Abstract
In the course of surveying for the carbapenem-hydrolyzing metallo-β-lactamase gene blaIMP in pathogenic bacteria by the PCR method, we detected a gene encoding a variant metallo-β-lactamase, designated IMP-3, which differed from IMP-1 by having low hydrolyzing activity for penicillins and carbapenems. PCR product direct sequencing of a 2.2-kb segment revealed that the gene blaIMP-3 was located on a cassette inserted within a class I integron in the pMS390 plasmid. The 741-bp nucleotide sequence of blaIMP-3 was identical to that of blaIMP-1, except for seven base substitutions. Among these were two, at nucleotide positions 314 and 640, which caused amino acid alterations. Hybrid bla genes were constructed from blaIMP-3 and blaIMP-1 by recombinant DNA techniques, and β-lactamases encoded by these genes were compared with those of the parents IMP-3 and IMP-1 under the same experimental conditions. The kinetic parameters indicated that the inefficient hydrolysis of benzylpenicillin, ampicillin, imipenem, and ceftazidime by IMP-3 was due to the substitution of glycine for serine at amino acid residue 196 in the mature enzyme. This alteration corresponded to the presence of guanine instead of an adenine at nucleotide position 640 of the blaIMP-3 gene. This indicated that extension of the substrate profile in the metallo-β-lactamase IMP-1 compared to IMP-3 is the result of a one-step single-base mutation, suggesting that the gene blaIMP-3 is an ancestor of blaIMP-1.
β-Lactamases are enzymes that hydrolyze β-lactam antibiotics, conferring resistance to a variety of these antibiotics for most pathogenic bacteria. These enzymes have been classified phylogenetically based on their functional and molecular characteristics (5).
Molecular class B metallo-β-lactamases belonging to functional group 3a subclass B1 are characteristic in their broad substrate spectrum, which extends to most β-lactam antibiotics, except for monobactams, and have activities as penicillinases, cephalosporinases, and carbapenemases (5, 15, 23). They have been reported in Bacillus cereus, alkalophilic Bacillus sp., Bacteroides fragilis, Pseudomonas aeruginosa, Serratia marcescens, and Klebsiella pneumoniae (23). Among this group of metallo-β-lactamases, ESP from P. aeruginosa GN17203, IMP-1 from S. marcescens TN9106, and DK4 from K. pneumoniae are all plasmid mediated and were found to be the same enzyme because the nucleotide sequences of their genes are identical (14, 22; GenBank accession number D29636).
Genes blaESP and blaIMP, respectively encoding ESP and IMP-1 β-lactamase, were identified in the cassettes inserted in the integrons on plasmids (24). Both cassettes had the same nucleotide sequence, but they were found to be inserted into different integrons, class 1 integron 0 (In0) for the blaESP cassette (2, 14) and the class 3 integron for the blaIMP cassette (1). This fact suggested that the blaIMP (blaESP) cassette has been disseminated among different integrons. Since In0 is reported to be widespread among clinical isolates of gram-negative bacilli and has insertions of single or multiple drug resistance genes (17), we surveyed clinical isolates for In0 bearing blaIMP (blaESP) by the PCR method, followed by an assay of β-lactamase activity. In the process of these assays, we demonstrated the presence of a novel metallo-β-lactamase which was mediated by Shigella flexneri plasmid pMS390 (21). The β-lactamase, originally named MET-1 is a cephalosporinase, showing preferential hydrolysis of cephalosporins rather than penicillins or carbapenems. We examined the nucleotide sequence of the gene blaMET to identify the amino acid substitutions affecting the substrate specificity in the MET-1 β-lactamase. On the basis of our findings, the gene and the enzyme were renamed blaIMP-3 and IMP-3 β-lactamase, respectively, to mesh with prior discoveries and nomenclature.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Plasmids pMS390 from S. flexneri JS19622 and pMS350 from P. aeruginosa GN17203 are the starting plasmids used for this study. Plasmid pMS390 was transferred by conjugation to an Escherichia coli K-12 ML4905 strain for enzyme analysis (21), and the gene was cloned in E. coli K-12 JM83 for sequencing (this work). The metallo-β-lactamase gene blaESP of pMS350 (29) had been earlier cloned and sequenced (13, 14). Since the nucleotide sequence of blaESP was identical to that of blaIMP from S. marsescens strain TN9106 (22), hereafter in this work the abbreviation blaIMP is used for blaESP from pMS350.
The plasmid and host strain used for direct cloning of the PCR product were pT7Blue T-Vector with an ampicillin resistance marker and E. coli NovaBlue with a tetracycline resistance marker (Novagen), respectively.
Cloning vectors used throughout were pHSG397 and pHSG398, which were multicopy vectors with a chloramphenicol resistance marker (27). E. coli K-12 JM83 was used as the host strain (28). The HindIII site on the multicloning region of pHSG398 was deleted for this experiment using the deletion kit (Takara Shuzo Co., Ltd.), forming the vector pHSG398ΔHd.
Recombinant plasmids pMS400, pMS500, pMS401, pMS501, pMS402, and pMS502 were constructed for these experiments.
Antibacterial agents.
The β-lactam antibiotics used and their sources were as follows: cephaloridine and cephalothin, Shionogi & Co., Ltd.; cefotaxime, Hoechst Marion Roussel Ltd.; ceftazidime, Nippon Glaxo Ltd.; benzylpenicillin, Meiji Seika Kaisha, Ltd.; ampicillin, Toyama Chemical Co., Ltd.; imipenem, Banyu Pharmaceutical Co., Ltd.; and aztreonam, Bristol-Myers Squibb K. K.
Susceptibility tests.
MICs were determined by an agar dilution method with sensitivity disk agar (Nissui Pharmaceutical Co.) (29).
Purification of β-lactamase and assay of activity.
Preparation and purification of β-lactamases using E. coli JM83 strains harboring recombinant plasmids were performed according to a previously reported method (21).
β-Lactamase activity was determined spectrophotometrically at 30°C in 50 mM morpholinepropanesulfonic acid (MOPS) buffer (pH 7.0). The kinetic parameters were determined according to a procedure reported previously (21). Statistical data were obtained by the online analysis (UV absorption method and Lineweaver-Burk plot analysis on the computer) system by measuring the rate of hydrolysis (kcat) and Km values more than three times. When Km values were too large for this system, they were obtained from Michaelis-Menten graphs by measuring the initial hydrolysis rates.
PCR amplification of the DNA segment.
Primer sequences for PCR amplification were CGGATGAAGGCACGAAC (forward) and AAGCAGACTTGACCTGA (reverse), constructed for detection of the blaIMP cassette inserted in the integron In0 (14). The forward primer was part of the 5′ conserved sequence of the In0 located 5 nucleotides upstream from the promoter region for blaIMP (18), and the reverse primer was part of the 3′ conserved sequence of the integron downstream from the blaIMP gene cassettes (2, 17).
The PCR procedure consisted of 25 cycles of denaturation at 94°C for 1.5 min, annealing at 55°C for 1.5 min, and amplification at 72°C for 1.5 min, followed by an additional 7 min at 72°C using Premix Taq reagent (Takara Shuzo Co., Ltd.) with the Program Template Control System PC-700 (ASTEC Co., Ltd.).
Nucleotide sequencing.
The PCR-amplified DNA segment was sequenced by a direct sequencing method using the Autosequencer ABI377 in the Gene Analysis Center of Takara Shuzou Co., Ltd.
Cloning of the PCR-amplified segment.
The PCR product obtained by agarose gel electrophoresis was purified by adsorbing the DNA to a silica matrix using a DNA purification kit (Gene Mate; ISC BioExpress) and was ligated with pT7Blue T-Vector (Novagen) according to the manufacturer's protocol. Recombinant plasmids were introduced into the host strain E. coli NovaBlue, and the transformants were obtained as white colonies on a Luria agar plate containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (50 μg/ml), IPTG (isopropyl-β-d-thiogalactopyranoside) (50 μg/ml), tetracycline (15 μg/ml), and ampicillin (50 μg/ml).
RESULTS
Identification and nucleotide sequencing of the β-lactamase gene of pMS390.
A DNA segment, approximately 2.2 kb in size, was obtained from the pMS390-containing E. coli ML4905 cells after PCR amplification, using primers for detection of the In0 sequence including the gene cassettes. The nucleotide sequence was determined by the PCR product direct-sequencing method. Within this segment, the gene composed of 741 bp was detected. The sequence of the gene (DDBJ accession number AB010417) was identical to that of blaIMP (1, 14) (DDBJ accession number D78375) except for seven nucleotide substitutions. The gene was designated blaIMP-3 to correspond with the renaming of the metallo-β-lactamase mediated by pMS390 (21), which was originally named MET-1 and is now known as IMP-3 β-lactamase.
Two of the seven substitutions caused changes in amino acids between IMP-3 and IMP-1 β-lactamases. Substitution of an A with a G at both nucleotide positions 314 and 640 resulted in the replacement of the amino acid: Glu with Gly and Ser with Gly at amino acid positions 87 and 196, respectively, in the mature metallo-β-lactamase (23).
Cloning of the PCR-amplified segment.
Three preparations of 2.2-kb segments, each obtained by independent PCR amplification, were ligated with the pT7Blue vector, and the recombinant plasmids were introduced by transformation into the host strain E. coli NovaBlue. The transformants were examined for cefotaxime resistance, which is conferred by the IMP-3 β-lactamase. The MIC of cefotaxime was raised from less than 0.5 μg/ml for the host strain to 64 μg/ml for transformants.
The nucleotide sequences of the recombinant plasmids obtained from the three independent experiments were examined by the PCR direct-sequencing method. Two of the plasmids had no misincorporation of nucleotides in the blaIMP-3 genes, and one of the two was selected for this experiment. The 2.2-kb segment was cleaved out at the BamHI and XbaI sites flanking the insert in the multicloning region of the vector plasmid, subcloned into another vector (pHSG397), and introduced into E. coli JM83 cells by transformation. The transformants were selected for chloramphenicol resistance (marker of the vector). The recombinant plasmid thus obtained was named pMS400.
Preparation of hybrid metallo-β-lactamase genes from blaIMP-3 and blaIMP-1.
The kcat (per second) of penicillin relative to that of cephaloridine by highly purified β-lactamases was reported to be rather low for IMP-3 (21) but high for IMP-1 (16, 23). As this difference is expected to be due to either or both of the two amino acid alterations between the two enzymes, we prepared hybrid genes from blaIMP-3 and blaIMP-1 and compared them with those of the parents IMP-3 and IMP-1 under the same experimental conditions.
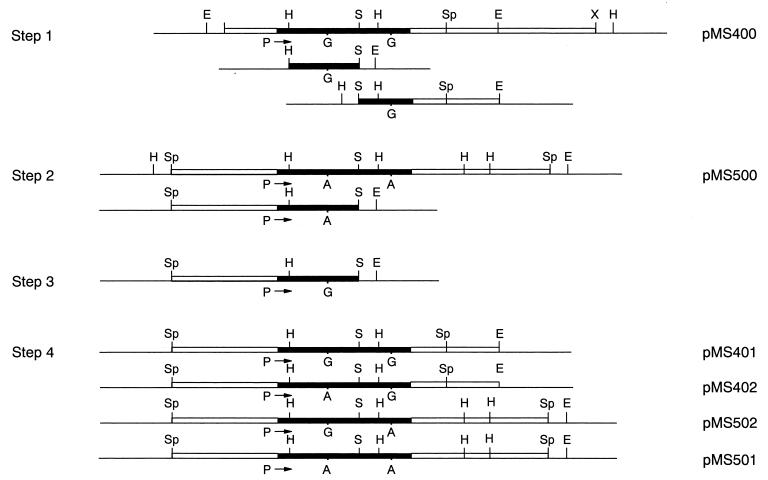
The hybrid and parent genes were constructed from pMS400 and pMS500, the recombinant plasmids of pHSG397, with segments containing blaIMP-3 and blaIMP-1, respectively (Fig. 1). The SphI segment of pMS500 was obtained from P. aeruginosa plasmid pMS350 (14).
FIG. 1.
Construction of the hybrid and parent metallo-β-lactamase genes. The procedure is explained in Materials and Methods. The horizontal single lines represent multicloning regions of the vector plasmids flanking the inserts. The EcoRI (E) and HindIII (H) sites located in the multicloning regions are indicated above the lines. Inserts are shown by boxes, in which the regions of metallo-β-lactamase genes are shaded in black. The sites of the restriction enzymes are indicated above the boxes. Abbreviations not used in the text are B (BamHI) and X (XbaI). The sites indicated under the boxes are P (for promoter) (14), G (for guanine), and A (for adenine).
First, the left (HindIII-SmaI [H-S]) and the right (SmaI-EcoRI [S-E]) segments of pMS400, respectively, including the nucleotide at position 314 or 640, were subcloned into pHSG398 (step 1). Second, the left (SphI-SmaI [Sp-S]) segment of pMS500 was subcloned into pHSG398ΔHd (step 2). Third, the H-S segment of the recombinant plasmid obtained in step 2 was cut out and replaced with the H-S segment of blaIMP-3 obtained in step 1 (step 3). Fourth, the S-E segment of pMS400 carrying the right part of blaIMP-3 (from step 1) was inserted into the step-3 plasmid, forming pMS401 which contained the whole blaIMP-3 gene. The same S-E segment was inserted into the plasmid obtained at step 2, forming a hybrid plasmid pMS402 consisting of the left part of blaIMP-1 and the right part of blaIMP-3.
The right S-E segment of pMS500 was obtained using the EcoRI site in the multicloning region of the vector and inserted into the step-3 plasmid. A hybrid plasmid pMS502 was thus obtained, and consisted of the left part of blaIMP-3 and the right part of blaIMP-1. The same S-E segment was inserted into the step-2 plasmid, thereby forming plasmid pMS501 which contained the whole blaIMP-1 gene.
The resulting four bla genes, from the two hybrids and their parents, were capable of expression under the common promoter derived from pMS500 provided by the integron portion (18).
Susceptibility to β-lactam antibiotics conferred by β-lactamases with amino acid substitutions.
With the common background of the vector pHSG398ΔHd and the host strain E. coli K-12 JM83, MICs of various β-lactam antibiotics were examined and compared among the four strains producing different β-lactamases encoded by hybrid or parent bla genes (Table 1). The MIC of imipenem was rather low in the E. coli host carrying pMS500, as was expected from previous findings using E. coli clones carrying the blaIMP-1 gene of P. aeruginosa plasmid pMS350 (13). A distinct rise in the MIC of ampicillin was observed for strains carrying pMS501 and pMS502, in which the β-lactamases had the amino acid Ser at position 196, in comparison to MICs observed for strains carrying pMS401 and pMS402, in which the β-lactamases had the amino acid Gly instead of Ser at that position. The MICs of cephalothin for strains carrying pMS401 or pMS402 were fourfold higher than those for strains carrying pMS501 or pMS502. Neither amino acid substitution caused distinct changes in the MICs of other β-lactams. Amino acid replacement at position 87 of the β-lactamases did not affect the MIC levels, as seen in the two strains bearing pMS401 or pMS402 and in those bearing pMS501 or pMS502. The results suggested that the low MIC of ampicillin and high MIC of cephalothin for strains carrying pMS401 and pMS402 resulted from a single amino acid alteration from Ser to Gly at position 196.
TABLE 1.
Resistance levels conferred by the parent and hybrid metallo-β-lactamasesa
| Plasmid | β-Lactamase | Amino acid residue at position:
|
MIC (μg/ml) ofb:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 87 | 196 | LOR | CEF | CTX | CAZ | AMP | IMP | ATM | ||
| pMS401 | IMP-3 | Gly | Gly | 64 | 512 | 64 | 64 | 8 | 1 | <0.5 |
| pMS402 | Hybrid | Glu | Gly | 64 | 512 | 64 | 64 | 8 | 1 | <0.5 |
| pMS502 | Hybrid | Gly | Ser | 32 | 128 | 32 | 64 | 64 | 2 | <0.5 |
| pMS501 | IMP-1 | Glu | Ser | 32 | 128 | 32 | 64 | 64 | 2 | <0.5 |
| pHSG398 Δ Hd | 2 | 8 | <0.5 | <0.5 | 4 | <0.5 | <0.5 | |||
| None | 2 | 8 | <0.5 | <0.5 | 4 | <0.5 | <0.5 | |||
Host strain: E. coli K-12 JM83.
LOR, cephaloridine; CEF, cephalothin; CTX, cefotaxime; CAZ, ceftazidime; AMP, ampicillin; IMP, imipenem; ATM, aztreonam.
Kinetic parameters of β-lactamases with amino acid substitutions.
β-Lactamase activities by enzymes encoded by the four kinds of bla genes were assayed spectrophotometrically using highly purified enzymes. Kinetic parameters for the hydrolysis of various β-lactam antibiotics were determined under the same assay conditions (Table 2). It was confirmed that the kinetic parameters of IMP-3 and IMP-1 were in good agreement with those previously estimated in an E. coli strain carrying pMS390 (for both kcat and Km) and in a P. aeruginosa strain carrying pMS350 (for Km), respectively (21, 29). Aztreonam-hydrolyzing activities were undetectable in all four enzymes (kcat < 0.06 s−1).
TABLE 2.
Kinetic parameters for hydrolysis of various β-lactam antibiotics by the hybrid and parent metallo-β-lactamases
| Plasmid | Enzyme | Amino acid at position:
|
Kinetic parameter | Antibiotica
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 87 | 196 | LOR | CEF | CTX | CAZ | PEN | AMP | IMP | |||
| pMS401 | IMP-3 | Gly | Gly | kcat (s−1) | 221 ± 15b | 223 ± 22 | 40.1 ± 2.6 | 4.5 ± 0.4b | 14.3 ± 0.8b | 7.4 ± 1.2b | 92.3 ± 2b |
| Km (μM) | 248 ± 39b | 9.9 ± 1.3 | 3.1 ± 0.3 | 128 ± 17b | 370 ± 59b | 464 ± 145b | 1140 ± 40b | ||||
| kcat/Km (μM−1 s−1) | 0.89 | 22.5 | 12.9 | 0.035 | 0.039 | 0.016 | 0.08 | ||||
| pMS402 | Hybrid | Glu | Gly | kcat (s−1) | 268 ± 5b | 384 ± 11 | 54.8 ± 5.3 | 3.6 ± 0.2b | 21.6 ± 1.0b | 11.8 ± 1.4b | 132 ± 8b |
| Km (μM) | 215 ± 17b | 9.8 ± 0.3 | 3.0 ± 0.3 | 118 ± 19b | 464 ± 60b | 437 ± 105b | 1280 ± 120b | ||||
| kcat/Km (μM−1 s−1) | 1.25 | 39.2 | 18.3 | 0.031 | 0.047 | 0.027 | 0.10 | ||||
| pMS502 | Hybrid | Gly | Ser | kcat (s−1) | 38.4 ± 1.2 | 43.2 ± 1.7 | 16.5 ± 1.3 | 11.3 ± 0.3 | 282 ± 19 | 87.0 ± 2.7 | 90.8 ± 5.3 |
| Km (μM) | 7.1 ± 0.1 | 2.3 ± 0.1 | 1.3 ± 0.2 | 31.8 ± 1.5 | 216 ± 13 | 110 ± 4.9 | 33.2 ± 1.9 | ||||
| kcat/Km (μM−1 s−1) | 5.4 | 18.8 | 12.7 | 0.36 | 1.3 | 0.8 | 2.7 | ||||
| pMS501 | IMP-1 | Glu | Ser | kcat (s−1) | 62.0 ± 3.0 | 65.0 ± 2.9 | 22.5 ± 1.9 | 16.3 ± 0.9 | 461 ± 17 | 162 ± 15 | 127 ± 11 |
| Km (μM) | 7.2 ± 0.4 | 2.0 ± 0.1 | 1.4 ± 0.1 | 46.0 ± 1.4 | 241 ± 10 | 140 ± 9 | 30.0 ± 3.9 | ||||
| kcat/Km (μM−1 s−1) | 8.6 | 32.5 | 16.1 | 0.35 | 1.9 | 1.2 | 4.2 | ||||
PEN, benzylpenicillin; see footnote b of Table 1 for other abbreviations.
Obtained from Michaelis-Menten graph; see Materials and Methods.
No significant difference in kinetic parameters was observed between the enzyme pairs of pMS401 and pMS402 or between those of pMS501 and pMS502, though the enzymes of the former pair were clearly distinguishable from those of the latter. This indicated that the differences in kinetic parameters were caused by the substitution of Gly for Ser at position 196 but not of Gly for Glu at position 87.
As indicated in Table 2, the single amino acid alteration from Ser to Gly at position 196 affected enzyme activities as reflected in the values of either kcat or Km, or both, and lowered hydrolysis efficiencies (kcat/Km) more than 1 order of magnitude for ceftazidime, benzylpenicillin, ampicillin, and imipenem.
DISCUSSION
The IMP-3 (renamed from MET-1) β-lactamase of plasmid pMS390 had been previously purified and revealed to be a cephalosporinase-type metallo-β-lactamase with low hydrolyzing activity for penicillins and carbapenems (21). In contrast, the IMP-1 β-lactamase, which is widespread among clinical strains, differed in its broad substrate spectrum of β-lactam antibiotics, including effective hydrolysis of penicillins and carbapenems (16, 22, 29). Sequence analysis of the gene blaIMP-3 revealed that the enzymes were identical except for two amino acid substitutions caused by two base replacements in the genes.
Hybrid bla genes obtained from blaIMP-1 and blaIMP-3 were constructed under a common promoter by recombination DNA techniques using a common vector and host strain of E. coli. Kinetic parameters for hydrolysis of various β-lactam antibiotics by the purified enzymes were estimated under the same assay conditions. The kinetic parameters of hybrid and parent β-lactamases indicated that the more potent hydrolysis of ceftazidime, benzylpenicillin, ampicillin, and imipenem could be acquired by a single amino acid substitution, i.e., Ser for Gly at position 196. The other substitution, Glu for Gly at position 87, had no effect on the substrate profiles examined so far, although further detailed analysis of enzyme kinetics, including affinity to metallo ions, remains to be carried out.
The amino acid sequences at the comparable regions of group 3a subclass B1 metallo-β-lactamases have been identified (23). The amino acid corresponding to that at position 196 in β-lactamases from B. cereus, Bacillus sp., and B. fragilis is reported to be Gly, the same amino acid found in the newly identified IMP-3 β-lactamase, which differs from IMP-1 β-lactamase. This amino acid lies just ahead of His, which has been identified as interacting with a Zn2+ cofactor (23). The association of the changed amino acid with the His amino acid remains unexplored, and the coincidence may be interesting to examine in terms of its affect on metallo ion interaction.
The results indicated that only a single-step mutation of the base G to A at nucleotide position 640 of blaIMP-3 extended the substrate profile of the IMP-3 to that of the IMP-1-type β-lactamase and conferred resistance to ampicillin in E. coli. Evolution of β-lactamases from a narrow to an extended spectrum of substrates was well analyzed in TEM- and SHV-type enzymes belonging to the class A β-lactamases (3, 4, 8, 9, 15). These β-lactamases, called extended-spectrum β-lactamases (ESBLs), have been conferred with hydrolyzing activities against the extended-spectrum β-lactam antibiotics by point mutations. Extension of the substrate spectrum by amino acid substitutions also has been reported in an OXA-type β-lactamase belonging to the class D enzymes (6, 7, 10). In a class C β-lactamase of Enterobacter cloacae, the substrate spectrum expansion was caused by a duplication of three amino acids which was attributed to a tandem duplication of a 9-nucleotide sequence in the parent enzyme (20). None of the ESBLs derived from class A, C, and D enzymes have hydrolyzing activities for carbapenems (5).
The metallo-β-lactamase IMP-1, which belongs to the class B β-lactamases, was characteristic in its carbapenem-hydrolyzing activity and had already been noted as an ESBL when it was first identified in a P. aeruginoisa plasmid (29) and later in gram-negative rods (11, 12, 19, 25, 26). Therefore, IMP-3 (originally designated MET-1) β-lactamase mediated by plasmid pMS390 could be considered an ancestor of IMP-1 β-lactamase. The gene could be mobilized as part of a cassette among integrons located on various plasmids, becoming widely disseminated among gram-negative bacteria, and conferring resistance to almost all β-lactam antibiotics.
ACKNOWLEDGMENTS
This work was supported by a grant-in-aid for Scientific Research (08670300) from the Ministry of Education, Science, Sports, and Culture, Japan, and by a grant for Molecular Characterization of Antibiotic Resistance from the Ministry of Health and Welfare, Japan.
REFERENCES
- 1.Arakawa Y, Murakami M, Suzuki K, Wacharotayankun R, Ohsuka S, Ito H, Kato N, Ohta M. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1995;39:1612–1615. doi: 10.1128/aac.39.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissonnette L, Roy P H. Characterization of In0 of Pseudomonas aeruginosa plasmid pVS1, an ancestor of integrons of multiresistance plasmids and transposons of gram-negative bacteria. J Bacteriol. 1992;174:1248–1257. doi: 10.1128/jb.174.4.1248-1257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanzquez J, Morosini M I, Negri M C, Gonzalez-Leiza M, Baquero F. Single amino acid replacements at positions altered in naturally occurring extended-spectrum TEM β-lactamases. Antimicrob Agents Chemother. 1995;39:145–149. doi: 10.1128/aac.39.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K, Jacoby G A. Nomenclature of TEM β-lactamases. J Antimicrob Chemother. 1977;39:1–3. doi: 10.1093/jac/39.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danel F, Hall L M C, Gur D, Livermore D M. OXA-14, another extended-spectrum variant of OXA-10 (PSE-2) β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;39:1881–1884. doi: 10.1128/aac.39.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danel F, Hall L M C, Gur D, Livermore D M. OXA-15, an extended-spectrum variant of OXA-2 β-lactamase, isolated from a Pseudomonas aeruginosa strain. Antimicrob Agents Chemother. 1997;41:785–790. doi: 10.1128/aac.41.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1995;246:375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 9.Du Bois S K, Marriott M S, Amyes S B. TEM- and SHV-derived extended-spectrum β-lactamases: relationship between selection, structure and function. J Antimicrob Chemother. 1995;35:7–22. doi: 10.1093/jac/35.1.7. [DOI] [PubMed] [Google Scholar]
- 10.Hall L M C, Livermore D M, Gur D, Akova M, Akalin H E. OXA-11, an extended-spectrum variant of OXA-10 (PSE-2) β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993;37:1637–1644. doi: 10.1128/aac.37.8.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirakawa Y, Izumikawa K, Yamaguchi T, Takemura H, Tanaka H, Yoshida R, Matsuda J, Nakano M, Tomono K, Maesaki S, Kaku M, Yamada Y, Kamihira S, Kohno S. Rapid detection and evaluation of clinical characteristics of emerging multiple-drug-resistant gram-negative rods carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1998;42:2006–2011. doi: 10.1128/aac.42.8.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito H, Arakawa Y, Ohsuka S, Wacharotayankun R, Kato N, Ohta M. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob Agents Chemother. 1995;39:824–829. doi: 10.1128/aac.39.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyobe S, Tsunoda M, Mitsuhashi S. Cloning and expression in Enterobacteriaceae of the extended-spectrum β-lactamase gene from a Pseudomonas aeruginosa plasmid. FEMS Microbiol Lett. 1994;121:175–180. doi: 10.1111/j.1574-6968.1994.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 14.Iyobe S, Minami S, Yamada H. Insertion of a carbapenemase gene cassette into an integron of a Pseudomonas aeruginosa plasmid. J Antimicrob Chemother. 1996;38:1114–1115. doi: 10.1093/jac/38.6.1114. [DOI] [PubMed] [Google Scholar]
- 15.Jacoby G A, Medeiros A A. More extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1991;35:1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laraki N, Franceschini N, Rossolini G M, Santucci P, Meunier C, De Pauw E, Amicosante G, Frere J M, Galleni M. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob Agents Chemother. 1999;43:902–906. doi: 10.1128/aac.43.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levesque C, Piche L, Larose C, Roy P H. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob Agents Chemother. 1995;39:185–191. doi: 10.1128/aac.39.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levesque C, Brassard S, Lapointe J, Roy P H. Diversity and relative strength of tandem promoters for the antibiotic-resistance genes of several integrons. Gene. 1994;142:49–54. doi: 10.1016/0378-1119(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 19.Minami S, Akama M, Araki H, Watanabe Y, Narita H, Iyobe S, Mitsuhashi S. Imipenem and cephem resistant Pseudomonas aeruginosa carrying plasmids for class B β-lactamases. J Antimicrob Chemother. 1996;37:433–444. doi: 10.1093/jac/37.3.433. [DOI] [PubMed] [Google Scholar]
- 20.Nukaga M, Haruta S, Tanimoto K, Kogure K, Taniguchi K, Tamaki M, Sawai T. Molecular evolution of a class C β-lactamase extending its substrate specificity. J Biol Chem. 1995;270:5729–5735. doi: 10.1074/jbc.270.11.5729. [DOI] [PubMed] [Google Scholar]
- 21.O'Hara K, Haruta S, Sawai T, Tsunoda M, Iyobe S. Novel metallo β-lactamase mediated by a Shigella flexneri plasmid. FEMS Microbiol Lett. 1998;162:201–206. doi: 10.1111/j.1574-6968.1998.tb12999.x. [DOI] [PubMed] [Google Scholar]
- 22.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. Molecular characterization of an enterobacterial metallo β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen B A, Bush K. Carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Recchia G D, Hall R M. Gene casettes: a new class of mobile element. Microbiology. 1995;141:3015–3027. doi: 10.1099/13500872-141-12-3015. [DOI] [PubMed] [Google Scholar]
- 25.Senda K, Arakawa Y, Nakashima K, Ito H, Ichiyama S, Shimokata K, Kato N, Ohta M. Multifocal outbreaks of metallo-β-lactamase-producing Pseudomonas aeruginosa resistant to broad-spectrum β-lactams, including carbapenems. Antimicrob Agents Chemother. 1996;40:349–353. doi: 10.1128/aac.40.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senda K, Arakawa Y, Ichiyama S, Nakashima K, Ito H, Ohsuka S, Shimokata K, Kato N, Ohta M. PCR detection of metallo-β-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum β-lactams. J Clin Microbiol. 1996;34:2909–2913. doi: 10.1128/jcm.34.12.2909-2913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeshita S, Sato M, Oba M, Masahashi W, Hashimoto-Gotoh T. High-copy number and low-copy number plasmid vectors for lacZα-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61:63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- 28.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis, and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]