Abstract
Purpose
Periprosthetic joint infection (PJI) is one of the most debilitating complications following joint replacement surgery. Synovial biomarkers, such as Calprotectin, have become valuable in the diagnosis of PJI. This meta-analysis aimed to investigate the role of synovial Calprotectin as a diagnostic test in PJI.
Methods
This meta-analysis was conducted with adherence to PRISMA guidelines. PubMed, Cochrane, Web of Science, and Google Scholar were searched until February 2022. Inclusion criteria were as follows: all studies in which the patients with joint replacements were evaluated for PJI; synovial Calprotectin was the biomarker of choice to diagnose PJI; standardized guidelines were used as the gold standard for the diagnosis; and a comparison between the guidelines and Calprotectin results was made. Diagnostic parameters such as sensitivity, specificity, diagnostic odds ratio (DOR), positive predictive value, negative predictive value, and area under the curve (AUC) were calculated for the included studies to evaluate synovial Calprotectin for PJI diagnosis.
Results
The total number of the included patients was 618 from eight studies. The pooled sensitivity, specificity, and diagnostic odds ratio of Calprotectin test were 92% (95%CI: 84%-98%), 93% (95%CI: 84%-99%), and 187.61 (95%CI: 20.21–1741.18), respectively. The results showed that the negative and positive likelihood ratios of the Calprotectin test were 0.07 (95%CI: 0.02–0.22) and 9.91 (95%CI: 4.11–23.93), respectively. The SROC showed that the area under the curve for Calprotectin test was 0.935.
Conclusion
Synovial Calprotectin is a valuable biomarker as it provides a reliable and rapid diagnosis of PJI. It has the potential to be used in clinical practice due to its high sensitivity and specificity that are comparable to the other utilized biomarkers. Another advantage is its low cost relative to other biomarkers.
Keywords: Periprosthetic joint infection, Arthroplasty, Diagnosis, Calprotectin, Diagnosis, Synovial
Introduction
Periprosthetic joint infection (PJI) is defined as an infection of prosthesis and the surrounding soft tissues and is considered one of the most debilitating complications following joint replacementsurgery. PJI contributes to around 14% of all knee and hip revision arthroplasties [1], leading to an enormous healthcare and economic burden that adds up to $1.62 billion in the USA alone [2]. However, the accurate and timely diagnosis of PJI remains quite challenging with wide variation based on the standard adopted guidelines.
Efforts to standardize the diagnosis of PJI yielded various guidelines of pre-operative and intra-operative criteria by the Musculoskeletal Infection Society (MSIS) and International Consensus Meetings (ICMs) [3–5]. In 2018, a new validated and updated version of the MSIS criteria was defined by Parvizi et al. with a higher sensitivity of 97.7% compared to the original MSIS (79.3%) and ICM definition (86.9%), with a similar specificity of 99.5% [4]. However, the inclusion of microbial cultures in these criteria remains a setback due to their poor reliability (sensitivity and specificity), particularly with low-grade micro-organism infections [6].
While serologic markers such as CRP, D-dimer, ESR have been widely used in the diagnosis of PJI, they are highly influenced by various systemic and confounding factors [7, 8]. The emergence of new diagnostic modalities has made synovial biomarkers of particular interest, including synovial WBC, leukocyte esterase, Alpha-Defensin, and Calprotectin, which have shown promising potential as diagnostic tools in PJI.
Calprotectin, also known as cystic fibrosis antigen, is a protein complex mainly secreted by neutrophils as part of the inflammatory response and plays a role in leukocyte migration and stimulation [9]. Different testing methods have also been explored to detect synovial Calprotectin, including enzyme-linked immunosorbent assay (ELISA) and lateral flow testing, showing promising results [10, 11]. Several studies have reported the efficacy of synovial Calprotectin in the diagnosis of PJI; however, further understanding of the underlying pathophysiology and diagnostic accuracy is warranted. Therefore, high-quality evidence is needed to highlight the reliability of synovial Calprotectin as a diagnostic tool in PJI.
This meta-analysis aimed to investigate the role of synovial Calprotectin as a diagnostic test in PJI and measure its reliability and validity in terms of sensitivity, specificity, diagnostic odds ratio (DOR), positive predictive value, negative predictive value, and area under the curve (AUC).
Materials and methods
This systematic review and meta-analysis were conducted with strict adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. The focus was studies that compared Calprotectin, as a biomarker to diagnose PJI, with gold standard criteria such as the MSIS and ICM-2018.
Information sources and search strategy
Electronic databases of PubMed, Cochrane, Web of Science, and Google Scholar were searched from inception till February 2022. The following keywords were used: “Periprosthetic joint infection” OR “Prosthesis-related infections” AND “Synovial” AND “Calprotectin.” Two independent reviewers screened the titles and abstracts, and the full-text review was done for the eligible studies as per the below-mentioned criteria.
Eligibility criteria
All articles were included if the following criteria were met:
Patients with joint replacements being evaluated for PJI.
Synovial fluid aspiration was done for PJI diagnosis.
Standardized diagnostic criteria, such as MSIS and ICM-2018, were used to diagnose PJI.
Calprotectin was used as a biomarker to diagnose PJI.
A comparison between Calprotectin and the diagnostic criteria was done.
We only included accessible articles that were published in English.
Exclusion criteria
Studies that did not use standardized criteria were excluded. We also excluded studies that did not use Calprotectin among the biomarkers for PJI diagnoses. Patients who had a first-stage revision before being investigated for PJI with Calprotecin were excluded.
Data collection process and data items
We collected the following data items: Author’s name, study year, country of origin, age, sex, number of participants, diagnostic criteria, detection method, Calprotectin cutoff point, Calprotectin sensitivity, Calprotectin specificity, Calprotectin positive predictive value, Calprotectin negative predictive value, Area Under the Curve, Calprotectin concentration in septic and aseptic joints.
Risk of bias in individual studies
Two of the authors evaluated the methodological quality of the included studies using the QUADAS-2 tool, which is composed of four key domains; patient selection, index test, reference standard, and flow and timing [13]. Signaling questions were applied to evaluate the risk of bias and clinical applicability. The risk of bias is judged as “low,” “high,” or “unclear” (when insufficient data are reported to permit a judgment). Any disagreement between the two authors was resolved by a discussion with a senior author.
Statistical analysis
For all the studies, we constructed a 2 × 2 contingency table, then the sensitivity, specificity, diagnostic odds ratio, positive and negative predictive values were calculated for each study. Moreover, we pooled the prevalence of the disease in the included studies using a random effect model with double arcsine transformation to calculate the diagnostic parameters that need prevalence to be calculated (PPV and NPV). When more than one threshold was used by any of the included studies, the threshold with the largest Yourdon index was used in the analysis. The mentioned diagnostic parameters were pooled using a random effect model. In addition, the summarized receiver operating characteristic (SROC) curve was constructed using these diagnostic parameters. The heterogeneity of the included studies was investigated using the Cochrane Q and I2 statistic. All the mentioned analyses except the SROC were conducted using Meta XL, version 5.3 (EpiGear International, Queensland, Australia). The SROC was generated using MetaDTA: Diagnostic Test Accuracy Meta-Analysis v2.01 [14].
Results
Study Selection
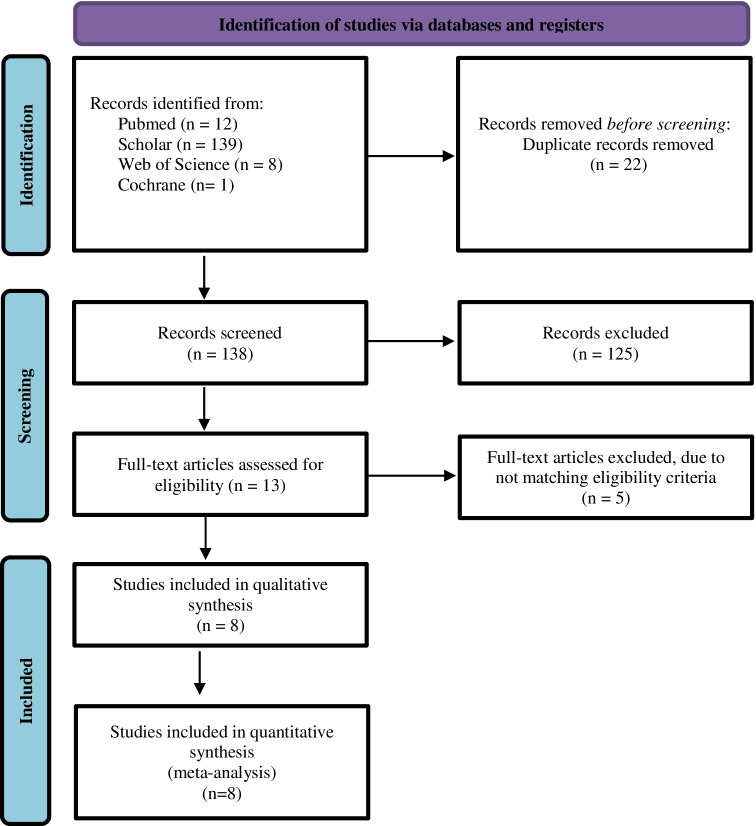
The search yielded 160 articles, 22 of which were duplicates that were removed manually and electronically. After screening using title/abstract, 125 were excluded. The remaining 13 articles were screened using a full-text form, and five of them were excluded. Finally, eight articles were included in this study. The detailed selection process is described in Fig. 1.
Fig. 1.
Search strategy flowchart
Characteristics of the included studies
A total number of 618 patients from eight studies were included in this meta-analysis. The percentage of septic patients in the included studies according to the ICM or MSIS criteria was 39.5% (244/618) while 60.5% of them were aseptic patients (374/618). Four studies specified the number of septic and aseptic patients according to gender using the ICM/MSIS criteria. Among the male patients in these studies, 19.7% were septic (55/127), while 40.7% of the female patients were septic (83/204) Two of the included studies used ELISA as a detection technique, two studies used point of care and ELISA tests, three studies used lateral flow immunoassay, and one study used immunoturbidimetric Calprotectin immunoassay. Salari et al. [15], WouthuyzenBakker et al. 2017 [16], WouthuyzenBakker et al. 2018a [17] and Grassi et al. [18] used the value of more than or equal 50 mg/l as their cutoff point for positive tests. On the other hand, the cutoff point for positive tests varied between Trotter et al. [10], Zhan et al. [11], and Grzelecki et al. [19], which were > = 14 mg/l, > 173 ug/ml, and > = 1.5 mg/l, respectively. In addition, Warren et al. [20] used two cutoff points for positive Calprotectin tests which were more than or equal 50 mg/l and more than or equal 14 mg/l. The more than or equal 50 mg/l showed higher Youden index value; hence, it was the one that was used in the analysis. Grassi et al. reported all parameters for both ELISA and POC test. However, the POC test they used was a protoyle, and therefore, the parameter calculated for ELISA test was included in the analysis. The characteristics of the included studies are described in Table 1.
Table 1.
Studies characteristics
| Study | Country | Study design | Participants (M/F) | Age | Detection method | Gold standard | Cutoff point | Septic Joints | Aseptic Joints | S* | SP* | AUC | PLR | NLR | PPV | NPV | Calprotectin concentration in septic Vs. aseptic joints |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Salari, 2019 ✚ |
Italy | Cohort |
72 (36/40) |
69 | ELISA | ICM 2018 | > = 50 mg/L | Knee 28 | Knee 44 | 100% | 95% | 0.996 | 22 | 0 | - | - |
320 mg/L Vs 5.5 mg/L |
| Warren, 2021 | USA | Cohort |
123 (57/66) |
Septic 66.9 ± 10.6 Aseptic 65.4 ± 10.6 |
POC + ELISA |
MSIS |
^ > = 50 mg/L > = 14 mg/L |
Knee 53 | Knee 70 | 98.1% | 95.7% | 0.969 | - | - | 94.50% | 98.50% | - |
| Wouthuyzn-Bakker, 2017 | Netherl-ands | Pilot |
61 (25/36) |
Septic 65 (24–87) Aseptic 60 (23–90) |
Lateral Flow Immunoassay | MSIS | > = 50 mg/L |
Knee 5 Hip 11 Shoulder 3 |
Knee 5 Hip 34 Shoulder 2 Ankle 1 |
89% | 90% | 0.94 | 8.9 | 0.1 | 81% | 95% |
991 mg/L Vs 11 mg/L |
| Wouthuyzn-Bakker, 2018 | Netherl-ands | Cohort | 52 | - | Lateral Flow Immunoassay | MSIS | > = 50 mg/L |
Knee 5 Hip 8 Shoulder 2 |
Knee 12 Hip 24 Shoulder 1 |
86.7% | 91.7% | 0.94 | 10.9 | 0.14 | 81.3% | 94.4% |
859 mg/L Vs 7 mg/L |
| Zhng, 2020 | China | Cohort |
63 (20/43) |
Septic 64 (54–83) Aseptic 57 (41–86) |
ELISA | MSIS | 173 μg/ml |
Knee 6 Hip 15 |
Knee 12 Hip 30 |
92.2% | 976% | 0.993 | 39.6 | 0.049 | 95.2% | 97.6% |
776 μg/m Vs 54.5 μg/m |
|
Trotter, 2020 # |
UK | Pilot |
69 (37/32) |
74.3 (45–89) | Lateral Flow Immunoassay | ICM 2018 | > = 14 mg/L |
Knee 9 Hip 15 |
Knee 8 Hip 37 |
75% | 75.56% | 0.78 | - | - | 62.07% | 85% | - |
| Grzelecki, 2021 | Poland | Cohort |
85 (25/60) |
Septic 65.5 ± 10 Aseptic 68.3 ± 12 |
Immunoturbidimetric Calprotectin Immunoassay | ICM 2018 | 1.5 mg/L |
Knee 25 Hip 20 |
Knee 25 Hip 15 |
95.6% | 95% | - | - | - | 95.50% | 95.00% |
20.4 mg/L Vs 0.7 mg/L |
| Italy | Cohort |
93 (42/51) |
77 |
ELISA + POC |
ICM 2018 | > = 50 mg/L | Knee 39 | Knee 50 |
ELISA 92.3% POC 97.4% |
ELISA 100% POC 94% |
ELISA 0.962 POC 0.957 |
ELISA - POC 16.239 |
ELISA 0.077 POC 0.027 |
ELISA 100% POC 92.7% |
ELISA 94.3% POC 97.9% |
ELISA 290.6 mg/L Vs 6.5 mg/ L |
✚ 4 patients were excluded from the analysis due to inconclusive results and not all minor criteria were considered for all patients
# Minor criteria of ICM 2018 were not considered
S*: Sensitivity
SP*: Specificity
PLR: Positive likelihood ratio
NLR: Negative likelihood ratio
^ Two cutoff points were used, the > = 50 mg/L was used in the analysis
POC used in the study is a prototype. Thus, ELIZA parameters were used in the analysis
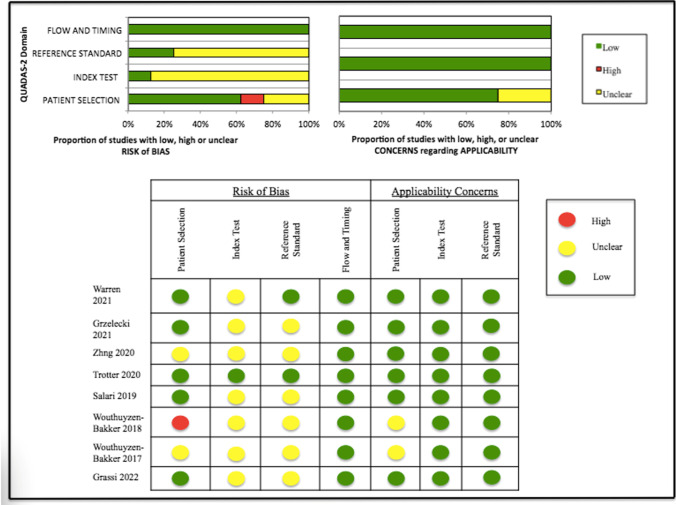
Quality Assessment
Figure 2 illustrates the quality assessment of the included studies using QUADAS-2 tool criteria.
Fig. 2.
Quality assessment of the included studies using QUADAS-2 tool criteria
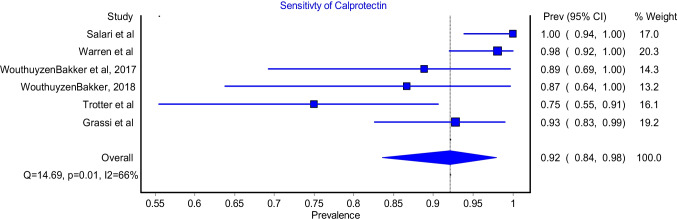
Sensitivity
The Calprotectin test sensitivity model included six studies. The model showed that the Calprotectin test had a sensitivity of 92% (Fig. 3; 95%CI: 84%-98%). The model had significant heterogeneity (Fig. 3; I2 = 66%, P-value = 0.01). The highest sensitivity was reported by Salari et al., and it was 99.6%, whereas the lowest sensitivity was reported by Trotter et al. and it was 75.0%.
Fig. 3.
Sensitivity for Calprotectin test
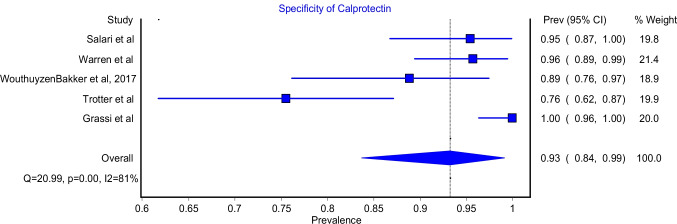
Specificity
The specificity of the Calprotectin test model included five studies. The model showed that the Calprotectin test had a specificity of 93% (Fig. 4; 95%CI: 84%-99%). The model had significant heterogeneity (Fig. 4; I2 = 81%, P-value = 0.00). The highest specificity was reported by Grassi et al. who reported a 100%% specificity for the test. On the other hand, the lowest specificity was reported by Trotter et al. and it was 75.6%.
Fig. 4.
Specificity for Calprotectin test
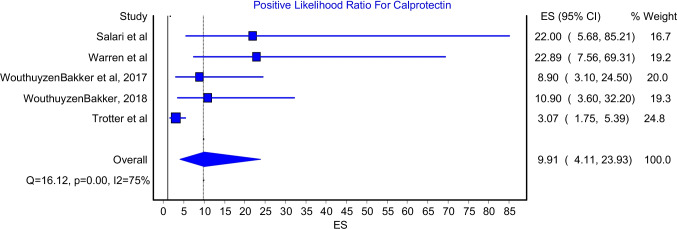
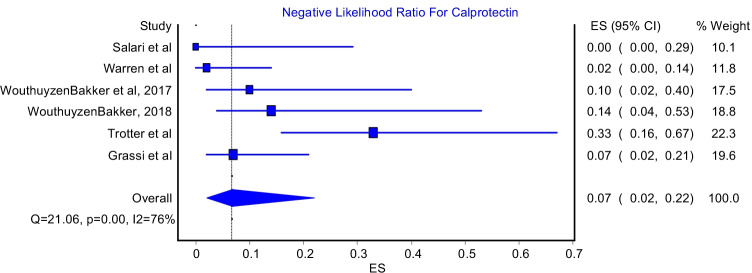
Positive and negative likelihood ratio
The positive likelihood ratio model included five studies while the negative likelihood ratio model included six studies. The pooled positive likelihood ratio was 9.91 (Fig. 5; 95%CI: 4.11–23.93). This model had significant heterogeneity (Fig. 5; I2 = 75%, P-value = 0.00). The highest positive likelihood ratio was reported by Zhang et al. who reported a value of 39.6, while the lowest positive likelihood ratio was reported by Trotter et al. and it was 3.07. Moreover, the pooled negative likelihood ratio was 0.07 (Fig. 6; 95%CI: 0.02–0.22). This model had significant heterogeneity (Fig. 6; I2 = 76%, P-value = 0.00). The lowest negative likelihood ratio was reported by Salari et al. who reported a value of 0. In contrast, the highest negative likelihood ratio was reported by Trotter et al. which was 33.0.
Fig. 5.
Positive likelihood ratio for Calprotectin test
Fig. 6.
Negative likelihood ratio for Calprotectin test
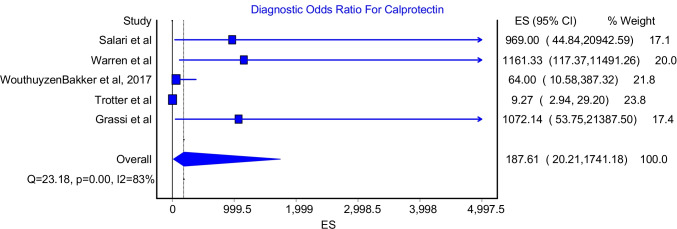
Diagnostic Odds Ratio
The diagnostic odds ratio model for the Calprotectin test included five studies. The pooled diagnostic odds ratio was 187.61 (Fig. 7; 95%CI: 20.21–1741.18). This model showed significant heterogeneity (Fig. 7; I2 = 83%, P-value = 0.00). The highest diagnostic odds ratio was reported by Warren et al. and Grassi et al., which were 1161.33 and 1072.14, respectively, whereas the lowest diagnostic odds ratio was reported by Trotter et al. which was 9.27.
Fig. 7.
Diagnostic odds ratio for Calprotectin test
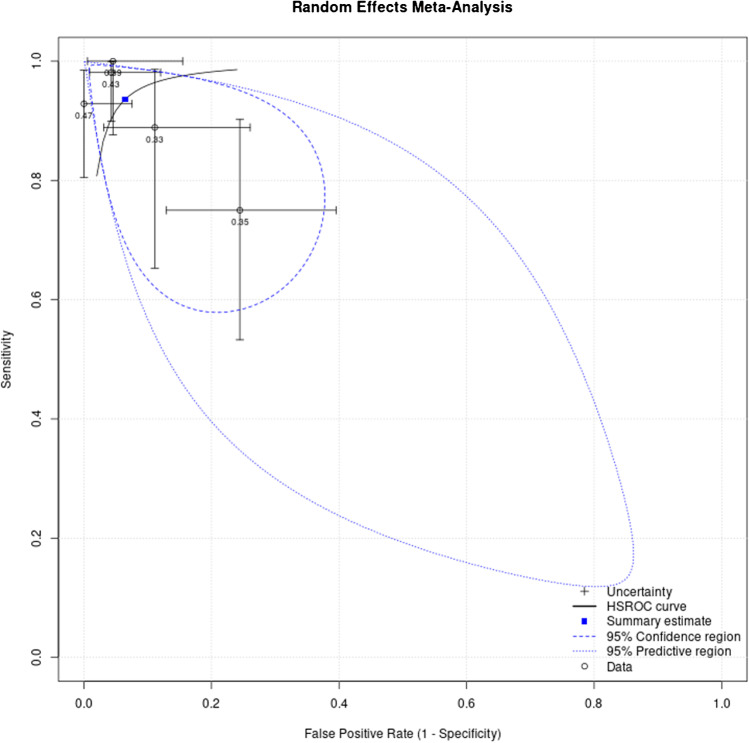
The Summary of Receiver Operating Characteristics Curve
The Summary of Receiver Operating Characteristics Curve (SROC) model for the Calprotectin test included five studies (Table 2). The SROC showed that the area under the curve (AUC) for the Calprotectin test was 0.935 (Fig. 8). The highest AUC was reported by Salari et al. which was 0.996 while the lowest was reported by Trotter et al. at 0.78. Moreover, the pooling of the studies that were included in the SROC revealed that the sensitivity and specificity for the Calprotectin test were 93.6% (95%CI: 83.5%-97.7%) and 93.5% (95%CI: 84.5%-97.5%), respectively. The positive and negative likelihood ratio for the Calprotectin test in the studies that were included in the SROC was 14.469 (95%CI: 5.571–37.579) and 0.068 (95%CI: 0.024–0.192), respectively. Additionally, the diagnostic odds ratio for the Calprotectin test in the studies that were included in the SROC was 212.457 (95%CI: 33.992–1327.901).
Table 2.
Meta-analysis for studies that were included in summary of ROC
| Parameter | Estimate | 2.5% CI | 97.5% CI |
|---|---|---|---|
| Sensitivity | 0.936 | 0.835 | 0.977 |
| Specificity | 0.935 | 0.845 | 0.975 |
| False positive rate | 0.065 | 0.025 | 0.155 |
| Random effects correlation | 1.000 | ||
| Θ | -0.027 | ||
| Λ | 5.359 | ||
| Β | -0.027 | ||
| σθ | 0.000 | ||
| σα | 3.344 | ||
| Diagnostic odds ratio | 212.457 | 33.992 | 1327.901 |
| Likelihood ratio + ve | 14.469 | 5.571 | 37.579 |
| Likelihood ratio -ve | 0.068 | 0.024 | 0.192 |
| logit(sensitivity) | 2.688 | 1.625 | 3.751 |
| logit(specificity) | 2.671 | 1.697 | 3.645 |
| Prevalence | 0.39 | 0.35 | 0.44 |
| Accuracy | 0.935 |
Fig. 8.
Meta-analysis for summary of ROC
Discussion
This meta-analysis reported an excellent pooled diagnostic value of Calprotectin in the diagnosis of PJI in comparison with gold standard methods. The pooled sensitivity and specificity of Calprotectin were 92% (Fig. 2; 95%CI: 84%-98%) and 93% (Fig. 3; 95%CI: 84%-99%), respectively. Moreover, Calprotectin was shown to provide a good diagnostic accuracy for PJI with a pooled positive likelihood ratio of 9.91 (Fig. 4; 95%CI: 4.11–23.93) and pooled negative likelihood ratio of 0.07 (Fig. 5; 95%CI: 0.02–0.22).
Eight articles with a total of 618 arthroplasties were included in this current review. High heterogeneity was reported among the included articles in terms of the type of arthroplasty (TKA, THA, TSA), diagnostic tools (ELISA, lateral flow immunoassay, immunoturbidimetric immunoassay), and cutoff point for diagnosis. Comparing individual studies, Calprotectin was found to have a better diagnostic power in the studies that included total knee replacements alone. Salari et al. reported 100% sensitivity and 95% specificity for the diagnosis of infected TKA in comparison with ICM 2018 with an AUC of 0.996. Similarly, Warren et al. compared Calprotectin to the MSIS criteria in the diagnosis of PJI in TKA; they reported high sensitivity and specificity of 98% and 96%, respectively. In addition, Grassi et al., who used the ICM 2018 criteria as a reference, reported a sensitivity and specificity of 92.3% and 100%, respectively. On the other hand, using Calprotectin in a mixed cohort of patients yielded a relatively lower diagnostic value. In the study by Wouthzyn-Bakker (2017), the diagnostic accuracy of Calprotectin was assessed in the diagnosis of PJI in 10 TKAs, 45 THAs, 5 TSAs, and 1 total ankle arthroplasty using lateral flow immunoassay with cutoff value of 50 mg/L. The authors reported 89% sensitivity and 90% specificity in comparison with the MSIS criteria. In comparison with the ICM 2018 criteria, Trotter et al. reported an AUC as low as 0.78 for Calprotectin in diagnosing PJI in TKA and THA with a sensitivity and specificity of 75%. The authors used lateral flow immunoassay with a cutoff point of 14 mg/L. As a new method used to detect PJI, however, there is no consensus about the most accurate diagnostic tool and threshold for Calprotectin. Five of the included studies used 50 mg/L as a cutoff using either ELISA (Salari, Warren), lateral flow immunoassay (Wouthuzyn-Bakker 2017 and 2018), or both methods (Grassi). Higher sensitivity and specificity were reported using ELISA with a higher PLR and a lower NLR. Grzelecki et al. used Immunoturbidimetric Calprotectin immunoassay with a threshold of 1.5 mg/L in diagnosing hip and knee PJI. They reported 95% sensitivity and specificity. This demonstrated that the method used can affect the diagnostic accuracy and lateral flow immunoassay might be inferior to other methods. Moreover, with the available literature, a threshold for diagnosis cannot be determined and more studies are needed.
Low cost, availability, and previous utilization for other pathologies are considered advantages for the use of Calprotectin in diagnosing PJI. In comparison with other available biomarkers, Calprotectin showed promising and comparable results. A pooled sensitivity and specificity of Alpha-Defensin of 95% and 96%, respectively, were reported in two recent meta-analyses [21, 22]. The meta-analysis by Wyatt et al. [23] reported pooled diagnostic sensitivity and specificity of leukocyte esterase for PJI were 0.81and 0.97, respectively. Furthermore, IL-6 showed a pooled sensitivity of 83% and a pooled specificity of 91% in the meta-analysis by Yoon et al. [24]. Future comparative controlled studies are needed to draw a solid conclusion on the value of Calprotectin in comparison with other available biomarkers in the diagnosis of PJI.
A recently published meta-analysis by Xing et al. investigated the role of Calprotectin in diagnosing PJI. However, this article did not account for the fact that the majority of the included studies used different cutoff points. Moreover, they did not specify which cutoff point was used when they conducted the analysis on the studies that reported different cutoff points. On the other hand, our study accounted for this limitation by using the Youden Index. This index guided our decision on which cutoff value to use in our analysis. Using Youden to guide us on which cutoff point to use in the analysis explains the variation in the results between our article and Xing’s meta-analysis. In addition, this meta-analysis included eight studies with a total number of 618 patients, a 15% larger sample size when compared the Xing’s meta-analysis. The larger sample size results in lower standard of error and hence lower confidence intervals across all the analyses. The new study made huge effect on the conference intervals, which is reflected on the reliability of our results.
Limitations
Several limitations should be acknowledged in this study. First, the low number of the included studies hindered our ability to perform sensitivity analysis for different Calprotectin cutoff points or testing techniques. Second, since there is no standard technique or cutoff point for testing synovial fluid Calprotectin, different studies used different techniques and different values, which can impact the diagnostic accuracy. Accordingly, future large-scale prospective randomized trials are required to address these problems. The study by Wouthuyzen-Bakker [17] included some of the patients who were recruited in their previous study in 2017 [16], which might have created some crosspoints in our analysis. However, not all the patients were included and the sequel study included a significant number of patients. Another limitation is the fact that most of the included studies did not mention any information about blinding, sampling point time, and adjustment for confounding variables, which increases the risk of both confounding and selection biases. Finally, our analysis revealed high heterogeneity among the included studies, which can be explained by different cutoff points and Calprotectin testing techniques used by the included studies.
Conclusion
Based on this meta-analysis, Synovial Calprotectin is a reliable and valid biomarker for PJI. It has the potential to be used in clinical practice due to its high sensitivity and specificity that are comparable to the other utilized biomarkers. Another advantage is its low cost relative to other tests. The role of Calprotectin in PJI diagnosis still needs to be elucidated in randomized trials.
Funding
Open Access funding provided by the Qatar National Library.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of Interest
The authors have no financial or proprietary interests in any material discussed in this article.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Joint Registry for England, Wales, Northern Ireland, and the Isle of Man: 15th Annual Report (2018). https://www.hqip.org.uk/resource/national-joint-registry-15th-annual-report-2018/. Accessed 10 Sep 2021
- 2.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic Burden of Periprosthetic Joint Infection in the United States. J Arthroplasty. 2012;27(8 Suppl):61–5.e1. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1–10. doi: 10.1093/cid/cis966. [DOI] [PubMed] [Google Scholar]
- 4.Parvizi J, Tan TL, Goswami K, et al. The 2018 Definition of Periprosthetic Hip and Knee Infection: An evidence-based and validated criteria. J Arthroplasty. 2018;33(5):1309–1314.e2. doi: 10.1016/j.arth.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 5.Parvizi J, Zmistowski B, Berbari EF, et al. New Definition for Periprosthetic Joint Infection: From the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Related. 2011;469(11):2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parvizi J, Erkocak OF, Della Valle CJ. Culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(5):430–436. doi: 10.2106/JBJS.L.01793. [DOI] [PubMed] [Google Scholar]
- 7.Matsen Ko L, Parvizi J. Diagnosis of Periprosthetic Infection: Novel Developments. Orthop Clin North Am. 2016;47(1):1–9. doi: 10.1016/j.ocl.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Saleh A, George J, Faour M, et al. Serum biomarkers in periprosthetic joint infections. Bone Joint Res. 2018;7(1):85–93. doi: 10.1302/2046-3758.71.BJR-2017-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stríz I, Trebichavský I. Calprotectin: A pleiotropic molecule in acute and chronic inflammation. Physiol Res. 2004;53(3):245–253. [PubMed] [Google Scholar]
- 10.Trotter, A.J., Dean, R., Whitehouse, C.E., Mikalsen, J., Hill C., Brunton-Sim, R., Kay, G.L., Shakokani, M., Durst, A.Z.E., Wain, J., McNamara, I., O'Grady, J., (2020) Preliminary Evaluation of a Rapid Lateral Flow Calprotectin Test for the Diagnosis of Prosthetic Joint Infection. Bone and Joint Research, 8;9(5):202–210. 10.1302/2046-3758.95.BJR-2019-0213.R1 [DOI] [PMC free article] [PubMed]
- 11.Zhang, Z., Cai, Y., Bai, G., Zhang, C., Li, W., Yang, B., Zhang, W., (2020) The value of Calprotectin in Synovial Fluid for the Diagnosis of Chronic Prosthetic Joint Infection. Bone and Joint Research, 11;9(8):450–457. 10.1302/2046-3758.98.BJR-2019-0329.R2 [DOI] [PMC free article] [PubMed]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-analyses: The PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 14.Patel A, Cooper N, Freeman S, Sutton A. Graphical Enhancements to Summary Receiver Operating Characteristic Plots to Facilitate the Analysis and Reporting of Meta-analysis of Diagnostic Test accuracy Data. Res Synth Methods. 2020;12(1):34–44. doi: 10.1002/jrsm.1439. [DOI] [PubMed] [Google Scholar]
- 15.Salari P, Grassi M, Cinti B, Onori N, Gigante A. Synovial Fluid Calprotectin for the Preoperative Diagnosis of Chronic Periprosthetic Joint Infection. J Arthroplasty. 2020;35(2):534–537. doi: 10.1016/j.arth.2019.08.052. [DOI] [PubMed] [Google Scholar]
- 16.Wouthuyzen-Bakker M, Ploegmakers J, Kampinga G, Wagenmakers-Huizenga L, Jutte P, Muller Kobold A. Synovial Calprotectin: A potential biomarker to exclude a prosthetic joint infection. Bone Joint J. 2017;99-B(5):660–665. doi: 10.1302/0301-620X.99B5.BJJ-2016-0913.R2. [DOI] [PubMed] [Google Scholar]
- 17.Wouthuyzen-Bakker M, Ploegmakers J, Ottink K, Kampinga G, Wagenmakers-Huizenga L, Jutte P, Kobold A. Synovial Calprotectin: An Inexpensive Biomarker to Exclude a Chronic Prosthetic Joint Infection. J Arthroplasty. 2018;33(4):1149–1153. doi: 10.1302/0301-620X.99B5.BJJ-2016-0913.R2. [DOI] [PubMed] [Google Scholar]
- 18.Grassi M., Salari P., Farinelli L., D'Anzeo M., Onori N., Gigante A., (2022) Synovial Biomarkers to Detect Chronic Periprosthetic Joint Infection: A pilot study to compare Calprotectin rapid test, Calprotectin ELISA immunoassay and leukocyte esterase test. J Arthroplasty, 6:S0883–5403(21)00985–2. 10.1016/j.arth.2021.12.040 [DOI] [PubMed]
- 19.Grzelecki D, Walczak P, Szostek M, Grajek A, Rak S, Kowalczewski J. Blood and Synovial Fluid Calprotectin as Biomarkers to Diagnose Chronic Hip and Knee Periprosthetic Joint Infections. Bone Joint J. 2021;103-B(1):46–55. doi: 10.1302/0301-620X.103B1.BJJ-2020-0953.R1. [DOI] [PubMed] [Google Scholar]
- 20.Warren J, Anis H, Bowers K, Pannu T, Villa J, Klika A, Colon-Franco J, Piuzzi N, Higuera C. Diagnostic Utility of a Novel Point-of-Care Test of Calprotectin for Periprosthetic Joint Infection After Total Knee Arthroplasty. J Bone Joint Surg. 2021;103(11):1009–1015. doi: 10.2106/JBJS.20.01089. [DOI] [PubMed] [Google Scholar]
- 21.Yuan J, Yan Y, Zhang J, Wang B, Feng J. Diagnostic Accuracy of Alpha-Defensin in Periprosthetic Joint Infection: A systematic review and meta-analysis. Int Orthop. 2017;41(12):2447–2455. doi: 10.1007/s00264-017-3647-3. [DOI] [PubMed] [Google Scholar]
- 22.Suen K, Keeka M, Ailabouni R, Tran P. Synovasure ‘Quick Test’ is not as Accurate as the Laboratory-Based Alpha-Defensin Immunoassay. Bone Joint J. 2018;100-B(1):66–72. doi: 10.1302/0301-620X.100B1.BJJ-2017-0630.R1. [DOI] [PubMed] [Google Scholar]
- 23.Wyatt, M.C., Beswick, A.D., Kunutsor, S.K., Wilson, M.J., Whitehouse, M.R., Blom, A.W., (2016) The Alpha-Defensin Immunoassay and Leukocyte Esterase Colorimetric Strip Test for the Diagnosis of Periprosthetic Infection: A systematic review and meta-analysis. Journal of Bone and Joint Surgery Am, 15;98(12):992–1000. 10.2106/JBJS.15.01142 [DOI] [PMC free article] [PubMed]
- 24.Yoon JR, Yang SH, Shin YS. Diagnostic accuracy of Interleukin-6 and Procalcitonin in Patients with Periprosthetic Joint Infection: A systematic review and meta-analysis. Int Orthop. 2018;42(6):1213–1226. doi: 10.1007/s00264-017-3744-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.