Abstract
Mycobacterium avium is a common cause of systemic bacterial infection in patients with AIDS. Infection with M. avium has been linked to bacterial colonization of domestic water supplies and commonly occurs through the gastrointestinal tract. Acanthamoeba castellanii, a waterborne protozoan, may serve as an environmental host for M. avium. It has been shown that growth of M. avium in amoebae enhances invasion and intracellular replication of the bacteria in human macrophages and intestinal epithelial cell line HT-29 as well as in mice. We determined that growth of M. avium within A. castellanii influenced susceptibility to rifabutin, azithromycin, and clarithromycin. No significant activity against M. avium was seen with rifabutin, azithromycin, and clarithromycin when used to treat monolayers on both day 1 and day 4 after infection. When tested in a macrophage-like cell line (U937), all compounds showed significant anti-M. avium activity. Growth of M. avium in amoebae appears to reduce the effectiveness of the antimicrobials. These findings may have significant implications for prophylaxis of M. avium infection in AIDS.
Disseminated infection caused by organisms of the Mycobacterium avium complex is a common finding in patients with advanced states of AIDS (11, 12). Recent evidence supports the concept that M. avium's major route of infection in this population of patients is through the intestinal tract (9; F. Torriani, J. N. Maslow, R. Kornbluth, R. D. Arbeit, J. A. McCutchan, P. Hasegawa, L. Keays, D. Havlir, and The California Collaborative Treatment Group, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. I91, p. 221, 1995). M. avium has been shown to colonize the intestinal lumen before dissemination (9; Torriani et al., 35th ICAAC). In addition, studies with experimental mice indicated that M. avium can invade the intact intestinal mucosa and gain access to the lamina propria (5).
M. avium is an opportunistic pathogen encountered in water and soil (10). We have recently reported that AIDS isolates of M. avium can infect Acanthamoeba castellanii (an environmental amoeba) and grow intracellularly at both 37 and 30°C (8). In addition, it was found that amoeba-grown M. avium was capable of invading intestinal mucosal cells with significantly increased efficiency compared with that of M. avium grown on 7H10 agar.
Infection with M. avium grown within A. castellanii, as well as M. avium grown within amoebae and released from the amoeba host prior to oral infection in mice, was associated with a significant increase in invasion of the gastrointestinal mucosa and an augmented number of CFU per gram of tissue in the liver and spleen of infected mice in comparison with the bacterial load in mice infected with M. avium grown on 7H11 agar (8).
These findings suggest the possibility that environmental amoebae are part of the mechanisms of pathogenesis of M. avium in AIDS patients. Theoretically, M. avium and environmental amoebae can inhabit the same environmental site. Since it is plausible that at least in some cases M. avium would infect environmental amoebae such as Acanthamoeba and subsequently be ingested by the human host in association with amoebae, the susceptibility of intracellular (within amoebae) M. avium to prophylactic antibiotics should be examined. Previous studies have demonstrated that another environmental bacterium, Legionella pneumophila, grown within amoebae is more resistant to high concentrations of rifampin than is L. pneumophila grown on plates (3).
Therefore, we sought to investigate the influence of amoeba infection on the susceptibility of M. avium to antibiotics used as prophylaxis of M. avium infection, such as rifabutin, clarithromycin, and azithromycin.
M. avium strain 101 (serovar 1) was originally isolated from the blood of an AIDS patient. Mycobacteria were cultured on Middlebrook 7H11 agar (Difco Laboratories, Detroit, Mich.) for 10 days at 37°C. Transparent colonies were resuspended in Hanks' balanced salt solution (HBSS) and washed twice, and the final suspension was adjusted to 3 × 107 bacteria/ml by using the McFarland turbidity standard. A sample obtained from the bacterial suspension was plated onto 7H11 agar to confirm the number of CFU per milliliter. Before infection of the monolayers, the final suspension was vortex agitated for 2 min to disperse bacteria. M. avium 101 was previously shown to be virulent in mice and to infect A. castellanii (8). Staphylococcus aureus isolated from a patient was cultured on Luria-Bertani agar for 24 h.
A. castellanii obtained from Jeffrey Cirillo (University of Nebraska, Lincoln) was cultured in 75-cm2 tissue culture flasks in 712 broth. The amoebae were kept in the dark at room temperature as reported previously (7, 8).
Human monocyte cell line U937 was obtained from the American Type Culture Collection (Manassas, Va.) and cultured in RPMI 1640 supplemented with 5% inactivated fetal bovine serum at 37°C and 5% CO2. Cell maturation was induced by treating the monolayers with phorbol myristate acetate as previously described (4). The medium was replenished every 3 days. Cell viability was determined by staining of the monolayer with trypan blue. Only monolayers with more than 90% viable cells were used for the assays.
A. castellanii was harvested by scraping the flasks with a rubber policeman. The resulting suspension was centrifuged at 800 × g for 10 min, and the cells were resuspended in high-salt buffer (7, 8) to a concentration of 2 × 105 cells/ml. To prepare monolayers, a 24-well tissue culture plate was used. Acanthamoeba was seeded and incubated for 1 h to allow the amoebae to adhere to the plastic. The Acanthamoeba monolayers were then infected with 106 bacteria and incubated for 4 h at 37°C. After the period of infection, monolayers were washed twice with HBSS to remove extracellular bacteria. The removal of extracellular bacteria by this method has been established previously (6, 8).
Baseline wells were lysed by adding 1 ml of 1% Triton X-100. Previous study has demonstrated that M. avium is resistant to this concentration of Triton X-100 (8). Lysed monolayers were diluted in water, and the number of viable bacteria was determined as previously described (8).
Monolayers of macrophage cell line U937 (106 cells) were infected as described for amoebae, except that macrophages were lysed by incubating the cells with sterile water for 10 min (4).
The monolayers used in experimental assays were monitored as previously described to ensure that all the monolayers had approximately the same number of cells (4, 8). Infection of Acanthamoeba monolayers with S. aureus (multiplicity of infection of 10) was carried out for 1 h at 37°C with a subsequent wash (three times) of extracellular bacteria with HBSS.
Rifabutin was provided by Farmitalia Carlos Erba (Milan, Italy), azithromycin was provided by Pfizer Research Laboratories, Inc. (Groton, Conn.), and clarithromycin was supplied by Abbott (Chicago, Ill.). The antimicrobials were prepared as recommended by the manufacturers. Rifabutin was dissolved in ethanol; azithromycin and clarithromycin were dissolved in dimethyl sulfoxide. All compounds were subsequently diluted in HBSS to the desired concentration. The MICs of rifabutin, azithromycin, and clarithromycin for M. avium strain 101, as determined by the radiometric method (BACTEC; Johnston Laboratories, Cockeysville, Md.) as described previously (4), were 2, 32, and 4 μg/ml, respectively (data not shown).
To compare the responses of M. avium to treatment with antimicrobials when within either amoebae or macrophages, infected monolayers were treated from day 1 postinfection with rifabutin (2 and 4 μg/ml), clarithromycin (2 and 4 μg/ml), and azithromycin (2 and 4 μg/ml) for 4 days with the drug and medium was replenished daily. These concentrations had been shown to be bacteriostatic for intracellular M. avium (12). On day 5, cells were lysed and the viable bacteria in the lysates were quantitated.
As a variant of the assay, Acanthamoeba monolayers were infected with M. avium for 4 h, extracellular bacteria were removed by washing, and the monolayers were kept for 4 days before the initiation of treatment with antimicrobials (Fig. 1B). Acanthamoeba monolayers were treated for 4 days and then lysed. Monolayers infected with S. aureus for 1 h were treated with antimicrobials for 4 h at 37°C and then lysed with 1 ml of 1% Triton X-100.
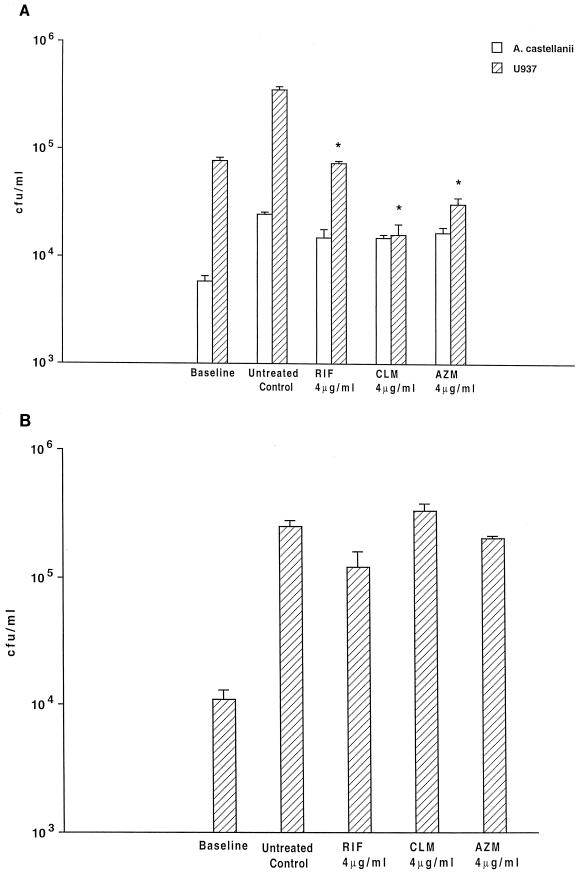
FIG. 1.
Activities of rifabutin (RIF), clarithromycin (CLM), and azithromycin (AZM) against intracellular M. avium were examined according to two different protocols. (A) A. castellanii and macrophages (U937) were infected as described in the text, and treatment was initiated 4 h after infection; asterisks show that P was <0.05 for the comparison between the number of bacteria in the untreated control and the number of organisms within macrophages following all three antibiotic treatments. No statistically significant difference was observed among the Acanthamoeba groups. (B) A. castellanii was infected with M. avium, and 4 days after infection, treatment with antimicrobials was initiated and continued for 4 more days. No statistically significant difference was observed between the untreated control and the groups treated with antimicrobials.
In order to determine whether intracellular M. avium that showed resistance to drug treatment when inside amoebae would have a resistant phenotype, Acanthamoeba was infected with M. avium 101 for 4 days and then lysed. Released bacteria were cultured on 7H11 agar for 8 days, and then their susceptibility to antibiotics was determined as described previously (4).
The assays were repeated three times, and the results were expressed as means ± standard errors of the means. The statistical significance of the differences between experimental groups and control at the same time point was analyzed by the Mann-Whitney nonparametric test.
M. avium-infected A. castellanii monolayers treated with rifabutin, clarithromycin, or azithromycin from the day after infection, for 4 days, showed no significant reduction in CFU per milliliter (Fig. 1A). Similarly, monolayers in which treatment was delayed for 4 days also showed no reduction in the number of intracellular bacteria compared with untreated controls (Fig. 1B). In contrast, all three compounds were found to be significantly active against M. avium within U937 macrophages. Treatment with rifabutin (both at 2 and at 4 μg/ml) resulted in approximately 97% inhibition of growth after 4 days (P < 0.05). Clarithromycin when added at 2 μg/ml was associated with 93% inhibition, and at 4 μg/ml it led to 96% inhibition of growth of intracellular M. avium, while azithromycin at 4 μg/ml resulted in an 88% inhibition of growth of intracellular bacteria. Only data for 4 μg/ml are shown (Fig. 1A).
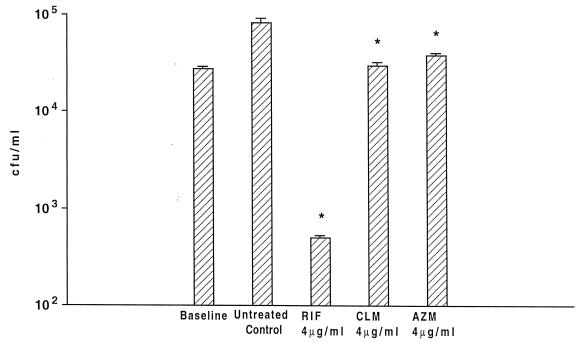
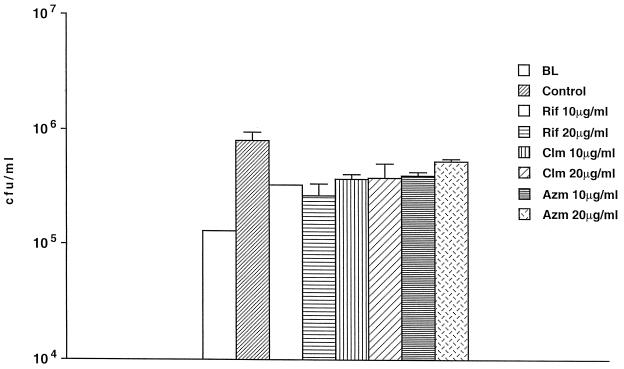
The lack of effect of antimicrobials could be due to an impaired ability to enter Acanthamoeba. To determine whether antimicrobials could penetrate A. castellanii and not be degraded intracellularly, we infected A. castellanii monolayers with a clinical strain of S. aureus and treated the monolayers with rifabutin (2 or 4 μg/ml), clarithromycin (2 or 4 μg/ml), or azithromycin (2 or 4 μg/ml). As shown in Fig. 2 (only data for 4 μg/ml are shown), all the concentrations used of the three compounds were associated with significant killing or inhibition of growth of the intracellular S. aureus. Rifabutin was bactericidal while clarithromycin and azithromycin were bacteriostatic (rifabutin MIC, 0.5 μg/ml; clarithromycin MIC, 0.5 μg/ml; and azithromycin MIC, 2 μg/ml), showing that activity can be observed within amoebae. We then used 10 and 20 μg of the antimicrobials per ml in the medium (Fig. 3). The effect of the compounds on the bacterial growth was similar to the effect obtained with concentrations of 2 and 4 μg/ml (Fig. 1A). Even the effect of 2 or 4 μg/ml was small if the inoculum used was greater than 104 organisms (data not shown).
FIG. 2.
Activities of rifabutin (RIF), clarithromycin (CLM), and azithromycin (AZM) against S. aureus within A. castellanii. P was <0.05 for the comparisons between the number of bacteria in untreated control and the number of bacteria within Acanthamoeba treated with the three antibiotics.
FIG. 3.
Effect of antibiotics against intra-amoeba M. avium at a 10-fold increase in concentration. Acanthamoeba was infected with M. avium and after 4 h treated with antibiotics for 4 days. P was >0.05 for the comparison between the number of bacteria within untreated control (4-day control) and the number of bacteria within Acanthamoeba treated with antibiotics. BL, baseline, time zero; control, untreated. Rif, rifabutin; Clm, clarithromycin; Azm, azithromycin.
To rule out the presence of selection of a resistant phenotype of Acanthamoeba, M. avium was used to infect Acanthamoeba, then retrieved from amoebae, and grown on culture medium in vitro and subsequently the MIC was determined. Table 1 shows that M. avium passaged in vitro is still susceptible to the drugs used.
TABLE 1.
MICs determined by the BACTEC method for M. avium 101 grown within Acanthamoeba
| Antibiotic | MIC typea | MIC (μg/ml) for M. avium culture
|
||
|---|---|---|---|---|
| Cultured in amoebae (day 1) and passaged on 7H11 | Cultured in amoebae (day 4) and passaged on 7H11 | Control (7H11 culture) | ||
| Azithromycin | MIC99 | 39 | 41 | 26b |
| MIC99.9 | 52 | 67 | 49b | |
| Clarithromycin | MIC99 | 3.3 | 3.6 | 1.9b |
| MIC99.9 | 4.9 | 5.1 | 3.0b | |
| Rifabutin | MIC99 | 0.6 | 0.5 | 0.5b |
| MIC99.9 | 0.8 | 0.6 | 0.6b | |
MIC99, MIC at which 99% of the isolates tested are inhibited; MIC99.9, MIC at which 99.9% of the isolates tested are inhibited. The data represent the numbers obtained in a representative experiment (BACTEC assay was carried out twice). The numbers were obtained by using the T100 method as previously described (4).
P > 0.05 compared with the MICs of antibiotics against M. avium grown in amoebae and passaged on 7H11 medium.
We showed that M. avium living within Acanthamoeba has increased resistance to antimicrobials usually prescribed as prophylaxis for M. avium disease in AIDS patients, such as rifabutin, clarithromycin, and azithromycin (13), compared with M. avium organisms residing within U937 macrophages. This finding could be intuitively explained by either decreased uptake of the antimicrobials into the amoebae in comparison with the uptake of macrophages, inactivation of the compound within amoebae, or even a change in the phenotype of the bacterium. If a change in the M. avium phenotype is the explanation for the resistance phenomenon, it must occur early in the infection, because Acanthamoeba-infected monolayers showed resistance to rifabutin and macrolides both when the monolayer was exposed to the antibiotics 4 h after infection and when intracellular bacteria were allowed to adapt to the intracellular environment for 4 days before the initiation of therapy. This hypothesis has some support from previous data showing that L. pneumophila undergoes surface changes once inside Acanthamoeba polyphaga (2), becoming more resistant to biocide inactivation (1) including chlorine treatment (J. S. Navratil, R. H. Palmer, S. States, J. M. Kuchta, R. M. Wadowsky, and R. B. Yee, Abstr. 90th Annu. Meet. Am. Soc. Microbiol. 1990, abstr. Q-82, p. 302, 1990) than cells grown in vitro.
Because amoebae live in natural sources of water and therefore are constantly exposed to hypoosmolarity, the cytoplasmic membrane is more resistant to decrease in osmolarity and the amoeba is capable of controlling the effects of osmolarity either by encysting or by pumping salts across the membranes. These two properties may be associated with decreased permeability to drugs as well as decreased intracellular concentration.
Enhanced resistance of M. avium to antimicrobials either by location within amoebae or by passage within amoebae may impact the efficacy of the prophylactic use of antimicrobials. Using concentrations of antibiotics that are active against M. avium in human macrophages (12), we have determined that infection of Acanthamoeba by M. avium results in decreased activity of rifabutin and macrolides against the bacterium, although there is no evidence that the intracellular bacterium, when released from the amoeba, will maintain a phenotype of resistance to antimicrobials for several generations. The mechanism of bacterial resistance and the hypothetical impact on prophylaxis of M. avium disease in AIDS patients warrant further investigation.
Acknowledgments
We thank Karen Allen for preparing the manuscript.
This work was supported by research funds from the Kuzell Institute.
REFERENCES
- 1.Barker J, Brown M R, Collier P J, Farrell I, Gilbert P. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: physiological status and susceptibility to chemical inactivation Appl. Environ Microbiol. 1992;58:2420–2425. doi: 10.1128/aem.58.8.2420-2425.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker J, Lambert P A, Brown M R. Influence of intra-amoebic and other growth conditions on the surface properties of Legionella pneumophila. Infect Immun. 1993;61:3503–3510. doi: 10.1128/iai.61.8.3503-3510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker J, Scaife H, Brown M R. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob Agents Chemother. 1995;39:2684–2688. doi: 10.1128/aac.39.12.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez L E, Inderlied C B, Kolonoski P, Wu M, Barbara-Burnham L, Young L S. Activities of Bay Y 3118, levofloxacin, and ofloxacin alone or in combination with ethambutol against Mycobacterium avium complex in vitro, in human macrophages, and in beige mice. Antimicrob Agents Chemother. 1996;40:546–551. doi: 10.1128/aac.40.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez L E, Petrofsky M, Kolonoski P, Young L S. An animal model of Mycobacterium avium complex disseminated infection after colonization of the intestinal tract. J Infect Dis. 1992;165:75–79. doi: 10.1093/infdis/165.1.75. [DOI] [PubMed] [Google Scholar]
- 6.Bermudez L E, Young L S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988;140:3006–3013. [PubMed] [Google Scholar]
- 7.Cirillo J D, Falkow S, Tompkins L S. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect Immun. 1994;62:3254–3261. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cirillo J D, Falkow S, Tompkins L S, Bermudez L E. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect Immun. 1997;65:3759–3767. doi: 10.1128/iai.65.9.3759-3767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damsker B, Bottone E J. Mycobacterium avium-Mycobacterium intracellulare from the intestinal tracts of patients with the acquired immunodeficiency syndrome: concepts regarding acquisition and pathogenesis. J Infect Dis. 1985;151:179–181. doi: 10.1093/infdis/151.1.179. [DOI] [PubMed] [Google Scholar]
- 10.Falkinham J O., III . Molecular epidemiology techniques for the study of Mycobacterium avium complex infection. In: Korvick J A, Benson C A, editors. Mycobacterium avium complex: progress in research and treatment. New York, N.Y: Marcel Dekker, Inc.; 1996. pp. 23–44. [Google Scholar]
- 11.Horsburgh C R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 12.Inderlied C B, Kemper C A, Bermudez L E. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korvick J, Benson C. Advances in the treatment and prophylaxis of Mycobacterium avium complex in individuals infected with human immunodeficiency virus. In: Korvick J, Benson C, editors. Mycobacterium avium-complex: progress in research and treatment. New York, N.Y: Marcel Dekker, Inc.; 1996. pp. 241–262. [Google Scholar]