Abstract
Combating antimicrobial resistance (AMR) is an on-going global grand challenge, as recognized by several UN Sustainable Development Goals. Silver nanoparticles (Ag NPs) are well-known for their efficacy against antimicrobial resistance, and a plethora of green synthesis methodologies now exist in the literature. Herein, this review evaluates recent advances in biological approaches for Ag NPs, and their antimicrobial potential of Ag NPs with mechanisms of action are explored deeply. Moreover, short and long-term potential toxic effects of Ag NPs on animals, the environment, and human health are briefly discussed. Finally, we also provide a summary of the current state of the research and future challenges on a biologically mediated Ag-nanostructures-based effective platform for alleviating AMR.
Keywords: antimicrobial resistance, silver NP’s, green synthesis, environmental
1. Introduction
Microbial infections cause a variety of chronic diseases and account for around 10 million deaths each year, most of which occur in tropical nations. The developed world is not immune either. Antibiotics have been utilized to treat bacterial infections because of their cost-effectiveness and potent results. Antibiotic abuse and overuse, on the other hand, have aided the development and spread of resistance mechanisms among bacteria, resulting in the creation of multidrug-resistant (MDR) microorganisms. The annual cost of multidrug-resistant (MDR) microorganisms in the United States is estimated to be around $20 billion [1,2]. Clinicians have no effective alternative to treat infected patients due to the development of multidrug-resistant bacteria and super bugs. Antibiotic overuse promotes the emergence and evolution of strains with a wide range of genotypic and phenotypic characteristics, jeopardizing antibiotics’ therapeutic value. The ability to form biofilms is one of the most efficacious mechanisms available, which is associated with about 65–80% of human infections. Bacteria can form a biofilm on a surface and proliferate as a colony where cells aggregate together and surround themselves with a self-produced extracellular matrix that makes cells 100–1000 times less susceptible to antibiotics than planktonic cells [3]. Antimicrobial drug resistance has been identified by the World Health Organization as one of the top three global public health problems. According to WHO data, nearly 80% of MDR or XDR bacteria are caused by antibiotic abuse and overuse, and these infections are linked to serious side effects [4]. As a result, alternative therapeutic approaches for microbial pathogens are required. Nanoparticles have vital biological uses, such as antibacterial, drug delivery, and bioimaging, because of their unique physicochemical characteristics, such as high specific surface area, optical properties, antimicrobial activity, catalytic activity, electronic properties, and magnetic properties [5,6,7,8,9]. Silver exhibits strong toxicity against a wide range of microbes (anti-bacterial applications). Silver compounds are known to be effective against both aerobic and anaerobic bacteria. Silver precipitates bacterial cellular proteins and blocks the microbial respiratory chain system. Before silver nanoparticles, silver nitrate was used as an effective antibacterial agent that follows various mechanisms of action, including direct penetration inside the bacteria, inhibition of DNA by interaction with bacterial membrane proteins, and an attack on an electron transport chain in mitochondria [5,10,11].
Although there have been numerous papers written on this topic in the past [5,10,12] and recently [10], this article presents the most up-to-date information with year-by-year comparisons that are no more than three years old. Furthermore, it presents a toxicity evaluation of Ag NPs with crucial future difficulties. As a result, given its comprehensive scope and up-to-date analysis, this article will be extremely useful and serve as an effective single platform for readers and researchers interested in the subject of nanotechnology.
Methods of AgNPs Synthesis
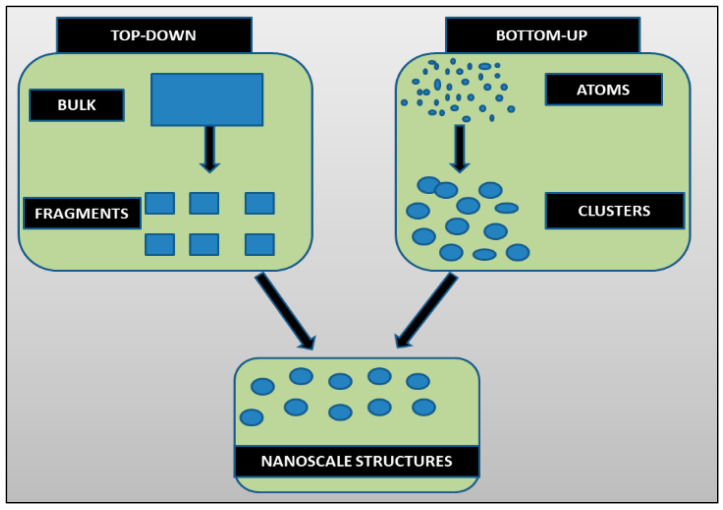
Traditionally, two approaches are used to synthesize nanomaterials: i. ‘Top-down’ and ii. ‘Bottom-up’ (Figure 1). The top-down approach generates nanoparticles using size reduction of bulk materials techniques, i.e., pulse laser ablation, pulse wire discharge method evaporation—condensation, ball milling, etc. In the bottom-up approach, chemical and biological methods are used to synthesize NPs by a self-assembly phenomenon of atoms to new nuclei that grow in the particles of nanoscale dimensions [11].
Figure 1.
“Top-down” and “bottom-up” approaches for the synthesis of nanoparticles.
In physical processes, the synthesis of AgNPs is achieved by evaporation–condensation method, which operates tube furnace at atmospheric pressure. AgNPs are also being produced with laser ablation of metallic bulk materials in solution. The biggest advantage of laser ablation over the conventional method is the removal of chemical reagents in solutions. Hence, pure colloids can be created by this technique, which will be valuable for additional applications [5]. Furthermore, for the preparation of Ag NPs, physical vapor deposition methods such as sputtering are considered to be a green and safe option [12,13].
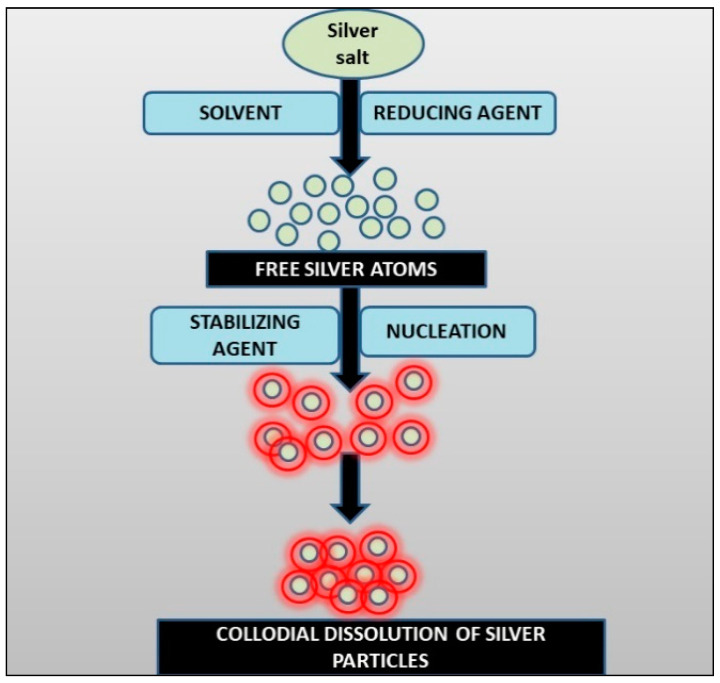
Chemical reduction of metal salt solutions is one of the most common methods for the synthesis of AgNPs [5]. For the most part, the chemical synthesis for AgNPs has three fundamental components: i. reducing agents; ii. precursors; and iii. stabilizing/capping agents. The resultant nanoparticles tend to nucleate and grow to produce a colloidal solution (Figure 2) [14].
Figure 2.
Schematic of Ag-NPs synthesis by the chemical reduction method.
The use of high temperatures, hazardous chemicals, and pressure, and the formation of dangerous by-products, are all downsides of chemical-physical approaches for AgNPs synthesis, necessitating the search for safer alternatives. The biological production of NPs is gaining importance in this way. This list of disadvantages is continuous with difficult separation procedures, high pressure, and energy requirement. Their large-scale production is difficult, chemical purification of nanoparticles is needed, and controlling the accumulating parameters is also hard to achieve [15,16].
To address these issues, scientists have been looking for greener alternatives, such as naturally occurring sources and their products, that can be utilized to synthesize NPs. The development of biological methods for the synthesis of Ag-NPs is becoming a major branch of nanotechnology. The bioreduction of the Ag+ ions, which utilizes nitrate, can be attained by metabolic processes. Nitrate is consumed as a main source of nitrogen by cyanobacteria, as described in the following equations:
| NO3− + 2H+ + 2e− = NO2− + H2O |
| NO2− + 8H+ + 6e− = NH4+ + 2H2O |
It is suggested that Ag+ ions could also be reduced by an intracellular electron donor [5].
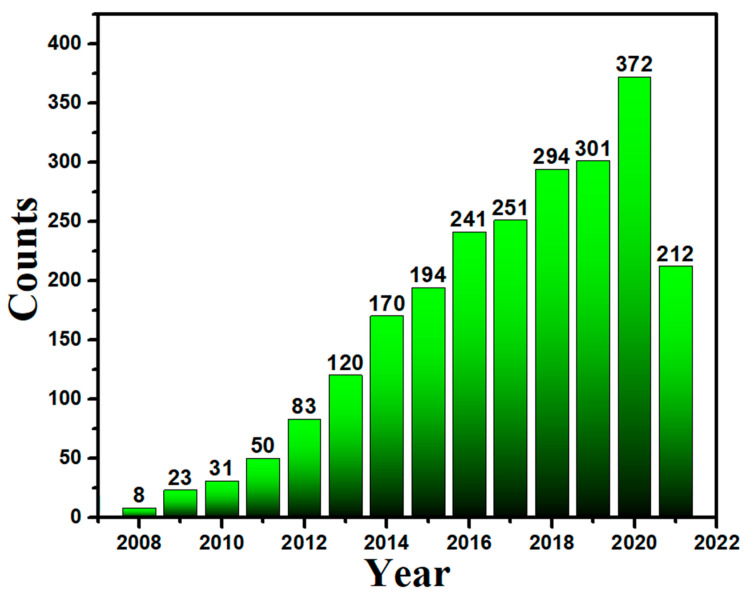
The biosynthesis approach is cost-efficient and environmentally friendly. The purpose of this review article is to disseminate information about the significance of biosynthesized AgNPs to mitigate the contemporary AMR issue in the pharmaceutical industry. Moreover, this study also involves the detailed concepts of biological approaches and explores the advantages of green approaches over the physical or chemical approaches, which foster one to follow these cost-effective and benign biological approaches. We also collected paper publication data from PubMed (Figure 3), which indicates the continuous increase of interest of researchers in biological approaches for Ag NPs.
Figure 3.
A histogram shows the frequency of paper publication on biological approaches for Ag NPs.
2. Synthesis of Ag-NPs via Biological Methods
Green synthesis can be divided into two categories:
-
(a)
The use of microorganisms such as yeasts, bacteria, fungus, and actinomycetes in the production of chemicals; and
-
(b)
Extract of different parts of plants.
The next sections explain biological synthesis employing bacteria, fungus, and plant extracts.
2.1. AgNPs Synthesis via Bacteria
Bacteria can reduce metal ions and can produce NPs. Therefore, there are numerous bacterial species that are being exploited for this purpose [17], for example:
Geobacter spp., Arthrobacter gangotriensis;
Bacillus cereus, Antarctica Bacillus amyloliquefaciens;
Corynebacterium sp. SH09, and Shewanellaoneidensis;
Pseudomonas proteolytica, Aeromonas sp. SH10 Phaeocystis;
Escherichia coli, Lactobacillus case;
Bacillus cecembensis, Enterobacter cloacae, and Bacillus indicus.
There are two methods for Ag NPs synthesis using microbes: i. intracellular method and ii. extracellular method. The synthesis of Ag NPs via different bacterial species is summarized in Table 1.
Table 1.
Synthesis of AgNPs from various bacterial species.
| Year | S/N | Bacterial Species | Method | Size (nm) | Morphology | References |
|---|---|---|---|---|---|---|
| 2021 | 1. | Serratia nematodiphila | Intracellular | 10–31 | Spherical | [18] |
| 2. | Cyanobacteria Spirulina platensis and actinobacteria Streptomyces spp. 211A | Intracellular | 7–15 | - | [19] | |
| 3. | Klebsiella pneumonia | Extracellular | 1–6 | Spherical | [20] | |
| 4. | Bacillus indicus | Extracellular | - | Spherical | [21] | |
| 5. | Bacillus subtilis (MTCC441) | Intracellular | 10–100 | Spherical | [22] | |
| 6. | Endosymbiotic Bacterium | Intracellular | 10–60 | Cubic, spherical, hexagonal, crystalline, and oval | [23] | |
| 7. | Penicillium glabrum (MTCC 1985) | Extracellular | 26–32 | Spherical | [24] | |
| 8. | Bacillus strain CS 11 | Extracellular | 45 ± 0.15 | FCC, Spherical | [25] | |
| 2020 | 9. | Marine Ochrobactrum sp. | Intracellular | 35–85 | Spherical | [26] |
| 10. | Exiguobacterium mexicanum | Extracellular | 5–40 | - | [27] | |
| 11. | Actinobacteria | Intracellular | 5–50 | Spherical | [28] | |
| 12. | Lactobacillus strains | Intracellular | 15–500 | Cluster triangular, hexagonal, and crystalline | [29] | |
| 13. | Pseudomonas proteolytica, Bacillus cecembensis | Extracellular | 6–13 | Spherical | [30] | |
| 14. | Rhodococcus sp. | Intracellular | 5–50 | Spherical | [26] | |
| 15. | Bacillus sp. | Extracellular | 5–15 | Crystalline | [31] | |
| 16. | Bacillus licheniformis | Extracellular | 8–63 | Spherical | [32] | |
| 17. | Shewanellao neidensis | Intracellular | 4 ± 1.5 | Spherical | [33] | |
| 18. | Gluconacetobacter xylinus | Intracellular | 40–100 | Spherical | [34] | |
| 19. | Bacillus subtilis | Extracellular | 5–60 | Spherical | [35] | |
| 20. | Nocardiopsissp.MBRC-1 | Intracellular | 45 ± 0.15 | Spherical | [36] | |
| 21. | Pseudomonas stutzeri AG259 | Extracellular | 35–200 | Cluster equilateral triangular, and hexagonal | [37] | |
| 2019 | 22. | Klebsiella pneumonia, Escherichia coli, and Enterobacter cloacae | Extracellular | 28–122 | Spherical | [38] |
| 23. | Aeromonas sp. THG-FG1.2 | Extracellular | 8–16 | fcc spherical | [39] | |
| 24. | Escherichia coli DH5a | Extracellular | 10–100 | Spherical | [40] | |
| 25. | Pseudomonas putida NCIM 2650 | Extracellular | 70 | Spherical | [41] | |
| 26. | Vibrio alginolyticus | Intracellular | 50–100 | Crystalline, spherical | [42] | |
| 27. | Lactobacillus casei | Intracellular | 20–50 | Spherical | [43] | |
| 28. | Deinococcus radiodurans | Extracellular | 4–50 | Spherical | [44] | |
| 29. | Bacillus pumilus, B. persicus, and B. licheniformis | Extracellular | 77–92 | Spherical | [41] | |
| 30. | Staphylococcus aureus | Extracellular | 160–180 | Spherical | [45] |
2.1.1. Intracellular Method and Mechanism
The intracellular approach involves the deposition of silver within the cell, which initiates the synthesis of Ag NPs while maintaining microbial growth. The live cells with nanoparticles are retrieved after the bacteria have grown to their maximum potential. To secrete the NPs, the collected cell requires special treatment. Bacterial extracellular secretions are isolated and employed in the synthesis mechanism during the extracellular process. Microorganisms that are resistant to silver ions are more likely to make AgNPs, and the mechanism of resistance differs depending on the organism [46].
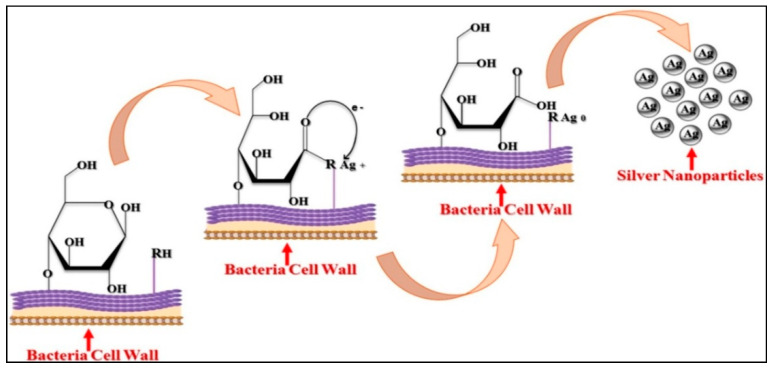
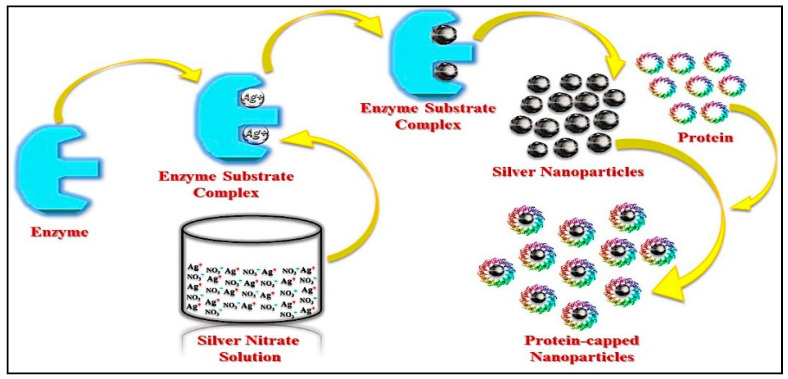
To exemplify intracellular synthesis, LactobacillusA09, where Ag+ reduction took place on the bacterial cell surface, was used to explain intracellular synthesis. Lactobacillus A09 bacteria have many anionic surface groups on their cell walls, which act as Ag-NPs ions biosorption sites. The pH of the solution gradually fell after Ag+ adsorption on Lactobacillus A09, and competition between proton and silver, which binds to negatively charged sites, occurred. Due to an increase in pH, the monosaccharide rings in bacterial cell walls are disrupted, and the oxidized monosaccharide rings are converted to open-chain aldehydes. Negatively charged Ag+ adsorption sites on the cell surface originate from the dissociation of protons from protonated anionic functional groups (–RH). While forming the aldehyde group, the two electrons are released from alcohol, which can reduce Ag+ ions to elemental Ag0. Few of the steps in the bacterial production of Ag-NPs are mediated by the opening of the glucose ring (Figure 4) [47].
Figure 4.
Non-enzymatic intracellular synthesis of AgNPs by bacteria [47].
2.1.2. Extracellular Method and Mechanism
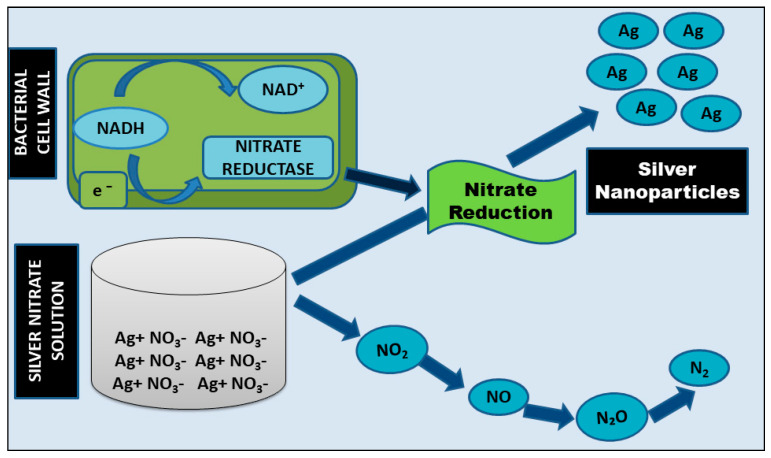
Streptomyces sp.LK3 and Bacillus licheniformis are two bacteria that generate Ag-NPs by reducing silver ion (Ag+) via an extracellular process driven by reduced nicotinamide adenine dinucleotide (NADH)-dependent nitrate reductase. Nitrate ions (NO3) in silver nitrate (AgNO3) are reduced to nitrite (NO2) by taking two protons and then releasing two electrons and water during the reduction process. Elemental silver is formed when the electrons liberated in this process are transferred to the silver (Ag0). This method may be reliant on electron transport channels and enzymatic metal reduction activities (Figure 5) [48].
Figure 5.
Extracellular enzymatic synthesis of Ag-NPs by bacteria.
2.2. Synthesis by Using Fungi
The employment of fungi in the production of metal/metal oxide NPs is also a reasonably methodical strategy for producing monodispersed NPs with distinct morphologies (Table 2). For the generation of metal and metal oxide nanoparticles, fungi are good biological agents, due to the presence of numerous intracellular enzymes [9]. The advantages of fungi over other microbes include large production of proteins and enzymes, and fast and sustainable synthesis of nanoparticles. Fungi are frequently utilized as a stabilizing and reducing agent due to their heavy metal tolerance and propensity to absorb metals. Moreover, the production of fungi on a large scale is easy (“nano factories”) and can produce nanoparticles of desired size and morphology [40].
Table 2.
Synthesis of metallic NPs from various fungal species.
| Year | S/N | Fungal Species | Method | Size | Morphology | References |
|---|---|---|---|---|---|---|
| 2021 | 1. | Endophytic fungus | Intracellular | 10–25 | Polydispersedspherical, hexagonal, and spherical | [49] |
| 2. | Trichoderma viride | Extracellular | 5–40 | Spherical | [50] | |
| 3. | Schizophyllum commune | Intracellular and extracellular | 51–93 | Spherical | [51] | |
| 4. | Humicola sp. | Extracellular | 5–25 | Spherical | [52] | |
| 5. | Penicillium citrinum | Extracellular | - | Uniform spherical | [53] | |
| 6. | Rhizopus stolonifer | Extracellular | 9.47 | Spherical | [54] | |
| 7. | Cladosporium cladosporioides | Intracellular | 10–100 | Spherical | [55] | |
| 8. | Fusarium semitectum | Extracellular | 1–50 | Ellipsoid, polydispersed spherical | [56] | |
| 9. | Filamentous fungus | Extracellular | 58.35 ± 17.88 | - | [57] | |
| 10. | Aspergillus flavus | Extracellular | 8.92 | - | [58] | |
| 11. | Cladosporium sphaerospermum | Extracellular | 15.1 ± 1 | Spherical | [55] | |
| 12. | Arthroderma fulvum | Intracellular | 20.56 | Spherical | [59] | |
| 13. | Sclerotinia sclerotiorum MTCC 8785 | Extracellular | 10–15 | Spherical | [60] | |
| 2020 | 14. | Penicillium brecompactum | Intracellular | 23–105 | Crystalline spherical | [61] |
| 15. | Rhizoctonia solani | Intracellular | 2–22 | Spherical | [62] | |
| 16. | Rhizopus nigricans | Extracellular | 35–40 | Round | [61] | |
| 17. | Alternaria alternate | Extracellular | 32.5 | Polydispersed, spherical | [63] | |
| 18. | Aspergillus niger | Extracellular | 1–20 | Polydispersed, spherical | [64] | |
| 19. | Penicillium hrysogenumad Aspergillus oryzae | Extracellular | 6–100, 14–76 | Spherical | [65] | |
| 20. | Cryphonectria sp. | Extracellular | 30–70 | - | [66] | |
| 21. | Penicillium sp. | Extracellular | 25–30 | Spherical | [67] | |
| 22. | Penicillium sp. | Extracellular | 58.35 ± 17.88 | - | [68] | |
| 23. | Aspergillus fumigates | Extracellular | 5–25 | Spherical | [69] | |
| 2019 | 24. | Guignardia mngifera | Extracellular | 5–30 | Spherical | [70] |
| 25. | Cariolus versicolor | Intracellular | 25–75 | Spherical | [71] | |
| 26. | Duddingtonia flagrans | Extracellular | 30–409 | Spherical | [72] | |
| 27. | Isaria fumosorosea | Extracellular | 51.31–111.02 | Spherical | [73] | |
| 28. | Penicillium purpurogenum | Intracellular | 8–10 | Spherical | [70] | |
| 29. | Fusarium solani | Extracellular | 5–35 | Spherical | [15] | |
| 30. | Trichoderma harzianum | Extracellular | 34.77 | Ellipsoidal, spherical | [74] | |
| 31. | Aspergillus fumigates | Extracellular | 5–25 | Spherical | [72] | |
| 32. | Endophytic fungus | Extracellular | 25–30 | Spherical | [75] | |
| 33. | Phoma glomerata | Extracellular | 60–80 | Spherical | [15] | |
| 34. | Trichoderma reesei | Extracellular | 5–50 | Random | [76] | |
| 35. | Fusarium acuminatum | Extracellular | 13 | Spherical | [77] |
Mechanism of Synthesis
The specific mechanism of the synthesis of AgNPs using is still not known. However, in general, the mechanism can be extracellular or intracellular. In the mycelial culture, the metal precursor is incorporated in the biomass, in the intracellular approach. Following synthesis, Ag-NPs extraction is required. To disrupt the biomass and liberate the nanoparticles, the extraction technique includes centrifugation, chemical treatment, and filtering.
In extracellular synthesis, metal precursor is added to a filtrate that solely includes fungal biomolecules in extracellular synthesis, leading to the generation of free NPs in the dispersion solution. This method is widely utilized, and it eliminates the requirement for AgNPs to be extracted from cells [40]. Extracellular nanoparticle formation is found to follow processes in which Ag ions are converted to elemental silver (Ag0) by an enzyme present in the fungal filtrate. [40]. Mainly reductases are involved in the fungal NP synthesis. For example, Aspergillus flavus releases a 32-kDa reductase protein that lowers Ag+ ions during the formation of Ag-NPs, as described in Figure 6 [47].
Figure 6.
Fungal synthesis of Ag-NPs [47].
In comparison to bacteria, several studies have demonstrated the appropriateness and possibility of employing fungus for large-scale NP synthesis. AgNPs were recently created utilizing A. flavus fungus and antibiotics to increase biocidal efficacy against multidrug-resistant bacteria, resulting in antibiotics coupled with AgNPs being more effective [11].
2.3. Synthesis of AgNPs by Using Plants
The utilization of plant extracts for AgNPs synthesis provides a number of merits over chemical, physical, and microbiological approaches. The removal of hazardous reducing and capping chemicals, radiation and high temperature, microbial strain, and expensive media for microbial growth are just a few of the positives. The time required for the synthesis of AgNPs is also less using plants. For example, when compared to microbes, neem leaf extract is faster at removing metals. Plant-synthesized AgNPs remain constant for a longer time and have application in the biomedical field. Different factors such as temperature, pH, reaction period, changing ratio, and concentration of plant extract and precursor (AgNO3) can be controlled to synthesize AgNPs on a large scale with various shapes and sizes, which is difficult or impossible to do in microbial synthesis (Table 3) [78].
Table 3.
Ag NPs synthesized from diverse plants.
| Year | S/N | Plant Name | Size (nm) | Morphology | References |
|---|---|---|---|---|---|
| 2021 | 1. | Phaseolus vulgaris | 10–20 | Spherical | [79] |
| 2. | Ficus Benjamina | 20–30 | – | [80] | |
| 3. | Magnolia Kobus | 50–500 | FCC | [20] | |
| 4. | Pinus thunbergii | 5–50 | Triangular, hexagonal | [81] | |
| 5. | Ficus panda | 12–36 | Spherical | [82] | |
| 6. | Dalbergia sissoo | 5–55 | Spherical | [83] | |
| 7. | Musa balbisiana, Azadirachta indica, and Ocimum tenuiflorum | 100 | Spherical, triangular, and cuboidal | [84] | |
| 8. | Buniumpersicum | 20–50 | Spherical | [85] | |
| 9. | Acalypha Indica | 20–30 | - | [86] | |
| 10. | Medicago Sativa | 2–20 | Spherical | [87] | |
| 11. | Sesuvium portulacastrum L. | 5–20 | Spherical | [88] | |
| 12. | Cyamopsis tetragonaloba | 8 | Spherical | [89] | |
| 13. | Pine, persimmon, ginkgo, magnolia, and Platanus | 15–500 | _ | [90] | |
| 14. | Rosa Damascena | - | Spherical | [91] | |
| 2020 | 15. | Camellia Sinensis | 4 | Spheroidal | [92] |
| 16. | Sorghum bicolor | 10 | Spheroidal | [93] | |
| 17. | Jatropha gossypifolia | 62 | Spherical | [94] | |
| 18. | Coffee Arabica | 20–30 | Spherical, Ellipsoidal | [41] | |
| 19. | Prunus yedoensis | 20–70 | Spherical and oval | [95] | |
| 20. | Emblica Officinalis | 10–20 | - | [96] | |
| 21. | Vitex negundo | 10–30 | Spheroidal | [30] | |
| 22. | Cinnamomum camhora | 55–80 | Triangular or spherical | [97] | |
| 23. | Mimosa pudica | 25–60 | Spherical | [98] | |
| 24. | Camellia Sinensis | 20 | Spheroidal | [92] | |
| 25. | Euphorbia lacteal | 186 | Spherical | [99] | |
| 26. | Azadirachta Indica | 50–100 | Spherical | [100] | |
| 27. | Morinda citrifolia | 30–55 | Spherical | [101] | |
| 28. | Jatropha curcas | 10–20 | Spherical | [97] | |
| 29. | Bauhinia variegate | 38–65 | Spherical, triangle, truncated triangles, and decahedrons | [102] | |
| 30. | Pinus thunbergii | 20–100 | Spheroidal | [103] | |
| 31. | Pulicaria glutinosa | 40–60 | Spherical | [28] | |
| 32. | Nyctanthes arbor-tristis | 50–80 | Spherical | [104] | |
| 33. | Terminalia chebula | 25 | Spherical, ovoid | [105] | |
| 34. | Dioscorea bulbifera | 8–20 | FCC | [106] | |
| 35. | Elaeagnus latifolia | 30–50 | Spherical | [107] | |
| 36. | Vigna sp. L. | 24.35 | Spherical | [108] | |
| 37. | Piper nigrum | 5–50 | FCC | [109] | |
| 2019 | 38. | Musa acuminata | - | Agglomerated form | [110] |
| 39. | Amaranthus retroflexus | 10–32 | Spherical | [111] | |
| 40. | Tribulus Terrestris L. | 16–28 | Spherical | [112] | |
| 41. | Cassia auriculata | 20–40 | Spherical | [113] | |
| 42. | Adenium obesum | 10–30 | Spherical | [40] | |
| 43. | Coleus aromaticus | 40–50 | Spherical | [114] | |
| 44. | Artocarpus heterophyllus Lam | 10.78 | Irregular | [115] | |
| 45. | Vigna radiate | 5–30 | Spherical, oval | [116] | |
| 46. | Zingiber officinale | 10–20 | - | [117] | |
| 47. | Lantana Camara | 14–27 | Spherical | [118] | |
| 48. | Aloe vera | 20 | Spherical | [119] | |
| 49. | Hevea brasiliensis | 2–100 | Spherical | [120] | |
| 50. | Dodonaea viscosa | 16 | Spheroidal | [121] | |
| 51. | Murraya koenigii | 20–35 | Spheroidal | [15] | |
| 52. | Jatropha curcas | 73 | Spherical | [122] | |
| 53. | Pedilanthus tithymaloides | 123 | Spherical | [46] | |
| 54. | Euphorbia prostrate | 25–80 | Rod | [123] | |
| 55. | Syzygium aromaticum | - | - | [124] | |
| 56. | Tinospora cordifolia | 55–80 | Aggregated | [125] | |
| 57. | Solanum torvum | 14 | Spheroidal | [126] | |
| 58. | Murraya koenigii | 10–25 | Spheroidal | [15] | |
| 59. | Ocimum tenuiflorum | 7–15 | Spherical and ovoid | [75] |
Mechanism of Synthesis
Plants have various biomolecules (lcarbohydrates, proteins, terpenoids, flavonoids, flavones, terpenes, phenolics, polysaccharides, saponins, tannins, alkaloids, and coenzymes) have the greatest capacity to convert metal salt to NPs. Several plants are used for synthesis, including Coriander (Coriandrum sativum), Oat (Avena sativa), alfalfa (Medicago sativa), aloe vera (Aloe barbadensis Miller), Neem (Azadirachta indica), Mustard (Brassica juncea), and lemongrass (Cymbopogon fexuosus), Tulsi (Ocimum sanctum), and Lemon (Citrus limon).
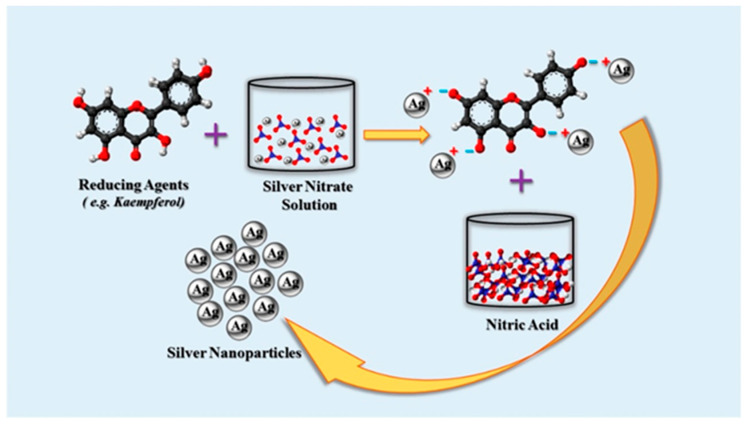
Plant extracts have the ability to serve as both reducing and stabilizing agents during the production of AgNPs. Phytochemicals include functional groups that can reduce Ag+ ions to AgNPs, such as hydroxyl, amino, ketone, carboxyl, and aldehyde groups. For instance, the flavonoids naringin and kaempferol, as well as their glycosides, are found in Punica granatum peel extract. All substances with hydroxyl (–OH) groups can cause Ag+ ions to be reduced, resulting in the creation of AgNPs, as illustrated in Figure 7 [47,48].
Figure 7.
Plant-based synthesis of Ag-NPs by reaction of AgNO3 with phytochemicals [47].
2.4. Key Factors for Efficient, Economical, and Reliable Preparation of AgNPs
The proportions of plant extracts and metal salts, ambient duration, temperature, pH, and other parameters all play a role in the quick, sustainable, and scalable manufacturing of AgNPs. These variables also influence the form and size of obtained NPs.
Temperature and metal salt content impacted extracellular production of AgNPs employing culture supernatant of Pseudoduganella eburnea MAHUQ-39, as per Huq [127]. The optimal conditions for the efficient and sustainable formation of AgNPs utilizing P. eburnea were determined to be 30 °C temperature, 1 mM AgNO3 (final concentration), and 24 h incubation duration. The impact of temperature, ionic strength, and contact time on the ecofriendly synthesis of AgNPs via bark extracts of A. indica and F. benghalensis was studied by Nayak et al. [128], who found that an 80 °C temperature, a pH of 10, and a 30 min incubation time are the best conditions for quick and reliable production.
Mittal et al. [129] extracted the natural herb Potentilla fulgens for the ecofriendly synthesis of AgNPs and discovered that assorted physicochemical variables such as plant extract and metal ion concentration levels, incubation duration and temperature, and the pH of the reaction time all had a significant impact on the rate of synthesis as well as the shape, size, and yield. They tested various plant extract concentrations (1 to 200 mg in 50 mL water) and discovered that 4 mg extract in 50 mL water produced the maximum quantity of AgNPs. They additionally employed different concentrations of AgNO3 ranging from 0.5 to 5 mM and discovered that the production of AgNPs enhanced as the AgNO3 amount grew from 0.5 to 1 mM, after which the absorbance dropped again. They discovered that 45 °C is the optimal temperature for the highest yield, and that higher temperatures improved the rate of synthesis of finer NPs. The pH of the precursor solution had an impact on the synthesis. They discovered that finer NPs developed at an alkaline pH, while bigger NPs emerged at an acidic pH.
Hamouda et al. [130] used an aqueous extract of Oscillatoria limnetica to analyze the influence of plant extracts and AgNO3 doses on the synthesis of NPs. They found that the quantities of the aqueous leaf extract of Oscillatoria limnetica and AgNO3 affected the size and shape of obtained AgNPs. These characteristics have a substantial impact on microbe-mediated synthesis, just as they do on plant-mediated synthesis.
Much additional recent research has shown the influence of plant extract and metal salt concentration, incubation duration, temperature, and pH on the quick and stable synthesis of homogeneous AgNPs with a high yield employing both plants and microorganisms [131,132,133].
3. Antimicrobial Activity of Ag NPs
The fast growth of drug resistance to new antibiotics, as well as rapid mutations, has resulted in the creation of antimicrobial substances and alternate therapies [46]. It is reported that more than 70% of infections by bacteria are resistant to one or more antibiotics that are used to treat the infection. Metals such as copper (Cu), titanium (Ti), silver (Ag), gold (Au), and zinc (Zn) are known to show antimicrobial activity [134]. AgNPs can cause structural damage, produce ROS, disrupt DNA replication, and react with the thiol group of enzymes, among other biocidal effects (Table 4). The antagonistic AgNPs influence the enzymes and proteins of bacteria regardless of their Gram characteristics, according to these investigations. Antibiotics that target a specific method of microbial suppression, on the other hand, do not have this problem [135]. Silver compounds are known to be effective against aerobic and anaerobic bacteria by precipitation of cellular proteins of bacteria, and the microbial respiratory chain system has been blocked. Antiviral activity of AgNPs with a large surface-to-volume ratio (size ≤ 100 nm) against HIV-infected cells has been demonstrated [46]. They are also effective against multidrug-resistant organisms such as Streptococcus pyogenes, methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, Pseudomonas aeruginosa, and vancomycin-resistant Staphylococcus aureus [46].
Table 4.
Ag NPs prepared via bacteria, fungi, and plants.
| Year | S/N | Organism | Biological Entity | Size (nm) | Morphology | Method | References |
|---|---|---|---|---|---|---|---|
| 2021 | 1. | Fungi | Penicillium sp. | 25 | Spherical | Agar well diffusion method | [53] |
| 2. | Fungi | Arthroderma fulvum | 15.5 | Spherical | Brothmicro-dilution method | [18] | |
| 3. | Fungi | Penicillium aculeatum | 4–55 | Spherical | Disk diffusion method | [37] | |
| 4. | Bacteria | Acinetobacter baumannii | 37–168 | Spherical | Broth micro-dilution method | [136] | |
| 5. | Plant | Artocarpus altilis | 20–45 | Spherical | Agar well diffusion method | [81] | |
| 6. | Plant | Convolvulus arvensis | 28 | Spherical | Disc diffusion and broth macro-dilution method | [137] | |
| 7. | Plant | Erythrina suberosa | 15–34 | Spherical | Agar cup and broth micro-dilution methods | [37] | |
| 8. | Plant | Psidium guajava | 20–25 | Spherical | Agar well diffusion 1method | [89] | |
| 9. | Plant | Nelumbo Nucifera | 12.9 | Quasi–Spherical | Broth dilution method | [138] | |
| 10. | Plant | Boerhaavia diffusa | 25 | Spherical | Agar well diffusion | [20] | |
| 11. | Plant | Alpinia katsumadai | 12.6 | Quasi-Spherical | Broth dilution method | [104] | |
| 12. | Fungi | Curvularia lunata | 64.3 | Spherical | Disk diffusion assay | [139] | |
| 13. | Fungi | Pleurotus ostreatus | 10–40 | Spherical | Disk diffusion and broth micro-dilution methods | [140] | |
| 2020 | 14. | Fungi | Rhodotorula glutinis | 15–220 | Spherical | Agar well diffusion and broth microdilution methods | [109] |
| 15. | Bacteria | Pseudomonas deceptionensis | 127 | Spherical | Agar well diffusion method | [141] | |
| 16 | Bacteria | Acinetobacter baumannii | 37–168 | Spherical | Broth micro-dilution method | [142] | |
| 17. | Bacteria |
Phenerochaete
Chrysosporium |
- | Spherical and oval | Agar well diffusion method | [143] | |
| 18. | Bacteria | Bacillus endophyticus | 4.8–6.6 | Spherical | Agar well diffusion method | [144] | |
| 19. | Plant | tea | 10–20 | Spherical | Disk and broth dilution methods | [145] | |
| 20. | Plant | Eucalyptus globules | 1.9–4.3 | Spherical | Agar well diffusion and broth dilution methods | [109] | |
| 21. | Plant | Mimusops elengi | 55–83 | Spherical | Disk diffusion method | [146] | |
| 2019 | 22. | Fungi | Saccharomyces cerevisiae | 5–50 | Spherical | Brothmicro-dilution method | [147] |
| 23. | Fungi | Guignardia mangiferae | 5–30 | Spherical | Agar well | [70] | |
| 24. | Fungi | Penicillium polonicum | 10–15 | Spherical | Agar well diffusion | [148] | |
| 25. | Fungi | Raphanus sativus | 4–30 | Spherical | Disk diffusion method | [149] | |
| 26. | Fungi | Ganoderma applanatum | 133 | Spherical | Agar welldiffusionmethod | [150] | |
| 27. | Bacteria | Weissella oryzae | 150 | Spherical | Disk diffusion method | [43] | |
| 28. | Bacteria | Ochrobactrum anthropi | 38–85 | Spherical | Agar well diffusion method | [46] | |
| 29. | Plant | Elephantopus scaber | 37 | Spherical | Agar well diffusion method | [46] | |
| 30. | Plant | Ocimum sanctum | ~15 | Spherical | Disc and dilution approach | [151] | |
| 31. | Plant | Musa paradisiacal | 23.7 | Spherical | Agar well diffusion and broth dilution approaches | [46] | |
| 32. | Plant | Dalbergia spinosa | 18 | Spherical | Disk diffusion and broth microdilution methods | [15] | |
| 33. | Plant | Emblica officinalis | 15 | Spherical | Disk diffusion method | [152] |
3.1. Mechanism of Action
Antibacterial research on silver nanoparticles is generally done in vitro on solid media or liquid cultures of microorganisms. The antibacterial mode of action of AgNPs, as well as their influence on other bodily components, must be investigated in vivo. The antibacterial activity of AgNPs produced from plant extracts has been well demonstrated; however, the specific mechanism is unknown [97].
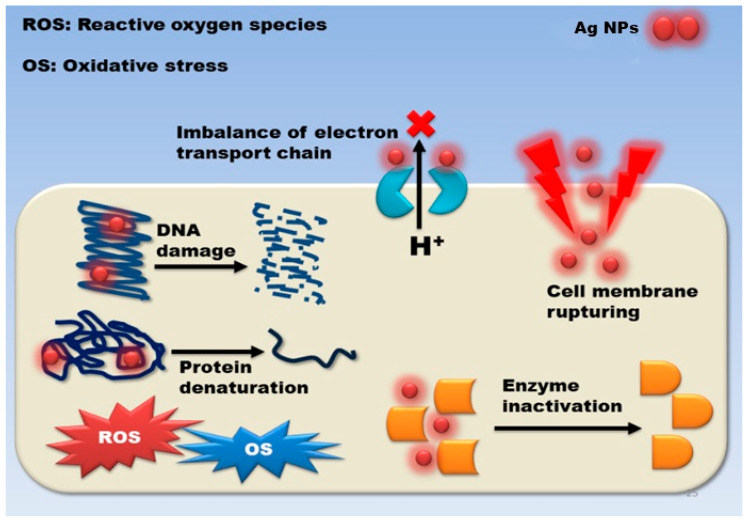
Despite this, a lot of studies have attempted to figure out how they work, and three distinct processes have been postulated so far: damage to cell membranes and cell walls penetrating and damaging cells within cells oxidative stress, as depicted in Figure 8.
Figure 8.
Silver nanoparticles’ antibacterial mechanism of action is described in general terms [79].
3.2. Damage to the Cell Wall and Membrane
The fundamental purpose of the cell wall and membrane is to protect microorganisms from external threats and to maintain homeostasis while allowing nutrients to be transported within the cell. AgNPs exhibit high antibacterial activity against Gram-negative bacteria as compared to Gram-positive bacteria because the peptidoglycan layer present in the Gram-positive bacterial cell wall, which acts as a natural barrier, is thick and thus averts the diffusion of the NPs. Due to adhesion between AgNPs and microorganisms, interaction occurs between the microbial cell wall and the surface of the Ag-NPs. The electrostatic attraction between the negative charge on the microbial cell membrane and the positive or less negative charge on AgNPs determines the outcome of this interaction. Following such attraction and contact, the NPs produce morphological changes in the membrane’s structure, resulting in membrane permeability and respiratory functions being disrupted by membrane depolarization, which ultimately disrupts cell integrity and cell death. It was found that when membrane permeability increases and the cell wall is disrupted, cellular content such as DNA, enzymes, organelles, ions, metabolites, and the energy reserve seeps into the environment. As a result, damage to cell membranes and cell walls, intracellular penetration and damage, and oxidative stress, as depicted in Figure 8 [46].
3.3. Intracellular Penetration and Damage
AgNPs can permeate the cell and affect essential activities such as DNA and protein interaction, depending on the degree of membrane damage. One of the recognized mechanisms for AgNPs antibacterial action is silver ion release from the NPs, which has a detrimental impact on microorganisms. Silver ions were shown to cause the transition of bacteria’s DNA from a naturally relaxed state to a compacted one, in which the molecule of DNA loses its reproduction ability. Furthermore, X-ray examination reveals the presence of sulphur, indicating that silver ions interact with protein thiol groups, resulting in the inhibition of enzyme function. AgNPs can cause DNA degradation and/or denaturation, in addition to changing their configuration. Ag+ ions are also attached to DNA via physical attractions and interact with the nucleoside component of the nucleotide, according to research. The base-pairing inside complimentary strands is altered as a result of the hydrogen bond breakage [46].
The intracellular action of Ag NPs is not limited to DNA destruction. The effects of Ag NPs on proteins and protein synthesis have been discovered through proteomic investigations. Previous research has shown that silver nanoparticles and Ag+ ions formed from AgNPs are reactive with protein thiol groups. Cysteine amino acids are found with thiol or thiolate groups as their functional group. Cysteine is an inadequate amino acid; however, it is a highly conserved residue in functional protein locations. It is important in biological processes because of its high-affinity metal-binding capability, nucleophilic participation in catalytic events, and ability to form disulfide bonds, which is essential for protein folding and 3-D structure.
3.4. Oxidative Stress
The term “reactive oxygen species” (ROS) refers to oxygen-containing compounds with high redox potential. When circumstances are normal, the generation of ROS inside the cell is balanced, as is its antioxidant capacity. However, due to an imbalance between the antioxidant mechanism and the inappropriate release of ROS, the redox balance of cells favors oxidation, resulting in oxidative stress. AgNPs cause cellular oxidative stress, and cells respond by exhibiting defensive responses that include enzymatic and non-enzymatic defense mechanisms to counteract this stress. When oxidative stress overwhelms these defensive systems, ROS and free radicals cause damage to the cell wall and macromolecules, including proteins, lipids, and DNA.
DNA damage includes deletions, mutations, single and double-strand breaks, adduct accumulation, and protein cross-linking. Studies have shown that oxidation-mediated DNA fragmentation occurs following exposure to metal oxide NPs. Cells strive to repair damaged DNA in response to DNA damage. Failure to repair can lead to cell death. There is also a possibility of the generation of ROS, which is mediated by Ag+ ions produced by AgNPs, which may disrupt the bacterial electron transport chain as well as proton motive force. This leads to enzyme inhibition involved in the reactions. Researchers also found that, in addition to the breakdown of membrane functions, ROS generation also causes protein leakage through increased membrane permeability. These leaked proteins by the cells interact with AgNPs, which eventually leads to cell death. Oxidative stress can change gene expression, in addition to its direct effects on cell walls and components. On the treatment of Pseudomonas cells with AgNPs, translation of ribosomal proteins S2 and L9, alkyl hydroperoxide reductase C (AhpC), keto-hydroxyglutarate aldolase (KHGA), and thiol-specific antioxidant (TSA) was deemed to be overexpressed.
Thus, the essential aspect of NPs is their method of action, which is influenced by their size; dissolving efficiency; ionic strength of the medium; synthesis and treatment variables; and the kind of stabilizing agent used.
AgNPs have been shown to impede protein expression along with cell wall production in the literature, providing strong evidence for protein breakdown of the exterior cell surface and increased ATP permeability, leading to apoptosis [153].
Furthermore, the size and morphology of AgNPs were shown to boost the production of Ag+ ions due to their larger surface area, thus influencing their potency towards microbial illness. AgNPs’ aqueous solubility significantly affects their antibacterial activity. The potential efficacy might be boosted if the aqueous solubility is significant [154]. AgNPs smaller than 10 nm are thought to be capable of directly penetrating cell walls, entering bacterial cells, and causing cell lysis [155]. As a result, the findings might be useful in determining if AgNPs can be used as an alternative antibacterial agent to prevent dangerous microorganisms and alleviate microbial disease illnesses.
4. Toxicology of Silver Nanoparticles to Human Health
Although nanotechnology has been exploited in a large number of commercial products overall the world recently, there is still a lot of information lacking regarding the increase of human, animal, and ecological exposure to AgNPs and their short and long-term potential lethal effects [156]. Silver can enter the human body and shows lethal effects on human health. Previous literature indicated that Ag+ ions alter the cell membrane permeability to K+ and then to Na+. This reduces the mitochondrial function or ATP activity and increases membrane leakage [156,157]. Silver nanoparticles were found to have a significant cytotoxic effect on peripheral blood mononuclear cells (PBMCs) when levels were over 15 ppm, and Phyto-haemagglutinin-induced cytokine production was remarkably inhibited by silver nanoparticles [157]. Additionally, lactate dehydrogenase (LDH) leakage was notably elevated in cells exposed to AgNPs (10–50 µg mL−1). A study proved the significant decrease in reduced glutathione (GSH) level, and increase in reactive oxygen species (ROS) levels, which signifies that AgNPs cytotoxicity (15, 100 nm) is mediated by oxidative stress in liver cells. Silver nanoparticles also exhibit severe effects on the male reproductive system. Studies suggest that silver nanoparticles can cross the blood-testes barrier and be deposited in there. They show potential adverse effects on sperm cells. Silver also accumulates and shows some toxic effects in organs and tissues. When overused, it can accumulate in the liver, skin, corneas, mucous membranes, kidneys, nails, gingiva, spleen, and other places. It can cause effects such as producing reactive oxygen species, and cell activation that is more toxic to tissue, which gradually leads to cell death [158].
5. Future Challenges
Synthetic methods involving fungus, bacteria, and other creatures are challenging since strain separation and growth are required. These processes are also difficult owing to the need to maintain the culture media, as well as the physical and chemical conditions. Plants are selected primarily because they are simple to extract and plentiful. As earlier explained, AgNP’s essential properties depend on their morphology and size. Therefore, future challenges lie in how these biological procedures can be used to produce other shapes such as triangular, cuboidal, truncated, ellipsoidal, pyramidal, decahedral, and oval shapes. Scaling up NP production from laboratory to commercial scale is not an easy task and has many difficulties and uncertainties. There are two further challenges. First, cost, dependability, waste, energy consumption, recycling potential, material safety, and hazard level should all be considered throughout manufacturing. Second, when nanomaterials scale up, their characteristics may alter. When working with huge volumes, the level of control may be compromised.
6. Summary
In this review paper, the research trends, worldwide use, synthesis, characteristics, and future challenges of Ag NPs have all been thoroughly assessed. Three methods are often employed to synthesize Ag-NPs: physical, chemical, and biological. The physical approach has several drawbacks, including high energy consumption, a large quantity of space needed, and a significant time to achieve thermal stability. Using a chemical technique, AgNPs may be easily prepared. On the other and, the usage of costly and dangerous chemicals is a major cause of environmental concern. Biological AgNP synthesis approaches are gaining popularity since they are ecologically friendly/green, cost-effective, and have no negative effects on the environment. Recent studies have revealed that AgNPs have good physical, chemical, biological, electrical, optical, thermal, and catalytic properties, making them appropriate for a wide range of essential applications. Concurrently, it IS crucial to acknowledge that AgNPs are dangerous, which must be considered when they are used in consumer items. Green synthesis should take into account three factors: simplicity, time consumption, and cost. It is also worth considering how this process may be enhanced to create shapes other than spheres. AgNPs discharged into the environment should be investigated from their origins, techniques, and transportation through their effects, utilizing better prototypes than those now available.
Acknowledgments
The authors gratefully acknowledge Chandigarh University, Mohali, Sri Guru Granth Sahib World University, Punjab (India), University of York, UK, National University of Singapore, Singapore and University of Montpellier, France for providing necessary resources.
Author Contributions
Conceptualization, J.S.; formal analysis, J.S. and M.B.; investigation, J.S.; resources, S.P., H.K. and J.S.; data curation, S.P., H.K. and J.S.; writing—original draft preparation, S.P., H.K.; writing—review and editing, J.S., A.S.M. and S.R.; visualization, S.P., H.K. and J.S.; supervision, J.S.; funding acquisition, J.S. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mba I.E., Nweze E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2021:37. doi: 10.1007/s11274-021-03070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pimentel C., Le C., Tuttobene M.R., Subils T., Martinez J., Sieira R., Papp-Wallace K.M., Keppetipola N., Bonomo R.A., Actis L.A., et al. Human pleural fluid and human serum albumin modulate the behavior of a hypervirulent and multidrug-resistant (MDR) acinetobacter Baumannii representative strain. Pathogens. 2021;10:471. doi: 10.3390/pathogens10040471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaro F., Morón Á., Díaz S., Martín-González A., Gutiérrez J.C. Metallic nanoparticles—friends or foes in the battle against antibiotic-resistant bacteria? Microorganisms. 2021;9:364. doi: 10.3390/microorganisms9020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan D.J., Okeke I.N., Laxminarayan R., Perencevich E.N., Weisenberg S. Non-prescription antimicrobial use worldwide: A systematic review. Lancet Infect. Dis. 2011;11:692–701. doi: 10.1016/S1473-3099(11)70054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbasi E., Milani M., Aval S.F., Kouhi M., Akbarzadeh A., Nasrabadi H.T., Nikasa P., Joo S.W., Hanifehpour Y., Nejati-Koshki K., et al. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit. Rev. Microbiol. 2016;42:173–180. doi: 10.3109/1040841X.2014.912200. [DOI] [PubMed] [Google Scholar]
- 6.Mostafavi E., Medina-Cruz D., Vernet-Crua A., Chen J., Cholula-Díaz J.L., Guisbiers G., Webster T.J. Green nanomedicine: The path to the next generation of nanomaterials for diagnosing brain tumors and therapeutics? Expert Opin. Drug Deliv. 2021;18:715–736. doi: 10.1080/17425247.2021.1865306. [DOI] [PubMed] [Google Scholar]
- 7.Iqbal T., Raza A., Zafar M., Afsheen S., Kebaili I., Alrobei H. Plant-mediated green synthesis of zinc oxide nanoparticles for novel application to enhance the shelf life of tomatoes. Appl. Nanosci. 2022;12:179–191. doi: 10.1007/s13204-021-02238-z. [DOI] [Google Scholar]
- 8.Dhumale V.A., Gangwar R.K., Pande N. Importance of gold nanoparticles for detection of toxic heavy metal ions and vital role in biomedical applications. Mater. Res. Innov. 2021;25:354–362. doi: 10.1080/14328917.2020.1825770. [DOI] [Google Scholar]
- 9.Sridhar K., Inbaraj B.S., Chen B.H. Recent advances on nanoparticle based strategies for improving carotenoid stability and biological activity. Antioxidants. 2021;10:713. doi: 10.3390/antiox10050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huq M.A., Ashrafudoulla M., Rahman M.M., Balusamy S.R., Akter S. Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers. 2022;14:742. doi: 10.3390/polym14040742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafique M., Sadaf I., Rafique M.S., Tahir M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017;45:1272–1291. doi: 10.1080/21691401.2016.1241792. [DOI] [PubMed] [Google Scholar]
- 12.Chu C., Hu X., Yan H., Sun Y. Surface functionalization of nanostructured Cu/Ag-deposited polypropylene fiber by magnetron sputtering. E-Polymers. 2021;21:140–150. doi: 10.1515/epoly-2021-0020. [DOI] [Google Scholar]
- 13.Valerini D., Tammaro L., Vitali R., Guillot G., Rinaldi A. Sputter-deposited ag nanoparticles on electrospun pcl scaffolds: Morphology, wettability and antibacterial activity. Coatings. 2021;11:345. doi: 10.3390/coatings11030345. [DOI] [Google Scholar]
- 14.Natsuki J. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015;4:325. doi: 10.11648/j.ijmsa.20150405.17. [DOI] [Google Scholar]
- 15.Qais F.A., Shafiq A., Khan H.M., Husain F.M., Khan R.A., Alenazi B., Alsalme A., Ahmad I. Antibacterial effect of silver nanoparticles synthesized using Murraya koenigii (L.) against multidrug-resistant pathogens. Bioinorg. Chem. Appl. 2019;2019 doi: 10.1155/2019/4649506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MODAN E.M., PLĂIAȘU A.G. Advantages and Disadvantages of Chemical Methods in the Elaboration of Nanomaterials. Ann. “Dunarea Jos” Univ. Galati. Fascicle IX Metall. Mater. Sci. 2020;43:53–60. doi: 10.35219/mms.2020.1.08. [DOI] [Google Scholar]
- 17.Singh J., Dutta T., Kim K.H., Rawat M., Samddar P., Kumar P. “Green” synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018:16. doi: 10.1186/s12951-018-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawadi S., Katuwal S., Gupta A., Lamichhane U., Thapa R., Jaisi S., Lamichhane G., Bhattarai D.P., Parajuli N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021;2021 doi: 10.1155/2021/6687290. [DOI] [Google Scholar]
- 19.Koul B., Poonia A.K., Yadav D., Jin J.O. Microbe-mediated biosynthesis of nanoparticles: Applications and future prospects. Biomolecules. 2021;11:886. doi: 10.3390/biom11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sivakumar T. A modern review of silver nanoparticles mediated plant extracts and its potential bioapplications. Int. J. Bot. Stud. 2021;6:170–175. [Google Scholar]
- 21.De Silva C., Nawawi N.M., Karim M.M.A., Gani S.A., Masarudin M.J., Gunasekaran B., Ahmad S.A. The mechanistic action of biosynthesised silver nanoparticles and its application in aquaculture and livestock industries. Animals. 2021;11:2097. doi: 10.3390/ani11072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Bendary M.A., Afifi S.S., Moharam M.E., Abo El-Ola S.M., Salama A., Omara E.A., Shaheen M.N.F., Hamed A.A., Gawdat N.A. Biosynthesis of silver nanoparticles using isolated Bacillus subtilis: Characterization, antimicrobial activity, cytotoxicity, and their performance as antimicrobial agent for textile materials. Prep. Biochem. Biotechnol. 2020;0:54–68. doi: 10.1080/10826068.2020.1789992. [DOI] [PubMed] [Google Scholar]
- 23.Monowar T., Rahman M.S., Bhore S.J., Sathasivam K.V. Endophytic bacteria enterobacter hormaechei fabricated silver nanoparticles and their antimicrobial activity. Pharmaceutics. 2021;13:511. doi: 10.3390/pharmaceutics13040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordienko M.G., Palchikova V.V., Kalenov S.V., Lebedev E.A., Belov A.A., Menshutina N.V. The alginate–chitosan composite sponges with biogenic Ag nanoparticles produced by combining of cryostructuration, ionotropic gelation and ion replacement methods. Int. J. Polym. Mater. Polym. Biomater. 2020;0:1–11. doi: 10.1080/00914037.2020.1798439. [DOI] [Google Scholar]
- 25.Vala A.K., Trivedi H., Gosai H., Panseriya H., Dave B. Biosynthesized Silver Nanoparticles and Their Therapeutic Applications. 1st ed. Elsevier B.V.; Amsterdam, The Netherlands: 2021. [Google Scholar]
- 26.Ghosh V. Marine Bioresources as Potential Source for Synthesis of Nanoparticles. Encycl. Mar. Biotechnol. 2020:1521–1534. doi: 10.1002/9781119143802.ch64. [DOI] [Google Scholar]
- 27.Orizola J., Ríos-Silva M., Muñoz-Villagrán C., Vargas E., Vásquez C., Arenas F. In vitro biosynthesis of Ag, Au and Te-containing nanostructures by Exiguobacterium cell-free extracts. BMC Biotechnol. 2020;20:1–12. doi: 10.1186/s12896-020-00625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan M., Al-Hamoud K., Liaqat Z., Shaik M.R., Adil S.F., Kuniyil M., Alkhathlan H.Z., Al-Warthan A., Siddiqui M.R.H., Mondeshki M., et al. Synthesis of au, ag, and au–ag bimetallic nanoparticles using pulicaria undulata extract and their catalytic activity for the reduction of 4-nitrophenol. Nanomaterials. 2020;10:1885. doi: 10.3390/nano10091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Syame S.M., Mansour A.S., Khalaf D.D., Ibrahim E.S., Gaber E.S. Green Synthesis of Silver Nanoparticles Using Lactic Acid Bacteria: Assessment of Antimicrobial Activity. World’s Vet. J. 2020;10:625–633. doi: 10.54203/scil.2020.wvj75. [DOI] [Google Scholar]
- 30.Patil S.P., Kumbhar S.T. Vitex negundo assisted green synthesis of metallic nanoparticles with different applications: A mini review. Futur. J. Pharm. Sci. 2020;6 doi: 10.1186/s43094-020-00111-4. [DOI] [Google Scholar]
- 31.Gloria Martin K.D., Vergara Padilla K.G. Sunlight Mediated Synthesis of Silver Nanoparticles by Bacillus sp and Its Antibacterial Property. Orient. J. Chem. 2020;36:419–424. doi: 10.13005/ojc/360309. [DOI] [Google Scholar]
- 32.Yorseng K., Siengchin S., Ashok B., Rajulu A.V. Nanocomposite egg shell powder with in situ generated silver nanoparticles using inherent collagen as reducing agent. J. Bioresour. Bioprod. 2020;5:101–107. doi: 10.1016/j.jobab.2020.04.003. [DOI] [Google Scholar]
- 33.Awwad A.M., Salem N.M., Aqarbeh M.M., Abdulaziz F.M. Green synthesis, characterization of silver sulfide nanoparticles and antibacterial activity evaluation. Chem. Int. 2019;6:42–48. doi: 10.31221/osf.io/8byuc. [DOI] [Google Scholar]
- 34.Ramakrishnan A., Gokul R., Pandimadevi M. Preparation and characterisation of nanofibres from bio cellulose and neem-AgNP bio composites for wound healing. Int. J. Biomed. Nanosci. Nanotechnol. 2020;4:80. doi: 10.1504/IJBNN.2020.107187. [DOI] [Google Scholar]
- 35.Arifin D.C.V., Saragih D.I., Santosa S.J. Antibacterial activity of silver nanoparticles synthesized using tyrosine as capping and reducing agent. Int. J. Emerg. Trends Eng. Res. 2020;8:2414–2421. doi: 10.30534/ijeter/2020/34862020. [DOI] [Google Scholar]
- 36.Mamangkey J., Suryanto D., Munir E., Mustopa A.Z. Antibacterial and Antioxidant Activity of Newly Keratinolytic Bacteria, Azotobacter chroococcum B4. Int. J. PharmTech Res. 2020;13:123–127. doi: 10.20902/IJPTR.2019.130215. [DOI] [Google Scholar]
- 37.Geoprincy G., Vidhya Srri B.N., Poonguzhali U., Nagendra Gandhi N., Renganathan S. A review on green synthesis of silver nanoparticles. Asian J. Pharm. Clin. Res. 2013;6:8–12. [Google Scholar]
- 38.Salouti M., Derakhshan F.K. Phytosynthesis of Nanoscale Materials. Elsevier; Amsterdam, The Netherlands: 2019. [Google Scholar]
- 39.Kobayashi R.K.T., Nishio E.K., Scandorieiro S., Saikawa G.I.A., Da Rocha S.P.D., Nakazato G. Metallic Nanoparticles as a Potential Antimicrobial for Catheters and Prostheses. Elsevier; Amsterdam, The Netherlands: 2019. [Google Scholar]
- 40.Baruah D., Yadav R.N.S., Yadav A., Das A.M. Alpinia nigra fruits mediated synthesis of silver nanoparticles and their antimicrobial and photocatalytic activities. J. Photochem. Photobiol. B Biol. 2019;201:111649. doi: 10.1016/j.jphotobiol.2019.111649. [DOI] [PubMed] [Google Scholar]
- 41.Akl B., Nader M., El-Saadony M. Biosynthesis of Silver Nanoparticles by Serratia marcescens ssp sakuensis and its Antibacterial Application against some Pathogenic Bacteria. J. Agric. Chem. Biotechnol. 2020;11:1–8. doi: 10.21608/jacb.2020.76656. [DOI] [Google Scholar]
- 42.Elangovan M., Muju G., Anantharaman P. Biosynthesis of Silver Nanoparticles from Platymonas sp. and Its Antibacterial Activity Against Biofouling Causing Bacterial Strains. J. Biol. Act. Prod. Nat. 2019;9:269–277. doi: 10.1080/22311866.2019.1666741. [DOI] [Google Scholar]
- 43.Arya G., Mankamna Kumari R., Pundir R., Chatterjee S., Gupta N., Kumar A., Chandra R., Nimesh S. Versatile biomedical potential of biosynthesized silver nanoparticles from Acacia nilotica bark. J. Appl. Biomed. 2019;17:115–124. doi: 10.32725/jab.2019.010. [DOI] [PubMed] [Google Scholar]
- 44.Hu X., Saravanakumar K., Jin T., Wang M.H. Mycosynthesis, characterization, anticancer and antibacterial activity of silver nanoparticles from endophytic fungus Talaromyces purpureogenus. Int. J. Nanomed. 2019;14:3427–3438. doi: 10.2147/IJN.S200817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minh Dat N., Linh V.N.P., Huy L.A., Huong N.T., Tu T.H., Phuong N.T.L., Nam H.M., Thanh Phong M., Hieu N.H. Fabrication and antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus of silver nanoparticle decorated reduced graphene oxide nanocomposites. Mater. Technol. 2019;34:369–375. doi: 10.1080/10667857.2019.1575555. [DOI] [Google Scholar]
- 46.Roy A., Bulut O., Some S., Mandal A.K., Yilmaz M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019;9:2673–2702. doi: 10.1039/C8RA08982E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ratan Z.A., Haidere M.F., Nurunnabi M., Shahriar S.M., Ahammad A.J.S., Shim Y.Y., Reaney M.J.T., Cho J.Y. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers. 2020;12:855. doi: 10.3390/cancers12040855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Rubaye H.I., Al-Rubaye B.K., Al-Abodi E.E., Yousif E.I. Green Chemistry Synthesis of Modified Silver Nanoparticles. J. Phys. Conf. Ser. 2020;1664:1–26. doi: 10.1088/1742-6596/1664/1/012080. [DOI] [Google Scholar]
- 49.Seetharaman P.K., Chandrasekaran R., Periakaruppan R., Gnanasekar S., Sivaperumal S., Abd-Elsalam K.A., Valis M., Kuca K. Functional attributes of myco-synthesized silver nanoparticles from endophytic fungi: A new implication in biomedical applications. Biology. 2021;10:473. doi: 10.3390/biology10060473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan I.H., Javaid A., Ahmed D. Trichoderma viride Controls Macrophomina phaseolina through its DNA disintegration and Production of Antifungal Compounds. Int. J. Agric. Biol. 2021;25:888–894. doi: 10.17957/IJAB/15.1743. [DOI] [Google Scholar]
- 51.Štular D., Savio E., Simončič B., Šobak M., Jerman I., Poljanšek I., Ferri A., Tomšič B. Multifunctional antibacterial and ultraviolet protective cotton cellulose developed by in situ biosynthesis of silver nanoparticles into a polysiloxane matrix mediated by sumac leaf extract. Appl. Surf. Sci. 2021;563 doi: 10.1016/j.apsusc.2021.150361. [DOI] [Google Scholar]
- 52.Al-hosaini K.A., Azhar A. Silver Nanoparticle and their Antimicrobial Properties. EC Pharmacol. Toxicol. 2021;9:24–31. [Google Scholar]
- 53.Dawoud T.M., Yassin M.A., El-Samawaty A.R.M., Elgorban A.M. Silver nanoparticles synthesized by Nigrospora oryzae showed antifungal activity. Saudi J. Biol. Sci. 2021;28:1847–1852. doi: 10.1016/j.sjbs.2020.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-khattaf F.S. Gold and silver nanoparticles: Green synthesis, microbes, mechanism, factors, plant disease management and environmental risks. Saudi J. Biol. Sci. 2021;28:3624–3631. doi: 10.1016/j.sjbs.2021.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kahraman T., Elif Korcan S., Liman R., Hakkı Ciğerci İ., Acikbas Y., Konuk M., Uysal Akkuş G. Synthesis, Characterization, and Optimization of Green Silver Nanoparticles Using Neopestalotiopsis clavispora and Evaluation of Its Antibacterial, Antibiofilm, and Genotoxic Effects. EuroBiotech J. 2021;5:109–122. doi: 10.2478/ebtj-2021-0020. [DOI] [Google Scholar]
- 56.Rai M., Bonde S., Golinska P., Trzcińska-Wencel J., Gade A., Abd-Elsalam K., Shende S., Gaikwad S., Ingle A.P. Fusarium as a novel fungus for the synthesis of nanoparticles: Mechanism and applications. J. Fungi. 2021;7:139. doi: 10.3390/jof7020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santos T.S., Silva T.M., Cardoso J.C., de Albuquerque-Júnior R.L.C., Zielinska A., Souto E.B., Severino P., da Costa Mendonça M. Biosynthesis of Silver Nanoparticles Mediated by Entomopathogenic Fungi: Antimicrobial Resistance, Nanopesticides, and Toxicity. Antibiotics. 2021;10:852. doi: 10.3390/antibiotics10070852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El-Mekkawy R.M., Almanaa T.N., Yassin M.A., Rabie G., Saleh N. Silver nanoparticles (AgNPs) biosynthesized by Aspergillus flavus KF946095; their characterization and antibacterial activity. J. Pure Appl. Microbiol. 2021;15:105–113. doi: 10.22207/JPAM.15.1.05. [DOI] [Google Scholar]
- 59.Singh A., Gaurav S.S., Shukla G., Rani P. Evaluation of mycosilver nanofungicides as potential control agent against phytophthora infestans. Plant Cell Biotechnol. Mol. Biol. 2021;22:157–168. [Google Scholar]
- 60.Guilger-Casagrande M., Germano-Costa T., Bilesky-José N., Pasquoto-Stigliani T., Carvalho L., Fraceto L.F., de Lima R. Influence of the capping of biogenic silver nanoparticles on their toxicity and mechanism of action towards Sclerotinia sclerotiorum. J. Nanobiotechnol. 2021;19:1–18. doi: 10.1186/s12951-021-00797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gnanasangeetha D., Suresh M. A review on green synthesis of metal and metal oxide nanoparticles. Nat. Environ. Pollut. Technol. 2020;19:1789–1800. doi: 10.46488/NEPT.2020.v19i05.002. [DOI] [Google Scholar]
- 62.Potbhare A.K., Chouke P.B., Mondal A., Thakare R.U., Mondal S., Chaudhary R.G., Rai A.R. Rhizoctonia solani assisted biosynthesis of silver nanoparticles for antibacterial assay. Mater. Today Proc. 2019;29:939–945. doi: 10.1016/j.matpr.2020.05.419. [DOI] [Google Scholar]
- 63.Sopan Namdev N., Pravin Onkar P. Green Synthesis of Silver Nanoparticles: An Eco- Friendly Approach. Nano Biomed. Eng. 2020;12:281–296. doi: 10.5101/nbe.v12i4.p281-296.Sopan. [DOI] [Google Scholar]
- 64.Çağrı Mehmetoğlu A., Sezer E., Erol S. Development of antimicrobial whey protein-based film containing silver nanoparticles biosynthesised by Aspergillus Niger. Int. J. Food Sci. Technol. 2021;56:965–973. doi: 10.1111/ijfs.14749. [DOI] [Google Scholar]
- 65.Rizwan M., Amin S., Kudaibergenova B.M., Rauf A., Siddique M., Ullah K., Bawazeer S., Farooq U., Mabkhot Y.N., Ramadan M.F. Green synthesis and antimicrobial potential of silver Nanoparticles with Boerhavia procumbens extract. J. Pure Appl. Microbiol. 2020;14:1437–1451. doi: 10.22207/JPAM.14.2.42. [DOI] [Google Scholar]
- 66.Huang W., Yan M., Duan H., Bi Y., Cheng X., Yu H. Synergistic Antifungal Activity of Green Synthesized Silver Nanoparticles and Epoxiconazole against Setosphaeria turcica. J. Nanomater. 2020;2020 doi: 10.1155/2020/9535432. [DOI] [Google Scholar]
- 67.Almaary K.S., Sayed S.R.M., Abd-Elkader O.H., Dawoud T.M., El Orabi N.F., Elgorban A.M. Complete green synthesis of silver-nanoparticles applying seed-borne Penicillium duclauxii. Saudi J. Biol. Sci. 2020;27:1333–1339. doi: 10.1016/j.sjbs.2019.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soliman A.M., Abdel-Latif W., Shehata I.H., Fouda A., Abdo A.M., Ahmed Y.M. Green Approach to Overcome the Resistance Pattern of Candida spp. Using Biosynthesized Silver Nanoparticles Fabricated by Penicillium chrysogenum F9. Biol. Trace Elem. Res. 2021;199:800–811. doi: 10.1007/s12011-020-02188-7. [DOI] [PubMed] [Google Scholar]
- 69.Pandey S., De Klerk C., Kim J., Kang M., Fosso-Kankeu E. Eco friendly approach for synthesis, characterization and biological activities of milk protein stabilized silver nanoparticles. Polymers. 2020;12:1418. doi: 10.3390/polym12061418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guilger-Casagrande M., de Lima R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019;7:1–16. doi: 10.3389/fbioe.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-bahrani R.M. Green synthesized of silver nanoparticles and study their properties and applications. J. Biotechnol. Res. Cent. 2019;13:5–9. [Google Scholar]
- 72.Barbosa A.C.M.S., Costa Silva L.P., Ferraz C.M., Tobias F.L., De Araújo J.V., Loureiro B., Braga G.M.A.M., Veloso F.B.R., Soares F.E.D.F., Fronza M., et al. Nematicidal activity of silver nanoparticles from the fungus Duddingtonia flagrans. Int. J. Nanomed. 2019;14:2341–2348. doi: 10.2147/IJN.S193679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Madhankumar R., Sivasankar P., Kalaimurugan D., Murugesan S. Antibacterial and Larvicidal Activity of Silver Nanoparticles Synthesized by the Leaf Extract of Andrographis serpyllifolia Wight. J. Clust. Sci. 2020;31:719–726. doi: 10.1007/s10876-019-01679-5. [DOI] [Google Scholar]
- 74.Noshad A., Iqbal M., Folkers L., Hetherington C., Khan A., Numan M., Ullah S. Antibacterial Effect of Silver Nanoparticles (AgNPs) Synthesized from Trichoderma Harzianum against Clavibacter Michiganensis. J. Nano Res. 2019;58:10–19. doi: 10.4028/www.scientific.net/JNanoR.58.10. [DOI] [Google Scholar]
- 75.Bagur H., Poojari C.C., Melappa G., Rangappa R., Chandrasekhar N., Somu P. Biogenically Synthesized Silver Nanoparticles Using Endophyte Fungal Extract of Ocimum tenuiflorum and Evaluation of Biomedical Properties. J. Clust. Sci. 2020;31:1241–1255. doi: 10.1007/s10876-019-01731-4. [DOI] [Google Scholar]
- 76.Gemishev O.T., Panayotova M.I., Panayotov V.T. Biosynthesis of silver nanoparticles by cell-free extract from Trichoderma reesei—Study on the influence of growth media. IOP Conf. Ser. Mater. Sci. Eng. 2021;1117:012007. doi: 10.1088/1757-899X/1117/1/012007. [DOI] [Google Scholar]
- 77.Avilala J., Golla N. Antibacterial and Antiviral Properties of Silver Nanoparticles Synthesized By Marine Actinomycetes. Int. J. Pharm. Sci. Res. 2019;10:1223–1228. doi: 10.13040/IJPSR.0975-8232.10(3).1223-28. [DOI] [Google Scholar]
- 78.Lee S.H., Jun B.H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019;20:865. doi: 10.3390/ijms20040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rani P., Kumar V., Pal P., Singh A., Zhang W. Highly stable AgNPs prepared via a novel green approach for catalytic and photocatalytic removal of biological and non-biological pollutants. Environ. Int. 2020;143:105924. doi: 10.1016/j.envint.2020.105924. [DOI] [PubMed] [Google Scholar]
- 80.Wani I.A. Review—Recent Advances in Biogenic Silver Nanoparticles & NanoComposite Based Plasmonic-Colorimetric and Electrochemical Sensors. ECS J. Solid State Sci. Technol. 2021;10:047003. doi: 10.1149/2162-8777/abf2df. [DOI] [Google Scholar]
- 81.Jain R., Mendiratta S., Kumar L., Srivastava A. Green synthesis of iron nanoparticles using Artocarpus heterophyllus peel extract and their application as a heterogeneous Fenton-like catalyst for the degradation of Fuchsin Basic dye. Curr. Res. Green Sustain. Chem. 2021;4:100086. doi: 10.1016/j.crgsc.2021.100086. [DOI] [Google Scholar]
- 82.Safipour Afshar A., Saeid Nematpour F. Evaluation of the Cytotoxic Activity of Biosynthesized Silver Nanoparticles Using Berberis vulgaris Leaf Extract. Jentashapir J. Cell. Mol. Biol. 2021;12:1–7. doi: 10.5812/jjcmb.112437. [DOI] [Google Scholar]
- 83.Swara B.J., Al-barzinji I.M. Effects of Nano Silver Particles and Gibberellic Acid on Growth and Some Physiological Characteristics of Dalbergia Sissoo Roxb. Sci. J. Univ. Zakho. 2021;9:102–108. doi: 10.25271/sjuoz.2021.9.2.789. [DOI] [Google Scholar]
- 84.Arif R., Uddin R. A review on recent developments in the biosynthesis of silver nanoparticles and its biomedical applications. Med. Devices Sens. 2021;4:1–20. doi: 10.1002/mds3.10158. [DOI] [Google Scholar]
- 85.Bahattab O., Khan I., Bawazeer S., Rauf A., Qureshi M.N., Al-Awthan Y.S., Muhammad N., Khan A., Akram M., Islam M.N., et al. Synthesis and biological activities of alcohol extract of black cumin seeds ( Bunium persicum )-based gold nanoparticles and their catalytic applications. Green Process. Synth. 2021;10:440–455. doi: 10.1515/gps-2021-0041. [DOI] [Google Scholar]
- 86.Vivehananthan K., Weligodage H. Effect of Different Process Parameters on the formation of Silver Nanoparticles using Crude and Modified Neem ( Azadirachta indica ) Leaf Extracts. USJP-Acad. J. 2021;1:266–276. doi: 10.31357/ait.v1i2.4855. [DOI] [Google Scholar]
- 87.Chen Z., Niu J., Guo Z., Sui X., Xu N., Kareem H.A., Hassan M.U., Zhang Q., Cui J., Wang Q. Integrating transcriptome and physiological analyses to elucidate the essential biological mechanisms of graphene phytotoxicity of alfalfa (Medicago sativa L.) Ecotoxicol. Environ. Saf. 2021;220:112348. doi: 10.1016/j.ecoenv.2021.112348. [DOI] [PubMed] [Google Scholar]
- 88.Lashin I., Fouda A., Gobouri A.A., Azab E., Mohammedsaleh Z.M., Makharita R.R. Antimicrobial and in vitro cytotoxic efficacy of biogenic silver nanoparticles (Ag-nps) fabricated by callus extract of solanum incanum L. Biomolecules. 2021;11:341. doi: 10.3390/biom11030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma Y., Kawatra A., Sharma V., Dhull D., Kaushik S., Yadav J.P., Kaushik S. In-vitro and in-silico evaluation of the anti-chikungunya potential of Psidium guajava leaf extract and their synthesized silver nanoparticles. VirusDisease. 2021;32:260–265. doi: 10.1007/s13337-021-00685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vyavahare S., Padole N.N. A REVIEW: SILVER NANOPARTICLES IN WOUND HEALING. Eur. J. Pharm. Med. Res. 2021;8:212–218. [Google Scholar]
- 91.Kyzioł A., Łukasiewicz S., Sebastian V., Kuśtrowski P., Kozieł M., Majda D., Cierniak A. Towards plant-mediated chemistry – Au nanoparticles obtained using aqueous extract of Rosa damascena and their biological activity in vitro. J. Inorg. Biochem. 2021;214 doi: 10.1016/j.jinorgbio.2020.111300. [DOI] [PubMed] [Google Scholar]
- 92.Göl F., Aygün A., Seyrankaya A., Gür T., Yenikaya C., Şen F. Green synthesis and characterization of Camellia sinensis mediated silver nanoparticles for antibacterial ceramic applications. Mater. Chem. Phys. 2020;250 doi: 10.1016/j.matchemphys.2020.123037. [DOI] [Google Scholar]
- 93.Al-Shaheen M.A.S., Owaid M.N., Muslim R.F. Synthesis and Characterization of Zinc Nanoparticles by Natural Organic Compounds Extracted from Licorice Root and their Influence on Germination of Sorghum bicolor Seeds. Jordan J. Biol. Sci. 2020;13:559–565. [Google Scholar]
- 94.Tehri N., Vashishth A., Gahlaut A., Hooda V. Biosynthesis, antimicrobial spectra and applications of silver nanoparticles: Current progress and future prospects. Inorg. Nano-Metal Chem. 2020;52:1–19. doi: 10.1080/24701556.2020.1862212. [DOI] [Google Scholar]
- 95.Fahmy S.A., Preis E., Bakowsky U., Azzazy H.M.E.S. Platinum Nanoparticles: Green Synthesis and Biomedical Applications. Molecules. 2020;25:4981. doi: 10.3390/molecules25214981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meena R.K., Meena R., Arya D.K., Jadoun S., Hada R., Kumari R. Synthesis of Silver Nanoparticles by Phyllanthus emblica Plant Extract and Their Antibacterial Activity. Mater. Sci. Res. India. 2020;17:136–145. doi: 10.13005/msri/170206. [DOI] [Google Scholar]
- 97.Anees Ahmad S., Sachi Das S., Khatoon A., Tahir Ansari M., Afzal M., Saquib Hasnain M., Kumar Nayak A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020;3:756–769. doi: 10.1016/j.mset.2020.09.002. [DOI] [Google Scholar]
- 98.Balogun S.W., James O.O., Sanusi Y.K., Olayinka O.H. Green synthesis and characterization of zinc oxide nanoparticles using bashful (Mimosa pudica), leaf extract: A precursor for organic electronics applications. SN Appl. Sci. 2020;2:1–8. doi: 10.1007/s42452-020-2127-3. [DOI] [Google Scholar]
- 99.Nguyen D.H., Lee J.S., Park K.D., Ching Y.C., Nguyen X.T., Phan V.H.G., Thi T.T.H. Green silver nanoparticles formed by Phyllanthus urinaria, Pouzolzia zeylanica, and scoparia dulcis leaf extracts and the antifungal activity. Nanomaterials. 2020;10:542. doi: 10.3390/nano10030542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manik U.P., Nande A., Raut S., Dhoble S.J. Green synthesis of silver nanoparticles using plant leaf extraction of Artocarpus heterophylus and Azadirachta indica. Results Mater. 2020;6:100086. doi: 10.1016/j.rinma.2020.100086. [DOI] [Google Scholar]
- 101.Jeyaprakash K., AlSalhi M.S., Devanesan S. Anticancer and antioxidant efficacy of silver nanoparticles synthesized from fruit of Morinda citrifolia Linn on Ehrlich ascites carcinoma mice. J. King Saud Univ.-Sci. 2020;32:3181–3186. doi: 10.1016/j.jksus.2020.09.005. [DOI] [Google Scholar]
- 102.Tag H.M. Green Synthesis and Characterization of Silver Nanoparticles Using Bauhinia Variegate Leaves Aqueous Extract. Biomed. J. Sci. Tech. Res. 2020;29 doi: 10.26717/BJSTR.2020.29.004862. [DOI] [Google Scholar]
- 103.Bhardwaj K., Dhanjal D.S., Sharma A., Nepovimova E., Kalia A., Thakur S., Bhardwaj S., Chopra C., Singh R., Verma R., et al. Conifer-derived metallic nanoparticles: Green synthesis and biological applications. Int. J. Mol. Sci. 2020;21:9028. doi: 10.3390/ijms21239028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maktumsab M.T., Guled M.B. Plant archives. Plant Arch. 2017;17:261–266. [Google Scholar]
- 105.Ankegowda V.M., Kollur S.P., Prasad S.K., Pradeep S., Dhramashekara C., Jain A.S., Prasad A., Srinivasa C., Sridhara Setty P.B., Gopinath S.M., et al. Phyto-Mediated Synthesis of Silver Nanoparticles Using Terminalia chebula Fruit Extract and Evaluation of Its Cytotoxic and Antimicrobial Potential. Molecules. 2020;25:5042. doi: 10.3390/molecules25215042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mikhailova E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020;11:84. doi: 10.3390/jfb11040084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akintelu S.A., Bo Y., Folorunso A.S. A review on synthesis, optimization, mechanism, characterization, and antibacterial application of silver nanoparticles synthesized from plants. J. Chem. 2020;2020 doi: 10.1155/2020/3189043. [DOI] [Google Scholar]
- 108.Salmen S.H., Alharbi S.A. Silver nanoparticles synthesized biogenically from Aloe fleurentiniorum extract: Characterization and antibacterial activity. Green Chem. Lett. Rev. 2020;13:1–5. doi: 10.1080/17518253.2019.1707883. [DOI] [Google Scholar]
- 109.Hassan O.M., Ibraheem I.J., Adil B.H., Obaid A.S., Salih T.A., Obaid A.S. Synthesis of Silver Nanoparticles by ecofriendly nvironmental method using Piper nigrum, Ziziphus spina-christi, and Eucalyptusglobulus extract. J. Phys. Conf. Ser. 2020;1530 doi: 10.1088/1742-6596/1530/1/012139. [DOI] [Google Scholar]
- 110.Valsalam S., Agastian P., Esmail G.A., Ghilan A.K.M., Al-Dhabi N.A., Arasu M.V. Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J. Photochem. Photobiol. B Biol. 2019;201:111670. doi: 10.1016/j.jphotobiol.2019.111670. [DOI] [PubMed] [Google Scholar]
- 111.Bahrami-Teimoori B., Nikparast Y., Hojatianfar M., Akhlaghi M., Ghorbani R., Pourianfar H.R. Characterisation and antifungal activity of silver nanoparticles biologically synthesised by Amaranthus retroflexus leaf extract. J. Exp. Nanosci. 2017;12:129–139. doi: 10.1080/17458080.2017.1279355. [DOI] [Google Scholar]
- 112.Hamidi A., Taghavizadeh Yazdi M.E., Amiri M.S., Hosseini H.A., Darroudi M. Biological synthesis of silver nanoparticles in Tribulus terrestris L. extract and evaluation of their photocatalyst, antibacterial, and cytotoxicity effects. Res. Chem. Intermed. 2019;45:2915–2925. doi: 10.1007/s11164-019-03770-y. [DOI] [Google Scholar]
- 113.Bhuvaneswari T.S., Thirugnanam T., Thirumurugan V. Phytomediated synthesis of silver nanoparticles using Cassia auriculata L: Evaluation of antibacterial and antifungal activity. Asian J. Pharm. Pharmacol. 2019;5:326–331. doi: 10.31024/ajpp.2019.5.2.16. [DOI] [Google Scholar]
- 114.Boomi P., Ganesan R.M., Poorani G., Gurumallesh Prabu H., Ravikumar S., Jeyakanthan J. Biological synergy of greener gold nanoparticles by using Coleus aromaticus leaf extract. Mater. Sci. Eng. C. 2019;99:202–210. doi: 10.1016/j.msec.2019.01.105. [DOI] [PubMed] [Google Scholar]
- 115.Chandhru M., Logesh R., Rani S.K., Ahmed N., Vasimalai N. One-pot green route synthesis of silver nanoparticles from jack fruit seeds and their antibacterial activities with escherichia coli and salmonella bacteria. Biocatal. Agric. Biotechnol. 2019;20:101241. doi: 10.1016/j.bcab.2019.101241. [DOI] [Google Scholar]
- 116.Chaudhary S., Rohilla D., Umar A., Kaur N., Shanavas A. Synthesis and characterizations of luminescent copper oxide nanoparticles: Toxicological profiling and sensing applications. Ceram. Int. 2019;45:15025–15035. doi: 10.1016/j.ceramint.2019.04.239. [DOI] [Google Scholar]
- 117.Eisa W.H., Zayed M.F., Anis B., Abbas L.M., Ali S.S.M., Mostafa A.M. Clean production of powdery silver nanoparticles using Zingiber officinale: The structural and catalytic properties. J. Clean. Prod. 2019;241:118398. doi: 10.1016/j.jclepro.2019.118398. [DOI] [Google Scholar]
- 118.Aritonang H.F., Koleangan H., Wuntu A.D. Synthesis of silver nanoparticles using aqueous extract of medicinal plants’ (impatiens balsamina and lantana camara) fresh leaves and analysis of antimicrobial activity. Int. J. Microbiol. 2019;2019 doi: 10.1155/2019/8642303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Izwanie N., Basri H., Harun Z. Biosynthesized nanoparticles from aloe vera: A brief review towards membrane technology. Malays. J. Fundam. Appl. Sci. 2019;15:895–902. [Google Scholar]
- 120.Kalaiselvi D., Mohankumar A., Shanmugam G., Nivitha S., Sundararaj P. Green synthesis of silver nanoparticles using latex extract of Euphorbia tirucalli: A novel approach for the management of root knot nematode, Meloidogyne incognita. Crop Prot. 2019;117:108–114. doi: 10.1016/j.cropro.2018.11.020. [DOI] [Google Scholar]
- 121.Anandan M., Poorani G., Boomi P., Varunkumar K., Anand K., Chuturgoon A.A., Saravanan M., Gurumallesh Prabu H. Green synthesis of anisotropic silver nanoparticles from the aqueous leaf extract of Dodonaea viscosa with their antibacterial and anticancer activities. Process Biochem. 2019;80:80–88. doi: 10.1016/j.procbio.2019.02.014. [DOI] [Google Scholar]
- 122.Nayak S., Sajankila S.P., Rao C.V., Hegde A.R., Mutalik S. Biogenic synthesis of silver nanoparticles using Jatropha curcas seed cake extract and characterization: Evaluation of its antibacterial activity. Energy Sources Part A Recover. Util. Environ. Eff. 2019;43:3415–3423. doi: 10.1080/15567036.2019.1632394. [DOI] [Google Scholar]
- 123.Thakur B.K., Kumar A., Kumar D. Green synthesis of titanium dioxide nanoparticles using Azadirachta indica leaf extract and evaluation of their antibacterial activity. S. Afr. J. Bot. 2019;124:223–227. doi: 10.1016/j.sajb.2019.05.024. [DOI] [Google Scholar]
- 124.Ajitha B., Reddy Y.A.K., Jeon H.J., Ahn C.W. Synthesis of silver nanoparticles in an eco-friendly way using Phyllanthus amarus leaf extract: Antimicrobial and catalytic activity. Adv. Powder Technol. 2018;29:86–93. doi: 10.1016/j.apt.2017.10.015. [DOI] [Google Scholar]
- 125.Labanni A., Zulhadjri Z., Handayani D., Ohya Y., Arief S. The effect of monoethanolamine as stabilizing agent in Uncaria gambir Roxb. mediated synthesis of silver nanoparticles and its antibacterial activity. J. Dispers. Sci. Technol. 2020;41:1480–1487. doi: 10.1080/01932691.2019.1626249. [DOI] [Google Scholar]
- 126.Ezealisiji K.M., Siwe-Noundou X., Maduelosi B., Nwachukwu N., Krause R.W.M. Green synthesis of zinc oxide nanoparticles using Solanum torvum (L) leaf extract and evaluation of the toxicological profile of the ZnO nanoparticles–hydrogel composite in Wistar albino rats. Int. Nano Lett. 2019;9:99–107. doi: 10.1007/s40089-018-0263-1. [DOI] [Google Scholar]
- 127.Huq M.A. Green synthesis of silver nanoparticles using pseudoduganella eburnea MAHUQ-39 and their antimicrobial mechanisms investigation against drug resistant human pathogens. Int. J. Mol. Sci. 2020;21:1510. doi: 10.3390/ijms21041510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nayak D., Ashe S., Rauta P.R., Kumari M., Nayak B. Bark extract mediated green synthesis of silver nanoparticles: Evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C. 2016;58:44–52. doi: 10.1016/j.msec.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 129.Mittal A.K., Tripathy D., Choudhary A., Aili P.K., Chatterjee A., Singh I.P., Banerjee U.C. Bio-synthesis of silver nanoparticles using Potentilla fulgens Wall. ex Hook. and its therapeutic evaluation as anticancer and antimicrobial agent. Mater. Sci. Eng. C. 2015;53:120–127. doi: 10.1016/j.msec.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 130.Hamouda R.A., Hussein M.H., Abo-elmagd R.A., Bawazir S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-49444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sukweenadhi J., Setiawan K.I., Avanti C., Kartini K., Rupa E.J., Yang D.C. Scale-up of green synthesis and characterization of silver nanoparticles using ethanol extract of Plantago major L. leaf and its antibacterial potential. S. Afr. J. Chem. Eng. 2021;38:1–8. doi: 10.1016/j.sajce.2021.06.008. [DOI] [Google Scholar]
- 132.Nahar K., Yang D.C., Rupa E.J., Khatun K., Al-Reza S.M. Eco-friendly synthesis of silver nanoparticles from clerodendrum viscosum leaf extract and its antibacterial potential. Nanomed. Res. J. 2020;5:276–287. doi: 10.22034/NMRJ.2020.03.008. [DOI] [Google Scholar]
- 133.Khatami M., Zafarnia N., Heydarpoor Bami M., Sharifi I., Singh H. Antifungal and antibacterial activity of densely dispersed silver nanospheres with homogeneity size which synthesized using chicory: An in vitro study. J. Mycol. Med. 2018;28:637–644. doi: 10.1016/j.mycmed.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 134.Adibkia K., Dizaj S.M., Zarrintan M.H., Lotfipour F., Barzegar-Jalali M. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C. 2014;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 135.Borase H.P., Salunke B.K., Salunkhe R.B., Patil C.D., Hallsworth J.E., Kim B.S., Patil S.V. Plant extract: A promising biomatrix for ecofriendly, controlled synthesis of silver nanoparticles. Appl. Biochem. Biotechnol. 2014;173:1–29. doi: 10.1007/s12010-014-0831-4. [DOI] [PubMed] [Google Scholar]
- 136.Hetta H.F., Al-Kadmy I.M.S., Khazaal S.S., Abbas S., Suhail A., El-Mokhtar M.A., Ellah N.H.A., Ahmed E.A., Abd-ellatief R.B., El-Masry E.A., et al. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2021;11:1–11. doi: 10.1038/s41598-021-90208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jahan I., IȘILDAK I. Lemon Peel Extract for Synthesizing Non-Toxic Silver Nanoparticles through One-Step Microwave-Accelerated Scheme. Dergipark. Org. Tr. 2021;4:1–10. [Google Scholar]
- 138.Siddiqui S., Rai P.K., Singh R., Kumar D. Evaluation of antimicrobial activity and biofilm inhibition potential of Nelumbo nucifera Seed Extract Encapsulated Silver Nanoparticle. Int. J. Pharm. Res. 2021;13 doi: 10.31838/ijpr/2021.13.02.494. [DOI] [Google Scholar]
- 139.Abdel-Azeem A., Nada A.A., O’Donovan A., Thakur V.K., Elkelish A. Mycogenic silver nanoparticles from endophytic Trichoderma atroviride with antimicrobial activity. J. Renew. Mater. 2020;8:171–185. doi: 10.32604/jrm.2020.08960. [DOI] [Google Scholar]
- 140.Martínez-Flores H.E., Contreras-Chávez R., Garnica-Romo M.G. Effect of Extraction Processes on Bioactive Compounds from Pleurotus ostreatus and Pleurotus djamor: Their Applications in the Synthesis of Silver Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2021;31:1406–1418. doi: 10.1007/s10904-020-01820-2. [DOI] [Google Scholar]
- 141.Torabfam M. Microwave - assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies. Green Process. Synth. 2020;9:283–293. doi: 10.1515/gps-2020-0024. [DOI] [Google Scholar]
- 142.Behdad R., Pargol M., Mirzaie A., Karizi S.Z., Noorbazargan H., Akbarzadeh I. Efflux pump inhibitory activity of biologically synthesized silver nanoparticles against multidrug-resistant Acinetobacter baumannii clinical isolates. J. Basic Microbiol. 2020;60:494–507. doi: 10.1002/jobm.201900712. [DOI] [PubMed] [Google Scholar]
- 143.Estevez M.B., Raffaelli S., Mitchell S.G., Faccio R., Alborés S. Biofilm eradication using biogenic silver nanoparticles. Molecules. 2020;25:2023. doi: 10.3390/molecules25092023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Salisu I.B., Abubakar A.S., Abdullahi M. A Novel Biosynthesis, Characterization and Antimicrobial Activity of Silver Nanoparticles Using Leaves Extract of Aloe vera Plant. Int. J. Sci. Res. 2014;3:311–314. [Google Scholar]
- 145.Magdy A., Sadaka E., Hanafy N., El-Magd M.A., Allahloubi N., El Kemary M. Green tea ameliorates the side effects of the silver nanoparticles treatment of Ehrlich ascites tumor in mice. Mol. Cell. Toxicol. 2020;16:271–282. doi: 10.1007/s13273-020-00078-6. [DOI] [Google Scholar]
- 146.Biswal S.K., Behera M., Rout A.S., Tripathy A. Green synthesis of silver nanoparticles using raw fruit extract of mimusops elengi and their antimicrobial study. Biointerface Res. Appl. Chem. 2021;11:10040–10051. doi: 10.33263/BRIAC113.1004010051. [DOI] [Google Scholar]
- 147.Babele P.K., Singh A.K., Srivastava A. Bio-Inspired Silver Nanoparticles Impose Metabolic and Epigenetic Toxicity to Saccharomyces cerevisiae. Front. Pharmacol. 2019;10:1–15. doi: 10.3389/fphar.2019.01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lagashetty A., Patil M.K., Ganiger S.K. Green Synthesis, Characterization, and Thermal Study of Silver Nanoparticles by Achras sapota, Psidium guajava, and Azadirachta indica Plant Extracts. Plasmonics. 2019;14:1219–1226. doi: 10.1007/s11468-019-00910-3. [DOI] [Google Scholar]
- 149.Al-Daami Q., Al-Mashhedy L. Hypoglycemic effect by assay some glucoregulatory enzymes and hematological parameters using silver nanoparticles of peel Raphanus sativus L aqueas extract in male Rats. J. Phys. Conf. Ser. 2019;1294 doi: 10.1088/1742-6596/1294/6/062047. [DOI] [Google Scholar]
- 150.Jogaiah S., Kurjogi M., Abdelrahman M., Hanumanthappa N., Tran L.S.P. Ganoderma applanatum-mediated green synthesis of silver nanoparticles: Structural characterization, and in vitro and in vivo biomedical and agrochemical properties. Arab. J. Chem. 2019;12:1108–1120. doi: 10.1016/j.arabjc.2017.12.002. [DOI] [Google Scholar]
- 151.Priyadarshini S., Sulava S., Bhol R., Jena S. Green synthesis of silver nanoparticles using Azadirachta indica and Ocimum sanctum leaf extract. Curr. Sci. 2019;117:1300–1307. doi: 10.18520/cs/v117/i8/1300-1307. [DOI] [Google Scholar]
- 152.Vanti G.L., Kurjogi M., Basavesha K.N., Teradal N.L., Masaphy S., Nargund V.B. Synthesis and antibacterial activity of solanum torvum mediated silver nanoparticle against Xxanthomonas axonopodis pv.punicae and Ralstonia solanacearum. J. Biotechnol. 2020;309:20–28. doi: 10.1016/j.jbiotec.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 153.Park J., Lim D.H., Lim H.J., Kwon T., Choi J.S., Jeong S., Choi I.H., Cheon J. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem. Commun. 2011;47:4382–4384. doi: 10.1039/c1cc10357a. [DOI] [PubMed] [Google Scholar]
- 154.Jacob J.M., John M.S., Jacob A., Abitha P., Kumar S.S., Rajan R., Natarajan S., Pugazhendhi A. Bactericidal coating of paper towels via sustainable biosynthesis of silver nanoparticles using Ocimum sanctum leaf extract. Mater. Res. Express. 2019;6 doi: 10.1088/2053-1591/aafaed. [DOI] [Google Scholar]
- 155.Saravanan M., Arokiyaraj S., Lakshmi T., Pugazhendhi A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb. Pathog. 2018;117:68–72. doi: 10.1016/j.micpath.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 156.Tran Q.H., Nguyen V.Q., Le A. Corrigendum: Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives (Adv. Nat. Sci: Nanosci. Nanotechnol. 4 033001) Adv. Nat. Sci. Nanosci. Nanotechnol. 2018;9:049501. doi: 10.1088/2043-6254/aad12b. [DOI] [Google Scholar]
- 157.Panyala N.R., Peña-Méndez E.M., Havel J. Silver or silver nanoparticles: A hazardous threat to the environment and human health? J. Appl. Biomed. 2008;6:117–129. doi: 10.32725/jab.2008.015. [DOI] [Google Scholar]
- 158.Castellini C., Ruggeri S., Mattioli S., Bernardini G., Macchioni L., Moretti E., Collodel G. Long-term effects of silver nanoparticles on reproductive activity of rabbit buck. Syst. Biol. Reprod. Med. 2014;60:143–150. doi: 10.3109/19396368.2014.891163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.