Abstract
With the industrial development and progressive increase in environmental pollution, the mankind overexposure to heavy metals emerges as a pressing public health issue. Excessive intake of heavy metals, such as arsenic (As), manganese (Mn), mercury (Hg), aluminium (Al), lead (Pb), nickel (Ni), bismuth (Bi), cadmium (Cd), copper (Cu), zinc (Zn), and iron (Fe), is neurotoxic and it promotes neurodegeneration. Astrocytes are primary homeostatic cells in the central nervous system. They protect neurons against all types of insults, in particular by accumulating heavy metals. However, this makes astrocytes the main target for heavy metals neurotoxicity. Intake of heavy metals affects astroglial homeostatic and neuroprotective cascades including glutamate/GABA-glutamine shuttle, antioxidative machinery and energy metabolism. Deficits in these astroglial pathways facilitate or even instigate neurodegeneration. In this review, we provide a concise outlook on heavy metal-induced astroglio-pathies and their association with major neurodegenerative disorders. In particular, we focus on astroglial mechanisms of iron-induced neurotoxicity. Iron deposits in the brain are detected in main neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis. Accumulation of iron in the brain is associated with motor and cognitive impairments and iron-induced histopathological manifestations may be considered as the potential diagnostic biomarker of neurodegenerative diseases. Effective management of heavy metal neurotoxicity can be regarded as a potential strategy to prevent or retard neurodegenerative pathologies.
Keywords: Heavy metals, Astrocytes, Neurotoxicity, Neurodegeneration, Glutamate
1. Introduction
Humans are exposed to pathological load of metals through contaminated food, contaminated environment (water and air) or through occupational exposure (Liu et al., 2019a; Bai et al., 2019; Jan et al., 2015; Qu et al., 2012; Sarah et al., 2019); excess of metals in the body often results in neurotoxicity and associated neurological disorders. Excessive accumulation of metallic elements, such as arsenic (As), manganese (Mn), mercury (Hg), lead (Pb), aluminium (Al), nickel (Ni), bismuth (Bi), cadmium (Cd), zinc (Zn), copper (Cu) and iron (Fe), are known to be neurotoxic and increase the risk for neurodegenerative diseases, particularly of Alzheimer’s disease (AD) and Parkinson’s disease (PD) (Supino-Viterbo et al., 1977; Kirkley et al., 2017; Pamphlett and Kum, 2018; Rahman et al., 2019; Ijomone et al., 2020; Sudhakaran et al., 2019).
Metal poisioning has been recognized throughout history of mankind. Lead toxicity, which results in memory impairments, has been known since antiquity (Waldron, 1973), while adverse effects caused by water drinking from lead-furnished water pipes have been proposed as a biological background for the fall of the Roman empire (Nriagu, 1983). The first clinical description of an acute Pb poisoning that included paralysis was produced by Nicander of Colophon in the 2nd century BCE (Waldron, 1973). Chronic mercury poisoning caused erethism mercurialis (also known as mad hatter syndrome in the 17th century France) characterised with psychotic symptoms and impaired memory (O’Carroll et al., 1995). Mercury poisoning resulting in neurodegeneration was observed on the shores of Minamata bay, which gave the name to Minamata disease (McAlpine and Araki, 1958). Today, metal pollution of food chains rise significant concerns (Rahman et al., 2014; Wang et al., 2017; Wu et al., 2013). In particular iron oxides are ubiquitous in both natural and industrial environments, with iron being fundamental for metabolic and physiological activities. At the same time, a loss of iron homeostasis or an excessive load with iron (resulting from dietary intake or from medical procedures such as iron implants or usage of iron oxide nanoparticles) is harmful for health (Okereafor et al., 2020; Li et al., 2020; Vogel et al., 2016; Xia et al., 2020a; 2020b).
Metal toxicity affects the brain development and impairs cognition, memory and learning in humans and in animal models (Hussien et al., 2018; Mason et al., 2014). Clinically, chronic superfluous intake of metals usually instigate neurological symptoms such as dizziness, headaches, motor impairments and cognitive and memory deficits (Wu et al., 2013; Peres et al., 2015). These symptoms may also signal the beginning of a neurodegenerative process (Bauer et al., 2015; Buckner, 2004), which results from integration of multiple factors, including genetic background, adverse lifestyle and environmental pressures (Eid et al., 2019). Levels of some heavy metals such as Mn, Hg and Cd are increased in the plasma and cerebrospinal fluid (CSF) of AD-patients (Gerhardsson et al., 2008; Basun et al., 1991). Heavy metals have been reported to increase the presence of AD-relevant proteins, such as β-amyloid, Tau and ApoE4 both in vitro and in vivo (Moyano et al., 2020; Godfrey et al., 2003; Olivieri et al., 2000; Al Kahtani, 2020). In addition, heavy metals elevate β-amyloid load by decreasing the clearance of β-amyloid from the brain (Gu et al., 2011; Kim et al., 2014). Recent and growing evidence reports that disturbed iron homeostasis is an early presentation in the AD (Kim et al., 2018; Mandel et al., 2007), while the level of iron in the hippocampus and cortex of AD-patients is increased compared with healthy subjects (Corrigan et al., 1993; Gu et al., 1998). Abnormal level of iron causes the suppression of function of several enzymes that require iron as a co-modulator, forming toxic oxidizing species, and stimulating β-amyloidogenesis (Qian and Wang, 1998). Several common genetic polymorphisms that cause the aberrant iron homeostasis are frequently associated with AD (Crespo et al., 2014; Nandar and Connor, 2011), although the mechanistic links remain unclear. Environmental overexposure to Pb is another well known risk factor for AD (Chin-Chan et al., 2015). In animal models, the oral intake of excessive Pb increases the cerebral level of β-amyloid, as well as levels of the pro-inflammatory interleukin-1 (IL-1) and tumour necrosis factor α (TNF-α); these changes were associated with the impaired cognitive capacity (Li et al., 2014).
The over-intake of heavy metals represents a high risk for PD, resulting from the loss of dopaminergic neurones in the substantia nigra (Mu et al., 2020). Significant association between PD and exposure to Cu, Mn and Fe in workers with more than 20 years of occupational history has been identified in a population-based case-control study in Detroit (Gorell et al., 1997; Powers et al., 2003; Fukushima et al., 2013). In animal models, chronic exposure to Mn, Cd or Hg triggers neuroinflammation and impairs the function of mitochondria, thus, producing the PD-like neurological symptoms (Hammond et al., 2020; Zhang et al., 2017a; Qu et al., 2013; Han et al., 2017). Substantia nigra pars compacta has the highest levels of iron in the human brain and, hence, iron is considered as a risk factor for PD (Jiang et al., 2019). In PD patients, iron levels are increased in parietal and prefrontal cortices; increase in iron can be a predictor of poor cognitive outcome, and its elevation in the putamen predicts poorer motor function (Thomas et al., 2020). Overload with iron triggers production of reactive oxygen species (ROS) and pro-inflammatory factors, thus, further exacerbating neuroinflammation and brain pathology (Bjørklund et al., 2019; Heneka et al., 2010; Neal and Richardson, 2018).
Astrocytes are the homeostatic cells of the central nervous system (CNS); in particular they are fundamental for the ionostasis of the nervous tissue (Verkhratsky and Nedergaard, 2014, 2018). Through an extended family of plasmalemmal transporters astrocytes control concentrations of ions in the interstitial fluids (Verkhratsky and Rose, 2020; Rose and Verkhratsky, 2016). In particular, astroglial transporters remove excess of heavy metals from the brain parenchyma, thus, protecting neurones against toxicity. Of note, microglial cells also contribute to this protection (Zheng et al., 2010). Accumulation of heavy metals, however, damages astrocytes and affects their homoeostatic and neuroprotective cascades, of which most important are associated with glutamate-glutamine transport and anti-oxidative support.
2. Astrocytes glutamate homoeostatic cascade as the main target for metal neurotoxicity
2.1. The homeostasis of glutamate
Glutamate and γ-aminobutoric acid (GABA) are respectively major excitatory and inhibitory neurotransmitters in the brain. Both share the same biosynthetic pathway deriving from glucose, which makes catabolism of them strictly astroglia-dependent. Astrocytes are the only cells in the brain capable of producing de novo glutamate (and by proxy, GABA) from glucose (Hertz et al., 1999). The key enzymes for this process are pyruvate carboxylase (which produce α-ketoglutarate) and glutamine synthetase (which converts glutamate to glutamine); both these enzymes are expressed exclusively in astrocytes (Norenberg and Martinez-Hernandez, 1979; Schousboe et al., 2014; Shank et al., 1985; Rose et al., 2013). Glutamine is a non-toxic precursor for glutamate and hence it can be safely transported to neurones where it is converted (by phosphate-activated glutaminase) to glutamate in excitatory terminals (Hertz, 2013); in inhibitory terminals glutamate is converted to GABA (Rose et al., 2013). This final conversion is mediated by glutamate decarboxylase (Bak et al., 2006). These enzymatic cascades are coordinated with astroglial plasmalemmal transporters and operate in concert as an astroglial glutamate/GABA-glutamine shuttle, which controls extracellular levels of glutamate and supplies neurones with glutamine. Control over extracellular glutamate is of paramount importance to prevent glutamate excitotoxicity which appears as the major neuronal killer in conditions of brain pathology (Choi, 1992).
Glutamate, secreted during neurotransmission is taken up by astrocytes via sodium-dependent excitatory amino acid transporters 1 and 2 (EAAT1/SLC1A3 and EAAT2/SLC1A2, also known, in rodent experiments as glutamate-aspartate transporter GLAST and glutamate transporter-1, GLT1). These transporters are almost exclusively astroglial with some variations between brain regions (Danbolt, 2001; Zhou and Danbolt, 2013); activity of these transporters is regulated by transmembrane Na+ gradients (Kirischuk et al., 2007). Expression of glutamate transporters varies across the brain; the EAAT1 dominates cerebellum, retina and circumventricular organs (Lehre and Danbolt, 1998; Berger and Hediger, 2000; Rauen et al., 1996), whereas EAAT2 demonstrates higher expression in other regions. The average density of astroglial transporters is exceptionally high with EAAT1 reaching 4700/μm2 in Bergmann glia and 2300/μm2 in astrocytes of the CA1 hippocampal area; the density of EAAT2 is ~8500/μm2 in the hippocampus and ~740/μm2 in the cerebellum (Lehre and Danbolt, 1998). At the ultrastructural level most of transporters are concentrated at the peri-synaptic astroglial processes (Chaudhry et al., 1995). After entering astrocytes, glutamate is mainly converted to glutamine by glutamine synthetase; glutamine is subsequently transported to neurones. This transport is mediated by sodium-coupled neutral amino acid transporters; astroglial SNAT3/SLC38A3 and SNAT5/SLC38A5 mediate export of glutamine, while neuronal SNAT1/SLC38A1, SNAT2/SLC38A2 and SNAT4/SLC38A4 are responsible for glutamine import (Verkhratsky and Nedergaard, 2018; Verkhratsky and Rose, 2020). Proper functional activity of glutamate/GABA-glutamine shuttle is critical for neurotransmission and changes in expression or activity of its components lead to various pathologies including neurodegeneration (Rose et al., 2013).
2.2. Astrocytes define glutamate excitotoxicity
In pathological conditions, decreased expression and/or inefficiency of EAATs results in the increased level of extracellular glutamate with the subsequent excitotoxicity (Olloquequi et al., 2018). Mechanistically, an excess of extracellular glutamate depolarises neurones, which leads to opening of Ca2+ permeable NMDA receptors and voltage-gated Ca2+ channels, subsequently causing an overload of the cytoplasm with Ca2+. This pathological Ca2+ signalling initiates oxidative stress, mitochondrial damage, massive activation of proteolytic enzymes etc., ultimately instigating necrotic or apoptotic cell death (Peng and Jou, 2010; Martínez-Ruiz et al., 2011; Działo et al., 2013). Excitotoxicity contributes to neurodegenerative diseases. For example, neurotoxic β-amyloid may increase extracellular glutamate, thus, triggering pathological Ca2+ signalling (Busche et al., 2008; Kuchibhotla et al., 2008). Pharmacological inhibition of NMDA receptors with memantine was reported to normalise cognitive performance, decrease β-amyloid load and plaque deposition in clinical and animal studies of AD (Danysz and Parsons, 2012). In PD, the expression and function of EAAT1 is significantly decreased, thus, instigating excitotoxicity and subsequent death of dopaminergic neurones (Sominsky et al., 2015). Aberrant Ca2+ signalling and failed [Ca2+]i homeostasis induced by excitotoxicity can lead to the malfunction of mitochondrial bioenergetics and the increased level of ROS, damaging dopaminergic neurones (Cieri et al., 2017; Surmeier et al., 2017). Excitotoxicity is the key pathogenetic step in amyotrophic lateral sclerosis (ALS); in this pathology excessive glutamate in the extracellular space results from substantial down-regulation of astroglial EAAT2 transporters that results in the insufficient glutamate uptake (Mathis et al., 2017). Treatment with riluzole (2-amino-6-tri-fluoromethoxy benzothiazole; the only partially effective monotherapy in ALS) counteracts glutamate excitotoxicity by inhibiting glutamate release from presynaptic terminals and up-regulating expression of astroglial plasmalemmal glutamate transporters (Zarei et al., 2015; Carbone et al., 2012).
2.3. Heavy metals affect astroglial plasmalemmal glutamate transporters, hence, instigating neurodegeneration
In the brain, Mn ions (Mn2+/Mn3+) cross the blood-brain barrier (BBB) and are preferentially accumulated by astrocytes through the plasmalemmal divalent metal transporter-1 (DMT1) and by binding to and internalising with transferrin receptor (TFR) (Fitsanakis et al., 2006; 2007;; Erikson and Aschner, 2006). The main pathological effect of Mn is the disturbance of the glutamate/GABA-glutamine shuttle at multiple levels. Excess of intra-astroglial Mn decreases the activity of glutamine synthetase and down-regulates expression of EAAT1 and EAAT2 (Deng et al., 2012; Lee et al., 2013; Johnson et al., 2018). Suppression of plasmalemmal glutamate transporters expression in astrocytes is mediated by a transcription factor Yin Yang 1 (YY1) activated by the Mn-sensitive nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) cascade (Karki et al., 2014, 2015). As a result, dysfunctional glutamine catabolism and elevated extracellular glutamate induce excitotoxicity, neuronal damage and contribute to neurodegenerative process (Sidoryk-Wegrzynowicz and Aschner, 2013; Miyata et al., 1983; Lu et al., 2018). Astrocytic plasmalemmal glutamate transporters are similarly vulnerable to other heavy metals, such as Pb and Hg, both of which dysregulate glutamate homeostasis by inhibiting the expression and/or function of astroglial plasmalemmal glutamate transporters (Struzyńska et al., 2005). Hippocampal structures seem to be more sensitive to Pb, which evokes deficits in cognition and learning (Gilbert et al., 1999). Additionally, the overexposure to Hg inhibits the mRNA expression and the related functions of EAATs by stimulating astrocytic ROS (Allen et al., 2001; Mutkus et al., 2005). Similarly, arsenite inhibits glutamate clearance by suppressing expression of glutamine synthetase, EAAT-1 and EAAT2 in astrocytes; impairment of glutamate/GABA-glutamine shuttle represents a leading mechanism of As-induced neurotoxicity (Zhao et al., 2012).
3. Astroglial zink and neurodegeneration
Zinc is the second most abundant trace element in mammalian tissues and is indispensable for normal brain functions (Paoletti et al., 2009). Zinc dyshomeostasis is associated with several neurological disorders including depression, schizophrenia, AD, ALS and ageing-related cognitive decline (Levenson and Tassabehji, 2007; Grabrucker et al., 2011; Adlard et al., 2014). Astrocytes can rapidly accumulate zinc through zinc-transporters (ZnT) including Zn-regulated and iron-regulated transporter proteins 14 (ZIP14) and ZnT3, thus, protecting neurones against Zn toxicity (Nolte et al., 2004; Bishop et al., 2010; Sun et al., 2012). Over-accumulation of Zn in the brain can trigger the aggregation of β-amyloid and formation of associated senile plaques, thus contributing to the AD-type neurodegeneration (Hancock et al., 2014). Chronic exposure to Zn can promote the deposition of β-amyloid and an increase in S100A6 (an acidic Ca2+/Zn2+-binding protein) in APP/PS1 transgenic mice; however, exogenous S100A6 is capable of decreasing the aggregation of β-amyloid by buffering/binding Zn in astrocytes and attenuating the AD-related cognitive deficits (Tian et al., 2019).
4. Iron in brain pathology: the role of glia
Iron is the most abundant metal in the brain responsible for normal physiological functions and developmental processes (Ashraf et al., 2018). The level of iron is gradually increasing with ageing in the substantia nigra, in basal ganglia and cortex (Ramos et al., 2014), albeit the reason for this specific accumulation remains unknown. Excessive accumulation of iron in the brain has been regarded as the major high risk for the neurodegenerative diseases, including AD, PD and ALS (Belaidi and Bush, 2016; Liu et al., 2019b; Kwakye et al., 2019; Stephenson et al., 2014).
In physiological conditions, iron contributes to oxygen transportation, mitochondrial respiration, myelin formation, DNA replication and cell signalling (Dev and Babitt, 2017). Homeostatic iron control is fundamental for human health, because both the deficiency and overload of iron are harmful. Iron deficiency is one of the most abundant nutritional deficient diseases. The most common disease is the iron deficiency anaemia, which occurs in infants, adolescents, pregnant women and it appears in many clinical conditions, such as gastrectomy and inflammatory bowel disease (Wan et al., 2019). In contrast, iron overload usually develops in patients with chronic liver or kidney diseases, or results from the iatrogenic treatments including excessive therapeutic supplementation and haemodialysis, or caused by the excessive dietary intake and nutritional supplements (Rostoker and Vaziri, 2017a; 2017b;; Lu et al., 2020; Ceylan et al., 2019).
4.1. Iron transport and homeostasis in the brain
Iron from food comes in two forms, the heme iron and non-heme iron, with the latter accounting for 90% of the total iron. Non-heme iron is mainly taken in the brush border of duodenal enterocytes (Zhang et al., 2017b), the cytochrome b of the cellular membranes of these cells reduces Fe3+ to Fe2+, while the latter is transported by plasmalemmal DMT1. The heme iron absorption proceeds through the uptake of the heme carrier protein 1 (HCP-1) (Shayeghi et al., 2005; Krishnamurthy et al., 2007), while the export of the intracellular iron occurs through the ferroportin1 (Fpn1) (Troadec et al., 2010). After Fe2+ comes into the circulation, it is oxidized to Fe3+ by the ferroxidases including hephaestin (HEPH) and ceruloplasmin (CP); Fe3+ is transported by binding to transferrin (Tf) (Chen et al., 2004; Hellman and Gitlin, 2002). Several pathways translocate iron across the BBB: (i) TF-bound Fe3+ by virtue of TFR mainly crosses the luminal membrane of the endothelium; (ii) TF-bound Fe3+ can also be transported by trans-cytosis; (iii) Fe2+ is mainly transported by DMT1 localised on the luminal membrane of endothelial cells; (iv) Fpn1 is responsible for the export of iron from endothelial cells to the extracellular space of the brain parenchyma (Jiang et al., 2019; Qian and Ke, 2019). After entering the brain, iron binds to TF, which is mainly secreted by epithelial cells of the choroid plexus (Leitner and Connor, 2012). Compared with the peripheral tissues, the concentration of non-transferrin-bound iron (NTBI) is higher in the CSF and interstitial fluid (IF), because citrate and ascorbate secreted by astrocytes help to maintain iron in the reduced Fe2+ status (Knutson, 2019; Ji and Kosman, 2015). The glia limitans vascularis formed by astroglial endfeet covering blood vessels plays a crucial role in regulating iron homeostasis in the brain through expressing DMT1 to uptake Fe2+ (Dringen et al., 2007; Simpson et al., 2015). Early immunohistochemical studies failed to detect TFR or DMT1 is in the adult mouse astrocytes (Moos, 1996; Moos and Morgan, 2004). Recently, however, expression of TFR and DMT1 have been demonstrated in astrocytes in vitro as well as in vivo (Lis et al., 2004; Pelizzoni et al., 2013; Rathore et al., 2012; Urrutia et al., 2013; Zarruk et al., 2015; Xu et al., 2019; Qian and Wang, 1998; Hoepken et al., 2004). Therefore, astrocytes are able to accumulate Fe3+ and Fe2+ by the TFR and DMT1 pathways, respectively (Tulpule et al., 2010). In addition, the uptake of NTBI into astrocytes can be mediated by ZIP14 (Bishop et al., 2010).
4.2. Iron overload and neurodegeneration
Patients with PD have higher iron content in the substantia nigra pars compacta as seen by magnetic resonance imaging (MRI) (Ulla et al., 2013; Wieler et al., 2015). Similarly, iron is accumulated in neurones of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced monkey and mouse models of PD (He et al., 2003; Wang et al., 2009; Jiang et al., 2003; Youdim, 2003). Increase in iron concentration in dopaminergic neurones aggravates the oxidative stress in which iron reacts with H2O2 produced by dopamine metabolism with subseqeunet generation of OH− radicals that can damage proteins, nucleic acids, and membrane phospholipids (Dias et al., 2013; Melis et al., 2013). Furthermore, ROS can induce additional release of iron from mitochondria, thus further stimulating production of ROS (Ward et al., 2014). This vicious circle and excessive accumulation of ROS are arguably instrumental in instigating cellular death by oxidizing proteins (Melis et al., 2013; Ward et al., 2014). This type of cell death is known as ferroptosis, as the overload with iron causes the overwhelming oxidative damage and the lethal increase in lipid hydroperoxides (Reed and Pellecchia, 2012).
Abnormal iron accumulation is also considered as an early hallmark for AD. Iron elevation in the AD brain was first reported in 1953 (Goodman, 1953), and its association with β-amyloid and neurofibrillary tangles or with ferritin in peripheral glial cells was also documented (Kim et al., 2018; Mandel et al., 2007; Ward et al., 2014). The level of iron in deep grey matter and the neocortex reported by 3T MRI was higher in AD-patients when compared to healthy control subjects (Damulina et al., 2020). Elevated iron in AD-patients is also related to the degree of cognitive impairments (Derry and Kent, 2017). Abnormally increased iron in the brain of AD patients can interact with H2O2 to produce highly active hydroxyl radicals, which damage cellular structures (Levi and Tiranti, 2019). In AD patients, TFR is reported to be increased in the hippocampus (Morris et al., 1994), although the level of Fpn is reduced in many cerebral regions (Raha et al., 2013), which may indicate the enhanced uptake of iron which accumulates in in neuronal cells. However, the low levels of plasma iron are also reported in AD-patients (Faux et al., 2014; Camaschella, 2013), which may be attributed to the abnormal loading and desaturation of TF (Hare et al., 2015). Hence, the relationship between iron overload and AD pathology still requires further research.
Through MRI or quantitative susceptibility mapping (QSM), iron accumulation can also be observed in the motor cortex in ALS (Bhattarai et al., 2020; Ignjatović et al., 2013). Increased ferritin and the decreased TFR were reported in plasma of ALS patients (Mitchell et al., 2010; Qureshi et al., 2008). Similarly, increases in serum iron, ferritin and saturated TF were found in ALS patients (Veyrat-Durebex et al., 2014); serum ferritin is even considered as a biomarker for ALS progression (Yu et al., 2018). In addition, the level of iron is also increased in the CSF of ALS patients (Hozumi et al., 2011), which may translate into iron-induced oxidative stress and ROS generation (Ignjatović et al., 2012). Iron overload in the brain may stimulate neuroinflammation by activating TNF α converting enzyme (Lee et al., 2015). Finally, mutations of genes encoding the regulation related proteins of iron, such as homeostatic iron regulator (HFE) and SLC11A2 (encoding DMT1) genes, have been observed in ALS (Nandar et al., 2013; 2014; Blasco et al., 2011).
4.3. Iron toxicity and neuroglia
In the CNS, astrocytes accumulate Fe2+ through plasmalemmal transporters DMT1 and ZIP14 (Codazzi et al., 2015; Bishop et al., 2010). Astrocytes can also accumulate Fe3+ bound to TFR, the expression of which was found in cultured astrocytes (Zarruk et al., 2015) and recently confirmed in vivo (Xia et al., 2020a; 2020b). Astrocytes also contribute to maintaining the pool of Fe2+ in the brain by secreting acidic interstitial buffers (Hohnholt and Dringen, 2013; Pelizzoni et al., 2013; Ji and Kosman, 2015). Iron is stored as ferritin in astrocytes and is released by Fpn and the ferroxidase CP (Wu et al., 2004); the deficiency of CP can also lead to the iron overload in the brain and neurotoxicity (Jeong and David, 2003).
In iron-induced chronic seizure models, astrocytic EAAT expression is persistently decreased in the hippocampus, whereas the neuroactive androgen steroid dehydroepiandrosterone can exert an antiepileptic action by up-regulating these transporters (Mishra et al., 2013). With ageing, the permeability of the BBB is increased, and iron deposition is also increased in astrocytes from the cortex, hippocampus and basal ganglia (Farrall and Wardlaw, 2009; Block et al., 2007). However, iron overloaded astrocytes may become a trigger of neurotoxicity that contributes to the pathogenesis of age-dependent neurodegeneration, which involves iron-induced oxidative stress and mitochondrial malfunction (Dringen et al., 2007; Schipper et al., 2009). In the frontal cortex, iron dextran increases the glial fibrillary acidic protein (GFAP)-positive astroglial profiles (Liang et al., 2020), which is indicative of reactive astrogliosis (Fig. 1).
Fig. 1.
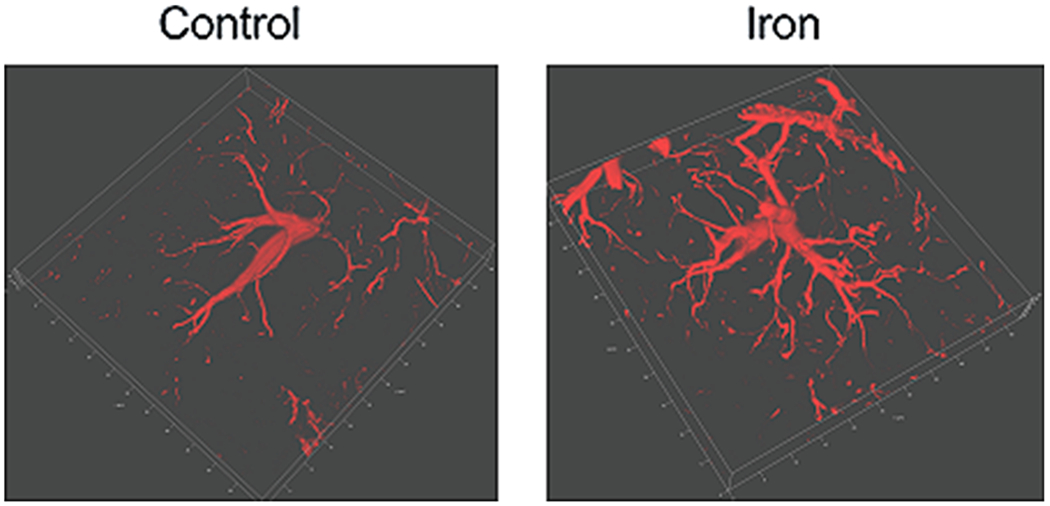
3D-images of GFAP labelled astrocytes in frontal cortex. After treatment with dextran (control) or 2 mg/kg/day iron dextran for 6 days, 3D-images of GFAP residing in astrocytes were taken in the mouse frontal cortex indicating a development of reactive astrogliosis.
As shown in Fig. 2, the iron overload can impair the brain-wide glymphatic system (Liang et al., 2020), responsible for the clearance of waste proteins through a paravascular pathway (Iliff and Nedergaard, 2013). In the mice model of depression induced by chronic unpredictable mild stress (CUMS), the function of glymphatic system is suppressed by down-regulation of the expression of the astrocytic water channel aquaporin 4 (AQP4) (Xia et al., 2017). Injection of iron dextran further worsens the operation of the glymphatic system and exacerbates the depressive-like behaviours induced by CUMS; in turn, this triggers neuronal apoptosis (Liang et al., 2020). Hence, the iron supplements for major depressive patients should be monitored. Coincidentally, iron oxide nanoparticles (widely applied in biological and medical fields) are also reported to cause toxic damage to human astrocytes (Valdiglesias et al., 2016; Coccini et al., 2017).
Fig. 2.
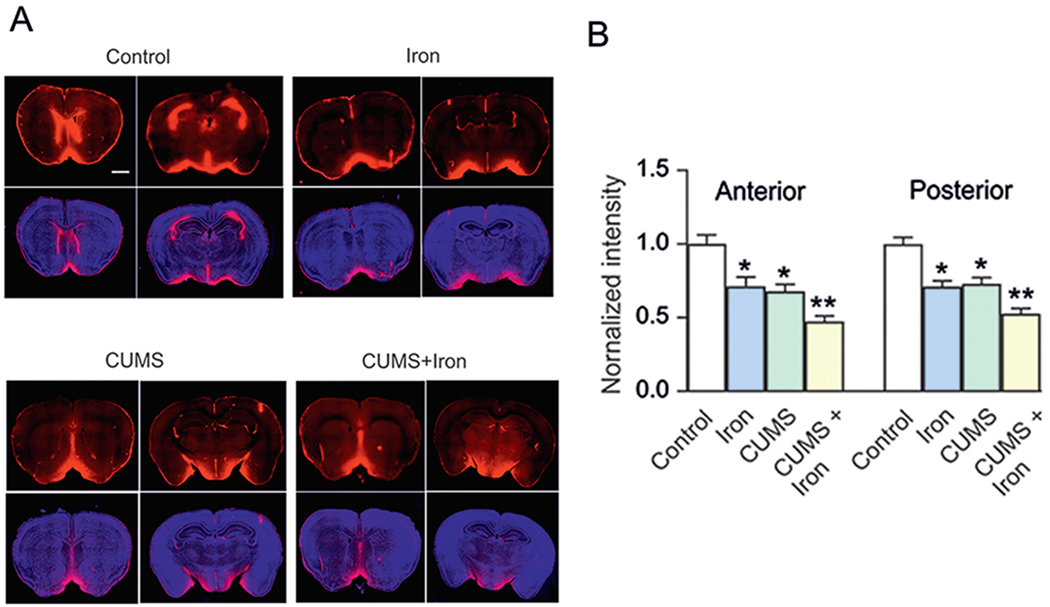
Excess iron aggravates malfunction of glymphatic system in chronic unpredictable mild stress-treated mice. (A, B) Mice were pre-treated with or without chronic unpredictable mild stress (CUMS) for 6 weeks; in the last week the mice were randomly separated to be injected with dextran or iron dextran for 6 days. The fluorescence tracer (OA555, 45 kDa) was injected intracisternally. (A) Representative images indicated the fluorescence tracer penetration into the brain; OA555 (red) and DAPI (blue; cell nuclei label) were stained simultaneously, in anterior and posterior brain slices. Scale bar, 1 mm. (B) Thirty minutes after injection, the animals were perfusion fixed and the whole-slice fluorescence was calculated. The fluorescence intensities of OA555 normalised to the intensity of the control group were assessed. Scale bar, 50 μm. Data are presented as mean ± SEM, n = 6. *p < 0.05, statistically significant difference compared to the control group. **p < 0.05, statistically significant difference compared to any other group (reproduced from Liang et al., 2020 with permission).
Microglial cells, responsible for innate brain immunity, are also involved in iron homeostasis. Microglia are the most efficient in accumulating iron in the brain, followed by astrocytes, and then neurones (Bishop et al., 2011). However, this may differ between brain regions (Reinert et al., 2019), because expression of iron transporters varies greatly in neural cells from different parts of the brain (Rouault, 2013). Astrocytes are well known to regulate the transport of iron to other neural cells (Dringen et al., 2007), and microglia supplies iron to oligodendrocytes to ensure their demand for this ion (Zhang et al., 2006). Accumulation of iron in microglia gradually increases with age through the elevation of ferritin (Lopes et al., 2008). Ferritin positive dystrophic microglia are associated with β-amyloid plaques and neuofibrillary tangles (Streit et al. 2014). Aberrant iron homeostasis increases the release of proinflammatory cytokines from microglia in vitro (Wang et al. 2013). Neuromelanin, a protein that stores iron in neurones can be phagocytosed by microglia, which can increase production of proinflammatory cytokines and ROS, thus exacerbating neurodegeneration (Rathnasamy et al. 2013). Accumulation of iron in microglia is consistently observed in the neurodegeneration-prone brain regions, providing a high correlation between the pathological phenomena of neurodegeneration and the increase of iron in microglia (Andersen et al. 2014).
5. Conclusion and future directions
Rising environmental contamination and increased presence of heavy metals in general life along with related neurotoxicity are gaining increasing attention. Astrocytes are key protectors of neurones in the CNS, but under the exposure to excessive heavy metals, astrocytes may become the main targets for metal toxicity. Heavy metals, such as Mn, Pb, Hg and iron, all can destroy the integrity of nervous tissue and affect glial-neuronal interactions. In particular, heavy metals severely disrupt glutamate homeostasis through affecting expression and efficacy of glutamate/GABA-glutamine shuttle at multiple levels including suppression of glutamine synthetase activity and down-regulation of plasmalemmal glutamate transporters. Heavy metal-induced glutamate excitotoxicity evokes pathological Ca2+ signalling and damages intracellular Ca2+ homoeostasis, triggers oxidative stress, destroys mitochondria, and instigates cell death. The neurotoxicity caused by heavy metals in astrocytes can play a deteriorative role in facilitating neurodegenerative diseases. Among these heavy metals, some are key trace elements for physiological and developmental processes, like iron, so its homoeostasis is essential for proper operation of the CNS. Iron overload in neurodegenerative disorders is widely reported, while MRI images support the accumulation of iron in brains of patients suffering from AD, PD and ALS. Iron-mediated neurotoxicity is also associated with reactive astrogliosis and impairments of th eglymphatic system, which may also contribute to the progression of neurodegenerative diseases.
Future research need to consider several issues:: (i) monitoring of heavy metals in the CNS should receive more attention, especially for metals of the iatrogenic origin; (ii) the reasons for the metals deposition in specific brain regions are little known, although these depositions may directly contribute to the occurrence of neurodegenerative diseases, such as PD; (iii) the ways to effectively reduce the neurotoxicity induced by heavy metals using antioxidants, anti-inflammation agents, and/or by (epi)genetic modulation; (iv) understanding the relationship between the accumulation of heavy metal(s) and brain ageing and whether the effective clearance of excessive metal can slow the ageing process and improve the cognitive longevity.
Acknowledgements
BL was supported by Grant No. 81871852 from the National Natural Science Foundation of China, Grant No. XLYC1807137 from LiaoNing Revitalization Talents Program, Grant No. 20151098 from the Scientific Research Foundation for Returned Scholars of Education Ministry of China, Grant No. 202078 from Liaoning BaiQianWan Talents Program, and Grant No. 2020703 from “ChunHui” Program of Education Ministry of China. RZ was supported by grants from the Slovenian Research Agency (P3 310, P1-0055, J3 4051, J3 4146, L3 3654; J3 3236, J3 6790, J3 6789, J3 7605). V.P.’s work is supported by a grant from the National Institute of General Medical Sciences of the National Institutes of Health (R01GM123971). VP is an Honorary Professor at University of Rijeka, Croatia.
References
- Adlard PA, Sedjahtera A, Gunawan L, Bray L, Hare D, Lear J, Doble P, Bush AI Finkelstein DI, Cherny RA, 2014. A novel approach to rapidly prevent age-related cognitive decline. Aging Cell 13 (2), 351–359. 10.1111/acel.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Kahtani MA, 2020. Effect of both selenium and biosynthesized nanoselenium particles on cadmium-induced neurotoxicity in albino rats. Hum. Exp. Toxicol 39 (2), 159–172. 10.1177/0960327119880589. [DOI] [PubMed] [Google Scholar]
- Allen JW, Mutkus LA, Aschner M, 2001. Methylmercury-mediated inhibition of 3H-D-aspartate transport in cultured astrocytes is reversed by the antioxidant catalase. Brain. Res 902 (1), 92–100. 10.1016/s0006-8993(01)02375-7. [DOI] [PubMed] [Google Scholar]
- Andersen HH, Johnsen KB, Moos T, 2014. Iron deposits in the chronically inflamed central nervous system and contributes to neurodegeneration. Cell Mol Life Sci. 71 (9), 1607–1622. 10.1007/s00018-013-1509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf A, Clark M, So PW, 2018. The aging of iron man. Front. Aging. Neurosci 10, 65. 10.3389/fnagi.2018.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Zhao Q, Wang W, Wang X, Jia J, Cui B, Liu X, 2019. Arsenic and heavy metals pollution along a salinity gradient in drained coastal wetland soils: depth distributions, sources and toxic risks. Ecol. Indie 96, 91–98. [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS, 2006. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 98 (3), 641–653. 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Basun H, Forssell LG, Wetterberg L, Winblad B, 1991. Metals and trace elements in plasma and cerebrospinal fluid in normal aging and Alzheimer’s disease. J. Neural. Transm. Park. Dis. Dement. Sect 3 (4, 231–258. [PubMed] [Google Scholar]
- Bauer E, Toepper M, Gebhardt H, Gallhofer B, Sammer G, 2015. The significance of caudate volume for age-related associative memory decline. Brain. Res 1622, 137–148. 10.1016/j.brainres.2015.06.026. [DOI] [PubMed] [Google Scholar]
- Belaidi AA, Bush AI, 2016. Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J. Neurochem 139 (Suppl. 1), 179–197. 10.1111/jnc.13425. [DOI] [PubMed] [Google Scholar]
- Berger UV, Hediger MA, 2000. Distribution of the glutamate transporters GLAST and GLT-1 in rat circumventricular organs, meninges, and dorsal root ganglia. J. Comp. Neurol 421 (3), 385–399. 10.1002/(sici)1096-9861(20000605)421:3385::aid-cne73.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Bhattarai A, Chen Z, Ward P, Talman P, Mathers S, Phan TG, Chapman C, Howe J, Lee S, Lie Y, Egan GF, Chua P, 2020. Serial assessment of iron in the motor cortex in limb-onset amyotrophic lateral sclerosis using quantitative susceptibility mapping. Quant. Imaging. Med. Surg 10 (7), 1465–1476. 10.21037/qims-20-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GM, Dang TN, Dringen R, Robinson SR, 2011. Accumulation of non-transferrin-bound iron by neurons, astrocytes, and microglia. Neurotox. Res 19 (3), 443–451. 10.1007/s12640-010-9195-x. [DOI] [PubMed] [Google Scholar]
- Bishop GM, Scheiber IF, Dringen R, Robinson SR, 2010. Synergistic accumulation of iron and zinc by cultured astrocytes. J. Neural. Transm. (Vienna) 117 (7), 809–817. 10.1007/s00702-010-0420-9. [DOI] [PubMed] [Google Scholar]
- Bjørklund G., Hofer T., Nurchi VM., Aaseth J., 2019. Iron and other metals in the pathogenesis of Parkinson’s disease: Toxic effects and possible detoxification. J. Inorg. Biochem 199 10.1016/j.jinorgbio.2019.110717. [DOI] [PubMed] [Google Scholar]
- Blasco H, Vourc’h P, Nadjar Y, Ribourtout B, Gordon PH, Guettard YO, Camu W, Praline J, Meininger V, Andres CR, Corcia P, French ALS study group 2011. Association between divalent metal transport 1 encoding gene (SLC11A2) and disease duration in amyotrophic lateral sclerosis. J. Neurol. Sci 303(1–2), 124–127. doi: 10.1016/j.jns.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS, 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci 8 (1), 57–69. 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Buckner RL, 2004. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 44 (1), 195–208. 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O, 2008. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321 (5896), 1686–1689. 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- Camaschella C, 2013. Iron and hepcidin: a story of recycling and balance. Hematology. Am. Soc. Hematol. Educ. Program 2013, 1–8. 10.1182/asheducation-2013.1.1. [DOI] [PubMed] [Google Scholar]
- Carbone M, Duty S, Rattray M, 2012. Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochem. Int 60 (1), 31–38. 10.1016/j.neuint.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceylan H, Budak H, Kocpinar EF, Baltaci NG, Erdogan O, 2019. Examining the link between dose-dependent dietary iron intake and Alzheimer’s disease through oxidative stress in the rat cortex. J. Trace. Elem. Med. Biol 56, 198–206. 10.1016/j.jtemb.2019.09.002. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J, 1995. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron 15 (3), 711–720. 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Chen H, Attieh ZK, Su T, Syed BA, Gao H, Alaeddine RM, Fox TC, Usta J, Naylor CE, Evans RW, McKie AT, Anderson GJ, Vulpe CD, 2004. Hephaestin is a ferroxidase that maintains partial activity in sex-linked anemia mice. Blood 103 (10), 3933–3939. 10.1182/blood-2003-09-3139. [DOI] [PubMed] [Google Scholar]
- Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B, 2015. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front. Cell. Neurosci 9, 124. 10.3389/fncel.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, 1992. Excitotoxic cell death. J. Neurobiol 23 (9), 1261–1276. 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Cieri D, Brini M, Calì T, 2017. Emerging (and converging) pathways in Parkinson’s disease: keeping mitochondrial wellness. Biochem. Biophys. Res. Commun 483 (4), 1020–1030. 10.1016/j.bbrc.2016.08.153. [DOI] [PubMed] [Google Scholar]
- Coccini T, Caloni F, Ramírez Cando LJ, De Simone U, 2017. Cytotoxicity and proliferative capacity impairment induced on human brain cell cultures after shortand long-term exposure to magnetite nanoparticles. J. Appl. Toxicol 37 (3), 361–373. 10.1002/jat.3367. [DOI] [PubMed] [Google Scholar]
- Codazzi F, Pelizzoni I, Zacchetti D, Grohovaz F, 2015. Iron entry in neurons and astrocytes: a link with synaptic activity. Front. Mol. Neurosci 8, 18. 10.3389/fnmol.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan FM, Reynolds GP, Ward NI, 1993. Hippocampal tin, aluminum and zinc in Alzheimer’s disease. Biometals 6 (3), 149–154. 10.1007/BF00205853. [DOI] [PubMed] [Google Scholar]
- Crespo ÂC, Silva B, Marques L, Marcelino E, Maruta C, Costa S, Timóteo A, Vilares A, Couto FS, Faustino P, Correia AP, Verdelho A, Porto G, Guerreiro M, Herrero A, Costa C, de Mendonça A, Costa L, Martins M, 2014. Genetic and biochemical markers in patients with Alzheimer’s disease support a concerted systemic iron homeostasis dysregulation. Neurobiol. Aging 35(4), 777–785. doi: 10.1016/j.neurobiolaging.2013.10.078. [DOI] [PubMed] [Google Scholar]
- Damulina A, Pirpamer L, Soellradl M, Sackl M, Tinauer C, Hofer E, Enzinger C, Gesierich B, Duering M, Ropele S, Schmidt R, Langkammer C, 2020. Cross-sectional and Longitudinal Assessment of Brain Iron Level in Alzheimer Disease Using 3-T MRI. Radiology 192541. Advance online publication, doi: 10.1148/radiol.2020192541. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, 2001. Glutamate uptake. Prog. Neurobiol 65 (1), 1–105. 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons CG, 2012. Alzheimer’s disease, [β-amyloid, glutamate, NMDA receptors and memantine–searching for the connections. Br. J. Pharmacol 167 (2), 324–352. 10.1111/j.1476-5381.2012.02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Xu Z, Xu B, Xu D, Tian Y, Feng W, 2012. The protective effects of riluzole on manganese-induced disruption of glutamate transporters and glutamine synthetase in the cultured astrocytes. Biol. Trace. Elem. Res 148 (2), 242–249. 10.1007/s12011-012-9365-1. [DOI] [PubMed] [Google Scholar]
- Derry PJ, Kent TA, 2017. Correlating quantitative susceptibility mapping with cognitive decline in Alzheimer’s disease. Brain 140 (8), 2069–2072. 10.1093/brain/awx167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev S, Babitt JL, 2017. Overview of iron metabolism in health and disease. Hemodial. Int 21 Suppl 1 (Suppl. 1), S6–S20. 10.1111/hdi.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias V, Junn E, Mouradian MM, 2013. The role of oxidative stress in Parkinson’s disease. J. Parkinsons. Dis 3 (4), 461–491. 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Bishop GM, Koeppe M, Dang TN, Robinson SR, 2007. The pivotal role of astrocytes in the metabolism of iron in the brain. Neurochem. Res 32 (11), 1884–1890. 10.1007/s11064-007-9375-0. [DOI] [PubMed] [Google Scholar]
- Działo J, Tokarz-Deptuła B, Deptuła W, 2013. Excitotoxicity and Wallerian degeneration as a processes related to cell death in nervous system. Arch. Ital. Biol 151 (2), 67–75. 10.4449/aib.v151i2.1471. [DOI] [PubMed] [Google Scholar]
- Eid A, Mhatre I, Richardson JR, 2019. Gene-environment interactions in Alzheimer’s disease: a potential path to precision medicine. Pharmacol. Ther 199, 173–187. 10.1016/j.pharmthera.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson KM, Aschner M, 2006. Increased manganese uptake by primary astrocyte cultures with altered iron status is mediated primarily by divalent metal transporter. Neurotoxicology 27 (1), 125–130. 10.1016/j.neuro.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Farrall AJ, Wardlaw JM, 2009. Blood-brain barrier: ageing and microvascular disease-systematic review and meta-analysis. Neurobiol. Aging 30 (3), 337–352. 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Faux NG, Rembach A, Wiley J, Ellis KA, Ames D, Fowler CJ, Martins RN, Pertile KK, Rumble RL, Trounson B, Masters CL, AIBL Research Group., Bush AI, 2014. An anemia of Alzheimer’s disease. Mol. Psychiatry. 19(11), 1227–1234. doi: 10.1038/mp.2013.178. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Piccola G, Aschner JL, Aschner M, 2006. Characteristics of manganese (Mn) transport in rat brain endothelial (RBE4) cells, an in vitro model of the blood-brain barrier. Neurotoxicology 27 (1), 60–70. 10.1016/j.neuro.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Piccola G, Marreilha dos Santos AP, Aschner JL, Aschner M, 2007. Putative proteins involved in manganese transport across the blood-brain barrier. Hum. Exp. Toxicol 26 (4), 295–302. 10.1177/0960327107070496. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Tan X, Luo Y, Wang P, Song J, Kanda H, Hayakawa T, Kumagai T, Kakamu T, Tsuji M, Hidaka T, Mori Y, 2013. Heavy metals in blood and urine and its relation to depressive symptoms in Parkinson’s disease patients. Fukushima. J. Med. Sci 59 (2), 76–80. 10.5387/fms.59.76. [DOI] [PubMed] [Google Scholar]
- Gerhardsson L, Lundh T, Minthon L, Londos E, 2008. Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord 25 (6), 508–515. 10.1159/000129365. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Mack CM, Lasley SM, 1999. The influence of developmental period of lead exposure on long-term potentiation in the adult rat dentate gyrus in vivo. Neurotoxicology 20 (1), 57–69. [PubMed] [Google Scholar]
- Godfrey ME, Wojcik DP, Krone CA, 2003. Apolipoprotein E genotyping as a potential biomarker for mercury neurotoxicity. J. Alzheimers. Dis 5 (3), 189–195. 10.3233/jad-2003-5303. [DOI] [PubMed] [Google Scholar]
- Goodman L, 1953. Alzheimer’s disease; a clinico-pathologic analysis of twenty-three cases with a theory on pathogenesis. J. Nerv. Ment. Dis 118 (2), 97–130. [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ, 1997. Occupational exposures to metals as risk factors for Parkinson’s disease. Neurology 48 (3), 650–658. 10.1212/wnl.48.3.650. [DOI] [PubMed] [Google Scholar]
- Grabrucker AM, Rowan M, Garner CC, 2011. Brain-delivery of zinc-ions as potential treatment for neurological diseases: mini review. Drug. Deliv. Lett 1 (1), 13–23. 10.2174/2210303111101010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Wei X, Monnot AD, Fontanilla CV, Behl M, Farlow MR, Zheng W, Du Y, 2011. Lead exposure increases levels of β-amyloid in the brain and CSF and inhibits LRP1 expression in APP transgenic mice. Neurosci. Lett 490 (1), 16–20. 10.1016/j.neulet.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Owen AD, Toffa SE, Cooper JM, Dexter DT, Jenner P, Marsden CD, Schapira AH, 1998. Mitochondrial function, GSH and iron in neurodegeneration and Lewy body diseases. J. Neurol. Sci 158 (1), 24–29. 10.1016/s0022-510x(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Hammond SL, Bantle CM, Popichak KA, Wright KA, Thompson D, Forero C, Kirkley KS, Damale PU, Chong E, Tjalkens RB, 2020. NF-κB signaling in astrocytes modulates brain inflammation and neuronal injury following sequential exposure to manganese and MPTP during development and aging. Toxicol. Sci Advance online publication, 10.1093/toxsci/kfaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Yang X, Chen X, Li Z, Fang M, Bai B, Tan D, 2017. Hydrogen sulfide may attenuate methylmercury-induced neurotoxicity via mitochondrial preservation. Chem. Biol. Interact 263, 66–73. 10.1016/j.cbi.2016.12.020. [DOI] [PubMed] [Google Scholar]
- Hancock SM, Finkelstein DI, Adlard PA, 2014. Glia and zinc in ageing and Alzheimer’s disease: a mechanism for cognitive decline? Front. Aging. Neurosci 6, 137. 10.3389/fnagi.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare DJ, Doecke JD, Faux NG, Rembach A, Volitakis I, Fowler CJ, Grimm R, Doble PA, Cherny RA, Masters CL, Bush AI, Roberts BR, 2015. Decreased plasma iron in Alzheimer’s disease is due to transferrin desaturation. ACS. Chem. Neurosci 6 (3), 398–402. 10.1021/cn5003557. [DOI] [PubMed] [Google Scholar]
- He Y, Thong PS, Lee T, Leong SK, Mao BY, Dong F, Watt F, 2003. Dopaminergic cell death precedes iron elevation in MPTP-injected monkeys. Free. Radic. Biol. Med 35 (5), 540–547. 10.1016/s0891-5849(03)00385. [DOI] [PubMed] [Google Scholar]
- Hellman NE, Gitlin JD, 2002. Ceruloplasmin metabolism and function. Annu. Rev. Nutr 22, 439–458. 10.1146/annurev.nutr.22.012502.114457. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Rodríguez JJ, Verkhratsky A, 2010. Neuroglia in neurodegeneration. Brain. Res. Rev 63 (1–2), 189–211. 10.1016/j.brainresrev.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Hertz L, 2013. The glutamate-glutamine (GABA) cycle: importance of late postnatal development and potential reciprocal interactions between biosynthesis and degradation. Front. Endocrinol (Lausanne). 4, 59. 10.3389/fendo.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Dringen R, Schousboe A, Robinson SR, 1999. Astrocytes: glutamate producers for neurons. J. Neurosci. Res 57 (4), 417–428. [PubMed] [Google Scholar]
- Hoepken HH, Korten T, Robinson SR, Dringen R, 2004. Iron accumulation, iron-mediated toxicity and altered levels of ferritin and transferrin receptor in cultured astrocytes during incubation with ferric ammonium citrate. J. Neurochem 88, 1194–1202. 10.1046/j.1471-4159.2003.02236.x. [DOI] [PubMed] [Google Scholar]
- Hohnholt MC, Dringen R, 2013. Uptake and metabolism of iron and iron oxide nanoparticles in brain astrocytes. Biochem. Soc. Trans 41 (6), 1588–1592. doi. 10.1042/BST20130114. [DOI] [PubMed] [Google Scholar]
- Hozumi I, Hasegawa T, Honda A, Ozawa K, Hayashi Y, Hashimoto K, Yamada M, Koumura A, Sakurai T, Kimura A, Tanaka Y, Satoh M, Inuzuka T, 2011. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J. Neurol. Sci 303(1–2), 95–99. 10.1016/j.jns.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Hussien HM, Abd-Elmegied A, Ghareeb DA, Hafez HS, Ahmed H, El-Moneam NA, 2018. Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food. Chem. Toxicol 111, 432–444. 10.1016/j.fct.2017.11.025. [DOI] [PubMed] [Google Scholar]
- Ignjatović A, Stević Z, Lavrnić D, Nikolić-Kokić A, Blagojević D, Spasić M, Spasojević I, 2012. Inappropriately chelated iron in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Amyotroph. Lateral. Scler 13 (4), 357–362. 10.3109/17482968.2012.665929. [DOI] [PubMed] [Google Scholar]
- Ignjatović A, Stević Z, Lavrnić S, Daković M, Baĉić G, 2013. Brain iron MRI: a biomarker for amyotrophic lateral sclerosis. J. Magn. Reson. Imaging. 38 (6), 1472–1479. 10.1002/jmri.24121. [DOI] [PubMed] [Google Scholar]
- Ijomone OM, Miah MR, Akingbade GT, Bucinca H, Aschner M, 2020. Nickel-Induced Developmental Neurotoxicity in C. elegans Includes Cholinergic, Dopaminergic and GABAergic Degeneration, Altered Behaviour, and Increased SKN-1 Activity. Neurotox. Res 37 (4), 1018–1028. 10.1007/s12640-020-00175-3. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Nedergaard M, 2013. Is there a cerebral lymphatic system? Stroke 44 (6 Suppl 1), S93–S95. 10.1161/STROKEAHA.112.678698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QM, 2015. Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci 16 (12), 29592–29630. 10.3390/ijms161226183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SY, David S, 2003. Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J. Biol. Chem 278 (29), 27144–27148. 10.1074/jbc.M301988200. [DOI] [PubMed] [Google Scholar]
- Ji C, Kosman DJ, 2015. Molecular mechanisms of non-transferrin-bound and transferring-bound iron uptake in primary hippocampal neurons. J. Neurochem 133 (5), 668–683. 10.1111/jnc.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Qian ZM, Xie JX, 2003. Sheng. Li. Xue. Bao. [Acta physiologica Sinica] 55 (5), 571–576. [PubMed] [Google Scholar]
- Jiang H, Song N, Jiao Q, Shi L, Du X, 2019. Iron pathophysiology in Parkinson Diseases. Adv. Exp. Med. Biol 1173, 45–66. 10.1007/978-981-13-9589-5_4. [DOI] [PubMed] [Google Scholar]
- Johnson J Jr, Pajarillo E, Taka E, Reams R, Son DS, Aschner M, Lee E, 2018. Valproate and sodium butyrate attenuate manganese-decreased locomotor activity and astrocytic glutamate transporters expression in mice. Neurotoxicology 64, 230–239. 10.1016/j.neuro.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Kim C, Smith K, Son DS, Aschner M, Lee E, 2015. Transcriptional Regulation of the Astrocytic Excitatory Amino Acid Transporter 1 (EAAT1) via NF-κB and Yin Yang 1 (YY1). J. Biol. Chem 290 (39), 23725–23737. 10.1074/jbc.M115.649327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Webb A, Smith K, Johnson J Jr, Lee K, Son DS, Aschner M, Lee E, 2014. Yin Yang 1 is a repressor of glutamate transporter EAAT2, and it mediates manganese-induced decrease of EAAT2 expression in astrocytes. Mol. Cell. Biol 34 (7), 1280–1289. 10.1128/MCB.01176-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AC, Lim S, Kim YK, 2018. Metal ion effects on Aβ and Tau aggregation. Int. J. Mol. Sci 19 (1), 128. 10.3390/ijms19010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Park JD, Choi BS, 2014. Mercury-induced amyloid-beta (Aβ) accumulation in the brain is mediated by disruption of Aβ transport. J. Toxicol. Sci 39 (4), 625–635. 10.2131/jts.39.625. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Kettenmann H, Verkhratsky A, 2007. Membrane currents and cytoplasmic sodium transients generated by glutamate transport in Bergmann glial cells. Pflugers. Arch 454 (2), 245–252. 10.1007/s00424-007-0207-5. [DOI] [PubMed] [Google Scholar]
- Kirkley KS, Popichak KA, Afcali MF, Legare ME, Tjalkens RB, 2017. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity.J. Neuroinflammation 14 (1), 99. 10.1186/s12974-017-0871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson MD, 2019. Non-transferrin-bound iron transporters. Free Radical Biol. Med 133, 101–111. 10.1016/j.freeradbiomed.2018.10.413. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Xie T, Schuetz JD, 2007. The role of transporters in cellular heme and porphyrin homeostasis. Pharmacol. Ther 114 (3), 345–358. 10.1016/j.pharmthera.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ, 2008. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 59 (2), 214–225. 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakye GF, Jiménez JA, Thomas MG, Kingsley BA, McIIvin M, Saito MA, Korley EM, 2019. Heterozygous huntingtin promotes cadmium neurotoxicity and neurodegeneration in striatal cells via altered metal transport and protein kinase C delta dependent oxidative stress and apoptosis signaling mechanisms. Neurotoxicology 70, 48–61. 10.1016/j.neuro.2018.10.012. [DOI] [PubMed] [Google Scholar]
- Lee E, Sidoryk-Wegrzynowicz M, Farina M, Rocha JB, Aschner M, 2013. Estrogen attenuates manganese-induced glutamate transporter impairment in rat primary astrocytes. Neurotox. Res 23 (2), 124–130. 10.1007/s12640-012-9347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Shin JH, Gwag BJ, Choi EJ, 2015. Iron accumulation promotes TACE-mediated TNF-α secretion and neurodegeneration in a mouse model of ALS. Neurobiol. Dis 80, 63–69. 10.1016/j.nbd.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC, 1998. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J. Neurosci 18 (21), 8751–8757. 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson CW, Tassabehji NM, 2007. Role and Regulation of Copper and Zinc Transport Proteins in the Central Nervous System, in: Lajtha A. (Ed.), Handbook of Neurochemistry and Molecular Neurobiology. New York, Plenum. 257–284. [Google Scholar]
- Leitner DF, Connor JR, 2012. Functional roles of transferrin in the brain. Biochim. Biophys. Acta 1820, 393–402. 10.1016/j.bbagen.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Levi S, Tiranti V, 2019. Neurodegeneration with brain iron accumulation disorders: valuable models aimed at understanding the pathogenesis of iron deposition. Pharmaceuticals (Basel) 12 (1), 27. 10.3390/ph12010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu F, Song L, Zhang P, Qiao M, Zhao Q, Li W, 2014. The effects of early life Pb exposure on the expression of IL1-β, TNF-α and Aβ in cerebral cortex of mouse pups. J. Trace. Elem. Med. Biol 28 (1), 100–104. 10.1016/j.jtemb.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang X, Wang J, Huang R, Wan D, 2020. Regulation of iron homeostasis and related diseases. Mediators. Inflamm 2020, 6062094. 10.1155/2020/6062094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Lu Y, Li Z, Li S, Chen B, Zhang M, Chen B, Ji M, Gong W, Xia M, Verkhratsky A, Wu X, Li B, 2020. Iron Aggravates the Depressive Phenotype of Stressed Mice by Compromising the Glymphatic System. Neurosci. Bull 10.1007/s12264-020-00539-x. Advance online publication, 10.1007/s12264-020-00539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis A, Barone TA, Paradkar PN, Plunkett RJ, Roth JA, 2004. Expression and localization of different forms of DMT1 in normal and tumor astroglial cells. Braimn Res. Mol Brain Res 122, 62–70. 10.1016/j.molbrainres.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Liu C, Liang MC, Soong TW, 2019a. Nitric oxide, iron and neurodegeneration. Front. Neurosci 13, 114. 10.3389/fnins.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Pan G, Zhang Y, Xu J, Ma R, Shen Z, Dong S, 2019b. Risk assessment of soil heavy metals associated with land use variations in the riparian zones of a typical urban river gradient. Ecotoxicol. Environ. Saf 181, 435–444. 10.1016/j.ecoenv.2019.04.060. [DOI] [PubMed] [Google Scholar]
- Lopes KO, Sparks DL, Streit WJ, 2008. Microglial dystrophy in the aged and Alzheimer’s disease brain is associated with ferritin immunoreactivity. Glia 56 (10), 1048–1060. 10.1002/glia.20678. [DOI] [PubMed] [Google Scholar]
- Lu C, Meng Z, He Y, Xiao D, Cai H, Xu Y, Liu X, Wang X, Mo L, Liang Z, Wei X, Ao Q, Liang B, Li X, Tang S, Guo S, 2018. Involvement of gap junctions in astrocyte impairment induced by manganese exposure. Brain. Res. Bull 140, 107–113. 10.1016/j.brainresbull.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Lu M, Liu Y, Shao M, Tesfaye GC, Yang S, 2020. Associations of Iron Intake, Serum Iron and Serum Ferritin with Bone Mineral Density in Women: The National Health and Nutrition Examination Survey, 2005–2010. Calcif. Tissue. Int 106 (3), 232–238. 10.1007/s00223-019-00627-9. [DOI] [PubMed] [Google Scholar]
- Mandel S, Amit T, Bar-Am O, Youdim MB, 2007. Iron dysregulation in Alzheimer’s disease: multimodal brain permeable iron chelating drugs, possessing neuroprotective-neurorescue and amyloid precursor protein-processing regulatory activities as therapeutic agents. Prog. Neurobiol 82 (6), 348–360. 10.1016/j.pneurobio.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Martínez-Ruiz A, Cadenas S, Lamas S, 2011. Nitric oxide signaling: classical, less classical, and nonclassical mechanisms. Free. Radic. Biol. Med 51 (1), 17–29. 10.1016/j.freeradbiomed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Mason LH, Harp JP, Han DY, 2014. Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed. Res. Int 2014 10.1155/2014/840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis S, Couratier P, Julian A, Corcia P, Le Masson G, 2017. Current view and perspectives in amyotrophic lateral sclerosis. Neural. Regen. Res 12 (2), 181–184. 10.4103/1673-5374.200794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine D, Araki S, 1958. Minamata disease: an unusual neurological disorder caused by contaminated fish. Lancet 2 (7047), 629–631. 10.1016/s0140-6736(58)90348-9. [DOI] [PubMed] [Google Scholar]
- Melis JP, van Steeg H, Luijten M, 2013. Oxidative DNA damage and nucleotide excision repair. Antioxid. Redox. Signal 18 (18), 2409–2419. 10.1089/ars.2012.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M, Singh R, Mukherjee S, Sharma D, 2013. Dehydroepiandrosterone’s antiepileptic action in FeCl3-induced epileptogenesis involves upregulation of glutamate transporters. Epilepsy. Res 106 (1–2), 83–91. 10.1016/j.eplepsyres.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Mitchell RM, Simmons Z, Beard JL, Stephens HE, Connor JR, 2010. Plasma biomarkers associated with ALS and their relationship to iron homeostasis. Muscle. Nerve 42 (1), 95–103. 10.1002/mus.21625. [DOI] [PubMed] [Google Scholar]
- Miyata S, Nakamura S, Nagata H, Kameyama M, 1983. Increased manganese level in spinal cords of amyotrophic lateral sclerosis determined by radiochemical neutron activation analysis. J. Neurol. Sci 61 (2), 283–293. 10.1016/0022-510x(83)90012-6. [DOI] [PubMed] [Google Scholar]
- Moos T, 1996. Immunohistochemical localization of intraneuronal transferrin receptor immunoreactivity in the adult mouse central nervous system. J Comp. Neurol 375, 675–692. 10.1002/(SICI)1096-9861. [DOI] [PubMed] [Google Scholar]
- Moos T, Morgan EH, 2004. The metabolism of neuronal iron and its pathogenic role in neurological disease: review. Ann. NY Acad. Sci 1012, 14–26. 10.1196/annals.1306.002. [DOI] [PubMed] [Google Scholar]
- Morris CM, Candy JM, Kerwin JM, Edwardson JA, 1994. Transferrin receptors in the normal human hippocampus and in Alzheimer’s disease. Neuropathol. Appl. Neurobiol 20 (5), 473–477. 10.1111/j.1365-2990.1994.tb00998.x. [DOI] [PubMed] [Google Scholar]
- Moyano P, García JM, García J, Anadon MJ, Naval MV, Frejo MT, Sola E, Pelayo A, Pino JD, 2020. Manganese increases Aβ and Tau protein levels through proteasome 20S and heat shock proteins 90 and 70 alteration, leading to SN56 cholinergic cell death following single and repeated treatment. Ecotoxicol. Environ. Saf 203, 110975. Advance online publication. 10.1016/j.ecoenv.2020.110975. [DOI] [PubMed] [Google Scholar]
- Mu MD, Qian ZM, Yang SX, Rong KL, Yung WH, Ke Y, 2020. Therapeutic effect of a histone demethylase inhibitor in Parkinson’s disease. Cell. Death. Dis 11 (10), 927. 10.1038/s41419-020-03105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutkus L, Aschner JL, Syversen T, Aschner M, 2005. Methylmercury alters the in vitro uptake of glutamate in GLAST and GLT-1-transfected mutant CHO-K1 cells. Biol. Trace. Elem. Res 107 (3), 231–245. 10.1385/BTER:107:3:231. [DOI] [PubMed] [Google Scholar]
- Nandar W, Connor JR, 2011. HFE gene variants affect iron in the brain. J. Nutr 141 (4), 729S–739S. 10.3945/jn.110.130351. [DOI] [PubMed] [Google Scholar]
- Nandar W, Neely EB, Simmons Z, Connor JR, 2014. H63D HFE genotype accelerates disease progression in animal models of amyotrophic lateral sclerosis. Biochim. Biophys. Acta 1842(12 Pt A), 2413–2426. doi: 10.1016/j.bbadis.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Nandar W, Neely EB, Unger E, Connor JR, 2013. A mutation in the HFE gene is associated with altered brain iron profiles and increased oxidative stress in mice. Biochim. Biophys. Acta 1832 (6), 729–741. 10.1016/j.bbadis.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Neal M, Richardson JR, 2018. Epigenetic regulation of astrocyte function in neuroinflammation and neurodegeneration. Biochim. Biophys. Acta. Mol. Basis. Dis 1864 (2), 432–443. 10.1016/j.bbadis.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte C, Gore A, Sekler I, Kresse W, Hershfinkel M, Hoffmann A, Kettenmann H, Moran A, 2004. ZnT-1 expression in astroglial cells protects against zinc toxicity and slows the accumulation of intracellular zinc. Glia 48 (2), 145–155. 10.1002/glia.20065. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A, 1979. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 161 (2), 303–310. doi. 10.1016/00068993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Nriagu JO, 1983. Saturnine gout among Roman aristocrats. Did lead poisoning contribute to the fall of the Empire? N. Engl. J. Med 308 (11), 660–663. 10.1056/NEJM198303173081123. [DOI] [PubMed] [Google Scholar]
- O’Carroll RE, Masterton G, Dougall N, Ebmeier KP, Goodwin GM, 1995. The neuropsychiatric sequelae of mercury poisoning. The Mad Hatter’s disease revisited. Br. J. Psychiatry 167 (1), 95–98. 10.1192/bjp.167.1.95. [DOI] [PubMed] [Google Scholar]
- Okereafor U, Makhatha M, Mekuto L, Uche-Okereafor N, Sebola T, Mavumengwana V, 2020. Toxic metal implications on agricultural soils, plants, animals, aquatic life and human health. Int. J. Environ. Res. Public Health 17 (7), 2204. 10.3390/ijerph17072204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri G, Brack C, Müller-Spahn F, Stähelin HB, Herrmann M, Renard P, Brockhaus M, Hock C, 2000. Mercury induces cell cytotoxicity and oxidative stress and increases beta-amyloid secretion and tau phosphorylation in SHSY5Y neuroblastoma cells. J. Neurochem 74 (1), 231–236. 10.1046/j.1471-4159.2000.0740231.x. [DOI] [PubMed] [Google Scholar]
- Olloquequi J, Cornejo-Córdova E, Verdaguer E, Soriano FX, Binvignat O, Auladell C, Camins A, 2018. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: therapeutic implications. J. Psychopharmacol 32 (3), 265–275. 10.1177/0269881118754680. [DOI] [PubMed] [Google Scholar]
- Pamphlett R, Kum Jew S, 2018. Inorganic mercury in human astrocytes, oligodendrocytes, corticomotoneurons and the locus ceruleus: implications for multiple sclerosis, neurodegenerative disorders and gliomas. Biometals 31 (5), 807–819. 10.1007/s10534-018-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Vergnano AM, Barbour B, Casado M, 2009. Zinc at glutamatergic synapses. Neuroscience 158 (1), 126–136. 10.1016/j.neuroscience.2008.01.061. [DOI] [PubMed] [Google Scholar]
- Pelizzoni I, Zacchetti D, Campanella A, Grohovaz F, Codazzi F, 2013. Iron uptake in quiescent and inflammation-activated astrocytes: a potentially neuroprotective control of iron burden. Biochim. Biophys. Acta 1832 (8), 1326–1333. 10.1016/j.bbadis.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng TI, Jou MJ, 2010. Oxidative stress caused by mitochondrial calcium overload. Ann. N.Y. Acad. Sci 1201, 183–188. 10.1111/j.1749-6632.2010.05634.x. [DOI] [PubMed] [Google Scholar]
- Peres TV, Eyng H, Lopes SC, Colie D, Gonçalves FM, Venske DK, Lopes MW, Ben J, Bornhorst J, Schwerdtle T, Aschner M, Farina M, Prediger RD, Leal RB, 2015. Developmental exposure to manganese induces lasting motor and cognitive impairment in rats. Neurotoxicology. 50, 28–37. 10.1016/j.neuro.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Powers KM, Smith-Weller T, Franklin GM, Longstreth WT Jr, Swanson PD, Checkoway H, 2003. Parkinson’s disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology 60 (11), 1761–1766. 10.1212/01.wnl.0000068021.13945.7f. [DOI] [PubMed] [Google Scholar]
- Qian ZM, Ke Y, 2019. Brain iron transport. Biol. Rev. Camb. Philos. Soc 94 (5), 1672–1684. 10.1111/brv.12521. [DOI] [PubMed] [Google Scholar]
- Qian ZM, Wang Q, 1998. Expression of iron transport proteins and excessive iron accumulation in the brain in neurodegenerative disorders. Brain. Res. Brain. Res. Rev 27 (3), 257–267. 10.1016/s0165-0173(98)00012-5. [DOI] [PubMed] [Google Scholar]
- Qu CS, Ma ZW, Yang J, Liu Y, Bi J, Huang L, 2012. Human exposure pathways of heavy metals in a lead-zinc mining area, Jiangsu Province, China. PLoS One 7 (11). 10.1371/journal.pone.0046793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M, Nan X, Gao Z, Guo B, Liu B, Chen Z, 2013. Protective effects of lycopene against methylmercury-induced neurotoxicity in cultured rat cerebellar granule neurons. Brain. Res 1540, 92–102. 10.1016/j.brainres.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Qureshi M, Brown RH Jr, Rogers JT, Cudkowicz ME, 2008. Serum ferritin and metal levels as risk factors for amyotrophic lateral sclerosis. Open. Neurol. J 2, 51–54. 10.2174/1874205X00802010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha AA, Vaishnav RA, Friedland RP, Bomford A, Raha-Chowdhury R, 2013. The systemic iron-regulatory proteins hepcidin and ferroportin are reduced in the brain in Alzheimer’s disease. Acta. Neuropathol. Commun 1, 55. 10.1186/2051-5960-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Al-Qenaie S, Rao MS, Khan KM, Guillemin GJ, 2019. Memantine is protective against cytotoxicity caused by lead and quinolinic acid in cultured rat embryonic hippocampal cells. Chem. Res. Toxicol 32 doi.(6) doi. 1134–1143. 10.1021/acs.chemrestox.8b00421. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Rahman MM, Reichman SM, Lim RP, Naidu R, 2014. Heavy metals in Australian grown and imported rice and vegetables on sale in Australia: health hazard. Ecotoxicol. Environ. Saf 100, 53–60. 10.1016/j.ecoenv.2013.11.024. [DOI] [PubMed] [Google Scholar]
- Ramos P, Santos A, Pinto NR, Mendes R, Magalhães T, Almeida A, 2014. Iron levels in the human brain: a post-mortem study of anatomical region differences and age-related changes. J. Trace Elem. Med. Biol 28 (1), 13–17. 10.1016/j.jtemb.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Rathnasamy G, Ling EA, Kaur C, 2013. Consequences of iron accumulation in microglia and its implications in neuropathological conditions. CNS Neurol. Disord. Drug. Targets 12 doi.(6), doi. 785–798. 10.2174/18715273113126660169. [DOI] [PubMed] [Google Scholar]
- Rathore KI, Redensek A, David S, 2012. Iron homeostasis in astrocytes and microglia is differentially regulated by TNF-α and TGF-β1. Glia 60, 738–750. 10.1002/glia.22303. [DOI] [PubMed] [Google Scholar]
- Rauen T, Rothstein JD, Wässle H, 1996. Differential expression of three glutamate transporter subtypes in the rat retina. Cell. Tissue. Res 286 (3), 325–336. doi. 10.1007/s004410050702. [DOI] [PubMed] [Google Scholar]
- Reed JC, Pellecchia M, 2012. Ironing out cell death mechanisms. Cell. 149 (5), 963–965. 10.1016/j.cell.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Reinert A, Morawski M, Seeger J, Arendt T, Reinert T, 2019. Iron concentrations in neurons and glial cells with estimates on ferritin concentrations. BMC Neurosci. 20 (1), 25. 10.1186/s12868-019-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Verkhratsky A, 2016. Principles of sodium homeostasis and sodium signalling in astroglia. Glia 64, 1611–1627. 10.1002/glia.22964. [DOI] [PubMed] [Google Scholar]
- Rose CF, Verkhratsky A, Parpura V, 2013. Astrocyte glutamine synthetase: pivotal in health and disease. Biochem. Soc. Trans 41 (6), 1518–1524. 10.1042/BST20130237. [DOI] [PubMed] [Google Scholar]
- Rostoker G, Vaziri ND, 2017. Iatrogenic iron overload and its potential consequences in patients on hemodialysis. Presse. Med 46 (12 Pt 2), e312–e328. 10.1016/j.1pm.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Rostoker G, Vaziri ND, 2017. Impact of iatrogenic iron overload on the course of hepatitis C in the dialysis population: a plea for caution. Hemodial. Int. International Symposium on Home Hemodialysis 21 Suppl 1, S68–S77. 10.1111/hdi.12557. [DOI] [PubMed] [Google Scholar]
- Rouault TA, 2013. Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat. Rev. Neurosci 14 (8), 551–564. 10.1038/nrn3453. [DOI] [PubMed] [Google Scholar]
- Sarah R, Tabassum B, Idrees N, Hashem A, Abd Allah EF, 2019. Bioaccumulation of heavy metals in Channa punctatus (Bloch) in river Ramganga (U.P.), India. Saudi. J. Biol. Sci 26 (5), 979–984. 10.1016/j.sjbs.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper HM, Song W, Zukor H, Hascalovici JR, Zeligman D, 2009. Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J. Neurochem 110 doi.(2) doi.469–485. 10.1111/j.1471-4159.2009.06160.x. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC, 2014. Glutamate metabolism in the brain focusing on astrocytes. Adv. Neurobiol 11, 13–30. 10.1007/978-3-319-08894-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank RP, Bennett GS, Freytag SO, Campbell GL, 1985. Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain. Res 329 (1–2), 364–367. 10.1016/00068993(85)90552-9. [DOI] [PubMed] [Google Scholar]
- Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT, 2005. Identification of an intestinal heme transporter. Cell. 122(5), 789–801. 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Aschner M, 2013. Manganese toxicity in the central nervous system: the glutamine/glutamate-γ-aminobutyric acid cycle. J. Intern. Med 273 (5), 466–477. 10.llll/joim.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson IA, Ponnuru P, Klinger ME, Myers RL, Devraj K, Coe CL, Lubach GR, Carruthers A, Connor JR, 2015. A novel model for brain iron uptake: introducing the concept of regulation. J. Cereb. Blood Flow Metab 35, 48–57. 10.1038/jcbfm.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sominsky L, Walker AK, Hodgson DM, 2015. Editorial: Neuroinflammation and behavior. Front. Neurosci 9, 201. 10.3389/fhins.2015.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson E, Nathoo N, Mahjoub Y, Dunn JF, Yong VW, 2014. Iron in multiple sclerosis: roles in neurodegeneration and repair. Nat. Rev. Neurol 10 (8), 459–468. 10.1038/nrneurol.2014.118. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Xue QS, Tischer J, Bechmann I, 2014. Microglial pathology. A Acta Neuropathol. Commun 2, 142. 10.1186/s404780140142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struzyhska L, Chalimoniuk M, Sulkowski G, 2005. Changes in expression of neuronal and glial glutamate transporters in lead-exposed adult rat brain. Neurochem. Int 47 (5), 326–333. 10.1016/j.neuint.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Sudhakaran S, Athira SS, Mohanan PV, 2019. Zinc oxide nanoparticle induced neurotoxic potential upon interaction with primary astrocytes. Neurotoxicology 73, 213–227. 10.1016/j.neuro.2019.04.008. [DOI] [PubMed] [Google Scholar]
- Sun XY, Wei YP, Xiong Y, Wang XC, Xie AJ, Wang XL, Yang Y, Wang Q, Lu YM, Liu R, Wang JZ, 2012. Synaptic released zinc promotes tau hyperphosphorylation by inhibition of protein phosphatase 2A (PP2A). J. Biol. Chem 287 (14), 11174–11182. 10.1074/jbc.Mlll.309070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supino-Viterbo V, Sicard C, Risvegliato M, Rancurel G, Buge A, 1977. Toxic encephalopathy due to ingestion of bismuth salts: clinical and EEG studies of 45 patients. J. Neurol. Neurosurg. Psychiatry. 40 (8), 748–752. 10.1136/jnnp.40.8.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Schumacker PT, Guzman JD, Ilijic E, Yang B, Zampese E, 2017. Calcium and Parkinson’s disease. Biochem. Biophys. Res. Commun 483 (4), 1013–1019. 10.1016/j.bbrc.2016.08.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Leyland LA, Schrag AE, Lees AJ, Acosta-Cabronero J, Weil RS, 2020. Brain iron deposition is linked with cognitive severity in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 91 (4), 418–425. 10.1136/jnnp-2019-322042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian ZY, Wang CY, Wang T, Li YC, Wang ZY, 2019. Glial S100A6 degrades β-amyloid aggregation through targeting competition with zinc ions. Aging Dis. 10 (4), 756–769. 10.14336/AD.2018.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troadec MB, Ward DM, Lo E, Kaplan J, De Domenico I, 2010. Induction of FPN1 transcription by MTF-1 reveals a role for ferroportin in transition metal efflux. Blood. 116 (22), 4657–4664. 10.1182/blood-2010-04-278614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulpule K, Robinson SR, Bishop GM, Dringen R, 2010. Uptake of ferrous iron by cultured rat astrocytes. J. Neurosci. Res 88, 563–571. 10.1002/jnr.22217. [DOI] [PubMed] [Google Scholar]
- Ulla M, Bonny JM, Ouchchane L, Rieu I, Claise B, Durif F, 2013. Is R2* a new MRI biomarker for the progression of Parkinson’s disease? A longitudinal follow-up. PloS One 8 (3). 10.1371/journal.pone.0057904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia P, Aguirre P, Esparza A, Tapia V, Mena NP, Arredondo M, Gonzalez Billault C, Ndhez MT, 2013. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J. Neurochem 126, 541–549. 10.1111/jnc.12244. [DOI] [PubMed] [Google Scholar]
- Valdiglesias V, Fernandez-Bertolez N, ΚΠίς G, Costa C, Costa S, Fraga S, Bessa MJ, Pasaro E, Teixeira JP, Laffon B, 2016. Are iron oxide nanoparticles safe? Current knowledge and future perspectives. J. Trace. Elem. Med. Biol 38, 53–63. 10.1016/j.jtemb.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M, 2014. Astroglial cradle in the life of the synapse. Philos. Trans. R. Soc. Lond. B. Biol. Sci 369 (1654), 20130595. 10.1098/rstb.2013.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M, 2018. Physiology of astroglia. Physiol. Rev 98 (1), 239–389. 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Rose CR, 2020. Na+ dependent transporters: The backbone of astroglial homeostatic function. Cell Calcium 85. 10.1016/j.ceca.2019.102136. [DOI] [PubMed] [Google Scholar]
- Veyrat-Durebex C, Corcia P, Mucha A, Benzimra S, Mallet C, Gendrot C, Moreau C, Devos D, Piver E, Pages JC, Maillot F, Andres CR, Vourc’h P, Blasco H, 2014. Iron metabolism disturbance in a French cohort of ALS patients. Biomed. Res. Int 2014, 485723. 10.1155/2014/485723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Charrier JG, Wu D, McFall AS, Li W, Abid A, Kennedy IM, Anastasio C, 2016. Physicochemical properties of iron oxide nanoparticles that contribute to cellular ROS-dependent signaling and acellular production of hydroxyl radical. Free. Radic. Res 50 (11), 1153–1164. 10.3109/10715762.2016.1152360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron HA, 1973. Lead poisoning in the ancient world. Med. Hist 17 (4), 391–399. 10.1017/s0025727300019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan D, Wu Q, Ni H, Liu G, Ruan Z, Yin Y, 2019. Treatments for iron deficiency (ID): prospective organic iron fortification. Curr. Pharm. Des 25 (3), 325–332. 10.2174/1381612825666190319111437. [DOI] [PubMed] [Google Scholar]
- Wang J, Song N, Jiang H, Wang J, Xie J, 2013. Pro-inflammatory cytokines modulate iron regulatory protein 1 expression and iron transportation through reactive oxygen/nitrogen species production in ventral mesencephalic neurons. Biochim. Biophys. Acta 1832 (5), 618–625. 10.1016/j.bbadis.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu HM, Yang HD, Du XX, Jiang H, Xie JX, 2009. Rgl reduces nigral iron levels of MPTP-treated C57BL6 mice by regulating certain iron transport proteins. Neurochem. Int 54 (1), 43–48. 10.1016/j.neuint.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Wang S, Wu W, Liu F, Liao R, Hu Y, 2017. Accumulation of heavy metals in soil-crop systems: a review for wheat and corn. Environ Sci Pollut. Res. Int 24 (18), 15209–15225. 10.1007/s11356-017-8909-5. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L, 2014. The role of iron in brain ageing and neurodegenerative disorders. Lancet. Neurol 13 (10), 1045–1060. 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieler M, Gee M, Martin WR, 2015. Longitudinal midbrain changes in early Parkinson’s disease: iron content estimated from R2*/MRI. Parkinsonism Relat. Disord 21 (3), 179–183. 10.1016/j.parkreldis.2014.ll.017. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Leenders AG, Cooperman S, Meyron-Holtz E, Smith S, Land W, Tsai RY, Berger UV, Sheng ZH, Rouault TA, 2004. Expression of the iron transporter ferroportin in synaptic vesicles and the blood-brain barrier. Brain. Res 1001 (1–2), 108–117. 10.1016/j.brainres.2003.10.066. [DOI] [PubMed] [Google Scholar]
- Wu ML, Deng JF, Lin KP, Tsai WJ, 2013. Lead, mercury, and arsenic poisoning due to topical use of traditional Chinese medicines. Am. J. Med 126 (5), 451–454. 10.1016/j.amjmed.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Xia M, Guan W, Ji M, Li S, Li Z, Chen B, Zhang M, Liang S, Chen B, Gong W, Dong C, Wen G, Zhan X, Zhang D, Li X, Verkhratsky A, Li B, 2020. Astrocytes express DMT1 and transferrin receptors, which transport iron thus activating Ca2+ signalling: possible role in neuroprotection against iron overload? BioRxiv 2020.07.06.190652. 10.1101/2020.07.06.190652. [DOI] [Google Scholar]
- Xia M, Liang S, Li S, Li Z, Zhang M, Chen B, Dong C, Chen B, Ji M, Gong W, Guan D, Verkhratsky A, Li B, 2020. Iatrogenic Iron Promotes Neurodegeneration and Activates Self-protection of Neural Cells against Exogenous Iron Attacks. BioRxiv. 2020.03.21.001925. 10.1101/2020.03.21.001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Yang L, Sun G, Qi S, Li B, 2017. Mechanism of depression as a risk factor in the development of Alzheimer’s disease: the function of AQP4 and the glymphatic system. Psychopharmacology (Berl). 234 (3), 365–379. 10.1007/S00213-016-4473-9. [DOI] [PubMed] [Google Scholar]
- Xu M, tan X, Li N, Wu H, Wang Y, Xie J, Wang J, 2019. Differential regulation of estrogen in iron metabolism in astrocytes and neurons. J. Cell. Pathol 234, 4232–4242. 10.1002/jcp.27188. [DOI] [PubMed] [Google Scholar]
- Youdim MB, 2003. What have we learnt from CDNA microarray gene expression studies about the role of iron in MPTP induced neurodegeneration and Parkinson’s disease? J. Neural. Transm. Suppl 65, 73–88. 10.1007/978-3-7091-0643-3_5. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang N, Qi F, Wang X, Zhu Q, Lu Y, Zhang H, Che F, Li W, 2018. Serum ferritin is a candidate biomarker of disease aggravation in amyotrophic lateral sclerosis. Biomed. Rep 9 (4), 333–338. 10.3892/br.2018.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei S, Carr K, Reiley L, Diaz K, Guerra O, Altamirano PF, Pagani W, Lodin D, Orozco G, Chinea A, 2015. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int 6, 171. 10.4103/2152-7806.169561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarruk JG, Berard JL, Passos dos Santos R, Kroner A, Lee J, Arosio P, David S, 2015. Expression of iron homeostasis proteins in the spinal cord in experimental autoimmune encephalomyelitis and their implications for iron accumulation. Neurobiol. Dis 81, 93–107. 10.1016/j.nbd.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Zhang X, Surguladze N, Slagle-Webb B, Cozzi A, Connor JR, 2006. Cellular iron status influences the functional relationship between microglia and oligodendrocytes. Glia 54 (8), 795–804. 10.1002/glia.20416. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wan D, Zhou X, Long C, Wu X, Li L, He L, Huang P, Chen S, Tan B, Yin Y, 2017a. Biochem. Biophys. Res. Commun 490 (4), 1210–1214. 10.1016/j.bbrc.2017.06.187. [DOI] [PubMed] [Google Scholar]
- Zhang F, Xing S, Li Z, 2017b. Antagonistic effects of lycopene on cadmium-induced hippocampal dysfunctions in autophagy, calcium homeostatis and redox. Oncotarget 8 (27), 44720–44731. 10.18632/oncotarget.18249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Liao Y, Jin Y, Li G, Lv X, Sun G, 2012. Effects of arsenite on glutamate metabolism in primary cultured astrocytes. Toxicol. In. Vitro 26 (1), 24–31. doi. 10.1016/j.tiv.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Zheng Z, White C, Lee J, Peterson TS, Bush AI, Sun GY, Weisman GA, Petris MJ, 2010. Altered microglial copper homeostasis in a mouse model of Alzheimer’s disease. J. Neurochem 114 (6), 1630–1638. 10.1111/j.1471-4159.2010.06888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Danbolt NC, 2013. GABA and glutamate transporters in brain. Front. Endocrinol 4, 165. 10.3389/fendo.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]


