The antibiotic fosfomycin has been used in Japan for 21 years, and although it can be used as a single agent, it is more active when used in combination with various other antibiotics (3). Fosfomycin enters the cells of fosfomycin-susceptible bacteria by means of two different transport uptake systems: GlpT and UhpT (10).
In our studies, we measured the MICs of fosfomycin for 412 randomly selected Pseudomonas aeruginosa clinical isolates, collected in 1996 from across Japan, and investigated a new mechanism of resistance found among fosfomycin-resistant isolates.
Fosfomycin MICs were determined by agar dilution with an inoculum of 500 cells to the nutrient agar surface (Difco Laboratories) (4, 9). The enzymatic inactivation of fosfomycin using crude extracts with and without cofactor (40 mM ATP) was determined by measuring residual fosfomycin (9). The transfer frequency of fosfomycin resistance was determined as described previously (9), using P. aeruginosa PAO2142Rp (8) as recipient.
The distribution of MICs of fosfomycin for the 412 isolates of P. aeruginosa exhibited a cluster of the majority of strains centered around an MIC value of 6.25 μg/ml and a second significant cluster centered at MICs of more than 12,800 μg/ml. Compared with the 1975 report of Goto et al. (4), our results suggested that the susceptibility of P. aeruginosa to fosfomycin has remained almost unchanged for 21 years.
In 1977, although 65% of 60 fosfomycin-resistant gram-negative isolates transferred some other demonstrable antibiotic resistance to Escherichia coli K-12 in 1977 (2), there were no occurrences of the transfer of fosfomycin resistance. Of the 67 fosfomycin-resistant isolates in our study with MICs greater than 800 μg/ml, none transferred this resistance to the recipient strain PAO2142Rp. Thus, our data suggest that transferable plasmid-encoded fosfomycin resistance has not emerged even in recent Japanese P. aeruginosa isolates.
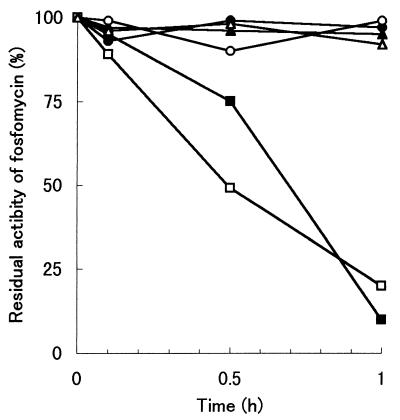
Crude extracts from two fosfomycin-resistant isolates, CU252 and CU358, completely inactivated fosfomycin but only in the presence of ATP (Fig. 1).
FIG. 1.
Inactivation of fosfomycin by crude extracts of P. aeruginosa CU252 and CU358 with and without ATP. ●, heated crude extract (CU252); ■, crude extract (CU252) in the presence of ATP; ▴, crude extract (CU252) without ATP; ○, heated crude extract (CU358); □, crude extract (CU358) in the presence of ATP; ▵, crude extract (CU358) without ATP.
Fosfomycin resistance in clinical isolates is caused mainly by an alteration of the chromosomally encoded GlpT transport system. The plasmid-mediated fosfomycin glutathione S-transferase genes fosA and fosB, found in only a low percentage of strains (1, 6, 7), catalyze the addition of glutathione to fosfomycin (10).
The fosfomycin inactivation mechanism reported here appears to be new, as it was nontransferable and ATP dependent. It will be interesting to compare it with the mechanism in fosC and in fomA and fomB cloned into E. coli from fosfomycin-producing Pseudomonas syringae and Streptomyces wedmorensis (5), respectively. The correlation between fosfomycin resistance of P. aeruginosa and the mechanism of resistance, including enzyme characterization, will be the subject of our future studies.
Acknowledgments
This study was supported by a grant from Ministry of Health and Welfare, Japan, 1999, for molecular characterization of antibiotic resistance and development of methods for the rapid detection of drug-resistant bacteria.
REFERENCES
- 1.Arca P, Reguera G, Hardisson C. Plasmid-encoded fosfomycin resistance in bacteria isolated from the urinary tract in a multicentre survey. J Antimicrob Chemother. 1997;40:393–399. doi: 10.1093/jac/40.3.393. [DOI] [PubMed] [Google Scholar]
- 2.Baquero F, Lopez-Brea M, Valls A, Canedo T. Fosfomycin and plasmidic resistance. Chemotherapy. 1977;23(Suppl. 1):133–140. doi: 10.1159/000222039. [DOI] [PubMed] [Google Scholar]
- 3.Gatermann S, Schulz E, Marre R. The microbiological efficacy of combination of fosfomycin and vancomycin against clinically relevant staphylococci. Infection. 1989;17:35–37. doi: 10.1007/BF01643498. [DOI] [PubMed] [Google Scholar]
- 4.Goto S, Dogasaki I, Kaneko Y, Ogawa M, Takita T, Kuwahara S. In vitro and in vivo antibacterial activity of fosfomycin. Chemotherapy. 1975;23:1653–1661. [Google Scholar]
- 5.Kobayashi S, Kuzuyama T, Seto H. Characterization of the fomA and fomB gene products from Streptomyces wedmorensis, which confer fosfomycin resistance on Escherichia coli. Antimicrob Agents Chemother. 2000;44:647–650. doi: 10.1128/aac.44.3.647-650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Hara K. Two different types of fosfomycin resistance in clinical isolates of Klebsiella pneumoniae. FEMS Microbiol Lett. 1993;114:9–16. doi: 10.1111/j.1574-6968.1993.tb06543.x. [DOI] [PubMed] [Google Scholar]
- 7.O'Hara K, Kotake J, Omiya K, Kono M. Fosfomycin-inactivating enzyme from clinically isolated Pseudomonas aeruginosa. Chemotherapy. 1988;36:905–910. [Google Scholar]
- 8.O'Hara K, Kawabe T, Taniguchi K, Ohnuma M, Nakagawa M, Naitou Y, Sawai T. A simple assay for determining aminoglycoside inactivation in intact cells of Pseudomonas aeruginosa. Microbios. 1997;90:177–186. [PubMed] [Google Scholar]
- 9.Shimizu M, Nonomiya T, Shigenobu F, O'Hara K, Sawai T. Fosfomycin resistance in Escherichia coli in Japan. J Antibiot. 1998;51:889–892. doi: 10.7164/antibiotics.51.889. [DOI] [PubMed] [Google Scholar]
- 10.Suárez J E, Mendoza M C. Plasmid-encoded fosfomycin resistance. Antimicrob Agents Chemother. 1991;35:791–795. doi: 10.1128/aac.35.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]