Abstract
Background
Motor abnormalities, such as psychomotor agitation and retardation, are widely recognized as core features of depression. However, it is not currently known whether motor abnormalities connote risk for depression.
Methods
Using data from the Adolescent Brain Cognitive Development (ABCD) Study, a nationally representative sample of youth (N = 10,835, 9–11 years old), the present paper examines whether motor abnormalities are associated with 1) depression symptoms in early adolescence, 2) familial risk for depression (familial risk loading), and 3) future depression symptoms. Motor abnormality measures included traditional (DSM) motor signs such as psychomotor agitation and retardation as well as other motor domains such as developmental motor delays and dyscoordination.
Results
Traditional motor abnormalities were less prevalent (agitation = 3.2%, retardation = 0.3%) than nontraditional domains (delays = 13.79%, coordination = 35.5%) among adolescents. Motor dysfunction was associated with depression symptoms (Cohen’s d = 0.02 to 0.12). Familial risk for depression was related to motor abnormalities (Cohen’s d = 0.08 to 0.27), with the exception of motor retardation. Family vulnerability varied in sensitivity to depression risk (e.g., retardation: 0.53%; dyscoordination: 32.05%). Baseline endorsement of motor abnormalities predicted future depression symptoms at 1-year follow-up.
Conclusions
These findings suggest that motor signs reflect a novel, promising future direction for examining vulnerability to depression risk in early adolescence.
Keywords: Adolescence, Clinical risk, Depression, Development, Familial risk, Motor
Major depressive disorder is one of the leading causes of disability in the world, indicating that depression is both a serious public health concern and an economic burden (1). Although treatments exist, they have modest response rates (approximately 40%–50% remission) (2,3). In response to this problem, a growing literature suggests that early identification of this disorder and targeted interventions may reduce the rates and course of depression (4,5). Current approaches to assessing risk in depression focus on familial risk, emotion, cognition, or psychosocial factors (6, 7, 8). However, these risk assessments omit motor signs, despite psychomotor slowing and agitation being characteristic features of depression (9), and the sensitivity of motor behaviors signal changes in underlying neural circuitry (10, 11, 12). As a result, motor abnormalities may be a valuable early marker of vulnerability (11,13). Indeed, pediatric practice has long depended on the use of early motor milestones as a sensitive marker of disturbances in development (14). In other psychopathology, such as psychosis, distinct motor abnormalities have served as strong indicators of early vulnerability as well as emerging brain dysfunction (15, 16, 17, 18, 19, 20, 21, 22). There is some evidence to suggest that motor abnormalities may predict a severe course of depression in older adults (11,23, 24, 25) and that motor abnormalities show promise for the notion of an early biomarker application (26). However, it is not known whether motor abnormalities are associated with vulnerability for depression in early adolescence. Early adolescence is a critical developmental period when symptoms first emerge, making it an ideal period for identifying markers of vulnerability for the disorder (27,28). Unfortunately, the relationship of motor abnormalities to depression in adolescence remains poorly characterized (15). In addition, it is not clear which motor signs might be most relevant in adolescence. Finally, motor signs may be a marker that could predict a symptom course starting in early adolescence.
Current neurobiological models of depression suggest that many depression symptoms may be related to a long-term downregulation of dopamine impacting interrelated, parallel circuits (e.g., affective, associative, and motor) (10,11,29). Recent literature also suggests that disturbances in these parallel circuits may have synergistic impacts on clinical deficits across emotion, cognition, and motor behaviors (30). Regardless of the exact source of the pathophysiology (31), it is clear that any such disturbance may also impact motor behavior, alongside cognitive and affective circuits. As a result, motor signs can be a clinically significant risk marker that should be considered alongside traditional cognitive, heritability, and affective markers.
Among the adult literature, motor signs have been viewed as core components of depression for decades (9). Extant research highlights the potential for motor abnormalities to be crucial behavioral markers that relate to greater symptom severity (23,24,27), worsening course (25,32), lower quality of life (24), and worse response to antidepressant treatment (33,34). However, in developmental literature, it remains unknown whether these motor abnormalities are merely epiphenomena or reflect a marker of vulnerability for depression (15). Furthermore, it is not clear whether psychomotor agitation and psychomotor retardation are the ideal markers of vulnerability in early adolescence or whether other motor markers may be more relevant earlier in development.
Early depression vulnerability may be marked by delays in meeting developmental motor milestones (e.g., sitting upright, rolling over, walking) (35). Indeed, infants of depressed mothers exhibited developmental motor delays (36,37), and such delays have predicted symptoms several decades later in adulthood (16,38). While these studies provide strong evidence of developmental motor delays as a vulnerability marker, it is not clear whether developmental motor delays confer additional sensitivity to vulnerability measures over traditional motor signs or reflect the same general vulnerability. In addition to developmental motor delays, developmental coordination disorder (a childhood movement disorder) has been related to high levels of childhood depression (39,40) and future depression in adulthood (41,42). In a parallel line of work, adults with depression with psychotic features have also shown motor dyscoordination (43), which suggests that dyscoordination may be relevant marker of vulnerability for this disorder. Therefore, an expanded list of motor abnormalities may be a useful marker of symptoms, onset, or risk.
In addition to a limited set of motor abnormalities, there has been no comprehensive or comparative assessment of motor abnormalities and vulnerability to depression in a single sample. Comparing motor abnormality prevalence across current depression, familial risk for depression, and future depression may indicate whether motor abnormalities reflect inherited mechanisms (familial risk loading for depression) or the presence of active disease processes (current depression) (44). Although some extant literature suggests the heritability of specific motor signs (27), little is known whether motor abnormalities reflect a stable vulnerability or track early emerging symptoms in adolescence. Examining multiple facets of vulnerability in a single study will thus aid in assessing whether motor abnormalities may be potential psychiatric endophenotypes of depression vulnerability.
Large, representative, and longitudinal samples of adolescents, such as the Adolescent Brain Cognitive Development (ABCD) Study, provide a unique opportunity to assess the relevance and sensitivity of motor signs to depression vulnerability. To date, studies using the ABCD dataset have largely focused on the contribution of health behaviors [i.e., sleep (45), body mass index (46), exercise (47,48)], environment [i.e., stress (49), socioeconomic status (50,51)], or individual traits [i.e., familial risk loading (52, 53, 54), race (50,51)] to depression. Far fewer of these studies have examined specific depression symptoms over time (55), and none have examined the potential relationship of motor abnormalities to concurrent depression, familial risk for depression, or future risk for depression. The current study leverages the nationally representative samples of young adolescents in the ABCD Study to answer several heretofore unanswered questions about the relevance of motor signs in this critical developmental period. First, we determined the prevalence of motor abnormalities in adolescents in general and in those with depression using both traditional (psychomotor agitation, psychomotor retardation) and novel (developmental motor delays, dyscoordination) motor measures, thus examining the utility of expanding the traditional motor signs to include developmental motor delays from early life and dyscoordination. We hypothesized that motor signs will be present in adolescence and more prevalent among individuals with emerging depression or risk for depression. Importantly, given the age of the sample and the age window for when depression typically has its onset (56), many individuals in ABCD will have not yet passed through the peak risk window for full depression onset. Therefore, in the current study, we examined depressive symptoms dimensionally and hypothesized that individuals who endorsed a motor sign will have a greater number of depression symptoms. Second, we tested whether motor abnormalities are familial vulnerability factors for depression. This analysis provided insight into which motor abnormalities track emerging symptoms or mechanisms related to inherited risk and whether motor abnormalities represent a general depression vulnerability endophenotype. We hypothesized that motor signs are related to higher rates of familial vulnerability. Finally, we examined the potential utility of motor abnormalities at baseline to predict depression symptoms at the 1-year follow-up. We expected youth with motor abnormalities at baseline to demonstrate higher rates of depression at follow-up.
Methods and Materials
Participants
The ABCD Study included 21 sites across the United States that collected participants (aged 9–11 years) with a broad demographic diversity range. All research protocols were in line with the ethical guidelines laid out by each respective institutional review board. These guidelines included obtaining both parents’ informed consent and the children’s assent. The ABCD Study aimed to track development from childhood through adolescence to understand factors that may alter healthy development. In the ABCD Study, self-assessments were comprehensive and included various measures, such as automated current diagnoses, dimensional assessments of current psychopathology symptoms, and familial risk loading for psychopathology. The present paper takes advantage of this comprehensive approach by examining each of these clinical characterizations of depression vulnerability to examine how each might relate to motor signs. Data were used from ABCD baseline time point for motor and baseline depression vulnerability data and 1-year follow-up waves for the longitudinal analyses (https://dx.doi.org/10.15154/1522640).
Measures
Youth Psychopathology Assessments
The Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime Version (K-SADS-PL) is a semistructured parent-child interview designed to assess present and lifetime psychopathology (57). K-SADS-PL measures affective and psychotic impairments on both diagnosis-specific and global levels and is highly reliable and well validated; all questions were asked of each participant. The depression symptoms sum was calculated as a total count of symptoms that were endorsed (current or in the past), resulting in a possible score of 0–35 (see the Supplement) (2). In addition, this assessment produces a spectrum of depression diagnoses that are automated and not fully assessed by a clinician. Therefore, they are not recommended for inclusion in studies. However, such diagnoses show similar patterns of findings as the results below. As a result, we have included these diagnostics in supplemental material but emphasize caution in their reference and interpretation. For a comparison of specificity, total social anxiety symptoms were also compared with motor symptoms.
Motor Assessments
Measurements of motor abnormalities are not a central domain of the ABCD Study. However, 4 items within clinical scales assess past and current motor abnormalities. These items include 1) early motor developmental delays (ABCD Developmental History Questionnaire), 2) current symptoms coordination (Child Behavioral Checklist), 3) psychomotor retardation (K-SADS), and 4) psychomotor agitation (K-SADS) (Supplement). All motor variables were coded dichotomously (absence or presence).
Family History Assessment
The Family History Assessment Module Screener (58) screens for the presence/absence of psychopathological symptoms in first-degree biological relatives and was completed by parents. A total number of first-degree relatives (parents or siblings) endorsed as having depression were used as a proximal measure of familial loading of risk.
Data Analyses
First, we used a linear regression analyses to examine current depression symptoms and rates of motor abnormalities (i.e., psychomotor agitation, psychomotor retardation, developmental motor delays, dyscoordination), where depression symptoms was the dependent variable, and stimulant medication status was a covariate given the impact of stimulants on motor behavior. Confidence intervals were assessed with 1000 iterations of bootstrapped samples. In developmental symptoms, no correction for current medication was applied, and differences were assessed with a χ2 analysis. Second, for the dependent variable of familial risk, general linear models were run for each motor abnormality. Diagnostic test characteristics are reported for all motor measures (Table 1) (59). Third, we tested whether motor abnormalities at baseline predicted depression symptoms at a 1-year follow-up in 2 stepwise regressions. All current symptom analyses excluded individuals on antipsychotics (n = 74). Supplemental analyses that included these individuals and did not correct for stimulant medication use yielded similar results (Supplement). In addition, post hoc parallel analyses were conducted for total social anxiety symptoms to examine possible specificity (Supplement). Finally, for each set of analyses (baseline depression, family history of depression, depression at follow-up) we ran multivariate models that include all motor abnormality variables to examine the unique contribution of each motor abnormality to the depression outcome variable. All models described above were considered significant if they passed Bonferroni correction for 4 model comparisons (p < .0125) (Supplement). Analyses were run in SPSS (version 27; IBM Corp.) and visualized in R (version 4.0.3; R Foundation for Statistical Computing).
Table 1.
Demographic Metrics: Prevalence Among the Whole Sample, Depression Diagnoses, and Familial History Categories
| Characteristic | Whole Sample (N = 11,870) | Familial History of Depression |
Group Comparison Statistics | |
|---|---|---|---|---|
| Yes (n = 3639) | No (n = 8230) | |||
| Age, Months, Mean (SD) | 118.95 (7.46) | 119.03 (7.46) | 118.75 (7.46) | t11868 = 1.92, p = .06 |
| Sex, Female | 48.00% | 47.80% | 48.10% | χ21 = 0.14, p = .71 |
| Any Motor Sign | 26.9% | 31.35% | 24.85% | |
| Psychomotor Agitation | 1.5% | 1.30% | 2.00% | χ21,11800 = 26.04, p = .001 |
| Psychomotor Retardation | 0.3% | 0.50% | 0.20% | χ21,11800 = 9.08, p = .01 |
| Developmental Motor Delays | 8.8% | 8.40% | 2.60% | χ21,11662 = 5.62, p = .02 |
| Dyscoordination | 19.3% | 23.30% | 17.50% | χ22,11800 = 111.08, p < .001 |
Results
Prevalence of Motor Abnormalities and Depression Symptoms in Whole Sample
In the whole sample, 26.9% of individuals endorsed at least one motor sign, and 3% endorsed two or more motor signs: 1.5% endorsed psychomotor agitation, 0.3% endorsed psychomotor retardation, 8.8% endorsed developmental motor delays, and 19.3% endorsed dyscoordination (Table 1). The average depression symptoms endorsed in the whole sample was 0.74 (SD = 2.44) at baseline and 0.94 (SD = 2.71) at follow-up; change in number of symptoms endorsed averaged of 0.41 (SD = 3.20). Among individuals who endorsed at least one depression symptom, the average number of symptoms endorsed was 5.97 (SD = 4.08) at baseline and 5.79 (SD = 4.13) at follow-up. For individuals who endorsed at least one symptom at either time point, the average change in symptoms was 1.79 (SD = 6.03). Finally, 30.5% of the sample had a first-degree relative with a depression diagnosis.
Prevalence of Motor Abnormalities and Concurrent Depression Symptoms
Depression Symptoms and Psychomotor Agitation
Adolescents who endorsed psychomotor agitation endorsed more depression symptoms (mean = 1.55, SD = 3.84) than those who did not endorse psychomotor agitation (mean = 0.73, SD = 2.41; Cohen’s d = 0.34; B = 0.08, SE = 0.02, p < .001).
Depression Symptoms and Psychomotor Retardation
Adolescents who endorsed psychomotor retardation endorsed more depression symptoms (mean = 2.51, SD = 4.40) than those without psychomotor retardation (mean = 0.76, SD = 2.43; Cohen’s d = 0.73; B = 0.13, SE = 0.03, p < .001).
Depression Symptoms and Developmental Motor Milestones Delays
Adolescents with developmental motor delays endorsed more depression symptoms (mean = 1.18, SD = 3.23) than adolescents with no motor delays (mean = 0.07, SD = 2.43; Cohen’s d = 0.152; t11652 = 6.07, p < .001).
Depression Symptoms and Dyscoordination
Adolescents who endorsed dyscoordination endorsed more depression symptoms (mean = 1.49, SD = 3.50) than coordinated adolescents (mean = 0.56, SD = 2.07; B = 0.12, SE = 0.008, Cohen’s d = 0.39, p < .001).
Motor Abnormalities Relative Association With Depression Symptoms
A general linear model with simultaneously entered predictors demonstrated that motor abnormalities overall were related to depression symptoms even after accounting for the variance related to stimulant medication use (F5,11646 = 82.49, r2 = 0.03, p < .001). All four motor variables were related to number of depression symptoms endorsed—psychomotor agitation (B = 0.82; 95% CI, 0.41–0.59; t = 2.55; SE = 0.19; p = .007), psychomotor retardation (B = 1.37; 95% CI, 0.56–2.19; t = 3.32; SE = 0.41; p = .001), developmental motor delays (B = 0.43; 95% CI, 0.27–0.99; t = 5.45; SE = 0.09; p < .001), and dyscoordination (B = 0.86; 95% CI, 0.67–1.00; t = 15.20; SE = 0.06; p < .001).
Motor Abnormalities and Familial Risk for Depression
Familial Risk and Psychomotor Agitation
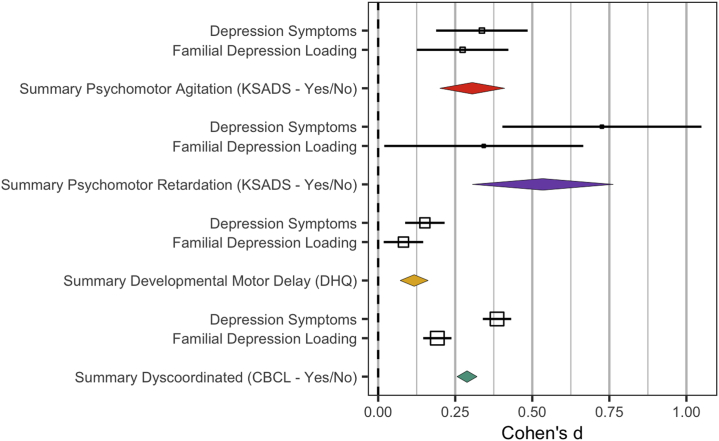
Endorsement of psychomotor agitation related to a greater familial risk for depression (B = 0.24, SE = 0.08, p < .001, Cohen’s d = 0.27; psychomotor agitation: mean = 1.55, SD = 3.84; no psychomotor agitation: mean = 0.73, SD = 2.41) (Figure 1).
Figure 1.
Effect size of motor abnormalities by current depression symptoms and depression familial vulnerability measures. Psychomotor agitation: green, psychomotor retardation: yellow, developmental motor delays: red, and dyscoordination symptoms: purple. Effect sizes above were transformed into raw, not model-corrected data; odds ratios (Table 2) and standard error were transformed to Cohen’s d using the Michaela package in R; error bars reflect the standard error; effect sizes were converted to common values using the R Michaela package (84) and visualized with the metaviz package (85). CBCL, Child Behavior Checklist; DHQ, Developmental History Questionnaire; K-SADS, Schedule for Affective Disorders and Schizophrenia for School-Age Children.
Familial Risk and Psychomotor Retardation
Psychomotor retardation in youth was unrelated to the number of relatives with a depression diagnosis (B = 0.28, SE = 0.15, p = .07, Cohen’s d = 0.34).
Familial Risk and Developmental Motor Milestones Delays
Individuals with delayed motor milestones had more first-degree relatives with depression (mean = 0.47, SD = 0.759) than individuals who did not experience delays (mean = 0.40, SD = 0.727, t11730 = 2.93, p < .001, Cohen’s d = 0.082).
Familial Risk and Dyscoordination
Endorsement of dyscoordination related to familial risk for depression (B = 0.22, SE = 0.029, p < .001, Cohen’s d = 0.19). Individuals who endorsed current dyscoordination had more first-degree relatives with depression (mean = 1.49, SD = 2.07) than those who did not endorse dyscoordination (mean = 0.56, SD = 2.867) (Figure 2).
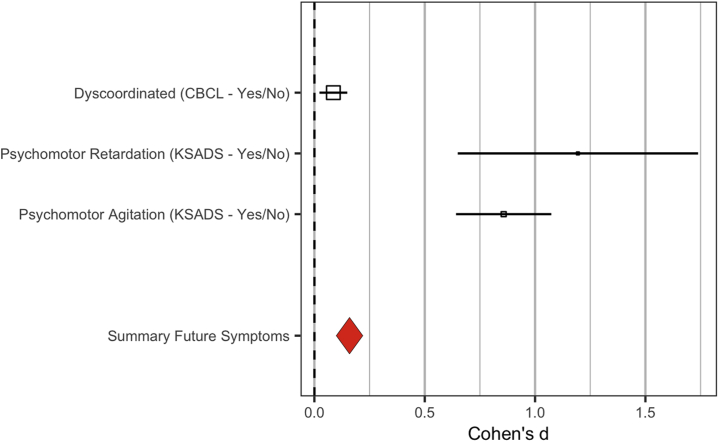
Figure 2.
Effect size of motor abnormalities at baseline on depression at 1-year follow-up. Effect sizes above are raw, not model-corrected data; odds ratios (Table 2) and standard error were calculated using the Michaela package in R; error bars reflect the standard error; effect sizes were converted to common values using the R Michaela package (84) and visualized with the metaviz package (85). CBCL, Child Behavior Checklist; K-SADS, Schedule for Affective Disorders and Schizophrenia for School-Age Children.
Motor Abnormalities Relative Contribution to Depression Familial Vulnerability—Familial Risk Loading
A general linear model with simultaneously entered predictors demonstrated that motor abnormalities overall were related to familial risk for depression, accounting for the variance related to stimulant medication use (F5,11646 = 33.18, p < .001, r2 = 0.013). Three of the four motor variables were each uniquely related to familial risk for depression—psychomotor agitation (B = 0.16; 95% CI, 0.036–0.26; t = 2.58; SE = 0.06, p = .007), developmental motor delays (B = 0.06, SE = 0.03, p = .02), and dyscoordination (B = 0.13, SE = 0.02, p < .001), while psychomotor retardation was not (B = 0.23, SE = 0.17, p = .17). The test characteristics of motor signs in predicting familial vulnerability to depression can be found in Table 2.
Table 2.
Test Characteristics of Motor Abnormalities for Detecting Depression Diagnoses
| Motor Abnormalities | Familial MDD, History/None | Overall Prevalence | Sensitivity | Specificity | PPV | NPV | OR |
|---|---|---|---|---|---|---|---|
| Any Motor Sign | 1141/2045 | 27.33% | 32.05% | 74.75% | 35.81% | 71.45% | 1.40 |
| 2+ Motor Signs | 111/166 | 3.17% | 4.39% | 97.33% | 40.07% | 71.45% | 1.67 |
| Psychomotor Agitation | 72/105 | 1.50% | 2.00% | 98.72% | 40.68% | 69.66% | 1.57 |
| Psychomotor Retardation | 19/18 | 0.31% | 0.53% | 99.78% | 51.35% | 69.57% | 2.41 |
| Developmental Motor Delays | 447/680 | 9.58% | 12.21% | 91.61% | 39.66% | 69.78% | 1.52 |
| Dyscoordination | 839/1432 | 19.25% | 23.32% | 82.54% | 36.94% | 71.05% | 1.44 |
MDD, major depressive disorder; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value.
Motor Abnormalities and Future Symptoms
Depression Symptoms at 1-Year Follow-up
In a stepwise regression, predicting dimensional depression symptoms at follow-up, the first step included baseline symptoms of depression and stimulant uses, and the second step included baseline motor abnormalities (motor agitation, motor retardation, and dyscoordination). The overall model was significant (F4,6448 = 38.18, p < .001), and the change between steps was significant (F-change4,6448 = 42.25, r2-change = 0.011, p < .001), indicating that motor abnormalities predicted depression symptoms at follow-up over and above baseline depression symptoms alone. Baseline depression symptoms significantly contributed to the model (β = 0.146; t = 8.89, partial r = 0.11, p < .001), and all four motor abnormalities each uniquely predicted follow-up depression symptoms in a second step (dyscoordination: t = 2.53, p = .011, β = 0.31; partial r = 0.10; psychomotor retardation: t = 6.80, p < .001, β = 0.088; partial r = 0.084; psychomotor agitation: t = 2.07, p = .038, β = 0.027; partial r = 0.026) (Table 2).
Discussion
Adolescents with motor abnormalities had greater depression vulnerability. Traditional (psychomotor agitation, psychomotor retardation) and novel (developmental motor milestones, dyscoordination) motor signs were related to depression. Similarly, familial risk loading was related to psychomotor agitation, developmental motor delays, and dyscoordination in adolescents. In a prospective model, motor abnormalities predicted future symptoms. Collectively, these analyses suggest that motor abnormalities are sensitive to depression in adolescence (35) and may provide added benefits when combined with the traditional depression risk metrics (i.e., cognitive and affective measures).
All motor abnormalities were endorsed by some adolescents in this sample, but traditional motor measures had a notably low prevalence compared with other motor abnormalities. Developmental motor delays have been reported to have a prevalence (7%) (60) similar to that in the current sample (9.6%). Similarly, the rates of dyscoordination in the current study (19.25%) was similar to the rate of clinically assessed dyscoordination in previous adolescent studies (27.72%) (61) and populations studies of Canadian children (23%) (62). Psychomotor agitation and psychomotor retardation among adolescents with a current depression diagnosis in the present study (0.3%–3.2%) (Supplement) were substantially lower than the prevalence among adults with the diagnosis (34%–63%) (11,63, 64, 65). This difference across development may suggest that motor abnormalities develop over the symptom course. Alternatively, psychomotor agitation and psychomotor retardation may be masked by neurodevelopmental processes, such that motor abnormalities become more salient in adulthood. Motor abnormalities may also take alternative forms in adolescence, as dyscoordination had a prevalence of 35.5% among individuals with depression, which was more consistent with the prevalence of adult motor disturbance (39,40,42,66). Traditional motor measures did, however, show high negative predictive value that may be useful to distinguish individuals without the diagnosis from a larger pool of potential patients. Concurrent symptoms also related to motor abnormalities, consistent with studies that have linked single motor measures to patient symptom severity in adults (16,23,24,38). In addition, supplemental analyses demonstrate that traditional motor metrics (psychomotor agitation and retardation) may have a less specific relationship to depression, as they relate to endorsements of psychomotor agitation, but novel motor measures (dyscoordination and motor milestones) did not show a similar relationship to social anxiety (Supplement). As a result, the addition of novel motor abnormalities, i.e., dyscoordination, increased sensitivity and specificity to disorder vulnerability beyond traditional measures.
Familial risk loading was related to the endorsement of psychomotor agitation, developmental motor delays, and dyscoordination; it had similar effect sizes to depression symptoms. Thus, motor abnormalities may not reflect the presence of depression alone but a general depression vulnerability. These findings are consistent with motor abnormalities in infants, children, and adolescents with familial risk for depression (27,36,37,67,68) but build upon these findings by demonstrating the relevance of multiple motor metrics to familial risk loading in adolescence. However, it is notable that the current study is unable to disambiguate environmental influences from genetic influences, and more studies (e.g., genome-wide association study, twin studies) are needed to test whether motor abnormalities are potential endophenotypes.
Endorsement of motor abnormalities at baseline predicted future depression symptoms independent of baseline depressive symptoms consistent with adult research (17,27,34,38,69). These results provide convincing evidence for the clinical significance of motor abnormalities, even with a relatively short follow-up period of 1 year. Notably, the age of the sample at follow-up is earlier than the peak onset window for disorder (56), suggesting that motor abnormalities may connote early (and perhaps more chronic) risk for depression. In supplemental analyses (Supplement) (17, 57, 58, 59), a discriminant function yielded similar accuracy as a previously published motor discriminant function in adult samples that discriminated healthy individuals from those with depression at follow-up (64). Importantly, many of the motor abnormalities provided a unique predictive contribution to the model, suggesting that expanding motor measure may improve sensitivity to predicting future disorder onset and course. The variability of the motor abnormalities in terms of specificity and sensitivity to depression may explain why each motor measure showed an added benefit in classifying individuals having depression. As a result, motor measurements, both traditional and novel, show promise as early markers of risk for disorder onset and course.
Generally, motor abnormalities were related to depression, but psychomotor retardation had a limited relationship to baseline familial risk, contrary to adult literature, which emphasizes the importance of psychomotor retardation (13,23,24,32,37,70, 71, 72, 73, 74, 75, 76, 77). However, these studies often measure psychomotor retardation directly (e.g., actigraphy) rather than via self-reports, which may underestimate the prevalence of this symptom in the ABCD Study. Psychomotor retardation did, however, show utility in predicting concurrent and future symptom course at 1-year follow-up, highlighting its potential in clinical utility. These latter findings are consistent with reports that psychomotor retardation may highlight a subgroup of the most severe patients or only mark individuals at risk for severe course (17,27,34,38,69). Taken together, psychomotor retardation may have limited utility as an early clinical vulnerability feature but may connote risk for a more severe course and thus warrant early screening to identify those most in need of intervention.
The exact mechanisms of motor disturbance in depression are currently still debated. Some models suggest that hyperactivity in cortical structures, subgenual anterior cingulate cortex and basal lateral amygdala signals the nucleus accumbens to drive a downregulation of dopamine input to cortical regions in the ventral tegmental area (10,11,29,30). This hypodopaminergic state leads to long-term decrease in dopaminergic gain, which may manifest as decreased volition, movement, and hedonics among other motoric, cognitive, and affective symptoms (30). Some researchers suggest that reduced metabolism of catecholamine leads to psychomotor agitation type motor signs (11), or reduction of dopamine in particular leads to psychomotor retardation (11,30). Others propose that tuning of excitability of these circuits, in general (30) or among interneurons in particular (10), during development will result in motor, cognitive, and affective symptoms. This model suggests that motor signs may be a risk marker that may have sensitivity to depression risk alongside traditional cognitive and affective measures (11). The current study provides support for this model and suggests that motor may provide additional sensitivity to emerging psychopathology in early adolescence.
Although the study had several notable strengths, it is important to note key limitations. First, these analyses may overestimate the sensitivity of psychomotor agitation and psychomotor retardation as they are included in the symptom sum. However, these motor signs were independent of the measure used to assess risk for this disorder, which also showed a comparable effect size. Second, all of the current motor behavior measures were single items, self-report of motor signs, limiting the sensitivity of motor measures (relative to controlled laboratory assessments) to ones that are salient to participants. This limitation is particularly notable as many motor behavioral measures are readily available, e.g., force variability and velocity scaling (13,78). Existing literature demonstrates that behavioral measures are more sensitive at identifying motor symptoms than observation or self-report measures alone (79,80). It is also notable that the motor items were included in both self- and parental reports, and it is possible that parental reports of motor milestone delays and dyscoordination were less stringent than self-reports of psychomotor slowing and agitation. However, it is also possible that the parental reports may be a more sensitive measure of dyscoordination as their assessment reflects a number of observations of this phenomenon in several of contexts. In addition, parental report of abnormalities may be biased by parental psychopathology or concerns regarding psychopathology in the child. Next, it is notable that the small effect sizes and test characteristics suggest that self- and parental reports of motor behavior alone will not be sufficient to assess risk for depression. Instead, motor may confer some additional benefit as a part of a larger risk battery. However, the current effect sizes (odds ratios [ORs]: 1.40–2.41) are within a similar range to other risk markers of depression (81) including genome-wide associations of depression (OR = 1.35) (82), single nucleotide polymorphisms/single candidate genes (OR = 1.15) (82), severe irritability (OR = 1.33) (83), and current parental depression (maternal OR = 1.99; paternal OR = 1.45) (53). Finally, although depression in the current paper is treated as a singular outcome, we recognize that the depression is heterogeneous that reflects a number of complex profiles. Future studies should consider examining the specific features of depression rather than aggregating over this heterogeneous group.
In conclusion, motor abnormalities show promise as an early marker of vulnerability to depression, as these markers 1) discriminated between individuals with depression and the general population, 2) predicted familial risk loading, and 3) prospectively predicted worsening symptoms and onset. This study also demonstrated the utility of expanding motor abnormality metrics beyond psychomotor agitation and psychomotor retardation to increase the sensitivity to a broader set of more developmentally relevant motor issues, including developmental motor delays and dyscoordination.
Acknowledgments and Disclosures
This work was supported by the National Institutes of Mental Health (Grant Nos. R01MH094650, R01MH112545–01, R01MH103231, R01MH112545, R01MH094650, R01MH118741, and R21/R33MH103231 [to VAM]; T32MH126368 [to KSFD]; R01 MH119771 [to SS]) and Swiss National Science Foundation (Grant Nos. 182469 and 184717 [to SW]). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 years and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners (Grant Nos. U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025).
ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the National Institutes of Health or ABCD consortium investigators.
A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/Consortium_Members.pdf. The ABCD data repository grows and changes over time. DOIs can be found at https://nda.nih.gov/generalquery.html?q=query=studies%20%7Eand%7E%20orderBy=id%20%7Eand%7E%20orderDirection=Ascending.
Data used in the preparation of this work were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the National Institute of Mental Health Data Archive.
Unrelated to the current work, SW has been awarded honoraria from Lundbeck, Janssen, and Sunovion. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.06.011.
Supplementary Material
References
- 1.James S., Abate D., Abate K., Abay S., Abbafati C., Abbasi N., Abbastabar H., GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins K.A., Westra H.A., Dozois D.J.A., Burns D.D. Gaps in accessing treatment for anxiety and depression: Challenges for the delivery of care. Clin Psychol Rev. 2004;24:583–616. doi: 10.1016/j.cpr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Tolin D.F. Is cognitive-behavioral therapy more effective than other therapies? A meta-analytic review. Clin Psychol Rev. 2010;30:710–720. doi: 10.1016/j.cpr.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Harrington R., Clark A. Prevention and early intervention for depression in adolescence and early adult life. Eur Arch Psychiatry Clin Neurosci. 1998;248:32–45. doi: 10.1007/s004060050015. [DOI] [PubMed] [Google Scholar]
- 5.Avenevoli S., Merikangas K.R. Implications of high-risk family studies for prevention of depression. Am J Prev Med. 2006;31(6 suppl 1):S126–S135. doi: 10.1016/j.amepre.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Pelkonen M., Marttunen M., Aro H. Risk for depression: A 6-year follow-up of Finnish adolescents. J Affect Disord. 2003;77:41–51. doi: 10.1016/s0165-0327(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 7.Dobson K.S., Dozois D.J.A. In: Risk Factors in Depression. Dobson K.S., Dozois D.J.A., editors. Elsevier; San Diego: 2008. Chapter 1 - Introduction: assessing risk and resilience factors in models of depression; pp. 1–16. [Google Scholar]
- 8.Thapar A., Collishaw S., Pine D.S., Thapar A.K. Depression in adolescence. Lancet. 2012;379:1056–1067. doi: 10.1016/S0140-6736(11)60871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The American Psychiatric Association . 5th ed. American Psychiatric Publishing; Washington, D.C.: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 10.Grace A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17:524–532. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobin C., Sackeim H.A. Psychomotor symptoms of depression. Am J Psychiatry. 1997;154:4–17. doi: 10.1176/ajp.154.1.4. [DOI] [PubMed] [Google Scholar]
- 12.Schrijvers D., Hulstijn W., Sabbe B.G.C. Psychomotor symptoms in depression: A diagnostic, pathophysiological and therapeutic tool. J Affect Disord. 2008;109:1–20. doi: 10.1016/j.jad.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Shankman S.A., Mittal V.A., Walther S. An examination of psychomotor disturbance in current and remitted MDD: An RDoC study. J Psychiatr Brain Sci. 2020;5 doi: 10.20900/jpbs.20200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sices L. Use of developmental milestones in pediatric residency training and practice: Time to rethink the meaning of the mean. J Dev Behav Pediatr. 2007;28:47–52. doi: 10.1097/DBP.0b013e31803084c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damme T.V., Simons J., Sabbe B., van West D. Motor abilities of children and adolescents with a psychiatric condition: A systematic literature review. World J Psychiatry. 2015;5:315–329. doi: 10.5498/wjp.v5.i3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Os J., Jones P., Lewis G., Wadsworth M., Murray R. Developmental precursors of affective illness in a general population birth cohort. Arch Gen Psychiatry. 1997;54:625–631. doi: 10.1001/archpsyc.1997.01830190049005. [DOI] [PubMed] [Google Scholar]
- 17.Emck C., Bosscher R.J., Van Wieringen P.C., Doreleijers T., Beek P.J. Gross motor performance and physical fitness in children with psychiatric disorders. Dev Med Child Neurol. 2011;53:150–155. doi: 10.1111/j.1469-8749.2010.03806.x. [DOI] [PubMed] [Google Scholar]
- 18.Walker E.F. Developmentally moderated expressions of the neuropathology underlying schizophrenia. Schizophr Bull. 1994;20:453–480. doi: 10.1093/schbul/20.3.453. [DOI] [PubMed] [Google Scholar]
- 19.Mittal V.A., Bernard J.A., Northoff G. What can different motor circuits tell us about psychosis? An RDoC perspective. Schizophr Bull. 2017;43:949–955. doi: 10.1093/schbul/sbx087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittal V.A., Walker E.F. Movement abnormalities predict conversion to Axis I psychosis among prodromal adolescents. J Abnorm Psychol. 2007;116:796–803. doi: 10.1037/0021-843X.116.4.796. [DOI] [PubMed] [Google Scholar]
- 21.Mittal V.A., Tessner K.D., McMillan A.L., Delawalla Z., Trotman H.D., Walker E.F. Gesture behavior in unmedicated schizotypal adolescents. J Abnorm Psychol. 2006;115:351–358. doi: 10.1037/0021-843X.115.2.351. [DOI] [PubMed] [Google Scholar]
- 22.Mittal V.A., Hasenkamp W., Sanfilipo M., Wieland S., Angrist B., Rotrosen J., Duncan E.J. Relation of neurological soft signs to psychiatric symptoms in schizophrenia. Schizophr Res. 2007;94:37–44. doi: 10.1016/j.schres.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Finazzi M.E., Mesquita M.E., Lopes J.R., Fu L.I., Oliveira M.G., Del Porto J.A. Motor activity and depression severity in adolescent outpatients. Neuropsychobiology. 2010;61:33–40. doi: 10.1159/000262178. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien J.T., Gallagher P., Stow D., Hammerla N., Ploetz T., Firbank M., et al. A study of wrist-worn activity measurement as a potential real-world biomarker for late-life depression. Psychol Med. 2017;47:93–102. doi: 10.1017/S0033291716002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vares E.A., Salum G.A., Spanemberg L., Caldieraro M.A., Fleck M.P. Depression dimensions: Integrating clinical signs and symptoms from the perspectives of clinicians and patients. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Leemput I.A., Wichers M., Cramer A.O.J., Borsboom D., Tuerlinckx F., Kuppens P., et al. Critical slowing down as early warning for the onset and termination of depression. Proc Natl Acad Sci U S A. 2014;111:87–92. doi: 10.1073/pnas.1312114110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leventhal A.M., Pettit J.W., Lewinsohn P.M. Characterizing major depression phenotypes by presence and type of psychomotor disturbance in adolescents and young adults. Depress Anxiety. 2008;25:575–592. doi: 10.1002/da.20328. [DOI] [PubMed] [Google Scholar]
- 28.Mittal V.A., Wakschlag L.S. Research domain criteria (RDoC) grows up: Strengthening neurodevelopment investigation within the RDoC framework. J Affect Disord. 2017;216:30–35. doi: 10.1016/j.jad.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obeso J.A., Rodriguez-Oroz M.C., Stamelou M., Bhatia K.P., Burn D.J. The expanding universe of disorders of the basal ganglia. Lancet. 2014;384:523–531. doi: 10.1016/S0140-6736(13)62418-6. [DOI] [PubMed] [Google Scholar]
- 30.Robison A.J., Thakkar K.N., Diwadkar V.A. Cognition and reward circuits in schizophrenia: Synergistic, not separate. Biol Psychiatry. 2020;87:204–214. doi: 10.1016/j.biopsych.2019.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cullen K.R., Gee D.G., Klimes-Dougan B., Gabbay V., Hulvershorn L., Mueller B.A., et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders J.B., Bremmer M.A., Comijs H.C., Deeg D.J.H., Beekman A.T.F. Gait speed and the natural course of depressive symptoms in late life; an independent association with chronicity? J Am Med Dir Assoc. 2016;17:331–335. doi: 10.1016/j.jamda.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Ulbricht C.M., Dumenci L., Rothschild A.J., Lapane K.L. Changes in depression subtypes among men in STAR∗D: A latent transition analysis. Am J Mens Health. 2018;12:5–13. doi: 10.1177/1557988315607297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulbricht C.M., Rothschild A.J., Lapane K.L. Functional impairment and changes in depression subtypes for women in STAR∗D: A latent transition analysis. J Womens Health (Larchmt) 2016;25:464–472. doi: 10.1089/jwh.2015.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beauchaine T.P., Constantino J.N. Redefining the endophenotype concept to accommodate transdiagnostic vulnerabilities and etiological complexity. Biomark Med. 2017;11:769–780. doi: 10.2217/bmm-2017-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cornish A.M., McMahon C.A., Ungerer J.A., Barnett B., Kowalenko N., Tennant C. Postnatal depression and infant cognitive and motor development in the second postnatal year: The impact of depression chronicity and infant gender. Infant Behav Dev. 2005;28:407–417. [Google Scholar]
- 37.Vameghi R., Akbari S.A.A., Sajjadi H., Sajedi F., Alavimajd H. Correlation between mothers’ depression and developmental delay in infants aged 6-18 months. Glob J Health Sci. 2015;8:11–18. doi: 10.5539/gjhs.v8n5p11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colman I., Ploubidis G.B., Wadsworth M.E.J., Jones P.B., Croudace T.J. A longitudinal typology of symptoms of depression and anxiety over the life course. Biol Psychiatry. 2007;62:1265–1271. doi: 10.1016/j.biopsych.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Campbell W.N., Missiuna C., Vaillancourt T. Peer victimization and depression in children with and without motor coordination difficulties. Psychol Schs. 2012;49:328–341. [Google Scholar]
- 40.Draghi T.T.G., Cavalcante Neto J.L., Tudella E. Symptoms of anxiety and depression in schoolchildren with and without developmental coordination disorder. J Health Psychol. 2021;26:1519–1527. doi: 10.1177/1359105319878253. [DOI] [PubMed] [Google Scholar]
- 41.Poole K.L., Schmidt L.A., Missiuna C., Saigal S., Boyle M.H., Van Lieshout R.J. Childhood motor coordination and adult psychopathology in extremely low birth weight survivors. J Affect Disord. 2016;190:294–299. doi: 10.1016/j.jad.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 42.Hill E.L., Brown D. Mood impairments in adults previously diagnosed with developmental coordination disorder. J Ment Health. 2013;22:334–340. doi: 10.3109/09638237.2012.745187. [DOI] [PubMed] [Google Scholar]
- 43.Owoeye O., Kingston T., Scully P.J., Baldwin P., Browne D., Kinsella A., et al. Epidemiological and clinical characterization following a first psychotic episode in major depressive disorder: Comparisons with schizophrenia and bipolar I disorder in the Cavan-Monaghan first episode psychosis study (CAMFEPS) Schizophr Bull. 2013;39:756–765. doi: 10.1093/schbul/sbt075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raballo A., Poletti M. Advances in early identification of children and adolescents at risk for psychiatric illness. Curr Opin Psychiatry. 2020;33:611–617. doi: 10.1097/YCO.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 45.Goldstone A., Javitz H.S., Claudatos S.A., Buysse D.J., Hasler B.P., de Zambotti M., et al. Sleep disturbance predicts depression symptoms in early adolescence: Initial findings from the adolescent brain cognitive development study. J Adolesc Health. 2020;66:567–574. doi: 10.1016/j.jadohealth.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray J.C., Schvey N.A., Tanofsky-Kraff M. Demographic, psychological, behavioral, and cognitive correlates of BMI in youth: Findings from the Adolescent Brain Cognitive Development (ABCD) study. Psychol Med. 2020;50:1539–1547. doi: 10.1017/S0033291719001545. [DOI] [PubMed] [Google Scholar]
- 47.Gorham L.S., Barch D.M. White matter tract integrity, involvement in sports, and depressive symptoms in children. Child Psychiatry Hum Dev. 2020;51:490–501. doi: 10.1007/s10578-020-00960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karcher N.R., Barch D.M. The ABCD study: Understanding the development of risk for mental and physical health outcomes. Neuropsychopharmacology. 2021;46:131–142. doi: 10.1038/s41386-020-0736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffman E.A., Clark D.B., Orendain N., Hudziak J., Squeglia L.M., Dowling G.J. Stress exposures, neurodevelopment and health measures in the ABCD study. Neurobiol Stress. 2019;10:100157. doi: 10.1016/j.ynstr.2019.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Assari S., Boyce S., Bazargan M., Caldwell C.H. African Americans’ diminished returns of parental education on adolescents’ depression and suicide in the Adolescent Brain Cognitive Development (ABCD) study. Eur J Investig Health Psychol Educ. 2020;10:656–668. doi: 10.3390/ejihpe10020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mennies R.J., Birk S.L., Norris L.A., Olino T.M. The main and interactive associations between demographic factors and psychopathology and treatment utilization in youth: A test of intersectionality in the ABCD study. Res Child Adolesc Psychopathol. 2021;49:5–17. doi: 10.1007/s10802-020-00687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai Y., Elsayed N., Barch D. Contributions from resting state functional connectivity and familial risk to early adolescent-onset MDD: Results from the Adolescent Brain Cognitive Development study. J Affect Disord. 2021;287:229–239. doi: 10.1016/j.jad.2021.03.031. [DOI] [PubMed] [Google Scholar]
- 53.Pagliaccio D., Alqueza K.L., Marsh R., Auerbach R.P. Brain volume abnormalities in youth at high risk for depression: Adolescent brain and cognitive development study. J Am Acad Child Adolesc Psychiatry. 2020;59:1178–1188. doi: 10.1016/j.jaac.2019.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmer C.E., Loughnan R.J., Makowski C., Thompson W., Barch D., Jernigan T., et al. Delineating genetic and familial risk for psychopathology in the ABCD study. medRxiv. 2020 doi: 10.1101/2020.09.08.20186908. [DOI] [Google Scholar]
- 55.Funkhouser C.J., Chacko A.A., Correa K.A., Kaiser A.J.E., Shankman S.A. Unique longitudinal relationships between symptoms of psychopathology in youth: A cross-lagged panel network analysis in the ABCD study. J Child Psychol Psychiatry. 2021;62:184–194. doi: 10.1111/jcpp.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 57.Kaufman J., Birmaher B., Brent D.A., Ryan N.D., Rao U. K-SADS-PL. J Am Acad Child Adolesc Psychiatry. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Rice J.P., Reich T., Bucholz K.K., Neuman R.J., Fishman R., Rochberg N., et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 59.Ranganathan P., Aggarwal R. Common pitfalls in statistical analysis: Understanding the properties of diagnostic tests - Part 1. Perspect Clin Res. 2018;9:40–43. doi: 10.4103/picr.PICR_170_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valla L., Wentzel-Larsen T., Hofoss D., Slinning K. Prevalence of suspected developmental delays in early infancy: Results from a regional population-based longitudinal study. BMC Pediatr. 2015;15:215. doi: 10.1186/s12887-015-0528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sittiprapaporn P., editor. Learning Disabilities. InTech; Rijeka: 2012. pp. 217–238. [Google Scholar]
- 62.Kirby A: Overlapping Conditions: Overlapping Conditions Management: Services for Individuals With Developmental Coordination Disorder. Children With Developmental Coordination Disorder. London: Wiley, 242–265.
- 63.Bryan C.J., Songer T.J., Brooks M.M., Thase M.E., Gaynes B.N., Klinkman M., et al. A comparison of baseline sociodemographic and clinical characteristics between major depressive disorder patients with and without diabetes: A STAR∗D report. J Affect Disord. 2008;108:113–120. doi: 10.1016/j.jad.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Caligiuri M.P., Ellwanger J. Motor and cognitive aspects of motor retardation in depression. J Affect Disord. 2000;57:83–93. doi: 10.1016/s0165-0327(99)00068-3. [DOI] [PubMed] [Google Scholar]
- 65.Parker G., Hadzi-Pavlovic D., Brodaty H., Boyce P., Mitchell P., Wilhelm K., et al. Psychomotor disturbance in depression: Defining the constructs. J Affect Disord. 1993;27:255–265. doi: 10.1016/0165-0327(93)90049-p. [DOI] [PubMed] [Google Scholar]
- 66.Zhao Q., Ma Y.T., Lui S.S.Y., Liu W.H., Xu T., Yu X., et al. Neurological soft signs discriminate schizophrenia from major depression but not bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:72–78. doi: 10.1016/j.pnpbp.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 67.Mehler-Wex C., Kölch M. Depression in children and adolescents. Dtsch Arztebl Int. 2008;105:149–155. doi: 10.3238/arztebl.2008.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kounali D., Zammit S., Wiles N., Sullivan S., Cannon M., Stochl J., et al. Common versus psychopathology-specific risk factors for psychotic experiences and depression during adolescence. Psychol Med. 2014;44:2557–2566. doi: 10.1017/S0033291714000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fried E.I., Nesse R.M. Depression is not a consistent syndrome: An investigation of unique symptom patterns in the STAR∗D study. J Affect Disord. 2015;172:96–102. doi: 10.1016/j.jad.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walther S., Höfle O., Federspiel A., Horn H., Hügli S., Wiest R., et al. Neural correlates of disbalanced motor control in major depression. J Affect Disord. 2012;136:124–133. doi: 10.1016/j.jad.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 71.Cantisani A., Stegmayer K., Bracht T., Federspiel A., Wiest R., Horn H., et al. Distinct resting-state perfusion patterns underlie psychomotor retardation in unipolar vs. bipolar depression. Acta Psychiatr Scand. 2016;134:329–338. doi: 10.1111/acps.12625. [DOI] [PubMed] [Google Scholar]
- 72.Matthews K., Coghill D., Rhodes S. Neuropsychological functioning in depressed adolescent girls. J Affect Disord. 2008;111:113–118. doi: 10.1016/j.jad.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 73.Reichert M., Lutz A., Deuschle M., Gilles M., Hill H., Limberger M.F., Ebner-Priemer U.W. Improving motor activity assessment in depression: Which sensor placement, analytic strategy and diurnal time frame are most powerful in distinguishing patients from controls and monitoring treatment effects. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krane-Gartiser K., Henriksen T.E.G., Vaaler A.E., Fasmer O.B., Morken G. Actigraphically assessed activity in unipolar depression: A comparison of inpatients with and without motor retardation. J Clin Psychiatry. 2015;76:1181–1187. doi: 10.4088/JCP.14m09106. [DOI] [PubMed] [Google Scholar]
- 75.Bracht T., Federspiel A., Schnell S., Horn H., Höfle O., Wiest R., et al. Cortico-cortical white matter motor pathway microstructure is related to psychomotor retardation in major depressive disorder. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Razavi N., Horn H., Koschorke P., Hügli S., Höfle O., Müller T., et al. Measuring motor activity in major depression: The association between the Hamilton Depression Rating Scale and actigraphy. Psychiatry Res. 2011;190:212–216. doi: 10.1016/j.psychres.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 77.Stange J.P., Zulueta J., Langenecker S.A., Ryan K.A., Piscitello A., Duffecy J., et al. Let your fingers do the talking: Passive typing instability predicts future mood outcomes. Bipolar Disord. 2018;20:285–288. doi: 10.1111/bdi.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Damme K.S.F., Osborne K.J., Gold J.M., Mittal V.A. Detecting motor slowing in clinical high risk for psychosis in a computerized finger tapping model. Eur Arch Psychiatry Clin Neurosci. 2020;270:393–397. doi: 10.1007/s00406-019-01059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cortese L., Caligiuri M.P., Malla A.K., Manchanda R., Takhar J., Haricharan R. Relationship of neuromotor disturbances to psychosis symptoms in first-episode neuroleptic-naïve schizophrenia patients. Schizophr Res. 2005;75:65–75. doi: 10.1016/j.schres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 80.van Harten P.N., Walther S., Kent J.S., Sponheim S.R., Mittal V.A. The clinical and prognostic value of motor abnormalities in psychosis, and the importance of instrumental assessment. Neurosci Biobehav Rev. 2017;80:476–487. doi: 10.1016/j.neubiorev.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 81.Funder D.C., Ozer D.J. Evaluating effect size in psychological research: Sense and nonsense. Adv Methods Pract Psychol Sci. 2019;2:156–168. [Google Scholar]
- 82.Flint J., Kendler K.S. The genetics of major depression. Neuron. 2014;81:484–503. doi: 10.1016/j.neuron.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stringaris A., Cohen P., Pine D.S., Leibenluft E. Adult outcomes of youth irritability: A 20-year prospective community-based study. Am J Psychiatry. 2009;166:1048–1054. doi: 10.1176/appi.ajp.2009.08121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lam P.H., Chiang J.J. michaela—open r package for converting effect sizes. https://github.com/phoebehlam/michaela Available at: Accessed October 2020.
- 85.Schild A.H.E., Voracek M. Finding your way out of the forest without a trail of bread crumbs: Development and evaluation of two novel displays of forest plots. Res Synth Methods. 2015;6:74–86. doi: 10.1002/jrsm.1125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.