Abstract
25-hydroxyvitamin D (25(OH)D), the precursor of the active form of vitamin D, is recognized as the optimal indicator of vitamin D status. Vitamin D3 undergoes conversion through a multitude of enzymatic reactions described within the paper and vitamin D levels are dependent on many factors, including the vitamin D binding protein (DBP). The free hormone hypothesis postulates that protein-bound hormones are not biologically available and that unbound hormones are biologically active. The majority of circulating 25(OH)D and 1,25-dihydroxyvitamin D is tightly bound to DBP and albumin, with less than 1% circulating in an unbound form. As a result, factors affecting DBP alter the interpretation of 25(OH)D levels. The aim of this review is to assess the current methodology used to measure total and free 25(OH)D, and DBP. Additionally, we analyze the effects of other endocrine hormones and disease processes on DBP levels and subsequently, the interpretation of 25(OH)D levels.
1.0. Introduction:
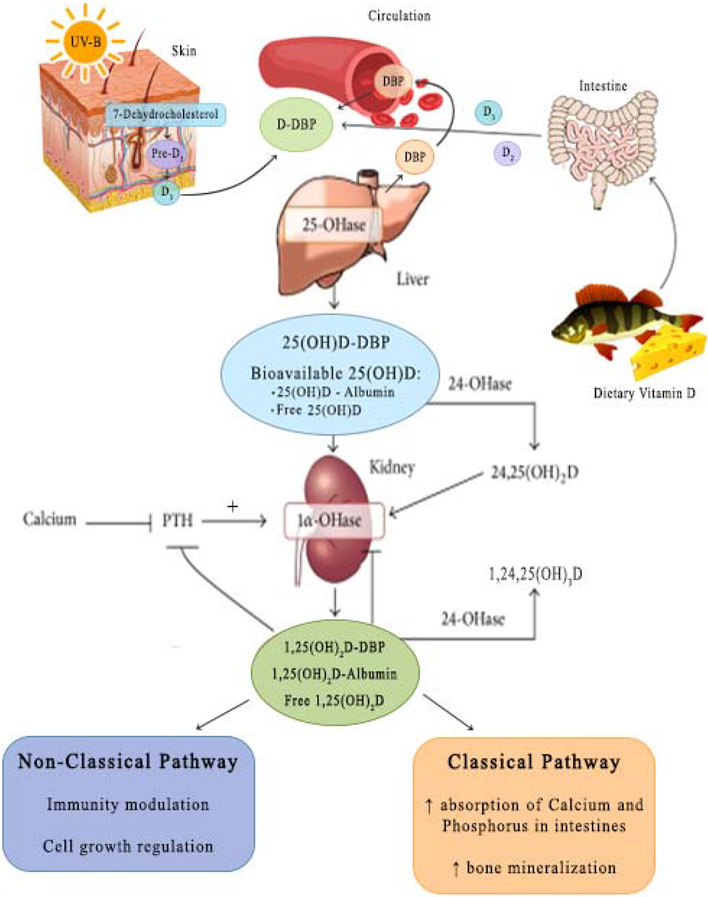
25-hydroxyvitamin D [25(OH)D], the precursor of the active form of vitamin D, is recognized as the optimal indicator of vitamin D status. Vitamin D3 is produced in the skin from 7-dehydrocholesterol through a two-step process. Pre-vitamin D3 is synthesized non-enzymatically in the skin from 7-dehydrocholesterol during exposure to ultraviolet rays in sunlight. Pre-vitamin D3 undergoes a temperature-dependent reaction forming vitamin D3. It is then transported to the liver bound to vitamin D-binding protein (DBP) and metabolized to 25(OH)D, through an enzymatic process involving 25-hydroxylase. Alternatively, vitamin D, either as D2 or D3, can enter the body from its absorption in the intestine. In either case D2 or D3 is metabolized to 25-hydroxyvitamin D2 or 25-hydroxyvitamin D3, respectively, by the action of 25-hydroxylases, the most specific of which is CYP2R1. 25-hydroxyvitamin D2 and D3 enter the circulation bound to DBP (Please see Figure 1). Within the renal tubular cell, 25(OH)D is released from the binding protein, following uptake of the DBP-25(OH)D complex by megalin/cubilin in renal tubules and the parathyroid glands. [1]. After dissociation, 1-alpha-hydroxylase and 24-alpha hydroxylase act on 25(OH)D, producing 1,25 dihydroxyvitamin D and 24, 25-dihydroxyvitamin D [2].
Figure 1.
Vitamin D metabolism: Abbreviations: 25(OH)D: 25-hydroxyvitamin D, DBP: Vitamin D binding protein, PTH: parathyroid hormone, 25-OHase: 25-hydroxylases, 24-OHase: 24-hydroxylases, 1α-OHase: 1-alpha-hydroxylase
Low 25(OH)D levels exist in many chronic conditions such as end-stage liver disease, nephrotic syndrome, and in critical illness where intact parathyroid hormone levels are not elevated. The variations in the 25(OH)D levels in these conditions result from variations in the binding of 25(OH)D to DBP (Please see Figure 1). Vitamin D and its metabolites exist in blood both bound and unbound. It is the unbound or free form of these metabolites, which is active in most cells. This is a classic feature of both sex binding globulin regulation of sex hormones and thyroxine binding globulin regulation of thyroid hormones. In this article we evaluate conditions that affect DBP and 25(OH)D levels.
2.0. DBP and free 25(OH)D:
The majority of circulating 25(OH)D and 1,25-dihydroxyvitamin D is tightly bound to DBP, with a smaller amount (10–15%) bound to albumin. Less than 1% of circulating vitamin D metabolites exists in a free, unbound form [3]. The free hormone hypothesis postulates that protein-bound hormones are biologically inactive while unbound hormones are biologically free to exert their physiologic activity [3]. This hypothesis applies to lipophilic molecules, such as 25(OH)D, in cells that have the megalin/cubulin transport system [1]. Specifically in this case, binding of DBP impairs delivery of 25(OH)D to vitamin D-activating 1-alpha-hydroxylase in target cells. Thus, it is the action of unbound, free 25(OH)D that exerts its physiologic activity. In the epithelial renal cells in the proximal tubule express both megalin and cubulin, which facilitate the entry of the 25(OH)D-DBP complex into the cell. Once internalized, through megalin mediated endocytosis, 25(OH)D dissociates from DBP and then it can be further metabolized to the free, biological active form [4].
DBP is a polymorphic single chain serum glycoprotein that is primarily produced by the liver. DBP is responsible for binding 85% of the circulating 25(OH)D. DBP has three main roles in vitamin D physiology: protecting vitamin D from biodegradation, limiting its access to target tissues, and reabsorbing vitamin D in the kidneys [5]. The complex of DBP with 25(OH)D is filtered in the kidney glomerulus and then reabsorbed at the brush border of the tubular epithelial cells. Additional roles for DBP include transporting fatty acids, protecting complement C5a from degradation, macrophage activation, neutrophil chemotaxis, and functioning as an actin scavenger. For example, tissue injury results in the release of intracellular actin, which can further damage the microarchitecture of the cell. DBP works in association with other serum proteins to protect the cell from actin-mediated damage [6].
2.1. Measurement of total 25OH vitamin D
There are several methods for measurement of vitamin D in plasma. Methods used to measure total 25(OH)D include RIA, HPLC, LC-MS, and chemiluminescence assay by IDS. The first assay for measuring total 25(OH)D was based on a competitive protein binding method, using DBP. The major limitation of this assay was that in addition to total 25(OH)D, other vitamin D metabolites including 24, 25-dihydroxyvitamin D were detected. These other vitamin D metabolites circulate as less than 10-15% of the concentration of total 25(OH)D and are usually biologically inactive [5]. In 1985, a radioimmunoassay was developed to detect 25-hydroxyvitamin D. This assay also recognized both 25(OH)D and 25(OH)D3. However, like the first assay developed, the RIA assay also could not exclude other vitamin D metabolites without initial purification and thus, overestimated the total 25(OH)D levels. Thus, to remove the effects of interfering vitamin D metabolites, liquid chromatography tandem mass spectroscopy (LC-MS) was applied for direct measurement of total 25(OH)D [7]. The LC-MS is considered the most accurate of all methodologies and is currently regarded as the gold standard for measurement. To ensure accurate measurement, we encourage endocrinologists to ensure that laboratory participates in an external quality program. The inactive 3-epimer form, which is synthesized in significant concentrations in infants, is removed through new methodologies prior to MS [8].
2.2. Measurement of DBP: monoclonal vs. polyclonal antibodies
Immunonephelometry with both monoclonal and polyclonal antibodies has been used to assay DBP. DBP is a multifunctional plasma protein with 3 major electrophoretic variants (Gc2, Gc1s, Gc1f) that differ by amino acid substitutions and extent of glycosylation. The majority of studies initially utilized the monoclonal antibody enzyme-linked immunosorbent assay. DBP levels measured by a commercial enzyme linked immunosorbent assay that uses two monoclonal antibodies in a sandwich may be different when compared to the methods using polyclonal antibodies. These differences may due to the monoclonal sandwich immunoassay recognizing only one epitope near the polymorphic region of the protein and having different affinities for the different variants [9]. In contrast, a polyclonal antibody can interact at multiple epitopes. For example, the observations that DBP concentrations measured using the monoclonal assay were markedly lower in black compared to white participants. Whereas, a recent publication reported that DBP levels did not vary with race when the polyclonal assay was utilized [10]. In 2015, Hoofnagle et al. [11] and Nielson et al. [12] developed an alternative methodology, LC-MS, in which plasma proteins are proteolytically cleaved into peptides that can be more accurately detected and quantified. The concentrations determined by tandem mass spectrometry mirrored those obtained with the polyclonal immunoassay [11, 12]. There is a lack of standardization of measuring VDBP and as a result, it is difficult to compare the different assays and studies.
2.3. Free 25(OH)D: calculation vs. direct measurement
Free 25(OH)D levels can be directly measured by centrifugal ultrafiltration and a newer commercially available enzyme-linked immunosorbent assay. Centrifugal ultrafiltration is the gold standard but not commonly utilized, as it is technically difficult, expensive, and requires a very sensitive assay methodology [13]. Utilizing the ELISA assay, a direct 2-step process, free 25(OH)D was captured by an antibody during a first incubation and after washing, a biotin-labeled 25(OH)D analog is allowed to react with the unoccupied antibody binding sites. After incubation with a streptavidin-peroxidase conjugate, bound enzyme is quantitated colorimetrically. The level of free 25-vitamin D is inversely proportional to the resultant signal measured [14]. Comparison of accuracy between the two mechanisms is limited as there are multiple affecting the results and methodologies. The choice of which assay/laboratory to utilize should be determined by availability of required equipment, technical expertise, as well as ensuring that the laboratory provides adequate quality control mechanisms ensuring accuracy [13].
Free plasma 25(OH)D can also be determined by calculation through two different methods. One method involves the knowledge of total 25(OH)D, albumin, and DBP. The second method utilizes knowledge of bioavailable 25(OH)D, defined as the fraction of hormone that is both free and albumin-bound. Free, bioavailable, and DBP-bound 25(OH)D are calculated using equations that have been adopted and validated from equations utilized for the calculation for free and total testosterone. Both methodologies utilize the same affinity binding constants and produced calculated free 25(OH)D levels that are highly correlated. For example, Bikle et al. calculated free 25(OH)D levels are determined using the following sample equation: [(Free fraction) 1/F = 1 + Ka dbp[DBP]f + Ka alb[Alb]f, with DBP affinity constant (Ka) for 25(OH)D= 7 x 108 M−1, Albumin (Ka) for 25(OH)D = 6 x 105 M−1, and F* total 25(OH)D = free 25(OH)D.] The equations to estimate free 25(OH)D rely on the albumin concentration, albumin-binding affinity for 25(OH)D, DBP, and total 25(OH)D concentration. Inaccuracies in measurement of any of these parameters can affect the results of free 25(OH)D, and indeed those measurements have varied especially for DBP but also for the assumption of constancy for the DBP affinity constant for the different DBP alleles and different physiologic and pathologic conditions [13-15].
Recent studies have demonstrated that calculated free 25(OH)D overestimates measured free 25(OH)D levels. Shiel et al reported that change in serum intact PTH is more strongly associated with change in measured free 25(OH)D than total 25(OH)D, which demonstrates that measured free 25(OH)D is an accurate surrogate of vitamin D activity [16].
3.0. Genetics, Medications and Smoking
3.1. Genetic
Gene sequencing has uncovered many variations in the DBP gene. The genetic effects on DBP and 25(OH)D are complicated and are still evolving at time of this review. Over 120 variants of DBP have been found and of these, 3 main phenotypic variants have been described-Gc1F, Gc2, and Gc1S. The variants have different characteristics that can alter 25(OH)D levels. Genetic variants are associated with different serum concentrations and affinity for 25(OH)D [17], and likely have effects on binding to 25(OH)D [18].
In regards to race, allelic differences were reported to be correlated with different DBP concentrations in the white population but were not shown to be correlated in the black population[9]. Powe et al confirmed this finding by conducting a randomized, placebo controlled trial in which it was shown that variations in race altered DBP levels and no change in DBP occurred with replacement of vitamin D. In addition, the black subjects were found to have lower DBP levels than non-black subjects, resulting in similar concentrations of estimated bioavailable 25(OH)D [19]. However, this result has been recently refuted in that differences between black and white populations, when measured by a polyclonal assay rather than a monoclonal assay disappeared, [11][12] as described earlier. Aloia et al also found that black Americans had low levels of total 25(OH)D and DBP, but the free 25(OH)D was not different when directly measurement [20]. However, a recent report indicates that free 25(OH)D levels are lower in black men when compared to white men, when measured directly or calculated from DBP values based on an assay using polyclonal antibodies [12].
The effects of DBP phenotype and sex on different measures of serum 25(OH)D were evaluated in 472 subjects in a recent randomized, placebo controlled trial. Results of this study showed that serum total 25(OH)D and DBP concentrations were lower in subjects with the Gc2/Gc2 phenotype compared to phenotypes with the Gc1S allele, and lower in males than in females. However, the differences with DBP phenotype and sex were reduced when directly measured free 25(OH)D was determined. Thus, the effects of vitamin D supplementation were not dependent on DBP phenotype or sex [21]. As DBP phenotypes affect both DBP levels and affinity of DBP, we suggest that the measured free 25(OH)D should be considered when comparing vitamin D status among races.
3.2. Medications
Tenofovir: DBP, intact PTH, total 25(OH)D and skeletal markers were measured in individuals with HIV treated with tenofovir, lamivudine and efavirenz. Serum samples from 134 subjects were collected at the beginning of the study and 24 and 48 weeks after initiation of treatment. It was found that DBP and iPTH levels were increased and 25(OH)D levels remained unchanged. It was concluded that antiretroviral therapy possibly causes elevation of DBP and thus lowers calculated bioavailable 25(OH)D, resulting in an increase in iPTH [22].
Oral contraceptive: Use of hormone contraceptives (HC) may have effects on 25(OH)D levels due to an increase in DBP. Recent study in 2016 further showed increases in 25(OH) D in women using estrogen containing HC [23]. In a study by Moller et al. in 2013, 25(OH)D levels and DBP were measured in 75 Caucasian women: 23 that did not use HC and 52 that did use HC. Results from all 75 subjects showed that 25(OH)D was significantly higher and positively correlated with DBP concentrations [24]. Wilson et al. in a study in 2015 also found that DBP concentrations were increased by HC use [25].
Aspirin: Li et al investigated DBP levels in 18 patients before and after aspirin treatment of cerebral thrombotic events via Western blot. Their study demonstrated DBP levels significantly increased post-aspirin treatment (114.04 ± 16.69) relative to pre-treatment (66.33 ± 5.61), whereas actin showed the opposite trend (p < 0.01 for both comparisons). Further investigation is needed to determine if changes in DBP affect total 25(OH)D levels [26].
3.3. Smoking
A proteomic approach was used to identify differentially expressed proteins in plasma samples from healthy cigarette smokers and healthy nonsmokers. Plasma DBP was found to have been down-regulated and DBP levels were lower in smokers compared to those of nonsmokers. The authors concluded that smoking influences the profile of the human plasma proteome but did not speculate on the possible specific role of DBP as a biomarker of smoking related disease [27].
4.0. Hormones affecting DBP:
4.1. Sex hormones and DBP
DBP is up regulated by estrogen; lower levels of estrogen lead to lower levels of DBP. In a 2015 study by Pop et al., estradiol, 25(OH)D, DBP, iPTH and albumin were measured in 165 females from ages 26 to 75 years. Pre-menopausal women had a higher serum DBP, estradiol, and 25(OH)D levels than postmenopausal women. The calculated free 25(OH)D was also lower than that of control subjects, but to a much lesser degree than total 25(OH)D [28].
Pirani et al. studied the in vitro effects of estradiol on the receptor-mediated endocytosis of DBP. Estradiol treatment stimulated the uptake of labeled DBP by hepatocytes isolated from the female animals but not by cells from male animals. This results suggests that the estradiol effect is dependent on the presence of estrogen receptors [29].
During pregnancy, estrogen levels rise and DBP levels are increased. Several studies had high concentrations of DBP and 25(OH)D during pregnancy, but calculated free remained relatively stable [30, 31]. On the other hand, Bikle et al. [32] found that both the total and calculated free concentrations of 1,25 (OH)2D increased during pregnancy despite the increase in DBP. In 2016, DBP levels were measured with respect with estradiol fluctuations that occur during the menstrual cycle. Franasiak et al. collected data from infertile women undergoing in vitro fertilization (IVF). Interestingly, they found that DBP did not vary over early, mid and late follicular phases when estrogen was at baseline and at peak. [33]. Females were found to have 10% higher DBP levels than men in a 2007 study that evaluated DBP in healthy subjects [34]. In 2011, Blanton et al. showed that the patients with type 1 diabetes mellitus and the healthy male subjects had low DBP compared to healthy females [35].
4.2. Growth hormone
Treatment with growth hormone (GH) has been shown to increase VDBP levels. Brixen et al. demonstrated that short-term use of recombinant GH in normal men initially increased serum DBP and IGF-1 levels[36]. Altinova et al demonstrated that acromegaly patients had elevated serum DBP levels, normal total 25(OH)D but lower calculated free 25(OH)D compared with normal controlled subjects [37].
4.3. Parathyroid hormone
Low total 25(OH)D levels are common in patients with primary hyperparathyroidism (PHPT), but the mechanism is not fully understood. In 2013, Wang et al reported that levels of 25(OH)D, albumin, and DBP were lower in postmenopausal women with PHPT [38]. In their 2016 study, they demonstrated that total 25(OH)D and DBP were 20% lower in PHPT patients compared with control subjects but the calculated free 25(OH)D were no different from those of the control subjects [39]. These findings were confirmed by a study in 2016 by Battista et al., which showed that PHPT patients had low DBP [40]. The findings support the concept that the low DBP levels contribute to the low total 25(OH)D levels but found a lower calculated 25(OH)D in patients with PHPT.
4.4. Insulin, insulin resistance and obesity
Vitamin D is commonly deficient in patients with obesity. Compared to healthy controls, total and measured 25(OH)D levels were found to be lower in patients with obesity but did not correlate with DBP levels [41]. Walsh et al. found that DBP, total 25(OH)D and measured free 25(OH)D were lower in obese and overweight subjects but did not correlate with bone density and bone turnover [42]. A cross-sectional study was performed in 63 obese children and 21 lean controls. Children with insulin resistance showed higher levels of calculated bioavailable 25(OH)D levels compared to non-insulin resistant children, but total 25(OH)D levels were similar between obese and control populations. The study also showed an inverse correlation between insulin resistance and DBP levels. It was concluded that it is likely that insulin resistance causes lower DBP and thus changes in the free, active form of 25(OH)D [43].
Out of concern for low bone mass, Botella-Carretero investigated bioavailable 25(OH)D in women after bariatric surgery. 91 women were followed for a mean of 7 years after surgery. No significant changes were noted in DBP levels in the cohort of women [44].
5.0. Chronic Conditions affecting DBP:
5.1. End stage liver disease
Low 25(OH)D levels are a common finding in patients with end-stage liver disease (ESLD). A study of 82 patients with cirrhosis demonstrated that those with low albumin had low DBP with measured free 25(OH)D levels remaining normal [45]. Not only are low levels of DBP found in patient’s with ESLD, but it had increased after liver transplant [46]. Patients with ESLD are known to have bone disease that is not related to hyperparathyroidism. On bone biopsy, pathology is more consistent with osteoporosis than osteomalacia. Moreover, vitamin D supplementation does not reverse the bone disease [47, 48]. 25(OH)D, calcium and PTH levels, and overall bone health were assessed in 158 patients with ESLD. Regardless of vitamin D status, bone disease was present in 64.6% of patients. The author proposed that calculated free 25(OH)D and DBP levels are better indicators of vitamin D deficiency that total 25(OH)D to confirm true vitamin D deficiency in ESLD [49].
In an earlier study, Bikle et al found that patients with liver disease had reduced levels of albumin, DBP and total 25(OH)D compared to normal subjects. However, the levels of calculated free 25(OH)D were equivalent in the patients and normal subjects[50].
5.2. Renal disease
Nephrotic syndrome is defined by proteinuria. In patients affected by nephrotic syndrome, DBP is excreted at higher rates in the urine. A study compared 14 children with idiopathic nephrotic syndrome and 10 healthy and age matched controls, the authors found that DBP levels were below normal range and 10 of 14 patients had 25(OH)D levels below 7 ng/ml. They also found a negative correlation between serum DBP and urinary protein excretion [51]. It was proposed that DBP loss in urine contributes to the low 25(OH)D levels found in nephrotic syndrome. However, a recent study found that serum DBP levels were lower but not significant when compared with controls (P>0.05). This suggests that the loss of DBP in urine may not alter serum changes in DBP [52]. Possible causes of the discrepancy between the studies may be related to other factors such as duration of renal disease, extent of renal disease, and level of vitamin D stores [53]. Aggarwal et al found that calculated bioavailable 25(OH)D level were reduced and correlated with bone density and bone marker in patient with nephrotic syndrome [52].
Another study showed urinary DBP loss occurs in proteinuria and improves with treatment of proteinuria; however, the loss of protein was not associated with changes in 25(OH)D [54]. Furthermore, urinary DBP loss was evaluated in patients with steroid-resistant and steroid-sensitive nephrotic syndromes. Subjects with steroid resistant nephrotic syndrome had significantly higher urinary DBP. This study highlighted a noninvasive method to detect response to treatment in nephrotic syndrome [55]. Concentration of DBP in the urine was found to be correlated with the degree of proteinuria in patients with chronic kidney disease and undergoing hemodialysis, and healthy subjects [5]. One group of researchers has used urinary DBP as a measure of diabetic nephropathy. The study findings considered urinary DBP to be a novel biochemical marker for early diabetic nephropathy [56].
5.3. Critical illness
Vitamin D modulates receptors of immune cells as well as immune cascades. Studies have shown that 25(OH)D regulates immune responses of the pro-inflammatory bacterial endotoxin in rodent models of sepsis. Serum DBP falls during systemic inflammatory response and theoretically is related to a fall in 25(OH)D and was discovered in a study performed on patients undergoing orthopedic surgery who were found to have elevated acute phase reactants [57]. A study performed on intensive care unit patients showed that direct bactericidal activity was positively associated with 25(OH)D concentrations, and DBP was decreased in patients with sepsis in comparison to subjects without sepsis [58]. Madden et al enrolled 511 children in pediatric intensive care units and found that levels of DBP and total 25(OH)D were lower than those reported in healthy children [59]. The lower DBP levels increased bioavailability of 25(OH)D and the calculated bioavailable 25(OH)D levels were also inversely associated with illness severity. We suggest that total 25(OH)D might not be a reliable indicator in the critical care situation, but the ability of 25(OH)D bound to DBP to influence immune responses might be a better marker of benefit.
5.4. Other chronic diseases
Vitamin D deficiency is a common finding in patients with cystic fibrosis (CF). 28 healthy adults, 25 clinically stable adults and children with CF, and 27 adults experiencing a CF exacerbation underwent testing for serum concentrations of 25(OH)D, free 25(OH)D, albumin, and DBP. It was noted that patients with CF had lower albumin and DBP levels compared to normal healthy subjects, which is likely attributed to nutritional challenges common to CF. Both calculated and measure free 25(OH)D levels were lower in CF patients [60].
DBP has been implicated in contributing to the pathogenesis of multiple sclerosis (MS). Vitamin D deficiency has been indicated as an environmental factor causing MS. In a 2014 study, serum DBP and albumin levels were measured in 28 subjects with MS and 24 healthy subjects. DBP levels were higher in subjects with MS than healthy controls. DBP levels were higher in subjects with MS than healthy controls, and as a result the authors found that estimated bioavailable 25(OH)D levels were lower in patients with MS compared to controls. [61]. However, a recent study in 2016 showed that there were no differences in DBP levels healthy controls and patients with MS and lower calculated 25(OH)D levels were found in MS patients [62]. It is clear that further studies are needed to delineate the levels of DBP in patients with MS.
DBP plays a role in the immune system as noted earlier. In 2014, Zhang et al. found that patients with aggressive gingival disease had higher plasma DBP concentrations but lower gingival fluid DBP concentrations than healthy controls. A correlation was made between decreased gingival fluid DBP levels and increased plasma DBP levels in patients with periodontitis [63]
Asthma has been linked to common variants of DBP. A study performed on 467 subjects confirmed that two common variants of DBP were correlated with asthma susceptibility [64]. Thus, DBP has potential for roles as a marker of diseases such as asthma and gingival disease as a risk factor for a disease such as MS, and as causal for vitamin D deficiency in CF.
5.5. Psychiatric disorder:
Total 25(OH)D levels are lower in chronic psychiatric patients suggesting possible correlation. Yee et al compared 31 first episode psychiatric (FEP) patients and 31 healthy controls in Singapore; they found that DBP levels is higher in FEP patients compared with health subjects, the calculated free 25(OH)D is lower compared with health control even the total 25(OH)D remained unchanged. The calculated free 25(OH)D, not total 25(OH)D correlated with negative symptoms in FEP patients [65]
6.0. Summary and Conclusion:
A complex relationship exists between DBP and total and free 25(OH)D as discussed above. In Table 1, we summarize the many endocrine hormones and disease and theirs effects on DBP, which then affect the interpretation of total plasma 25(OH)D levels. Further investigation is needed to better understand this intricate relationship. When evaluating patients with low 25(OH)D levels, we should now consider the possibility that there are factors affecting DBP, which may alter the interpretation of total plasma 25(OH)D levels. This possibility should encourage clinician to consider the various clinical situations (please see table 1) and may necessitate the need for measuring free 25(OH)D measurements or iPTH with corresponding calcium levels if free 25(OH)D is not available.
Table 1.
Summary of DBP and vitamin D metabolites levels in several clinical conditions.
| CONDITION | DBP | 25 (OH) D | FREE 25(OH)D | REFERENCES |
|---|---|---|---|---|
| Nephrotic Syndrome | Low | Low | Normal | Grymonprez (51)* Aggarwal (52)* |
| ESLD | Low | Low | High/Normal | Lai (45)** Reese (46)* Corey (49)** Bikle (50)* |
| Critical Illness | Low | Low | Low/Normal/High | Kempker (58)N/A Madden (59)* |
| PHPT | Low | Low | Low/Normal | Wang (38, 39)* Battista (40)* |
| Smoker | Low | Bortner (27)N/A | ||
| Menopausal | Low | Low | Low | Pop (28)* |
| Cystic Fibrosis | Low | Low | Low | Lee (60)* |
| Acromegaly | High | Normal | Low | Altinova (37)* |
| Pregnancy | High | High/ Normal | Normal | Zhang (30) * Park (31)* |
| OCP | High | High/ Normal | Normal | Moller (24)* Wilson (25)** Harmon (23)** |
| Psychosis | High | Normal | Low | Yee (65)* |
| MS | High/Normal | High | Low | Rinaldi (61)N/A Behrens (62)* |
| Obesity | Low/Normal | Low | Low/Normal | Holmlund-Suila (41)** Walsh (42)** Giudice (43)* |
Abbreviations: ESLD: end-stage liver disease, PHPT: primary hyperparathyroidism, OCP: Oral contraceptive pills, MS: multiple sclerosis. N/A: no free 25(OH)D
calculated free 25(OH)D
directly measured free 25(OH)D
ACKNOWLEGMENT
The authors are grateful to Sherry Wang BA, who assisted in data management and the design for figure 1, and Dr. Vincent A. Rifici, who assisted in preparing the manuscript.
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D
- DBP
Vitamin D binding protein
- PHPT
Primary hyperparathyroidism
- RIA
radioimmunoassay
- HPLC
high performance liquid chromatography
- LC-MS
liquid chromatography-mass spectrometry
- iPTH
intact PTH
REFERENCES:
- 1.Chun RF, Peercy BE, Orwoll ES, et al. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014; 144 Pt A: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357: 266–281. [DOI] [PubMed] [Google Scholar]
- 3.Yousefzadeh P, Shapses SA, Wang X. Vitamin D Binding Protein Impact on 25-Hydroxyvitamin D Levels under Different Physiologic and Pathologic Conditions. Int J Endocrinol. 2014; 2014: 981581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowling MJ, Kemmis CM, Taffany DA, Welsh J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr. 2006; 136: 2754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalousova M, Dusilova-Sulkova S, Zakiyanov O, et al. Vitamin D Binding Protein Is Not Involved in Vitamin D Deficiency in Patients with Chronic Kidney Disease. Biomed Res Int. 2015; 2015: 492365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol Metab. 2000; 11: 320–327. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008; 87: 1087s–91s. [DOI] [PubMed] [Google Scholar]
- 9.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013; 369: 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denburg MR, Hoofnagle AN, Sayed S, et al. Comparison of Two ELISA Methods and Mass Spectrometry for Measurement of Vitamin D-Binding Protein: Implications for the Assessment of Bioavailable Vitamin D Concentrations Across Genotypes. J Bone Miner Res. 2016; 31: 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoofnagle AN, Eckfeldt JH, Lutsey PL. Vitamin D-Binding Protein Concentrations Quantified by Mass Spectrometry. N Engl J Med. 2015; 373: 1480–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielson CM, Jones KS, Chun RF, et al. Free 25-Hydroxyvitamin D: Impact of Vitamin D Binding Protein Assays on Racial-Genotypic Associations. J Clin Endocrinol Metab. 2016;101: 2226–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bikle DD, Gee E, Halloran B, et al. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986; 63: 954–959. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz JB, Lai J, Lizaola B, et al. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014; 99:1631–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab. 1985; 61: 969–975. [DOI] [PubMed] [Google Scholar]
- 16.Shieh A, Chun RF, Ma C, et al. , Effects of High-Dose Vitamin D2 Versus D3 on Total and Free 25-Hydroxyvitamin D and Markers of Calcium Balance. J Clin Endocrinol Metab. 2016; 101: 3070–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhan I Vitamin d binding protein and bone health. Int J Endocrinol. 2014; 2014: 561214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnsen MS, Grimnes G, Figenschau Y, et al. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D. Scand J Clin Lab Invest. 2014; 74: 177–183. [DOI] [PubMed] [Google Scholar]
- 19.Ponda MP, McGee D, Breslow JL. Vitamin D-binding protein levels do not influence the effect of vitamin D repletion on serum PTH and calcium: data from a randomized, controlled trial. J Clin Endocrinol Metab. 2014; 99: 2494–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aloia J, Mikhail M, Dhaliwal R, et al. Free 25(OH)D and the Vitamin D Paradox in African Americans. J Clin Endocrinol Metab. 2015; 100: 3356–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sollid ST, Hutchinson MY, Berg V, et al. Effects of vitamin D binding protein phenotypes and vitamin D supplementation on serum total 25(OH)D and directly measured free 25(OH)D. Eur J Endocrinol. 2016;174: 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh E, Fraenkel L, Han Y, et al. Longitudinal Increase in Vitamin D Binding Protein Levels after Initiation of Tenofovir/Lamivudine/Efavirenz among Individuals with HIV. Aids. 2016; 30:1935–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harmon QE, Umbach DM, Baird DD. Use of Estrogen-Containing Contraception Is Associated With Increased Concentrations of 25-Hydroxy Vitamin D. J Clin Endocrinol Metab. 2016; 101: 3370–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moller UK., Streym S, Jensen LT, et al. Increased plasma concentrations of vitamin D metabolites and vitamin D binding protein in women using hormonal contraceptives: a cross-sectional study. Nutrients. 2013; 5: 3470–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson RT, Bortner JD Jr., Roff A, et al. Genetic and environmental influences on plasma vitamin D binding protein concentrations. Transl Res. 2015; 165: 667–676. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Gao J, Ma Y, et al. Vitamin D-Binding Protein Acts in the Actin Scavenge System and Can Have Increased Expression During Aspirin Therapy. Curr Neurovasc Res. 2016;13: 184–192. [DOI] [PubMed] [Google Scholar]
- 27.Bortner JD Jr., Richie JP Jr., Das A, et al. Proteomic profiling of human plasma by iTRAQ reveals down-regulation of ITI-HC3 and VDBP by cigarette smoking. J Proteome Res. 2011; 10: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 28.Pop CL, Shapses SA, Chang B, Sun W, Wang X. Vitamin D binding protein in healthy pre- and postmenopausal women: relationship with estradiol concentrations. Endocr. Pract. 2015;21: 936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pirani T, Chen J, Vieira A. Effects of estradiol on the endocytic transport of vitamin D carrier protein in hepatocytes. Biochim Biophys Acta. 2013; 1830: 3421–3426. [DOI] [PubMed] [Google Scholar]
- 30.Zhang JY, Lucey AJ, Horgan R, et al. Impact of pregnancy on vitamin D status: a longitudinal study. Br J Nutr. 2014; 112: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 31.Park H, Brannon PM, West AA, et al. Vitamin D Metabolism Varies among Women in Different Reproductive States Consuming the Same Intakes of Vitamin D and Related Nutrients. J Nutr, 2016;146:1537–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bikle DD, Gee E, Halloran B, Haddad JG. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J Clin Invest. 1984; 74:1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franasiak JM, Wang X, Molinaro TA, et al. Free vitamin D does not vary through the follicular phase of the menstrual cycle. Endocrine. 2016; 53: 322–326. [DOI] [PubMed] [Google Scholar]
- 34.Bolland MJ, Grey AB, Ames RW, et al. Age-, gender-, and weight-related effects on levels of 25-hydroxyvitamin D are not mediated by vitamin D binding protein. Clin Endocrinol (Oxf). 2007; 67: 259–264. [DOI] [PubMed] [Google Scholar]
- 35.Blanton D, Han Z, Bierschenk L, et al. Reduced serum vitamin D-binding protein levels are associated with type 1 diabetes. Diabetes. 2011; 60: 2566–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brixen K, Nielsen HK, Bouillon R et al. Effects of short-term growth hormone treatment on PTH, calcitriol, thyroid hormones, insulin and glucagon. Acta Endocrinol (Copenh). 1992; 127: 331–336. [DOI] [PubMed] [Google Scholar]
- 37.Altinova AE, Ozkan C, Akturk M, et al. Vitamin D-binding protein and free vitamin D concentrations in acromegaly. Endocrine. 2016; 52: 374–379. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Shapses SA, Wei S, et al. Vitamin D-binding protein levels in female patients with primary hyperparathyroidism. Endocr Pract. 2013;19: 609–613. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Shapses SA , Al-Hraishawi H. Free and Bioavailable 25-Hydroxyvitamin D Levels in Patients with Primary Hyperparathyroidism. Endocr Pract. 2016. Sep 28. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40.Battista C, Guarnieri V, Carnevale V, et al. Vitamin D status in primary hyperparathyroidism: effect of genetic background. Endocrine. 2016. May 6 [Epub ahead of print].. [DOI] [PubMed] [Google Scholar]
- 41.Holmlund-Suila E, Pekkinen M, Ivaska KK, et al. Obese young adults exhibit lower total and lower free serum 25-hydroxycholecalciferol in a randomized vitamin D intervention. Clin Endocrinol (Oxf). 2016;5:378–385. [DOI] [PubMed] [Google Scholar]
- 42.Walsh JS, Evans AL, Bowles S, et al. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am J Clin Nutr. 2016; 103:1465–1471. [DOI] [PubMed] [Google Scholar]
- 43.Miraglia del Giudice E, Grandone A, Cirillo G, et al. Bioavailable Vitamin D in Obese Children: The Role of Insulin Resistance. J Clin Endocrinol Metab. 2015; 100: 3949–3955. [DOI] [PubMed] [Google Scholar]
- 44.Botella-Carretero JI, Lafuente C, Montes-Nieto R, et al. Serum Bioavailable Vitamin D Concentrations and Bone Mineral Density in Women After Obesity Surgery. Obes Surg. 2016:26:2732–2737. [DOI] [PubMed] [Google Scholar]
- 45.Lai JC, Bikle DD, Lizaola B, et al. Total 25(OH) vitamin D, free 25(OH) vitamin D and markers of bone turnover in cirrhotics with and without synthetic dysfunction. Liver Int. 2015; 35: 2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reese PP, Bloom RD, Feldman HI, et al. Changes in vitamin D binding protein and vitamin D concentrations associated with liver transplantation. Liver Int. 2012; 32: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matloff DS, Kaplan MM, Neer RM, et al. , Osteoporosis in primary biliary cirrhosis: effects of 25-hydroxyvitamin D3 treatment. Gastroenterology. 1982; 83: 97–102. [PubMed] [Google Scholar]
- 48.Herlong HF, Recker RR, Maddrey WC. Bone disease in primary biliary cirrhosis: histologic features and response to 25-hydroxyvitamin D. Gastroenterology. 1982; 83: 103–108. [PubMed] [Google Scholar]
- 49.Corey RL, Whitaker MD, Crowell MD, et al. Vitamin D deficiency, parathyroid hormone levels, and bone disease among patients with end-stage liver disease and normal serum creatinine awaiting liver transplantation. Clin Transplant. 2014; 28: 579–584. [DOI] [PubMed] [Google Scholar]
- 50.Bikle DD, Halloran BP, Gee E, et al. Free 25-hydroxyvitamin D levels are normal in subjects with liver disease and reduced total 25-hydroxyvitamin D levels. J Clin Invest. 1986; 78: 748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grymonprez A, Proesmans W, Van Dyck M, et al. Vitamin D metabolites in childhood nephrotic syndrome. Pediatr Nephrol. 1995; 9: 278–281. [DOI] [PubMed] [Google Scholar]
- 52.Aggarwal A, Yadav AK, Ramachandran R, et al. Bioavailable vitamin D levels are reduced and correlate with bone mineral density and markers of mineral metabolism in adults with nephrotic syndrome. Nephrology (Carlton). 2016;21:483–489. [DOI] [PubMed] [Google Scholar]
- 53.Colston K, Williams NJ, Cleeve HJ. Studies on vitamin D binding protein in the nephrotic syndrome. Clin Chem. 1985; 31: 718–721. [PubMed] [Google Scholar]
- 54.Doorenbos CR, de Cuba MM, Vogt L, et al. Antiproteinuric treatment reduces urinary loss of vitamin D-binding protein but does not affect vitamin D status in patients with chronic kidney disease. J Steroid Biochem Mol Biol. 2012; 128: 56–61. [DOI] [PubMed] [Google Scholar]
- 55.Bennett MR, Pordal A, Haffner C, et al. Urinary Vitamin D-Binding Protein as a Biomarker of Steroid-Resistant Nephrotic Syndrome. Biomark Insights. 2016;11: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shoukry A, Bdeer Sel A, El-Sokkary RH. Urinary monocyte chemoattractant protein-1 and vitamin D-binding protein as biomarkers for early detection of diabetic nephropathy in type 2 diabetes mellitus. Mol Cell Biochem. 2015; 408: 25–35. [DOI] [PubMed] [Google Scholar]
- 57.Waldron JL, Ashby HL, Cornes MP, et al. Vitamin D: a negative acute phase reactant. J Clin Pathol. 2013; 66: 620–622. [DOI] [PubMed] [Google Scholar]
- 58.Kempker JA, Tangpricha V, Ziegler TR, Martin GS. Vitamin D in sepsis: from basic science to clinical impact. Crit Care. 2012; 16: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madden K, Feldman HA, Chun RF, et al. Critically Ill Children Have Low Vitamin D-Binding Protein, Influencing Bioavailability of Vitamin D. Ann Am Thorac Soc. 2015; 12: 1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee MJ, Kearns MD, Smith EM, et al. Free 25-Hydroxyvitamin D Concentrations in Cystic Fibrosis. Am J Med Sci. 2015; 350: 374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rinaldi AO, Sanseverino I, Purificato C, et al. Increased circulating levels of vitamin D binding protein in MS patients. Toxins. 2015; 7: 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Behrens JR, Rasche L, Giess RM, et al. Low 25-hydroxyvitamin D, but not the bioavailable fraction of 25-hydroxyvitamin D, is a risk factor for multiple sclerosis. Eur J Neurol. 2016; 23: 62–67. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Meng H, Xu L, et al. Vitamin d-binding protein levels in plasma and gingival crevicular fluid of patients with generalized aggressive periodontitis. Int J Endocrinol. 2014; 2014: 783575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang H, Chi X, Zhang X, Wang J. Increased serum VDBP as a risk predictor for steroid resistance in asthma patients. Respir Med. 2016; 114: 111–116. [DOI] [PubMed] [Google Scholar]
- 65.Yee JY, Chi X, Zhang X, Wang J. Association between serum levels of bioavailable vitamin D and negative symptoms in first-episode psychosis. Psychiatry Res. 2016; 243: 390–394. [DOI] [PubMed] [Google Scholar]