Abstract
Mushrooms are known not only for their taste but also for beneficial effects on health attributed to plethora of constituents. All mushrooms belong to the kingdom of fungi, which also includes yeasts and molds. Each year, hundreds of new metabolites of the main fungal sterol, ergosterol, are isolated from fungal sources. As a rule, further testing is carried out for their biological effects, and many of the isolated compounds exhibit one or another activity. This study aims to review recent literature (mainly over the past 10 years, selected older works are discussed for consistency purposes) on the structures and bioactivities of fungal metabolites of ergosterol. The review is not exhaustive in its coverage of structures found in fungi. Rather, it focuses solely on discussing compounds that have shown some biological activity with potential pharmacological utility.
Keywords: ergosterol, ergosteroids, fungi, mushrooms, anticancer, antiviral, cytotoxicity
1. Introduction
Fungi are a rich source of chemical compounds with a wide spectrum of biological activity [1]. To survive in the environment in which they exist, they need to protect themselves from fungal infections. Therefore, it is not surprising that antimicrobial or antiviral compounds beneficial to humans can be isolated from many fungi [2]. A large number of currently used drugs have their origins in fungi [3]. Steroids occupy an important place among fungal constituents. The vast majority of them are ergosterol metabolites. The latter is the main sterol of fungi involved in the regulation of membrane fluidity and structure as well as performing immunological functions [4]. Fungal ergosterol derivatives are often referred to as “ergostane-type steroids” [5,6,7,8,9,10,11,12] or “ergosteroids” [13,14,15,16,17]. One should bear in mind, however, that the application of the term “ergosteroids” can be confusing, as it was also suggested by Lardy et al. [18] to structurally different dehydroepiandrosterone derivatives based on their mode of action (influence on energy metabolism).
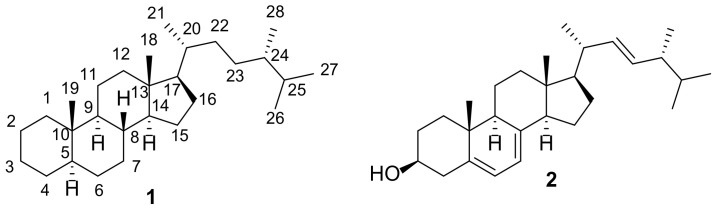
Ergostane-type steroids are characteristic not only of fungi but also of plants [19,20,21] and sponges [22]. These steroids are not a focus of the present paper. The purpose of this review is to highlight current knowledge on the structures and biological activities of fungal constituents, built on an ergostane skeleton 1 (Figure 1) or structures of which can be traced back to it. Currently, there are a number of reviews in this area dedicated to certain aspects or groups of ergostanes. A nice review on chemistry, biology, and medicinal aspects of rearranged ergostane-type natural products has been published recently by Heretsch et al. [23]. A detailed literature survey by Merdivan and Lindequist was dedicated to the consideration of biological activities of a single compound (ergosterol 5α,8α-endoperoxide) [24]. Many reviews discuss ergostane-type steroids as a part of fungal compositional diversity constituents [25,26,27,28,29,30,31,32].
Figure 1.
5α-Ergostane skeleton 1 and structure of ergosterol (2).
2. Sterols
2.1. Ergosterol
Detailed studies of the biological effects of fungi have shown that some of them can be attributed to ergosterol (2) [33,34,35,36,37,38]. That is why ergosterol itself has attracted considerable attention as a potential lead for the development of new therapeutics. Its anticancer properties were investigated on the lungs [39], liver [40,41], breast [42], human gastric [43], and prostate [44] cancer cell lines.
Ergosterol treatment of mice inoculated with breast cancer cells prolonged mouse survival [42]. Suppression of cancer cell viability was explained by apoptosis and by up-regulating Foxo3 and Foxo3 downstream molecules Bim, Fas, and Fas L.
The antitumor potential of ergosterol was studied upon its application with amphotericin B [40]. The latter is a macrolide antifungal agent that is also used to reverse chemotherapeutic drug resistance. The combined treatment of liver cancer cell lines with ergosterol followed by amphotericin B resulted in a significant decrease of their viability as a result of necrotic cell death.
Experiments on reversing multidrug resistance in cancer cells were also performed using drug-sensitive human gastric carcinoma cell line SGC7901 and its adriamycin-resistant counterpart SGC7901/Adr. Ergosterol at concentrations below 5 μM has been shown to enhance the cytotoxicity of adriamycin on SGC7901/Adr cells [43].
In experiments with Hep2 cancer cells, it was shown that ergosterol inhibited cell growth with IC50 value of 40 μM/mL [41]. The observed effect was explained by the pro-oxidant properties of ergosterol on the Hep2 cells.
Different effects have been noted for androgen-dependent LNCaP and androgen-independent DU-145 prostate cancer cells [44]. While ergosterol exerted an antiproliferative action on LNCaP, it promoted cell proliferation on DU-145. The authors [44] suggested that the observed difference may be related to the ability of ergosterol to act as a ligand for the androgen receptor.
Experiments with rats fed with a diet containing 0.1% ergosterol have shown a certain bladder carcinogenesis-preventing effect [45]. It was supposed that the observed effect is due to an androgen receptor expression-reducing action of brassicasterol (metabolite of ergosterol) on bladder epithelial cells.
Several studies have reported the anti-inflammatory effects of ergosterol. Its treatment of RAW 264.7 macrophages inhibited lipopolysaccharide-induced inflammation by suppressing the production of tumor necrosis factor-α and expression of cyclooxygenase-2 [46]. The inhibitory effect of ergosterol on degranulation of mucosal-type murine bone marrow-derived mast cells [47] or basophilic leukemia (RBL-2H3) cells [48] was associated with inhibition of β-hexosaminidase and histamine release in antigen-stimulated cells and was of interest for the treatment of allergic diseases dependent on mast cells.
Pretreatment of mice with ergosterol at doses of 25 and 50 mg/kg reduced lipopolysaccharide-induced histopathological changes in the lungs [49]. In addition, inhibition of inflammatory cells and pro-inflammatory cytokines, including tumor necrosis factor-α and interleukin-6, was observed. Similar effects were found on cigarette smoke-induced chronic obstructive pulmonary disease (COPD) in mice [50]. Besides inhibiting pro-inflammatory cytokines, ergosterol restored the activities of superoxide dismutase and reduced the content of malondialdehyde in serum and in the lung. Another study of ergosterol’s protective effect against the cigarette smoke extract-induced COPD suggested that protective effects may be related to the NF-κB/p65 signaling pathway [51].
The transcription factor Nrf2 plays an important role in controlling the expression of antioxidant genes, which ultimately leads to anti-inflammatory effects. Activation of the Nrf2 signaling pathway by ergosterol was shown to enhance cardiomyocyte resistance to oxidative stress in lipopolysaccharide- or isoproterenol-induced myocardial injury [52,53]. Oral administration of ergosterol (25 mg/kg/day) to mice for two weeks effectively delayed the progression of osteoarthritis through a mechanism involving activation of the Nrf2 pathway in primary chondrocytes [54].
Diabetic nephropathy is a chronic loss of kidney function in patients with diabetes mellitus. Ergosterol has been shown to attenuate kidney damage in diabetic mice [55,56]. It restored blood glucose and serum insulin levels and improved most biochemical and renal functional parameters. Xiong et al. [57] considered ergosterol as a potential hypoglycemic agent for the treatment of type 2 diabetes mellitus based on the discovery that it could promote glucose transporter type 4 translocation and expression, as well as glucose uptake via the PI3K (phosphatidylinositol 3-kinase) and Akt (protein kinase B) pathways. Hyperglycemia promotes the formation of advanced glycation end products (AGE) by crosslinking proteins and carbohydrates. Ergosterol prevented the suppression of oxidative stress in HSC-T6 cells and prevented age-related diseases such as liver fibrosis and diabetes [58].
An inhibitory effect of ergosterol against human recombinant aromatase (IC50 8.1 μM) was observed in aromatase inhibitory assay [59]. Potential beneficial effects against ethanol hepatotoxicity were predicted by density functional theory calculations based on the ability of ergosterol to scavenge the •CH(OH)CH3 radical [60].
The following pharmacokinetic parameters were measured after a single oral administration (100 mg/kg) of ergosterol to rats: the area under the plasma concentration versus time curve from time 0 h to 36 h (AUC0–36) was 22.3 μg h mL−1, peak plasma concentration (Cmax) was 2.27 μg/mL, the elimination half-life (t1/2) was 5.90 h, and time to Cmax (Tmax) was 8.00 h [61].
Ergosterol is an easily crystallized compound with low water and oil solubility. To increase its bioavailability, nano-sized delivery vehicles were suggested to overcome this limitation. Poly(lactide-co-glycolide) nanoparticle encapsulation allowed a 4.9-fold increase of oral bioavailability compared to free ergosterol [62]. The relative oral bioavailability of ergosterol-loaded nanostructured lipid carriers prepared using glyceryl monostearate and decanoyl/octanoyl glycerides by hot emulsification-ultrasonication was 277% higher than that of ergosterol itself [63].
In addition to being used as an active ingredient, ergosterol has also been tested as part of other drug delivery systems. The study of cellular uptake and in vitro cytotoxicity of cyclic arginine-glycine-aspartic and octa-arginine peptide-modified ergosterol-combined cisplatin liposomes showed their stability in serum and the strongest anti-lung cancer effect [39]. The encapsulation of chlorin e6 in self-assembled ergosterol nanoparticles resulted in a novel supramolecularly assembled photosensitizer [64]. When applied to cancer cells 4T1 and MCF-7, it showed remarkable in vitro phototoxicity with cell inhibition of about 73% and 92%, respectively. Evident in vitro antiproliferative activity was demonstrated for a mixture of sterols (consisting mainly of ergosterol and 22,23-dihydroergosterol) from popular edible mushroom Flammulina velutipes [65]. Encapsulation of the mixture increased the relative bioavailability of ergosterol and 22,23-dihydroergosterol to 163 and 244%, respectively.
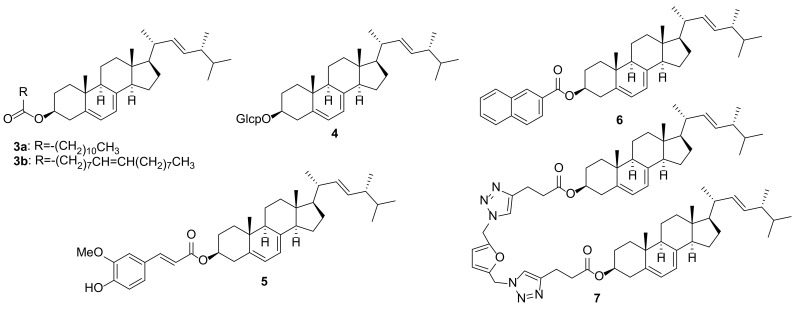
Another way to increase the bioavailability of ergosterol is the preparation of its derivatives. Direct esterification of ergosterol and lauric acid led to the coupling product ergosterol laurate (3a) (Figure 2) with solubility in vegetable oil above 5.7 g/100 mL, while for ergosterol it was below 0.9 g/100 mL [66]. Esters of unsaturated fatty acids, ergosterol oleate (3b), ergosterol linoleate, and ergosterol linolenate were prepared by transesterification reaction using Proteus vulgaris K80 lipase [67]. Their solubility in the tricaprylin solvent was 11–16 times higher than that of the initial sterol. Another ergosterol ester, α-linolenic acid derivative, was prepared using Candida sp. 99-125 lipase as a biocatalyst [68].
Figure 2.
Structures of ergosterol O-derivatives.
The glucopyranosyl derivative 4 showed slightly higher activity in inhibiting LPS-induced NO production than ergosterol (1) (IC50 16.6 and 14.3 μM, respectively) [69]. On the other hand, COX-1 enzyme inhibitory activity of 4 was weaker compared with that of the aglycone 1 [70].
Ergosterol adduct, ferulate 5, was studied for the HMG-CoA reductase inhibitory activity, which was 1.93 times higher than that of oryzanol [71]. Another adduct 6, derived from 2-naphthoic acid and ergosterol, showed stronger anti-tumor [72] and antidepressant [73] activities in vivo compared to ergosterol.
The antiproliferative effects of some ergosterol dimers have been studied against the HT29 and MCF-7 cancer cell lines [74]. The most effective was dimer 7 for the HT29 cancer cell line with an IC50 value of 160 μM. Unfortunately, the results of comparing the activity with ergosterol itself were not reported.
2.2. Other Fungal Sterols
Sterol fraction of fungi is typically a mixture of sterols [75]. As a rule, ergosterol has been considered to be its dominant component. However, this is not true in all cases. There are at least four other taxon-specific sterols (cholesterol, 24-methylenecholesterol, 24-ethylcholesterol, and brassicasterol), which may be the main sterols in some fungal species [76]. Research on the biological or pharmaceutical uses of ergostane sterols has received much less attention compared to ergosterol or functionalized ergostanes. Only sterols that have attracted attention as objects for the further in-depth study will be considered here.
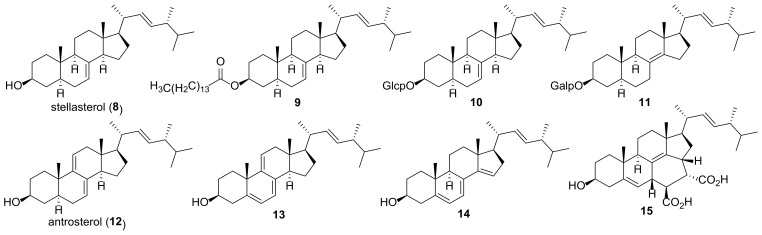
5,6-Dihydroergosterol or stellasterol (8) (Figure 3) is widely found as a minor ergostane constituent of many fungi, including sclerotia of Polyporus umbellatus [77], mycelium of Cordyceps jiangxiensis [78], Stereum insigne [79], Eurotium rubrum [80], fruiting bodies of Stropharia rugosoannulata [81], Amauroderma amoiensis [82], Amauroderma subresinosum [83], Lasiosphaera fenzlii [84], Cortinarius xiphidipus [85], Pleurotus eryngii [59], Trametes versicolor [86]. For practical purposes, a more suitable source of stellasterol (8) is its chemical synthesis from ergosterol [69,87].
Figure 3.
Structures of some fungal sterols and their derivatives.
Andrade et al. studied the effect of the purified Marthasterias glacialis extract and stellasterol (8) as its sterol constituent on inflammation in LPS-treated RAW 264.7 cells [88] and against human breast cancer (MCF-7) and human neuroblastoma (SH-SY5Y) cell lines [89]. The maximum anti-inflammatory effect was achieved when used in combination with unsaturated fatty acids [88]. In experiments with cancer cells, treatment with the extract markedly affected their growth, with stellasterol being responsible for the cell cycle arrest [89]. Yang et al. reported decreased NO production in LPS-treated RAW 264.7 cells with IC50 value of 15.1 μM and inhibition of iNOS and COX-2 [90].
The oxygen radical antioxidant capacity (ORAC) assay of components of the edible mushroom Meripilus giganteus revealed the highest antioxidant activity (4.94 mmol TE/g) for stellasterol (8) [91].
The study of the mechanism of anti-diabetic activity of the cosmopolitan woody polypore fungus Ganoderma austral showed that this may be due to its major component, stellasterol [92]. Its IC50 as an α-glucosidase inhibitor (315 μM) was close to that of acarbose (208 μM), which is an anti-diabetic drug used to treat diabetes mellitus.
Stellasterol was also isolated from fruiting bodies of Ganoderma lucidum as pentadecanoate ester (9), which at a dose 100 mg/kg bw demonstrated moderate anti-inflammatory activity (60% inhibition) in carrageenan-induced paw edema [93].
Kim et al. conducted an extensive study of the effects of synthetically obtained stellasterol glucoside (10) and its analogs on skin inflammation [69,94,95,96]. It has been shown that 10 exhibits strong inhibitory activity against the production of nitric oxide (NO), which is a molecular mediator involved in inflammation. In addition, glucoside 10 suppressed the production of Th2-type chemokines CCL17 and CCL22. It was not cytotoxic up to a concentration of 100 μM, which makes it possible to consider 10 as a potential therapeutic agent for atopic dermatitis. Further studies in this area led to the discovery of galactosyl Δ8(14)-ergostenol (11) as the best candidate for the treatment of arthritis [97].
Ergostatrienol 12 (also named as antrosterol or EK100) is a quite common steroid in fungal sources. In particular, it was isolated from Antrodia camphorate [98,99,100], Coprinus setulosus [101], Cordyceps militaris [102], Ganoderma resinaceum [103], Nigrospora sphaerica [104], Xylaria nigripes [105].
Shih et al. showed that antrosterol (12) may be useful in the treatment of type 2 diabetes associated with hyperlipidemia [98]. Its use has led to a decrease in blood glucose and total cholesterol and triglyceride levels, an increase in the GLUT4 protein in skeletal muscle, and an improvement in insulin resistance.
The anti-inflammatory properties of Antrodia camphorata mycelium, used in traditional Chinese medicine, are at least partially determined by the presence of antrosterol as one of its constituents. Similar to the action of corticosteroids, compound 12 reduced the expression of IL-6 and IL-1β in macrophages [106]. The mechanism of anti-inflammatory effect of 12 has also been studied by Kuo et al. [107]. Authors explained the observed effect by an increase in the activity of antioxidant enzymes such as catalase, superoxide dismutase, and glutathione peroxidase in liver tissue, and the reduction of the expression of iNOS and cyclooxygenase-2. The studies [108,109] also noted a decrease in the expression of the inflammatory factor NF-κB and inflammatory cytokines IL-6 and TNF-α. The mechanism of anti-inflammatory action of 12 was also investigated in LPS-stimulated RAW264.7 cells and Drosophila [102].
In experimental acute ischemic stroke model, antrosterol (12) reduced ischemic brain damage by decreasing the expression of p65NF-κB and caspase 3 and promoted neurogenesis and neuroprotection by activating PI3k/Akt-associated inhibition of GSK3 and activation of β-catenin [110]. Compound 12 was proposed as a potential therapeutic agent in intracerebral hemorrhage [111]. It had an inhibitory effect on the activation of the microglial c-Jun N-terminal kinase and attenuated the expression of brain cyclooxygenase, activation of matrix metalloproteinase and brain injuries in a model of intracerebral hemorrhage in mice. Long-term daily administration of 12 was shown to be safe and can be used as a potential ergogenic aid [112].
Hu et al. showed a strong cytotoxic effect of 12 against human U2OS lung osteosarcoma cells with IC50 value of 0.93 μM [105].
Cholesterol is a vital component of eukaryotic cells and its trafficking is an important issue for their proper functioning. 9-Dehydroergosterol (13) has proven to be a very convenient biochemical tool for studying cholesterol transport in living cells [113,114,115]. First of all, this is due to its own fluorescence because no additional moieties covalently attached to cholesterol are required. Second, 9-dehydroergosterol (13) mimics cholesterol very well, which is a consequence of its ability to stand upright in the membrane, almost identical to cholesterol.
Ano et al. found that extracts of dairy products fermented with Penicillum candidum have potent anti-inflammatory effect on microglia [116]. Repeated purification of the extracts led to the isolation of 9-dehydroergosterol (13) as an active principle responsible for the observed effect. Compound 13 significantly inhibited neurotoxicity and neuronal cell death induced by over-activated microglia, making it a valuable agent for the prevention of dementia.
Dendritic cells play a key role in regulating the balance between tolerance and immune response. It has been shown that 14-dehydroergosterol (14) induces the transformation of dendritic cells in the bone marrow of mice and differentiates them into a tolerogenic type [117]. It can be helpful in preventing chronic inflammatory and autoimmune diseases.
She et al. isolated from the mangrove-derived fungus Aspergillus sp. two steroids having a 6/6/6/6/5 pentacyclic steroidal system [118]. Ergosterdiacid A (15) was supposed to be a natural Diels-Alder product derived from fumaric acid and ergostatetraene 14. In vitro experiments showed that adduct 15 was active against Mycobacterium tuberculosis tyrosine phosphatase B (IC50 15.1 μM) and had a strong anti-inflammatory effect by suppressing NO production at 4.5 μM.
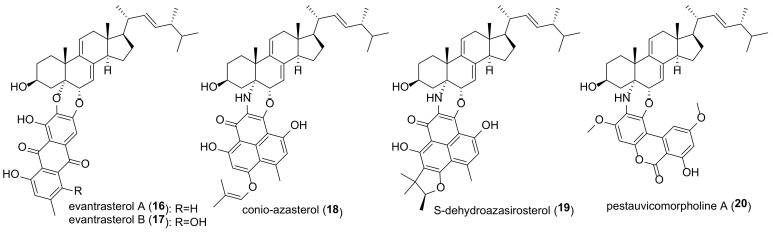
A number of hybrids of 9-dehydroergosterol with polyketides have been isolated from natural sources. Two anthraquinone derivatives, evantrasterol A and B (16 and 17) (Figure 4), have been found in the endophytic fungus Emericella variecolor [119].
Figure 4.
Structures of natural hybrids of 9-dehydroergosterol with polyketides.
Elsebai et al. isolated nitrogenous metabolites of phenalenone, conio-azasterol (18), and S-dehydroazasirosterol (19), from the marine endophytic fungus Coniothyrium cereal [120]. Another nitrogenous hybrid of 9-dehydroergosterol fused through the morpholine ring with alternariol, pestauvicomorpholine A (20), was isolated from the fermentation product of the fungus Pestalotiopsis uvicola [121]. No cytotoxicity was detected for any of the tested compounds 16–20.
3. Endoperoxides
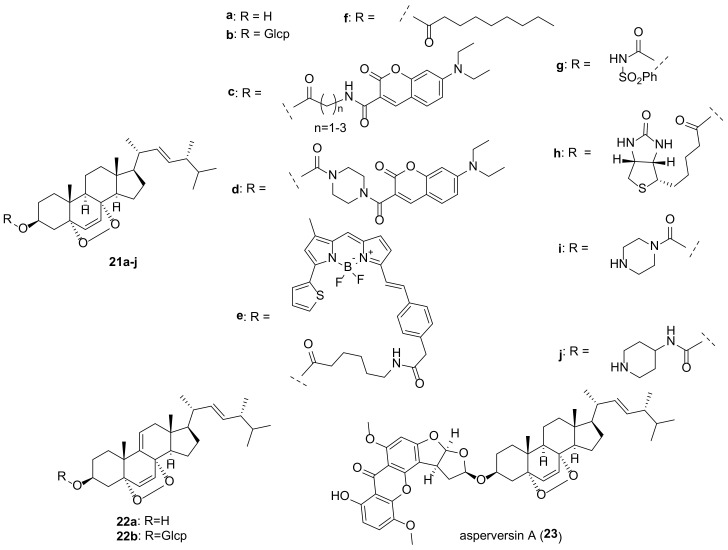
Compounds containing a peroxide group are quite widespread among various natural substances, and steroids are not an exception [27]. Two 5α,8α-endoperoxides, ergosterol peroxide (EP, 21a) and 9,11-dehydroergosterol peroxide (DHEP, 22a) (Figure 5), are the most typical representatives of fungal steroids. Publications up to 2016 on the biological activity of EP (5a) have been thoroughly reviewed by Merdivan and Lindequist [24], and only the more recent literature regarding this compound will be discussed here.
Figure 5.
Structures of fungal 5α,8α-endoperoxides and their O-derivatives.
Biological studies of endoperoxides 21a and 22a have been aimed primarily at assessing their cytotoxic potential. Both compounds revealed quite high level of cytotoxicity in a wide range of cancer cells (Table 1). It should be noted that measurements of cell toxicity often vary significantly from laboratory to laboratory. Thus, for EP and cell line MCF-7 the values of IC50 varied from IC50 1.18 μM [122] to 151 μM [123].
Table 1.
Cytotoxicity of fungal endoperoxides on different cell lines.
| Compound | Cell Line | Origin * | Effect [Ref.] |
|---|---|---|---|
| 21a | 4T1 | Mouse breast cancer | IC50 9.06 μM [138] |
| A549 | Lung carcinoma | IC50 17.04 μM [138], IC50 17.2 μM [84], IC50 > 20 μM [139], IC50 23 μM [125], IC50 57 μM [140] | |
| B 16 | Murine melanoma | IC50 78.77 μM [141] | |
| B16F10 | Murine melanoma | IC50 55.8 μM [142] | |
| BGC823 | Gastric cancer | IC50 35.23 μg/mL [137] | |
| Eca-109 | Esophageal carcinoma | IC50 23.17 μg/mL [137] | |
| DU145 | Prostate cancer | IC50 21 μg/mL [133] | |
| HCT116 | Colorectal carcinoma | IC50 80.72 μM [142] | |
| HeLa | Cervical carcinoma | IC50 13.6 μM [84], IC50 > 20 μM [139], IC50 31 μM [125], IC50 > 50 μM [143], IC50 > 50 μM [138] | |
| Hep 3B | Hepatocellular carcinoma | IC50 35.2 μg/mL [144] | |
| HepG2 | Liver carcinoma | IC50 13.19 μM [138], IC50 > 20 μM [139], IC50 23.15 μM [145], IC50 23.5 μM [146], IC50 34 μM [147], IC50 46.9 μM [144], IC50 113 μM [123] | |
| HL-60 | Promyelocytic leukemia | IC50 39.4 μM [143] | |
| HT-29 | Colon adenocarcinoma | IC50 25.47 μM [137], IC50 > 50 μM [138] | |
| J5 | Hepatocellular carcinoma | IC50 33 μM [125] | |
| L1210 | Mouse lymphotic leukemia | IC50 36.40 μM [138] | |
| LNCap | Prostate cancer | IC50 15 μg/mL [133], IC50 35.53 μg/mL [141] | |
| LS180 | Colon adenocarcinoma | IC50 17.3 μg/mL [148] | |
| MDA-MB-231 | Breast carcinoma | IC50 12.82 μM [122], EC50 18 μM [149], IC50 24.75 μM [146], IC50 44.6 μM [147] | |
| MCF-7 | Breast cancer | IC50 1.18 μM [122], IC50 9.01 μM [138], IC50 26 μM [140], IC50 26.06 μM [145,146], IC50 29 μM [125], IC50 38.2 μM [143], IC50 40 μM [124], IC50 98.12 μM [142], IC50 > 100 μM [126,144], IC50 151 μM [123] | |
| MGC-803 | Gastric carcinoma | IC50 15.2 μM [84] | |
| NCI 60 panel | significant activity against most tumor cell lines tested [132] | ||
| PC3 | Prostate cancer | IC50 42 μg/mL [133] | |
| PC-3M | Prostatic carcinoma | IC50 23.15 μM [123] | |
| RCC | Renal carcinoma | IC50 30 μM [134] | |
| SK-Hep1 | Liver cancer | IC50 19.25 μM [145], IC50 19.71 μM [146] | |
| SUM-149 | Breast cancer | EC50 9 μM [149], EC50 20 μM [150] | |
| T-47D | Breast cancer | EC50 19 μM [149] | |
| 21b | A549 | Lung carcinoma | IC50 14.21 μM [151] |
| HCT-15 | Colon adenocarcinoma | IC50 17.49 μM [151] | |
| SK-MEL-2 | Skin melanoma | IC50 9.01 μM [151] | |
| SK-OV-3 | Ovary malignant ascites | IC50 15.11 μM [151] | |
| U87 | Glioblastoma | 20.1% inhibition at 100 μM [152] | |
| 21c | HepG2 | Liver carcinoma | IC50 12.34 (n = 1), 9.46 (n = 2), 6.74 (n = 3) μM [145] |
| MCF-7 | Breast cancer | IC50 14.80 (n = 1), 13.70 (n = 2), 7.45 (n = 3) μM [145] | |
| SK-Hep1 | Liver cancer | IC50 10.43 (n = 1), 11.70 (n = 2), 5.92 (n = 3) μM [145] | |
| 21d | HepG2 | Liver carcinoma | 6.60 μM [145] |
| MCF-7 | Breast cancer | 10.62 μM [145] | |
| SK-Hep1 | Liver cancer | 8.10 μM [145] | |
| 21e | MDA-MB-231 | Breast carcinoma | EC50 7 μM [149] |
| SUM-149 | Breast cancer | EC50 2 μM [149] | |
| T-47D | Breast cancer | EC50 16 μM [149] | |
| 21f | HCT-116 | Colon carcinoma | IC50 0.21 μM [153] |
| 21g | SUM-149 | Breast cancer | EC50 12 μM [150] |
| 21h | MDA-MB-231 | Breast carcinoma | EC50 10 μM [149] |
| SUM-149 | Breast cancer | EC50 4 μM [149] | |
| T-47D | Breast cancer | EC50 > 10 μM [149] | |
| 21i | HepG2 | Liver carcinoma | IC50 0.85 μM [146] |
| MCF-7 | Breast cancer | IC50 3.26 μM [146] | |
| MDA-MB-231 | Breast carcinoma | IC50 4.12 μM [146] | |
| SK-Hep1 | Liver cancer | IC50 1.75 μM [146] | |
| 21j | HepG2 | Liver carcinoma | IC50 2.83 μM [146] |
| MCF-7 | Breast cancer | IC50 4.62 μM [146] | |
| MDA-MB-231 | Breast carcinoma | IC50 3.99 μM [146] | |
| SK-Hep1 | Liver cancer | IC50 0.92 μM [146] | |
| 22a | 4T1 | Mouse breast cancer | IC50 9.31 μM [138] |
| A375 | Malignant melanoma | IC50 9.46 μg/mL [131] | |
| A549 | Lung carcinoma | IC50 9.7 μM [84], IC50 10.77 μM [138], IC50 49 μM [125], IC50 63 μM [140], IC50 103.74 μM [154], IC50 121.9 μM [155], No cytotoxicity [156] | |
| Calu-6 | Lung carcinoma | IC50 71.2 μM [155] | |
| Colo201 | Colorectal adenocarcinoma | IC50 13.02 μg/mL [131] | |
| H1264 | Lung carcinoma | IC50 92.3 μM [155] | |
| H1299 | Lung carcinoma | IC50 50.6 μM [155] | |
| HeLa | Cervical carcinoma | IC50 7.6 μM [84], IC50 8.58 μM [130], IC50 35.82 μM [138], IC50 37 μM [125] | |
| Hep 3B | Hepatocellular carcinoma | IC50 16.7 μg/mL [127] | |
| HepG2 | Liver carcinoma | IC50 10.93 μM [138], IC50 44.5 μM [147], IC50 64.95 μM[154] | |
| HGC27 | Gastric carcinoma | IC50 26.47 μM [16] | |
| HT-29 | Colon adenocarcinoma | IC50 30.76 μM [138] | |
| J5 | Hepatocellular carcinoma | IC50 36 μM [125] | |
| L1210 | Mouse lymphotic leukemia | IC50 29.31 μM [138] | |
| MCF-7 | Breast cancer | IC50 3.3 μM [140], IC50 8.40 μM [138], IC50 16.89 μg/mL [131], IC50 34 μM [125], IC50 67.89 μg/mL [131], IC50 > 100 μM [126] | |
| MDA-MB-231 | Breast carcinoma | IC50 72.68 μM [154], IC50 99 μM [16], IC50 328 μM [147] | |
| MGC-803 | Gastric carcinoma | IC50 7.8 μM [84] | |
| Panc-28 | Pancreatic adenocarcinoma | No cytotoxicity [156] | |
| SW620 | Colorectal adenocarcinoma | IC50 32.87 μg/mL [131] | |
| 22b | A549 | Lung carcinoma | No cytotoxicity [156] |
| A549 | Lung carcinoma | IC50 15.42 μM [151] | |
| HCT-15 | Colon adenocarcinoma | IC50 19.32 μM [151] | |
| Panc-28 | Pancreatic adenocarcinoma | No cytotoxicity [156] | |
| SK-MEL-2 | Skin melanoma | IC50 12.96 μM [151] | |
| SK-OV-3 | Ovary malignant ascites | IC50 18.26 μM [151] | |
| 27 | A549 | Lung carcinoma | IC50 5.26 μg/mL [12] |
| MCF-7 | Breast cancer | IC50 5.15 μg/mL [12] | |
| 28 | A549 | Lung carcinoma | IC50 0.26 μg/mL [157] |
| HSC-3 | Oral squamous cell carcinoma | IC50 1.72 μg/mL [157] | |
| HSC-4 | Oral squamous cell carcinoma | IC50 1.94 μg/mL [157] | |
| MKN45 | Stomach adenocarcinoma | IC50 0.34 μg/mL [157] |
* Human, if not stated otherwise.
Attempts have been made to understand the cytotoxicity mechanism for 21a, and some authors have concluded that more than one mechanism is at work. Obviously, the peroxide bridge plays a crucial role, bearing in mind that ergosterol is not cytotoxic. It was assumed that induction of apoptosis is the main cause of cytotoxicity [24]. Homolytic cleavage of the peroxide moiety in a reducing medium leads to the formation of reactive oxygen species (ROS), which are powerful internal stimuli for apoptosis. This has been confirmed, in particular, in experiments with MCF-7 cells [124]. Their treatment with 21a at concentrations of 40–80 μg/mL led to an increase in the production of ROS in a dose-dependent manner and to the induction of apoptosis. The inhibitory properties of 21a against A549 lung cancer cells were mediated by mitochondria-dependent apoptosis and autophagy [125]. EP also reduced LPS/ATP-induced proliferation and migration of A549 cells. A synergistic effect was observed when using EP with kinase inhibitor Sorafenib.
Based on ID50 values for the MCF-7 cell line (1.18 μM) compared to the MDA-MB-231 cell line (12.82 μM), EP (21a) was hypothesized to target estrogen receptors [122]. Its possible role as an ERα antagonist was suggested by Kim et al. based on the suppression of the increase in the viability of MCF-7 cells caused by 17β-estradiol [126].
Ergosterol peroxide (21a) and 9,11-dehydroergosterol peroxide (22a) were often isolated from the same fungal material, and on the whole both compounds exhibit similar biological properties. DHEP (22a) was slightly more cytotoxic than EP (21a) on the Hep 3B cell viability (IC50 16.7 and 19.4 μg/mL, respectively) [127]. In experiments with BV-2 microglia cells, compound 22a did not damage cell viability, although EP was cytotoxic to these cells [128]. Kobori et al. showed that 22a selectively inhibits the growth of HT29 human colon adenocarcinoma cells without affecting normal human WI38 fibroblasts [129]. The inhibition was attributed to the induction of expression of an inhibitor of cyclin-dependent kinase 1A, thus causing cell cycle arrest and apoptosis. The rather strong cytotoxic effect of 22a (IC50 8.58 μM) on HeLa human cervical carcinoma cells was associated with the regulated expression of stathmin 1, a protein that is critical for the regulation of the cell cytoskeleton [130]. The mechanisms of 22a cytotoxicity in A375 melanoma cells have been shown to be caspase-dependent and mediated via the mitochondrial pathway and include targeting of the induced differentiation protein of myeloid leukemia cells Mcl-1, release of cytochrome c, and activation of caspase-9 and -3 [131].
In experiments with a large number of cell lines EP possessed cytotoxic activity at the level of 1 μM and was more active in comparison with DHEP [132]. On the other hand, in the aromatase inhibitory assay 9(11)-double-bond enhances the inhibitory activity (IC50 > 100 μM vs. 32.6 μM for EP and DHEP, respectively) [59].
EP was thought to be one of the main compounds responsible for the antiproliferative effect of an ethanolic extract of the native New Zealand mushroom Hericium novae-zealandiae [133]. Two possible mechanisms of the observed effect have been proposed: apoptosis based on upregulation of CASP3, CASP8, CASP9, and anti-inflammation, as follows from downregulation of IL6 and upregulation of IL24.
Studying the cytotoxic effects on renal cell carcinoma cells, Zhang et al. found that EP treatment suppressed cell growth, colonization, migration and invasion, arrested the cell cycle, and triggered apoptosis [134]. This also means that several mechanisms can act for the same effect.
A similar situation with multiple pathways was observed in experiments with ovarian cancer cells [135]. Their treatment with 21a inhibited nuclear β-catenin, thus decreasing the expression levels of cyclin D1 and c-Myc. Meanwhile, the level of protein tyrosine phosphatase SHP2 was increased in the treated cells, while the activity of Src kinase was suppressed. Thus, the antitumor effect of 21a on ovarian cancer cells is due to both the β-catenin and STAT3 signaling pathways.
Significant inhibition of the formation of experimental lung metastases in vivo was found for EP (21a) [136]. The effect was attributed to inhibition of the NF-κB and STAT3 inflammatory pathways in 4T1 breast cancer cells.
EP was more effective than cisplatin in a mouse tumor model, inhibiting CT26 cell growth and improving the survival of tumor mice with no obvious side effects [137]. The growth of tumor cells of the gastrointestinal tract was suppressed due to the induction of apoptosis by the stress of the endoplasmic reticulum and mitochondria-dependent pathway.
Compound 21a can be used as a radiosensitizer in the treatment of cervical cancer to reduce the toxic effects that occur after ionizing radiation therapy. Loss of viability of the cervical cell lines HeLa and CaSki was observed with increasing dose of 21a [158].
Biological effects of EP (21a) and its Δ9,11-counterpart 22a are not limited to their cytotoxic and anticancer properties. A detailed study on the bioactivity of the components of the truffle Reddellomyces parvulosporus revealed a number of EP activities, including anti-tyrosinase, anti-urease, anti-α-glucosidase, and anti-α-amylase ones [159]. Tyrosinase is an enzyme involved in the biosynthesis of melanin in humans, and its inhibitors are of interest for preventing excessive melanin production, as being active ingredients of skin whitening agents. Tyrosinase inhibitory activity (IC50: 202.37 μg/mL) of EP was also detected by Bai et al. [160].
Ng et al. reported the antidiabetic effect of 21a that was due to the upregulation of glucose absorption and modulation of the PI3K/Akt, MAPK, and GLUT-4 signaling pathways [161].
EP was tested for its antileishmania activity against Leishmania donovani promastigotes and showed good activity with IC50 values of 9.43 μM [162]. The EP trypanocidal activity has been associated with its interaction with CYP51 [163]. The key structural moiety responsible for this is the peroxide bridge, which mediates interaction with the CYP51 heme binding site. At a later stage, this can cause the appearance of free radicals through homolytic cleavage at the O-O site, the pharmacophore responsible for the biological activity of 21a.
Zhou et al. studied the immunoregulatory effect on inflammation caused by influenza A virus in human alveolar epithelial cells A549. EP (21a) was found to have anti-inflammatory effects and prevent virus-induced apoptosis by attenuating retinoic acid-inducible gene I signaling in infected cells [164].
Oral administration of EP to piglets infected with porcine delta-coronavirus resulted in a reduction in diarrhea, relief of intestinal damage, and a decrease in viral load in feces and tissues [165]. Wang et al. demonstrated that ergosterol peroxide prevents infection by suppressing porcine delta-coronavirus-induced autophagy via the p38 signaling pathway [166,167].
DHEP (22a) was found to exhibit strong anti-inflammatory effect in lipopolysaccharide-stimulated RAW264.7 cells [168,169,170]. It suppressed the production of NO even at 12.5 μM and pro-inflammatory cytokines interleukin 6 at 25 μM [168].
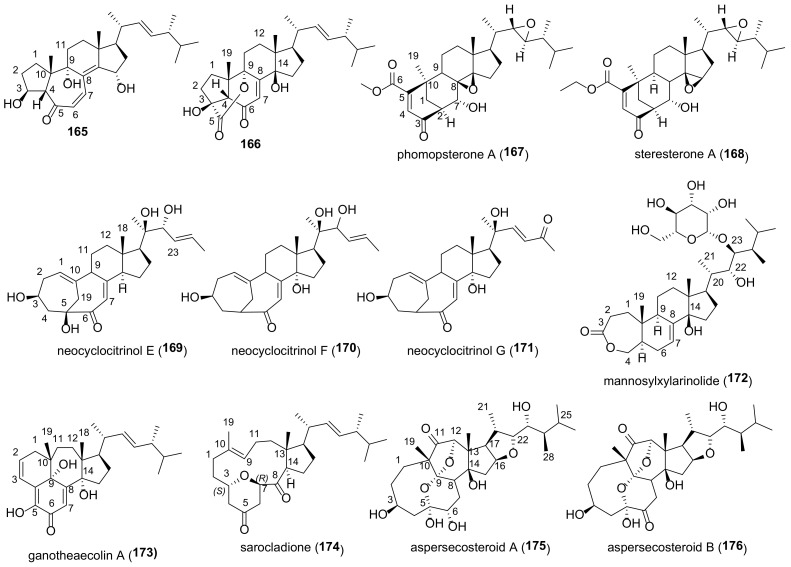
With age, mesenchymal stem cells in bone marrow tend to differentiate more into adipocytes than into osteocytes. Compounds 21a and 22a have been shown to inhibit the differentiation of mesenchymal stem cells toward adipocytes, which may be useful for the treatment of postmenopausal osteoporosis [171].
In experiments with 3T3-L1 mouse embryonic fibroblast cells, it was shown that EP inhibits triglyceride synthesis and reduces the accumulation of lipid droplets by suppressing adipogenesis [172].
The endoperoxides 21a and 22a were tested for their antibacterial activity [173,174,175,176,177]. The presence of a 9,11-double bond contributed to the increase in activity [173,177]. Thus, Δ9,11-derivative 22a was more effective against M. tuberculosis H37Rv in comparison with 21a (MIC 16 μg/mL and 64 μg/mL, respectively) [173]. Antitubercular activity of the fungus Gliocladium sp. MR41., was tested on M. tuberculosis. It was found to be due to EP (21a) with MIC 0.78 μg/mL [178].
Kim et al. isolated glucosides 21b and 22b from the Korean wild fungus Xerula furfuracea and tested their effects on adipogenesis and osteogenesis in a mouse mesenchymal stem cell line [10]. Both compounds were found to inhibit the differentiation of stem cells into adipocytes, which is of interest in the treatment of syndromes associated with menopause.
Significant antifungal and cytotoxic activities were reported for EP decanoate (21f) [153]. In disk diffusion test against Candida albicans culture, its MIC value was found to be 8.3 μg/disc that was comparable to clotrimazole (MIC 5.1 μg/disc). Compound 21f showed also very good cytotoxicity against the HCT-116 cell line with IC50 value of 0.21 μM compared to doxorubicin (IC50 0.06 μM).
In an attempt to improve antitumor activity, a number of derivatives of endoperoxide 21a have been studied. Ergosterol peroxide sulfonamide 21g was found to be more effective in reducing cancer cell viability than the parental endoperoxide 21a [150]. Significantly, its toxicity to normal human BJ fibroblasts was minimal, indicating that 21g targets cancer cells.
A series of EP analogs containing BODIPY or a biotin moiety was obtained by Rivas et al. as probes for cellular localization studies [149]. They demonstrated that EP is distributed across the cytosol with significant accumulation in the endoplasmic reticulum. In addition, the resulting compounds were tested for antiproliferative activity in breast cancer cell models. The most active ones were analogs 21e and 21h (Table 1).
Several adducts of EP with 7-N,N-diethylaminocoumarins have been obtained by Bu et al. [145]. Analogues 21c and 21d exhibited increased cytotoxicity compared to 21a, which was explained by their localization mainly in mitochondria, as followed from fluorescence imaging. In addition, the piperazine derivative 21d suppressed the formation, invasion, and migration of cell colonies, induced arrest of HepG2 cells in the G2/M phase, and increased the level of intracellular reactive oxygen species.
A number of EP 3-carbamate derivatives were obtained by Hu et al. [146]. They exhibited antiproliferative activity, which was 6–28 times stronger than that of the initial endoperoxide 21a (Table 1). The most active compounds 21i and 21j contain piperazinyl and piperidinyl fragments.
A steroid-xanthone heterodimer, asperversin A (23), was isolated from the culture of Aspergillus versicolor, an endophytic fungus isolated from the marine brown alga Sargassum thunbergii [179]. Compound 23 was tested for biological activities against some bacterial and fungal strains with no noticeable effect.
Further structural modifications of steroidal molecule with retention of the 5α,8α-endoperoxide scaffold included changes in the carbon skeleton of the side chain [180,181]. Thus, 7-dehydrocholesterol peroxide, its acetate and hemisuccinate showed improved anticancer activity and selectivity over the corresponding derivatives of EP [180].
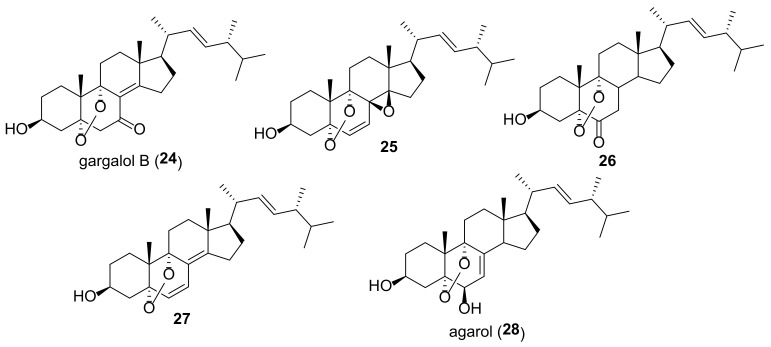
In comparison with the compounds 21a and 22a, 5α,9α-endoperoxides have been studied much less due to their lower availability. Compounds 24 and 25 (Figure 6) were isolated from the edible mushroom Grifola gargal and evaluated in the osteoclast-forming assay [182]. They inhibited osteoclast formation, which may be of interest for the prevention of osteoporosis. Endoperoxide 26, isolated from the fruiting bodies of Stropharia rugosoannulata, protected neuronal cells by attenuating endoplasmic reticulum stress caused by thapsigargin, an inhibitor of the Ca2+-ATPase [81]. A significant cytotoxicity (Table 1) against A549 and MCF-7 cells was noted for the endoperoxide 27, isolated from the fruiting body of a medicinal macro fungus Ganoderma lingzhi [12]. Agarol (28) was isolated as a tumoricidal substance from the mushroom Agaricus blazei [157]. Its cytotoxicity was evaluated against four cancer lines (Table 1). Agarol (28) was shown to induce apoptosis by increasing generation of ROS and release of apoptosis-inducing factor from the mitochondria to the cytosol.
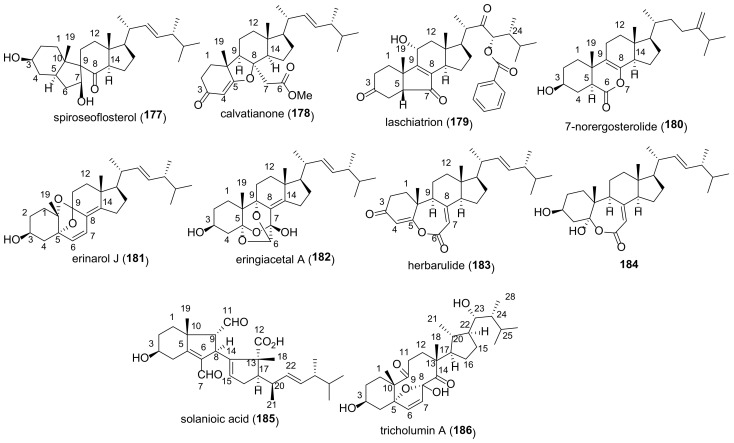
Figure 6.
Structures of fungal 5α,9α-endoperoxides.
4. Epoxides
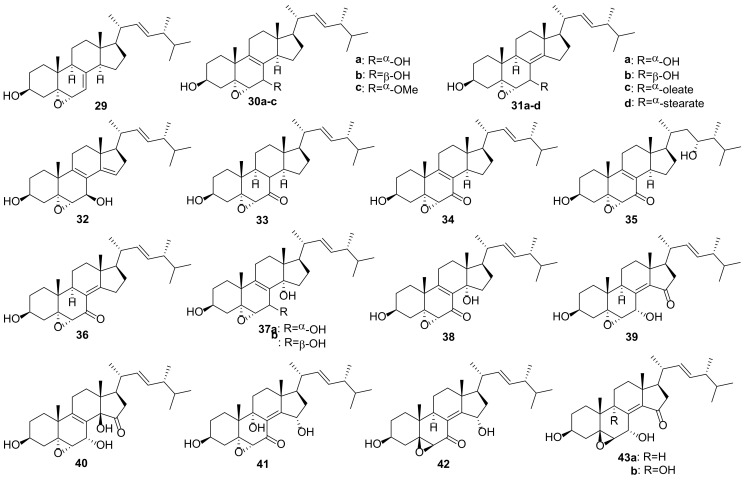
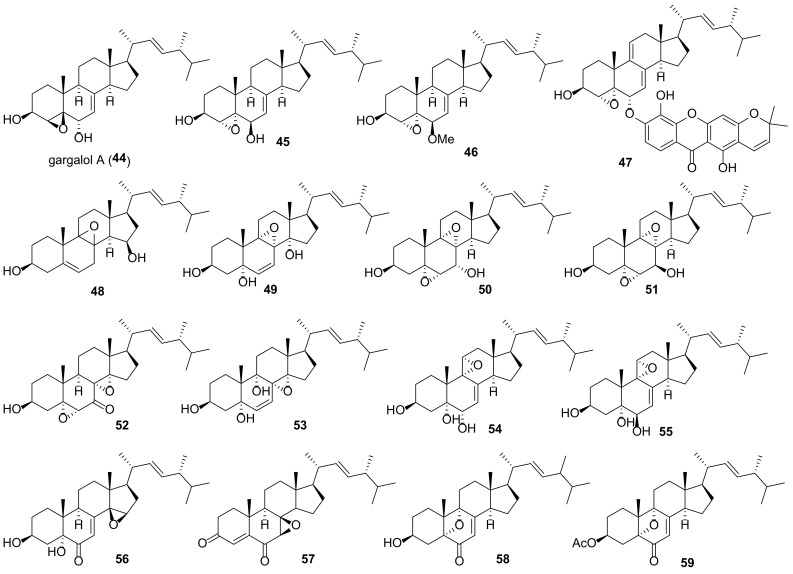
The majority of compounds of this group are 5α,6α epoxides (Figure 7). Almost all of them contain a hydroxy- or keto group at C-7, Δ8(9)-, or Δ8(14)-double bond, and some 5α,6α-epoxides have a functionalized ring D. Other epoxides (4,5-, 5β,6β-, 8,9-, 8,14-, and 9,11-derivatives) are much less common in fungi (Figure 8). Compounds 29–59 were tested in various assays, including AChE inhibitory, cytotoxic, α-glucosidase inhibition, NO production inhibition, etc., (Table 2).
Figure 7.
Structures of fungal 5,6-epoxides.
Figure 8.
Structures of other fungal epoxides.
Table 2.
Sources and biological activity of fungal epoxides.
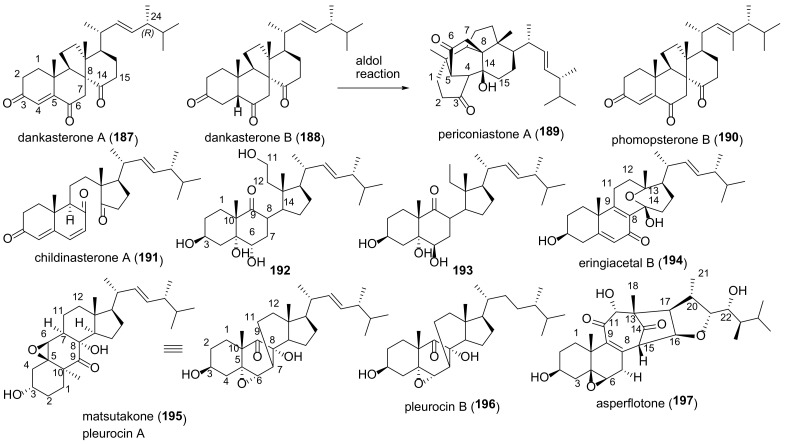
| Compound | Fungal Source [Ref.] | Assays (Activity) [Ref.] |
|---|---|---|
| 29 | Hericium erinaceus [187,188], Chaetomium sp. M453 [189], Colletotrichum sp. YMF432 [190], Cordyceps sinensis [191], Stereum insigne CGMCC5.57 [79] | AChE inhibitory assay (IC50 67.8 μM) [190], nematicidal and antibacterial assays (no activity) [79] |
| 30a | Amauroderma subresinosum [83], Ganoderma lucidum [147], G. resinaceum [103], Grifola frondosa [154], Omphalia lapidescens [15], Simplicillium sp. YZ-11 [192], Stropharia rugosoannulata [193], Pleurotus eryngii [6] | α-glucosidase inhibition assay (IC50 > 100 μM) [154], cytotoxic assay (HGC-27, IC50 11.69 μM) [15], (MCF-7, IC50 24.3 μM; NCI-H460, IC50 19.8 μM; SF-268, IC50 15.5 μM) [194], (A549, IC50 35.99 μM; HepG2, IC50 25.81 μM; MDA-MB-231, IC50 29.73 μM) [154], (HepG2, IC50 22.1 μM; MDA-MB-231, IC50 20.3 μM) [147], lettuce hypocotyl growth assay (65–80% inhibition) [193], NO production inhibition assay (IC50 12.4 μM) [6], (IC50 19.77 μM) [103] |
| 30b | Ganoderma resinaceum [103], Stropharia rugosoannulata [81] | anti-fungal assay (MIC 250 μM) [81], NO production inhibition assay (IC50 17.23 μM) [103], osteoclast-forming assay [81] |
| 30c | Amauroderma amoiensis [82], Ganoderma resinaceum [103] | NO production inhibition assay (IC50 3.24 μM) [103] |
| 31a | Cortinarius glaucopus [195], Ganoderma lucidum [147], G. resinaceum [103], G. sinense [196], Grifola frondosa [154], Hygrophorus russula [183], Leptographium qinlingensis [197], Omphalia lapidescens [15], Simplicillium sp. YZ-11 [192], Stropharia rugosoannulata [193], Phellinus linteus [198], Pleurotus eryngii [6], Termitomyces microcarpus [132] | α-glucosidase inhibition assay (IC50 > 100 μM) [154], cytotoxic assay (HGC-27, IC50 18.97 μM) [15], (MCF-7, IC50 > 50 μM; NCI-H460, IC50 > 50 μM); SF-268, IC50 > 50 μM)-194], (A549, IC50 69.11 μM; HepG2, IC50 38.87 μM; MDA-MB-231, IC50 46.76 μM) [154], (A549, IC50 15.3 μg/mL; XF498, IC50 15.1 μg/mL) [183], (HepG2, IC50 50.6 μM; MDA-MB-231, IC50 46.7 μM) [147], HNE inhibitory assay (IC50 28.2 μM) [198], lettuce hypocotyl growth assay (61–78% inhibition) [193], NCI 60 panel [132], NO production inhibition assay (IC50 > 30 μM) [6], (IC50 23.34 μM) [103], (IC50 > 40 μM) [196] |
| 31b | Ganoderma resinaceum [103], Hericium erinaceus [187,188], Sparassis crispa [186,199], Phellinus linteus [198], Pleurotus eryngii [6] | cytotoxic assay (MCF-7, IC50 > 50 μM) [194], (NCI-H460, IC50 > 50 μM) [194], (SF-268, IC50 > 50 μM) [194], NO production inhibition assay (IC50 14.3 μM) [6], (IC50 17.23 μM) [103], PCSK9 mRNA expression (inhibition, IC50 8.23 μM) [186] |
| 31c | Hericium erinaceum [200] | PPAR transactivation assay (EC50 8.2 μM) [200] |
| 31d | Hericium erinaceum [200] | PPAR transactivation assay (EC50 6.4 μM) [200] |
| 32 | Pleurotus eryngii [59] | aromatase inhibitory assay (IC50 17.3 μM) [59] |
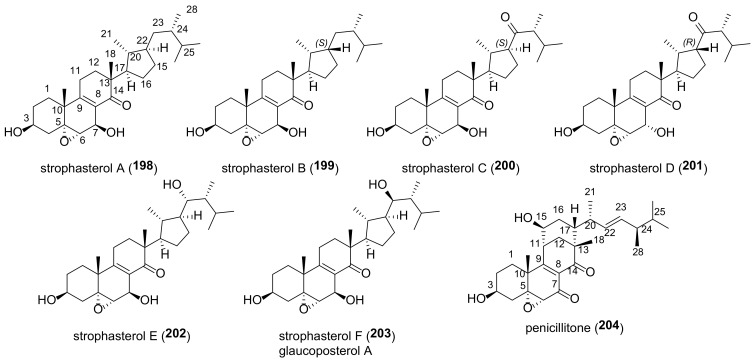
| 33 | Hericium erinaceum [187], Omphalia lapidescens [15] | cytotoxic assay (HGC-27, IC50 29.34 μM) [15], HNE inhibitory assay (IC50 75.1 μM) [198], TNF-α secretion assay (inhibition value of 37.5% at 10 μM) [187] |
| 34 | Grifola gargal [182] | osteoclast-forming assay [182] |
| 35 | Amauroderma subresinosum [83] | AChE inhibitory assay (20.9% at 100 μM) [83] |
| 36 | Omphalia lapidescens [15] | cytotoxic assay (HGC-27, IC50 23.41 μM) [15] |
| 37a | Pleurotus eryngii [201] | cytotoxic assay (RAW264.7, IC50 > 30 μM) [201] |
| 37b | Stropharia rugosoannulata [81] | osteoclast-forming assay [81] |
| 38 | Grifola gargal [182] | cytotoxic assay (HepG2, IC50 200.9 μM; MDA-MB-231, IC50 189.4 μM) [147], osteoclast-forming assay [182] |
| 39 | Amauroderma subresinosum [83], Polyporus ellisii [184] | cytotoxic assay (HL-60, IC50 32.1 μM; SMMC-7721, A549, MCF-7, SW480, IC50 > 40 μM) [184] |
| 40 | Pleurotus eryngii [201] | cytotoxic assay (RAW264.7, IC50 > 30 μM) [201], NO production inhibition assay (IC50 13.2 μM) [201] |
| 41 | Polyporus ellisii [184] | cytotoxic assay (HL-60, IC50 1.5 μM; SMMC-7721, IC50 3.9 μM; A549, IC50 2.7 μM; MCF-7, IC50 3.1 μM; SW480, IC50 2.9 μM) [184] |
| 42 | Phomopsis sp. [202] | α-glucosidase inhibition assay (IC50 > 100 μM) [202] |
| 43a | Polyporus ellisii [184], Phomopsis sp. [202] | antibacterial assay (MIC 28.2 μM against Micrococcus tenuis) [202], cytotoxic assay (HL-60, IC50 32.1 μM; SMMC-7721, A549, MCF-7, SW480, IC50 > 40 μM) [184] |
| 43b | Ganoderma resinaceum [103], Polyporus ellisii [184], Phomopsis sp. [202] | cytotoxic assay (HL-60, IC50 18.8 μM; SMMC-7721, A549, MCF-7, SW480, IC50 > 40 μM) [184] |
| 44 | Grifola gargal [182] | osteoclast-forming assay [182] |
| 45 | Pleurotus eryngii [6] | NO production inhibition assay (IC50 > 30 μM) [6] |
| 46 | Ganoderma lucidum [147] | cytotoxic assay (HepG2, IC50 138.3 μM; MDA-MB-231, IC50 176.1 μM) [147] |
| 47 | Amauroderma amoiensis [82] | AChE inhibitory assay (14.63% inhibition at 100 μM) [82] |
| 48 | Trametes versicolor [168] | (NO inhibitory activity at 12.5 μM, IL-6 inhibitory effect at 25 μM) [168] |
| 49 | Hericium erinaceus [187,188] | TNF-αsecretion assay (37.5% inhibition at 10 μM) [187] |
| 50 | Hericium erinaceus [187,188], Phellinus linteus [198], Stropharia rugosoannulata [193] | HNE inhibitory assay (IC50 35.2 μM) [198], inhibition of lettuce hypocotyl growth (no activity) [193] |
| 51 | Ganoderma lucidum [147], Hericium erinaceum [187] | NO production inhibition assay (moderate activity) [187] |
| 52 | Aspergillus awamori [203], Omphalia lapidescens [15] | cytotoxic assay (HGC-27, IC50 58.43 μM) [15], (A549, IC50 64 μM) [203] |
| 53 | Hericium erinaceum [187], Pleurotus eryngii [6] | NO production inhibition assay (IC50 > 30 μM) [6] |
| 54 | Omphalia lapidescens [15] | cytotoxic assay (HGC-27, IC50 15.37 μM) [15] |
| 55 | Pleurotus eryngii [201] | cytotoxic assay (RAW264.7, IC50 > 30 μM) [201] |
| 56 | Talaromyces stipitatus [204] | cytotoxic assay (Hep3B, IC50 4.75 μM; HepG2, IC50 8.85 μM; Huh-7, IC50 13.78 μM) [204] |
| 57 | Aspergillus penicillioides [205], Ganoderma lingzhi [12] | antibacterial assay (MIC 32 μg/mL against Vibrio anguillarum) [205], cytotoxic assay (A549, IC50 8.57 μM; MCF-7, IC50 6.09 μM) [12] |
| 58 | Chaetomium sp. [189] | AChE inhibitory assay (20–60% inhibition at 50 μg/mL) [189] |
| 59 | Colletotrichum sp. [206] | AChE inhibitory assay (18.2% inhibition at 100 μg/mL) [206] |
Bae et al. noted that the presence of an epoxy group in the tetracyclic skeleton of ergosterol derivatives increases their cytotoxic properties [183]. Isolation of a series of 5α,6α-epoxides from the macrofungus Omphalia lapidescens allowed to establish some structure activity relationship correlations [15]. The greatest cytotoxicity against a human gastric cancer cell line, HGC-27, was noted for the compound 30a and 31a containing an α-oriented hydroxyl group at C-7 and Δ8(9)- or Δ8(14)-double bond (Table 2). The transition to 7-ketones 33 and 36 led to a decrease in activity, and of both compounds, the derivative 33 without a double bond in the BC cycles was less active. The diepoxide 52 showed the least activity, which indicates the importance of the double bond for cytotoxic activity.
Epoxides 41, 43a, and 43b, isolated from the culture of Basidiomycete Polyporus ellisii, were evaluated for cytotoxicity against five human cancer cell lines [184]. The first two compounds were practically inactive, while epoxide 41 exhibited strong activity against all tested cell lines with IC50 in the range from 1.5 to 3.9 μM (Table 2).
Ferreira et al. performed virtual screening experiments on low-molecular weight fungal constituents as potential MDM2 inhibitors [185]. The latter is an important negative regulator of the p53 tumor suppressor, and its inhibitors have significant anti-tumor activity. From the compounds studied, epoxide 29 returned one of the best docking scores.
Epoxide 31b was found to exhibit potent inhibitory activity on the expression of mRNA of proprotein convertase subtilisin/kexin type 9 (PCSK9) [186]. The latter affects the low density lipoprotein receptor on the surface of liver cells, resulting in high level of low density lipoprotein cholesterol (LDL-C). PCSK9 inhibitors have been proposed as novel LDL-C-lowering agents for the treatment of hyperlipidemia. Compound 31b showed activity with IC50 values of 8.23 μM, which was comparable with berberine (IC50 8.04 μM) used as a positive control.
A number of epoxides were tested for their anti-inflammatory activity. As a rule, inhibition of TNF-α and NO production in LPS-stimulated RAW264.7 macrophage cells was used to evaluate anti-inflammatory effects. Epoxide 30c showed superior inhibitory activity on NO production with IC50 value of 3.24 μM [103]. In the same experiment, the positive control L-NMMA, nitric oxide synthase inhibitor, revealed IC50 value of 49.86 μM. TNF-α secretion decreased after treatment of macrophage cells with epoxide 49, which at 10 μM exhibited activity with inhibition value of 37.5% [187]. This was comparable to the positive control (52.5% at 1 μM) exerted by celecoxib, the cyclooxygenase-specific inhibitor.
Human neutrophil elastase (HNE) is a serine protease that can degrade extracellular matrix proteins such as collagen, fibronectin, etc. Inhibition of this enzyme can prevent the loss of skin elasticity, thereby preventing skin aging. Yoo et al. reported the HNE-inhibitory properties of Phellinus linteus mycelium components [198]. All three tested epoxides 31a, 34, and 50 showed significant activity with ID50 ranging from 28.2 to 75.1 μM.
Epoxides 30a, 31a, and 33 were isolated after anaerobic incubation of ergosterol peroxide (EP, 21a) with rat intestinal flora [207]. Two of them (30a and 33) were found to be more active against human colorectal cancer cells than the original EP. This means that EP’s strong anti-tumor properties may be (at least in part) due to its metabolic products.
A number of ergostane-type sterol fatty acid esters, including epoxides 31c and 31d, were isolated from the mushroom Hericium erinaceum and evaluated for their PPAR transactivational effects using a luciferase reporter system [200]. Oleyl and linoleyl esters 31c and 31d proved to be the most potent activators of the transcriptional activity of PPARs with EC50 values down to 6.4 μM.
5. Polyols
It should be kept in mind that the structures of ergostane-type steroids with hydroxyl and/or carbonyl group(s) given below do not fully reflect their diversity in fungal sources. A large number of compounds have been isolated before 2010; for a number of compounds isolated later, no data on biological activity are given, and for this reason they are not included in this review.
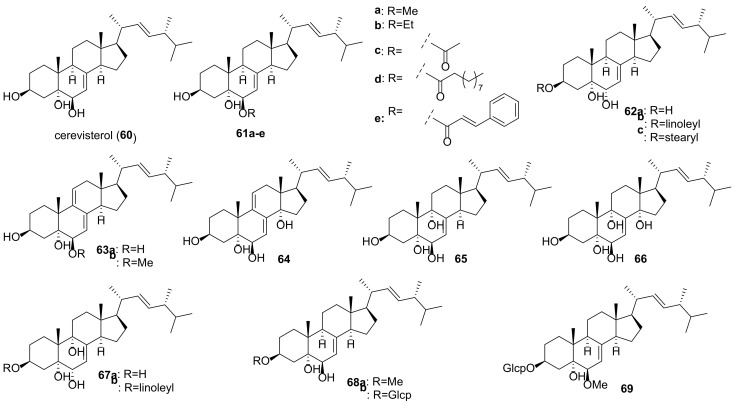
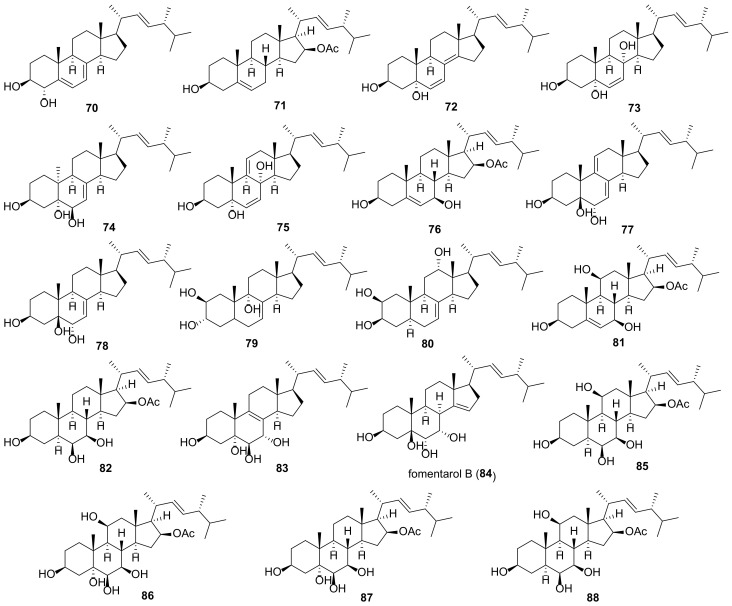
Many fungal ergostanes of this class are 5α-alcohols containing (an)other hydroxy (or a functionalized hydroxy) group(s) at C-6, C-9, and/or C-14 (Figure 9). 5α,6α Epoxides are their evident biosynthetic precursors. As a rule, rings A and B are trans-fused for most ergostanes of this group, with the exception of 5β-alcohols 77, 78, 84 (Figure 10). It should be noted that fomentarol B (84) has a cis-junction of ring B and C, which is rare among the ergostane type steroids [208].
Figure 9.
Structures of fungal steroids with a 5α,6-diol fragment and their O-derivatives.
Figure 10.
Structures of other fungal polyols.
Cerevisterol (60) is probably the best studied among 5α,6β-dihydroxy derivatives, as it is widespread in the fungal kingdom (Table 3). It should be noted that data on its cytotoxicity are inconsistent and sometimes contradictory. Thus, cerevisterol (60) showed significant activity with IC50 values of 1.1–1.9 μM against the BT-549, KB, SK-MEL, and SKOV-3 cancer cell lines [209]. On the other hand, it was practically inactive toward A549, HeLa, HepG2, and MCF-7 cells [210]. This inconsistence may be partly due to the diverse cell lines used by different authors. But a large difference was also observed in experiments with the same cell lines (e.g., reported IC50 values for HepG2 varied from 14.5 μM [211] to 174.6 μM [147]).
Table 3.
Sources and biological activity of fungal alcohols.
| Compound | Fungal Source [Ref.] | Assays (Activity) [Ref.] |
|---|---|---|
| 60 | Aspergillus fumigatus [213], A. versicolor [179], Cladosporium sp. [217], Clitocybe nebularis [214], Eurotium rubrum [80], Fomes fomentarius [208], Fusarium chlamydosporum [209,218], F. equiseti [219], F. solani [216], Ganoderma sinense [196,220], Glomerella sp. [215], Gomphus clavatus [221], Hericium erinaceum [222,223], Hypholoma lateritium [224], Lentinus polychrous [225], Leptographium qinlingensis [197], Leucocalocybe mongolica [210], Meripilus giganteus [91], Morchella esculenta [226], Omphalia lapidescens [15], Penicillium brasilianum [227], Pleurotus eryngii [6], P. tuber-regium [228], Polyporus umbellatus [77,211], Termitomyces microcarpus [132], Trametes gibbosa and T. elegans [212], Tricholoma populinum [229], Xylaria nigripes [105] | AChE inhibitory assay (0.4% inhibition at 100 μg/mL) [80], antibacterial assay (no activity against Streptococcus agalactiae, Staphylococcus epidermidis, Moraxella catarrhalis, Haemophilus influenzae, and Proteus mirabilis) [214], (S. typhi, S. aureus and A. niger, MICs 25 μg/mL each, E. faecalis, MIC 50 μg/mL) [212], (Bacillus subtilis and Escherichia coli, MICs 64 μg/mL each; Staphylococcus aureus, MIC 32 μg/mL) [213], cytotoxic assay (A549, IC50 94.75 μM; HeLa, IC50 74.13 μM; HepG2, IC50 46.58 μM; MCF-7, IC50 63.76 μM) [210], (T47D, 50.2% inhibition at 30 μM) [229], (BT-549, 1.4 μM; KB, 1.90 μM; SK-MEL, 1.70 μM; SKOV-3, 1.1 μM) [209], (Caco-2, IC50 37.56 μM; MCF-7, IC50 32.4 μM; MDA-MB-231, IC50 41.5 μM) [219], (HGC-27, IC50 37.71 μM) [15], (MCF-7, IC50 37.2 μM; PC-3, IC50 80 μM) [221], (HepG2, IC50 14.5 μM) [211], (HepG2, IC50 174.6 μM; MDA-MB-231, IC50 148.8 μM) [147], (SW1990, IC50 32.81 μM; Vero, IC50 > 100 μM) [220], NF-κB inhibitory assay (IC50 5.1 μM) [226], HIV-inhibitory assay (IC50 9.3 μM) [230], HNE inhibitory assay (IC50 77.5 μM) [198], DPPH free radical-scavenging assay (IC50 11.38 μM) [222], GIRK channel inhibitory assay (13% inhibition at 10 μM) [224], lipoxygenase inhibitory assay (IC50 5.46 μM) [218], NO production inhibition assay (IC50 > 40 μM) [196], (IC50 > 30 μM) [6], ORAC assay (antioxidant activity 1.94 mmol TE/g) [91], PTP1B inhibitory activity assay (IC50 7.5 μg/mL) [77], toxicity to Pinus armandi seedlings assay (lethal rate 95% at 30 μg/mL) [197], trap activity assay (reduction to 28.1% from 332% in control cells) [223] |
| 61a | Aspergillus penicillioides [205], A. ustus [231], Aspergillus versicolor [179], Eurotium rubrum [80], Ganoderma lucidum [232], G. sinense [233], Hericium erinaceum [223], Omphalia lapidescens [15], Penicillium brasilianum [227], Pleurotus eryngii [6], Tricholoma populinum [229], Xylaria nigripes [105] | AChE inhibitory assay (2.7% inhibition at 100 μg/mL) [80], cytotoxic assay (T47D, 23.7% inhibition at 30 μM; MDA-MB-231, 54.7% inhibition at 30 μM) [229], (U2OS, IC50 6.0 μM) [105], (HGC-27, IC50 4.17 μM) [15], [15], (HL-60, IC50 22.4 μM; LLC, IC50 55.3 μM; MCF-7, IC50 > 100 μM) [232], HIV-inhibitory assay (IC50 3.8 μM) [230], HNE inhibitory assay (IC50 14.6 μM) [198], neuroprotective activity assay (20.9% increase in cell viability against Aβ25-35-induced injury in SH-SY5Y neuroblastoma cells at the concentration 10 μM) [105], NO production inhibition assay (IC50 20.4 μM) [6], (108.2% inhibitory rate at 10 μM) [230], trap activity assay (reduction to 74.8% from 332% in control cells) [223] |
| 61b | Fomes fomentarius [208], Omphalia lapidescens [15] | cytotoxic assay (HGC-27, IC50 25.50 μM) [15] |
| 61c | Eurotium rubrum [80], Hericium erinaceum [223] | AChE inhibitory assay (17.9% inhibition at 100 μg/mL) [80], trap activity assay (reduction to 81.8% from 332% in control cells) [223] |
| 61d | Fusarium chlamydosporum [218] | lipoxygenase inhibitory assay (IC50 3.06 μM) [218] |
| 61e | Hericium erinaceum [223] | ORAC assay (antioxidant activity 8.01 mmol TE/g at 10 μM) [223] |
| 62a | Eurotium rubrum [80], Fomes fomentarius [208], Hericium erinaceum [223], Hygrophorus russula [183], Omphalia lapidescens [15] | AChE inhibitory assay (2.4% inhibition at 100 μg/mL) [80], cytotoxic assay (HGC-27, IC50 > 100 μM) [15], (HepG2, IC50 196.9 μM; MDA-MB-231, IC50 114.2 μM) [147], (A549, >30 μg/mL; XF498, >30 μg/mL) [183], trap activity assay (reduction to 138.9% from 332% in control cells) [223] |
| 62b | Hericium erinaceum [200] | PPAR transactivation assay (EC50 18.7 μM) [200] |
| 62c | Hericium erinaceum [200] | PPAR transactivation assay (EC50 20.6 μM) [200] |
| 63a | Ganoderma lucidum [147], Pleurotus eryngii [6] | cytotoxic assay (HepG2, IC50 62.5 μM; MDA-MB-231, IC50 56.3 μM) [147], NO production inhibition assay (IC50 29.8 μM) [6] |
| 63b | Ganoderma sinense [220] | cytotoxic assay (SW1990, IC50 5.05 μM; Vero, IC50 22.59 μM) [220] |
| 64 | Fomes fomentarius [208], Ganoderma lucidum [147], Hericium erinaceum [187] | cytotoxic assay (HepG2, IC50 156.4 μM; MDA-MB-231, IC50 168.9 μM) [147], TNF-α secretion assay (33.7% inhibition at 10 μg/mL) [187] |
| 65 | Clitocybe nebularis [214], Fomes fomentarius [208], Hericium erinaceum [223], Hygrophorus russula [183], Leptographium qinlingensis [197], Naematoloma fasciculare [151], Stropharia rugosoannulata [81], Tricholoma populinum [229] | antibacterial assay (no activity against Streptococcus agalactiae, Staphylococcus epidermidis, Haemophilus influenzae, and Proteus mirabilis, marginal activity against Moraxella catarrhalis) [214], anti-fungal assay (MIC 500 μM) [81], cytotoxic assay (MCF-7, MDA-MB-231, T47D, no activity) [229], (HepG2, IC50 129.7 μM; MDA-MB-231, IC50 148.2 μM) [147], (A549, 17.1 μg/mL; XF498, 16.5 μg/mL) [183], (A549, 10.83 μM; HCT-15, 13.2 μM; SK-MEL-2, 10.39 μM; SK-OV-3, 12.16 μM;) [151] |
| 66 | Ganoderma lucidum [147] | cytotoxic assay (HepG2, IC50 286.4 μM; MDA-MB-231, IC50 216.5 μM) [147] |
| 67a | Omphalia lapidescens [15] | cytotoxic assay (HGC-27, IC50 12.71 μM) [15], (HepG2, IC50 184.6 μM; MDA-MB-231, IC50 224.2 μM) [147] |
| 67b | Hericium erinaceum [200] | PPAR transactivation assay (EC50 22.3 μM) [200] |
| 68a | Omphalia lapidescens [15] | cytotoxic assay (HGC-27, IC50 26.74 μM) [15] |
| 68b | Fomes fomentarius [208] | cytotoxic assay (A549, IC50 29.8 μM; MCF-7, IC50 26.1 μM; NUGC-3, IC50 24.1 μM) [208] |
| 69 | Pleurotus eryngii [6] | NO production inhibition assay (IC50 > 30 μM) [6] |
| 70 | Hericium erinaceus [187,188] | TNF-α secretion assay (25% inhibition at 10 μg/mL) [187] |
| 71 | Penicillium granulatum [234] | cytotoxic assay (no activity) [234] |
| 72 | Hericium erinaceum [187] | TNF-α secretion assay (36.7% inhibition at 10 μg/mL) [187] |
| 73 | Coprinus setulosus [101], Ganoderma lipsiense [235], G. resinaceum [103], Xylaria nigripes [105] | antigiardial assay (93.6% inhibition against Giardia duodenalis throphozoites) [235], NO production inhibition assay (IC50 27.6 μM) [105], (IC50 22.76 μM) [103], tyrosinase inhibitory assay (IC50 6.9 μM) [236] |
| 74 | Eurotium rubrum [80] | AChE inhibitory assay (23.1% inhibition at 100 μg/mL) [80] |
| 75 | Ganoderma resinaceum [103] | NO production inhibition assay (IC50 22.76 μM) [103] |
| 76 | Penicillium granulatum [234] | cytotoxic assay (no activity) [234] |
| 77 | Omphalia lapidescens [16] | cytotoxic assay (GES-1, IC50 > 50 μM; HGC-27, IC50 12.28 μM; MDA-MB-231, IC50 11.33 μM) [16] |
| 78 | Omphalia lapidescens [16], Pleurotus eryngii [6] | cytotoxic assay (GES-1, IC50 28.0 μM; HGC-27, IC50 > 50 μM; MDA-MB-231, IC50 24.85 μM) [16], NO production inhibition assay (IC50 > 30 μM) [6] |
| 79 | Ganoderma duripora [237], Ganoderma lucidum [232,238], Phellinus linteus [198] | cytotoxic assay (HL-60, IC50 12.7 μM; LLC, IC50 45.2 μM; MCF-7, IC50 > 100 μM) [232], (A549, MCF-7, PC-3, IC50 > 50 μM) [238], HNE inhibitory assay (IC50 > 100 μM) [198] |
| 80 | Lasiodiplodia pseudotheobromae [11] | AChE inhibitory assay (no activity) [11], α-glucosidase inhibition assay (no activity) [11] |
| 81 | Penicillium granulatum [234] | cytotoxic assay (A549, IC50 5.5 μM) [234] |
| 82 | Penicillium granulatum [234] | cytotoxic assay (A549, BEL-7402, SHG-44, IC50 > 20 μM; ECA-109, IC50 9.2 μM; HepG2, IC50 7.0 μM) [234] |
| 83 | Omphalia lapidescens [16] | cytotoxic assay (GES-1, HGC-27, MDA-MB-231, IC50 > 50 μM) [16] |
| 84 | Fomes fomentarius [208], Omphalia lapidescens [16] | cytotoxic assay (MDA-MB-231, IC50 140.86 μM) [16], NO production inhibition assay (98.77% inhibitory activity at 50 μM) [208] |
| 85 | Penicillium chrysogenum [239], Penicillium granulatum [240] | anti-fungal assay (8 mm diameter at 20 μg/disk) [239], cytotoxic assay (HeLa, IC50 15 μg/mL; NCI-H460, IC50 40 μg/mL; SW1990, IC50 31 μg/mL) [239], (HepG2, IC50 8.2 μM) [240] |
| 86 | Penicillium granulatum [234] | cytotoxic assay (no activity) [234] |
| 87 | Penicillium granulatum [234] | cytotoxic assay (A549, IC50 8.0 μM; BEL-7402, IC50 8.5 μM; ECA-109, IC50 8.3 μM; HepG2, IC50 6.7 μM; SHG-44, IC50 4.8 μM) [234] |
| 88 | Penicillium granulatum [234] | cytotoxic assay (no activity) [234] |
The results of studies of antimicrobial activity also vary quite a lot. Thus, in the course of searching for biologically active constituents of wood decaying mushrooms, Trametes gibbosa and Trametes elegans, Agyare et al. isolated cerevisterol (60) as a compound responsible for their antimicrobial activity [212]. It inhibited the growth of a number of bacteria with MICs ranging from 25 to 50 µg/mL (ciprofloxacin MICs were between 0.31 and 3.50 µg/mL). The sub-inhibitory concentration of 60 (3 µg/mL) modified the activity of commonly used antibiotics (either potentiating or reducing). Similar results with respect to antimicrobial activity of 60 were obtained by Zhou et al. [213]. On the other hand, no antimicrobial activity for cerevisterol (60) was reported in works [214,215].
To access the anti-inflammatory activity of cerevisterol (60), Lee et al. measured the levels of NO and PGE2 and the production of cytokines TNF-α, IL-1, and IL-6 in LPS-stimulated macrophages [216]. It was shown that 60 suppressed the LPS-induced production of NO and PGE2 and decreased the expression of pro-inflammatory cytokines.
Yoo et al. studied the HNE-inhibitory potency of ergostanes isolated from the mycelium of Phellinus linteus [198]. Methyl ether 61a revealed the highest activity among all tested compounds with an IC50 14.6 μM, which was comparable with the positive control (epigallocatechin gallate, IC50 12.5 μM). The corresponding alcohol 60 was five times less active than 61a.
Kim et al. studied the inhibitory activity of steroids isolated from Hericium erinaceum against tartrate-resistant acid phosphatase (TRAP) [223]. The latter has become a promising target for the development of new therapeutics for the treatment of osteoporosis and other bone-related diseases. Compounds 60, 61a, 61c, 62a at a concentration of 10 μM reduced TRAP activity in osteoclasts differentiated from RAW 264.7 cells, from 322% in control cells to 28–139% in treated cells.
Compared to 5α,6-diols, other fungal polyols (Figure 10) have been relatively less studied. As mentioned above, many ergostane steroids are found in both mushrooms and plants. In particular, this applies to triol 73 found in various fungal species [101,103,105,235]. Among sixty-three compounds isolated from bamboo Sinocalamus affinis and studied as inhibitors of estrogen biosynthesis, triol 73 showed the highest activity with an IC50 value of 0.5 μM [241]. It reduced the level of expression of aromatase mRNA in granulosa-like cells of human ovaries without affecting the catalytic activity of aromatase. This discovery makes the steroid 73 an interesting lead compound in the development of new agents for the treatment of estrogen-dependent cancers.
Studying the cytotoxicity of compounds isolated from the fruiting bodies of a medicinal mushroom Ganoderma lucidum, Min et al. selected the 2β,3α,9α-triol 79 for a more detailed evaluation [232]. Treatment with 79 in a dose-dependent manner inhibited the growth of HL-60 human premyelocytic leukemia cells with the IC50 value of 12.7 μg/mL. The effect was attributed to the induction of the apoptotic process, including activation of DNA fragmentation and caspase-3 activity.
6. Hydroxyketones
This group of ergostanes in the present review is divided into compounds containing two (Figure 11), three (Figure 12), and four or more (Figure 13) functional groups in the cyclic part of the steroid molecule. It should be borne in mind that such a classification is rather arbitrary and does not cover all the aspects that are relevant to these steroids.
Figure 11.
Structures of fungal hydroxyketones with two functional groups.
Figure 12.
Structures of fungal hydroxyketones with three functional groups.
Figure 13.
Structures of fungal hydroxyketones with four or more functional groups.
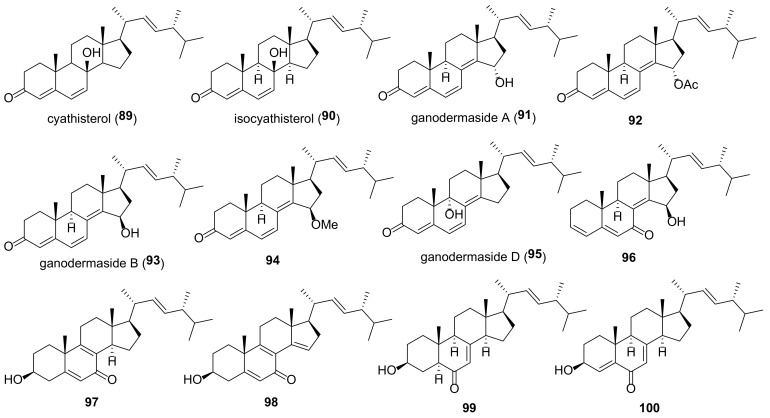
The first 8β-hydroxyergosta-3-one type of steroid, cyathisterol (89), was isolated from the fruiting body of Caluatia cyathiformis [242]. Later, Ji et al. isolated from an algicolous strain of Aspergillus ustus a very similar but not identical compound called isocyathisterol (90) [231]. A detailed NMR study allowed to determine the configuration of all stereocenters in 90. The authors concluded that the difference between the compounds 89 and 90 was in the C-9 and/or C-14 configuration.
Li et al. reported theoretical and experimental results on the properties of isocyathisterol (90) as inhibitor of isocitrate dehydrogenase IDH1 [233]. Mutations in this enzyme are associated with certain brain tumors, that makes IDH1 inhibitors as potential anticancer therapeutics for glioma patients. Based on the results of molecular virtual screening, isocyathisterol (90) had a low equilibrium dissociation constant of 18.40 μM, which confirmed the strongest binding to the IDH1 mutant. Kinetic studies showed that 90 inhibited the mutant enzyme in a noncompetitive manner.
Qi et al. isolated from spores of a medicinal mushroom Ganoderma lucidum a number of steroids possessing a 4,6,8(14),22-tetraene-3-one unit [243,244]. The obtained compounds called as ganodermasides A-D 91, 93, 110, 95 were tested for their antiaging effect on the yeast replicative lifespan assay (Table 4). All of them increased the average lifespan compared to negative control and exhibited effect similar to the known anti-aging substance, resveratrol.
Table 4.
Sources and biological activity of fungal hydroxyketones.
| Compound | Fungal Source [Ref.] | Assays (Activity) [Ref.] |
|---|---|---|
| 89 | Calvatia cyathiformis [242] | |
| 90 | Aspergillus ustus [231], Calvatia nipponica [126], Ganoderma sinense [233], Stereum hirsutum [17], Tricholoma imbricatum [245] | antibacterial assay (against E. coli, S. aureus, and A. salina with inhibitory zones of 6.7, 5.7, and 5.1 mm, respectively, at 30 μg/disk) [231], cytotoxic assay (A549, IC50 12.3 μM; HL-60, IC50 18.7 μM; K562, IC50 27.2 μM; MCF-7, IC50 23.8 μM; SMMC-7721, IC50 15.7 μM; SW480, IC50 19.1 μM) [245], (MCF-7, IC50 > 100 μM) [126], (A549, IC50 19.1 μM; HL-60, IC50 14.6 μM; MCF-7, IC50 20.4 μM; SMMC-7721, IC50 19.0 μM; SW480, IC50 25.7 μM) [17] |
| 91 | Ganoderma lucidum [243,244], Talaromyces stipitatus [204] | cytotoxic assay (Hep3B, IC50 9.67 μM; HepG2, IC50 11.83 μM) [204], lifespan assay (number of divisions of K6001 yeast strain cells before death: 8.2 in control, 8.9 at 1 μM, 11.4 at 10 μM, 9.4 at 100 μM) [244] |
| 92 | Polyporus ellisii [184] | cytotoxic assay (A549, HL-60, MCF-7, SMMC-7721, SW480, IC50 > 40 μM; HL-60, IC50 22.8 μM) [184] |
| 93 | Ganoderma lucidum [243,244], Talaromyces stipitatus [204] | cytotoxic assay (Hep3B, IC50 12.59 μM; HepG2, IC50 18.95 μM; Huh-7, IC50 32.81 μM) [204], lifespan assay (number of divisions of K6001 yeast strain cells before death: 8.2 in control, 9.1 at 1 μM, 11.1 at 10 μM, 9.6 at 100 μM) [244] |
| 94 | Polyporus ellisii [184] | cytotoxic assay (A549, HL-60, MCF-7, SMMC-7721, SW480, IC50 > 40 μM; HL-60, IC50 17.8 μM) [184] |
| 95 | Ganoderma lucidum [243], Phomopsis sp. [246] | antifungal assay (MIC 64 μg/mL against Fusarium avenaceum, MIC 128 μg/mL against Hormodendrum compactum) [246], lifespan assay (number of divisions of K6001 yeast strain cells before death: 7.5 in control, 10.0 at 3 μM, 10.7 at 10 μM, 9.2 at 30 μM) [243] |
| 96 | Chaetomium globosum [247] | cytotoxic assay (A549, MG-63, SMMC-7721, IC50 > 50 μg/mL) [247] |
| 97 | Colletotrichum sp. [206], Penicillium brasilianum [227], Pleurotus eryngii [6], Tricholoma imbricatum [245] | cytotoxic assay (A549, IC50 21.7 μM; HL-60, IC50 7.9 μM) [245], NO production inhibition assay (IC50 12.4 μM) [6] |
| 98 | Tricholoma imbricatum [245] | cytotoxic assay (HL-60, IC50 25.7 μM; SMMC-7721, IC50 27.3 μM; SW480, IC50 37.7 μM) [245] |
| 99 | Fomes fomentarius [208], Grifola frondosa [48], Phellinus linteus [198] | β-hexosaminidase release assay (no activity) [48], HNE inhibitory assay (IC50 > 100 μM) [198], NO production inhibition assay (IC50 32.87 μM) [208] |
| 100 | Hericium erinaceum [187] | TNF-α secretion assay (24.6% inhibition at 10 μg/mL) [187] |
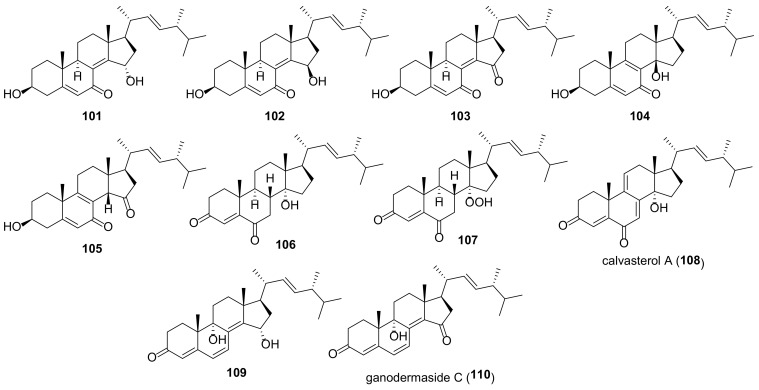
| 101 | Tricholoma imbricatum [245] | cytotoxic assay (A549, IC50 12.4 μM; HL-60, IC50 12.2 μM; K562, IC50 13.8 μM; MCF-7, IC50 17.8 μM; SMMC-7721, IC50 27.6 μM; SW480, IC50 19.7 μM) [245] |
| 102 | Chaetomium globosum [247], Phomopsis sp. [202], Tricholoma imbricatum [245] | α-glucosidase inhibition assay (IC50 > 100 μM) [202], cytotoxic assay (A549, IC50 20.72 μg/mL; MG-63, IC50 15.34 μg/mL; SMMC-7721, IC50 19.20 μg/mL) [247], (A549, IC50 27.3 μM; HL-60, IC50 23.6 μM) [245] |
| 103 | Tricholoma imbricatum [245] | cytotoxic assay (A549, IC50 36.7 μM; HL-60, IC50 16.6 μM; K562, IC50 19.9 μM; MCF-7, IC50 21.3 μM; SMMC-7721, IC50 23.5 μM) [245] |
| 104 | Pleurotus eryngii [248] | NO production inhibition assay (weak activity) [248] |
| 105 | Tricholoma imbricatum [245] | cytotoxic assay (A549, IC50 12.7 μM; HL-60, IC50 7.7 μM) [245] |
| 106 | Stereum hirsutum [17] | cytotoxic assay (A549, IC50 11.0 μM; HL-60, IC50 3.1 μM; MCF-7, IC50 12.3 μM; SMMC-7721, IC50 9.0 μM; SW480, IC50 13.4 μM) [17] |
| 107 | Stereum hirsutum [17] | cytotoxic assay (A549, HL-60, MCF-7, SMMC-7721, SW480, IC50 > 40 μM) [17] |
| 108 | Gymnoascus reessii [249], Polyporus ellisii [198], Phomopsis sp. [246] | antifungal assay (MIC 64 μg/mL against Fusarium avenaceum, MIC 256 μg/mL against Aspergillus niger and Trichophyton gypseum) [246], antimalarial assay (IC50 3.4 μg/mL against Plasmodium falciparum) [249], cytotoxic assay (KB, IC50 3.8 μM; MCF-7, IC50 7.9 μM; NCI-H187, IC50 1.9 μM; Vero, IC50 3.3 μM) [249], HNE inhibitory assay (IC50 20.5 μM) [198], |
| 109 | Ganoderma resinaceum [103], Omphalia lapidescens [15], Talaromyces stipitatus [204] | cytotoxic assay (Hep3B, IC50 5.26 μM; HepG2, IC50 6.29 μM; Huh-7, IC50 16.23 μM) [204], (HGC-27, IC50 16.93 μM) [15] |
| 110 | Ganoderma lucidum [243] | lifespan assay (number of divisions of K6001 yeast strain cells before death: 7.5 in control, 8.8 at 3 μM, 10.8 at 10 μM, 9.4 at 30 μM) [243] |
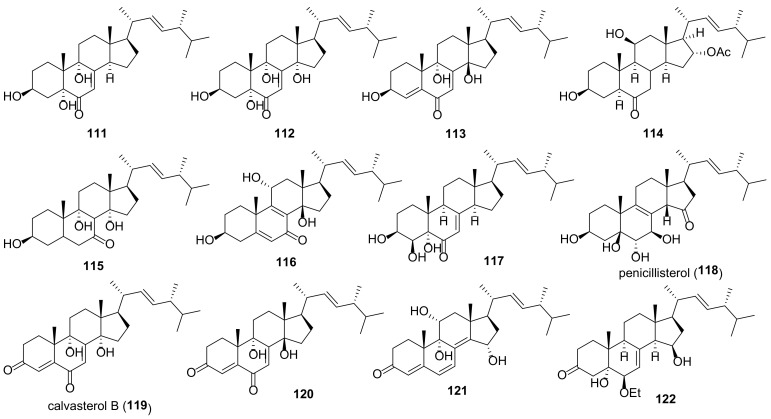
| 111 | Colletotrichum sp. [206], Ganoderma sinense [196], Pleurotus eryngii [250], Psathyrella candolleana [251], Volvariella volvacea [123] | cytotoxic assay (HepG2, IC50 5.90 μM; SGC-7901, IC50 12.03 μM) [123], (A549, HL-60, MCF-7, SMMC-7721, SW480, IC50 > 40 μM) [251], (RAW264.7, IC50 > 100 μM) [250], NO production inhibition assay (IC50 28.5 μM) [196], (IC50 100 μM) [250] |
| 112 | Volvariella volvacea [123] | cytotoxic assay (HepG2, IC50 20.27 μM) [123] |
| 113 | Ganoderma resinaceum [103] | NO production inhibition assay (IC50 35.19 μM) [103] |
| 114 | Gliomastix sp. [252] | antiviral assay (EV-71 virus, IC50 17.8 μM) [252], cytotoxic assay (HL-60, IC50 1.75 μM; DU-145, IC50 7.37 μM; HeLa, IC50 12.1 μM; MOLT-4, IC50 6.53 μM) [252] |
| 115 | Ganoderma philippii [253] | AChE inhibitory assay (35.8% inhibition at 50 μg/mL) [253] |
| 116 | Ganoderma resinaceum [103] | NO production inhibition assay (IC50 32.87 μM) [103] |
| 117 | Pleurotus eryngii [6] | NO production inhibition assay (IC50 18.1 μM) [6] |
| 118 | Penicillium purpurogenum [254] | cytotoxic assay (A549, HepG2, MCF-7, IC50 > 100 μM) [254] |
| 119 | Gymnoascus reessii [249], Phomopsis sp. [246], Talaromyces sp. [255] | antifungal assay (MIC 128 μg/mL against Candida albicans, MIC 256 μg/mL against Aspergillus niger and Hormodendrum compactum) [246], antimalarial assay (IC50 3.4 μg/mL against Plasmodium falciparum) [249], cytotoxic assay (KB, IC50 20.4 μM; MCF-7, IC50 > 50 μM; NCI-H187, IC50 12.5 μM; Vero, IC50 19.3 μM) [249] |
| 120 | Stereum hirsutum [17], Phomopsis sp. [246] | antifungal assay (MIC 64 μg/mL against Candida albicans and Hormodendrum compactum, MIC 128 μg/mL against Aspergillus niger) [246], cytotoxic assay (A549, IC50 27.8 μM; HL-60, IC50 14.4 μM; MCF-7, IC50 > 40 μM; SMMC-7721, IC50 32.0 μM; SW480, IC50 > 40 μM) [17] |
| 121 | Lasiodiplodia pseudotheobromae [11] | AChE inhibitory assay (no activity) [11], α-glucosidase inhibition assay (no activity) [11] |
| 122 | Phomopsis sp. [246] | antifungal assay (MIC 128 μg/mL against Candida albicans and Fusarium avenaceum, MIC 256 μg/mL against Hormodendrum compactum) [246] |
A number of ergosterol metabolites including hydroxyketones 91, 93, 109 were isolated from a non-pathogenic filamentous fungus Talaromyces stipitatus [204]. Compounds 91, 93, 109 showed remarkable cytotoxic activities against hepatoma cell lines with IC50 values ranging down to 5.26 μM.
7. Ketones
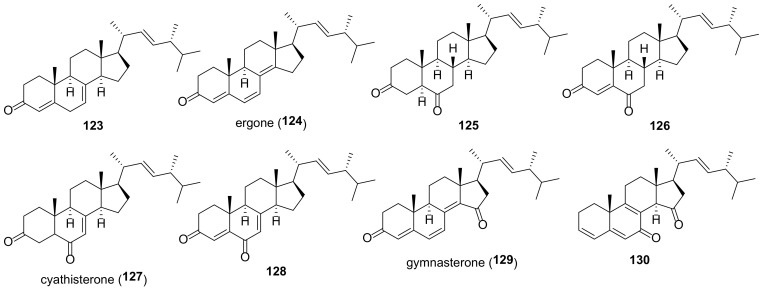
Most compounds of this group of ergostane-type steroids contain keto functions at C-3 and C-6, as well as a number of double bonds (Figure 14). Ergone (124) is probably the best studied among them [256]. It is found in many fungal sources (Table 5), usually with a content of less than 10 μg/g of mushroom fruit bodies. Polyporus umbellatus, in comparison with other mushrooms, contains the highest amount of this compound, which, under optimized conditions, can reach 86.9 μg/g [257]. For practical purposes, ergone (124) can be easily obtained through a three-step chemical synthesis from ergosterol [258]. Ergone has been reported to possess various activities (Table 5), including cytotoxic, anti-bacterial [205], anti-inflammatory [228,259], anti-malarial [249], diuretic [260] abilities, and protective effects of early renal injury [261,262].
Figure 14.
Structures of fungal ketones.
Table 5.
Sources and biological activity of fungal ketones.
| Compound | Fungal Source [Ref.] | Assays (Activity) [Ref.] |
|---|---|---|
| 123 | Gymnoascus reessii [249] | antimalarial assay (IC50 3.3 μg/mL against Plasmodium falciparum) [249], cytotoxic assay (KB, IC50 32.5 μM; MCF-7, IC50 > 50 μM; NCI-H187, IC50 16.3 μM; Vero, IC50 17.0 μM) [249] |
| 124 | Antrodia cinnamomea [263], Aspergillus penicillioides [205], A. ustus [231], Colletotrichum sp. [190], Cortinarius xiphidipus [85], Fulviformes fastuosus [264], Ganoderma sinense [220,233], Gymnoascus reessii [249], Hygrophorus russula [183], Lentinus polychrous [225], Leucocalocybe mongolica [210], Mahonia fortune [265], Nigrospora sphaerica [104], Phellinus pini [90], Pleurotus tuber-regium [228], Polyporus umbellatus [266,267], Talaromyces sp. [268], Xylaria sp. [259] | antibacterial assay (MIC 16 μg/mL against Edwardsiella tarda and Micrococcus luteus) [205], antimalarial assay (IC50 4.5 μg/mL against Plasmodium falciparum) [249], cytotoxic assay (A549, IC50 98.56 μM; HeLa, IC50 53.19 μM; HepG2, IC50 34.02 μM; MCF-7, IC50 45.92 μM) [210], (HepG2, IC50 68.32 μM; RD, IC50 1.49 μM) [264], (LNCap, IC50 34.7 μM; MCF-7, IC50 57.5 μM; N2A, IC50 20.8 μM; Saos-2, IC50 27.8 μM) [85], (KB, IC50 48.1 μM; NCI-H187, IC50 58.8 μM) [269], (HL60, IC50 30 μM; K562, IC50 350 μM) [104], (KB, IC50 40.9 μM; MCF-7, IC50 > 50 μM; NCI-H187, IC50 47.9 μM; Vero, IC50 49.2 μM) [249], (MDA-MB-231, IC50 33 μM) [268], (A549, IC50 18.8 μg/mL; XF498, IC50 24.6 μg/mL) [183], (AGS, IC50 56.1 μM; Hela229, IC50 67 μM; Hep3B, IC50 12.7 μM; HT-29, IC50 18.4 μM;) [267], (HepG2, IC50 10 μM) [270], (LU-1, IC50 10.21 μg/mL) [271], NO production inhibition assay (IC50 28.96 μM) [259], (IC50 29.7 μM) [90] |
| 125 | Stereum hirsutum [17] | cytotoxic assay (A549, MCF-7, SMMC-7721, SW480, IC50 > 40 μM; HL-60, IC50 34.3 μM) [17] |
| 126 | Stereum hirsutum [17], Xerula furfuracea [10] | cytotoxic assay (A549, HL-60, MCF-7, SMMC-7721, SW480, IC50 > 40 μM) [17] |
| 127 | Apiospora montagnei [269], Gymnoascus reessii [249] | cytotoxic assay (NCI-H187, IC50 14.8 μM) [269], (KB, MCF-7, NCI-H187, Vero, IC50 > 50 μM) [249] |
| 128 | Polyporus ellisii [198] | HNE inhibitory assay (IC50 55.2 μM) [198] |
| 129 | Phomopsis sp. [202], Polyporus ellisii [184], Talaromyces stipitatus [204] | α-glucosidase inhibition assay (IC50 > 100 μM) [202], cytotoxic assay (Hep3B, IC50 36.27 μM; HepG2, IC50 36.51 μM) [204] |
| 130 | Tricholoma imbricatum [245] | cytotoxic assay (A549, IC50 22.8 μM; SMMC-7721, IC50 19.5 μM) [245] |
Attempts were made to study the mechanism of its action. A strong anticancer effect of 124 to HepG2 cells was associated with the induction of G2/M cell cycle arrest and apoptosis in a caspase-dependent manner [270].
Wang et al. studied the effect of ergone (124) on lipopolysaccharide-induced acute lung injury [272]. Pretreatment of mice with 124 was found to reduce neutrophil recruitment, regulate the release of inflammatory cytokines, reduce pulmonary edema, and correct pulmonary insufficiency. The observed effects were associated with inhibition of the NLRP3 signaling pathway.
Ergone (124) was found to inhibit signaling pathways STAT3 and Src in head and neck cancer-initiating cells [263] that results in the reduction of their stemness properties and tumorigenicity and is of interest for the treatment of head and neck squamous cell carcinoma.
The variety of pharmacological activities prompted scientists to study pharmacokinetic properties of ergone. Fan et al. investigated the interactions between ergone and human serum albumin [273]. The latter is a carrier protein for many endogenous and exogenous molecules in blood and greatly affects the pharmacokinetics of drugs. Fluorescence spectroscopy revealed the binding of ergone to albumin, in which hydrogen bonds and hydrophobic interactions play a dominant role.
The following pharmacokinetic parameters were measured after a single oral administration (20 mg/kg) of ergone to rats: the area under the plasma concentration versus time curve from time 0 h to indefinite time (AUC0–∞) was 19.6 μg h mL−1, peak plasma concentration (Cmax) was 1.5 μg/mL, the elimination half-life (t1/2) was 5.90 h, and time to Cmax (Tmax) was 3.8 h [266].
To improve the therapeutic effect of ergone, several drug delivery systems has been proposed [274,275]. The folate receptor is known to be overexpressed in a wide variety of cancers, which is the basis for the development of tumor-targeted drug delivery systems. One of them uses the most abundant protein in plasma, albumin. Folate-modified ergone bovine serum albumin nanoparticles showed increased cellular uptake, targeting ability and cytotoxicity toward KB cells [274]. An in vivo experiment showed a higher antitumor effect and less toxicity of ergone nanoparticles compared to free ergone. Another delivery system was based on the encapsulation of ergone in PEGylated liposomes [275]. Pharmacokinetic studies have shown that encapsulation provides a longer residence time of ergone in the blood, which leads to a more effective in vivo antitumor effect.
8. Fungal Steroids with a Transformed Side Chain
The metabolic transformations of the ergosterol side chain are not as diverse as those of the tetracyclic skeleton. As a rule, they include hydrogenation of the Δ22-double bond, its epoxidation, and hydroxylation of the terminal fragment (in most cases at C-25), as well as subsequent secondary transformations of the introduced functional groups.
Many steroids of this class of ergostanes are 25-hydroxy derivatives (Figure 15). Compounds 131–140 were tested in inflammatory, cytotoxic, and antibacterial assays, but showed no particular activity (Table 6).
Figure 15.
Structures of fungal 25-hydroxy steroids.
Table 6.
Sources and biological activity of fungal steroids with a transformed side chain.
| Compound | Fungal Source [Ref.] | Assays (Activity) [Ref.] |
|---|---|---|
| 131 | Ganoderma sinense [196] | NO production inhibition assay (IC50 17.7 μM) [196] |
| 132 | Ganoderma sinense [196] | NO production inhibition assay (IC50 32.4 μM) [196] |
| 133 | Ganoderma sinense [196] | NO production inhibition assay (IC50 19.8 μM) [196] |
| 134 | Fusarium chlamydosporum [218] | lipoxygenase inhibitory assay (IC50 7.23 μM) [218] |
| 136 | Psathyrella candolleana [251] | cytotoxic assay (A549, HL-60, MCF-7, SMMC-7721, SW480, IC50 > 40 μM) [251] |
| 136 | Psathyrella candolleana [251] | cytotoxic assay (A549, IC50 23.4 μM; HL-60, IC50 32.3 μM; MCF-7, IC50 28.3 μM) [251] |
| 137 | Psathyrella candolleana [251] | cytotoxic assay (MCF-7, IC50 22.3 μM; SMMC-7721, IC50 29.3 μM) [251] |
| 138 | Conocybe siliginea [282] | NO production inhibition assay (IC50 > 40 μM) [282] |
| 139 | Conocybe siliginea [282] | NO production inhibition assay (IC50 > 40 μM) [282] |
| 140 | Aspergillus alabamensis [283] | antimicrobial assay (MIC 32 μg/mL against Edwardsiella ictaluri, MIC 64 μg/mL against Vibrio alginolyticus) [283] |
| 141 | Mahonia fortune [265] | antibacterial assay (MIC 100 μg/mL against Staphylococcus aureus) [265] |
| 142 | Hymenoscyphus fraxineus [284] | antibacterial assay (MIC 16.7 μg/mL against Bacillus subtilis, Micrococcus luteus and Staphylococcus aureus) [284], cytotoxic assay (L929, IC50 24 μg/mL) [284] |
| 143 | Aspergillus flocculosus [13] | antibacterial assay (MIC 3.3 μg/mL against Candida albicans, 3.3 μg/mL against Pseudomonas aeruginosa, 1.6 μg/mL against Enterobacter aerogenes) [13] |
| 144 | Trichoderma sp. [230] | HIV-inhibitory assay (IC50 41.6 μM) [230], NO production inhibition assay (10% inhibition at 10 μM) [230] |
| 145 | Aspergillus flocculosus [276] | cytotoxic assay (A549, IC50 55.0 μM; HepG2, IC50 23.6 μM) [276], IL-6 immune-suppressive activity assay (IC50 2.02 μM) [276], NO inhibitory activity assay (IC50 1.11 μM) [276] |
| 146 | Aspergillus flocculosus [277], Chaetomium globosum [285] | cytotoxic assay (A549, HepG2, THP-1, IC50 > 80 μM) [277], IL-6 immune-suppressive activity assay (IC50 22 μM) [277] |
| 147 | Aspergillus sp. [279] | antiviral assay (no activity against H3N2 and EV71 viruses) [279] |
| 148 | Aspergillus flocculosus [278] | cytotoxic assay (A549, HepG2, THP-1, IC50 > 80 μM) [278], IL-6 immune-suppressive activity assay (IC50 24 μM), TNF-α secretion assay (IC50 28 μM) [278] |
| 149 | Favolaschia calocera [281] | antifungal assay (active in the agar diffusion test) [281] |
| 150 | Favolaschia calocera [281] | antifungal assay (active in the agar diffusion test) [281] |
| 151 | Albatrellus confluens [286] | cytotoxic assay (HL-60, PANC-1, A549, SK-BR-3, SMMC-7721, no activity) [286] |
| 152 | Aspergillus ustus [280] | immunosuppressive assay (ConA-induced T-cell proliferation, IC50 22.49 μM; LPS-induced B-cell proliferation, IC50 22.49 μM) [280] |
| 153 | Aspergillus ustus [280] | immunosuppressive assay (ConA-induced T-cell proliferation, IC50 69.68 μM; LPS-induced B-cell proliferation, IC50 69.68 μM) [280] |
| 154 | Penicillium janthinellum [246] | antibacterial assay (MICs 25.0–50.0 μM against three pathogenic Vibrio spp.) [246] |
| 155 | Penicillium janthinellum [246] | antibacterial assay (MICs 25.0–50.0 μM against three pathogenic Vibrio spp.) [246] |
| 156 | Phoma sp. [287] | PTP inhibitory activity assay (PTP1B, IC50 25 μM each) [287] |
The epoxide 143 (Figure 16) was isolated from a halotolerant fungus Aspergillus flocculosus PT05-1 cultured in a hypersaline medium [13]. It exhibited a moderate antibacterial and antifungal activity and a weak cytotoxicity against HL-60 and BEL-7402 cell lines.
Figure 16.
Structures of steroids with a transformed side chain.
An ochratoxin-ergosteroid heterodimer, ochrasperfloroid (145), was isolated from the sponge-derived fungus Aspergillus flocculosus 16D-1 [276]. It showed potent inhibitory effects on IL-6 production in LPS-induced cells and NO production in LPS-activated macrophages (Table 6). Fungi of Aspergillus genus have been the source of three more steroids with the same side chain, including asperfloroid (146) [277], asperflosterol (148) [278], and compound 147 [279]. Anti-inflammatory properties were identified for asperfloroid (146) and asperflosterol (148) (Table 6).
Three 18,22-cyclosterols, including aspersteroid B (152) and aspersteroid C (153), were isolated from the culture extract of Aspergillus ustus NRRL 275 [280]. Both compounds exhibited no cytotoxicity against MCF-7, HeLa, A549, and HT-29 cells. When analyzing the immunosuppressive effect on the proliferation of T- and B-lymphocytes in vitro, they showed activity from moderate to weak.
Two bis-epoxides, favolon (149) and favolon C (150), were isolated from the cultures of basidiomycete Favolaschia calocera BCC 36684 [281]. They were evaluated for a number of activities such as antimalarial, antitubercular, cytotoxic, but a positive result was obtained only in the antifungal assay.
A pair of steroidal epimers, penijanthoids A and B (154 and 155), were isolated from the marine-derived fungus Penicillium janthinellum [246]. Both compounds showed weak anti-Vibrio activity against three pathogenic Vibrio spp.
9. Ergostanes with a Rearranged Tetracyclic Skeleton
Due to their intriguing structural complexity and promising biological activities, ergostanes with a rearranged tetracyclic carbon skeleton have become very attractive targets for chemists and biologists. A recent review [23] has covered this area quite thoroughly, but for consistency and completeness some results will be briefly discussed here.
Most ergostanes with a modified skeleton are highly functionalized compounds bearing three and more functional groups. A certain exception are aromatic 1(10→6)abeo-ergostane-type steroids 157–160 (Figure 17). Two of them, 157 and 158, exhibited significant cytotoxicity toward murine colorectal CT26 and human leukemia K562 cancer cell lines (Table 7). Citreoanthrasteroid B (158) was also tested for the neuroprotective effects on PC12 cells injured by glutamate (15 mM) [288]. Compound 158 showed potential neuroprotective activities by inhibiting the death of injured PC12 cells with EC50 value of 24.2 μM.
Figure 17.
Structures of 1(10→6)abeo-ergostane-type steroids.
Table 7.
Sources and biological activity of fungal steroids with a rearranged tetracyclic carbon skeleton.
| Compound | Fungal Source [Ref.] | Assays (Activity) [Ref.] |
|---|---|---|
| 157 | Antrodia camphorata [310], Aspergillus ustus [231], Gibberella zeae [311] | cytotoxic assay (CT26, IC50 15.3 μM; K562, IC50 19.9 μM) [310] |
| 158 | Antrodia camphorata [310], Penicillium citreo-viride [312], Phyllosticta capitalensis [288] | cytotoxic assay (CT26, IC50 18.2 μM; K562, IC50 12.5 μM) [310], neuroprotective activity assay (EC50 24.2 μM) [288] |
| 159a | Aspergillus ustus [231] | |
| 159b | Aspergillus ustus [231] | |
| 160 | Penicillium citreo-viride [312] | |
| 161 | Aspergillus ustus [280] | antimicrobial assay (Candida albicans, MIC50 17.24 μg/mL; Escherichia coli, MIC50 17.24 μg/mL; Staphylococcus aureus, MIC50 15.51 μg/mL) [280], cytotoxic assay (A549, IC50 40.32 μM; Hela, IC50 26.09 μM; HT-29, IC50 43.58 μM; MCF-7, IC50 32.03 μM) [280], immunosuppressive assay (ConA-induced T-cell proliferation, IC50 23.61 μM; LPS-induced B-cell proliferation, IC50 23.61 μM) [280] |
| 162a | Malbranchea filamentosa [289] | cytotoxic assay (A549, IC50 38.6 μM; Hela, IC50 28.1 μM) [289] |
| 162b | Malbranchea filamentosa [289] | cytotoxic assay (A549, Hela, no activity) [289] |
| 162c | Malbranchea filamentosa [289] | cytotoxic assay (Hela, IC50 76.9 μM) [289] |
| 163 | Aspergillus flocculosus [290] | anti-inflammatory assay [290] |
| 164 | Aspergillus flocculosus [290] | anti-inflammatory assay [290] |
| 165 | Phomopsis sp. [202], Polyporus ellisii [184] | α-glucosidase inhibition assay (IC50 > 100 μM) [202], cytotoxic assay (A549, IC50 > 40 μM; HL-60, IC50 17.1 μM; MCF-7, IC50 23.3 μM; SMMC-7721, IC50 21.3 μM; SW480, IC50 16.3 μM) [184] |
| 166 | Lasiodiplodia pseudotheobromae [11] | |
| 167 | Phomopsis sp. [7] | NO production inhibition assay (IC50 > 25 μM) [7] |
| 168 | Stereum hirsutum [17] | cytotoxic assay (A549, HL-60, MCF-7, SMMC-7721, SW480, IC50 > 40 μM) [17] |
| 169 | Chaetomium sp. [189] | cytotoxic assay (A549, HL-60, MCF-7, SMMC-7721, SW480, IC50 > 40 μM) [189] |
| 170 | Chaetomium sp. [189] | cytotoxic assay (A549, HL-60, MCF-7, SMMC-7721, SW480, IC50 > 40 μM) [189] |
| 171 | Chaetomium sp. [189] | cytotoxic assay (A549, HL-60, MCF-7, SMMC-7721, SW480, IC50 > 40 μM) [189] |
| 172 | Xylaria sp. [313] | |
| 173 | Ganoderma theaecolum [291] | neurite outgrowth-promoting assay in PC12 cells (stimulated cell differentiation with a maximum effect at 10 μM) [291] |
| 174 | Sarocladium kiliense [292] | cytotoxic assay (Bel-7402, ECA-109, HeLa, PANC-1, SHG-44, no activity) [292] |
| 175 | Aspergillus flocculosus [278] | cytotoxic assay (A549, HepG2, THP-1, IC50 > 80 μM) [278], IL-6 immune-suppressive activity assay (IC50 21 μM), TNF-α secretion assay (IC50 28 μM) [278] |
| 176 | Aspergillus flocculosus [278] | cytotoxic assay (A549, HepG2, THP-1, IC50 > 80 μM) [278], IL-6 immune-suppressive activity assay (IC50 26 μM), TNF-α secretion assay (IC50 31 μM) [278] |
| 177 | Butyriboletus roseoflavus [294] | cytotoxic assay (HepG2, IC50 9.1 μM; Huh7/S, IC50 6.2 μM; L02, IC50 22.8 μM) [294] |
| 178 | Calvatia nipponica [126] | cytotoxic assay (MCF-7, IC50 > 100 μM) [126] |
| 179 | Favolaschia calocera [281], Favolaschia sp. [295] | antifungal assay (activity against Candida albicans, Cryptococcus neoformans, etc. at concentrations of 10–50 μg/mL) [295] |
| 180 | Aspergillus flocculosus [13], Aspergillus ochraceus [296] | antibacterial assay (MIC 1.9 μg/mL against Candida albicans, 7.5 μg/mL against Pseudomonas aeruginosa and Enterobacter aerogenes) [13], cytotoxic assay (BEL-7402, IC50 17.7 μM; HL-60, IC50 12.4 μM) [13], (NCI-H460, IC50 5.0 μg/mL; SMMC-7721, IC50 7.0 μg/mL; SW1990, IC50 28.0 μg/mL) [296] |
| 181 | Hericium erinaceum [187] | NO production inhibition assay (38.4% inhibition at 10 μg/mL) [187], TNF-α secretion assay (43.3% inhibition at 10 μg/mL) [187] |
| 182 | Pleurotus eryngii [250] | cytotoxic assay (RAW264.7, IC50 25.6 μM) [250], NO production inhibition assay (IC50 19.9 μM) [250] |
| 183 | Antrodia camphorate [298], Gymnoascus reessii [249], Stereum hirsutum [17] | cytotoxic assay (A549, HL-60, MCF-7, SMMC-7721, SW480, IC50 > 40 μM) [17], (KB, MCF-7, IC50 > 50 μM; NCI-H187, IC50 22.6 μM; Vero, IC50 43.8 μM) [249] |
| 184 | Ganoderma resinaceum [103] | NO production inhibition assay (56.37% inhibition at 50 μM) [103] |
| 185 | Rhizoctonia solani [300] | antibacterial assay (MIC 1 μg/mL against the Gram-positive bacteria Bacillus subtilis, Staphylococcus aureus, and MRSA; MIC 16 μg/mL against the yeast Candida albicans; MIC 64 μg/mL against the Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa) [300] |
| 186 | Trichoderma asperellum [301] | antibacterial assay (against V. harveyi, V. splendidus, and P. citrea with inhibitory zones of 10, 7.5, and 8.0 mm, respectively, at 50 μg/disk) [301], antifungal assay (MIC 12 μg/mL against Glomerella cingulate) [301] |
| 187 | Antrodia camphorate [310], Arthrinium sp. [314], Aspergillus penicillioides [205], Colletotrichum sp. [206], Conocybe siliginea [315], Gymnascella dankaliensis [303], Neosartorya fennelliae, N. tsunodae [316], Pestalotiopsis sp. [139], Phomopsis sp. [7], Pleosporales sp. [317], Stereum hirsutum [17], Talaromyces purpurogenus [318], Talaromyces sp. [255] | cytotoxic assay (P388, ED50 2.2 μg/mL) [303], (A549, IC50 4.4 μM; HL-60, IC50 2.3 μM; MCF-7, IC50 2.7 μM; SMMC-7721, IC50 3.3 μM; SW480, IC50 3.5 μM) [17], (K562, IC50 > 20 μM; ST26, IC50 6.7 μM) [310], (A549, IC50 21.3 μM; HL-60, IC50 7.9 μM; MCF-7, IC50 23.8 μM; SMMC-7721, IC50 > 40 μM; SW480, IC50 14.2 μM) [318], iNOS inhibitory assay (IC50 6.58 μM) [7], NO production inhibition assay (IC50 13.04 μM) [7] |
| 188 | Antrodia camphorate [310], Calvatia nipponica [126], Gymnascella dankaliensis [303], Stereum hirsutum [17] | cytotoxic assay (P388, ED50 2.8 μg/mL) [303], (MCF-7, IC50 > 100 μM) [126], (A549, IC50 16.6 μM; HL-60, IC50 15.6 μM; MCF-7, IC50 17.2 μM; SMMC-7721, IC50 16.3 μM; SW480, IC50 17.3 μM) [17], (K562, IC50 23.1 μM; ST26, IC50 8.4 μM) [310] |
| 189 | Periconia sp. [304] | antibacterial assay (MIC 4 μg/mL against Staphylococcus aureus, MIC 32 μg/mL against Enterococcus faecalis; MIC > 100 μg/mL against all four Gram-negative bacteria tested) [304], NO production inhibition assay (IC50 > 40 μM) [304] |
| 190 | Phomopsis sp. [7] | iNOS inhibitory assay (IC50 1.49 μM) [7], NO production inhibition assay (IC50 4.65 μM) [7] |
| 191 | Daldinia childiae [306] | cytotoxic assay (MCF-7, SMMC-7721, SW480, IC50 > 40 μM) [306], NO production inhibition assay (IC50 21.2 μM) [306] |
| 192 | Pleurotus eryngii [6] | NO production inhibition assay (IC50 10.3 μM) [6] |
| 193 | Pleurotus eryngii [201] | NO production inhibition assay (NO produced 57.8% at 30 μM) [201] |
| 194 | Pleurotus eryngii [248] | NO production inhibition assay (IC50 13.0 μM) [248] |
| 195 | Tricholoma matsutake [319], Pleurotus eryngii [248] | AChE inhibitory assay (62.8% inhibition at 50 μg/mL) [319], NO production inhibition assay (IC50 25 μM) [248] |
| 196 | Pleurotus eryngii [248] | NO production inhibition assay (IC50 23.6 μM) [248] |
| 197 | Aspergillus flocculosus [277] | cytotoxic assay (A549, HepG2, THP-1, IC50 > 80 μM) [277], IL-6 immune-suppressive activity assay (IC50 22 μM) [277] |
| 198 | Stropharia rugosoannulata [307] | |
| 199 | Stropharia rugosoannulata [307] | |
| 200 | Stropharia rugosoannulata [307] | |
| 201 | Cortinarius glaucopus [195], Stropharia rugosoannulata [307] | |
| 202 | Pleurotus eryngii [201] | cytotoxic assay (RAW 264.7, IC50 > 30 μM) [201] |
| 203 | Pleurotus eryngii [201] | cytotoxic assay (RAW 264.7, IC50 > 30 μM) [201] |
| 204 | Penicillium purpurogenum [254] | cytotoxic assay (A549, IC50 5.57 μM; HepG2, IC50 4.44 μM; MCF-7, IC50 5.98 μM) [254], IL-6 immune-suppressive activity assay (96.7% inhibition at 5 μg/mL) [254], NO production inhibition assay (70.7% inhibition at 5 μg/mL) [254] |
Another 1(10→6)abeo-steroid, aspersteroid A (161), was isolated from the culture extract of Aspergillus ustus [280]. It exhibited moderate cytotoxicity on four cancer cell lines, antimicrobial activity against Gram-negative and Gram-positive bacteria and immunosuppressive activities against the proliferation of T and B lymphocyte cells in vitro (Table 7).
Three anthrasteroid glycosides, malsterosides A-C (162a–c), were isolated from the fungus Malbranchea filamentosa [289]. The sugar moiety in the side chain of all glycosides was found to be D-mannose and the glycoside 162c contained N-acetyl-D-glucosamine at the C-3 position. Cytotoxicity studies were performed with the A549 and Hela cancer cell lines. A moderate cytotoxicity in both lines was noted for malsteroside A (162a).
Two 1(10→6)-abeo-14,15-secosteroids, asperfloketals A (163) and B (164), were found in the sponge-associated fungus Aspergillus f locculosus 16D-1 [290]. They exhibited no cytotoxicity against three tested cancer cell lines. Promising results were obtained in anti-inflammatory assays. Compounds 163 and 164 displayed stronger activity in the CuSO4-induced transgenic fluorescent zebrafish than ibuprofen used as a positive control.
A-nor-B-homo steroid 165 (Figure 18) containing a 10(5→4)-abeo-ergostane fragment was isolated from culture of basidiomycete Polyporus ellisii [184] and from the mangrove-derived fungus Phomopsis sp. MGF222 [202]. Compound 165 exhibited inhibitory activities against four out of five human cancer cell lines tested except A549 [184] (Table 7). It was also tested for the antibacterial activities against seven pathogenic bacteria and for the inhibitory activities against α-glucosidase, but no effect was observed [202].
Figure 18.
Structures of ergostanes with a rearranged A-ring.
Another A-nor steroid 166 was isolated from the fungus of Lasiodiplodia pseudotheobromae [11]. A distinguished structural feature of this compound is an additional δ-lactone ring between C-3 and C-9.
Two nearly identical steroids 167 and 168 featured a bicyclo[3.3.1]nonane motif were discovered in the fungi Phomopsis sp. TJ507A [7] and Stereum hirsutum [17]. The only difference in their structures is the presence of a methoxy group in phomopsterone A (167) instead of an ethoxy one in steresterone A (168). Compound 167 was tested for NO inhibitory activity. Steresterone A (168) was evaluated for the cytotoxicity against five human cancer cell lines. Both compounds showed no activity in the respective tests.
Three C25 steroids, neocyclocitrinols E-G (169–171) were isolated from endophytic fungus Chaetomium sp. M453 [189]. All compounds were tested for AChE inhibitory activities and cytotoxicity, however, no effect was found.
Cheng et al. isolated from Ganoderma theaecolum ganotheaecolin A (173), having a naphtho[1,8-ef]azulene ring system steroid [291]. At a concentration of 10 μM, it showed activity to promote neurite growth in PC12 cells, comparable to that of nerve growth factor used as control.
A new steroid sarocladione (174) bearing a 5,10:8,9-diseco moiety was isolated from the deep-sea-derived fungus Sarocladium kiliense [292]. The initially proposed configuration at C-3 and C-7 proved to be incorrect and was revised to 3S,7R through the chemical synthesis [293]. Cytotoxic studies of compound 174 revealed no apparent cellular toxicities.
Lin et al. isolated from the sponge-derived fungus Aspergillus flocculosus 16D-1 two 11(9→10)-abeo-5,10-secosteroids, aspersecosteroids A (175) and B (176) [278], a characteristic structural feature of which was the presence of a dioxatetraheterocyclic ring system. Both compounds were non-cytotoxic at the concentrations up to 40 μM and showed a strong inhibitory effect on the production of TNF-α and IL-6.
Spiroseoflosterol (177) (Figure 19), having a unique spiro[4.5]decan-6-one moiety, was isolated from the fruiting bodies of Butyriboletus roseoflavus [294]. It showed a strong cytotoxic effect on HepG2 cell line (IC50 9.1 μM), which was comparable to that of sorafenib (IC50 5.5 μM) used as a positive control. Moreover, spiroseoflosterol (177) was active against sorafenib-resistant Huh7/S cells with an IC50 value of 6.2 μM, that makes it a promising candidate for antihepatoma drug development.
Figure 19.
Structures of ergostanes with a rearranged B-ring.
Calvatianone (178), featuring a contracted tetrahydrofuran B-ring, was found in a rare mushroom Calvatia nipponica [126]. It showed a weak cytotoxicity against MCF-7 with IC50 > 100 μM (Table 7).
Another compound with a five-membered B ring, laschiatrion (179), was isolated from fermentations of Favolaschia sp. [281,295]. It was not active in antibacterial and cytotoxic assays, but exhibited antifungal activity in the agar diffusion test [281].
7-Nor-ergosterolide (180), featuring a pentalactone B-ring system, was found in the culture extract of an endophytic fungus Aspergillus ochraceus EN-31 [296] and a halotolerant fungus Aspergillus flocculosus PT05-1 [13]. Compound 180 showed pronounced cytotoxic and antibacterial properties.
A characteristic structural feature of erinarol J (181), isolated from the dried fruiting bodies of Hericium erinaceum, is the presence of 6,8-dioxabicyclo[3.2.1]oct-2-ene moiety [187]. Biotests have shown potent anti-inflammatory activity of 181 due to the inhibition of TNF-α secretion and NO production.
The first natural 5,6-secosteroid, eringiacetal A (182), was isolated from the fruiting bodies of mushroom Pleurotus eryngii [250]. Biological assays showed its modest cytotoxicity and ability to inhibit NO production.
Herbarulide (183) was first isolated from the endophytic fungus Pleospora herbarum as a compound having a campestane side chain [297]. Later the same structure was assigned to one of the constituents of the Taiwanese fungus Antrodia camphorate [298]. The correct structure of herbarulide (183) was proposed by Chen and Liu who isolated it from the fungus Stereum hirsutum [17]. The assignment was based rather on the assumption that the C-24 stereocenter of the starting ergosterol will remain unchanged during the transformations in the cyclic part. Finally, the correct structure of 183 was confirmed by its chemical synthesis [299]. Compound 184, structurally very close to herbarulide (183), was isolated from the fruiting bodies of Ganoderma resinaceum [103].
Solanioic acid (185) is a degraded and rearranged steroid isolated from laboratory cultures of the fungus Rhizoctonia solani [300]. An important feature of its biological activity is antibacterial effect against methicillin-resistant Staphylococcus aureus. The latter is a cause of infection that is difficult to treat due to resistance to many antibiotics.
Tricholumin A (186) was isolated from the alga-endophytic fungus Trichoderma asperellum [301]. The only structural element of the parent ergosterol that remained after a number of metabolic stages of its biosynthesis is cycle A. The rest of the molecule, including a fragment of the side chain, has undergone deep transformations. Inhibitory properties of 186 against harmful microalgae and weak antibacterial activity against five aquatic pathogens were found.
Dankasterone A (187) (Figure 20) was first isolated from a fungal strain of Gymnascella dankaliensis derived from the sponge Halichondria japonica [302]. The initial erroneous assignment of stereochemistry at C-24 was corrected from S to R in a follow-up work by these authors [303]. Subsequently, compound 187 was repeatedly isolated from fungal sources as one of the ergostane constituents (Table 7). The only structural difference between 187 and dankasterone B (188) is the saturated ring A. From the endophytic fungus Phomopsis sp. TJ507A was also isolated phomopsterone B (190) differing from 187 by the presence of a methyl group at C-23 [7]. Dankasterone A (187) showed promising anticancer activities with IC50 down to 2.3 μM on a range of cancer cell lines (Table 7). Structure activity relationship studies of dankasterones A and B showed that the Δ4-double bond is essential for high cytotoxicity against the cancer cell lines tested. Carbonyl groups in dankasterone B (188) were other structural elements important for the high biological activity, because products of its NaBH4 reduction were not cytotoxic [17]. Phomopsterone B (190) was tested for inflammatory activity and showed promising results in iNOS inhibitory and NO production inhibition assays [7].
Figure 20.
Structures of ergostanes with a rearranged C-ring.
At first glance, the carbon skeleton of periconiastone A (189) [304] looks completely different from that of dankasterone B (188). In fact, compound 189 is available from 188 in one step via the intramolecular aldol reaction [305], which is also evidently realized in the course of its biosynthesis. So far, periconiastone A (189) has been tested for anti-inflammatory and antibacterial activities. Positive results were obtained in an antibacterial assay against Gram-positive bacteria [304].
An 8,14-seco-steroid, childinasterone A (191), was isolated from fruiting bodies of the ascomycete Daldinia childiae [306]. It showed no activity in cytotoxic studies and exhibited strong inhibition of NO production (IC50 value of 21.2 μM versus 41.5 μM for L-NMMA used as a positive control).
9,11-Secosteroids are quite common in sea sponges [22], but rather rare in fungal sources. The first such an ergostane 192 was isolated from king trumpet mushroom Pleurotus eryngii [6]. Compound 192 exhibited NO inhibitory activity similar to that of L-NMMA and revealed no cytotoxicity. Another 9,11-secoergostane (193), found in the fruiting bodies of Pleurotus eryngii, displayed similar profile of biological activity [6].
Three steroids with a rearranged ring B, eringiacetal B (194), matsutakone (195), and pleurocin B (196), were isolated from the fruiting bodies of Pleurotus eryngii by Tanaka et al. [248]. All three compounds revealed inhibitory activity on production of NO which was stronger than that of L-NMMA. The 13,14-seco-13,14-epoxysteroid, eringiacetal B (194), was most active with an IC50 of 13.0 μM compared to 23.9 μM for the L-NMMA positive control.
An 8(14→15)-abeo-steroid, asperflotone (197), was obtained from the solid culture of Aspergillus flocculosus 16D-1 [277]. Its characteristic structural feature is a rearranged bicyclo[4.2.1]non-2-ene ring system. Compound 197 was tested on three cancer cell lines with no cytotoxic effects. In immune-suppressive activity assay, asperflotone (197) exhibited inhibitory effects on IL-6 secretion.
The 15(14→22)abeo-steroid framework is common for ergostanes 198–203 (Figure 21), collectively referred to as strophasterols. It took some effort to establish the correct structures of these structurally related compounds. Strophasterols A–D (198–201) were first isolated from the mushroom Stropharia rugosoannulata [307]. The structure of strophasterin A (198) was established by X-ray crystallographic analysis. Comparison of the NMR data made it possible to assign the structure of 199 as the C-22 isomer of strophasterol A that was later confirmed by X-ray analysis [193]. Structure of strophasterol C (200) was proposed based on NOE correlations by Aung et al., who isolated it from the basidiomycete Cortinarius glaucopus together with glaucoposterol A (203) [195]. Additional evidence for the structure of 200 was obtained by its chemical synthesis [308]. Two more steroids with a strophastane skeleton, strophasterol E (202) and strophasterol F (203), were isolated from the fruiting bodies of Pleurotus eryngii [201]. Their structures were determined by X-ray analysis of the corresponding tris-p-bromobenzoate derivatives. Structural elucidation of strophasterol D (201) was done by comparing it with a synthetically prepared sample [309]. This work also showed that glaucoposterol A and strophasterol F are the same compound (203).
Figure 21.
Structures of ergostanes with a rearranged D-ring.
So far, the biological activity of strophasterols has been studied only marginally. Strophasterol A (198) showed a dose-dependent inhibitory effect on the toxicity of thapsigargin. The latter is known to disrupt the balance of the Ca2+ concentration in the endoplasmic reticulum that is especially harmful to neuronal cells. Under the action of strophasterol A (198), an increase in cell viability by 10.3% compared with the control was noted [307]. Strophasterols E and F were tested for anti-inflammatory activity, but showed no promising results [201].
A 15(14→11)-abeo-ergostane, penicillitone (204), was isolated from the culture of the fungus Penicillium purpurogenum SC0070 [254]. It was evaluated for cytotoxicity against three cancer lines and showed good potency with IC50 ranging from 4.44 to 5.98 μM. In addition, compound 204 was active in the inflammatory assay on the production of TNF-α and IL-6. At the concentration of 5 μM it reduced their secretion by 70.7% and 96.6%, respectively. For comparison, inhibition rates of the positive control dexamethasone at 100 μM were 87.3% and 96.7%, respectively. This makes promising further in-depth study of penicillitone (204) as an anti-inflammatory or antitumor agent.
10. Degraded Sterols
The progressive degradation of ergostane-type steroids through 5,6- and 9,10-oxidative cleavages leads to the loss of ring A and the formation of highly degraded sterols (Figure 22). The most common and best studied among them is demethylincisterol A3 (206). It demonstrated a potent activity against many cancer lines (Table 8). Cytotoxicity-guided investigation of Chinese mangrove Rhizophora mucronata endophytic Pestalotiopsis sp. yielded 206 as the most active compound with IC50 values reaching nanomolar order [139].
Figure 22.
Structures of degraded sterols.
Table 8.
Sources and biological activity of fungal degraded sterols.
| Compound | Fungal Source [Ref.] | Assays (Activity) [Ref.] |
| 205 | Fusarium solani [328] | cytotoxic assay (A549, HL-60, MCF-7, SMMC-7721, SW480, IC50 > 40 μM) [328], COX-2 inhibitory assay (IC50 > 20 μM) [328] |
| 206 | Agrocybe chaxingu [322], Amauroderma amoiensis [82], Aspergillus sp. [321], Colletotrichum sp. [206], Gymnascella dankaliensis [329], Omphalia lapidescens [16], Pestalotiopsis sp. [139,320], Pleosporales sp. [317], Termitomyces microcarpus [132], Tricholoma imbricatum [245], Xylaria allantoidea [330] | AChE inhibitory assay (<10% inhibition at 50 μg/mL) [82], antibacterial assay (MIC 12.5 μM against S. aureus, 3.13 μM against S. epidermidis, 3.13 μM against B. cereus) [321], cytotoxic assay (A549, IC50 11.14 nM; Hela, IC50 0.17 nM; HepG2, IC50 14.16 nM) [139],(A549, IC50 27.2 μM; HL-60, IC50 18.1 μM; K562, IC50 13.6 μM; MCF-7, IC50 10.9 μM; SMMC-7721, IC50 21.7 μM; SW480, IC50 19.2 μM) [245], (GES-1, IC50 7.81 μM; HGC-27, IC50 51.16 μM; MDA-MB-231, IC50 16.48 μM) [16], (HeLa, IC50 2.24 μg/mL; HCT-116, IC50 2.51 μg/mL; HT-29, IC50 3.50 μg/mL; MCF-7, IC50 3.77 μg/mL; Vero, IC50 3.65 μg/mL) [330], (P388, ED50 1.0 μg/mL) [329], osteoclast differentiation assay (at 4.8 μM suppressed the rate of osteoclast formation to 55%) [322], protein tyrosine phosphatase assay (IC50 6.75 μg/mL) [320] |
| 207 | Amauroderma amoiensis [82], Armillariella tabescens [170], Aspergillus aculeatinus [331], Aspergillus sp. [332], Pyropolyporus fomentarius [333], Tricholoma imbricatum [245] | AChE inhibitory assay (46.3% inhibition at 50 μg/mL) [82], cytotoxic assay (A549, IC50 7.1 μM; HL-60, IC50 22.1 μM; K562, IC50 17.1 μM; MCF-7, IC50 18.9 μM; SMMC-7721, IC50 19.3 μM; SW480, IC50 16.7 μM) [245], (A549, IC50 18.2 μM; HL-60, IC50 23.9 μM; K562, IC50 > 40 μM; MCF-7, IC50 16.9 μM; SMMC-7721, IC50 27.3 μM; SW480, IC50 >40 μM) [333], NO production inhibition assay (IC50 36.48 μM) [170] |
| 208 | Agrocybe chaxingu [322] | osteoclast differentiation assay (at 4.8 μM suppressed the rate of osteoclast formation to 6.7%) [322] |
| 209 | Agrocybe chaxingu [323] | osteoclast differentiation assay (at 3.1 μg/mL suppressed the rate of osteoclast formation to 66%) [323] |
| 210 | Agrocybe chaxingu [323] | |
| 211 | Agrocybe chaxingu [323], Cordyceps jiangxiensis [326], Tricholoma imbricatum [245], Xylaria allantoidea [330] | cytotoxic assay (A549, IC50 7.9 μM; MCF-7, IC50 10.2 μM) [245], (HeLa, IC50 50.17 μg/mL; Vero, IC50 76.57 μg/mL) [330], (A549, IC50 2.93 μM; SGC-7901, IC50 1.38 μM) [326], osteoclast differentiation assay (at 3.1 μg/mL suppressed the rate of osteoclast formation to 0%) [323] |
| 212 | Agrocybe chaxingu [323] | |
| 213 | Hericium alpestre [334] | cytotoxic assay (A549, IC50 71.1 μM; HeLa, IC50 69.6 μM; HT-29, IC50 54.8 μM) [334] |
| 214 | Antrodia camphorate [335] | cytotoxic assay (A-2058, IC50 31.1 μM; B16F10, IC50 26.69 μM; Huh-7, IC50 43.03 μM; MCF-7, IC50 77.59 μM) [335] |
| 215 | Ganoderma capense [8] | cytotoxic assay (BGC823, Daoy, HCT116, HepG2, NCI-H1650, IC50 > 50 μM) [8] |
| 216 | Ganoderma sinense [220] | cytotoxic assay (SW1990, Vero, IC50 > 100 μM) [220] |
| 217 | Daedaleopsis tricolor [336] | cytotoxic assay (A-549, HL-60, MCF-7, SMMC-7721, SW480, IC50 > 40 μM) [336] |
| 218 | Lenzites betulinus [337] | PTP1B inhibitory activity assay (IC50 21.5 μg/mL) [337] |
| 219 | Antrodiella albocinnamomea [327] | PTP1B inhibitory activity assay (no activity against DPP-IV and PTP1B at 50 μg/mL) [327] |
| 220 | Antrodiella albocinnamomea [327] | PTP1B inhibitory activity assay (IC50 1.1 μg/mL in a mixture with 10–46) [327] |
| 221 | Antrodiella albocinnamomea [327] | PTP1B inhibitory activity assay (IC50 1.1 μg/mL in a mixture with 10–45) [327] |
| 222 | Phomopsis tersa [338] | cytotoxic assay (A549, HepG2, MCF-7, SF-268, IC50 > 100 μM) [338] |
| 223 | Tricholoma matsutake [319] | AChE inhibitory assay (40.3% inhibition at 50 μg/mL) [319] |
Luo et al. examined a collection of secondary metabolites of endophytic fungi in search for inhibitors of SH2 containing protein tyrosine phosphatase-2 (SHP2) [320]. The latter is an oncogenic phosphatase participating in many signaling cascades and identified as a potential therapeutic target for cancer. It was found that demethylincisterol A3 (206) inhibited the protein tyrosine phosphatase activity of SHP2 with an IC50 of 6.75 μg/mL. In comparison, sodium orthovanadate used as a positive control showed an IC50 value of 114 μg/mL.
Demethylincisterol A3 (206) revealed significant antibacterial activities against a number of pathogenic bacteria with MICs values ranging from 3.13 to 12.5 μM (MICs of the positive control ciprofloxacin varied from 0.78 to 1.56 μM) [321].
Agrocybe chaxingu extract was shown to have a very strong osteoclast suppression effect, useful in the prevention and control of osteoporosis. In search of the active components of this mushroom, Kawagishi et al. isolated a number of degraded sterols 208–212, collectively called as chaxines [322,323]. The initially assigned 2′S,5′S stereochemistry of the A ring of chaxine B (209) was erroneous and was subsequently revised to 2′R,5′S [324,325]. Chaxines A-C were evaluated in the osteoclast-forming assay and were shown to suppress the rate of osteoclast formation with no cytotoxicity [322,323].
Chaxine C (211) was also isolated from traditional Chinese medicinal mushroom Cordyceps jiangxiensis under the name jiangxienone and showed promising results in inhibiting cancer cells [326]. Its IC50 values against A549 and SGC-7901 cells were six-fold lower than that of cisplatin.
Albocisterols A-C (219–221) isolated from cultures of Antrodiella albocinnamomea were tested for inhibitory activities against protein tyrosine phosphatase [327]. A mixture of compounds 220 and 221 exhibited significant activity with IC50 value of 1.1 μg/mL (IC50 1.2 μg/mL for ursolic acid used as a positive control). The corresponding C-27 alcohol, albocisterol A (219), was inactive at 50 μg/mL.
11. Conclusions
Fungi have been a traditional object of human practical interest throughout history. At first this was due to the nutritional value of mushrooms. Currently, fungi are attracting special attention as a source of a large number of biologically active compounds belonging to different classes: polyketides, terpenoids, peptides, alkaloids, etc., [339]. A wide variety of fungi secondary metabolites, their low content in natural material and the complexity of structural identification have led to the rapid development of research in this area only in the last two–three decades through the use of highly efficient methods of instrumental analysis and separation of complex natural compositions. A special place among fungi constituents is occupied by the metabolic products of ergosterol, the most important fungal sterol. Many of them are discussed in this review and some appear promising as leads for new medicines. At the same time, it is obvious that the described results not only characterize the achieved high level of research in this area, but also indicate directions for further scientific search, which is necessary for a better understanding of the content of the fungal metabolome and will allow revealing more fully the possibilities of practical use of its components in human healthcare.
Acknowledgments
We would like to acknowledge the Molecules journal for providing the APC waiver.
Abbreviations
| AChE | acetylcholinesterase |
| bw | body weight |
| ConA | concanavalin A |
| COX | cyclooxygenase |
| DHEP | 9,11-dehydroergosterol peroxide |
| DPP-IV | dipeptidyl peptidase IV |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| ED50 | median effective dose |
| EP | ergosterol peroxide |
| GIRK | G protein-coupled inwardly-rectifying potassium channel |
| Galp | galactopyranosyl |
| Glcp | glucopyranosyl |
| HNE | human neutrophil elastase |
| IC50 | half maximal inhibitory concentration |
| IDH | isocitrate dehydrogenase |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| LDL-C | low density lipoprotein cholesterol |
| L-NMMA | NG-methyl-L-arginine acetate salt |
| LPS | lipopolysaccharide |
| MDM2 | mouse double minute 2 homolog |
| MIC | minimum inhibitory concentration |
| MRSA | methicillin resistant Staphylococcus aureus |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | nitric oxide |
| NOE | nuclear Overhauser effect |
| ORAC | Oxygen Radical Antioxidant Capacity |
| PCSK9 | proprotein convertase subtilisin/kexin type 9 |
| PEG | poly(ethylene glycol) |
| PGE2 | prostaglandin E2 |
| PTP | protein tyrosine phosphatase |
| PTP1B | protein tyrosine phosphatase 1B |
| ROS | reactive oxygen species |
| PPAR | peroxisome proliferator-activated receptor |
| SHP2 | SH2-containing protein tyrosine phosphatase-2 |
| TE | Trolox equivalent |
| TNF-α | tumor necrosis factor alpha |
| TRAP | tartrate-resistant acid phosphatase |
Author Contributions
V.N.Z., P.D., and V.A.K. contributed equally. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support by the Belarusian Foundation for Fundamental Research (projects X22ChI-025 and X22MC-004) is greatly appreciated.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Öztürk M., Tel-Çayan G., Muhammad A., Terzioğlu P., Duru M.E. Mushrooms: A Source of Exciting Bioactive Compounds. In: Atta-ur R., editor. Studies in Natural Products Chemistry. Volume 45. Elsevier; Amsterdam, The Netherlands: 2015. pp. 363–456. [DOI] [Google Scholar]
- 2.Lindequist U., Niedermeyer T.H.J., Jülich W.D. The pharmacological potential of mushrooms. eCAM. 2005;2:285–299. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanjai S., Manmohan C., Inder Pal S. Fungal bioactive compounds in pharmaceutical research and development. Curr. Bioact. Compd. 2019;15:211–231. doi: 10.2174/1573407214666180622104720. [DOI] [Google Scholar]
- 4.Rodrigues M.L. The multifunctional fungal ergosterol. mBio. 2018;9:e01755-18. doi: 10.1128/mBio.01755-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang B.-B., Han X.-L., Jiang Q., Liao Z.-X., Wang H.-S. Cytotoxic cholestane-type and ergostane-type steroids from the aerial parts of Euphorbia altotibetic. Steroids. 2013;78:38–43. doi: 10.1016/j.steroids.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Kikuchi T., Maekawa Y., Tomio A., Masumoto Y., Yamamoto T., In Y., Yamada T., Tanaka R. Six new ergostane-type steroids from king trumpet mushroom (Pleurotus eryngii) and their inhibitory effects on nitric oxide production. Steroids. 2016;115:9–17. doi: 10.1016/j.steroids.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Hu Z., Wu Y., Xie S., Sun W., Guo Y., Li X.-N., Liu J., Li H., Wang J., Luo Z., et al. Phomopsterones A and B, two functionalized ergostane-type steroids from the endophytic fungus Phomopsis sp. TJ507A. Org. Lett. 2017;19:258–261. doi: 10.1021/acs.orglett.6b03557. [DOI] [PubMed] [Google Scholar]
- 8.Tan Z., Zhao J.L., Liu J.M., Zhang M., Chen R.D., Xie K.B., Chen D.W., Dai J.G. Lanostane triterpenoids and ergostane-type steroids from the cultured mycelia of Ganoderma capense. J. Asian Nat. Prod. Res. 2018;20:844–851. doi: 10.1080/10286020.2017.1399879. [DOI] [PubMed] [Google Scholar]
- 9.Happi G.M., Wouamba S.C.N., Ismail M., Kouam S.F., Frese M., Lenta B.N., Sewald N. Ergostane-type steroids from the Cameroonian ‘white tiama’ Entandrophragma angolense. Steroids. 2020;156:108584. doi: 10.1016/j.steroids.2020.108584. [DOI] [PubMed] [Google Scholar]
- 10.Lee S.R., Choi J.H., Ryoo R., Kim J.-C., Pang C., Kim S.-H., Kim K.H. Ergostane-type steroids from Korean wild mushroom Xerula furfuracea that control adipocyte and osteoblast differentiation. J. Microbiol. Biotechnol. 2020;30:1769–1776. doi: 10.4014/jmb.2006.06013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang Y., Zhang M., Yu M., Wang J., Zhu H., Chen C., Zhang Y. Four new ergostane-type steroids from Lasiodiplodia pseudotheobromae. Tetrahedron Lett. 2020;61:151737. doi: 10.1016/j.tetlet.2020.151737. [DOI] [Google Scholar]
- 12.Yu J.H., Yu S.J., Liu K.L., Wang C., Liu C., Sun J.Y., Zhang H. Cytotoxic ergostane-type steroids from Ganoderma lingzhi. Steroids. 2021;165:108767. doi: 10.1016/j.steroids.2020.108767. [DOI] [PubMed] [Google Scholar]
- 13.Zheng J., Wang Y., Wang J., Liu P., Li J., Zhu W. Antimicrobial ergosteroids and pyrrole derivatives from halotolerant Aspergillus flocculosus PT05-1 cultured in a hypersaline medium. Extremophiles. 2013;17:963–971. doi: 10.1007/s00792-013-0578-9. [DOI] [PubMed] [Google Scholar]
- 14.Han J.-J., Bao L., Tao Q.-Q., Yao Y.-J., Liu X.-Z., Yin W.-B., Liu H.-W. Gloeophyllins A–J, cytotoxic ergosteroids with various skeletons from a Chinese Tibet fungus Gloeophyllum abietinum. Org. Lett. 2015;17:2538–2541. doi: 10.1021/acs.orglett.5b01080. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Dai O., Peng C., Su H.G., Miao L.L., Liu L.S., Xiong L. Polyoxygenated ergosteroids from the macrofungus Omphalia lapidescens and the structure-cytotoxicity relationship in a human gastric cancer cell line. Phytochem. Lett. 2018;25:99–104. doi: 10.1016/j.phytol.2018.04.005. [DOI] [Google Scholar]
- 16.Liu F., Chen J.-F., Wang Y., Guo L., Zhou Q.-M., Peng C., Xiong L. Cytotoxicity of lanostane-type triterpenoids and ergosteroids isolated from Omphalia lapidescens on MDA-MB-231 and HGC-27 cells. Biomed. Pharmacother. 2019;118:109273. doi: 10.1016/j.biopha.2019.109273. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Z.-Z., Han K.-Y., Li Z.-H., Feng T., Chen H.-P., Liu J.-K. Cytotoxic ergosteroids from the fungus Stereum hirsutum. Phytochem. Lett. 2019;30:143–149. doi: 10.1016/j.phytol.2019.02.007. [DOI] [Google Scholar]
- 18.Lardy H., Partridge B., Kneer N., Wei Y. Ergosteroids: Induction of thermogenic enzymes in liver of rats treated with steroids derived from dehydroepiandrosterone. Proc. Natl. Acad. Sci. USA. 1995;92:6617–6619. doi: 10.1073/pnas.92.14.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glotter E. Withanolides and related ergostane-type steroids. Nat. Prod. Rep. 1991;8:415–440. doi: 10.1039/np9910800415. [DOI] [PubMed] [Google Scholar]
- 20.Chen L.X., He H., Qiu F. Natural withanolides: An overview. Nat. Prod. Rep. 2011;28:705–740. doi: 10.1039/c0np00045k. [DOI] [PubMed] [Google Scholar]
- 21.Xu Q.Q., Wang K.W. Natural bioactive new withanolides. Mini Rev. Med. Chem. 2020;20:1101–1117. doi: 10.2174/1389557518666171129164056. [DOI] [PubMed] [Google Scholar]
- 22.Aiello A., Fattorusso E., Menna M. Steroids from sponges: Recent reports. Steroids. 1999;64:687–714. doi: 10.1016/S0039-128X(99)00032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duecker F.L., Reuß F., Heretsch P. Rearranged ergostane-type natural products: Chemistry, biology, and medicinal aspects. Org. Biomol. Chem. 2019;17:1624–1633. doi: 10.1039/C8OB02325E. [DOI] [PubMed] [Google Scholar]
- 24.Merdivan S., Lindequist U. Ergosterol peroxide: A mushroom-derived compound with promising biological activities-a review. Int. J. Med. Mushrooms. 2017;19:93–105. doi: 10.1615/IntJMedMushrooms.v19.i2.10. [DOI] [PubMed] [Google Scholar]
- 25.Choi J.-H. Biologically functional molecules from mushroom-forming fungi. Biosci. Biotechnol. Biochem. 2018;82:372–382. doi: 10.1080/09168451.2018.1431519. [DOI] [PubMed] [Google Scholar]
- 26.Clericuzio M., Mellerio G.G., Finzi P.V., Vidari G. Secondary metabolites isolated from Tricholoma species (Basidiomycota, Tricholomatacee): A review. Nat. Prod. Commun. 2018;13:1213–1224. doi: 10.1177/1934578X1801300926. [DOI] [Google Scholar]
- 27.Vil V.A., Gloriozova T.A., Poroikov V.V., Terent’ev A.O., Savidov N., Dembitsky V.M. Peroxy steroids derived from plant and fungi and their biological activities. Appl. Microbiol. Biotechnol. 2018;102:7657–7667. doi: 10.1007/s00253-018-9211-2. [DOI] [PubMed] [Google Scholar]
- 28.Vil V.A., Terent’ev A.O., Savidov N., Gloriozova T.A., Poroikov V.V., Pounina T.A., Dembitsky V.M. Hydroperoxy steroids and triterpenoids derived from plant and fungi: Origin, structures and biological activities. J. Steroid Biochem. Mol. Biol. 2019;190:76–87. doi: 10.1016/j.jsbmb.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Dai Z.B., Wang X., Li G.H. Secondary metabolites and their bioactivities produced by Paecilomyces. Molecules. 2020;25:5077. doi: 10.3390/molecules25215077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao J., Gao M., Fei B., Huang G., Diao Q. Nature-derived anticancer steroids outside cardica glycosides. Fitoterapia. 2020;147:104757. doi: 10.1016/j.fitote.2020.104757. [DOI] [PubMed] [Google Scholar]
- 31.Ha J.W., Kim J., Kim H., Jang W., Kim K.H. Mushrooms: An important source of natural bioactive compounds. Nat. Prod. Sci. 2020;26:118–131. doi: 10.20307/nps.2020.26.2.118. [DOI] [Google Scholar]
- 32.Savidov N., Gloriozova T.A., Poroikov V.V., Dembitsky V.M. Highly oxygenated isoprenoid lipids derived from fungi and fungal endophytes: Origin and biological activities. Steroids. 2018;140:114–124. doi: 10.1016/j.steroids.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Loria-Kohen V., Lourenco-Nogueira T., Espinosa-Salinas I., Marin F.R., Soler-Rivas C., Ramirez de Molina A. Nutritional and functional properties of edible mushrooms: A food with promising health claims. J. Pharm. Nutr. Sci. 2014;4:187. doi: 10.6000/1927-5951.2014.04.03.4. [DOI] [Google Scholar]
- 34.Nowak R., Nowacka-Jechalke N., Pietrzak W., Gawlik-Dziki U. A new look at edible and medicinal mushrooms as a source of ergosterol and ergosterol peroxide—UHPLC-MS/MS analysis. Food Chem. 2022;369:130927. doi: 10.1016/j.foodchem.2021.130927. [DOI] [PubMed] [Google Scholar]
- 35.Shao S., Hernandez M., Kramer J.K., Rinker D.L., Tsao R. Ergosterol profiles, fatty acid composition, and antioxidant activities of button mushrooms as affected by tissue part and developmental stage. J. Agric. Food Chem. 2010;58:11616–11625. doi: 10.1021/jf102285b. [DOI] [PubMed] [Google Scholar]
- 36.Schneider I., Kressel G., Meyer A., Krings U., Berger R.G., Hahn A. Lipid lowering effects of oyster mushroom (Pleurotus ostreatus) in humans. J. Funct. Foods. 2011;3:17–24. doi: 10.1016/j.jff.2010.11.004. [DOI] [Google Scholar]
- 37.Gil-Ramirez A., Ruiz-Rodriguez A., Marin F.R., Reglero G., Soler-Rivas C. Effect of ergosterol-enriched extracts obtained from Agaricus bisporus on cholesterol absorption using an in vitro digestion model. J. Funct. Foods. 2014;11:589–597. doi: 10.1016/j.jff.2014.08.025. [DOI] [Google Scholar]
- 38.Morales D., Tejedor-Calvo E., Jurado-Chivato N., Polo G., Tabernero M., Ruiz-Rodriguez A., Largo C., Soler-Rivas C. In vitro and in vivo testing of the hypocholesterolemic activity of ergosterol- and β-glucan-enriched extracts obtained from shiitake mushrooms (Lentinula edodes) Food Funct. 2019;10:7325–7332. doi: 10.1039/c9fo01744e. [DOI] [PubMed] [Google Scholar]
- 39.Wu M., Huang T., Wang J., Chen P., Mi W., Ying Y., Wang H., Zhao D., Huang S. Antilung cancer effect of ergosterol and cisplatin-loaded liposomes modified with cyclic arginine-glycine-aspartic acid and octa-arginine peptides. Medicine. 2018;97:e11916. doi: 10.1097/md.0000000000011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin Y.-C., Lee B.-H., Alagie J., Su C.-H. Combination treatment of ergosterol followed by amphotericin B induces necrotic cell death in human hepatocellular carcinoma cells. Oncotarget. 2017;8:72727–72738. doi: 10.18632/oncotarget.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sankaran M., Isabella S., Amaranth K. Anti proliferative potential of ergosterol: A unique plant sterol on Hep2 cell line. Int. J. Pharma Res. Health Sci. 2017;5:1736–1742. doi: 10.21276/ijprhs.2017.04.04. [DOI] [Google Scholar]
- 42.Li X., Wu Q., Xie Y., Ding Y., Du W.W., Sdiri M., Yang B.B. Ergosterol purified from medicinal mushroom Amauroderma rude inhibits cancer growth in vitro and in vivo by up-regulating multiple tumor suppressors. Oncotarget. 2015;6:17832–17846. doi: 10.18632/oncotarget.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu X., Jiang D., Li F., Wu Z., Huang Y., Song S., Wang Z. Ergosterol reverses multidrug resistance in SGC7901/Adr cells. Pharmazie. 2014;69:396–400. doi: 10.1691/ph.2014.3866. [DOI] [PubMed] [Google Scholar]
- 44.Munoz-Fonseca M.B., Vidal-Limon A., Fernandez-Pomares C., Rojas-Duran F., Hernandez-Aguilar M.E., Espinoza C., Trigos A., Suarez-Medellin J. Ergosterol exerts a differential effect on AR-dependent LNCaP and AR-independent DU-145 cancer cells. Nat. Prod. Res. 2021;35:4857–4860. doi: 10.1080/14786419.2020.1737054. [DOI] [PubMed] [Google Scholar]
- 45.Yazawa Y., Ikarashi N., Hoshino M., Kikkawa H., Sakuma F., Sugiyama K. Inhibitory effect of ergosterol on bladder carcinogenesis is due to androgen signaling inhibition by brassicasterol, a metabolite of ergosterol. J. Nat. Med. 2020;74:680–688. doi: 10.1007/s11418-020-01419-4. [DOI] [PubMed] [Google Scholar]
- 46.Kuo C.-F., Hsieh C.-H., Lin W.-Y. Proteomic response of LAP-activated RAW 264.7 macrophages to the anti-inflammatory property of fungal ergosterol. Food Chem. 2011;126:207–212. doi: 10.1016/j.foodchem.2010.10.101. [DOI] [Google Scholar]
- 47.Kageyama-Yahara N., Wang P., Wang X., Yamamoto T., Kadowaki M. The inhibitory effect of ergosterol, a bioactive constituent of a traditional Japanese herbal medicine saireito on the activity of mucosal-type mast cells. Biol. Pharm. Bull. 2010;33:142–145. doi: 10.1248/bpb.33.142. [DOI] [PubMed] [Google Scholar]
- 48.Kawai J., Higuchi Y., Hirota M., Hirasawa N., Mori K. Ergosterol and its derivatives from Grifola frondosa inhibit antigen-induced degranulation of RBL-2H3 cells by suppressing the aggregation of high affinity IgE receptors. Biosci. Biotechnol. Biochem. 2018;82:1803–1811. doi: 10.1080/09168451.2018.1490169. [DOI] [PubMed] [Google Scholar]
- 49.Zhang S.-Y., Xu L.-T., Li A.-X., Wang S.-M. Effects of ergosterol, isolated from Scleroderma polyrhizum Pers., on lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation. 2015;38:1979–1985. doi: 10.1007/s10753-015-0178-1. [DOI] [PubMed] [Google Scholar]
- 50.Wang H., Zhang T., Li Y., Wang S. Effects of ergosterol on COPD in mice via JAK3/STAT3/NF-κB pathway. Inflammation. 2017;40:884–893. doi: 10.1007/s10753-017-0533-5. [DOI] [PubMed] [Google Scholar]
- 51.Sun X., Feng X., Zheng D., Li A., Li C., Li S., Zhao Z. Ergosterol attenuates cigarette smoke extract-induced COPD by modulating inflammation, oxidative stress and apoptosis in vitro and in vivo. Clin. Sci. 2019;133:1523–1536. doi: 10.1042/cs20190331. [DOI] [PubMed] [Google Scholar]
- 52.Xu J., Lin C., Wang T., Zhang P., Liu Z., Lu C. Ergosterol attenuates LPS-induced myocardial injury by modulating oxidative stress and apoptosis in rats. Cell. Physiol. Biochem. 2018;48:583–592. doi: 10.1159/000491887. [DOI] [PubMed] [Google Scholar]
- 53.Xie Q., Li S., Gao Y., Jin L., Dai C., Song J. Ergosterol attenuates isoproterenol-induced myocardial cardiotoxicity. Cardiovasc. Toxicol. 2020;20:500–506. doi: 10.1007/s12012-020-09574-6. [DOI] [PubMed] [Google Scholar]
- 54.Cai D., Yan H., Liu J., Chen S., Jiang L., Wang X., Qin J. Ergosterol limits osteoarthritis development and progression through activation of Nrf2 signaling. Exp. Ther. Med. 2021;21:194. doi: 10.3892/etm.2021.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong Z., Sun Y., Wei G., Li S., Zhao Z. Ergosterol ameliorates diabetic nephropathy by attenuating mesangial cell proliferation and extracellular matrix deposition via the TGF-β1/Smad2 signaling pathway. Nutrients. 2019;11:483. doi: 10.3390/nu11020483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu C., Zhao S., Zhu C., Gao Q., Bai J., Si J., Chen Y. Ergosterol ameliorates renal inflammatory responses in mice model of diabetic nephropathy. Biomed. Pharmacother. 2020;128:110252. doi: 10.1016/j.biopha.2020.110252. [DOI] [PubMed] [Google Scholar]
- 57.Xiong M., Huang Y., Liu Y., Huang M., Song G., Ming Q., Ma X., Yang J., Deng S., Wen Y., et al. Antidiabetic activity of ergosterol from Pleurotus ostreatus in KK-Ay mice with spontaneous type 2 diabetes mellitus. Mol. Nutr. Food Res. 2018;62:1700444. doi: 10.1002/mnfr.201700444. [DOI] [PubMed] [Google Scholar]
- 58.Tai C.-J., Choong C.-Y., Lin Y.-C., Shi Y.-C., Tai C.-J. The anti-hepatic fibrosis activity of ergosterol depended on upregulation of PPARgamma in HSC-T6 cells. Food Funct. 2016;7:1915–1923. doi: 10.1039/c6fo00117c. [DOI] [PubMed] [Google Scholar]
- 59.Kikuchi T., Motoyashiki N., Yamada T., Shibatani K., Ninomiya K., Morikawa T., Tanaka R. Ergostane-type sterols from king trumpet mushroom (Pleurotus eryngii) and their inhibitory effects on aromatase. Int. J. Mol. Sci. 2017;18:2479. doi: 10.3390/ijms18112479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Medina M.E., Galano A., Trigos A. Scavenging ability of homogentisic acid and ergosterol toward free radicals derived from ethanol consumption. J. Phys. Chem. B. 2018;122:7514–7521. doi: 10.1021/acs.jpcb.8b04619. [DOI] [PubMed] [Google Scholar]
- 61.Zhao Y.-Y., Cheng X.-L., Liu R., Ho C.C., Wei F., Yan S.-H., Lin R.-C., Zhang Y., Sun W.-J. Pharmacokinetics of ergosterol in rats using rapid resolution liquid chromatography-atmospheric pressure chemical ionization multi-stage tandem mass spectrometry and rapid resolution liquid chromatography/tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011;879:1945–1953. doi: 10.1016/j.jchromb.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H.-Y., Firempong C.K., Wang Y.-W., Xu W.-Q., Wang M.-M., Cao X., Zhu Y., Tong S.-S., Yu J.-N., Xu X.-M. Ergosterol-loaded poly(lactide-co-glycolide) nanoparticles with enhanced in vitro antitumor activity and oral bioavailability. Acta Pharmacol. Sin. 2016;37:834–844. doi: 10.1038/aps.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong Z., Iqbal S., Zhao Z. Preparation of ergosterol-loaded nanostructured lipid carriers for enhancing oral bioavailability and antidiabetic nephropathy effects. AAPS PharmSciTech. 2020;21:64. doi: 10.1208/s12249-019-1597-3. [DOI] [PubMed] [Google Scholar]
- 64.Cheng J., Zhao H., Yao L., Li Y., Qi B., Wang J., Yang X. Simple and multifunctional natural self-assembled sterols with anticancer activity-mediated supramolecular photosensitizers for enhanced antitumor photodynamic therapy. ACS Appl. Mater. Interfaces. 2019;11:29498–29511. doi: 10.1021/acsami.9b07404. [DOI] [PubMed] [Google Scholar]
- 65.Yi C., Fu M., Cao X., Tong S., Zheng Q., Firempong C.K., Jiang X., Xu X., Yu J. Enhanced oral bioavailability and tissue distribution of a new potential anticancer agent, Flammulina velutipes sterols, through liposomal encapsulation. J. Agric. Food Chem. 2013;61:5961–5971. doi: 10.1021/jf3055278. [DOI] [PubMed] [Google Scholar]
- 66.He W.-S., Yin J., Xu H.-S., Qian Q.-Y., Jia C.-S., Ma H.-L., Feng B. Efficient synthesis and characterization of ergosterol laurate in a solvent-free system. J. Agric. Food Chem. 2014;62:11748–11755. doi: 10.1021/jf504516q. [DOI] [PubMed] [Google Scholar]
- 67.Park S.H., Kim H.K. Antibacterial activity of emulsions containing unsaturated fatty acid ergosterol esters synthesized by lipase-mediated transesterification. Enzyme Microb. Technol. 2020;139:109581. doi: 10.1016/j.enzmictec.2020.109581. [DOI] [PubMed] [Google Scholar]
- 68.He W.S., Li L., Zhao J., Xu H., Rui J., Cui D., Li H., Zhang H., Liu X. Candida sp. 99-125 lipase-catalyzed synthesis of ergosterol linolenate and its characterization. Food Chem. 2019;280:286–293. doi: 10.1016/j.foodchem.2018.12.076. [DOI] [PubMed] [Google Scholar]
- 69.Park H.G., Lee T.H., Chang F., Kwon H.J., Kim J., Kim H. Synthesis of ergosterol and 5,6-dihydroergosterol glycosides and their inhibitory activities on lipopolysaccharide-induced nitric oxide production. Bull. Korean Chem. Soc. 2013;34:1339–1344. doi: 10.5012/bkcs.2013.34.5.1339. [DOI] [Google Scholar]
- 70.Dissanayake A.A., Zhang C.-R., Mills G.L., Nair M.G. Cultivated maitake mushroom demonstrated functional food quality as determined by in vitro bioassays. J. Funct. Foods. 2018;44:79–85. doi: 10.1016/j.jff.2018.02.031. [DOI] [Google Scholar]
- 71.Aziz S., Elfahmi, Soemardji A.A. Molecular docking, synthesis and biological evaluation of ergosteryl-ferulate as a HMG-Coa reductase inhibitor. Pak. J. Pharm. Sci. 2020;33:997–1003. [PubMed] [Google Scholar]
- 72.Lin M., Li H., Zhao Y., Cai E., Zhu H., Gao Y., Liu S., Yang H., Zhang L., Tang G. 2-Naphthoic acid ergosterol ester, an ergosterol derivative, exhibits anti-tumor activity by promoting apoptosis and inhibiting angiogenesis. Steroids. 2017;122:9–15. doi: 10.1016/j.steroids.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 73.Lin M., Li H., Zhao Y., Cai E., Zhu H., Gao Y., Liu S., Yang H., Zhang L., Tang G., et al. Ergosteryl 2-naphthoate, an ergosterol derivative, exhibits antidepressant effects mediated by the modification of GABA ergic and glutamatergic systems. Molecules. 2017;22:565. doi: 10.3390/molecules22040565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karatavuk A.O., Karabulut H.R.F. Synthesis of novel dimers containing cholesterol and ergosterol using click reaction and their anti-proliferative effects. Mon. Chem. 2020;151:837–844. doi: 10.1007/s00706-020-02594-6. [DOI] [Google Scholar]
- 75.Weete J.D. Structure and Function of Sterols in Fungi. In: Paoletti R., Kritchevsky D., editors. Advances in Lipid Research. Volume 23. Elsevier; Amsterdam, The Netherlands: 1989. pp. 115–167. [DOI] [Google Scholar]
- 76.Weete J.D., Abril M., Blackwell M. Phylogenetic distribution of fungal sterols. PLoS ONE. 2010;5:e10899. doi: 10.1371/journal.pone.0010899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee H.S., Hwang I.H., Kim J.A., Choi J.Y., Jang T.-S., Osada H., Ahn J.S., Na M., Lee S.H. Isolation of protein tyrosine phosphatase 1B inhibitory constituents from the sclerotia of Polyporus umbellatus fries. Bull. Korean Chem. Soc. 2011;32:697–700. doi: 10.5012/bkcs.2011.32.2.697. [DOI] [Google Scholar]
- 78.Xiao J.-H., Sun Z.-H., Pan W.-D., Zhong J.-J. Secondary metabolite steroids isolated from medicinal entomogenous mushroom Cordyceps jiangxiensis mycelium. Nat. Prod. Indian J. 2011;7:118–123. [Google Scholar]
- 79.Tian M.-Q., Wu Q.-L., Wang X., Zhang K.-Q., Li G.-H. A new compound from Stereum insigne CGMCC5.57. Nat. Prod. Res. 2017;31:932–937. doi: 10.1080/14786419.2016.1255889. [DOI] [PubMed] [Google Scholar]
- 80.Qiao M.-F., Yi Y.-W., Deng J. Steroids from an endophytic Eurotium rubrum strain. Chem. Nat. Compd. 2017;53:678–681. doi: 10.1007/s10600-017-2089-x. [DOI] [Google Scholar]
- 81.Wu J., Fushimi K., Tokuyama S., Ohno M., Miwa T., Koyama T., Yazawa K., Nagai K., Matsumoto T., Hirai H., et al. Functional-food constituents in the fruiting bodies of Stropharia rugosoannulata. Biosci. Biotechnol. Biochem. 2011;75:1631–1634. doi: 10.1271/bbb.110308. [DOI] [PubMed] [Google Scholar]
- 82.Zhang S.S., Ma Q.Y., Zou X.S., Dai H.F., Huang S.Z., Luo Y., Yu Z.F., Luo H.R., Zhao Y.X. Chemical constituents from the fungus Amauroderma amoiensis and their in vitro acetylcholinesterase inhibitory activities. Planta Med. 2013;79:87–91. doi: 10.1055/s-0032-1327951. [DOI] [PubMed] [Google Scholar]
- 83.Wang Q., Wang Y.G., Ma Q.Y., Huang S.Z., Kong F.D., Zhou L.M., Dai H.F., Zhao Y.X. Chemical constituents from the fruiting bodies of Amauroderma subresinosum. J. Asian Nat. Prod. Res. 2016;18:1030–1035. doi: 10.1080/10286020.2016.1183650. [DOI] [PubMed] [Google Scholar]
- 84.Gao J., Wang L.W., Zheng H.C., Damirin A., Ma C.M. Cytotoxic constituents of Lasiosphaera fenzlii on different cell lines and the synergistic effects with paclitaxel. Nat. Prod. Res. 2016;30:1862–1865. doi: 10.1080/14786419.2015.1075526. [DOI] [PubMed] [Google Scholar]
- 85.Torres S., Cajas D., Palfner G., Astuya A., Aballay A., Pérez C., Hernández V., Becerra J. Steroidal composition and cytotoxic activity from fruiting body of Cortinarius xiphidipus. Nat. Prod. Res. 2017;31:473–476. doi: 10.1080/14786419.2016.1185717. [DOI] [PubMed] [Google Scholar]
- 86.Borlagdan M.S., De Castro M.E.G., van Altena I.A., Ragasa C.Y. Sterols from Trametes versicolor. Res. J. Pharm. Biol. Chem. Sci. 2017;8:740–744. [Google Scholar]
- 87.Giroux S., Corey E.J. An efficient, stereocontrolled synthesis of the 25-(R)-diastereomer of dafachronic acid A from β-ergosterol. Org. Lett. 2008;10:801–802. doi: 10.1021/ol702936f. [DOI] [PubMed] [Google Scholar]
- 88.Pereira D.M., Correia-da-Silva G., Valentao P., Teixeira N., Andrade P.B. Anti-inflammatory effect of unsaturated fatty acids and ergosta-7,22-dien-3-ol from Marthasterias glacialis: Prevention of CHOP-mediated ER-stress and NF-κB activation. PLoS ONE. 2014;9:e88341. doi: 10.1371/journal.pone.0088341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pereira D.M., Correia-da-Silva G., Valentao P., Teixeira N., Andrade P.B. Palmitic acid and ergosta-7,22-dien-3-ol contribute to the apoptotic effect and cell cycle arrest of an extract from Marthasterias glacialis L. in neuroblastoma cells. Mar. Drugs. 2014;12:54. doi: 10.3390/md12010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hong Y.J., Jang A.R., Jang H.J., Yang K.S. Inhibition of nitric oxide production, iNOS and COX-2 expression of ergosterol derivatives from Phellinus pini. Nat. Prod. Sci. 2012;18:147–152. [Google Scholar]
- 91.Sárközy A., Béni Z., Dékány M., Zomborszki Z.P., Rudolf K., Papp V., Hohmann J., Ványolós A. Cerebrosides and steroids from the edible mushroom Meripilus giganteus with antioxidant potential. Molecules. 2020;25:1395. doi: 10.3390/molecules25061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Budipramana K., Junaidin J., Wirasutisna K.R., Pramana Y.B., Sukrasno S. An integrated in silico and in vitro assays of dipeptidyl peptidase-4 and α-glucosidase inhibition by stellasterol from Ganoderma austral. Sci. Pharm. 2019;87:21. doi: 10.3390/scipharm87030021. [DOI] [Google Scholar]
- 93.Joseph S., Janardhanan K.K., George V., Baby S. A new epoxidic ganoderic acid and other phytoconstituents from Ganoderma lucidum. Phytochem. Lett. 2011;4:386–388. doi: 10.1016/j.phytol.2011.08.011. [DOI] [Google Scholar]
- 94.Jung M., Lee T.H., Oh H.J., Kim H., Son Y., Lee E.H., Kim J. Inhibitory effect of 5,6-dihydroergosteol-glucoside on atopic dermatitis-like skin lesions via suppression of NF-κB and STAT activation. J. Dermatol. Sci. 2015;79:252–261. doi: 10.1016/j.jdermsci.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 95.Kim T.K., Cho Y.K., Park H., Lee T.H., Kim H. Comparison of the inhibitory activities of 5,6-dihydroergosterol glycoside α- and β-anomers on skin inflammation. Molecules. 2019;24:371. doi: 10.3390/molecules24020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park Y.S., Moon H.J., Ahn K.H., Lee T.H., Kim H. Comparative study of the effect of 5,6-dihydroergosterol and 3-epi-5,6-dihydroergosterol on chemokine expression in human keratinocytes. Molecules. 2020;25:522. doi: 10.3390/molecules25030522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moon H., Ko M., Park Y., Kim J., Yoon D., Lee E., Lee T., Kim H. Δ8(14)-Ergostenol glycoside derivatives inhibit the expression of inflammatory mediators and matrix metalloproteinase. Molecules. 2021;26:4547. doi: 10.3390/molecules26154547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuo Y.H., Lin C.H., Shih C.C. Ergostatrien-3β-ol from Antrodia camphorata inhibits diabetes and hyperlipidemia in high-fat-diet treated mice via regulation of hepatic related genes, glucose transporter 4, and AMP-activated protein kinase phosphorylation. J. Agric. Food Chem. 2015;63:2479–2489. doi: 10.1021/acs.jafc.5b00073. [DOI] [PubMed] [Google Scholar]
- 99.Chang Y.Y., Liu Y.C., Kuo Y.H., Lin Y.L., Wu Y.S., Chen J.W., Chen Y.C. Effects of antrosterol from Antrodia camphorata submerged whole broth on lipid homeostasis, antioxidation, alcohol clearance, and anti-inflammation in livers of chronic-alcohol fed mice. J. Ethnopharmacol. 2017;202:200–207. doi: 10.1016/j.jep.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 100.Kuo Y.H., Lin T.Y., You Y.J., Wen K.C., Sung P.J., Chiang H.M. Antiinflammatory and antiphotodamaging effects of ergostatrien-3β-ol, isolated from Antrodia camphorata, on hairless mouse skin. Molecules. 2016;21:1213. doi: 10.3390/molecules21091213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma Q.-Y., Yang S., Huang S.-Z., Kong F.-D., Xie Q.-Y., Dai H.-F., Yu Z.-F., Zhao Y.-X. Ergostane steroids from Coprinus setulosus. Chem. Nat. Compd. 2018;54:710–713. doi: 10.1007/s10600-018-2451-7. [DOI] [Google Scholar]
- 102.Hsieh W.T., Hsu M.H., Lin W.J., Xiao Y.C., Lyu P.C., Liu Y.C., Lin W.Y., Kuo Y.H., Chung J.G. Ergosta-7,9 (11),22-trien-3β-ol interferes with LPS docking to LBP, CD14, and TLR4/MD-2 co-receptors to attenuate the NF-κB inflammatory pathway in vitro and Drosophila. Int. J. Mol. Sci. 2021;22:6511. doi: 10.3390/ijms22126511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shi Q., Huang Y., Su H., Gao Y., Peng X., Zhou L., Li X., Qiu M. C28 steroids from the fruiting bodies of Ganoderma resinaceum with potential anti-inflammatory activity. Phytochemistry. 2019;168:112109. doi: 10.1016/j.phytochem.2019.112109. [DOI] [PubMed] [Google Scholar]
- 104.Metwaly A.M., Kadry H.A., El-Hela A.A., Mohammad A.-E.I., Ma G., Cutler S.J., Ross S.A. Nigrosphaerin A a new isochromene derivative from the endophytic fungus Nigrospora sphaerica. Phytochem. Lett. 2014;7:1–5. doi: 10.1016/j.phytol.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xiong J., Huang Y., Wu X.-Y., Liu X.-H., Fan H., Wang W., Zhao Y., Yang G.-X., Zhang H.-Y., Hu J.-F. Chemical constituents from the fermented mycelia of the medicinal fungus Xylaria nigripes. Helv. Chim. Acta. 2016;99:83–89. doi: 10.1002/hlca.201500231. [DOI] [Google Scholar]
- 106.Kao S.-T., Kuo Y.-H., Wang S.-D., Hong H.-J., Lin L.-J. Analogous corticosteroids, 9A and EK100, derived from solid-state-cultured mycelium of Antrodia camphorata inhibit proinflammatory cytokine expression in macrophages. Cytokine. 2018;108:136–144. doi: 10.1016/j.cyto.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 107.Huang G.-J., Huang S.-S., Lin S.-S., Shao Y.-Y., Chen C.-C., Hou W.-C., Kuo Y.-H. Analgesic effects and the mechanisms of anti-inflammation of ergostatrien-3β-ol from Antrodia camphorata submerged whole broth in mice. J. Agric. Food Chem. 2010;58:7445–7452. doi: 10.1021/jf1013764. [DOI] [PubMed] [Google Scholar]
- 108.Tsai T.-C., Tung Y.-T., Kuo Y.-H., Liao J.-W., Tsai H.-C., Chong K.-Y., Chen H.-L., Chen C.-M. Anti-inflammatory effects of Antrodia camphorata, a herbal medicine, in a mouse skin ischemia model. J. Ethnopharmacol. 2015;159:113–121. doi: 10.1016/j.jep.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 109.Chao T.Y., Hsieh C.C., Hsu S.M., Wan C.H., Lian G.T., Tseng Y.H., Kuo Y.H., Hsieh S.C. Ergostatrien-3β-ol (EK100) from Antrodia camphorata attenuates oxidative stress, inflammation, and liver injury in vitro and in vivo. Prev. Nutr. Food Sci. 2021;26:58–66. doi: 10.3746/pnf.2021.26.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y.H., Chern C.M., Liou K.T., Kuo Y.H., Shen Y.C. Ergostatrien-7,9(11),22-trien-3β-ol from Antrodia camphorata ameliorates ischemic stroke brain injury via downregulation of p65NF-κ-B and caspase 3, and activation of Akt/GSK3/catenin-associated neurogenesis. Food Funct. 2019;10:4725–4738. doi: 10.1039/c9fo00908f. [DOI] [PubMed] [Google Scholar]
- 111.Hsueh P.J., Wang M.H., Hsiao C.J., Chen C.K., Lin F.L., Huang S.H., Yen J.L., Tsai P.H., Kuo Y.H., Hsiao G. Ergosta-7,9(11),22-trien-3β-ol alleviates intracerebral hemorrhage-induced brain injury and bv-2 microglial activation. Molecules. 2021;26:2970. doi: 10.3390/molecules26102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Y.M., Sung H.C., Kuo Y.H., Hsu Y.J., Huang C.C., Liang H.L. The effects of ergosta-7,9(11),22-trien-3β-ol from Antrodia camphorata on the biochemical profile and exercise performance of mice. Molecules. 2019;24:1225. doi: 10.3390/molecules24071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Robalo J.R., do Canto A.M.T.M., Carvalho A.J.P., Ramalho J.P.P., Loura L.M.S. Behavior of fluorescent cholesterol analogues dehydroergosterol and cholestatrienol in lipid bilayers: A molecular dynamics study. J. Phys. Chem. B. 2013;117:5806–5819. doi: 10.1021/jp312026u. [DOI] [PubMed] [Google Scholar]
- 114.Pourmousa M., Rog T., Mikkeli R., Vattulainen l., Solanko L.M., Wustner D., List N.H., Kongsted J., Karttunen M. Dehydroergosterol as an analogue for cholesterol: Why it mimics cholesterol so well-or does it? J. Phys. Chem. B. 2014;118:7345–7357. doi: 10.1021/jp406883k. [DOI] [PubMed] [Google Scholar]
- 115.Chattopadhyay A., Biswas S.C., Rukmini R., Saha S., Samanta A. Lack of environmental sensitivity of a naturally occurring fluorescent analog of cholesterol. J. Fluoresc. 2021;31:1401–1407. doi: 10.1007/s10895-021-02767-4. [DOI] [PubMed] [Google Scholar]
- 116.Ano Y., Kutsukake T., Hoshi A., Yoshida A., Nakayama H. Identification of a novel dehydroergosterol enhancing microglial anti-inflammatory activity in a dairy product fermented with Penicillium candidum. PLoS ONE. 2015;10:e0116598. doi: 10.1371/journal.pone.0116598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ano Y., Ikado K., Shindo K., Koizumi H., Fujiwara D. Identification of 14-dehydroergosterol as a novel anti-inflammatory compound inducing tolerogenic dendritic cells. Sci. Rep. 2017;7:13903. doi: 10.1038/s41598-017-14446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu Z., Dong Z., Qiu P., Wang Q., Yan J., Lu Y., Wasu P.-A., Hong K., She Z. Two new bioactive steroids from a mangrove-derived fungus Aspergillus sp. Steroids. 2018;140:32–38. doi: 10.1016/j.steroids.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 119.Liangsakul J., Srisurichan S., Pornpakakul S. Anthraquinone-steroids, evanthrasterol A and B, and a meroterpenoid, emericellic acid, from endophytic fungus, Emericella variecolor. Steroids. 2016;106:78–85. doi: 10.1016/j.steroids.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 120.Elsebai M.F., Ghabbour H.A., Mehiri M. Unusual nitrogenous phenalenone derivatives from the marine-derived fungus Coniothyrium cereale. Molecules. 2016;21:178. doi: 10.3390/molecules21020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hou G.M., Xu X.M., Wang Q., Li D.Y., Li Z.L. Hybrid of dehydroergosterol and nitrogenous alternariol derivative from the fungus Pestalotiopsis uvicola. Steroids. 2018;138:43–46. doi: 10.1016/j.steroids.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 122.El-Sherif N.F., Ahmed S.A., Ibrahim A.K., Habib E.S., El-Fallal A.A., El-Sayed A.K., Wahba A.E. Ergosterol peroxide from the egyptian red lingzhi or reishi mushroom, Ganoderma resinaceum (Agaricomycetes), showed preferred inhibition of MCF-7 over MDA-MB-231 breast cancer cell lines. Int. J. Med. Mushrooms. 2020;22:389–396. doi: 10.1615/IntJMedMushrooms.2020034223. [DOI] [PubMed] [Google Scholar]
- 123.Chen P., Qin H.-J., Li Y.-W., Ma G.-X., Yang J.-S., Wang Q. Study on chemical constituents of an edible mushroom Volvariella volvacea and their antitumor activity in vitro. Nat. Prod. Res. 2020;34:1417–1422. doi: 10.1080/14786419.2018.1509324. [DOI] [PubMed] [Google Scholar]
- 124.Govindharaj M., Arumugam S., Nirmala G., Bharadwaj M., Murugiyan K. Effect of marine basidiomycetes Fulvifomes sp.-derived ergosterol peroxide on cytotoxicity and apoptosis induction in MCF-7 cell line. J. Fungi. 2019;5:16. doi: 10.3390/jof5010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu H.-Y., Yang F.-L., Li L.-H., Rao Y.K., Ju T.-C., Wong W.-T., Hsieh C.-Y., Pivkin M.V., Hua K.-F., Wu S.-H. Ergosterol peroxide from marine fungus Phoma sp. induces ROS-dependent apoptosis and autophagy in human lung adenocarcinoma cells. Sci. Rep. 2018;8:17956. doi: 10.1038/s41598-018-36411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee S., Lee D., Ryoo R., Kim J.-C., Park H.B., Kang K.S., Kim K.H. Calvatianone, a sterol possessing a 6/5/6/5-fused ring system with a contracted tetrahydrofuran B-ring, from the fruiting bodies of Calvatia nipponica. J. Nat. Prod. 2020;83:2737–2742. doi: 10.1021/acs.jnatprod.0c00673. [DOI] [PubMed] [Google Scholar]
- 127.Chen Y.K., Kuo Y.H., Chiang B.H., Lo J.M., Sheen L.Y. Cytotoxic activities of 9,11-dehydroergosterol peroxide and ergosterol peroxide from the fermentation mycelia of Ganoderma lucidum cultivated in the medium containing leguminous plants on Hep 3B cells. J. Agric. Food Chem. 2009;57:5713–5719. doi: 10.1021/jf900581h. [DOI] [PubMed] [Google Scholar]
- 128.Lee C., Kim S., Li W., Bang S., Lee H., Lee H.-J., Noh E.-Y., Park J.-E., Bang W.Y., Shim S.H. Bioactive secondary metabolites produced by an endophytic fungus Gaeumannomyces sp. JS0464 from a maritime halophyte Phragmites communis. J. Antibiot. 2017;70:737–742. doi: 10.1038/ja.2017.39. [DOI] [PubMed] [Google Scholar]
- 129.Kobori M., Yoshida M., Ohnishi-Kameyama M., Takei T., Shinmoto H. 5α,8α-Epidioxy-22E-ergosta-6,9(11),22-trien-3β-ol from an edible mushroom suppresses growth of HL60 leukemia and HT29 colon adenocarcinoma cells. Biol. Pharm. Bull. 2006;29:755–759. doi: 10.1248/bpb.29.755. [DOI] [PubMed] [Google Scholar]
- 130.Cui Y.J., Guan S.H., Feng L.X., Song X.Y., Ma C., Cheng C.R., Wang W.B., Wu W.Y., Yue Q.X., Liu X., et al. Cytotoxicity of 9,11-dehydroergosterol peroxide isolated from Ganoderma lucidum and its target-related proteins. Nat. Prod. Commun. 2010;5:1183–1186. [PubMed] [Google Scholar]
- 131.Zheng L., Wong Y.S., Shao M., Huang S., Wang F., Chen J. Apoptosis induced by 9,11-dehydroergosterol peroxide from Ganoderma Lucidum mycelium in human malignant melanoma cells is Mcl-1 dependent. Mol. Med. Rep. 2018;18:938–944. doi: 10.3892/mmr.2018.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Njue A.W., Omolo J.O., Cheplogoi P.K., Langat M.K., Mulholland D.A. Cytotoxic ergostane derivatives from the edible mushroom Termitomyces microcarpus (Lyophyllaceae) Biochem. Syst. Ecol. 2018;76:12–14. doi: 10.1016/j.bse.2017.11.006. [DOI] [Google Scholar]
- 133.Chen Z., Bishop K.S., Tanambell H., Buchanan P., Smith C., Quek S.Y. Characterization of the bioactivities of an ethanol extract and some of its constituents from the New Zealand native mushroom Hericium novae-zealandiae. Food Funct. 2019;10:6633–6643. doi: 10.1039/c9fo01672d. [DOI] [PubMed] [Google Scholar]
- 134.He L., Shi W., Liu X., Zhao X., Zhang Z. Anticancer action and mechanism of ergosterol peroxide from Paecilomyces cicadae fermentation broth. Int. J. Mol. Sci. 2018;19:3935. doi: 10.3390/ijms19123935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tan W., Pan M., Liu H., Tian H., Ye Q., Liu H. Ergosterol peroxide inhibits ovarian cancer cell growth through multiple pathways. OncoTargets Ther. 2017;10:3467–3474. doi: 10.2147/ott.s139009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shin M.-K., Sasaki F., Ki D.-W., Win N.N., Morita H., Hayakawa Y. Anti-metastatic effects of ergosterol peroxide from the entomopathogenic fungus Ophiocordyceps gracilioides on 4T1 breast cancer cells. J. Nat. Med. 2021;75:824–832. doi: 10.1007/s11418-021-01520-2. [DOI] [PubMed] [Google Scholar]
- 137.Yang Y., Luo X., Yasheng M., Zhao J., Li J., Li J. Ergosterol peroxide from Pleurotus ferulae inhibits gastrointestinal tumor cell growth through induction of apoptosis via reactive oxygen species and endoplasmic reticulum stress. Food Funct. 2020;11:4171–4184. doi: 10.1039/c9fo02454a. [DOI] [PubMed] [Google Scholar]
- 138.Zhao F., Xia G., Chen L., Zhao J., Xie Z., Qiu F., Han G. Chemical constituents from Inonotus obliquus and their antitumor activities. J. Nat. Med. 2016;70:721–730. doi: 10.1007/s11418-016-1002-4. [DOI] [PubMed] [Google Scholar]
- 139.Zhou J., Li G., Deng Q., Zheng D., Yang X., Xu J. Cytotoxic constituents from the mangrove endophytic Pestalotiopsis sp. induce G(0)/G(1) cell cycle arrest and apoptosis in human cancer cells. Nat. Prod. Res. 2018;32:2968–2972. doi: 10.1080/14786419.2017.1395431. [DOI] [PubMed] [Google Scholar]
- 140.Xiao L.-G., Zhang Y., Zhang H.-L., Li D., Gu Q., Tang G.-H., Yu Q., An L.-K. Spiroconyone A, a new phytosterol with a spiro [5,6] ring system from Conyza japonica. Org. Biomol. Chem. 2020;18:5130–5136. doi: 10.1039/d0ob00666a. [DOI] [PubMed] [Google Scholar]
- 141.Shaker S., Sun T.-T., Wang L.-Y., Ma W.-Z., Wu D.-L., Guo Y.-W., Dong J., Chen Y.-X., Zhu L.-P., Yang D.-P., et al. Reactive oxygen species altering the metabolite profile of the marine-derived fungus Dichotomomyces cejpii F31-1. Nat. Prod. Res. 2021;35:41–48. doi: 10.1080/14786419.2019.1611816. [DOI] [PubMed] [Google Scholar]
- 142.Le B.V., Nguyen T.M.N., Yang S.Y., Kim J.H., Le T.V., Huong P.T.T., Nguyen V.T., Nguyen X.C., Nguyen H.N., Chau V.M., et al. A new rearranged abietane diterpene from Clerodendrum inerme with antioxidant and cytotoxic activities. Nat. Prod. Res. 2018;32:2001–2007. doi: 10.1080/14786419.2017.1360885. [DOI] [PubMed] [Google Scholar]
- 143.Nguyen T.T., Nguyen D.H., Zhao B.T., Le D.D., Min B.S., Kim Y.H., Woo M.H. Triterpenoids and sterols from the grains of Echinochloa utilis Ohwi & Yabuno and their cytotoxic activity. Biomed. Pharmacother. 2017;93:202–207. doi: 10.1016/j.biopha.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 144.Hung D.X., Kuo P.-C., Tuan N.N., Van Trung H., Tan Thanh N., Thi Ha N., Long Giang B., Quang Trung N., Thi Ngan N., Hai H.V., et al. Triterpenoids and steroids from the fruiting bodies of Hexagonia tenuis and their cytotoxicity. Nat. Prod. Res. 2021;35:251–256. doi: 10.1080/14786419.2019.1624963. [DOI] [PubMed] [Google Scholar]
- 145.Bu M., Li H., Wang H., Wang J., Lin Y., Ma Y. Synthesis of ergosterol peroxide conjugates as mitochondria targeting probes for enhanced anticancer activity. Molecules. 2019;24:3307. doi: 10.3390/molecules24183307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bu M., Cao T., Li H., Guo M., Yang B.B., Zeng C., Hu L. Synthesis of 5α,8α-ergosterol peroxide 3-carbamate derivatives and a fluorescent mitochondria-targeting conjugate for enhanced anticancer activities. ChemMedChem. 2017;12:466–474. doi: 10.1002/cmdc.201700021. [DOI] [PubMed] [Google Scholar]
- 147.Chen S., Yong T., Zhang Y., Su J., Jiao C., Xie Y. Anti-tumor and anti-angiogenic ergosterols from Ganoderma lucidum. Front. Chem. 2017;5:85. doi: 10.3389/fchem.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nowak R., Drozd M., Mendyk E., Lemieszek M., Krakowiak O., Kisiel W., Rzeski W., Szewczyk K. A new method for the isolation of ergosterol and peroxyergosterol as active compounds of Hygrophoropsis aurantiaca and in vitro antiproliferative activity of isolated ergosterol peroxide. Molecules. 2016;21:946. doi: 10.3390/molecules21070946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ling T., Lang W.H., Martinez-Montemayor M.M., Rivas F. Development of ergosterol peroxide probes for cellular localisation studies. Org. Biomol. Chem. 2019;17:5223–5229. doi: 10.1039/c9ob00145j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Martínez-Montemayor M.M., Ling T., Suárez-Arroyo I.J., Ortiz-Soto G., Santiago-Negrón C.L., Lacourt-Ventura M.Y., Valentín-Acevedo A., Lang W.H., Rivas F. Identification of biologically active Ganoderma lucidum compounds and synthesis of improved derivatives that confer anti-cancer activities in vitro. Front. Pharm. 2019;10:115. doi: 10.3389/fphar.2019.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim K.H., Choi S.U., Noh H.J., Zee O., Lee K.R. Cytotoxic ergosterol derivatives from the mushroom Naematoloma fasciculare. Nat. Prod. Sci. 2014;20:76–79. [Google Scholar]
- 152.Shi X.-W., Li X.-J., Gao J.-M., Zhang X.-C. Fasciculols H and I, two lanostane derivatives from chinese mushroom Naematoloma fasciculare. Chem. Biodivers. 2011;8:1864–1870. doi: 10.1002/cbdv.201000203. [DOI] [PubMed] [Google Scholar]
- 153.Ibrahim S.R.M., Mohamed G.A., Haari R.A.A., Abd-Elmoneim A., El-Kholy S., Asfour H.Z., Zayed M.F. Fusaristerol A: A new cytotoxic and antifungal ergosterol fatty acid ester from the endophytic fungus Fusarium sp. associated with Mentha longifolia roots. Pharmacogn. Mag. 2018;14:308–313. doi: 10.4103/pm.pm_113_18. [DOI] [Google Scholar]
- 154.Chen S., Yong T., Xiao C., Su J., Zhang Y., Jiao C., Xie Y. Pyrrole alkaloids and ergosterols from Grifola frondosa exert anti-α-glucosidase and anti-proliferative activities. J. Funct. Foods. 2018;43:196–205. doi: 10.1016/j.jff.2018.02.007. [DOI] [Google Scholar]
- 155.Lee S., Lee S., Roh H.-S., Song S.-S., Ryoo R., Pang C., Baek K.-H., Kim K.H. Cytotoxic constituents from the sclerotia of Poria cocos against human lung adenocarcinoma cells by inducing mitochondrial apoptosis. Cells. 2018;7:116. doi: 10.3390/cells7090116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Su Z., Wang P., Yuan W., Li S. Chemical constituents from the fruit body of Chlorophyllum molybdites. Nat. Prod. Commun. 2013;8:1227–1228. doi: 10.1177/1934578x1300800910. [DOI] [PubMed] [Google Scholar]
- 157.Shimizu T., Kawai J., Ouchi K., Kikuchi H., Osima Y., Hidemi R. Agarol, an ergosterol derivative from Agaricus blazei, induces caspase-independent apoptosis in human cancer cells. Int. J. Oncol. 2016;48:1670–1678. doi: 10.3892/ijo.2016.3391. [DOI] [PubMed] [Google Scholar]
- 158.Meza-Menchaca T., Poblete-Naredo I., Albores-Medina A., Pedraza-Chaverri J., Quiroz-Figueroa F.R., Cruz-Gregorio A., Zepeda R.C., Melgar-Lalanne G., Lagunes I., Trigos Á. Ergosterol peroxide isolated from oyster medicinal mushroom, Pleurotus ostreatus (Agaricomycetes), potentially induces radiosensitivity in cervical cancer. Int. J. Med. Mushrooms. 2020;22:1109–1119. doi: 10.1615/IntJMedMushrooms.2020036673. [DOI] [PubMed] [Google Scholar]
- 159.Cayan F., Tel-Cayan G., Deveci E., Duru M.E., Tuerk M. A detailed study on multifaceted bioactivities of the extracts and isolated compounds from truffle Reddellomyces parvulosporus. Int. J. Food Sci. Technol. 2022;57:1411–1419. doi: 10.1111/ijfs.15190. [DOI] [Google Scholar]
- 160.Li F., Guo S., Zhang S., Peng S., Cao W., Ho C.-T., Bai N. Bioactive constituents of F. esculentum bee pollen and quantitative analysis of samples collected from seven areas by HPLC. Molecules. 2019;24:2705. doi: 10.3390/molecules24152705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu S.-J., Tung Y.-J., Ng L.-T. Anti-diabetic effects of Grifola frondosa bioactive compound and its related molecular signaling pathways in palmitate-induced C2C12 cells. J. Ethnopharmacol. 2020;260:112962. doi: 10.1016/j.jep.2020.112962. [DOI] [PubMed] [Google Scholar]
- 162.Wonkam A.K.N., Ngansop C.A.N., Tchuenmogne M.A.T., Tchegnitegni B.T., Bitchagno G.T.M., Awantu A.F., Bankeu J.J.K., Boyom F.F., Sewald N., Lenta B.N. Chemical constituents from Baphia leptobotrys Harms (Fabaceae) and their chemophenetic significance. Biochem. Syst. Ecol. 2021;96:104260. doi: 10.1016/j.bse.2021.104260. [DOI] [Google Scholar]
- 163.Meza-Menchaca T., Ramos-Ligonio A., Lopez-Monteon A., Limon A.V., Kaluzhskiy L.A., Shkel T.V., Strushkevich N.V., Jimenez-Garcia L.F., Moreno L.T.A., Gallegos-Garcia V., et al. Insights into ergosterol peroxide’s trypanocidal activity. Biomolecules. 2019;9:484. doi: 10.3390/biom9090484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou B., Liang X., Feng Q., Li J., Pan X., Xie P., Jiang Z., Yang Z. Ergosterol peroxide suppresses influenza A virus-induced pro-inflammatory response and apoptosis by blocking RIG-I signaling. Eur. J. Pharmacol. 2019;860:172543. doi: 10.1016/j.ejphar.2019.172543. [DOI] [PubMed] [Google Scholar]
- 165.Duan C., Wang J., Liu Y., Zhang J., Si J., Hao Z., Wang J. Antiviral effects of ergosterol peroxide in a pig model of porcine deltacoronavirus (PDCoV) infection involves modulation of apoptosis and tight junction in the small intestine. Vet. Res. 2021;52:86. doi: 10.1186/s13567-021-00955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Duan C., Liu Y., Hao Z., Wang J. Ergosterol peroxide suppresses porcine deltacoronavirus (PDCoV)-induced autophagy to inhibit virus replication via p38 signaling pathway. Vet. Microbiol. 2021;257:109068. doi: 10.1016/j.vetmic.2021.109068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Duan C., Ge X., Wang J., Wei Z., Feng W.-h., Wang J. Ergosterol peroxide exhibits antiviral and immunomodulatory abilities against porcine deltacoronavirus (PDCoV) via suppression of NF-κB and p38/MAPK signaling pathways in vitro. Int. Immunopharmacol. 2021;93:107317. doi: 10.1016/j.intimp.2020.107317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jin M., Zhou W., Jin C., Jiang Z., Diao S., Jin Z., Li G. Anti-inflammatory activities of the chemical constituents isolated from Trametes versicolor. Nat. Prod. Res. 2019;33:2422–2425. doi: 10.1080/14786419.2018.1446011. [DOI] [PubMed] [Google Scholar]
- 169.Lee S., Lee D., Jang T.S., Kang K.S., Nam J.-W., Lee H.-J., Kim K.H. Anti-inflammatory phenolic metabolites from the edible fungus Phellinus baumii in LPS-stimulated RAW264.7 cells. Molecules. 2017;22:1583. doi: 10.3390/molecules22101583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lee S., Lee D., Park J.Y., Seok S., Jang T.S., Park H.B., Shim S.H., Kang K.S., Kim K.H. Antigastritis effects of Armillariella tabescens (Scop.) Sing. and the identification of its anti-inflammatory metabolites. J. Pharm. Pharmacol. 2018;70:404–412. doi: 10.1111/jphp.12871. [DOI] [PubMed] [Google Scholar]
- 171.Lee S., Choi E., Yang S.-M., Ryoo R., Moon E., Kim S.-H., Kim K.H. Bioactive compounds from sclerotia extract of Poria cocos that control adipocyte and osteoblast differentiation. Bioorg. Chem. 2018;81:27–34. doi: 10.1016/j.bioorg.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 172.Jeong Y.-U., Park Y.-J. Ergosterol peroxide from the medicinal mushroom Ganoderma lucidum inhibits differentiation and lipid accumulation of 3T3-L1 adipocytes. Int. J. Mol. Sci. 2020;21:460. doi: 10.3390/ijms21020460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cateni F., Doljak B., Zacchigna M., Anderluh M., Piltaver A., Scialino G., Banfi E. New biologically active epidioxysterols from Stereum hirsutum. Bioorg. Med. Chem. Lett. 2007;17:6330–6334. doi: 10.1016/j.bmcl.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 174.Zhai M.M., Qi F.M., Li J., Jiang C.X., Hou Y., Shi Y.P., Di D.L., Zhang J.W., Wu Q.X. Isolation of secondary metabolites from the soil-derived fungus Clonostachys rosea YRS-06, a biological control agent, and evaluation of antibacterial activity. J. Agric. Food Chem. 2016;64:2298–2306. doi: 10.1021/acs.jafc.6b00556. [DOI] [PubMed] [Google Scholar]
- 175.Sadorn K., Saepua S., Boonyuen N., Laksanacharoen P., Rachtawee P., Prabpai S., Kongsaeree P., Pittayakhajonwut P. Allahabadolactones A and B from the endophytic fungus, Aspergillus allahabadii BCC45335. Tetrahedron. 2016;72:489–495. doi: 10.1016/j.tet.2015.11.056. [DOI] [Google Scholar]
- 176.Kornsakulkarn J., Saepua S., Laksanacharoen P., Rachtawee P., Thongpanchang C. Chaetone G, a new dibenzo[b,e]oxepinone from the insect pathogenic fungus Aschersonia luteola BCC 31749. Tetrahedron Lett. 2016;57:305–307. doi: 10.1016/j.tetlet.2015.12.001. [DOI] [Google Scholar]
- 177.Wang A., Li P., Han P., Gu G., Shan T., Lai D., Zhou L. New nitrogen-containing metabolites from cultures of rice false smut pathogen Villosiclava virens. Nat. Prod. Res. 2021;35:272–281. doi: 10.1080/14786419.2019.1627354. [DOI] [PubMed] [Google Scholar]
- 178.Uc-Cachon A., Gamboa-Angulo M.M., Borges-Argaez R., Reyes-Estebanez M., Said-Fernandez S., Molina-Salinas G. Antitubercular activity of the fungus Gliocladium sp. MR41 strain. Iran. J. Pharm. Res. 2019;18:860–866. doi: 10.22037/ijpr.2019.1100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Miao F.-P., Li X.-D., Liu X.-H., Cichewicz R.H., Ji N.-Y. Secondary metabolites from an algicolous Aspergillus versicolor strain. Mar. Drugs. 2012;10:131–139. doi: 10.3390/md10010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tian N.-N., Li C., Tian N., Zhou Q.-X., Hou Y.-J., Zhang B.-W., Wang X.-S. Syntheses of 7-dehydrocholesterol peroxides and their improved anticancer activity and selectivity over ergosterol peroxide. New J. Chem. 2017;41:14843–14846. doi: 10.1039/C7NJ04100D. [DOI] [Google Scholar]
- 181.Bu M., Cao T., Li H., Guo M., Yang B.B., Zhou Y., Zhang N., Zeng C., Hu L. Synthesis and biological evaluation of novel steroidal 5α,8α-endoperoxide derivatives with aliphatic side-chain as potential anticancer agents. Steroids. 2017;124:46–53. doi: 10.1016/j.steroids.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 182.Wu J., Choi J.-H., Yoshida M., Hirai H., Harada E., Masuda K., Koyama T., Yazawa K., Noguchi K., Nagasawa K., et al. Osteoclast-forming suppressing compounds, gargalols A, B, and C, from the edible mushroom Grifola gargal. Tetrahedron. 2011;67:6576–6581. doi: 10.1016/j.tet.2011.05.091. [DOI] [Google Scholar]
- 183.Lee I.-S., Kim J.-P., Na M.-K., Jung H.-J., Min B.-S., Bae K.-H. Cytotoxicity of ergosterol derivatives from the fruiting bodies of Hygrophorus russula. Nat. Prod. Sci. 2011;17:85–89. [Google Scholar]
- 184.Wang S., Zhang L., Liu L.-Y., Dong Z.-J., Li Z.-H., Liu J.-K. Six novel steroids from culture of basidiomycete Polyporus ellisii. Nat. Prod. Bioprospect. 2012;2:240–244. doi: 10.1007/s13659-012-0058-4. [DOI] [Google Scholar]
- 185.Froufe H.J.C., Abreu R.M.V., Ferreira I.C.F.R. Virtual screening of low molecular weight mushrooms compounds as potential Mdm2 inhibitors. J. Enzyme Inhib. Med. Chem. 2013;28:569–575. doi: 10.3109/14756366.2012.658787. [DOI] [PubMed] [Google Scholar]
- 186.Bang S., Chae H.-S., Lee C., Choi H.G., Ryu J., Li W., Lee H., Jeong G.-S., Chin Y.-W., Shim S.H. New aromatic compounds from the fruiting body of Sparassis crispa (Wulf.) and their inhibitory activities on proprotein convertase subtilisin/kexin type 9 mRNA expression. J. Agric. Food Chem. 2017;65:6152–6157. doi: 10.1021/acs.jafc.7b02657. [DOI] [PubMed] [Google Scholar]
- 187.Li W., Zhou W., Cha J.Y., Kwon S.U., Baek K.-H., Shim S.H., Lee Y.M., Kim Y.H. Sterols from Hericium erinaceum and their inhibition of TNF-α and NO production in lipopolysaccharide-induced RAW 264.7 cells. Phytochemistry. 2015;115:231–238. doi: 10.1016/j.phytochem.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 188.Li W., Bang S.H., Lee C., Ma J.Y., Shim S.H., Kim Y.H. Sterols, aromatic compounds, and cerebrosides from the Hericium erinaceus fruiting body. Biochem. Syst. Ecol. 2017;70:254–259. doi: 10.1016/j.bse.2016.12.011. [DOI] [Google Scholar]
- 189.Yu F.-X., Li Z., Chen Y., Yang Y.-H., Li G.-H., Zhao P.-J. Four new steroids from the endophytic fungus Chaetomium sp. M453 derived of Chinese herbal medicine Huperzia serrata. Fitoterapia. 2017;117:41–46. doi: 10.1016/j.fitote.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 190.Li Z., Ma N., Zhao P.-J. Acetylcholinesterase inhibitory active metabolites from the endophytic fungus Colletotrichum sp. YMF432. Nat. Prod. Res. 2019;33:1794–1797. doi: 10.1080/14786419.2018.1434648. [DOI] [PubMed] [Google Scholar]
- 191.Bok J.W., Lermer L., Chilton J., Klingeman H.G., Towers G.H. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry. 1999;51:891–898. doi: 10.1016/s0031-9422(99)00128-4. [DOI] [PubMed] [Google Scholar]
- 192.Yan B.-F., Fang S.-T., Li W.-Z., Liu S.-J., Wang J.-H., Xia C.-H. A new minor diketopiperazine from the sponge-derived fungus Simplicillium sp. YZ-11. Nat. Prod. Res. 2015;29:2013–2017. doi: 10.1080/14786419.2015.1027890. [DOI] [PubMed] [Google Scholar]
- 193.Wu J., Kobori H., Kawaide M., Suzuki T., Choi J.H., Yasuda N., Noguchi K., Matsumoto T., Hirai H., Kawagishi H. Isolation of bioactive steroids from the Stropharia rugosoannulata mushroom and absolute configuration of strophasterol B. Biosci. Biotechnol. Biochem. 2013;77:1779–1781. doi: 10.1271/bbb.130216. [DOI] [PubMed] [Google Scholar]
- 194.Zhang Y.M., Li H.Y., Hu C., Sheng H.F., Zhang Y., Lin B.R., Zhou G.X. Ergosterols from the culture broth of marine Streptomyces anandii H41-59. Mar. Drugs. 2016;14:84. doi: 10.3390/md14050084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Aung H.T., Porta A., Clericuzio M., Takaya Y., Vidari G. Two new ergosterol derivatives from the basidiomycete Cortinarius glaucopus. Chem. Biodivers. 2017;14:e1600421. doi: 10.1002/cbdv.201600421. [DOI] [PubMed] [Google Scholar]
- 196.Mei R.-Q., Zuo F.-J., Duan X.-Y., Wang Y.-N., Li J.-R., Qian C.-Z., Xiao J.-P. Ergosterols from Ganoderma sinense and their anti-inflammatory activities by inhibiting NO production. Phytochem. Lett. 2019;32:177–180. doi: 10.1016/j.phytol.2019.06.006. [DOI] [Google Scholar]
- 197.Li X.-J., Gao J.-M., Chen H., Zhang A.-L., Tang M. Toxins from a symbiotic fungus, Leptographium qinlingensis associated with Dendroctonus armandi and their in vitro toxicities to Pinus armandi seedlings. Eur. J. Plant Pathol. 2012;134:239–247. doi: 10.1007/s10658-012-9981-9. [DOI] [Google Scholar]
- 198.Lee I.-S., Bae K., Yoo J.K., Ryoo I.-J., Kim B.Y., Ahn J.S., Yoo I.-D. Inhibition of human neutrophil elastase by ergosterol derivatives from the mycelium of Phellinus linteus. J. Antibiot. 2012;65:437–440. doi: 10.1038/ja.2012.42. [DOI] [PubMed] [Google Scholar]
- 199.Bang S., Lee C., Ryu J., Li W., Koh Y.-S., Jeon J.-H., Lee J., Shim S.H. Simultaneous determination of the bioactive compounds from Sparassis crispa (Wulf.) by HPLC-DAD and their inhibitory effects on LPS-stimulated cytokine production in bone marrow-derived dendritic cell. Arch. Pharm. Res. 2018;41:823–829. doi: 10.1007/s12272-018-1054-y. [DOI] [PubMed] [Google Scholar]
- 200.Li W., Zhou W., Song S.B., Shim S.H., Kim Y.H. Sterol fatty acid esters from the mushroom Hericium erinaceum and their ppar transactivational effects. J. Nat. Prod. 2014;77:2611–2618. doi: 10.1021/np500234f. [DOI] [PubMed] [Google Scholar]
- 201.Kikuchi T., Isobe M., Uno S., In Y., Zhang J., Yamada T. Strophasterols E and F: Rearranged ergostane-type sterols from Pleurotus eryngii. Bioorg. Chem. 2019;89:103011. doi: 10.1016/j.bioorg.2019.103011. [DOI] [PubMed] [Google Scholar]
- 202.Zhu X.-C., Huang G.-L., Mei R.-Q., Wang B., Sun X.-P., Luo Y.-P., Xu J., Zheng C.-J. One new α,β-unsaturated 7-ketone sterol from the mangrove-derived fungus Phomopsis sp.MGF222. Nat. Prod. Res. 2020;35:3970–3976. doi: 10.1080/14786419.2020.1752210. [DOI] [PubMed] [Google Scholar]
- 203.Gao H., Hong K., Chen G.-D., Wang C.-X., Tang J.-S., Yu Y., Jiang M.-M., Li M.-M., Wang N.-L., Yao X.-S. New oxidized sterols from Aspergillus awamori and the endo-boat conformation adopted by the cyclohexene oxide system. Magn. Reson. Chem. 2010;48:38–43. doi: 10.1002/mrc.2536. [DOI] [PubMed] [Google Scholar]
- 204.Zhang M., Deng Y., Liu F., Zheng M., Liang Y., Sun W., Li Q., Li X.N., Qi C., Liu J., et al. Five undescribed steroids from Talaromyces stipitatus and their cytotoxic activities against hepatoma cell lines. Phytochemistry. 2021;189:112816. doi: 10.1016/j.phytochem.2021.112816. [DOI] [PubMed] [Google Scholar]
- 205.Chi L.-P., Yang S.-Q., Li X.-M., Li X.-D., Wang B.-G., Li X. A new steroid with 7β,8β-epoxidation from the deep sea-derived fungus Aspergillus penicillioides SD-311. J. Asian Nat. Prod. Res. 2021;23:884–891. doi: 10.1080/10286020.2020.1791096. [DOI] [PubMed] [Google Scholar]
- 206.Lei H.-M., Ma N., Wang T., Zhao P.-J. Metabolites from the endophytic fungus Colletotrichum sp. F168. Nat. Prod. Res. 2021;35:1077–1083. doi: 10.1080/14786419.2019.1638383. [DOI] [PubMed] [Google Scholar]
- 207.Lee J.S., Ma C.M., Park D.K., Yoshimi Y., Hatanaka M., Hattori M. Transformation of ergosterol peroxide to cytotoxic substances by rat intestinal bacteria. Biol. Pharm. Bull. 2008;31:949–954. doi: 10.1248/bpb.31.949. [DOI] [PubMed] [Google Scholar]
- 208.Zang Y., Xiong J., Zhai W.-Z., Cao L., Zhang S.-P., Tang Y., Wang J., Su J.-J., Yang G.-X., Zhao Y., et al. Fomentarols A-D, sterols from the polypore macrofungus Fomes fomentarius. Phytochemistry. 2013;92:137–145. doi: 10.1016/j.phytochem.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 209.Ibrahim S.R.M., Elkhayat E.S., Mohamed G.A.A., Fat’hi S.M., Ross S.A. Fusarithioamide A, a new antimicrobial and cytotoxic benzamide derivative from the endophytic fungus Fusarium chlamydosporium. Biochem. Biophys. Res. Commun. 2016;479:211–216. doi: 10.1016/j.bbrc.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 210.Wang X., Bao H., Bau T. Investigation of the possible mechanism of two kinds of sterols extracted from Leucocalocybe mongolica in inducing HepG2 cell apoptosis and exerting anti-tumor effects in H22 tumor-bearing mice. Steroids. 2020;163:108692. doi: 10.1016/j.steroids.2020.108692. [DOI] [PubMed] [Google Scholar]
- 211.Zhao Y.Y., Chao X., Zhang Y., Lin R.C., Sun W.J. Cytotoxic steroids from Polyporus umbellatus. Planta Med. 2010;76:1755–1758. doi: 10.1055/s-0030-1249926. [DOI] [PubMed] [Google Scholar]
- 212.Appiah T., Agyare C., Luo Y., Boamah E.V., Boakye D.Y. Antimicrobial and resistance modifying activities of cerevisterol isolated from Trametes species. Curr. Bioact. Compd. 2020;16:115–123. doi: 10.2174/1573407214666180813101146. [DOI] [Google Scholar]
- 213.Zhou F., Zhang H., Liu R., Zhang D. Isolation and biological evaluation of secondary metabolites of the endophytic fungus Aspergillus fumigatus from Astragalus membranaceus. Chem. Nat. Compd. 2013;49:568–570. doi: 10.1007/s10600-013-0675-0. [DOI] [Google Scholar]
- 214.Yazdani M., Beni Z., Dekany M., Papp V., Lazar A., Burian K., Hohmann J., Vanyolos A. Isolation and characterization of chemical constituents from the mushroom Clitocybe nebularis. J. Res. Pharm. 2020;24:908–913. doi: 10.35333/jrp.2020.250. [DOI] [Google Scholar]
- 215.Guo K., Fang H., Gui F., Wang Y., Xu Q., Deng X. Two new ring A-cleaved lanostane-type triterpenoids and four known steroids isolated from endophytic fungus Glomerella sp. F00244. Helv. Chim. Acta. 2016;99:601–607. doi: 10.1002/hlca.201600039. [DOI] [Google Scholar]
- 216.Alam B.M., Chowdhury N.S., Sohrab H.M., Rana S.M., Hasan C.M., Lee S.-H. Cerevisterol alleviates inflammation via suppression of MAPK/NF-κB/AP-1 and activation of the Nrf2/HO-1 signaling cascade. Biomolecules. 2020;10:199. doi: 10.3390/biom10020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pang X., Lin X., Wang J., Liang R., Tian Y., Salendra L., Luo X., Zhou X., Yang B., Tu Z., et al. Three new highly oxygenated sterols and one new dihydroisocoumarin from the marine sponge-derived fungus Cladosporium sp. SCSIO41007. Steroids. 2018;129:41–46. doi: 10.1016/j.steroids.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 218.Al-Rabia M.W., Mohamed G.A., Ibrahim S.R.M., Asfour H.Z. Anti-inflammatory ergosterol derivatives from the endophytic fungus Fusarium chlamydosporum. Nat. Prod. Res. 2021;35:5011–5020. doi: 10.1080/14786419.2020.1762185. [DOI] [PubMed] [Google Scholar]
- 219.Wang H., Liu T., Xin Z. A new glucitol from an endophytic fungus Fusarium equiseti Salicorn 8. Eur. Food Res. Technol. 2014;239:365–376. doi: 10.1007/s00217-014-2230-z. [DOI] [Google Scholar]
- 220.Bao F., Yang K., Wu C., Gao S., Wang P., Chen L., Li H. New natural inhibitors of hexokinase 2 (HK2): Steroids from Ganoderma sinense. Fitoterapia. 2018;125:123–129. doi: 10.1016/j.fitote.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 221.Makropoulou M., Aligiannis N., Gonou-Zagou Z., Pratsinis H., Skaltsounis A.-L., Fokialakis N. Antioxidant and cytotoxic activity of the wild edible mushroom Gomphus clavatus. J. Med. Food. 2012;15:216–221. doi: 10.1089/jmf.2011.0107. [DOI] [PubMed] [Google Scholar]
- 222.Lu Q.-Q., Tian J.-M., Wei J., Gao J.-M. Bioactive metabolites from the mycelia of the basidiomycete Hericium erinaceum. Nat. Prod. Res. 2014;28:1288–1292. doi: 10.1080/14786419.2014.898145. [DOI] [PubMed] [Google Scholar]
- 223.Li W., Lee S.H., Jang H.D., Ma J.Y., Kim Y.H. Antioxidant and anti-osteoporotic activities of aromatic compounds and sterols from Hericium erinaceum. Molecules. 2017;22:108. doi: 10.3390/molecules22010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vanyolos A., Orvos P., Chuluunbaatar B., Talosi L., Hohmann J. GIRK channel activity of Hungarian mushrooms: From screening to biologically active metabolites. Fitoterapia. 2019;137:104272. doi: 10.1016/j.fitote.2019.104272. [DOI] [PubMed] [Google Scholar]
- 225.Fangkrathok N., Sripanidkulchai B., Umehara K., Noguchi H. Bioactive ergostanoids and a new polyhydroxyoctane from Lentinus polychrous mycelia and their inhibitory effects on E2-enhanced cell proliferation of T47D cells. Nat. Prod. Res. 2013;27:1611–1619. doi: 10.1080/14786419.2012.742079. [DOI] [PubMed] [Google Scholar]
- 226.Kim J.-A., Lau E., Tay D., Carcache De Blanco E.J. Antioxidant and NF-κB inhibitory constituents isolated from Morchella esculenta. Nat. Prod. Res. 2011;25:1412–1417. doi: 10.1080/14786410802425746. [DOI] [PubMed] [Google Scholar]
- 227.Tang H.-Y., Zhang Q., Li H., Gao J.-M. Antimicrobial and allelopathic metabolites produced by Penicillium brasilianum. Nat. Prod. Res. 2015;29:345–348. doi: 10.1080/14786419.2014.940347. [DOI] [PubMed] [Google Scholar]
- 228.Liu Y.-W., Mei H.-C., Su Y.-W., Fan H.-T., Chen C.-C., Tsai Y.-C. Inhibitory effects of Pleurotus tuber-regium mycelia and bioactive constituents on LPS-treated RAW 264.7 cells. J. Funct. Foods. 2014;7:662–670. doi: 10.1016/j.jff.2013.12.019. [DOI] [Google Scholar]
- 229.Kovacs B., Beni Z., Dekany M., Orban-Gyapai O., Sinka I., Zupko I., Hohmann J., Vanyolos A. Chemical analysis of the edible mushroom Tricholoma populinum: Steroids and sulfinyladenosine compounds. Nat. Prod. Commun. 2017;12:1583–1584. doi: 10.1177/1934578x1701201014. [DOI] [Google Scholar]
- 230.Zhao J.-L., Zhang M., Liu J.-M., Tan Z., Chen R.-D., Xie K.-B., Dai J.-G. Bioactive steroids and sorbicillinoids isolated from the endophytic fungus Trichoderma sp. Xy24. J. Asian Nat. Prod. Res. 2017;19:1028–1035. doi: 10.1080/10286020.2017.1285908. [DOI] [PubMed] [Google Scholar]
- 231.Liu X.-H., Miao F.-P., Liang X.-R., Ji N.-Y. Ergosteroid derivatives from an algicolous strain of Aspergillus ustus. Nat. Prod. Res. 2014;28:1182–1186. doi: 10.1080/14786419.2014.923996. [DOI] [PubMed] [Google Scholar]
- 232.Lee M.K., Hung T.M., Cuong T.D., Na M.K., Kim J.C., Kim E.-J., Park H.-S., Choi J.S., Lee I.S., Bae K.H., et al. Ergosta-7,22-diene-2β,3α,9α-triol from the fruit bodies of Ganoderma lucidum induces apoptosis in human myelocytic HL-60 cells. Phytother. Res. 2011;25:1579–1585. doi: 10.1002/ptr.3447. [DOI] [PubMed] [Google Scholar]
- 233.Zheng M., Tang R., Deng Y., Yang K., Chen L., Li H. Steroids from Ganoderma sinense as new natural inhibitors of cancer-associated mutant IDH1. Bioorg. Chem. 2018;79:89–97. doi: 10.1016/j.bioorg.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 234.Xie C.-L., Zhang D., Xia J.-M., Hu C.-C., Lin T., Lin Y.-K., Wang G.-H., Tian W.-J., Li Z.-P., Zhang X.-K., et al. Steroids from the deep-sea-derived fungus Penicillium granulatum MCCC 3A00475 induced apoptosis via retinoid X receptor (RXR)-α pathway. Mar. Drugs. 2019;17:178. doi: 10.3390/md17030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Costa T.M., Lenzi J., Paganelli C.J., Filho H.H.D.S., Alberton M.D., Tavares L.B.B., de Oliveira D. Liposoluble compounds from Ganoderma lipsiense grown on solid red rice medium with antiparasitic and antibacterial properties. Biotechnol. Appl. Biochem. 2020;67:180–185. doi: 10.1002/bab.1851. [DOI] [PubMed] [Google Scholar]
- 236.Nhiem N.X., Yen H.T., Luyen B.T.T., Tai B.H., Van Hoan P., Thao N.P., Anh H.L.T., Ban N.K., Van Kiem P., Van Minh C., et al. Chemical components from the leaves of Trichosanthes baviensis and their tyrosinase inhibitory activity. Bull. Korean Chem. Soc. 2015;36:703–706. doi: 10.1002/bkcs.10086. [DOI] [Google Scholar]
- 237.Lian C., Wang C.F., Xiao Q., Xiao L., Xu Y., Liu J. The triterpenes and steroids from the fruiting body Ganoderma duripora. Biochem. Syst. Ecol. 2017;73:50–53. doi: 10.1016/j.bse.2017.06.005. [DOI] [Google Scholar]
- 238.Nguyen V.T., Tung N.T., Cuong T.D., Hung T.M., Kim J.A., Woo M.H., Choi J.S., Lee J.-H., Min B.S. Cytotoxic and anti-angiogenic effects of lanostane triterpenoids from Ganoderma lucidum. Phytochem. Lett. 2015;12:69–74. doi: 10.1016/j.phytol.2015.02.012. [DOI] [Google Scholar]
- 239.Gao S.-S., Li X.-M., Li C.-S., Proksch P., Wang B.-G. Penicisteroids A and B, antifungal and cytotoxic polyoxygenated steroids from the marine alga-derived endophytic fungus Penicillium chrysogenum QEN-24S. Bioorg. Med. Chem. Lett. 2011;21:2894–2897. doi: 10.1016/j.bmcl.2011.03.076. [DOI] [PubMed] [Google Scholar]
- 240.Niu S., Wang N., Xie C.-L., Fan Z., Luo Z., Chen H.-F., Yang X.-W. Roquefortine J, a novel roquefortine alkaloid, from the deep-sea-derived fungus Penicillium granulatum MCCC 3A00475. J. Antibiot. 2018;71:658–661. doi: 10.1038/s41429-018-0046-y. [DOI] [PubMed] [Google Scholar]
- 241.Guo J., Yuan Y., Lu D., Du B., Xiong L., Shi J., Yang L., Liu W., Yuan X., Zhang G., et al. Two natural products, trans-phytol and (22E)-ergosta-6,9,22-triene-3β,5α,8α-triol, inhibit the biosynthesis of estrogen in human ovarian granulosa cells by aromatase (CYP19) Toxicol. Appl. Pharmacol. 2014;279:23–32. doi: 10.1016/j.taap.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 242.Kawahara N., Sekita S., Satake M. Steroids from Calvatia cyathiformis. Phytochemistry. 1994;37:213–215. doi: 10.1016/0031-9422(94)85028-3. [DOI] [Google Scholar]
- 243.Weng Y., Lu J., Xiang L., Matsuura A., Zhang Y., Huang Q., Qi J. Ganodermasides C and D, two new anti-aging ergosterols from spores of the medicinal mushroom Ganoderma lucidum. Biosci. Biotechnol. Biochem. 2011;75:800–803. doi: 10.1271/bbb.100918. [DOI] [PubMed] [Google Scholar]
- 244.Weng Y., Xiang L., Matsuura A., Zhang Y., Huang Q., Qi J. Ganodermasides A and B, two novel anti-aging ergosterols from spores of a medicinal mushroom Ganoderma lucidum on yeast via UTH1 gene. Bioorg. Med. Chem. 2010;18:999–1002. doi: 10.1016/j.bmc.2009.12.070. [DOI] [PubMed] [Google Scholar]
- 245.Zhang F.L., Yang H.X., Wu X., Li J.Y., Wang S.Q., He J., Li Z.H., Feng T., Liu J.K. Chemical constituents and their cytotoxicities from mushroom Tricholoma imbricatum. Phytochemistry. 2020;177:112431. doi: 10.1016/j.phytochem.2020.112431. [DOI] [PubMed] [Google Scholar]
- 246.Wu S.H., Huang R., Miao C.P., Chen Y.W. Two new steroids from an endophytic fungus Phomopsis sp. Chem. Biodivers. 2013;10:1276–1283. doi: 10.1002/cbdv.201200415. [DOI] [PubMed] [Google Scholar]
- 247.Zhang C.-Y., Ji X., Gui X., Huang B.-K. Chemical constituents from an endophytic fungus Chaetomium globosum Z1. Nat. Prod. Commun. 2013;8:1217–1218. doi: 10.1177/1934578x1300800907. [DOI] [PubMed] [Google Scholar]
- 248.Kikuchi T., Horii Y., Maekawa Y., Masumoto Y., In Y., Tomoo K., Sato H., Yamano A., Yamada T., Tanaka R. Pleurocins A and B: Unusual 11(9→7)-abeo-ergostanes and eringiacetal B: A 13,14-seco-13,14-epoxyergostane from fruiting bodies of Pleurotus eryngii and their inhibitory effects on nitric oxide production. J. Org. Chem. 2017;82:10611–10616. doi: 10.1021/acs.joc.7b01259. [DOI] [PubMed] [Google Scholar]
- 249.Kitchawalit S., Kanokmedhakul K., Kanokmedhakul S., Soytong K. A new benzyl ester and ergosterol derivatives from the fungus Gymnoascus reessii. Nat. Prod. Res. 2014;28:1045–1051. doi: 10.1080/14786419.2014.903478. [DOI] [PubMed] [Google Scholar]
- 250.Kikuchi T., Masumoto Y., In Y., Tomoo K., Yamada T., Tanaka R. Eringiacetal A, 5,6-seco-(5S,6R,7R,9S)-5,6:5,7:6,9-triepoxyergosta-8(14),22-diene-3β,7β-diol, an unusual ergostane sterol from the fruiting bodies of Pleurotus eryngii. Eur. J. Org. Chem. 2015;2015:4645–4649. doi: 10.1002/ejoc.201500382. [DOI] [Google Scholar]
- 251.Liu Y.P., Pu C.J., Wang M., He J., Li Z.H., Feng T., Xie J., Liu J.K. Cytotoxic ergosterols from cultures of the basidiomycete Psathyrella candolleana. Fitoterapia. 2019;138:104289. doi: 10.1016/j.fitote.2019.104289. [DOI] [PubMed] [Google Scholar]
- 252.He W.-J., Zhou X.-J., Qin X.-C., Mai Y.-X., Lin X.-P., Liao S.-R., Yang B., Zhang T., Tu Z.-C., Wang J.-F., et al. Quinone/hydroquinone meroterpenoids with antitubercular and cytotoxic activities produced by the sponge-derived fungus Gliomastix sp. ZSDS1-F7. Nat. Prod. Res. 2017;31:604–609. doi: 10.1080/14786419.2016.1207076. [DOI] [PubMed] [Google Scholar]
- 253.Yang S., Ma Q.Y., Kong F.D., Xie Q.Y., Huang S.Z., Zhou L.M., Dai H.F., Yu Z.F., Zhao Y.X. Two new compounds from the fruiting bodies of Ganoderma philippii. J. Asian Nat. Prod. Res. 2018;20:249–254. doi: 10.1080/10286020.2017.1326911. [DOI] [PubMed] [Google Scholar]
- 254.Xue J., Wu P., Xu L., Wei X. Penicillitone, a potent in vitro anti-inflammatory and cytotoxic rearranged sterol with an unusual tetracycle core produced by Penicillium purpurogenum. Org. Lett. 2014;16:1518–1521. doi: 10.1021/ol500418f. [DOI] [PubMed] [Google Scholar]
- 255.Hwang H., Kwon H.C., Kwon J. Chemical constituents isolated from the moss-derived fungus Talaromyces sp. J. Korean Magn. Reson. Soc. 2020;24:123–128. doi: 10.6564/JKMRS.2020.24.4.123. [DOI] [Google Scholar]
- 256.Chen H., Chen D.Q., Li Q.F., Li P.F., Chen H., Zhao Y.Y. Research progress on pharmacology, pharmacokinetics and determination of ergosta-4,6,8 (14),22-tetraen-3-one. China J. Chin. Mater. Med. 2014;39:3905–3909. [PubMed] [Google Scholar]
- 257.Lee W.Y., Park Y., Ahn J.K. Improvement of ergone production from mycelial culture of Polyporus umbellatus. Mycobiology. 2007;35:82–86. doi: 10.4489/myco.2007.35.2.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Boehme R.M., Kempfle M.A. Synthesis of fluorescent 4,6,8(14)-trien-3-one steroids via 3,5,7-trien-3-ol ethers. Important probes for steroid-protein interactions. Steroids. 1994;59:265–269. doi: 10.1016/0039-128x(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 259.Quang D.N., Bach D.D. Ergosta-4,6,8(14),22-tetraen-3-one from Vietnamese Xylaria sp. possessing inhibitory activity of nitric oxide production. Nat. Prod. Res. 2008;22:901–906. doi: 10.1080/14786410701642706. [DOI] [PubMed] [Google Scholar]
- 260.Yuan D., Mori J., Komatsu K.I., Makino T., Kano Y. An anti-aldosteronic diuretic component (drain dampness) in Polyporus sclerotium. Biol. Pharm. Bull. 2004;27:867–870. doi: 10.1248/bpb.27.867. [DOI] [PubMed] [Google Scholar]
- 261.Zhao Y.Y., Zhang L., Mao J.R., Cheng X.H., Lin R.C., Zhang Y., Sun W.J. Ergosta-4,6,8(14),22-tetraen-3-one isolated from Polyporus umbellatus prevents early renal injury in aristolochic acid-induced nephropathy rats. J. Pharm. Pharmacol. 2011;63:1581–1586. doi: 10.1111/j.2042-7158.2011.01361.x. [DOI] [PubMed] [Google Scholar]
- 262.Zhao Y.Y., Zhang L., Long F.Y., Cheng X.L., Bai X., Wei F., Lin R.C. UPLC-Q-TOF/HSMS/MS(E)-based metabonomics for adenine-induced changes in metabolic profiles of rat faeces and intervention effects of ergosta-4,6,8(14),22-tetraen-3-one. Chem. Biol. Interact. 2013;201:31–38. doi: 10.1016/j.cbi.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 263.Chang C.-W., Chen Y.-S., Chen C.-C., Chan I.-O., Chen C.-C., Sheu S.-J., Lin T.-W., Chou S.-H., Liu C.-J., Lee T.-C., et al. Targeting cancer initiating cells by promoting cell differentiation and restoring chemosensitivity via dual inactivation of STAT3 and Src activity using an active component of Antrodia cinnamomea mycelia. Oncotarget. 2016;7:73016–73031. doi: 10.18632/oncotarget.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Fernando D., Adhikari A., Nanayakkara C., de Silva E.D., Wijesundera R., Soysa P. Cytotoxic effects of ergone, a compound isolated from Fulviformes fastuosus. BMC Complement. Altern. Med. 2016;16:484. doi: 10.1186/s12906-016-1471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wang Z.-R., Li G., Ji L.-X., Wang H.-H., Gao H., Peng X.-P., Lou H.-X. Induced production of steroids by co-cultivation of two endophytes from Mahonia fortunei. Steroids. 2019;145:1–4. doi: 10.1016/j.steroids.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 266.Zhao Y.-Y., Cheng X.-L., Wei F., Bai X., Lin R.-C. Ultra performance liquid chromatography coupled with electrospray and atmospheric pressure chemical ionization (ESCi)-quadrupole time-of-flight mass spectrometry with novel mass spectrometryElevated Energy (MSE) data collection technique: Determination and pharmacokinetics, tissue distribution and biliary excretion study of ergone in rat. J. Sep. Sci. 2012;35:1619–1626. doi: 10.1002/jssc.201200131. [DOI] [PubMed] [Google Scholar]
- 267.Lee W.Y., Park Y., Ahn J.-K., Park S.-Y., Lee H.-J. Cytotoxic activity of ergosta-4,6,8(14),22-tetraen-3-one from the sclerotia of Polyporus umbellatus. Bull. Korean Chem. Soc. 2005;26:1464–1466. doi: 10.5012/bkcs.2005.26.9.1464. [DOI] [Google Scholar]
- 268.Yuan W.-H., Teng M.-T., Sun S.-S., Ma L., Yuan B., Ren Q., Zhang P. Active metabolites from endolichenic fungus Talaromyces sp. Chem. Biodivers. 2018;15:e1800371. doi: 10.1002/cbdv.201800371. [DOI] [PubMed] [Google Scholar]
- 269.Arthan S., Tantapakul C., Kanokmedhakul K., Soytong K., Kanokmedhakul S. A new xanthone from the fungus Apiospora montagnei. Nat. Prod. Res. 2017;31:1766–1771. doi: 10.1080/14786419.2017.1290622. [DOI] [PubMed] [Google Scholar]
- 270.Zhao Y.Y., Shen X., Chao X., Ho C.C., Cheng X.L., Zhang Y., Lin R.C., Du K.J., Luo W.J., Chen J.Y., et al. Ergosta-4,6,8(14),22-tetraen-3-one induces G2/M cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Biochim. Biophys. Acta. 2011;1810:384–390. doi: 10.1016/j.bbagen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 271.Nguyen T.H., Ho V.D., Do T.T., Bui H.T., Phan V.K., Sak K., Raal A. A new lignan glycoside from the aerial parts and cytotoxic investigation of Uvaria rufa. Nat. Prod. Res. 2015;29:247–252. doi: 10.1080/14786419.2014.971790. [DOI] [PubMed] [Google Scholar]
- 272.Zhang Y., Zhao T., Wang H., Wang J., Wang R., Bu H., Hu L., Hu D., Wang S. Effects of ergosterone on lipopolysaccharideinduced acute lung injury and nucleo side-binding oligomerization domain, leucine-rich repeats and pyrin domain containing protein 3 inflammatory signaling pathway in mice. Mater. Express. 2021;11:38–45. doi: 10.1166/mex.2021.1884. [DOI] [Google Scholar]
- 273.Sun Y., Zhao Y., Li G., Yang S., Hu X., Fan J. Studies of interaction between ergosta-4,6,8(14),22-tetraen-3-one (ergone) and human serum albumin by molecular spectroscopy and modeling. J. Chin. Chem. Soc. 2011;58:602–610. doi: 10.1002/jccs.201190094. [DOI] [Google Scholar]
- 274.Liang X., Sun Y., Liu L., Ma X., Hu X., Fan J., Zhao Y. Folate-functionalized nanoparticles for controlled ergosta-4,6,8(14),22-tetraen-3-one delivery. Int. J. Pharm. 2013;441:1–8. doi: 10.1016/j.ijpharm.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 275.Sun Y., Ji Z., Zhao Y., Liang X., Hu X., Fan J. Enhanced distribution and anti-tumor activity of ergosta-4,6,8(14),22-tetraen-3-one by polyethylene glycol liposomalization. J. Nanosci. Nanotechnol. 2013;13:1435–1439. doi: 10.1166/jnn.2013.6009. [DOI] [PubMed] [Google Scholar]
- 276.Gu B.-B., Jiao F.-R., Wu W., Liu L., Jiao W.-H., Sun F., Wang S.-P., Yang F., Lin H.-W. Ochrasperfloroid, an ochratoxin-ergosteroid heterodimer with inhibition of IL-6 and NO production from Aspergillus flocculosus 16D-1. RSC Adv. 2019;9:7251–7256. doi: 10.1039/c8ra10539a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Gu B.B., Wu W., Jiao F.R., Jiao W.H., Li L., Sun F., Wang S.P., Yang F., Lin H.W. Asperflotone, an 8(14->15)-abeo-ergostane from the sponge-derived fungus Aspergillus flocculosus 16D-1. J. Org. Chem. 2019;84:300–306. doi: 10.1021/acs.joc.8b02679. [DOI] [PubMed] [Google Scholar]
- 278.Gu B.-B., Wu W., Jiao F.-R., Jiao W.-h., Li L., Sun F., Wang S.-P., Yang F., Lin H.-W. Aspersecosteroids A and B, two 11(9→10)-abeo-5,10-secosteroids with a dioxatetraheterocyclic ring system from Aspergillus flocculosus 16D-1. Org. Lett. 2018;20:7957–7960. doi: 10.1021/acs.orglett.8b03530. [DOI] [PubMed] [Google Scholar]
- 279.Tao H., Li Y., Lin X., Zhou X., Dong J., Liu Y., Yang B. A new pentacyclic ergosteroid from fungus Aspergillus sp. ScSiO41211 derived of mangrove sediment sample. Nat. Prod. Commun. 2018;13:1629–1631. [Google Scholar]
- 280.Liu L., Duan F.F., Gao Y., Peng X.G., Chang J.L., Chen J., Ruan H.L. Aspersteroids A–C, three rearranged ergostane-type steroids from Aspergillus ustus NRRL 275. Org. Lett. 2021;23:9620–9624. doi: 10.1021/acs.orglett.1c03863. [DOI] [PubMed] [Google Scholar]
- 281.Palasarn S., Intereya K., Boonpratuang T., Thongpanchang C., Isaka M. Ergostane triterpenoids from the cultures of basidiomycete Favolaschia calocera BCC 36684 and stereochemical elucidation of favolon. Phytochem. Lett. 2022;47:168–173. doi: 10.1016/j.phytol.2022.01.002. [DOI] [Google Scholar]
- 282.Pu C.J., Peng Y.L., Li Z.H., He J., Huang R., Feng T., Liu J.K. Two highly oxygenated ergosterols from cultures of the basidiomycete Conocybe siliginea. Nat. Prod. Res. 2019;33:3037–3043. doi: 10.1080/14786419.2018.1516217. [DOI] [PubMed] [Google Scholar]
- 283.Yang S.Q., Li X.M., Li X., Chi L.P., Wang B.G. Two new diketomorpholine derivatives and a new highly conjugated ergostane-type steroid from the marine algal-derived endophytic fungus Aspergillus alabamensis EN-547. Mar. Drugs. 2018;16:114. doi: 10.3390/md16040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Surup F., Halecker S., Nimtz M., Rodrigo S., Schulz B., Steinert M., Stadler M. Hyfraxins A and B, cytotoxic ergostane-type steroid and lanostane triterpenoid glycosides from the invasive ash dieback ascomycete Hymenoscyphus fraxineus. Steroids. 2018;135:92–97. doi: 10.1016/j.steroids.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 285.Ji J.-C., Wei P.-P., Han X.-Y., Li Z.-H., Ai H.-L., Lei X.-X. Secondary metabolites of the endophytic fungus Chaetomium globosum isolated from Coptis chinensis. Nat. Prod. Commun. 2021;16:1934578X211044574. doi: 10.1177/1934578x211044574. [DOI] [Google Scholar]
- 286.Guo H., Li Z.-H., Feng T., Liu J.-K. One new ergostane-type steroid and three new phthalide derivatives from cultures of the basidiomycete Albatrellus confluens. J. Asian Nat. Prod. Res. 2015;17:107–113. doi: 10.1080/10286020.2014.951925. [DOI] [PubMed] [Google Scholar]
- 287.Chen Z.-M., Fan Q.-Y., Chen H.-P., Li Z.-H., Feng T., Liu J.-K. A novel C25 sterol peroxide from the endophytic fungus Phoma sp. EA-122. Z. Nat. C. 2015;70:93–96. doi: 10.1515/znc-2014-4217. [DOI] [PubMed] [Google Scholar]
- 288.Zhu X., Liu Y., Hu Y., Lv X., Shi Z., Yu Y., Jiang X., Feng F., Xu J. Neuroprotective activities of constituents from Phyllosticta capitalensis, an endophyte fungus of Loropetalum chinense var. rubrum. Chem. Biodivers. 2021;18:e2100314. doi: 10.1002/cbdv.202100314. [DOI] [PubMed] [Google Scholar]
- 289.Wakana D., Itabashi T., Kawai K.-I., Yaguchi T., Fukushima K., Goda Y., Hosoe T. Cytotoxic anthrasteroid glycosides, malsterosides A–C, from Malbranchea filamentosa. J. Antibiot. 2014;67:585–588. doi: 10.1038/ja.2014.43. [DOI] [PubMed] [Google Scholar]
- 290.Jiao F.-R., Gu B.-B., Zhu H.-R., Zhang Y., Liu K.-C., Zhang W., Han H., Xu S.-H., Lin H.-W. Asperfloketals A and B, the first two ergostanes with rearranged a and d rings: From the sponge-associated Aspergillus flocculosus 16D-1. J. Org. Chem. 2021;86:10954–10961. doi: 10.1021/acs.joc.0c02049. [DOI] [PubMed] [Google Scholar]
- 291.Luo Q., Yang Z.-L., Yan Y.-M., Cheng Y.-X. Ganotheaecolin A, a neurotrophic conjugated ergosterol with a naphtho[1,8-ef]azulene scaffold from Ganoderma theaecolum. Org. Lett. 2017;19:718–721. doi: 10.1021/acs.orglett.7b00012. [DOI] [PubMed] [Google Scholar]
- 292.Fan S.-Q., Xie C.-L., Xia J.-M., Xing C.-P., Luo Z.-H., Shao Z., Yan X.-J., He S., Yang X.-W. Sarocladione, a unique 5,10:8,9-diseco-steroid from the deep-sea-derived fungus Sarocladium kiliense. Org. Biomol. Chem. 2019;17:5925–5928. doi: 10.1039/c9ob01159e. [DOI] [PubMed] [Google Scholar]
- 293.Ning Y., Tian H., Gui J. Biogenesis-guided synthesis and structural revision of sarocladione enabled by ruthenium-catalyzed endoperoxide fragmentation. Angew. Chem. Int. Ed. 2021;60:11222–11226. doi: 10.1002/anie.202101451. [DOI] [PubMed] [Google Scholar]
- 294.Su L.H., Geng C.A., Li T.Z., Huang X.Y., Ma Y.B., Zhang X.M., Wu G., Yang Z.L., Chen J.J. Spiroseoflosterol, a rearranged ergostane-steroid from the fruiting bodies of Butyriboletus roseoflavus. J. Nat. Prod. 2020;83:1706–1710. doi: 10.1021/acs.jnatprod.9b01282. [DOI] [PubMed] [Google Scholar]
- 295.Anke T., Werle A., Kappe R., Sterner O. Laschiatrion, a new antifungal agent from a Favolaschia species (Basidiomycetes) active against human pathogens. J. Antibiot. 2004;57:496–501. doi: 10.7164/antibiotics.57.496. [DOI] [PubMed] [Google Scholar]
- 296.Cui C.-M., Li X.-M., Meng L., Li C.-S., Huang C.-G., Wang B.-G. 7-Nor-ergosterolide, a pentalactone-containing norsteroid and related steroids from the marine-derived endophytic Aspergillus ochraceus EN-31. J. Nat. Prod. 2010;73:1780–1784. doi: 10.1021/np100386q. [DOI] [PubMed] [Google Scholar]
- 297.Krohn K., Biele C., Aust H.J., Draeger S., Schulz B. Herbarulide, a ketodivinyllactone steroid with an unprecedented homo-6-oxaergostane skeleton from the endophytic fungus Pleospora herbarum. J. Nat. Prod. 1999;62:629–630. doi: 10.1021/np980422a. [DOI] [PubMed] [Google Scholar]
- 298.Tong Z.B., Cui X.H., Wang J., Zhang C.L., Zhang Y.Y., Ren Z.J. Constituents from solid-cultured Antrodia camphorata. Nat. Prod. Res. 2017;31:2564–2567. doi: 10.1080/14786419.2017.1320785. [DOI] [PubMed] [Google Scholar]
- 299.Duecker F.L., Heinze R.C., Mueller M., Zhang S., Heretsch P. Synthesis of the alleged structures of fortisterol and herbarulide and structural revision of herbarulide. Org. Lett. 2020;22:1585–1588. doi: 10.1021/acs.orglett.0c00180. [DOI] [PubMed] [Google Scholar]
- 300.Ratnaweera P.B., Williams D.E., Patrick B.O., de Silva E.D., Andersen R.J. Solanioic acid, an antibacterial degraded steroid produced in culture by the fungus Rhizoctonia solani isolated from tubers of the medicinal plant Cyperus rotundus. Org. Lett. 2015;17:2074–2077. doi: 10.1021/acs.orglett.5b00596. [DOI] [PubMed] [Google Scholar]
- 301.Song Y.-P., Shi Z.-Z., Miao F.-P., Fang S.-T., Yin X.-L., Ji N.-Y. Tricholumin A, a highly transformed ergosterol derivative from the alga-endophytic fungus Trichoderma asperellum. Org. Lett. 2018;20:6306–6309. doi: 10.1021/acs.orglett.8b02821. [DOI] [PubMed] [Google Scholar]
- 302.Amagata T., Doi M., Tohgo M., Minoura K., Numata A. Dankasterone, a new class of cytotoxic steroid produced by a Gymnascella species from a marine sponge. Chem. Commun. 1999;14:1321–1322. doi: 10.1039/A903840J. [DOI] [Google Scholar]
- 303.Amagata T., Tanaka M., Yamada T., Doi M., Minoura K., Ohishi H., Yamori T., Numata A. Variation in cytostatic constituents of a sponge-derived Gymnascella dankaliensis by manipulating the carbon source. J. Nat. Prod. 2007;70:1731–1740. doi: 10.1021/np070165m. [DOI] [PubMed] [Google Scholar]
- 304.Gao W., Chai C., He Y., Li F., Hao X., Cao F., Gu L., Liu J., Hu Z., Zhang Y. Periconiastone A, an antibacterial ergosterol with a pentacyclo[8.7.0.01,5.02,14.010,15]heptadecane system from Periconia sp. TJ403-rc01. Org. Lett. 2019;21:8469–8472. doi: 10.1021/acs.orglett.9b03270. [DOI] [PubMed] [Google Scholar]
- 305.Duecker F.L., Heinze R.C., Heretsch P. Synthesis of swinhoeisterol A, dankasterone A and B, and periconiastone A by radical framework reconstruction. J. Am. Chem. Soc. 2020;142:104–108. doi: 10.1021/jacs.9b12899. [DOI] [PubMed] [Google Scholar]
- 306.Zhao Z.Z., Chen H.P., Huang Y., Zhang S.B., Li Z.H., Feng T., Liu J.K. Bioactive polyketides and 8,14-seco-ergosterol from fruiting bodies of the ascomycete Daldinia childiae. Phytochemistry. 2017;142:68–75. doi: 10.1016/j.phytochem.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 307.Wu J., Tokuyama S., Nagai K., Yasuda N., Noguchi K., Matsumoto T., Hirai H., Kawagishi H. Strophasterols A to D with an unprecedented steroid skeleton: From the mushroom Stropharia rugosoannulata. Angew. Chem. Int. Ed. Engl. 2012;51:10820–10822. doi: 10.1002/anie.201205351. [DOI] [PubMed] [Google Scholar]
- 308.Sato S., Kuwahara S. Synthesis of strophasterols C, E, and F. Org. Lett. 2020;22:1311–1315. doi: 10.1021/acs.orglett.9b04628. [DOI] [PubMed] [Google Scholar]
- 309.Sato S., Taguchi Y., Kuwahara S. Synthesis and stereochemistry of glaucoposterol A and strophasterol D. Tetrahedron. 2020;76:131129. doi: 10.1016/j.tet.2020.131129. [DOI] [Google Scholar]
- 310.Lee T.-H., Chen C.-C., Chen J.-J., Liao H.-F., Chang H.-S., Sung P.-J., Tseng M.-H., Wang S.-Y., Ko H.-H., Kuo Y.-H. New and cytotoxic components from Antrodia camphorata. Molecules. 2014;19:21378–21385. doi: 10.3390/molecules191221378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Liu X.-H., Tang X.-Z., Miao F.-P., Ji N.-Y. A new pyrrolidine derivative and steroids from an algicolous Gibberella zeae strain. Nat. Prod. Commun. 2011;6:1243–1246. doi: 10.1177/1934578x1100600908. [DOI] [PubMed] [Google Scholar]
- 312.Nakada T., Yamamura S. Three new metabolites of hybrid strain KO 0231, derived from Penicillium citreo-viride IFO 6200 and 4692. Tetrahedron. 2000;56:2595–2602. doi: 10.1016/s0040-4020(00)00157-5. [DOI] [Google Scholar]
- 313.Tang G.-H., Lu N., Li W., Wu M., Chen Y.-Y., Zhang H.-Y., He S.-Y. Mannosylxylarinolide, a new 3,4-seco-ergostane-type steroidal saponin featuring a β-D-mannose from the endophytic fungus Xylaria sp. J. Asian Nat. Prod. Res. 2020;22:397–403. doi: 10.1080/10286020.2018.1563075. [DOI] [PubMed] [Google Scholar]
- 314.Elissawy A.M., Ebada S.S., Ashour M.L., Ozkaya F.C., Ebrahim W., Singab A.N.B., Proksch P. Spiroarthrinols a and B, two novel meroterpenoids isolated from the sponge- derived fungus Arthrinium sp. Phytochem. Lett. 2017;20:246–251. doi: 10.1016/j.phytol.2017.05.008. [DOI] [Google Scholar]
- 315.Yang X.-Y., Li Z.-H., Dong Z.-J., Feng T., Liu J.-K. Three new sesquiterpenoids from cultures of the basidiomycete Conocybe siliginea. J. Asian Nat. Prod. Res. 2015;17:671–675. doi: 10.1080/10286020.2014.939072. [DOI] [PubMed] [Google Scholar]
- 316.Kumla D., Aung T.S., Buttachon S., Dethoup T., Gales L., Pereira J.A., Inacio A., Costa P.M., Lee M., Sekeroglu N., et al. A new dihydrochromone dimer and other secondary metabolites from cultures of the marine sponge-associated fungi Neosartorya fennelliae KUFA 0811 and Neosartorya tsunodae KUFC 9213. Mar. Drugs. 2017;15:375. doi: 10.3390/md15120375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Jiao Y., Li G., Wang H.-Y., Liu J., Li X.-B., Zhang L.-L., Zhao Z.-T., Lou H.-X. New metabolites from endolichenic fungus Pleosporales sp. Chem. Biodivers. 2015;12:1095–1104. doi: 10.1002/cbdv.201400279. [DOI] [PubMed] [Google Scholar]
- 318.Wang W., Wan X., Liu J., Wang J., Zhu H., Chen C., Zhang Y. Two new terpenoids from Talaromyces purpurogenus. Mar. Drugs. 2018;16:150. doi: 10.3390/md16050150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zhao Z.-Z., Chen H.-P., Wu B., Zhang L., Li Z.-H., Feng T., Liu J.-K. Matsutakone and matsutoic acid, two (nor)steroids with unusual skeletons from the edible mushroom Tricholoma matsutake. J. Org. Chem. 2017;82:7974–7979. doi: 10.1021/acs.joc.7b01230. [DOI] [PubMed] [Google Scholar]
- 320.Chen C., Liang F., Chen B., Sun Z., Xue T., Yang R., Luo D. Identification of demethylincisterol A3 as a selective inhibitor of protein tyrosine phosphatase Shp2. Eur. J. Pharmacol. 2017;795:124–133. doi: 10.1016/j.ejphar.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 321.Chen M., Wang K.L., Liu M., She Z.G., Wang C.Y. Bioactive steroid derivatives and butyrolactone derivatives from a gorgonian-derived Aspergillus sp. fungus. Chem. Biodivers. 2015;12:1398–1406. doi: 10.1002/cbdv.201400321. [DOI] [PubMed] [Google Scholar]
- 322.Kawagishi H., Akachi T., Ogawa T., Masuda K., Yamaguchi K., Yazawa K., Takahashi M. Chaxine A, an osteoclast-forming suppressing substance, from the mushroom Agrocybe chaxingu. Heterocycles. 2006;69:253–258. [Google Scholar]
- 323.Choi J.-H., Ogawa A., Abe N., Masuda K., Koyama T., Yazawa K., Kawagishi H. Chaxines B, C, D, and E from the edible mushroom Agrocybe chaxingu. Tetrahedron. 2009;65:9850–9853. doi: 10.1016/j.tet.2009.09.064. [DOI] [Google Scholar]
- 324.Hirata Y., Nakazaki A., Kawagishi H., Nishikawa T. Biomimetic synthesis and structural revision of chaxine B and its analogues. Org. Lett. 2017;19:560–563. doi: 10.1021/acs.orglett.6b03724. [DOI] [PubMed] [Google Scholar]
- 325.Niki M., Hirata Y., Nakazaki A., Wu J., Kawagishi H., Nishikawa T. Biomimetic synthesis of chaxine and its related compounds. J. Org. Chem. 2020;85:4848–4860. doi: 10.1021/acs.joc.9b03482. [DOI] [PubMed] [Google Scholar]
- 326.Xiao J.H., Sun Z.H., Pan W.D., Lü Y.H., Chen D.X., Zhong J.J. Jiangxienone, a new compound with potent cytotoxicity against tumor cells from traditional Chinese medicinal mushroom Cordyceps jiangxiensis. Chem. Biodivers. 2012;9:1349–1355. doi: 10.1002/cbdv.201100244. [DOI] [PubMed] [Google Scholar]
- 327.Chen Z.-M., Yang X.-Y., Fan Q.-Y., Li Z.-H., Wei K., Chen H.-P., Feng T., Liu J.-K. Three novel degraded steroids from cultures of the Basidiomycete Antrodiella albocinnamomea. Steroids. 2014;87:21–25. doi: 10.1016/j.steroids.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 328.Chen K., Sun W., Bie Q., Liu X., Chen C., Liu J., Xue Y., Wang J., Luo Z., Zhu H., et al. Fusopoltide A and fusosterede A, A polyketide with a pentaleno[1,2-c]pyran ring system and A degraded steride, from the fungus Fusarium solani. Tetrahedron Lett. 2018;59:2679–2682. doi: 10.1016/j.tetlet.2018.05.082. [DOI] [Google Scholar]
- 329.Amagata T., Tanaka M., Yamada T., Chen Y.-P., Minoura K., Numata A. Additional cytotoxic substances isolated from the sponge-derived Gymnascella dankaliensis. Tetrahedron Lett. 2013;54:5960–5962. doi: 10.1016/j.tetlet.2013.08.044. [DOI] [Google Scholar]
- 330.McCloskey S., Noppawan S., Mongkolthanaruk W., Suwannasai N., Senawong T., Prawat U. A new cerebroside and the cytotoxic constituents isolated from Xylaria allantoidea SWUF76. Nat. Prod. Res. 2017;31:1422–1430. doi: 10.1080/14786419.2016.1258559. [DOI] [PubMed] [Google Scholar]
- 331.Wu J., Zhang H., He L.-M., Xue Y.-Q., Jia J., Wang S.-B., Zhu K.-K., Hong K., Cai Y.-S. A new fusicoccane-type norditerpene and a new indone from the marine-derived fungus Aspergillus aculeatinus WHUF0198. Chem. Biodivers. 2021;18:e2100562. doi: 10.1002/cbdv.202100562. [DOI] [PubMed] [Google Scholar]
- 332.An C.L., Kong F.D., Ma Q.Y., Xie Q.Y., Yuan J.Z., Zhou L.M., Dai H.F., Yu Z.F., Zhao Y.X. Chemical constituents of the marine-derived fungus Aspergillus sp. SCS-KFD66. Mar. Drugs. 2018;16:468. doi: 10.3390/md16120468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zhang F.L., Shi C., Sun L.T., Yang H.X., He J., Li Z.H., Feng T., Liu J.K. Chemical constituents and their biological activities from the mushroom Pyropolyporus fomentarius. Phytochemistry. 2021;183:112625. doi: 10.1016/j.phytochem.2020.112625. [DOI] [PubMed] [Google Scholar]
- 334.Li L.-N., Wang L., Guo X.-L. Chemical constituents from the culture of the fungus Hericium alpestre. J. Asian Nat. Prod. Res. 2019;21:735–741. doi: 10.1080/10286020.2018.1483346. [DOI] [PubMed] [Google Scholar]
- 335.Huang H.-C., Liaw C.-C., Yang H.-L., Hseu Y.-C., Kuo H.-T., Tsai Y.-C., Chien S.-C., Amagaya S., Chen Y.-C., Kuo Y.-H. Lanostane triterpenoids and sterols from Antrodia camphorata. Phytochemistry. 2012;84:177–183. doi: 10.1016/j.phytochem.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 336.Zhao J.-Y., Feng T., Li Z.-H., Dong Z.-J., Zhang H.-B., Liu J.-K. Sesquiterpenoids and an ergosterol from cultures of the fungus Daedaleopsis tricolor. Nat. Prod. Bioprospect. 2013;3:271–276. doi: 10.1007/s13659-013-0065-0. [DOI] [Google Scholar]
- 337.Wen C.-N., Chen H.-P., Zhao Z.-Z., Hu D.-B., Li Z.-H., Feng T., Liu J.-K. Two new γ-lactones from the cultures of basidiomycete Lenzites betulinus. Phytochem. Lett. 2017;20:9–12. doi: 10.1016/j.phytol.2017.03.004. [DOI] [Google Scholar]
- 338.Chen S., Liu Z., Chen Y., Tan H., Zhu S., Liu H., Zhang W. Phosteoid A, a highly oxygenated norsteroid from the deep-sea-derived fungus Phomopsis tersa FS441. Tetrahedron Lett. 2020;61:151555. doi: 10.1016/j.tetlet.2019.151555. [DOI] [Google Scholar]
- 339.Liu Z., Zhao J.-Y., Sun S.-F., Li Y., Liu Y.-B. Fungi: Outstanding source of novel chemical scaffolds. J. Asian Nat. Prod. Res. 2020;22:99–120. doi: 10.1080/10286020.2018.1488833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.