Abstract
Background:
SARS-CoV-2 infection can lead to multisystem inflammatory syndrome in children (MIS-C). We sought to investigate risk factors for admission to the intensive care unit (ICU) and explored changes in disease severity over time.
Methods:
We obtained data from chart reviews of children younger than 18 years with confirmed or probable MIS-C who were admitted to 15 hospitals in Canada, Iran and Costa Rica between Mar. 1, 2020, and Mar. 7, 2021. Using multivariable analyses, we evaluated whether admission date and other characteristics were associated with ICU admission or cardiac involvement.
Results:
Of 232 children with MIS-C (median age 5.8 yr), 130 (56.0%) were male and 50 (21.6%) had comorbidities. Seventy-three (31.5%) patients were admitted to the ICU but none died. We observed an increased risk of ICU admission among children aged 13–17 years (adjusted risk difference 27.7%, 95% confidence interval [CI] 8.3% to 47.2%), those aged 6–12 years (adjusted risk difference 25.2%, 95% CI 13.6% to 36.9%) or those with initial ferritin levels greater than 500 μg/L (adjusted risk difference 18.4%, 95% CI 5.6% to 31.3%). Children admitted to hospital after Oct. 31, 2020, had numerically higher rates of ICU admission (adjusted risk difference 12.3%, 95% CI −0.3% to 25.0%) and significantly higher rates of cardiac involvement (adjusted risk difference 30.9%, 95% CI 17.3% to 44.4%). At Canadian sites, the risk of ICU admission was significantly higher for children admitted to hospital between December 2020 and March 2021 than those admitted between March and May 2020 (adjusted risk difference 25.3%, 95% CI 6.5% to 44.0%).
Interpretation:
We observed that age and higher ferritin levels were associated with more severe MIS-C. We observed greater severity of MIS-C later in the study period. Whether emerging SARS-CoV-2 variants pose different risks of severe MIS-C needs to be determined.
Multisystem inflammatory syndrome in children (MIS-C)1 manifests as immune dysregulation after SARS-CoV-2 infection.2 The syndrome has no pathognomonic features. Thus, the diagnostic criteria of the Royal College of Paediatrics and Child Health (RCPCH), the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) differ, but they all include fever, evidence of systemic inflammation and involvement of at least 1 organ or system.3
Our primary objective was to assess initial clinical or laboratory features that predict severe illness in MIS-C. We also sought to explore changes in overall disease severity and cardiac involvement over time as it was the impression of many investigators that severity of MIS-C increased through pandemic waves.
Methods
This was part of a larger study. Investigators from 15 pediatric hospitals (13 in Canada and 1 each in San José, Costa Rica, and Tehran, Iran; Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210873/tab-related-content) included consecutive children aged younger than 18 years who were admitted to hospital from Mar. 1, 2020, through Mar. 7, 2021, and who fulfilled the WHO criteria for MIS-C, namely fever (not otherwise defined) for at least 3 days; elevated C-reactive protein, erythrocyte sedimentation rate or procalcitonin level; illness involving 2 or more systems, with no other obvious microbial cause of inflammation; and “evidence of COVID-19 ([reverse transcription polymerase chain reaction], antigen test or serology positive [for SARS-CoV-2 infection]), or likely contact with patients with COVID-19.”4 Given that asymptomatic individuals can transmit SARS-CoV-2,5 any child residing in a community with ongoing SARS-CoV-2 transmission was considered to have “likely contact.” In addition, we included patients who otherwise fulfilled WHO criteria but had fever for less than 3 days, if they received corticosteroids or intravenous immunoglobulin (IVIG) as treatment for MIS-C before the third day of fever. We excluded patients with a likely alternative diagnosis. We identified cases by screening admission lists, reviewing charts of children admitted to hospital with positive SARS-CoV-2 test results and communicating with the clinical services likely to admit or be consulted on MIS-C cases.
Co-investigator physicians (11 sites) or their supervised research assistants (4 sites) collected study data using REDCap electronic data capture tools, hosted at the University of Alberta. We used chart review to collect primary reason for admission, demographics, comorbidities, clinical presentation and course, coinfections, treatments and complications (Appendix 1). We designed the initial case report form for acute SARS-CoV-2 cases and continually modified it over time to clarify any confusing questions. Multisystem inflammatory syndrome in children was not described when the study started, so the MIS-C questions were added in May 2020. If investigators were aware of patients who met the MIS-C criteria and were admitted before May 2020, they entered them once the MIS-C questions were added.
We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.6
Definitions
We considered patients to have confirmed MIS-C if, in addition to fulfillment of MIS-C clinical criteria, SARS-CoV-2 or antibodies to SARS-CoV-2 were detected at any point. We considered patients to have probable MIS-C if they fulfilled MIS-C clinical criteria but SARS-CoV-2 tests were negative or not performed. Definitions of other clinical manifestations of SARS-CoV-2 infection are shown in Appendix 2, Supplementary Table 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210873/tab-related-content.
Outcomes
Our outcomes were hospital length of stay, need for intensive care unit (ICU) admission and cardiac involvement. As criteria for ICU admission varied by site, we assessed a “critical disease” outcome (defined as ICU admission with use of vasopressors, or noninvasive or mechanical ventilation) in a sensitivity analysis.
Statistical analysis
We used descriptive statistics to compare demographic, clinical, laboratory and treatment data, as well as outcomes to identify differences between patients with confirmed and probable SARS-CoV-2 infections and between the participating countries. We analyzed categorical data using the χ2 or Fisher exact test and analyzed continuous data using the Kruskal–Wallis test for medians.
We used multivariable logistic or linear log-transformed regression analysis, as appropriate, to explore clinical and laboratory factors at hospital admission, and their association with outcomes. We assessed factors of interest in separate multivariable models, including age, sex, country, initial ferritin, leukocyte count, platelet count and organ system involvement on admission. We created a directed acyclic graph to inform the assessment of potential confounders, mediators and colliders for the outcome variables, and to sequentially build the models (Appendix 3, Supplementary Figure 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210873/tab-related-content).7 Rates of missing data in the included variables were low (< 4%) and we excluded missing data from the analysis. We assessed model fit using the Hosmer–Lemeshow goodness-of-fit test for logistic and R2 for linear regression. We also investigated potential overfitting (Appendix 4, Supplementary Methods, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210873/tab-related-content). We reported results of regression models as adjusted predicted probabilities of the outcome (absolute risks and risk differences) representing the average marginal effect across the total study population.8 We considered a risk difference that did not include the null (0%) in its confidence interval (CI) as statistically significant.
To evaluate temporal changes in severity, we compared outcomes for 2 time cohorts, Mar. 1–Oct. 31, 2020, and Nov. 1, 2020–Mar. 7, 2021, analyzing time as an exposure in separate explorative models. We arbitrarily chose these dates before data analysis to divide the cases into roughly equal groups. It was not possible to analyze cases by epidemic wave, as distinct waves did not occur in Costa Rica. We analyzed data from Canada separately for risk factors of ICU admission and cardiac involvement, dividing cases into 3-month periods from March 2020 (adding the 3 patients admitted to hospital in March 2021 to the fourth quarter). In addition, we used a sensitivity analysis to compare outcomes for confirmed and probable cases. We did not make adjustments for multiple comparisons.
We analyzed data using STATA 13 (StataCorp. College Station).
Ethics approval
The study was approved by the research ethics boards at the University of Alberta (Pro00099426) and all participating sites.
Results
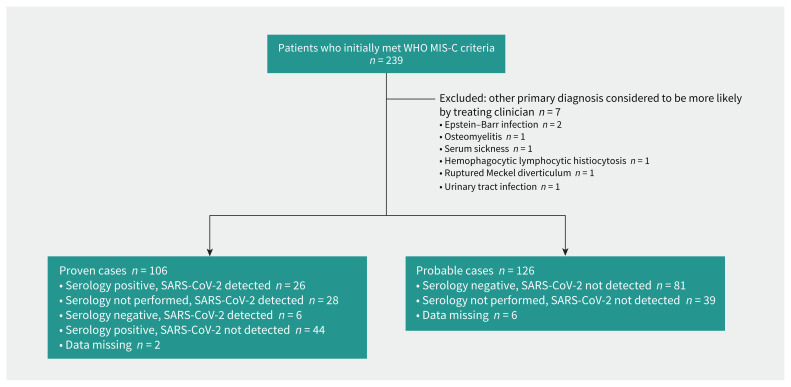
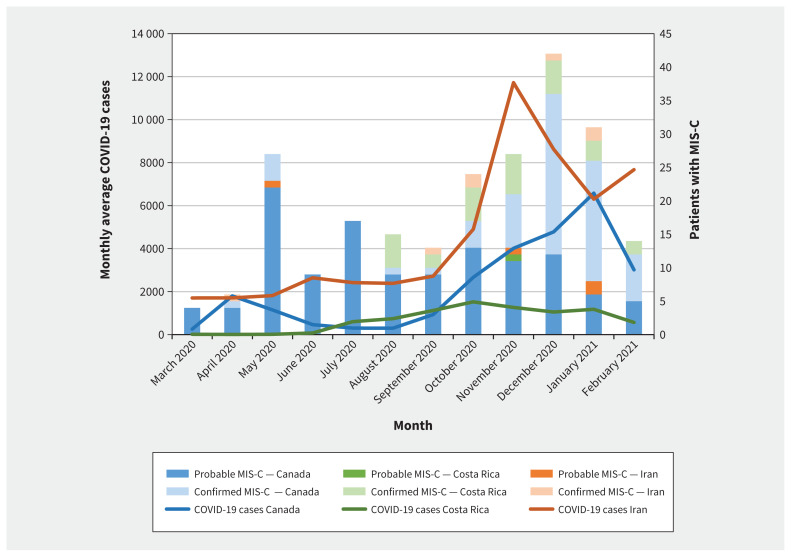
We included 232 children with MIS-C (106 confirmed, 126 probable) (Figure 1), of whom 130 (56.0%) were male and 50 (21.6%) had 1 or more comorbidities. The median age of patients was 5.8 (interquartile range [IQR] 3.0–9.5) years. Figure 2 shows the number of patients with MIS-C over time, in relation to the regional average number of COVID-19 cases. Data by country is in Appendix 2, Supplementary Table 2.
Figure 1:
Flowchart of study inclusion of patients admitted to hospital with confirmed or probable multisystem inflammatory syndrome in children (MIS-C). Note: WHO = World Health Organization.
Figure 2:
Patients with multisystem inflammatory syndrome in children (MIS-C) in Canada, Costa Rica and Iran from Mar. 1, 2020, and Mar. 7, 2021 (patients admitted in March 2021 included in February 2021 counts). Monthly COVID-19 case counts are averaged over the 5th, 15th and 25th of the month (https://coronavirus.jhu.edu/map.html).
Clinical features
Almost all patients had gastrointestinal involvement (89.2%), mainly abdominal pain, and mucocutaneous findings (84.5%) (Table 1). Children with confirmed MIS-C had a fever for a median total duration of 6 (IQR 4–8) days, compared with 7 (IQR 6–10) days for children with probable MIS-C.
Table 1:
Characteristics of patients admitted to hospital with multisystem inflammatory syndrome in children from Mar. 1, 2020, to Mar. 7, 2021
| Characteristic | No. (%) of patients* | ||
|---|---|---|---|
| Confirmed MIS-C n = 106 |
Probable MIS-C n = 126 |
All cases n = 232 |
|
| Age, yr, median (IQR) | 6.85 (4.01–9.66) | 4.93 (1.92–8.87) | 5.75 (3.02–9.49) |
| Age categories, yr | |||
| 0–5 | 46 (43.3) | 74 (58.7) | 120 (51.7) |
| 6–12 | 48 (45.3) | 40 (31.7) | 88 (37.9) |
| 13–18 | 12 (11.3) | 12 (9.5) | 24 (10.3) |
| Sex, male | 61 (57.5) | 69 (54.8) | 130 (56.0) |
| Country | |||
| Canada | 69 (65.1) | 121 (96.0) | 190 (81.9) |
| Costa Rica | 29 (27.4) | 1 (0.8) | 30 (12.9) |
| Iran | 8 (7.5) | 4 (3.2) | 12 (5.2) |
| ≥ 1 comorbidities†‡ | 22 (20.8) | 28 (22.2) | 50 (21.6) |
| Duration of fever before admission, d, median (IQR)§ | 4 (3–5) | 5 (3–6) | 4 (3–6) |
| Total duration of fever, d, median (IQR)§ | 6 (4–8) | 7 (6–10) | 6 (5–9) |
| Mucocutaneous signs | 89 (84.0) | 107 (84.9) | 196 (84.5) |
| Gastrointestinal involvement** | 100 (94.3) | 107 (84.9) | 207 (89.2) |
| Cardiac involvement†† | 81 (76.4) | 55 (43.7) | 136 (58.6) |
| Echocardiogram performed | 102 (96.2) | 124 (98.4) | 226 (97.4) |
| Normal or changes not compatible with MIS-C | 52 (49.1) | 83 (65.9) | 135 (58.2) |
| Changes compatible with MIS-C‡‡ | 50 (47.2) | 40 (31.7) | 90 (38.8) |
| Coronary artery z score ≥ 2.5 | 5 (4.7) | 15 (11.9) | 21 (9.1) |
| Abnormal coronary arteries§§ | 17 (16.0) | 8 (6.3) | 25 (10.8) |
| Coronary artery aneurysm | 1 (0.9) | 1 (0.8) | 2 (0.9) |
| Ascending aorta dilatation | 1 (0.9) | 7 (5.6) | 8 (3.4) |
| Poor systolic function | 20 (18.9) | 6 (4.8) | 26 (11.2) |
| Regurgitation of ≥ 1 valves | 18 (17.0) | 6 (4.8) | 24 (10.3) |
| Myocarditis | 2 (1.9) | 3 (2.4) | 5 (2.2) |
| Pericardial effusion | 10 (9.4) | 6 (4.8) | 16 (6.9) |
| Bundle branch block | 0 (0) | 1 (0.8) | 1 (0.4) |
| Prolonged Q-T interval | 1 (0.9) | 0 (0) | 1 (0.4) |
| Possible thrombus in ventricle | 0 (0) | 1 (0.8) | 1 (0.4) |
| NT-ProBNP or troponins elevated, despite no evidence of cardiac involvement on echocardiogram | 31 (29.2) | 15 (11.9) | 46 (19.8) |
| Abnormal coagulation profile | 103 (97.2) | 106 (84.1) | 209 (90.1) |
| Neurological complications | 5 (4.7) | 4 (3.2) | 9 (3.9) |
| Clinical hematological complications*** | 1 (0.9) | 2 (1.6) | 3 (1.3) |
| Contact with a person with proven SARS-CoV-2 infection | 42 (39.6) | 8 (6.3) | 50 (21.6) |
| Bacterial coinfection | 2 (1.9) | 10 (7.9) | 12 (5.2) |
| Viral coinfection | 6 (5.7) | 3 (2.4) | 9 (3.9) |
| Laboratory results | |||
| Peak C-reactive protein, mg/L, median (IQR) | 145 (89–206) | 122 (67–203) | 135 (83–204) |
| Ferritin, μg/L | |||
| Not measured | 3 (2.8) | 4 (3.2) | 7 (3.0) |
| Initial value, median (IQR) | 367 (208–649) | 207 (114–404) | 265 (144–482) |
| Peak value, median (IQR) | 463 (284–835) | 259 (122–555) | 376 (173–689) |
| Initial leukocyte count, × 109/L, median (IQR) | 11.6 (7.8–15.0) | 9.2 (6.5–12.8) | 10.6 (7.0–14.4) |
| Lowest neutrophil count, × 109/L, median (IQR) | 3.6 (2.5–5.7) | 2.7 (1.4–4.3) | 3.1 (1.8–4.8) |
| Highest neutrophil count, × 109/L, median (IQR) | 10.0 (6.9–14.1) | 9.6 (6.5–14.4) | 9.0 (6.5–14.2) |
| Platelet count, × 109/L | |||
| Initial value, median (IQR) | 250 (175–356) | 250 (175–356) | 225 (144–312) |
| Lowest value, median (IQR) | 218 (135–316) | 170 (115–245) | 191 (123–278) |
| Highest value, median (IQR) | 426 (295–591) | 451 (327–585) | 440 (304–589) |
| ALT, IU/L | |||
| Always ≤ 40 | 56 (52.8) | 69 (54.8) | 125 (53.9) |
| > 40 | 50 (47.2) | 57 (45.2) | 107 (46.1) |
| ALT when > 40, median (IQR) | 81 (55–130) | 49 (49–187) | 81 (54–136) |
| AST, IU/L | |||
| Always ≤ 50 | 58 (54.7) | 73 (57.9) | 131 (56.5) |
| > 50 | 48 (45.3) | 53 (42.1) | 101 (43.5) |
| AST when > 50, median (IQR) | 80 (58–127) | 87 (58–126) | 85 (58–126) |
| Creatinine, μmol/L | |||
| Not measured | 2 (1.9) | 2 (1.6) | 4 (1.7) |
| Increased for age††† | 3 (2.8) | 3 (2.4) | 6 (2.6) |
| Peak level for abnormal results, median (IQR) | 137 (107–184) | 259 (122–345) | 161 (122–259) |
| Required renal replacement therapy | 0 | 0 | 0 |
| Troponin, ng/L | |||
| Normal, not measured or not recorded‡‡‡ | 55 (51.9) | 111 (88.1) | 166 (71.6) |
| Abnormal§§§ | 51 (48.1) | 15 (11.9) | 66 (28.4) |
| NT-proBNP, pg/mL | |||
| Normal, not measured or not recorded‡‡‡ | 44 (41.5) | 109 (86.5) | 153 (65.9) |
| Peak level of abnormal result, median (IQR) | 3215 (1095–9269) | 1736 (880–4319) | 2685 (1056–6952) |
Note: ALT = alanine aminotransferase, AST = aspartate aminotransferase, IQR = interquartile range, MIS-C = multisystem inflammatory syndrome in children, NT-proBNP = N-terminal pro-brain natriuretic peptide.
Unless indicated otherwise. Patients with confirmed MIS-C had SARS-CoV-2 detected or positive serology.
Comorbidities among patients with confirmed MIS-C included asthma (n = 8, of whom 1 also had Familial Mediterranean Fever, 1 was living with obesity, 1 had idiopathic thrombocytopenic purpura and 1 had sickle cell disease), cancer (n = 4, of whom 2 had acute lymphoblastic leukemia and 2 had neuroblastoma), obesity (n = 2), autism (n = 2), seizure disorder (n = 2, of whom 1 had cerebral palsy), congenital heart disease (n = 1), Prader–Willi syndrome with hypertension (n = 1), chromosomal disorder with prematurity (n = 1) and renal tubular acidosis with hypertension and ovalocytosis (n = 1).
Comorbidities among patients with probable MIS-C included prematurity (n = 6), asthma (n = 6), periodic fever syndrome (n = 4), obesity (n = 2), global developmental delay (n = 3), sickle cell disease (n = 1), liver transplant (n = 1), congenital heart disease (n = 1), cerebral palsy (n = 1), Crohn disease (n = 1); 2 others had multiple comorbidities: (i) obesity, congenital heart disease and seizures and (ii) sickle cell disease, global developmental delay and congenital heart disease.
46 patients missing data on days of fever before admission, 70 patients missing data on total days of fever, 4 patients missing initial leukocyte count, 1 patient missing initial platelet count, 11 patients missing data on ALT.Signs include rash, conjunctivitis, strawberry tongue, red cracked lips or redness or swelling of extremities.
Gastrointestinal involvement includes vomiting, diarrhea or abdominal pain.
Cardiac involvement defined as features of myocardial dysfunction, pericarditis, valvulitis or coronary abnormalities (including echocardiographic findings or elevated troponin or NT-proBNP).
Some children had more than 1 change compatible with MIS-C.
Coronary arteries were reported to appear abnormal (dilated, inflamed or edematous), but z scores were not provided or were less than 2.5.Abnormal coagulation profile defined as elevated partial thromboplastin time or D-dimers, or international normalized ratio greater than 1.2.
Thrombosis or evidence of bleeding or disseminated intravascular coagulation; or received erythrocytes, platelets or fresh frozen plasma, tranexamic acid, antifibrinolytics, low-molecular-weight heparin treatment dose or other anticoagulants, such as direct oral anticoagulants.
Serum creatinine > 140 μmol/L for children younger than 7 days, 55 μmol/L for children aged 7–364 days, 100 μmol/L for children aged 1–12 years and 140 μmol/L for those aged 13–17 years (excluding children with chronic renal disease).14
Once these parameters were added to the case report form in May 2020, abnormal results were recorded. Abnormal results before this time and and all normal results were not consistently recorded.
We asked only if results were normal or abnormal, without specification of normal values. If result was reported as abnormal, we asked for actual value.
Bacterial coinfections were documented in 12 (5.2%) patients (4 had pharyngitis, 3 had colitis and 5 had urinary tract infections), and 142 (61.2%) received antibiotics, primarily as empiric therapy for sepsis. Viral coinfections were documented in 9 (3.9%) patients (rhinovirus n = 4; rhinovirus and enterovirus n = 3; rhinovirus, enterovirus and parainfluenza n = 1; adenovirus n = 1).
Complications
Cardiac involvement was common (n = 136, 58.6%) including among 76.4% of patients with confirmed MIS-C and 43.6% of those with probable MIS-C (Table 1). Six (2.6%) patients had acute kidney injury; none required renal replacement therapy. Hepatitis affected 120 (51.7%) patients, although none progressed to liver failure.
Most patients (n = 209, 90.1%) had abnormalities in 1 or more coagulation parameters, yet only 3 (1.3%) patients developed clinical hematological manifestations. One patient with confirmed MIS-C had extensive venous thrombosis involving the internal jugular vein, a lower extremity and the lungs. One patient with probable MIS-C developed pulmonary emboli and melena, and another had hematemesis with disseminated intravascular coagulation.
Other complications in patients with confirmed MIS-C included removal of a normal appendix (n = 3, including 1 patient whose appendix was removed 4 days before the hospital admission for MIS-C), anterior uveitis (n = 2), reversible encephalopathy (n = 2) and 1 case each of ileitis requiring total parenteral nutrition, mesenteric adenitis and orchitis. Complications in patients with probable MIS-C included aseptic meningitis (n = 5; none had a virus detected), removal of a normal appendix (n = 2) and 1 case each of ascites, pleural effusion, renal infarction, cholecystitis, orchitis and raised intracranial pressure. One patient with confirmed MIS-C and 3 patients with probable MIS-C received diagnoses of secondary hemophagocytic lymphohistiocytosis by the treating clinician.
For treatment, 194 (83.6%) patients received IVIG, 126 (54.3%) patients received corticosteroids and 10 (4.3%) patients received anakinra; 24 (10.3%) patients received none of these treatments (Table 2).
Table 2:
Hospital length of stay and level of care for patients admitted to hospital with multisystem inflammatory syndrome in children, according to time of admission
| Variable | No. (%)* of patients admitted Mar. 1 to Oct. 31, 2020 | No. (%)* of patients admitted Nov. 1, 2020, to Mar. 7, 2021 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Confirmed n = 28 |
Probable n = 92 |
All n = 120 |
Confirmed n = 78 |
Probable n = 34 |
All n = 112 |
|
| Duration of fever before admission, d, median (IQR) | 5 (3–6) | 5 (3–6) | 5 (3–6) | 4 (3–5) | 5 (3–6) | 4 (3–6) |
|
| ||||||
| Total duration of fever, d, median (IQR) | 7 (6–9) | 6.5 (5–9.5) | 7(5–9) | 5.5 (4–8) | 6 (6–10) | 6 (4–8) |
|
| ||||||
| Duration of admission, d, median (IQR) | 8 (6.5–10) | 5 (3–7) | 5 (4–8) | 6 (4–8) | 7 (4–12) | 6 (4–9) |
|
| ||||||
| Highest level of care | ||||||
|
| ||||||
| Ward admission | 18 (64.3) | 79 (85.9) | 97 (80.8) | 39 (50.0) | 23 (67.6) | 62 (55.4) |
|
| ||||||
| No supplemental oxygen | 14 (50.0) | 76 (82.6) | 90 (75.0) | 33 (42.3) | 20 (58.9) | 53 (47.3) |
|
| ||||||
| Supplemental oxygen | 4 (14.3) | 3 (3.3) | 7 (5.8) | 6 (7.7) | 3 (8.8) | 9 (8.0) |
|
| ||||||
| ICU admission | 10 (35.7) | 13 (14.1) | 23 (19.2) | 39 (50.0) | 11 (32.4) | 50 (44.6) |
|
| ||||||
| Observation with or without supplemental oxygen | 5 (17.8) | 2 (2.2) | 7 (5.8) | 11 (14.1) | 4 (11.8) | 15 (13.4) |
|
| ||||||
| Vasopressors with or without supplemental oxygen | 3 (10.7) | 6 (6.5) | 9 (7.5) | 19 (24.4) | 6 (17.6) | 25 (22.3) |
|
| ||||||
| Noninvasive ventilation and vasopressors | 0 (0) | 0 (0) | 0 (0) | 3 (3.8) | 1 (2.9) | 4 (3.6) |
|
| ||||||
| Mechanical ventilation, no vasopressors | 0 (0) | 1 (1.1) | 1 (0.8) | 1 (1.3) | 0 (0) | 1 (0.9) |
|
| ||||||
| Mechanical ventilation and vasopressors | 2 (7.1) | 1 (1.1) | 3 (3.3) | 4 (5.1) | 0 (0) | 4 (3.6) |
|
| ||||||
| Admission not for MIS-C | 0 (0) | 3 (3.3) | 3 (3.3) | 1 (1.3) | 0 (0) | 1 (0.9) |
|
| ||||||
| Treatment† | ||||||
|
| ||||||
| Intravenous immunoglobulin | 25 (89.3) | 69 (75.0) | 94 (78.3) | 68 (87.2) | 32 (94.1) | 100 (89.3) |
|
| ||||||
| Corticosteroids | 12 (42.8) | 37 (40.2) | 49 (40.8) | 57 (73.1) | 20 (58.8) | 77 (68.8) |
|
| ||||||
| Anakinra | 0 (0) | 3 (3.3) | 3 (3.3) | 3 (3.8) | 4 (11.8) | 7 (6.2) |
|
| ||||||
| None of the above | 2 (7.1) | 17 (18.5) | 19 (15.8) | 4 (5.1) | 1 (2.9) | 5 (4.5) |
Note: ICU = intensive care unit, IQR = interquartile range, MIS-C = multisystem inflammatory syndrome in children.
Unless indicated otherwise.
Many patients received a combination of these treatments.
The median length of hospital stay was 6 days, with 69.0% of patients discharged by day 7 and 84.9% discharged by day 10. A ferritin level on admission greater than 500 μg/L was associated with prolonged length of stay (Appendix 2, Supplementary Table 3).
Factors associated with ICU admission
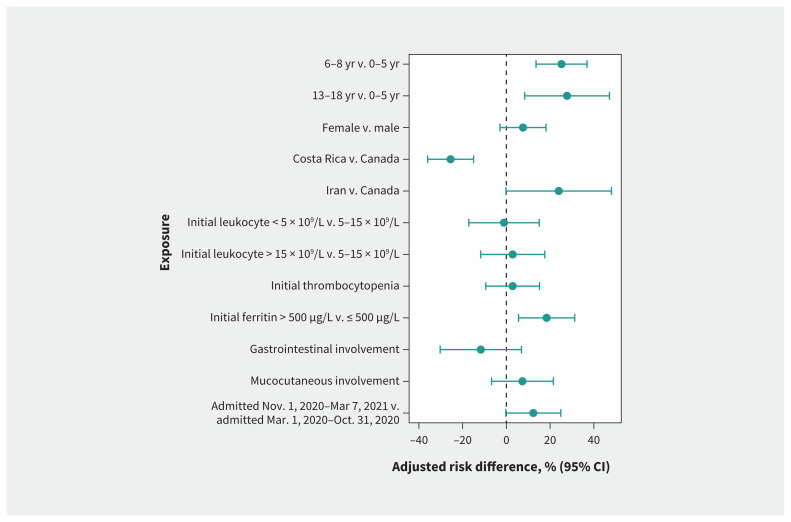
Overall, 73 children (31.5%) required ICU admission, of whom 47 (64.4%) required vasopressors with or without mechanical or noninvasive ventilation. No children required extracorporeal life support or died. Compared with the risk among children aged 0–5 years, the adjusted absolute risk of ICU admission was higher among children aged 6–12 years (43.6% v. 18.4%, adjusted risk difference 25.2%, 95% CI 13.6% to 36.9%) and children aged 13–17 (46.2% v. 18.4%, adjusted risk difference 27.7%, 95% CI 8.3% to 47.2%) (Figure 3 and Table 3). An initial ferritin greater than 500 μg/L was also associated with risk of ICU admission (adjusted risk difference 18.4%, 95% CI 5.6% to 31.3%).
Figure 3:
Adjusted risk differences for admission to the intensive care unit. Average marginal effects are presented with adjusted risk differences from multivariable logistic regressions. The reference categories used for adjusted risk differences are the absence of the symptom or the reported category. Models are adjusted for sex, age, country, presence of comorbidity, coinfection, treatment, confirmed case status and admission time. We used 9 different models, each assessing the 9 exposures. Note: CI = confidence interval.
Table 3:
Adjusted model estimates of risk factors for intensive care unit admission for patients admitted to hospital with multisystem inflammatory syndrome in children*
| Variable | Absolute risk, % (95% CI) | Risk difference, % (95% CI) | Relative risk (95% CI) |
|---|---|---|---|
| Age, yr | |||
| 0–5 | 18.4 (11.6 to 25.2) | – | – |
| 6–12 | 43.6 (34.4 to 52.8) | 25.2 (13.6 to 36.9) | 2.37 (1.34 to 3.40) |
| 13–18 | 46.2 (28.2 to 64.1) | 27.7 (8.3 to 47.2) | 2.51 (1.14 to 3.88) |
| Sex | |||
| Male | 28.0 (21.3 to 34.8) | – | – |
| Female | 35.6 (27.7 to 43.5) | 7.6 (−2.9 to 18.1) | 1.27 (0.85 to 1.69) |
| Country | |||
| Canada | 34.7 (28.5 to 40.9) | – | – |
| Costa Rica | 9.2 (1.0 to 17.4) | −25.5(−36.0 to −15.0) | 0.26 (0.03 to 0.51) |
| Iran | 58.7 (28.5 to 40.9) | 24.0 (2.3 to 50.9) | 1.69 (0.95 to 2.43) |
| Initial leukocyte count, × 109/L† | |||
| < 5 | 29.2 (14.6 to 43.9) | −1.1 (−17.2 to 15.0) | 0.96 (0.44 to 1.49) |
| 5–15 | 30.3 (24.1 to 36.6) | – | – |
| > 15 | 33.2 (20.2 to 46.2) | 2.9 (−11.8 to 17.5) | 1.10 (0.60 to 1.59) |
| Initial platelet count, × 109/L† | |||
| < 150 | 33.8 (23.6 to 44.0) | 2.9 (−9.4 to 15.2) | 1.09 (0.69 to 1.50) |
| ≥ 150 | 30.9 (24.8 to 37.1) | – | – |
| Initial ferritin, μg/L† | |||
| ≤ 500 | 26.6 (20.9 to 32.5) | – | – |
| > 500 | 45.1 (33.8 to 56.4) | 18.4 (5.6 to 31.3) | 1.69 (1.12 to 2.26) |
| Gastrointestinal involvement | 30.4 (25.2 to 35.6) | −11.7 (−30.3 to 6.9) | 0.72 (0.39 to 1.05) |
| Mucocutaneous involvement | 32.6 (27.0 to 38.1) | 7.4 (−6.8 to 21.5) | 1.29 (0.59 to 1.99) |
| Admission period | |||
| Mar. 1–Nov. 1, 2020 | 24.9 (16.8 to 33.0) | – | – |
| Nov. 1, 2020–Mar. 7, 2021 | 37.2 (28.9 to 45.6) | 12.3 (−0.3 to 25.0) | 1.50 (0.86 to 2.13) |
Note: CI = confidence interval.
Models are adjusted for sex, age, country, presence of comorbidity, coinfection, confirmed case status, treatment and admission time. Average marginal effects are presented with adjusted risk differences from multivariable logistic regressions.
Number of observations differing from complete data set of 232, leukocytes: 4 missing; platelets: 2 missing; ferritin: 7 missing.
Patients with MIS-C admitted to hospital between Nov. 1, 2020, and Mar. 7, 2021, were more likely to require ICU admission (n = 50 of 112, 45%) than those admitted between Mar. 1 and Oct. 31, 2020 (n = 23 of 120, 19%, p < 0.001) (Table 3), although the difference was not statistically significant after multivariable adjustment (adjusted risk difference 12.3%, 95% CI −0.3% to 25.0%) (Table 3). A stratified analysis of patients with confirmed or probable MIS-C (Appendix 2, Supplementary Table 4; Appendix 5, Supplementary Figure 2, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210873/tab-related-content) showed a statistically significant increased risk for ICU admission in the later time period (on or after Nov. 1, 2020) among children with probable MIS-C (adjusted risk difference 17.9%, 95% CI 22.7% to 33.6%), in contrast to children with confirmed MIS-C (adjusted risk difference 0.8%, 95% CI −20.4% to 22.0%). For sites in Canada, the risk of ICU admission was higher in the last quarter of the study period (December 2020 to March 2021) than in the first quarter (March to May 2020) (adjusted risk difference 25.3%, 95% CI 6.5% to 44.0%) (Appendix 2, Supplementary Table 5 and Appendix 6, Supplementary Figure 3, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210873/tab-related-content).
The sensitivity analysis in which we used critical disease as the outcome was consistent with the analysis of ICU admission, with the additional association of mucocutaneous symptoms with a higher risk for critical disease (adjusted risk difference 14.0%, 95% CI 4.6% to 23.3%) (Appendix 2, Supplementary Table 6; Appendix 7, Supplementary Figure 4, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210873/tab-related-content).
Factors associated with cardiac involvement
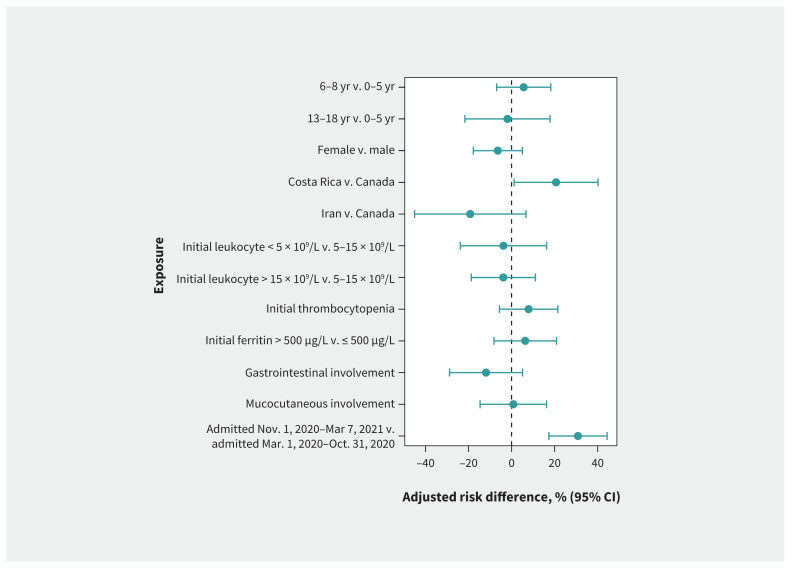
Among the 232 children, 136 (58.6%) had cardiac involvement, including 90 with changes compatible with MIS-C on echocardiography and 46 with elevated N-terminal pro–brain-type natriuretic peptide or troponin levels, despite normal echocardiography (Table 1). Compared with the risk among children admitted before Nov. 1, 2020, the adjusted absolute risk of cardiac involvement was significantly higher among children admitted on or after Nov. 1, 2020 (75.0% v. 44.1%; adjusted risk difference 30.9%, 95% CI 17.3% to 44.4%) (Figure 4; Appendix 2, Supplementary Table 7). For Canadian sites, the incidence of cardiac involvement also increased over time (Appendix 8, Supplementary Figure 5, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210873/tab-related-content). In a stratified analysis of patients with confirmed or probable MIS-C, we observed the association between being admitted on or after Nov. 1, 2020, and increased risk of cardiac involvement among patients with probable MIS-C (Appendix 2, Supplementary Table 8, Appendix 9, Supplementary Figure 6, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210873/tab-related-content).
Figure 4:
Adjusted risk differences for cardiac involvement. Average marginal effects are presented with adjusted risk differences from multivariable logistic regressions. The reference categories used for risk differences are the absence of the symptom or the reported category. Models are adjusted for sex, age, country, presence of comorbidity, coinfection, confirmed case status and admission time. We used 9 different models, each assessing the 9 exposures. Note: CI = confidence interval.
Interpretation
We found that older age and initial ferritin level were associated with an increased risk of ICU admission among children with MIS-C. Another large study also identified older age as a risk factor (adjusted odds ratio [OR] 2.6 for age 13–20 yr v. 0–5 yr; adjusted OR 1.9 for age 6–12 yr v. 0–5 yr).9 In the same study, peak inflammatory markers, brain natriuretic peptide, serum troponin and nadir platelet counts were predictive of ICU admission,9 but initial values are of more practical value than peak and nadir results.
We found evidence for greater severity of MIS-C later in the study period. Exploratory analyses showed that the adjusted risk for cardiac involvement increased in the second period of the study, with a trend toward a higher incidence of ICU admission. However, the severity appeared to be less than in cohorts from the United States observed from March to October 202010 and from March 2020 to January 2021.11 In these studies, 74%10 and 58% of patients,11 respectively, required ICU care, compared with 31% in the current study. Mechanical ventilation was required in 17% in 1 US cohort,10 compared with 4% in the current study. Similarly, vasopressors were required in 45% in this cohort,10 compared with 20% in the current study. Differences in case criteria, patient management and threshold for ICU admission may account for some of the variability. We cannot provide a clear explanation for the increased MIS-C severity in the later period of our study. The features of severe MIS-C were widely publicized by May 2020, so it seems unlikely that severe cases were missed early in the study period. SARS-CoV-2 variants of concern have replaced the wild-type virus. It is possible that the immune response to circulating variants alters the severity of COVID-19 and MIS-C when compared with wild-type virus.
Multisystem inflammatory syndrome in children is a new diagnosis, with differing diagnostic criteria that have not been validated. The criteria differ in their requirement for evidence of SARS-CoV-2 infection.3 In the absence of viral detection or positive serology, CDC criteria require likely exposure to SARS-CoV-2 within the previous 4 weeks. In contrast, WHO criteria do not specify the timing of exposure and do not clarify whether exposure must be to a specific person or whether exposure includes residence in a community with circulating cases (the definition applied in the current study).4 Finally, RCPCH criteria3 and a recent large international study12 did not require a positive SARS-CoV-2 test or history of exposure and, therefore, all of the 126 children with probable MIS-C in our study would have met these criteria. Most of these children lacked a history of contact with a person with proven SARS-CoV-2 infection. Identifying exposure can be difficult as infected contacts may be asymptomatic or may never have been tested. Confirmation of past infection is complicated as serology is not 100% sensitive13 and was not available until partway through the study. As more individuals are infected or vaccinated, the diagnostic utility of serology will decline. An international consensus on MIS-C diagnostic criteria is thus necessary to serve both clinical and research purposes and to allow for better comparison of cohorts.
Despite nearly one-third of patients with MIS-C requiring ICU care,14 recovery was typically rapid, with 85% of patients discharged within 10 days. The role of adjunctive glucocorticoids and the optimal glucocorticoid regimen has not been established, with recent large studies reporting discordant results.12,15 It is not known whether glucocorticoids could replace IVIG in low-income countries where IVIG is not readily available. Of note, MIS-C resolved without IVIG or glucocorticoids in 10% of patients in our study, mainly among those admitted to hospital early in the study period. It is not clear whether this is because these patients had mild MIS-C or because not all patients require treatment. Long-term follow-up will be important to determine the role of treatment.
Limitations
This study has all the limitations of a chart review. We did not have a formal process to verify the accuracy of data, but generated queries for data that were obviously incorrect (such as when the date of discharge was before the date of admission). We do not have long-term follow-up data, but an MIS-C case series from the US reported resolution of cardiac aneurysms in 45 of 57 patients (79%) and normalization of left ventricular function in 91% of 172 patients 30 days after the initial echocardiogram.10 In our study, fewer than half of the patients had a positive test for SARS-CoV-2. Diagnostic criteria from MIS-C have not been validated and differ globally. Patients with milder MIS-C may not have been admitted or recognized. Serology for SARS-CoV-2 was not available early in our study period. Race and ethnicity are not reliably recorded in health records in Canada. Although our cohort included data from 15 sites, the total sample size was modest and the use of full models to investigate multiple predictors can lead to small data bias and overfitting. However, our outcomes were common and their number satisfied the relaxed rule of 10 events per variable when using logistic regression.16 To assess the more detailed role of comorbidities, a large sample size would be necessary to perform a more elaborate analysis, taking the different and heterogeneous comorbidities into account.
Conclusion
Older age and a high initial level of serum ferritin predict the need for ICU admission among children admitted to hospital with MIS-C. Further research is needed to evaluate long-term outcomes of MIS-C with and without treatment, as well as changes in MIS-C severity and short- and long-term outcomes with emerging variants of concern.
Supplementary Material
Footnotes
Competing interests: Joanna Merckx reports a role as medical director of bioMerieux Canada and as a volunteer member of the Belgian Pediatric Task Force for COVID-19 during the conduct of the study. She is also an independent researcher, with a contract with Public Health Belgium, Sciensano for a study on the seroprevalence of SARS-CoV-2 in schools. E. Ann Yeh reports grants from Biogen, Roche, Horizon Therapeutics and Alexion; honoraria from Prime and Novartis; and participation on advisory boards with Roche and Horizon Therapeutics. She is also involved with the editorial board or is an editor with Multiple Sclerosis and Related Disorders, Multiple Sclerosis Journal, PLOS ONE and Neurology. Jesse Papenburg reports grants from AbbVie, Sanofi Pasteur, Merck and MedImmune; consulting fees from Merck; and honoraria from AbbVie, AstraZeneca and Seegene. He is a member of the National Advisory Committee on Immunization. Marie-Astrid Lefebvre reports an honorarium from Takeda Canada. Tammie Dewan reports grants from the Sick Kids Hospital Foundation, the Canadian Institutes for Health Research (CIHR) and the Department of Pediatrics at the University of Calgary. She is a past president of the Complex Care Section of the Canadian Pediatric Society. Jared Bullard reports grants from CIHR, the Manitoba Medical Service Foundation and Research Manitoba, as well as participation on provincial and hospital leadership committees. He is also secretary of the Association of Medical Microbiology and Infectious Diseases. Manish Sadarangani reports grants from GlaxoSmithKline, Merck, Moderna, Pfizer, Sanofi Pasteur, Seqirus, Symvivo and VBI Vaccines, as well as participating on 2 data safety monitoring boards for COVID-19 vaccine trials. Rupeena Purewal reports honoraria and consulting fees from Verity Pharmaceuticals. Kirk Leifso reports a grant from the Hospital for Sick Children. Cheryl Foo reports an unpaid position with IMPACT. No other competing interests were declared.
This article has been peer reviewed.
Contributors: The study was conceived and designed by Michelle Barton, Dara Petel, Sarah Tehseen and Joan Robinson. Suzette Cooke, Tala El Tal, Ari Bitnun, Shaun Morris, Peter Gill, Jesse Papenburg, Marie-Astrid Lefebvre, Rolando Ulloa-Gutierrez, Helena Brenes-Chacon, Adriana Yock-Corrales, Gabriela Ivankovich-Escoto, Alejandra Soriano-Fallas, Marcela Hernandezde Mezerville, Tammie Dewan, Lea Restivo, Alireza Nateghian, Behzad Haghighi Aski, Ali Manafi, Jared Bullard, Alison Lopez, Manish Sadarangani, Ashley Roberts, Michelle Barton, Dara Petel, Nicole Le Saux, Jennifer Bowes, Rupeena Purewal, Janell Lautermilch, Ann Bayliss, Jacqueline Wong, Kirk Leifso, Cheryl Foo and Joan Robinson obtained approval for data acquisition, or supervised or collected data themselves. Joanna Merckx performed the statistical analysis. All authors contributed to data interpretation. Joan Robinson drafted the initial manuscript. All authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was partially funded by a Janeway Foundation Research Grant to support data collection.
Data sharing: Requests for data should be submitted to the corresponding author.
References
- 1.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395:1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cirks BT, Rowe SJ, Jiang SY, et al. Sixteen weeks later: expanding the risk period for multisystem inflammatory syndrome in children. J Pediatric Infect Dis Soc 2021;10:686–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2021;39:3037–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19 [scientific brief]. Geneva: World Health Organization; 2020. Available: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed 2021 May 10). [Google Scholar]
- 5.Johansson MA, Quandelacy TM, Kada S, et al. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open 2021;4:e2035057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019;13(Suppl 1):S31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol 2014;43:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrams JY, Oster ME, Godfred-Cato SE, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health 2021;5:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldstein LR, Tenforde MW, Friedman KG, et al. Overcoming COVID-19 Investigators. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021;325:1074–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belay ED, Abrams J, Oster ME, et al. Trends in geographic and temporal distribution of US children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr 2021;175:837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McArdle AJ, Vito O, Patel H, et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med 2021;385:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Caeseele P, Bailey D, Forgie SE, et al. SARS-CoV-2 (COVID-19) serology: implications for clinical practice, laboratory medicine and public health. CMAJ 2020;192:E973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouldali N, Toubiana J, Antona D, et al. French Covid-19 Paediatric Inflammation Consortium. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA 2021;325:855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Son MBF, Murray N, Friedman K, et al. Overcoming COVID-19 Investigators. Multisystem inflammatory syndrome in children: initial therapy and outcomes. N Engl J Med 2021;385:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.