To the editor:
Despite an initial satisfactory increase in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antispike antibody (Ab) titer after completing a 3-dose regimen in the first set of vaccination in dialysis patients,1 Ab titer substantially decreases at 6 months in this population.2 Other authors suggested that for the B.1.1.529 Omicron variant, 3 vaccine doses might be insufficient in in-center hemodialysis patients.3
We assessed the dynamics of the anti–SARS-CoV-2 spike protein S1 total Ig Ab (Roche Elecsys immunoassay4) of both hemodialysis (n = 17) and peritoneal dialysis (n = 28) patients who received a 3-dose regimen of the mRNA BNT162b2 (Pfizer–BioNTech), followed by a fourth “booster” dose of mRNA vaccine (BNT162b2, n = 43, or mRNA-1273 [Moderna], n = 2), after a median of 7.6 [interquartile range: 7.1; 7.8] months after the third dose (Supplementary Figure S1). Patients with a breakthrough infection (symptomatic or not) before the fourth dose were excluded (Supplementary Figure S2).
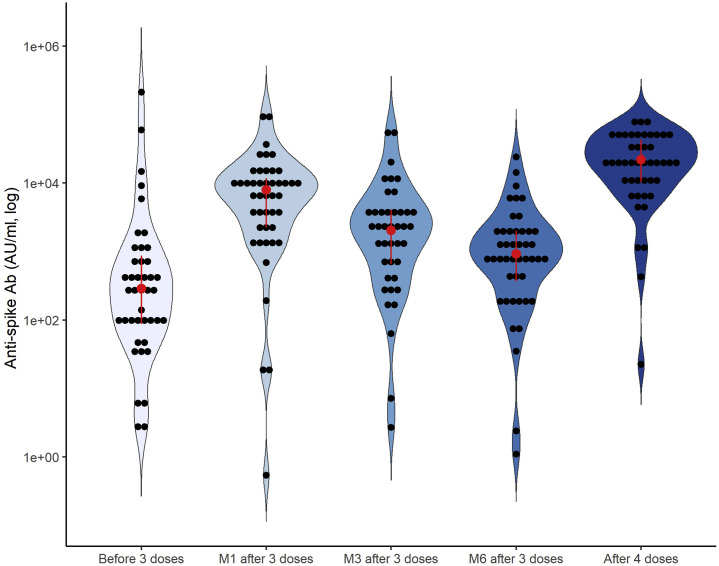
In patients (57.8% men, median age 72 [56; 79] years), 15.6% had a history of immunosuppression (Supplementary Table S1). At 15 [14; 22] days after the fourth “booster” dose, antispike Ab titer significantly increased from 923 [369; 2019] to 21,883 [10,234; 42,870] AU/ml (Figure 1 ; Supplementary Figure S3), which corresponds to a 19-fold increase (median) in antispike Ab titer. Ab titer after the fourth dose was 3.4-fold higher (median) than the Ab peak reached after the third dose. Dose 4 appeared well-tolerated (Supplementary Figure S4), and no serious adverse event was observed. After the fourth dose, only 2 patients developed a breakthrough infection (vs. 7 cases of coronavirus disease 2019 after the third dose; Supplementary Table S2).
Figure 1.
Kinetics of antispike antibodies. The figure shows the antispike antibody (Ab) levels before, and 1 (M1), 3 (M3), and 6 (M6) months after the third dose of the mRNA BNT162b2 vaccine, and after the fourth vaccine dose (BNT162b2 Pfizer–BioNech or mRNA-1273 Moderna) in dialysis patients. Each point represents individual data. Antibody titers lower than 1 cannot be plotted in the graph because of the logarithm scale. The red points and vertical lines indicate the median with interquartile range. Conversion factor: 1 AU/ml = 1.0288 BAU/ml.
To conclude, our finding shows that a 3-dose regimen of an mRNA-based vaccine with a fourth booster dose appears to produce an important antibody response in dialysis patients, with a significant increase in antispike Ab titer. Long-term follow-up studies are needed to assess if this vaccination strategy elicits a durable and robust protective immune response against SARS-CoV-2 in dialysis patients.
Data Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank patients for their participation in the study.
Author Contributions
PH and A-LF researched the idea, created the study design, acquired the data, and analyzed and interpreted the data. A-LF provided statistical analyses. SK performed the antispike serology testing. Each author contributed important intellectual content during manuscript drafting or revision.
Footnotes
Supplementary questionnaire on vaccine reactions and global tolerance after the fourth vaccine dose.
Table S1. Characteristics of the study population.
Table S2. Characteristics of the patients with breakthrough coronavirus disease 2019 (COVID-19) after the third or the fourth vaccine dose during the fifth wave pandemic.
Figure S1. Vaccination strategy in dialysis patients.
Figure S2. Flowchart.
Figure S3. Kinetics of the antispike antibodies.
Figure S4. Self-reported tolerance.
Supplementary Material
References
- 1.Bensouna I., Caudwell V., Kubab S., et al. SARS-CoV-2 antibody response after a third dose of the BNT162b2 vaccine in patients receiving maintenance hemodialysis or peritoneal dialysis. Am J Kidney Dis. 2022;79:185–192.e1. doi: 10.1053/j.ajkd.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Housset P., Kubab S., Pardon A., et al. Waning but persistent humoral response 6 months after the third dose of the mRNA BNT162b2 vaccine in hemodialysis and peritoneal dialysis patients. J Nephrol. 2022;35:783–785. doi: 10.1007/s40620-022-01276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr E.J., Wu M., Harvey R., et al. Omicron neutralising antibodies after COVID-19 vaccination in haemodialysis patients. Lancet. 2022;399:800–802. doi: 10.1016/S0140-6736(22)00104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muench P., Jochum S., Wenderoth V., et al. Development and validation of the Elecsys anti-SARS-CoV-2 immunoassay as a highly specific tool for determining past exposure to SARS-CoV-2. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.01694-20. e01694-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.