Abstract
Morphology-control, as a promising and effective strategy, is widely implemented to change surface atomic active sites and thus enhance the intrinsic electrocatalytic activity and selectivity. As a typical n-type semiconductor, a series of bismuth vanadate samples with tunable morphologies of clavate, fusiform, flowered, bulky, and nanoparticles were prepared to investigate the morphology effect. Among all the synthesized samples, the clavate shaped BiVO4 with high index facets of (112), (301), and (200) exhibited reduced extrinsic pseudocapacitance and enhanced redox response, which is beneficial for tackling the sluggish voltammetric response of the traditional nanoparticle on the electrode surface. Benefiting from the large surface-active area and favorable ion diffusion channels, the clavate shaped BiVO4 exhibited the best electrochemical sensing performance for paracetamol with a linear response in the range of 0.5–100 µmol and a low detection limit of 0.2 µmol. The enhanced electrochemical detection of paracetamol by bismuth vanadate nanomaterials with controllable shapes indicates their potential for applications as electrochemical sensors.
Keywords: BiVO4, morphology control, electrochemical sensor, paracetamol
1. Introduction
Drug detection is essential in the monitoring of drug molecules in bio-fluids and plays an important role in drug quality control [1,2]. Paracetamol, also known as acetaminophen, is one of the most popular analgesics/antipyretics and has been applied in effective treatment of pain and fever in adults and children [3,4]. Paracetamol distributes rapidly after oral administration and is easily excreted in the urine. Unlike other analgesic drugs, paracetamol does not produce gastrointestinal damage or untoward cardiorenal effects [5,6]. However, the hypersensitivity or overdose of paracetamol can lead to formation of some liver and nephrotoxic metabolites, such as acute liver necrosis [7]. Moreover, the hydrolytic degradation product of paracetamol is 4-amino-phenol that can be found in pharmaceutical preparations and can cause teratogenic effect and nephrotoxicity [8].
It is desirable to develop an efficient electrochemical catalyst for paracetamol for the quality control of pharmaceuticals, physiological function, and diagnosis in clinical medicine [9]. Semiconductors have been taken as effective photocatalytic and electrochemical sensors for direct detection of paracetamol [10,11,12,13]. Therein, transition metal oxide BiVO4, with an excellent charge transport property (hole diffusion length Lp = 70 nm) [14,15], has emerged as a highly promising electrocatalytic material with good chemical stability, environmental inertness, and low cost [16,17]. Medeiros et al. reported that BiVO4 nanoparticles could be used as a highly efficient and sensitive photoelectrochemical sensor for paracetamol detection [18]. Generally speaking, morphology optimization can further enhance the electrocatalytic performance of material oxides. Control of the size and shape of material oxides is essential to optimize their active areas and favorable ion diffusion channels [19]. As a result, many efforts have been made to engineer metal oxides on the nanoscale that have led to the understanding of their fundamental size- and shape-dependent properties [20]. For example, porous BiVO4 with a larger surface area and more reactive sites compared with nanoparticle shape could afford a faster electron transfer rate, as well as higher stability and reproducibility of the sensor [21]. The ability to control the particle morphology can provide a means to tune so-called structure-sensitive catalytic reactions [22,23]. It is highly desirable to be able to synthesize electrocatalytic materials with different morphologies in a facile and controllable manner for the purpose of improving the sensitivity of paracetamol detection, as well as investigating the morphology effect on the electrochemical sensing.
In this work, the BiVO4 electrodes with different morphologies were prepared for the purpose of investigating the influence of morphology on sensitivity of electrochemical sensing. The BiVO4 samples were synthesized by the microwave approach at 180 °C for 30 min. The addition of additives and the adjustment of the pH value of solutions could change the morphology of the BiVO4 samples effectively. Further, the influence of different morphology of BiVO4 on the electrochemical detection of paracetamol was explored. Among all synthesized BiVO4 samples, the clavate morphological of the BiVO4 electrode exhibited the best electrochemical sensing performance on paracetamol with the widest linear detection range (0.5–100 µM) and lowest detection limit (0.2 µM).
2. Experimental Section
2.1. Apparatus
A microwave chemistry working platform (Model: TOPEX+, PreeKem Scientific Instruments Co., Ltd., Shanghai, China) and high-performance liquid chromatography (Model: Essentia CTO-16L, Shimadzu, Shanghai, China) with a column of 2.1 mm × 10 cm (Model: ZORBAX SB-C18, Agilent, CA, USA) were used. Scanning electron microscopy (SEM) images were obtained by Apreo (Thermo Scientific, Waltham, MA, USA). Field emission transmission electron microscopy (TEM) images were recorded using a TecnaiTM G2 F30 (FEI Co. Ltd., Hillsboro, OA, USA). X-ray diffraction (XRD) characterization was carried out on a D2 PHASER (Bruker, Karlsruhe, Germany) with Cu-Kα as the radiation source (λ = 0.154 nm). X-ray photoelectron spectroscopy (XPS, K-Alpha+, Thermo Scientific, MA, USA) was used. The C1s binding energy of adventitious carbon contamination with 284.6 eV was selected as the reference. UV–Vis diffuse reflectance spectra were recorded with a UV–Vis spectrophotometer (Model: Frontier, PerkinElmer Inc., MA, USA). All the electrochemical experiments were carried out using an electrochemical workstation (CH Instrument 660E, Shanghai Chenhua Instrument Co., Ltd., Shanghai, China). The electrochemical experiment was performed using a conventional three-electrode system with the prepared BiVO4 as the working electrode, a graphite rod as the counter electrode, and a saturated Ag/AgCl electrode as the reference electrode.
2.2. Chemicals and Reagents
Bi(NO3)3.5H2O (99.0%), NH4VO3 (99.9%), KCl (99.8%), NaH2PO4 (99.0%), Na2HPO4 (99.0%), and Na3PO4 (96%) were obtained from Aladdin Reagent (Shanghai) Co., Ltd., Shanghai, China. HCl, Na2CO3 (99.5%), and NaOH (97%) were purchased from Macklin (Shanghai) Biochemical Technology Co., Ltd., Shanghai, China. The standard drug of paracetamol tablets (over the counter, OTC) was obtained from Taiji Pharmaceutical Industrial Co., Ltd., Sichuan, China. Phosphate buffered saline (PBS) was prepared (lab temperature at 26 ± 2 °C) by 0.010 M NaH2PO4, 0.010 M Na2HPO4, and 0.050 M KCl. All solutions were prepared using ultra-pure water supplied by a Milli-Q system (Millipore, Burlington, MA, USA) with a resistivity of 18.2 MΩ cm.
2.3. Preparation of Bismuth Vanadate
The amounts of 5 mM Bi(NO3)3 and 5 mM NH4VO3 were dissolved in 10 mL ultra-pure water. Different amounts of NaH2PO4, Na2HPO4, Na3PO4, and Na2CO3 were used as additives. The amount of 1.0 M NaOH and 36% HCl solutions were used to adjust the pH values of solutions. Then 20 mL amounts of prepared solutions with different pH values were transferred to 100 mL Teflon reactors. The microwave reaction was carried out at 180 °C for 30 min. Different morphologies of BiVO4 powders were collected by centrifugation at 12,000 rpm and dried in an oven at 80 °C for 24 h. The BiVO4 electrodes with different morphologies were prepared by the spin coating method; 5 mg BiVO4 powder was dispersed in 1 mL DI water by ultrasound for 10 min. Then the BiVO4 suspension was transferred to FTO substrates by spin coating. The as-prepared BiVO4/FTO electrodes were annealed in a muffle furnace with 200 °C for 2 h for stabilization. Finally, the BiVO4/FTO electrodes with different morphologies were obtained.
2.4. Determination of Paracetamol in Tablets
High performance liquid chromatography (HPLC) was used for the estimation of the content of paracetamol in standard drug of paracetamol tablets. The method was carried out on a Hichrom C18 (25 cm × 4.6 mm i.d., 5 µm) column with a mobile phase consisting of methanol and icy ultra-pure water containing formic acid (volume ratio of 50%/49.9%/0.1%) at a flow rate of 0.2 mL min−1. Detection was carried out at 257 nm. Standard stock solutions of 0, 10, 20, 50, and 100 µmol of paracetamol were prepared in PBS (pH = 7.4), respectively. The injection volume of solution was 50 μL.
3. Results and Discussion
3.1. Characterization of BiVO4 with Different Morphologies
The synthesis procedures with different conditions to obtain different morphologies of BiVO4 are summarized in Figure S1. The morphology of bismuth vanadate could be controlled with different additives, including NaH2PO4, Na2HPO4, Na3PO4, and Na2CO3. The pH values of the solutions were adjusted in the range of 2 to 10 with HCl or NaOH. Due to the differences in ionization and hydrolysis of phosphate, carbonate, and alkaline, the morphology of bismuth vanadate could form nanosheet, tetrahedron, cuboid, sphere, or irregular shapes. In this article, several representative morphologies of bismuth vanadate were selected, which were denoted as clavate, fusiform, flowered, bulky, and particle BiVO4, as illustrated in Figure 1. Certain bismuth vanadate samples were chosen as representatives to discuss the effect of morphology on electrochemical properties of BiVO4 materials.
Figure 1.
SEM images of BiVO4 with selected morphology: (A) clavate, (B) fusiform, (C) flowered, (D) bulky, and (E) particle. (F) Schematic diagram of shape control of the bismuth vanadate.
The particle BiVO4 was obtained by adding Na3PO4 to the precursor solution (Figure 1E). Then HCl was added drop by drop to adjust the pH value to 4. The Bi3+ ions and VO3− ions combined to form nanoparticles. The size of BiVO4 nanoparticles ranged from 30 to 300 nm. The clavate and fusiform topography were obtained by adding Na2HPO4 (Figure 1A,B). The pH values of solutions were adjusted to 9.5 and 3.6, respectively. The pH value of 0.1 M Na2HPO4 solution was about 9 (Ka1 = 7.1 × 10−3). Due to the similar pH values of solution, the clavate morphology BiVO4 was formed directly and rapidly. By contrast, the fusiform BiVO4 was formed by the aggregation of nanoparticles (Figure S2), which was due to the partial dissolution and structural reorganization during the process of adjusting the pH value of the solution. The flowered BiVO4 was formed with the addition of NaH2PO4. NaH2PO4 solution is acidic (Ka1 = 6.2 × 10−8). NaOH was added slowly to change the pH value of solution to 7.7. The BiVO4 gradually assumed a cross-linked flowered-sphere structure with a diameter of ca. 5 µm (Figure 1C). The bulky topography of BiVO4 could be obtained by adding NaHCO3 and adjusting the pH value to 6 (Figure 1D). The bulky BiVO4 showed a cube structure with a hole in the center.
Due to the different hydrolysis and ionization rates of carbonate and phosphate, the pH values of the solution were different, which further affected the nucleation rate of the Bi+ in solution. A further change in the pH of the solution would lead to the dissolution or reshaping of BiVO4 samples. As a result, the morphology control of BiVO4 could be achieved with different additives and pH values.
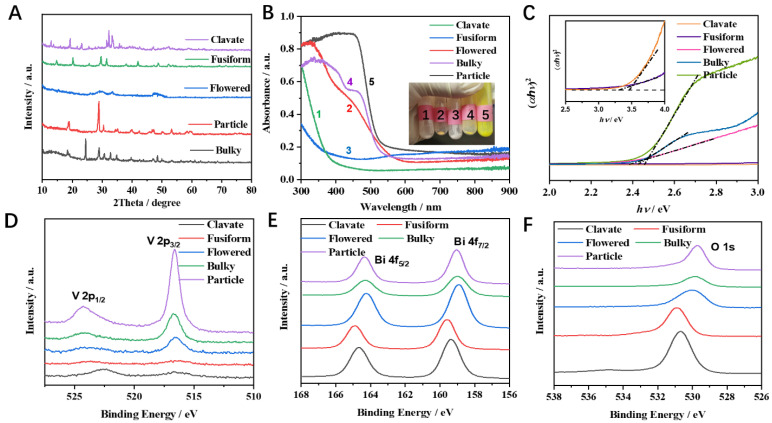
The selected BiVO4 samples were characterized with a powder X-ray diffractometer (Figure 2A). Bulky BiVO4 exhibited reflection planes (101), (200), (112), and (312) corresponding to the 2θ values of 18.3°, 24.3°, 32.7°, and 48.4°, respectively. These values are well matched with standard JCPDS file No. 14-0133, illustrating a tetragonal phase of bulky BiVO4. The clavate BiVO4 sample exhibited a tetragonal structure. Moreover, diffraction peaks of impurities (BiO2) were observed in clavate BiVO4. Particle BiVO4 exhibited sharp diffraction peaks at 18.6°, 28.6°, and 30.9°, which correspond to (101), (112), and (004) crystal planes, respectively (JCPDS file No. 48-0744). The fusiform morphological sample exhibited (011), (004), and (113) planes of orthorhombic structure as indicated by the 2θ of 19.1°, 29.8°, and 33.1° (JCPDS file No. 12-0293). Notably, there were no clear diffraction peaks in the flowered BiVO4 sample, indicating poor crystallization of the flowered BiVO4 sample. Overall, the selected BiVO4 samples exhibited different properties, which are summarized in Table 1.
Figure 2.
(A) XRD patterns, (B) UV–Vis spectra, (C) Kubelka–Munk plots of BiVO4. Inset of (B) is the photographs of clavate, fusiform, flowered, bulky, and particle from 1 to 5. High-resolution XPS spectra of (D) V 2p, (E) Bi 4f, and (F) O 1s of different morphologies BiVO4.
Table 1.
Properties of BiVO4 samples with different morphologies.
| Shape | Structure | Size | Color | Band Gap | Impurity |
|---|---|---|---|---|---|
| clavate | tetragonal | L = 5 µm W = 400 nm |
white | 3.45 eV | bismuth oxide |
| fusiform | orthorhombic | L = 5–10 µm W = 2–4 µm |
off-white | 3.30 eV | bismuth oxide |
| flowered | tetragonal | D = 5 µm | light-yellow | 2.38 eV | None |
| bulky | tetragonal | D = 600 nm | yellow | 2.40 eV | None |
| particle | tetragonal | D = 100–200 nm | yellow | 2.45 eV | None |
L = length, W = width, D = diameter.
The surface chemical states of selected BiVO4 samples were further investigated by XPS. As for the O 1s spectrum (Figure 2F), the peaks around 529.2 eV were clearly shown in particle, bulky, and flowered BiVO4 samples, which could be attributed to the lattice oxygen (O2−) in BiVO4 [24]. High-resolution XPS spectra of O 1s after peak fitting were shown in Figure S3. The peak at 529.2 eV was not observed in clavate and fusiform BiVO4 samples, while a new peak at 530.4 eV appeared, which could be ascribed to the lattice oxygen in bismuth oxide, suggesting the surface of clavate and fusiform BiVO4 were oxidized. The existence of the surface oxide layer in clavate and fusiform BiVO4 samples was further confirmed by high-resolution XPS spectra of Bi 4f (Figure 2E) and V 2p (Figure 2D). The Bi 4f of particle, bulky, and flowered BiVO4 samples showed two characteristic peaks at 164.8 eV and 159.1 eV that were attributed to Bi 4f5/2 and Bi 4f7/2, respectively. The Bi 4f of clavate and fusiform BiVO4 samples showed an apparent shift to high binding energy, attributed to the bismuth oxide. A similar phenomenon could be observed in the V 2p spectra. The V 2p peaks were not shown in clavate and fusiform BiVO4 samples due to the surface oxide layer that covered the single V orbit. XRD results show that the clavate and fusiform BiVO4, which were both synthesized with Na2HPO4, were much more unstable. The VO4 unit in BiVO4 easily formed a new bismuth oxide unit as well as oxygen vacancies on the surface of BiVO4.
3.2. Optical Analysis of BiVO4 Samples
The BiVO4 powders obtained from different precursors showed different colors (the inset of Figure 2B). The flowered, bulky, and particle BiVO4 showed yellow color with the band edge of optical absorption at around 550 nm. The clavate and fusiform BiVO4 exhibited a white color with a shift of optical absorption edge to about 400 nm (Figure 2B). The change of the color was due to the surface oxide layer of BiVO4. The UV–vis DRS data were combined with the Kubelka–Munk (K–M) relation to study the association of diffused reflectance with the absorption coefficient: F(R) = (1 − R)2/2R, where F(R) is the Kubelka–Munk function, and R is the absolute reflectance of the sample. The optical band gap of the prepared samples is calculated using Tauc’s equation: F(R) hυ = A(hυ − Eg)n, where n = 2 for a directly allowed transition, and n = 1/2 for an indirectly allowed transition, and A is a constant and hυ is photon energy [25]. According to the calculation, the band gap (Eg) of the selected BiVO4 was summarized in Table 1. The band gap of intrinsic BiVO4 is around 2.4 eV [26]. The particle, bulky, and flowered BiVO4 showed a comparable value of Eg with intrinsic BiVO4, while clavate and fusiform BiVO4 had a larger value of Eg due to the surface oxide layer. Generally speaking, BiVO4 shows excellent photoelectrochemical performance due to its suitable band gap. To investigate the influence of the solar light in photo-assisted detection of paracetamol, differential pulse voltammetry (DPV) of clavate BiVO4 on determination of paracetamol in the dark and under illumination was studied. As displayed in Figure S4, the photoresponse current on BiVO4 electrode increased from 17.5 to 21.0 mA cm−2, which indicates that solar light has a positive effect on improving the sensitivity of paracetamol detection.
3.3. Electrochemical Response at Various BiVO4 Electrodes
Electrochemical techniques have been widely explored in the detection of paracetamol in biological fluids and tablets due to their simple pretreatment procedure, high sensitivity, low time of analysis, and low costs over other analytical methods [27,28]. To understand the effect of morphology control on electrochemical performance, different morphological BiVO4 were examined in 0.01 M PBS with and without 100 µM paracetamol using CV. All the electrochemical experiments were carried out under dark conditions in order to exclude the influence of other factors.
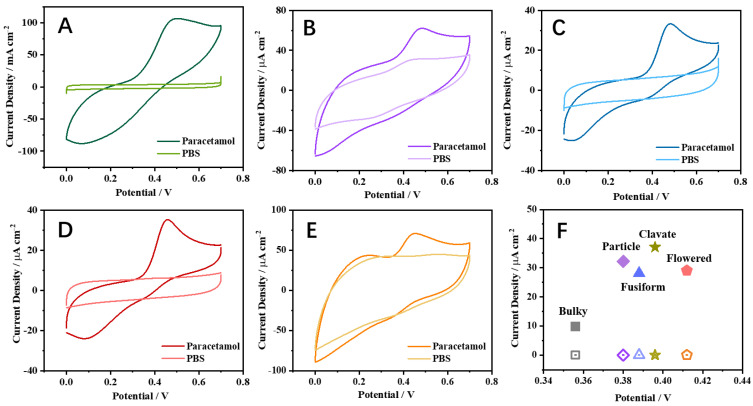
Figure 3A–E shows the CV response of the paracetamol re-dox process on different BiVO4 electrodes. The CV curves of all the BiVO4 electrodes displayed a strong anodic peak (Epa) at 0.48 V. The different peaks at BiVO4 electrodes could be observed in the process of backward scan with two small cathodic peaks (Epc), which were registered at 0.4 and 0.1 V, respectively. The chemical reaction was coupled to the electrochemical product for the oxidation of acetaminophen, i.e., N-acetyl-p-benzoquinoneimine (NAPQI) [29]. The competition between two forms of NAPQI (protonated and unprotonated species) has been proposed by Kissinger et al. [30]. The anodic peak currents in CV curves were higher than the cathodic ones, where the most probable coupled chemical reaction was the hydration of the NAPQI molecule, leading to a lower cathodic current. The limited cathodic current illustrates that the reduction of the radical intermediate is controlled by dynamics. In addition, the low cathodic current suggests that the oxidation of paracetamol on the surface of the BiVO4 electrode is more efficient than its reduction.
Figure 3.
CV curves at (A) clavate, (B) fusiform, (C) flowered, (D) bulky, and (E) particle BiVO4 in 0.01 M PBS with and without 100 µM paracetamol. Scan rate: 100 mV s−1. (F) The current densities at BiVO4 electrode in 0.01 M PBS without (hollow icon) and with (solid icon) 100 µM paracetamol from the DPV curve. The X−axis is the peak potential at different morphologies of BiVO4 from DPV curves.
In order to investigate the influence of morphology on the electrochemical performance, the compared CV results of BiVO4 with clavate, fusiform, flowered, and bulky, as well as the reference morphology of particle shape are shown in Figure 3A–E. The reference morphology of particle BiVO4 exhibited high electrochemical performance of 75 µA cm2 with 100 µM paracetamol when the applied potential was 0.5 V. However, the particle BiVO4 showed large capacitance and a sluggish voltammetric response. The pseudocapacitance could affect the redox peak during the voltammetric response, and the extrinsic pseudocapacitance arose at the electrode surfaces along with the gradual BiVO4 nanonization [31]. The bulky and flower BiVO4 exhibited low current density due to their low specific surface area and poor crystallinity, respectively (Figure 3C,D). It is noted that the fusiform BiVO4 with orthorhombic structure and clavate BiVO4 with tetragonal structure were both covered by bismuth oxides. However, the fusiform BiVO4 (Figure 3B) showed weaker catalytical performance compared with that of clavate BiVO4, illustrating the tetragonal structure is beneficial for catalytic performance. Compare to BiVO4 with other morphologies, the clavate BiVO4 (Figure 3A) exhibited the highest electrochemical performance with a maximum redox peak current of 100 mA cm−2 in the 0.1 M PBS solution containing 100 µM paracetamol, owing to the large specific surface area and surface oxygen vacancies, which could effectively improve the charge transfer characteristics and increase the charge diffusion coefficient of BiVO4. Moreover, the clavate BiVO4 could afford favorable channels for ion diffusion, which further increased the contact between the carriers and the drug molecules.
In order to suppress the influence of charging current and obtain higher sensitivity, the DPV was introduced into electrochemical analysis. The DPV experimental parameters were optimized at 50 ms pulse width, 50 mV pulse amplitude. The DPV curves of different BiVO4 samples in different concentrations of paracetamol solutions are shown in Figure S5, and the electrochemical performances on morphology-dependent BiVO4 electrodes in 0.01 M PBS without and with 100 µM paracetamol from DPV results are summarized in Figure 3F. Accordingly, clavate BiVO4 with high crystallinity showing the highest electrochemical signal was chosen to systematically investigate its paracetamol sensing performance.
3.4. Structural Characterization of Clavate BiVO4
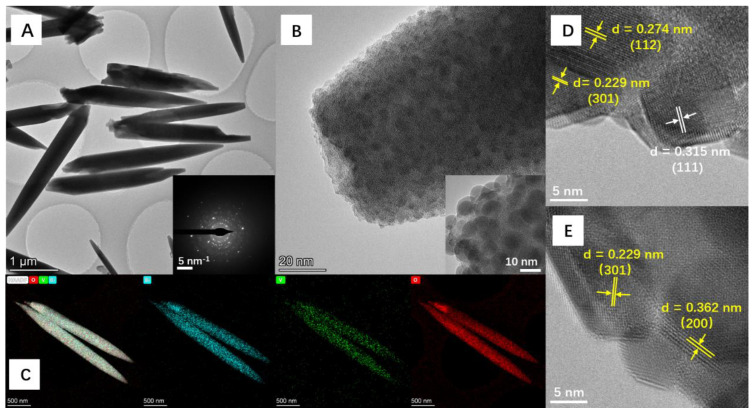
TEM characterizations were performed on the clavate BiVO4 with the length of several micrometers (Figure 4A,B). Figure 4D,E show the high magnification TEM images of clavate BiVO4 that show well-resolved lattice fringes with an interlayer spacing of 0.274, 0.229, and 0.362 nm corresponding to the (112), (301), and (200) planes of tetragonal BiVO4 (yellow color), respectively. Previous studies illustrated that BiVO4 with high-index planes promotes the catalytic activity compared with low-index (010), (110), and (101) facets [32,33]. Thus, the clavate BiVO4 with high index lanes (112), (301), and (200) at the surface could show good electrocatalytic performance. The high index lanes refer to a facet where one of the indexes is greater than 1 in (h, k, l). The lattice fringes with an interlayer spacing of 0.315 nm (white color) corresponded to the (111) crystal plane of BiO2, suggesting oxidization at the surface of BiVO4. The EDS data in Figure 4C depict the uniform distribution of Bi, V, and O elements. The TEM characterization proved the tetragonal structure of clavate BiVO4, and the surface of BiVO4 was partially covered by BiO2 nanoparticles.
Figure 4.
(A) TEM, (B,D,E) HRTEM, and (C) EDS images of clavate BiVO4. Inset of (A) is the SAED pattern, and the inset of (B) is the magnification of clavate BiVO4.
3.5. Electrochemical Investigation of Paracetamol on Clavate BiVO4
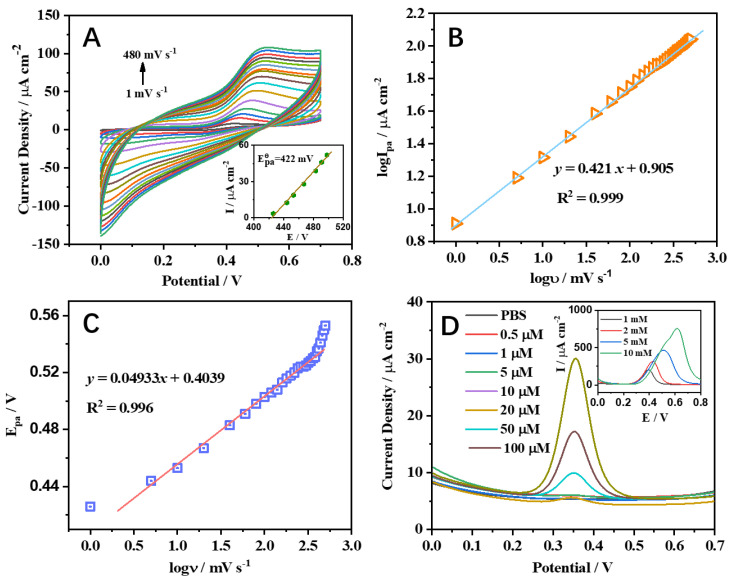
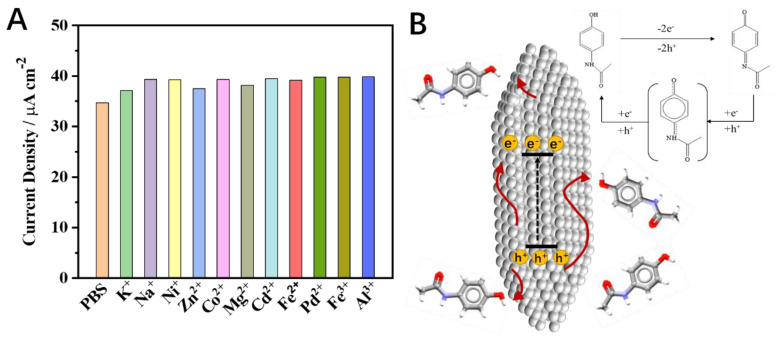
The effect of the clavate BiVO4 electrode on the electrochemical detection of paracetamol was further investigated. Cyclic voltammograms at the clavate BiVO4 electrode in 100 µM paracetamol with scan rates of 1 to 480 mV s−1 are shown in Figure 5A. The anodic peak potentials shifted with the increase of scan rate, and the anodic peak currents presented a linear dependence on the scan rate (Figure 5B). A linear regression equation was adopted for the anodic peak, logIpa = 0.421 logυ + 0.905, giving the correlation coefficient values of R2 = 0.999. It demonstrated that oxidation of paracetamol is an irreversible redox process with diffusion-controlled mass transport. The regression equation was obtained as Epa = 0.0493 logυ + 0.404 (R2 = 0.996, Figure 5C). The number of electrons involved in the reaction and the charge transfer coefficient could be calculated as 2 and 0.5, respectively. These results indicate that two electrons are involved in the electrochemical redox process of paracetamol. The paracetamol exhibited sluggish voltammetric response at the traditional electrode surface, which restricted the sensitivity of the electrochemical sensor. To solve this problem, DPV was used to improve the detection sensitivity. DPV curves of the clavate BiVO4 electrode for paracetamol determination are shown in Figure 5D. The oxidation DPV peak of paracetamol was observed at about +0.35 V on the clavate BiVO4 electrode. A linear correlation between the current density and the paracetamol concentration was obtained in the range from 5 × 10−7 to 1 × 10−5 M, which could be represented by a regression equation as follows: I = 4.65 × 10−2 c + 5.51 (R2 = 0.997). The detection limit of the sensor was calculated as 2 × 10−7 M (S/N = 3). The obtained DPV data revealed that the clavate BiVO4 electrode had more competitive analytical performance and a much lower detection limit compared with BiVO4 electrodes of other morphologies (Figure S4). The comparisons between the clavate BiVO4 electrode and some reported electrodes for paracetamol determination are summarized in Table S1. The clavate BiVO4 electrode exhibited analytical performances with acceptable sensitivity and a wide linear range. To evaluate the selectivity and stability of the clavate BiVO4 electrode, metal interference ions such as K+, Na+, Ni+, Zn2+, Co2+, Mg2+, Cd2+, Fe2+, Pd2+, Al3+, and Fe3+ were each added into a standard solution containing 100 µM paracetamol. As shown in Figure 6A, the addition of 0.10 M metal ion species did not affect the DPV current response of paracetamol on the clavate BiVO4 electrode. The result illustrates that the clavate BiVO4 electrode has excellent selectivity, even in the presence of a 1000-fold concentration of interference species.
Figure 5.
(A) CV curves at the clavate BiVO4 electrode in 100 µM paracetamol with different scan rates from 1 to 480 mV s−1. Inset of (A) is a plot of the peak current against peak potential with different scan rates. Dependence of (B) logυ−logIpa and (C) Ep−logυ at the clavate BiVO4 electrode. (D) DPV curves at clavate BiVO4 electrode with the concretion of paracetamol from 0 to 100 µM. Inset of (D) shows DPV voltammograms corresponding to the high concentration of paracetamol with a 1–10 mM range.
Figure 6.
(A) Anti-interference experiment of different ions in 100 µM paracetamol with different metal ions. The concentration of the metal ions is 0.10 M. The data of current density are collected by DPV. (B) Mechanism of the electrochemical oxidation and reduction of paracetamol.
The mechanism of the electrochemical oxidation and reduction of paracetamol is shown in Figure 6B. The applied voltage promotes the separation of electrons and holes in BiVO4. The oxidation peak of paracetamol could vary with the pH of the solution. Paracetamol converts to intermediate NAPQI easily when the pH of solution is 7.4. NAPQI can stably exist in the solution in a deprotonated form. The CV of paracetamol shows an oxidation peak and a relatively weak reduction peak. With the progress of the reaction, NAPQI gradually transforms into benzoquinone through other intermediates. The reduction peak of benzoquinone could be observed in CV.
3.6. Determination of Paracetamol in Pharmaceutical Samples
The analytical applicability of the clavate BiVO4 electrode was tested by determining the concentration of paracetamol in compound paracetamol tablets (Ⅱ) (250 mg per pill). One tablet was dissolved in 0.01 M PBS solution (pH = 7.4). The concentration of paracetamol in the measured solution with a calculated concentration of 45.0 µM was detected by HPLC and DPV methods, respectively. Compared with the HPLC results, the values obtained by the DPV method exhibited high credibility. Moreover, the DPV method showed good reliability and repeatability during three times tests. The recoveries with 96.1–101.9% are given in Table 2, indicating that the fabricated clavate BiVO4 sensor is accurate and sensitive enough for detecting paracetamol in pharmaceutical tablets. The above results show that the DPV method is a simple and reliable method to detect the content of paracetamol in drugs. The comparison between our work and some reported sensors for paracetamol determination were shown in Table S1. The clavate BiVO4 electrode shows good stability and reliability in electrochemical detection of paracetamol.
Table 2.
Analytical application of paracetamol in real samples.
| Sample | No. | Calculated/µM | Found by HPLC/µM | Found by DPV/µM | Recovery/% |
|---|---|---|---|---|---|
| Paracetamol tablets (Ⅱ) |
1 | 45.0 | 41.4 | 42.2 | 101.9 |
| 2 | 45.0 | 40.9 | 39.6 | 96.8 | |
| 3 | 45.0 | 40.8 | 39.2 | 96.1 |
4. Conclusions
In summary, the ability to control the morphology of BiVO4 has great development prospects in high sensitivity electrochemical analysis. The BiVO4 samples with different morphologies were obtained by adding different additives, and changing the pH values of the solution. The electrochemical properties of different morphology BiVO4 were systematically studied in this article. The clavate BiVO4 with high index lanes at the surface could solve the problem of the sluggish voltammetric response of the traditional nanoparticle on the electrode surface. The clavate BiVO4 also could afford favorable channels for ion diffusion, increasing the contact between the carriers and the drug molecules. Therefore, the clavate BiVO4 with a tetragonal structure exhibited the highest sensitivity and lowest detection limit among all selected BiVO4 electrodes. In the DPV mode, the clavate BiVO4 electrode showed linear responses over the concentration range of 0.5–100 µM (R2 = 0.998) for paracetamol detection, and the LOD value was found to be 0.2 µM. The study of the relationship between morphology and electrocatalytic performance could provide very important information on the reaction activity and selectivity on target.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nano12071173/s1, Figure S1: The synthesis produces of morphology-dependent BiVO4 by stirring, hydrothermal and micro wave methods. Figure S2: (A) TEM, (B) HRTEM and EDS mapping images of fusiform BiVO4. TEM images of (D) flowered and (E) particle BiVO4. Figure S3: High-resolution XPS spectra of V 2p, Bi 4f and O 1s. (A) clavate (B) fusiform (C) flowered, (D) bulky and (E) particle BiVO4. Figure S4: DPV curves of clavate BiVO4 in 0.01 M PBS with and without 100 μM paracetamol. The DPV test was performance under dark condition (labeled as dark) and in illumination condition (labeled as light). Figure S5: DPV curves at (A) fusiform (B) flowered, (C) bulky and (D) particle BiVO4 in 0.01 M PBS with 0–100 μM paracetamol. Table S1: Comparison between the present work and some reported sensors for paracetamol determination. References [34,35,36,37,38,39,40,41,42,43,44,45] are cited in the supplementary materials.
Author Contributions
Conceptualization and Writing—original draft, Y.L.; Data curation, X.X.; Funding acquisition, Y.L. and K.C.; Methodology, F.Z.; Software, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Foundation for Young Scientists of China (Grant No. 6210030074) and the National Natural Science Foundation of China (Grant No. 61975067).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon reasonable request to the authors.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alsultan A., Peloquin C.A. Therapeutic drug monitoring in the treatment of tuberculosis: An update. Drugs. 2014;74:839–854. doi: 10.1007/s40265-014-0222-8. [DOI] [PubMed] [Google Scholar]
- 2.Caldera F., Nistic R., Magnacca G., Matencio A., Monfared K., Trotta F. Magnetic composites of dextrin-based carbonate nanosponges and iron oxide nanoparticles with potential application in targeted drug delivery. Nanomaterials. 2022;12:754. doi: 10.3390/nano12050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateman D.N., Dear J.W. Acetylcysteine in paracetamol poisoning: A perspective of 45 years of use. Toxicol. Res. 2019;8:489–498. doi: 10.1039/C9TX00002J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCrae J.C., Morrison E.E., MacIntyre I.M., Dear J.W., Webb D.J. Long-term adverse effects of paracetamol-a review. Br. J. Clin. Pharmacol. 2018;84:2218–2230. doi: 10.1111/bcp.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goscianska J., Olejnik A., Ejsmont A., Galarda A., Wuttke S. Overcoming the paracetamol dose challenge with wrinkled mesoporous carbon spheres. J. Colloid Interf. Sci. 2021;15:673–682. doi: 10.1016/j.jcis.2020.10.137. [DOI] [PubMed] [Google Scholar]
- 6.Wang X., Wu Q., Liu A., Anadón A., Rodríguez J.L., Martínez-Larrañaga M.R., Yuan Z., Martínez M.A. Paracetamol: Overdose-induced oxidative stress toxicity, metabolism, and protective effects of various compounds In Vivo and In Vitro. Drug Metab. Rev. 2017;49:395–437. doi: 10.1080/03602532.2017.1354014. [DOI] [PubMed] [Google Scholar]
- 7.Boyd E.M., Eastham W.N. Liver necrosis from paracetamol. Br. Med. J. 1966;2:497–499. doi: 10.1111/j.1476-5381.1966.tb01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan Y., Liu J.H., Lu H.T., Zhang Q. Electrochemical behavior and voltammetric determination of paracetamol on Nafion/TiO2-graphene modified glassy carbon electrode. Colloids Surf. B. 2011;85:289–292. doi: 10.1016/j.colsurfb.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 9.Deroco P.B., Vicentini F.C., Fatibello-Filho O. An electrochemical Sensor for the Simultaneous determination of paracetamol and codeine using a glassy carbon electrode modified with nickel oxide nanoparticles and carbon black. Electroanalysis. 2015;27:2214–2220. doi: 10.1002/elan.201500156. [DOI] [Google Scholar]
- 10.Vinay M.M., Nayaka Y.A. Iron oxide (Fe2O3) nanoparticles modified carbon paste electrode as an advanced material for electrochemical investigation of paracetamol and dopamine. J. Sci. Adv. Mater. Dev. 2019;4:442–450. doi: 10.1016/j.jsamd.2019.07.006. [DOI] [Google Scholar]
- 11.Wu Q., Chen S., Guan L., Wu H. Highly sensitive photothermal fiber sensor based on MXene device and vernier effect. Nanomaterials. 2022;12:766. doi: 10.3390/nano12050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monsef R., Salavati-Niasari M. Electrochemical sensor based on a chitosan-molybdenum vanadate nanocomposite for detection of hydroxychloroquine in biological samples. J. Colloid Interface Sci. 2022;613:1–14. doi: 10.1016/j.jcis.2022.01.039. [DOI] [PubMed] [Google Scholar]
- 13.Zhao P., Ni M., Chen C., Zhou Z., Li X., Li C., Xie Y., Fei J. Stimuli-enabled switch-like paracetamol electrochemical sensor based on thermosensitive polymer and MWCNTs-GQDs composite nanomaterial. Nanoscale. 2019;11:7394–7403. doi: 10.1039/C8NR09434A. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.H., Lee J.S. Elaborately modified BiVO4 photoanodes for solar water splitting. Adv. Mater. 2019;31:1806938. doi: 10.1002/adma.201806938. [DOI] [PubMed] [Google Scholar]
- 15.Baral B., Reddy K.H., Parida K.M. Construction of M-BiVO4/T-BiVO4 isotype heterojunction for enhanced photocatalytic degradation of norfloxacine and oxygen evolution reaction. J. Colloid Interface Sci. 2019;554:278–295. doi: 10.1016/j.jcis.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Wang L., Bian Z. Photocatalytic degradation of paracetamol on Pd-BiVO4 under visible light irradiation. Chemosphere. 2020;239:124815. doi: 10.1016/j.chemosphere.2019.124815. [DOI] [PubMed] [Google Scholar]
- 17.Orimolade B.O., Zwane B.N., Koiki B.A., Rivallin M., Bechelany M., Mabuba N., Lesage G., Cretin M., Arotiba O.A. Coupling cathodic electro-fenton with anodic photo-electrochemical oxidation: A feasibility study on the mineralization of paracetamol. J. Environ. Chem. Eng. 2020;8:104394. doi: 10.1016/j.jece.2020.104394. [DOI] [Google Scholar]
- 18.da Silva Araújo M., Barretto T.R., Galvão J.C.R., Tarley C.R.T., Dall’Antônia L.H., de Matos R., Medeiros R.A. Visible light photoelectrochemical sensor for acetaminophen determination using a glassy carbon electrode modified with BiVO4 nanoparticles. Electroanalysis. 2021;33:663–671. doi: 10.1002/elan.202060031. [DOI] [Google Scholar]
- 19.Zaera F. Shape-controlled nanostructures in heterogeneous catalysis. ChemSusChem. 2013;6:1797–1820. doi: 10.1002/cssc.201300398. [DOI] [PubMed] [Google Scholar]
- 20.Joo J., Kim T., Lee J., Choi S., Lee K. Morphology-controlled metal sulfides and phosphides for electrochemical water splitting. Adv. Mater. 2019;31:1806682. doi: 10.1002/adma.201806682. [DOI] [PubMed] [Google Scholar]
- 21.Feng J., Li F., Liu L., Liu X., Qian Y., Ren X., Wang X., Qin W. Ultrasensitive photoelectrochemical immunosensor for procalcitonindetection with porous nanoarray BiVO4/CuxS platform as advanced signalamplification under anodic bias. Sens. Actuators B Chem. 2020;308:1276852. doi: 10.1016/j.snb.2020.127685. [DOI] [Google Scholar]
- 22.Santen R.A.V. Complementary structure sensitive and insensitive catalytic relationships. Acc. Chem. Res. 2009;42:57–66. doi: 10.1021/ar800022m. [DOI] [PubMed] [Google Scholar]
- 23.Tan X., Shen J., Semagina N., Secanell M. Decoupling structure-sensitive deactivation mechanisms of Ir/IrOx electrocatalysts toward oxygen evolution reaction. J. Catal. 2019;371:57–70. doi: 10.1016/j.jcat.2019.01.018. [DOI] [Google Scholar]
- 24.Lu H., Hao Q., Chen T., Zhang L., Chen D., Ma C., Yao W., Zhu Y. A high-performance Bi2O3/Bi2SiO5 p-n heterojunction photocatalyst induced by phase transition of Bi2O3. Appl. Catal. B Environ. 2018;237:59–67. doi: 10.1016/j.apcatb.2018.05.069. [DOI] [Google Scholar]
- 25.Lopez R., Gomez R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012;61:1–7. doi: 10.1007/s10971-011-2582-9. [DOI] [Google Scholar]
- 26.Gao Y., Li X., Hu J., Fan W., Wang F., Xu D., Ding J., Bai H., Shi W. Ag-Pi/BiVO4 heterojunction with efficient interface carrier transport for photoelectrochemical water splitting. J. Colloid Interface Sci. 2020;579:619–627. doi: 10.1016/j.jcis.2020.06.108. [DOI] [PubMed] [Google Scholar]
- 27.Wong A., Santos A.M., Fatibello-Filho O. Simultaneous determination of paracetamol and levofloxacin using a glassy carbon electrode modified with carbon black, silver nanoparticles and PEDOT:PSS film. Sens. Actuators B Chem. 2018;255:2264–2273. doi: 10.1016/j.snb.2017.09.020. [DOI] [Google Scholar]
- 28.Yang L., Zhang B., Xu B., Zhao F., Zeng B. Ionic liquid functionalized 3D graphene-carbon nanotubes–AuPd nanoparticles–molecularly imprinted copolymer based paracetamol electrochemical sensor: Preparation, characterization and application. Talanta. 2021;224:121845. doi: 10.1016/j.talanta.2020.121845. [DOI] [PubMed] [Google Scholar]
- 29.Atta N.F., Galal A., Ahmed Y.M., El-Ads E.H. Design strategy and preparation of a conductive layered electrochemical sensor for simultaneous determination of ascorbic acid, dobutamine, acetaminophen and amlodipine. Sens. Actuators B Chem. 2019;297:126648. doi: 10.1016/j.snb.2019.126648. [DOI] [Google Scholar]
- 30.Miner D.J., Rice J.R., Riggin R.M., Kissinger P.T. Voltammetry of acetaminophen and its metabolites. Anal. Chem. 1981;53:2258–2263. doi: 10.1021/ac00237a029. [DOI] [Google Scholar]
- 31.Augustyn V., Simon P., Dunn B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014;7:1597–1614. doi: 10.1039/c3ee44164d. [DOI] [Google Scholar]
- 32.Li P., Chen X., He H., Zhou X., Zhou Y., Zou Z. Polyhedral 30-faceted BiVO4 microcrystals predominantly enclosed by high-index planes promoting photocatalytic water-splitting activity. Adv. Mater. 2018;30:170311. doi: 10.1002/adma.201703119. [DOI] [PubMed] [Google Scholar]
- 33.Liu X., Chen W., Wang W., Jiang Y., Cao K., Jiao Z. F-regulate the preparation of polyhedral BiVO4 enclosed by high-Index facet and enhance its photocatalytic activity. J. Colloid Interface Sci. 2022;606:393–405. doi: 10.1016/j.jcis.2021.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Kang X., Wang J., Wu H., Liu J., Aksay I.A., Lin Y. A graphene-based electrochemical sensor for sensitive detection of paracetamol. Talanta. 2010;3:754–759. doi: 10.1016/j.talanta.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Atta N.F., Azab A.G.M. Electrochemical determination of paracetamol using gold nanoparticles-application in tablets and human fluids. Int. J. Electrochem. Sci. 2011;6:5082–5096. [Google Scholar]
- 36.Li M., Wang W., Chen Z., Song Z., Luo X. Electrochemical determination of paracetamol based on Au@graphene core-shell nanoparticles doped conducting polymer PEDOT nanocomposite. Sens. Actuat. B-Chem. 2018;260:778–785. doi: 10.1016/j.snb.2018.01.093. [DOI] [Google Scholar]
- 37.Kutluay A., Aslanoglu M. An electrochemical sensor prepared by sonochemical one-pot synthesis of multi-walled carbon nanotube-supported cobalt nanoparticles for the simultaneous determination of paracetamol and dopamine. Anal. Chim. Acta. 2014;839:59–66. doi: 10.1016/j.aca.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Li J., Liu J., Tan G., Jiang J., Peng S., Deng M., Qian D., Feng Y., Liu Y. High-sensitivity paracetamol sensor based on Pd/graphene oxide nanocomposite as an enhanced electrochemical sensing platform. Biosens. Bioelectron. 2014;54:468–475. doi: 10.1016/j.bios.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Luo J., Fan C., Wang X., Liu R., Liu X. A novel electrochemical sensor for paracetamol based on molecularly imprinted polymeric micelles. Sens. Actuat. B-Chem. 2013;188:909–916. doi: 10.1016/j.snb.2013.07.088. [DOI] [Google Scholar]
- 40.Bonyadi S., Ghanbari K., Ghiasi M. All-electrochemical synthesis of a three-dimensional mesoporous polymeric g-C3N4/PANI/CdO nanocomposite and its application as a novel sensor for the simultaneous determination of epinephrine, paracetamol, mefenamic acid, and ciprofloxacin. New J. Chem. 2020;44:3412. doi: 10.1039/C9NJ05954G. [DOI] [Google Scholar]
- 41.Wei R. Biosynthesis of Au–Ag alloy nanoparticles for sensitive electrochemical determination of paracetamol. Int. J. Electrochem. Sci. 2017;12:9131–9140. doi: 10.20964/2017.10.38. [DOI] [Google Scholar]
- 42.Asadpour-Zeynali K., Amini R. Nanostructured hexacyanoferrate intercalated Ni/Al layered double hydroxide modified electrode as a sensitive electrochemical sensor for paracetamol determination. Electroanalysis. 2017;29:635–642. doi: 10.1002/elan.201600175. [DOI] [Google Scholar]
- 43.Kumar Y., Pramanik P., Das D.K. Electrochemical detection of paracetamol and dopamine molecules using nano-particles of cobalt ferrite and manganese ferrite modified with graphite. Heliyon. 2019;5:e02031. doi: 10.1016/j.heliyon.2019.e02031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matt S.B., Raghavendra S., Shivanna M., Sidlinganahalli M., Siddalingappa D.M. Electrochemical detection of paracetamol by voltammetry techniques using pure zirconium oxide nanoparticle based modified carbon paste electrode. J. Inorg. Organomet. Polym. Mater. 2021;31:511–519. doi: 10.1007/s10904-020-01743-y. [DOI] [Google Scholar]
- 45.Zidan M., Tee T.W., Abdullah A.H., Zainal Z., Kheng G.J. Electrochemical oxidation of paracetamol mediated by nanoparticles bismuth oxide modified glassy carbon Electrode. Int. J. Electrochem. Sci. 2011;6:279–288. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon reasonable request to the authors.