Abstract
The release of pharmaceuticals into the environment induces adverse effects on the metabolism of humans and other living species, calling for advanced remediation methods. Conventional removal methods are often non-selective and cause secondary contamination. These issues may be partly solved by the use of recently-developped adsorbents such as molecularly imprinted polymers. Here we review the synthesis and application of molecularly imprinted polymers for removing pharmaceuticals in water. Molecularly imprinted polymers are synthesized via several multiple-step polymerization methods. Molecularly imprinted polymers are potent adsorbents at the laboratory scale, yet their efficiency is limited by template leakage and polymer quality. Adsorption performance of multi-templated molecularly imprinted polymers depends on the design of wastewater treatment plants, pharmaceutical consumption patterns and the population serviced by these wastewater treatment plants.
Keywords: Remediation, Pharmaceuticals, Contaminated water, Molecularly imprinted materials, Limitations
Introduction
Pharmaceuticals constitute a wide range of biologically active hydrophilic chemical compounds whose derivatives have substantial variability in structure, function, behavior, and activity (Mezzelani et al. 2018b; Monisha et al. 2022). They are generally metabolized by humans and animals' bodies subsequent to their intake. Some of them are completely degraded while others are partially disintegrated in the excrements. Finally, they enter the sewage system and eventually join the environment via sewage leakages or the discharge of wastewater from treatment facilities into aquatic systems, consequently degrading the quality of surface and subsurface water and the health of living organisms (Yang et al. 2017). Additionally, they may enter the environment through the pharmaceutical manufacturing processes, broadly divided into fermentation, extraction, chemical synthesis, formulation, and packaging. Among them, chemical synthesis and fermentation generate significant amounts of wastewater with high levels of spent solvents, recalcitrant organics, and pharmaceutical residue as well as salts (Shi et al. 2017).
The advancements in analytical techniques have enabled the detection of pharmaceuticals' trace concentrations in assorted environmental matrices such as surface water, sub-surface water, drinking water, waste effluents, sediments, and biota (aus der Beek et al. 2016). The widespread presence of pharmaceuticals in aquatic environments confirms the inefficiency of conventional (waste)water treatment methods with an emergent need for the optimal removal of pharmaceuticals through alternative methods. One of the biggest challenges in the field of (waste)water treatment is the usage of low-cost technologies and guarantee for ensuring safety and health criteria in the recycled treated effluents.
In water pollution control and wastewater management, the adsorption process is a promising method because it has design and operational simplicity, higher efficacy, lower maintenance, and no undesirable byproducts (de Andrade et al. 2018; Wang et al. 2022; Zare et al. 2021; Maksoud et al. 2022). The non-conventional adsorption approach based on molecularly imprinted polymers offers a valid technology for the selective removal of specific target pollutants in (waste)water treatment. Molecularly imprinted polymers are constructed using molecular imprinting technology, which results in precise three-dimensional cavities aligned with the polymer substrates’ template molecules (Morin-Crini et al. 2022).
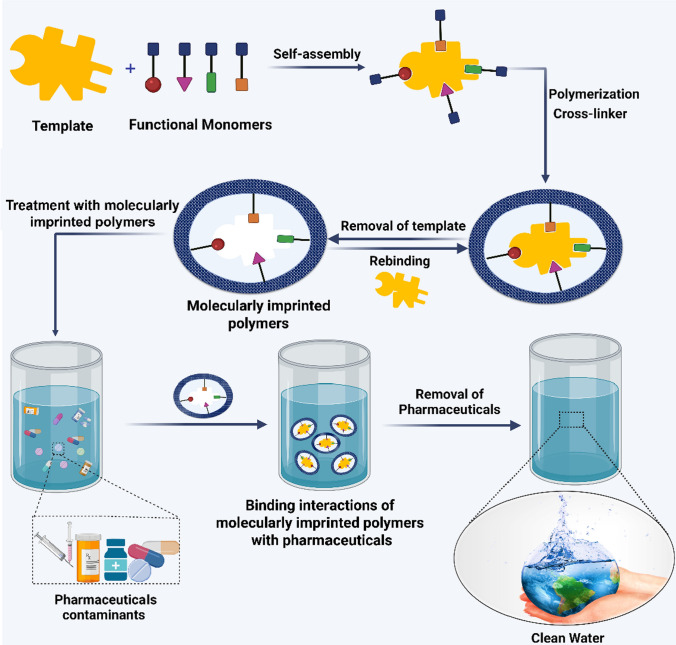
Herein, we review the negative impacts of pharmaceuticals on humans and the environment, the syntheses of molecularly imprinted polymers, and the advances and limitations of their deployment for adsorbing aqueous pharmaceuticals from synthetic solutions. We also provide future research directions which could be explored to advance the integration of molecularly imprinted polymers in real water purification processes (Fig. 1).
Fig. 1.
Fabrication of molecularly imprinted polymers and their application in the remediation of pharmaceuticals from contaminated water. First, the polymerization is performed in the presence of the target drug. Then the drug template is formed after the drug is withdrawn by leaching operation. The size of the template depends on the size of the chemical structure of the drug
Pharmaceuticals
Pharmaceuticals have specific biological activities due to their various functional groups and physicochemical properties. These compounds are commonly categorized as analgesic (anti-inflammatory and opioid), antibiotic, anticancer or cytostatic, antidepressant, antidiabetic, antiseizure, β-blocker, hormone, and lipid regulator, among others (Yang et al. 2017). The important categories of pharmaceuticals and their therapeutic applications are summarized in Table 1.
Table 1.
Common pharmaceuticals, their excretion mode, and therapeutic properties
| Pharmaceutical type | Emerging contaminants | Molecular formula | Molar mass (g mol−1) | Excretion | Therapeutic applications |
|---|---|---|---|---|---|
| Antibiotic | Amoxicillin | C16H19N3O5S | 365.40 | Urine | To treat bacterial infections |
| Antibiotic | Azithromycin | C38H76N2O14 | 748.99 | Urine | To treat some bacterial infections like middle ear infection, streptococcal sore throat, pneumonia, and diarrhea |
| Antibiotic | Cephalexin | C16H17N3O4S | 347.39 | Urine | To treat the infections of the respiratory, urinary, and genital tract |
| Antibiotic | Chlortetracycline | C22H23ClN2O8 | 478.88 | Urine | To treat the methicillin-resistant S. aureus |
| Antibiotic | Clarithromycin | C38H69NO13 | 747.96 | Bile, Urine | To treat some bacterial infections like Helicobacter pylori, strep throat, bronchitis, and sinusitis |
| Antibiotic | Ciprofloxacin | C17H18FN3O3 | 331.34 | Bile, Urine | To treat some severe and complex infections |
| Antibiotic | Erythromycin | C36H65NO13 | 733.93 | Bile, Urine | To treat bacterial infections |
| Antibiotic | Gatifloxacin | C19H22FN3O4 | 375.39 | Urine | To treat eye infections |
| Antibiotic | Lincomycin | C18H35ClN2O6S | 406.54 | Bile, Urine | To treat the gram-positive microbes’ infections (respiratory tract and urinary tract infections, osteomyelitis, sepsis, otitis, and infectious arthritis) |
| Antibiotic | Metronidazole | C6H9N3O3 | 171.15 | Feces, Urine | To treat bacterial vaginosis and pelvic inflammatory disease |
| Antibiotic | Norfloxacin | C16H18FN3O3 | 319.33 | Bile, Urine | To treat gonorrhea and the infections of the urinary tract, bladder, and gynecological disease |
| Antibiotic | Ofloxacin | C18H20FN3O4 | 361.37 | Feces, Urine | To treat cellulite, pneumonia, urinary tract infections, and prostatitis |
| Antibiotic | Oxytetracycline | C22H24N2O9 | 460.43 | Urine | To treat the infection of the chest and mouth |
| Antibiotic | Penicillin | C16H18N2O4S | 334.40 | Bile, Urine | To treat infections |
| Antibiotic | Roxithromycin | C41H76N2O15 | 837.05 | Feces, Urine | To treat the infections of the respiratory, urinary tract, and soft tissues |
| Antibiotic | Sulfadiazine | C10H10N4O2S | 250.28 | Urine | To treat vaginal infections |
| Antibiotic | Sulfamethoxazole | C10H11N3O3S | 253.28 | Urine, Breast milk | To treat urinary tract infections, bronchitis, and prostatitis |
| Antibiotic | Tetracycline | C22H24N2O8 | 444.43 | Urine | To treat a wide variety of infections such as acne |
| Antibiotic | Trimethoprim | C14H18N4O3 | 290.32 | Urine | To treat urinary tract infections |
| Anticancer | Bleomycin | C55H84N17O21S3+ | 1415.55 | Urine | To treat cancer |
| Anticancer | Cyclophosphamide | C7H15Cl2N2O2P.H2O | 261.08 | Bile, Urine | To treat cancer |
| Anticancer | Docetaxel | C43H53NO14 | 807.88 | Urine | To treat cancer |
| Anticancer | Etoposide | C29H32O13 | 588.56 | Bile, Urine | To treat cancer |
| Anticancer | Epirubicin | C27H29NO11 | 543.50 | Bile, Urine | To treat cancer |
| Anticancer | 5-Fluorouracil | C4H3FN2O2 | 130.08 | Bile, Urine | To treat cancer |
| Anticancer | Ibrance (Palbociclib) | C24H29N7O2 | 447.54 | Feces, Urine | To treat the breast cancer |
| Anticancer | Methotrexate | C20H22N8O5 | 454.43 | Bile, Urine | To treat cancer |
| Anticancer | Oxaliplatin | C8H14N2O4Pt | 397.29 | Feces, Urine | To treat the colon cancer |
| Anticancer | Vincristine | C46H56N4O10 | 824.96 | Feces, Urine | To treat the lymphoblastic leukemia |
| Antidepressant | Amitriptyline | C20H23N | 277.40 | Urine | To treat the psychiatric disorders |
| Antidepressant | Bupropion | C13H18ClNO | 239.74 | Feces, Urine | To treat the major depressive disorders |
| Antidepressant | Citalopram | C20H21FN2O | 324.39 | Urine | To treat the major depressive disorders |
| Antidepressant | Escitalopram | C20H21FN2O | 324.39 | Urine | To treat the disturbance of major depression or anxiety |
| Antidepressant | Fluoxetine | C17H18F3NO | 309.33 | Urine | To treat depression, panic disorder, obsessive–compulsive disorder, and bulimia nervosa |
| Antidepressant | Norfluoxetine | C16H16F3NO | 295.299 | Urine, Breast milk | Selective serotonin reuptake inhibitor |
| Antidepressant | Paroxetine | C19H20FNO3 | 329.37 | Urine | To treat depression and disturbance of panic, social anxiety, generalized anxiety, and obsession |
| Antidepressant | Sertraline | C17H17Cl2N | 306.23 | Urine | To treat the disorders of depression, obsession, panic, and social phobia |
| Antidepressant | Reboxetine | C19H23NO3 | 313.39 | Urine | To Treat the disturbance of depression, fear, and attention deficit hyperactivity |
| Antidepressant | Venlafaxine | C17H27NO2 | 277.40 | Urine | To treat the disorder of depression, panic, social anxiety, and generalized anxiety |
| Antidiabetic | Metformin | C4H11N5 | 129.16 | Urine | To control blood glucose |
| Anti-inflammatory analgesic | Acetaminophen | C8H9NO2 | 151.16 | Urine | To relieve pain and fever |
| Anti-inflammatory analgesic | Acetylsalicylic acid | C9H8O4 | 180.16 | Urine | To prevent platelet aggregation, stroke, and myocardial infarction |
| Anti-inflammatory analgesic | Diclofenac | C14H11Cl2NO2 | 296.15 | Bile, Urine | Pain reliever |
| Anti-inflammatory analgesic | Ibuprofen | C13H18O2 | 206.29 | Urine | To treat pain, inflammation, and fever |
| Anti-inflammatory analgesic | Indomethacin | C19H16ClNO4 | 357.79 | Feces, Urine | To mitigate pain, joint stiffness, and swelling |
| Anti-inflammatory analgesic | Ketoprofen | C16H14O3 | 254.28 | Urine | To relieve minor pains like headaches, toothaches, muscle aches, backaches, and menstrual periods |
| Anti-inflammatory analgesic | Naproxen | C14H14O3 | 230.26 | Urine | To relieve pain, fever, inflammatory diseases like rheumatoid arthritis |
| Anti-inflammatory analgesic | Paracetamol | C8H9NO2 | 151.16 | Urine | Painkiller |
| Anti-inflammatory analgesic | Salicylic acid | C7H6O3 | 138.13 | Urine | To treat skin problems like psoriasis and acne |
| Opioid analgesic | Morphine | C17H19NO3 | 285.34 | Feces, Urine | To reduce pain and anxiety and increase happiness |
| Antiseizure | Benzodiazepine | C9H8N2 | 144.17 | Urine | To treat anxiety and seizures |
| Antiseizure | Carbamazepine | C15H12N2O | 236.27 | Urine | To treat epilepsy, neuropathic pain, and bipolar disorder |
| Antiseizure | Ethosuximide | C7H11NO2 | 141.17 | Urine | To treat seizures |
| Antiseizure | Perampanel | C23H15N3O | 349.38 | Feces, Urine | To treat epilepsy and minor seizures |
| Antiseizure | Retigabine | C16H20Cl2FN3O2 | 303.33 | Feces, Urine | To treat partial-onset seizures |
| β-blocker | Metoprolol | C15H25NO3 | 267.36 | Urine | To relax blood vessels, slow heart rate, and decline blood pressure |
| β-blocker | Propranolol | C16H21NO2 | 259.34 | Urine | To treat high blood pressure, slow heart rate, and relieve anxiety signs |
| Hormone | Aldosterone | C21H28O5 | 360.45 | Urine | To treat hypertension and heart failure |
| Hormone (estrogen) | Diethylstilbestrol | C18H20O2 | 268.3 | Bile, Urine | To treat prostate cancer and to prevent premature delivery or miscarriage in women |
| Hormone (estrogen) |
Estradiol or 17β-Estradiol |
C18H24O2 | 272.38 | Feces, Urine | To treat menopause symptoms like hot flashes, to prevent osteoporosis in menopausal women |
| Hormone (estrogen) | Estriol | C18H24O3 | 288.38 | Urine | To control menopause symptoms like osteoporosis, frequent urinary tract infections, insomnia, vaginal dryness, and hot flashes |
| Hormone (estrogen) | Estrone | C18H22O2 | 270.37 | Urine | To manage symptoms of perimenopausal and postmenopausal |
| Hormone (estrogen) |
Ethinylestradiol or 17α-Ethynylestradiol |
C20H24O2 | 296.40 | Bile, Feces, Urine | To relieve moderate to severe vasomotor symptoms of menopause, to prevent postmenopausal osteoporosis, to treat moderate acne, and contraceptive side effects |
| Hormone | Levonorgestrel | C21H28O2 | 312.45 | Feces, Urine | To prevent pregnancy |
| Hormone | Progesterone | C21H30O2 | 314.46 | Bile, Urine | To treat abnormal uterine bleeding and intense premenstrual syndrome symptoms |
| Hormone | Testosterone | C19H28O2 | 288.42 | Bile, Urine | To develop the reproductive system in men and organs like prostate and testicles |
| Hormone | Cortisol | C21H30O5 | 362.46 | Urine | To maintain immune function, blood pressure, and anti-inflammatory operations |
| Hormone | Insulin | C257H383N65O77S6 | 5808 | Urine | To control the body's metabolism, provide energy, and diminish blood sugar |
| Hormone | Levothyroxine | C15H11I4NO4 | 776.87 | Feces, Urine | To treat hypothyroidism |
| Hormone | Triiodothyronine | C15H12I3NO4 | 650.98 | Feces, Urine | Affecting on the almost every physiological process in the body |
| Hormone | Somatotropin | C69H114N22O21S2 | 1651.9 | – | To treat short stature and growth failure, and to increase the growth hormone level |
| Lipid regulator | Atorvastatin | C33H35FN2O5 | 558.64 | Bile, Urine | To treat high cholesterol and diminish the risk of heart attack and stroke |
| Lipid regulator | Bezafibrate | C19H20ClNO4 | 361.82 | Urine | To treat hyperlipidemia |
| Lipid regulator | Clofibric acid | C10H11ClO3 | 214.64 | Urine | To treat dyslipidemia |
| Lipid regulator | Gemfibrozil | C15H22O3 | 250.33 | Bile, Urine | To treat dyslipidemia |
| Lipid regulator | Simvastatin | C25H38O5 | 418.57 | Feces, Urine | To treat dyslipidemia |
Analgesics have the potential to relieve pain selectively, obviously by changing sensory perception, and affect consciousness via supraspinal mechanisms without blocking the conduction of nerve impulses. They can be divided into anti-inflammatories and opioids that relieve pain by diminishing local inflammatory responses and affecting the brain, respectively. Anti-inflammatories analgesics are classified into three types: corticosteroids, nonsteroids, and immunomodulators (Fuller 2022). The anti-inflammatory analgesics (e.g. acetaminophen or paracetamol, acetylsalicylic acid or aspirin, and salicylic acid) and nonsteroidal anti-inflammatory analgesics (e.g. diclofenac, ibuprofen, indomethacin, ketoprofen, and naproxen) are consumed to alleviate pain, inflammation, and fever. The action mechanism of these drugs is via the synthesis inhibition of cyclooxygenase enzymes (COX1 and COX2) in the central nervous system and periphery that is responsible for the prostaglandins synthesis inducing the responses in local tissue containing inflammation and pain.
Antibiotics are substances obtained both naturally and synthetically. Natural antibiotics are derived from microorganisms such as fungi (Penicillin; penicillin or amoxicillin) and bacteria (Actinomycete; tetracycline or erythromycin). Antibiotics are classified into bactericidal antibiotics that kill pathogenic cells and bacteriostatic antibiotics that prevent pathogenic cells from growing (Kovalakova et al. 2020). Antibiotics have revolutionized current medicine and extended human life significantly. Besides remedying infectious illness, antibiotics enable the performance of various modern medical procedures. Nonetheless, antibiotic misuse has resulted in antimicrobial resistance against some infections (Kovalakova et al. 2020).
Anticancer or cytostatic medications are being increasingly dispensed with the increase in the number of cancer patients. Cytostatic drugs, comprising natural and synthetic compounds, are applied in cancer therapy and chemotherapy. The action mechanism of anticancer drugs entails intervening in cell division, inhibiting deoxyribonucleic acid (DNA) replication, and inducing cell death in the tumor. Additionally, preventing transcription and translation may occur in some cases by blocking the synthesis of nucleotides or intercalating in DNA. Generally, cancer patients have to consume anticancer drugs at near-fatal doses for preventing mutation and causing cell death, and consequently, the excretion of high concentrations of these drugs and their metabolites from the body are responsible for their entry into the environment (Zounkova et al. 2010).
Antidepressant pharmaceuticals are used to cure depression, anxiety disorder, and personality disturbance and are often referred to as psychotropic drugs that interfere with the serotonin or noradrenaline systems; serotonin and noradrenaline (norepinephrine), as neurotransmitters, are linked to emotion and mood. Serotonin reuptake-inhibiting antidepressants (e.g. citalopram, escitalopram, fluoxetine, norfluoxetine, paroxetine, and sertraline) or norepinephrine reuptake-inhibiting antidepressants (e.g. reboxetine) motivate the secretion of adrenocorticotrophic or cortisol hormone or induce an increase in serotonin or norepinephrine concentration within the central nervous via their reuptake inhibition (Schüle 2007).
Antiseizure medications prevent seizures by affecting the brain excitability mechanisms by intervening in the creation and deployment of epileptic hyperexcitability. The action mechanism of antiseizure drugs (e.g. retigabine, ethosuximide, and carbamazepine) comprise the modulation in voltage-gated potassium, calcium, and sodium channels, respectively. Antiseizure medications (e.g. benzodiazepines and perampanel) alter neurotransmitters release and the excitation or inhibition of neurotransmission. Among the antiseizure drugs, carbamazepine is the most frequently used antiepileptic medication for epilepsy treatment, as well as in the cure of psychiatric and neurological disturbances (Beydoun et al. 2020).
Diabetes mellitus, caused by elevated blood glucose levels due to the alteration in carbohydrate metabolism, is treated by using antidiabetic medications to control the glucose concentration. Antidiabetic pharmaceuticals are classified into insulin, stimulating insulin secretion, reducing insulin resistance, α-glucosidase inhibitors, peptide analogs, and glucose-stimulating diuretics. Insulin injection and metformin are the most often used means of controlling type 1 and type 2 diabetes, respectively (Foretz et al. 2019).
β-blockers (metoprolol and propranolol) are a group of drugs that act as competitive antagonists to bind with adrenergic receptors (Srinivasan 2019). β-blockers are often non-specific blockers that affect the beta-1 receptor located in the heart and arteries. So, β1-blockers can reduce heart rate and blood pressure, and they are the most common drugs consumed to treat cardiovascular illnesses.
Hormones are substances secreted by endocrine glands in very low concentrations that control and regulate the activity of specific cells in the target organ and are categorized based on their structure and the organs in which they are produced (Möstl and Palme 2002). According to their molecular structure, hormones are classified as follows: steroid hormones (adrenal cortex hormones: cortisol and aldosterone, ovarian and placental hormones: estrogen and progesterone, and testicular hormone: testosterone), eicosanoid hormones (e.g. prostaglandins), amino acids (e.g. thyroid hormones), and peptide hormones (e.g. insulin, growth hormone, and anti-urinary hormone). Based on the hormone-producing organs in the human body, we can mention hormones of the pituitary, thyroid, adrenal, sex, and so on (Patel et al. 2018).
Lipid regulator drugs (fibrates and statins) are applied to diminish plasma cholesterol levels for treating dyslipidemia, which is an imbalance in the metabolism of blood lipids that results in elevated triglycerides causing the development of atherosclerosis. Fibrates (bezafibrate, clofibric acid, and gemfibrozil) can increase high-density lipoprotein cholesterol, decrease low-density lipoprotein cholesterol, and diminish the triglycerides concentration in blood plasma (Staels et al. 1998). Indeed, fibrates have the capacity for altering the transcription of protein genes encoding responsible for lipoprotein metabolism and changing the expression of high-density lipoprotein. On the other hand, statins (atorvastatin and simvastatin) can inhibit 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase in the liver and modify lipid metabolism (Stancu and Sima 2001).
Cytotoxic effects of pharmaceuticals
Drugs are designed to maximize the intrinsic biological power at low concentrations and are developed for inducing a continued activity in biological systems through convenient absorption in living organisms and active interaction with them (Li et al. 2020). In addition, the non-biodegradability of pharmaceuticals leads to their extended biological activity for longer periods and such unique attributes and situations can potentially create severe biological risks to the ecosystem’s balance and human health (Rathi et al. 2021).
Pharmaceuticals can be released into the atmosphere via wastewaters from hospitals, drugs companies, research centers, agricultural activities for fisheries, poultry and livestock farming, human excretion and/or direct disposal of expired or unused drugs (Pereira et al. 2020). The wastewater discharges in surface water or reclaimed wastewater to irrigate agricultural land leads to water and soil contamination. Drugs contaminants are transferred from the surface water and soil to groundwater, plants and animals, to finally reach the human body via the food chain (Mackuľak et al. 2019). Thus, synergistic or antagonistic hazards with acute and long-term consequences can ensue because of the pharmaceutical transfer cycle. Several dangers that threaten the environment and human health instigated by pharmaceutical pollutants are described in the following sections.
Environmental risks
Pharmaceuticals are constructed for producing a biological response in specific organisms, while the biological response in non-specific organisms can be created by prolonged exposure to their environmental concentrations (Khan et al. 2020a). Most pharmaceuticals last long in the aquatic environment due to lipophilicity, so their cytotoxic effects are chronic rather than acute, even at low concentrations. Chronic pharmaceutical effects depend on the contaminant, concentration, exposure duration and the species. The ecotoxicology of pharmaceuticals on aquatic organisms includes the potential effects on a molecular, histological, developmental, and behavioral level, and also on reproduction and mortality (Godoy and Kummrow 2017).
Research has shown that antibiotics are among the most abundant pharmaceutical contaminants in the aquatic environment for most countries in the world. The overall hazardous effects of antibiotic pollutants are associated with developing antibiotic-resistant genes and bacteria, and growth inhibition in algae and cyanobacteria. For instance, the tetracycline antibiotics exhibit growth inhibition of Cyanophyta (Microcystis aeruginosa), Chlorophyta (Selenastrum capricornutum), and cyanobacteria (Anabaena flosaque) (Xu et al. 2021). The growth inhibition of algae by antibiotics transpires by affecting the chloroplast transcription, translation, and replication (Cheng et al. 2020). Also, antibiotics had toxic effects on the structure, growth, internal organs function, and enzymatic activity of fish. For example, exposure to erythromycin displays oxidative DNA damage in gilthead seabream (Sparus aurata) and rainbow trout (Oncorhynchus mykiss) (Rodrigues et al. 2019c).
Research in many countries, such as China, has revealed that more than half of the non-antibiotic contaminants in the aquatic environment are hormones- or endocrine-disrupting chemicals. The estrogens or estrogenic pollutants in water can induce chronic alterations in reproductive and nonreproductive organs and physiologically abnormal behaviors of aquatic organisms (Taisen et al. 2006). Also, defeminization of female fish takes place by 17α-ethynylestradiol pollutant by reducing the synthesis and secretion of estrogen (Aris et al. 2014). Undeniably, the fish population and ecosystem balance are implicated by gender changes of male fish.
Numerous studies have been reported to evaluate the toxicity of analgesics and anti-inflammatory drugs such as diclofenac, ibuprofen, ketoprofen, paracetamol, naproxen, etc. in the environment. The environmental risks of diclofenac have been investigated, and the results showed that diclofenac and its metabolites can cause a high risk due to the synergistic interactions with other pollutants, the creation of new drug-resistant strains, and the generation of novel emerging contaminants (Sathishkumar et al. 2020). Chronic effects of diclofenac are reported on DNA methylation level and reproductive ability in Gladioferens pectinatus (marine copepod) (Guyon et al. 2018). Diclofenac contaminants can lead to DNA damage, oxidative stress, the raised activity of cyclooxygenase, and diminished stability of the lysosomal membrane in the mussel Perna perna (Fontes et al. 2018).
The long-term exposure to ketoprofen and ibuprofen has a hazardous potential in Mytilus galloprovincialis through transcriptional changes and alteration of the cellular turn-over and immunological factors (Mezzelani et al. 2018a). Paracetamol, as the most abundant anti-inflammatory drug contaminant in the aquatic environment, can cause the genotoxicity in kidneys and gills of Rhamdia quelen catfish at a concentration of 2.5 μg L−1 (Perussolo et al. 2019), and the mortality in D. magna at the concentrations between 1.2 and 1.7 mg L−1 (Nunes et al. 2014). The acute toxicity of naproxen for B. calyciflorus and T. platyurus (Lethal Concentration 50, LC50) is reported at concentrations of 84.09 and 62.48 mg L−1, respectively. In contrast, naproxen chronic toxicity in the form of inhibition growth (half maximal Effective Concentration, EC50) occurred for T. platyurus and C. dubia, at concentrations of 0.56 and 0.33 mg L−1, respectively (Isidori et al. 2005).
Anticancer drugs or cytostatic pharmaceuticals, as dangerous compounds, cause irreparable damage to the environment because their effective removal has not been possible from wastewater; they are present in high concentrations (up to 2.12 × 10–4 mg L−1) in the aqueous media (Jureczko and Kalka 2020). Cytostatic contaminants, due to their mode of action on DNA damage by disturbing the repair activity and inhibiting the de novo synthesis, can endanger the organisms’ health (Kleinert et al. 2021).
Among the anticancer pharmaceuticals, 5-fluorouracil, cyclophosphamide, methotrexate, bleomycin, and vincristine are the most toxic and widely used drugs. The 5-fluorouracil inhibits the growth of Synechococcus leopoliensis (cyanobacteria) and Pseudokirchneriella subcapitata (algae) at concentrations of 1.20 and 0.13 mg L−1, respectively (Brezovšek et al. 2014). Exposure to environmental concentrations of 5-fluorouracil and cyclophosphamide pollutants (0.2–123 μg L−1) for Lithobates catesbeianus (tadpoles) can generate morphological changes and mutagenic effects in the gastrointestinal tract via formation of binuclei and micronuclei as well as melanocytes development (Araújo et al. 2019). The toxicity results for 5-fluorouracil and methotrexate to endemic Elliptio complanata (freshwater mussels) at the highest concentration (100 μg L−1) for 96 h indicate that methotrexate and 5-fluorouracil with a larger spectrum of effects can increase the dihydrofolate reductase and can decrease the DNA repair activity and lipid peroxidation (Kleinert et al. 2021).
A recent study about carbamazepine, an antiepileptic drug, has demonstrated its toxic effects on bivalves by creating oxidative stress at environmental concentrations (0.03–11.6 μg L−1). The ecotoxicity of carbamazepine combined with cetirizine on energy metabolism and oxidative stress of Ruditapes philippinarum (edible clam) could occur at low concentrations (Almeida et al. 2018). Recently, the mechanistic toxicity of fluoxetine, as an antidepressant drug, was examined in Pimephales promelas (adult fathead minnows) (Colville et al. 2022); molecular toxicity in the fish's brain and liver could be observed after 96 h. Exposure to fluoxetine concentration of 56.7 μg L−1 after 21 days led to hepatocyte vacuolation and considerable fertility decline (Feijão et al. 2020).
The toxicity of norfluoxetine, a fluoxetine metabolite, was evaluated on zebrafish larvae, and the results showed that the concentration range 0.64 × 10−6 to 400 × 10−6 mg L−1 could display remarkable influence on the embryos' malformations numbers. Sertraline hydrochloride, an extensively consumed antidepressant, could inhibit the growth of Chlorella vulgaris (green alga) and Microcystis aeruginosa (cyanobacterium), and diminish the photosynthetic efficiency by decreasing the concentration of chlorophyll in microcosm (Yang et al. 2019). Moreover, the sertraline hydrochloride could disturb the cyanobacteria balance by stimulating the specific cyanobacteria growth.
The chronic and acute toxicity of loratadine, as an antihistamine drug, and its metabolite desloratadine were examined in two crustaceans; Ceriodaphnia dubia and Thamnocephalus platyurus, the Pseudokirchneriella subcapitata (alga), and the Brachionus calyciflorus (rotifer) (Iesce et al. 2019). The bioassay results proved that loratadine could exert chronic and acute ecotoxicity, mainly in Ceriodaphnia dubia (EC50 = 28.14 μg L−1, LC50 = 600 μg L−1). Whereas desloratadine could display the same acute ecotoxicity among the mentioned organisms, with the most chronic effect of desloratadine being illustrated in Pseudokirchneriella subcapitata and Ceriodaphnia dubia.
Antiviral drugs with the potential of antiviral resistance development and ecosystem alterations have attracted global anxiety. Antiviral contaminants from antiviral drugs related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can cause ecotoxicological effects on bacteria, algae, water flea, planktonic crustaceans, and fish (Kumar et al. 2021). The cytotoxic assay of efavirenz, a highly toxic antiviral drug, exhibited the EC50 values of 34 and 26 μg L−1 for Raphidocelis subcapitata (algae) and Ceriodaphnia dubia (water flea), respectively (Almeida et al. 2021). In addition, the acute exposure of efavirenz drug (anti-human immunodeficiency virus, HIV; concentration = 20.6 ng L−1) to Oreochromis mossambicus tilapia fish represented decreasing the overall health, damaging the hepatocyte cells, and even death (Robson et al. 2017).
Blood lipid regulators and β-blockers are among the main groups of environmental pollutants, and these contaminants instigate the same influences in target and non-target organisms. For example, exposure to atorvastatin, a blood lipid regulator, could lead to the depletion of protein pools, lipid, and carbohydrates in Mytulis edulis (mussel) at a concentration of 1.2 μg L−1 (Falfushynska et al. 2019). In addition, lipid regulating agents have cytotoxic effects on the growth, lipid metabolism, and reproductivity of numerous fish. Propranolol, a widely used β-blocker, could decline the heart rate of Daphnia magna (Jeong et al. 2018) and could create raised abnormal cells growth (125 µg L−1), decreased hatching rate, and increased mortality rate (100% at 12.5 mg L−1) in Paracentrotus lividus (zebrafish) (Ribeiro et al. 2015).
A substantial scientific concern is currently related to pharmaceutical transformation products, which pose a remarkable risk to the ecosystem; pharmaceutical transformation products can be generated during the water treatment process or in the environment through biodegradation, photodegradation, and oxidation. The pharmaceutical transformation products not only can be as harmful as the primary pristine drugs but even more dangerous. For example, the polar transformation of the antiviral drug acyclovir generates the carboxy-acyclovir and N-(4-carbamoyl-2-imino-5-oxoimidazolidin)formamide-n-methoxy-acid during the (waste)water treatment by ozonation (Schlüter-Vorberg et al. 2015). The ecotoxicity results of transformation products demonstrated that carboxy-acyclovir could considerably diminish the fertility of D. magna (40% at 102 mg L−1) and carboxy-acyclovir and N-(4-carbamoyl-2-imino-5-oxoimidazolidin)formamide-n-methoxy-acid could prevent the green algae growth, whereas no ecotoxicity is reported for acyclovir up to 100 mg L−1 (Borgatta et al. 2015).
The toxic effects of salicylic acid, as a well-known metabolite of non-steroidal anti-inflammatory drugs, on early life stages of Cyprinus carpio (common carp) comprise lipid peroxidation, decreasing weight, and hatching disturbance (Zivna et al. 2015). Salicylic acid could cause the neurotoxic effect, metabolism disruption, decreased respiratory capacity, and induced oxidative stress in M. galloprovincialis (mussel) (Freitas et al. 2019). Investigation of the temperature influence on the salicylic acid cytotoxicity on M. galloprovincialis revealed more toxicity at higher temperatures (control = 17 ± 1 °C) (Freitas et al. 2020). Due to the magnitude of pharmaceutical risks on the environment, especially the aquatic environment and aquatic organisms, the additional information in this field is provided in Table 2.
Table 2.
Toxicological effects of pharmaceuticals and their metabolites in the aquatic environment
| Category | Chemicals | Species | Class | Ecotoxic effects | References |
|---|---|---|---|---|---|
| Antibiotic | Anhydrotetracycline | Chlorella vulgaris | Algae | Alteration in cell permeability and oxidative stress | (Xu et al. 2019) |
| Antibiotic | Cephalexin | Lemna gibba | Plant | Decreasing the population | (Liu et al. 2020b) |
| Antibiotic | Chlortetracycline | Vibrio fischeri | Bacteria | Luminescence inhibition | (Wei et al. 2019) |
| Antibiotic | Ciprofloxacin | Pseudokirchneriella subcapitata | Algae | Toxic effects on the antioxidant system | (Nie et al. 2013) |
| Antibiotic | Ciprofloxacin | Zebrafish | Fish | Cardiovascular toxicity | (Shen et al. 2019) |
| Antibiotic | Ciprofloxacin | Lemna gibba | Plant | Decreasing the population | (Liu et al. 2020b) |
| Antibiotic | Chlortetracycline | Oreochromis niloticus | Fish | Growth inhibition | (Liu et al. 2020b) |
| Antibiotic | Clarithromycin | Pseudokirchneriella subcapitata | Algae | Decreasing the population | (Liu et al. 2020b) |
| Antibiotic | Erythromycin | Sparus aurata | Fish | Inducting the oxidative stress | (Rodrigues et al. 2019a) |
| Antibiotic | Erythromycin | Pseudokirchneriella subcapitata | Algae | Toxic effects on the antioxidant system | (Nie et al. 2013) |
| Antibiotic | Erythromycin | Sparus aurata | Fish | Histopathological influences in liver and gills | (Rodrigues et al. 2019c) |
| Antibiotic | Erythromycin | Oncorhynchus mykiss | Rainbow trout | DNA damage | (Rodrigues et al. 2019b) |
| Antibiotic | Erythromycin | Synechococcus leopoliensis | Algae | Decreasing the population | (Liu et al. 2020b) |
| Antibiotic | Ofloxacin | Microcystis aeruginosa | Algae | Decreasing the population | (Liu et al. 2020b) |
| Antibiotic | Oxytetracycline | Sparus aurata | Fish | Histopathological influences in liver and gills | (Rodrigues et al. 2019c) |
| Anticancer | Norfloxacin | Microcystis aeruginosa | Algae | Decreasing the population | (Liu et al. 2020b) |
| Antidepressant | Norsertraline | Scardinius erythrophthalmus | Fish | Bioaccumulation in the liver | (Arnnok et al. 2017) |
| Antiepileptic | Carbamazepine | Gammarus pulex | Crustaceans | Behavioral alterations | (Liu et al. 2020b) |
| Antiepileptic | Carbamazepine | Danio rerio | Fish | Diminished steroid production | (Fraz et al. 2018) |
| Anti-inflammatory analgesic | Paracetamol | Rhamdia quelen | Catfish | Histopathological alterations of the gills and kidneys | (Perussolo et al. 2019) |
| β-blocker | Propanolol | Daphnia magna | Planktonic crustacean | Decreased heart rate | (Jeong et al. 2015) |
| Hormone | 17α-Ethynylestradiol | – | Fish | Defeminization of female fish | (Aris et al. 2014) |
| Lipid regulator | Atorvastatin | Mytilus edulis | Mussel | Metabolic disruption | (Falfushynska et al. 2019) |
| Lipid regulator | Gemfibrozil | Danio rerio | Fish | Genetic changes | (Liu et al. 2020b) |
| Lipid regulator | Gemfibrozil | Danio rerio | Fish | Diminished steroid production | (Fraz et al. 2018) |
Human risks
Pharmaceuticals and their metabolites or transformation products are active chemicals that affect the environment or aquatic species, make their way into the food chain, and ultimately imperil humans as the end consumers. The spread of antibiotic-resistant bacteria due to inappropriate antibiotic consumption is extremely troublesome. On the other hand, the antibiotic-resistant genes are transferred between antibiotic-resistant bacteria through horizontal gene transfer and subsequently transported into the human body via trophic transfer (Khan et al. 2020b).
Antibiotic-resistant bacteria containing antibiotic-resistant genes can reach the human body through irrigation by using treated (waste)water or in other words, via the food chain (Kumar and Pal 2018). Additionally, antibiotic-resistant bacteria in drinking water can harm human health by disrupting the balance of intestinal microbiota. As a result of antibiotic-resistant gene transfer, antibiotic resistance develops in humans, so antibiotics' therapeutic benefits are lessened in the human body (Ma et al. 2018). Antibiotic resistance can result in prolonged morbidity and even mortality as a consequence of pathogenicity, and increased intensity and outbreaks of disease.
Human mortality associated with antibiotic resistance has been announced up to 25,000 each year by European Centre for disease prevention and control. Additionally, the Centers for Disease Control and Prevention declared that ~ 2 million people are infected with antibiotic-resistant bacteria each year leading to at least 23,000 deaths. Further, it is estimated that by 2050, the world death rate will surpass 10 million individuals annually if antibiotic-resistant genes and antibiotic-resistant bacteria are not addressed (Parry and Threlfall 2008).
Another adverse effect of antibiotic contaminants is bioaccumulation. Prolonged exposure of vertebrates and invertebrates to antibiotic contaminants causes a biological accumulation of antibiotics in their tissues and muscles. So, the consumption of aquatic organisms with bioaccummulated antibiotics generates a significant risk to human health.
Research has shown that children, especially those who are susceptible to the toxic xenobiotics load, can endanger by consuming fish treated with a legal oxytetracycline dose (Limbu et al. 2018). Furthermore, the direct consumption of antibiotic residues via drinking water can affect human health. For example, tetracycline residue may result in nephropathy, joint illness, endocrine disturbance, mutagenicity, central nervous system dysfunction, and altering photosensitivity.
Anticancer or cytostatic drugs despite their impressive influences in treating cancer and saving human lives, have many unpleasant side effects (Jureczko and Kalka 2020). Anemia, alopecia, and nausea or vomiting are among the undesirable effects of anticancer drugs. Moreover, the rapidly dividing of cells in bone marrow, mucous membranes, and hair can be disrupted by cytostatic pharmaceuticals. The most vulnerable groups to the DNA harm induced by chemotherapy pharmaceuticals are the fetuses, babies, and children due to the many rapidly growing cells they possess (Jureczko and Kalka 2020).
Chemotherapy-induced mutations could trigger embryonic mortality or congenital impairments in a fetus, and intense illnesses like autism, diabetes, idiopathic arthritis, and even cancer in babies or children. Cytostatic pollutants can generate serious hazards in non-patients and cause carcinogenic, mutagenic, and genotoxic effects (Zounková et al. 2007). It is widely established that exposure to low concentrations of cytostatic pollutants can have irreversible health consequences.
Although the risks of endocrine-disrupting compounds on human health have been nearly ignored in the scientific community due to a shortage of studies on their possible harmful influences, endocrine-disrupting compounds exposure at low levels (ng L−1) could detrimentally impact the human physiological function and ultimately human populations (Wee and Aris 2017). The endocrine-disrupting compounds can cause the following dangerous effects: abnormalities of the central nervous system (behavioral and neurological alteration), thyroid (hypothyroidism), pancreas (hyperinsulinemia), reproductive system (early puberty, diminished fertility, disrupted embryonic growth, and breast or prostate cancer), and metabolic system (obesity, diabetes, cardiovascular and autoimmune illnesses). For example, the results of exposure to ibuprofen in young men demonstrated that it can change the endocrine system and testicular physiology by selective transcriptional suppression in the testes, hence inducing compensated hypogonadism (Kristensen et al. 2018).
The relationship between mortality and β-blockers toxicity has been investigated in Finland on 595 people who underwent treatment for hypertension or other cardiac diseases (Kriikku et al. 2021). Examination of the post-mortem toxicological profile of metoprolol/propranolol-positive individuals exhibited that propranolol compared with metoprolol can be associated with more drug abuse, suicide, and lethal poisoning. Given the destructive effects of pharmaceutical contaminants and their metabolites on human health and the environment, it is necessary to develop more efficient methods for eliminating them from the aquatic environment.
Molecular imprinting polymers and their role in water decontamination
The removal of pollutants from water is very important due to their adverse effects on human health and the environment. So far, many methods such as coagulation, sedimentation, ion exchange, among others, have been proposed to eliminate contaminants from water. However, the typically employed methods are usually nonselective and often lead to secondary contamination impacts. Thus, it appears that the innovative newer approaches for the selective removal of toxic contaminants from water sources are of great economic and environmental importance. Consequently, current research efforts for tailor-made materials, with high selectivity and specificity and long-term stability, as well as the development of modern, efficient, eco-friendly, and cost-effective water purification technologies are the needs of the hour.
Molecular imprinting is a method to produce pattern-shaped holes in material matrices with prearranged selectivity and high affinity. It is one of the most innate nature-mimicking approaches wherein the idea of creating selective substrate recognition positions in a matrix entails a molecular pattern by the casting technique. First, the pattern molecule makes variable interactions with one or more functional materials in the pre-arrangement step. Then, the locking of interactions between pattern molecules and functional materials leads to the creation of a matrix containing the location-selectable identification sites for the pattern. Thus, this method can create specific binding sites for the location of selected molecules and is similar to the “Fisher Lock and Key Model” model for the enzyme.
A “molecular key” can be anything from drugs or metal ions to proteins. The size of the molecular key is an important factor in the preparation of molecularly imprinted polymers as the formation of molecularly imprinted polymers is very difficult by increasing of molecular key. Therefore, the use of molecular mold materials in the removal of drugs can be considered in many ways which are discussed in the following sections encompassing the methods of making molecular mold materials, their advantages and disadvantages, limitations, and prospects for their deployment in water treatment.
Molecularly imprinted polymers
Molecularly imprinted polymers, newer kinds of tailor-made materials with strong molecular recognition ability, are obtained by a copolymerization process following a self-assembly step between the cross-linkers and functional monomers in the appropriate solvent with the presence of the template molecule (Liu et al. 2021b). Molecularly imprinted polymers exhibit unique characteristic features such as specific target recognition, structural predictability, and practicability, which collectively ensure their broad applications in various fields, including chemical sensing, drug delivery, artificial antibodies, biotechnology, separation science, and water treatment (Li and Yang 2021). Molecularly imprinted polymers are highly cross-linked polymers with great physical stability that can be harnessed under harsh environmental conditions, such as extreme pH, high temperatures and pressures, and reactions in organic solvents. These advantages make molecularly imprinted polymers utmost suited for various environmental remediation applications.
General procedures for the preparation of molecularly imprinted polymers
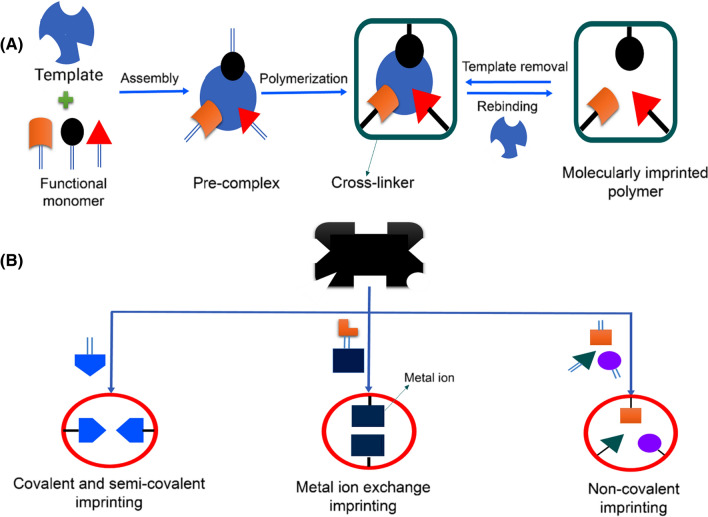
Molecularly imprinted polymers are usually produced via three main successive steps, as depicted in Fig. 2A: (1) formation of a pre-polymerization complex by self-assembling functional monomers into a selected template through metal ion coordination, covalent or non-covalent bonds; (2) polymerization and crosslinking reactions of the template-monomer complex in the presence of an initiator and an appropriate cross-linker; and (3) removal of the template molecules using suitable solvents for creating available binding sites (i.e. cavities). The choice of the appropriate chemical reagent(s) for the template, monomer, initiator, and cross-linker is vital in the synthesis of molecularly imprinted polymers with highly specific cavities designed for the template molecule.
Fig. 2.
Molecularly imprinted polymers synthesis including (1) formation of a pre-polymerization complex by self-assembling functional monomers (2) polymerization and crosslinking reactions of the template-monomer complex (3) removal of the template molecules using suitable solvents (A) and main tactics for producing molecularly imprinted polymers based on the nature of interactions between the functional monomers and templates (B)
The most critical requirement for selecting a monomer is that it needs to have functional groups capable of interacting with the template but not participating in the polymerization with the template. Meanwhile, the template should have characteristic properties such as solubility under imprinting conditions, strong interaction with functional monomers, and availability in large quantities at a competitively low price. The choice of the cross-linker must also be taken into consideration because the binding capacity and stability of the molecularly imprinted polymers increase with the degree of cross-linking. The initiator is used in the imprinting process to initiate the polymerization of the cross-linking agent by means of heat and light (Pichon and Chapuis-Hugon 2008).
Furthermore, the ratios of template/monomer and monomer/crosslinker also play important roles in preserving the selectivity, efficiency, and required rigidity of the molecularly imprinted polymers. The standard ratio of the template to functional monomer in most of the studies is 1:4, while the optimum ratio of monomer-crosslinker is 1:2 (Azizi and Bottaro 2020). Some common functional monomers, target templates, cross-linkers, and initiators used in molecular imprinting are summarized in Table 3 (Pan et al. 2018; Sajini and Mathew 2021).
Table 3.
Common functional monomers, target templates, cross-linkers, and initiators used in molecularly imprinted polymers
| Essential elements | Typical example |
|---|---|
| Functional monomers |
Covalent: 4-Vinyl benzene boric acid; 4-vinyl aniline; 4-vinyl benzaldehyde; tert-butylp-vinylphenylcarbonate Semi-covalent: 3-Isocyanatopropyltriethoxysilane Non-covalent: Acrylic acid; methacrylic acid; trifluoromethyl acrylic acid; itaconic acid; 2-vinylpyridine; p-vinylbenzoic acid; 1-vinylimidazole; allylamine; 4-vinylpyridine; styrene; 4-ethylstyrene; methyl methacrylate; acrylamide; methacrylamide; 2-hydroxyethyl methacrylate; 2-acrylamido-2-methy-1-propane sulfonic; 3-aminopropyltriethoxysilane; trans-3-(3-pyridyl)-acrylic acid; methylvinydiethoxysilane; glycidoxypropyltrimethoxysilane; 3-methylacryloxyprolyl trimethoxysilane Ligand exchange: Fe2+/methacrylic acid complex; Cu(II)-iminodiacetate-derivatized vinyl monomer |
| Target templates |
Biomacromolecules: Bovine serum albumin; adenosine; lysozyme; 3,5-cyclic monophosphate Organic molecules: Pesticides: Benzimidazole fungicides; 2,4-dichlorophenoxyacetic acid; atrazine. Explosive: 2,4,6-trinitrotoluene Endocrine-disrupting chemicals: Oestradiol; bisphenol A; polycyclic aromatic hydrocarbon; oestrone. Amino acids and peptides: alanine; tyrosine; N-terminal histidine sequence of dipeptides; tripeptides; cinchona alkaloids; helical peptides. Pharmaceuticals: quinolones; tetracycline; sulphonamides; propranolol; digoxin. Sugars: d-glucose; d-fructose; d-galactose Ions: CH3Hg(I); Sr(II); Fe(III); Pb(II); Hg(II); As(III); Cu(II); Cd(II); Cr(III); Ni(II); Th(IV); PO43−; UO22+; Eu(III) Cells and viruses: Bovine leukemia virus; gut-homing; tobacco mosaic virus; dengue virus |
| Cross-linkers |
Covalent: Dicumyl peroxide; bis-(1-(tert-butylperoxy)-1-methylethyl)-benzene; triallyl isocyanurate Non-covalent: Divinylbenzene; N,N′-methylenediacrylamide; ethylene glycol dimethacrylate; 1,3-diisopropenyl benzene; 2,6-bisacryloylamidopyridine; pentaerythritol tetraacrylate; N,N′-1,4-phenylenediacrylamine; 3,5-bis(acryloylamido)benzoic acid; trimethylpropane trimethacrylate; N,O-bisacryloyl-phenylalaninol; tetra-methylene dimethacrylate; 1,4-diacryloyl piperazine; glycidilmethacrylate; N,O-bismethacryloyl ethanolamine |
| Initiators | 4,4′-Azo(4-cyanovaleric acid); potassium persulfate; azobisdimethylvaleronitrile; 2,2-azobis(2,4-dimethylvaleronitrile); dimethylacetal of benzyl; benzoylperoxide; azobisisobutyronitrile; 2,2-azobis(2-methylbytyronitrile) |
Based on the nature of interactions between the functional monomers and templates in the pre-polymerization step, molecular imprinting approaches can be classified into covalent, non-covalent, semi-covalent, and metal-ion exchange (Fig. 2B). Covalent imprinting was initially reported for designing artificial receptors (Pan et al. 2018). Covalent interactions in this type of imprinting can be the establishment of Schiff base bonds (Wulff and Vietmeier 1989), acetal (Wulff and Wolf 1986), ketals (Shea and Dougherty 1986), or boronic ester bonds (Wulff and Schauhoff 1991).
To implement covalent imprinting, the template molecule prerequisite is to have at least one functional group amenable to being derivatized; and aldehydes, amines, ketones, carboxylic acids, or alcohols are some of the common examples found in the literature (Herrera-Chacón et al. 2021). The covalent-based molecularly imprinted polymers display the utmost homogenous structures with well-defined binding sites and cavities owing to the fixed stoichiometric ratio of template molecules to functional monomers arising from the specificity of the bond formation (Azizi and Bottaro 2020).
However, the application of covalent imprinting is restricted by the following drawbacks: (i) the limited choice of an appropriate monomer-template complex that can form a covalent bond with reversible behavior (Elugoke et al. 2021); (ii) prolonged synthesis time due to slow rebinding kinetics; and (iii) the need for extreme effort to remove the template because of strong covalent interactions (Sajini and Mathew 2021).
The non-covalent imprinting approach, as the name suggests, is based on the non-covalent interactions (e.g. hydrogen bonding, electrostatic attraction, charge transfer, the van der Waals forces, dipole–dipole and/or π-stacking) between the template and functional monomers, which has been established by Mosbach’s research group (Sajini and Mathew 2021). The non-covalent-based molecularly imprinted polymers are more flexible in the selection of templates and monomers and are easier to prepare. Furthermore, this imprinting type is universal due to the more favorable rebinding kinetics, simplicity in the removal of templates, and broad applications (Liu et al. 2021b).
The main disadvantage of non-covalent imprinting is the low selectivity of extraction due to the presence of many non-specific binding sites in the ensued molecularly imprinted polymers caused by the excess of functional monomers. To reduce non-selective binding, semi-covalent imprinting, an intermediate strategy that combines the advantages of both, the covalent and the non-covalent synthesis, has been used (Adumitrăchioaie et al. 2018; Gao et al. 2020; Ndunda 2020; Sajini and Mathew 2021). In this method, the template-monomer complex is linked together by covalent bonds, but after the polymerization step, the covalent features are replaced by non-covalent functionalities so that the removal of template molecule occurs via non-covalent interaction (Azizi and Bottaro 2020).
Similar to the semi-covalent imprinting process, metal-ion exchange imprinting appears as an alternative to improve the performance of existing methods. This methodology of imprinting aims to utilize metal ions to create ionic bonds (stronger than electrostatic interactions or hydrogen bonds) that strengthen the interaction between the template molecule, and monomer, while also combining the benefits of covalent and non-covalent methods. The strength of the interactions and the stability of the template-monomer complexes can be adjusted by varying the metal, its oxidation state, and/or the ligand characteristics (Herrera-Chacón et al. 2021). Despite its evident potential, this approach is limited to strategies containing complex-forming groups with metals.
The selection of the polymerization method for imprinting is an important factor as it greatly affects the shape and size of the required Molecularly imprinted polymers (Farooq et al. 2018). Bulk polymerization, also known as traditional polymerization, is reported as the most extensively used technique for the preparation of molecularly imprinted polymers due to its simplicity (Sajini and Mathew 2021). The principle of this method involves the mixing of the template molecule, functional monomer, initiator, and crosslinker in a container with subsequent application of light, heat, or radiation. The resultant polymer monolith is then crushed, ground into small particles mainly in the 25–50 µm size range, and sieved (Pichon and Chapuis-Hugon 2008). The last step consists of sedimentation to remove fine particles.
The major disadvantages of bulk polymerization are the difficulty in removing the template from the interior part of the molecularly imprinted polymer particles, the low yields, and fewer binding sites due to the grinding damage (He et al. 2021; Sajini and Mathew 2021), other methods of polymerization, such as suspension polymerization, precipitation polymerization, in-situ polymerization, and sol–gel polymerization, are exploited to obtain spherical and monodispersed particles of molecularly imprinted polymers. An example of morphological differences of curcumin molecularly imprinted polymers synthesized by different polymerization methods (Suwanwong et al. 2014). The principle as well as the associated advantages and disadvantages of various polymerization methods are summarized in Table 4.
Table 4.
Comparison of polymerization methods for the synthesis of molecularly imprinted polymers
| Method of imprinting | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Bulk polymerization | Functional monomers, templates, initiators, crosslinking agents are uniformly mixed in a certain ratio in a non-polar solvent and sealed in a vacuum for crosslinking polymerization. The resulting bulk polymer is then subjected to crushing and sieving so that the template molecule can be removed from the polymer | Simplicity in synthesis; no requirement for expensive or sophisticated instrumentation; high purity of the produced molecularly imprinted polymers | Low performance; difficulty in template elution; irregular particle in size and shape; time-consuming; fewer binding sites |
| In-situ polymerization | The synthesis of molecularly imprinted polymers is conducted directly in the column by simple polymerization | In-situ preparation, one-step; continuity and uniformity; good porosity; rapid response to the template is assured | Poor selectivity; slow flow rate; short service life; high column pressure; extensive optimization is required for each new template system |
| Precipitation polymerization | The precursors are dissolved in a suitable solvent for reactive polymerization and the Molecularly imprinted polymers precipitate out of the reaction system after the formation of solid microspheres that are insoluble in the reaction medium | Imprinted microspheres with uniform size and large specific surface area; high performance; single preparative step | A large amount of template and solvents; high dilution factor; high requirements for solvent viscosity |
| Suspension polymerization | The reaction precursors, including functional monomers, templates, porogens, cross-linkers, and other substances, dissolved in the selected solvent, followed by the addition of the dispersant. The mixture is further sealed and stirred at high speed | Uniform spherical particles; large scale assembly possible; high reproducibility | Long preparation period; big particle size; specialist surfactant polymers required; poor recognition; the presence of a surfactant and a stabilizer is mandatory |
| Emulsion polymerization | The template, cross-linker, and functional monomer are first emulsified in water, followed by the addition of stabilizers to the disperse phase to eliminate diffusion in the continuous phase | Uniform particle size; the abundance of binding sites on the molecularly imprinted polymer surface | Complex and expensive synthesis; low purity of the products |
| Surface imprinting polymerization | Functional monomers are attached to the surface of the matrix carriers (e. g. SiO2, carbon dots, carbon nanotubes, metal–organic framework) by suitable techniques such as chelation or grafting. Then the template is introduced to the monomer to form a pre-polymerization complex, which is further cross-linked on the matrix carrier surface in the presence of the initiator, forming surface molecularly imprinted polymer | Easy to prepare; large imprinted surface; abundant binding sites; controlled size and shape; good reproducibility; high selectivity and sensitivity | The limited yield on large scale preparation |
| Sol–gel polymerization | Inorganic precursor and template molecules are dissolved in low molecular weight solvent, and the gel is formed by hydrolysis (water) and condensation polymerization | Eco-friendly reaction solvent; good thermal and chemical stability; simple preparation under mild operating conditions; | The difficulty in forming porous silicon; lack of polymerization method and the functional monomer; low sensitivity; the need for high pH hydrolysis |
Currently, the aforementioned polymerization methods (Table 4) are being modified to improve the properties of the molecularly imprinted polymers which include strategies deploying dual functional monomers (Zhao et al. 2019; Ut Dong et al. 2021), ultrasound-assisted precipitation polymerization (Abdullah et al. 2019), the use of a dual template (Lu et al. 2019), microwave-assisted emulsion polymerization (Chen et al. 2015), and microwave-assisted heating (Fu et al. 2015). Furthermore, the traditional polymerization techniques are also upgraded by newer techniques, including green chemistry concepts, with the use of deep eutectic solvents (Li and Row 2019), supercritical carbon dioxide (Byun et al. 2013), and ionic liquids (Viveiros et al. 2018; Liu et al. 2021a).
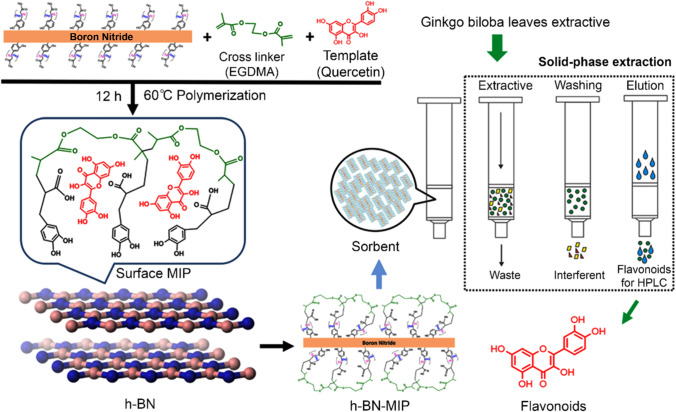
For example, Li and Row (Li and Row 2019) described the fabrication of hexagonal boron nitride-molecularly imprinted polymer nanoparticles from deep eutectic solvents containing hexagonal boron nitride, template (quercetin), porogen (methanol), initiator (2,2-azobisisobutyronitrile), and crosslinking agent (ethylene glycoldimethacrylate) and used it for the improved solid-phase extraction of flavonoids from Ginkgo biloba leaves (Fig. 3). The application of deep eutectic solvents resulted in the enhanced recognition capability of hexagonal boron nitride-molecularly imprinted polymer for flavonoids compared to conventional molecularly imprinted polymer nanoparticles because the recognition sites of the monomers are protected via the hydrogen bond of the deep eutectic solvents before the molecularly imprinted polymer is polymerized.
Fig. 3.
Formation of hexagonal boron nitride (h-BN)-molecularly imprinted polymers and the solid-phase extraction process. The h-BN was mixed with EGDMA as a crosslinking agent, AIBN as an initiator, methanol as a porogen, and quercetin as a template. Afterward, the product was leached by methanol in a Soxhlet extractor. Reprinted with permission of Springer from ref. (Li and Row 2019). h-BN hexagonal boron nitride; h-BN-MIP hexagonal boron nitride-molecularly imprinted polymer; MIP molecularly imprinted polymer; EGDMA ethylene glycol methacrylate; AIBN 2,2-azobisisobutyronitrile; HPLC high-performance liquid chromatography
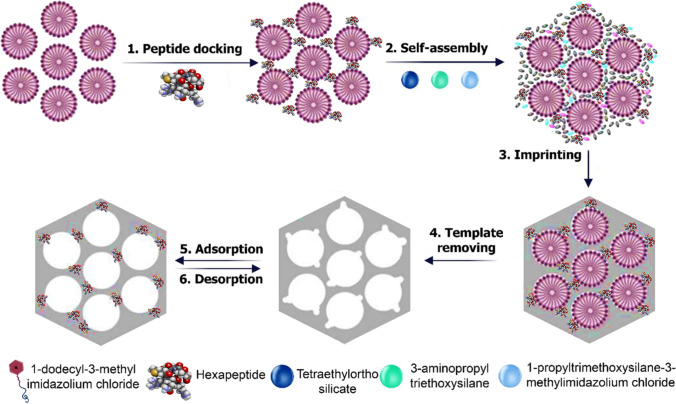
Shichao Ding et al. (2018) applied a novel imprinting strategy with amphiphilic ionic liquids as a surfactant to prepare well-defined peptide-imprinted mesoporous silica for specific recognition of an immunostimulating hexapeptide from human casein (Fig. 4). Herein, the ionic liquid, 1-dodecyl-3-methylimidazolium chloride, was selected as a surfactant, which assisted the imprinted template (Val–Glu–Pro–Ile–Pro–Tyr) to form micelles (mesoporous template) in the pre-polymerization system. Due to the multiple weak interactions between the imprinted template, the ionic liquids, and the hole of molecularly imprinted polymer, the dual-template was easily removed.
Fig. 4.
Synthesis of peptide imprinted mesoporous silica by 1-dodecyl-3-methylimidazolium chloride amphiphilic ionic liquid as the surfactant through docking oriented imprinting approach. Reprinted with permission of IOP from ref. (Ding et al. 2018)
Removal of pharmaceuticals by molecularly imprinted polymers
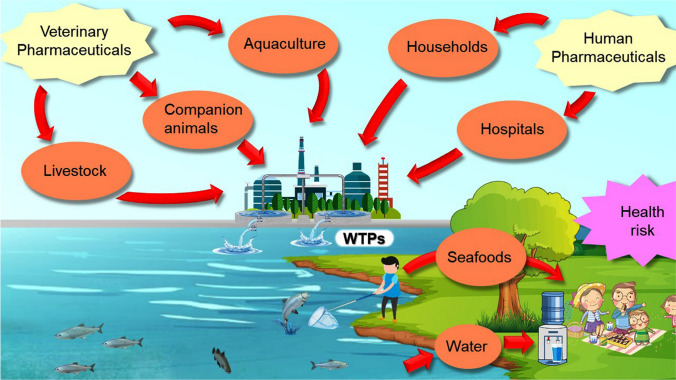
In recent years, the widespread use of pharmaceutical drugs has raised serious concerns about potential unfavorable effects on human health due to their improper disposal into the environment (Metwally et al. 2021). The pharmaceuticals and their metabolites can infiltrate the aquatic environment via different routes as illustrated in Fig. 5 for various pathways and the fates of pharmaceuticals discharged into the aquatic environment.
Fig. 5.
Routes and fate of discharged pharmaceuticals into the aquatic environment. The pharmaceuticals and their metabolites can infiltrate the aquatic environment. After metabolism in the animal and human body followed by excretion, pharmaceuticals or their metabolites have been significantly discharged as domestic wastewater. They head to wastewater treatment plants and sometimes can transform into more persistent and toxic compounds by additional chemical reactions with the existing substances in wastewaters and the materials used in the wastewater treatment process. The majority of pharmaceuticals are water-soluble with high polarity, therefore they can easily sidestep wastewater treatment plants and enter ground and surface water. WTPs wastewater treatment plants
As can be seen, after metabolism in the animal and human body followed by excretion, pharmaceuticals or their metabolites have been significantly discharged as domestic wastewater (Quesada et al. 2019). In this context, they head to wastewater treatment plants and sometimes can transform into more persistent and toxic compounds by additional chemical reactions with the existing substances in wastewaters and the materials used in the wastewater treatment process (Mackuľak et al. 2019). The majority of pharmaceuticals are water-soluble with high polarity, therefore they can easily sidestep wastewater treatment plants and enter ground and surface water (Sharma et al. 2019). If pharmaceutical drugs and their residues in contaminated food and water sources are inadvertently ingested, they can cause disturbance in the enzymatic and hormonal systems of animals and humans (Husin et al. 2021).
In the last decade, molecularly imprinted polymers and their composites have been garnering widespread attention in the removal of pharmaceutical residues from aqueous solutions in view of their high stability, good adsorption capacity, and superior selectivity towards target pollutants (Madikizela et al. 2018a). Compared to other materials commonly used for several pharmaceutical treatments such as biochar, activated carbon, and resin, the adsorption capacity of molecularly imprinted polymers is not significantly inferior, but the selectivity is much superior (Yu et al. 2022). The recent applications of some novel molecularly imprinted polymers, in the removal of pharmaceutical residues from wastewaters, are summarized in Table 5.
Table 5.
Application of recent molecularly imprinted polymers in the removal of pharmaceuticals from wastewater
| Pharmaceuticals | Target pollutant |
Molecularly imprinted polymers | Particle size (nm)/specific surface area (m2 g−1) | Removal method and conditions | Qm or R-MIP (mg g−1 or %) a |
Imprinting factor (IF) b | Synthesis method |
References |
|---|---|---|---|---|---|---|---|---|
| Antibiotics | Norfloxacin | MIP-MOF/CF | 364/~ 200 |
Adsorption: m = 10 mg pH = 7 t = 3 h V = 100 mL |
456 | 1.93 | Surface imprinting combined with the solvothermal method | (Liu et al. 2021d) |
| CoFe2O4@TiO2-MMIP | –/88.9 |
Adsorption: m = 40 mg pH = 6–8 t = 60 min V = 100 mL |
14.26 | – | Surface imprinting combined with the solvothermal method | (Fang et al. 2021) | ||
| Kitasamycin | MIP | –/– |
Adsorption: m = 10 mg pH = 8–10 t = 3 h V = 100 mL |
127.06 | 2.97 | Surface imprinting | (Chen et al. 2021) | |
| Ciprofloxacin | BiPO4@GO-MIP | –/– |
Adsorption: m = 20 mg pH = 6–8 t = 15 min V = 100 mL |
252 | 9.33 | Surface imprinting | (Kumar et al. 2020) | |
| Tetracycline | ZnO@NH2-UiO-66 | –/152.99 |
Photocatalysis: Visible light t = 30 min 50 W xenon lamp m = 10 mg V = 1 mL |
61.9% | – | Post-synthetic modification method | (Du et al. 2020) | |
| Ag/Ag3VO4/g-C3N4 | –/97.6 |
Adsorption: m = 50 mg t = 1.5 h V = 100 mL |
16.51 | 1.08 |
Surface imprinting and photo-induced polymerization |
(Sun et al. 2019) | ||
|
Photocatalysis: λ > 420 nm t = 120 min C = 20 mg/L |
90.18% | – | ||||||
| Tylosin | MIP | –/– |
Adsorption: m = 16 mg pH = 8.5 t = 8 h V = 10 mL |
74.3 | 1.73 | Precipitation polymerization | (Zeng et al. 2021b) | |
| Chloramphenicol | Si@MIP-CAP | ~ 439/58.99 |
Adsorption: m = 5 mg pH = 7 t = 24 h V = 1 mL |
32.26 | 2.27 | Precipitation polymerization | (Mohamed Idris et al. 2020) | |
| Sulfonamides | MIP | –/144.78 |
Adsorption: m = 20 mg pH = 7 t = 3 h V = 2 mL |
3.33 | 1.44 | Surface polymerization | (Zhu et al. 2019) | |
| MIP | –/– |
Adsorption: m = 20 mg pH = 7 t = 1 h V = 50 mL |
12.48 | 1.4 | Prepolymerization | (Fan et al. 2020) | ||
| Antiseptics | Triclosan | TCS-CTS-Fe0-MIP | –/– |
Adsorption: m = 10 mg pH = 6–9 t = 6 h V = 100 mL |
20.86 | 1.38 | Surface polymerization | (Chen et al. 2017) |
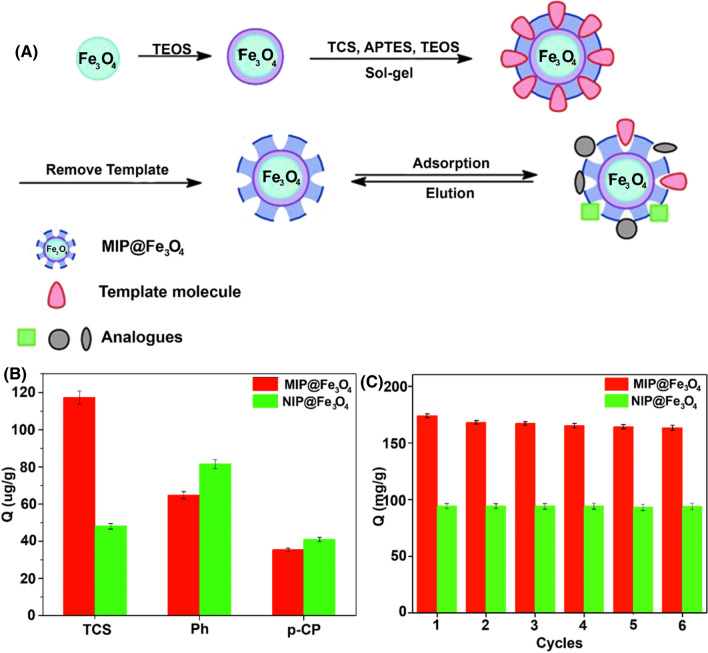
| MIP@Fe3O4 | 200/- |
Adsorption: m = 20 mg pH = 6–9 t = 20 min V = 10 mL |
0.218 | 2.46 | Surface imprinting and sol–gel | (Kong et al. 2020) | ||
| Antidepressant | Sertraline | MIP | –/193 |
Adsorption: m = 5 g pH = 6–8 t = 20 h |
72.6 | 3.7 | Bulk Polymerization | (Gornik et al. 2021) |
| Anticonvulsant | Carbamazepine | Fe3O4@CTS@MIP | 500/265.8 |
Adsorption: m = 4 mg pH = 9 t = 3 h V = 4 mL T = 30 °C |
76.34 | 4.83 | Reversible addition-fragmentation chain transfer polymerization | (Wang et al. 2019) |
| 4VNIA-MIP | 24.65/384.9 |
Adsorption: m = 10 mg t = 2 h min V = 50 mL |
28.40 | 2.72 | Two-step polymerization | (He et al. 2020) | ||
|
Non-steroidal anti-inflammatory drugs |
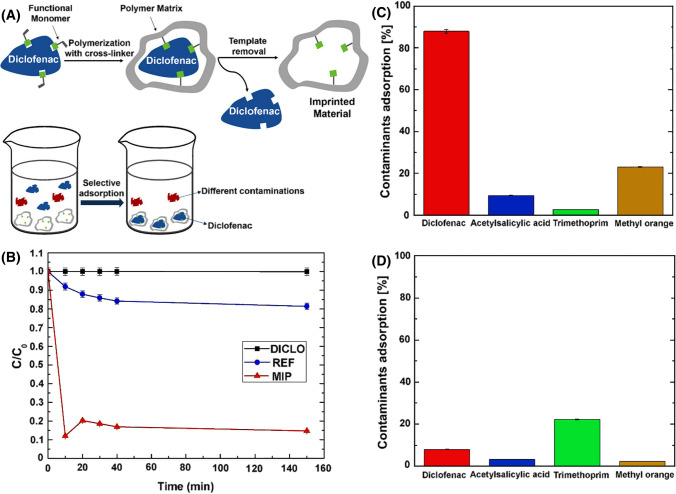
Diclofenac | MIP | –/320 |
Adsorption:m = 5 mg t = 10 min C = 10–4 Mol/L V = 6 mL |
32.5 | – | Bulk polymerization | (Cantarella et al. 2019) |
| MIP | 200/– |
Adsorption: m = 10 mg pH = 7 t = 120 min V = 20 mL |
5.5 | – |
Precipitation polymerization method |
(Amaly et al. 2021) | ||
| MIP-TiO2 | > 100/49.07 |
Adsorption: m = 10 mg pH = 6.5 t = 2 h V = 10 mL |
0.16 mg/g | 1.83 | Surface imprinting technology | (Bi et al. 2021) | ||
|
Photocatalysis: λ < 400 nm (300 W xenon lamp) m = 80 mg C = 1 mg g−1 V = 200 mL T = 1 h in dark + 10 min irradiation |
~ 89% | |||||||
|
Potassium diclofenac |
MIP-BC | –/47.98 |
Adsorption: m = 12.5 mg pH = 6 t = 5 min V = 5 mL |
1.82 | 5.5 | Bulk polymerization | (Da Silva et al. 2019) | |
| MIP-SDS | –/43.36 | 1.12 | 3.2 | |||||
| MIP-TTX-100 | –/42.49 | 0.51 | 1.45 | |||||
| Naproxen | Fe3O4@MIP-ChCl-BuIM | –/– |
Adsorption: m = 30 mg pH = 5 t = 20 h V = 10 mL |
90.91 | – | Bulk Polymerization | (Husin et al. 2021) | |
| Ketoprofen | MIP | 2–50/209 |
Adsorption: m = 8 mg pH = 5 t = 45 min V = 10 mL |
8.7 | – | Bulk polymerization | (Madikizela et al. 2018d) | |
| Others | Sildenafil | MOG@HP-MIP | 30 − 100/219.6 |
Adsorption: m = 5 mg t = 30 min V = 1 mL |
17.8 | 2.5 | Surface imprinting technology | (Wan et al. 2021) |
| Cyproheptadine | MMIP | 300 ± 20/11.50 |
Adsorption: m = 20 mg pH = 8 t = 1 h V = 10 mL |
48.89 | 4.74 | Surface imprinting technology | (Cao et al. 2021) | |
| Amitriptyline | 47.14 | 5.63 | ||||||
| Desloratadine | 47.33 | 5.69 | ||||||
| 8-Chloroazatadine | 46.89 | 6.91 | ||||||
|
Ketotifen fumarate |
42.14 | 5.68 | ||||||
| 1-Praziquantel enantiomers | MMIPPy | –/– |
Adsorption: m = 75 mg pH = 6.5 t = 10 min V = 1 mL |
1322 | – | Surface imprinting technology | (Do Nascimento et al. 2019) | |
| 2-Praziquantel enantiomers | 1508 |
MIM molecularly imprinted materials; MIP molecularly imprinted polymer; MMIP magnetic molecularly imprinted polymer; MIP-MOF/CF molecular imprinting with metal–organic framework particles anchored to porous carbon foam; Si@MIP-CAP silica-grafted molecularly imprinted polymers for chloramphenicol adsorption; TCS triclosan; CTS chitosan; MIP-ChCl-BuIM Molecularly imprinted polymer-choline-imidazole based deep eutectic solvent; MOG@HP-MIP hierarchically porous molecularly imprinted polymers in metal–organic gel; MMIPPy magnetic molecularly imprinted polypyrrole; MIP-BC MIP-SDS MIP-TTX-100 benzalkonium chloride Sodium dodecyl sulfate and Triton ×-100 molecularly imprinted polymers; g-C3N4 graphitic carbon nitrides; NH2-UiO-66 Amino-functionalized zirconium-based metal–organic; 4VNIA-MIP 4-Vinyl benzoic acid molecularly imprinted polymers; m adsorbent dosage; t time; V volume; T temperature min minute; h hour
aQm or R–MIP, Maximum adsorption capacity (mg g−1) or removal efficiency of molecularly imprinted polymers (or %)
bIF, Imprinting factor, a ratio between adsorption capacity of molecularly-imprinted and nonimprinted materials
It has been well recognized that different types of pharmaceuticals including antibiotics, antiseptics, antidepressants, analgesics, antiretroviral, anticonvulsant, hormones, and non-steroidal anti-inflammatory drugs were found in wastewaters (Khulu et al. 2021, 2022; Metwally et al. 2021). Among them, antibiotics are considered as a new class of environmental pollutants, which can cause potential damage to ecosystem survival since continuous exposure leads to the development of bacterial resistance to existing antibiotics (Yang et al. 2018; Hu et al. 2021a,b). The removal of antibiotics by molecularly imprinted polymers has received a lot of research interest due to the difficulty of the former being biodegraded and their strong durability (Liu et al. 2021b; Zhao et al. 2021; Li and Yang 2021).
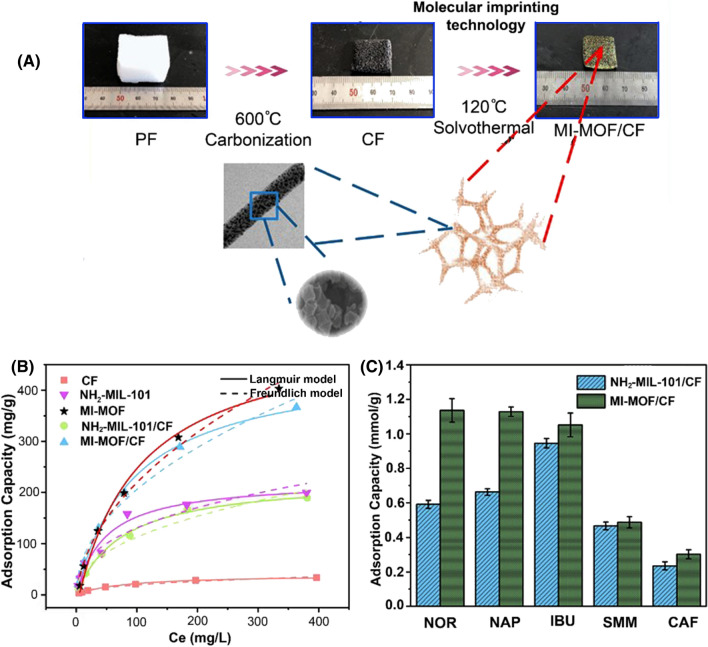
For highly efficient norfloxacin removal from the aqueous solution, a combined strategy involving molecular imprinting technology, and an in-situ growth strategy was applied to prepare advanced metal–organic frameworks (MOFs) particles of aminated porous chromium-benzenedicarboxylate (named NH2-MIL-101) anchored on the molecular imprinting metal–organic frameworks/carbon foam (MI-MOF/CF) (Fig. 6) (Liu et al. 2021d). The as-prepared MI-MOF/CF with the average particle size of 364 nm and developed surface area of about 200 m2 g−1 exhibited a far superior maximum capacity of 456 mg g−1 within 60 min for norfloxacin, compared to pristine nonimprinted material. Importantly, a specific recognition ability of the active sites played a crucial role in the selective pharmaceuticals’ adsorption ability of MI-MOF/CF, along with particle size and large surface area. Moreover, the MI-MOF/CF displayed a sustained good adsorption performance and stability over four consecutive cycles that carry a great value for industrial-scale application.
Fig. 6.
Synthesis process for the MI-MOF/CF composite (A), adsorption isotherms of NOR (B), and adsorption performances of MOF/CF and MI-MOF/CF on NOR, IBU, NAP, SMM, and CAF (C). Reprinted with permission of Elsevier from ref. (Liu et al. 2021d). Ce equilibrium concentration; PF polyurethane foam; CF carbon foam; MI-MOF molecular imprinting metal–organic framework; MI-MOF/CF molecular imprinting metal–organic frameworks/carbon foam; NH2-MIL-101 aminated porous chromium-benzenedicarboxylate; NH2-MIL-101/CF aminated porous chromium-benzenedicarboxylate/carbon foam; NOR norfloxacin; NAP naproxen; IBU ibuprofen; SMM sulfadimethoxine; CAF caffeine
Another newer kind of magnetic molecularly imprinted polymer based on CoFe2O4@TiO2 for adsorption and photocatalytic degradation of norfloxacin was synthesized via surface imprinting technology (Fang et al. 2021). As reported, CoFe2O4@TiO2-molecularly imprinted polymer had selective adsorption ability towards norfloxacin with a maximum capacity of 14.26 mg g−1. The removal efficiency was enhanced by additional photocatalytic reaction based on TiO2 under ultraviolet irradiation and reached 99.4% after 60 min. Interestingly, the magnetic molecularly imprinted polymer exhibited excellent stability, a high level of in-situ photocatalytic regeneration, and recycling performance after nine consecutive reuses with insignificant loss of the adsorption efficiency.
Nowadays, the synthesis of green molecularly imprinted polymers in water solutions instead of using toxic and volatile, chloroform, or acetonitrile has attracted considerable attention due to the introduction of environmentally friendly and benign protocols. The secondary pollution burden to the environment can be caused by the polymerization process using organic solvents, and the fabricated molecularly imprinted polymers show a poor recognition for target pollutant molecules in water-rich media (Madikizela et al. 2018c).
Ionic liquids composed only of cations and anions are one of the good choices to synthesize more eco-friendly molecularly imprinted polymers in water solutions due to their non-toxicity and water solubility (Elencovan et al. 2022). In highly polar media, they can form multiple interactions with target molecules thanks to their diversity of functional groups (Peng et al. 2019).
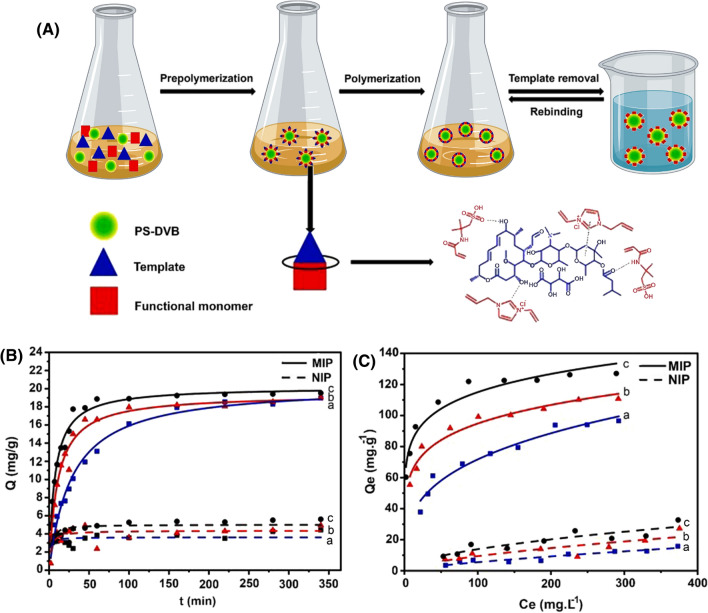
By using 2-acrylamide-2-methyl propane sulfonic acid and 1-allyl-3-vinyl imidazolium chloride ionic liquid on the surface of poly (styrene–divinylbenzene) particles, an innovative and greener molecularly imprinted polymer was successfully fabricated in water for the selective removal of kitasamycin in mixed solution (Chen et al. 2021). The molecularly imprinted polymer revealed a significant affinity towards kitasamycin with the maximum adsorption capacity of 127.06 mg g−1, in which about 90% of kitasamycin bounded onto the molecularly imprinted polymer surface within the first 30 min (Fig. 7). The powerful affinity and selectivity of the prepared molecularly imprinted polymer for kitasamycin are attributed to its molecule structure, π-π interaction, and hydrogen bond between the molecularly imprinted polymer’s surface and the target pollutant.
Fig. 7.
Synthetic procedure for molecularly imprinted polymer by 1-allyl-3-vinyl imidazolium chloride ionic liquid and 2-acrylamide-2-methyl propane sulfonic acid as a co-functional monomer on the surface of PS-DVB (A), kinetic adsorption curves (B), and adsorption isotherm curves of MIP and NIP towards kitasamycin at 25 ℃ (a), 35 ℃ (b) and 45 ℃ (c), respectively (C). Reprinted with permission of Elsevier from ref. (Chen et al. 2021). MIP molecularly imprinted polymer; PS-DVB poly (styrene–divinylbenzene); NIP non-imprinted polymer; Q adsorption capacity; Ce equilibrium concentration
Furthermore, a variety of molecularly imprinted polymers as adsorbents and photocatalysts have been developed for the removal of other antibiotics, such as ciprofloxacin (Kumar et al. 2020), tetracycline (Sun et al. 2019; Du et al. 2020), tylosin (Zeng et al. 2021b), chloramphenicol (Mohamed Idris et al. 2020), and sulfonamides (Zhu et al. 2019; Fan et al. 2020) in wastewater. The synthesis methods including imprinting factor and the results of molecularly imprinted polymers’ main characterization, the removal method, and conditions for other’s antibiotics treatment as well as their removal performance are presented in Table 5.
Antiseptics, also known as antimicrobial substances, which have the ability to safely destroy bacteria within the body, are widely used in healthcare to reduce the possibility of infection. Among commonly used antiseptics, triclosan is widespread throughout the world in pharmaceuticals and personal care products due to its multi-purpose appliance (Kong et al. 2020). However, triclosan has biological toxicity such as biological inhibition and has been frequently detected in wastewater because of its chemical stability and ability to bioaccummulate (Alshishani et al. 2019; Magro et al. 2020).
Moreover, toxic dioxins and chloroform can be formed from the decomposition of triclosan in the chlorination under the effect of sunlight (Li et al. 2018). For triclosan removal from the environmental water, a water-compatible triclosan imprinted material coated on the surface of magnetic nano-zero-valent iron was synthesized by Chen et al. (2017) via surface polymerization. The stable triclosan imprinted material had a good recyclable adsorption performance of about 20 mg g−1 in a wide pH range (6.0–9.0) with a high enrichment factor and selectivity in complex water samples.
Later, a molecularly imprinted polymer layer on magnetic Fe3O4 nanoparticles has been developed by Kong et al. (2020) based on combined surface imprinting and sol–gel technique (Fig. 8). The ensuing molecularly imprinted polymers @Fe3O4 nanoparticles with uniform core–shell structure exhibited a quite impressive imprinting factor of 2.46, good selectivity, and a fast adsorption rate with only 20 min to establish adsorption equilibrium. However, the obtained adsorption capacity of 0.218 mg g−1 is still not alluring when compared to previously documented molecularly imprinted polymers. To enhance removal performance based on the selectivity of molecularly imprinted polymers for antiseptics in general and triclosan in particular, more advanced molecularly imprinted polymers encompassing much higher adsorption capacities should be developed and additional treatment techniques should be applied along with adsorption mechanism.
Fig. 8.
Synthetic route for MIP@Fe3O4 via sol–gel condensation reaction (A), the binding specificity of MIP@Fe3O4 and NIP@Fe3O4 towards TSC, Ph, and p-CP, (B) and reusability of MIP@Fe3O4 and NIP @Fe3O4 (C). Reprinted with permission of Wiley–VCH from ref. (Kong et al. 2020). MIP molecularly imprinted polymer; NIP non-imprinted polymer; TEOS tetraethylorthosilicate; APTES 3-aminopropyltriethoxysilane; TCS triclosan; Ph phenol; p-CP p-chlorophenol; Q adsorption capacity
The most prescribed class of antidepressants, selective serotonin reuptake inhibitors including sertraline, metabolite norsertraline, paroxetine, fluoxetine, escitalopram, and bupropion have been regularly detected in surface waters and wastewater (Mole and Brooks 2019; Castillo-Zacarías et al. 2021). In aquatic organisms, they cause changes in growth processes, and feeding and survivorship behavior (Sehonova et al. 2019; Stoczynski and van den Hurk 2020). Recently, a molecularly imprinted polymer based on methacrylic acid as functional monomers and sertraline as a template has been used for removal of not only targeted template but for the whole class of selective serotonin reuptake inhibitors has been reported by Gornik et al. (2021); maximum imprinting factor was found to be 3.7, and the maximum capacity for sertraline was estimated at 72.6 mg g−1. The molecularly imprinted polymers can form more non-specific interactions with the targets due to their high surface area and pore volume, thus improving the overall removal efficiency in wastewater treatment.
A widely used anticonvulsant drug, carbamazepine, has complex body metabolic processes and can be excreted from the urine (Hassan et al. 2021; Kefayati et al. 2021) and the drug and its metabolites can be uncovered in the environment through different treatment processes of wastewater treatment plants (Björlenius et al. 2018). For undertaking the removal of carbamazepine, a synthesized molecularly imprinted polymer via reversible addition-fragmentation chain transfer polymerization coated on the surface of the chain transfer agent-modified magnetic chitosan nanoparticles has been developed by Wang et al. (2019). The prepared molecularly imprinted polymer showed a desirable adsorption capacity of 76.34 mg g−1, high adsorption selectivity, and a high imprinting factor of 4.83.
It should be emphasized that the adsorption selectivity and imprinting factor play a significant role in the removal of carbamazepine in real aqueous environments because the removal is often disrupted by co-existing organic matter while using advanced oxidation processes or other adsorbents such as activated carbon (He et al. 2020). To screen for the most appropriate monomer, thereby addressing carbamazepine removal disruptions, ten different functional monomers were constructed via molecular simulation by He et al. (2020) to choose more stable, low-energy conformations of the template-monomer interaction systems. It was found that the molecularly imprinted polymer based on 4-vinyl benzoic acid was the best fit for carbamazepine removal with a maximum adsorption capacity of 28.40 mg g−1. The selective adsorption was ensured by electrostatic forces comprising hydrogen bonding and van der Waals forces, including the p–p conjugate (He et al. 2020).
Non-steroidal anti-inflammatory drugs such as diclofenac, potassium diclofenac, naproxen, and ketoprofen, with their concentration up to μg L−1, may be enough to cause toxic effects that can often be located in water sources (Qiao et al. 2021). As persistent toxic pollutants in the environment, diclofenac and its derivatives can cause serious damage to human livers, kidneys, and affect the growth of microorganisms even at very low concentrations (Amaly et al. 2021; Bi et al. 2021).
In 2019, a diclofenac-selective molecularly imprinted polymer was synthesized based on methacrylic acid as functional monomers and diclofenac sodium salt as template molecule by Cantarella et al. via a simple bulk polymerization process for removal of these drugs from water (Cantarella et al. 2019). The prepared molecularly imprinted polymer exhibited a good adsorption capacity of 32.5 mg g−1 with extreme selectivity towards diclofenac due to the imprinting effect (Fig. 9). The outstanding adsorption performance of the molecularly imprinted polymer with ~ 90% of diclofenac removal from an aqueous solution was recorded after only 10 min wherein the molecularly imprinted polymer also showed good reusability after a simple regeneration step. The low-cost synthesis procedure with less time and solvent consumption and the first use of hydrophilic methacrylic acid as a monomer has opened up the prospect of large-scale production of the molecularly imprinted polymer with excellent compatibility in water.
Fig. 9.
Synthetic route for the diclofenac-selective molecularly imprinted polymer (A), the time profile of diclofenac adsorbed by MIP and REF as a reference (B), adsorption performance in 10 min of MIP (C) and REF (D), respectively, towards diclofenac, acetylsalicylic acid, trimethoprim and methyl orange. Reprinted with permission of Elsevier from ref. (Cantarella et al. 2019). MIP molecularly imprinted polymer; REF non-imprinted polymer; DICLO diclofenac; C0 initial concentration
Subsequently, recyclable nanospheres with an average size of 200 nm were fabricated with methacrylic acid as functional monomer and N,N-methylene bis(acrylamide) as a crosslinker for removal of diclofenac by Amaly et al. (2021). Accordingly, a high adsorption capacity of 5.5 mg g−1 and adsorption efficiency of ~ 82% of diclofenac can be attained even after seven regeneration cycles. To increase the efficiency and shorten processing time, the photodegradation of diclofenac in water was applied using a molecularly imprinted film on TiO2 particles in 2021 (Bi et al. 2021). The stable film exhibited excellent photodegradation activity towards diclofenac, about 89% of diclofenac was removed from 200 mL solution (1 mg/L) and complete mineralization of ~ 17% after only 10 min of irradiation.
To evaluate the influence of surfactants on the performance of molecularly imprinted polymers towards potassium diclofenac, different adsorbents were synthesized via the double-imprinted process deploying various kinds of surfactants including Triton ×-100, sodium dodecyl sulfate, and a mixture of alkyl benzyl dimethyl ammonium chlorides (Da Silva et al. 2019). The surfactants could interact with the template and the polymer network to create a more connected hierarchically imprinted structure. Thus, all the obtained surfactant-based molecularly imprinted polymers had better affinity and selectivity for potassium diclofenac compared to those of bare molecularly imprinted polymers and non-molecularly imprinted polymers.
Among non-steroidal anti-inflammatory drugs, naproxen is one of the most widely used pharmaceuticals to relieve pain from various conditions (Friedman et al. 2018). However, naproxen can cause serious side effects, such as gastric vomiting, bleeding, ulcers, and kidney and central nervous system problem if it reaches the body through contaminated water (Kwak et al. 2018; Quesada et al. 2019). A molecularly imprinted polymer-coated with Fe3O4 nanoparticles was successfully prepared by Husin et al. using choline-imidazole-based deep eutectic solvent for the selective removal of naproxen from the aqueous environment (Husin et al. 2021). Due to hydrogen bonding between adsorbents and adsorbates and strong formation of stable complexes through the π–π interaction, this molecularly imprinted polymer displayed a better adsorption capacity of 90.91 mg g−1 towards naproxen compared to Fe3O4@ molecularly imprinted polymer. The details for the removal of the last studied non-steroidal anti-inflammatory (ketoprofen) (Madikizela et al. 2018d) and other drugs including sildenafil (Wan et al. 2021), cyproheptadine (Cao et al. 2021), amitriptyline (Cao et al. 2021), desloratadine (Cao et al. 2021), 8-chloroazatadine (Cao et al. 2021), ketotifen fumarate (Cao et al. 2021), praziquantel enantiomers (Do Nascimento et al. 2019) are presented in Table 5. The main inferences that can be drawn from Table 5 comprise:
Molecularly imprinted materials have promising perspectives to be used in wastewater treatment due to their high selectivity towards target pharmaceuticals, compared to traditional adsorption materials
The deployment of molecularly imprinted polymers for pharmaceutical removal may be more cost-effective compared to other removal techniques, such as photochemical, ozonolysis, or electro-decomposition methods because of co-existing organic matter in the water environment. The interaction of the chemicals in those techniques with other organic substances besides target molecules requires a large number of treatment agents which increases the treatment cost
The combination of using molecularly imprinted polymers and other removal techniques should be exploited to enhance the removal performance
Studies on the usage of molecularly imprinted polymers, especially molecularly imprinted polymers prepared via nano-imprinting or surface imprinting with larger surface areas and higher selectivity for more kinds of drugs need to be promoted in the future
Limitations of molecularly imprinted polymers
Based on the above discussions, it is clear that many molecularly imprinted polymers are endowed with excellent adsorption capabilities. As a result, they could selectively sequester a range of pharmaceuticals from synthetic solutions under adsorption-related parametric variability. Despite these encouraging adsorption performances of molecularly imprinted polymers for pharmaceuticals, their known adsorptive capabilities are limited to laboratory-scale systems containing only a few adsorbates.
In reality, these molecularly imprinted polymers need to display highly stable, profoundly efficient, and reproducible adsorption performances for the uptake of at least one target pharmaceutical in extremely complex contaminated aqueous milieus. Additional research and development efforts are warranted to address a number of limitations for them to be able to embody this breadth and depth of morphological excellence and phenomenological performance in taking up pharmaceutical micropollutants by adsorptive pathways from highly competitive aqueous environments.
During the realization of this review, more than 150 publications were perused and analyzed. However, certain key limitations which are still associated with the use of molecularly imprinted polymers for adsorbing pharmaceutical micropollutants are reported in relatively few articles (Wu et al. 2017; Madikizela et al. 2018b; Tian et al. 2019; Cantarella et al. 2019; Li et al. 2019; Zhang et al. 2020; Azizi and Bottaro 2020; Bagheri and Ghaedi 2020; Yuan et al. 2020; Fan et al. 2020; Mohamed Idris et al. 2020; Yahaya et al. 2021; Fang et al. 2021; Liu et al. 2021a; Li and Yang 2021). These limitations, which may be specific to certain molecularly imprinted polymers, are summarized below:
Target adsorbates which are directly utilized as molecular templates to synthesize molecularly imprinted polymers can consume large quantities of standard products (Zhang et al. 2020)
Template leakage can be persistent during the sample preparation and this can result in inaccurate quantification of trace adsorbates/analytes (Zhang et al. 2020)
When the number of adsorbates/analytes increase from one to more chemically complex combinations of molecules (such as aqueous mixtures of norfloxacin, ibuprofen, ciprofloxacin, phenol, and carbamazepine), removal efficiencies resulting under highly competitive adsorption onto magnetic molecularly imprinted polymers can decrease significantly (Fang et al. 2021)
Besides the complexity and number of steps involved in the time- and solvent-consuming precipitation polymerization approach to construct molecularly imprinted polymers (Dai et al. 2012; Manzo et al. 2015; Madikizela and Chimuka 2016a; Cantarella et al. 2019), their swelling ratio in aqueous solutions is often entirely different from the swelling behavior observed in the organic solvent in which they were synthesized, thus making them incapable of differentiating target species from non-target ones (Shen et al. 2013; Cantarella et al. 2019)
In a research study, graphene oxide–functionalized molecularly imprinted polymer particles were prepared for cefadroxil extraction using a new imprinting approach. A dispersive solid-phase extraction method based on these particles, combined with ultra-high-performance liquid chromatography with photodiode array detection, was formulated for the determination of cefadroxil in water samples wherein a narrow linear range was obtained because the particles had attained maximum saturation adsorption (Chen and Ye 2017)
Adsorption performances of a multi-templated molecularly imprinted polymer differed because of factors related to the wastewater treatment plant design, consumption of pharmaceuticals, and the population serviced by these wastewater treatment plants (Madikizela and Chimuka 2016b)
In certain situations, the selectivity of molecularly imprinted polymers can be significantly impaired during the treatment of aqueous samples, and this can then lead to the extraction of non-targeted species (CARO et al. 2004; Madikizela and Chimuka 2016a; Madikizela et al. 2018b). This could also result in the impairment or blockage of pores that are present on the surface of the molecularly imprinted polymer because of extensive washing performed to remove interfering species that eventually influence the reusability of the molecularly imprinted polymers (Madikizela et al. 2018b)
The need to extract water-soluble compounds becomes necessary in the wake of their selective rebinding from water samples (Azizi and Bottaro 2020)
Given that Molecularly imprinted polymers are normally constructed in organic solvents and this approach lowers their efficiency in aqueous samples (Azizi and Bottaro 2020), optimal water compatibility of hydrophobic molecularly imprinted polymers has to be ensured if they are intended for use in treating pharmaceutical-contaminated aqueous streams
Molecularly imprinted polymers prepared by the free radical polymerization method can have binding sites which are inaccessible and heterogeneous, and such a morphology can hamper the mass transfer of species involved resulting in low selectivity (Hu et al. 2012; Azizi and Bottaro 2020). Accordingly, an optimal homogeneity of molecularly imprinted polymers has to be safeguarded (Azizi and Bottaro 2020)
Although at a higher cost, the need to have precise construction of microscopic binding cavities motivates additional research efforts in this direction (Li and Yang 2021). This is because there are different bonding interactions between the templates and molecularly imprinted polymers which have to be sustained so that the aqueous micropollutants (such as pharmaceuticals) can be effectively detected in the aqueous systems (Li and Yang 2021)
Molecularly imprinted polymers have been widely synthesized using a single template molecule. Such materials enable the selective uptake and segregation of only one specific target adsorbate. Hence, they can be inadequate for use in more complex real aqueous media which can comprise a significantly higher number of potential analytes including pharmaceuticals. There are reports in the literature that indicate that wastewaters generated from medical and hospital facilities contain a complex mixture of pharmaceuticals having different stereochemistries and toxicity. For example, a study was conducted for preliminary characterization of Turkish hospital wastewaters wherein an assessment was made on their environmental risk and toxicity; 14 pharmaceuticals in hospital wastewater were categorized as “high risk” with hazard quotients greater than 10 (Yilmaz et al. 2017). Diclofenac, ofloxacin, clarithromycin, ciprofloxacin, trimethoprim, and sulfapyridine found in the hospital wastewaters had hazard quotients greater than 100, with ofloxacin having a hazard quotient of 9090 and thus being the most hazardous species (Yilmaz et al. 2017). Despite the decreases in the hazard quotients observed in the treated hospital wastewater, the environmental (pollution) load remained a serious issue (Yilmaz et al. 2017). Thus, advanced treatment processes would still be required to scavenge completely the persistent pharmaceuticals.
One recent study, which involved the long-term characterization of medical laboratory wastewaters generated in diagnosis and examination units, revealed that such wastewaters are extremely toxic (Basturk et al. 2021). These wastewaters contained the following pharmaceutical micropollutants (highest four concentrations are provided in brackets and have units μg L–1): acetaminophen (paracetamol), acetylsalicylic acid, allopurinol (1.14), atenolol, caffeine (2.68), carbamazepine, cephalexin, ciprofloxacin, clarithromycin, erythromycin (2.33), iomeprol, morphine and sulfamethoxazole (1.82) (Basturk et al. 2021). The results of two-year monitoring of a specific study Site Pilote de Bellecombe (SIPIBEL) indicated that 11 pharmaceuticals could still be detected in hospital and urban wastewaters even after treatment and they comprise paracetamol (12.2), sulfamethoxazole (5.9), ciprofloxacin (35.3), vancomycin (50.0), ketoprofen (9.3), diclofenac (5.0), ibuprofen (0.5), salicylic acid (13.3), atenolol (4.1), propranolol (0.6) and carbamazepine (0.6) (values in brackets are the respective limit of quantification in ng L–1 of the pharmaceutical) (Wiest et al. 2018). In another study, nine pharmaceuticals were detected in the real hospital wastewater samples (average concentration given in brackets in μg L–1): paracetamol (29.886), diclofenac (0.110), naproxen (0.824), ciprofloxacin (2.386), ampicillin (8.256), carbamazepine (1.042), cyclophosphamide (2.234), warfarin (1.950), and tris(2-butoxyethyl)phosphate (2.578) (Top et al. 2020).
The above discussion leads to the following two key points. First, given in the case of a single template molecularly imprinted polymer to be used as an adsorbent, the presence of several pharmaceuticals within the same aqueous milieu and them exposed to the same adsorbent surfaces imply that competitive adsorption will be a complex phenomenon. This complexity will influence the competitive equilibrium adsorption capacity of the adsorbent for the target pharmaceutical. For example, the removal efficiencies of norfloxacin, ciprofloxacin, ibuprofen, carbamazepine, and phenol by a magnetic molecularly imprinted polymer were 91.1%, 85.9%, 34.0%, 3.4%, and 2.9%, respectively, when unitary aqueous systems were considered for adsorption experiments (Fang et al. 2021). However, when the latter five compounds co-existed, the adsorptive removal efficiencies were 56.3%, 52.8%, 19.2%, 3.2%, and 4.3% for norfloxacin, ciprofloxacin, ibuprofen, carbamazepine, and phenol, respectively (Fang et al. 2021).
Second, in the case of a dual or multiple-template molecularly imprinted polymer to be used as an adsorbent, the extent of competitive adsorption can become even more intricate. This further complexity will still affect the competitive equilibrium adsorption capacities of the adsorbent for the target pharmaceuticals. Indeed, with a view of extending the real-world use of molecularly imprinted polymers for micropollutant adsorption, new multiple-template molecularly imprinted polymers have to be synthesized (Madikizela et al. 2016; Sun et al. 2018; Kechagia et al. 2018; Wang et al. 2020; Liu et al. 2020a, 2021c; Wu et al. 2020; Deng et al. 2021, a; Zeng et al. 2021a; Mohiuddin et al. 2021). These multi-template molecularly imprinted polymers can foster the concomitant selective recognition and efficient separation of more than one micropollutant from contaminated water/aqueous or other liquid samples. They have delivered excellent adsorption performance at the laboratory scale of adsorption system analysis.
In a nutshell, the point here is that competitive adsorption, which has been evoked in many other adsorption-related studies (Turk Sekulic et al. 2019; Kim et al. 2021; Shin et al. 2021; Almuntashiri et al. 2021), is a crucial parameter that has to be absolutely considered. A thorough consideration of competitive adsorption becomes all the more necessary when designing and engineering molecularly imprinted polymers intended for purifying real contaminated water (e.g. drinking water) and real wastewaters in large-scale water treatment processes applying adsorption.
Real pharmaceutical-contaminated waters and pharmaceutical-containing wastewaters in the aqueous media will be requiring treatment in large-scale water treatment processes. These aqueous media will comprise complex mixtures of potentially harmful pharmaceutical molecules. Encompassing the latter two facts, it then becomes an absolute necessity to ensure that nearly all of the pharmaceuticals are scavenged down to and below their respective accepted safe limits. This need stems from two crucial premises.
First, the duty of care is to stop the release of any residual pharmaceuticals in the natural receiving water bodies. Second, an obligation to abide by environmental protection laws that regulate the quality and intensity of such releases after the final stage(s) of advanced water treatment. Along the same lines, it becomes a more substantial challenge to fully elucidate and understand the interrelationships and influences of the potential dominating molecular interactions which can onset and operate in such multi-pharmaceutical adsorbate-multi-template adsorbent-type molecularly imprinted polymers. As of now, a major limitation exists in comprehending these molecular interactions the multi-template molecularly imprinted polymers can exhibit in real contaminated aqueous environments. Conceivably, adequate documentation of these molecular interactions can enable a more optimized design and tuning of the selected multi-template adsorbent-type molecularly imprinted polymers for use in real water treatment systems containing multiple pharmaceuticals.
At this point, the competitive adsorption that can get onset and affect the removal efficiency of each pharmaceutical becomes a combined material science & engineering and process engineering challenge. As it appears, multiple-template molecularly imprinted polymers can become a class of adsorbents choice for use in large-scale water treatment systems given their prima facie ability to sequester more than one target compound at reasonably high adsorption capacities and removal efficiencies. Yet, before this goal may actualize, the feasibility of designing multiple-template adsorbent-type molecularly imprinted polymers which can sustain a prolonged and stable multiple adsorption cycle usage in harsh water/wastewater treatment environments should be first tackled as a material science & engineering challenge.
It will be challenging to work out the actual mode(s) of multi-template molecularly imprinting on a specific adsorbent ‘base’ and ensure chemical integrity and adsorption site delineation. ‘Chemical integrity’ pertains to the selective binding of one specific target pharmaceutical at a dedicated adsorption site location programmed to host molecules of that target pharmaceutical. And then, to make that specific binding permanent and protected enough against any subsequent preferential rebinding by another more ‘competitive’ target pharmaceutical species.
Apart from this challenge related to the chemical morphology of the multiple-template adsorbent-type molecularly imprinted polymers, it will be interesting, and possibly useful, to map and delineate the chemical molecular imprinting of specific ‘segments of active surface adsorption sites’ developed on the surface of the material. Such a ‘molecularly imprinted map’ of chemically stimulated adsorption sites may be generated using suitable analytical methods. These maps can then be analyzed to gauge the relative propensity and probability of binding of the target pharmaceuticals on the surface of the multiple-template adsorbent-type molecularly imprinted polymers.
Depending on the optimal adsorption dynamics and net removal efficiency sought for each target micropollutant, the map may be ‘redrawn’ by reapportioning the molecular imprinting with suitable templates. In this manner, an optimally functional assembly of the multiple-template molecular imprinting may be reached for the specific adsorption-based water/wastewater treatment operations designed to scavenge the target pharmaceutical(s) using the selected multiple-template adsorbent-type molecularly imprinted polymers.
The process engineering challenge resides, first, in designing economic and relatively benign large-scale production schemes for the selected multiple-template adsorbent-type molecularly imprinted polymers. At this point, it is also crucial to select and scale up an imprinting strategy. For example, boronate affinity imprinting, stimuli-responsive imprinting, fragment template imprinting, or dummy template imprinting (Bhogal et al. 2021) will best meet the requirements of the real-scale water/wastewater treatment processes. Second, the large-scale handling, dispersion, and physicochemical stabilization of the selected multiple-template adsorbent-type molecularly imprinted polymers within the successive unit operations of the industrial water/wastewater treatment systems have to be optimized. Third, both technically and economically feasible large-scale recovery and regeneration schemes for the saturated or spent multiple-template adsorbent-type molecularly imprinted polymers need to be worked out and optimally implemented.
At this stage, the use of multiple-template adsorbent-type molecularly imprinted polymers that have strong magnetic properties will be extremely desirable. Having magnetic properties under certain controlled conditions of process operations can imply that these spent magnetic multiple-template adsorbent-type molecularly imprinted polymers can be recovered from the treated effluent streams quasi entirely by applying an appropriately designed external magnetic field. In this way, the application of the centrifugation and filtration unit operations for the separation and recovery of micropollutant-loaded adsorbent-type molecularly imprinted polymers can be avoided (Aylaz et al. 2021). Fourth, green and environmentally compatible disposal routes have to be designed for regenerating as much as possible the completely exhausted multiple-template adsorbent-type molecularly imprinted polymers.
Conclusion
In this review, we have discussed the toxicity impact of pharmaceuticals on the human body and ecosystem and their removal by molecularly imprinted polymers. Subsequently, we illustrated various procedures for the preparation of molecularly imprinted polymers, and their advantages and disadvantages; molecularly imprinted polymers have a high potential for water decontamination, owning to high selectivity and strong affinity. They have proven to be effective sorbents for selective drug uptake of a group of structurally analogous compounds. Integration of nanoparticles into Molecularly imprinted polymers can preserve their distinguished selectivity and maybe oblige innovative performance e.g. high uptake ability. To date, huge steps have been made to bring the imprinting technology one step closer to water purification appliances.
Despite these encouraging adsorption performances of molecularly imprinted polymers for pharmaceuticals, their known adsorptive capabilities are limited to laboratory-scale systems containing only a few adsorbates. In addition, other limitations that can be specific to one molecularly imprinted polymer may include, template leakage that can be persistent during sample preparation which can result in inaccurate quantification of trace adsorbates/analytes.
The need to extract water-soluble compounds becomes necessary in the wake of their selective rebinding from water samples, molecularly imprinted polymers have been widely synthesized using a single template molecule. Such materials enable the selective uptake and segregation of only one specific target adsorbate. Hence, they can be insufficient for use in more complex real aqueous media which can comprise a significantly higher number of potential analytes including pharmaceuticals. However, we are optimistic about the future potential of molecularly imprinted polymers as selective adsorbents for water decontamination.
Acknowledgements
S.W.J. and Y.V. would like to acknowledge the financial support from the Korea Environment Industry & Technology Institute (KEITI) through the Technology Development Project for Safety Management of Household Chemical Product Program, funded by the Korea Ministry of Environment (MOE) (2020002970005, 1485017845).
Author contributions
Writing—original draft; revision: Sections “Introduction” and “Introduction of pharmaceuticals”: ZF and MT, Section “Molecular imprinting polymers and their role in water decontamination”: ENZ and OM, Section “Molecularly imprinted polymers”: VTL, VDD, SWJ and YV, Section “Limitations of molecularly imprinted polymers”: AM and MS, Section “Conclusion”: ENZ, Scheme: ENZ and RSV Writing—review and editing; revision: ENZ and RSV.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdullah BA, Talpur FN, et al. Synthesis of ultrasonic-assisted lead ion imprinted polymer as a selective sorbent for the removal of Pb2+ in a real water sample. Microchem J. 2019;146:1160–1168. doi: 10.1016/j.microc.2019.02.037. [DOI] [Google Scholar]
- Adumitrăchioaie A, Tertiş M, Cernat A, et al. Electrochemical methods based on molecularly imprinted polymers for drug detection. A review. Int J Electrochem Sci. 2018;13:2556–2576. doi: 10.20964/2018.03.75. [DOI] [Google Scholar]
- Almeida Â, Calisto V, Esteves VI, et al. Effects of single and combined exposure of pharmaceutical drugs (carbamazepine and cetirizine) and a metal (cadmium) on the biochemical responses of R. philippinarum. Aquat Toxicol. 2018;198:10–19. doi: 10.1016/j.aquatox.2018.02.011. [DOI] [PubMed] [Google Scholar]
- Almeida LC, Mattos AC, Dinamarco CPG, et al. Chronic toxicity and environmental risk assessment of antivirals in Ceriodaphnia dubia and Raphidocelis subcapitata. Water Sci Technol. 2021;84:1623–1634. doi: 10.2166/wst.2021.347. [DOI] [PubMed] [Google Scholar]
- Almuntashiri A, Hosseinzadeh A, Volpin F, et al. Removal of pharmaceuticals from nitrified urine. Chemosphere. 2021;280:130870. doi: 10.1016/j.chemosphere.2021.130870. [DOI] [PubMed] [Google Scholar]
- Alshishani A, Saaid M, Basheer C, Saad B. High performance liquid chromatographic determination of triclosan, triclocarban and methyl-triclosan in wastewater using mini-bar micro-solid phase extraction. Microchem J. 2019;147:339–348. doi: 10.1016/j.microc.2019.03.044. [DOI] [Google Scholar]
- Amaly N, Istamboulie G, El-Moghazy AY, Noguer T. Reusable molecularly imprinted polymeric nanospheres for diclofenac removal from water samples. J Chem Res. 2021;45:102–110. doi: 10.1177/1747519820925998. [DOI] [Google Scholar]
- Araújo APC, Mesak C, Montalvão MF, et al. Anti-cancer drugs in aquatic environment can cause cancer: insight about mutagenicity in tadpoles. Sci Total Environ. 2019;650:2284–2293. doi: 10.1016/j.scitotenv.2018.09.373. [DOI] [PubMed] [Google Scholar]
- Aris AZ, Shamsuddin AS, Praveena SM. Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: a review. Environ Int. 2014;69:104–119. doi: 10.1016/j.envint.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Arnnok P, Singh RR, Burakham R, et al. Selective uptake and bioaccumulation of antidepressants in fish from effluent-impacted Niagara river. Environ Sci Technol. 2017;51:10652–10662. doi: 10.1021/acs.est.7b02912. [DOI] [PubMed] [Google Scholar]
- aus der Beek T, Weber FA, Bergmann A, et al. Pharmaceuticals in the environment-global occurrences and perspectives. Environ Toxicol Chem. 2016;35:823–835. doi: 10.1002/etc.3339. [DOI] [PubMed] [Google Scholar]
- Aylaz G, Kuhn J, Lau ECHT, et al. Recent developments on magnetic molecular imprinted polymers (MMIPs) for sensing, capturing, and monitoring pharmaceutical and agricultural pollutants. J Chem Technol Biotechnol. 2021;96:1151–1160. doi: 10.1002/jctb.6681. [DOI] [Google Scholar]
- Azizi A, Bottaro CS. A critical review of molecularly imprinted polymers for the analysis of organic pollutants in environmental water samples. J Chromatogr A. 2020;1614:460603. doi: 10.1016/j.chroma.2019.460603. [DOI] [PubMed] [Google Scholar]
- Bagheri AR, Ghaedi M. Green preparation of dual-template chitosan-based magnetic water-compatible molecularly imprinted biopolymer. Carbohydr Polym. 2020;236:116102. doi: 10.1016/j.carbpol.2020.116102. [DOI] [PubMed] [Google Scholar]
- Basturk I, Varank G, Murat-Hocaoglu S, et al. Characterization and treatment of medical laboratory wastewater by ozonation: optimization of toxicity removal by central composite design. Ozone Sci Eng. 2021;43:213–227. doi: 10.1080/01919512.2020.1794794. [DOI] [Google Scholar]
- Beydoun A, DuPont S, Zhou D, et al. Current role of carbamazepine and oxcarbazepine in the management of epilepsy. Seizure. 2020;83:251–263. doi: 10.1016/j.seizure.2020.10.018. [DOI] [PubMed] [Google Scholar]
- Bhogal S, Kaur K, Mohiuddin I, et al. Hollow porous molecularly imprinted polymers as emerging adsorbents. Environ Pollut. 2021;288:117775. doi: 10.1016/j.envpol.2021.117775. [DOI] [PubMed] [Google Scholar]
- Bi L, Chen Z, Li L, et al. Selective adsorption and enhanced photodegradation of diclofenac in water by molecularly imprinted TiO2. J Hazard Mater. 2021;407:124759. doi: 10.1016/j.jhazmat.2020.124759. [DOI] [PubMed] [Google Scholar]
- Björlenius B, Ripszám M, Haglund P, et al. Pharmaceutical residues are widespread in Baltic Sea coastal and offshore waters—screening for pharmaceuticals and modelling of environmental concentrations of carbamazepine. Sci Total Environ. 2018;633:1496–1509. doi: 10.1016/j.scitotenv.2018.03.276. [DOI] [PubMed] [Google Scholar]
- Borgatta M, Decosterd LA, Waridel P, et al. The anticancer drug metabolites endoxifen and 4-hydroxy-tamoxifen induce toxic effects on Daphnia pulex in a two-generation study. Sci Total Environ. 2015;520:232–240. doi: 10.1016/j.scitotenv.2015.03.040. [DOI] [PubMed] [Google Scholar]
- Brezovšek P, Eleršek T, Filipič M. Toxicities of four anti-neoplastic drugs and their binary mixtures tested on the green alga Pseudokirchneriella subcapitata and the cyanobacterium Synechococcus leopoliensis. Water Res. 2014;52:168–177. doi: 10.1016/j.watres.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Byun HS, Yang DS, Cho SH. Synthesis and characterization of high selective molecularly imprinted polymers for bisphenol A and 2,4-dichlorophenoxyacetic acid by using supercritical fluid technology. Polymer (Guildf) 2013;54:589–595. doi: 10.1016/j.polymer.2012.11.079. [DOI] [Google Scholar]
- Cantarella M, Carroccio SC, Dattilo S, et al. Molecularly imprinted polymer for selective adsorption of diclofenac from contaminated water. Chem Eng J. 2019;367:180–188. doi: 10.1016/j.cej.2019.02.146. [DOI] [Google Scholar]
- Cao Y, Sheng T, Yang Z, et al. Synthesis of molecular-imprinting polymer coated magnetic nanocomposites for selective capture and fast removal of environmental tricyclic analogs. Chem Eng J. 2021;426:128678. doi: 10.1016/j.cej.2021.128678. [DOI] [Google Scholar]
- Caro E, Marce R, Cormack P, et al. A new molecularly imprinted polymer for the selective extraction of naproxen from urine samples by solid-phase extraction. J Chromatogr B. 2004;813:137–143. doi: 10.1016/j.jchromb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Castillo-Zacarías C, Barocio ME, Hidalgo-Vázquez E, et al. Antidepressant drugs as emerging contaminants: Occurrence in urban and non-urban waters and analytical methods for their detection. Sci Total Environ. 2021;757:143722. doi: 10.1016/j.scitotenv.2020.143722. [DOI] [PubMed] [Google Scholar]
- Chen X, Ye N. A graphene oxide surface–molecularly imprinted polymer as a dispersive solid-phase extraction adsorbent for the determination of cefadroxil in water samples. RSC Adv. 2017;7:34077–34085. doi: 10.1039/C7RA02985C. [DOI] [Google Scholar]
- Chen H, Son S, Zhang F, et al. Rapid preparation of molecularly imprinted polymers by microwave-assisted emulsion polymerization for the extraction of florfenicol in milk. J Chromatogr B. 2015;983–984:32–38. doi: 10.1016/j.jchromb.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lei X, Dou R, et al. Selective removal and preconcentration of triclosan using a water-compatible imprinted nano-magnetic chitosan particles. Environ Sci Pollut Res. 2017;24:18640–18650. doi: 10.1007/s11356-017-9467-6. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang L, Liu Y, et al. Highly selective removal of kitasamycin from the environment by molecularly imprinted polymers: Adsorption performance and mechanism. Colloids Surf A Physicochem Eng Asp. 2021;625:126926. doi: 10.1016/j.colsurfa.2021.126926. [DOI] [Google Scholar]
- Cheng D, Ngo HH, Guo W, et al. A critical review on antibiotics and hormones in swine wastewater: water pollution problems and control approaches. J Hazard Mater. 2020;387:121682. doi: 10.1016/j.jhazmat.2019.121682. [DOI] [PubMed] [Google Scholar]
- Colville C, Alcaraz AJ, Green D, et al. Characterizing toxicity pathways of fluoxetine to predict adverse outcomes in adult fathead minnows (Pimephales promelas) Sci Total Environ. 2022;817:152747. doi: 10.1016/j.scitotenv.2021.152747. [DOI] [PubMed] [Google Scholar]
- Da Silva RCS, Santos MN, Pires BC, et al. Assessment of surfactants on performance of molecularly imprinted polymer toward adsorption of pharmaceutical. J Environ Chem Eng. 2019;7:103037. doi: 10.1016/j.jece.2019.103037. [DOI] [Google Scholar]
- Dai CM, Zhang J, Zhang YL, et al. Selective removal of acidic pharmaceuticals from contaminated lake water using multi-templates molecularly imprinted polymer. Chem Eng J. 2012;211–212:302–309. doi: 10.1016/j.cej.2012.09.090. [DOI] [Google Scholar]
- de Andrade JR, Oliveira MF, da Silva MGC, Vieira MGA. Adsorption of pharmaceuticals from water and wastewater using nonconventional low-cost materials: a review. Ind Eng Chem Res. 2018;57:3103–3127. doi: 10.1021/acs.iecr.7b05137. [DOI] [Google Scholar]
- Deng D, He Y, Li M, et al. Preparation of multi-walled carbon nanotubes based magnetic multi-template molecularly imprinted polymer for the adsorption of phthalate esters in water samples. Environ Sci Pollut Res. 2021;28:5966–5977. doi: 10.1007/s11356-020-10970-2. [DOI] [PubMed] [Google Scholar]
- Ding S, Li Z, Cheng Y, et al. Enhancing adsorption capacity while maintaining specific recognition performance of mesoporous silica: a novel imprinting strategy with amphiphilic ionic liquid as surfactant. Nanotechnology. 2018;29:375604 . doi: 10.1088/1361-6528/aace10. [DOI] [PubMed] [Google Scholar]
- Do Nascimento TA, De Oliveira HL, Borges KB. Magnetic molecularly imprinted polypyrrole as a new selective adsorbent for pharmaceutically active compounds. J Environ Chem Eng. 2019;7:103371. doi: 10.1016/j.jece.2019.103371. [DOI] [Google Scholar]
- Du Q, Wu P, Sun Y, et al. Selective photodegradation of tetracycline by molecularly imprinted ZnO@NH2-UiO-66 composites. Chem Eng J. 2020;390:124614. doi: 10.1016/j.cej.2020.124614. [DOI] [Google Scholar]
- Elencovan V, Joseph J, Yahaya N, et al. Exploring a novel deep eutectic solvents combined with vortex assisted dispersive liquid–liquid microextraction and its toxicity for organophosphorus pesticides analysis from honey and fruit samples. Food Chem. 2022;368:130835. doi: 10.1016/j.foodchem.2021.130835. [DOI] [PubMed] [Google Scholar]
- Elugoke SE, Adekunle AS, Fayemi OE, et al. Molecularly imprinted polymers (MIPs) based electrochemical sensors for the determination of catecholamine neurotransmitters—review. Electrochem Sci Adv. 2021;1:1–43. doi: 10.1002/elsa.202000026. [DOI] [Google Scholar]
- Falfushynska H, Sokolov EP, Haider F, et al. Effects of a common pharmaceutical, atorvastatin, on energy metabolism and detoxification mechanisms of a marine bivalve Mytilus edulis. Aquat Toxicol. 2019;208:47–61. doi: 10.1016/j.aquatox.2018.12.022. [DOI] [PubMed] [Google Scholar]
- Fan Y, Zeng G, Ma X. Effects of prepolymerization on surface molecularly imprinted polymer for rapid separation and analysis of sulfonamides in water. J Colloid Interface Sci. 2020;571:21–29. doi: 10.1016/j.jcis.2020.03.027. [DOI] [PubMed] [Google Scholar]
- Fang L, Miao Y, Wei D, et al. Efficient removal of norfloxacin in water using magnetic molecularly imprinted polymer. Chemosphere. 2021;262:128032. doi: 10.1016/j.chemosphere.2020.128032. [DOI] [PubMed] [Google Scholar]
- Farooq S, Nie J, Cheng Y, et al. Molecularly imprinted polymers’ application in pesticide residue detection. Analyst. 2018;143:3971–3989. doi: 10.1039/c8an00907d. [DOI] [PubMed] [Google Scholar]
- Feijão E, Cruz de Carvalho R, Duarte IA, et al. Fluoxetine arrests growth of the model diatom Phaeodactylum tricornutum by increasing oxidative stress and altering energetic and lipid metabolism. Front Microbiol. 2020;11:1803. doi: 10.3389/fmicb.2020.01803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes MK, Gusso-Choueri PK, Maranho LA, et al. A tiered approach to assess effects of diclofenac on the brown mussel Perna perna: a contribution to characterize the hazard. Water Res. 2018;132:361–370. doi: 10.1016/j.watres.2017.12.077. [DOI] [PubMed] [Google Scholar]
- Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol. 2019;15:569–589. doi: 10.1038/s41574-019-0242-2. [DOI] [PubMed] [Google Scholar]
- Fraz S, Lee AH, Wilson JY. Gemfibrozil and carbamazepine decrease steroid production in zebrafish testes (Danio rerio) Aquat Toxicol. 2018;198:1–9. doi: 10.1016/j.aquatox.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Freitas R, Silvestro S, Coppola F, et al. Biochemical and physiological responses induced in Mytilus galloprovincialis after a chronic exposure to salicylic acid. Aquat Toxicol. 2019;214:105258. doi: 10.1016/j.aquatox.2019.105258. [DOI] [PubMed] [Google Scholar]
- Freitas R, Silvestro S, Pagano M, et al. Impacts of salicylic acid in Mytilus galloprovincialis exposed to warming conditions. Environ Toxicol Pharmacol. 2020;80:103448. doi: 10.1016/j.etap.2020.103448. [DOI] [PubMed] [Google Scholar]
- Friedman BW, Cisewski D, Irizarry E, et al. A randomized, double-blind, placebo-controlled trial of naproxen with or without orphenadrine or methocarbamol for acute low back pain. Ann Emerg Med. 2018;71:348–356.e5. doi: 10.1016/j.annemergmed.2017.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Chen L, Li J, Zhang Z. Current status and challenges of ion imprinting. J Mater Chem A. 2015;3:13598–13627. doi: 10.1039/C5TA02421H. [DOI] [Google Scholar]
- Fuller BB. Antioxidants and anti-inflammatories. In: Draelos ZD, editor. Cosmet. Dermatology, Third Edition, Chapter 37. Hoboken: Wiley; 2022. pp. 366–387. [Google Scholar]
- Gao M, Gao Y, Chen G, et al. Recent Advances and future trends in the detection of contaminants by molecularly imprinted polymers in food samples. Front Chem. 2020;8:1–20. doi: 10.3389/fchem.2020.616326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy AA, Kummrow F. What do we know about the ecotoxicology of pharmaceutical and personal care product mixtures? A critical review. Crit Rev Environ Sci Technol. 2017;47:1453–1496. doi: 10.1080/10643389.2017.1370991. [DOI] [Google Scholar]
- Gornik T, Shinde S, Lamovsek L, et al. Molecularly imprinted polymers for the removal of antidepressants from contaminated wastewater. Polymers (Basel) 2021;13:1–20. doi: 10.3390/polym13010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Smith KF, Charry MP, et al. Effects of chronic exposure to benzophenone and diclofenac on DNA methylation levels and reproductive success in a marine copepod. J Xenobiotics. 2018;8:7674. doi: 10.4081/xeno.2018.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AA, Tanimu A, Alhooshani K. Iron and cobalt-containing magnetic ionic liquids for dispersive micro-solid phase extraction coupled with HPLC-DAD for the preconcentration and quantification of carbamazepine drug in urine and environmental water samples. J Mol Liq. 2021;336:116370. doi: 10.1016/j.molliq.2021.116370. [DOI] [Google Scholar]
- He Q, Liang JJ, Chen LX, et al. Removal of the environmental pollutant carbamazepine using molecular imprinted adsorbents: molecular simulation, adsorption properties, and mechanisms. Water Res. 2020;168:115164. doi: 10.1016/j.watres.2019.115164. [DOI] [PubMed] [Google Scholar]
- He S, Zhang L, Bai S, et al. Advances of molecularly imprinted polymers (MIP) and the application in drug delivery. Eur Polym J. 2021;143:110179. doi: 10.1016/j.eurpolymj.2020.110179. [DOI] [Google Scholar]
- Herrera-Chacón A, Cetó X, del Valle M. Molecularly imprinted polymers—towards electrochemical sensors and electronic tongues. Anal Bioanal Chem. 2021;413:6117–6140. doi: 10.1007/s00216-021-03313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Fan Y, Zhang Y, et al. Molecularly imprinted polymer coated solid-phase microextraction fiber prepared by surface reversible addition–fragmentation chain transfer polymerization for monitoring of Sudan dyes in chilli tomato sauce and chilli pepper samples. Anal Chim Acta. 2012;731:40–48. doi: 10.1016/j.aca.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Hu Y, Jiang L, Sun X, et al. Risk assessment of antibiotic resistance genes in the drinking water system. Sci Total Environ. 2021;800:149650. doi: 10.1016/j.scitotenv.2021.149650. [DOI] [PubMed] [Google Scholar]
- Hu Y, Jin L, Zhao Y, et al. Annual trends and health risks of antibiotics and antibiotic resistance genes in a drinking water source in East China. Sci Total Environ. 2021;791:148152. doi: 10.1016/j.scitotenv.2021.148152. [DOI] [PubMed] [Google Scholar]
- Husin NA, Muhamad M, Yahaya N, et al. Application of a new choline-imidazole based deep eutectic solvents in hybrid magnetic molecularly imprinted polymer for efficient and selective removal of naproxen from aqueous samples. Mater Chem Phys. 2021;261:124228. doi: 10.1016/j.matchemphys.2021.124228. [DOI] [Google Scholar]
- Iesce MR, Lavorgna M, Russo C, et al. Ecotoxic effects of loratadine and its metabolic and light-induced derivatives. Ecotoxicol Environ Saf. 2019;170:664–672. doi: 10.1016/j.ecoenv.2018.11.116. [DOI] [PubMed] [Google Scholar]
- Isidori M, Lavorgna M, Nardelli A, et al. Ecotoxicity of naproxen and its phototransformation products. Sci Total Environ. 2005;348:93–101. doi: 10.1016/j.scitotenv.2004.12.068. [DOI] [PubMed] [Google Scholar]
- Jeong TY, Kim HY, Kim SD. Multi-generational effects of propranolol on Daphnia magna at different environmental concentrations. Environ Pollut. 2015;206:188–194. doi: 10.1016/j.envpol.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Jeong TY, Yoon D, Kim S, et al. Mode of action characterization for adverse effect of propranolol in Daphnia magna based on behavior and physiology monitoring and metabolite profiling. Environ Pollut. 2018;233:99–108. doi: 10.1016/j.envpol.2017.10.043. [DOI] [PubMed] [Google Scholar]
- Jureczko M, Kalka J. Cytostatic pharmaceuticals as water contaminants. Eur J Pharmacol. 2020;866:172816. doi: 10.1016/j.ejphar.2019.172816. [DOI] [PubMed] [Google Scholar]
- Kechagia M, Samanidou V, Kabir A, Furton KG. One-pot synthesis of a multi-template molecularly imprinted polymer for the extraction of six sulfonamide residues from milk before high-performance liquid chromatography with diode array detection. J Sep Sci. 2018;41:723–731. doi: 10.1002/jssc.201701205. [DOI] [PubMed] [Google Scholar]
- Kefayati H, Yamini Y, Shamsayei M, Abdi S. Molecularly imprinted polypyrrole@CuO nanocomposite as an in-tube solid-phase microextraction coating for selective extraction of carbamazepine from biological samples. J Pharm Biomed Anal. 2021;204:114256. doi: 10.1016/j.jpba.2021.114256. [DOI] [PubMed] [Google Scholar]
- Khan HK, Rehman MYA, Malik RN. Fate and toxicity of pharmaceuticals in water environment: an insight on their occurrence in South Asia. J Environ Manage. 2020;271:111030. doi: 10.1016/j.jenvman.2020.111030. [DOI] [PubMed] [Google Scholar]
- Khan NA, Ahmed S, Farooqi IH, et al. Occurrence, sources and conventional treatment techniques for various antibiotics present in hospital wastewaters: a critical review. TrAC Trends Anal Chem. 2020;129:115921. doi: 10.1016/j.trac.2020.115921. [DOI] [Google Scholar]
- Khulu S, Ncube S, Kgame T, et al. Synthesis, characterization and application of a molecularly imprinted polymer as an adsorbent for solid-phase extraction of selected pharmaceuticals from water samples. Polym Bull. 2021;79:1287–1307. doi: 10.1007/s00289-021-03553-9. [DOI] [Google Scholar]
- Khulu S, Ncube S, Nuapia Y, et al. Multivariate optimization of a two-way technique for extraction of pharmaceuticals in surface water using a combination of membrane assisted solvent extraction and a molecularly imprinted polymer. Chemosphere. 2022;286:131973. doi: 10.1016/j.chemosphere.2021.131973. [DOI] [PubMed] [Google Scholar]
- Kim S, Gholamirad F, Yu M, et al. Enhanced adsorption performance for selected pharmaceutical compounds by sonicated Ti3C2TX MXene. Chem Eng J. 2021;406:126789. doi: 10.1016/j.cej.2020.126789. [DOI] [Google Scholar]
- Kleinert C, Poirier-Larabie S, Gagnon C, et al. Occurrence and ecotoxicity of cytostatic drugs 5-fluorouracil and methotrexate in the freshwater unionid Elliptio complanata. Comp Biochem Physiol C Toxicol Pharmacol. 2021;244:109027. doi: 10.1016/j.cbpc.2021.109027. [DOI] [PubMed] [Google Scholar]
- Kong X, Li F, Li Y, et al. Molecularly imprinted polymer functionalized magnetic Fe3O4 for the highly selective extraction of triclosan. J Sep Sci. 2020;43:808–817. doi: 10.1002/jssc.201900924. [DOI] [PubMed] [Google Scholar]
- Kovalakova P, Cizmas L, McDonald TJ, et al. Occurrence and toxicity of antibiotics in the aquatic environment: a review. Chemosphere. 2020;251:126351. doi: 10.1016/j.chemosphere.2020.126351. [DOI] [PubMed] [Google Scholar]
- Kriikku P, Pelkonen S, Kaukonen M, Ojanperä I. Propranolol and metoprolol: two comparable drugs with very different post-mortem toxicological profiles. Forensic Sci Int. 2021;327:110978. doi: 10.1016/j.forsciint.2021.110978. [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Desdoits-Lethimonier C, Mackey AL, et al. Ibuprofen alters human testicular physiology to produce a state of compensated hypogonadism. Proc Natl Acad Sci USA. 2018 doi: 10.1073/pnas.1715035115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Pal D. Antibiotic resistance and wastewater: correlation, impact and critical human health challenges. J Environ Chem Eng. 2018;6:52–58. doi: 10.1016/j.jece.2017.11.059. [DOI] [Google Scholar]
- Kumar S, Karfa P, Majhi KC, Madhuri R. Photocatalytic, fluorescent BiPO4@ graphene oxide based magnetic molecularly imprinted polymer for detection, removal and degradation of ciprofloxacin. Mater Sci Eng C. 2020;111:110777. doi: 10.1016/j.msec.2020.110777. [DOI] [PubMed] [Google Scholar]
- Kumar M, Mazumder P, Mohapatra S, et al. A chronicle of SARS-CoV-2: Seasonality, environmental fate, transport, inactivation, and antiviral drug resistance. J Hazard Mater. 2021;405:124043. doi: 10.1016/j.jhazmat.2020.124043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak K, Ji K, Kho Y, et al. Chronic toxicity and endocrine disruption of naproxen in freshwater waterfleas and fish, and steroidogenic alteration using H295R cell assay. Chemosphere. 2018;204:156–162. doi: 10.1016/j.chemosphere.2018.04.035. [DOI] [PubMed] [Google Scholar]
- Li X, Row KH. Preparation of deep eutectic solvent-based hexagonal boron nitride-molecularly imprinted polymer nanoparticles for solid phase extraction of flavonoids. Microchim Acta. 2019;186:753. doi: 10.1007/s00604-019-3885-8. [DOI] [PubMed] [Google Scholar]
- Li N, Yang H. Construction of natural polymeric imprinted materials and their applications in water treatment: a review. J Hazard Mater. 2021;403:123643. doi: 10.1016/j.jhazmat.2020.123643. [DOI] [PubMed] [Google Scholar]
- Li Q, Yu J, Chen W, et al. Degradation of triclosan by chlorine dioxide: reaction mechanism,2,4-dichlorophenol accumulation and toxicity evaluation. Chemosphere. 2018;207:449–456. doi: 10.1016/j.chemosphere.2018.05.065. [DOI] [PubMed] [Google Scholar]
- Li J, Ji F, Ng DHL, et al. Bioinspired Pt-free molecularly imprinted hydrogel-based magnetic Janus micromotors for temperature-responsive recognition and adsorption of erythromycin in water. Chem Eng J. 2019;369:611–620. doi: 10.1016/j.cej.2019.03.101. [DOI] [Google Scholar]
- Li Y, Zhang L, Ding J, Liu X. Prioritization of pharmaceuticals in water environment in China based on environmental criteria and risk analysis of top-priority pharmaceuticals. J Environ Manag. 2020;253:109732. doi: 10.1016/j.jenvman.2019.109732. [DOI] [PubMed] [Google Scholar]
- Limbu SM, Zhou L, Sun SX, et al. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ Int. 2018;115:205–219. doi: 10.1016/j.envint.2018.03.034. [DOI] [PubMed] [Google Scholar]
- Liu L, Yang M, He M, et al. Magnetic solid phase extraction sorbents using methyl-parathion and quinalphos dual-template imprinted polymers coupled with GC-MS for class-selective extraction of twelve organophosphorus pesticides. Microchim Acta. 2020;187:503. doi: 10.1007/s00604-020-04465-7. [DOI] [PubMed] [Google Scholar]
- Liu N, Jin X, Feng C, et al. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: a proposed multiple-level system. Environ Int. 2020;136:105454. doi: 10.1016/j.envint.2019.105454. [DOI] [PubMed] [Google Scholar]
- Liu H, Jin P, Zhu F, et al. A review on the use of ionic liquids in preparation of molecularly imprinted polymers for applications in solid-phase extraction. TrAC Trends Anal Chem. 2021;134:116132. doi: 10.1016/j.trac.2020.116132. [DOI] [Google Scholar]
- Liu Y, Lian Z, Li F, et al. Review on molecular imprinting technology and its application in pre-treatment and detection of marine organic pollutants. Mar Pollut Bull. 2021;169:112541. doi: 10.1016/j.marpolbul.2021.112541. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu Y, Liu Z, et al. Ultra-durable, multi-template molecularly imprinted polymers for ultrasensitive monitoring and multicomponent quantification of trace sulfa antibiotics. J Mater Chem B. 2021;9:3192–3199. doi: 10.1039/D1TB00091H. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen G, Lu X. In-situ growth of molecularly imprinted metal–organic frameworks on 3D carbon foam as an efficient adsorbent for selective removal of antibiotics. J Mol Liq. 2021;340:117232. doi: 10.1016/j.molliq.2021.117232. [DOI] [Google Scholar]
- Lu W, Liu J, Li J, et al. Dual-template molecularly imprinted polymers for dispersive solid-phase extraction of fluoroquinolones in water samples coupled with high performance liquid chromatography. Analyst. 2019;144:1292–1302. doi: 10.1039/c8an02133c. [DOI] [PubMed] [Google Scholar]
- Ma Z, Wu H, Zhang K, et al. Long-term low dissolved oxygen accelerates the removal of antibiotics and antibiotic resistance genes in swine wastewater treatment. Chem Eng J. 2018;334:630–637. doi: 10.1016/j.cej.2017.10.051. [DOI] [Google Scholar]
- Mackuľak T, Černanský S, Fehér M, et al. Pharmaceuticals, drugs, and resistant microorganisms—environmental impact on population health. Curr Opin Environ Sci Health. 2019;9:40–48. doi: 10.1016/j.coesh.2019.04.002. [DOI] [Google Scholar]
- Madikizela LM, Chimuka L. Synthesis, adsorption and selectivity studies of a polymer imprinted with naproxen, ibuprofen and diclofenac. J Environ Chem Eng. 2016;4:4029–4037. doi: 10.1016/j.jece.2016.09.012. [DOI] [Google Scholar]
- Madikizela LM, Chimuka L. Determination of ibuprofen, naproxen and diclofenac in aqueous samples using a multi-template molecularly imprinted polymer as selective adsorbent for solid-phase extraction. J Pharm Biomed Anal. 2016;128:210–215. doi: 10.1016/j.jpba.2016.05.037. [DOI] [PubMed] [Google Scholar]
- Madikizela LM, Mdluli PS, Chimuka L. Experimental and theoretical study of molecular interactions between 2-vinyl pyridine and acidic pharmaceuticals used as multi-template molecules in molecularly imprinted polymer. React Funct Polym. 2016;103:33–43. doi: 10.1016/j.reactfunctpolym.2016.03.017. [DOI] [Google Scholar]
- Madikizela LM, Tavengwa N, Pakade V. Molecularly imprinted polymers for pharmaceutical compounds: synthetic procedures and analytical applications. Recent Res Polym. London: IntechOpen; 2018. [Google Scholar]
- Madikizela LM, Tavengwa NT, Chimuka L. Applications of molecularly imprinted polymers for solid-phase extraction of non-steroidal anti-inflammatory drugs and analgesics from environmental waters and biological samples. J Pharm Biomed Anal. 2018;147:624–633. doi: 10.1016/j.jpba.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Madikizela LM, Tavengwa NT, Tutu H, Chimuka L. Green aspects in molecular imprinting technology: from design to environmental applications. Trends Environ Anal Chem. 2018;17:14–22. doi: 10.1016/j.teac.2018.01.001. [DOI] [Google Scholar]
- Madikizela LM, Zunngu SS, Mlunguza NY, et al. Application of molecularly imprinted polymer designed for the selective extraction of ketoprofen from wastewater. Water SA. 2018;44:406–418. doi: 10.4314/wsa.v44i3.08. [DOI] [Google Scholar]
- Magro C, Mateus EP, Paz-Garcia JM, Ribeiro AB. Emerging organic contaminants in wastewater: understanding electrochemical reactors for triclosan and its by-products degradation. Chemosphere. 2020;247:125758. doi: 10.1016/j.chemosphere.2019.125758. [DOI] [PubMed] [Google Scholar]
- Maksoud MIAA, Fahim RA, Bedir AG, et al. Engineered magnetic oxides nanoparticles as efficient sorbents for wastewater remediation: a review. Environ Chem Lett. 2022;20:519–562. doi: 10.1007/s10311-021-01351-3. [DOI] [Google Scholar]
- Manzo V, Ulisse K, Rodríguez I, et al. A molecularly imprinted polymer as the sorptive phase immobilized in a rotating disk extraction device for the determination of diclofenac and mefenamic acid in wastewater. Anal Chim Acta. 2015;889:130–137. doi: 10.1016/j.aca.2015.07.038. [DOI] [PubMed] [Google Scholar]
- Metwally MG, Benhawy AH, Khalifa RM, et al. Application of molecularly imprinted polymers in the analysis of waters and wastewaters. Molecules. 2021;26:1–38. doi: 10.3390/molecules26216515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzelani M, Gorbi S, Fattorini D, et al. Long-term exposure of Mytilus galloprovincialis to diclofenac, Ibuprofen and Ketoprofen: insights into bioavailability, biomarkers and transcriptomic changes. Chemosphere. 2018;198:238–248. doi: 10.1016/j.chemosphere.2018.01.148. [DOI] [PubMed] [Google Scholar]
- Mezzelani M, Gorbi S, Regoli F. Pharmaceuticals in the aquatic environments: evidence of emerged threat and future challenges for marine organisms. Mar Environ Res. 2018;140:41–60. doi: 10.1016/j.marenvres.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Mohamed Idris Z, Hameed BH, Ye L, et al. Amino-functionalised silica-grafted molecularly imprinted polymers for chloramphenicol adsorption. J Environ Chem Eng. 2020;8:103981. doi: 10.1016/j.jece.2020.103981. [DOI] [Google Scholar]
- Mohiuddin I, Bhogal S, Grover A, et al. Simultaneous determination of amitriptyline, nortriptyline, and clomipramine in aqueous samples using selective multi-template molecularly imprinted polymers. Environ Nanotechnol Monit Manag. 2021;16:100527. doi: 10.1016/j.enmm.2021.100527. [DOI] [Google Scholar]
- Mole RA, Brooks BW. Global scanning of selective serotonin reuptake inhibitors: occurrence, wastewater treatment and hazards in aquatic systems. Environ Pollut. 2019;250:1019–1031. doi: 10.1016/j.envpol.2019.04.118. [DOI] [PubMed] [Google Scholar]
- Monisha RS, Mani RL, Sivaprakash B, et al. Green remediation of pharmaceutical wastes using biochar: a review. Environ Chem Lett. 2022;20:681–704. doi: 10.1007/s10311-021-01348-y. [DOI] [Google Scholar]
- Morin-Crini N, Lichtfouse E, Fourmentin M, et al. Removal of emerging contaminants from wastewater using advanced treatments. A review. Environ Chem Lett. 2022 doi: 10.1007/s10311-021-01379-5. [DOI] [Google Scholar]
- Möstl E, Palme R. Hormones as indicators of stress. Domest Anim Endocrinol. 2002;23:67–74. doi: 10.1016/S0739-7240(02)00146-7. [DOI] [PubMed] [Google Scholar]
- Ndunda EN. Molecularly imprinted polymers—a closer look at the control polymer used in determining the imprinting effect: a mini review. J Mol Recognit. 2020;33:1–11. doi: 10.1002/jmr.2855. [DOI] [PubMed] [Google Scholar]
- Nie XP, Liu BY, Yu HJ, et al. Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole exposure to the antioxidant system in Pseudokirchneriella subcapitata. Environ Pollut. 2013;172:23–32. doi: 10.1016/j.envpol.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Nunes B, Antunes SC, Santos J, et al. Toxic potential of paracetamol to freshwater organisms: a headache to environmental regulators? Ecotoxicol Environ Saf. 2014;107:178–185. doi: 10.1016/j.ecoenv.2014.05.027. [DOI] [PubMed] [Google Scholar]
- Pan J, Chen W, Ma Y, Pan G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem Soc Rev. 2018;47:5574–5587. doi: 10.1039/c7cs00854f. [DOI] [PubMed] [Google Scholar]
- Parry CM, Threlfall EJ. Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr Opin Infect Dis. 2008;21:531–538. doi: 10.1097/QCO.0b013e32830f453a. [DOI] [PubMed] [Google Scholar]
- Patel S, Homaei A, Raju AB, Meher BR. Estrogen: the necessary evil for human health, and ways to tame it. Biomed Pharmacother. 2018;102:403–411. doi: 10.1016/j.biopha.2018.03.078. [DOI] [PubMed] [Google Scholar]
- Peng Q, Wu Y, Cong H, et al. Preparation of monodisperse porous polymeric ionic liquid microspheres and their application as stationary phases for HPLC. Talanta. 2019;208:120462. doi: 10.1016/j.talanta.2019.120462. [DOI] [PubMed] [Google Scholar]
- Pereira A, Silva L, Laranjeiro C, et al. Selected pharmaceuticals in different aquatic compartments: part I—source. Fate Occur Mol. 2020;25:1026. doi: 10.3390/molecules25051026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussolo MC, Guiloski IC, Lirola JR, et al. Integrated biomarker response index to assess toxic effects of environmentally relevant concentrations of paracetamol in a neotropical catfish (Rhamdia quelen) Ecotoxicol Environ Saf. 2019;182:109438. doi: 10.1016/j.ecoenv.2019.109438. [DOI] [PubMed] [Google Scholar]
- Pichon V, Chapuis-Hugon F. Role of molecularly imprinted polymers for selective determination of environmental pollutants—a review. Anal Chim Acta. 2008;622:48–61. doi: 10.1016/j.aca.2008.05.057. [DOI] [PubMed] [Google Scholar]
- Qiao L, Sun R, Yu C, et al. Novel hydrophobic deep eutectic solvents for ultrasound-assisted dispersive liquid-liquid microextraction of trace non-steroidal anti-inflammatory drugs in water and milk samples. Microchem J. 2021;170:106686. doi: 10.1016/j.microc.2021.106686. [DOI] [Google Scholar]
- Quesada HB, Baptista ATA, Cusioli LF, et al. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: a review. Chemosphere. 2019;222:766–780. doi: 10.1016/j.chemosphere.2019.02.009. [DOI] [PubMed] [Google Scholar]
- Rathi BS, Kumar PS, Show PL. A review on effective removal of emerging contaminants from aquatic systems: current trends and scope for further research. J Hazard Mater. 2021;409:124413. doi: 10.1016/j.jhazmat.2020.124413. [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Torres T, Martins R, Santos MM. Toxicity screening of diclofenac, propranolol, sertraline and simvastatin using Danio rerio and Paracentrotus lividus embryo bioassays. Ecotoxicol Environ Saf. 2015;114:67–74. doi: 10.1016/j.ecoenv.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Robson L, Barnhoorn IEJ, Wagenaar GM. The potential effects of efavirenz on Oreochromis mossambicus after acute exposure. Environ Toxicol Pharmacol. 2017;56:225–232. doi: 10.1016/j.etap.2017.09.017. [DOI] [PubMed] [Google Scholar]
- Rodrigues S, Antunes SC, Correia AT, et al. Assessment of toxic effects of the antibiotic erythromycin on the marine fish gilthead seabream (Sparus aurata L.) by a multi-biomarker approach. Chemosphere. 2019;216:234–247. doi: 10.1016/j.chemosphere.2018.10.124. [DOI] [PubMed] [Google Scholar]
- Rodrigues S, Antunes SC, Correia AT, Nunes B. Toxicity of erythromycin to Oncorhynchus mykiss at different biochemical levels: detoxification metabolism, energetic balance, and neurological impairment. Environ Sci Pollut Res. 2019;26:227–239. doi: 10.1007/s11356-018-3494-9. [DOI] [PubMed] [Google Scholar]
- Rodrigues S, Antunes SC, Nunes B, Correia AT. Histopathological effects in gills and liver of Sparus aurata following acute and chronic exposures to erythromycin and oxytetracycline. Environ Sci Pollut Res. 2019;26:15481–15495. doi: 10.1007/s11356-019-04954-0. [DOI] [PubMed] [Google Scholar]
- Sajini T, Mathew B. A brief overview of molecularly imprinted polymers: highlighting computational design, nano and photo-responsive imprinting. Talanta Open. 2021;4:100072. doi: 10.1016/j.talo.2021.100072. [DOI] [Google Scholar]
- Sathishkumar P, Meena RAA, Palanisami T, et al. Occurrence, interactive effects and ecological risk of diclofenac in environmental compartments and biota—a review. Sci Total Environ. 2020;698:134057. doi: 10.1016/j.scitotenv.2019.134057. [DOI] [PubMed] [Google Scholar]
- Schlüter-Vorberg L, Prasse C, Ternes TA, et al. Toxification by transformation in conventional and advanced wastewater treatment: the antiviral drug acyclovir. Environ Sci Technol Lett. 2015;2:342–346. doi: 10.1021/acs.estlett.5b00291. [DOI] [Google Scholar]
- Schüle C. Neuroendocrinological mechanisms of actions of antidepressant drugs. J Neuroendocrinol. 2007;19:213–226. doi: 10.1111/j.1365-2826.2006.01516.x. [DOI] [PubMed] [Google Scholar]
- Sehonova P, Hodkovicova N, Urbanova M, et al. Effects of antidepressants with different modes of action on early life stages of fish and amphibians. Environ Pollut. 2019;254:112999. doi: 10.1016/j.envpol.2019.112999. [DOI] [PubMed] [Google Scholar]
- Sharma BM, Bečanová J, Scheringer M, et al. Health and ecological risk assessment of emerging contaminants (pharmaceuticals, personal care products, and artificial sweeteners) in surface and groundwater (drinking water) in the Ganges River Basin, India. Sci Total Environ. 2019;646:1459–1467. doi: 10.1016/j.scitotenv.2018.07.235. [DOI] [PubMed] [Google Scholar]
- Shea KJ, Dougherty TK. Molecular recognition on synthetic amorphous surfaces. the influence of functional group positioning on the effectiveness of molecular recognition. J Am Chem Soc. 1986;108:1091–1093. doi: 10.1021/ja00265a046. [DOI] [Google Scholar]
- Shen X, Xu C, Ye L. Molecularly imprinted polymers for clean water: analysis and purification. Ind Eng Chem Res. 2013;52:13890–13899. doi: 10.1021/ie302623s. [DOI] [Google Scholar]
- Shen R, Yu Y, Lan R, et al. The cardiovascular toxicity induced by high doses of gatifloxacin and ciprofloxacin in zebrafish. Environ Pollut. 2019;254:112861. doi: 10.1016/j.envpol.2019.07.029. [DOI] [PubMed] [Google Scholar]
- Shi X, Leong KY, Ng HY. Anaerobic treatment of pharmaceutical wastewater: a critical review. Bioresour Technol. 2017;245:1238–1244. doi: 10.1016/j.biortech.2017.08.150. [DOI] [PubMed] [Google Scholar]
- Shin J, Kwak J, Lee YG, et al. Competitive adsorption of pharmaceuticals in lake water and wastewater effluent by pristine and NaOH-activated biochars from spent coffee wastes: contribution of hydrophobic and π-π interactions. Environ Pollut. 2021;270:116244. doi: 10.1016/j.envpol.2020.116244. [DOI] [PubMed] [Google Scholar]
- Srinivasan AV. Propranolol: a 50-year historical perspective. Ann Indian Acad Neurol. 2019;22:21. doi: 10.4103/aian.AIAN_201_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staels B, Dallongeville J, Auwerx J, et al. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–2093. doi: 10.1161/01.CIR.98.19.2088. [DOI] [PubMed] [Google Scholar]
- Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5:378–387. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoczynski L, van den Hurk P. Effects of selective serotonin reuptake inhibitor sertraline on hybrid striped bass predatory behavior and brain chemistry. Aquat Toxicol. 2020;226:105564. doi: 10.1016/j.aquatox.2020.105564. [DOI] [PubMed] [Google Scholar]
- Sun XY, Ma RT, Chen J, Shi YP. Synthesis of magnetic molecularly imprinted nanoparticles with multiple recognition sites for the simultaneous and selective capture of two glycoproteins. J Mater Chem B. 2018;6:688–696. doi: 10.1039/C7TB03001K. [DOI] [PubMed] [Google Scholar]
- Sun L, Li J, Li X, et al. Molecularly imprinted Ag/Ag3VO4/g-C3N4 Z-scheme photocatalysts for enhanced preferential removal of tetracycline. J Colloid Interface Sci. 2019;552:271–286. doi: 10.1016/j.jcis.2019.05.060. [DOI] [PubMed] [Google Scholar]
- Suwanwong Y, Kulkeratiyut S, Prachayasittikul V, Boonpangrak S. Effects of polymerization methods and functional monomers on curcumin imprinted polymer properties. Sep Sci Technol. 2014;49:1086–1095. doi: 10.1080/01496395.2013.871036. [DOI] [Google Scholar]
- Taisen I, Hajime W, Yoshinao K. Application of ecotoxicogenomics for studying endocrine disruption in vertebrates and invertebrates. Environ Health Perspect. 2006;114:101–105. doi: 10.1289/ehp.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Yu K, Li L, et al. Fabrication of dual-template molecularly imprinted mesoporous silica for simultaneous rapid and efficient detection of bisphenol A and diethylstilbestrol in environmental water samples. Anal Methods. 2019;11:4761–4768. doi: 10.1039/C9AY01368G. [DOI] [Google Scholar]
- Top S, Akgün M, Kıpçak E, Bilgili MS. Treatment of hospital wastewater by supercritical water oxidation process. Water Res. 2020;185:116279. doi: 10.1016/j.watres.2020.116279. [DOI] [PubMed] [Google Scholar]
- Turk Sekulic M, Boskovic N, Slavkovic A, et al. Surface functionalised adsorbent for emerging pharmaceutical removal: adsorption performance and mechanisms. Process Saf Environ Prot. 2019;125:50–63. doi: 10.1016/j.psep.2019.03.007. [DOI] [Google Scholar]
- Ut Dong T, Thi H, Pham T, et al. Synergetic effect of dual functional monomers in molecularly imprinted polymer preparation for selective solid phase extraction of ciprofloxacin. Polymers (Basel) 2021;13:2788. doi: 10.3390/polym13162788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveiros R, Rebocho S, Casimiro T. Green strategies for molecularly imprinted polymer development. Polymers (Basel) 2018;10:306. doi: 10.3390/polym10030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Gao H, Yan G, et al. Metal-organic gel-modulated synthesis of hierarchically porous molecularly imprinted polymers for efficient removal of sildenafil from water. ACS Omega. 2021;6:7478–7486. doi: 10.1021/acsomega.0c06000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Cui Y, Hu F, et al. Selective recognition and enrichment of carbamazepine in biological samples by magnetic imprinted polymer based on reversible addition-fragmentation chain transfer polymerization. J Chromatogr A. 2019;1591:62–70. doi: 10.1016/j.chroma.2019.01.057. [DOI] [PubMed] [Google Scholar]
- Wang D, Liu Y, Xu Z, et al. Multitemplate molecularly imprinted polymeric solid-phase microextraction fiber coupled with HPLC for endocrine disruptor analysis in water samples. Microchem J. 2020;155:104802. doi: 10.1016/j.microc.2020.104802. [DOI] [Google Scholar]
- Wang XT, Deng X, Zhang TD, et al. Biocompatible self-healing hydrogels based on boronic acid-functionalized polymer and laponite nanocomposite for water pollutant removal. Environ Chem Lett. 2022;20:81–90. doi: 10.1007/s10311-021-01350-4. [DOI] [Google Scholar]
- Wee SY, Aris AZ. Endocrine disrupting compounds in drinking water supply system and human health risk implication. Environ Int. 2017;106:207–233. doi: 10.1016/j.envint.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Wei CJ, Li XY, Xie YF, Wang XM. Direct photo transformation of tetracycline and sulfanomide group antibiotics in surface water: kinetics, toxicity and site modeling. Sci Total Environ. 2019;686:1–9. doi: 10.1016/j.scitotenv.2019.04.041. [DOI] [PubMed] [Google Scholar]
- Wiest L, Chonova T, Bergé A, et al. Two-year survey of specific hospital wastewater treatment and its impact on pharmaceutical discharges. Environ Sci Pollut Res. 2018;25:9207–9218. doi: 10.1007/s11356-017-9662-5. [DOI] [PubMed] [Google Scholar]
- Wu Y, Ma Y, Pan J, et al. Porous and magnetic molecularly imprinted polymers via pickering high internal phase emulsions polymerization for selective adsorption of λ-cyhalothrin. Front Chem. 2017;5:1–10. doi: 10.3389/fchem.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Xing W, Meng M, et al. Multiple-functional molecularly imprinted nanocomposite membranes for high-efficiency selective separation applications: an imitated core-shell TiO2@PDA-based MIMs design. Compos B Eng. 2020;198:108123. doi: 10.1016/j.compositesb.2020.108123. [DOI] [Google Scholar]
- Wulff G, Schauhoff S. Enzyme-analog-built polymers. 27. Racemic resolution of free sugars with macroporous polymers prepared by molecular imprinting. Selectivity dependence on the arrangement of functional groups versus spatial requirements. J Org Chem. 1991;56:395–400. doi: 10.1021/jo00001a071. [DOI] [Google Scholar]
- Wulff G, Vietmeier J. Enzyme-analogue built polymers, 26. Enantioselective synthesis of amino acids using polymers possessing chiral cavities obtained by an imprinting procedure with template molecules. Die Makromol Chem. 1989;190:1727–1735. doi: 10.1002/macp.1989.021900724. [DOI] [Google Scholar]
- Wulff G, Wolf G. Zur Chemie von Haftgruppen, VI. Über die Eignung verschiedener Aldehyde und Ketone als Haftgruppen für Monoalkohole. Chem Ber. 1986;119:1876–1889. doi: 10.1002/cber.19861190610. [DOI] [Google Scholar]
- Xu D, Xiao Y, Pan H, Mei Y. Toxic effects of tetracycline and its degradation products on freshwater green algae. Ecotoxicol Environ Saf. 2019;174:43–47. doi: 10.1016/j.ecoenv.2019.02.063. [DOI] [PubMed] [Google Scholar]
- Xu L, Zhang H, Xiong P, et al. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: a review. Sci Total Environ. 2021;753:141975. doi: 10.1016/j.scitotenv.2020.141975. [DOI] [PubMed] [Google Scholar]
- Yahaya N, Zain NNM, Miskam M, Kamaruzaman S. Molecularly imprinted polymer composites in wastewater treatment. In: Mathew MP, Beena, Nair, Archana S, Thomas S, editors. Molecularly imprinted polymer composites. 1. Amsterdam: Elsevier; 2021. pp. 381–413. [Google Scholar]
- Yang Y, Ok YS, Kim KH, et al. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: a review. Sci Total Environ. 2017;596–597:303–320. doi: 10.1016/j.scitotenv.2017.04.102. [DOI] [PubMed] [Google Scholar]
- Yang Y, Song W, Lin H, et al. Antibiotics and antibiotic resistance genes in global lakes: a review and meta-analysis. Environ Int. 2018;116:60–73. doi: 10.1016/j.envint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Yang Z, Lu T, Zhu Y, et al. Aquatic ecotoxicity of an antidepressant, sertraline hydrochloride, on microbial communities. Sci Total Environ. 2019;654:129–134. doi: 10.1016/j.scitotenv.2018.11.164. [DOI] [PubMed] [Google Scholar]
- Yilmaz G, Kaya Y, Vergili I, et al. Characterization and toxicity of hospital wastewaters in Turkey. Environ Monit Assess. 2017;189:55. doi: 10.1007/s10661-016-5732-2. [DOI] [PubMed] [Google Scholar]
- Yu M, Li H, Xie J, et al. A descriptive and comparative analysis on the adsorption of PPCPs by molecularly imprinted polymers. Talanta. 2022;236:122875. doi: 10.1016/j.talanta.2021.122875. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Yang Y, Zhu G. Molecularly imprinted porous aromatic frameworks for molecular recognition. ACS Cent Sci. 2020;6:1082–1094. doi: 10.1021/acscentsci.0c00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare EN, Mudhoo A, Khan MA, et al. Water decontamination using bio-based, chemically functionalized, doped, and ionic liquid-enhanced adsorbents. Environ Chem Lett. 2021;19:3075–3114. doi: 10.1007/s10311-021-01207-w. [DOI] [Google Scholar]
- Zeng G, Liu Y, Ma X, Fan Y. Fabrication of magnetic multi-template molecularly imprinted polymer composite for the selective and efficient removal of tetracyclines from water. Front Environ Sci Eng. 2021;15:107. doi: 10.1007/s11783-021-1395-5. [DOI] [Google Scholar]
- Zeng H, Yu X, Wan J, Cao X. Synthesis of molecularly imprinted polymers based on boronate affinity for diol-containing macrolide antibiotics with hydrophobicity-balanced and pH-responsive cavities. J Chromatogr A. 2021;1642:461969. doi: 10.1016/j.chroma.2021.461969. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cao X, Zhang Z, et al. Synthesis of dummy-template molecularly imprinted polymer adsorbents for solid phase extraction of aminoglycosides antibiotics from environmental water samples. Talanta. 2020;208:120385. doi: 10.1016/j.talanta.2019.120385. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Zhao H, Huang W, et al. Dual functional monomer surface molecularly imprinted microspheres for polysaccharide recognition in aqueous solution. Anal Methods. 2019;11:2800–2808. doi: 10.1039/C9AY00132H. [DOI] [Google Scholar]
- Zhao X, Wang Y, Zhang P, et al. Recent advances of molecularly imprinted polymers based on cyclodextrin. Macromol Rapid Commun. 2021;42:1–19. doi: 10.1002/marc.202100004. [DOI] [PubMed] [Google Scholar]
- Zhu G, Cheng G, Wang L, et al. A new ionic liquid surface-imprinted polymer for selective solid-phase-extraction and determination of sulfonamides in environmental samples. J Sep Sci. 2019;42:725–735. doi: 10.1002/jssc.201800759. [DOI] [PubMed] [Google Scholar]
- Zivna D, Sehonova P, Plhalova L, et al. Effect of salicylic acid on early life stages of common carp (Cyprinus carpio) Environ Toxicol Pharmacol. 2015;40:319–325. doi: 10.1016/j.etap.2015.06.018. [DOI] [PubMed] [Google Scholar]
- Zounková R, Odráska P, Dolezalová L, et al. Ecotoxicity and genotoxicity assessment of cytostatic pharmaceuticals. Environ Toxicol Chem. 2007;26:2208–2214. doi: 10.1897/07-137R.1. [DOI] [PubMed] [Google Scholar]
- Zounkova R, Kovalova L, Blaha L, Dott W. Ecotoxicity and genotoxicity assessment of cytotoxic antineoplastic drugs and their metabolites. Chemosphere. 2010;81:253–260. doi: 10.1016/j.chemosphere.2010.06.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.