Abstract
Data on outcome of patients with mantle cell lymphoma (MCL) and COVID-19 infection are limited. The European MCL (EMCL) registry is a centralized registry of the EMCL network, collecting real-world information about treatments and disease courses. During the COVID-19 pandemic, additional data on MCL patients with COVID-19 infection were collected, aiming to identify risk factors for mortality from COVID-19. In our retrospective, multicenter, international study, we collected data from 63 MCL patients with a median age of 64 years (range, 44–84) in 9 countries with evidence of a COVID-19 infection between February 2020 and October 2021. The overall mortality rate was high (44.4%), especially in hospitalized patients (61%) and in patients with need for intensive care unit care (94%). Patients receiving rituximab had significantly poorer survival than patients not receiving rituximab (P = 0.04). Our data highlight the importance of prevention strategies and underline the need for effective vaccination in this vulnerable cohort.
INTRODUCTION
Mantle cell lymphoma (MCL) is a rare (3% to 10% of adult non-Hodgkin lymphomas [NHL] in Western countries) and usually aggressive, incurable subtype of NHL.1–3 Median age at diagnosis is 68 years, and most patients are diagnosed with advanced stage, requiring systemic treatment at the time of diagnosis.4 First-line therapy for MCL typically includes polychemotherapy in combination with a CD20 targeting antibody.5 Younger patients are typically treated with intensified chemoimmunotherapy (CIT) treatments, integrating cytarabine, and consolidation with high-dose therapy and autologous stem-cell transplantation.6 Elderly patients are treated with full or attenuated dose CIT, and rituximab maintenance is commonly delivered across all age groups.7 At relapse, targeting BTK with ibrutinib, acalabrutinib, or zanubrutinib8–10 is representing the most widely applicable and efficacious approach. Following BTKi failure, the use of novel therapeutic agents such as venetoclax,11 the re-use of CIT, or chimeric antigen receptor T-cell therapy12 are therapeutic options. However, with available standard treatment options, MCL is considered incurable and most patients will die of the disease. Besides the immunosuppressive treatment with chemotherapy or immunotherapy, several other factors, including hypogammaglobulinemia, neutropenia, and lymphopenia may contribute to immune deficiency and impact patients’ outcomes.13 Overall, the treatment of MCL is heterogenous, as therapies range from oral, mostly well tolerable regimens to toxic and intensive regimens.
The global pandemic with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to affect health delivery and outcomes on many different levels and has cost the lives of >5 million people worldwide since the first documented infections in December 2019.14 Multiple studies demonstrated that vulnerable populations such as patients at increased age, with comorbidities and cancer are at increased risk of severe disease and death.15–19 However, data on the outcome of patients with hematological malignancies, especially in the field of malignant lymphoma in patients with COVID-19 are still limited. Lymphoma patients are often immunosuppressed by their active disease per se, and lymphodepleting therapies including anti-CD20 monoclonal antibodies, chemotherapy, with targeted agents also leading to an additional immunosuppressive effect.20 The speed and degree of humoral and cellular immune recovery following prolonged immunosuppression following therapy interruption is unclear. The impact of different therapeutic approaches in patients with COVID-19 remains poorly defined, but active chemotherapy appears to be associated with increased risk of death in cancer patients with COVID-19.21 Furthermore, data on seroresponse in patients with hematologic malignancies following COVID-19 vaccination are limited. Recent systematic reviews suggest that active cancer treatment, particularly anti-CD20 therapy, is associated with lower vaccination response.22,23 We wanted to gain further insight by conducting this retrospective analysis. To our knowledge, this is the largest series with a specific focus on COVID-19 in MCL.
Various studies have reported dismal outcomes in lymphoma patients during the pandemic. In a retrospective multicenter cohort study with 89 patients diagnosed with NHL and evidence of a COVID-19 infection (including 10 cases of MCL), the 30-day overall survival (OS) was 71% with a median follow up of 33 days from admission.24 Seventy percentage of the patients were under any chemotherapy with 53% containing an anti-CD20 monoclonal antibody. Relapsed/refractory (r/r) lymphoma, age ≥70 years, and recent bendamustine were identified as risk factors for a poorer outcome in a multivariate analysis. Notably, survival of patients <70 years without r/r lymphoma was comparable to that of the general population. Further investigations showed that an age ≥70 years, r/r lymphoma, and recent administration of anti-CD20 monoclonal antibody therapy are risk factors for a prolonged hospitalization and death due to COVID-19 among lymphoma patients.25
A Spanish study demonstrated similar observations in 177 lymphoma patients with a COVID-19 infection, with an overall mortality rate of 34.5% after median follow up of 27 days.26 Age ≥70 years and active disease were significant predictors of death. Surprisingly, active treatment did not modify mortality risk and no differences were found between the different therapeutic regimens.
In contrast to this, the most recent report from the COVID-19 and cancer consortium (CCC-19) found that specific anticancer therapies were associated with high 30-day all-cause mortality.27 Among 1097 patients with hematological neoplasms, the highest 30-day mortality of 50% was observed in patients receiving DNA methyltransferase inhibitor therapy, most of them diagnosed with a myelodysplastic syndrome and second-highest 30-day mortality of 36% among patients receiving R-CHOP-like therapies, most of them diagnosed with diffuse large B-cell lymphoma. An Indian study underlined the preceding findings, that elderly patients with hematological malignancy and severe COVID-19 infection have the worst outcomes, especially when the disease is not in remission.28
A recently developed prognostic model by Visco et al helps to predict the outcome in COVID-19-infected lymphoma patients by investigating four items (age, gender, lymphocyte, and platelet count) that are associated with risk of death.29 By rating these four items, the authors defined three risk groups for probability of death due to COVID-19 infection: 0–1 point low risk, 2–3 points intermediate risk, and 4–5 points high risk. The four items were rated differently: age > 65 = 2 points, male gender = 1 point, absolute lymphocyte count < 650 × 109/L = 1 point, Plt < 100 × 109/L = 1 point. The score is not yet internationally validated, but its use might help to better understand disease courses in COVID-19 patients.
METHODS
This is a retrospective, international study of the European MCL (EMCL) network. We collected data from 63 MCL patients from 9 countries (Croatia, France, Italy, Portugal, Spain, Germany, United Kingdom, Ireland, and Netherlands) with evidence of a COVID-19 infection between February 2020 and October 2021. Investigators at each center were asked to collect the following data: baseline demographics, treatment status, remission status, date of COVID-19 diagnosis, associated symptoms, need for hospitalization, COVID-19 disease course and outcome, COVID-19-specific treatment, hemoglobin level, platelet count, and lymphocyte count at COVID-19 infection. Severe COVID-19 infection was defined as hospitalization with need of oxygen therapy of any kind as well as admission to an intensive care unit (ICU); mild COVID-19 infection was defined as ambulatory care or hospitalization without need for oxygen.
Different data sources were joined for our analysis. First, we used primary data of the EMCL registry, where 26 patients with a documented COVID-19 infection were identified. All of them gave written informed consent for data collection and analysis at registry enrollment. We additionally included 37 patients identified at local hospitals by investigators, sharing anonymized data after obtaining informed consent.
OS analysis according to the remission status was performed in 56 patients. Patients with complete remission (CR) or partial remission (PR) according to Lugano criteria were documented as “responding patients” and patients with stable disease (SD) or progressive disease (PD) were documented as “active disease.” Regarding the different therapeutic approaches, OS analysis could be performed in 59 patients.
We aimed to validate the recently developed prognostic model to predict outcome by Visco et al,29 and therefore applied it to patients with all 4 items available (age, gender, lymphocyte, and platelet count, Suppl. Table S1).
Statistical analysis
Quantitative data are expressed as medians with interquartile ranges. Categorical variables are given as frequencies and percentages. Overall survival was measured from the diagnosis of COVID-19 infection to last follow up or death. The probability of OS was estimated using the Kaplan-Meier method, and differences compared using the log-rank test. Cox proportional hazard regression models were used to identify predictors of OS. Covariates considered in this analysis were age (≥ 65 y versus below), remission status (responding patients versus active disease) and current lymphoma treatment at time of infection. All statistical analysis were conducted using GraphPad Prism 8.4.3.
RESULTS
Patient characteristics
A total of 63 patients with a median age of 64 years (interquartile ranges 44–84) at time of COVID-19 infection were included from 9 countries: Croatia (19), France (9), Portugal (8), Italy (8), Spain (7), Germany (5), UK (3), Ireland (2), and Netherlands (2). Seventy-six percent (n = 48) of the patients were male. The majority of patients were diagnosed with COVID-19 during the so called “second wave” of infections between October 2020 and January 2021 (62%, n = 39). Patient characteristics are summarized in Table 1.
Table 1.
Baseline Characteristics of the MCL Patients With COVID-19 Infection
| Characteristics | All Patients (n = 63) | Deceased (n = 28) |
|---|---|---|
| Male (all patients), n (%) | 48 (76) | 24 (50) |
| Female (all patients), n (%) | 15 (24) | 4 (26.6) |
| Age (y), median (range) at infection onset | 64 (43–84) | |
| ≤65, n (%) | 32 (50.8) | 13 (40.6) |
| ≥65, n (%) | 31 (49.2) | 15 (48.3) |
| Current treatment at time of COVID-19 infection, n (%) | ||
| Induction/consolidationa | 23 (36.5) | 13 (56.5) |
| Rituximab maintenance | 15 (24) | 7 (47) |
| Novel drugsb | 15 (24) | 7 (47) |
| None | 6(9.5) | 1 (16) |
| Unknown | 4 (6) | 0 |
| Remission status at time of COVID-19 infection, n (%) | ||
| Responding (CR, PR)c | 44 (70) | 19 (43) |
| Active disease (SD, PD)d | 13 (21) | 7 (54) |
| Unknown | 6 (9) | 2 (33.3) |
| MCL treatment paused due to COVID-19 infection, n (%) | ||
| Yes | 6 (10) | 0 |
| No | 45 (71) | |
| Not applicable or unknown | 12 (19) | |
| Overall mortality, n (%) | ||
| Death due to COVID-19 infection | 24 (86) | 24 (86) |
| Death due to lymphoma | 3 (11) | 3 (100) |
| Death for other reasons/unknown | 1 (3.5) | 1 (100) |
| Days between COVID-19 infection and death (range) | 26 (1–344) | |
| COVID-19 disease course, n (%) | ||
| Ambulatory care for COVID-19 infection | 17 (27) | 0 |
| Hospitalization because of COVID-19 infection | 46 (73) | 28 (61) |
| Mild infectione | 29 (46) | 4 (13.7) |
| Severe infectionf | 34 (54) | 24 (70.5) |
| ICU admission | 16 (35) | 15 (94) |
| Median number of days of between infection and hospital admission (range) | 1.5 (0–365) | |
| Median number of days between hospitalization and death (range) | 22 (4–211) | |
| Time between hospital admission and discharge in days (range), n = 15 | 21.5 (4–66) | |
| Airway management in hospitalized patients (n = 46), n (%) | ||
| None | 9 (20) | 2 (22) |
| Nasal oxygen support | 17 (37) | 8 (50) |
| CPAP | 2 (4,4) | 2 (100) |
| Ventilator | 14 (30) | 13 (93) |
| ECMO | 1 (2) | 1 (100) |
| Unknown | 4 (89) | 2 (50) |
| COVID-19-specific treatment in all patients, n (%) | ||
| Corticosteroids | 27 (43) | 20 (71) |
| Antibiotics | 18 (,29) | 11 (39) |
| Remdesivir | 15 (24) | 10 (36) |
| Convalescent plasma | 12 (19) | 6 (21) |
| Virus monoclonal antibodies | 1 (2) | 1 (4) |
| Anti-IL6 treatment | 1 (2) | 1 (4) |
| COVID-19 sequelae in COVID-19 survivors (n = 35), n (%) | ||
| Reduced bone marrow function | 7 (20) | |
| Fatigue | 15 (43) | |
| Pulmonary toxicity acute/chronic | 4 (11)/3(9) | |
| Number of vaccinated patients | 9 (14.2) | 0 |
| COVID-19 infection after vaccination | 2 (22.2) | 0 |
aInduction: R-CHOP (6), R-Bendamustine (5), R-CHOP/R-HAD + ASCT (5), R-BAC (4); consolidation: Post allogeneic transplantation (2, one patient 52 d after TX, unknown for the other patient), R-BEAM + ASCT (1).
bNovel drugs: Ibrutinib (5), Lenalidomide (3), Ibrutinib+Venetoclax (2), Ibrutinib+Obinutuzumab (1), Ibrutinib+Rituximab (3), R² (Rituximab + Lenalidomide, 1).
cResponding patients: 48% CR (n = 30), 22% PR (n = 14).
dActive disease: 8% SD (n = 5), 13% PD (n = 8).
eMild infection = no need for hospitalization or hospitalization without need for oxygen therapy.
fSevere infection = need for hospitalization and oxygen therapy.
CPAP = continuous positive airway pressure; CR = complete remission; ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; MCL = mantle cell lymphoma; PD = progressive disease; PR = partial remission; SD = stable disease.
Eighty-seven percent (n = 55) of the patients were on some form of treatment at the time of COVID-19 infection. In total, 12 different therapeutic approaches were administered for MCL at the time of the COVID-19 infection, as well as “no treatment” (n = 6). For OS analysis, we grouped them into induction/consolidation therapy (36.5%), maintenance therapy (24%), and “novel drugs” (24%, see footnotes later, Table 1). The treatment was not stopped in the majority of patients due to the COVID-19 infection (n = 45, 71%).
Regarding the remission status, 70% of the patients were responding (CR or PR) and 21% of the patients had active disease (SD or PD).
Nine patients were vaccinated (14%). In 2 patients, the COVID-19 infection occurred after vaccination and 7 patients were vaccinated following recovery after COVID-19 infection. Overall, 7 patients were receiving rituximab monotherapy or in combination and 2 patients were not receiving any treatment. Seventy-eight percent of the vaccinated patients were in CR. Information on time between COVID-19 vaccination and infection as well as response to vaccination were not collected.
Forty-seven percent (n = 8) of the ambulatory patients (n = 17) were undergoing induction or consolidation therapy at COVID-19 infection, followed by 23.5% not under treatment, 18% on rituximab maintenance, and 12% on therapy with novel drugs. Sixty-five percent of these patients were responding to MCL therapy (n = 11), 23.5% (n = 4) had active disease (data not shown).
COVID-19 disease course
Most patients were diagnosed with COVID-19 in the context of symptoms. Seventy-three percent were hospitalized due to the COVID-19 infection after median of 1.5 days (range 0–365) of COVID-19 diagnostic test positivity via polymerase chain reaction testing. Patients had a median number of 21.5 days (range 4–66) of hospital admission and the median follow up was 57 days (range 4–366).
Of the hospitalized patients, 74% had a severe COVID-19 infection with need for oxygen therapy of any kind (including nasal oxygen support, continuous positive airway pressure, ventilator and extracorporeal membrane oxygenation [ECMO]). Thirty-five percent of the patients were admitted to ICU. Only 20% of all hospitalized patients did not require oxygen therapy.
COVID-19-specific treatment most commonly included the use of corticosteroids (administered in 43% of all patients), antibiotics (administered in 29%), the antiviral agent remdesivir (administered in 24%), and convalescent plasma (administered in 19%). Combination of treatments was common, as shown in the 27 patients receiving corticosteroids, of whom 52% also received antibiotics, 48% also received remdesivir, and 37% also received convalescent plasma. Of the 14 patients requiring mechanical ventilation, 93% received corticosteroids, 57% received antibiotics, 50% received remdesivir, and 35% received plasma.
In contrast to this, most patients in the ambulatory setting (n = 17) were not treated with any of the above mentioned therapies except for 3 patients who received antibiotics (17.6%).
Of the vaccinated patients, only 55% were hospitalized and all of them were discharged.
Of the patients who survived the COVID-19 infection, persisting symptoms typical for long COVID syndrome were observed, including fatigue (40%), reduced bone marrow function (20%) and pulmonary symptoms (10%). Information about onset of symptoms after COVID-19 infection were not collected.
Overall survival and risk factors of fatality
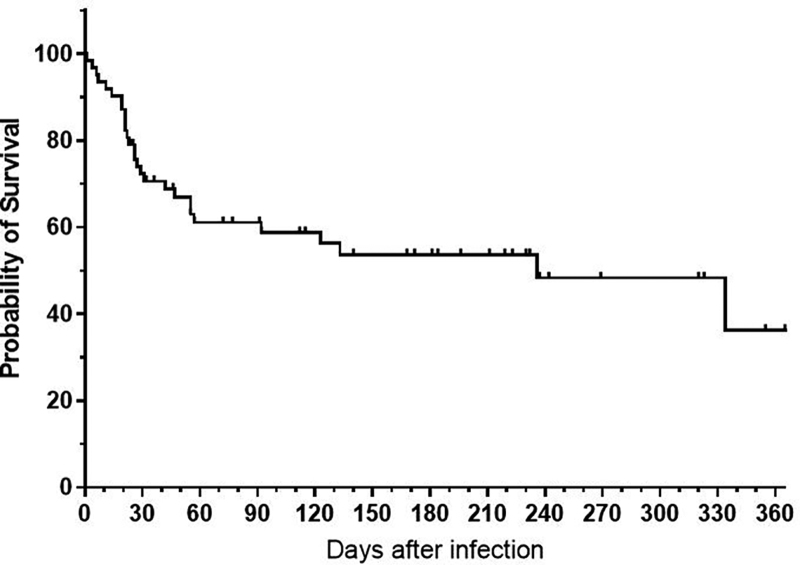
Overall, 28 patients died after a median follow up of 26 days (range 1–344). The overall mortality rate was 44.4% and 71.4% of the deaths occurred within the first 30 days. The median overall survival of the entire cohort after COVID-19 infection is shown in Figure 1. Median age at death due to COVID-19 infection was 65.8 years.
Figure 1.
OS after COVID-19 infection (all patients). Overall survival of all patients is shown, including deaths due to lymphoma (n = 3) and other reasons (n = 1). The 3 patients who died due to lymphoma died after 1, 21, and 334 d. The patient who died for other reasons died after 236 d. OS = overall survival.
The vast majority of deaths were related to the COVID-19 infection (86%, n = 24); 11% of the patients died because of the lymphoma (n = 3) after 1, 21, and 334 days, and one patient died due to other reasons after 236 days.
For patients classified with severe COVID-19 infection, the mortality was high (70.6%). Of all the patients admitted to an ICU ward, 93.8% died. Especially in patients requiring continuous positive airway pressure, ventilator or ECMO therapy, outcome was predominantly fatal (13/14 patients on a ventilator died).
All patients without need for hospitalization (n = 17) survived the COVID-19 infection. Most of them (11/17) were in CR (41%, n = 7) or PR (23.5%, n = 4) at the time of infection. Suppl. Figure S1 shows the OS superiority of this group compared with hospitalized patients.
In univariate analysis, age ≥65 years was not significantly associated with an increased mortality (hazard ratio 0.87, 1.26–6.07, P = 0.89).
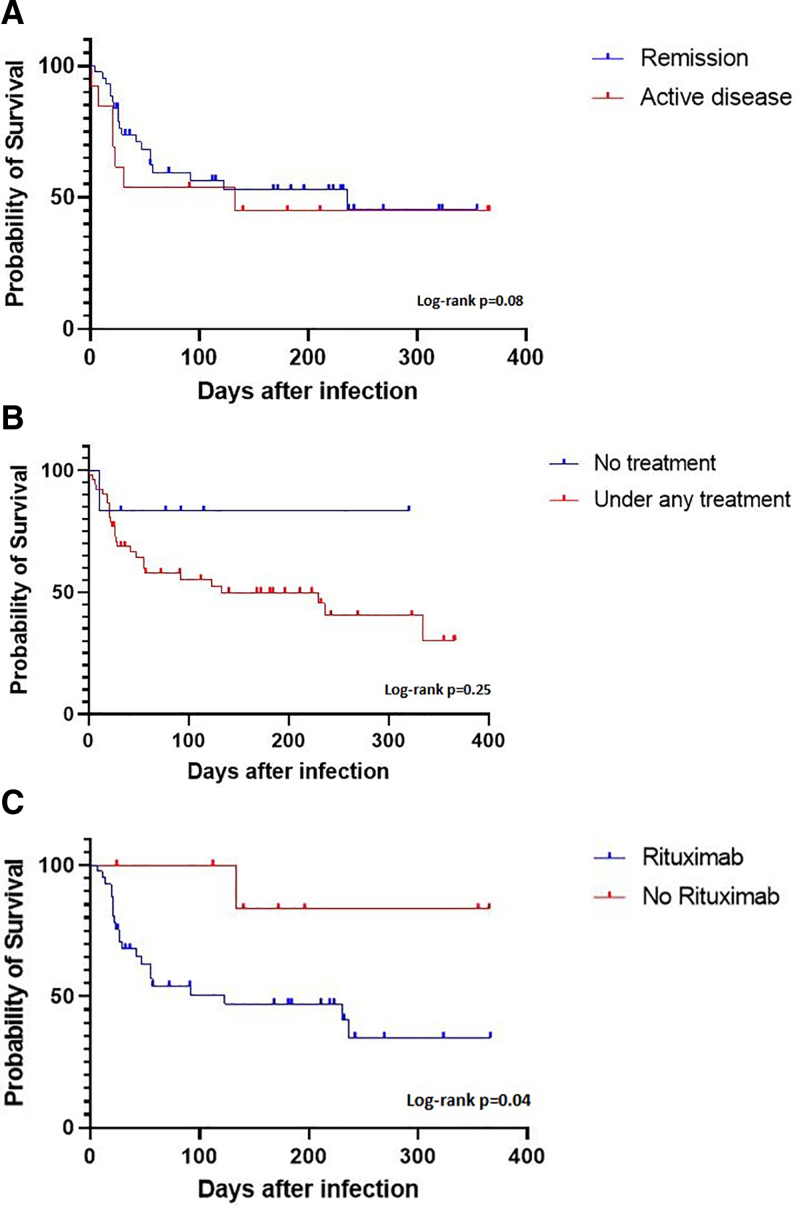
There was only a trend in OS regarding the current remission status (remission versus active disease) at diagnosis of the COVID-19 infection (P = 0.08, Figure 2A). Seventy-seven percent (n = 34) of the patients in remission (n = 44) received any rituximab-containing regimen, namely CIT (45%, n = 20) or maintenance therapy (32%, n = 14, see footnotes later, Figure 2A). Patients with progressive disease had the poorest survival (n = 8, data not shown).
Figure 2.
(A) OS and current remission status at time of COVID-19 infection (n = 56). Patients in Remission (CR or PR): n = 44, patients with active disease (SD or PD): n = 13; P = 0.08. Of the 44 patients in remission, 20 (45%) received rituximab-containing induction or consolidation therapy, 14 (32%) patients were under rituximab maintenance, 6 (14%) were treated with novel agents, and 4 (9%) patients were not under treatment. Of the 13 patients with active disease, 9 (69%) received rituximab-containing induction or consolidation therapy and 4 (31%) received novel agents. (B) OS and current lymphoma treatment at time of COVID-19 infection (n = 59): 6 patients not under treatment and 53 patients under either induction/ consolidation, maintenance or novel drugs (see Table 1 for more details). (C). OS in patients treated with Rituximab within the past 6 mo versus patients without Rituximab: N = 49 patients evaluable, 41 of these received any Rituximab-containing regime within the past 6 months (P = 0.04). CR = complete remission; OS = overall survival; PD = progressive disease; PR = partial remission; SD = stable disease.
Patients without active treatment had numerically but not significantly superior outcome (only 1 of 6 untreated patients died in PR after R-BAC [rituximab, bendamustine, cytarabine], aged 73) as compared to patients receiving induction, consolidation, maintenance, or novel drugs (Figure 2B). Moreover, patients receiving any rituximab-containing regimen within the past 6 months had a significantly lower OS compared with patients not receiving rituximab within 6 months (P = 0.04; hazard ratio, 0.35; 95% CI, 0.129-0.9, Figure 2C).
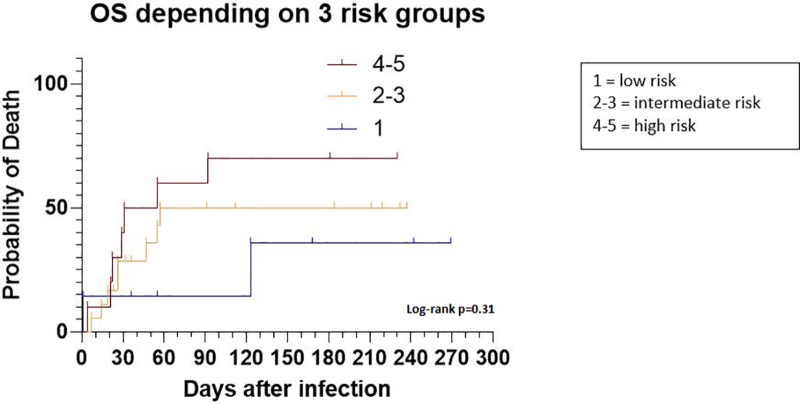
When applying the prognostic model developed by Visco et al to a subgroup of 35 patients in our series (Suppl. Table S1), patients with the highest score of 4–5 points had the highest probability of death at 60 days (55%) as compared to patients with an intermediate (50%) or low score (14%) although these differences in survival were not significant (P = 0.31, Figure 3).
Figure 3.
Application of a prognostic model for lymphoma patients with COVID-19 (n = 35). No statistical difference between the three groups (P = 0.31).
DISCUSSION
In this study, to the best of our knowledge we present data on the largest retrospective cohort of MCL patients with a COVID-19 infection to date and were able to analyze outcome and survival in a real-world scenario.
We observed an overall mortality rate of 44.4%, 71.4% occurring within 30 days from the onset of COVID-19 infection. This is a higher mortality rate than reported in other pooled lymphoma cohorts, which usually varies between 13 and 34%.24,26,29–31 These prior findings are based on large cohorts and our series is of limited size. Notably, MCL patients with need for hospitalization are overrepresented in our series, which is a potential bias. In the aforementioned studies, age ≥70 years, refractory or relapsed lymphoma or active disease were associated with mortality.32 We could not demonstrate a significant correlation between survival and age or remission status in our series. However, age >65 years still influenced the risk of death, as we could demonstrate by applying the prognostic model by Visco et al in 35 patients with complete data. Again, due to the small sample size, we were not able to reproduce statistical significant differences here. However, this score adds new and important aspects to risk assessment of lymphoma patients with a COVID-19 infection. Age and gender are known risk factors but taking into account the lymphocyte and platelet count is a new and promising approach. Hence, we support the utilization of this prognostic model in patients with lymphoma. It may be used for risk stratification, for example, for patients in need of ventilator or ECMO support, to facilitate decision-making.
Regalado et al stated that in terms of mortality risk, no significant differences were found between the therapeutic regimens.26 In our series, differences between the main therapeutic strategies (induction/consolidation, maintenance, novel drugs, no treatment) were also not significant. However, the difference in OS was significant for patients receiving or not receiving rituximab, highlighting the potential additive detrimental effect of anti-CD20 therapy. Chatzikonstantinou et al recently showed similar findings for a large cohort of CLL patients, many of whom were also receiving anti-CD20 antibodies similarly to the patients in our series.30 These results highlight that recent exposure to anti-CD20 antibody—likely resulting in long lasting lymphodepletion—significantly impact survival. Overall, data regarding the risk of severe COVID-19 disease course among rituximab treated MCL patients is lacking. Notably, most MCL patients (71%) in the whole series did not have their treatment paused due to the pandemic.
Although our series is dominated by a “pre vaccination cohort,” 9 patients were vaccinated. The hospitalization rate in the vaccinated cohort was numerically lower than in the entire cohort (55% versus 73%), and importantly, all vaccinated patients were discharged, and no deaths were reported among them (data not shown). These findings support the role of early and diligent vaccination policy in this vulnerable cohort and is of specific interest, as a hospital admission with prolonged hospitalization (in this cohort: median of 21 d) can be anticipated. Furthermore, additional sequelae after COVID-19 infection have been observed, known as long COVID syndrome. Among the various described symptoms, dyspnea, headaches, and cognitive symptoms are common.33 It was not our intention to further investigate the prevalence of long COVID in our cohort. However, we checked if three relevant symptoms, that typically affect hematological patients under treatment regardless of COVID-19 infection, were present: fatigue, impaired bone marrow function and pulmonary toxicity. Twenty percent of the survivors had reduced bone marrow function, 43% had fatigue, and about 10% had pulmonary toxicity. This is particularly relevant in this cohort, as an adequate bone marrow function and normal pulmonary capacity are typically required prior to many therapeutic approaches in lymphoma. Hence, even if patients survive a COVID-19 infection, the presence of COVID-19 sequelae could theoretically preclude subsequent treatment, leading to further impaired outcome.
Limitations
There are some limitations that must be acknowledged. First, hospitalized patients are likely to be slightly overrepresented in our series, as patients with a mild COVID-19 infection were probably either undiagnosed due to a lack of widespread testing or managed by simple measures in the community. Second, as we included patients from different countries and centers, diagnosed in different waves of the pandemic with different disease management, a degree of heterogeneity in our series is expected. Our results may therefore be influenced by differences in treatment strategies. Additionally, only a small number of patients in this series were vaccinated (n = 9). This is due to the fact that most data were collected during early waves, where vaccinations were not available for many patients. Data on outcome and survival must be interpreted carefully in these patients, as it is not known how many vaccination doses they received and what the time sequence of vaccination(s) and COVID-19 infection was. Also, we have no additional information on the onset and severity of the long COVID symptoms. Furthermore, data on performance status and lymphoma disease stage were only available in patients enrolled in the EMCL registry (missing in 59%) and data on comorbidities, body mass index or smoking, which may be confounders for overall mortality, were not collected.
CONCLUSION
Overall, with our data, we are able to add more information on the rare subgroup of MCL patients. We demonstrated a frequent fatal outcome especially in patients with need for hospitalization and undergoing current or recent rituximab therapy. Interestingly, MCL treatment was not paused in the majority of patients due to the COVID-19 infection.
However, finding the right balance between controlling the disease and not exposing MCL patients to the risk of COVID-19 remains a challenge and requires patient specific decision-making as well as comprehensive patient education. To optimize COVID-19 specific treatments with, for example, monoclonal antibodies or oral antiviral agents, patients need to report symptoms early and physicians must be aware of appropriate treatment strategies. The role of novel long-acting monoclonal antibodies for pre-exposure prevention of COVID-19 (eg, tixagevimab in combination with cilgavimab) still needs to be defined but could be a promising preventive approach in patients with a compromised immune system. Even though vaccine responses are often poor in lymphoma patients, vaccinations remain a cornerstone of COVID-19 prevention. Additional studies in vaccinated MCL cohorts are needed to understand the COVID-19 disease course better, which is a goal of the EMCL network for 2022.
ACKNOWLEDGMENTS
We thank all physicians, study nurses, and assistant physicians who helped to recruit patients and document their data.
AUTHOR CONTRIBUTIONS
The study conception and design was performed by MGdS, EG, MD, GH, AO, and M-KT on behalf of the EMCL network; data collection was performed by all authors; material preparation and analysis were performed by M-KT and SE. The article was drafted by M-KT, and all authors commented on the article. All authors read and approved the final article.
DISCLOSURES
M-KT received speakers’ honoraria from Astra Zeneca, Shire Takeda, Hexal. CV served as a advisory board or scientific advisor for Roche, Gilead, Incyte, Beigene, AbbVie, Janssen, Takeda, Novartis, and received research funding from Jannsen, Gentili. SK received honoraria and advisory board for Roche, Sandoz, and Octapharm/Celtrion. OH received grants from Celgene, Alexion, AB Science, and Inatherys. SL received miscellaneous support from Janssen, Gilead, Roche, Abbvie, Sanofi, Novartis, Actelion, Pfizer, honorarium from Gilead, and a research grant from Janssen. RD received personal fees from Takeda, Novartis, and Biotest, nonfinancial support from Gilead. MGdS received research support, travel to scientific conferences, advisory boards from Gilead; research support from Astrazeneca; travel to scientific conferences, advisory boards from Abbvie; advisory boards from JC; travel to scientific conferences, advisory boards, trial steering committee from Roche; advisory boards from Takeda; advisory boards from Bristol Myers Squibb; advisory boards from ADC Therapeutics; and advisory board from Celgene. EG received honoraria from Gilead/Kite, Janssen, Genmab; served as consulting or advisory role in Gilead/Kite; and research Funding from Janssen. TE received honorarium, advisory board honorarium from Roche; received honorarium; research support; and travel to scientific conferences from Gilead; advisory board honorarium from KITE; honorarium from Janssen; honorarium and Travel to scientific conferences from Abbvie; AstraZeneca: Honorarium, Research funding, Loxo Oncology: Advisory Board Honorarium, Trial steering committee, Beigene: Advisory Board Honorarium, Research funding, Incyte: Advisory Board Honorarium, Secura Bio: Advisory Board Honorarium. MD is a HemaSphere editor, receives funding by Abbvie, Bayer, Celgene, Gilead/Kite, Janssen; speaker’s honoraria from Amgen, Astra Zeneca, Bayer, Celgene, Gilead/Kite, Incyte, Janssen, Novartis, Roche; advisory honoraria from Astra Zeneca, Bayer, Beigene, Celgene, Genmab, Gilead/Kite, Incyte, Janssen, Lilly/Loxo, Morphosys, Novartis, Roche. GH receives funding by Roche, Janssen Pharmaceuticals, Celgene and Gilead and speaker’s or advisory honoraria from Janssen, Roche, Celgene, Gilead, Novartis, Incyte, Genmab, Morphosys, Abbvie, and Astra. All the other authors have no conflicts of interest to disclose.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
The data that support the findings of this study are available from the corresponding author, M.-K. Tilch, upon reasonable request.
Obtained at local ethics committees for each participating country in the EMCL network.
Patients registered in the EMCL registry gave written informed consent for data collection and evaluation at registry enrollment. Data of patients not enrolled were used in an anonymized way after obtaining informed consent.
REFERENCES
- 1.Sant M, Allemani C, Tereanu C, et al. ; HAEMACARE Working Group. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116:3724–3734. [DOI] [PubMed] [Google Scholar]
- 2.Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105:1684–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu S, Wang M, Lairson DR, et al. Trends and variations in mantle cell lymphoma incidence from 1995 to 2013: a comparative study between Texas and National SEER areas. Oncotarget. 2017;8:112516–112529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain P, Dreyling M, Seymour JF, et al. High-risk mantle cell lymphoma: definition, current challenges, and management. J Clin Oncol. 2020;38:4302–4316. [DOI] [PubMed] [Google Scholar]
- 5.Dreyling M, Campo E, Hermine O, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals Oncol. 2017;28:iv62–iv71. [DOI] [PubMed] [Google Scholar]
- 6.Hermine O, Hoster E, Walewski J, et al. ; European Mantle Cell Lymphoma Network. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016;388:565–575. [DOI] [PubMed] [Google Scholar]
- 7.Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367:520–531. [DOI] [PubMed] [Google Scholar]
- 8.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam CS, Opat S, Simpson D, et al. Zanubrutinib for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 2021;5:2577–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witzig TE, Inwards D. Acalabrutinib for mantle cell lymphoma. Blood. 2019;133:2570–2574. [DOI] [PubMed] [Google Scholar]
- 11.Eyre TA, Walter HS, Iyengar S, et al. Efficacy of venetoclax monotherapy in patients with relapsed, refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor therapy. Haematologica. 2019;104:e68–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maschmeyer G, De Greef J, Mellinghoff SC, et al. ; European Conference on Infections in Leukemia (ECIL). Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European Conference on Infections in Leukemia (ECIL). Leukemia. 2019;33:844–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saini KS, Tagliamento M, Lambertini M, et al. Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur J Cancer. 2020;139:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannakoulis VG, Papoutsi E, Siempos II. Effect of cancer on clinical outcomes of patients with COVID-19: a meta-analysis of patient data. JCO Glob Oncol. 2020;6:799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharafeldin N, Bates B, Song Q, et al. Outcomes of COVID-19 in patients with cancer: report from the national covid cohort collaborative (N3C). J Clin Oncol. 2021;39:2232–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136:1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–145. [DOI] [PubMed] [Google Scholar]
- 20.Bonuomo V, Ferrarini I, Dell’Eva M, et al. COVID-19 (SARS-CoV-2 infection) in lymphoma patients: A review. World J Virol. 2021;10:312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park R, Lee SA, Kim SY, et al. Association of active oncologic treatment and risk of death in cancer patients with COVID-19: a systematic review and meta-analysis of patient data. Acta Oncol. 2021;60:13–19. [DOI] [PubMed] [Google Scholar]
- 22.Gong I, Vijenthira A, Betschel S, Hicks LK, Cheung M. COVID-19 vaccine response in patients with hematologic malignancy: a systematic review and meta-analysis. Blood. 2021;138(Supplement 1):4113–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guven DC, Sahin TK, Kilickap S, et al. Antibody responses to COVID-19 vaccination in cancer: a systematic review. Front Oncol. 2021;11:759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamure S, Duléry R, Di Blasi R, et al. Determinants of outcome in Covid-19 hospitalized patients with lymphoma: A retrospective multicentric cohort study. EClinicalMedicine. 2020;27:100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duléry R, Lamure S, Delord M, et al. Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am J Hematol. 2021;96:934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regalado-Artamendi I, Jiménez-Ubieto A, Hernández-Rivas JÁ, et al. Risk factors and mortality of COVID-19 in patients with lymphoma: a multicenter study. HemaSphere. 2021;5:e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grivas P, Khaki AR, Wise-Draper TM, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021;32:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borah P, Mirgh S, Sharma SK, et al. ; AIIMS Hematology Alumni Group. Effect of age, comorbidity and remission status on outcome of COVID-19 in patients with hematological malignancies. Blood Cells Mol Dis. 2021;87:102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visco C, Marcheselli L, Mina R, et al. A prognostic model for patients with lymphoma and COVID-19: a multicentre cohort study. Blood Adv. 2021;6:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatzikonstantinou T, Kapetanakis A, Scarfò L, et al. COVID-19 severity and mortality in patients with CLL: an update of the international ERIC and Campus CLL study. Leukemia. 2021;35:3444–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yigenoglu TN, Ata N, Altuntas F, et al. The outcome of COVID-19 in patients with hematological malignancy. J Med Virol. 2021;93:1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avivi I, Arcaini L, Ferretti V, et al. High dose therapy and autologous stem cell transplantation in marginal zone lymphoma: an EBMT-FIL-gimeto retrospective study. Blood. 2014;124:2526–2526. [Google Scholar]
- 33.Taquet M, Dercon Q, Luciano S, et al. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18:e1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.