Abstract
Nowadays, many studies focus on the potential of bamboo as a source of bioactive compounds and natural antioxidants for nutraceutical, pharmaceutical, and food sources. This study is a pioneering effort to determine the total phenolic content, total flavonoid content and free radical scavenging activity, as well as the phenolic identification and quantification of Bambusa beecheyana. The study was conducted by using ethanol, methanol, and water for solvent extraction by applying cold maceration, Soxhlet, and ultrasonic-assisted extraction techniques. The results showed that Soxhlet and ultrasonic-assisted Bambusa beecheyana culm extracts had an increase in the extract’s dry yield (1.13–8.81%) but a constant p-coumaric acid (4) content (0.00035 mg/g) as compared to the extracts from the cold maceration. The ultrasonic-assisted extraction method required only a small amount (250 mL) of solvent to extract the bamboo culms. A significant amount of total phenolics (107.65 ± 0.01 mg GAE/g) and flavonoids (43.89 ± 0.05 mg QE/g) were found in the Soxhlet methanol culm extract. The extract also possessed the most potent antioxidant activity with an IC50 value of 40.43 µg/mL as compared to the positive control, ascorbic acid. The UHPLC–ESI–MS/MS analysis was carried out on the Soxhlet methanol extract, ultrasonic-assisted extract at 40 min, and cold methanol extract. The analysis resulted in the putative identification of a total of five phenolics containing cinnamic acid derivatives. The two cinnamic acid derivatives, p-coumaric acid (4) and 4-methoxycinnamic acid (5), were then used as markers to quantify the concentration of both compounds in all the extracts. Both compounds were not found in the water extracts. These results revealed that the extract from Soxhlet methanol of Bambusa beecheyana could be a potential botanical source of natural antioxidants. This study provides an important chemical composition database for further preclinical research on Bambusa beecheyana.
Keywords: Bambusa beecheyana, total phenolic content, total flavonoid content, free radical scavenging activity, cinnamic acid derivatives
1. Introduction
Similar to corn, wheat, rye, oat, sugarcane, barley, and rice, bamboo is a grass that belongs to the Poaceae family, where it covers over 1250 species from 75 genera. It is normally found within tropical, subtropical, and temperate parts of all countries [1]. In addition, with a total area of over 10 million hectares in southern Asia and approximately 5 million hectares in China, Asia is the largest bamboo reservoir in the world [2]. In Asia, bamboo is a common main ingredient for vegetable dishes, salads, and pickles. Bamboo has been described as a great supply of nutrients, minerals, amino acids, and dietary fiber. These contain phenolic compounds which contribute to their significant antioxidant potential [3,4,5,6,7,8]. However, the scientific study on the chemical composition and potential application of Bambusa beecheyana species is still very limited. Therefore, various pieces of research need to be done to determine the potential of this species as a nutraceutical and food source.
Given the wide range of bioactive constituents that might present in many plant species, a standard and integrated approach to extracting these potential bioactive components is required. Hence, selecting an appropriate extraction method is important in producing potential bioactive compounds that are rich in phenolics, especially bioactive flavonoids. Conventional extraction methods such as maceration and Soxhlet are common methods used to produce botanical extracts, while modern extraction methods such as ultrasonic-assisted extraction (UAE) have extensively been used in the optimization of phenolic extraction. Both maceration and Soxhlet required a longer time and more solvents for the extraction process compared to UAE. However, conventional methods have a greater advantage in terms of extract yield compared to UAE due to the large amount of sample used in the extraction process [9,10]. Various extraction techniques were used in the production of botanical extracts depending on the sample type, desired bioactivity, and targeted and non-targeted metabolites.
Maceration is a technique where the plant materials are soaked in a solvent and placed in a closed container for at least three days [11]. Ethanol, methanol, and water are commonly used solvents for the extraction of Bambusa species. Bioactive compounds such as phenolic, flavonoid, alkaloid, terpenoid, and tannin were found in various studies on Bambusa species [12,13,14,15,16]. Meanwhile, Soxhlet is a technique used widely for the extraction of thermally stable compounds. It is a continuous cycle extraction through the matrix by boiling and condensation and the sample in collected in the hot solvent [17]. Phenolic and flavonoid compounds were mainly found in the previous study [12,18]. Moreover, ultrasonic-assisted extraction uses ultrasonic waves to disrupt the plant matrix in order to accommodate the release of bioactive compounds [19]. Likewise, phenolic and flavonoid compounds were found in the extract [20]. However, there was a limited study on the use of ultrasonic-assisted to extract the plant material from Bambusa species. All the extraction methods have shown to be good extraction methods used to extract phenolic and flavonoid compounds; thus, they have a promising antioxidant activity that are contributed from those compounds. Methanol is a commonly used solvent even though it is identified as a toxic solvent. Due to its high polarity, methanol influences the extract yields and resulting antioxidant activities [21].
In the present study, different extraction methods and solvents have been used to determine the total phenolic and flavonoid content as well as antioxidant activity of Bambusa beecheyana culm extracts. The effect of the different extraction techniques on the extraction yield, total phenolic content, total flavonoid content, and the antioxidant activity of Bambusa beecheyana culm extracts was revealed for the first time in this study. UHPLC-ESI-QTOF-MS/MS analysis was conducted to identify the potential biomarkers. Moreover, in-depth bioactive compounds were characterized and isolated using HPLC, GC/MS, UV-Vis, FTIR, and NMR.
2. Results
2.1. Extraction of Bambusa beecheyana Culms
Bambusa beecheyana culms were first extracted with ethanol, methanol, and water using cold maceration to measure its extraction efficiency. p-coumaric acid and 4-methoxycinnamic acid are known as cinnamic acid derivatives. Both derivatives were known to be strong antioxidants [22]. Therefore, the determination of both compounds was significant in this experiment. However, there was no significant change in dry yield (1.06–2.00%) for p-coumaric acid (4) (0.00039 to 0.00059 mg/g), but a noticeable change in 4-methoxycinnamic acid (5) content (0.00093–0.00278 mg/g) after cold maceration extraction (see Table 1). After this, the Bambusa beecheyana culms were extracted using Soxhlet and ultrasonic-assisted extraction (UAE) with the same solvents, which were methanol, ethanol, and water, respectively (see Table 2). This increased the extract’s dry yield (1.13–8.81%), but had the same trend in p-coumaric acid (4), and 4-methoxycinnamic acid (5) content. The p-coumaric acid (4) showed a constant content of 0.00035 mg/g.
Table 1.
Yield percentage of Bambusa beecheyana extracts.
| Sample | Dry Yield (%) | p-Coumaric Acid (4) (mg/g) | %RSD | 4-Methoxycinnamic Acid (5) (mg/g) | %RSD |
|---|---|---|---|---|---|
| Maceration | |||||
| BBER * | 1.06 ± 0.0004 | 0.00059 ± 1.67 × 10−11 | 0.0000056 | 0.00278 ± 1.67 × 10−11 | 0.0000012 |
| BBMR * | 1.20 ± 0.0028 | 0.00039 ± 1.67 × 10−11 | 0.0000086 | 0.00093 ± 2.17 × 10−10 | 0.0000474 |
| BBHR * | 2.00 ± 0.0204 | ND * | ND * | ND * | ND * |
| Soxhlet | |||||
| BBES * | 4.12 ± 0.0010 | 0.00035 ± 1.67 × 10−11 | 0.0000096 | 0.00081 ± 1.17 × 10−10 | 0.0000283 |
| BBMS * | 5.09 ± 0.0011 | 0.00035 ± 6.67 × 10−11 | 0.0000039 | 0.00069 ± 2.17 × 10−10 | 0.0000065 |
| BBHS * | 4.35 ± 0.0007 | ND * | ND * | ND * | ND * |
| Ultrasonic-assisted | |||||
| BBEU20 * | 2.06 ± 0.0052 | ND * | ND * | 0.00002 ± 6.67 × 10−11 | 0.0005000 |
| BBEU40 * | 2.93 ± 0.0045 | ND * | ND * | 0.00007 ± 1.67 × 10−11 | 0.0000455 |
| BBEU60 * | 1.13 ± 0.0019 | 0.00035 ± 1.67 × 10−11 | 0.0000094 | 0.00032 ± 1.67 × 10−11 | 0.0000103 |
| BBMU20 * | 1.85 ± 0.0034 | ND * | ND * | 0.00023 ± 6.44× 10−7 | 0.1446180 |
| BBMU40 * | 2.84 ± 0.0027 | 0.00035 ± 1.67 × 10−11 | 0.0000094 | 0.00087 ± 2.17 × 10−10 | 0.0000502 |
| BBMU60 * | 2.42 ± 0.0014 | 0.00035 ± 1.67 × 10−11 | 0.00000096 | 0.00062 ± 2.17 × 10−10 | 0.0000681 |
| BBHU20 * | 6.64 ± 0.0035 | ND * | ND * | ND * | ND * |
| BBHU40 * | 8.81 ± 0.0025 | ND * | ND * | ND * | ND * |
| BBHU60 * | 7.77 ± 0.0011 | ND * | ND * | ND * | ND * |
* BBER—cold maceration ethanol extract, BBMR—cold maceration methanol extract, BBHR—cold maceration water extract; BBES—Soxhlet ethanol extract, BBMS—Soxhlet methanol extract, BBHS—Soxhlet water extract; BBEU20—20 min ultrasonic-assisted ethanol extract, BBEU40—40 min ultrasonic-assisted ethanol extract, BBEU60—60 min ultrasonic-assisted ethanol extract; BBMU20—20 min ultrasonic-assisted methanol extract, BBMU40—40 min ultrasonic-assisted methanol extract, BBMU60—60 min ultrasonic-assisted methanol extract; BBHU20—20 min ultrasonic-assisted water extract, BBHU40—40 min ultrasonic-assisted water extract, BBHU60—60 min ultrasonic-assisted water extract. ND—not detected.
Table 2.
Validation data from calibration curves of compounds (4) and (5).
| Compounds | Regression Equation | Correlation Coefficient (R2) | Linear Range (mg/mL) | Detection Limit (mg/mL) | Quantitation Limit (mg/mL) | Purity (%) |
|---|---|---|---|---|---|---|
| p-coumaric acid (4) | y = 4 × 107×−14.4 | 0.9924 | 0.000000–0.000008 | 1.10 × 10−7 | 3.32 × 10−7 | 99 |
| 4-methoxycinnamic acid (5) | y = 8 × 107× + 14.5 | 0.9835 | 0.000000–0.000008 | 5.48 × 10−7 | 1.66 × 10−7 | 96 |
The percentage of p-coumaric acid (4) was consistent with the respective extracts, although the percentage of dry yield varied among the extracts. Furthermore, the extraction methods and solvents used in these studies do not affect the percentage of the p-coumaric acid (4). The lower content of the p-coumaric acid (4) is affected by the lignification in the culms of the bamboo. In this study, the young bamboo culm was used; the consistent and lower content of p-coumaric acid (4) is due to lignification at the early stage of the development of the culm. The content of p-coumaric acid (4) will increase eventually at the later stage when the bamboo matures [23]. From the results, both p-coumaric acid (4) and 4-methoxycinnamic acid (5) were not detected in the water extracts for all three extraction techniques but were only found in the organic solvents of different polarities. To our advantage, the UAE method required only a small amount (250 mL) of solvent to extract the bamboo culms.
2.2. Isolation and Purification of p-Coumaric Acid (4), and 4-Methoxycinnamic Acid (5)
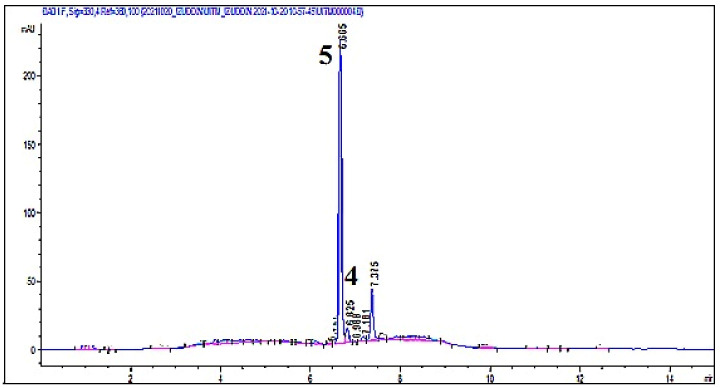
Two cinnamic acid derivatives, p-coumaric acid (4), and 4-methoxycinnamic acid (5) were isolated from the cold maceration ethanol extract (BBER) and the HPLC chromatogram as shown in Figure 1, with the retention time of 6.825 and 6.665, respectively. The HPLC chromatograms of both p-coumaric acid and 4-methoxycinnamic acid are shown in (Figures S1 and S2, Supplementary Material). Both compounds were separated using reversed-phase prep-HPLC. BBER was chosen because the peaks for both compounds were more significant than other extracts.
Figure 1.
HPLC chromatogram of cold maceration ethanol extract (BBER).
2.3. NMR Analysis of p-Coumaric Acid (4) and 4-Methoxycinnamic Acid (5)
The 1H-NMR spectrum of p-coumaric acid (4) [24,25] established the presence of four aromatic protons at δ 7.56 (2H, d, J = 8.64) and δ 6.91 (2H, d, J = 8.60). This showed the typical pattern for para-substituted aromatic moiety. Another two protons were assigned to the trans-olefinic protons at δ 7.61 (1H, d, J = 16.00) and δ 6.35 (1H, d, J = 16.00). According to its 13C-NMR spectrum, downfield signals at δ 174.0, δ 157.7, δ 144.3, and δ 130.0 were assigned carbon of the aromatic ring. The three high-field signals appearing at δ 126.2, δ 115.8, and δ 114.8 were assigned to the carbon atoms bearing carboxylic groups.
The 1H-NMR spectrum of 4-methoxycinnamic acid (5) [26] established the presence of four aromatic protons at δ 7.55 (2H, d, J = 8.56) and δ 6.91 (2H, d, J = 8.56). This showed the typical pattern for para-substituted aromatic moiety. Another two protons were assigned to the trans-olefinic proton at δ 7.61 (1H, d, J = 15.96) and δ 6.35 (1H, d, J = 15.96). A single sharp peak at δ 3.73 ppm was assigned to the methoxy. According to its 13C-NMR spectrum, a high-field signal at δ 50.6 was indicative for the presence of methoxy. Downfield signals at δ 167.4, δ 160.1, δ 144.6, and δ 130.0 were assigned carbon of the aromatic ring. The three high-field signals appearing at δ 125.8, δ 115.8, and δ 114.2 were assigned to the carbon atoms bearing carboxylic groups.
2.4. Contents of Bioactive Markers, p-Coumaric Acid (4), and 4-Methoxycinnamic Acid (5) in the Exctracts of Bambusa beecheyana
The results of regression analysis on calibration curves and detection limits are presented in Table 2. The detection limit was evaluated based on a signal-to-noise ratio of 3 (S/N = 3), the detection limit was 0.002 mg/mL. The quantitation limit was evaluated based on a signal-to-noise ratio of 10 (S/N = 10), the quantitation limit was 0.006 mg/mL.
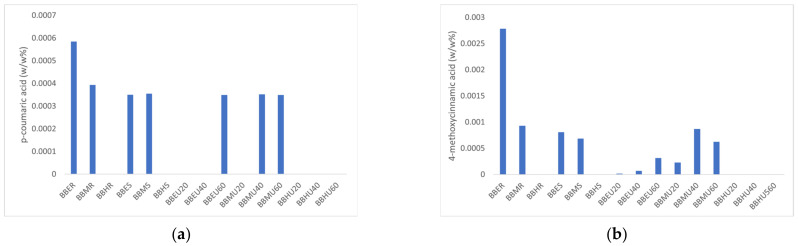
The samples were injected directly and separated under the optimum condition. The chromatogram of one of the extracts is shown in Figure 1. The HPLC chromatograms of other extracts are shown in (Figures S3–S16, Supplementary Material). The calculated content of 4- p-coumaric acid (4) and 4-methoxycinnamic acid (5) are shown in Figure 2. From the results, BBER has the highest content of compounds (4) and (5), which were 0.00059 mg/g ± 1.67 x 10−11 and 0.00278 mg/g ± 1.67 × 10−11, respectively. BBES, BBMS, BBEU60, BBMU40, and BBMU60 have the lowest content of compound (4) which is 0.00035 mg/g ± 1.67 × 10−11, 0.00035 mg/g ± 1.67 × 10−11, 0.00035 mg/g ± 1.67 × 10−11, 0.00035 mg/g ± 1.67 × 10−11, and 0.00035 mg/g ± 1.67 × 10−11 while BBEU20 has the lowest content of compound (5) which is 0.00002 mg/g ± 6.67 × 10−11. The uncertainty of the results was measured as %RSD with a confidence interval (95%) of 0.00008 for p-coumaric acid and 0.0006 for 4-methoxycinnamic acid. Both compounds were not detected in water extracts.
Figure 2.
Content of compounds (4) and (5) in the extracts of Bambusa beecheyana. The results are reported in w/w%: (a) p-coumaric acid (4); (b) 4-methoxycinnamic acid (5).
2.5. Total Phenolic Content (TPC)
The results were derived from a calibration curve (y = 0.0004x − 0.0163, R2 = 0.9996) of gallic acid (200–1000 µg/mL) and expressed in gallic acid equivalents (GAE) per gram of weight of dry extracts. From Table 3, BBMS has the highest total phenolic content which is 107.65 mg GAE/g, while 60 min BBHU60 has the lowest total phenolic content which is 27.89 mg GAE/g. From the results, methanolic extracts have the most phenolic compounds as compared to other extracts.
Table 3.
Total phenolic and flavonoid content and DPPH scavenging activity of Bambusa beecheyana extracts using different extraction methods and solvent.
| Extracts | TPC (mg GAE/g) | TFC (mg QE/g) | DPPH (IC50 µg/mL) |
|---|---|---|---|
| Cold maceration | |||
| BBER | 44.50 ± 0.03 a,b | 28.22 ± 0.03 1 | 95.93 ± 0.02 I |
| BBMR | 60.15 ± 0.03 a | 27.73 ± 0.05 1 | 63.32 ± 0.04 I |
| BBHR | 40.30 ± 0.02 b | 12.38 ± 0.04 2 | 1931.38 ± 0.01 II |
| Soxhlet | |||
| BBES | 97.25 ± 0.02 a | 40.00 ± 0.01 1 | 87.12 ± 0.03 I |
| BBMS | 107.65 ± 0.01 a | 48.89 ± 0.05 2 | 40.43 ± 0.02 I |
| BBHS | 68.95 ± 0.03 b | 22.39 ± 0.03 3 | 1670.71 ± 0.03 II |
| Ultrasonic-assisted | |||
| BBEU20 | 42.65 ± 0.04 a,d | 25.40 ± 0.02 1 | 573.56 ± 0.02 I,II |
| BBEU40 | 55.35 ± 0.01 a,b | 34.45 ± 0.04 2 | 557.20 ± 0.03 I,II |
| BBEU60 | 69.60 ± 0.03 b,c | 36.07 ± 0.02 2 | 463.54 ± 0.02 I,II |
| BBMU20 | 58.30 ± 0.01 a,b | 34.46 ± 0.03 2 | 235.71 ± 0.02 I |
| BBMU40 | 85.35 ± 0.01 c | 35.43 ± 0.01 2 | 45.01 ± 0.03 I |
| BBMU60 | 81.85 ± 0.01 c | 37.20 ± 0.01 2 | 94.27 ± 0.02 I |
| BBHU20 | 42.40 ± 0.04 a | 25.32 ± 0.03 1 | 982.13 ± 0.01 II,III |
| BBHU40 | 45.79 ± 0.07 a,d | 38.32 ± 0.01 2 | 1418.35 ± 0.03 III |
| BBHU60 | 27.89 ± 0.03 d | 17.01 ± 0.01 3 | 1279.95 ± 0.03 III |
| Positive control | |||
| Ascorbic acid | - | - | 45.50 ± 0.01 |
The experiment was done in triplicate and the data expressed as mean SEM, with . Data within rows with a common superscript alphabet are not significantly different from others chemical at TPC (p < 0.05), superscript number are not significantly different from others chemical at TFC (p < 0.05) and superscript roman numerals are not significantly different from others chemical at DPPH (p < 0.05) (two-way ANOVA, followed by Tukey’s test).
2.6. Total Flavonoid Content (TFC)
The results were derived from a calibration curve (y = 0.0197x − 0.0889, R2 = 0.9964) of quercetin (25–125 µg/mL) and expressed in quercetin equivalents (QE) per gram of weight of dry extracts. From Table 3, BBMS has the highest total flavonoid content which is 48.89 mg QE/g, while BBHR has the lowest total flavonoid content which is 12.38 mg QE/g. The results were the same trend as TPC where methanolic extracts have the highest TFC. From the results, it is shown that the TFC is significantly lower than the TPC, from which it can be suggested that flavonoid compounds were not the major class of phenolic constituents in Bambusa beecheyana extracts.
2.7. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
DPPH is a stable free radical compound and has a strong absorption at 510 nm. DPPH free radical scavenging assay is a relatively rapid and efficient method to evaluate free radical scavenging activity [27]. The decreases in absorbance of DPPH radical can be observed by changes in color from purple to yellow. This shows that there is an interaction between the antioxidants present in the extracts and free radicals from DPPH [28]. DPPH free radical scavenging assay IC50 value can be determined as follows: 4250 µg/mL, inactive; 4100–250 µg/mL, weakly active; 450–100 µg/mL, moderately active; 10–50 µg/mL, strongly active; <10 µg/mL, very strongly active [29]. Based on the results shown in Table 3, BBMS exhibits moderate antioxidant activity, while BBER, BBMR, BBES, BBMU20, BBMU40, BBMU60 exhibit weak antioxidant activity.
2.8. Potential Bioactive Markers from Bambusa beecheyana Extracts
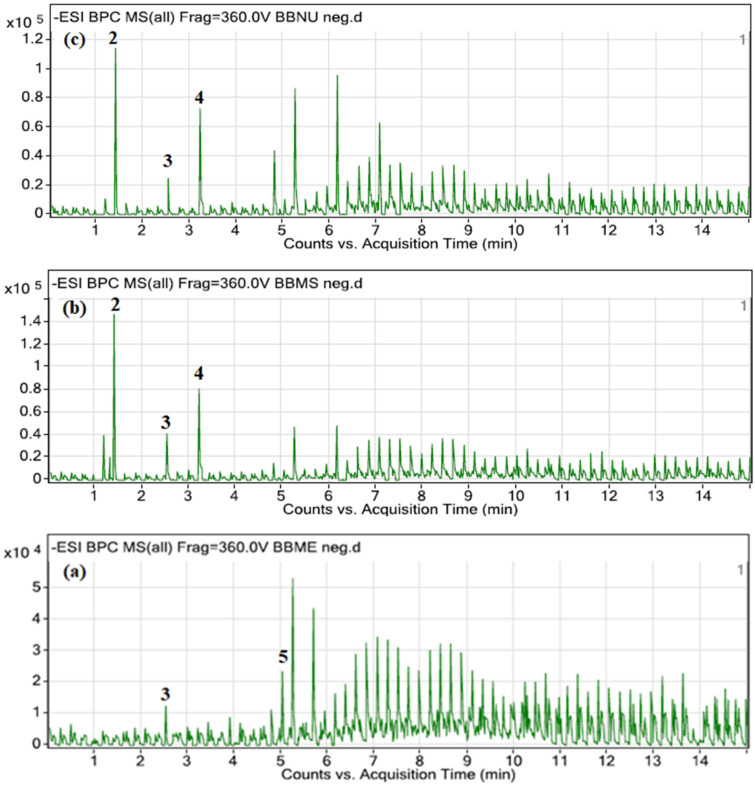
Liquid chromatography coupled with mass spectrometry (LC-MS) has been widely used to identify organic compounds, where it is more sensitive and accurate. Three extracts from each extraction method that possess the highest total phenolic and flavonoid content and DPPH radical scavenging activity were chosen to be further analyzed by using UHPLC-ESI-QTOF-MS/MS to identify the potential bioactive markers. The compounds were identified by utilizing their MS/MS spectra from the library and comparison with literature data. Table 4 shows a list of potential bioactive chemical markers found in those three extracts. Chromatograms of the three extracts are shown in Figure 3.
Table 4.
List of selected cinnamic acid derivatives tentatively found in BBMR, BBMS, and BBMU40.
| No. | RT (min) | Experimental m/z | Calculated m/z | Error (ppm) | Molecular Formula | MS/MS Product Ions |
Tentative Identification | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.036 | 193.0624 | 194.0697 | −2.27 | C10H10O4 | 146.0504, 134.8932, 106.0429 | Ferulic acid | [30] |
| 2 | 1.801 | 147.0585 | 148.0658 | −2.24 | C9H8O2 | 134.0152, 106.0415 | Cinnamic acid | [31] |
| 3 | 2.605 | 137.0179 | 138.0251 | 0.18 | C7H6O3 | 93.0263 | 2-hydroxybenzoic acid | [30] |
| 4 | 3.295 | 163.0325 | 164.0397 | −2.77 | C9H8O3 | 146.0453, 134.8934, 106.029 | p-Coumaric acid | [30] |
| 5 | 5.090 | 177.0480 | 178.0552 | −1.72 | C10H10O3 | 146.0464, 134.0172, 106.0429 | 4-methoxycinnamic acid | [32] |
Figure 3.
Total ion chromatogram (TIC) of: (a) BBMR, (b) BBMS, and (c) BBMU40.
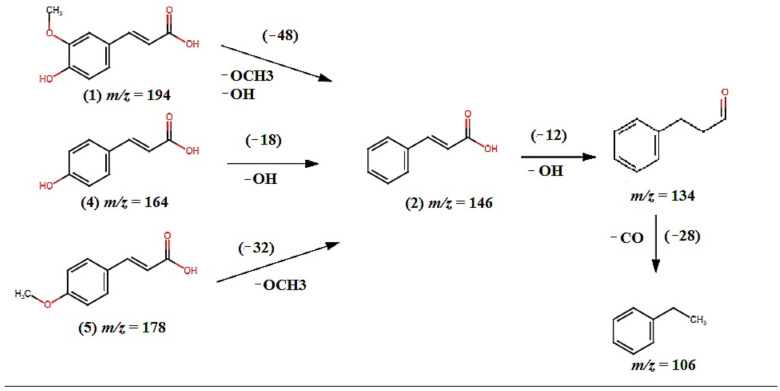
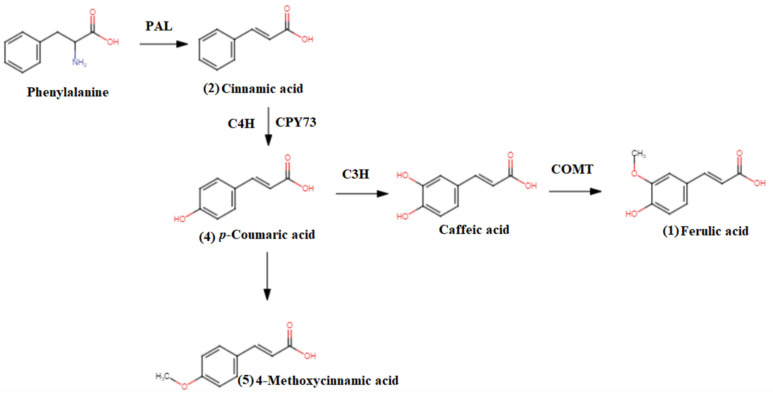
From the analysis, a total of five phenolic compounds were putatively identified based on the MS/MS data in comparison with the literature. The base peak chromatograms showed that most of the prominent peaks were attributed to the presence of those phenolic compounds. Ferulic acid (1), cinnamic acid (2), p-coumaric acid (4), and 4-methoxycinnamic acid (5) were identified as cinnamic acid and its derivatives. They have pseudomolecular ion peaks at m/z 193.0624, m/z 147.0585, m/z 163.0325, and m/z 177.0480, respectively. The fragment ion at m/z 146.0504 is due to the loss of hydroxyl and methoxy groups (Figure 4). This was proven by previous research done by [30,31,32]. Compound (3) was identified as hydroxybenzoic acid with pseudomolecular ion peak at m/z 137.0179 based on a comparison with a previous report [30]. This could support the high TPC and TFC values, and hence the potent antioxidant activities of the three Bambusa beecheyana extracts. The MS fragmentation of ferulic acid (1), p-coumaric acid (4) and 4-methoxycinnamic acid (5) matched the biosynthetic pathways (see Figure 5) of cinnamic acid, where it was part of the phenylpropanoid pathway. Phenyl ammonia lyase (PAL) converted phenylalanine to cinnamic acid. Then, cinnamic acid-4-hydroxylase hydroxylated the cinnamic acid to yield p-coumaric acid (4) [33]. From the pathways, it was clearly shown that ferulic acid (1), p-coumaric acid (4) and 4-methoxycinnmaic acid (5) were all the products of the cinnamic acid biosynthetic pathway. This could support the substantial amount of TPC and TFC values, and hence the potent antioxidant activities of the three Bambusa beecheyana extracts.
Figure 4.
Fragmentation pathways for compound ferulic acid (1), cinnamic acid (2), p-coumaric acid (4), and 4-methoxycynammic acid (5).
Figure 5.
Biosynthetic pathways of cinnamic acid.
3. Discussions
Extraction is a crucial part of extracting the bioactive compounds from plants. Conventional and modern extraction methods have been widely used in the industry to achieve common goals which are: first, extracting targeted bioactive compounds from a complex plant sample; second, increasing the selectivity of the analytical method; third, increasing bioassay sensitivity; fourth, converting bioactive compounds into a more suitable form for detection and separation; and finally, providing a strong and repeatable method that is independent of variations in the sample matrix [34]. This is the pioneering study on different extraction methods that were applied to extract Bambusa beecheyana culms.
The dry yield extracts were mainly affected by the time and solvent used in each extraction. Cold maceration and Soxhlet required a longer time and cold maceration required a larger volume of solvents. Meanwhile, UAE required a shorter amount of time and a smaller volume of solvents for extracting plant materials. In the present study, cold maceration was first used as the extraction method to obtain the bamboo culm extracts. No significant differences were observed in dry yield and p-coumaric acid (4), but there was a noticeable change in 4-methoxycinnamic acid (5) content after cold maceration extraction. The bamboo culms were extracted using Soxhlet, and ultrasonic-assisted extraction (UAE) with the same solvents increased the extract’s dry yield (1.13–8.81%), but the same trend in p-coumaric acid (4) and 4-methoxycinnamic acid (5) content. However, the dry yield percentage of UAE extracts is varied, whereas water extracts have the highest dry yield and ethanol extracts have the lowest dry yield. The same trend was reported by [35]. More polar solvents were expected to extract a higher number of hydrophilic compounds, thus resulting in a higher dry yield.
In addition, the TPC and TFC of Bambusa beecheyana extracts were determined in this study. Methanol extracts possessed the highest amount of both TPC and TFC, while water extracts possessed the lowest TPC and TFC. Despite methanol being known as a toxic solvent, it is a widely used solvent due to its ability to extract different types of chemical constituents and produce higher yields of plant extract. A previous study reported that possibly the content of water extract was mainly non-phenol compounds such as carbohydrates and terpene [36]. The results (see Figures S17 and S18, Supplementary Material) have shown a positive correlation between the TPC and scavenging activity of the extracts (R2 = 0.3294) with p < 0.05, meanwhile the correlation between TFC and scavenging activity of the extracts (R2 = 0.4538) also showed a positive correlation with p < 0.05. In addition, higher TPC is attributed to the potent antioxidant activity which has been reported in a previous study [37]. Furthermore, the antioxidant activity of the phenolic compounds was also influenced by their structural composition. This is mainly due to the capacity of phenolics to scavenge free radicals [38]. The antioxidant activity of phenolic compounds may also be related to the electron-donating ability of the carboxylic acid group. In addition, the presence of hydroxy and methoxy groups in the phenolic compounds will also enhance the free radical scavenging activity based on the number and position of both groups [39]. The ortho position in an aromatic ring paired with the presence of di-active groups, especially with the presence of the hydroxyl group, will possess high free radical scavenging activity due to the hydroxyl group having lower bond dissociation energies [40]. This trend can be seen in these extracts: BBER, BBMR, BBES, BBMS, and BBMU40 showed a potent DPPH scavenging activity with IC50 values of 95.93 ± 0.02 µg/mL, 63.32 ± 0.04 µg/mL, 87.12 ± 0.03 µg/mL, 40.43 ± 0.02 µg/mL, and 45.01 ± 0.03 µg/mL, respectively. However, secondary metabolites such as vitamin C and E and traces of mineral elements such as selenium, copper, zinc, manganese, and iron also contributed to the antioxidant activity of bamboo extract [3].
Table 5 shows the comparison of TPC, TFC, and DPPH scavenging activity of Bambusa beecheyana and various species of bamboo. The values varied based on the species and demographic of the bamboo. The study of bamboo species is still limited. From the table, Bambusa arundinacea has the highest TPC and TFC among the Bambusa genus, which is 647.76 mg GAE/g and 247.85 mg QE/g, respectively. The Bambusa genus has great potential as a medicinal plant. It has promising antioxidant activity due to the phenolic and flavonoid content which is a source of antioxidants. Further research on the potential of the Bambusa genus as a great source of antioxidants can be done in the future.
Table 5.
Comparison of TPC, TFC, and DPPH scavenging activity of Bambusa beecheyana and various species of bamboos.
| Raw Materials |
Extraction Methods | Results | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC (mg GAE/g) | TFC (mg QE/g) | DPPH (IC50 µg/mL) | |||||||||
| Et. * | Me. * | Wt. * | Et. * | Me. * | Wt. * | Et. * | Me. * | Wt. * | |||
| Bambusa beecheyana | maceration | 44.50 ± 0.03 |
60.15 ± 0.03 |
40.30 ± 0.02 |
28.22 ± 0.03 |
27.73 ± 0.05 |
12.38 ± 0.04 |
95.93 ± 0.02 |
63.32 ± 0.04 |
1931.38 ± 0.01 |
This study |
| Soxhlet | 97.25 ± 0.02 |
107.65 ± 0.01 |
68.95 ± 0.03 |
40.00 ± 0.01 |
48.89 ± 0.05 |
22.39 ± 0.03 |
87.12 ± 0.03 |
40.43 ± 0.02 |
1670.71 ± 0.03 |
||
| ultrasonic- assisted |
42.65 ± 0.04 |
58.3 ± 0.01 |
42.4 ± 0.04 |
25.4 ± 0.02 |
34.46 ± 0.03 |
25.32 ± 0.03 |
573.56 ± 0.02 |
235.71 ± 0.02 |
982.13 ± 0.01 |
||
| 55.35 ± 0.01 |
85.35 ± 0.01 |
45.79 ± 0.07 |
35.45 ± 0.04 |
35.43 ± 0.01 |
38.32 ± 0.01 |
557.2 ± 0.03 |
45.01 ± 0.03 |
1418.35 ± 0.03 |
|||
| 69.6 ± 0.03 |
81.85 ± 0.01 |
27.89 ± 0.03 |
36.07 ± 0.02 |
37.2 ± 0.01 |
17.01 ± 0.01 |
463.54 ± 0.02 |
94.27 ± 0.02 |
1279.95 ± 0.03 |
|||
| Bambusa tulda | maceration | 126 ± 3.4 | - | - | 40 ± 0.2 | - | - | 360 ± 1.4 | - | [12] | |
| Soxhlet | - | 164 ± 3.8 | - | - | 68 ± 0.9 | - | - | 404 ± 4.3 | - | ||
| Bambusa arundinacea | maceration | 14.6 | 2.79 | - | 6.71 | 2.54 | - | 273 | 964 | [13] | |
| ultrasonic-assisted | - | 647.76 ± 5.77 | - | - | 247.85 ± 3.79 | - | - | - | - | [20] | |
| Bambusa vulgaris | maceration | 44 ± 0.1 | - | 27 ± 0.5 | 22 ± 0.3 | - | 12 ± 1 | 490 ± 60 | - | 400 ± 20 | [14] |
| Bambusa nutan | maceration | - | 15.35 ± 0.55 | - | - | - | - | - | 123.45 | - | [16,18] |
| - | - | 180.45 | - | - | - | - | - | 85.81 | |||
| Soxhlet | - | 230.07 | - | - | 139.11 | - | - | 57.89 | - | ||
| Phyllostachys bambusoides | maceration | - | - | - | - | - | - | 882.08 | - | - | [41] |
| Gigantochloa levis | maceration | 2500 | - | - | - | - | - | 86.4 ± 1.05 | - | - | [42] |
* Et.—ethanol extract, Me.—methanol extract, Wt.—water.
Additionally, putative identification of potentially bioactive compounds was done by using UHPLC-ESI-QTOF-MS/MS. From the analysis, cinnamic acid and its derivatives were found in Bambusa beecheyana extract; three of its derivatives are p-coumaric acid (4), 4-methoxycinnamic acid (5), and ferulic acid (1). Two of the derivatives were successfully isolated from the extract: p-coumaric acid (4) and 4-methoxycinammic acid (5). Cinnamic acid is a phenolic compound which is known to be an antioxidant [43] and anticancer [44]. While its methoxylated derivatives are potentially hepatoprotective, antidiabetic, neuroprotective, and chemopreventive [45]. In this study, the contents p-coumaric acid (4) and 4-methoxycinnamic acid (5) isolated from the extract were not significantly high, whereas BBES had the highest content of both compounds which were 0.00059 mg/g ± 0.03 and 0.00278 mg/g ± 0.02, respectively; thus, the high TPC value and the antioxidant activity of the extracts were also contributed by other phenolic and secondary metabolites present in the extract. Therefore, further investigation can be done in the future to explore more medicinal potential of Bambusa beecheyana extracts.
4. Materials and Methods
4.1. Raw Material
The young culms of Bambusa beecheyana were collected from the plantation of Carbon Xchange Sdn. Bhd, Kuching. The plant authentication was carried out by Mr. Tinjan Anak Kuda from Jabatan Hutan and the herbarium voucher specimen (UiTM3040) was kept in UiTM Sarawak, Samarahan 2 Campus. The young culms of Bambusa beecheyana were dried under shade for 14 days, respectively, prior to mechanical grinding into a fine powder. The raw materials were stored in a chiller at 5 °C until needed.
4.2. Extraction Method of Bambusa beecheyana Culm
500 g, 300 g, and 50 g of powdered young bamboo culm were weighed. Each sample was extracted using solvents which were deionized water, ethanol, and methanol.
For cold extraction, the powdered bamboo (500 g) was soaked in ethanol (2 L) for 72 h [14]. Another 300 g of powdered young bamboo culm was weighed and extracted using Soxhlet extractor for six hours where 250 mL of solvent was used [46]. Meanwhile, using ultrasonic-assisted extract, the powdered young bamboo culm (50 g) was weighed and extracted using a bath sonicator and soaked in ethanol (2 L) for 20, 40, and 60 min. The water bath temperature was maintained at 60 °C. Then, all the extracts were filtered using vacuum filter. The extracts were then evaporated by using a rotary evaporator to separate the solvents from the samples. Finally, the crude extract was weighed. All the steps were repeated using methanol and deionized water.
4.3. Total Phenolic Content
The TPC was determined by using Folin–Ciocalteu method described by [15] with a slight modification. An amount of 5 mg of the sample was dissolved in 5 mL of ethanol. A total of 300 µL of the sample was taken out and placed into another vial. Then, 2250 µL of Folin–Ciocalteu reagent was added to it. The solution mixture was let stand for 5 min. An amount of 2250 µL of 6% of sodium carbonate was gently mixed into the vial. After 60 min, the absorbance values were measured by UV-Vis spectrophotometer (Lambda 25, Perkin Elmer, Waltham, MA, USA) with detection of 765 nm by using gallic acid (200, 400, 600, 800 and 1000 µg/mL) as the reference standard. The results were expressed as mg GAE/g extract.
4.4. Total Flavonoid Content
The TFC of the extracts was determined by using aluminum chloride colorimetric method as described by [47] with a slight modification. Briefly, 5 mL of sample was mixed with 5 mL of 2% aluminum chloride in a vial. The vial was shaken and left for 10 min. The analysis was carried out using UV-Vis spectrophotometer with the detection of 415 nm by using quercetin (25, 50, 75, 100 and 125 µg/mL) as the reference standard. The results were expressed as mg QE/g extract.
4.5. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Scavenging Activity Assay
A sample of 6 mg was dissolved in ethanol as a stock solution. It was diluted to concentrations of 50, 125, 250, 500, and 1000 µL/mL. An amount of 1 mL of 2,2-diphenyl-1-picrylhydrazyl (DPPH) was added into a vial containing a mixture of 1 mL of sample solutions at different concentrations and 3 mL of ethanol. The solution mixture was allowed to react for 60 min. The absorbance was measured at 517 nm using UV-Vis spectrophotometer with ethanol as blank, ascorbic acid as positive control, and 1 mL ethanol plus 3 mL DPPH as a negative control. The percentage of inhibition was calculated by using the following formula:
| % inhibition = [(Abc − Abs)/Abc] × 100% |
Abc is the absorbance of negative control and Abs is the absorbance of samples.
This method was adapted from [27,48] with slight modifications. The results were expressed in IC50 value.
4.6. UHPLC-ESI-QTOF-MS/MS Analysis
The UHPLC-ESI-QTOF-MS/MS analysis was carried out using a 1290 Infinity UHPLC-ESI-QTOF-MS/MS system attached to a 6550 iFunnel Quadrupole Time of Flight mass spectrometer (Agilent, Santa Clara, CA, USA). Analyte separation was carried out using a Zorbax Eclipse Plus C18 column (ø 4.6 × 100 mm, 3.5 µm, Agilent, Santa Clara, CA, USA) with a mobile phase consisting of LCMS grade water (solvent A) and acetonitrile (solvent B), flowing at 1.0 mL/min. The programmed gradient consisted of 0 min (90% A), 10 min (0% A), and 15 min (0% A). The MS analysis was done with the parameters set as follows: negative and positive ion mode, fixed collision energies of 10, 20, 30, 40, and 50 eV, a gas temperature of 200 °C and a flow rate of 14 L/min, sheath gas temperature and flow rate of 350 °C and 11 (arbitrary units), respectively. Finally, the mass resolution was set to a full scan from 100 to 1700 amu. The identification of potential bioactive markers was done by comparing the data from MS/MS with the literature.
4.7. Isolation of Bioactive Compounds Using Preparative HPLC
The crude fraction was purified on a preparative HPLC (1260 Infinity, Agilent, Santa Clara, CA, USA) system with Zorbax Eclipse XDB-C18 (Agilent, Santa Clara, CA, USA) column (ø 9.4 mm × 250 mm; 5 µm). The mobile phase consisted of water (solvent A) and acetonitrile (solvent B). The gradient elution program was as follows: 0–10 min, 10% B; 10–15 min, 100% B. The flow rate was set at 10 mL/min with UV detection at 330 nm to yield compounds (4) (2.0 mg) and (5) (4.0 mg).
4.8. Quantification and Optimization of Compounds (4) and (5)
The analysis was done by HPLC (1260 Infinity Quaternary LC VL, Agilent, Santa Clara, CA, USA), with Zorbax Eclipse Plus C18 (Agilent, Santa Clara, CA, USA) column (ø 4.6 mm × 100 mm; 3.5 µm).
4.8.1. Preparation of Standard Solutions
Standard stock solutions for compounds (4) and (5) were prepared in methanol at a concentration of 0.002, 0.004, 0.006, and 0.008 mg/mL. All the standard solutions were filtered using a membrane filter before being injected into HPLC.
The dried extracts (6 mg) were dissolved in methanol (6 mL). All the sample solutions were filtered using a 0.20 µm membrane filter before being injected into HPLC. The uncertainty of the results was measured in terms of %RSD.
4.8.2. Chromatographic Conditions
The analysis was carried out by a C18 reversed-phase column. The mobile phase was water (Solvent A) and acetonitrile (Solvent B). Before using the mobile phase, it was filtered using a membrane filter and de-aerated ultrasonically. The gradient elution program was as follows: 0–10 min, 10% B; 10–15 min, 100% B. Compounds (4) and (5) were quantified by DAD following RP-HPLC separation at 330 nm. The flow rate and injection volumes were 1.0 mL/min and 10 µL, respectively. The peaks of the analytes were confirmed by comparing the retention time and UV spectra of the compounds isolated. Quantification was carried out by the integration of the peak using a standard method. The operations were carried out at ambient temperature. This method was adapted and modified from [49].
4.9. Analytical Data
The characterization of two cinnamic acid derivatives; p-coumaric acid (4) and 4-methoxycinnamic acid (5) were done as follows: determination of UV wavelength was carried out using UV-Vis spectrophotometer (Lambda 25, Perkin Elmer, Waltham, MA, USA). The mass spectrometry analysis was carried out using GC (Clarus 680, Perkin Elmer, Waltham, MA, USA) coupled with MS (Clarus Sq 8T, Perkin Elmer, Waltham, MA, USA). The characterization of functional groups was performed on FTIR-ATR (Frontier, Perkin Elmer, Waltham, MA, USA). NMR was carried out using Bruker 400 MHz (USA).
(E)-3-(4-hydroxyphenyl)prop-2-enoic acid (4) [24,25]. Yellowish solid. UV λmax (MeOH) 330 nm; EIMS m/z 164.08; IR(ATR) νmax/cm 3347, 2925, 2853, 1631; 1H NMR (400 MHz, acetone-d6) 7.61 (d, J = 16.0 Hz, 1H), 7.56 (d, J = 8.64 Hz, 2H), 6.91 (d, J = 8.60 Hz, 2H), 6.35 (d, J = 16.0 Hz, 1H); 13C NMR (400 MHz, acetone-d6) 174.0 (C), 157.7 (C), 144.3 (CH), 130.0 (CH), 126.2 (C), 115.8 (CH), 114.8 (CH).
(E)-3-(4-methoxyphenyl)prop-2-enoic acid (5) [26]. Yellowish solid. UV λmax (MeOH) 330 nm; EIMS m/z 178.02; IR(ATR) νmax/cm 3369, 2920, 2850, 1685, 1633, 1282; 1H NMR (400 MHz, acetone-d6) 7.61 (d, J = 15.9 Hz, 1H), 7.55 (d, J = 8.56 Hz, 2H), 6.91 (d, J = 8.56 Hz, 2H), 6.35 (d, J = 15.96 Hz, 1H), 3.73 (s, 3H); 13C NMR (400 MHz, acetone-d6) 167.4 (C), 160.1 (C), 144.6 (CH), 130.0 (CH), 125.8 (C), 115.8 (CH), 114.2 (CH), 50.6 (CH3).
4.10. Statistical Analysis
Microsoft Excel 365 was used for the analysis of TPC, TFC, and DPPH scavenging activity and quantification of compounds (4) and (5). The results were expressed as the mean ± standard deviation of three replicates. Two-way ANOVA followed by Tukey’s comparison method was used to determine the significant difference between the extraction methods and the solvents used. p-values less than 0.05 were considered significant.
5. Conclusions
Extraction technique and solvent selection are important steps in sample preparation for nutraceuticals and food sources. Soxhlet and ultrasonic-assisted Bambusa beecheyana culms extracts showed an increase in dry yield but a constant p-coumaric acid (4) content as compared to the extracts from the cold maceration. A substantial amount of total phenolics (107.65 ± 0.01 mg GAE/g) and flavonoids (43.89 ± 0.05 mg QE/g) were found in the Soxhlet methanol culm extract. The extract also possesses the most potent antioxidant activity with an IC50 value of 40.43 µg/mL as compared to the positive control, ascorbic acid. From the UHPLC-ESI-QTOF-MS/MS analysis, a total of five phenolics were putatively identified and this analysis has led us to identifying cinnamic acid derivatives, p-coumaric acid (4) and 4-methoxycinnamic acid (5), which later were isolated. They were then used as markers to quantify the concentration of both compounds in all the extracts. Both compounds were found in the organic extracts (methanol and ethanol) but not in the water extracts. This indicated that organic solvents have the ability to extract different types of phytochemicals and produce higher yields of plant extract. This study highlighted the usage of the different extraction techniques and suitable solvents in bamboo culm extraction, which could benefit the application of bamboo culm extracts in preclinical research to support their medicinal benefits.
Acknowledgments
The authors would like to acknowledge Carbon Xchange (Sarawak) Sdn. Bhd., and Universiti Teknologi MARA, Sarawak Branch for technical assistance and support given throughout the research conducted on Bambusa beecheyana.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27072359/s1, Figure S1: Chromatogram of p-coumaric acid, Figure S2: Chromatogram of 4-methoxycinnamic acid, Figure S3: Chromatogram of BBMR, Figure S4: Chromatogram of BBHR, Figure S5: Chromatogram of BBES, Figure S6: Chromatogram of BBMS, Figure S7: Chromatogram of BBHS, Figure S8: Chromatogram of BBEU20, Figure S9: Chromatogram of BBEU40, Figure S10: Chromatogram of BBEU60, Figure S11: Chromatogram of BBMU20, Figure S12: Chromatogram of BBMU40, Figure S13: Chromatogram of BBMU60, Figure S14: Chromatogram of BBHU20, Figure S15: Chromatogram of BBHU40, Figure S16: Chromatogram of BBHU60, Figure S17: Correlation graph of TPC and DPPH free radical scavenging activity, Figure S18: Correlation graph of TFC and DPPH free radical scavenging activity.
Author Contributions
Contributed to writing—original draft preparation resources, M.I.N.; Conceptualization writing, supervision, and funding acquisition, V.Y.M.J.; Conceptualization writing and supervision, L.F.K.; Review and editing, T.H.C., C.H.A., J.I., R.H., S.W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds p-coumaric acid and 4-methoxycinnamic acid are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liese W., Kohl M. Bamboo—The Plants and Its Uses. Springer International Publishing; Cham, Switzerland: 2015. [Google Scholar]
- 2.Zhou B.-Z., Fu M.-Y., Xie J.-Z., Yang X.-S., Li Z.-C. Ecological functions of bamboo forest: Research and application. J. For. Res. 2005;16:143–147. [Google Scholar]
- 3.Nirmala C., Bisht M.S., Bajwa H.K., Santosh O. Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci. Technol. 2018;77:91–99. doi: 10.1016/j.tifs.2018.05.003. [DOI] [Google Scholar]
- 4.Silva M.F., Menis-Henrique M.E., Felisberto M.H., Goldbeck R., Clerici M.T. Bamboo as an eco-friendly material for food and biotechnology industries. Curr. Opin. Food Sci. 2020;33:124–130. doi: 10.1016/j.cofs.2020.02.008. [DOI] [Google Scholar]
- 5.Nirmala C., David E., Sharma M.L. Changes in nutrient components during ageing of emerging juvenile bamboo shoots. Int. J. Food Sci. Nutr. 2007;58:612–618. doi: 10.1080/09637480701359529. [DOI] [PubMed] [Google Scholar]
- 6.Nongdam P., Tikendra L. The nutritional facts of bamboo shoots and their usage as important traditional foods of northeast India. Int. Sch. Res. Not. 2014;2014:679073. doi: 10.1155/2014/679073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visuphaka K. The role of bamboo as a potential food source in Thailand; Proceedings of the 2nd International Bamboo Workshop; Hangzhou, China. 6–14 October 1985. [Google Scholar]
- 8.Satya S., Bal L.M., Singhal P., Naik S.N. Bamboo shoot processing: Food quality and safety aspect (a review) Trends Food Sci. Technol. 2010;21:181–189. doi: 10.1016/j.tifs.2009.11.002. [DOI] [Google Scholar]
- 9.Yılmaz F.M., Görgüç A., Uygun Ö., Bircan C. Steviol glycosides and polyphenols extraction from Stevia rebaudiana Bertoni leaves using maceration, microwave-, and ultrasound-assisted techniques. Sep. Sci. Technol. 2021;56:936–948. doi: 10.1080/01496395.2020.1743311. [DOI] [Google Scholar]
- 10.Zannou O., Pashazadeh H., Ibrahim S.A., Koca I., Galanakis C.M. Green and Highly Extraction of Phenolic Compounds and Antioxidant Capacity from Kinkeliba (Combretum micranthum G. Don) by Natural Deep Eutectic Solvents (NADESs) using Maceration, Ultrasound-assisted Extraction and Homogenate-assisted Extraction. Arab. J. Chem. 2022;15:103752. doi: 10.1016/j.arabjc.2022.103752. [DOI] [Google Scholar]
- 11.Abubakar A.R., Haque M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020;12:1–10. doi: 10.4103/jpbs.JPBS_175_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pande H., Kumar B., Varshney V.K. Phenolic composition and antioxidant capacity of biomass residue (leaves) generated from Bambusa tulda plantations. Waste Biomass Valorization. 2017;8:2349–2362. doi: 10.1007/s12649-016-9683-1. [DOI] [Google Scholar]
- 13.Macwan C., Patel H.V., Kalia K. A comparative evaluation of in vitro antioxidant properties of Bamboo Bambusa arundinacea leaves extracts. J. Cell Tissue Res. 2010;10:2413. [Google Scholar]
- 14.Kong C.K., Tan Y.N., Chye F.Y., Sit N.W. Nutritional composition and biological activities of the edible shoots of Bambusa vulgaris and Gigantochloa ligulata. Food Biosci. 2020;36:100650. doi: 10.1016/j.fbio.2020.100650. [DOI] [Google Scholar]
- 15.Wani M.A., Prasad S., Prakash O. Qualitative phytochemical analysis of various parts of bamboo (Bambusa balcooa) for possible therapeutic usages in bovine reproductive disorders. J. Pharmacogn. Phytochem. 2019;8:217–221. [Google Scholar]
- 16.Tripathi Y.C., Jhumka Z., Anjum N. Evaluation of total polyphenol and antioxidant activity of leaves of Bambusa nutans and Bambusa vulgaris. J. Pharm. Res. 2015;9:271–277. [Google Scholar]
- 17.Lai M., Lü B. Comprehensive Sampling and Sample Preparation. Academic Press; Cambridge, MA, USA: 2012. Extraction techniques and applications: Biological, medical and environmental, forensics. [Google Scholar]
- 18.Pande H., Kumar B., Varshney V.K. HPLC-ESI-QTOF-MS analysis of phenolic compounds, antioxidant capacity and α-glucosidase inhibitory effect of Bambusa nutans leaves. Indian J. Chem. 2018;57B:988–996. [Google Scholar]
- 19.Idris F.N., Mohd Nadzir M. Comparative studies on different extraction methods of Centella asiatica and extracts bioactive compounds effects on antimicrobial activities. Antibiotics. 2021;10:457. doi: 10.3390/antibiotics10040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haldipur A.C., Srividya N. A comparative evaluation of in vitro antihyperglycemic potential of Bamboo seed rice (Bambusa arundinacea) and Garudan samba (Oryza sativa): An integrated metabolomics, enzymatic and molecular docking approach. J. Cereal Sci. 2021;99:103200. doi: 10.1016/j.jcs.2021.103200. [DOI] [Google Scholar]
- 21.Sultana B., Anwar F., Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–2180. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunia-Krzyżak A., Słoczyńska K., Popiół J., Koczurkiewicz P., Marona H., Pękala E. Cinnamic acid derivatives in cosmetics: Current use and future prospects. Int. J. Cosmet. Sci. 2018;40:356–366. doi: 10.1111/ics.12471. [DOI] [PubMed] [Google Scholar]
- 23.Tsuyama T., Shimada N., Motoda T., Matsushita Y., Kijidani Y., Fukushima K., Kamei I. Lignification in developing culms of bamboo Sinobambusa tootsik. J. Wood Sci. 2017;63:551–559. doi: 10.1007/s10086-017-1651-2. [DOI] [Google Scholar]
- 24.Ferreira J.C., Reis M.B., Coelho G.D., Gastaldello G.H., Peti A.P.F., Rodrigues D.M., Bastos J.K., Campo V.L., Sorgi C.A., Faccioli L.H., et al. Baccharin and p-coumaric acid from green propolis mitigate inflammation by modulating the production of cytokines and eicosanoids. J. Ethnopharmacol. 2021;278:114255. doi: 10.1016/j.jep.2021.114255. [DOI] [PubMed] [Google Scholar]
- 25.Shafiq N., Arshad U., Yaqoob N., Khan J., Khan A., Saleem K., Rashid M., Rafiq N., Ahmad R., Javaid I., et al. Structure-based experimental and theoretical analysis of Ricinus communis for their HepG2 human carcinoma cell line inhibitors. Process Biochem. 2021;104:152–160. doi: 10.1016/j.procbio.2021.03.012. [DOI] [Google Scholar]
- 26.Rodrigues M.P., Tomaz D.C., de Souza L.A., Onofre T.S., de Menezes W.A., Almeida-Silva J., Suarez-Fontes A.M., de Almeida M.R., da Silva A.M., Bressan G.C., et al. Synthesis of cinnamic acid derivatives and leishmanicidal activity against Leishmania braziliensis. Eur. J. Med. Chem. 2019;183:111688. doi: 10.1016/j.ejmech.2019.111688. [DOI] [PubMed] [Google Scholar]
- 27.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 28.Bandonienė D., Murkovic M., Pfannhauser W., Venskutonis P., Gruzdienė D. Detection and activity evaluation of radical scavenging compounds by using DPPH free radical and on-line HPLC-DPPH methods. Eur. Food Res. Technol. 2002;214:143–147. doi: 10.1007/s00217-001-0430-9. [DOI] [Google Scholar]
- 29.Phongpaichit S., Nikom J., Rungjindamai N., Sakayaroj J., Hutadilok-Towatana N., Rukachaisirikul V., Kirtikara K. Biological activities of extracts from endophytic fungi isolated from Garcinia plants. FEMS Immunol. Med. Microbiol. 2007;51:517–525. doi: 10.1111/j.1574-695X.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- 30.Ali A., Bashmil Y.M., Cottrell J.J., Suleria H.A., Dunshea F.R. LC-MS/MS-QTOF Screening and Identification of Phenolic Compounds from Australian Grown Herbs and Their Antioxidant Potential. Antioxidants. 2021;10:1770. doi: 10.3390/antiox10111770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B., Fan S., Hu J., Ma Y., Feng Y., Wang F., Wang X., Niu L. Phytochemical Analysis Using UPLC-MS/MS Combined with Network Pharmacology Methods to Explore the Biomarkers for the Quality Control of Lingguizhugan Decoction. Evid.-Based Complementary Altern. Med. 2021;2021:7849032. doi: 10.1155/2021/7849032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X., Zhong X.J., Zhou N., Cai N., Xu J.H., Wang Q.B., Li J.J., Liu Q., Lin P.C., Shang X.Y. Rapid characterizaiton of chemical constituents of the tubers of Gymnadenia conopsea by UPLC–Orbitrap–MS/MS Analysis. Molecules. 2020;25:898. doi: 10.3390/molecules25040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahn R.A., Durst F. Function and evolution of plant cytochrome P450. Recent Adv. Phytochem. 2000;34:151–190. [Google Scholar]
- 34.Azmir J., Zaidul IS M., Rahman M.M., Sharif K.M., Mohamed A., Sahena F., Jahurul M.H.A., Ghafoor K., Norulaini N.A.N., Omar AK M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013;117:426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- 35.Sim Y.Y., Ong WT J., Nyam K.L. Effect of various solvents on the pulsed ultrasonic assisted extraction of phenolic compounds from Hibiscus cannabinus L. leaves. Ind. Crops Prod. 2019;140:111708. doi: 10.1016/j.indcrop.2019.111708. [DOI] [Google Scholar]
- 36.Do Q.D., Angkawijaya A.E., Tran-Nguyen P.L., Huynh L.H., Soetaredjo F.E., Ismadji S., Ju Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buzgaia N., Lee S.Y., Rukayadi Y., Abas F., Shaari K. Antioxidant Activity, α-Glucosidase Inhibition and UHPLC–ESI–MS/MS Profile of Shmar (Arbutus pavarii Pamp) Plants. 2021;10:1659. doi: 10.3390/plants10081659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minatel I.O., Borges C.V., Ferreira M.I., Gomez H.A.G., Chen C.Y.O., Lima G.P.P. Phenolic Compounds: Biological Activity. IntechOpen; London, UK: 2017. Phenolic compounds: Functional properties, impact of processing and bioavailability; pp. 1–24. [Google Scholar]
- 39.Chen J., Yang J., Ma L., Li J., Shahzad N., Kim C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020;10:2611. doi: 10.1038/s41598-020-59451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bendary E., Francis R.R., Ali HM G., Sarwat M.I., El Hady S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013;58:173–181. doi: 10.1016/j.aoas.2013.07.002. [DOI] [Google Scholar]
- 41.Kim H.-J., Shin H.J. Screening of Scalp Cosmetic Material of Domestic Phyllostachys bambusoides Stem Extract; Proceedings of the 2020 KSBB Fall Meeting and International Symposium: Hybrid Conference; Seoul, South Korea. 21–23 October 2020. [Google Scholar]
- 42.Ilham A.M., Vimala S., Rashih A.A., Rohana S., Jamaluddin M., Juliza M. Antioxidant and antityrosinase properties of Malaysian bamboo leaf extracts. J. Trop. For. Sci. 2008;20:123–131. [Google Scholar]
- 43.De P., Baltas M., Bedos-Belval F. Cinnamic acid derivatives as anticancer agents-a review. Curr. Med. Chem. 2011;18:1672–1703. doi: 10.2174/092986711795471347. [DOI] [PubMed] [Google Scholar]
- 44.Sova M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini-Rev. Med. Chem. 2012;12:749–767. doi: 10.2174/138955712801264792. [DOI] [PubMed] [Google Scholar]
- 45.Rychlicka M., Rot A., Gliszczyńska A. Biological Properties, Health Benefits and Enzymatic Modifications of Dietary Methoxylated Derivatives of Cinnamic Acid. Foods. 2021;10:1417. doi: 10.3390/foods10061417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He W., Jiang S., Zhang Q., Pan M. Isolation and characterization of cellulose nanofibers from Bambusa rigida. BioResources. 2013;8:5678–5689. doi: 10.15376/biores.8.4.5678-5689. [DOI] [Google Scholar]
- 47.Chukwumah Y., Walker L.T., Verghese M. Peanut skin color: A biomarker for total polyphenolic content and antioxidative capacities of peanut cultivars. Int. J. Mol. Sci. 2009;10:4941–4952. doi: 10.3390/ijms10114941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mensor L.L., Menezes F.S., Leitão G.G., Reis A.S., Santos TC D., Coube C.S., Leitão S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 49.Zu Y., Li C., Fu Y., Zhao C. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J. Pharm. Biomed. Anal. 2006;41:714–719. doi: 10.1016/j.jpba.2005.04.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.