevention | References |
|---|---|---|---|---|---|
| Yellow fever | 1900 | Mosquito | Travel limitation in areas affected by yellow fever. Yellow fever control strategies include insecticide spraying, larval control including larvicide spraying, and bacterial toxins |
Vaccine valid for 10 years | [108,109] |
| SARS-CoV | 2002–2004 | Bats, palm civets |
During the viral incubation period (4–5 days), which is important for the prevention and control of the disease, there are no clinical symptoms | One of the treatment modalities used to reduce the replication of the virus and its spread is passive immunization with monoclonal antibodies | [110] |
| MERS-CoV | 2012 | Dromedary camels | Prevention includes washing hands often, cleaning surfaces regularly with an alcohol-based cleaner, | Interferons (IFNs). Different vaccines targeting SARS-CoV and MERS-CoV have been developed and tested in preclinical models. However, only a few of them have gone into clinical trials, and none of them have been approved by the FDA. |
|
| covering mouth and nose with a tissue when coughing or sneezing | The different vaccines include protein subunit vaccines (RBD-based vaccine), virus-like particle vaccines, DNA vaccines, viral vector vaccines, inactivated vaccines, and live attenuated vaccines | ||||
| A/H1N1 | 2009 | Pigs | Reducing the risk of human-to-human transmission: isolation and quarantine of infected patients. Use of the surgical template. Hand hygiene is the most important measure to reduce the risk of transmission. Hands should be washed frequently with soap and water, alcohol-based cloths, or antiseptic. Cleaning of contaminated surfaces or equipment should be performed with phenolic disinfectants, ammonia compounds, or alcohol | Antiviral drugs, including adamantanes (amantadine, rimantadine) and neuraminidase inhibitors (zanamivir, oseltamivir, peramivir, and laninamivir) are used to treat cases of influenza, even if they have side effects; antibiotics for the treatment or prevention of secondary bacterial pneumonia; parenteral nutrition; oxygen therapy or ventilatory support and vasopressors for shock. Vaccines: for subjects between the ages of 3 and 77. The immunization schedule consisted of two vaccinations, 21 days apart |
[111,112] |
| Ebola | 2013–2016 | Bats, NHPs, and small terrestrial mammals | Reducing the risk of wildlife-to-human: avoiding contact with infected fruit bats or monkeys/apes and the consumption of their raw meat. | Combination of three mono-clonal antibodies directed against the envelope glycoprotein (GP) of EBOV, liposomal-formulated interfering RNA, and inhibitors of RNA polymerase. | [113,114] |
| Reducing the risk of human-to-human transmission: avoiding direct or close contact with people with Ebola symptoms, particularly with their bodily fluids. Gloves and appropriate personal protective equipment should be worn when taking care of ill patients at home. Regular hand washing is required after visiting patients in hospital, as well as after taking care of patients at home | Two main vaccines have proved efficacious in preventing Ebola infection. Both vaccines express GP as the single EBOV component and are virally vectored in chimpanzee adenovirus and vesicular stomatitis virus (rVSV), respectively | ||||
| Outbreak containment measure: prompt and safe burial of the dead, identifying people who may have been in contact with someone infected with Ebola, monitoring the health of contacts for 21 days, the importance of separating the healthy from the sick to prevent further spread, the importance of good hygiene and maintaining a clean environment | |||||
| SARS-CoV-2 | 2019 | Probably from bats | Several practices are recommended with the aim to limit further transmission; they include handwashing, hand disinfection, wearing of face masks and gloves, disinfection of surfaces, and physical distance | Conservative fluid therapy and broad-spectrum antibiotics are given to patients as a protective measure to avoid opportunistic bacterial infections. However, ventilator support for respiration is provided to patients under extreme conditions. | [115,116] |
| Numerous FDA-approved antiviral drugs, plasma therapy, vaccines (live attenuated vaccine (LAV), inactivated virus, subunit vaccines, monoclonal antibody vaccine, virus vectors, protein vaccines, and DNA/RNA-based vaccines), and immunotherapies |
3. Transmission and Measures to Contain SARS-CoV-2 Spread
After the WHO declared a pandemic [117] on 11th March 2020, most countries, to avoid the pandemic spread and limit the number of casualties, introduced several strict nonpharmaceutical interventions [118], namely (1) improved diagnostic testing and contact tracing; (2) isolation and quarantine for infected people; and (3) measures aimed at reducing mobility and creating social distancing (containment, mitigation, and suppression).
Most countries decided on the following containment measures:
Physical distances > 1.5 m;
Wearing masks and gloves;
Stay-at-home orders;
School and workplace closures and activation of distance learning and smart working;
Closure of museums, commercial parks, gyms, and swimming pools;
Cancellation of public events;
Restrictions on size of crowds;
Seat limitation on public transport to ensure the right distance between passengers;
Restrictions on internal and international travel;
Measurements of body temperature at the entrance of closed areas (<37.5 °C);
Ensuring disinfection rules are followed in public areas such as public transport, shopping areas, schools, and universities;
Protecting healthcare workers with appropriate personal protection equipment (PPE).
The main objective of these interventions is to reduce the reproduction number (Rt) of the virus. The Rt is defined “as the mean number of secondary cases generated by a typical primary case at time t in a population calculated for the whole period over a 5-day moving average” [59]. Thus, Rt is an indicator measuring the transmission of SARS-CoV-2 before and after the interventions.
According to Ecclesiastes 1:9 [קהלת, Qohelet הַשָּֽׁמֶשׁתַּ֥חַת כָּל־חָדָ֖שׁ וְאֵ֥ין Latin Ecclesiastes Nihil sub sole novum], “There’s nothing new under the sun.” Table 5 [101,102,103,104,105] reports the containment measures adopted over the millennia to avoid disease spread.
Table 5.
Containment measures to avoid disease spread over the millennia.
| Years | Source | Measurements | References |
|---|---|---|---|
| 430–428 BCE | Plague of Athens | The containment strategies used included the application of purifications and incantations and the enforcement of abstinence from baths and many food items then considered noxious to diseased people | [119] |
| 541–755 | Plague of Justinian | The containment strategies included unspecified traditional public health measures and quarantine | [119] |
| Giovanni Boccaccio (1313–1375), in his book The Decameron | Written in Tuscan vernacular (Italian). The book is a collection of short stories told by a group of seven young women and three young men sheltering in a villa just outside Florence to escape the black death that afflicted that city. Boccaccio probably conceived his masterpiece of classical Italian Renaissance prose after the plague epidemic of 1348, which came to a standstill by 1353 | ||
| 1377, 1397 | “De ordinibus contra eos qui veniunt de loc ispestiferis anno 1397 factis” | Quarantine was first introduced in Dubrovnik on Croatia’s Dalmatian Coast | [120] |
| Orders made against those who come to the place of the pestiferous in 1397 | The Great Council of Ragusa specified again the 30-day duration of quarantine and determined the place | ||
| 1423, 1448 | Venetian Senate | First permanent plague hospital (lazaretto) was opened by the Republic of Venice in 1423 on the small island of Santa Maria di Nazareth.Prolonged the waiting period to 40 days, thus giving birth to the term “quarantine” | [121] |
| 1467 | Genoa adopted the Venetian system | [119] | |
| 1476 | In Marseille, France, a hospital for persons with leprosy was converted into a lazaretto | [121] | |
| 1480 | Marsilio Ficino, “Consilio contro la pestilentia” | When you converse, stay away from your partner at least two arms, and in the open place, and when it is suspicious, let us stay at least six fathoms longer, and out in the open, and let the wind not be reversed by him | |
| 1589 | Viceroy of Peru | Lima physicians advised the use of quarantine among all native communities to prevent further spread of the disease | [119] |
| 1663 | English quarantine regulations provided for the confinement (in the Thames estuary) of ships with suspected plague-infected passengers or crew | [121] | |
| 1665 | A journal of the plague year by Daniel Defoe | It was a rule with those who had thus two houses in their keeping or care, that if anybody was taken sick in a family, before the master of the family let the examiners or any other officer know of it, he immediately would send all the rest of his family, whether children or servants, as it fell out to be, to such other house which he had so in charge, and then giving notice of the sick person to the examiner, have a nurse or nurses appointed, and have another person to be shut up in the house with them (which many for money would do), so to take charge of the house in case the person should die | [122] |
| 1688 and 1691 | Quarantine to control yellow fever which first appeared in New York and Boston | [121] | |
| 1796 | United States introduced quarantine legislation in port cities threatened by yellow fever from the West Indies | [123] | |
| 1799 | In the harbor of Philadelphia, the first quarantine station was built after a previous yellow fever outbreak in 1793 | [123] | |
| 1878 | Release of the National Quarantine Act, which shifted quarantine power from single states to the federal government | [123] | |
| 1944 | The federal government quarantine authority was set up | [123] |
In the past, against some infectious diseases, medicine was ineffective [85] Thucydides in his History of Peloponnesian War (II, vii3-5) wrote “The doctors were unable to cope, since they were treating the disease for the first time and in ignorance: indeed, the more they came into contact with sufferers, the more liable they were to lose their own lives.” Indeed, the only possible way to escape the plague was to avoid any contact with infected persons and contaminated objects. The Italian poet Giovanni Boccaccio (1313–1375), in his book The Decameron (1349–1353), tells the story of ten people, seven women and three men, who entertain themselves with novels while in isolation from the plague of Florence in a villa in the countryside. In the first chapter, Boccaccio describes how the plague struck the city of Florence, how people reacted, and the staggering death toll. Boccaccio, echoing Thucydides, also wrote: “Neither a doctor’s advice nor the strength of medicine could do anything to cure this illness”.
Accordingly, the procedure of obligatory quarantine was introduced as a measure to isolate and separate people, animals, foods, and objects that may have been exposed to a contagious disease. Quarantine is from the Italian “quaranta”, meaning forty. For millennia, contagious diseases were believed to be a divine punishment for sinners. Thus, in the Old Testament, God destroyed the earth with water for 40 days (בראשית: Genesis 7:4); Noah waited for forty days after the tops of mountains were seen after the flood (בראשית: Genesis 8:5–7). Moses was on Mount Sinai for 40 days (Exodus שְׁמֹות 24:18): “Then Moses entered the cloud as he went on up the mountain. And he stayed on the mountain forty days and forty nights”. In the New Testament, Jesus was tempted for 40 days (Matthew 4:2, Mark 1:13, Luke 4:2). There were 40 days between Jesus’ resurrection and ascension (Acts 1:3). Eugenia Tognotti [121] and Gensini et al. [124] reviewed the origin of quarantine from the time of the Bible to nowadays.
Nevertheless, 40 days may derive from the Pythagorean theory of numbers; according to Pythagoreans, the number 40 was considered to be sacred. Hippocratic teaching in the 5th century BCE established that an acute illness only manifested itself within forty days [124]. Only during the epidemic of 1347–1352 was an organized institutional response to control disease set up. Quarantine was introduced, for the first time, in 1377 by the Rector of the seaport of Ragusa (Dubrovnik, Croatia), and the first stable plague hospital (lazaretto or quarantine station) was built by the Republic of Venice in 1423 on the island of Santa Maria di Nazareth [124]. The term lazaretto, usually referred to as Nazarethum or Lazarethum, is related to Lazarus, who was brought back to life by Jesus (John 11:1–45) and/or to the Order of Saint Lazarus of Jerusalem, a Catholic military order founded by crusaders around 1119 at a leper hospital in Jerusalem, as a hospital and military order of chivalry [125]. The Venetian system became a model for other European countries: in 1467, Genoa adopted the Venetian system, and in 1476, in Marseille, France, a hospital for persons with leprosy was converted into a lazaretto [126]. Afterward, quarantine became the foundation of a coordinated disease-control strategy that included different measures such as isolation, sanitary cordons, bills of health issued to ships (certification assuring the absence of disease), sanification (i.e., fumigation), and disinfection. Girolamo Fracastoro, Latin Hieronymus Fracastorius (1478–1553, Verona), physician, poet, astronomer, and geologist, was the first to propose, in 1546, a scientific germ theory of disease. In his book “On Contagion and Contagious Diseases”, he affirmed that each disease is caused by a different type of rapidly multiplying minute body and that these bodies are transferred from the infector to the infected in three ways: by direct contact; by carriers such as soiled clothing and linen; and through the air.
4. Boosting the Immune Response: How Vaccines Changed the Scenario
In the millennial history of mankind, vaccination is a relatively young intervention of primary prevention. For about 200 years, vaccination strategies have had a profound effect in shaping the natural history of infectious diseases. Smallpox eradication represents the most impressive success of a vaccination strategy. As discussed above, smallpox represented a dreadful menace throughout the centuries [127]. It was common knowledge that smallpox survivors acquired immunity to the disease, so the practice of variolation, consisting in having healthy individuals inhale dust from smallpox lesions, become common in Europe and in North America. At the end of the 18th century, there were anecdotes regarding immunity to smallpox in people previously infected with cowpox, a zoonotic pathogen [128]. In 1798, Edward Jenner published his first observations on the benefits of inoculating biological material from cowpox lesions in humans, to protect from smallpox, and for the first time, the term “vaccination” (from Latin vacca, English cow) was used [129]. Initially, vaccination was perpetuated by transferring fluids from individual to individual, but this practice reduced the strength and the duration of protection. The next step was to deliberately infect cows to mass-produce sufficient material (“lymph”) for vaccination [127]. However, this practice led to an increase in the frequency of transmission of secondary infections, including syphilis. This issue was resolved after the observation of bacterial inactivation by glycerin made by Robert Koch [130], so lymph was treated with glycerin before inoculation. At the end of the 19th century, Louis Pasteur made observations that strongly enhanced the development of vaccines. Studying chicken cholera, he noticed that chickens inoculated with cultures left out over a prolonged period were protected from subsequent infection with fresh material, suggesting the existence of protective immunity induced by the inoculation of “aged” material. These observations would lead Louis Pasteur to the production of antirabic vaccination, using material from an infected dog’s brain, exposed to dry air [131].
In the meantime, studies on the immune system contributed to unravel the mechanisms of host defense from pathogens. In particular, studies by Elie Metchnikoff in 1884 [132] introduced the concept of cellular immunity, and Paul Ehrlich published his theory of receptor of immunity in 1897, paving the way for the development of antitoxins against pathogens such as diphtheria. At the end of the 19th century, five human vaccines were in use: two live virus vaccines (smallpox and rabies) and three dead bacterial vaccines (typhoid, cholera, and plague.).
The first half of the 20th century saw the development of passive immunization, with the production of antitoxins for diphtheria and tetanus. At the same time, new vaccines against tuberculosis, bacillus Calmette-Guérin (BCG), yellow fever, typhus, influenza A, and pertussis were developed, and the first combination vaccine, against diphtheria, tetanus, and pertussis was produced in 1948. Progresses in cell culture lead the way to the techniques of virus attenuation through passages on tissues and cellular monolayers, thanks to the studies of Hugh and Mary Maitland in 1928 and Ernest William Goodpasture in 1931, who first used the chorioallantoic membranes of a fertile hen’s egg as a culture medium for sterile passage of viruses. In the 1900s, poliomyelitis (caused by the poliovirus) represented another threat to public health. The virus spreads from person to person and can invade an infected person’s brain and spinal cord, causing paralysis. Better hygiene conditions led to an increase in the age of infected children, which in the previous centuries were breastfed, protected by maternal antibodies. The older age of infected children led to frequent polio outbreaks. The first effective antipolio vaccine was a formaldehyde-inactivated (or “killed”) PV vaccine (IPV) developed by Jonas Salk in 1955. A second vaccine which was demonstrated to be both safe and effective was the oral (or “live”) PV vaccine (OPV) was developed by Albert Sabin in 1963 [133]. Jonas Salk and Albert Sabin decided not to patent their vaccines and therefore sacrificed billions of dollars in potential royalties, approximately USD 500 million for Salk and approximately USD 1.2 billion for Sabin. Nowadays, thanks to the vaccines, the virus remains endemic only in Afghanistan and Pakistan.
In more recent years, research started to focus on multiple vaccines, starting from live viruses attenuated by multiple passages on cultured cells, such as the vaccine against measles, mumps, and rubella. Exploiting the newly available techniques of molecular biology, newly designed vaccines started to be produced. Japanese researchers developed an acellular pertussis vaccine based on two of the main protective antigens of Bordetella pertussis. Research on polysaccharide vaccines led to the development of new vaccines against pneumococcus, meningococcus, and Haemophilus Influenzae type B. Recombinant DNA technology and the possibility to produce recombinant protein in vitro paved the way for the release of the anti-hepatitis B vaccine. Under the urgent need to battle COVID-19, different SARS-CoV-2 vaccines, including the inactivated virus vaccine, nucleic acid vaccine, adenovirus vector vaccine, and viral subunit vaccine, have been developed [134]. In the history of vaccines, COVID-19 vaccines are unique for the extraordinary rapidity of their production. In recent years, mRNA vaccines have started to attract great attention thanks to their potential to:
-
(1)
Speed up vaccine development;
-
(2)
Simplify vaccine production, scale-up, and quality control;
-
(3)
Be produced and scaled up in a predictable and consistent fashion regardless of the antigen;
-
(4)
Have improved safety and efficacy;
-
(5)
Challenge diseases impossible to prevent with other approaches;
-
(6)
Enable precise antigen design;
-
(7)
Generate proteins with a “native-like” presentation;
-
(8)
Express proteins stabilized in a more immunogenic conformation or expose key antigenic sites;
-
(9)
Deliver multiple mRNAs to the same cell;
-
(10)
Allow the generation of multiprotein complexes or protein antigens from different pathogens, thus creating a single vaccine against several targets.
Moreover, mRNA is characterized as:
-
(11)
Being noninfectious;
-
(12)
Being nonintegrating;
-
(13)
Being degradable by normal cellular processes soon after injection;
-
(14)
Decreasing the risk of toxicity and long-term side effects;
-
(15)
Not inducing vector-specific immunity;
-
(16)
Not competing with pre-existing or newly raised vector immunity that could interfere with subsequent vaccinations.
An mRNA vaccine is based on the principle that mRNA is an intermediate messenger to be translated to an antigen after the delivery into host cells via various routes. The mRNA is synthesized in the laboratory by transcribing a DNA template of the genetic sequence encoding the immunogen. In the case of SARS-CoV-2, the spike (S) protein is identified as the immunodominant antigen of the virus. The most important problem is that mRNA is unstable, easily recognized by the immune system, and rapidly degraded by nucleases after entering the body. mRNA vaccines do not enter the nucleus but need to pass through the cell membrane, a negatively charged phospholipid bilayer, to enter the cytoplasm and then be translated into the target protein. Different delivery systems for mRNA vaccines, such as viral and nonviral vector delivery systems, may be utilized. Vectors based upon lipids or lipid-like compounds are the most common nonviral gene carriers.
5. Conclusions
The deep knowledge of the history of the “plagues” that have struck humanity is not only precious in understanding the long-term sociological and demographic changes but in better understanding the evolution of infectious diseases over the centuries. Reading all the literary works carefully, we can recognize our present journeys under the COVID-19 pandemic, including the risks of inappropriate responses. Confinement measures such as social distancing and/or quarantine still remain the most efficient measure to contain the spread of the virus. As in the past, an “infodemia” is present, generating chaos and fear among the population. Moreover, technical knowledge on agents, hosts, and the environment alone, although essential, is not enough.
The COVID-19 pandemic is an example of how diseases unknown to medical science and to human immune systems may develop and spread quickly in a highly connected world. Even it is not possible to avoid every risk, there are ways to reduce or mitigate the chances of a future pandemic, such as investing in research and preparation, funding and implementing vaccine programs, and strengthening health systems. Along with vaccines and specific therapies, the best course of action in facing new pandemics remains social distancing, the practice of good hygiene, and the use of quarantine.
“And Darkness and Decay and the Red Death held illimitable dominion over all.”
(“The Mask of the Red Death: A Fantasy” E. A. Poe, 1842)
Author Contributions
Conceptualization, P.R.; methodology, L.S. and L.G.; data curation, L.S., F.M., M.B., M.C. and L.V.; writing—original draft preparation, P.R., L.V. and S.I.; writing—review and editing, P.R. and C.M.; visualization, C.M.; supervision, S.B. and V.M.; project administration, C.T. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by funds granted by the Italian Ministry of Health for Institutional Research (Ricerca Corrente) to IRCCS San Raffaele Roma, and it was supported by grants from PON03PE_00078_1 and PON03PE_00078_2.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandhi V.R., Shanmugasundaram S. A Missed Case of Osteoid Osteoma of the Acetabulum Treated with a Novel Computed Tomography-Guided Technique—A Case Report. J. Orthop. Case Rep. 2020;10:102–105. doi: 10.13107/jocr.2020.v10.i09.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang C., Li Y., Xiao S.Y. Differential expression of ACE2 in the respiratory tracts and its relationship to COVID-19 pathogenesis. EBioMedicine. 2020;60:103004. doi: 10.1016/j.ebiom.2020.103004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa L.B., Perez L.G., Palmeira V.A., Macedo e Cordeiro T., Ribeiro V.T., Lanza K., Simoes e Silva A.C. Insights on SARS-CoV-2 Molecular Interactions with the Renin-Angiotensin System. Front. Cell Dev. Biol. 2020;8:559841. doi: 10.3389/fcell.2020.559841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muus C., Luecken M.D., Eraslan G., Sikkema L., Waghray A., Heimberg G., Kobayashi Y., Vaishnav E.D., Subramanian A., Smillie C., et al. Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nat. Med. 2021;27:546–559. doi: 10.1038/s41591-020-01227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amenta E.M., Spallone A., Rodriguez-Barradas M.C., El Sahly H.M., Atmar R.L., Kulkarni P.A. Postacute COVID-19: An Overview and Approach to Classification. Open Forum Infect. Dis. 2020;7:ofaa509. doi: 10.1093/ofid/ofaa509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfe N.D., Dunavan C.P., Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rascovan N., Sjogren K.G., Kristiansen K., Nielsen R., Willerslev E., Desnues C., Rasmussen S. Emergence and Spread of Basal Lineages of Yersinia pestis during the Neolithic Decline. Cell. 2019;176:295–305.e10. doi: 10.1016/j.cell.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 9.A History of ‘Plague’: Illness as Metaphor. [(accessed on 8 February 2022)]. Available online: https://www.merriam-webster.com/words-at-play/plague-word-history-literary-metaphor.
- 10.Nunn J.F. Ancient Egyptian Medicine. University of Oklahoma Press; Norman, OK, USA: 2002. [Google Scholar]
- 11.Trevisanato S.I. The ‘Hittite plague’, an epidemic of tularemia and the first record of biological warfare. Med. Hypotheses. 2007;69:1371–1374. doi: 10.1016/j.mehy.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Norrie P. A History of Disease in Ancient Times. Palgrave Macmillan; Cham, Switzerland: 2016. How Disease Affected the End of the Bronze Age. [DOI] [Google Scholar]
- 13.Tsoucalas G., Laios K., Karamanou M., Androutsos G. Demystifying the epidemic among Achaeans during the Trojan War. Infez. Med. 2014;22:342–348. [PubMed] [Google Scholar]
- 14.Russell J.C. That Earlier Plague. Volume 5. Duke University Press; Durham, NC, USA: 1968. p. 11. [Google Scholar]
- 15.Kousoulis A.A., Economopoulos K.P., Poulakou-Rebelakou E., Androutsos G., Tsiodras S. The plague of Thebes, a historical epidemic in Sophocles’ Oedipus Rex. Emerg. Infect. Dis. 2012;18:153–157. doi: 10.3201/eid1801.AD1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Littman R.J. The plague of Athens: Epidemiology and paleopathology. Mt. Sinai J. Med. J. Transl. Pers. Med. 2009;76:456–467. doi: 10.1002/msj.20137. [DOI] [PubMed] [Google Scholar]
- 17.Cunha B.A. The cause of the plague of Athens: Plague, typhoid, typhus, smallpox, or measles? Infect. Dis. Clin. N. Am. 2004;18:29–43. doi: 10.1016/S0891-5520(03)00100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dirckx J.H. Pestilence narratives in classical literature: A study in creative imitation: II. Virgil, Ovid, Seneca, and Silius Italicus. Am. J. Dermatopathol. 2000;22:459–464. doi: 10.1097/00000372-200010000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Angeletti L.R., Gazzaniga V. The plague of Syracuse (396 B.C.E.) in Diodorus Siculus (XIV, 70) Ann. Ig. Med. Prev. Comunita. 2002;14:7–13. [PubMed] [Google Scholar]
- 20.Fears J.R. The plague under Marcus Aurelius and the decline and fall of the Roman Empire. Infect. Dis. Clin. N. Am. 2004;18:65–77. doi: 10.1016/S0891-5520(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 21.Iniesta I. Pandemics in ancient Greek and Roman coinage: Medical memories at the service of hope. Intern. Med. J. 2020;50:1574–1578. doi: 10.1111/imj.15111. [DOI] [PubMed] [Google Scholar]
- 22.Wagner D.M., Klunk J., Harbeck M., Devault A., Waglechner N., Sahl J.W., Enk J., Birdsell D.N., Kuch M., Lumibao C., et al. Yersinia pestis and the plague of Justinian 541–543 AD: A genomic analysis. Lancet Infect. Dis. 2014;14:319–326. doi: 10.1016/S1473-3099(13)70323-2. [DOI] [PubMed] [Google Scholar]
- 23.Ryan J.J., Klein K.A., Neuberger T.J., Leftwich J.A., Westin E.H., Kauma S., Fletcher J.A., DeVries G.H., Huff T.F. Role for the stem cell factor/KIT complex in Schwann cell neoplasia and mast cell proliferation associated with neurofibromatosis. J. Neurosci. Res. 1994;37:415–432. doi: 10.1002/jnr.490370314. [DOI] [PubMed] [Google Scholar]
- 24.Wheelis M. Biological warfare at the 1346 siege of Caffa. Emerg. Infect. Dis. 2002;8:971–975. doi: 10.3201/eid0809.010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowsky W.M. The impact of the black death upon Sienese government and society. Speculum. 1964;39:1–34. doi: 10.2307/2850126. [DOI] [PubMed] [Google Scholar]
- 26.Sgouridou M. The figure of the doctor and the science of medicine through Boccaccio’s “Decameron”. Infez. Med. 2014;22:62–68. [PubMed] [Google Scholar]
- 27.Giovanni Villani (Italian Historian) [(accessed on 26 February 2022)]. Available online: https://www.britannica.com/biography/Giovanni-Villani.
- 28.Spyrou M.A., Keller M., Tukhbatova R.I., Scheib C.L., Nelson E.A., Andrades Valtuena A., Neumann G.U., Walker D., Alterauge A., Carty N., et al. Phylogeography of the second plague pandemic revealed through analysis of historical Yersinia pestis genomes. Nat. Commun. 2019;10:4470. doi: 10.1038/s41467-019-12154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freemon F.R. Bubonic plague in the Book of Samuel. J. R. Soc. Med. 2005;98:436. doi: 10.1177/014107680509800923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampath S., Khedr A., Qamar S., Tekin A., Singh R., Green R., Kashyap R. Pandemics Throughout the History. Cureus. 2021;13:e18136. doi: 10.7759/cureus.18136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.A Visual History of Pandemics. [(accessed on 26 February 2022)]. Available online: https://www.weforum.org/agenda/2020/03/a-visual-history-of-pandemics.
- 32.Papagrigorakis M.J., Yapijakis C., Synodinos P.N., Baziotopoulou-Valavani E. DNA examination of ancient dental pulp incriminates typhoid fever as a probable cause of the Plague of Athens. Int. J. Infect. Dis. 2006;10:206–214. doi: 10.1016/j.ijid.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Saez A. The Antonine plague: A global pestilence in the II century d.C. Rev. Chil. Infectol. 2016;33:218–221. doi: 10.4067/S0716-10182016000200011. [DOI] [PubMed] [Google Scholar]
- 34.Sabbatani S., Manfredi R., Fiorino S. The Justinian plague (part one) Infez. Med. 2012;20:125–139. [PubMed] [Google Scholar]
- 35.Stenseth N.C., Atshabar B.B., Begon M., Belmain S.R., Bertherat E., Carniel E., Gage K.L., Leirs H., Rahalison L. Plague: Past, present, and future. PLoS Med. 2008;5:e3. doi: 10.1371/journal.pmed.0050003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glatter K.A., Finkelman P. History of the Plague: An Ancient Pandemic for the Age of COVID-19. Am. J. Med. 2021;134:176–181. doi: 10.1016/j.amjmed.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haensch S., Bianucci R., Signoli M., Rajerison M., Schultz M., Kacki S., Vermunt M., Weston D.A., Hurst D., Achtman M., et al. Distinct clones of Yersinia pestis caused the black death. PLoS Pathog. 2010;6:e1001134. doi: 10.1371/journal.ppat.1001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bramanti B., Dean K.R., Walloe L., Chr. Stenseth N. The Third Plague Pandemic in Europe. Proc. R. Soc. B. 2019;286:20182429. doi: 10.1098/rspb.2018.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spinney L. Smallpox and other viruses plagued humans much earlier than suspected. Nature. 2020;584:30–32. doi: 10.1038/d41586-020-02083-0. [DOI] [PubMed] [Google Scholar]
- 40.Behbehani A.M. The smallpox story: Life and death of an old disease. Microbiol. Rev. 1983;47:455–509. doi: 10.1128/mr.47.4.455-509.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eyler J.M. Smallpox in history: The birth, death, and impact of a dread disease. J. Lab. Clin. Med. 2003;142:216–220. doi: 10.1016/S0022-2143(03)00102-1. [DOI] [PubMed] [Google Scholar]
- 42.Becker A.M. Smallpox in Washington’s Army: Strategic Implications of the Disease during the American Revolutionary War. J. Mil. Hist. 2004;68:381–430. doi: 10.1353/jmh.2004.0012. [DOI] [Google Scholar]
- 43.Lederberg J. Infectious history. Science. 2000;288:287–293. doi: 10.1126/science.288.5464.287. [DOI] [PubMed] [Google Scholar]
- 44.Ferretti J., Kohler W. History of Streptococcal Research. In: Ferretti J.J., Stevens D.L., Fischetti V.A., editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. University of Oklahoma Health Sciences Center; Oklahoma City, OK, USA: 2016. [PubMed] [Google Scholar]
- 45.Sanchez-Sampedro L., Perdiguero B., Mejias-Perez E., Garcia-Arriaza J., Di Pilato M., Esteban M. The evolution of poxvirus vaccines. Viruses. 2015;7:1726–1803. doi: 10.3390/v7041726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plotkin S.A. Vaccination against measles in the 18th century. Clin. Pediatr. 1967;6:312–315. doi: 10.1177/000992286700600524. [DOI] [PubMed] [Google Scholar]
- 47.Smith K.A. Louis pasteur, the father of immunology? Front. Immunol. 2012;3:68. doi: 10.3389/fimmu.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baxter A.G. Louis Pasteur’s beer of revenge. Nat. Rev. Immunol. 2001;1:229–232. doi: 10.1038/35105083. [DOI] [PubMed] [Google Scholar]
- 49.Hajj Hussein I., Chams N., Chams S., El Sayegh S., Badran R., Raad M., Gerges-Geagea A., Leone A., Jurjus A. Vaccines Through Centuries: Major Cornerstones of Global Health. Front. Public Health. 2015;3:269. doi: 10.3389/fpubh.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Migliori G.B., Loddenkemper R., Blasi F., Raviglione M.C. 125 years after Robert Koch’s discovery of the tubercle bacillus: The new XDR-TB threat. Is “science” enough to tackle the epidemic? Eur. Respir. J. 2007;29:423–427. doi: 10.1183/09031936.00001307. [DOI] [PubMed] [Google Scholar]
- 51.Bos L. Beijerinck’s work on tobacco mosaic virus: Historical context and legacy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999;354:675–685. doi: 10.1098/rstb.1999.0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solomon T. Alexandre Yersin and the plague bacillus. J. Trop. Med. Hyg. 1995;98:209–212. [PubMed] [Google Scholar]
- 53.Hawgood B.J. Alexandre Yersin (1863–1943): Discoverer of the plague bacillus, explorer and agronomist. J. Med. Biogr. 2008;16:167–172. doi: 10.1258/jmb.2007.007017. [DOI] [PubMed] [Google Scholar]
- 54.Williamson J.D., Gould K.G., Brown K. Richard Pfeiffer’s typhoid vaccine and Almroth Wright’s claim to priority. Vaccine. 2021;39:2074–2079. doi: 10.1016/j.vaccine.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 55.Hawgood B.J. Waldemar Mordecai Haffkine, CIE (1860–1930): Prophylactic vaccination against cholera and bubonic plague in British India. J. Med. Biogr. 2007;15:9–19. doi: 10.1258/j.jmb.2007.05-59. [DOI] [PubMed] [Google Scholar]
- 56.Browning C.H. Emil Behring and Paul Ehrlich: Their contributions to science. Nature. 1955;175:570–575. doi: 10.1038/175570a0. [DOI] [PubMed] [Google Scholar]
- 57.Dimmock N.J., Easton A., Leppard K. Introduction to Modern Virology. 7th ed. Wiley-Blackwell; Hoboken, NJ, USA: 2015. [Google Scholar]
- 58.Gelpi A., Gilbertson A., Tucker J.D. Magic bullet: Paul Ehrlich, Salvarsan and the birth of venereology. Sex. Transm. Infect. 2015;91:68–69. doi: 10.1136/sextrans-2014-051779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Francis T. Transmission of Influenza by a Filterable Virus. Science. 1934;80:457–459. doi: 10.1126/science.80.2081.457.b. [DOI] [PubMed] [Google Scholar]
- 60.Is Influenza Due To A Filtrable Virus? J. Am. Med. Assoc. 1918;71:2154–2155. doi: 10.1001/jama.1918.02600520040012. [DOI] [Google Scholar]
- 61.Bennett J.W., Chung K.T. Alexander Fleming and the discovery of penicillin. Adv. Appl. Microbiol. 2001;49:163–184. doi: 10.1016/s0065-2164(01)49013-7. [DOI] [PubMed] [Google Scholar]
- 62.Freundlich M.M. Origin of the Electron Microscope. Science. 1963;142:185–188. doi: 10.1126/science.142.3589.185. [DOI] [PubMed] [Google Scholar]
- 63.Demeure C.E. In: Chapter 10. Live Vaccines against Plague and Pseudotuberculosis. Carniel E., Hinnebusch B.J., editors. Caister Academic Press; Norfolk, UK: 2012. [Google Scholar]
- 64.Coulanges P. 50th anniversary of the EV antiplague vaccine (Girard and Robic) Bull. Soc. Pathol. Exot. Fil. 1983;76:114–120. [PubMed] [Google Scholar]
- 65.Chung K.T. Azo dyes and human health: A review. J. Environ. Sci. Health Part C. 2016;34:233–261. doi: 10.1080/10590501.2016.1236602. [DOI] [PubMed] [Google Scholar]
- 66.Barberis I., Martini M., Iavarone F., Orsi A. Available influenza vaccines: Immunization strategies, history and new tools for fighting the disease. J. Prev. Med. Hyg. 2016;57:E41–E46. [PMC free article] [PubMed] [Google Scholar]
- 67.Wagle P.M. Recent advances in the treatment of bubonic plague. Ind. J. Med. Sci. 1948;2:489–494. [Google Scholar]
- 68.Avery O.T., Macleod C.M., McCarty M. Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types: Induction of Transformation by a Desoxyribonucleic Acid Fraction Isolated from Pneumococcus Type III. J. Exp. Med. 1944;79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schatz A., Bugie E., Waksman S.A. Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. Proc. Soc. Exp. Biol. Med. 1944;55:66–69. doi: 10.3181/00379727-55-14461. [DOI] [PubMed] [Google Scholar]
- 70.Rosen F.S. Isolation of poliovirus—John Enders and the Nobel Prize. N. Engl. J. Med. 2004;351:1481–1483. doi: 10.1056/NEJMp048202. [DOI] [PubMed] [Google Scholar]
- 71.Watson J.D., Crick F.H. Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature. 1953;171:737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 72.Achtman M., Zurth K., Morelli G., Torrea G., Guiyoule A., Carniel E. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA. 1999;96:14043–14048. doi: 10.1073/pnas.96.24.14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barre-Sinoussi F., Ross A.L., Delfraissy J.F. Past, present and future: 30 years of HIV research. Nat. Rev. Microbiol. 2013;11:877–883. doi: 10.1038/nrmicro3132. [DOI] [PubMed] [Google Scholar]
- 74.Maeda K., Das D., Kobayakawa T., Tamamura H., Takeuchi H. Discovery and Development of Anti-HIV Therapeutic Agents: Progress Towards Improved HIV Medication. Curr. Top. Med. Chem. 2019;19:1621–1649. doi: 10.2174/1568026619666190712204603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parkhill J., Wren B.W., Thomson N.R., Titball R.W., Holden M.T., Prentice M.B., Sebaihia M., James K.D., Churcher C., Mungall K.L., et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature. 2001;413:523–527. doi: 10.1038/35097083. [DOI] [PubMed] [Google Scholar]
- 76.Malenfant J.H., Joyce A., Choi M.J., Cossaboom C.M., Whitesell A.N., Harcourt B.H., Atmar R.L., Villanueva J.M., Bell B.P., Hahn C., et al. Use of Ebola Vaccine: Expansion of Recommendations of the Advisory Committee on Immunization Practices To Include Two Additional Populations—United States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022;71:290–292. doi: 10.15585/mmwr.mm7108a2. [DOI] [PubMed] [Google Scholar]
- 77.Lamb Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs. 2021;81:495–501. doi: 10.1007/s40265-021-01480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Syed Y.Y. Molnupiravir: First Approval. Drugs. 2022;82:455–460. doi: 10.1007/s40265-022-01684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mahase E. COVID-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713. doi: 10.1136/bmj.n2713. [DOI] [PubMed] [Google Scholar]
- 80.Lamb Y.N. Nirmatrelvir Plus Ritonavir: First Approval. Drugs. 2022;19:1–7. doi: 10.1007/s40265-022-01692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zink A.R., Reischl U., Wolf H., Nerlich A.G. Molecular analysis of ancient microbial infections. FEMS Microbiol. Lett. 2002;213:141–147. doi: 10.1111/j.1574-6968.2002.tb11298.x. [DOI] [PubMed] [Google Scholar]
- 82.Donoghue H.D. Paleomicrobiology of Human Tuberculosis. Microbiol. Spectr. 2016;4 doi: 10.1128/microbiolspec.PoH-0003-2014. [DOI] [PubMed] [Google Scholar]
- 83.Donoghue H.D. Insights into ancient leprosy and tuberculosis using metagenomics. Trends Microbiol. 2013;21:448–450. doi: 10.1016/j.tim.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Brier B. Infectious diseases in ancient Egypt. Infect. Dis. Clin. N. Am. 2004;18:17–27. doi: 10.1016/S0891-5520(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 85.McNeill W.H. Plagues and Peoples. Anchor Books Doubleday; New York, NY, USA: 1998. [Google Scholar]
- 86.Diamond J. Guns, Germs, and Steel: The Fates of Human Societies. W. W. Norton & Company Inc.; New York, NY, USA: 1999. [Google Scholar]
- 87.Spyrou M.A., Bos K.I., Herbig A., Krause J. Ancient pathogen genomics as an emerging tool for infectious disease research. Nat. Rev. Genet. 2019;20:323–340. doi: 10.1038/s41576-019-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andrades Valtuena A., Mittnik A., Key F.M., Haak W., Allmae R., Belinskij A., Daubaras M., Feldman M., Jankauskas R., Jankovic I., et al. The Stone Age Plague and Its Persistence in Eurasia. Curr. Biol. 2017;27:3683–3691.e8. doi: 10.1016/j.cub.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 89.Neukamm J., Pfrengle S., Molak M., Seitz A., Francken M., Eppenberger P., Avanzi C., Reiter E., Urban C., Welte B., et al. 2000-year-old pathogen genomes reconstructed from metagenomic analysis of Egyptian mummified individuals. BMC Biol. 2020;18:108. doi: 10.1186/s12915-020-00839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lalremruata A., Ball M., Bianucci R., Welte B., Nerlich A.G., Kun J.F., Pusch C.M. Molecular identification of falciparum malaria and human tuberculosis co-infections in mummies from the Fayum depression (Lower Egypt) PLoS ONE. 2013;8:e60307. doi: 10.1371/journal.pone.0060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nerlich A.G., Schraut B., Dittrich S., Jelinek T., Zink A.R. Plasmodium falciparum in ancient Egypt. Emerg. Infect. Dis. 2008;14:1317–1319. doi: 10.3201/eid1408.080235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zink A.R., Grabner W., Reischl U., Wolf H., Nerlich A.G. Molecular study on human tuberculosis in three geographically distinct and time delineated populations from ancient Egypt. Epidemiol. Infect. 2003;130:239–249. doi: 10.1017/S0950268802008257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raele D.A., Panzarino G., Sarcinelli G., Cafiero M.A., Maria Tunzi A., Dellu E. Genetic Evidence of the Black Death in the Abbey of San Leonardo (Apulia Region, Italy): Tracing the Cause of Death in Two Individuals Buried with Coins. Pathogens. 2021;10:1354. doi: 10.3390/pathogens10111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bramanti B., Wu Y., Yang R., Cui Y., Stenseth N.C. Assessing the origins of the European Plagues following the Black Death: A synthesis of genomic, historical, and ecological information. Proc. Natl. Acad. Sci. USA. 2021;118:e2101940118. doi: 10.1073/pnas.2101940118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guellil M., Kersten O., Namouchi A., Luciani S., Marota I., Arcini C.A., Iregren E., Lindemann R.A., Warfvinge G., Bakanidze L., et al. A genomic and historical synthesis of plague in 18th century Eurasia. Proc. Natl. Acad. Sci. USA. 2020;117:28328–28335. doi: 10.1073/pnas.2009677117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bos K.I., Schuenemann V.J., Golding G.B., Burbano H.A., Waglechner N., Coombes B.K., McPhee J.B., DeWitte S.N., Meyer M., Schmedes S., et al. A draft genome of Yersinia pestis from victims of the Black Death. Nature. 2011;478:506–510. doi: 10.1038/nature10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guellil M., Rinaldo N., Zedda N., Kersten O., Gonzalez Muro X., Stenseth N.C., Gualdi-Russo E., Bramanti B. Bioarchaeological insights into the last plague of Imola (1630–1632) Sci. Rep. 2021;11:22253. doi: 10.1038/s41598-021-98214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fornaciari G., Marchetti A. Intact Smallpox Virus Particles in an Italian Mummy of Sixteenth Century. Lancet. 1986;328:625. doi: 10.1016/S0140-6736(86)92443-8. [DOI] [PubMed] [Google Scholar]
- 99.Duggan A.T., Perdomo M.F., Piombino-Mascali D., Marciniak S., Poinar D., Emery M.V., Buchmann J.P., Duchene S., Jankauskas R., Humphreys M., et al. 17th Century Variola Virus Reveals the Recent History of Smallpox. Curr. Biol. 2016;26:3407–3412. doi: 10.1016/j.cub.2016.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muhlemann B., Vinner L., Margaryan A., Wilhelmson H., de la Fuente Castro C., Allentoft M.E., de Barros Damgaard P., Hansen A.J., Holtsmark Nielsen S., Strand L.M., et al. Diverse variola virus (smallpox) strains were widespread in northern Europe in the Viking Age. Science. 2020;369:eaaw8977. doi: 10.1126/science.aaw8977. [DOI] [PubMed] [Google Scholar]
- 101.Johnson N.P., Mueller J. Updating the accounts: Global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull. Hist. Med. 2002;76:105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 102.Tumpey T.M., Basler C.F., Aguilar P.V., Zeng H., Solorzano A., Swayne D.E., Cox N.J., Katz J.M., Taubenberger J.K., Palese P., et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 103.Taubenberger J.K., Morens D.M. 1918 Influenza: The mother of all pandemics. Emerg. Infect. Dis. 2006;12:15–22. doi: 10.3201/eid1209.05-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morens D.M., Taubenberger J.K., Fauci A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: Implications for pandemic influenza preparedness. J. Infect. Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hatchett R.J., Mecher C.E., Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc. Natl. Acad. Sci. USA. 2007;104:7582–7587. doi: 10.1073/pnas.0610941104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Merson M.H., O’Malley J., Serwadda D., Apisuk C. The history and challenge of HIV prevention. Lancet. 2008;372:475–488. doi: 10.1016/S0140-6736(08)60884-3. [DOI] [PubMed] [Google Scholar]
- 107.WHO. [(accessed on 6 February 2022)]. Available online: https://covid19.who.int/
- 108.Reno E., Quan N.G., Franco-Paredes C., Chastain D.B., Chauhan L., Rodriguez-Morales A.J., Henao-Martinez A.F. Prevention of yellow fever in travellers: An update. Lancet Infect. Dis. 2020;20:e129–e137. doi: 10.1016/S1473-3099(20)30170-5. [DOI] [PubMed] [Google Scholar]
- 109.Chen L.H., Wilson M.E. Yellow fever control: Current epidemiology and vaccination strategies. Trop. Dis. Travel Med. Vaccines. 2020;6:1. doi: 10.1186/s40794-020-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang C.Q., Chung P.K., Liu J.D., Chan D.K.C., Hagger M.S., Hamilton K. Health Beliefs of Wearing Facemasks for Influenza A/H1N1 Prevention: A Qualitative Investigation of Hong Kong Older Adults. Asia Pac. J. Public Health. 2019;31:246–256. doi: 10.1177/1010539519844082. [DOI] [PubMed] [Google Scholar]
- 112.Cauchemez S., Ferguson N.M., Wachtel C., Tegnell A., Saour G., Duncan B., Nicoll A. Closure of schools during an influenza pandemic. Lancet Infect. Dis. 2009;9:473–481. doi: 10.1016/S1473-3099(09)70176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Delgado R., Simon F. Transmission, Human Population, and Pathogenicity: The Ebola Case in Point. Microbiol. Spectr. 2018;6 doi: 10.1128/microbiolspec.MTBP-0003-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Undurraga E.A., Carias C., Meltzer M.I., Kahn E.B. Potential for broad-scale transmission of Ebola virus disease during the West Africa crisis: Lessons for the Global Health security agenda. Infect. Dis. Poverty. 2017;6:159. doi: 10.1186/s40249-017-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shah V.K., Firmal P., Alam A., Ganguly D., Chattopadhyay S. Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past. Front. Immunol. 2020;11:1949. doi: 10.3389/fimmu.2020.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kampf G., Bruggemann Y., Kaba H.E.J., Steinmann J., Pfaender S., Scheithauer S., Steinmann E. Potential sources, modes of transmission and effectiveness of prevention measures against SARS-CoV-2. J. Hosp. Infect. 2020;106:678–697. doi: 10.1016/j.jhin.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.International Outbreak of Novel SARS-CoV-2 Coronavirus Infection. [(accessed on 26 February 2022)]. Available online: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-international-outbreak.
- 118.Castaldi S., Romano L., Pariani E., Garbelli C., Biganzoli E. COVID-19: The end of lockdown what next? Acta Biomed. 2020;91:236–238. doi: 10.23750/abm.v91i2.9605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Afari F. A Brief International History of Pandemics. [(accessed on 26 February 2022)]. Available online: https://www.graduateinstitute.ch/communications/news/brief-international-history-pandemics.
- 120.Grmek M.D., Buchet C. Man, Health and the Sea. Honoré Champion; Paris, France: 1997. The beginnings of maritime quarantine; pp. 39–60. (In French) [Google Scholar]
- 121.Tognotti E. Lessons from the history of quarantine, from plague to influenza A. Emerg. Infect. Dis. 2013;19:254–259. doi: 10.3201/eid1902.120312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Defoe D. A Journal of the Plague Year. Int. J. Epidemiol. 2006;35:1066. doi: 10.1093/ije/dyl122. [DOI] [Google Scholar]
- 123.Bassareo P.P., Melis M.R., Marras S., Calcaterra G. Learning from the past in the COVID-19 era: Rediscovery of quarantine, previous pandemics, origin of hospitals and national healthcare systems, and ethics in medicine. Postgrad. Med. J. 2020;96:633–638. doi: 10.1136/postgradmedj-2020-138370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gensini G.F., Yacoub M.H., Conti A.A. The concept of quarantine in history: From plague to SARS. J. Infect. 2004;49:257–261. doi: 10.1016/j.jinf.2004.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Savona-Ventura C. The Order of St Lazarus in the Kingdom of Jerusalem. J. Monast. Mil. Orders. 2008;1:55–64. [Google Scholar]
- 126.Sehdev P.S. The origin of quarantine. Clin. Infect. Dis. 2002;35:1071–1072. doi: 10.1086/344062. [DOI] [PubMed] [Google Scholar]
- 127.Barquet N., Domingo P. Smallpox: The triumph over the most terrible of the ministers of death. Ann. Intern. Med. 1997;127:635–642. doi: 10.7326/0003-4819-127-8_Part_1-199710150-00010. [DOI] [PubMed] [Google Scholar]
- 128.Pead P.J. Benjamin Jesty: New light in the dawn of vaccination. Lancet. 2003;362:2104–2109. doi: 10.1016/S0140-6736(03)15111-2. [DOI] [PubMed] [Google Scholar]
- 129.Jenson A.B., Ghim S.J., Sundberg J.P. An inquiry into the causes and effects of the variolae (or Cow-pox. 1798) Exp. Dermatol. 2016;25:178–180. doi: 10.1111/exd.12925. [DOI] [PubMed] [Google Scholar]
- 130.Plotkin S. History of vaccination. Proc. Natl. Acad. Sci. USA. 2014;111:12283–12287. doi: 10.1073/pnas.1400472111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Plotkin S.A. Vaccines: Past, present and future. Nat. Med. 2005;11:S5–S11. doi: 10.1038/nm1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Underhill D.M., Gordon S., Imhof B.A., Nunez G., Bousso P. Elie Metchnikoff (1845–1916): Celebrating 100 years of cellular immunology and beyond. Nat. Rev. Immunol. 2016;16:651–656. doi: 10.1038/nri.2016.89. [DOI] [PubMed] [Google Scholar]
- 133.De Jesus N.H. Epidemics to eradication: The modern history of poliomyelitis. Virol. J. 2007;4:70. doi: 10.1186/1743-422X-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021;21:475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]