Abstract
Background:
The evidence linking sugar-sweetened beverage (SSB) intake and mortality risk is conflicting, and associations between various SSB subtypes and mortality remain unclear.
Objective:
To examine the association between baseline SSB intake, subtypes of SSB intake, and mortality risk in women.
Design:
Prospective cohort study.
Participants/setting:
Participants of the California Teachers Study (n=100,314; median age 53) free of cardiovascular disease (CVD), cancer, and diabetes at baseline (1995–1996) were followed from 1995 to 2015. Baseline SSB intake was defined as caloric soft drinks (regular soft drinks, not diet soda), sweetened bottled waters or teas, and fruit drinks; and was derived from a self-administered food frequency questionnaire.
Main outcome measure:
Mortality was ascertained via annual linkage with state- and nationwide mortality records and the National Death Index over 20-years.
Statistical analysis:.
Multivariable-adjusted Cox proportional hazards models were used to generate hazard ratios (HR) and 95% confidence intervals (CI) for assessing associations between SSB intake and mortality. Rare/never consumers were the comparator group.
Results:
There were a total of 14,143 deaths over 20 years (30.5% from CVD; 29.2% from cancer). In women who consumed ≥7 servings/week of SSBs at baseline (4% of participants), the multivariable-adjusted HRs were not significant for all-cause, CVD-specific, or cancer-specific mortality. Consuming ≥7 servings/week of baseline caloric soft drink was associated with a higher risk of all-cause (HR = 1.26, 95% CI 1.10, 1.46; P trend = 0.02) and cancer-specific (HR= 1.33, 95% CI 1.08, 1.63; P trend = 0.08) mortality. In secondary analyses, consuming ≥1.5 cups/day of baseline SSBs was associated with all-cause mortality (HR = 1.12, 95% CI 1.02, 1.24; P trend = 0.01).
Conclusions:
Although the baseline frequency of total SSB intake was not significantly associated with mortality, consuming ≥7 servings/week of caloric soft drinks was associated with higher risk of all-cause and cancer-specific mortality. Findings support public health efforts to reduce caloric soft drink consumption.
Keywords: sugar-sweetened beverage, caloric soft drink, sugary drink, mortality, death
Introduction
Sugar-sweetened beverages (SSBs) are a leading source of added sugars consumed by adults in the United States (U.S.).1 SSBs are defined as manufactured carbonated and noncarbonated drinks containing caloric sweeteners or syrups, and include soft drinks (regular soft drinks, not diet soda), fruit drinks, sports and energy drinks, waters, and tea and coffee beverages with added sugars.1 Although overall intake of SSBs declined from 2003 to 2014 in the U.S.,2 recent data suggest that 49.3% of adults consume at least one SSB per day.3 According to the National Health and Nutrition Examination Survey (2011–2014),3 mean SSB intake in adult men and women is 6.9% (~179 kcal) and 6.1% (~113 kcal) of total caloric intake, respectively.
The 2020 U.S. Dietary Guidelines Advisory Committee1 recommends that consumption of added sugars be limited to <10% of total daily caloric intake. Moreover, the American Heart Association recommends restricting the intake of added sugars to no more than half of daily discretionary calories, which is equivalent to ~ 100 calories per day (~6 teaspoons of table sugar) for women and ~150 calories per day (~9 teaspoons of table sugar).4 However, in the U.S., a single serving of caloric soft drinks or sodas (1 can = 12 fluid ounce) has, on average, 140–150 calories and the equivalent of 10 teaspoons of table sugar.5
Consumption of SSBs has been associated with increased obesity and chronic disease risk,6–10 yet few studies have examined the association between SSBs and mortality risk.11–15 Furthermore, published studies have inconsistent findings and data regarding the effects of specific subtypes of SSBs are sparse.11,12 Therefore, we examined the association between SSB intake and risk of mortality from all-causes, CVD, and cancer. We hypothesized that higher levels of baseline SSB consumption are associated with an increased mortality risk.
Materials and Methods
Study population and design
The California Teachers Study (CTS) is an ongoing prospective cohort study of 133,477 active and retired female teachers and administrators initiated to document and study the risk and determinants of breast and other cancers. The baseline (1995–1996) 16-page questionnaire was mailed to 329,684 women who had been professional public school employees and were active members of the California State Teachers Retirement System.16 Two mailings were conducted and, originally, a total of 133,477 women enrolled in the study by returning the questionnaire where they responded to women’s health questions including: demographics; medical history; menstrual and reproductive events; use of exogenous estrogens, vitamins, and medications; screening behaviors; physical activity; height and weight; dietary intake; use of alcohol and tobacco; and exposure to potential environmental hazards. The cohort age at baseline was 22 to 104 years (median age 53). Information on certain lifestyle practices, risk factors, and chronic disease occurrence have continued to be collected and updated, such as participant level of education which was obtained after baseline, during fourth mail-in questionnaire follow-up (2005–2008). Deaths were detected at the annual follow-up. Information on date and cause of death was determined from state mortality records and the National Death Index. Cancer diagnoses were identified via linkage with the California Cancer Registry. Inpatient hospitalizations were identified via linkage with the Office of Statewide Health Planning and Development and included ambulatory surgery, and emergency department procedures and diagnoses performed in California. The CTS study was approved by the Institutional Review Boards at the participating institutions. This analysis was approved by the Institutional Review Boards of City of Hope and the University of California San Diego.
Assessment of Sugar-Sweetened Beverage Intake and Overall Diet
Dietary intake during the year prior to enrollment was assessed once using a validated 103-item self-administered food frequency questionnaire (FFQ) included in the 1995–1996 questionnaire, and adapted from a former version of the Block 95. This FFQ ascertained How Much - usual serving size (i.e., small, medium, large or extra-large serving) - and How Often - frequency of consumption (i.e., never or <1 time/month, 1 time/month, 2–3 times/month, 1 time/week, 2 times/week, 3–4 times/week, 5–6 times/week, once a day, or ≥2 times/day) - of 103 food and beverage items. The reproducibility and validity of the dietary assessment instrument in the cohort has been previously described.23
Estimation of SSB intake was determined from 3 items on the FFQ, specifically asked as: ‘First: mark the column to show How Often, on the average, you ate the food during the past year; second: mark the column to show How Much you usually eat of each food’ for ‘Regular soft drinks (not diet soda)’, ‘Snapple, Calistoga, sweetened bottled waters or iced teas’, and ‘Kool-Aid, Hi-C, or other drinks with added Vitamin C’. The use of brand names was included in the FFQ mailed to participants. These three beverage subtypes will be referred to as ‘caloric soft drinks’, ‘sweetened bottled waters or teas’ and ‘fruit drinks’. From the nine possible frequency categories, SSB consumption was collapsed into four categories: Rare or never, >rare/never to <1 serving/week, ≥1 to ≤6 servings/week, and ≥7 servings/week, as a semiquantitative categorization for the primary analyses, which included subtypes of SSBs. In secondary analyses, volume was used to categorize SSB intake into cups per day (1 cup = 8 fluid ounces (fl oz)). For this categorization, one serving of caloric soft drink equals 12 fl oz; 1 serving of sweetened water bottle or tea and fruit drink equals 8 fl oz. The five categories used for frequency of intake were: Rare or never, >rare/never to <0.5 cup/day, ≥0.5 to <1 cup/day, ≥1 to <1.5 cups/day, and ≥1.5 cups/day.
Estimation of all other food variables was similar to the SSB variables. They were calculated, reviewed, and then categorized based on their distributions.
Ascertainment of Death
Deaths were identified from annual linkage with California mortality files, the Social Security Death Index, and the National Death Index records through December 31, 2015, providing mortality data that included underlying cause of death. Participants without a death record were considered alive during the follow-up period. Using the International Classification of Diseases (ICD) 9th and 10th Revision codes, study endpoints were defined as: 1) CVD-specific mortality (ICD-9 codes 390–398, 402, 404, 410–429, and 430–438 and ICD-10 codes I00 to I09, I11, I13, and I20 to I51, I60 to I69) that includes diseases of the heart, hypertension, atherosclerosis, and cerebrovascular diseases; 2) cancer-specific mortality (ICD-9 codes 140–209 and ICD-10 codes C00 to C97), which only includes malignant neoplasms, excluding in situ and benign neoplasms; 3) other-cause mortality (ICD-9 codes 001–139, 240–389, 460–629, 680–759, 780–799, and E000-E999 and ICD-10 codes A00-B99, D50-H95, J00-N99, Q00-R99, and V00-Y99) included those death from any known causes, except for cancer and CVD; and 4) all-cause mortality, which is a combination of all of the above-mentioned causes of deaths.
Assessment of Covariates
The demographic, lifestyle, and clinical characteristics considered as possible confounders included: age, race/ethnicity, socioeconomic status (SES), marital status, smoking status, alcohol intake, family history of CVD, diabetes, or cancer (includes breast, endometrial, ovarian, cervical, lung, thyroid, colon, rectal, prostate, melanoma, skin cancers, leukemia, and Hodgkin’s lymphoma) in first degree relatives, moderate-to-vigorous physical activity (MVPA), aspirin use, multivitamin use, antihypertensive medication use, history of hypertension, menopausal status and menopausal hormone therapy use, oral contraceptive use, body mass index (BMI), total energy intake, and intake of a set of dietary factors: fruit and vegetable, red meat, processed meat, fish, refined carbohydrates, dietary fiber, and coffee/tea beverages, in lieu of a diet quality score which is currently unavailable. These data were collected at baseline (1995–1996), by self-report.
SES was determined by combining three 1990 U.S. block census data variables (occupation, education, and family income); where all block groups in the state were ranked by occupation (% adults employed in managerial/professional occupation), level of education (% of adults over the age of 25 completing at least a college degree), and median family income, corresponding to quartiles analogous the statewide adult population. A summary score was developed for SES with categories ranging from 1 (lowest) to 4 (highest). Alcohol intake was determined from frequency and number of drinks/week of beer, champagne and/or wine, and cocktails and/or liquor. MVPA was estimated using questionnaire-derived intensity, duration, and frequency of listed activities, on an average day. Participants were asked to report all of their daily activities, including sleep, by dividing up their 24-hour time windows into types of activities. The activity examples listed on the questionnaire were provided with the study population (active & recently retired teachers) in mind. BMI (kg/m2) was calculated as weight (kg) divided by height squared (m2), from self-reported weight and height.
Foods and beverages that were included in the models as covariates were adjusted for total energy by using the residual method,24 before including them in the model. With the residual method, the energy-adjusted intake estimate is the residual from a regression model in which the absolute dietary factor intake is the dependent variable and the total energy intake is the independent variable. Thereby, the residual is an estimate of dietary factor intake uncorrelated with total energy intake by removing the variation caused by total energy intake, and directly related to food selection and diet composition.24
Analytic sample
Of the 133,477 enrolled CTS participants, the final analytic sample included 100,314 women (age range 22–84). We first excluded n=20,889 participants due to pre-existing disease at baseline including history of cardiovascular disease (n=3,851), history of cancer (n=14,126), and history of diabetes (n=2,912); and n=1,693 that were age ≥85 years at baseline. We further excluded (in hierarchical manner) participants who: specified their data only be used for breast cancer research (n=22); returned incomplete or unreadable questionnaires (n=4); had extreme caloric intake values <1% or >99% of the population distribution (<600 [n=9,029] or >5000 [n=490] kcal/day); had incomplete FFQ data at baseline (defined as missing dietary data for ≥26 food items out of 103) including vitamin supplement use (n=1); were a missing death code and label (n=632); and/or had an undefined death code (n=403).
Statistical Analyses
Mean and standard error of mean, or proportion and frequency, were calculated for baseline characteristics of study participants in each SSB consumption category. Cox proportional hazard modeling was used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) of all and cause-specific mortality risk according to SSB consumption. We also examined the independent association between subtype of SSB and risk of all-cause and cause-specific mortality. Linear trend was modeled by assigning each participant the median intake in her respective SSB intake category and included as a continuous independent variable in the Cox proportional hazard model. The proportional hazards assumption was tested by inspecting the survival curves according to SSB consumption categories as well as testing time-varying covariates in the model.
In multivariable analysis, we first adjusted for potential confounding by sociodemographic and lifestyle factors including age, race/ethnicity (White, Asian/Pacific Islander, African-American, Hispanic, Native-American, or Mixed/Other; further categorized as White vs all other before including it in the model), SES (quartiles: 1st, 2nd, 3rd, 4th, or unknown), marital status (married, separated, divorced, widowed, never married, or unknown; further categorized as married, separated or divorced, widowed, and all other before including it in the model), smoking status (never, past, current cigarette use [1–12, 13–24, ≥25/day], or unknown use), alcohol intake (0, <20, or ≥20 grams/day), family history of CVD (yes or no), family history of cancer (yes or no), family history of diabetes (yes or no), MVPA (quintiles minutes/week: 0–30, 30–105, 105–210, 210–360, >360, or unknown), aspirin use (1–3 times/week, 4–6 times/week, daily, undetermined frequency, or unknown), multivitamin use (never, 1–3 times/week, 4–6 times/week, daily, undetermined frequency), use of at least one antihypertensive medication (daily, up to 6 times/week, regular use but undetermined frequency, not regularly taken, or unknown), history of hypertension (yes or no), menopausal status and menopausal hormone therapy use (premenopausal, perimenopausal/postmenopausal with never, past, or current hormone therapy use of estrogen, estrogen and progesterone, or other hormone combinations), and oral contraceptive use (never, past or current). We further adjusted for BMI, total energy intake, and intakes of several dietary factors: fruit and vegetable, red meat, processed meat, fish, refined carbohydrates, dietary fiber, and coffee/tea beverages as possible mediators. Two progressively adjusted multivariable Cox regression models after the age-adjusted model were fitted. Model 1 included age, race/ethnicity, socioeconomic status, marital status, smoking, alcohol intake, cardiovascular disease family history, cancer family history, diabetes family history, history of hypertension, physical activity, aspirin use, multivitamin use, use of at least one anti-hypertensive medication, menopausal status, menopausal hormone therapy use, and oral contraceptive use. Model 2 included variables in Model 1 and additionally adjusted for BMI, total energy intake, and intakes of fruit and vegetable, red meat, processed meat, fish, refined carbohydrates, dietary fiber, and coffee/tea beverages. Family histories of cardiovascular disease, cancer, and diabetes were removed from the model since they did not change the risk estimates. The final model, Model 2, included covariates that were considered potential and tested (if ≥10% change in HR) confounders in this exposure and outcome association, and had a P value ≤0.05. Additionally, the models examining the association between subtype of SSB consumption and risk of mortality, were reciprocally adjusted for the other beverage subtypes. That is, the sweetened water or tea analysis was adjusted for fruit drink and caloric soft drink, and vice versa. Multicollinearity was assessed via evaluation of tolerance and the variation inflation factor. Derived values of these two aspects did not suggest collinearity.
A secondary analysis was conducted to further assess amount of SSB and determine whether key information about the association was lost with our semiquantitative categorization. As a sensitivity analysis beyond the aforementioned primary analysis models, we also considered the impact of specific confounders. The supplemental material includes Model 3, which adjusted for variables in Model 1 plus BMI; and Model 4, which adjusted for variables in Model 2 with the exception of total energy intake, assessing the influence of over-adjustment by total energy intake. Another sensitivity analysis was also conducted to examine the possibility of reverse causality, excluding deaths that occurred within the first 2 and 4 years of follow-up. All P values presented are from 2-tailed analyses; P < 0.05 was considered statistically significant. Analyses were conducted with SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Table 1 provides the baseline characteristics of study participants according to SSB intake in semiquantitative frequency categories. Four percent of participants consumed ≥7 servings/week of SSBs with an average baseline SSB intake of 13.6 ± 0.05 fl oz (mean ± standard error). In comparison to rare/never consumers, participants that consumed ≥7 servings/week of SSBs were more likely to be younger, past or current smokers (34.8%), past or current oral contraceptive users (72.8%), and had the highest obesity rates (17.5%). They also had a higher daily intake of total energy, red and processed meat, and refined carbohydrates, and lower intakes of fruits and vegetables, compared to rare/never consumers. Foods and beverages that were used as covariates were adjusted for total energy before including them in the models. With regard to SSB subtype across all participants, 4.3%, 0.4%, and 3.1% consumed sweetened bottled waters or teas, fruit drinks, and caloric soft drinks daily, respectively.
During 20 years of follow-up representing 1,897,745 person-years, there were 14,143 (14.1%) CTS participants who died. Of these deaths, 30.5% were from CVD (73.8% heart disease-specific and 26.2% cerebrovascular disease-specific deaths), 29.2% were from cancer, and 40.3% were from other causes. Table 2 shows the association between baseline SSB consumption and mortality risk. After adjusting for sociodemographic, lifestyle, and dietary factors, compared with those who rarely/never consumed SSBs, those who consumed ≥7 servings/week of SSBs did not have a significantly different mortality than those who reported consuming SSBs rarely/never. The trend test did not show linearity in these associations (all P trend >0.05). Models that assessed the impact of BMI and over-adjustment of total energy intake are included in the supplemental material (Table 3).
With regard to SSB subtype, we observed a significant association between baseline caloric soft drink consumption and all-cause, cancer-specific and other-cause mortality (Figure 1). After adjusting for sociodemographic, lifestyle, and dietary factors, compared with participants who rarely/never consumed caloric soft drinks, those who consumed ≥7 servings/week of caloric soft drinks, had a 26% higher risk of all-cause mortality (95% CI: 1.10, 1.43; P trend = 0.02), a 33% greater risk cancer-specific mortality (95% CI: 1.08, 1.63; P trend = 0.08), and a 28% risk of other-cause mortality (95% CI: 1.02, 1.59; P trend = 0.15). There was no association between baseline caloric soft drinks and CVD-specific mortality after controlling for potential confounders. Details on the SSB subtype adjusted models are included in the supplemental material (Table 4).
Figure 1.
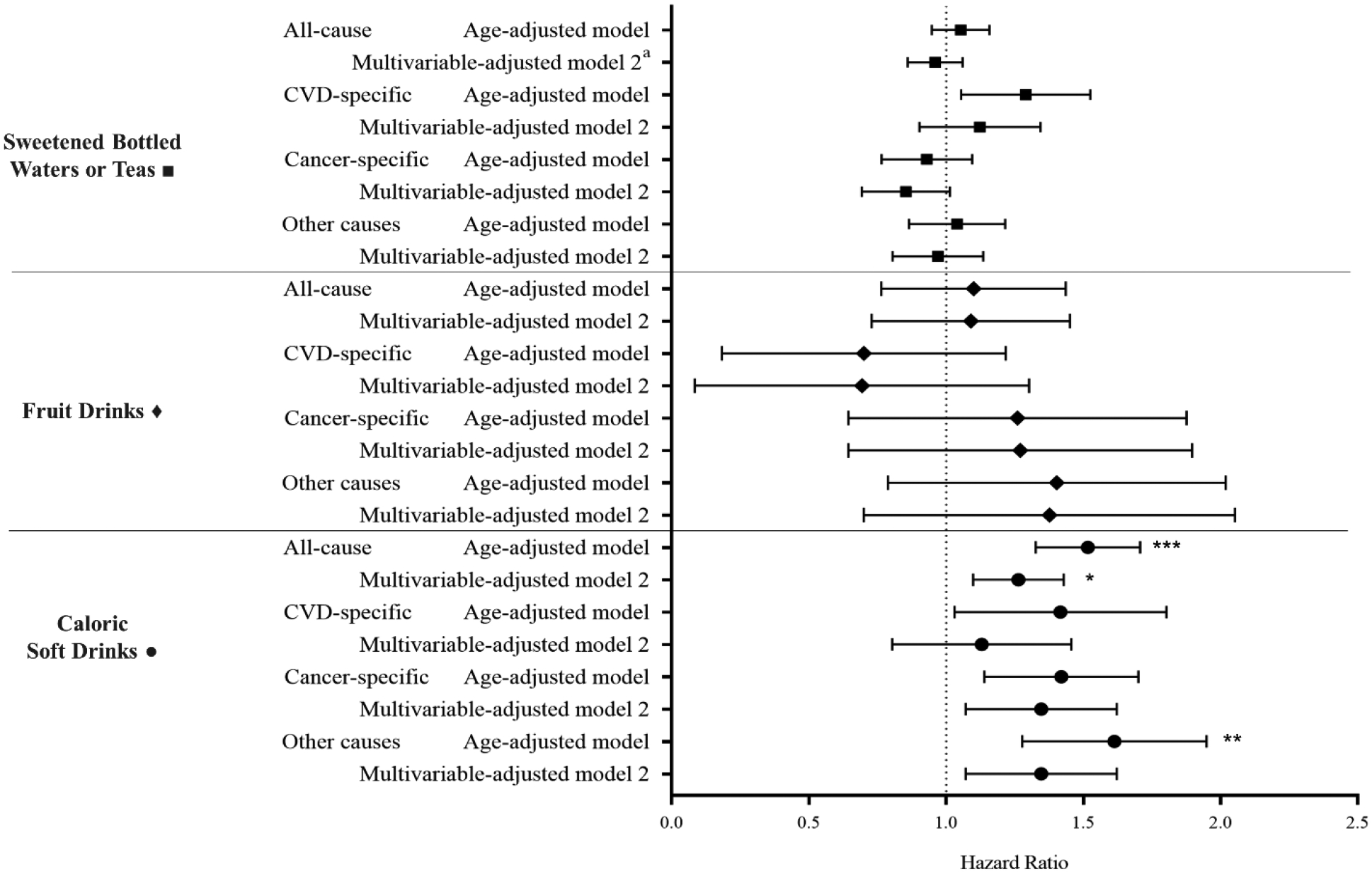
Association of specific sugar- sweetened beverage consumption and mortality risk. Hazard ratios comparing ≥7 sugar- sweetened beverage servings per week vs rare/never (reference) categories. Multivariable- adjusted (model 2) adjusted for: age, race/ethnicity, socioeconomic status, marital status, smoking status, alcohol intake, history of hypertension, physical activity, aspirin use, menopausal status, menopausal hormone therapy use, use of at least one anti-hypertensive medication, oral contraceptive use, body mass index, total energy, and intakes of fruit and vegetable, red meat, processed meat, fish, refined carbohydrates, dietary fiber, and coffee/tea beverage, and consumption of sugar- sweetened bottled waters or teas, fruit drinks, and caloric soft drinks (other than the main exposure, depending on model). *P for trend statistical significance at P<0.05. **P for trend statistical significance at P<0.01. *** P for trend statistical significance at P<0.0001.
Secondary analyses of associations between baseline total SSB intake in cups/day and mortality showed a statistically significant association between SSB intake and all-cause mortality (Table 5). Women who consumed ≥1.5 cups/day SSBs had a 12% higher risk of all-cause mortality (95% CI: 1.02, 1.24; P trend = 0.01), compared to women who reported consumption to be rare/never. There was no association between baseline total SSB volume consumption and CVD-specific or cancer-specific mortality. Observed associations with both servings/day and cups/day persisted in sensitivity analyses excluding deaths which occurred during the first 2 years (Tables 6 and 7) and 4 years (data not shown since results were similar to first 2 years) of follow-up.
Discussion
The present study found that when using the semiquantitative approach to calculating SSB intake, there was no significant association between baseline intake and all-cause mortality risk among adult women over a period of 20 years. However, when caloric soft drink intake was examined, there was a significant positive association with all-cause, cancer-specific, and other-cause mortality risk. In secondary analyses, there was a significant positive association between volume of baseline SSB intake and all-cause mortality.
The null finding regarding the association between baseline SSB serving consumption and mortality risk in the primary analysis is consistent with findings from the European Prospective Investigation into Cancer and Nutrition (EPIC)14 multinational cohort (HR = 1.06 [95 % CI: 0.95, 1.18]), although inconsistent with results from another longitudinal analysis of SSB consumption and mortality in a U.S. cohort,13 where researchers found a 14% increased risk (95% CI: 1.08, 1.20) in mortality among women who consumed 1–2 servings/day. However, the findings from the secondary analysis of the present study, baseline SSB intake by volume (cups/day) (HR = 1.12 [95% CI: 1.02, 1.24]) were highly concordant with these results from the U.S. cohort.13
The null finding on the association between baseline SSB intake and risk of CVD-specific mortality aligns with findings from the EPIC cohort.14 This is contrary to results in two other cohorts, where a positive association between baseline SSB intake and CVD mortality was determined.13,25 Furthermore, previous research in the CTS found that consuming ≥1 serving/day of SSB was associated with a higher risk of CVD, revascularization, and stroke.26 It is possible that the association for CVD-specific mortality does exist but that intermediate condition diagnoses such as hypertension, hypercholesterolemia, or type 2 diabetes, may have been associated with lower SSB intake at baseline.
The present study also found a null association with baseline SSB servings/day intake and cancer-specific mortality. The servings/day findings were comparable with those of the Singapore Chinese Health Study11 and the EPIC14 cohort, yet contradictory of the Nurses’ Health Study results.13 A recent systematic review and meta-analysis of 31,925 cases of cancer (51% in prospective studies) observed a null association between consumption of carbonated sweetened beverages and cancer risk (RR=1.03 [95% CI: 0.96; 1.11]). The authors reported no association with specific cancer types.27
A positive and dose-dependent association between consumption of ≥7 servings/week of caloric soft drink and all-cause mortality, when compared to rare/never consumers, was observed. In contrast to these results, The Leisure World Cohort Study12 and the Singapore Chinese Health Study11 found a null association between soft drink consumption and mortality risk examining sugar-added cola and non-cola soft drink intake in older Californian adults and Chinese adults in Singapore, respectively.11,12 However, the former study categorized soft drink intake differently (none, ≤1 can/week and >1 can/week; 1 can = 12 fl oz) than our present study, while the latter shared a similar approach (none, monthly, 1 serving/week, 2–6 servings/week and ≥1 serving/day; 1 serving = 237 ml or 1 cup).
Caloric soft drink consumption was significantly associated with cancer-specific mortality. Studies show that the relationship between cancer risk and SSB subtype varies by cancer type. For example, the association with pancreatic cancer risk was strong in women consuming >3 caloric soft drinks/week compared to those consuming <1/month (RR = 1.57 [95% CI: 1.02–2.41]),28 yet pooled cohort studies have shown null associations between caloric soft drink intake and risk of colon cancer and risk of lymphoma and leukemia.14,29–31 Additional studies addressing SSBs and caloric soft drinks associations with individual types of cancer are warranted to identify if a direct or indirect association exists, as analyzing total cancer may obscure specific associations.
A possible explanation for the caloric soft drink findings could be that a low number of deaths in the higher frequency of consumption categories for fruit drinks for all causes of mortality limiting the power to detect an association. In addition, volume and sugar dose vary across subtypes of SSB serving sizes in our study. Bottled sweetened teas contain phenolic compounds and bio-active flavonoids32 that may offer health benefits, reducing the risk of CVD, cancer, and overall mortality, and countering the negative effects of added sugar.32–34 The other-cause mortality findings are interpreted with caution since this outcome includes deaths from a variety of diseases and conditions. Further studies on the relationship between SSB consumption and death from specific diseases included in this category are needed.
Although there was a statistically significant association between baseline caloric soft drink intake and all-cause, cancer-specific and other-cause of mortality in the primary analysis, there was an attenuation of the measure of association after adjusting for common conditions (hypertension and obesity), suggesting that the association is not independent of these risk factors. Furthermore, it is possible that SSB consumption may serve as a surrogate of a suboptimal diet and unfavorable lifestyle, since frequent consumption of SSBs has been associated with suboptimal diets.49–51 However, the multivariable models controlled for measured lifestyle factors, including smoking, alcohol intake, fruit and vegetable intake, BMI, and MVPA.
The study had several strengths including a large sample size, prospective design, and extensive follow-up period. The secondary analysis disaggregated the last two semiquantitative categorizations of SSB intake, highlighting heavier (relative to rare/never) SSB consumers. Also, the sensitivity analysis investigated the possibility of reverse causality, and our analyses adjusted for potential confounders. Additionally, linkage with state mortality records derived well-defined and accurate ascertained endpoints, minimizing participant burden and reducing bias due to loss to follow-up.
There are also several limitations to this study. First, SSB intake data were collected from a single dietary assessment at baseline, which likely introduced random and systematic measurement error and also prevented examination of concurrent intakes. This also prevented the examination of trends in SSB intake. Of note, SSB consumption trends among U.S. adults rose in the late 1980’s to early-mid 2000’s (58% in 1988–1994 to 63% in 1999–2004),52 and alongside this, portion sizes have also changed substantially throughout the years - the average portion size of SSBs consumed in 1977 was 13.6 fl oz and increased to 21.0 fl oz by 1996.53 Correspondingly, dietary patterns have shifted and ultra-processed foods are more common in the global food system.54 In the U.S., the percentage of energy from ultra-processed foods was documented to be as high as 58% in the period 2007–2012.55 Therefore, the impact of SSBs might act synergistically with ultra-processed foods. However, recent national population data suggests that these consumption trends have been declining (61.5% in 2003 to 50.0% in 2014).2,56,57 Currently, calories from fruit drinks have significantly decreased among adults, and soda intake has significantly declined among 20- to 39- year- olds (171.0– 97.4 kcal) and 40- to 59- year- olds (104.7– 66.2 kcal).58 Therefore, an an attenuation in the magnitude of associations between SSB intake and mortality risk in the CTS cohort is expected. Nevertheless, differential exposure misclassification during follow-up could also affect the exposure-outcome association, depending on how the unmeasured exposure changes occurred.
Another limitation is that sources of sweeteners also changed throughout the years, from sucrose to high fructose corn syrup, yet we were unable to examine this shift. Further, self-reporting of baseline SSB and dietary intake is subject to social desirability bias and may be associated with under-reporting of SSB intake and poor diet quality. The inability to determine SSB added sugar quantity is a significant limitation. As well, artificially sweetened beverages and sugar-sweetened hot beverages were not evaluated as they were not included in the FFQ version used. Baseline SSB intake in the study population was relatively low and sparse compared to that in other populations,13,14 which may inflate our measures of association. Residual confounding from suboptimal measurement of variables and unmeasured variables is also possible. Lastly, generalizability is limited as the cohort was female and primarily non-Hispanic white.
Conclusions
Our findings contribute to the growing evidence on the association between frequent SSB consumption and poor health outcomes, and is supportive of efforts to limit SSB intake to improve health and reduce mortality. Additional studies with long-term follow-up and repeated measures using standardized SSB serving size units are warranted to better elucidate the association between SSB consumption and mortality risk.
Supplementary Material
Research snapshot:
Research Question:
Is there an association between sugar-sweetened beverage intake and mortality risk in women?
Key Findings:
In a prospective cohort of 100,314 women from the California Teachers Study, baseline total sugar-sweetened beverage intake was not significantly associated with mortality risk at 20-year follow up. In analyses of the subtypes of sugar-sweetened beverages, there was a positive association between caloric soft drink intake and risk of all-cause, cancer-specific, and other-causes of mortality.
Acknowledgements
The authors would like to thank the participants and staff of the California Teachers Study for their continuous involvement and valuable contributions. We also acknowledge the workforce of the California’s Office of Statewide Health Planning and Development and California Cancer Registry. The authors would like to thank the California Teachers Study Steering Committee that is responsible for the formation and maintenance of the Study within which this research was conducted. A full list of California Teachers Study team members is available at https://www.calteachersstudy.org/team.
Funding/support:
The California Teachers Study and the research reported in this manuscript were supported by the National Cancer Institute of the National Institutes of Health under award number U01-CA199277; P30-CA033572; P30-CA023100; UM1-CA164917; and R01-CA077398. Additionally, research described in this manuscript was supported by grant CA023100-29 from the University of California San Diego Moores Cancer Center (PI: Maria Elena Martinez), grant T32 HL079891-11 from the National Heart, Lung, and Blood Institute (PI: Matthew Allison), grant T32 DK007703-26 from the National Institute of Diabetes and Digestive and Kidney Diseases (PI: Frank Hu and Christopher Duggan); and the Harvard Chan Yerby Fellowship at Harvard T.H. Chan School of Public Health.
Biography
L.S. Pacheco, was a doctoral student in both the Department of Family Medicine and Public Health in the School of Medicine at University of California San Diego, La Jolla, CA, and the School of Public Health at San Diego State University, San Diego, CA, at the time of manuscript conception and development, and is currently a post-doctoral research fellow in the Department of Nutrition at Harvard T.H. Chan School of Public Health, Boston, MA.
J. V. Lacey Jr., is a professor and director in the Division of Health Analytics in the Department of Computational and Quantitative Medicine at City of Hope, Duarte, CA.
M.E. Martinez, is a professor in the Department of Family Medicine and Public Health in the School of Medicine at University of California San Diego, La Jolla, CA, and Chair for Cancer Research at the Moores Cancer Center at University of California San Diego, La Jolla, CA.
H. Lemus, is a lecturer in the School of Public Health at San Diego State University, San Diego, CA.
D.D. Sears, is a professor in the College of Health Solutions at Arizona State University, Phoenix, AZ, and an adjunct professor in the Departments of Medicine and Family Medicine and Public Health in the School of Medicine at University of California San Diego, La Jolla, CA.
M.R.G. Araneta, is a professor in the Department of Family Medicine and Public Health in the School of Medicine at University of California San Diego, La Jolla, CA, and Associate Dean of Diversity and Community Partnerships in the School of Medicine at University of California San Diego, La Jolla, CA.
C.A.M. Anderson is a professor and Dean of the Herbert Wertheim School of Public Health and Human Longevity Science at University of California San Diego, La Jolla, CA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of potential conflict of interest:
The authors declare no potential conflict of interest.
Additional disclosure:
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The opinions, findings, and conclusions expressed herein are those of the author and do not necessarily reflect the official views of the State of California, Department of Public Health, the National Cancer Institute, the National Institutes of Health, the Centers for Disease Control and Prevention or their Contractors and Subcontractors, or the Regents of the University of California, or any of its programs.
Contributor Information
Lorena Sonia Pacheco, Harvard T.H. Chan School of Public Health, Boston, MA..
James Vincent Lacey, Jr., City of Hope, Duarte, CA.
Maria Elena Martinez, University of California San Diego, La Jolla, CA.
Hector Lemus, San Diego State University, San Diego, CA..
Dorothy Dee Sears, Arizona State University, Phoenix, AZ; University of California San Diego, La Jolla, CA..
Maria Rosario G. Araneta, University of California San Diego, La Jolla, CA,.
Cheryl Ann Marie Anderson, University of California San Diego, La Jolla, CA..
References
- 1.Guidelines Advisory Committee D. Scientific Report of the 2020 Dietary Guidelines Advisory Committee Advisory Report to the Secretary of Agriculture and Secretary of Health and Human Services. Washington, D.C.; 2020. [Google Scholar]
- 2.Bleich SN, Vercammen KA, Koma JW, Li Z. Trends in beverage consumption among children and adults, 2003–2014. Obesity. 2018;26(2):432–441. doi: 10.1002/oby.22056 [DOI] [PubMed] [Google Scholar]
- 3.Rosinger A, Herrick K, Gahche J, Park S. Sugar-sweetened Beverage Consumption Among U.S. Adults, 2011–2014. NCHS Data Brief. 2017:1–8. https://www.cdc.gov/nchs/data/databriefs/db270_table.pdf#1. Accessed January 17, 2019. [PubMed] [Google Scholar]
- 4.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The american heart association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 5.United States Department of Agriculture. USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/. Accessed April 9, 2019.
- 6.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84(2):274–288. doi: 10.1093/ajcn/84.1.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-Sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–934. doi: 10.1001/jama.292.8.927 [DOI] [PubMed] [Google Scholar]
- 8.Keller A, Heitmann BL, Olsen N. Sugar-sweetened beverages, vascular risk factors and events: a systematic literature review. Public Health Nutr. 2014;18(7):1145–1154. doi: 10.1017/S1368980014002122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health. 2007;97(4):667–675. doi: 10.2105/AJPH.2005.083782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2012;345:e7492. doi: 10.1136/bmj.e7492 [DOI] [PubMed] [Google Scholar]
- 11.Odegaard AO, Koh W-P, Yuan J-M, Pereira MA. Beverage Habits and Mortality in Chinese Adults. J Nutr. 2015;145:595–604. doi: 10.3945/jn.114.200253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paganini-Hill A, Kawas CH, Corrada MM. Non-alcoholic beverage and caffeine consumption and mortality: the Leisure World Cohort Study. Prev Med (Baltim). 2007;44(4):305–310. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2034362/pdf/nihms22466.pdf. Accessed April 8, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malik VS, Li Y, Pan A, et al. Long-Term Consumption of Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Mortality in US Adults. Circulation. 2019;(139). doi: 10.1161/CIRCULATIONAHA.118.037401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullee A, Romaguera D, Pearson-Stuttard J, et al. Association Between Soft Drink Consumption and Mortality in 10 European Countries. JAMA Intern Med. September 2019. doi: 10.1001/jamainternmed.2019.2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collin LJ, Judd S, Safford M, Vaccarino V, Welsh JA. Association of Sugary Beverage Consumption With Mortality Risk in US Adults. JAMA Netw Open. 2019;2(5):e193121. doi: 10.1001/jamanetworkopen.2019.3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein L, Allen M, Anton-Culver H, et al. High breast cancer incidence rates among California teachers: results from the California Teachers Study (United States). Cancer Causes Control. 2002;13(7):625–635. http://www.ncbi.nlm.nih.gov/pubmed/12296510. Accessed February 2, 2019. [DOI] [PubMed] [Google Scholar]
- 17.USDA nutrient database. website: http//www.nal.usda.gov/fnic/foodcomp/.
- 18.USDA-NCC carotenoid database. ebsite: http//www.nal.usda.gov/fnic/foodcomp/data/car98/car98.html.
- 19.Pennington JAT, Schoen SA, Salmon GD, Young B, Johnson RD, Marts RW. Composition of core foods of the U.S. food supply, 1982–1991. III. copper, manganese, selenium, and iodine. J Food Compos Anal. 1995;8(2):171–217. doi: 10.1006/jfca.1995.1014 [DOI] [Google Scholar]
- 20.Wang H, Cao G, Prior RL. Total antioxidant capacity of fruits. J Agric Food Chem. 1996;44(3):701–705. doi: 10.1021/jf950579y [DOI] [Google Scholar]
- 21.Cao G, Sofic E, Prior RL. Antioxidant Capacity of Tea and Common Vegetables. J Agric Food Chem. 1996;44(11):3426–3431. doi: 10.1021/jf9602535 [DOI] [Google Scholar]
- 22.Horn-Ross PL, Barnes S, Lee M, et al. Assessing Phytoestrogen Exposure in Epidemiologic Studies: Development of a Database (United States). [DOI] [PubMed]
- 23.Horn-Ross PL, Lee VS, Collins CN, et al. Dietary assessment in the California Teachers Study: reproducibility and validity. Cancer Causes Control. 2008;19(6):595–603. doi: 10.1007/s10552-008-9124-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. http://www.ncbi.nlm.nih.gov/pubmed/3521261. Accessed February 5, 2019. [DOI] [PubMed] [Google Scholar]
- 25.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added Sugar Intake and Cardiovascular Diseases Mortality Among US Adults. JAMA Intern Med. 2014;174(4):516. doi: 10.1001/jamainternmed.2013.13563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacheco LS, Lacey JV, Martinez ME, et al. Sugar‐ Sweetened Beverage Intake and Cardiovascular Disease Risk in the California Teachers Study. J Am Heart Assoc. 2020;9(10). doi: 10.1161/JAHA.119.014883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyle P, Koechlin A, Autier P. Sweetened carbonated beverage consumption and cancer risk. Eur J Cancer Prev. 2014;23(5):481–490. doi: 10.1097/CEJ.0000000000000015 [DOI] [PubMed] [Google Scholar]
- 28.Schernhammer ES, Hu FB, Giovannucci E, et al. Sugar-Sweetened Soft Drink Consumption and Risk of Pancreatic Cancer in Two Prospective Cohorts. 2005. doi: 10.1158/1055-9965.EPI-05-0059 [DOI] [PubMed]
- 29.Zhang X, Albanes D, Beeson WL, et al. Risk of colon cancer and coffee, tea, and sugar-sweetened soft drink intake: pooled analysis of prospective cohort studies. J Natl Cancer Inst. 2010;102(11):771–783. doi: 10.1093/jnci/djq107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schernhammer ES, Bertrand KA, Birmann BM, Sampson L, Willett WC, Feskanich D. Consumption of artificial sweetener-and sugar-containing soda and risk of lymphoma and leukemia in men and women 1–4. Am J Clin Nutr. 2012;96:1419–1447. doi: 10.3945/ajcn.111.030833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pacheco LS, Anderson CAM, Lacey JV., et al. Sugar-sweetened beverages and colorectal cancer risk in the California Teachers Study. PLoS One. 2019;14(10). doi: 10.1371/journal.pone.0223638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CS, Wang Z-Y. Tea and Cancer. J Natl Cancer Inst. 1993;85(13):1038–1049. https://academic.oup.com/jnci/article-abstract/85/13/1038/947998. Accessed June 30, 2020. [DOI] [PubMed] [Google Scholar]
- 33.Hodgson JM, Croft KD. Tea flavonoids and cardiovascular health. Mol Aspects Med. 2010;31(6):495–502. doi: 10.1016/j.mam.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 34.Lambert JD, Yang CS. Mechanisms of Cancer Prevention by Tea Constituents. J Nutr. 2003;133(10):3262S–3267S. https://academic.oup.com/jn/article-abstract/133/10/3262S/4687607. Accessed June 30, 2020. [DOI] [PubMed] [Google Scholar]
- 35.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men. 2011. doi: 10.1056/NEJMoa1014296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma J, McKeown NM, Hwang S-J, Hoffman U, Jacques PF, Fox CS. Sugar-sweetened beverage consumption is associated with change of visceral adipose tissue over 6 years of follow-up. Circulation. 2016;133(4):370–377. doi: 10.1161/CIRCULATIONAHA.115.018704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2012;346(jan15 3):e7492–e7492. doi: 10.1136/bmj.e7492 [DOI] [PubMed] [Google Scholar]
- 38.Malik VS, Popkin BM, Bray GA, Després J-P, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33(11):2477–2483. doi: 10.2337/dc10-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89(4):1037–1042. doi: 10.3945/ajcn.2008.27140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernstein AM, de Koning L, Flint AJ, Rexrode KM, Willett WC. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr. 2012;95(5):1190–1199. doi: 10.3945/ajcn.111.030205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klement RJ, Fink MK. Dietary and pharmacological modification of the insulin/ IGF-1 system: exploiting the full repertoire against cancer. Nat Publ Gr. 2016;5:193. doi: 10.1038/oncsis.2016.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ludwig DS. The Glycemic Index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–2423. doi: 10.1001/jama.287.18.2414 [DOI] [PubMed] [Google Scholar]
- 43.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106(4):453–458. doi: 10.1172/JCI10762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhupathiraju SN, Tobias DK, Malik VS, et al. Glycemic index, glycemic load, and risk of type 2 diabetes: Results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr. 2014;100(1):218–250. doi: 10.3945/ajcn.113.079533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abe R, Yamagashi S. AGE-RAGE System and Carcinogenesis. Curr Pharm Des. 2008;14(10):940–945. [DOI] [PubMed] [Google Scholar]
- 46.Prasad A, Bekker P, Tsimikas S. Advanced Glycation End Products and Diabetic Cardiovascular Disease. Cardiol Rev. 2012;20(4):177–183. [DOI] [PubMed] [Google Scholar]
- 47.Malik VS, Hu FB. Fructose and Cardiometabolic Health: What the Evidence from Sugar-Sweetened Beverages Tells Us. J Am Coll Cardiol. 2015;66(14):1615–1624. doi: 10.1016/j.jacc.2015.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanhope KL, Medici V, Bremer AA, et al. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr. 2015;101(6):1144–1154. doi: 10.3945/ajcn.114.100461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodríguez-Monforte M, Flores-Mateo G, Sánchez E. Dietary patterns and CVD: A systematic review and meta-analysis of observational studies. Br J Nutr. 2015;114(9):1341–1359. doi: 10.1017/S0007114515003177 [DOI] [PubMed] [Google Scholar]
- 50.Anand SS, Hawkes C, De Souza RJ, et al. Food Consumption and its Impact on Cardiovascular Disease: Importance of Solutions Focused on the Globalized Food System A Report From the Workshop Convened by the World Heart Federation. J Am Coll Cardiol. 2015;66:1590–1614. doi: 10.1016/j.jacc.2015.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heidemann C, Schulze MB, Franco OH, Van Dam RM, Mantzoros CS, Hu FB. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation. 2008;118(3):230–237. doi: 10.1161/CIRCULATIONAHA.108.771881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bleich SN, Wang C, Wang Y, Gortmaker SL. Increasing consumption of sugar-sweetened beverages among US adults: 1988–1994 to 1999–2004. Am J Clin Nutr. 2009;89:372–381. doi: 10.3945/ajcn.2008.26883 [DOI] [PubMed] [Google Scholar]
- 53.Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. 2004;27(3):205–210. doi: 10.1016/j.amepre.2004.05.005 [DOI] [PubMed] [Google Scholar]
- 54.Monteiro CA, Moubarac J-C, Cannon G, Ng SW, Popkin B, Monteiro C. Ultra-processed products are becoming dominant in the global food system. 2013. doi: 10.1111/obr.12107 [DOI] [PubMed] [Google Scholar]
- 55.Baraldi LG, Martinez Steele E, Canella DS, Monteiro CA. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: Evidence from a nationally representative cross-sectional study. BMJ Open. 2018;8(3):20574. doi: 10.1136/bmjopen-2017-020574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kit BK, Fakhouri TH, Park S, Nielsen SJ, Ogden CL. Trends in sugar-sweetened beverage consumption among youth and adults in the United States: 1999–2010. Am J Clin Nutr. 2013;98(1):180–188. doi: 10.3945/ajcn.112.057943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han E, Powell LM. Consumption patterns of sugar-sweetened beverages in the United States. J Acad Nutr Diet. 2013;113(1):43–53. doi: 10.1016/j.jand.2012.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bleich SN, Vercammen KA, Koma JW, Li Z. Trends in Beverage Consumption Among Children and Adults, 2003–2014. Obesity. 2018;26(2):432–441. doi: 10.1002/oby.22056 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


