Vitamin D is one of the most frequently used medicinal products around the world. The dietary intake is irregular as few food items contain vitamin D and is usually well below human requirements, so that its synthesis in the skin is the most important source of vitamin D. The global supply of vitamin D is usually considered as a passive series of events, not controlled by enzymes or hormones. In the nearly hundred years after its discovery, we learned that vitamin D has a complex metabolism and steroid like hormonal action. Vitamin D is totally inactive and requires a complex metabolism, first in the liver (mostly but not exclusively by CYP2R1) into 25-hydroxyvitamin D (25OHD), followed by a second hydroxylation by CYP27B1 into 1,25-dihydroxyvitamin D [1,25(OH)2D]. CYP2R1 is usually considered to be constitutively expressed. Therefore, the production of 25OHD is considered to be mostly substrate dependent so that serum 25OHD reflects the global supply of vitamin D. CYP27B1 in the kidney is the unique source of circulating 1,25D and is tightly regulated by different ions and hormones so that it behaves as a classical feed-back regulated hormonal system. CYP27B1 is also widely expressed in many extra renal tissues so that 1,25(OH)2D also behaves in a paracrine/autocrine fashion. In these tissues its activity is regulated by a variety of mechanisms different from what happens in the kidney. Although there are probably around 50 known metabolites of vitamin D, measurement of serum 25OHD is clinically used to define the vitamin D status, whereas serum 1,25(OH)2D is used to assess the biological activity of the vitamin D endocrine system. All metabolites of vitamin D in serum are bound with relatively high affinity to a specific binding protein, vitamin D binding protein (DBP). This protein is highly polymorphic and circulates in serum in high concentrations so that the free concentrations of all vitamin D metabolites are very low. 1,25(OH)2D binds to the vitamin D receptor (VDR), present in most cells. This hormonal system functions as most steroid and thyroid hormones and regulates a very large number of genes involved in calcium and phosphate transport but also regulates a very large number genes (up to 10% of all genes of the some organisms such as the zebrafish) not involved in ion transport or bone metabolism (reviewed in (1)).
However, a few recent publications have challenged some aspects of our understanding of vitamin D metabolism including the concept of stable expression of the hepatic 25-hydroxylases (2,3). We review these new data regarding CYP2R1, discuss their potential implications, and extend this review to examine the overall metabolism of vitamin D to explore whether old dogmas still hold today.
Obesity and the metabolic syndrome are associated with low vitamin D status (1). Prospective studies suggest that low non-epimeric 25OHD or increased 3-epi-25OHD concentrations are associated with higher risk for type 2 diabetes (4). The causality (in whatever direction) between obesity/diabetes and low vitamin D status is, however, not proven. In a recent JBMR paper, Roizen et al. clearly demonstrated that the serum concentration of 25OHD is substantially lower (~-20%) in serum of obese mice (fed a high fat diet), compared with normal weight mice, whereas serum concentrations of vitamin D3 itself were similar in both groups (2). This is not a surprise as serum 25OHD concentrations in overweight or obese humans are virtually systematically lower than in normal subjects in many different areas of the world having different sun exposure or dietary habits (5). Their novel finding, however, was that the mRNA of major vitamin D-25-hydroxylase (CYP2R1) is markedly (~40 %) lower in livers of obese mice (fed a high fat diet) compared with livers from normal mice. They confirm that by finding lower protein expression (~50 % decrease) of CYP2R1. The gene expression of some other potential 25-hydroxylases (CYP27A1 and CYP3A4) as well as the major catabolizing enzyme (CYP24A1) were not changed by diet-induced obesity. Finally, the authors measured the 25-hydroxylase activity by incubating mouse liver homogenates with vitamin D2 and found a ~70% reduction in the overall enzymatic activity. As the substrate concentration was in the millimolar range such an assay is not specific for the high affinity, low capacity CYP2R1 but represents a combined activity of all 25-hydroxylases including those that hydrolyze vitamin D2 less well than D3. They also used the ratio of serum 25OHD to serum vitamin D concentrations as a marker of 25-hydroxylase activity and found a strong positive correlation between this ratio and liver mRNA expression of CYP2R1.
Aatsinki et al. addressed a similar question about the origin of fairly systematic low serum 25OHD concentrations in diabetic subjects compared to their euglycemic controls, by studying high fat diet induced obesity and type 2 diabetes in mice (3). In addition, they also studied the effect of 24 h fasting and of streptozotocin-induced type 1 diabetes. All these metabolic situations decreased the hepatic mRNA and protein concentration of CYP2R1. Fasting for 24h, type 1 diabetes or type 2 diabetes decreased the mRNA of CYP2R1 in liver by 80, 43 and 45%, respectively, and generated a decrease of about 30% in protein concentration (estimated by Western blot). In vitro measurement of total 25-hydroxylase activity indicated a more than 50% decrease during 12-24h fasting. In addition, these authors demonstrated that the decrease in CYP2R1 was mediated by PPARγ-coactivator-1α (PGC1α), the key control enzyme, induced by metabolic diseases such as fasting, or type1 or type2 diabetes. By using several in vitro and in vivo gene KO and overexpression experiments, they showed that the control of CYP2R1 gene expression by PGC1α required the presence of another nuclear receptor, Estrogen-related receptor α (ERRα), known to bind tightly to this and other nuclear receptors [such as VDR and Glucocorticoid receptor (GR)]. Activation of the GR receptor by dexamethasone also decreased hepatic CYP2R1 mRNA and protein concentrations (by 50 and 26%, respectively), again mediated by induction of PGC1α. PGC1α also induced hepatic and renal expression of CYP24A1 several-fold, again mediated by the GR-PGC1α-ERRα pathway [but much less than the 100-fold induction by 1,25(OH)2D]. Other major fat regulating nuclear receptors are less likely involved, as the ENCODE project did not find consensus sequences for nuclear receptor (VDR) binding sites in promoters of genes involved in fat metabolism in the liver, such as constitutive androstane receptor (CAR), pregnane X receptor (PXR) or peroxisome proliferator-activated receptor (PPAR) binding sites in the proximal promoter of mouse or human CYP2R1 (http://www.cbrc.jp/htbin/nph-tfsearch), but several binding sites for NFkB were identified(2).
Both studies thus clearly demonstrate that the major (CYP2R1) and global hepatic 25-hydroxylase activity is under tight control of metabolic signals induced by fasting, diabetes or exposure to high dose glucocorticoids. PGC1α and ERRα as well as the GR are involved in the regulation of CYP2R1 but additional mechanisms may also be involved. A recent abstract demonstrates that a high fat diet induces an epigenetic downregulation of CYP2R1 in the mouse liver, thereby causing decreased serum 25OHD, whereas CYP24A1 was upregulated(6). These observations are fully in line with previous studies cited above. However, these authors now add another mechanism by hypermethylation of the promotor regions of these CYP2R1 and CYP27B1 genes and hypomethylation of the CYP24A1 promoter. In addition, they observed a decreased expression of glutathione, and treatment of such mice with glutathione precursors could partially correct the abnormal expression of vitamin D regulatory genes. They concluded that high fat diet caused glutathione deficiency, changing the methylation pattern of vitamin D regulatory genes and causing low serum 25OHD concentrations. All these studies failed to report blood glucose levels in their obese animals so that the separate effects of obesity and diabetes cannot be fully estimated. There are many other remaining questions such as: (1) Are CYP2R1 and CYP24A1 expression in other tissues also under the same metabolic control? (2) Is DBP, the major transport protein of all D metabolites, also regulated by metabolic factors? DBP is indeed decreased in diabetic subjects or animals (7,8). Apart from obesity, fasting, and diabetes, many other diseases are associated with poor vitamin D status compared with healthy controls. Therefore, the question arises whether patients with chronic renal failure, liver cirrhosis, and acute illness also have low serum 25OHD due to metabolic control of CYP2R1. If so, it could at least partially explain why such patients and especially patients admitted to intensive care units require so much vitamin D (10-100 times the normal doses) to generate serum 25OHD concentrations above 20 ng/ml (9,10). On the other hand, DBP levels often drop in these circumstances as an acute phase reactant, and this is associated with reduced 25OHD concentrations.
Finally, the short and long-term effects (harm or benefit) of this metabolic regulation of CYPs involved in vitamin D metabolism are not known. The PGC1α-ERRα pathway is known to play a major role in hepatic gluconeogenesis, in energy homeostasis in general and in fat tissue in particular. Indeed, as PGC1α is a strong positive regulator of mitochondrial function (and energy production) one might wonder whether the new observations are linked to overall energy balance and may help to clarify why VDR or CYP27B1 null mice are resistant to high fat diet-induced obesity (by activating energy expenditure) (11,12), whereas in humans a low vitamin D status is strongly associated with obesity. Knock down of CYP2R1 in zebrafish did not affect bone homeostasis but generated a phenotype of abnormal visceral fat accumulation (13). A similar phenotype of increased visceral and subcutaneous fat accumulation was observed in zebrafish raised on a vitamin D deficient diet, probably related to the increased expression of adipogenic and lipid processing markers in their liver(14). These data clearly indicate that the link between vitamin D metabolism and energy homeostasis already occurred early in the evolution of vertebrates (15).
Variations in hepatic (or extra-hepatic) CYP2R1 expression may also play a role in the great variability of serum concentrations of 25OHD in healthy populations with similar food and lifestyle attitudes. Indeed, there is widespread variability in the response of serum 25OHD to comparable amounts of dietary vitamin D and/or vitamin D supplementation (16-18) and no compelling mechanism has been able to explain this variability.
25-Hydroxylase activity (CYP2R1).
The studies just reviewed dealt with mice and need to be confirmed in humans. Human and mouse CYP2R1 are structurally and functionally very similar (19) and in both species, serum 25OHD is lower in case of type 1 or type 2 diabetes. Therefore, these studies clearly demonstrate that the general belief of constitutive expression of liver 25-hydroxylase activity no longer holds true (Figure 1). Indeed, the major 25-hydroxylase, CYP2R1, is highly regulated by a variety of “clinical” conditions (obesity, starvation, type 1 or type 2 diabetes) and a number of regulatory factors are now clearly identified, albeit there are still major missing links. Genetic silencing mutations in CYP2R1 can cause rickets or osteomalacia (20,21), but no activating mutations are so far described. Null mutations of the same gene cause the same phenotype in cats (22). Polymorphisms in CYP2R1 have the greatest effect on inter-individual variations in serum 25OHD when comparing with other known polymorphisms (23). If confirmed in humans, serum 25OHD is not only reflecting access to vitamin D of nutritional and skin-produced vitamin D, but is also reflecting a complex metabolic regulation of its hepatic synthesis and the likely involvement of many hormones.
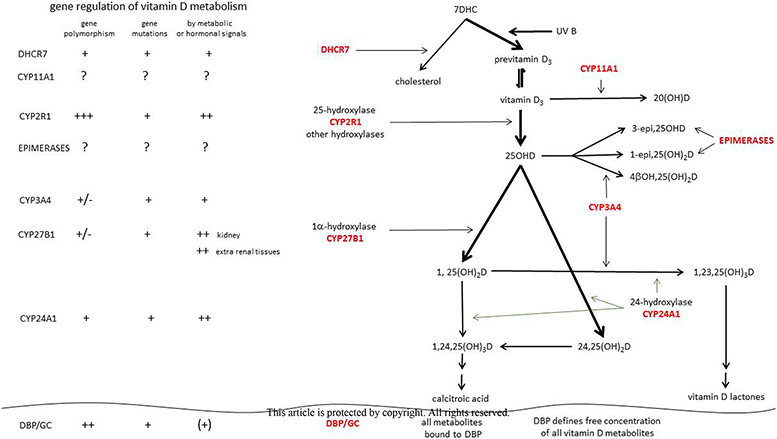
Figure 1.
Overview of the origin, metabolism and transport of vitamin D and its most important “old” and newly discovered metabolites and the major enzymes involved. The polymorphisms, mutations and metabolic or hormonal regulation of these major genes involved in vitamin D metabolism are also depicted.
These studies may also have practical implications for correcting a poor vitamin D status in obese or diabetic subjects. Intervention studies have shown that obese subjects need more vitamin D than normal weight subjects to achieve similar serum 25OHD concentrations as based on a comparison between an overview of such studies (5,16). However, vitamin D supplementation of vitamin D replete prediabetic subjects did not decrease their risk of progression to type 2 diabetes (24). Whether supplementation of more vitamin D deficient subjects may generate better results is yet unclear.
Now that the dogma of a non-regulated CYP2R1 has been challenged, one may also question other “dogmas” regarding vitamin D metabolism (Figure 1).
7-Dehydrocholesterol reductase (DHCR7).
DHC-7a-reductase (DHCR7) is a key determinant of the amount of the vitamin D precursor, 7-dehydrocholesterol (7-DHC), in the skin. Most reviews mention that older subjects may have lower 7-DHC concentrations in the skin but do not include regulation of DHCR7 as an important regulator of vitamin D status. DHCR7 is the last step in the Kandutsch-Russell pathway of cholesterol synthesis, converting 7DHC to cholesterol. As such, DHCR7 is essential for the presence or absence of 7-DHC in skin cells. In case of overexpression of this enzyme, as in the skin of the members of the feline species (including cats and dogs), the near absence of 7-DHC makes these animals unable to synthesize vitamin D, so that vitamin D is a true vitamin in these species (1). The opposite condition, genetic absence of DHCR7 causes Lemli-Smith-Opitz disease (25), mainly characterized by the consequences of too little cholesterol, steroids or bile acids. However, this disease increases the accumulation of 7DHC and thereby increases the effect of UVB on the synthesis of vitamin D. Therefore these patients usually have higher serum 25OHD concentrations than normal subjects (26). In humans, polymorphisms in DHCR7 have been associated with either reduced (27,28) or increased (29) 25OHD levels. However, the impact of these polymorphisms on enzyme function has not been demonstrated. The regulation of DHCR7 is incompletely understood. Cholesterol and vitamin D [but not 1,25(OH)2D] increase proteasomal degradation of DHCR7, as does UVB, leading to increased vitamin D production (30). AMPK, a key sensor and regulator of cellular energy homeostasis and protein kinase A are potent inhibitors of DHCR7, whereas CaMKII has a lower inhibitory effect (31,32). Most textbooks and reviews clearly state that the photochemical production of vitamin D in the skin is a non-enzymatic reaction. While this remains technically correct, recent data suggest that the activity of DHCR7 is under (cellular) metabolic and genetic control. By controlling substrate (7DHC) availability, these factors thus can influence inter-individual variations in photosynthesis of vitamin D. To what extent this has implications for the vitamin D status of humans is, however, unknown.
CYP27B1.
When Fraser and Kodicek (33) first identified the kidney as the source of 1,25(OH)2D in 1971, it was thought to be the sole source. However, anephric pregnant rats can produce 1,25(OH)2D (34). Similarly a case report of a woman with chronic kidney disease showed an increase in serum 1,25(OH)2D during pregnancy (35), and the human placenta was shown to be capable of 1,25(OH)2D production (36). Moreover, in non pregnant anephric humans36,37 and pigs 38 detectable levels of 1,25(OH)2D were found at baseline and could be further increased with vitamin D or 25OHD administration. A report by Barbour et al. (40) of an anephric patient with sarcoidosis with clearly detectable 1,25(OH)2D levels demonstrated a disease state in which extrarenal 1,25(OH)2D3 production occurred. The source was soon discovered to be the activated pulmonary alveolar macrophages from the involved lungs (41). At about the same time, a number of investigators were finding 1,25(OH)2D production by bone cells (42), melanocytes (43), and epidermal keratinocytes in vitro (44) and many other cells and tissues (45). With the cloning of the 25OHD-1α hydroxylase (CYP27B1) in 1997 by 4 groups(46-49) came the demonstration that there is only one gene and protein such that the renal and extrarenal enzyme is the same. (46,50). The cloning enabled the development of molecular probes and antibodies to CYP27B1 (51), facilitating the demonstration of its expression in many other tissues. However, it soon became apparent that the regulation of CYP27B1 activity in non-renal tissues differed from that in the kidney. This difference in regulation is clearly demonstrated in diseases such as sarcoidosis and other disorders that lead to unregulated increases in circulating 1,25(OH)2D and hypercalcemia. Four examples of CYP27B1 regulation in non-renal tissues follow a discussion of its regulation in the kidney.
Kidney.
CYP27B1 in the renal proximal convoluted tubule (PCT) is controlled principally by three hormones, parathyroid hormone (PTH), having a positive effect and FGF23 as well as 1,25(OH)2D itself (both having an inhibitory effect), responding at least in part to changes in ambient calcium and phosphate levels (review in (52)). Calcitonin can stimulate CYP27B1 activity in the proximal straight tubule (53). PTH and FGF23 act by binding to their respective receptors and activating their signaling pathways. Meyer et al.(54) identified a region in the enhancer region of CYP27B1 in renal DNA that was responsive to PTH, FGF23, and 1,25(OH)2D regulation. However, this region was not accessible to such regulation in the extrarenal tissues they tested including skin and immune cells. In these non-renal tissues, a different region of the CYP27B1 enhancer region was regulated by inflammatory factors, consistent with different regulatory mechanisms in non-renal tissues by the cytokines interferon-γ and tumor necrosis factor-α. Leptin may also (negatively) regulate CYP27B1 but probably mainly by its stimulatory effect on FGF23 production (55,56). These feedback loops provide very tight regulation of 1,25(OH)2D production by the PCT of the kidney, control that differs from that of CYP27B1 in other cell types including that of distal renal tubule cells where PTH has little effect (58).
Keratinocytes.
1,25(OH)2D has very little effect on CYP27B1 activity in keratinocytes (59). Rather, 1,25(OH)2D regulates its own levels in the keratinocyte by inducing CYP24A1, the catabolic enzyme for 1,25(OH)2D (59). Tumor necrosis factor-α (TNFα) (60) and interferon-γ (IFNγ) (61), on the other hand, are potent inducers of CYP27B1 activity in the keratinocyte as is TGFβ1 (62). 1,25(OH)2D induces the expression TLR2 and CD14 in keratinocytes, and activation of TLR2, but not TLR4 (by LPS), induces CYP27B1 (62).
Macrophages and Monocytes.
The production of 1,25(OH)2D by pulmonary alveolar macrophages is activated by IFNγ and TNFα, but not by IFNα and IFNβ, and is inhibited by dexamethasone(63,64), but not by 1,25(OH)2D. IL-1, IL-2 and IL-15 also stimulate CYP27B1 activity in peripheral blood mononuclear cells (PBMC), whereas IL-4 is suppressive (65,66). In contrast to Th1 cells, which produce IFNγ and IL-2, Th2 cells produce not only IL-4 but IFNβ that increases IL-10 to decrease CYP27B1 activity(67). Mononuclear cells express FGF receptors and αKlotho, and respond to FGF23 with a reduction in CYP27B1 expression (68).
Bone.
CYP27B1 in human mesenchymal stem cells from bone marrow is stimulated by PTH through mechanisms involving both the phosphorylation of CREB (an acute response) and through the expression of IGF1 and the activation of its receptor (longer term response) (69). 25OHD increases CYP27B1 expression in these cells, but that appears to be due to a combination of increased expression of the PTH/PTHrP receptor (70) and IGF1 (71) as 1,25(OH)2D decreases the expression of CYP27B1 in these cells (71). However, not all studies have found that PTH stimulated CYP27B1 in human osteoblasts (72).
Parathyroid gland.
The parathyroid gland expresses both FGF receptors and αKlotho (73). Unlike the kidney, FGF23 stimulates CYP27B1 expression in the parathyroid gland (74,75). Activation of the calcium sensing receptor in the parathyroid gland either by calcium or cinacalcet also increases CYP27B1 expression (75). Both FGF23 (73) and cinacalcet (75) reduce PTH secretion suggesting a link between PTH secretion and CYP27B1 expression.
These data clearly show that the production of 1,25(OH)2D is much more complex than the original dogma of the kidney being the single source of the active vitamin D hormone, regulated by two key hormones, PTH and FGF23. Moreover, recent data demonstrate that the renal and especially the extrarenal production of this hormone is extremely complex and regulated by a wide variety of mechanisms. The contribution of extra-renal 1,25(OH)2D production in normal physiology and disease states is a matter of debate. Extrarenal tissues can contribute to the serum concentration of 1,25(OH)2D in case of inflammatory diseases and pregnancy, but this is disputed in other situations, although as noted earlier, 1,25(OH)2D levels can be increased with vitamin D or 25OHD supplementation in anephric or end stage renal failure patients. That said, the prevailing view is that extrarenal 1,25(OH)2D production serves primarily a paracrine function in the tissue where it is produced rather than an endocrine function.
CYP24A1.
CYP24A1 is the main enzyme responsible for the catabolism of all vitamin D metabolites (Figure 1). It creates a multistep pathway resulting in a large number of metabolites with side chain modifications ultimately leading to calcitroic acid. It also plays an essential role (albeit species specific) in the formation of 25OHD lactones. Absence of this unique 24-hydroxylase (in contrast with multiple 25-hydroxylases) results in accumulation of 1,25(OH)2D and neonatal hypercalcemia (76). This is potentially lethal in mice and infants (infantile hypercalcemia). In addition, absence of this enzyme may first demonstrate its consequences by nephrocalcinosis or multiple kidney stones in adulthood (77,78). CYP24A1 null mice also have a problem with fracture repair as 24R,25(OH)2D is able to bind to a GPCR, Fam57B2, and thereby stimulates lactosylceramide production and fracture repair (79). Whether this also applies to humans with bi-allelic mutations has, however, so far not been reported (78). Polymorphism of the CYP24A1 gene is responsible for modest genetic variability of serum 25OHD (as one of the 8 genes known so far to result in genetically predisposed higher or lower serum 25OHD concentrations). CYP24A1 is under control of many hormones but mainly by 1,25(OH)2D (very strong upregulation) and FGF23 (also stimulatory effect) or calcium (80). Even 5α-dihydrotestosterone, by using the progesterone receptor, seems to be able to stimulate CYP24A1 (81).
Although incompletely understood, there must be other mechanisms to eliminate vitamin D metabolites, as serum 25OHD is only modestly increased in animals or humans with bi-allelic null mutations. The most likely candidates are CYP3A4 and a variety of enzymes capable of esterification of all vitamin D metabolites.
CYP11A1.
This enzyme is well-known as the rate-limiting enzyme in steroid synthesis, converting cholesterol to pregnenolone, the side chain cleavage reaction. However, Slominski et al. (82) have demonstrated that CYP11A1 also metabolizes vitamin D3 to 20(OH)D3 with subsequent further metabolism to a variety of metabolites including 1,20(OH)2D3, which have biologic activity comparable in some cases to 1,25(OH)2D3. 25OHD is not a substrate (Figure 1). The efficiency of 1,20(OH)2D production presumably by CYP27B1 acting on 20(OH)D is much lower than that of 1,25(OH)2D production from 25(OH)D. CYP11A1 is expressed in the skin and cultured keratinocytes (83) as well as better known steroid producing tissues such as the adrenals, ovary, testes, and placenta. At this point, little is known about how this enzyme is regulated in the skin and elsewhere with respect to its vitamin D metabolizing activity.
CYP3A4.
CYP3A4 is the major drug metabolizing enzyme (84). It is primarily expressed in the liver and intestinal mucosa. 1,25(OH)2D induces this enzyme in both liver and intestinal cells (85), although in vivo there is probably little induction in the liver given the low levels of VDR in that tissue. The enterohepatic circulation of 1,25(OH)2D and 25OHD may increase the levels of CYP3A4 more than would be expected based on serum levels (86,87). Lithocholic acid can also function as a ligand for VDR inducing CYP3A4 in the intestine (88). CYP3A4 can metabolize both 25OHD and 1,25(OH)2D as well as other vitamin products such as 1αOHD and D2. These hydroxylations occur in the 24 and 25 positions of the side chains (89) as well as the 23 position for 1,25(OH)2D (90). The induction of CYP3A4 by 1,25(OH)2D was at least as great as the induction of CYP24A1 in the intestine (91). Rifampin is a potent inducer of CYP3A4, and its use results in lower levels of 25OHD and 1,25(OH)2D. This could lead to drug induced osteomalacia (92). The major circulating product of CYP3A4 activity is 4β,25(OH)2D, which can reach levels comparable to 1,25(OH)2D following rifampin therapy (93) (Figure 1). Its biologic activity is not known.
Recently a publication has appeared describing two unrelated subjects with early onset of rickets for which none of the known mutations in the enzymes involved with vitamin D metabolism or VDR could be found (94). Both 25(OH)D and 1,25(OH)2D levels were low, whereas 4β,25(OH)2D levels were elevated. The authors used whole exome sequencing to find the same activating missense mutation in the CYP3A4. The authors labeled this mutation as vitamin D dependent rickets type 3. It can be treated with very large doses of vitamin D (94).
25OHD-3-epimerase.
The enzyme catalyzing the 3β-epimerization of (3α)25OHD remains poorly studied. This reaction does not appear to be reversible. The gene has yet to be identified. The enzymatic activity is broadly distributed, and resides in the microsomal fraction of cells (95). Circulating levels of 3-epi-25OHD can be substantial, ranging from 3.5-22% of the 25OHD levels in adults (96) and 8.7-61.1% in children (97). LC/tandem mass spectroscopy methods have been developed to separate the 3-epi form from 25OHD itself (98). The 3-epi-25OHD can be further metabolized by CYP27B1 to 3-epi-1,25(OH)2D (99). 3-epi-1,25(OH)2D has biologic activity, although in most studies its activity is less than 1,25(OH)2D (100), although its affinity for the VDR appears to be substantially less (101). Moreover, its ability to stimulate intestinal calcium absorption, differentiation of UMR 106 cells, or CYP24A1 induction is markedly reduced. Thus the 3-epi forms of 25OHD and 1,25(OH)2D cannot be ignored, but their biologic roles need further study.
1β-epimerase.
Substantial amounts of 1β,25(OH)2D are detectable in serum of normal subjects (about 16-33 % of the concentration of 1α,25(OH)2D). Its concentration shows a high correlation with serum 25OHD (r=0.85) but a lower correlation with 1,25(OH)2D. The origin (tissue?) or enzyme(s) involved have not yet been defined (102).
Vitamin D esterification.
The conversion of vitamin D into 25OHD is far from complete. Based on clinical supplementation trials (reviewed in(16)), only one out of three to ten molecules of vitamin D ultimately is converted into 25OHD. The same is true for the conversion of 25OHD into 1,25(OH)2D. The other 25OHD molecules can be converted by CYP24A1 into 24,25(OH)2D and a number of other metabolites (Figure). The fate of the other vitamin D (or 25OHD) molecules is unclear but esterification is most likely involved as part of the degradation pathway. This involves conjugations with sulphate (into vitamin D/25-hydroxyvitamin D3-3-sulfate), glycosides (e.g. vitamin D and 25-hydroxyvitamin D3-3-glucuronide), taurine or long chain fatty acids(103,104). The esterification of vitamin D is already found early in evolution as some glycosides of vitamin D are even found as toxic agents in plants(105) and most vitamin D found in fish liver is in the form of fatty acid esters(106). The regulation of these esterifications and the potential recovery of vitamin D metabolites by de-esterification (e.g. hepato-biliary-intestinal reclycling) are largely unexplored.
Vitamin D binding protein.
The serum vitamin D binding protein (DBP) is responsible for the transport of all vitamin D metabolites due to its high affinity for all metabolites and especially for 25OHD. It thereby regulates the free concentration of these metabolites as is best demonstrated by the extremely low serum concentrations of 25OHD and 1,25(OH)2D in animals or the single human subject with biallelic mutations in the DBP/GC gene (107). Up to now, most experts considered DBP as being stably expressed by hepatocytes with little or no regulation, apart from the stimulatory effects of estrogens(108). DBP concentrations, however, are slightly (~ 10 %) lower in homozygous DBP/GC2-2 carriers with a similar decrease in total 25OHD concentrations. Polymorphisms in DBP are responsible for part of the genetic variability of serum 25OHD concentrations in all populations tested so far. DBP in serum can be measured by mono and polyclonal antibodies and more recently also by mass spectroscopy, whereby careful attention must be given to assure equal measurements of all isoforms of DBP (109).
DBP concentrations are markedly decreased in liver diseases, nephrotic syndrome, and in patients with very severe acute illness or acute trauma due at least in part to its actin scavenging function (110). Therefore, DBP is not a passive but an active player in the overall vitamin D homeostasis and is probably under control of various metabolic signals (Figure 1).
Summary and perspective.
The dual origin of vitamin D, discovered about a century ago, first evolved into a rather simple metabolic schema of constitutive 25-hydroxylation of vitamin D in the liver to produce 25OHD, followed by a tightly regulated 1α-hydroxylation by a unique CYP27B1 in a unique organ (kidney) to generate 1,25(OH)2D as ligand of a nuclear receptor, VDR. All these metabolites are transported by a single serum binding protein and are finally catabolized by a unique nearly ubiquitous CYP24A1. The present picture is much more complex with a large number of enzymes, expressed in a variety of cells. Most of these genes contain genetic polymorphisms which may alter their function , and are regulated by hormones and/or metabolic signaling that can vary in different tissues of the body. Finally, the vitamin D endocrine system regulates a large number of vertebrate genes. These recent findings reveal that the vitamin D endocrine system is much more complex than initially thought and remains still incompletely understood.
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/jbmr.3884
Contributor Information
Roger Bouillon, Laboratory of Clinical and Experimental Endocrinology, Department of Chronic Diseases, Metabolism and Ageing, KU Leuven, Belgium..
Dan Bikle, University of California San Francisco and VA Medical Center, CA, USA..
References
- 1.Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, et al. Skeletal and extra-skeletal actions of vitamin D: Current evidence and outstanding questions. Endocr Rev. Oct 2018. Epub 2018/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roizen JD, Long C, Casella A, O'Lear L, Caplan I, Lai M, et al. Obesity Decreases Hepatic 25-Hydroxylase Activity Causing Low Serum 25-Hydroxyvitamin D. J Bone Miner Res. Feb 2019:e3686. Epub 2019/02/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aatsinki SM, Elkhwanky MS, Kummu O, Karpale M, Buler M, Viitala P, et al. Fasting-Induced Transcription Factors Repress Vitamin D Bioactivation, a Mechanism for Vitamin D Deficiency in Diabetes. Diabetes. May 2019;68(5):918–31. Epub 2019/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng JS, Imamura F, Sharp SJ, van der Schouw YT, Sluijs I, Gundersen TE, et al. Association of Plasma Vitamin D Metabolites With Incident Type 2 Diabetes: EPIC-InterAct Case-Cohort Study. J Clin Endocrinol Metab. Apr 2019;104(4):1293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassatne A, Chakhtoura M, Saad R, Fuleihan GE. Vitamin D supplementation in obesity and during weight loss: A review of randomized controlled trials. Metabolism. Mar 2019;92:193–205. Epub 2019/01/04. [DOI] [PubMed] [Google Scholar]
- 6.Parsanathan R, Jain S. Glutathione-deficiency induces epigenetic modifications of vitamin D-regulatory genes in diabetic mice: its role in 25OHD deficiency. Vitamin D workshop; New York City May 28th- June 1st 2019. [Google Scholar]
- 7.Nyomba BL, Bouillon R, Bidingija M, Kandjingu K, De Moor P. Vitamin D metabolites and their binding protein in adult diabetic patients. Diabetes. Aug 1986;35(8):911–5. [DOI] [PubMed] [Google Scholar]
- 8.Nyomba BL, Bouillon R, Lissens W, Van Baelen H, De Moor P. 1,25-Dihydroxyvitamin D and vitamin D-binding protein are both decreased in streptozotocin-diabetic rats. Endocrinology. Jun 1985;116(6):2483–8. [DOI] [PubMed] [Google Scholar]
- 9.Van den Berghe G, Van Roosbroeck D, Vanhove P, Wouters PJ, De Pourcq L, Bouillon R. Bone turnover in prolonged critical illness: effect of vitamin D. J Clin Endocrinol Metab. Oct 2003;88(10):4623–32. [DOI] [PubMed] [Google Scholar]
- 10.Amrein K, Papinutti A, Mathew E, Vila G, Parekh D. Vitamin D and critical illness: what endocrinology can learn from intensive care and vice versa. Endocr Connect. Dec 2018;7(12):R304–R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouillon R, Carmeliet G, Lieben L, Watanabe M, Perino A, Auwerx J, et al. Vitamin D and energy homeostasis: of mice and men. Nat Rev Endocrinol. Feb 2014;10(2):79–87. Epub 2013/11/19. [DOI] [PubMed] [Google Scholar]
- 12.Narvaez C, Matthews D, Broun E, Chan M, Welsh J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology. Feb 2009;150(2):651–61. Epub 2008/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng X, Shang G, Wang W, Chen X, Lou Q, Zhai G, et al. Fatty Acid Oxidation in Zebrafish Adipose Tissue Is Promoted by 1alpha,25(OH)2D3. Cell Rep. May 16 2017;19(7):1444–55. Epub 2017/05/18. [DOI] [PubMed] [Google Scholar]
- 14.Knuth M, Mahapatra D, Jima D, Kullman S. Understanding the link between vitamin D deficiency and obesity. Vitamin D Workshop; New York City May 28th-June 1th 2019. [Google Scholar]
- 15.Bouillon R, Suda T. Vitamin D: calcium and bone homeostasis during evolution. Bonekey Rep. Jan 8 2014;3:480. Epub 2014/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quesada-Gomez JM, Bouillon R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos Int. Aug 2018;29(8):1697–711. Epub 2018/05/02. [DOI] [PubMed] [Google Scholar]
- 17.Manios Y, Moschonis G, Lambrinou CP, Tsoutsoulopoulou K, Binou P, Karachaliou A, et al. A systematic review of vitamin D status in southern European countries. Eur J Nutr. Sep 2018;57(6):2001–36. Epub 2017/11/02. [DOI] [PubMed] [Google Scholar]
- 18.Durazo-Arvizu RA, Dawson-Hughes B, Kramer H, Cao G, Merkel J, Coates PM, et al. The Reverse J-Shaped Association Between Serum Total 25-Hydroxyvitamin D Concentration and All-Cause Mortality: The Impact of Assay Standardization. Am J Epidemiol. Apr 15 2017;185(8):720–6. Epub 2017/03/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase. J Biol Chem. Sep 26 2003;278(39):38084–93. Epub 2003/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thacher TD, Levine MA. CYP2R1 mutations causing vitamin D-deficiency rickets. J Steroid Biochem Mol Biol. Oct 2017;173:333–6. Epub 2016/07/31. [DOI] [PubMed] [Google Scholar]
- 21.Molin A, Wiedemann A, Demers N, Kaufmann M, Do Cao J, Mainard L, et al. Vitamin D-Dependent Rickets Type 1B (25-Hydroxylase Deficiency): A Rare Condition or a Misdiagnosed Condition? J Bone Miner Res. Sep 2017;32(9):1893–9. Epub 2017/05/27. [DOI] [PubMed] [Google Scholar]
- 22.Teshima T, Kurita S, Sasaki T, Matsumoto H, Niina A, Abe D, et al. A genetic variant of CYP2R1 identified in a cat with type 1B vitamin D-dependent rickets: a case report. BMC Vet Res. Feb 18 2019;15(1):62. Epub 2019/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manousaki D, Dudding T, Haworth S, Hsu YH, Liu CT, Medina-Gomez C, et al. Low-Frequency Synonymous Coding Variation in CYP2R1 Has Large Effects on Vitamin D Levels and Risk of Multiple Sclerosis. Am J Hum Genet. Aug 3 2017;101(2):227–38. Epub 2017/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N Engl J Med. Jun 7 2019. Epub 2019/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tint GS, Irons M, Elias ER, Batta AK, Frieden R, Chen TS, et al. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N Engl J Med. Jan 13 1994;330(2):107–13. Epub 1994/01/13. [DOI] [PubMed] [Google Scholar]
- 26.Movassaghi M, Bianconi S, Feinn R, Wassif CA, Porter FD. Vitamin D levels in Smith-Lemli-Opitz syndrome. Am J Med Genet A. Oct 2017;173(10):2577–83. Epub 2017/08/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, et al. Genome-wide association study of circulating vitamin D levels. Human molecular genetics. Jul 1 2010;19(13):2739–45. Epub 2010/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. Jul 17 2010;376(9736):180–8. Epub 2010/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuan V, Martineau AR, Griffiths CJ, Hypponen E, Walton R. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol Biol. Jul 9 2013;13:144. Epub 2013/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prabhu AV, Luu W, Sharpe LJ, Brown AJ. Cholesterol-mediated Degradation of 7-Dehydrocholesterol Reductase Switches the Balance from Cholesterol to Vitamin D Synthesis. The Journal of biological chemistry. Apr 15 2016;291(16):8363–73. Epub 2016/02/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prabhu AV, Luu W, Sharpe LJ, Brown AJ. Phosphorylation regulates activity of 7-dehydrocholesterol reductase (DHCR7), a terminal enzyme of cholesterol synthesis. J Steroid Biochem Mol Biol. Jan 2017;165(Pt B):363–8. Epub 2016/08/16. [DOI] [PubMed] [Google Scholar]
- 32.Prabhu AV, Luu W, Li D, Sharpe LJ, Brown AJ. DHCR7: A vital enzyme switch between cholesterol and vitamin D production. Prog Lipid Res. Oct 2016;64:138–51. Epub 2016/11/05. [DOI] [PubMed] [Google Scholar]
- 33.Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. Nov 21 1970;228(5273):764–6. [DOI] [PubMed] [Google Scholar]
- 34.Gray TK, Lester GE, Lorenc RS. Evidence for extra-renal 1 alpha-hydroxylation of 25-hydroxyvitamin D3 in pregnancy. Science. Jun 22 1979;204(4399):1311–3. [DOI] [PubMed] [Google Scholar]
- 35.Turner M, Barre PE, Benjamin A, Goltzman D, Gascon-Barre M. Does the maternal kidney contribute to the increased circulating 1,25-dihydroxyvitamin D concentrations during pregnancy? Mineral and electrolyte metabolism. 1988;14(4):246–52. [PubMed] [Google Scholar]
- 36.Weisman Y, Harell A, Edelstein S, David M, Spirer Z, Golander A. 1 alpha, 25-Dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in vitro synthesis by human decidua and placenta. Nature. Sep 27 1979;281(5729):317–9. [DOI] [PubMed] [Google Scholar]
- 37.Lambert PW, Stern PH, Avioli RC, Brackett NC, Turner RT, Greene A, et al. Evidence for extrarenal production of 1 alpha ,25-dihydroxyvitamin D in man. The Journal of clinical investigation. Mar 1982;69(3):722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dusso A, Lopez-Hilker S, Rapp N, Slatopolsky E. Extra-renal production of calcitriol in chronic renal failure. Kidney international. Sep 1988;34(3):368–75. [DOI] [PubMed] [Google Scholar]
- 39.Littledike ET, Horst RL. Metabolism of vitamin D3 in nephrectomized pigs given pharmacological amounts of vitamin D3. Endocrinology. Dec 1982;111(6):2008–13. [DOI] [PubMed] [Google Scholar]
- 40.Barbour GL, Coburn JW, Slatopolsky E, Norman AW, Horst RL. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. The New England journal of medicine. Aug 20 1981;305(8):440–3. [DOI] [PubMed] [Google Scholar]
- 41.Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. The Journal of clinical investigation. 1983;72(5):1856–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner RT, Puzas JE, Forte MD, Lester GE, Gray TK, Howard GA, et al. In vitro synthesis of 1alpha,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol by isolated calvarial cells. Proceedings of the National Academy of Sciences of the United States of America. Oct 1980;77(10):5720–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frankel TL, Mason RS, Hersey P, Murray E, Posen S. The synthesis of vitamin D metabolites by human melanoma cells. The Journal of clinical endocrinology and metabolism. Sep 1983;57(3):627–31. [DOI] [PubMed] [Google Scholar]
- 44.Bikle DD, Nemanic MK, Whitney JO, Elias PW. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry. 1986;25(7):1545–8. [DOI] [PubMed] [Google Scholar]
- 45.Bikle DD, Halloran BP, Riviere JE. Production of 1,25 dihydroxyvitamin D3 by perfused pig skin. The Journal of investigative dermatology. May 1994;102(5):796–8. [DOI] [PubMed] [Google Scholar]
- 46.Fu GK, Lin D, Zhang MY, Bikle DD, Shackleton CH, Miller WL, et al. Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Molecular endocrinology (Baltimore, Md). Dec 1997;11(13):1961–70. [DOI] [PubMed] [Google Scholar]
- 47.Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 1997;277(5333):1827–30. [DOI] [PubMed] [Google Scholar]
- 48.St-Arnaud R, Messerlian S, Moir JM, Omdahl JL, Glorieux FH. The 25-hydroxyvitamin D 1-alpha-hydroxylase gene maps to the pseudovitamin D-deficiency rickets (PDDR) disease locus. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1997;12(10):1552–9. [DOI] [PubMed] [Google Scholar]
- 49.Shinki T, Shimada H, Wakino S, Anazawa H, Hayashi M, Saruta T, et al. Cloning and expression of rat 25-hydroxyvitamin D3-1alpha-hydroxylase cDNA. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(24):12920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones G, Ramshaw H, Zhang A, Cook R, Byford V, White J, et al. Expression and activity of vitamin D-metabolizing cytochrome P450s (CYP1alpha and CYP24) in human nonsmall cell lung carcinomas. Endocrinology. Jul 1999;140(7):3303–10. [DOI] [PubMed] [Google Scholar]
- 51.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. The Journal of clinical endocrinology and metabolism. 2001;86(2):888–94. [DOI] [PubMed] [Google Scholar]
- 52.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. Jan 2014;55(1):13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawashima H, Torikai S, Kurokawa K. Calcitonin selectively stimulates 25-hydroxyvitamin D3-1 alpha-hydroxylase in proximal straight tubule of rat kidney. Nature. May 28 1981;291(5813):327–9. [DOI] [PubMed] [Google Scholar]
- 54.Meyer MB, Benkusky NA, Kaufmann M, Lee SM, Onal M, Jones G, et al. A kidney-specific genetic control module in mice governs endocrine regulation of the cytochrome P450 gene Cyp27b1 essential for vitamin D3 activation. The Journal of biological chemistry. Oct 20 2017;292(42):17541–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bouillon R, Decallonne B. The white adipose tissue connection with calcium and bone homeostasis. J Bone Miner Res. Aug 2010;25(8):1707–10. Epub 2010/07/09. [DOI] [PubMed] [Google Scholar]
- 56.Tsuji K, Maeda T, Kawane T, Matsunuma A, Horiuchi N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J Bone Miner Res. Aug 2010;25(8):1711–23. Epub 2010/03/05. [DOI] [PubMed] [Google Scholar]
- 57.Kim MS, Fujiki R, Kitagawa H, Kato S. 1alpha,25(OH)2D3-induced DNA methylation suppresses the human CYP27B1 gene. Molecular and cellular endocrinology. Feb 2007;265-266:168–73. [DOI] [PubMed] [Google Scholar]
- 58.Bajwa A, Forster MN, Maiti A, Woolbright BL, Beckman MJ. Specific regulation of CYP27B1 and VDR in proximal versus distal renal cells. Archives of biochemistry and biophysics. Sep 1 2008;477(1):33–42. [DOI] [PubMed] [Google Scholar]
- 59.Xie Z, Munson SJ, Huang N, Portale AA, Miller WL, Bikle DD. The mechanism of 1,25-dihydroxyvitamin D(3) autoregulation in keratinocytes. The Journal of biological chemistry. Oct 04 2002;277(40):36987–90. [DOI] [PubMed] [Google Scholar]
- 60.Bikle DD, Pillai S, Gee E, Hincenbergs M. Tumor necrosis factor-alpha regulation of 1,25-dihydroxyvitamin D production by human keratinocytes. Endocrinology. Jul 1991;129(1):33–8. [DOI] [PubMed] [Google Scholar]
- 61.Bikle DD, Pillai S, Gee E, Hincenbergs M. Regulation of 1,25-dihydroxyvitamin D production in human keratinocytes by interferon-gamma. Endocrinology. Feb 1989;124(2):655–60. [DOI] [PubMed] [Google Scholar]
- 62.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. The Journal of clinical investigation. Mar 2007;117(3):803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adams JS, Gacad MA. Characterization of 1 alpha-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. The Journal of experimental medicine. Apr 1 1985;161(4):755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pryke AM, Duggan C, White CP, Posen S, Mason RS. Tumor necrosis factor-alpha induces vitamin D-1-hydroxylase activity in normal human alveolar macrophages. Journal of cellular physiology. Mar 1990;142(3):652–6. [DOI] [PubMed] [Google Scholar]
- 65.Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, Wheelwright M, et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proceedings of the National Academy of Sciences of the United States of America. Dec 28 2010;107(52):22593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gyetko MR, Hsu CH, Wilkinson CC, Patel S, Young E. Monocyte 1 alpha-hydroxylase regulation: induction by inflammatory cytokines and suppression by dexamethasone and uremia toxin. Journal of leukocyte biology. Jul 1993;54(1):17–22. [DOI] [PubMed] [Google Scholar]
- 67.Teles RM, Graeber TG, Krutzik SR, Montoya D, Schenk M, Lee DJ, et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science. Mar 22 2013;339(6126):1448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bacchetta J, Sea JL, Chun RF, Lisse TS, Wesseling-Perry K, Gales B, et al. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004 2013;28(1):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geng S, Zhou S, Glowacki J. Age-related decline in osteoblastogenesis and 1alpha-hydroxylase/CYP27B1 in human mesenchymal stem cells: stimulation by parathyroid hormone. Aging cell. Dec 2011;10(6):962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou S, Geng S, Glowacki J. Histone deacetylation mediates the rejuvenation of osteoblastogenesis by the combination of 25(OH)D3 and parathyroid hormone in MSCs from elders. The Journal of steroid biochemistry and molecular biology. Jul 2013;136:156–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou S, LeBoff MS, Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology. Jan 2010;151(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, et al. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. Nov 2006;20(13):2417–9. [DOI] [PubMed] [Google Scholar]
- 73.Silver J, Naveh-Many T. FGF23 and the parathyroid. Advances in experimental medicine and biology. 2012;728:92–9. [DOI] [PubMed] [Google Scholar]
- 74.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. The Journal of endocrinology. Oct 2007;195(1):125–31. [DOI] [PubMed] [Google Scholar]
- 75.Ritter CS, Haughey BH, Armbrecht HJ, Brown AJ. Distribution and regulation of the 25-hydroxyvitamin D3 1alpha-hydroxylase in human parathyroid glands. The Journal of steroid biochemistry and molecular biology. May 2012;130(1-2):73–80. [DOI] [PubMed] [Google Scholar]
- 76.Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med. Aug 4 2011;365(5):410–21. Epub 2011/06/17. [DOI] [PubMed] [Google Scholar]
- 77.Tebben PJ, Milliner DS, Horst RL, Harris PC, Singh RJ, Wu Y, et al. Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1 mutations: effects of ketoconazole therapy. J Clin Endocrinol Metab. Mar 2012;97(3):E423–7. Epub 2012/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cools M, Goemaere S, Baetens D, Raes A, Desloovere A, Kaufman JM, et al. Calcium and bone homeostasis in heterozygous carriers of CYP24A1 mutations: A cross-sectional study. Bone. Dec 2015;81:89–96. Epub 2015/06/29. [DOI] [PubMed] [Google Scholar]
- 79.Martineau C, Naja RP, Husseini A, Hamade B, Kaufmann M, Akhouayri O, et al. Optimal bone fracture repair requires 24R,25-dihydroxyvitamin D3 and its effector molecule FAM57B2. J Clin Invest. Aug 1 2018;128(8):3546–57. Epub 2018/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. Jan 2016;96(1):365–408. Epub 2015/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee SR, Park MY, Yang H, Lee GS, An BS, Park BK, et al. 5alpha-dihydrotestosterone reduces renal Cyp24a1 expression via suppression of progesterone receptor. J Mol Endocrinol. Feb 2018;60(2):159–70. Epub 2018/02/01. [DOI] [PubMed] [Google Scholar]
- 82.Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. The Journal of steroid biochemistry and molecular biology. Oct 2014;144 Pt A:28–39. Epub 2013/11/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. The Journal of clinical endocrinology and metabolism. Jul 1996;81(7):2746–9. Epub 1996/07/01. [DOI] [PubMed] [Google Scholar]
- 84.Wang Z, Schuetz EG, Xu Y, Thummel KE. Interplay between vitamin D and the drug metabolizing enzyme CYP3A4. The Journal of steroid biochemistry and molecular biology. Jul 2013;136:54–8. Epub 2012/09/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thummel KE, Brimer C, Yasuda K, Thottassery J, Senn T, Lin Y, et al. Transcriptional control of intestinal cytochrome P-4503A by 1alpha,25-dihydroxy vitamin D3. Molecular pharmacology. Dec 2001;60(6):1399–406. Epub 2001/11/28. [DOI] [PubMed] [Google Scholar]
- 86.Arnaud SB, Goldsmith RS, Lambert PW, Go VL. 25-Hydroxyvitamin D3: evidence of an enterohepatic circulation in man. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. Jun 1975;149(2):570–2. Epub 1975/06/01. [DOI] [PubMed] [Google Scholar]
- 87.Gascon-Barre M Is there any physiological significance to the enterohepatic circulation of vitamin D sterols? Journal of the American College of Nutrition. 1986;5(3):317–24. Epub 1986/01/01. [DOI] [PubMed] [Google Scholar]
- 88.Jurutka PW, Thompson PD, Whitfield GK, Eichhorst KR, Hall N, Dominguez CE, et al. Molecular and functional comparison of 1,25-dihydroxyvitamin D(3) and the novel vitamin D receptor ligand, lithocholic acid, in activating transcription of cytochrome P450 3A4. Journal of cellular biochemistry. Apr 1 2005;94(5):917–43. Epub 2004/12/04. [DOI] [PubMed] [Google Scholar]
- 89.Gupta RP, He YA, Patrick KS, Halpert JR, Bell NH. CYP3A4 is a vitamin D-24- and 25-hydroxylase: analysis of structure function by site-directed mutagenesis. The Journal of clinical endocrinology and metabolism. Feb 2005;90(2):1210–9. Epub 2004/11/18. [DOI] [PubMed] [Google Scholar]
- 90.Xu Y, Hashizume T, Shuhart MC, Davis CL, Nelson WL, Sakaki T, et al. Intestinal and hepatic CYP3A4 catalyze hydroxylation of 1alpha,25-dihydroxyvitamin D(3): implications for drug-induced osteomalacia. Molecular pharmacology. Jan 2006;69(1):56–65. Epub 2005/10/07. [DOI] [PubMed] [Google Scholar]
- 91.Brodie MJ, Boobis AR, Hillyard CJ, Abeyasekera G, Stevenson JC, MacIntyre I, et al. Effect of rifampicin and isoniazid on vitamin D metabolism. Clin Pharmacol Ther. Oct 1982;32(4):525–30. Epub 1982/10/01. [DOI] [PubMed] [Google Scholar]
- 92.Shah SC, Sharma RK, Hemangini, Chitle AR. Rifampicin induced osteomalacia. Tubercle. Sep 1981;62(3):207–9. Epub 1981/09/01. [DOI] [PubMed] [Google Scholar]
- 93.Wang Z, Lin YS, Zheng XE, Senn T, Hashizume T, Scian M, et al. An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway. Molecular pharmacology. Apr 2012;81(4):498–509. Epub 2011/12/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roizen JD, Li D, O'Lear L, Javaid MK, Shaw NJ, Ebeling PR, et al. CYP3A4 mutation causes vitamin D-dependent rickets type 3. J Clin Invest. May 1 2018;128(5):1913–8. Epub 2018/02/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tuckey RC, Tang EKY, Maresse SR, Delaney DS. Catalytic properties of 25-hydroxyvitamin D3 3-epimerase in rat and human liver microsomes. Archives of biochemistry and biophysics. Mar 27 2019;666:16–21. Epub 2019/03/31. [DOI] [PubMed] [Google Scholar]
- 96.Lensmeyer G, Poquette M, Wiebe D, Binkley N. The C-3 epimer of 25-hydroxyvitamin D(3) is present in adult serum. The Journal of clinical endocrinology and metabolism. Jan 2012;97(1):163–8. Epub 2011/10/21. [DOI] [PubMed] [Google Scholar]
- 97.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. The Journal of clinical endocrinology and metabolism. Aug 2006;91(8):3055–61. Epub 2006/05/25. [DOI] [PubMed] [Google Scholar]
- 98.Dowling KG, Hull G, Sundvall J, Lamberg-Allardt C, Cashman KD. Improved accuracy of an tandem liquid chromatography-mass spectrometry method measuring 24R,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D metabolites in serum using unspiked controls and its application to determining cross-reactivity of a chemiluminescent microparticle immunoassay. J Chromatogr A. May 12 2017;1497:102–9. Epub 2017/04/05. [DOI] [PubMed] [Google Scholar]
- 99.Kamao M, Tatematsu S, Hatakeyama S, Sakaki T, Sawada N, Inouye K, et al. C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1alpha or C-24 hydroxylation. The Journal of biological chemistry. Apr 16 2004;279(16):15897–907. Epub 2004/02/06. [DOI] [PubMed] [Google Scholar]
- 100.Molnar F, Sigueiro R, Sato Y, Araujo C, Schuster I, Antony P, et al. 1alpha,25(OH)2-3-epi-vitamin D3, a natural physiological metabolite of vitamin D3: its synthesis, biological activity and crystal structure with its receptor. PloS one. Mar 31 2011;6(3):e18124. Epub 2011/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Masuda S, Kamao M, Schroeder NJ, Makin HL, Jones G, Kremer R, et al. Characterization of 3-epi-1alpha,25-dihydroxyvitamin D3 involved in 1alpha,25-dihydroxyvitamin D3 metabolic pathway in cultured cell lines. Biol Pharm Bull. Feb 2000;23(2):133–9. Epub 2000/03/08. [DOI] [PubMed] [Google Scholar]
- 102.Pauwels S, Jans I, Billen J, Heijboer A, Verstuyf A, Carmeliet G, et al. 1beta,25-Dihydroxyvitamin D3: A new vitamin D metabolite in human serum. J Steroid Biochem Mol Biol. Oct 2017;173:341–8. Epub 2017/02/15. [DOI] [PubMed] [Google Scholar]
- 103.Wong T, Wang Z, Chapron BD, Suzuki M, Claw KG, Gao C, et al. Polymorphic Human Sulfotransferase 2A1 Mediates the Formation of 25-Hydroxyvitamin D3-3-O-Sulfate, a Major Circulating Vitamin D Metabolite in Humans. Drug Metab Dispos. Apr 2018;46(4):367–79. Epub 2018/01/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fraser DR, Kodicek E. Investigations on vitamin D esters synthesized rats. Detection and identification. Biochem J. Jan 1968;106(2):485–90. Epub 1968/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gregory JF, 3rd. Nutritional Properties and significance of vitamin glycosides. Annu Rev Nutr. 1998;18:277–96. Epub 1998/10/24. [DOI] [PubMed] [Google Scholar]
- 106.Fraser DR. Chapter 2 - Evolutionary Biology: Mysteries of Vitamin D in Fish. In: Feldman D, editor. Vitamin D (Fourth Edition): Academic Press; 2018. p. 13–27. [Google Scholar]
- 107.Henderson CM, Fink SL, Bassyouni H, Argiropoulos B, Brown L, Laha TJ, et al. Vitamin D-Binding Protein Deficiency and Homozygous Deletion of the GC Gene. N Engl J Med. Mar 21 2019;380(12):1150–7. Epub 2019/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guha C, Osawa M, Werner PA, Galbraith RM, Paddock GV. Regulation of human Gc (vitamin D--binding) protein levels: hormonal and cytokine control of gene expression in vitro. Hepatology. 1995;21:1675–81. Epub 1995/06/01. [PubMed] [Google Scholar]
- 109.Bouillon R, Schuit F, Antonio L, Rastinejad F. Vitamin D binding protein: a historic overview. Frontiers in Endocrinology. 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van Baelen H, Bouillon R, De Moor P. Vitamin D-binding protein (Gc-globulin) binds actin. J Biol Chem. Mar 25 1980;255(6):2270–2. Epub 1980/03/25. [PubMed] [Google Scholar]