Abstract
Aim:
To investigate how combined electrographic and radiologic data inform outcomes in children after cardiac arrest.
Methods:
Retrospective observational study of children admitted to the pediatric intensive care unit (PICU) of a tertiary children’s hospital with diagnosis of cardiac arrest from 2009 to 2016. The first 20 minutes of electroencephalogram (EEG) background was blindly scored. Presence and location of magnetic resonance imaging (MRI) diffusion-weighted image (DWI) abnormalities were correlated with T2-weighted signal. Outcomes were categorized using Pediatric Cerebral Performance Category (PCPC) scores at hospital discharge, with “poor outcome” reflecting a PCPC score of 4–6. Logistic regression models examined the association of EEG and MRI variables with outcome.
Results:
41 children met inclusion criteria and had both post-arrest EEG monitoring within 72 hours after ROSC and brain MRI performed within 8 days. Among the 19 children with poor outcome, 10 children did not survive to discharge. Severely abnormal EEG background (p<0.0001) and any diffusion restriction (p<0.0001) were associated with poor outcome. The area under the ROC curve (AUC) for identifying outcome based on EEG background alone was 0.86, which improved to 0.94 with combined EEG and MRI data (p=0.02).
Conclusion:
Diffusion abnormalities on MRI within 8 days after ROSC add to the prognostic value of EEG background in children surviving cardiac arrest.
Introduction:
Cardiac arrest patients comprise 0.7–2% of all pediatric hospital admissions and 1.4–5.5% of all pediatric intensive care unit (PICU) admissions.1–3 The incidence of out-of-hospital pediatric cardiac arrest patients is 8 per 100,000 child person-years.4 Morbidity and mortality after pediatric cardiac arrest cases are high. Favorable neurologic outcomes are variable and are largely influenced by whether the cardiac arrest occurs in or out of a hospital setting.5,6 Neurologic injury is a commonly-cited cause of death after pediatric cardiac arrest, accounting for 20% of mortality.6 Prognostication is often guided by clinical examination and neurophysiologic and/or neuroimaging data. The ability to provide early but accurate prognostication is critical to families and healthcare providers.
In 2015 The American Clinical Neurophysiology Society published a consensus statement of indications for continuous electroencephalography (EEG) in critically-ill children, including monitoring for nonconvulsive seizures and prognostication after cardiac arrest.7 These guidelines were based on a growing body of evidence that EEG is a useful tool to prognosticate neurologic outcome after cardiac arrest. Specifically, electrographic seizures and status epilepticus on EEG after cardiac arrest are associated with unfavorable neurologic outcomes.8,9 Aside from these clinical events, both EEG background and characteristics are important markers of the recovering brain.10,11 In particular, severely abnormal EEG background (discontinuous or burst-suppression, or attenuated-featureless) is associated with poor neurologic outcome after cardiac arrest.8,10,12
Prognostication after pediatric cardiac arrest may be assisted by visualizing injury patterns on magnetic resonance imaging (MRI). Restricted diffusion on diffusion-weighted imaging (DWI) indicates acute injury, followed by T2 prolongation in the subacute time period.13,14 The evolution of apparent diffusion coefficient (ADC) values following injury also informs insult timing since DWI hyperintensity represents a combination of diffusion restriction and T2 shine-through.15,16 Regional patterns of diffusion restriction in areas of deep gray, cerebral cortex, and cerebellum have been associated with poor neurologic recovery after cardiac arrest.15,17,18 In adults, restricted diffusion anywhere in the brain after cardiac arrest correlates with a poorer outcome, particularly if seen in the hippocampi.19,20,21
Identifying reliable predictors of outcomes will benefit both the clinicians and parents in making difficult end-of-life decisions, in addition to providing a basis for possible interventions. Although EEG and MRI have been investigated independently, there is limited data on their combined utility. EEG is a strong predictor for outcomes, and it is unclear what level of prognostic significance MRI adds. Our objective is to investigate how using a multimodal approach with electrographic and radiologic data informs outcomes in children after cardiac arrest. We hypothesized that regional diffusion restriction on MRI combined with EEG characteristics would be superior to EEG’s ability alone to independently distinguish neurologic outcome after cardiac arrest in children.
Methods:
Patient Selection
We completed a retrospective chart review of PICU patients with return of spontaneous circulation (ROSC) after cardiac arrest under an institutional review board-approved human studies protocol that waived the need for informed consent. Participants were identified from St. Louis Children’s Hospital’s Virtual Pediatric Systems database from October 31, 2009 to December 31, 2016 using ICD-9 code 427.5 (cardiac arrest).22 Exclusion criteria were not having both EEG and brain MRI modalities performed, specifically patients who did not have EEG within 72 hours and brain MRI within 8 days after arrest. We also excluded patients where the medical record explicitly noted cardiopulmonary resuscitation (CPR) duration of 30 seconds or less. Two patients were excluded because MRI diffusion changes were more consistent with underlying metabolic disease. Children with acute traumatic brain injury (TBI) with concomitant cardiac arrest were included in this study.
We reviewed the medical record for demographic and clinical data including: age, gender, preexisting comorbidities, survival, intervals between arrest and EEG and MRI, and outcomes at discharge from hospital, as well as cardiac arrest location, etiology and duration. Patients who experienced cardiopulmonary arrest at another hospital were included as in-hospital arrest patients.
Clinical data
EEG was performed as clinically indicated by using either a Stellate Harmonia (Natus, Pleasanton, CA) for studies from 2009–2013 or Nihon Kohden digital EEG system (Nihon Kohden, Tokyo, Japan) for studies from 2014 onward. EEG was excluded from analysis if performed greater than 72 hours after cardiac arrest. Our institution did not have a post-cardiac arrest EEG monitoring protocol until 2016, with a formalized guideline enacted in 2017. EEG recordings were scored using the first 20 minutes of either a stat or continuous monitoring epoch by a board-certified pediatric epileptologist blinded to all clinical information (RMG). EEG background was scored using four categories as either (1) normal (including sedated sleep), (2) slow-disorganized, (3) discontinuous or burst-suppression, and (4) attenuated-featureless. Prior cardiac arrest EEG studies have used this system for assessing background activity in critically-ill children.9,10,23 This background data was then dichotomized into either favorable (normal or slow-disorganized) or unfavorable (discontinuous/burst-suppression or attenuated-featureless). Further EEG characteristics were obtained through review of clinical reports, including status epilepticus and seizures, as the full clinical report often included EEG data beyond the first 20 minutes.
Post-cardiac arrest MRI was ordered at the discretion of the treating medical team. Images were blindly reviewed for locations of injury by a board-certified pediatric neurologist with experience in neuroimaging (KPG) and compared to clinical reads by board-certified neuroradiologists. When discrepancies existed, a blinded, board-certified pediatric neuroradiologist adjudicated (AYM). Injury characteristics were categorized according to the presence of diffusion restriction in specified locations (cortical, white matter, deep gray, hippocampal or cerebellar). To characterize these findings as acute versus subacute injury, accompanying ADC values and T2-weighted signal changes were identified using echo-planar diffusion and T2/FLAIR sequences. The presence of intracranial hemorrhage was also recorded as a separate indicator of injury severity. Chronic neurologic injuries, as determined through imaging characteristics at presentation and documentation of prior injury in the medical record, were excluded from analysis. If multiple post-arrest MRIs were performed, only the first study was included.
Primary outcome measured was neurologic outcome as categorized by Pediatric Cerebral Performance Category (PCPC) scores at hospital discharge.24 A “good outcome” was defined as a PCPC score of 1–3 (normal, mild disability, moderate disability). A “poor outcome” was a PCPC score 4–6 (severe disability, coma or vegetative state, death). Chart review determined factors contributing to death.
Statistics
We performed a univariate analysis of demographic, neurophysiologic, neuroimaging findings and outcomes. Variables with p<0.05 were entered into logistic regression model. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for identification of a poor outcome. Sensitive analyses were completed for survivors and with a broader definition of poor outcome. Receiver operating characteristic (ROC) curves evaluated the performance of EEG background characteristics and MRI injury patterns in detecting poor outcomes. ROC curves were then compared with nested models. Statistical analysis was performed in SAS 9.4 (Cary, NC).
Data Availability: Anonymized data not published within this article will be made available by request to qualified investigators.
Results:
A total of 158 children were admitted to our PICU after cardiac arrest resuscitation between October 31, 2009 and December 31, 2016 and had post-arrest MRI or EEG performed. Only 41 met final inclusion criteria, including having both EEG and MRI performed, with MRI done within 8 days after ROSC (Fig.1). Of these, 35 (85%) were out-of-hospital arrest patients (Table). Post-arrest brain MRI was obtained with median of 3 days IQR [1–4] after cardiac arrest. Among the 41 children, 19 (46%) had a poor outcome. Of survivors (n=31, 76%), there was no difference in the proportion of children with poor outcome (PCPC 4–6) between in-hospital (33%) and out-of-hospital (28%) cardiac arrest cases.
Fig. 1: Inclusion and Exclusion.
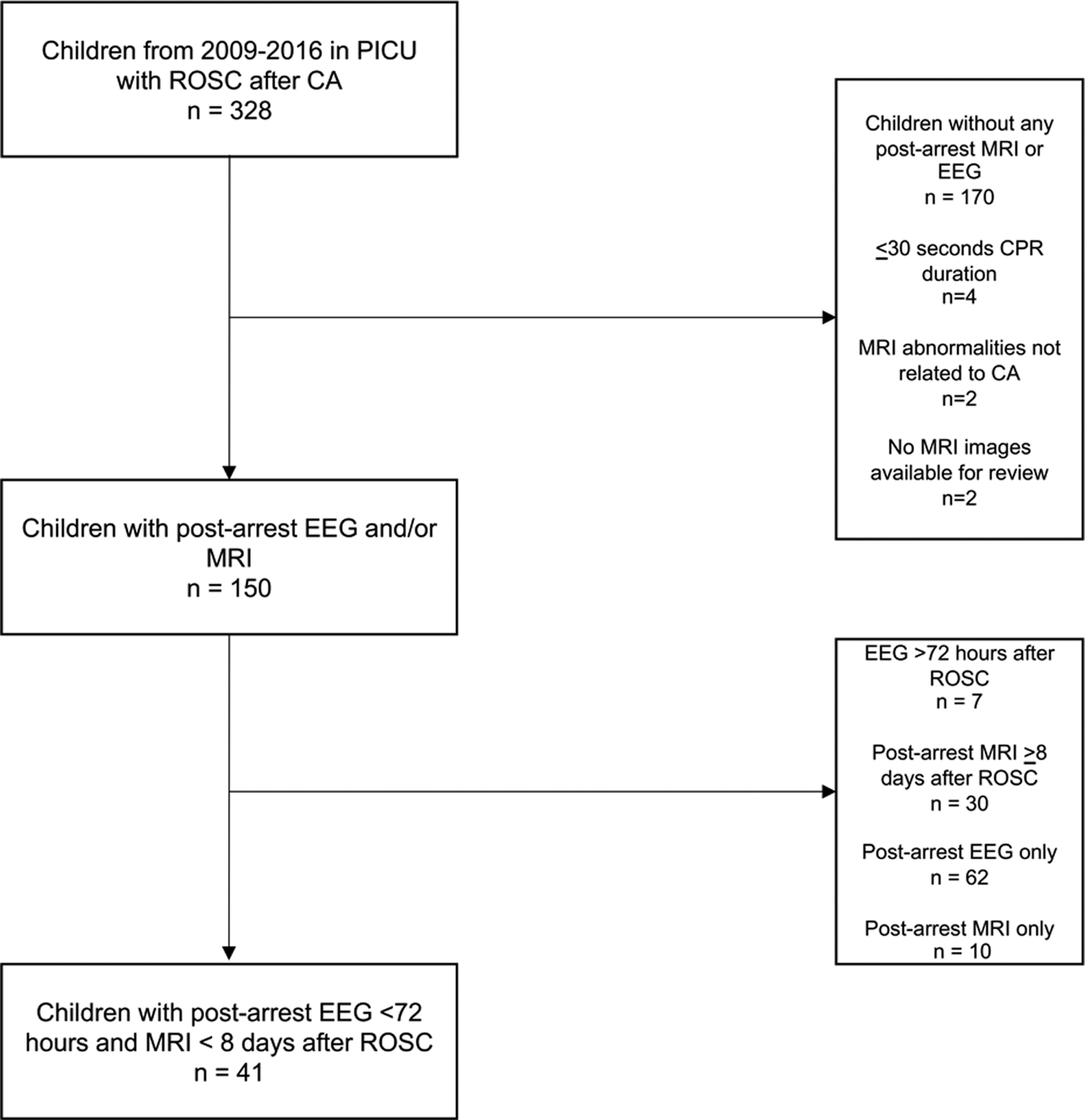
PICU=pediatric intensive care unit; ROSC=return of spontaneous circulation; CA=cardiac arrest; EEG=electroencephalogram; MRI= magnetic resonance imaging.
Table:
Patient Characteristics. TBI=traumatic brain injury; EEG=electroencephalogram; MRI= magnetic resonance imaging; PCPC=Pediatric Cerebral Performance Category.
| Clinical Variable | Total (n=41) | Good Outcome (n= 22) | Poor Outcome (n= 19) | P-value |
|---|---|---|---|---|
| Age in years, median [IQR] | 3.3 [0.3–10.4] | 2.9 [0.2–9.8] | 3.3 [0.6–12.2] | 0.35 |
| Male sex, n (%) | 28 (68) | 16 (73) | 12 (63) | 0.51 |
| Comorbidities, n (%) | ||||
| None | 34 (83) | 18 (82) | 16 (84) | 0.84 |
| Epilepsy | 2 (5) | 0 (0) | 2 (11) | 0.21 |
| Developmental delay | 2 (5) | 2 (9) | 0 (0) | 0.49 |
| Previous stroke | 2 (5) | 1 (4) | 1 (5) | >0.99 |
| Previous neurosurgical procedure | 1 (2) | 1 (4) | 0 (0) | >0.99 |
| Etiology of cardiac arrest, n (%) | 0.15 | |||
| Unknown | 7 (17) | 3 (14) | 4 (21) | |
| Asphyxia/respiratory | 21 (51) | 13 (59) | 8 (42) | |
| Cardiac | 2 (5) | 2 (9) | 0 (0) | |
| Near drowning | 6 (15) | 1 (4) | 5 (26) | |
| TBI | 2 (5) | 2 (9) | 0 (0) | |
| Other | 3 (7) | 1 (4) | 2 (11) | |
| Cardiac Arrest Location, n (%) | 0.67 | |||
| In-hospital | 6 (15) | 4 (18) | 2 (11) | |
| Out-of-hospital | 35 (85) | 18 (82) | 17 (89) | |
| Recorded arrest duration, n (%) | 17 (41) | 8 (36) | 9 (47) | 0.03 |
| Arrest duration in minutes, median [IQR] | 22 [5–30] | 4.5 [2–12.8] | 30 [22–30] | 0.01 |
| Arrest to EEG (hours), median [IQR] | 7.4 [4.8–20.4] | 12.5 [5.3–26.9] | 5.9 [4.3–10.1] | 0.05 |
| Abnormal EEG background, category 3 or 4, n (%) | 14 (34) | 0 (0) | 14 (74) | <0.0001 |
| Seizures, n (%) | 7 (17) | 3 (14) | 4 (21) | 0.68 |
| Status epilepticus, n (%) | 3 (7) | 0 (0) | 3 (16) | 0.08 |
| Arrest to MRI (days), median [IQR] | 3 [1–4] | 2 [1–4] | 4 [2.3–6] | 0.05 |
| Any hemorrhage on MRI, n (%) | 12 (29) | 4 (18) | 8 (42) | 0.17 |
| Any diffusion restriction on MRI, n (%) | 28 (68) | 9 (41) | 19 (100) | <0.0001 |
| Survived, n (%) | 31 (76) | 22 (100) | 9 (47) | <0.0001 |
| Outcomes by PCPC | ||||
| 1 = Normal | 6 (15) | |||
| 2= Mild disability | 12 (29) | |||
| 3= Moderate disability | 4 (10) | |||
| 4= Severe disability | 4 (10) | |||
| 5= Coma/vegetative state | 5 (12) | |||
| 6 = Deceased | 10 (24) |
In analysis of post-arrest EEG monitoring, 8 (20%) had normal background (category 1), 19 (46%) had slow-disorganized background (category 2), 13 (32%) had discontinuous or burst-suppression background (category 3) and 1 (2%) had attenuated-featureless background (category 4). EEG categories 3 or 4 had 74% sensitivity and 100% specificity for poor neurologic outcome (Supplemental Table).
In evaluating the MRI data, 28 (68%) had restricted diffusion indicating acute injury and 20 (49%) had T2-weighted signal commensurate with scan timing and deemed subacute injury related to the arrest. Furthermore, 12 (29%) had hemorrhage of which 11 (92%) represented only microhemorrhage on susceptibility weighted sequences. There were 19 children with deep gray diffusion injury and 18 with cortical diffusion injury. Diffusion injury in any region had 100% sensitivity and 59% specificity for poor neurologic outcome (Supplemental Table). Figure 2 shows examples of injury patterns.
Figure 2: Examples of post-arrest neuroimaging abnormalities.
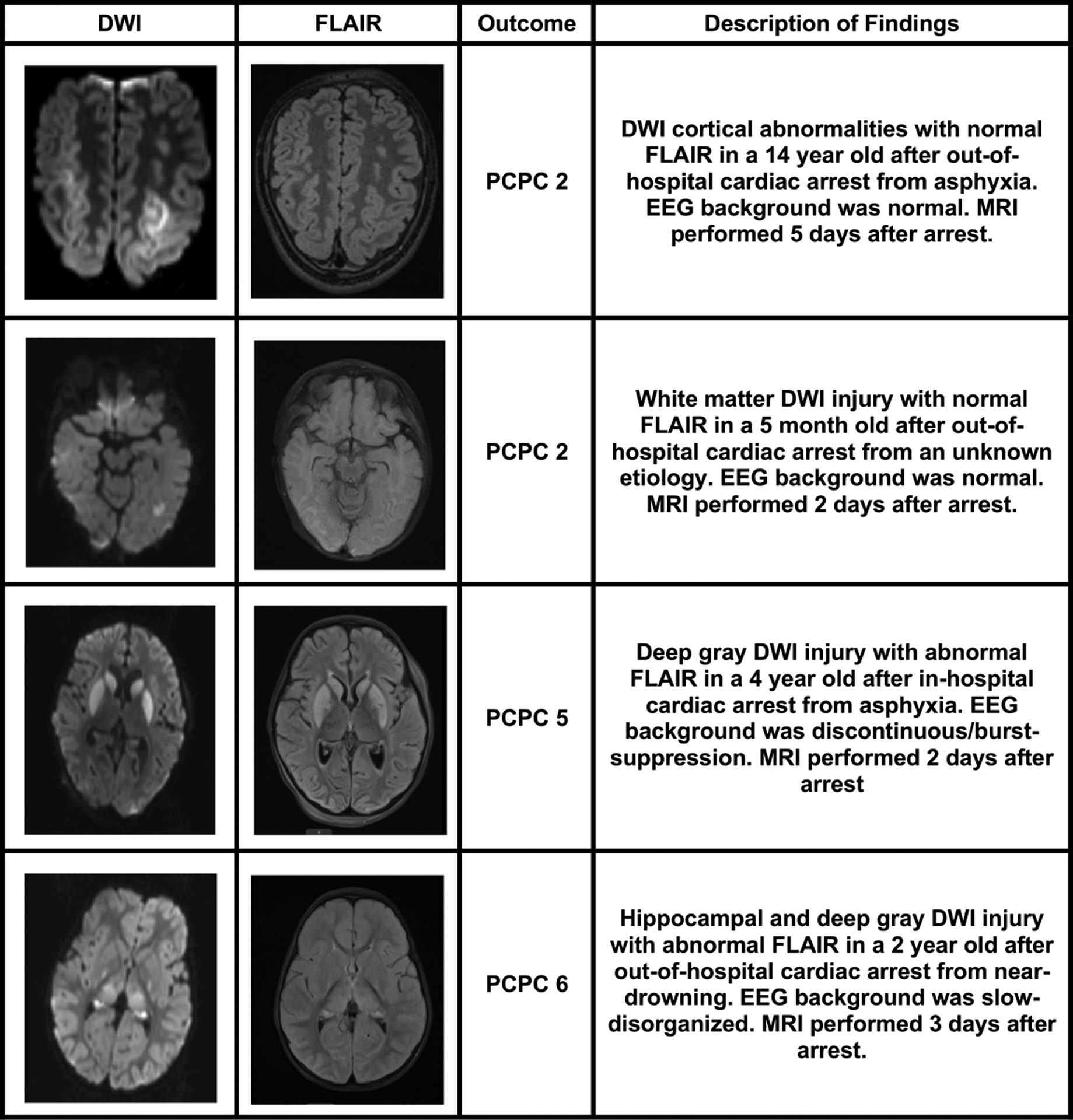
Four children with MRI performed after cardiac arrest are highlighted. MRI= magnetic resonance imaging; DWI=diffusion-weighted imaging; FLAIR=fluid-attenuated inversion recovery; PCPC=Pediatric Cerebral Performance Category.
Figure 3 illustrates the frequency of combined EEG background and MRI diffusion abnormalities with proportion of good and poor outcome for each combination. Initial EEG background ability to distinguish patients who ultimately had poor neurologic outcome increased with addition of diffusion abnormalities on imaging (AUC 0.86 and 0.94, respectively, p=0.02) (Fig. 4). Combined data performed better than MRI alone (AUC 0.79, p = 0.0008). Evaluating only survivors to ensure that abnormalities did not influence redirection of care, the AUC for combined initial EEG background and MRI within 8 days among survivors only remained high at 0.93. To confirm that these results were not dependent on how “poor outcome” was categorized, we completed a sensitivity analysis with poor outcome defined as PCPC 3–6. The results were nearly identical, with combined EEG and MRI within 8 days AUC 0.95, which was significantly higher than either than AUC for EEG alone (0.80; p=0.001) or MRI alone (0.86; p=0.02).
Fig. 3:
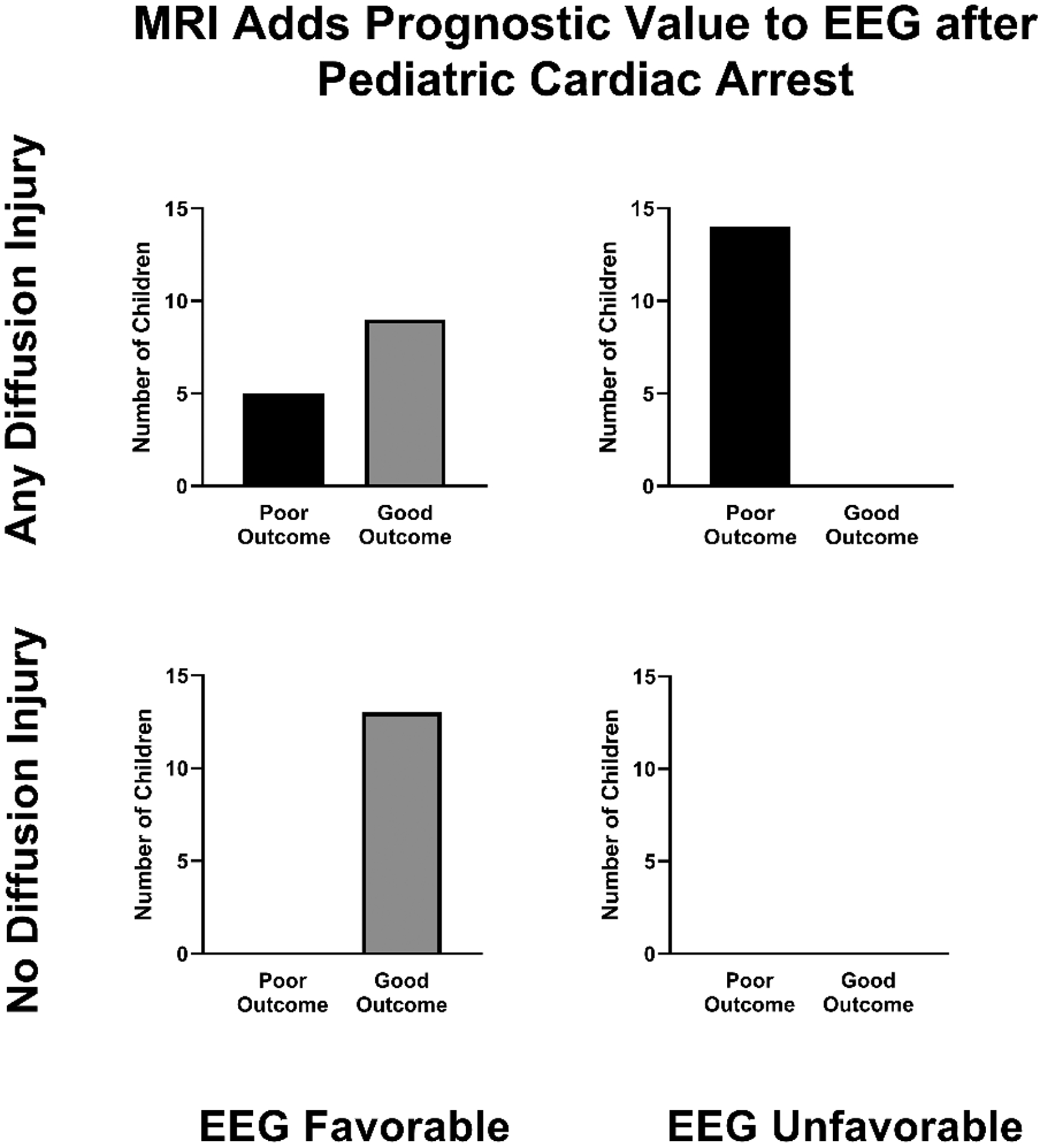
MRI diffusion abnormalities with EEG background in relation to outcome at hospital discharge. All children with poor outcome had MRI diffusion abnormalities, of which more had an unfavorable EEG background than favorable. No child with unfavorable EEG background had normal diffusion imaging. Favorable EEG background includes normal or slow-disorganized and unfavorable EEG background includes discontinuous/burst-suppression or attenuated-featureless. EEG=electroencephalogram; MRI= magnetic resonance imaging.
Fig. 4. Multimodal test results improve ability to discriminate poor outcome after pediatric cardiac arrest.
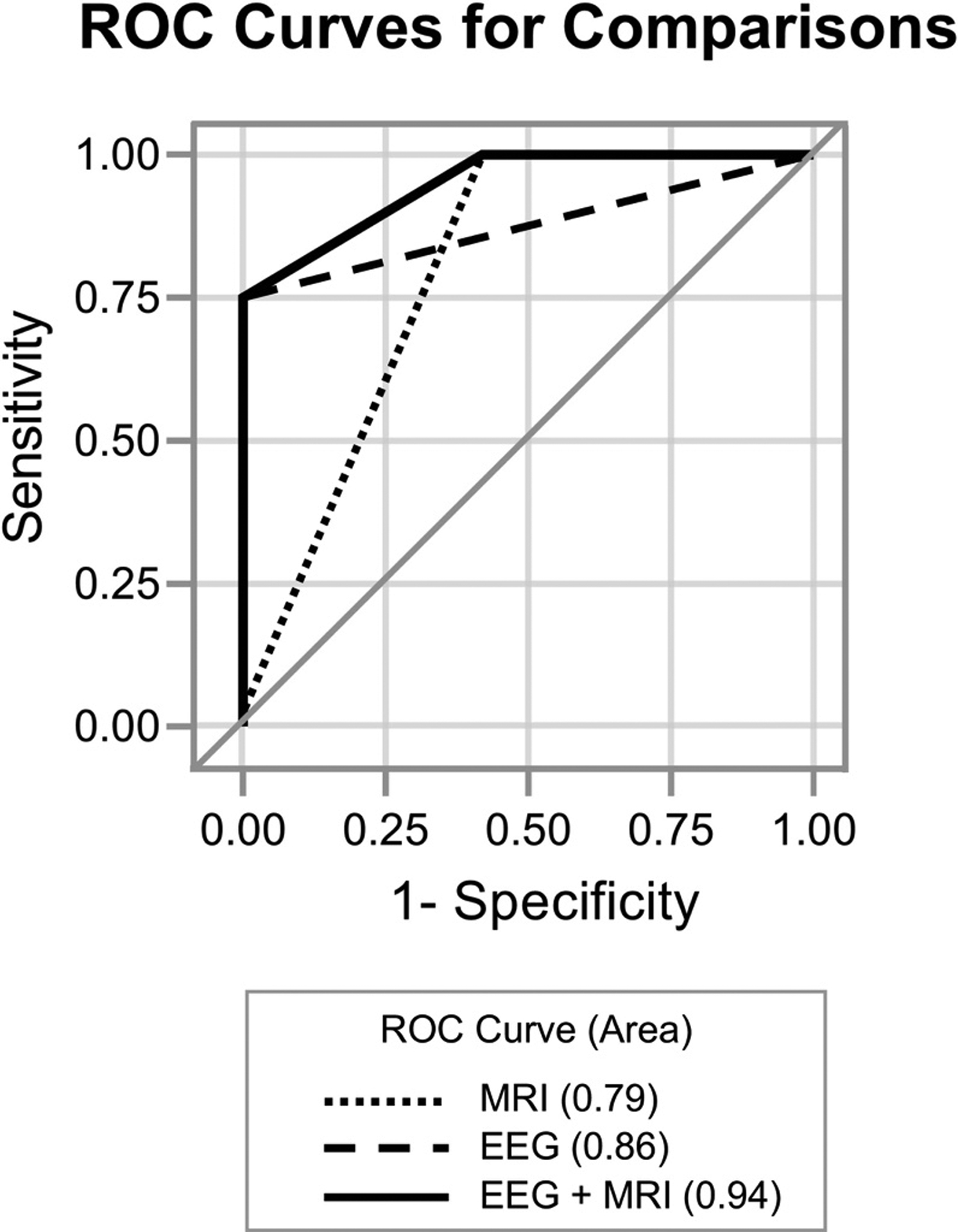
ROC curves depicting EEG suppressed background (Category 3 or 4, AUC=0.86), MRI diffusion abnormalities (AUC=0.79) and combined (EEG+MRI, AUC=0.94) were compared. A model combining EEG suppressed background with MRI diffusion abnormalities is better able to distinguish poor outcome than either EEG (p=0.02) or MRI alone (p=0.0008). EEG=electroencephalogram performed less than 72 hours after cardiac arrest; MRI=magnetic resonance imaging, post-arrest; AUC=area under the ROC curve.
We examined factors contributing to the death of the 10 non-survivors after cardiac arrest resuscitation. Brain death criteria were met in 1 child (10%) and 1 child had multisystemic organ dysfunction as cause of death. In 8 patients (80%), poor neurologic prognosis was cited as the reason for redirection of care. Of these, 6 had an abnormal EEG background category 3 or 4 and all 8 had diffusion abnormalities on their MRI.
Discussion:
This study found that combined multimodal EEG and MRI data had superior ability to discriminate outcome than either EEG or MRI alone in children who suffered cardiac arrest. EEG and MRI have been independently investigated for their prognostic value after pediatric cardiac arrest, but to our knowledge, the utility of their combined use in children has not been well studied.8–12,15,17–20,25,26 Consistent with previous reports, our data show severe attenuation or burst suppression on EEG background is highly associated with poor neurologic outcome, while non-attenuated EEG background outcomes were mixed. MRI signs of injury in the cortex, deep gray nuclei and/or hippocampus were also strongly correlated with poor outcome, regardless of EEG background. When these two modalities are combined, they are almost perfectly able to distinguish outcome categorization based on PCPC at hospital discharge.
Importantly, we demonstrate that “favorable” EEG background does not guarantee a good outcome. In another study of EEG after pediatric cardiac arrest, only 69% (43/62) of children with normal or slow EEG backgrounds ultimately had favorable outcomes.23 In our data, 81% of children with EEG background category 1 or 2 had good outcomes of PCPC 1–3 (22/27). None of the 5 children with EEG background 1 or 2 and poor outcome had severe pre-existing co-morbidity. The EEG background after cardiac arrest can assess both global and regional neuronal injury. However, the surface EEG cannot fully characterize and localize more discrete anatomical injury. Our results suggest that MRI adds prognostic information beyond EEG alone.
Commonly accepted markers of anoxic brain injury on MRI, whether global or localized, include restricted diffusion and T2 prolongation. In our data of brain MRI obtained less than 8 days after ROSC, diffusion restriction present in any region of the brain was associated with poor outcome (p<0.0001). Brain regions susceptible to injury on MRI after cardiac arrest in children have been previously reported to have an association with poor outcome, specifically basal ganglia, subcortical or deep white matter tracts, cortical ribbon and cerebellum.15,17,18,27–30 Patterns of injury after hypoxic-ischemic events are variable and depend both on the duration of anoxia and the degree of vulnerability of the involved tissue. The most common cause of pediatric cardiac arrest is asphyxia, and the resultant hypoxemia leading to cessation of cardiac output culminates in brain ischemia. Selective vulnerability occurs when specific areas in the brain have less metabolic reserve and ischemic tolerance, preferentially succumbing to irreversible injury when this lower ischemic threshold is reached. For example, areas with high metabolic rates such as the hippocampus or deep gray structures are at higher risk because they house receptors for excitatory neurotransmitters. During the biochemical cascade of an ischemic insult, many cytotoxic events take place, including an initial increase in intracellular calcium triggering excitatory neurotransmitter release, followed by receptor stimulation leading to a further increase in intracellular calcium: a vicious cycle which culminates in cell damage and selective neuronal death.31–34
In children who do not clearly have full neurologic recovery, brain MRI is important for prognostication and in family discussions about recovery and rehabilitation. Timing of imaging for this patient population has not been standardized, but our results suggest that obtaining MRI within 8 days after ROSC may assist with early prognostication.35,36,37 Brain injury identified later on MRI will be more likely to pose dilemmas of indeterminate chronicity of injury with unclear clinical significance. Another factor which affects MRI timing and confounds guidelines for timing include stage of brain maturation at the time of image interpretation. Early in life when myelination is nearly absent, T2-weighted sequences are of far less value and, in some cases, are supplanted by T1-weighted sequences for subacute injury.35 It is the subacute window that is most complicated, when diffusion may have normalized and changes on other sequences are nonspecific. In the setting of a very recent insult, DWI remains the most important sequence to assess injury and determine prognosis.13,14,16 Timing of imaging with EEG must first depend on how management will be affected, taking into account hemodynamic stability of a post-arrest patient, resource utilization, and MRI compatible EEG leads.38 These are some of the challenges in establishing uniform recommendations for cardiac arrest patients. Establishing the inherent prognostic value of coupling EEG data with MRI findings in pediatric cardiac arrest patients is an important step in improving current imaging recommendations.
This observational study has several limitations. First, is the potential for bias from self-fulfilling prophecy. We do not know how EEG, MRI and clinical exam findings may have influenced physicians to counsel about probability for poor neurologic functioning that led to a family choosing to redirect care. We also do not know the value systems of each of the families and what quality of life would be an acceptable outcome. However combined EEG background and MRI diffusion still hold high distinguishing capabilities when the analysis is limited to survivors. In addition to being a retrospective study, EEG and MRI were clinically obtained which introduces sampling bias into our data. Our EEG background data was acquired using the first 20 minutes, which can be impacted by use of sedating medications, especially if performed immediately following procedures. However, it has been suggested that during the first 72 hours after cardiac arrest, EEG background does not significantly change over time, which demonstrates the utility of an initial recording.10 Cardiac arrest patients in our study were chosen by ICD code, mitigating selection bias. We had heterogeneous etiologies of cardiac arrest, including traumatic brain injuries, drug overdoses and infectious causes, as well as the more common asphyxia, cardiac and near-drowning. This diversity allows for generalization of our results to a broader PICU cardiac arrest population, and we believe more accurately captures the patient population encountered in a tertiary care PICU. Lastly, we chose to define a good outcome as PCPC 1–3. We did not have pre-arrest PCPC scores available to use change in PCPC score to categorize outcome. Hypothetically, patients with a pre-arrest PCPC score of 4 who remain PCPC of 4 after arrest may be considered a good outcome and a child who progressed from PCPC of 1 to 3 post-arrest may be considered a poor outcome. However, we chose to include PCPC of 3 as a good outcome because a child with PCPC of 3 may still attend school with modifications, which many families may consider a good outcome. Importantly, our sensitivity analysis of a modified poor outcome definition of PCPC 3–6 did not change our results. By broadening this definition, however, it does limit the direct comparison of the proportion of good outcomes in our study population to other similar studies. This may explain why there was no difference in the proportion of children surviving with good outcomes between in-hospital and out-of-hospital cardiac arrest patients, unlike prior studies.5,6
Conclusions:
In this single center, retrospective convenience sample cohort, EEG and MRI independently identify poor neurologic outcome after ROSC in the PICU. Attenuated or suppressed EEG background and signs of brain injury on MRI are both associated with increased odds of poor neurologic outcomes at hospital discharge post-arrest. However, combined test results were nearly perfect in distinguishing outcome at hospital discharge when MRI is obtained within 8 days after ROSC. Particularly when added to other clinical factors, analyzing results of these tests together may hold synergistic and practical value in caring for this patient population. Prospective multi-center studies are needed to validate multimodal prognosis and refine neurologic outcomes after cardiac arrest in children.
Supplementary Material
Acknowledgements:
This work was supported by the National Institutes of Health [R01 NS097721] (SHF); [K23 NS099472] (KPG)
Footnotes
Conflicts of Interest: none
REFERENCES
- 1.Suominen P, Olkkola KT, Voipio V, Korpela R, Palo R, Räsänen J. Utstein style reporting of in-hospital paediatric cardiopulmonary resuscitation. Resuscitation. 2000;45(1):17–25. doi: 10.1016/S0300-9572(00)00167-2 [DOI] [PubMed] [Google Scholar]
- 2.Reis AG, Nadkarni V, Perondi MB, Grisi S, Berg RA. A Prospective Investigation Into the Epidemiology of In-Hospital Pediatric Cardiopulmonary Resuscitation Using the International Utstein Reporting Style. PEDIATRICS. 2002;109(2):200–209. doi: 10.1542/peds.109.2.200 [DOI] [PubMed] [Google Scholar]
- 3.Berg RA, Nadkarni VM, Clark AE, et al. Incidence and Outcomes of Cardiopulmonary Resuscitation in PICUs: Crit Care Med. Published online December 2015:1. doi: 10.1097/CCM.0000000000001484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and Outcomes From Out-of-Hospital Cardiac Arrest in Children: The Resuscitation Outcomes Consortium Epistry–Cardiac Arrest. Circulation. 2009;119(11):1484–1491. doi: 10.1161/CIRCULATIONAHA.108.802678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ichord R, Silverstein FS, Slomine BS, et al. Neurologic outcomes in pediatric cardiac arrest survivors enrolled in the THAPCA trials. Neurology. 2018;91(2):e123–e131. doi: 10.1212/WNL.0000000000005773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moler FW, Meert K, Donaldson AE, et al. In-hospital versus out-of-hospital pediatric cardiac arrest: A multicenter cohort study*: Crit Care Med. 2009;37(7):2259–2267. doi: 10.1097/CCM.0b013e3181a00a6a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman ST, Abend NS, Bleck TP, et al. Consensus Statement on Continuous EEG in Critically Ill Adults and Children, Part I: Indications. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2015;32(2):87–95. doi: 10.1097/WNP.0000000000000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic Status Epilepticus Is Associated With Mortality and Worse Short-Term Outcome in Critically Ill Children*: Crit Care Med. 2013;41(1):215–223. doi: 10.1097/CCM.0b013e3182668035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostendorf AP, Hartman ME, Friess SH. Early Electroencephalographic Findings Correlate With Neurologic Outcome in Children Following Cardiac Arrest: Pediatr Crit Care Med. 2016;17(7):667–676. doi: 10.1097/PCC.0000000000000791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abend NS, Xiao R, Kessler SK, Topjian AA. Stability of Early EEG Background Patterns After Pediatric Cardiac Arrest: J Clin Neurophysiol. 2018;35(3):246–250. doi: 10.1097/WNP.0000000000000458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ducharme-Crevier L, Press CA, Kurz JE, Mills MG, Goldstein JL, Wainwright MS. Early Presence of Sleep Spindles on Electroencephalography Is Associated With Good Outcome After Pediatric Cardiac Arrest: Pediatr Crit Care Med. 2017;18(5):452–460. doi: 10.1097/PCC.0000000000001137 [DOI] [PubMed] [Google Scholar]
- 12.Scarpino M, Lolli F, Lanzo G, et al. Neurophysiology and neuroimaging accurately predict poor neurological outcome within 24 hours after cardiac arrest: The ProNeCA prospective multicentre prognostication study. Resuscitation. 2019;143:115–123. doi: 10.1016/j.resuscitation.2019.07.032 [DOI] [PubMed] [Google Scholar]
- 13.Moseley ME, Kucharczyk J, Mintorovitch J, et al. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol. 1990;11(3):423–429. [PMC free article] [PubMed] [Google Scholar]
- 14.Hajnal JV, Bryant DJ, Kasuboski L, et al. Use of fluid attenuated inversion recovery (FLAIR) pulse sequences in MRI of the brain. J Comput Assist Tomogr. 1992;16(6):841–844. doi: 10.1097/00004728-199211000-00001 [DOI] [PubMed] [Google Scholar]
- 15.Oualha M, Gatterre P, Boddaert N, et al. Early diffusion-weighted magnetic resonance imaging in children after cardiac arrest may provide valuable prognostic information on clinical outcome. Intensive Care Med. 2013;39(7):1306–1312. doi: 10.1007/s00134-013-2930-z [DOI] [PubMed] [Google Scholar]
- 16.Lansberg MG, Thijs VN, O’Brien MW, et al. Evolution of Apparent Diffusion Coefficient, Diffusion-weighted, and T2-weighted Signal Intensity of Acute Stroke. Published online 2001:8. [PMC free article] [PubMed] [Google Scholar]
- 17.Fink EL, Panigrahy A, Clark RSB, et al. Regional Brain Injury on Conventional and Diffusion Weighted MRI is Associated with Outcome After Pediatric Cardiac Arrest. Neurocrit Care. 2013;19(1):31–40. doi: 10.1007/s12028-012-9706-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirschen MP, Licht DJ, Faerber J, et al. Association of MRI brain injury with outcome after pediatric out-of-hospital cardiac arrest. Neurology. Published online November 18, 2020:10.1212/WNL.0000000000011217. doi: 10.1212/WNL.0000000000011217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greer DM, Scripko PD, Wu O, et al. Hippocampal Magnetic Resonance Imaging Abnormalities in Cardiac Arrest are Associated with Poor Outcome. J Stroke Cerebrovasc Dis. 2013;22(7):899–905. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 20.Lopez Soto C, Dragoi L, Heyn CC, et al. Imaging for Neuroprognostication After Cardiac Arrest: Systematic Review and Meta-analysis. Neurocrit Care. Published online September 23, 2019. doi: 10.1007/s12028-019-00842-0 [DOI] [PubMed] [Google Scholar]
- 21.Wouters A, Scheldeman L, Plessers S, et al. Added Value of Quantitative Apparent Diffusion Coefficient Values for Neuroprognostication After Cardiac Arrest. Neurology. 2021;96(21):e2611–e2618. doi: 10.1212/WNL.0000000000011991 [DOI] [PubMed] [Google Scholar]
- 22.Wetzel RC, Sachedeva R, Rice TB. Are all ICUs the same? Paediatr Anaesth. 2011;21(7):787–793. doi: 10.1111/j.1460-9592.2011.03595.x [DOI] [PubMed] [Google Scholar]
- 23.Topjian AA, Sánchez SM, Shults J, Berg RA, Dlugos DJ, Abend NS. Early Electroencephalographic Background Features Predict Outcomes in Children Resuscitated From Cardiac Arrest*: Pediatr Crit Care Med. 2016;17(6):547–557. doi: 10.1097/PCC.0000000000000740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiser D, Tilford J, Roberson P. Relationship of illness severity and length of stay to functional outcomes in the pediatric intensive care unit: A multi-institutional study. Crit Care Med. 2000;28(4):1173–1179. [DOI] [PubMed] [Google Scholar]
- 25.Beuchat I, Sivaraju A, Amorim E, et al. MRI–EEG correlation for outcome prediction in postanoxic myoclonus: A multicenter study. Neurology. 2020;95(4):e335–e341. doi: 10.1212/WNL.0000000000009610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bevers MB, Scirica BM, Avery KR, Henderson GV, Lin AP, Lee JW. Combination of Clinical Exam, MRI and EEG to Predict Outcome Following Cardiac Arrest and Targeted Temperature Management. Neurocrit Care. 2018;29(3):396–403. doi: 10.1007/s12028-018-0559-z [DOI] [PubMed] [Google Scholar]
- 27.Christophe C, Fonteyne C, Ziereisen F, et al. Value of MR Imaging of the Brain in Children with Hypoxic Coma. Am J Neuroradiol. 2002;23(4):716–723. [PMC free article] [PubMed] [Google Scholar]
- 28.Manchester LC, Lee V, Schmithorst V, Kochanek PM, Panigrahy A, Fink EL. Global and Regional Derangements of Cerebral Blood Flow and Diffusion Magnetic Resonance Imaging after Pediatric Cardiac Arrest. J Pediatr. 2016;169:28–35.e1. doi: 10.1016/j.jpeds.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubowitz DJ, Bluml S, Arcinue E, Dietrich RB. MR of Hypoxic Encephalopathy in Children after Near Drowning: Correlation with Quantitative Proton MR Spectroscopy and Clinical Outcome. Am J Neuroradiol. 1998;19(9):1617–1627. [PMC free article] [PubMed] [Google Scholar]
- 30.Yacoub M, Birchansky B, Mlynash M, et al. The prognostic value of quantitative diffusion-weighted MRI after pediatric cardiopulmonary arrest. Resuscitation. 2019;135:103–109. doi: 10.1016/j.resuscitation.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 31.Arbelaez A, Castillo M, Mukherji SK. Diffusion-Weighted MR Imaging of Global Cerebral Anoxia. AJNR Am J Neuroradiol. 1999;20(6):999–1007. [PMC free article] [PubMed] [Google Scholar]
- 32.Beauchamp NJ, Bryan RN. Acute cerebral ischemic infarction: a pathophysiologic review and radiologic perspective. Am J Roentgenol. 1998;171(1):73–84. doi: 10.2214/ajr.171.1.9648768 [DOI] [PubMed] [Google Scholar]
- 33.Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic-ischemic brain damage. Ann Neurol. 1986;19(2):105–111. doi: 10.1002/ana.410190202 [DOI] [PubMed] [Google Scholar]
- 34.Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the Extracellular Concentrations of Glutamate and Aspartate in Rat Hippocampus During Transient Cerebral Ischemia Monitored by Intracerebral Microdialysis. J Neurochem. 1984;43(5):1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues K, Ellen Grant P. Diffusion-Weighted Imaging in Neonates. Neuroimaging Clin N Am. 2011;21(1):127–151. doi: 10.1016/j.nic.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 36.Smith AE, Friess SH. Neurological Prognostication in Children After Cardiac Arrest. Pediatr Neurol. 2020;108:13–22. doi: 10.1016/j.pediatrneurol.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology. 2000;217(2):331–345. doi: 10.1148/radiology.217.2.r00nv24331 [DOI] [PubMed] [Google Scholar]
- 38.Rubinos C, Alkhachroum A, Der-Nigoghossian C, Claassen J. Electroencephalogram Monitoring in Critical Care. Semin Neurol. 2020;40(06):675–680. doi: 10.1055/s-0040-1719073 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


