Abstract
It is of great significance to popularize and apply nanotechnology in forest plantations for the high-quality development of such areas. Camphor trees have good ecological and environmental benefits and are economic, which makes them worthy of widespread popularization and promotion. In this paper, we successfully synthesized bulk and rod-like TiO2 powder and used it to study the influence of camphor seed germination and seedling growth. The germination rate, germination potential, germination index activity index of camphorwood seed during germination were measured by TiO2 solution with different morphology. Meanwhile, the fresh weight, root length and seedling height of seedlings, as well as the activities of CAT, SOD and POD and MDA content in the seedlings were measured in detail. The difference in the promoting effect between bulk and rod TiO2 powder was compared. The possible reasons are also explained. The results showed that bulk and rod-like TiO2 solution improved the activities of SOD, POD and CAT, and increased the resilience of camphor seedlings. Moreover, the rod-like TiO2 solution has a stronger osmotic effect on seed, and has a better effect on promoting seed germination and seedling growth. The study on the influence of nano-TiO2 concentration also further showed that the treatment of nano-TiO2 solution with appropriate concentration could effectively promote seed germination and seedling growth, and enhance its adoptability to adversity; but excessive concentration will bring some side effects, which was not conducive to seed germination and seedling growth. In general, the results of this study provide a theoretical basis and technical guidance for the practical application of nanotechnology in camphor seedling and afforestation production.
Keywords: camphor tree, nano titanium dioxide, seed germination, seedling growth, concentration
1. Introduction
Camphor trees are evergreen in four seasons, with a strong vitality, low soil requirement and long survival period [1], and they can be used as street trees or landscape trees. They have a good ability to protect the environment by absorbing fumes from smoking and dust, helping to form headwaters, causing sand fixation, and making the environment more picturesque. In addition, the volatile oil made from camphor trees, which as a special aroma, has many benefits, such as temperature resistance, corrosion resistance and insect elimination, and can be used to make mothballs. The trunk and fruit can be used for medicine, and the medicine made from roots is called Tu Chen Xiang, which has the effects of promoting qi, promoting blood to reduce stasi, and removing arthralgia caused by wind, moisture or cold temperatures [2]. The latest research shows that camphor tree oil also has the function of reducing visceral fat and improving blood lipids [3]. Based on the above characteristics, the camphor tree was selected as the city tree of Loudi City, Hunan Province in 2004.
At present, camphor trees have the problems of low seed yield, low germination rate and low seedling rate in artificial cultivation, which have become major issues to solve in current large-scale promotion and planting work [4]. Improving the germination rate and seedling growth activity is the primary technical problem regarding the artificial cultivation of the camphor tree. Therefore, it is of great practical significance to seek effective seed-soaking methods to improve seed germination and seedling growth.
In recent years, nano-TiO2 has been broadly applied in the field of biology due to its special spatial conformation, micro-electromagnetic properties, certain hormone effects and antibacterial effects [5,6,7]. Some researchers found that the appropriate concentration of TiO2 solution could improve the effects of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) at the germination stage, thereby accelerating seed germination, improving the survival rate of seedlings and improving the stress resistance of plants, which is equivalent to improving seed vigor, promoting seed germination rate and seedling growth [8,9,10]. However, most of the existing studies focus on the regulation of nano-TiO2 in the growth of various microorganisms and lower plants, while there are few studies on the effect of nano-TiO2 on the growth of higher plants. The difference in the promoting effect of nano-TiO2 with a different structure and morphology on camphor seed germination is rarely reported.
Based on the above context, in this paper, bulk and rod-like nano-TiO2 powders were synthesized and configured into a certain concentration of solution for soaking camphor seed. Taking the germination rate, germination potential, germination index and vigor index of camphor seed as indexes, combined with the fresh weight, root length and seedling height of camphor seedlings; the activities of catalase (CAT), superoxide dismutase (SOD), peroxidase (POD); and the malondialdehyde (MDA) content in seedlings, the effects of TiO2 solutions with different structures and concentrations on the germination of camphor seed were investigated. The objective of this study is to develop the application of nano-TiO2 in the field of seedling cultivation, which is of great significance to the high-quality development of camphor artificial planting technology.
2. Materials and Methods
2.1. Synthesis of Bulk and Rod-like TiO2 Nanoparticles
All the chemicals were used as received.
There were some changes made to the synthesis of spherical nano-TiO2 according to the literature [11]. In a typical synthesis, 10 mL tetra-isopropyl orthotitanate was dissolved in 90 mL isopropoxide (TTIP) to form homogeneous solution. Then, 5 mL distilled water was added to the solution in terms of a molar ratio of Ti:H2O = 1:4. Nitric acid was used to adjust the pH to restrain the hydrolysis process of the solution. The solution was vigorously stirred for 50 min. After aging for 36 h, the sols were transformed into gels. The obtained gels were dried under 100 °C for 4 h to evaporate water and organic material to the maximum extent. Then, the dry gel was sintered at 400 °C for 2 h. The dried powder was ground by agate mortar, using pestle to remove agglomerates to obtain spherical TiO2 nanoparticles, and marked as TiO2-bulk.
There were some changes made to the synthesis of rod-like TiO2 according to the literature [12]. Firstly, 1:6 molar ratio of bulk TiO2 (<5 µm, 0.05 moL/L) to sodium hydroxide (NaOH, 1.0 moL/L) solution was dissolved in 300 mL DI water, with a mild stirring at room temperature; and in a 500 mL glass beaker with vigorous stirring for 90 min at 100 °C. After 120 min, the precipitate was collected and centrifuged at 3000 r/min for 5 min. This process was repeated 3 times using ethanol. The remainder was dried in a furnace at 80 °C for 12 h. After drying the substance, it was hand ground in agate mortar with a pestle to turn it into powder form. The powder was annealed at 600 °C for 2 h and marked as TiO2-rod.
The structures and morphologies of TiO2-bulk and TiO2-rod were characterized by XRD and SEM.
2.2. Test on Camphor Seed Germination
The seed of Chinese fir were cleaned with pure water 5 times, disinfected with 0.1% (mass fraction) potassium permanganate solution for 3 h; and then cleaned with pure water to clear and remove, while standing for 3 h, the floating inferior seed. The treated full seed were immersed in nano-TiO2 solution (TiO2-bulk and TiO2-rod) with diverse concentration (50, 100, 200 and 500 mg/L) at 45 °C for 24 h, pure water treatment was used as the control group. Thirty seeds with full particles and uniform sizes were selected from each group. After immersion, those 30 seeds were cultured in an artificial climate chamber in a disposable Petri dish, which had a diameter of 9 cm and two layers of pure water infiltration filter paper. Culture conditions: at 25 °C, illumination 12 h, darkness 12 h, humidity 75%, illumination 4200 lx.
2.3. Determination of Seed Germination Index and Morphological Index
The number of seed germinations was observed every 12 h after the experiment. Seed germination was marked by a 2 mm radicle breaking through of the seed coat, and was discarded following 7 consecutive days without new seed germination. The germination rate, germination potential, germination index and vigor index of seed were calculated according to standard [13]. Under the same conditions for 15 days, the root length, seedling height and fresh weight of all camphor seedlings treated with different concentrations of nano-TiO2 solution were measured. Formulas of germination rate (G), germination potential (GP), germination index (GI) and vigor index (VI) are as follows:
| G = (amount of seeds germinated/amount of seed samples) × 100% |
| GP = (amount of seeds reaching germination peak/amount of seed samples) × 100% |
| GI = ∑ (Gt/Dt) |
| VI = GI × S |
In the formulas, Gt is the amount of germination in time t, Dt is the corresponding germination days, and S is the length of seedling root (cm).
2.4. Determination of Physiological Indexes of Seedling
Preparation of enzyme solution: 0.4 g germinated camphor seedlings were washed and dried, and put into the ice bath bowl. Subsequently, 4 mL phosphate-buffered solution (0.005 moL/L PBS pH 7.8) was added for rapid grinding to homogenize, and then 6 mL phosphate-buffered solution was added for flushing to the centrifuge tube. At 4 °C, 10,000 r/min—1 for 30 min, the supernatant acted as the enzyme solution to be tested and was placed in a 4 °C refrigerator. Superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and malondialdehyde (MDA) were measured according to literature [14] and the process explained below:
For SOD activity measurement: fresh Camphor tree seedlings (0.60 g) were ground thoroughly with a cold mortar and pestle in potassium phosphate buffer (pH 7.0, 50 mM) with 0.1 mM EDTA. The homogenate was centrifuged at 20,000 rmp for 40 min at 4 °C. The supernatant was crude enzyme extraction. Activity of SOD was measured by the photochemical method with nitro-blue tetrazolium. One unit of SOD activity was defined as the amount of enzyme required to give 50% inhibition of the rate of nitro-blue tetrazolium.
POD activity measurement: fresh Camphor tree seedlings (1.0 g) were grinded in an ice bath in 5 mL borate buffer (pH 8.7, 50 mM) containing sodium hydrogen sulfite (5 mM) and Polyvinyl Pyrrolidone (0.1 g). Centrifuging the homogenate at 10,000 rmp at 4 °C for 40 min to obtain the enzyme extraction. A substrate mixture, which contains acetate buffer (0.1 mM, pH 5.4), ortho-dianisidine (0.25% in ethyl alcohol) and 0.75% H2O2 (0.1 mM) was added to the enzyme extract (0.1 mL). POD activity was determined based on the change in absorbance of the brown guaiacol at 460 nm. AT activity was determined using UV-VIS spectra by measuring the decrease in absorbance at 240 nm for 1 min following the decomposition of H2O2. The reaction mixture contained 50 mM phosphate buffer (pH 7.0), 15 mM H2O2 and 0.1 mL enzyme extract. CAT activity was calculated from the extinction coefficient (40 mM−1 cm−1) of H2O2.
MDA contents measurement: fresh Camphor tree seedling sample (1.0 g) was added to a total of 15 mL aqueous solution, which consisted of 7.5% trichloroacetic acid (w/v) with 0.1% (w/v) of ethylenediaminetetraacetic acid and 0.1% (w/v) of propyl gallate. The mixture was homogenized with an homogenizer (Scientz-150, Ningbo, China) for 1 min at 20,000 rpm, and the volume was adjusted to 30 mL with the addition of trichloroacetic acid. The homogenate was filtered through 150 mm filter paper, and a specific volume reacted with the thiobarbituric acid reagent. HPLC was used for MDA quantification. Briefly, a total of 1 mL of extract and 3 mL of thiobarbituric acid reagent (40 mM dissolved in 2 M acetate buffer at pH 2.0) were mixed in a test tube and heated in a boiling water bath for 35 min. The reaction mixture was chilled prior to the addition of 1 mL of methanol, and 20 μL of the sample were injected into a Varian C18 HPLC column (5 μm, 150 × 4.6 mm) and held at 30 °C. The mobile phase consisting of 50 mM KH2PO4 buffer solution, methanol, and acetonitrile (72:17:11, v/v/v, pH 5.3) was pumped isocratically at 1 mL min−1.
3. Results
3.1. XRD and SEM Characterization of Nano-TiO2 with Different Morphologies
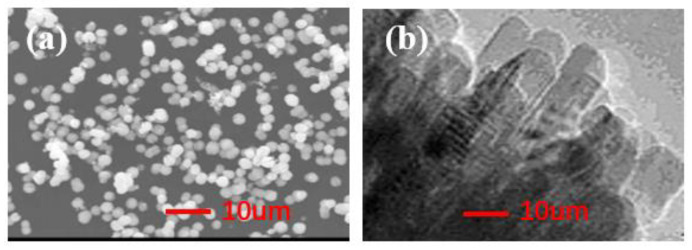
XRD and SEM characterization of bulk-TiO2 and rod-like TiO2 powders, as shown in Figure 1 and Figure 2.
Figure 1.
SEM characterization of TiO2-bulk (a) and TiO2-rod (b).
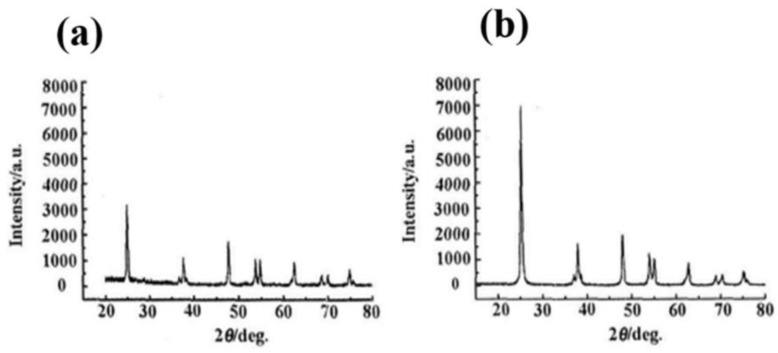
Figure 2.
The XRD patterns of TiO2-bulk (a) and TiO2-rod (b).
Figure 1 shows the SEM image of obtained TiO2 nanoparticles of a spherical and rod morphology. The TiO2-bulk sphere has a single shape and uniform dispersion. Additionally, the clear TiO2-bulk nanostructures are seen to have a grain size of ~200 nm. Moreover, it is clear show that TiO2-bulk spheres consist of a number of crystallites, which are seen by the TEM image. Thus the SEM characterization indicates the successful synthesis of TiO2 with different morphologies [15].
The XRD patterns of obtained TiO2 nanoparticles of spherical and rod morphology are shown in Figure 2a,b, respectively. The synthesized nanoTiO2 both showed a crystalline nature with 2θ peaks at 2θ = 25.25° (101), 2θ = 37.8° (004), 2θ = 47.9° (200), 2θ = 53.59° (105) and 2θ = 62.36° (204). All the peaks in the XRD patterns could be indexed as anatase phases of TiO2 and the diffraction data were in good agreement with JCPDS files # 21-1272 [15]. So, the SEM and XRD characterizations illustrate the successful synthesis of desired TiO2-bulk and TiO2-rod nanomaterials.
3.2. Germination Rate, Germination Potential, Germination Index and Vigor Index of Camphor Seed Treated with Nano-TiO2 Solutions with Different Morphologies
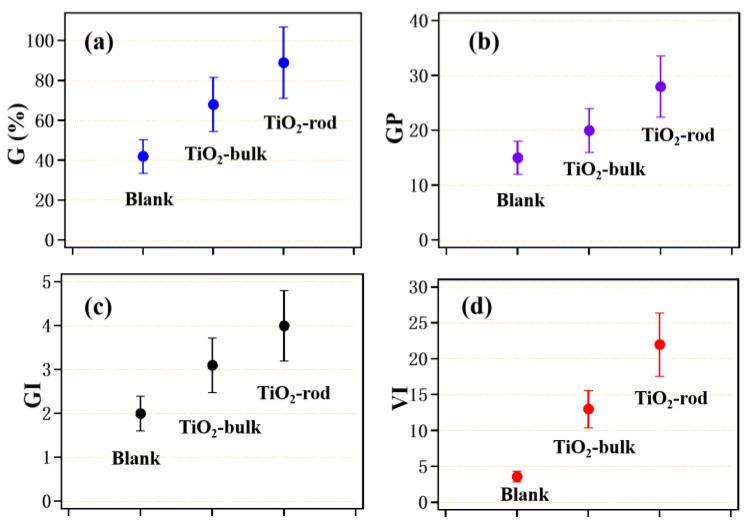
The germination rate, germination potential, germination index and vigor index of camphor seeds after soaking with 100 mg/L bulkTiO2 and rod TiO2 are shown in Figure 3.
Figure 3.
Germination rate (a); germination potential (b); germination index (c) and vigor index (d) of camphor seed soaked with liquid bulkTiO2 and rod TiO2 (concentration of 100 mg/L).
Figure 3 shows that the indexes of camphor seed soaked in bulk TiO2 and rod-like TiO2 solutions increased to different degrees. The germination rate is increased from 42 ± 8.1% of the blank sample to 68 ± 12.4% and 89 ± 14.5%, respectively. The germination potential is increased from 15 ± 3.4 of the blank sample to 20 ± 4.1 and 28 ± 0.52, respectively. The germination index is increased from 2.0 ± 0.4 of the blank sample to 3.1 ± 0.5 and 4 ± 0.7, respectively. The vigor index is increased from 3.6 ± 0.7 of the blank sample to 13.02 ± 2.7 and 22 ± 4.1, respectively.
It has been reported that plant seed soaked in nano titanium dioxide solution can absorb nanoparticles to varying degrees. The absorbed nanoparticles can promote germination in seeds [16]. Specifically, there are two reasons that liquid-phase TiO2 solution promotes camphor tree seed germination. One reason is that the concentration of nano-TiO2 enhances the activity of water to help the seeds absorb water, activate seed cells and tissues, stimulate organism function, and promote the germination and growth of Chinese fir seed. On the other hand, the seed enzyme activity is stimulated, making the free water in the seed become bound water, thereby promoting its germination. The promoting effect of rod-like TiO2 solution on seed germination is clearer than that of bulk TiO2. The reason for this may be that the shape of rod-like TiO2 in the radial direction is easier to penetrate in the organizational structure of the seed, helping the seed to absorb more water and better promote seed germination [17,18,19].
3.3. Fresh Weight, Root Length, Seedling Height, CAT, SOD, POD Activity and MDA Content of Seedlings in Nano-TiO2 Solution with Different Morphologies
Fresh weight, root length, seedling height, CAT, SOD, POD activity and MDA content of germinated camphor seedlings soaked with 100 mg/L bulk TiO2 and rod TiO2 are shown in Table 1.
Table 1.
Fresh weight, root length, seedling height, CAT, SOD, POD activity and MDA content of germinated camphor seedlings soaked with bulk TiO2 and rod TiO2.
| Solution | Fresh Weight | Root Length | Seedling Height | CTA | SOD | POD | MDA |
|---|---|---|---|---|---|---|---|
| mg | cm | cm | U/(g·min) | U/g | U/(g·min) | umoL/g | |
| Blank sample | 35.4 ± 2.3 | 1.8 ± 0.3 | 2.8 ± 0.4 | 5.2 ± 1.0 | 78.6 ± 12.2 | 48.6 ± 6.1 | 4.3 ± 0.8 |
| TiO2-bulk | 48.1 ± 4.2 | 4.2 ± 0.7 | 2.9 ± 0.3 | 11.3 ± 2.1 | 300.0 ± 42.5 | 69.7 ± 9.5 | 2.5 ± 0.4 |
| TiO2-rod | 58.7 ± 6.0 | 5.5 ± 0.9 | 2.8 ± 0.5 | 14.7 ± 2.4 | 393 ± 40.1 | 88.5 ± 11.8 | 2.1 ± 0.4 |
Table 1 shows that, compared with the blank sample, the fresh weight and root length of camphor seed germination seedlings after impregnation show a clear promotion, and the promotion effect of rod-like TiO2 solution is better than bulk TiO2 solution. However, the effect of different morphologies of TiO2 solutions on increasing seedling height is not clear. The contents of CTA, SOD and POD in the seedlings germinated after impregnation increase at different rates, while the content of MDA decreases.
Increasing the activities of CAT, SOD and POD can effectively eliminate excessive free radicals in plants, reduce membrane lipid peroxidation, and maintain the integrity of the structure and function of the membrane system [20,21]. In this study, the CAT, SOD and POD activities of camphor seed germination seedlings soaked in bulk TiO2 solution and rod TiO2 solution were significantly increased, because TiO2 can induce oxygen-free radicals. Oxygen-free radicals produced at suitable concentrations act as signals to induce the gene expression of the antioxidant enzyme system, thereby promoting the synthesis of several protective enzymes and improving the antioxidant capacity of wood seedlings [22,23]. MDA is one of the main products of lipid peroxidation in plant biofilm systems. It can cross-link with biological macromolecules, such as proteins and nucleic acids, changing the configuration of biological macromolecules and even making them lose their functions. Therefore, the amount of MDA can be used as an important indicator of the intensity of membrane lipid peroxidation and the damage degree of membrane system. In this study, different morphologies of TiO2 solution showed a good inhibitory effect on MDA in seedlings, which is another piece of evidence that it increases seedling growth.
3.4. Fresh Weight, Root Length and Seedling Height of Seedlings Treated with Different Concentrations of TiO2—Rod Solution
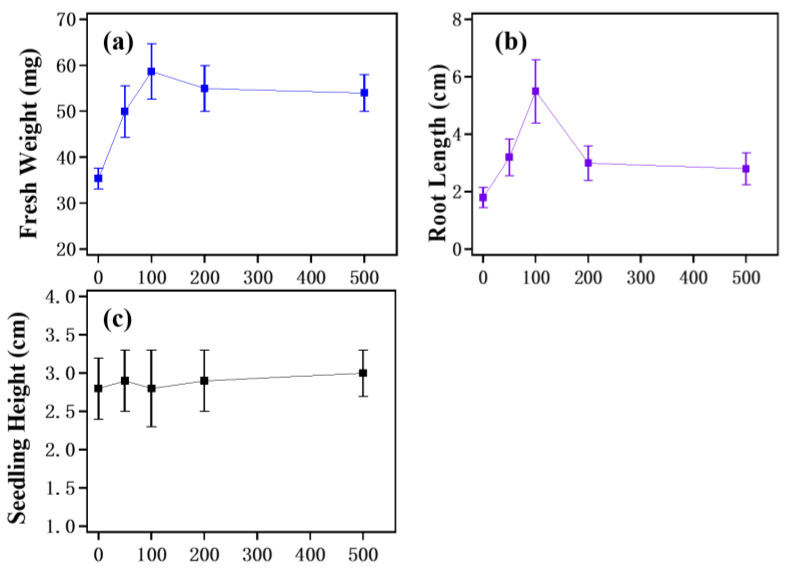
In order to further investigate the effect of the concentration of rod-like TiO2 in the impregnation solution, rod-like TiO2 with a good increasing effect on the germination and seedlings of cinnamomum camphor was taken as the object, and 0, 50, 100, 200 and 500 mg/L TiO2 solutions were selected for comparison. The fresh weight, root length and seedling height of camphor seedlings increased germination, as shown in Figure 4.
Figure 4.
Fresh weight (a), root length (b) and seedling height (c) of camphor seeds germinated after soaking with different concentrations of rod-like TiO2.
In Figure 4, compared with the blank sample, the fresh weight and root length of camphor seed germination seedlings soaked with different concentrations of rod-like TiO2 solution show a clear increase. However, when the solution concentration is greater than 100 mg/L, the seedling fresh weight stops increasing and has a promoting effect on root length. Different concentrations of rod-like TiO2 solution do not significantly promote seedling height.
As discussed in Section 3.3, different morphologies of TiO2 solution can promote the synthesis of several protective enzymes in seedlings and improve the antioxidant capacity of cinnamomum camphora seedlings. However, when the concentration of nanomaterials is too high, a large number of oxygen free radicals will be produced, destroying the cell structure and weakening these promoting effects [24]. Additionally, when the solution concentration is too high, camphor seeds will produce a cumulative effect in the process of seed soaking, resulting in a different sensitivity in the process of seedling growth and more complex results [7].
There are, of course, current records of the increasing effect of TiO2 solution with different morphologies on the seed germination and seedling growth of cinnamomum camphora, which are mostly based on the existing literature, and offer no more original evidence. In order to have a deeper understanding of the reasons behind this, the cross-disciplinary cooperation of scientists in the field of materials, forest experts and researchers other disciplines is necessary.
4. Conclusions
In this paper, bulk and rod-like TiO2 powders were successfully synthesized and their effects on the seed germination and seedling growth of cinnamomum camphora were studied. The germination rate, germination potential, germination index and vigor index of cinnamomum camphora seeds treated with different morphologies of TiO2 solution were measured in detail. At the same time, the fresh weight, root length, seedling height, CAT, SOD, POD activity and MDA content in seedlings were recorded in detail. The data showed that bulk and rod-like TiO2 solutions significantly improved the SOD, POD, CAT activity and stress resistance of camphor seedlings. In particular, rod-like TiO2 solutions have a stronger osmotic effect on seeds, which is more effective for promoting seed germination and seedling growth than bulk TiO2 solutions. However, the mechanism requires the interdisciplinary cooperation and a further in-depth study. Overall, the results of this study provide a theoretical basis and technical guidance for nano-technology (especially nano-TiO2) in camphor seedlings and afforestation production, as well as better technical support for the promotion of camphor tree planting and high-value utilization.
Author Contributions
Conceptualization, J.S.; methodology, validation, investigation, formal analysis, Y.Z., L.Z. and Y.L.; writing—original draft preparation, Y.Z. and L.Z; writing—review and editing, Y.Z., L.Z., Y.L. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Innovation Platform Open Fund project of Hunan Education Department, grant number 20K074.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rohadi D., Setyawati T., Maryani R., Riwukaho M., Gilmour D., Boroh P., Septiani Y., Lukas E. Strategies for Sustaining Sandalwood Resources in East Nusa Tenggara Indonesia. IUFRO Headquarters; Vienna, Austria: 2012. [Google Scholar]
- 2.Zhou Y., Yan W. Conservation and applications of camphor tree (Cinnamomum camphora) in China: Ethnobotany and genetic resources. Genet. Resour. Crop Evol. 2015;63:1049–1061. doi: 10.1007/s10722-015-0300-0. [DOI] [Google Scholar]
- 3.Fu J., Wang B., Gong D., Zeng C., Jiang Y., Zeng Z. Camphor Tree Seed Kernel Oil Reduces Body Fat Deposition and Improves Blood Lipids in Rats. J. Food Sci. 2015;80:H1912–H1917. doi: 10.1111/1750-3841.12943. [DOI] [PubMed] [Google Scholar]
- 4.Shi X., Dai X., Liu G., Zhang J., Ning G., Bao M. Cyclic secondary somatic embryogenesis and efficient plant regeneration in camphor tree (Cinnamomum camphora L.) Vitr. Cell. Dev. Biol. Plant. 2010;46:117–125. doi: 10.1007/s11627-009-9272-0. [DOI] [Google Scholar]
- 5.Haider A.J., Anbari R.H.A., Kadhim G.R., Salame C.-T. Exploring potential Environmental applications of TiO2 Nanoparticles. Energy Procedia. 2017;119:332–345. doi: 10.1016/j.egypro.2017.07.117. [DOI] [Google Scholar]
- 6.Ni M., Li F., Wang B., Xu K., Zhang H., Hu J., Tian J., Shen W., Li B. Effect of TiO2 Nanoparticles on the Reproduction of Silkworm. Biol. Trace Element Res. 2014;164:106–113. doi: 10.1007/s12011-014-0195-1. [DOI] [PubMed] [Google Scholar]
- 7.Saleem H., Zaidi S.J. Recent Developments in the Application of Nanomaterials in Agroecosystems. Nanomaterials. 2020;10:2411. doi: 10.3390/nano10122411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castiglione M.R., Giorgetti L., Geri C., Cremonini R. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J. Nanopart. Res. 2010;13:2443–2449. doi: 10.1007/s11051-010-0135-8. [DOI] [Google Scholar]
- 9.Mushtaq Y.K. Effect of nanoscale Fe3O4, TiO2 and carbon particles on cucumber seed germination. J. Environ. Sci. Health Part A. 2011;46:1732–1735. doi: 10.1080/10934529.2011.633403. [DOI] [PubMed] [Google Scholar]
- 10.García-Sánchez S., Gala M., Žoldák G. Nanoimpact in Plants: Lessons from the Transcriptome. Plants. 2021;10:751. doi: 10.3390/plants10040751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vijayalakshmi R., Rajendran V. Synthesis and characterization of nano-TiO2 via different methods. Arch. Appl. Sci. Res. 2012;4:1183–1190. [Google Scholar]
- 12.Danish R., Ahmed F., Arshi N., Anwar M., Koo B.H. Facile synthesis of single-crystalline rutile TiO2 nano-rods by solution method. Trans. Nonferrous Met. Soc. China. 2014;24:s152–s156. doi: 10.1016/S1003-6326(14)63303-3. [DOI] [Google Scholar]
- 13.Shao C., Wang D., Tang X., Zhao L., Li Y. Stimulating effects of magnetized arc plasma of different intensities on the germination of old spinach seeds. Math. Comput. Model. 2013;58:814–818. doi: 10.1016/j.mcm.2012.12.022. [DOI] [Google Scholar]
- 14.Liu W., Yu K., He T., Li F., Zhang D., Liu J. The Low Temperature Induced Physiological Responses of Avena nuda L., a Cold-Tolerant Plant Species. Sci. World J. 2013;2013:658793. doi: 10.1155/2013/658793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li B., Wang X., Yan M., Li L. Preparation and characterization of nano-TiO2 powder. Mater. Chem. Phys. 2003;78:184–188. doi: 10.1016/S0254-0584(02)00226-2. [DOI] [Google Scholar]
- 16.Feizi H., Moghaddam P.R., Shahtahmassebi N., Fotovat A. Impact of Bulk and Nanosized Titanium Dioxide (TiO2) on Wheat Seed Germination and Seedling Growth. Biol. Trace Element Res. 2011;146:101–106. doi: 10.1007/s12011-011-9222-7. [DOI] [PubMed] [Google Scholar]
- 17.Zheng L., Hong F., Lu S., Liu C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005;104:83–91. doi: 10.1385/BTER:104:1:083. [DOI] [PubMed] [Google Scholar]
- 18.Monica R.C., Cremonini R. Nanoparticles and higher plants. Caryologia. 2009;62:161–165. doi: 10.1080/00087114.2004.10589681. [DOI] [Google Scholar]
- 19.Hruby M., Cigler P., Kuzel S. Contribution to understanding the mechanism of titanium action in plant. J. Plant Nutr. 2002;25:577–598. doi: 10.1081/PLN-120003383. [DOI] [Google Scholar]
- 20.Navarro E., Baun A., Behra R., Hartmann N.B., Filser J., Miao A.J., Quigg A., Santschi P.H., Sigg L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology. 2008;17:372–386. doi: 10.1007/s10646-008-0214-0. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y.S., Tian S.P., Xu Y. Effects of high oxygen concentration on pro- and anti-oxidant enzymes in peach fruits during postharvest periods. Food Chem. 2005;91:99–104. doi: 10.1016/j.foodchem.2004.05.053. [DOI] [Google Scholar]
- 22.Hong F., Zhou J., Liu C., Yang F., Wu C., Zheng L., Yang P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem. Res. 2005;105:269–279. doi: 10.1385/BTER:105:1-3:269. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J., Kirkham M. Drought-Stress-Induced Changes in Activities of Superoxide Dismutase, Catalase, and Peroxidase in Wheat Species. Plant Cell Physiol. 1994;35:785–791. doi: 10.1093/oxfordjournals.pcp.a078658. [DOI] [Google Scholar]
- 24.Li J., Guo L., Cui H., Cui B., Liu G. Research Progress on Uptake and Transport of Nanopesticides in Plants. Chin. Bull. Bot. 2020;55:513–528. [Google Scholar]