Abstract
p120-catenin (p120) is an important regulator in the function and stability of E-cadherin. However, the role of p120 in the epidermis is unclear. Previous studies have shown that globally knockout of p120 caused increased epidermal proliferation but little changes in epidermal differentiation and permeability. In the present study, we generated a conditional knockout mouse model and examined epidermal proliferation, differentiation and permeability. The results showed that conditional knockout of p120 in the epidermis caused not only increased epidermal proliferation but also decreased epidermal differentiation and increased permeability. These data suggest that p120 is required for suppressing epidermal proliferation, promoting epidermal differentiation and maintaining permeability barrier function of the epidermis.
Keywords: barrier function, differentiation, epidermis, p120-catenin, proliferation
1 |. INTRODUCTION
p120 is a cytoplasmic protein essential for the regulation of the cadherin complex. p120 consists of an N-terminal regulatory domain, a central domain and a C-terminal tail (Carnahan, Rokas, Gaucher, & Reynolds, 2010; Fukumoto, Shintani, Reynolds, Johnson, & Wheelock, 2008; Ishiyama et al., 2010; Reynolds, 2007). It is localized at adherens junctions, thereby playing roles in the maintenance of cell-cell adhesion and intercellular permeability of the epithelium (Gentil-dit-Maurin et al., 2010; Konstantoulaki, Kouklis, & Malik, 2003; Ozaki et al., 2010). Other roles of p120 include stabilizing cell-cell transmembrane cadherin molecules at the cell membrane (Davis, Ireton, & Reynolds, 2003; Ireton et al., 2002; Ishiyama et al., 2010; Peifer & Yap, 2003; Reynolds, 2007; Xiao, Oas, Chiasson, & Kowalczyk, 2007), regulating the activity on actin dynamics associated with barrier function, lamellipodia formation, and cell migration through modulation of the activities of small GTPases RhoA, Rac, and Cdc42 (Anastasiadis et al., 2000; Grosheva, Shtutman, Elbaum, & Bershadsky, 2001; Hatanaka, Simons, & Murakami, 2011; Johnson et al., 2010; Reynolds & Roczniak-Ferguson, 2004; Yanagisawa et al., 2008) or by binding to cortactin (Boguslavsky et al., 2007) and transcriptional repressor Kaiso (Daniel & Reynolds, 1999; Park et al., 2006), regulating cell proliferation (Bantis et al., 2004; Jiang et al., 2012; Liu et al., 2009; Perez-Moreno et al., 2006) and probably differentiation (Lee, Ji, Furuta, Park, & McCrea, 2014). Decreased expression of p120 or E-cadherin has been shown in epithelial cancers (Jiang, Liao, Shrestha, Ji, et al., 2015; Jiang, Liao, Shrestha, Li, et al., 2015; Li et al., 2012; Yuan et al., 2012; Xu et al., 2013). Previous studies have shown that newborn mice globally lacking p120 had increased epidermal proliferation but normal differentiation and permeability. In the present study, we generated a p120 conditional knockout (p120cKO) mouse model in a spatial and temporal fashion. In this model, p120 deletion specifically in keratinocytes was induced by tamoxifen administration at the age of 28 weeks. This model allowed us to examine the role of p120 in the epidermal proliferation, differentiation, and permeability during the adult stage.
2 |. MATERIALS AND METHODS
2.1 |. Generation of p120cKO mouse model
In this study, we used mice in which the p120 gene was deleted specifically in their keratinocytes, with the deletion being initiated by tamoxifen administration. Mice were designated as p120-floxed/ Cre-recombinase (Cre) mice which were produced by breeding mice with floxed exons 3-8 of p120 (designated p120-floxed mice, a gift from Dr. Albert Reynolds) to keratin 14 (K14)-Cre-ERT2 mice (designated K14-Cre mice, a gift from Dr. Pierre Chambon) expressing tamoxifen-regulated Cre targeted to keratinocytes using the K14 promoter in the San Francisco VA Medical Center. Because Cre in K14-Cre mice is under the control of the K14 promoter that is specifically active in the squamous epithelial basal layer and the activity of Cre is induced by tamoxifen, the p120-floxed gene segment was excised through Cre-mediated recombination only in the squamous epithelia basal layer of tamoxifen-induced p120-floxed/Cre mice (Perez-Moreno et al., 2006). p120-floxed mice lacking the Cre were used as negative controls. All mice were received tamoxifen. The study was approved by the Institutional Animal Care and Use Committee of the San Francisco VA Medical Center, USA.
2.2 |. Quantitative real-time PCR
Mice were sacrificed, the skin immediately removed, and the total RNAs were extracted from the tissue using RNA-STAT 60 (Tel-Test, Inc., Friendwood, TX). Extracted RNAs were quantified by spectrophotometer and stored at −80°C. One microgram of RNA was reverse transcribed using random hexamers with the TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA). The amount of cDNA was then quantified by quantitative real time PCR, performed on a PE Biosystems model 7900 HT sequence detector. The PCR amplification was done using SYBR Green Universal PCR Master Mix (Applied Biosystems) and specific primers. Results of mRNA levels were normalized to mitochondrial ribosomal protein L19.
2.3 |. Cell Lysate preparation and western analysis
Cells were washed twice with phosphate-buffered saline (PBS) and then incubated in PBS containing 1% NP-40 and the complete protease inhibitors (Roche Diagnostics, Indianapolis, IN) or radio-immunoprecipitation assay (RIPA) lysis buffer containing 50 mM HEPES, pH 7.4, 1% Triton X-100, 0.1% SDS, 150 mM NaCl, 1 mM EDTA, and the complete protease inhibitors (Roche Diagnostics) for 5 min. Cells were scraped into microcentrifuge tubes, incubated at 4 °C for 30 min, and pelleted by centrifugation. The supernatant was collected. Equal amounts of protein were electrophoresed through reducing SDS-PAGE and electroblotted onto polyvinylidene fluoride microporous membranes (Immobilon-P, 0.45 μM, Merk Millipore, Billerica, MA). After incubation in blocking buffer (100 mM Tris base, 150 mM NaCl, 5% nonfat milk, and 0.5% Tween 20), the blot was incubated overnight at 4°C with specific primary antibodies against p120 (Santa Cruz Biotechnology, Santa Cruz, CA, Cat# sc-13957, RRID:AB_2086386), keratin 1 (K1) (Covance Research Products Inc, Princeton, NJ, Cat# PRB-165P-100, RRID:AB_291583), keratin 6 (K6) (Santa Cruz Biotechnology Cat# sc-166074, RRID:AB_2134713), K14 (Covance Research Products Inc Cat# PRB-155P, RRID:AB_292096), involucrin (Covance Research Products Inc Cat# PRB-140C-200, RRID:AB_291569), loricrin (Covance Research Products Inc Cat# PRB-145P-100, RRID:AB_10064155), filaggrin (Covance Research Products Inc Cat# PRB-417P-100, RRID:AB_10064149) and proliferating cell nuclear antigen (PCNA) (Santa Cruz Biotechnology Cat# sc-7907, RRID:AB_2160375). After washes in the blocking buffer, the membranes were incubated for 1 hr with the appropriate anti-IgG secondary antibody conjugated to horseradish peroxidase (GE Healthcare Life Sciences, Pittsburgh, PA) in the blocking buffer. After a second series of washes, bound antibody complexes were visualized using the SuperSignal ULTRA chemiluminescent kit (Thermo Fisher Scientific Inc., Waltham, MA) and subsequent exposure to X-ray films.
2.4 |. Barrier function assays
The integrity of the permeability barrier in p120cKO mice was assessed by transepidermal water loss (TEWL) recovery rate (du Plessis et al., 2013; Rogiers & Group, 2001). The probe was kept in contact with the skin for four to five times in order to obtain a stable TEWL value. Twenty-two p120-floxed mice and twenty-four p120cKO mice with four to five measurements in each were used for testing permeability barrier function. Measurements of TEWL were taken immediately after the epidermal barrier is disrupted by cellophane tape stripping and repeated at 2 hr later to evaluate recovery rate. The TEWL recovery rate was calculated using the following formula: ([TEWL immediately after stripping-TEWL at indicated time]/TEWL immediately after stripping) *100%. All data were compared with data from simultaneously studied controls.
2.5 |. Immunohistochemistry
Skins were fixed overnight with buffered 4% PFA at 4 °C and embedded in paraffin. Skin sections (10 μm) were stained with hematoxylin and eosin(H&E) or processed for immunohistochemistry. Slides were blocked with PBS, 0.3% Triton X-100, 1% BSA, 5% normal goat serum. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide in PBS. Nonspecific binding was blocked with 10 mM Tris buffer (pH 7.6) containing 4% bovine serum albumin, 0.5% fish gelatin, 0.1% Tween-20, and 500 mM NaCl. The sections were then incubated with specific antibodies against p120, K1, K6, K14, involucrin, loricrin, filaggrin and PCNA. The binding of the antibody was detected with biotinylated donkey anti-goat IgG (Vector labs, Burlingame, CA), followed by ABC-peroxidase (Vector labs) reagent. Peroxidase activity was revealed with diaminobenzidine substrate (Vector Labs) followed by counterstaining with hematoxylin. The immunochemical staining was carried out at The Second Xiangya Hospital of Central South University in China.
3 |. RESULTS
3.1 |. Conditional knockout of p120in keratinocytes
We used 4-month-old mice in which p120 was deleted specifically in their keratinocytes, with the ablation being initiated by tamoxifen administration. Tamoxifen (1 mg per mouse) was injected intraperitoneally to 4-week-old mice three times per week to induce deletion of p120 from p120-floxed/Cre mice. After tamoxifen injection, a nearly complete loss of p120 in epidermal keratinocytes was determined by immunostaining, PCR and western analysis (Figure 1a–1c). DNA was isolated from the epidermis, dermis, tongue mucosa, tongue muscle, heart, lung, stomach, liver, spleen and kidney and analyzed by PCR to verify the specificity of p120 deletion in the squamous epithelium. The results showed that the excised band was present only in the tongue, epidermis and hair follicles but not in other tissues, indicating that p120 is specifically deleted in keratinocytes (Figure 1d).
FIGURE 1.
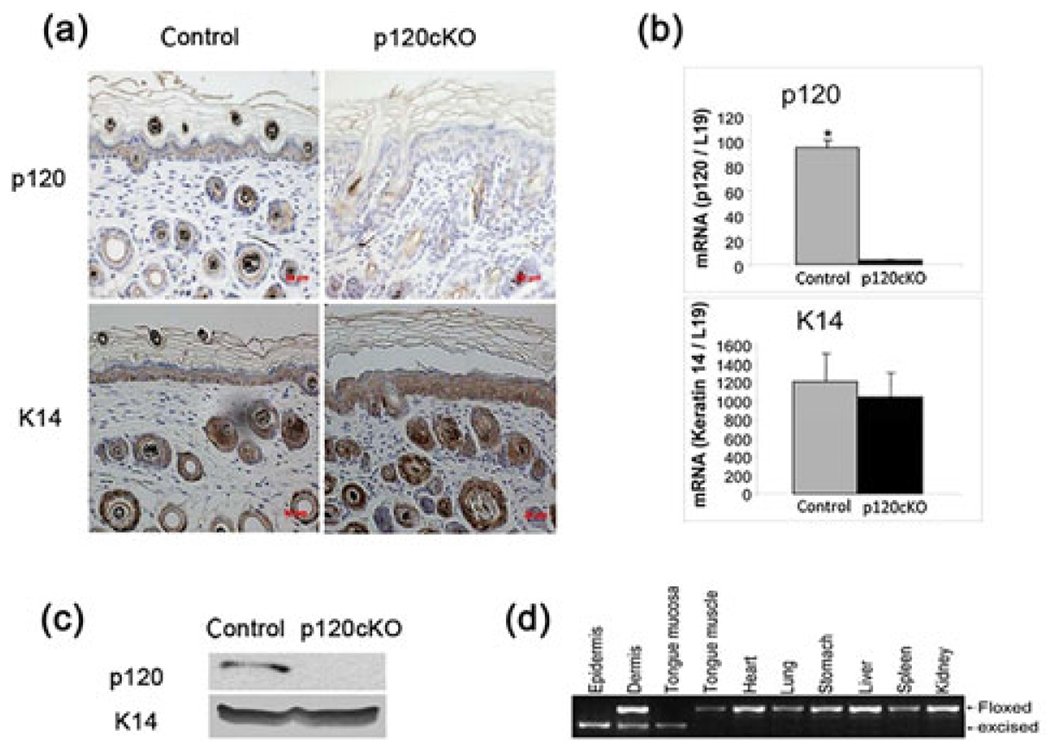
Conditional deletion of p120 in keratinocytes of p120cKO mice. Four-month-old p120cKO mice, with the deletion in keratinocytes being initiated by tamoxifen administration at four-week-old, were examined while p120-floxed littermates lacking the Cre were used as controls. After tamoxifen injection, sections of epidermal tissue from both groups were processed for H&E histochemistry and immunohistochemical staining with antibodies against p120 or K14. PCR and western analysis of p120 in keratinocytes were also performed. (a) A representative field of epidermal sections immunostained with p120 and K14 antibodies. (b and c) Quantitation of p120 mRNA and protein isolated from the epidermis. (d) The specificity of p120 deletion verified by PCR analysis of the epidermis and other tissues. *p < 0.05. Error bars: mean ± SD
3.2 |. Conditional knockout of p120 stimulated epidermal proliferation
To confirm the previous findings showing that p120 is required for epidermal proliferation, we examined the expression levels of K6 and PCNA in the epidermis of p120cKO mice by immunohistochemistry, PCR and western analysis. The results showed that the expression of K6 and PCNA in the skin of p120cKO mice was significantly higher than that in floxed control mice (Figures 2a and 2c), while K6 was barely detected in the epidermis and epidermal keratinocytes isolated from floxed control mice. In addition, the mRNA level of K6 in p120cKO mice was also increased compared to floxed mice (Figure 2b). These results indicate that p120 suppresses epidermal proliferation.
FIGURE 2.
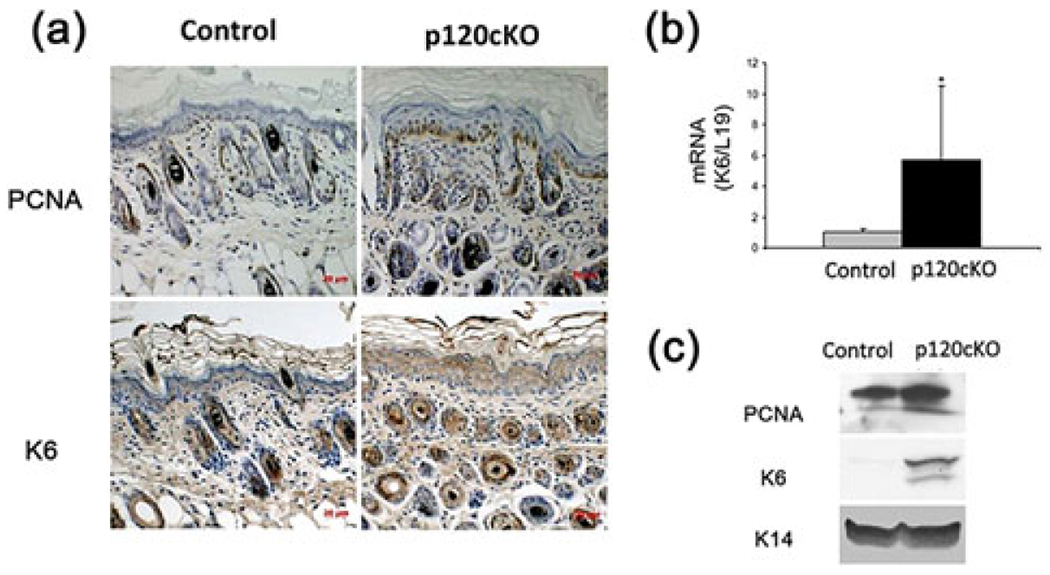
Conditional knockout of p120 stimulated proliferation of the skin. The epidermal tissue was removed from mice described in Figure 1 and sections of paraffin-embedded epidermis from two groups of mice were processed for H&E histochemistry and immunohistochemical staining with antibody for PCNA and K6, markers of cell proliferation and inflammation, respectively. Total cell lysates isolated from the mouse epidermis were used to determine mRNA and protein levels of PCNA and K6. (a) The figures show the representative sections of epidermis immunostained with PCNA and K6 antibodies. (b) Quantitation of the K6 mRNA levels in cells was shown in the bar graph. (c) The protein levels of PCNA, K6 and K14 were determined by western analysis. *p < 0.05. Error bars: mean ± SD
3.3 |. Conditional knockout of p120 suppressed epidermal differentiation
To determine the role of p120 in epidermal differentiation, the levels of epidermal differentiation markers including K1, involucrin, filaggrin, and loricrin in p120cKO mice were measured by immunohistochemistry and western analysis. The results showed that protein levels of these markers in p120cKO mice were markedly reduced compared to floxed mice (Figures 3a and 3b). These results indicate that p120 is required for epidermal differentiation.
FIGURE 3.
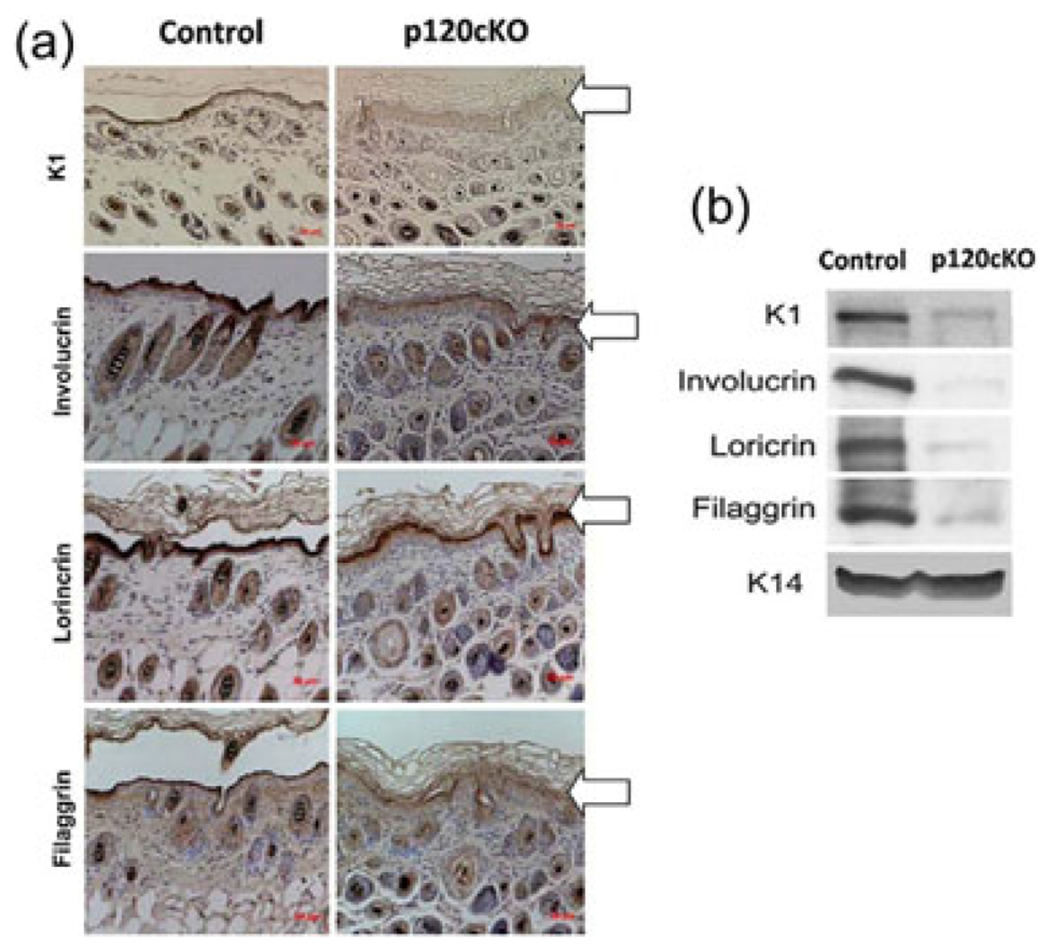
Conditional knockout of p120 suppressed epidermal differentiation. The skin was removed from the mice and sections of paraffin-embedded epidermis were processed for H&E and immunohistochemical staining. The antibodies used in the immunochemical staining included K1, involucrin, loricrin, and filaggrin. (a) The figure shows a representative field of epidermis indicated by arrows immunostained brown with different antibodies against K1, involucrin, loricrin, or filaggrin and counterstained blue with hematoxylin. (b) Total cell lysates were isolated from the epidermis of mice. The protein levels of K1, involucrin, loricrin, and filaggrin in the lysates were determined by western analysis. K14 was used as a loading control
3.4 |. Conditional knockout of p120 impaired permeability barrier function
To examine the role of p120 in permeability barrier function, TEWL on the back skin of p120cKO mice was measured. This method is non-invasive and allows us to determine the rate of barrier recovery following tape stripping to disrupt the barrier. Two hours after repeated tape stripping, a significant delay in permeability barrier recovery rates was demonstrated in lesional skin of p120cKO mice compared to the control group (Figure 4). These data suggest that p120 is required for the initial but not the latter response to epidermal barrier disruption.
FIGURE 4.
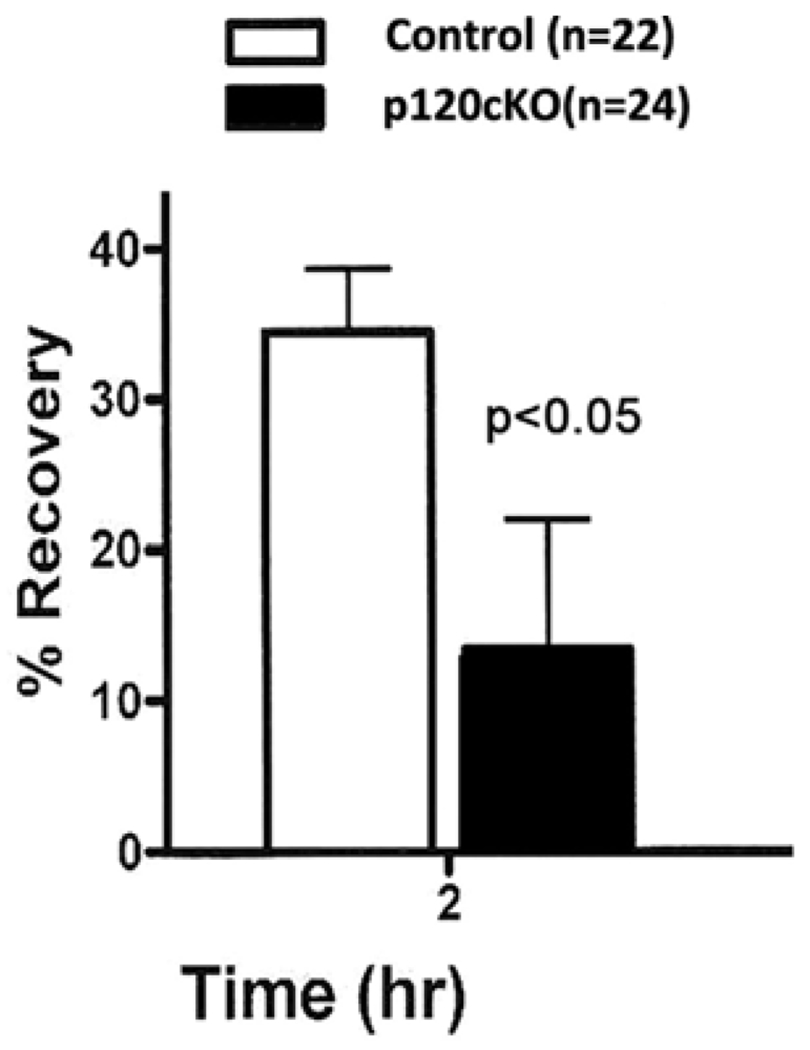
Conditional knockout of p120 impaired the permeability barrier function. The integrity of the epidermal permeability barrier was assessed by TEWL recovery rate, as shown in the bar graph. Measurements of TEWL for each section was obtained after being disrupted by cellophane tape stripping and repeated at 2 hr later to evaluate recovery rate. In each mouse, the TEWL recovery rate was calculated using the following formula: ([TEWL immediately after stripping-TEWL at indicated time]/TEWL immediately after stripping) *100%. *p < 0.05. Error bars: mean ±SD
4 |. DISCUSSION
Previous studies by Perez-Moreno et al. (2006) using global p120 knockout mice in which p120 was deleted in the embryonic stage showed that the adult mice were markedly smaller than their wild-type littermates. The newborn p120cKO mice had reduced intercellular adherens junction components but no discernible defect in epidermal differentiation and barrier function or intercellular adhesion. The adult p120cKO mice displayed notable epidermal hyperplasia and chronic inflammation. However, the differentiation in adult mice was not examined in their studies. In the present study, we used a tamoxifen-induced conditional p120 knockout model, which allowed us to examine epidermal proliferation, differentiation and barrier function during the adult stage in these mice. Four-month-old p120cKO mice displayed increased proliferation, decreased differentiation and impaired barrier function in the epidermis, suggesting that p120 is required for suppressing proliferation, promoting differentiation and barrier function in the epidermis. Previous studies have indicated that p120 suppresses epidermal proliferation and inflammation via inhibiting the NFkB pathway (Perez-Moreno et al., 2006). However, the mechanism by which p120 mediates epidermal differentiation is unclear. Further studies are required to determine the mechanism.
The major function of the skin is to serve as a protective barrier preventing the loss of fluids from the body. This skin barrier resides in the stratum corneum layer of the epidermis, which is composed of two components, protein-enriched nonviable corneocytes and lipid-enriched intercellular membranes. The epidermal permeability barrier plays a crucial role in human physical, chemical and biological cutaneous functions. An increased TEWL is considered as one of the indicators of impaired barrier function. In the present studies, barrier disruption was achieved by repeated applications of cellophane tape on mice back skin. The data showed that p120cKO mice displayed delayed repair of the epidermal barrier initially after disruption, suggesting that p120 is acting as impetus to repair disrupted permeability barrier. Given that p120 is a stabilizer for the E-cadherin complex (Ishiyama & Ikura, 2012) and is involved in calcium induced keratinocyte differentiation as previously reported (Xie & Bikle, 2007). It is reasonably probable that p120 promotes epidermal differentiation and barrier function via the E-cadherin complex. On the other hand, p120 suppresses epidermal proliferation likely via inhibiting the NFkB pathway according to the studies by Perez-Moreno et al. (2006).
The results from the present studies are discrepant from the ones reported by Perez-Moreno et al. regarding the epidermal differentiation and barrier function. Perez-Moreno et al. showed that loss of p120 in the epidermis displayed epidermal hyperplasia and chronic inflammation observed in adult mice but had no effect on epidermal differentiation and barrier function observed in newborn mice (Perez-Moreno et al., 2006). A possible explanation for these discrepant findings might be that we used adult mice in our study. In contrast to the newborn mice that used in their study there is a definitely delayed impact on the epidermal differentiation and barrier function by p120.
Funding information
National Natural Science Foundation of China, Grant numbers: 81072219, 81272973, 81471055, 81672646; National institutes of Health, Grant numbers: 1R03DE018001, 1R21DE019529-01A2, R01AR050023; Department of Veterans Affairs, Grant number: Bx001066
Abbreviations:
- p120
p120 catenin
- p120cKO
p120 conditional knockout
- Cre
Cre-recombinase
- K14
keratin 14
- PBS
phosphate-buffered saline
- RIPA
radio-immunoprecipitation assay
- K1
keratin 1
- K6
keratin 6
- PCNA
proliferating cell nuclear antigen
- TEWL
transepidermal water loss
- H&E
hematoxylin and eosin
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to this work
REFERENCES
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, & Reynolds AB (2000). Inhibition of RhoA by p120 catenin. Nature Cell Biology, 2(9), 637–644. [DOI] [PubMed] [Google Scholar]
- Bantis A, Giannopoulos A, Gonidi M, Liossi A, Aggelonidou E, Petrakakou E, … Athanassiadou P (2004). Expression of p120, Ki-67 and PCNA as proliferation biomarkers in imprint smears of prostate carcinoma and their prognostic value. Cytopathology, 15(1), 25–31. [DOI] [PubMed] [Google Scholar]
- Boguslavsky S, Grosheva I, Landau E, Shtutman M, Cohen M, Arnold K, … Bershadsky A (2007). P120 catenin regulates lamellipodial dynamics and cell adhesion in cooperation with cortactin. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 10882–10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan RH, Rokas A, Gaucher EA, & Reynolds AB (2010). The molecular evolution of the p120-catenin subfamily and its functional associations. PLoS ONE, 5(12), e15747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, & Reynolds AB (1999). The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Molecular and Cellular Biology, 19(5), 3614–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Ireton RC, & Reynolds AB (2003). A core function for p120-catenin in cadherin turnover. Journal of Cell Biology, 163(3), 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis J, Stefaniak A, Eloff F, John S, Agner T, Chou TC, … Holness L (2013). International guidelines for the in vivo assessment of skin properties in non-clinical settings: Part 2. transepidermal water loss and skin hydration. Skin Research and Technology: Official Journal of International Society for Bioengineering and the Skin, 19(3), 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto Y, Shintani Y, Reynolds AB, Johnson KR, & Wheelock MJ (2008). The regulatory or phosphorylation domain of p120 catenin controls E-cadherin dynamics at the plasma membrane. Experimental Cell Research, 314(1), 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentil-dit-Maurin A, Oun S, Almagro S, Bouillot S, Courcon M, Linnepe R, … Tillet E (2010). Unraveling the distinct distributions of VE- and N-cadherins in endothelial cells: A key role for p120-catenin. Experimental Cell Research, 316(16), 2587–2599. [DOI] [PubMed] [Google Scholar]
- Grosheva I, Shtutman M, Elbaum M, & Bershadsky AD (2001). P120 catenin affects cell motility via modulation of activity of Rho-family GTPases: A link between cell-cell contact formation and regulation of cell locomotion. Journal of Cell Science, 114(Pt 4), 695–707. [DOI] [PubMed] [Google Scholar]
- Hatanaka K, Simons M, & Murakami M (2011). Phosphorylation of VE-cadherin controls endothelial phenotypes via p120-catenin coupling and Rac1 activation. American Journal of Physiology-Heart and Circulatory Physiology, 300(1), H162–H172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, … Reynolds AB (2002). A novel role for p120 catenin in E-cadherin function. Journal of Cell Biology, 159(3), 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama N, & Ikura M (2012). The three-dimensional structure of the cadherin-catenin complex. Sub-cellular Biochemistry, 60, 39–62. [DOI] [PubMed] [Google Scholar]
- Ishiyama N, Lee SH, Liu S, Li GY, Smith MJ, Reichardt LF, & Ikura M (2010). Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell, 141(1), 117–128. [DOI] [PubMed] [Google Scholar]
- Jiang G, Wang Y, Dai S, Liu Y, Stoecker M, Wang E, & Wang E (2012). P120-catenin isoforms 1 and 3 regulate proliferation and cell cycle of lung cancer cells via beta-catenin and Kaiso respectively. PLoS ONE, 7(1), e30303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liao L, Shrestha C, Ji S, Chen Y, Peng J, … Xie Z (2015a). Reduced expression of E-cadherin and p120-catenin and elevated expression of PLC-gamma1 and PIKE are associated with aggressiveness of oral squamous cell carcinoma. International Journal of Clinical and Experimental Pathology, 8(8), 9042–9051. [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liao L, Shrestha C, Li D, Li M, Mu Y, … Xie Z (2015b). Inhibition of 4-nitroquinoline-1-oxide-induced oral carcinogenesis by dietary calcium. International Journal of Clinical and Experimental Pathology, 8(4), 3529–3542. [PMC free article] [PubMed] [Google Scholar]
- Johnson E, Seachrist DD, DeLeon-Rodriguez CM, Lozada KL, Miedler J, Abdul-Karim FW, & Keri RA (2010). HER2/ErbB2-induced breast cancer cell migration and invasion require p120 catenin activation of Rac1 and Cdc42. Journal of Biological Chemistry, 285(38), 29491–29501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantoulaki M, Kouklis P, & Malik AB (2003). Protein kinase C modifications of VE-cadherin, p120, and beta-catenin contribute to endothelial barrier dysregulation induced by thrombin. American Journal of Physiology-Lung Cellular and Molecular Physiology, 285(2), L434–L442. [DOI] [PubMed] [Google Scholar]
- Lee M, Ji H, Furuta Y, Park JI, & McCrea PD (2014). P120-catenin regulates REST and CoREST, and modulates mouse embryonic stem cell differentiation. Journal of Cell Science, 127(Pt 18), 4037–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Zhang GH, Yang XM, Li SS, Liu X, Yang QT, … Ye J (2012). Reduced E-cadherin expression is associated with lymph node metastases in laryngeal squamous cell carcinoma. Auris, Nasus, Larynx, 39(2), 186–192. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dong QZ, Zhao Y, Dong XJ, Miao Y, Dai SD, … Wang EH (2009). P120-catenin isoforms 1A and 3A differently affect invasion and proliferation of lung cancer cells. Experimental Cell Research, 315(5), 890–898. [DOI] [PubMed] [Google Scholar]
- Ozaki C, Yoshioka M, Tominaga S, Osaka Y, Obata S, & Suzuki ST (2010). P120-Catenin is essential for N-cadherin-mediated formation of proper junctional structure, thereby establishing cell polarity in epithelial cells. Cell Structure and Function, 35(2), 81–94. [DOI] [PubMed] [Google Scholar]
- Park JI, Ji H, Jun S, Gu D, Hikasa H, Li L, … McCrea PD (2006). Frodo links Dishevelled to the p120-catenin/Kaiso pathway: Distinct catenin subfamilies promote Wnt signals. Developmental Cell, 11(5), 683–695. [DOI] [PubMed] [Google Scholar]
- Peifer M, & Yap AS (2003). Traffic control: P120-catenin acts as a gatekeeper to control the fate of classical cadherins in mammalian cells. Journal of Cell Biology, 163(3), 437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, & Fuchs E (2006). P120-catenin mediates inflammatory responses in the skin. Cell, 124(3), 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AB (2007). P120-catenin: Past and present. Biochimica et Biophysica Acta, 1773(1), 2–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AB, & Roczniak-Ferguson A (2004). Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene, 23(48), 7947–7956. [DOI] [PubMed] [Google Scholar]
- Rogiers V, & Group E (2001). EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacology and Applied Skin Physiology, 14(2), 117–128. [DOI] [PubMed] [Google Scholar]
- Xiao K, Oas RG, Chiasson CM, & Kowalczyk AP (2007). Role of p120-catenin in cadherin trafficking. Biochimica et Biophysica Acta, 1773(1), 8–16. [DOI] [PubMed] [Google Scholar]
- Xie Z, & Bikle DD (2007). The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. Journal of Biological Chemistry, 282(12), 8695–8703. [DOI] [PubMed] [Google Scholar]
- Xu L, Jiang Y, Zheng J, Xie G, Li J, Shi L, & Fan S (2013). Aberrant expression of beta-catenin and E-cadherin is correlated with poor prognosis of nasopharyngeal cancer. Human Pathology, 44(7), 1357–1364. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Huveldt D, Kreinest P, Lohse CM, Cheville JC, Parker AS, … Anastasiadis PZ (2008). A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion, and predicts metastatic disease. Journal of Biological Chemistry, 283(26), 18344–18354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YL, Wang YM, Liu H, Qin GF, Tang AG, & Duan Y (2012). Aberrant expression of E-cadherin in lung tissues of patients with probable lung cancer. Asian Pacific Journal of Cancer Prevention: APJCP, 13(10), 5149–5153. [DOI] [PubMed] [Google Scholar]


