Pathological Potential of Neuroglia
The pathological potential of neuroglia was widely recognised and acknowledged by neurologists and neuroanatomists at end of the nineteenth and the beginning of the twentieth century. Contribution of neuroglia to the diseases was described, and numerous pathological morphological types of glial cells have been characterised in detail (Achucarro 1910; Alzheimer 1910; Frommann 1878; Nissl 1899). By 1920 the universal involvement of neuroglia in neuropathology was universally accepted; neurologists agreed that “the appearance of neuroglia serves as a delicate indicator of the action of noxious influences upon the central nervous system” (del Rio-Hortega and Penfield 1927); the concept of reactive gliosis has been formulated and generally recognised (del Río-Hortega and Penfield 1927; Penfield 1928b). The widespread role and importance of neuroglia in neurological and neuropsychiatric diseases were somewhat forgotten in the course of the twentieth century. However, the recently passed decade witnessed much revival in the interest in glia in neuropathology as the neuroglial cells are firmly considered as key players in pathophysiology of all disorders of the nervous system (both central and peripheral), and neuropharmacology regards neuroglia as a legitimate target for new therapeutic strategies (Burda and Sofroniew 2014; Ferrer 2018; Giaume et al. 2007; Parpura et al. 2012; Pekny et al. 2016; Sofroniew 2014b; Verkhratsky et al. 2012b, 2016a, 2017; Verkhratsky and Parpura 2016; Zeidan-Chulia et al. 2014).
Astrogliopathology: General Principles
Astrocytes are primary homeostatic cells of the central nervous system (CNS; see previous chapter); in addition, astrocytes contribute to brain defence. Astrocytic contribution to neuropathology can be primary (when cell-autonomous changes drive the pathologic progression) or secondary, when astrocytes respond to lesions or to various pathological changes in the nervous tissue. Current classification (Fig. 1) distinguishes the following forms of astrogliopathology: (i) reactive astrogliosis, (ii) astrocytic atrophy with loss of function, (iii) pathological remodelling of astrocytes and (iv) astrodegeneration (Fig. 1, (Verkhratsky et al. 2017). These pathological groups cover multiple pathological phenotypes which are yet to be fully characterised; furthermore pathological changes in astrocytes can occur together or in isolation; they are sometimes specific to disease stages and they are affected by age and systemic pathologies.
Fig. 1.
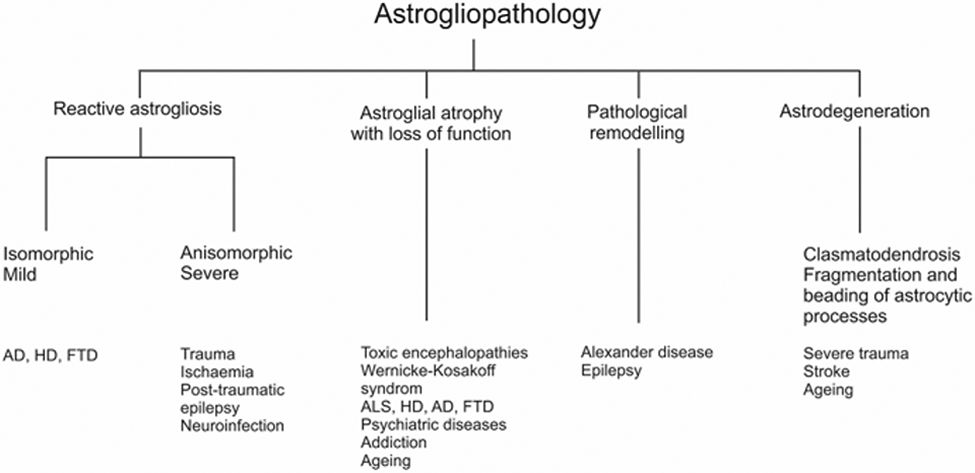
Classification of astrocytic pathological changes. AD Alzheimer’s disease, ALS amyotrophic lateral sclerosis, FTD fronto-temporal dementia, HD Huntington’s disease
Reactive Astrogliosis
Reactive astrogliosis is a specific and evolutionary conserved (from arthropods to humans) response of astrocytes to polyaetiological brain lesions, from trauma and infection to neurodegeneration. Reactive astrogliosis is a process whereby, in response to pathology, astrocytes launch genetic programmes that result in biochemical, morphological, metabolic and physiological remodelling (Escartin et al. 2021). This remodelling leads to either gain or loss or modification of astrocytic functions, all aimed at neuroprotection and preservation of the nervous tissue integrity. Astrogliotic remodelling of astrocytes leads to an emergence of multiple context-specific reactive phenotypes, characteristic for particular, age, type of pathology and brain region. These multiple phenotypes differ in specific molecular profile, functions and distinct impact on diseases (Pekny et al. 2016; Sofroniew 2014a; Sofroniew 2020; Verkhratsky et al. 2017). Reactive astrogliosis is flexible to adapt functional and biochemical reprogramming of astrocytes to the nature and strength of the insult with an ultimate goal to mount maximal protection. Within the framework of the same pathology and even within the same affected areas, astrocytes remain heterogeneous in their expression of transcription factors, inflammatory agents and signalling molecules, arguably associated with distinct reactive phenotypes (Garcia et al. 2010; Herrmann et al. 2008).
Reactive astrogliosis contributes to many neurological diseases. In particular, prominent astrogliosis occurs in disorders associated with direct lesion to the nervous tissue by physical, biological or chemical agents. These conditions include neurotrauma (Burda et al. 2016; Faulkner et al. 2004), systemic inflammation and sepsis (Shulyatnikova and Verkhratsky 2019; Tremblay et al. 2020), microbial or viral neuroinfection (Soung and Klein 2018; Zorec et al. 2019), toxic encephalopathies (Li et al. 2021; O’Callaghan et al. 2014), autoimmune inflammation of the nervous tissue including multiple sclerosis (Voskuhl et al. 2009; Wheeler and Quintana 2019), cancerous growth (Henrik Heiland et al. 2019) and neurodegenerative diseases (Verkhratsky et al. 2010). Histopathologically reactive astrogliosis is characterised by morphological hypertrophy, changes in the thickness of processes, sometimes associated with retraction of distal leaflets (Plata et al. 2018); furthermore, reactivity is manifested by an up-regulation of two major cytoskeletal intermediate filaments, glial fibrillary acidic protein (GFAP) and vimentin (Hol and Pekny 2015; Pekny and Pekna 2014; Sofroniew 2014a).
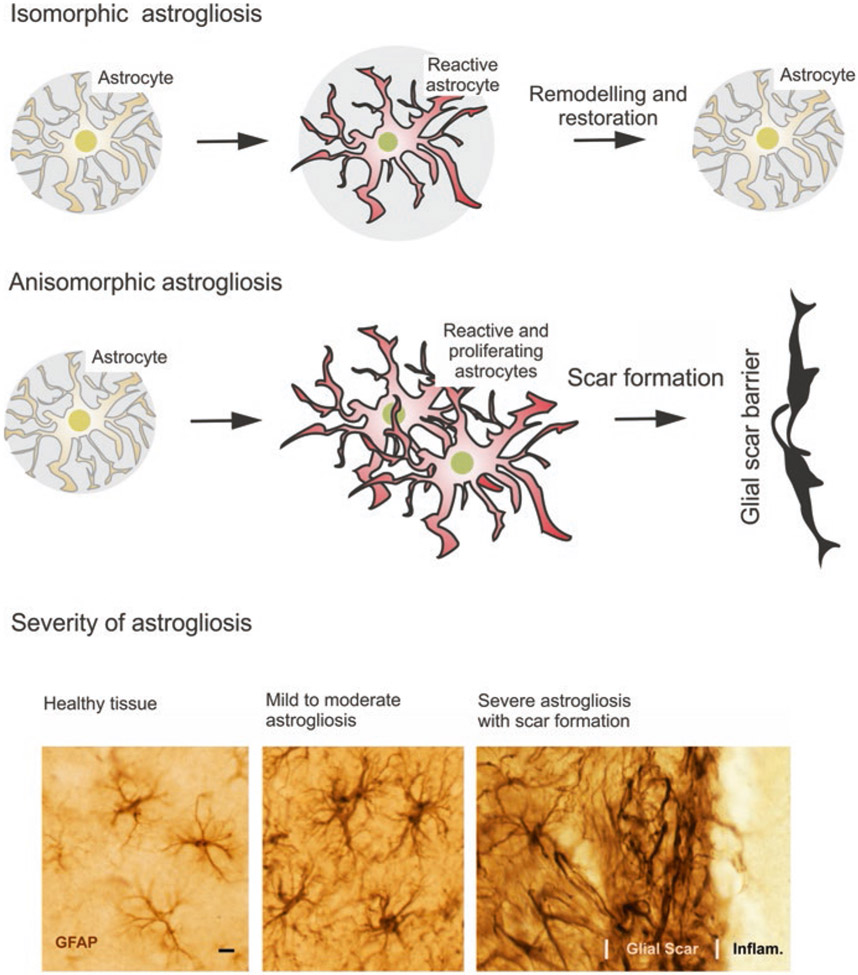
There are several classifications of reactive astrogliosis (Fig. 2). According to morphological changes, astrogliosis is classified into isomorphic and anisomorphic astrogliosis. In isomorphic astrogliosis, astrocytes become hypertrophic; however, they do not move and do not proliferate and the reach of their individual territorial domains remains unchanged (Escartin et al. 2021; Wilhelmsson et al. 2006). Isomorphic astrogliosis is fully reversible, and after the resolution of pathology, astrocytes return to physiological morphology; the isomorphic astrogliosis is indispensable for post-lesion regeneration (Anderson et al. 2016). In anisomorphic astrogliosis astrocytes start to proliferate, and they migrate towards the site of lesion, assemble into astroglial palisades and form the glial scar (Pekny et al. 2016; Sofroniew 2020). Another classification divides astrogliosis according to the severity of changes. According to this classification astrogliosis is classified into (i) mild to moderate astrogliosis, (ii) severe diffuse astrogliosis and (iii) severe astrogliosis with compact scar formation (Sofroniew 2009, 2014a). Although morphological presentation of reactive astrocytes can be similar in different pathological contexts generally following the above classification, their molecular signatures are quite distinct and disease-specific. Different astrocytic transcriptomes associate with different conditions and diseases including neurotrauma (Anderson et al. 2016), stroke (Zamanian et al. 2012), animal models of multiple sclerosis (Itoh et al. 2018) or neurodegenerations; in the latter group astrocytes in Huntington’s disease (Al-Dalahmah et al. 2020) are distinct from astrocytes in Alzheimer’s disease (Kamphuis et al. 2015). Similarly, astrocytic reactive phenotypes can be different in different stages of the same disease (Wheeler et al. 2020; Zamanian et al. 2012).
Fig. 2.
Classification of reactive astrogliosis; see text for explanation. (Modified from Verkhratsky and Butt (2013) and Sofroniew (2009))
Fundamentally, astrogliosis is a defensive response of astrocytes aimed at (i) neuroprotection and trophic support of neural cells tissue, (ii) isolation of the lesioned area, (iii) reconstruction of the compromised blood-brain barrier and (iv) facilitating the post-lesion regeneration of the nervous tissue (Sofroniew 2020). The ultimate result of severe astrogliosis, the scar formation, is essentially defensive reponse to isolate the damaged part of the nervous tissue and save the whole at the expense of its part (Pekny et al. 2016; Verkhratsky et al. 2017; Verkhratsky and Butt 2013). Inhibition of astroglial reactivity often exacerbates the damage to the nervous tissue and worsens neurological outcomes (Pekny et al. 2016). For example, suppression of astrogliotic response increases the size of the traumatic lesions and augments neurological deficit (Okada et al. 2006). Genetic deletion of GFAP and vimentin, both of which are critical for mounting reactive astrocyte remodelling, facilitates the evolution of brain ischaemia (Li et al. 2008) and potentiates posttraumatic synaptic loss (Pekny et al. 1999). Furthermore, inhibition of astroglial reactivity results in higher accumulation of β-amyloid and reduced microglial association with senile plaques in the animal model of Alzheimer’s disease (AD); all these changes seem to exacerbate AD-type pathology (Kraft et al. 2013).
Reactive astrogliosis is instigated by multiple factors. Conceptually, astrocytes may sense and integrate numerous molecular cues that signal the damage and provide some information about the nature of this damage. Such molecular cues can have multiple origin and nature. They can be released by damaged cells, they can be associated with accumulation of pathological material (β-amyloid being a well-known example), they can be blood-borne (blood cells or proteins, such as albumin or thrombin) and they can be associated with invading pathogens (bacteria, viruses or prions) or systemic immune factors. Astrogliosis can also be stimulated by certain neurotransmitters and hormones (Fig. 3; (Pekny et al. 2016, Sofroniew 2020)). Conceptually, all factors associated with the instigation of astrogliosis can be classified into (Tang et al. 2012) damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs). The DAMPs are molecules released from immune-responsive microglia or other stressed, damaged or dying cells or factors coming from the circulation through the compromised blood-brain barrier. These factors may include cytokines, chemokines, endothelins, blood-borne proteins, etc. Astrocytes express a wide pattern of receptors that can be activated by DAMPs (Verkhratsky and Nedergaard 2018). The archetypal DAMP is represented by ATP, which is massively released from damaged cells; in pathological contexts, ATP mainly acts on astrocytes through activation of P2X7 purinoceptors, although other classes of purinoceptors may also contribute (Franke et al. 2012). The PAMPs are exogenous agents associated with pathogens such as bacteria, viruses or prions; these factors stimulate Toll-like receptors (TLRs) widely expressed in astrocytes (Jack et al. 2005; Kielian 2006). In addition, astrocytes express nucleotide-binding oligomerisation domain (NOD)-like receptors (NLRs), double-stranded RNA-dependent protein kinase, scavenger receptors, mannose receptor and receptors for complement components and mediators, such as CXCL10, CCL2, interleukin-6 and B-cell-activating factor of the TNF family, all of which are contributing to the regulation of reactive astrogliosis (Farina et al. 2007).
Fig. 3.
Instigators of reactive astrogliosis. Numerous agents, including damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPS, pathogens), the former originating from various cells in the nervous tissue or from blood. All these agents can activate various astrocytic receptors which launch astrogliotic programmes. Abbreviations: TNF-α tumour necrosis factor α, INF-γ interferon γ, TGF-β transforming growth factor β, FGF fibroblast growth factor, IFG insulin growth factor, NO nitric oxide, ROS reactive oxygen species, LPS lipopolysaccharide
Intracellularly, initiation of reactive astrogliosis is associated with Ca2+ signalling. This signalling is an important part of astrocytic intracellular excitability, mediated by cytosolic ions and second messengers (Verkhratsky et al. 2020b, c). Exposure of astrocytes to various DAMPs and PAMPs is frequently associated with initiation of Ca2+ signals mainly originating from Ca2+ release from the intracellular endoplasmic reticulum (ER) Ca2+ store. This release is mediated by inositol-1,4,5,-trisphosphate (InsP3) receptor type 2, which is predominant in astrocytes (Verkhratsky et al. 2012a). Similarly, pharmacological inhibition of Ca2+ release from the ER suppressed astrocytic reactivity in response to β-amyloid (Alberdi et al. 2013)
Despite being an intrinsically defensive response, reactive astrocytes may, in certain conditions, acquire maladaptive features which may exacerbate or even cause damage to the nervous tissue (Pekny et al. 2016; Sofroniew 2020). First, astrocytic reactivity may interfere and downregulate essential homeostatic functions such as K+ buffering or glutamate homeostasis. In particular, failure of glutamate homeostasis seems to be a converging point in the pathophysiology of various neurological diseases, such as toxic encephalopathies (Li et al. 2021), hepatic encephalopathy (Montana et al. 2014; Obara-Michlewska et al. 2015), epilepsy (Bedner et al. 2015) or amyotrophic lateral sclerosis (Rossi et al. 2008; Valori et al. 2014). In addition, reactive astrocytes may be associated with the release of potentially damaging molecules through pathological gain of function, when existing homeostatic cascades start to overproduce particular agents. For example, in Alzheimer’s disease astrocytes overexpress monoamine oxidase-B (MAO-B) to produce GABA from puterscin; this overproduction of GABA counteracts neuronal hyperexcitability closely associated with AD progression (Garaschuk and Verkhratsky 2019; Ghatak et al. 2019). Increase in MAO-B activity, however, results in overproduction of hydrogen peroxide that initiates neuronal damage and death (Chun et al. 2020). Similarly, astrocytic overproduction of complement C3 (which otherwise is a legitimate physiological ligand) leads to morphological and functional neuronal defects (Lian et al. 2015).
To summarise, reactive astrogliosis is an intrinsic physiological astrocyte programme aimed at neuroprotection, at maintenance of tissue homeostasis and at preservation of integrity of nervous tissue. In certain conditions, however, and in particular in conditions of chronic and severe stress, reactive astrocytes may acquire maladaptive properties contributing to the damage of the CNS. In both conditions, reactive astrocytes remain an important part of disease progression often defining the neurological outcome of neuropathological process.
Pathological Remodelling of Astrocytes
The second group of astrogliopathologies is represented by pathological remodelling of astrocytes. This class of pathological changes covers astrocytic abnormalities associated with an acquisition of aberrant molecular cascades or functional properties, which drive pathology (Ferrer 2018; Pekny et al. 2016). The best examples of pathological astrocytic remodelling are represented by primary genetic astrogliopathies linked to expression of mutated genes. Alexander disease, a genetic leukomalacia, stems from astrocytic expression of sporadically mutated GFAP gene, which affects, in a yet unknown way, astrocyte function which ultimately results in severe damage to the white matter (Messing et al. 2012). Another example of pathological remodelling of astrocytes occurs in Duchenne muscular dystrophy (DMD) associated with expression of mutated dystrophin gene. Although major clinical presentation of DMD is associated with muscular weakness and cardiomyopathy, most of the patients show psychosocial abnormalities and impaired cognitive abilities. In the CNS dystrophin is expressed mainly in astrocytes (Hendriksen et al. 2016), and its mutations are linked to aberrant CNS cytoarchitecture, abnormalities in dendrites and loss of neurones. All these cytopathologies cause a general detrimental neurobehavioural profile, including attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorders and obsessive-compulsive disorder (Anderson et al. 2012; Hendriksen et al. 2018; Ricotti et al. 2015). At the cellular level, expression of mutant dystrophin gene resulted in an aberrant cytoskeleton arrangement and deficient homeostatic capabilities of astrocytes derived from stem cells isolated from DMD patients. In particular, glutamate clearance was severely affected in these astrocytes (Patel et al. 2019). Astroglial pathological remodelling is also central for several other leukodystrophies including vanishing white matter disease, megalencephalic leukoencephalopathy with subcortical cysts and Aicardi-Goutières syndrome (Brignone et al. 2014; Dooves et al. 2016; Jorge and Bugiani 2019). Finally, pathological remodelling of astrocytes has been suggested to occur in mesial temporal lobe epilepsy, characterised by aberrant astrocytic morphology, reduced gap junctional coupling and downregulation of Kir4.1 channel expression; all these changes converge into deficient K+ homeostasis that facilitates generation of seizures (Bedner et al. 2015).
Astroglial Atrophy, Asthenia and Loss of Function
This class of pathological changes includes cell-autonomous astrocytic changes, which do not involve reactivity (i.e. they are not instigated by lesion to the CNS) while being associated with diminished astrocytic function. First, this astrocytic insufficiency is linked to cellular atrophy manifested by decrease in astrocytic morphological profile, with corresponding decrease in astrocytic territorial domain and diminished astrocytic synaptic coverage. This morphological atrophy associated with decrease astrocytic homeostatic support is observed in numerous neuropathological contexts. In particular, morphological atrophy of astrocytes has been detected in diseases of cognition such as neurodegenerative diseases (Heneka et al. 2010; Rodriguez et al. 2009; Verkhratsky et al. 2010) and psychiatric diseases (Dietz et al. 2020; Verkhratsky et al. 2014; Verkhratsky and Parpura 2016; Windrem et al. 2017). Major neuropsychiatric disorders, such as schizophrenia, major depressive disorder and addictive disorders are all associated with reduction of astrocytic density and decrease in astrocytic morphological profiles as revealed by multiple markers (Cotter et al. 2001; Czeh and Di Benedetto 2013; Czeh and Nagy 2018; Miguel-Hidalgo 2009; Rajkowska et al. 2002; Rajkowska and Stockmeier 2013; Scofield et al. 2016). Another pathological feature, the astrocytic asthenia, which is manifested by failures of astroglial homeostatic cascades, is also frequently present in diseases of the brain. In particular, severe decrease in glutamate clearance due to ~80% decrease in expression of astrocytic plasmalemmal glutamate transporters is a leading cause of Wernicke-Korsakoff encephalopathy, associated with massive excitotoxic neuronal death (Hazell 2009; Hazell et al. 2009). Deficits in astroglial glutamate clearance and failure in glutamate-glutamine/GABA shuttle are likely responsible for abnormal neurotransmission as well as for excitotoxic neuronal death, both resulting in psychotic symptoms (Sanacora and Banasr 2013). Decreased expression of plasmalemmal glutamate transporters and decreased glutamate clearance from the extracellular space/synaptic cleft are common features of many addictive disorders, with astrocytic plasmalemmal glutamate transporters representing a promising drug target (Roberts-Wolfe and Kalivas 2015). Neuronal death in amyotrophic lateral sclerosis similarly reflects astrocytic loss of function being a consequence of insufficient astroglial function in extracellular glutamate clearance (Rossi et al. 2008; Valori et al. 2014).
Atrophy of astrocytes linked to decreased synaptic connectivity and synaptic efficacy contributes to cognitive deficiency in both normal ageing and senile dementia. Ageing is the main risk factor for neurodegenerative diseases underlying senile dementia, including Alzheimer’s disease. At the same time normal physiological brain ageing with mostly preserved cognitive capacity differs fundamentally from neurodegenerative pathology: in the former the number of neurones is largely preserved, whereas in the latter neurones undergo massive death, which underlies severe cognitive impairment (Pakkenberg and Gundersen 1997; Verkhratsky et al. 2004; von Bartheld et al. 2016; West 1993). Astrocytic numbers seem to be preserved in physiological ageing, whereas the data on astrocytic morphology are controversial and detailed analysis of astrocytic profiles is scarce (Olabarria et al. 2010; Pakkenberg and Gundersen 1997; Verkhratsky et al. 2020a). Most of our knowledge of the state of astrocytes in the ageing brain rests on the analysis of the expression of GFAP, the presumed universal marker of astrocytes (Hol and Pekny 2015). Expression of GFAP is generally increased in the aged brain, which was considered as a sign of astrogliosis and age-dependent inflammation (David et al. 1997; Goss et al. 1991; Hardy et al. 2018; Nichols et al. 1993). Morphometry of aged astrocytes, however, revealed rather contradictory results with both increase and decrease in size and complexity of GFAP-positive astrocytic profiles being observed (see Verkhratsky et al. (2020a) for details and references). All these results, however, need a critical revisit, because GFAP is not an ideal marker of astrocytes (see Verkhratsky and Nedergaard (2018) for detailed discussion). First, in a healthy brain, the majority of astrocytes do not express GFAP at the level of immunocytochemical detection. Second, the proportion of GFAP-positive cells depends on age and brain region. Third, increases in GFAP immunorecativity does not necessarily report reactive changes; in the suprachiasmatic nucleus and the intergeniculate leaflet, for example, GFAP expression undergoes substantial circadian changes (Moriya et al. 2000). Fourth, GFAP labels only cytoskeleton associated with primary astrocytic processes; the peripheral leaflets are always GFAP-negative, and therefore GFAP cannot accurately reveal astrocytic morphology. Finally, GFAP expression changes under various types of environmental stimulation: physical exercise or environmental enrichment increases GFAP-positive profiles, and this increase is beneficial for nervous tissue (Diniz et al. 2016; Rodriguez et al. 2013; Sampedro-Piquero et al. 2014). Thus, age-dependent changes in GFAP expression and GFAP-positive profiles do not reveal much about astrocytic ageing.
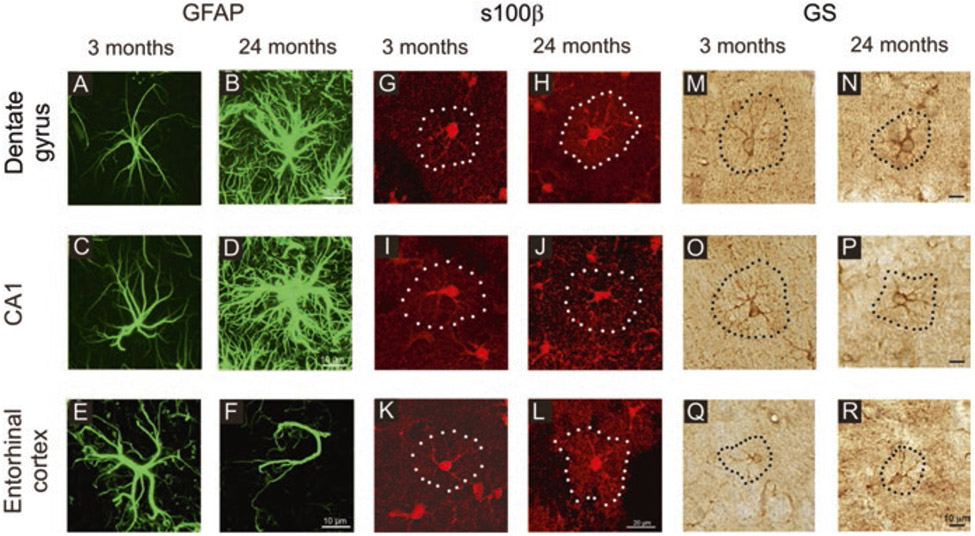
Labelling of astrocytes with other markers showed more complex age-dependent changes. Staining of astrocytes with Golgi black reaction did not identify age-dependent morphological changes (Castiglioni Jr. et al. 1991). Immunohistochemical analysis of astroglial profiles labelled with antibodies against GFAP, glutamine synthetase and protein s100β demonstrated complex region- and marker-dependent and age-dependent changes ranging from atrophy to hypertrophy (Rodriguez et al. 2014). The GFAP-labelled astrocytes showed hypertrophy in the CA1 region and in the dentate gyrus of old hippocampus but marked atrophy in the entorhinal cortex (EC). Astrocytes positive for glutamine synthetase were smaller in old hippocampus but larger in the old entorhinal cortex, while s100β-positive profiles from old animals demonstrated an increase in the entorhinal cortex and almost no change in the dentate gyrus and no changes in the CA1 region (Fig. 4).
Fig. 4.
Age-dependent remodelling of astroglial profiles in different brain areas. Confocal images showing glial fibrillary acidic protein (GFAP) (A to F), s100β (G to L) and glutamine synthetase (GS) (M to R) immunolabelled astrocytes in the dentate gyrus and CA1 hippocampal areas as well as in the entorhinal cortex of mice at 3 and 24 months. (Reproduced, with permission from Verkhratsky et al. (2020a))
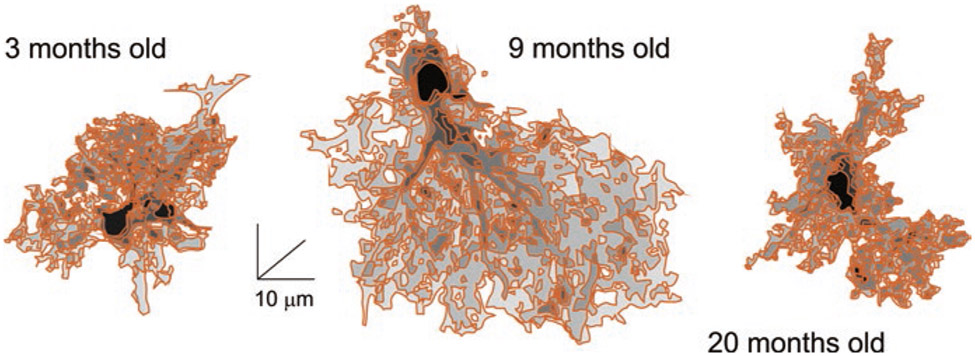
Morphology of astrocytes probed with intracellular injection of the fluorescent dye Alexa Fluor® 594 revealed age-dependent changes in astrocytic morphology. Two-photon imaging with subsequent 3D reconstruction of astrocytes perfused with the dye showed a significant increase in the size and complexity of astrocytes in development from youth to adulthood, whereas astrocytes in the old brains were smaller and less complex and significant decrease in size and complexity of astrocytes in old animals, with substantial reduction on the volume of peripheral processes (Fig. 5, (Popov et al. 2020)). These changes in peripheral processes affected synaptic coverage and synaptic homeostasis; in particular, astrocytic extracellular glutamate and K+ clearance are both compromised in old animals, leading to depression of long-term potentiation reflecting on deficient memory (Popov et al. 2020).
Fig. 5.
Reconstructions of hippocampal protoplasmic astrocytes from young, adult and old mice. (Reproduced, with permission from Verkhratsky et al. (2020a))
Astrocytic atrophy is also present in neurodegenerative diseases. In AD, atrophic astrocytes appear in the brain together with reactive astrocytes. Subpopulations of atrophic astrocytes have been found in transgenic AD mouse models (Beauquis et al. 2013; Olabarria et al. 2010) and confirmed in stem cell-derived astrocytes from AD patients in vitro (Jones et al. 2017; Mohamet et al. 2018) and in vivo in derived astrocytes grafted in the mouse brain (Preman et al. 2020). In AD mouse models the total number of astrocytes does not change with age (Olabarria et al. 2010, 2011). At the same time at the early, pre-plaque, stages, astrocytes in entorhinal and prefrontal cortices and hippocampus demonstrate morphological atrophy (Beauquis et al. 2013; Kulijewicz-Nawrot et al. 2012; Olabarria et al. 2010; Yeh et al. 2012). There is a specific temporal pattern in the emergence of atrophic astrocytes in mouse AD models (Rodriguez et al. 2016; Verkhratsky et al. 2019). First, atrophic astrocytic profiles (as visualised by antibodies against GFAP, s100β or glutamine synthetase) appear in the entorhinal cortex (they are present already in 1-month-old mice); subsequently, atrophic astrocytes appear in the prefrontal cortex (3–4 months of age) and finally in the hippocampus (in 6- to 9-month-old animals). Appearance of atrophic astrocytes thus precedes formation of β-amyloid deposits.
At the later stages (12- to 18-month-old animals) of AD, the emergence of β-amyloid plaques in the hippocampus instigates astrogliotic remodelling; reactive astrocytes migrate towards and surround senile plaques and β-amyloid infested blood vessels; at the same time atrophic astrocytes are positioned distantly to β-amyloid depositions (Olabarria et al. 2010; Verkhratsky et al. 2016b). Conversely, in entorhinal and prefrontal cortices, extracellular β-amyloid depositions are not accompanied with astroglial reactivity (Verkhratsky et al. 2016b). Failed astrogliosis represents a loss of function, which defines vulnerability of different brain regions to AD pathology. Indeed, in humans AD starts in entorhinal and prefrontal cortices before this disease spreads to the hippocampus.
Astrocytic atrophy and loss of function can contribute to AD pathophysiology being responsible for early synaptic dysfunction and cognitive deficits. Atrophic astrocytes provide diminished synaptic coverage, which translates in decreased support of synapses by astroglial cradle. First and foremost, this affects K+ buffering and glutamate homeostasis which both are critical for normal synaptic connectivity. In addition, astrocytes are fundamental for synaptogenesis not only in the developing but also in the adult brain, and astrocytic atrophy may impair the formation of new synapses associated with learning and neuronal plasticity. Early stages of AD are associated with synaptopathy (Coleman et al. 2004; Terry 2000), which might be directly linked to diminished astrocytic support. Astroglial asthenia and loss of function may also account for deficient support associated with the lactate shuttle. Finally, a failure of astrogliotic defence together with a loss of homeostatic capacity of astrocytes (the glial paralysis) can be directly linked to neuronal death and brain atrophy clinically manifested as senile dementia (Verkhratsky et al. 2015).
Astrodegeneration or Clasmatodendrosis
Insults to the brain as well as chronic brain pathologies stress astrocytes, which can undergo degenerative changes and necrotic or apoptotic death. Morphologically, astrodegeneration is manifested by clasmatodendrosis (from Greek “kλάσμα”, fragment, “δένδρoν”, tree, “ωσις”, process). This process has been initially characterised by Alois Alzheimer and also described and named by Santiago Ramón y Cajal (Penfield 1928a). Clasmatodendrosis appears as fragmentation of astroglial processes, vanishing of distal processes, and swelling and vacuolisation of the cell body. Clasmatodendrosis has been visualised in vitro and in tissue, and it was observed in several forms of neuropathology including ischaemia, infectious encephalopathies, stroke, dementia and psychiatric diseases (Hulse et al. 2001; Sahlas et al. 2002; Tachibana et al. 2019). Clasmatodendrotic astrocytes have been also identified in the brains of old mice (Mercatelli et al. 2016).
Envoi
We have outlined the presently ascribed roles of astroglia in nervous system pathology. As per human need to organize and stratify, we pigeonholed the roles into the present-day classification of astrogliopathology. While we have no doubt that this classification will develop further, likely by the time one reads these lines as the volume gets published, we deem it necessary and sufficient for further discussion of more detailed chapters that follow in this volume and delve into the role of astroglia in a variety of psychiatric conditions and diseases.
Acknowledgements
BL’s work is supported by the National Natural Science Foundation of China (grant number 8187185), LiaoNing Revitalization Talents Program (grant number XLYC1807137), Scientific Research Foundation for Returned Scholars of Education Ministry of China (grant number 20151098), LiaoNing Thousand Talents Program (grant number 202078) and “ChunHui” Program of Education Ministry of China (grant number 2020703). CS’s work is supported by a grant from the Italian Ministry of Education, University and Research (2015KP7T2Y_002) and a grant from Sapienza University of Rome (RM11916B7A8D0225). VP’s work is supported by a grant from the National Institute of General Medical Sciences of the National Institutes of Health (R01GM123971). VP is an Honorary Professor at University of Rijeka, Croatia.
Contributor Information
Alexei Verkhratsky, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK; Achucarro Center for Neuroscience, IKERBASQUE, Basque Foundation for Science, Bilbao, Spain.
Baoman Li, Practical Teaching Center, School of Forensic Medicine, China Medical University, Shenyang, China.
Caterina Scuderi, Department of Physiology and Pharmacology “Vittorio Erspamer”, SAPIENZA University of Rome, Rome, Italy.
Vladimir Parpura, Department of Neurobiology, The University of Alabama at Birmingham, Birmingham, AL, USA.
References
- Achucarro N (1910) Some pathological findings in the neuroglia and in the ganglion cells of the cortex in senile conditions. Bull Gov Hosp Insane 2:81–90 [Google Scholar]
- Al-Dalahmah O, Sosunov AA, Shaik A, Ofori K, Liu Y, Vonsattel JP, Adorjan I, Menon V, Goldman JE (2020) Single-nucleus RNA-seq identifies Huntington disease astrocyte states. Acta Neuropathol Commun 8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberdi E, Wyssenbach A, Alberdi M, Sanchez-Gomez MV, Cavaliere F, Rodriguez JJ, Verkhratsky A, Matute C (2013) Ca2+ -dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid beta-treated astrocytes and in a model of Alzheimer’s disease. Aging Cell 12:292–302 [DOI] [PubMed] [Google Scholar]
- Alzheimer A (1910) Beiträge zur Kenntnis der pathologischen Neuroglia und ihrer Beziehungen zu den Abbauvorgängen im Nervengewebe. In: Nissl F, Alzheimer A (eds) Histologische und histopathologische Arbeiten über die Grosshirnrinde mit besonderer Berücksichtigung der pathologischen Anatomie der Geisteskrankheiten, vol 3. Gustav Fischer, Jena, pp 401–562 [Google Scholar]
- Anderson JL, Head SI, Morley JW (2012) Duchenne muscular dystrophy and brain function. In: Muscular dystrophy. InTech; [DOI] [PubMed] [Google Scholar]
- Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauquis J, Pavia P, Pomilio C, Vinuesa A, Podlutskaya N, Galvan V, Saravia F (2013) Environmental enrichment prevents astroglial pathological changes in the hippocampus of APP transgenic mice, model of Alzheimer’s disease. Exp Neurol 239:28–37 [DOI] [PubMed] [Google Scholar]
- Bedner P, Dupper A, Huttmann K, Muller J, Herde MK, Dublin P, Deshpande T, Schramm J, Haussler U, Haas CA, Henneberger C, Theis M, Steinhauser C (2015) Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain 138:1208–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignone MS, Lanciotti A, Visentin S, De Nuccio C, Molinari P, Camerini S, Diociaiuti M, Petrini S, Minnone G, Crescenzi M, Laudiero LB, Bertini E, Petrucci TC, Ambrosini E (2014) Megalencephalic leukoencephalopathy with subcortical cysts protein-1 modulates endosomal pH and protein trafficking in astrocytes: relevance to MLC disease pathogenesis. Neurobiol Dis 66:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Bernstein AM, Sofroniew MV (2016) Astrocyte roles in traumatic brain injury. Exp Neurol 275(Pt 3):305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81:229–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglioni AJ Jr, Legare ME et al. (1991) Morphological changes in astrocytes of aging mice fed normal or caloric restricted diets. Age 14:102–106 [Google Scholar]
- Chun H, Im H, Kang YJ, Kim Y, Shin JH, Won W, Lim J, Ju Y, Park YM, Kim S, Lee SE, Lee J, Woo J, Hwang Y, Cho H, Jo S, Park JH, Kim D, Kim DY, Seo JS, Gwag BJ, Kim YS, Park KD, Kaang BK, Cho H, Ryu H, Lee CJ (2020) Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H2O2− production. Nat Neurosci 23:1555–1566 [DOI] [PubMed] [Google Scholar]
- Coleman P, Federoff H, Kurlan R (2004) A focus on the synapse for neuroprotection in Alzheimer disease and other dementias. Neurology 63:1155–1162 [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I (2001) Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry 58:545–553 [DOI] [PubMed] [Google Scholar]
- Czeh B, Di Benedetto B (2013) Antidepressants act directly on astrocytes: evidences and functional consequences. Eur Neuropsychopharmacol 23:171–185 [DOI] [PubMed] [Google Scholar]
- Czeh B, Nagy SA (2018) Clinical findings documenting cellular and molecular abnormalities of glia in depressive disorders. Front Mol Neurosci 11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JP, Ghozali F, Fallet-Bianco C, Wattez A, Delaine S, Boniface B, Di Menza C, Delacourte A (1997) Glial reaction in the hippocampal formation is highly correlated with aging in human brain. Neurosci Lett 235:53–56 [DOI] [PubMed] [Google Scholar]
- del Río-Hortega P, Penfield WG (1927) Cerebral cicatrix: the reaction of neuroglia and microglia to brain wounds. Bull Johns Hopkins Hosp 41:278–303 [Google Scholar]
- Dietz AG, Goldman SA, Nedergaard M (2020) Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry 7:272–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz DG, de Oliveira MA, de Lima CM, Foro CA, Sosthenes MC, Bento-Torres J, da Costa Vasconcelos PF, Anthony DC, Diniz CW (2016) Age, environment, object recognition and morphological diversity of GFAP-immunolabeled astrocytes. Behav Brain Funct 12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooves S, Bugiani M, Postma NL, Polder E, Land N, Horan ST, van Deijk AL, van de Kreeke A, Jacobs G, Vuong C, Klooster J, Kamermans M, Wortel J, Loos M, Wisse LE, Scheper GC, Abbink TE, Heine VM, van der Knaap MS (2016) Astrocytes are central in the pathomechanisms of vanishing white matter. J Clin Invest 126:1512–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A Steinhauser C, Volterra A, Carmignoto G, Agarwal A, Allen NJ, Araque A, Luis Barbeito L, Barzilai A, Bergles DE, Bonvento G, Butt AM, Chen W-T, Cohen-Salmon M, Cunningham C, Deneen B, De Strooper B, Díaz-Castro B, Farina C, Freeman M, Gallo V, Goldman JE, Goldman SA, Götz M, Gutiérrez A, Haydon PG, Heiland DH, Hol EM, Holt MG, Iino M, Kastanenka KV, Kettenmann H, Khakh BS, Koizumi S, Lee CJ, Liddelow SA, MacVicar BA, Magistretti P, Messing A, Mishra A, Molofsky AV, Murai K, Norris CM, Okada S, SHR O, Oliveira JF, Panatier A, Parpura V, Pekna M, Pekny M, Pellerin L, Perea G, Pérez-Nievas BG, Pfrieger FW, Poskanzer KE, Quintana FJ, Ransohoff RM, Riquelme-Perez M, Robel S, Rose CR, Rothstein J, Rouach N, Rowitch DH, Semyanov A, Sirko S, Sontheimer H, Swanson RA, Vitorica J, Wanner I-B, Wood LB, Wu J, Zheng B, Zimmer ER, Zorec R, Sofroniew MV, Verkhratsky A (2021) Reactive astrocyte nomenclature, definitions and future directions. Nat Neurosci 24(3):312–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28:138–145 [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24:2143–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I (2018) Astrogliopathy in Tauopathies. Neuroglia 1:126–150 [Google Scholar]
- Franke H, Verkhratsky A, Burnstock G, Illes P (2012) Pathophysiology of astroglial purinergic signalling. Purinergic Signal 8:629–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommann C (1878) Untersuchungen über die Gewebsveränderungen bei der Multiplen Sklerose des Gehirns und Rückenmarks. Verlag von Gustav Fischer, Jena [Google Scholar]
- Garaschuk O, Verkhratsky A (2019) GABAergic astrocytes in Alzheimer’s disease. Aging (Albany NY) 11:1602–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AD, Petrova R, Eng L, Joyner AL (2010) Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J Neurosci 30:13597–13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak S, Dolatabadi N, Trudler D, Zhang X, Wu Y, Mohata M, Ambasudhan R, Talantova M, Lipton SA (2019) Mechanisms of hyperexcitability in Alzheimer’s disease hiPSC-derived neurons and cerebral organoids vs isogenic controls. Elife 8:e50333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Kirchhoff F, Matute C, Reichenbach A, Verkhratsky A (2007) Glia: the fulcrum of brain diseases. Cell Death Differ 14:1324–1335 [DOI] [PubMed] [Google Scholar]
- Goss JR, Finch CE, Morgan DG (1991) Age-related changes in glial fibrillary acidic protein mRNA in the mouse brain. Neurobiol Aging 12:165–170 [DOI] [PubMed] [Google Scholar]
- Hardy RN, Simsek ZD, Curry B, Core SL, Beltz T, Xue B, Johnson AK, Thunhorst RL, Curtis KS (2018) Aging affects isoproterenol-induced water drinking, astrocyte density, and central neuronal activation in female Brown Norway rats. Physiol Behav 192:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell AS (2009) Astrocytes are a major target in thiamine deficiency and Wernicke’s encephalopathy. Neurochem Int 55:129–135 [DOI] [PubMed] [Google Scholar]
- Hazell AS, Sheedy D, Oanea R, Aghourian M, Sun S, Jung JY, Wang D, Wang C (2009) Loss of astrocytic glutamate transporters in Wernicke encephalopathy. Glia 58:148–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen RGF, Schipper S, Hoogland G, Schijns OEMG, Dings JTA, Aalbers MW, Vles JSH (2016) Dystrophin distribution and expression in human and experimental temporal lobe epilepsy. Front Cell Neurosci 10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen RGF, Vles JSH, Aalbers MW, Chin RFM, Hendriksen JGM (2018) Brain-related comorbidities in boys and men with Duchenne muscular dystrophy: a descriptive study. Eur J Paediatr Neurol 22:488–497 [DOI] [PubMed] [Google Scholar]
- Heneka MT, Rodriguez JJ, Verkhratsky A (2010) Neuroglia in neurodegeneration. Brain Res Rev 63:189–211 [DOI] [PubMed] [Google Scholar]
- Henrik Heiland D, Ravi VM, Behringer SP, Frenking JH, Wurm J, Joseph K, Garrelfs NWC, Strahle J, Heynckes S, Grauvogel J, Franco P, Mader I, Schneider M, Potthoff AL, Delev D, Hofmann UG, Fung C, Beck J, Sankowski R, Prinz M, Schnell O (2019) Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat Commun 10:2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV (2008) STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci 28:7231–7243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol EM, Pekny M (2015) Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol 32:121–130 [DOI] [PubMed] [Google Scholar]
- Hulse RE, Winterfield J, Kunkler PE, Kraig RP (2001) Astrocytic clasmatodendrosis in hippocampal organ culture. Glia 33:169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Itoh Y, Tassoni A, Ren E, Kaito M, Ohno A, Ao Y, Farkhondeh V, Johnsonbaugh H, Burda J, Sofroniew MV, Voskuhl RR (2018) Cell-specific and region-specific transcriptomics in the multiple sclerosis model: focus on astrocytes. Proc Natl Acad Sci U S A 115:E302–E309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP (2005) TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 175:4320–4330 [DOI] [PubMed] [Google Scholar]
- Jones VC, Atkinson-Dell R, Verkhratsky A, Mohamet L (2017) Aberrant iPSC-derived human astrocytes in Alzheimer’s disease. Cell Death Dis 8:e2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge MS, Bugiani M (2019) Astroglia in leukodystrophies. Adv Exp Med Biol 1175:199–225 [DOI] [PubMed] [Google Scholar]
- Kamphuis W, Kooijman L, Orre M, Stassen O, Pekny M, Hol EM (2015) GFAP and vimentin deficiency alters gene expression in astrocytes and microglia in wild-type mice and changes the transcriptional response of reactive glia in mouse model for Alzheimer’s disease. Glia 63:1036–1056 [DOI] [PubMed] [Google Scholar]
- Kielian T (2006) Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res 83:711–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AW, Hu X, Yoon H, Yan P, Xiao Q, Wang Y, Gil SC, Brown J, Wilhelmsson U, Restivo JL, Ciriito JR, Holtzman DM, Kim J, Pekny M, Lee JM (2013) Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB J 27:187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulijewicz-Nawrot M, Verkhratsky A, Chvatal A, Sykova E, Rodriguez JJ (2012) Astrocytic cytoskeletal atrophy in the medial prefrontal cortex of a triple transgenic mouse model of Alzheimer’s disease. J Anat 221:252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Xia M, Zorec R, Parpura V, Verkhratsky A (2021) Astrocytes in heavy metal neurotoxicity and neurodegeneration. Brain Res 1752:147234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M (2008) Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab 28:468–481 [DOI] [PubMed] [Google Scholar]
- Lian H, Yang L, Cole A, Sun L, Chiang AC, Fowler SW, Shim DJ, Rodriguez-Rivera J, Taglialatela G, Jankowsky JL, Lu HC, Zheng H (2015) NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron 85:101–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercatelli R, Lana D, Bucciantini M, Giovannini MG, Cerbai F, Quercioli F, Zecchi-Orlandini S, Delfino G, Wenk GL, Nosi D (2016) Clasmatodendrosis and beta-amyloidosis in aging hippocampus. FASEB J 30:1480–1491 [DOI] [PubMed] [Google Scholar]
- Messing A, Brenner M, Feany MB, Nedergaard M, Goldman JE (2012) Alexander disease. J Neurosci 32:5017–5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ (2009) The role of glial cells in drug abuse. Curr Drug Abuse Rev 2:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamet L, Jones VC, Dayanithi G, Verkhratsky A (2018) Pathological human astroglia in Alzheimer’s disease: opening new horizons with stem cell technology. Future Neurology 13:87–99 [Google Scholar]
- Montana V, Verkhratsky A, Parpura V (2014) Pathological role for exocytotic glutamate release from astrocytes in hepatic encephalopathy. Curr Neuropharmacol 12:324–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya T, Yoshinobu Y, Kouzu Y, Katoh A, Gomi H, Ikeda M, Yoshioka T, Itohara S, Shibata S (2000) Involvement of glial fibrillary acidic protein (GFAP) expressed in astroglial cells in circadian rhythm under constant lighting conditions in mice. J Neurosci Res 60:212–218 [DOI] [PubMed] [Google Scholar]
- Nichols NR, Day JR, Laping NJ, Johnson SA, Finch CE (1993) GFAP mRNA increases with age in rat and human brain. Neurobiol Aging 14:421–429 [DOI] [PubMed] [Google Scholar]
- Nissl F (1899) Über einige Beziehungen zwischen Nervenzellerkrankungen und gliösen Erscheinungen bei verschiedenen Psychosen. Arch Psychiatr 32:1–21 [Google Scholar]
- O’Callaghan JP, Kelly KA, VanGilder RL, Sofroniew MV, Miller DB (2014) Early activation of STAT3 regulates reactive astrogliosis induced by diverse forms of neurotoxicity. PLoS One 9:e102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara-Michlewska M, Ruszkiewicz J, Zielinska M, Verkhratsky A, Albrecht J (2015) Astroglial NMDA receptors inhibit expression of Kir4.1 channels in glutamate-overexposed astrocytes in vitro and in the brain of rats with acute liver failure. Neurochem Int 88:20–25 [DOI] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H (2006) Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12:829–834 [DOI] [PubMed] [Google Scholar]
- Olabarria M, Noristani HN, Verkhratsky A, Rodriguez JJ (2010) Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia 58:831–838 [DOI] [PubMed] [Google Scholar]
- Olabarria M, Noristani HN, Verkhratsky A, Rodriguez JJ (2011) Age-dependent decrease in glutamine synthetase expression in the hippocampal astroglia of the triple transgenic Alzheimer’s disease mouse model: mechanism for deficient glutamatergic transmission? Mol Neurodegener 6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ (1997) Neocortical neuron number in humans: effect of sex and age. J Comp Neurol 384:312–320 [PubMed] [Google Scholar]
- Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A (2012) Glial cells in (patho)physiology. J Neurochem 121:4–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AM, Wierda K, Thorrez L, van Putten M, De Smedt J, Ribeiro L, Tricot T, Gajjar M, Duelen R, Van Damme P, De Waele L, Goemans N, Tanganyika-de Winter C, Costamagna D, Aartsma-Rus A, van Duyvenvoorde H, Sampaolesi M, Buyse GM, Verfaillie CM (2019) Dystrophin deficiency leads to dysfunctional glutamate clearance in iPSC derived astrocytes. Transl Psychiatry 9:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallen A, Perlmann T, Lendahl U, Betsholtz C, Berthold CH, Frisen J (1999) Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol 145:503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Pekna M (2014) Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 94:1077–1098 [DOI] [PubMed] [Google Scholar]
- Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol 131:323–345 [DOI] [PubMed] [Google Scholar]
- Penfield W (1928a) Neuroglia and microglia-the interstitial tissue of the central nervous system. In: Cowdry EV (ed) Special cytology, the form and function of the cell in health and disease. Hoeber, New York, pp 1033–1068 [Google Scholar]
- Penfield WG (1928b) Neuroglia and microglia. the interstitial tissue of the central nervous system. In: Cowdry EV (ed) Special cytology, vol 2. Paul B. Hober Inc., New York, pp 1033–1068 [Google Scholar]
- Plata A, Lebedeva A, Denisov P, Nosova O, Postnikova TY, Pimashkin A, Brazhe A, Zaitsev AV, Rusakov DA, Semyanov A (2018) Astrocytic atrophy following status epilepticus parallels reduced Ca2+ activity and impaired synaptic plasticity in the rat hippocampus. Front Mol Neurosci 11:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov A, Brazhe A, Denisov P, Sutyagina O, Lazareva N, Verkhratsky A, Semyanov A (2020) Astrocytes dystrophy in ageing brain parallels impaired synaptic plasticity. Aging Cell 20(3):e13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preman P, Julia TCW, Calafate S, Snellinx A, Alfonso-Triguero M, Corthout N, Munck S, Thal DT, Goate AM, De Strooper B, Arranz AM (2020) Human iPSC-derived astrocytes transplanted into the mouse brain display three morphological responses to amyloid-β plaques. bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Makkos Z, Meltzer H, Overholser J, Stockmeier C (2002) Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophr Res 57:127–138 [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Stockmeier CA (2013) Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14:1225–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricotti V, Mandy WPL, Scoto M, Pane M, Deconinck N, Messina S, Mercuri E, Skuse DH, Muntoni F (2015) Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev Med Child Neurol 58:77–84 [DOI] [PubMed] [Google Scholar]
- Roberts-Wolfe DJ, Kalivas PW (2015) Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets 14:745–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Butt AM, Gardenal E, Parpura V, Verkhratsky A (2016) Complex and differential glial responses in Alzheimer’s disease and ageing. Curr Alzheimer Res 13:343–358 [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Olabarria M, Chvatal A, Verkhratsky A (2009) Astroglia in dementia and Alzheimer’s disease. Cell Death Differ 16:378–385 [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Terzieva S, Olabarria M, Lanza RG, Verkhratsky A (2013) Enriched environment and physical activity reverse astrogliodegeneration in the hippocampus of AD transgenic mice. Cell Death Dis 4:e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Yeh CY, Terzieva S, Olabarria M, Kulijewicz-Nawrot M, Verkhratsky A (2014) Complex and region-specific changes in astroglial markers in the aging brain. Neurobiol Aging 35:15–23 [DOI] [PubMed] [Google Scholar]
- Rossi D, Brambilla L, Valori CF, Roncoroni C, Crugnola A, Yokota T, Bredesen DE, Volterra A (2008) Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ 15:1691–1700 [DOI] [PubMed] [Google Scholar]
- Sahlas DJ, Bilbao JM, Swartz RH, Black SE (2002) Clasmatodendrosis correlating with periventricular hyperintensity in mixed dementia. Ann Neurol 52:378–381 [DOI] [PubMed] [Google Scholar]
- Sampedro-Piquero P, De Bartolo P, Petrosini L, Zancada-Menendez C, Arias JL, Begega A (2014) Astrocytic plasticity as a possible mediator of the cognitive improvements after environmental enrichment in aged rats. Neurobiol Learn Mem 114:16–25 [DOI] [PubMed] [Google Scholar]
- Sanacora G, Banasr M (2013) From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry 73:1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Li H, Siemsen BM, Healey KL, Tran PK, Woronoff N, Boger HA, Kalivas PW, Reissner KJ (2016) Cocaine self-administration and extinction leads to reduced glial fibrillary acidic protein expression and morphometric features of astrocytes in the nucleus accumbens core. Biol Psychiatry 80:207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulyatnikova T, Verkhratsky A (2019) Astroglia in sepsis associated encephalopathy. Neurochem Res 45(1):83–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2014a) Astrogliosis. Cold Spring Harb Perspect Biol 7:a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2014b) Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist 20:160–172 [DOI] [PubMed] [Google Scholar]
- Sofroniew MV (2020) Astrocyte reactivity: subtypes, states, and functions in CNS innate immunity. Trends Immunol 41:758–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soung A, Klein RS (2018) Viral encephalitis and neurologic diseases: focus on astrocytes. Trends Mol Med 24:950–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Mohri I, Hirata I, Kuwada A, Kimura-Ohba S, Kagitani-Shimono K, Fushimi H, Inoue T, Shiomi M, Kakuta Y, Takeuchi M, Murayama S, Nakayama M, Ozono K, Taniike M (2019) Clasmatodendrosis is associated with dendritic spines and does not represent autophagic astrocyte death in influenza-associated encephalopathy. Brain Dev 41:85–95 [DOI] [PubMed] [Google Scholar]
- Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT (2012) PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev 249:158–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD (2000) Cell death or synaptic loss in Alzheimer disease. J Neuropathol Exp Neurol 59:1118–1119 [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Madore C, Bordeleau M, Tian L, Verkhratsky A (2020) Neuropathobiology of COVID-19: the role for glia. Front Cell Neurosci 14:592214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valori CF, Brambilla L, Martorana F, Rossi D (2014) The multifaceted role of glial cells in amyotrophic lateral sclerosis. Cell Mol Life Sci 71:287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Augusto-Oliveira M, Pivoriunas A, Popov A, Brazhe A, Semyanov A (2020a) Astroglial asthenia and loss of function, rather than reactivity, contribute to the ageing of the brain. Pflugers Arch 12:1–22 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Butt AM (2013) Glial physiology and pathophysiology. Wiley-Blackwell, Chichester [Google Scholar]
- Verkhratsky A, Marutle A, Rodriguez-Arellano JJ, Nordberg A (2015) Glial asthenia and functional paralysis: a new perspective on neurodegeneration and Alzheimer’s disease. Neuroscientist 21:552–568 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Mattson MP, Toescu EC (2004) Aging in the mind. Trends Neurosci 27:577–578 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M (2018) Physiology of astroglia. Physiol Rev 98:239–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Olabarria M, Noristani HN, Yeh CY, Rodriguez JJ (2010) Astrocytes in Alzheimer’s disease. Neurotherapeutics 7:399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Parpura V (2016) Astrogliopathology in neurological, neurodevelopmental and psychiatric disorders. Neurobiol Dis 85:254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Rodrigues JJ, Pivoriunas A, Zorec R, Semyanov A (2019) Astroglial atrophy in Alzheimer’s disease. Pflugers Arch 471:1247–1261 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Rodriguez JJ, Parpura V (2012a) Calcium signalling in astroglia. Mol Cell Endocrinol 353:45–56 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Rodriguez JJ, Steardo L (2014) Astrogliopathology: a central element of neuropsychiatric diseases? Neuroscientist 20:576–588 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Semyanov A, Zorec R (2020b) Physiology of astroglial excitability. Function 1:zqaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Sofroniew MV, Messing A, deLanerolle NC, Rempe D, Rodriguez JJ, Nedergaard M (2012b) Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro 4:AN20120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Steardo L, Parpura V, Montana V (2016a) Translational potential of astrocytes in brain disorders. Prog Neurobiol 144:188–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Untiet V, Rose CR (2020c) Ionic signalling in astroglia beyond calcium. J Physiol 598:1655–1670 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Zorec R, Parpura V (2017) Stratification of astrocytes in healthy and diseased brain. Brain Pathol 27:629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Zorec R, Rodriguez JJ, Parpura V (2016b) Astroglia dynamics in ageing and Alzheimer’s disease. Curr Opin Pharmacol 26:74–79 [DOI] [PubMed] [Google Scholar]
- von Bartheld CS, Bahney J, Herculano-Houzel S (2016) The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comp Neurol 524:3865–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV (2009) Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 29:11511–11522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ (1993) Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging 14:287–293 [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Clark IC, Tjon EC, Li Z, Zandee SEJ, Couturier CP, Watson BR, Scalisi G, Alkwai S, Rothhammer V, Rotem A, Heyman JA, Thaploo S, Sanmarco LM, Ragoussis J, Weitz DA, Petrecca K, Moffitt JR, Becher B, Antel JP, Prat A, Quintana FJ (2020) MAFG-driven astrocytes promote CNS inflammation. Nature 578:593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MA, Quintana FJ (2019) Regulation of astrocyte functions in multiple. Sclerosis Cold Spring Harb Perspect Med 9(1):a029009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M (2006) Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A 103:17513–17518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Osipovitch M, Liu Z, Bates J, Chandler-Militello D, Zou L, Munir J, Schanz S, McCoy K, Miller RH, Wang S, Nedergaard M, Findling RL, Tesar PJ, Goldman SA (2017) Human iPSC glial mouse chimeras reveal Glial contributions to schizophrenia. Cell Stem Cell 21(195-208):e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CY, Vadhwana B, Verkhratsky A, Rodriguez JJ (2012) Early astrocytic atrophy in the entorhinal cortex of a triple transgenic animal model of Alzheimer’s disease. ASN Neuro 3:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32:6391–6410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan-Chulia F, Salmina AB, Malinovskaya NA, Noda M, Verkhratsky A, Moreira JC (2014) The glial perspective of autism spectrum disorders. Neurosci Biobehav Rev 38:160–172 [DOI] [PubMed] [Google Scholar]
- Zorec R, Zupanc TA, Verkhratsky A (2019) Astrogliopathology in the infectious insults of the brain. Neurosci Lett 689:56–62 [DOI] [PubMed] [Google Scholar]