Abstract
Objective:
Pharmacological treatments that can concomitantly address cigarette smoking and heavy drinking stand to improve healthcare delivery for these highly prevalent co-occurring conditions. This superiority trial tested the combination of varenicline plus naltrexone versus varenicline alone for smoking cessation and drinking reduction among heavy drinking smokers.
Methods:
This was a phase 2, randomized, double-blind, clinical trial. Participants (n=165) were daily smokers who drank heavily and received either (a) varenicline tartrate 1 mg twice daily plus naltrexone 50 mg once daily, or (b) varenicline tartrate 1 mg twice daily plus matched placebo pills for 12-weeks. Primary outcomes were (a) 7-day point prevalence of nicotine abstinence bioverified by breath carbon monoxide (CO) reading of ≤ 5 ppm at the 26-week follow-up, and (b) drinks per drinking day during the 12-week treatment phase.
Results:
Smoking abstinence at week 26 was significantly higher in the varenicline plus placebo condition than the varenicline plus naltrexone condition (n=37 [45.1%] versus n=22 [26.5%]; χ2[1] = 6.22, p=.015). For drinks per drinking day, there was a trend towards a main effect of medication [β=0.86, SE=0.44, t(1,250)=1.94, p=.054], favoring the combination of varenicline plus naltrexone over varenicline alone across the 12-week treatment phase.
Conclusions:
These findings suggest that smoking cessation and drinking reduction can be concomitantly targeted with pharmacotherapy and that while varenicline alone may be sufficient as a smoking cessation aid in heavy drinking smokers, the combination of varenicline plus naltrexone may confer benefits with regards to drinking outcomes, particularly during the 12-week period of active medication treatment.
Trial Registration:
Keywords: Smoking cessation, Heavy drinking, varenicline, naltrexone, clinical trial
Introduction
Cigarette smoking (1) and heavy alcohol use (2) are leading causes of morbidity and mortality in the U.S. and worldwide. Alcohol use and smoking have high rates of co-use, such that approximately 20-25% of current smokers are considered heavy drinkers (3, 4). Heavy drinking smokers experience more negative heath consequences, such as greater risk for various cancers (5), and lower likelihood of successful smoking cessation (4, 6-8). Notably, both smoking (9) and heavy drinking (10, 11) have been associated with worse outcomes for COVID-19, highlighting the importance of treating both conditions. It is estimated that smokers are up to four times more likely to experience a smoking lapse during drinking episodes (7). While heavy drinking smokers constitute a sizeable subgroup with higher risk of negative consequences and failed quit attempts, there are no available pharmacological treatments tailored to heavy drinking smokers (12, 13).
Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, is the most effective pharmacotherapy for smoking cessation (14), with an estimated 1 in 8 patients reaching smoking abstinence at 1-year follow-up (15). Studies have shown that in addition to its smoking cessation benefits, varenicline may also reduce alcohol consumption (16-18). Naltrexone, an opioid receptor antagonist, is an FDA-approved pharmacotherapy for alcohol use disorder (19, 20), which has been shown to increases smoking cessation rates, particularly among heavy drinking smokers (21, 22). While varenicline and naltrexone each have FDA-approval for nicotine and alcohol use disorder, respectively, there is evidence that their clinical benefit may extend across the two disorders.
Towards improving treatments for heavy drinking smokers, our laboratory has conducted an experimental medicine study in which we compared the effects of varenicline only, naltrexone only, the combination of varenicline and naltrexone, and matched placebo (23). The combination of varenicline and naltrexone was superior to placebo, and at times superior to monotherapy, in attenuating cigarette craving, cigarette and alcohol subjective ‘high’, and reducing ad-lib consumption of cigarettes and alcohol. The combination of varenicline and naltrexone was associated with the greatest reduction in neural activation of the bilateral anterior cingulate cortex during exposure to cigarette cues (24). These findings suggested that a clinical trial of the combination of varenicline and naltrexone for heavy drinking smokers was warranted. Testing combination pharmacotherapy for addiction is an understudied area, with potential to reach additive or even synergistic effects on craving and substance use (25). Further, the concurrent treatment of smoking and heavy alcohol use holds tremendous promise to patients and health care systems alike (26, 27).
The current study was a double-blind, parallel group, placebo-controlled randomized trial of treatment with varenicline alone compared to varenicline plus naltrexone, in outpatient heavy drinking smokers seeking treatment for smoking cessation and drinking reduction. The primary aim in this superiority trial was to test the hypothesis that the combination of varenicline plus naltrexone would be superior to varenicline alone with regards to (a) bioverified smoking cessation rates at 26-weeks, and (b) reductions in drinks per drinking day during the 12-week treatment phase and at 26-weeks.
Methods
Trial Design
This study was a 26-week randomized clinical trial (ClinicalTrials.gov identifier: NCT02698215) of varenicline alone versus varenicline plus naltrexone for smoking cessation and drinking reduction in a community sample of heavy drinking smokers. After screening and randomization to one of the two study medication arms, participants set a quit date for smoking during a 30-45-minute counseling visit with a master’s level clinician. Consistent with guidelines for the clinical usage of varenicline tartare, patients were instructed to quit smoking after reaching the target dose of the study medication. Study visits occurred at 4, 8, and 12 weeks post-quit date with post-treatment follow-up visits at weeks 16 and 26. This trial was approved by the Institutional review board of the University of California, Los Angeles (UCLA) and was monitored by a Data and Safety Monitoring Board. All study participants provided written informed consent upon discussing the study medications with a licensed physician. Participants were enrolled/randomized/followed in the study between July, 2015 and December, 2019. Data analysis was conducted from January to April 2020.
Setting and Participants
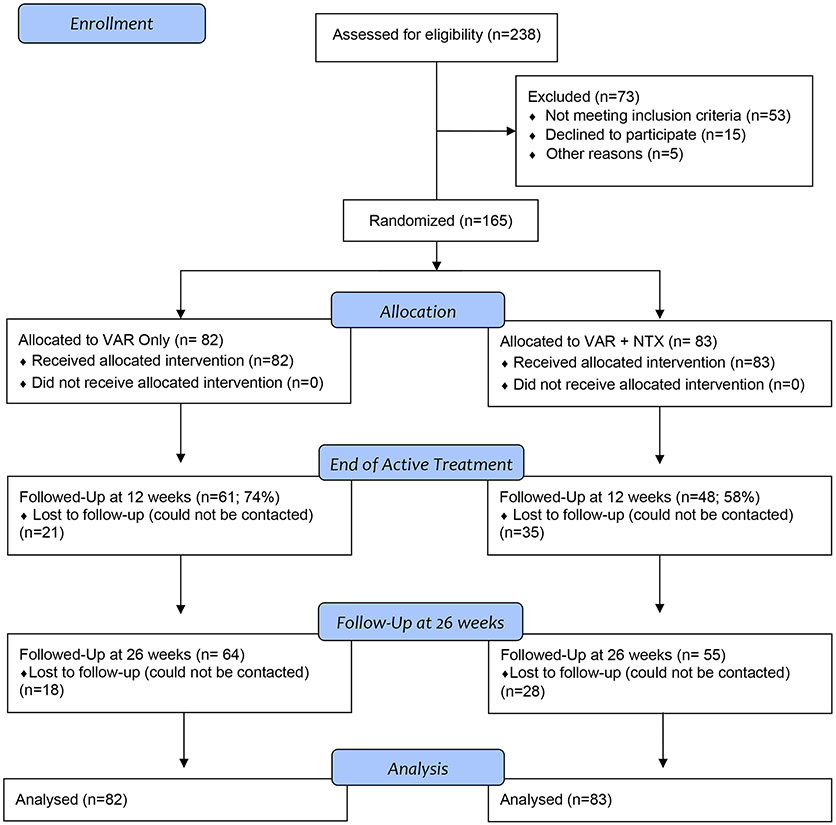
The trial was conducted at an outpatient research facility at UCLA. Participants were recruited through print, radio, social media, and mass transit advertisements. After initial screening for eligibility over the telephone or online, participants were invited for an in-person intake appointment. Eligible participants were men and women aged 21-65 seeking treatment for smoking cessation and expressing a desire to reduce or quit drinking. Participants were required to smoke ≥5 cigarettes per day and to have a carbon monoxide reading ≥ 4 ppm to verify smoker status (28). While six participants did not meet the baseline CO level requirement, their smoking status as confirmed by a cotinine test indicating they were regular smokers. Cotinine was assessed using a urine cotinine test (NicAlert™), a rapid test utilizing semiquantitative immunoassay technology to detect tobacco from cigarettes with a score of 3 or higher (100-200ng/mL) indicating recent tobacco exposure. Participants were also required to meet the definition of heavy drinking, by the National Institute on Alcohol Abuse and Alcoholism (NIAAA)(29): for men, > 14 drinks per week or ≥ 5 drinks per occasion at least once per month over the past 12 months; for women, > 7 drinks per week or ≥ 4 drinks per occasion at least once per month over the past 12 months. Exclusion criteria were clinically significant alcohol withdrawal, indicated by a score ≥ 10 on the Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar)(30) and lifetime history of psychotic or bipolar disorders. Participants with current substance use disorder or major depressive disorder with suicidal ideation were excluded from the trial. Women of childbearing age had to be practicing effective contraception and could not be pregnant or nursing. A physical exam including vital signs, E.K.G., and laboratory exams were conducted to ensure medical eligibility. Participants with any abnormalities were excluded and referred to their personal physicians for evaluation. See Figure 1.
Figure 1.
CONSORT Diagram for trial enrollment and analysis.
Interventions
A stratified randomization list was developed by the study statistician and was based on sex, drinks per drinking day, and cigarettes per day. Participants were randomized to one of two interventions: (1) 2 mg of varenicline tartrate plus 50 mg of naltrexone, or (2) 2 mg of varenicline tartrate plus matching placebo pills. Pfizer, Inc. supplied the varenicline tartrate for the study and the UCLA Research Pharmacy prepared all study medications in blister packs, which were dispensed once monthly for the 12-week medication treatment phase. Participants, providers, and research staff were kept blind to medication assignment for the duration of the study. Medication titration for varenicline tartrate followed recommended procedures in its FDA-indication for smoking cessation, namely 0.5 mg once daily for 3 days, 0.5 mg twice daily for 4 days, and 1 mg twice daily for the remainder of the 12-week treatment. For naltrexone, participants took 25 mg once daily for the first 5 days and 50 mg for the remainder of the 12-week treatment. Study medications were tapered off after week 12. Participants were instructed to take both medications at the same time and individuals reporting any significant side effects were counseled via telephone by the study physician. Medication compliance was monitored through pill counts returned from blister packs at each study visit. Dose reductions were allowed and participants who discontinued the medication could continue to attend research sessions. At study randomization, participants received a 30-45-minute counseling session tailored to heavy drinking smokers (31). At the counseling session all participants set a quit date for smoking cessation and discussed a drinking goal, such as abstinence or drinking reduction. The quit date was set to occur within 3 days after completing the varenicline titration schedule to reduce experimental error associated with highly variable quit dates. Following randomization participants attended the following research sessions: weeks 4, 8, 12, 16, 26. Each session consisted of research assessments, carbon monoxide recordings, and medication pill count. Participants completed brief telephone assessments at weeks 2 and 6 to promote medication compliance and retention in the study.
Assessments
At the in-person intake visit, a series of assessments were conducted for eligibility and individual differences. These assessments include the Structured Clinical Interview for DSM-5 (SCID5)(32), the Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar)(30), the Fagerström Test of Nicotine Dependence (33), and the Columbia Suicide Severity Rating Scale (34). At intake, all participants were required to have a breath alcohol concentration (BrAC) of 0.00 g/dl and to test negative for all drugs of abuse (except for cannabis). Blood pressure and heart rate were assessed at intake and at each visit. Side effects were elicited in open ended fashion at each study visit and were regularly reviewed by the study physician.
The Timeline Followback (TLFB) instrument was used to derive the drinking outcomes of interest. The TLFB consists of a calendar-assisted interview in which the participant reports on their use of alcohol over the specified assessment period (i.e., typically 4 weeks but up to 10 weeks in the 26-week assessment). A trained interviewer asked participants whether they consumed any alcohol on each calendar day within the assessment period covered by the visit and records the number of drinks consumed as standard average drinks (i.e., 12 ounces of regular beer, 5 ounces of wine, 1.5 ounces of distilled spirts). Those drinking estimates were then converted into the desired drinking outcomes over a given assessment period (i.e., baseline, 4, 8, 12, 16 and 26 weeks). The primary outcome of drinks per drinking day is computed by dividing the number of drinks consumed in total during the assessment period over the number of drinking days during the same period of time. The TLFB has high reliability and is widely used in clinical trials for AUD (35, 36).
Outcome Measures
For smoking cessation, the a-priori outcome was a 7-day point prevalence of nicotine abstinence bioverified through a carbon monoxide reading of ≤ 5 ppm, which is considered optimal for smoking cessation trials (37). The primary a-priori drinking outcome was drinks per drinking day. The rationale for selecting drinks per drinking day was based on the sample comprised of heavy drinking smokers for whom the percent of heavy drinking days variable may not be ideal. Specifically, the heavy drinking smoker population may not have as many heavy drinking days as samples required to have moderate-to-severe AUD, which is typical for AUD clinical trials. As such, drinks per drinking day was selected a-priori (i.e., at trial registration) given that it captures reductions in drinking, as opposed to abstinence, yet it does not rely on a high prevalence of heavy drinking days. Instead, any reduction in drinking on days in which participants consumed alcohol would be detectable. Secondary drinking outcomes were percent heavy drinking days, drinking days, and percent days abstinent. These secondary outcomes were intended to detect additional medication effects and standard in clinical trials for AUD (38).
Statistical Power and Data Analysis
The trial was powered to detect a medium effect size (Cohen d=0.5) for the between group difference on the primary outcomes. With over 64 participants per group, power to detect such an effect was ≥ 80% for a two-tailed test and an α level of .05. Analyses were conducted in SAS Statistical Software Version 9.4 (39). All analyses were of the intention-to-treat type. Since all participants took the first dose of the study medication under observation during the randomization visit, all participants were included in the subsequent analyses. The dichotomous primary outcome for smoking cessation at the 26-week follow up was analyzed using a Chi-Square test comparing smoking cessation rates for the two medication conditions. For the purpose of these analyses, all individuals who dropped out from the trial were considered to have returned to smoking, per guidelines for smoking cessation trials (40). Sensitivity analyses were conducted for the smoking cessation outcome, testing alternative assumptions about missing data, as opposed to relying solely on the assumption that data missingness should be scored as a failed cessation attempt; see Supplementary Materials. For the drinking outcomes, all of which were continuous, a series of multilevel models, via Proc Mixed, were conducted for each outcome separately, during the 12-week treatment period and for the entire 26-weeks of the trial, which is consistent with pharmacotherapy trials for excessive drinking (38, 41). These models tested whether the two medication groups differed in alcohol use over the 12-week medication period and across the entire 26-weeks of the trial. All models for drinking outcomes controlled for baseline drinking levels. Sensitivity analyses were conducted for the drinking outcomes to assess the effects of missing data assumptions (42); Supplementary Materials.
Results
Recruitment and disposition of study participants are summarized in Figure 1 (CONSORT Diagram). A total of 165 heavy drinking smokers were randomized in the trial and 119 completed the final follow-up at week 26, for a 72% retention rate. The difference in dropout between the two medication conditions was not statistically significant, χ2(1, N=165) = 2.85, p=0.92. Adverse events, recorded in an open-ended fashion at each study visit, are presented in Supplementary Materials. There were no significant differences in adverse events across the two medication groups across the various symptom categories (p > .06). All individuals randomized took the first medication dose under observation and were included in the final analysis. A total of 5 participants (4 male and 1 female) had dose reductions such that for all of them, the varenicline arm was reduced from 2mg/day to 1mg/day. All 5 participants undergoing the dose reduction remained in the study. One participant decided to discontinue the study medications upon discussing adverse events (mainly nausea) with the study physician. Participant demographics, smoking and drinking characteristics are presented in Table 1. Medication groups did not differ significantly on any baseline characteristic reported in Table 1.
Table 1.
Sample Characteristics at Baseline by Treatment Condition
| Variable | Varenicline + Placebo Condition (n=82) |
Varenicline + Naltrexone Condition (n=83) |
||
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Mean | SD | Mean | SD | |
| Age | 42.07 | 11.75 | 41.24 | 12.42 |
| N | % | N | % | |
| Gender | ||||
| Male | 50 | 60.98 | 52 | 62.65 |
| Female | 32 | 39.02 | 31 | 37.35 |
| Race | ||||
| White | 29 | 35.37 | 27 | 32.53 |
| African American | 36 | 43.90 | 40 | 48.19 |
| American Indian/Alaskan | 0 | 0 | 2 | 2.41 |
| Asian | 1 | 1.22 | 3 | 3.61 |
| Pacific Islander | 1 | 1.22 | 2 | 2.41 |
| Mixed Race | 7 | 8.54 | 5 | 6.02 |
| Another Race | 8 | 9.76 | 4 | 4.82 |
| Ethnicity | ||||
| Hispanic or Latino | 13 | 15.85 | 12 | 14.46 |
| Married or Living Together | 17 | 20.73 | 23 | 27.71 |
| Employment Status | ||||
| Full-Time or Part-Time | 47 | 57.32 | 55 | 66.27 |
| Unemployed | 24 | 29.27 | 17 | 20.48 |
| Income | ||||
| Less than $30,000 | 45 | 54.88 | 51 | 61.45 |
| Between $30,000 - $45,000 | 17 | 20.73 | 15 | 18.07 |
| Greater than $45,000 | 20 | 24.39 | 17 | 20.48 |
| Smoking Characteristics | ||||
| Mean | SD | Mean | SD | |
| Smoking Daysa | 29.39 | 1.49 | 28.94 | 3.37 |
| Cigarettes Per Smoking Daya | 14.10 | 7.79 | 14.21 | 8.39 |
| Expired Alveolar CO Level | 9.68 | 7.34 | 11.65 | 6.25 |
| Cotinine (ng/ml) | 5.37 | 1.05 | 5.50 | 1.14 |
| FTND Scoreb | 4.61 | 2.35 | 4.64 | 2.26 |
| Drinking Characteristics | ||||
| Mean | SD | Mean | SD | |
| Drinking Daysa | 19.63 | 7.92 | 20.71 | 8.08 |
| Drinks per Drinking Daya | 6.44 | 3.76 | 6.50 | 4.65 |
| Heavy Drinking Daysa | 12.30 | 8.84 | 12.87 | 9.55 |
| N | % | N | % | |
| AUD Severityc | ||||
| None | 24 | 29.27 | 24 | 29.27 |
| Mild | 22 | 26.83 | 27 | 32.93 |
| Moderate | 23 | 28.05 | 19 | 23.17 |
| Severe | 13 | 15.85 | 12 | 14.63 |
FTND = Fagerström Test for Nicotine Dependence
Assessed by Timeline Follow-Back (TLFB) interview for the past 30 days
DSM-5 current AUD severity status based on the SCID-5; 1 participant has missing data for the SCID-5.
The overall medication compliance across both medication conditions, based on pill count, was 64.12% (SD=0.37). There was a significant effect of medication group on compliance, such that the VAR + PLAC group had an overall compliance of 68.46% whereas the VAR + NTX has an overall compliance of 59.83% [F(1,164)=4.88, p=0.29], and that is after controlling for the large effect of dropout status on compliance [F(1,164)=180.61, p<.0001]. Thus, over and above the effect of medication group on dropout, individuals in the VAR + PLAC condition reported higher medication compliance on the basis of pill count across the 12-weeks of active medication treatment.
Medication Effects on Smoking Cessation
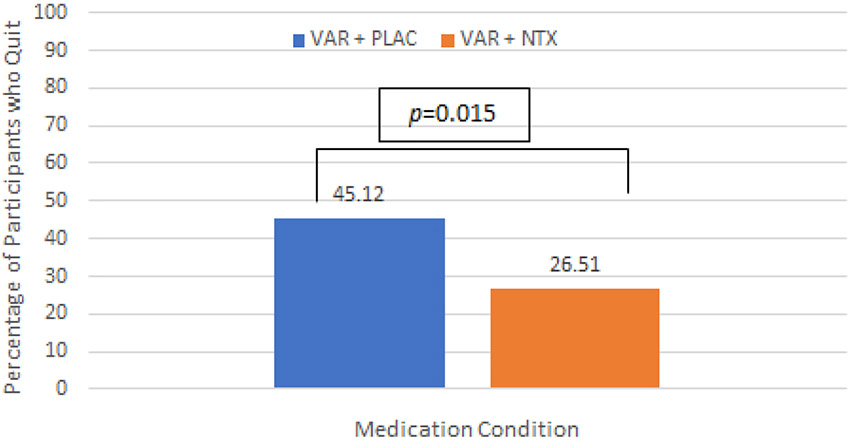
The primary outcome for the trial was 7-day point prevalence of nicotine abstinence bioverified by CO reading of ≤ 5 ppm at the 6-month follow-up. The overall quit rate for the study at the 26-week assessment was 35.76% such that of the 165 participants analyzed across both medication conditions, 59 of them quit smoking. Analyses within medication groups revealed that in the VAR + PLAC condition, 37 individuals (45.12%) had quit smoking at week 26, whereas 45 participants (54.88%) did not. In the VAR + NTX condition, 22 individuals (26.51%) had quit smoking (i.e., CO ≤ 5 ppm) at week 26, whereas 61 participants (73.49%) did not. The difference in quit rate at the 6-month follow-up between medication conditions was statistically significant [χ2(1, N=165) = 6.22, p=0.015], and favored the varenicline alone condition. See Figure 2. This pattern of results was also seen at the 12-week assessment, when participants were still on active medication. Specifically, the quit rate was 53.66% (44/82 participants quit) in the VAR + PLAC condition, compared to 38.55% quit rate (32/83 participants quit in the VAR + NTX condition, [χ2(1, N=165) = 3.79, p=0.051] at the 12-week assessment visit. See Supplementary Figure S1.
Figure 2.
Percentage of participants in each medication group, VAR + PLAC and VAR + NTX, who quit smoking (bioverified by CO reading of ≤ 5 ppm) at the 26-week assessment. Smoking abstinence at week 26 was significantly higher in the VAR + PLAC condition than the VAR + NTX condition (χ2[1] = 6.22, p=.015).
To test for sex effects, we ran a multivariate logistic regression which confirmed the main effect of medication [χ2(1, N=165) = 6.77, p=.009], and found no effect of sex (p=0.99), or medication × sex interaction (p=0.35), on abstinence at week 26. Further, we tested AUD severity as a predictor of smoking cessation outcome at 26-weeks and found no significant effect of AUD severity [χ2(1, N=164) = 1.52, p=0.68]. Likewise, there was no AUD severity × medication effect on smoking cessation outcomes at 26-weeks [Wald χ2(1, N=164) = 2.99, p=0.39]. Lastly, sensitivity analyses tested the effects of missing data assumptions, specifically missing at random (MAR) and missing not at random (MNAR), on smoking cessation outcomes at week 26. These results are presented in Supplementary Materials. The results of this sensitivity analyses were consistent across different assumptions for missing data such that significantly higher smoking quit rates were observed in the VAR+PLAC condition.
Medication Effects on Drinking Outcomes
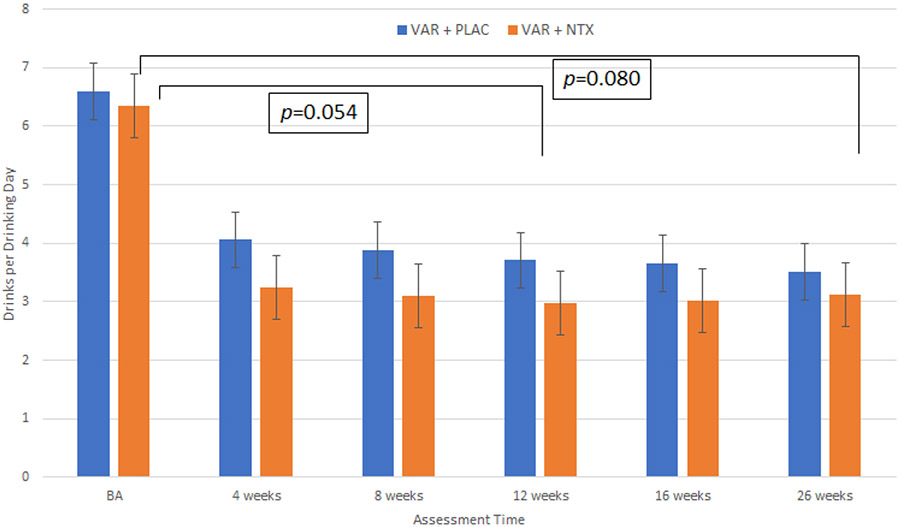
For all drinking outcomes, the course of drinking reduction showed a steep change from baseline to the 4-week follow-up and these changes were generally maintained at 8-, 12-week, 16-week, and 26-week follow-ups. As such, the effect of time on drinking outcomes was captured by three time-variables, the first capturing weekly change during the baseline to 4-week period, the second capturing weekly change from 4 to 12-weeks in the trial, and the third capturing weekly change from 12 to 26-weeks in the trial. Analyses of the primary drinking outcome of drinks per drinking day demonstrated a trend-level medication effect favoring the VAR + NTX condition, medication effect at across the entire trial, including the active medication period (weeks 4, 8, 12) and the follow-up period (weeks 16 and 26) [β=0.82 SE=0.47, t(1,484)=1.76, p=.080]. Sensitivity analyses that investigated the influence of different missing data handling assumptions on the findings were conducted and supported this main effect of medication (Supplementary Materials). Analyses also revealed a stronger trend-level main effect of medication condition over the course of the 12-week period [β=0.86, SE=0.44, t(1,250)=1.94, p=.054], favoring the combination of VAR + NTX over VAR alone. As shown in Figure 3, participants in the VAR + NTX condition consumed approximately 1 fewer drinks per drinking day, on average, during the 12-weeks of medication treatment. This effect was more pronounced in the change from baseline to the 4-week assessment, when the quit attempt took place. In support, there was significant effect for the first time variable (baseline to week 4), [average change per week; β=−0.78, SE=0.11, t(1,138)=−6.89, p<.0001] but not for the second time variable in the model (weeks 4 to 12), [β=−0.03, SE=0.04, t(1,250)=−0.76, p=.45], both of which were entered simultaneously in the single model testing medication effects on drinks per drinking day. Results for the third time variable in the model (weeks 12 to 26) [average change per week; β=0.04, SE=0.05, t(1,138)=0.83, p=.41] indicated no significant effct. Variance components further indicated that individual change rates exhibited significant variation during the first but not for the second- or third-time epochs. There was no medication condition × time interaction for either time 1 (p=0.34) or time 2 (p=0.50), or time 3 (p=.85). AUD severity was a significant predictor of outcome, with higher severity predicting more drinks per drinking day (p<.001).
Figure 3.
Results displayed are mean drinks per drinking day and standard errors at each follow-up time point. Analyses of drinks per drinking day across the entire trial, including the active medication period (weeks 4, 8, 12) and the follow-up period (weeks 16 and 26) were also performed and revealed a similar, albeit weaker, trend-level medication effect favoring the VAR + NTX condition, medication effect [β=0.82 SE=0.47, t(1,484)=1.76, p=.080]. As with the primary models, these analyses account for the effects of time, medication × time, and AUD severity.
Several variables were used to further probe the main effect of medication on drinks per drinking day. First, regarding the effect of smoking cessation outcome on subsequent drinking in the trial, analyses indicated that the effect of medication on drinks per drinking day was significant (p=0.050) in a model that accounted for whether participants had quit successfully smoking at the 4-week assessment (i.e., first assessment following the planned quit date) but there was no significant medication × smoking cessation interaction on drinking outcomes (p=0.47). Second, regarding sex effects, there was a main effect of sex [β=−1.66 SE=0.57, t(1,487)=−2.90, p=.04], such that overall, females reported fewer drinks per drinking days than males at the 4-week follow-up. However, there was not a sex × medication (p=0.15) or sex × medication × time interaction for either the first (p=0.39) or second (p=0.31) time variable. Third, while AUD severity was a significant covariate in all the drinking outcome models, there was no evidence of medication × AUD severity interaction on drinks per drinking day (p=0.88).
Results of medication effects on secondary drinking outcomes were largely not statistically significant for the time frames of primary interest and are reported in detail in Supplementary Materials. Of note, for percent heavy drinking days across the entire 26-weeks, there was no significant effect of the any of the Time variables (p’s > .666), nor Time × Medication interactions (p’s > .619). The overall medication effect was also not significant [β=0.056, SE=0.059, t(1,484)=0.95, p=0.344]
Discussion
This clinical trial tested the combination of varenicline plus naltrexone versus varenicline plus placebo for smoking cessation and drinking reduction in heavy drinking smokers. Based on preliminary studies, it was hypothesized that in this superiority trial, the combination of varenicline plus naltrexone would be superior to varenicline alone as a smoking cessation aid. Results for smoking cessation were contrary to the initial hypothesis, as we found that varenicline alone led to a 42% quit rate in this sample of heavy drinking smokers, compared to a 25% quit rate for the combination of varenicline plus naltrexone. The observed quit rate for varenicline alone in this sample is highly encouraging as this is the first large scale trial of varenicline efficacy focused solely on heavy drinking smokers. Notably, preliminary findings of varenicline for heavy drinking smokers have also been positive (43). If supported by large scale RCTs with a placebo arm, varenicline may be especially indicated for smoking cessation among heavy drinking smokers. In addition, a recent study found that dual nicotine replacement therapy (NRT) may be as effective as varenicline and have better tolerability (44), such that comparing varenicline to dual NRT in heavy drinking smokers may be warranted.
In essence, we found that there was no benefit of adding naltrexone to varenicline treatment for smoking cessation. In fact, adding naltrexone had an iatrogenic effect on smoking cessation rates. Given that in this sample individuals reported a desire to quit smoking and reduce, as opposed to quit, drinking, it is possible that naltrexone may work to reduce smoking while not being beneficial to quit smoking entirely. This is consistent with findings that naltrexone reduced the number of cigarettes per day during the pre-quit phase of smoking cessation (45) but did not improve smoking abstinence rates (46), compared to placebo. This is the first trial of the combination of VAR + NTX and heavy drinking smokers did worse on the combination than on varenicline alone. Further research exploring the bases for this iatrogenic effect is warranted. In brief, given that varenicline has been found to reduce drinking in trials for alcohol use disorder (16, 17), it is possible that its effects on both drinking and smoking present an optimal alternative for this unique subgroup of smokers. Large scale RCTs of varenicline versus placebo are needed to fully ascertain the clinical efficacy of varenicline in heavy drinking smokers.
Regarding the drinking outcomes in this study, there were significant reductions in drinking across both conditions; however, for our primary outcome of drinks per drinking day, there was evidence for some benefit of combining varenicline and naltrexone, as compared to varenicline alone. This medication by time effect was trending at week 12, and further reduced in significance when extending the analyses to week 26. Analyses of the secondary outcomes did not offer additional support for the benefit of the combination of varenicline and naltrexone for drinking reduction. It was expected that the addition of naltrexone to a varenicline regimen would have unique benefits on drinking outcomes given its established literature for AUD (20, 47). Naltrexone is especially useful in reducing heavy drinking among individuals who continue to drink (48) (as opposed to complete abstinence), and these effects are thought to be achieved via naltrexone-induced blunting of the rewarding effects of alcohol (49). To that end, this study sample was ideally-suited for naltrexone treatment as they were interested in reducing their drinking yet their primary reason for treatment seeking was smoking cessation. Nevertheless, this is a superiority trial and exceeding the effects of an establish pharmacotherapy such as varenicline imposes a high bar for clinical testing. The fact that the combination of varenicline and naltrexone was superior to varenicline alone by showing trend-level reductions in drinks per drinking day during the active medication phase suggests that combination pharmacotherapy could considered in samples with AUD whose primary treatment goal is drinking-related. However, it is also notable that the medication effect was reduced at the 26-week follow-up period. This effect should be verified in samples of non-smokers, as recent studies suggest that nicotine-use may predict a more positive clinical response to naltrexone (50, 51).
Another important area in which this study advances the literature is the concurrent treatment of smoking and heavy alcohol use. While the scientific literature is largely comprised of single-disorder treatment with stringent exclusion criteria (52), it has been long recognized that co-occurrence and comorbidity are the norm in clinical care settings. As such, concurrent treatments across disorders holds tremendous promise to patients and health care systems alike (26, 27). This study suggests that combined outcomes for smoking cessation and drinking reduction are achievable. In reference to the tolerability of both varenicline and naltrexone, this study found no significant differences in open-ended adverse events reported by participants between the two medication conditions. Furthermore, this clinical trial was able to recruit a diverse range of participants with African American’s being the most common race reported in our sample. Given the past difficulties recruiting minorities for participation in clinical trials (53), recruitment for this study was conducted in a culturally-sensitive manner, so as to create an inclusive environment for African American participants (54), and participants from diverse racial and ethnic backgrounds. As clinical studies are tasked expediting the translation to clinical practice (55), studying representative samples with co-occurring health conditions may ultimately improve the uptake of science-based treatments in clinical settings.
This study has several limitations that should be considered when interpreting its results. Most notably, in this superiority trial there was not a placebo arm, nor a naltrexone-alone condition. While we believe that varenicline is a first-line treatment for smoking cessation, its efficacy in heavy drinking smokers should be verified in a placebo-controlled design. This is the first large scale RCT to test the efficacy of varenicline in heavy drinking smokers, a sizeable and treatment-resistant subgroup (13). Likewise, for the purpose of improving treatment for alcohol-related problems, a comparison of the combination of varenicline plus naltrexone versus placebo, and versus naltrexone only, would be ideal in confirming the benefits of this pharmacotherapy combination. More generally, the potential to combine promising pharmacotherapies for AUD with naltrexone, has been aptly demonstrated elsewhere and should be carefully considered (56). An additional limitation was the use of an open-ended adverse event reporting system, which reduced the ability to compare adverse effect profiles to other studies with varenicline and naltrexone that may have used standardized side effects measures. Lastly, retention for this trial reached 72% for the 6-month follow-up and medication compliance was significantly higher for the monotherapy group. While these are acceptable retention and compliance rate, particularly for a group with co-occurring daily smoking and heavy drinking, combination pharmacotherapy should consider issues of tolerability and compliance. Our preliminary study of naltrexone and varenicline combination used a lower naltrexone dose, of 25 mg/day (23) and other studies have suggested a potential benefit of lower doses of naltrexone (57). Previous studies have also found support for the 50 mg/day dose used in this study. In combination with nicotine replacement therapy, 50 mg/day of naltrexone reduced the likelihood of relapse among participants who smoked during the first week in treatment (58). Other human laboratory (59) and clinical trials (60) have supported the use of 50 mg/day of naltrexone to improve smoking outcomes. On the other hand, studies have found that 100 mg/doses of naltrexone may be necessary to boost the clinical utility of this medication for smoking cessation (61). It has recently been shown that 100 mg doses of naltrexone may have unique clinical benefits through the blockade of kappa opioid receptors (62). On balance, future studies seeking to optimize dose selection may be especially critical in the context of combination pharmacotherapy.
Conclusions
This superiority trial tested the combination of varenicline plus naltrexone versus varenicline alone in a sample of heavy drinking smokers and found that combination pharmacotherapy was associated with a trend-towards lower drinks per drinking day during the active medication treatment period, which was slightly attenuated when including the post-treatment period. Regarding smoking cessation, varenicline alone was superior to combination pharmacotherapy yielding a 45% nicotine abstinence rate at the 6-month follow mark. Together, these results suggest that smoking cessation and drinking reduction can be concomitantly targeted and that while varenicline alone may be sufficient as a smoking cessation aid in heavy drinking smokers, the combination of varenicline plus naltrexone may confer unique benefits with regards to drinking outcomes but not smoking cessation.
Supplementary Material
Acknowledgement
This research was supported by R01DA041226 from the National Institute on Drug Abuse. Support for data analysis and manuscript preparation provided by K24AA025704. RG was funded by the Tobacco-Related Disease Research Program (T30DT0950). Pizer provided the study medication. The funder had no role in the design, analysis, interpretation, or writing of the report.
Footnotes
Disclosures
All authors report no financial relationships with commercial interests.
References
- 1.Control CfD, Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000-2004. MMWR Morbidity and mortality weekly report. 2008;57:1226. [PubMed] [Google Scholar]
- 2.Spillane S, Shiels MS, Best AF, Haozous EA, Withrow DR, Chen Y, Berrington de González A, Freedman ND. Trends in Alcohol-Induced Deaths in the United States, 2000-2016. JAMA network open. 2020;3:e1921451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson DA. Drinking as a risk factor for sustained smoking. Drug Alcohol Depend. 2000;59:235–249. [DOI] [PubMed] [Google Scholar]
- 4.Toll BA, Cummings KM, O'Malley SS, Carlin-Menter S, McKee SA, Hyland A, Wu R, Hopkins J, Celestino P. Tobacco quitlines need to assess and intervene with callers' hazardous drinking. Alcohol Clin Exp Res. 2012;36:1653–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebbert JO, Janney CA, Sellers TA, Folsom AR, Cerhan JR. The association of alcohol consumption with coronary heart disease mortality and cancer incidence varies by smoking history. J Gen Intern Med. 2005;20:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6 Suppl 2:S57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahler CW, Spillane NS, Metrik J. Alcohol use and initial smoking lapses among heavy drinkers in smoking cessation treatment. Nicotine Tob Res. 2010;12:781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahler CW, Borland R, Hyland A, McKee SA, Thompson ME, Cummings KM. Alcohol consumption and quitting smoking in the International Tobacco Control (ITC) Four Country Survey. Drug Alcohol Depend. 2009;100:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vardavas CI, Nikitara K. COVID-19 and smoking: A systematic review of the evidence. Tobacco induced diseases. 2020;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esper A, Burnham E, Moss M. The effect of alcohol abuse on ARDS and multiple organ dysfunction. Minerva anestesiologica. 2006;72:375. [PubMed] [Google Scholar]
- 11.Testino G. Are patients with alcohol use disorders at increased risk for Covid-19 infection? Alcohol and Alcoholism (Oxford, Oxfordshire). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yardley MM, Mirbaba MM, Ray LA. Pharmacological Options for Smoking Cessation in Heavy-Drinking Smokers. CNS Drugs. 2015;29:833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roche DJ, Ray LA, Yardley MM, King AC. Current insights into the mechanisms and development of treatments for heavy drinking cigarette smokers. Current addiction reports. 2016;3:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387:2507–2520. [DOI] [PubMed] [Google Scholar]
- 15.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2007:CD006103. [DOI] [PubMed] [Google Scholar]
- 16.Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med. 2013;7:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Malley SS, Zweben A, Fucito LM, Wu R, Piepmeier ME, Ockert DM, Bold KW, Petrakis I, Muvvala S, Jatlow P, Gueorguieva R. Effect of Varenicline Combined With Medical Management on Alcohol Use Disorder With Comorbid Cigarette Smoking: A Randomized Clinical Trial. JAMA Psychiatry. 2018;75:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Bejczy A, Löf E, Walther L, Guterstam J, Hammarberg A, Asanovska G, Franck J, Isaksson A, Söderpalm B. Varenicline for treatment of alcohol dependence: a randomized, placebo-controlled trial. Alcohol Clin Exp Res. 2015;39:2189–2199. [DOI] [PubMed] [Google Scholar]
- 19.Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. [DOI] [PubMed] [Google Scholar]
- 20.Donoghue K, Elzerbi C, Saunders R, Whittington C, Pilling S, Drummond C. The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: a meta-analysis. Addiction. 2015;110:920–930. [DOI] [PubMed] [Google Scholar]
- 21.Fridberg DJ, Cao D, Grant JE, King AC. Naltrexone improves quit rates, attenuates smoking urge, and reduces alcohol use in heavy drinking smokers attempting to quit smoking. Alcohol Clin Exp Res. 2014;38:2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King A, Cao D, Vanier C, Wilcox T. Naltrexone decreases heavy drinking rates in smoking cessation treatment: an exploratory study. Alcohol Clin Exp Res. 2009;33:1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED. Varenicline, low dose naltrexone, and their combination for heavy-drinking smokers: human laboratory findings. Psychopharmacology (Berl). 2014;231:3843–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED. Varenicline, naltrexone, and their combination for heavy-drinking smokers: preliminary neuroimaging findings. Am J Drug Alcohol Abuse. 2015;41:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee MR, Leggio L. Combined pharmacotherapies for the management of alcoholism: rationale and evidence to date. CNS Drugs. 2014;28:107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evers KE, Quintiliani LM. Advances in multiple health behavior change research. Transl Behav Med. 2013;3:59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prochaska JJ, Prochaska JO. A Review of Multiple Health Behavior Change Interventions for Primary Prevention. American journal of lifestyle medicine. 2011;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker TB, Piper ME, Stein JH, Smith SS, Bolt DM, Fraser DL, Fiore MC. Effects of Nicotine Patch vs Varenicline vs Combination Nicotine Replacement Therapy on Smoking Cessation at 26 Weeks: A Randomized Clinical Trial. Jama. 2016;315:371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NIAAA: The physicians' guide to helping patietns with alcohol problems. Bathesda, MD, National Institutes of Health; 1995. [Google Scholar]
- 30.Sullivan J, Sykora K, Schneiderman J, Naranjo C, Sellers E. Assessment of alcohol withdrawal: The revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-AR). British Journal of Addictions. 1989;84:1353–1357. [DOI] [PubMed] [Google Scholar]
- 31.Kahler CW, Metrik J, LaChance HR, Ramsey SE, Abrams DB, Monti PM, Brown RA. Addressing heavy drinking in smoking cessation treatment: a randomized clinical trial. J Consult Clin Psychol. 2008;76:852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders - Patient edition (SCID-I/P, version 2.0). . New York, Biometrics Research Department, New York State Psychiatric Institute.; 1995. [Google Scholar]
- 33.Heatherton TF, Kozlowski LT, Frecker RC, FAGERSTROM KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction. 1991;86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 34.Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students' recent drinking history: utility for alcohol research. Addict Behav. 1986;11:149–161. [DOI] [PubMed] [Google Scholar]
- 36.Hoeppner BB, Stout RL, Jackson KM, Barnett NP. How good is fine-grained Timeline Follow-back data? Comparing 30-day TLFB and repeated 7-day TLFB alcohol consumption reports on the person and daily level. Addict Behav. 2010;35:1138–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marrone GF, Shakleya DM, Scheidweiler KB, Singleton EG, Huestis MA, Heishman SJ. Relative performance of common biochemical indicators in detecting cigarette smoking. Addiction. 2011;106:1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witkiewitz K, Finney JW, Harris AH, Kivlahan DR, Kranzler HR. Guidelines for the Reporting of Treatment Trials for Alcohol Use Disorders. Alcohol Clin Exp Res. 2015;39:1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Institute S: Base SAS 9.4 procedures guide, SAS Institute; 2015. [Google Scholar]
- 40.Hall SM, Delucchi KL, Tsoh JY, Velicer WF, Kahler CW, Moore JR, Hedeker D, Niaura R. Statistical analysis of randomized trials in tobacco treatment: longitudinal designs with dichotomous outcome. Nicotine & Tobacco Research. 2001;3:193–202. [DOI] [PubMed] [Google Scholar]
- 41.Falk DE, Ryan ML, Fertig JB, Devine EG, Cruz R, Brown ES, Burns H, Salloum IM, Newport DJ, Mendelson J, Galloway G, Kampman K, Brooks C, Green AI, Brunette MF, Rosenthal RN, Dunn KE, Strain EC, Ray L, Shoptaw S, Ait-Daoud Tiouririne N, Gunderson EW, Ransom J, Scott C, Leggio L, Caras S, Mason BJ, Litten RZ. Gabapentin Enacarbil Extended-Release for Alcohol Use Disorder: A Randomized, Double-Blind, Placebo-Controlled, Multisite Trial Assessing Efficacy and Safety. Alcohol Clin Exp Res. 2019;43:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enders CK: Applied missing data analysis, Guilford press; 2010. [Google Scholar]
- 43.Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology. 2011;215:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tulloch HE, Pipe AL, Els C, Clyde MJ, Reid RD. Flexible, dual-form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: a randomized controlled trial. BMC Med. 2016;14:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King A, Cao D, Zhang L, Rueger SY. Effects of the opioid receptor antagonist naltrexone on smoking and related behaviors in smokers preparing to quit: a randomized controlled trial. Addiction. 2013;108:1836–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kahler CW, Cioe PA, Tzilos GK, Spillane NS, Leggio L, Ramsey SE, Brown RA, O'Malley SS. A Double-Blind Randomized Placebo-Controlled Trial of Oral Naltrexone for Heavy-Drinking Smokers Seeking Smoking Cessation Treatment. Alcohol Clin Exp Res. 2017;41:1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets. 2010;9:13–22. [DOI] [PubMed] [Google Scholar]
- 48.Ray LA, Krull JL, Leggio L. The Effects of Naltrexone Among Alcohol Non-Abstainers: Results from the COMBINE Study. Front Psychiatry. 2010;1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ray LA, Green R, Roche DJO, Magill M, Bujarski S. Naltrexone effects on subjective responses to alcohol in the human laboratory: A systematic review and meta-analysis. Addict Biol. 2019;24:1138–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anton RF, Latham PK, Voronin KE, Randall PK, Book SW, Hoffman M, Schacht JP. Nicotine-Use/Smoking Is Associated with the Efficacy of Naltrexone in the Treatment of Alcohol Dependence. Alcohol Clin Exp Res. 2018;42:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green R, Bujarski S, Lim AC, Venegas A, Ray LA. Naltrexone and alcohol effects on craving for cigarettes in heavy drinking smokers. Exp Clin Psychopharmacol. 2019;27:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blanco C, Olfson M, Okuda M, Nunes EV, Liu SM, Hasin DS. Generalizability of clinical trials for alcohol dependence to community samples. Drug Alcohol Depend. 2008;98:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmotzer GL. Barriers and facilitators to participation of minorities in clinical trials. Ethnicity & disease. 2012;22:226–230. [PubMed] [Google Scholar]
- 54.Otado J, Kwagyan J, Edwards D, Ukaegbu A, Rockcliffe F, Osafo N. Culturally Competent Strategies for Recruitment and Retention of African American Populations into Clinical Trials. Clinical and translational science. 2015;8:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ray LA, Grodin EN, Leggio L, Bechtholt AJ, Becker H, Feldstein Ewing SW, Jentsch JD, King AC, Mason BJ, O'Malley S, MacKillop J, Heilig M, Koob GF. The future of translational research on alcohol use disorder. Addict Biol. 2020:e12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anton RF, Myrick H, Wright TM, Latham PK, Baros AM, Waid LR, Randall PK. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Malley SS, Krishnan-Sarin S, McKee SA, Leeman RF, Cooney NL, Meandzija B, Wu R, Makuch RW. Dose-dependent reduction of hazardous alcohol use in a placebo-controlled trial of naltrexone for smoking cessation. Int J Neuropsychopharmacol. 2009;12:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krishnan-Sarin S, Meandzija B, O'Malley S. Naltrexone and nicotine patch smoking cessation: a preliminary study. Nicotine Tob Res. 2003;5:851–857. [DOI] [PubMed] [Google Scholar]
- 59.Epstein AM, King AC. Naltrexone attenuates acute cigarette smoking behavior. Pharmacology Biochemistry and Behavior. 2004;77:29–37. [DOI] [PubMed] [Google Scholar]
- 60.King AC, Cao D, O'Malley SS, Kranzler HR, Cai X, deWit H, Matthews AK, Stachoviak RJ. Effects of naltrexone on smoking cessation outcomes and weight gain in nicotine-dependent men and women. Journal of clinical psychopharmacology. 2012;32:630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Malley SS, Cooney JL, Krishnan-Sarin S, Dubin JA, McKee SA, Cooney NL, Blakeslee A, Meandzija B, Romano-Dahlgard D, Wu R, Makuch R, Jatlow P. A controlled trial of naltrexone augmentation of nicotine replacement therapy for smoking cessation. Arch Intern Med. 2006;166:667–674. [DOI] [PubMed] [Google Scholar]
- 62.de Laat B, Nabulsi N, Huang Y, O'Malley SS, Froehlich JC, Morris ED, Krishnan-Sarin S. Occupancy of the kappa opioid receptor by naltrexone predicts reduction in drinking and craving. Mol Psychiatry. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.