Abstract
Antimicrobial peptides are proposed to act as the first line of mucosal host defense by exerting broad-spectrum microbicidal activity against pathogenic microbes. Pleurocidin, a new 25-residue linear antimicrobial peptide, was recently isolated from the skin secretions of winter flounder (Pleuronectes americanus). The present study identifies the cDNA and gene encoding pleurocidin. The pleurocidin gene comprises four exons. Its upstream region demonstrates consensus binding sequences for transcription factors found in host defense genes in mammals, including sequences identical to the NF-IL6 and alpha and gamma interferon response elements. Pleurocidin is predicted to exist as a 68-residue prepropeptide that undergoes proteolytic cleavage of its amino-terminal signal and carboxy-terminal anionic propiece to form the active, mature peptide. Transmission electron microscopy localized pleurocidin to the mucin granules of skin and intestinal goblet cells. Significant synergy was shown to occur between pleurocidin and d-cycloserine targeting Mycobacterium smegmatis. Pleurocidin was functionally active at physiologic concentrations of magnesium and calcium; however, high concentrations of these divalent cations ablated pleurocidin's activity against a standard test strain, Escherichia coli D31. Pleurocidin was tested against bacterial and fungal clinical isolates and showed broad-spectrum antimicrobial activity. Together, these data support the hypothesis that pleurocidin participates in innate mucosal immunity, and it may prove to be a beneficial therapeutic agent.
Increasing evidence suggests that endogenous peptides with antimicrobial properties play an important role in host defense. These peptides possess marked microbicidal activity and have been isolated from a variety of cells of myeloid lineage and mucosal surfaces in most species tested thus far (4, 5, 8, 12). The recent focus has been on mucus-derived peptides and their roles in innate host defense at organism-environment interfaces, such as the integument and the respiratory and digestive epithelia. The multitude of peptides discovered at mucosal surfaces include human β-defensin 1 (HBD-1) in urogenital tissues (43) and bronchoalveolar lavage fluid (39), HBD-2 at sites of inflammation (24, 39), cryptdins from the Paneth cells (18), tracheal antimicrobial peptide (TAP) and lingual antimicrobial peptide (LAP) from cows (10, 33), and magainin and PGLa from frogs (46). Each peptide class confers a broad spectrum of antimicrobial activity and cationic charge at physiologic pH. Many peptides show high interspecies cDNA and protein sequence homology, frequently across evolutionarily diverse phyla.
We recently discovered pleurocidin, a novel 25-residue linear antimicrobial peptide in the skin mucous secretions of the winter flounder, Pleuronectes americanus (6). The sequence of the mature molecule, GWGSFFKKAAHVGKHVGKAALTHYL, shows sequence homology with the dermaseptin (tree frog) and ceratotoxin (medfly) classes of antimicrobial peptides. Pleurocidin is a highly basic molecule (pI = 10.02) and is predicted to form an amphipathic α-helix and to kill bacteria by irreversibly rupturing their membranes.
Here we extend our examination of pleurocidin to the cDNA and genomic levels and demonstrate its broad-spectrum antimicrobial activity against a number of clinical isolates. Transmission electron microscopy (TEM) reveals that pleurocidin is produced within the goblet cells of the flounder small intestine. We also examine the antimicrobial properties of pleurocidin and reveal multiple facets of its microbicidal character targeting pathogenic organisms. These data suggest a role for pleurocidin as a molecular agent of fish mucosal host defense.
MATERIALS AND METHODS
cDNA library construction, clone selection, and sequence.
mRNA was obtained by removing a 3- by 4-cm section of flounder skin, quick freezing it in liquid nitrogen for 1 min, pulverizing it in a liquid nitrogen mortar, and further grinding it with a hand-held blender (three 10-s bursts), followed by an established procedure for CsCl purification of RNA from tissue (2). The purified mRNA was subsequently used in the ZAP Express cDNA synthesis kit (Stratagene, La Jolla, Calif.) to produce inserts with 5′ EcoRI and 3′ XhoI restriction sites. These inserts were then ligated into pBK-CMV phagemid vectors, packaged using the restriction minus Gigapack III Gold packaging extract (Stratagene), amplified in an XL1-Blue MRF′ host strain, and screened with a combination of two degenerative end-radiolabeled (with γ-32P) oligonucleotide probes (degenerative sense, 5′-TTYTTYAARAARGCNGCNCAYGT-3′; inosine degenerative sense, 5′-TTYTTYAARAARGCIGCICA-3′). Inosine (boldface) was used in the latter oligonucleotide because it can base pair with A, C, G, or T, thus decreasing the overall degeneracy. Putative clones from the tertiary screen were in vivo excised with ExAssist helper phage using an XLOLR strain with kanamycin selection.
Dideoxynucleotide sequencing (Sequenase 2.0 kit; Amersham, Cleveland, Ohio) of three clones, using T3 and T7 primers to their respective pBK-CMV phagemid promoter region, revealed a 330-bp cDNA. The 5′ end sequence was confirmed by primer extension. Test winter flounder skin total RNA was hybridized with an excess of a single-stranded DNA oligonucleotide primer (*5′-TGGCAGTGAACTTCATTCTTGCGAATAC AAAGTGGGCTT-3′) radiolabeled at the 5′ terminus (asterisk). Reverse transcriptase was then used to extend the primer to produce cDNA. The length of this cDNA fragment was determined by polyacrylamide gel electrophoresis.
Genomic library construction, clone selection, and sequence.
Flounder genomic DNA was isolated by a modification of established protocols (2). Flounder testis (1.25 g) was snap-frozen in liquid nitrogen, ground with a liquid nitrogen mortar and pestle, suspended in 15 ml of digestion buffer (100 mM NaCl, 10 mM Tris-Cl [pH 8], 25 mM EDTA [pH 8], 0.5% sodium dodecyl sulfate, 100 μg of proteinase K/ml), and incubated at 50°C for 18 h. The DNA was purified by phenol extraction (25:24:1 phenol-chloroform-isoamyl alcohol), precipitated with ammonium acetate, and eluted with TE buffer (10 mM Tris-Cl [pH 8], 0.1 mM EDTA [pH 8]), yielding 57 mg of genomic DNA. The genomic DNA (1 mg) was partially digested with Sau3AI (BamHI compatible), size fractionated in a 5 to 25% (wt/vol) NaCl gradient, and centrifuged at 37,000 rpm (55,000 × g) for 4.5 h at 25°C in a Beckman SW41 rotor. The DNA was precipitated and resuspended in 100 μl of TE buffer. Agarose (0.5% [wt/vol]) gel electrophoresis determined the fractions containing digested genomic DNA in the range of 9 to 23 kb. These correctly sized DNA fragments were ligated into an equimolar concentration of Lambda DASH II-BamHI arms (Stratagene). Packaging into Gigapack III Gold extract (Stratagene), amplification in the XL1-Blue MRA′ (P2) strain (a P2 lysogenic strain which allows only recombinant phage to grow), and screening followed procedures similar to the cDNA library screening described above. The radiolabeled primers used for genomic library screening were as follows: 5AC-90c sense, 5′-GTCCTCATGGTTGAACCTGGA-3′; 5AC-90c antisense, 5′-GTCAAAAACAGTACTGGTGAT-3′; 5AC-95a sense, 5′-TGATTAGCATGTTCCTACAA-3′; and 5AC-95b sense, 5′-ACAGTTGGCAAGCATGTTGGC-3′. Clones of interest were digested with XhoI, EcoRI, HindIII, and XbaI endonucleases and subjected to Southern hybridization. Positive bands were excised and subcloned into Bluescript II SK (Stratagene), and the sequences of putative subclones were determined. The sequences were analyzed by BLAST (Basic Local Alignment Search Tool) searches against GenBank using the MacVector software package (Oxford Molecular Group).
Solid-phase pleurocidin synthesis.
Pleurocidin was synthesized using solid-phase technology and purified on a reverse-phase high-performance liquid chromatography preparatory column as described previously (6). Further production and purification of solid-phase synthesized pleurocidin was performed by Robert Donnelly (Molecular Biology Core Research Facility, University of Medicine and Dentistry of New Jersey). The naturally occurring and unmodified synthetic peptides show identical MICs and minimal bactericidal concentrations (MBCs) against a standard test strain, Escherichia coli D31 (data not shown).
TEM and immunogold staining.
Sections of winter flounder skin and small intestine (5 mm long by 2 mm wide by 1 mm thick) were fixed in 1.0 ml of Karnovski's fixative (4% paraformaldehyde–2% gluteraldehyde with 1× phosphate-buffered saline [PBS]) for 6 h at room temperature, washed three times with PBS, and embedded in Spurr's or Lowacryl embedding medium. Sections (800 Å thick) were fixed to nickel grids. Immunolabeling with colloidal gold conjugates followed modifications of standard methods (Nanoprobes, Inc., Stony Brook, N.Y.). The grids were submerged in distilled H2O for 7 to 8 min, incubated with 1% bovine serum albumin (BSA) in 1× PBS, pH 7.4, for 5 min at room temperature to block nonspecific protein binding sites, and incubated with a 1:5,000 dilution of preimmune serum (control) or primary polyclonal antiserum for 18 to 24 h at 4°C (the antibodies are described in reference 6). The grids were then washed with 1% BSA in PBS (three times for 1 min each time) and incubated with a 1:200 dilution of colloidal-gold-conjugated secondary goat anti-rabbit antibody for 1 h at room temperature. A final washing step in PBS preceded 1% glutaraldehyde-PBS postfixation (3 min at room temperature). The processed grids were stained in uranyl acetate-lead citrate for 15 to 20 s and examined with a Philips 300 transmission electron microscope.
Collection, typing, and conventional antibiotic testing of clinical bacterial and fungal isolates.
Seventeen isolates of Candida albicans, Klebsiella pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa were obtained from patients symptomatic for urinary and respiratory tract infections, venous catheter microbial colonization, and an infected hip wound and bone. The isolates were typed by a qualified clinical microbiologist (Veterans Administration Medical Center, Houston, Tex.). The bacterial isolates were subjected to antimicrobial susceptibility testing using susceptibility cards (bioMérieux Vitek, Inc., Hazelwood, Mo.) according to the manufacturer's instructions, with MIC breakpoints based on NCCLS guidelines. The following 31 antibiotics were tested: amikacin, ampicillin, ampicillin-sublactam, aztreonam, cefazolin, cefotaxime, ceftazidime, ceftizoxime, ceftriaxone, cefuroxime-axetil, cefuroxime-sodium, cephalothin, ciprofloxacin, clindamycin, erythromycin, gentamicin, gentamicin 500, imipenem, nitrofurantoin, ofloxacin, oxacillin, penicillin G, piperacillin, rifampin, streptomycin 2000, tetracycline, ticarcillin, ticarcillin-CA, tobramycin, trimeth-sulfa, and vancomycin. Percent resistance to standard antibiotics was determined by the following formula: (r/t) × 100, where r is the number of antibiotics to which the test bacterium was resistant and t is the total number of antibiotics tested. Antibiotic resistance was not correlated with bacterial etiology.
Microbes and culture conditions.
The clinical isolates C. albicans, K. pneumoniae, S. aureus, and P. aeruginosa and the test strain E. coli D31 were maintained on Trypticase soy agar plates with or without 5% sheep blood (Becton Dickinson and Co., Cockeysville, Md.) and cultured for 18 h in Mueller Hinton broth (MHB). Mycobacterium smegmatis (MC2155) was maintained on Trypticase soy agar plates and cultured for 60 to 72 h in basal salts minimal essential medium (1× basal salts, 1.25% glycerol, 0.5 mM CaCl2, 0.5 mM MgCl2, and 0.01% Tween).
Antimicrobial CFU microassays.
The microdilution assay for MIC and MBC of Steinberg and Lehrer (41) was modified as follows. Briefly, 5 × 105 CFU/ml were incubated for 18 h at 37°C in a final volume of 55 μl of MHB with serial twofold dilutions of pleurocidin (0.2 to 100 μg/ml) in 0.01% acetic acid–0.2% BSA using 96-well polypropylene microtiter plates. Visual verification of microbial sedimentation as well as absorbance readings (600 nm) confirmed the MIC. The MBC was determined by streaking a 5-μl aliquot of the microtiter plate reaction mixture onto an MHB agar plate for the three serial dilution wells above and below the determined MIC. The lowest concentration of pleurocidin that ablated bacterial colony growth on the agar plate was deemed the MBC.
Synergy assay.
M. smegmatis (MC2155) (40) was adopted as a test strain to determine the synergy between standard antibiotics and pleurocidin. Vancomycin, d-cycloserine, ethambutol, and isonicotinic acid hydrazide (INH) were tested from 0.2 to 100 μg/ml in a checkerboard fashion against pleurocidin (0.2 to 100 μg/ml) using modifications of the CFU microassay described above. The adjustments were as follows: 5.5 μl of pleurocidin, diluted in 0.01% acetic acid–0.2% BSA, and 5.5 μl of antibiotic, diluted in distilled water, were incubated with 5 × 105 CFU of M. smegmatis/ml in 44.5 μl of basal salts minimal essential medium. Since growth of M. smegmatis requires 60 to 72 h to reach stationary phase, minimal essential medium was used to reduce contaminant microbial growth. Controls were used for both standard antibiotics and pleurocidin, as well as for bacterial growth (i.e., without antibiotics).
Atomic absorption.
Mucus from a 25-cm-long winter flounder was aspirated, diluted 1:1 in double-distilled deionized H2O, and frozen at −20°C until use. Calcium and magnesium concentrations were measured by flame aspiration atomic absorption spectrophotometry (603 atomic absorption spectrophotometer; Perkin-Elmer Foster City, Calif.) at three serial dilutions (1:50, 1:100, and 1:200). The heavy metal lanthanum, at 2% final concentration, was used to aid in the dissociation of bound calcium.
Statistics.
Bacterial colony counts (CFU assay) were performed at least in triplicate for each independent experiment. Comparison of the pleurocidin MIC with conventional antibiotic resistance was performed using a paired t test for each species of bacterium (SigmaStat; SPSS Inc., Chicago, Ill.). Unless otherwise stated, MICs are represented as geometric means.
Nucleotide sequence accession numbers.
The GenBank accession numbers of the pleurocidin gene and upstream promoter sequence and the cDNA are AF210241 and AF210242, respectively.
RESULTS
Cloning and characterization of the pleurocidin gene and cDNA.
A cDNA library was constructed from winter flounder skin mRNA. End-radiolabeled degenerative oligonucleotide probes, derived from reverse translation of mature pleurocidin protein sequence, were used to screen the skin cDNA library. A single clone hybridized, and primer extension followed by dideoxynucleotide sequencing determined the length (317 nucleotides) and sequence of pleurocidin cDNA (Fig. 1).
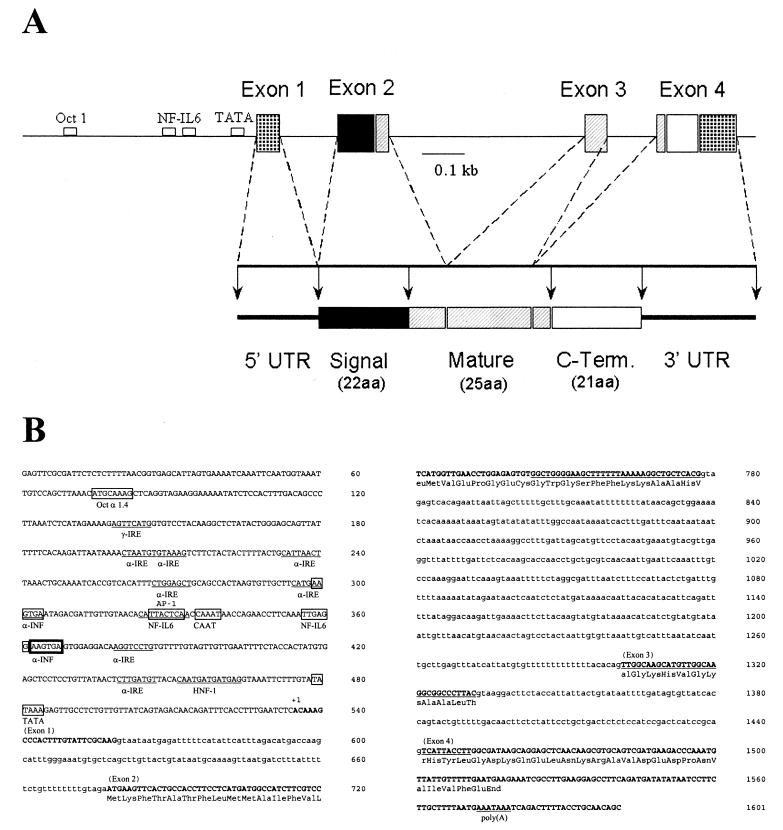
FIG. 1.
cDNA and prepropeptide sequences of pleurocidin. The 317-nucleotide cDNA sequence of pleurocidin is shown, with the polyadenylation signal in boldface. Prepropleurocidin is also shown, with the mature sequence underlined. The pre (signal) sequence is N terminal and is 22 amino acids in length. The propeptide is C terminal to the mature region, anionic, and 21 amino acids in length. The start and stop codons are in boldface.
Both a signal region and a propeptide sequence can be deduced from the cDNA of pleurocidin. Figure 1 shows the mature peptide region (6), a predicted 22-residue signal region NH2-terminal to the mature piece, and a predicted 21-residue propiece COOH-terminal to the mature region. The mature peptide region has a highly cationic charge density; it contains four lysine residues (Fig. 1), which may aid in the initial binding to bacterial membranes (14). The propeptide is negatively charged, containing three aspartic acid and two glutamic acid residues but only three basic residues (two lysine and one arginine).
A winter flounder DASH II lambda phage genomic library was created from flounder testis genomic DNA and screened to obtain clones of the pleurocidin gene. Radiolabeled oligonucleotide screening of the genomic library was performed similarly to cDNA library screening. A second screening of the genomic library was performed with radiolabeled pleurocidin cDNA, which identified several putative clones. Two clones were analyzed by dideoxynucleotide sequencing, which determined the sequence of the pleurocidin gene and the upstream promoter sequence (Fig. 2B). The pleurocidin gene consists of four exons and three introns (Fig. 2A), which is similar to the gene structure of the mammalian antimicrobial peptide PR-39 (16). The signal region is encoded by exons 1 and 2, the mature peptide is encoded by exons 2 to 4, and the propeptide is encoded only by exon 4. The 5′ untranslated region (UTR) and 3′ UTR are found in exons 1 and 4, respectively. Analysis of the 5′ flanking region indicates the presence of TATA and CAAT boxes at −56 and −200, respectively (MacVector; Oxford Molecular Group). Further analysis suggests the presence of binding sites for numerous transcription factors, including NF-IL6, gamma interferon (IFN-γ) response element, IFN-α response element, Oct 1, and HNF-1.
FIG. 2.
Analysis of pleurocidin gene. (A) Restriction map of pleurocidin gene, including 535 bp of upstream sequence. Also shown are the structures of the cDNA and the putative precursor of pleurocidin derived from the cDNA sequence. aa, amino acids. (B) Nucleotide sequence of pleurocidin gene. Exons (boldface capital letters) were determined by comparison with the cDNA sequence. Primer extension determined by comparison with the cDNA sequence. Primer extension determined the transcriptional start site (+1). The upstream consensus sites for transcription factor binding proteins are boxed. The sequence of the mature peptide is underlined. Intronic sequences are lowercased. Boldfaced box, overlap of consensus binding sites.
Ultrastructural localization of pleurocidin in flounder skin and intestine.
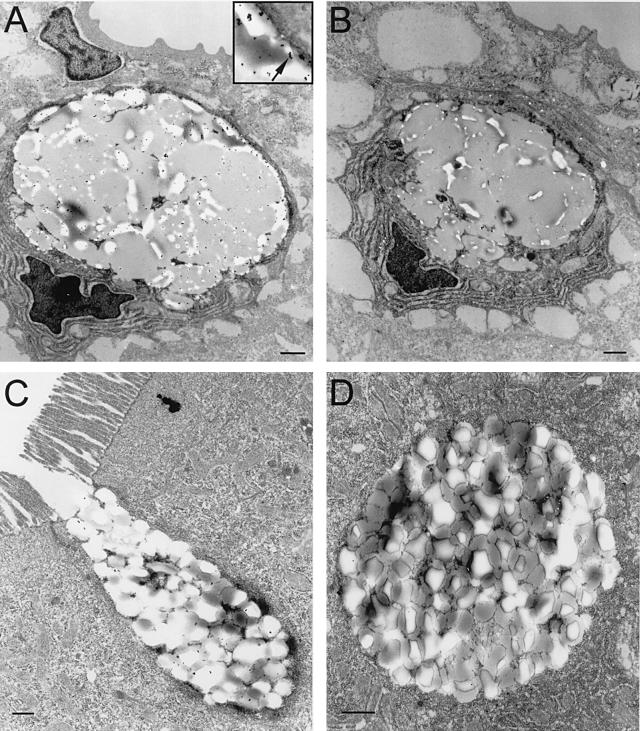
Winter flounder skin was examined for cellular localization of pleurocidin by immunolabeling and analyzed by TEM. The immunogold electron microscopy exhibited labeling (gold particles) in the mucus-producing cells of the flounder skin. The photomicrograph in Fig. 3A shows a low-magnification image of a section of flounder skin with one intact mucous cell. Gold particles are seen evenly dispersed throughout the mucin granules within the cytoplasm and concentrated at the periphery. Under this magnification, the labeling indicates preferential location of pleurocidin near the mucin granule membranes rather than in the intragranule domain. Figure 3A (inset) is a high-magnification electron micrograph of the goblet cell with representative gold particles indicated. A control, using preimmune serum, is shown in Fig. 3B and indicates the specificity of the labeling.
FIG. 3.
Immunogold TEM of flounder skin and intestine. (A) Flounder skin incubated with primary antiserum. (B) Preimmune serum skin control. (C) Flounder intestinal goblet cell incubated with primary antiserum. (D) Intestinal cell control. All antibody dilutions were 1:5,000 in 1% BSA–1× PBS. The arrow in the inset in panel A indicates representative gold particles (high magnification). Bars = 2 μm.
To determine if pleurocidin is expressed in other mucosal tissues, sections of winter flounder small intestine were similarly examined by TEM. Notably, a high level of labeling (gold particles) is seen within the goblet cells of tissue obtained from the proximal portion of the flounder small intestine (Fig. 3C). A preimmune serum control is shown under high magnification and is not immunoreactive with anti-pleurocidin (Fig. 3D). Immunohistochemistry of flounder intestine using the alkaline phosphatase-conjugated-secondary-antibody method (6) confirms this finding (data not shown). Sections of heart, striated muscle, gills, stomach, liver, and spleen did not stain for pleurocidin using immunohistochemistry. Pleurocidin is therefore not skin specific and likely also contributes to digestive tract host defense.
Human pathogens display varied sensitivities to pleurocidin.
Seventeen bacterial and fungal isolates were typed and cultured from clinically infected patients. To determine antimicrobial susceptibility, each isolate was tested with Vitek susceptibility cards containing conventional antibiotics to which respective standard typed strains are sensitive. The resistance of clinical isolates to conventional antibiotics ranged from 5 to 73% of antibiotics tested, with the most resistant isolates being K. pneumoniae 10808 and S. aureus 8580 (Table 1). To determine the activity of pleurocidin, the clinical isolates described above were incubated with pleurocidin in a CFU microassay (41). Most notably, all four isolates of the opportunistic fungus C. albicans were highly susceptible to pleurocidin. Although several strains that were highly resistant to standard antibiotics were also insensitive to pleurocidin (e.g., K. pneumoniae 10808 and S. aureus 8580; P < 0.05), there was not a significant correlation between standard antibiotic resistance and the pleurocidin MIC. This would suggest that pleurocidin acts via a different mechanism than the other tested antibiotics.
TABLE 1.
MICs and MBCs of pleurocidin targeting clinical isolates which exhibit resistance to conventional antibiotics
| Clinical isolate | Pleurocidin MICa (μg/ml) | Antibiotic resistance (%)b |
|---|---|---|
| P. aeruginosa 8613-1 | 28.0 | 25 |
| P. aeruginosa 11122 | 62.5 | 36 |
| P. aeruginosa 11173 | 37.3 | 38 |
| P. aeruginosa 11291 | 50.0 | 38 |
| P. aeruginosa 11300 | 37.3 | 25 |
| K. pneumoniae 8617 | 12.6 | 9 |
| K. pneumoniae 8660 | 15.4 | 5 |
| K. pneumoniae 8961 | 43.8 | 55 |
| K. pneumoniae 10808 | >100c | 73 |
| S. aureus 8534 | 62.5 | 13 |
| S. aureus 8538 | 62.5 | 11 |
| S. aureus 8542 | 82.9 | 11 |
| S. aureus 8580 | 74.7 | 73 |
| C. albicans 8557 | 24.8 | NDd |
| C. albicans 8562 | 15.4 | ND |
| C. albicans 11280 | 18.7 | ND |
| C. albicans 11301 | 24.8 | ND |
MIC was equal to MBC for all strains.
Percent resistance to conventional antibiotics tested per manufacturer's instructions (bioMérieux Vitek, Inc.).
Resistant to pleurocidin.
ND, not determined.
Pleurocidin acts synergistically with d-cycloserine.
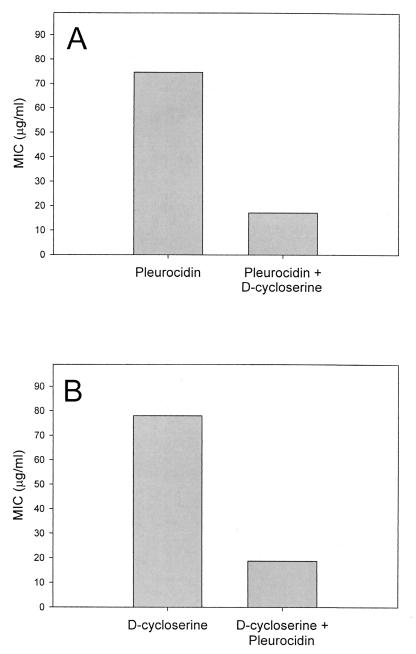
Pleurocidin was subjected to a CFU microassay in the presence and absence of antibiotics effective against Mycobacterium spp. to identify antimicrobial substances synergistic against M. smegmatis. M. smegmatis was chosen as a model for the clinically relevant Mycobacterium tuberculosis because of its low infection risk and relatively short incubation time (40). Pleurocidin was tested in a checkerboard fashion with d-cycloserine, vancomycin, ethambutol, or INH against M. smegmatis. In combination, the MICs of pleurocidin and d-cycloserine were each reduced fourfold (Fig. 4). Investigation of vancomycin, ethambutol, and INH indicated that their microbicidal effects were merely additive (data not shown). The MBC was equivalent to the MIC in all analyses. Antagonism was not detected for any combination tested.
FIG. 4.
Synergy of pleurocidin and d-cycloserine against M. smegmatis. (A) MIC of pleurocidin, alone and in combination with 185 μM (0.25× MIC) d-cycloserine. (B) MIC of d-cycloserine, alone and in combination with 5.2 μM (0.25× MIC) pleurocidin (n = 4).
Pleurocidin is insensitive to physiologic concentrations of magnesium and calcium.
Magnesium and calcium were added to determine their effects on the MICs of pleurocidin. Magnesium and calcium have been reported to inhibit the mammalian antimicrobial peptide defensin (20, 36), possibly by acting to increase bacterial membrane stabilization (7), thus regulating the microbicidal function. We noticed a similar trend in that an increased calcium or magnesium concentration resulted in deceased antimicrobial activity against the standard test strain, E. coli D31 (Table 2). NaCl, added at concentrations 15-fold greater than those of either MgCl2 or CaCl2, did not affect the microbicidal activity of pleurocidin, demonstrating that chloride ion was not the inhibiting factor. Winter flounder mucus calcium and magnesium concentrations, as measured by atomic absorption, were 0.68 mM magnesium and 0.36 mM calcium (data not shown). These values are within the functional range of pleurocidin and comparable to concentrations in human tracheal secretory gland cells (35).
TABLE 2.
Effect of increasing concentration of common cations on MICs and MBCs of pleurocidin against E. coli D31
| Salt | Concn (mM)a | MIC (μg/ml)b |
|---|---|---|
| MgCl2 | 0 | 3.1 |
| 1 | 6.3 | |
| 5 | 25 | |
| 10 | >100 | |
| CaCl2 | 0 | 3.1 |
| 1 | 25 | |
| 5 | 50 | |
| 10 | >100 | |
| NaCl | 0 | 3.1 |
| 50 | 3.1 | |
| 100 | 3.1 | |
| 150 | 3.1 |
Assays were performed in MHB supplemented with MgCl2, CaCl2, or NaCl to the final indicated concentrations.
Expressed as the most common MIC of ≥6 trials. MIC was equal to MBC for all trials.
DISCUSSION
Ultrastructural analyses were performed to identify the cellular distribution or compartmentalization of mature pleurocidin. Immunogold TEM confirmed immunohistochemical data in localizing pleurocidin to the flounder skin mucous cells, and the finding of pleurocidin in goblet cells of the small intestine lends credence to pleurocidin's role in mucosal immunity. This is not surprising, since the gut, like the epidermis, is an interface between the organism and the environment. However, immunogold TEM may also offer an additional explanation for pleurocidin's location and activity. The localization of pleurocidin primarily on the outer margins of mucin granules within the skin mucous cells deserves attention, as this may suggest regional accumulation of the peptide. Whether pleurocidin is stored as an active mature peptide or an inactive proform has yet to be determined.
Many antimicrobial peptides are formed from precursor proteins in which an anionic propeptide is involved in cellular trafficking and charge neutralization of the mature peptide (13, 26, 34, 42, 45). Although most antimicrobial peptides have a propeptide NH2 terminal to the mature peptide, pleurocidin is predicted to have an anionic propeptide COOH terminal to the mature region. This configuration, although rare, is also found in preprotachyplesins from the hemocytes of horseshoe crabs (17) and in preprostyelins from tunicates (47). The four cationic residues in the mature peptide region and the four anionic residues in the proregion of pleurocidin suggest that pleurocidin's propeptide is involved in charge neutralization of the mature region. Posttranslational enzymatic cleavage (13) could thus release the propiece and activate pleurocidin.
Genomic analysis of the pleurocidin gene reveals a four-exon structure, where the mature peptide is encoded by exons 2, 3, and 4, with the C-terminal propiece encoded by exon 4. Computer-based analysis (MacVector) of the 5′ flanking region indicated the presence of typical transcriptional promoter elements, including TATA and CAAT boxes. Also noted were consensus binding sequences for a number of transcription factors found in the promoter regions of host response genes in mammals, including NF-IL6 and IFN-α and -γ response elements. These consensus sequences have been found in the promoter regions of other fish genes, including the transferrin gene in salmon (19), the interferon-inducible Mx genes in trout (21), the insulinlike growth factor gene in trout (37), and the antifreeze protein gene in flounder (25). Activation of such transcription factors and binding to their consensus sequences have also been observed in fish (25, 31, 32). This suggests that the flounder may regulate pleurocidin gene expression in response to infection and inflammation, in a manner similar to that seen for antimicrobial peptide genes from amphibians (27, 38), insects (11), and mammals (9).
In previous studies (6), we characterized the activity of pleurocidin against standard test strains of nonpathogenic bacteria. We show here that pleurocidin notably exhibits both anticandidal and antibacterial activity against clinical isolates, which may decrease the chance of candidal superinfections normally associated with antibacterial treatment of conditions such as urinary tract infections, bacterial meningitis, and bacterial vaginosis (15, 29, 30). Pleurocidin may prove beneficial in the treatment or prevention of sequelae.
Antimicrobial peptides have been shown to act in concert with other microbicidal agents. NP-1, a β-sheet rabbit neutrophil peptide, acts synergistically with BPI (bactericidal/ permeability-increasing protein) when acting against E. coli J5 (22) and with fluconazole when acting against Cryptococcus neoformans (1). The antimicrobial peptides magainin-2 and PGLa from the skin of Xenopus laevis show a markedly increased effect when combined to target E. coli or tumor cells (44). The present study reveals synergy between a standard antibiotic and pleurocidin targeting M. smegmatis. Mycobacterium spp. have extraordinarily thick mycolic acid outer walls that are not easily penetrated by perforating molecules such as antimicrobial peptides. Although pleurocidin alone did not show appreciable antibacterial activity against M. smegmatis, its bactericidal activity was enhanced by the presence of d-cycloserine. d-Cycloserine acts as an inhibitor of cell wall synthesis and has been shown to increase mycobacterial susceptibility to other agents (28). Although d-cycloserine is an effective antimicrobial agent, it is rarely prescribed due to adverse neurological effects. Pleurocidin may therefore prove useful in reducing the toxicity of d-cycloserine.
In further characterizing the action of pleurocidin, we discovered that divalent magnesium and calcium (but not monovalent sodium) inhibit its antimicrobial activity. The actions of several other antimicrobial peptides are inhibited by high concentrations of monovalent and divalent salts (3, 20, 36). Stabilizing divalent cations, such as magnesium and calcium, are intimately associated with lipopolysaccharides in the outer leaflets of gram-negative bacteria (7). Candidacidal activity by NP-1 is inhibited by increasing the Ca2+ concentration of the incubation mixtures but is relatively unaffected by Mg2+ (36). Although calcium is not involved in the initial effects on the plasma membrane, varying the calcium concentration altered the later effects of defensin-treated K562 tumor cell lysis (23). Since the concentrations of both magnesium and calcium in seawater (10.0 and 52.3 mM, respectively) are much higher than physiologic concentrations in mucus, pleurocidin is likely active within the mucus proper and not at the environment interface. The reported concentrations of magnesium and calcium in human body fluids are on the order of 1 mM, which is within the active, and thus possibly therapeutic, antimicrobial range of pleurocidin.
In summary, we have characterized the gene, antimicrobial activity, and ultrastructural location of the antimicrobial peptide pleurocidin. Although we have established the antimicrobial spectrum of pleurocidin against several pathogenic clinical isolates and further characterized its activity, additional studies are necessary to determine its therapeutic benefits.
ACKNOWLEDGMENTS
We thank Francis Walter Kemp for his excellent work on atomic absorption measurements; Peddrick Weis, Noel Espina, and Michael R. Condon for invaluable technical assistance; and Richard D. Howland for statistical guidance.
This work was supported in part by the National Oceanic and Atmospheric Administration, Office of Sea Grant and Extramural Programs, Department of Commerce (grant NA36RG050; Project R/N-95003).
Footnotes
New Jersey Sea Grant Publication NJSG-00-441.
REFERENCES
- 1.Alcouloumre M S, Ghannoum M A, Ibrahim A S, Selsted M E, Edwards J E J. Fungicidal properties of defensin NP-1 and activity against Cryptococcus neoformans in vitro. Antimicrob Agents Chemother. 1993;37:2628–2632. doi: 10.1128/aac.37.12.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1996. [Google Scholar]
- 3.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson J M. Human β-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Investig. 1998;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevins C L. Antimicrobial peptides as agents of mucosal immunity. Ciba Found Symp. 1994;186:250–269. doi: 10.1002/9780470514658.ch15. [DOI] [PubMed] [Google Scholar]
- 5.Boman H G. Peptide antibiotics: holy or heretic grails of innate immunity? Scand J Immunol. 1996;43:475–482. doi: 10.1046/j.1365-3083.1996.d01-76.x. [DOI] [PubMed] [Google Scholar]
- 6.Cole A M, Weis P, Diamond G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J Biol Chem. 1997;272:12008–12013. doi: 10.1074/jbc.272.18.12008. [DOI] [PubMed] [Google Scholar]
- 7.Coughlin R T, Tonsager S, McGroarty E J. Quantitation of metal cations bound to membranes and extracted lipopolysaccharide of Escherichia coli. Biochemistry. 1983;22:2002–2007. doi: 10.1021/bi00277a041. [DOI] [PubMed] [Google Scholar]
- 8.Diamond G, Bevins C L. β-Defensins: endogenous antibiotics of the innate host defense response. Clin Immunol Immunopathol. 1998;88:221–225. doi: 10.1006/clin.1998.4587. [DOI] [PubMed] [Google Scholar]
- 9.Diamond G, Kaiser V, Rhodes J, Russell J P, Bevins C L. Transcriptional regulation of β-defensin gene expression in tracheal epithelial cells. Infect Immun. 2000;68:113–119. doi: 10.1128/iai.68.1.113-119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy W L, Bevins C L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci USA. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engstrom Y, Kadalayil L, Sun S C, Samakovlis C, Hultmark D, Faye I. Kappa B-like motifs regulate the induction of immune genes in Drosophila. J Mol Biol. 1993;232:327–333. doi: 10.1006/jmbi.1993.1392. [DOI] [PubMed] [Google Scholar]
- 12.Ganz T, Lehrer R I. Defensins. Curr Opin Immunol. 1994;6:584–589. doi: 10.1016/0952-7915(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 13.Ganz T, Liu L, Valore E V, Oren A. Posttranslational processing and targeting of transgenic human defensin in murine granulocyte, macrophage, fibroblast, and pituitary adenoma cell lines. Blood. 1993;82:641–650. [PubMed] [Google Scholar]
- 14.Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–354. [PubMed] [Google Scholar]
- 15.Gelfand M S, McGee Z A, Kaiser A B, Tally F P, Moses J. Candidal meningitis following bacterial meningitis. South Med J. 1990;83:567–570. doi: 10.1097/00007611-199005000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Gudmundsson G H, Magnusson K P, Chowdhary B P, Johansson M, Andersson L, Boman H G. Structure of the gene for porcine peptide antibiotic PR-39, a cathelin gene family member: comparative mapping of the locus for the human peptide antibiotic FALL-39. Proc Natl Acad Sci USA. 1995;92:7085–7089. doi: 10.1073/pnas.92.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwanaga S, Kawabata S, Muta T. New types of clotting factors and defense molecules found in horseshoe crab hemolymph: their structures and functions. J Biochem (Tokyo) 1998;123:1–15. doi: 10.1093/oxfordjournals.jbchem.a021894. [DOI] [PubMed] [Google Scholar]
- 18.Jones D E, Bevins C L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992;267:23216–23225. [PubMed] [Google Scholar]
- 19.Kvingedal A M. Characterization of the 5′ region of the Atlantic salmon (Salmo salar) transferrin-encoding gene. Gene. 1994;150:335–339. doi: 10.1016/0378-1119(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 20.Lehrer R I, Ganz T, Szklarek D, Selsted M E. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J Clin Investig. 1988;81:1829–1835. doi: 10.1172/JCI113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong J C, Trobridge G D, Kim C H, Johnson M, Simon B. Interferon-inducible Mx proteins in fish. Immunol Rev. 1998;166:349–363. doi: 10.1111/j.1600-065x.1998.tb01275.x. [DOI] [PubMed] [Google Scholar]
- 22.Levy O, Ooi C E, Weiss J, Lehrer R I, Elsbach P. Individual and synergistic effects of rabbit granulocyte proteins on Escherichia coli. J Clin Investig. 1994;94:672–682. doi: 10.1172/JCI117384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lichtenstein A. Mechanism of mammalian cell lysis mediated by peptide defensins. Evidence for an initial alteration of the plasma membrane. J Clin Investig. 1991;88:93–100. doi: 10.1172/JCI115310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Wang L, Jia H P, Zhao C, Heng H H Q, Schutte B C, McCray P B, Ganz T. Structure and mapping of the human β-defensin HBD-2 gene and its expression at sites of inflammation. Gene. 1998;222:237–244. doi: 10.1016/s0378-1119(98)00480-6. [DOI] [PubMed] [Google Scholar]
- 25.Miao M, Chan S L, Hew C L, Fletcher G L. Identification of nuclear proteins interacting with the liver-specific enhancer B element of the antifreeze protein gene in winter flounder. Mol Mar Biol Biotechnol. 1998;7:197–203. [PubMed] [Google Scholar]
- 26.Michaelson D, Rayner J, Couto M, Ganz T. Cationic defensins arise from charge-neutralized propeptides: a mechanism for avoiding leukocyte autocytotoxicity? J Leukoc Biol. 1992;51:634–639. doi: 10.1002/jlb.51.6.634. [DOI] [PubMed] [Google Scholar]
- 27.Miele R, Ponti D, Boman H G, Barra D, Simmaco M. Molecular cloning of a bombinin gene from Bombina orientalis: detection of NF-κB and NF-IL6 binding sites in its promoter. FEBS Lett. 1998;431:23–28. doi: 10.1016/s0014-5793(98)00718-2. [DOI] [PubMed] [Google Scholar]
- 28.Rastogi N, Goh K S, David H L. Enhancement of drug susceptibility of Mycobacterium avium by inhibitors of cell envelope synthesis. Antimicrob Agents Chemother. 1990;34:759–764. doi: 10.1128/aac.34.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redondo-Lopez V, Meriwether C, Schmitt C, Opitz M, Cook R, Sobel J D. Vulvovaginal candidiasis complicating recurrent bacterial vaginosis. Sex Transm Dis. 1990;17:51–53. [PubMed] [Google Scholar]
- 30.Romero-Vivas J, Rodriguez-Creixems M, Bouza E, Hellin T, Guerrero A, Martinez-Beltran J, Garcia de la Torre M. Evaluation of aztreonam in the treatment of severe bacterial infections. Antimicrob Agents Chemother. 1985;28:222–226. doi: 10.1128/aac.28.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross D A, Lyles M, Ledford B E, Magor B G, Wilson M R, Miller N W, Clem L W, Middleton D A, Warr G W. Catfish Oct2 binding affinity and functional preference for octamer motifs, and interaction with OBF-1. Dev Comp Immunol. 1999;23:199–211. doi: 10.1016/s0145-305x(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 32.Rycyzyn M A, Wilson M R, Bengten E, Warr G W, Clem L W, Miller N W. Mitogen and growth factor-induced activation of a STAT-like molecule in channel catfish lymphoid cells. Mol Immunol. 1998;35:127–136. doi: 10.1016/s0161-5890(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 33.Schonwetter B S, Stolzenberg E D, Zasloff M A. Epithelial antibiotics induced at sites of inflammation. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 34.Scocchi M, Skerlavaj B, Romeo D, Gennaro R. Proteolytic cleavage by neutrophil elastase converts inactive storage proforms to antibacterial bactenecins. Eur J Biochem. 1992;209:589–595. doi: 10.1111/j.1432-1033.1992.tb17324.x. [DOI] [PubMed] [Google Scholar]
- 35.Sebille S, Pereira M, Millot J M, Jacquot J, Delabroise A M, Arnaud M, Manfait M. Extracellular Mg2+ inhibits both histamine-stimulated Ca(2+)-signaling and exocytosis in human tracheal secretory gland cells. Biochem Biophys Res Commun. 1998;246:111–116. doi: 10.1006/bbrc.1998.8494. [DOI] [PubMed] [Google Scholar]
- 36.Selsted M E, Harwig S S, Ganz T, Schilling J W, Lehrer R I. Primary structures of three human neutrophil defensins. J Clin Investig. 1985;76:1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shamblott M J, Leung S, Greene M W, Chen T T. Characterization of a teleost insulin-like growth factor II (IGF-II) gene: evidence for promoter CCAAT/enhancer-binding protein (C/EBP) sites, and the presence of hepatic C/EBP. Mol Mar Biol Biotechnol. 1998;7:181–190. [PubMed] [Google Scholar]
- 38.Simmaco M, Boman A, Mangoni M L, Mignogna G, Miele R, Barra D, Boman H G. Effect of glucocorticoids on the synthesis of antimicrobial peptides in amphibian skin. FEBS Lett. 1997;416:273–275. doi: 10.1016/s0014-5793(97)01216-7. [DOI] [PubMed] [Google Scholar]
- 39.Singh P K, Jian H P, Wiles K, Hesselberth J, Liu L, Conway B D, Greenberg E P, Valore E V, Welsh M J, Ganz T, Tack B F, McCray P B J. Production of β-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snapper S B, Melton R E, Mustafa S, Kieser T, Jacobs W R J. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990;4:1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg D A, Lehrer R I. Designer assays for antimicrobial peptides. Disputing the “one-size-fits-all” theory. Methods Mol Biol. 1997;78:169–186. doi: 10.1385/0-89603-408-9:169. [DOI] [PubMed] [Google Scholar]
- 42.Valore E V, Martin E, Harwig S S, Ganz T. Intramolecular inhibition of human defensin HNP-1 by its propiece. J Clin Investig. 1996;97:1624–1629. doi: 10.1172/JCI118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valore E V, Park C H, Quayle A J, Wiles K R, McCray P B, Ganz T. Human β-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Investig. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westerhoff H V, Zasloff M, Rosner J L, Hendler R W, De Waal A, Vaz Gomes A, Jongsma P M, Riethorst A, Juretic D. Functional synergism of the magainins PGLa and magainin-2 in Escherichia coli, tumor cells and liposomes. Eur J Biochem. 1995;228:257–264. [PubMed] [Google Scholar]
- 45.Zanetti M, Litteri L, Gennaro R, Horstmann H, Romeo D. Bactenecins, defense polypeptides of bovine neutrophils, are generated from precursor molecules stored in the large granules. J Cell Biol. 1990;111:1363–1371. doi: 10.1083/jcb.111.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zasloff M. Antibiotic peptides as mediators of innate immunity. Curr Opin Immunol. 1992;4:3–7. doi: 10.1016/0952-7915(92)90115-u. [DOI] [PubMed] [Google Scholar]
- 47.Zhao C Q, Liaw L, Lee I H, Lehrer R I. cDNA cloning of three cecropin-like antimicrobial peptides (Styelins) from the tunicate, Styela clava. FEBS Lett. 1997;412:144–148. doi: 10.1016/s0014-5793(97)00769-2. [DOI] [PubMed] [Google Scholar]