Abstract
Abacavir (1592U89) is a nucleoside reverse transcriptase inhibitor with potent activity against human immunodeficiency virus type 1 (HIV-1) when used alone or in combination with other antiretroviral agents. The present study was conducted to determine the multiple-dose pharmacokinetics and pharmacodynamics of abacavir in HIV-1-infected subjects following oral administration of daily doses that ranged from 600 to 1,800 mg, with and without zidovudine. Seventy-nine subjects received abacavir monotherapy for 4 weeks (200, 400, or 600 mg every 8 hours [TID] and 300 mg every 12 h [BID]) and thereafter received either zidovudine (200 mg TID or 300 mg BID) or matching placebo with abacavir for 8 additional weeks. Pharmacokinetic parameters were calculated for abacavir after administration of the first dose and at week 4 and for abacavir, zidovudine, and its glucuronide metabolite at week 12. The concentrations of abacavir in cerebrospinal fluid were determined in a subset of subjects. Steady-state plasma abacavir concentrations were achieved by week 4 of monotherapy and persisted to week 12. At steady state, abacavir pharmacokinetic parameters (area under the plasma concentration-time curve for a dosing interval [AUCtau] and peak concentration [Cmax]) were generally proportional to dose over the range of a 600- to 1,200-mg total daily dose. Coadministration of zidovudine with abacavir produced a small and inconsistent effect on abacavir pharmacokinetic parameters across the different doses. At the clinical abacavir dose (300 mg BID) zidovudine coadministration had no effect on the abacavir AUCtau, which is most closely associated with efficacy. Zidovudine pharmacokinetics appeared to be unaffected by abacavir. Statistically significant but weak relationships were found for the change in the log10 HIV-1 RNA load from the baseline to week 4 versus total daily AUCtau and Ctau (P < 0.05). The incidence of nausea was significantly associated with total daily AUCtau and Cmax. In conclusion, abacavir has predictable pharmacokinetic characteristics following the administration of multiple doses.
Abacavir (1592U89) is a novel 2′-deoxyguanosine analog with potent in vitro and in vivo activities against human immunodeficiency virus (HIV) type-1 (HIV-1) (3). Abacavir is phosphorylated by a unique intracellular activation pathway to the active moiety, carbocyclic guanosine triphosphate, which inhibits HIV reverse transcriptase (6). Studies have shown that abacavir penetrates the cerebrospinal fluid (CSF) (3, 10; J. R. Ravitch, S. S. Good, J. E. Humpreys, J. W. Poli, W. H. Robertson, and J. L. Jarrett, Abstr. 5th Conf. Retroviruses Opportunistic Infections, abstr. 636, p. 199, 1998), has potency against HIV strains resistant to other nucleoside reverse transcriptase inhibitors (NRTIs) (3), and is slow to develop cross-resistance with other NRTIs (15; J. W. Mellors, R. Pauwels, P. Stoffels, B. Larder, N. Graham, V. Miller, R. Lanier, F. Peeters, and K. Hertogs, Abstr. 5th Conf. Retroviruses Opportunistic Infections, abstr. 687, p. 208, 1998).
Abacavir has demonstrated favorable safety and pharmacokinetic characteristics in phase I studies (7, 8). Orally administered abacavir has a mean absolute bioavailability of 83%, and the extent of absorption is not affected by food (2). The main route of abacavir elimination is metabolism to the 5′-carboxylate and the 5′-glucuronide metabolites, and 83% of a radiolabeled oral dose is recovered in urine, with less than 2% recovered unchanged in the urine (10). Clinical studies have reported that abacavir produced marked decreases in plasma HIV-1 RNA levels and concomitant increases in CD4+-cell counts when administered alone or in combination with other NRTIs or protease inhibitors (11, 13; P. A. Bart, G. Pantaleo, H. McDade, W. Spreen, H. Steel, G. Martin, P. Meylan, J. P. Chave, C. Graziosi, C. Welbon, S. Gallant, and G. P. Rizzardi, Abstr. 5th Conf. Retroviruses Opportunistic Infections, abstr. 365, p. 147, 1998; D. Kelleher, J. Mellors, M. Lederman, D. Haas, E. Cooney, J. Horton, J. Stanford, and R. Haubrich, Abstr. 12th World AIDS Conference, abstr. 12210, p. 53, 1998).
Abacavir has demonstrated few drug interactions in patients. No clinically significant pharmacokinetic interactions were found between abacavir and zidovudine (16), lamivudine (16), or amprenavir (J. A. McDowell, B. M. Sadler, J. Millard, P. Nunnally, and N. Mustafa, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-62, p. 13, 1997) in HIV-1-infected adults. Abacavir did not affect ethanol concentrations, although ethanol produced moderate changes in the absorption and elimination of abacavir (10a). In vitro studies indicate that abacavir is unlikely to inhibit human liver microsomal cytochrome P450 enzymes (CYP-3A4, 2D6, and 2C9) at clinically relevant concentrations or inhibit drugs that are metabolized by these enzymes (4; J. R. Ravitch, B. J. Bryant, M. J. Reese, C. C. Boehlert, J. S. Walsh, J. P. McDowell, and B. M. Sadler, Abstr. 5th Conf. Retroviruses Opportunistic Infections, abstr. 634, p. 199, 1998). The potent antiretroviral activity of abacavir, the favorable pharmacokinetic characteristics of abacavir, and the absence of drug interactions indicate that abacavir should be easily incorporated into combination antiretroviral regimens.
In order to fully characterize the pharmacokinetics of abacavir in patients, a phase II study was conducted to evaluate four multiple-dose regimens of abacavir as monotherapy and as combination therapy. The objective of the present study (Glaxo Wellcome protocol CNAA-2001) was to determine the multiple-dose pharmacokinetics and pharmacodynamics of abacavir in HIV-1-infected subjects following oral administration of daily doses that ranged from 600 to 1,800 mg, with and without zidovudine (ZDV). The clinical efficacy and safety results of this study have been reported elsewhere (11).
(Preliminary data from this study were presented in part at the XI International AIDS Conference, Vancouver, British Columbia, Canada, July 1996.)
MATERIALS AND METHODS
Study population and design.
Details about the study population and design have been reported elsewhere (11) and are summarized here. Sixty-eight male subjects and 11 female subjects who were ≥13 years of age, who were confirmed to have HIV-1 infection, and who had CD4+ counts of 200 to 550 cells/mm3 were enrolled in the study at 1 of 13 study centers in the United States and Europe. Subjects were excluded if they had previously received ZDV for >12 weeks. Subjects who had received prior ZDV therapy must have discontinued treatment 2 weeks before they received abacavir. The study was conducted on an outpatient basis. All study sites had investigational review board approval to perform the study, and all patients gave written informed consent prior to their participation.
Subjects were sequentially enrolled into four cohorts that received oral abacavir at 200 mg three times a day (TID) (cohort I; 19 subjects), 400 mg TID, 300 mg twice a day (BID), and 600 mg TID (cohorts II, III, and IV, respectively, each with 20 subjects) for 4 weeks. Thereafter, subjects were assigned in a randomized, double-blind manner to receive a daily dose of either ZDV or ZDV placebo in combination with the previously assigned abacavir dose for an additional 8 weeks. Subjects received ZDV at 200 mg TID (cohorts I, II, and IV) or at 300 mg BID (cohort III). Study drugs were supplied as abacavir caplets that contained 100 mg of abacavir free base as the succinate salt and Retrovir capsules that contained 100 mg of ZDV. Due to limited in vivo animal safety data relative to abacavir at the time that the study was initiated, extended therapy for greater than 12 weeks was not possible. At the end of the 12-week period abacavir was discontinued, and subjects were treated with other antiretroviral agents at the discretion of the investigator.
To determine the single-dose and steady-state pharmacokinetics of abacavir, subjects were required to report to the study center on day 1 and at the end of weeks 4 and 12. On these days, subjects were instructed to fast for 8 h before administration of each of the scheduled morning doses and the collection of samples. Study drugs were administered under supervision at the study center. Subjects took the study drugs with 200 ml of water and fasted for an additional 3 h postdosing.
Sample collection.
Blood samples (4 ml) for pharmacokinetic evaluations were collected at three evaluation times: on day 1 (for abacavir concentrations), at the end of week 4 (for abacavir concentrations), and at the end of week 12 (for abacavir and ZDV concentrations). On day 1, blood samples were collected predosing and then at 0.25, 0.5, 0.75, 1.0, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, and 12 h postdosing. For the steady-state pharmacokinetic assessments (weeks 4 and 12), blood samples were collected, after an overnight fast, before dosing and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, and 8 h after administration of the morning dose. One-milliliter samples of CSF were obtained from a subset of subjects at 1.5 h postdosing at the week 4 and/or week 12 pharmacokinetic evaluations. Plasma samples were obtained via centrifugation. All plasma and CSF samples were stored at −40°C until analysis.
Analytical methods.
Plasma and CSF samples were assayed for abacavir concentrations by a validated reverse-phase high-performance liquid chromatography (HPLC) assay with UV detection over a quantifiable range of 25 to 5,000 ng/ml. Briefly, plasma samples (0.2 ml) were mixed with 0.1 ml of 10% trichloroacetic acid and were then centrifuged at 8,800 × g for 10 min. Supernatant (0.1 ml) was then injected onto a Rainin (4.6 by 250 mm) C18 Microsorb MV column. The mobile phase consisted of 40% methanol and 0.3% (vol/vol) triethylamine at a flow rate of 1.0 ml/min. Abacavir was detected by measurement of the UV absorbance at 284 nm. CSF samples were analyzed by HPLC with UV detection following direct injection of the samples onto the column. The retention time for abacavir was approximately 9 min under these conditions. For the plasma samples, the interday variability (coefficient of variation) was <12% and the bias of the assay was <3%. For the CSF samples, the interday variability was <8% and the bias of the assay was <4%.
The plasma ZDV concentration was quantitated with a commercially available radioimmunoassay (ZDV-Trac kit; IncStar, Inc., Stillwater, Minn.). The quantifiable range for ZDV was 0.27 to 270 μg/ml. The interday variability was <13%, and the bias was <10%. The concentration of the glucuronide metabolite of ZDV (GZDV) was determined as the difference from the total ZDV concentration after treatment of the sample with β-glucuronidase, as described earlier (14).
Pharmacokinetic analyses.
Noncompartmental analysis was performed with plasma concentration data by using WinNonlin (version 1.2) with model 200 for extravascular input (Scientific Consulting Inc., Cary, N.C.). The peak concentration (Cmax) and the time to Cmax (Tmax) were obtained by direct inspection of the plasma concentration-time curves. The terminal rate constant (λz) was calculated from the slope of linear regression of the logarithm of the concentration in plasma as a function of time in the terminal elimination phase, and the apparent elimination half-life (t1/2) was calculated as ln(2)/λz. For the single-dose pharmacokinetics (day 1), the area under the concentration-time curve (AUC) from the time of dosing to the last time point with a quantifiable concentration (AUClast) was calculated by using the log-linear trapezoidal rule. The AUC from time zero to infinity (AUC0–∞) was then determined as AUClast + Clast/λz, where Clast is the last measurable concentration. Apparent clearance from plasma (CL/F) was calculated as dose divided by AUC0–∞. For multiple-dose pharmacokinetics, the AUC for a dosing interval (AUCtau), where tau is 8 h for the TID dosing regimens (cohorts I, II, and IV) and 12 h for the BID dosing regimen (cohort III), was calculated. Because plasma samples were not collected past 8 h postdosing, the area under the plasma concentration-time curve from 8 to 12 h postdosing for cohort IV was determined from the last measurable concentration by using λz. Adjustments were also made to the calculation interval for tau to correspond to the times when the first and last plasma samples were actually collected. The concentration in plasma at the end of a dosing interval (Ctau), where tau is 8 or 12 h, was also determined. The total daily AUC (TD-AUC) was calculated by obtaining the product of AUCtau and the dosing interval (two and three for the BID and TID dosing regimens, respectively).
Statistical analyses.
To compare pharmacokinetic parameters between the three evaluation times, analyses of variance (ANOVA) were performed by the SAS Mixed Linear Models procedure (version 6.0.9; SAS Institute Inc.). The model included subject as a random effect and treatment cohort, periods, and treatment · periods as fixed effects. Following log transformation, geometric least-squares mean values and 95% confidence intervals (CIs) were calculated for each pharmacokinetic parameter. Pairwise comparisons of parameters were performed by calculating the ratios of the geometric least-squares means for the test treatment to those for the reference treatment and the corresponding 90% CIs. Two one-sided tests were performed to evaluate the 90% CIs for the geometric least-squares mean ratios (12). Nonparametric methods were used to compute the 95% CIs for the untransformed median Tmax values. The estimated median differences in Tmax between any two evaluations and the associated 90% CIs were computed by the Wilcoxon signed rank test.
For single- to multiple-dose comparisons, the ratios of the geometric least-squares means for week 4 to those for the first dose (day 1) were calculated for AUCtau, Cmax, Ctau, t1/2, and CL/F. The pharmacokinetic parameter estimate for week 4 was not considered significantly different from that for the first dose if the 90% CI includes 1. Attainment of steady state was assessed by comparing the ratios of the geometric least-squares means for week 12 to those for week 4 by using data for subjects who did not receive ZDV during the combination therapy phase. Steady-state conditions were considered achieved if the 90% CI for the geometric least-squares mean ratio of the pharmacokinetic parameters included 1. The effect of ZDV coadministration on steady-state parameters was similarly assessed by comparing the ratios of the geometric least-squares mean for week 12 to those for week 4 for subjects who had received ZDV during the combination therapy phase.
Dose proportionality for pharmacokinetic parameters was evaluated by using the power model described previously (8). The estimated mean slope was calculated with the model for day 1 and week 4. The 90% CI of the mean slope value was determined, and the primary criterion for dose proportionality for the dose-dependent parameters (AUC0–∞, AUClast, and Cmax) was the inclusion of 1 within the range of the CIs, indicating that the slope did not deviate significantly from 1.
A secondary method, ANOVA with the SAS Mixed Linear Model, was also used to assess dose proportionality. For this analysis, the dose-dependent parameters were normalized to a 600-mg total daily dose (either 300 mg for day 1 data or 300 mg BID for multiple-dose data) and were then log transformed. The geometric least-squares means and 95% CIs were computed for each parameter for each dose group. Two one-sided tests were performed to evaluate 90% CIs for the parameters for each dose group relative to those for the appropriate reference dose. The pharmacokinetic parameters for each dose group were considered proportional to those for the reference dose if the 90% CI included 1.
SAS PROC MIXED was used to evaluate relationships between covariates (regimen, race, gender, entry age, weight, height, creatinine clearance) and the geometric least-squares means of log-transformed AUCtau (dose normalized), Cmax (dose normalized), untransformed λz, and Ctau at day 1, week 4, and week 12. The model included cohort, period, sex, race, period · sex, and cohort · sex as fixed effects and subject as a random effect.
Pharmacodynamic analyses.
Five adverse events (headache, nausea, dizziness, vomiting, and rash) were selected for analysis on the basis of their incidence and potential for pharmacokinetic dependence during abacavir monotherapy. TD-AUC and Cmax values were calculated and were empirically categorized to four and two intervals, respectively. For a specific adverse event, the number of subjects and the incidence of the event were categorized according to one of the TD-AUC or Cmax intervals. The Mantel-Haenszel chi-square test with modified ridit scores was used to evaluate the association between the adverse event incidence and drug exposure. Logistic regression analyses (SAS PROC LOGISTIC) were also applied to the data to estimate relationships between TD-AUC, Cmax, and Ctau at week 4 and each adverse event. Odds ratios with their 95% CIs were obtained by logistic regression for per unit change in TD-AUC (in microgram · hour per milliliter) or Cmax (in micrograms per milliliter) and 1/10-unit change in Ctau (in micrograms per milliliter).
The association between week 4 measurements of TD-AUC, Cmax, or Ctau and (i) the change in the log10 HIV-1 RNA viral load from the baseline to week 4 or (ii) the increase in the CD4+-cell count from the baseline to week 4 was estimated by the slope of activity versus pharmacokinetic parameters by using the SAS Linear Regression model. Spearman statistics were used to determine the correlation of the pharmacokinetic parameters with HIV-1 load and CD4+-cell count data at week 4. Further analysis by sigmoid Emax modeling (where Emax is the maximal effect) was attempted, but no significant relationships were found.
RESULTS
Single- to multiple-dose assessments.
Comparison of single- and multiple-dose data reveals similar curves, but with somewhat higher concentrations in plasma being achieved after the administration of multiple doses (data not shown). Mean Cmax values, which were dose related, were achieved between 0.75 and 1.5 h.
Comparison of abacavir pharmacokinetic parameters after the administration of a single dose and at week 4, after the administration of multiple doses (Table 1), revealed statistically significant differences in all cohorts for AUCtau, Cmax, and CL/F between day 1 and week 4, indicating the accumulation of abacavir after administration of the first dose. In addition, Ctau and t1/2 values for cohort I at week 4 were significantly increased relative to the values on day 1. Comparisons of pharmacokinetic parameters between week 4 and week 12 (for subjects randomized to receive placebo) revealed no statistically significant differences (Table 1).
TABLE 1.
Pharmacokinetic parameters of abacavir with and without ZDV following single- and multiple-dose oral administrations
| Abacavir dose cohort | Abacavir pharmacokinetic parametera
|
||||
|---|---|---|---|---|---|
| AUCtau (μg · h/ml)b | Cmax (μg/ml) | Ctau (μg/ml) | t1/2 (h) | CL/F (ml/min) | |
| I (200 mg TID) | |||||
| Day 1 (n = 19) | 2.49 (2.14–2.90) | 1.40 (1.20–1.64) | 0.02 (0.01–0.03) | 1.07 (0.90–1.27) | 1,340 (1,150–1,562) |
| Wk 4 (n = 18) | 3.70 (3.28–4.18)c | 2.02 (1.77–2.30)c | 0.04 (0.03–0.05)c | 1.43 (1.27–1.60)c | 901 (798–1,017)c |
| Wk 12 (n = 10) | 3.63 (3.05–4.32) | 1.87 (1.53–2.29) | 0.05 (0.03–0.07) | 1.41 (1.24–1.61) | 917 (771–1,091) |
| Wk 12 (n = 7) + ZDV | 2.76 (2.24–3.39) | 1.67 (1.31–2.12) | 0.03 (0.02–0.05) | 1.27 (1.08–1.48) | 1,207 (980–1,485) |
| II (400 mg TID) | |||||
| Day 1 (n = 18) | 6.51 (5.56–7.61) | 2.82 (2.40–3.30) | 0.07 (0.05–0.10) | 1.31 (1.10–1.57) | 1,024 (875–1,198) |
| Wk 4 (n = 18) | 7.41 (6.56–8.36)c | 3.48 (3.05–3.97)c | 0.08 (0.05–0.10) | 1.30 (1.16–1.46) | 900 (797–1,016)c |
| Wk 12 (n = 8) | 7.84 (6.46–9.52) | 3.62 (2.89–4.54) | 0.08 (0.05–0.12) | 1.25 (1.08–1.45) | 850 (700–1,032) |
| Wk 12 (n = 9) + ZDV | 7.67 (6.38–9.21) | 4.15 (3.35–5.14) | 0.08 (0.06–0.12) | 1.32 (1.15–1.52) | 870 (724–1,045) |
| III (300 mg BID) | |||||
| Day 1 (n = 19) | 4.26 (3.65–4.96) | 2.32 (1.99–2.71) | 0.01 (0.01–0.02) | 1.38 (1.16–1.64) | 1,174 (1,007–1,368) |
| Wk 4 (n = 20) | 5.80 (5.17–6.50)c | 2.89 (2.55–3.27)c | 0.01 (0.01–0.02) | 1.42 (1.28–1.59) | 862 (769–968)c |
| Wk 12 (n = 9) | 5.79 (4.82–6.96) | 3.00 (2.42–3.71) | 0.01 (0.01–0.02) | 1.30 (1.14–1.50) | 864 (719–1,037) |
| Wk 12 (n = 10) + ZDV | 6.03 (5.07–7.18) | 3.13 (2.55–3.83) | 0.01 (0.01–0.02) | 1.37 (1.20–1.56) | 829 (696–986) |
| IV (600 mg TID) | |||||
| Day 1 (n = 20) | 11.70 (10.08–13.58) | 4.12 (3.54–4.80) | 0.20 (0.14–0.28) | 1.61 (1.36–1.90) | 855 (736–992) |
| Wk 4 (n = 17) | 13.16 (11.62–14.91)c | 4.80 (4.19–5.50)c | 0.20 (0.14–0.27) | 1.42 (1.26–1.60) | 760 (671–861)c |
| Wk 12 (n = 6) | 13.01 (10.40–16.28) | 6.55 (5.04–8.51) | 0.15 (0.09–0.24) | 1.36 (1.15–1.62) | 769 (614–962) |
| Wk 12 (n = 9) + ZDV | 12.53 (10.44–15.05) | 5.24 (4.23–6.49) | 0.24 (0.16–0.35) | 1.56 (1.36–1.79) | 798 (664–958) |
Values are given as geometric least-squares means (95% CIs).
AUC0–∞ for day 1.
Significantly different from the value on day 1 (P < 0.05).
Steady-state assessments.
A comparison of log-transformed ratios of abacavir pharmacokinetic parameters at week 12 (restricted to subjects randomized to receive ZDV; data shown in Table 1) and those at week 4 (without ZDV; data not shown in Table 1) indicated a number of statistically significant differences (Table 2). Changes in the values at week 12 relative to those at week 4 were a 26% decrease in the mean AUCtau for cohort I (consistent with the increased CL/F), increases in the mean Cmaxs for cohorts II and III (by 16 and 23%, respectively), a 23% decrease in the mean Ctau for cohort II, and a 13% increase in the median t1/2 for cohort III. These changes were variable and unrelated to dose.
TABLE 2.
Comparison of ratios of log-transformed geometric least-squares means for abacavir pharmacokinetic parameters at week 12 (with ZDV) with those at week 4 (without ZDV)a
| Abacavir dose cohort | AUCtau (μg · h/ml) | Cmax (μg/ml) | Ctau (μg/ml) | t1/2 (h) | CL/F (ml/min) |
|---|---|---|---|---|---|
| I (200 mg TID) | 0.74 (0.66–0.84)b | 0.88 (0.74–1.05) | 0.76 (0.54–1.07) | 0.93 (0.82–1.06) | 1.34 (1.19–1.52)b |
| II (400 mg TID) | 0.99 (0.89–1.09) | 1.16 (1.00–1.35)b | 0.67 (0.49–0.92)b | 0.93 (0.83–1.03) | 1.01 (0.91–1.12) |
| III (300 mg BID) | 1.12 (1.00–1.25) | 1.23 (1.05–1.45)b | 1.30 (0.97–1.75) | 1.13 (1.00–1.27)b | 0.89 (0.80–1.00)b |
| IV (600 mg TID) | 0.92 (0.83–1.02) | 1.05 (0.90–1.23) | 1.06 (0.80–1.40) | 1.03 (0.92–1.15) | 1.09 (0.98–1.21) |
Comparison of week 12 versus week 4 data was enhanced by within-subject comparison. No correction was performed for multiple pairwise comparisons. Values are given as geometric least-squares means (95% CIs).
Statistically different from the value at week 4 (P < 0.05).
Selected pharmacokinetic parameters estimated for ZDV and its glucuronide metabolite (GZDV) are shown in Table 3. Geometric least-squares mean values for AUCtau and Cmax for ZDV were similar across the cohorts that received 200-mg doses of ZDV TID (cohorts I, II, and IV). In these cohorts, AUCtau ranged from 0.92 to 1.09 h · μg/ml, and Cmax ranged from 0.75 to 0.81 μg/ml. As expected, Cmax and AUCtau values for subjects in cohort III, who had received 300 mg of ZDV BID, were approximately 50% higher than those for the subjects in the other cohorts. For GZDV, AUCtau and Cmax estimates increased between cohort that received the lowest abacavir dose (cohort I) and the cohort that received the highest abacavir dose (cohort IV).
TABLE 3.
Selected pharmacokinetic parameters for ZDV and GZDV during coadministration with abacavir
| Abacavir dose cohort | Pharmacokinetic parametersa
|
||
|---|---|---|---|
| AUCtau (μg · h/ml) | Cmax (μg/ml) | Tmax | |
| I (200 mg TID; n = 6) | |||
| ZDV | 0.92 (0.70–1.21) | 0.75 (0.48–1.18) | 0.63 (0.27–1.50) |
| GZDV | 4.65 (3.29–6.58) | 2.97 (1.89–4.66) | 0.77 (0.50–1.50) |
| II (400 mg TID; n = 9) | |||
| ZDV | 1.09 (0.87–1.36) | 0.79 (0.54–1.14) | 0.90 (0.25–1.92) |
| GZDV | 7.28 (5.49–9.67) | 4.74 (3.28–6.85) | 1.02 (0.50–2.42) |
| III (300 mg BID; n = 8) | |||
| ZDV | 1.71 (1.35–2.16) | 1.30 (0.88–1.93) | 0.80 (0.47–1.03) |
| GZDV | 8.34 (6.17–11.26) | 5.01 (3.39–7.40) | 1.25 (0.47–1.65) |
| IV 600 mg TID; n = 5) | |||
| ZDV | 1.09 (0.81–1.46) | 0.81 (0.49–1.33) | 0.63 (0.50–2.00) |
| GZVD | 10.73 (7.33–15.69) | 6.12 (3.74–10.03) | 1.00 (0.63–2.00) |
ZDV was administered at 200 mg TID for cohorts I, II, and IV and 300 mg BID for cohort III. AUCtau and Cmax values are reported as geometric least-squares means (95% CIs), and Tmax values are reported as the median and range.
Dose proportionality.
Power model analysis indicated that the dose-dependent parameters for day 1 and week 4 did not increase proportionally with dose across all four cohorts (Table 4).
TABLE 4.
Power model assessment of dose proportionality for selected abacavir pharmacokinetic parameters at week 4
| Abacavir parameter | Estimated slope (90% CI) |
|---|---|
| AUCtau (μg · h/ml) | 1.13a (1.01, 1.26) |
| Cmax (μg/ml) | 0.78a (0.64, 0.92) |
| Ctau (μg/ml) | 1.71a (1.24, 2.17) |
Significant deviation from proportionality (P < 0.05).
The secondary analysis of dose proportionality separately compared the data for each cohort with those for cohort III. These results, for which data were normalized to a dose of 300 mg BID, indicated that after 4 weeks of dosing the AUCtau and Cmax values for cohorts I and II were proportional to those for the 300-mg-BID treatment, but those for cohort IV were proportional for AUCtau but not for Cmax (Table 5). None of the cohorts exhibited Ctau values that were dose proportional to those for cohort III.
TABLE 5.
ANOVA assessment of selected abacavir pharmacokinetic parameters at week 4
| Parametera | Treatment (mg TID) | Geometric least-squares mean | Mean ratiob (90% CI) |
|---|---|---|---|
| AUCtau (μg · h/ml) | 200 | 11.11 | 0.96 (0.83, 1.10) |
| 400 | 11.12 | 0.96 (0.83, 1.10) | |
| 600 | 13.16 | 1.14 (0.99, 1.31) | |
| Cmax (μg/ml) | 200 | 6.05 | 1.05 (0.90, 1.22) |
| 400 | 5.23 | 0.90 (0.78, 1.05) | |
| 600 | 4.80 | 0.83c (0.71, 0.97) | |
| Ctau (μg/ml) | 200 | 0.12 | 4.34c (2.92, 6.47) |
| 400 | 0.11 | 4.24c (2.85, 6.31) | |
| 600 | 0.20 | 7.31c (4.89, 10.94) |
Values were normalized to a dose of 300 mg BID.
Geometric least-squares mean ratios for parameter values for test treatments (200, 400, and 600 mg TID) versus those for the reference treatment (300 mg BID). Geometric least-squares means for the 300-mg-BID treatment were 11.59 μg · h/ml, 5.78 μg/ml, and 0.03 μg/ml for AUCtau, Cmax, and Ctau, respectively.
Significant deviation from proportionality (P < 0.05).
CSF analysis.
All 11 CSF samples collected were assayed for abacavir concentrations. There were seven samples from week 4 and four samples from week 12. Two samples were from the cohort II (one sample from week 4 and one sample from week 12), and all others were from cohort I. Of these 11 CSF samples, three samples were excluded: one contained blood and sampling times were missing for two samples.
Six of the reported values for abacavir CSF concentrations were for week 4 for cohort I, with only one sample obtained at week 12 (0.06 μg/ml). Another determination (0.40 μg/ml) was obtained at week 4 from cohort II. The six samples for week 4 for cohort I had concentrations that averaged 0.14 μg/ml (or 0.5 μM), with a coefficient of variation of 21%, and that ranged from 0.09 to 0.19 μg/ml. The ratio of the concentration in CSF to the concentration in plasma for these six samples averaged 0.42, with a coefficient of variation of 154% and a range of 0.08 to 1.73.
Covariant analysis.
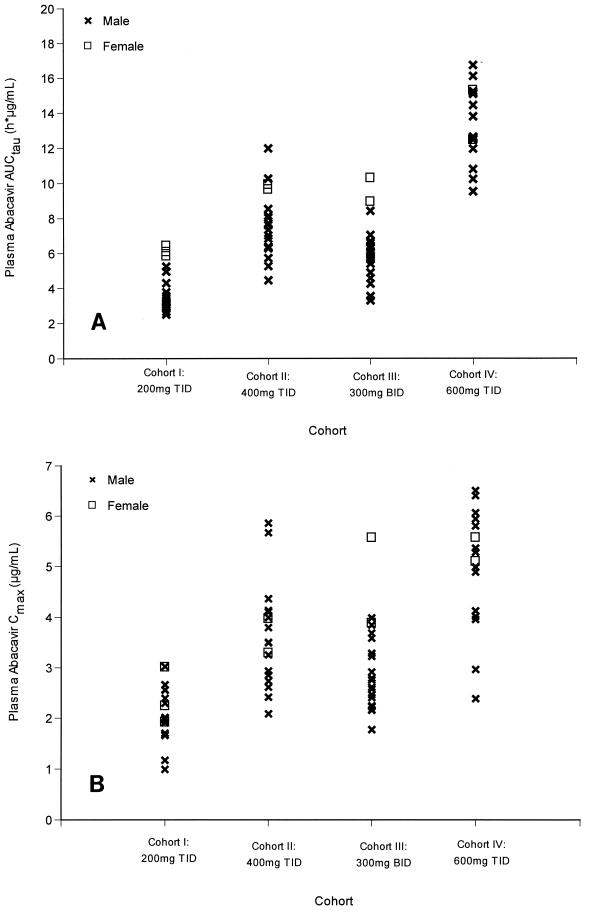
No significant associations were found between the covariates (age, weight, height, or creatinine clearance) and the single-dose or steady-state pharmacokinetic parameters. When gender was analyzed for its influence on pharmacokinetic parameters at week 4, significant associations with AUCtau and Cmax values were observed (Fig. 1A and B). Women (n = 11) exhibited a 54% greater mean AUCtau and a 30% greater mean Cmax relative to the values for men (n = 68) (AUCtau, 15.69 versus 10.20 μg · h/ml; Cmax, 6.45 versus 4.97 μg/ml) (P < 0.05).
FIG. 1.
(A) AUCtau values for women (n = 11) and men (n = 68) in each cohort at steady state (week 4). The mean AUCtau was 54% higher for women (15.67 μg · h/ml) than men (10.20 μg · h/ml). (B) Cmax values for women (n = 11) and men (n = 68) in each cohort at steady state (week 4). The mean Cmax was 30% higher for women (6.45 μg/ml) than for men (4.97 μg/ml).
Pharmacodynamic analyses.
The categorical Mantel-Haenszel analysis indicated that nausea was significantly associated with TD-AUC (P = 0.002) and Cmax (P = 0.001). Consistent with the Mantel-Haenszel analysis, logistic regression analysis indicated that the incidence of nausea was significantly associated with both TD-AUC (odds ratio, 1.05 [95% CI, 1.01 to 1.09]) and Cmax (odds ratio, 1.73 [95% CI, 1.20 to 2.61]) (Table 6).
TABLE 6.
Logistic regression analysis of adverse events versus pharmacokinetic parameters
| Adverse event | Odds ratio (95% CI)a
|
||
|---|---|---|---|
| TD-AUC | Cmax | Ctau | |
| Headache | 1.01 (0.97–1.05) | 1.10 (0.77–1.56) | 1.04 (0.64–1.65) |
| Nausea | 1.05b (1.01–1.09) | 1.73b (1.20–2.61) | 1.18 (0.75–1.88) |
| Vomiting | 1.00 (0.91–1.08) | 0.97 (0.41–1.99) | 0.65 (0.09–1.84) |
| Rash | 1.04 (0.97–1.10) | 1.33 (0.76–2.32) | 1.27 (0.59–2.44) |
| Dizziness | 0.94 (0.79–1.04) | 0.37 (0.08–1.03) | 0.74 (0.13–1.97) |
Odds ratios represent the increase in the occurrence of an adverse event for a 1-μg · h/ml increase in TD-AUC and a 1-μg/ml increase in Cmax or a 0.1-μg/ml increase in Ctau compared to the values for subjects without the adverse event.
Statistically significant association (P < 0.05).
Spearman analysis indicated that both the Ctau and the TD-AUC were significantly associated with the reduction in the log10 HIV-1 RNA load from the baseline to week 4 (P < 0.05) but that changes in the CD4+-cell count were not associated with any of the pharmacokinetic parameters evaluated. Selected week 4 pharmacokinetic parameters failed to reveal a statistically significant model to either the change in CD4+ cell count from baseline to week 4 nor to the change in baseline to week 4 in log10 HIV-1 RNA viral load (data not shown).
DISCUSSION
The results of this study indicate that steady-state plasma abacavir concentrations were achieved following 4 weeks of dosing and persisted for the duration of the study. At steady state, abacavir pharmacokinetic parameters (AUCtau and Cmax) were proportional to dose over the range of a 600- to 1,200-mg total daily dose. At steady state, coadministration of ZDV produced small and inconsistent effects on abacavir pharmacokinetics. The most prominent effect was on the lowest dose studied (cohort I, 200 mg TID), and the least prominent effect was on the highest single dose studied (600 mg TID). AUCtau decreased by 26% in cohort I but not in any of the other cohorts. Thus, it is unlikely that ZDV produced a clinically significant effect on abacavir pharmacokinetic parameters, and ZDV pharmacokinetics appeared to be unaffected by abacavir. CSF abacavir concentrations in subjects in cohort I exceeded the drug's in vitro mean 50% inhibitory concentration (IC50) for clinical isolates of HIV-1 (3). Statistically significant associations were observed between the incidence of nausea and AUCtau and Cmax. A 54% greater mean AUCtau and a 30% greater mean Cmax were observed for women than for men.
Consistent with the good systemic bioavailability of abacavir (83% in humans; ∼76 to 100% in preclinical studies) (2, 3; S. S. Good, B. S. Owens, M. B. Faletto, W. B. Mahony, and B. A. Domin, Abstr. 34th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 186, p. 92, 1994), detectable concentrations of abacavir appeared in plasma within minutes of oral administration, and maximal concentrations were achieved in less than 2 h. In the present study, Ctau ranged from 0.01 to 0.24 μg/ml (0.036 to 0.86 μM) and exceeded the mean IC50 of 0.07 μg/ml for clinical HIV-1 isolates from antiretroviral naive patients (3).
The observed multiple-dose-associated increases in Cmax and AUCtau values indicate the accumulation of abacavir. The accumulation could have been influenced to some extent by irregular compliance with the dosage regimens in this outpatient study. It seems more likely, however, that the accumulation reflects a first-pass effect on metabolism. The saturation observed in the increases in mean AUCtau and mean Cmax toward steady state also suggest a saturable first-pass effect. It is also possible that a second phase of abacavir elimination exists and is yet to be recognized. Plasma abacavir concentrations measured 8 h after dosing challenge the lower limits of sensitivity of the analytical method used. Increased assay sensitivity or pharmacokinetic data for higher doses may be necessary to further address the issue.
In earlier experiments with single escalating doses (8), AUCtau and Cmax values for 100 mg and, to a lesser extent, 300 mg of abacavir were less than proportional to dose, in contrast to the values for the three higher doses (600, 900, and 1,200 mg). In the present study, the results of pairwise comparisons normalized to a dose of 300 mg BID by ANOVA indicated dose proportionality for nearly all parameters at steady state.
Coadministration of ZDV had only small and inconsistent effects on the steady-state pharmacokinetics of abacavir that did not increase with dose. A preliminary study (8) had shown that the bioavailability of low doses of abacavir (less than 300 mg) is less than that of higher doses. Reduced low-dose bioavailability may be further compromised by ZDV coadministration. The absence of any statistically significant influence of ZDV on the pharmacokinetic parameters for higher doses of abacavir and the sporadic effects within the cohorts that received lower doses suggest that any possible effect is not of clinical significance.
The pharmacokinetic profiles of ZDV in the absence of abacavir were not determined during this study; thus, unequivocal statements concerning the effects of abacavir on ZDV pharmacokinetics cannot be made. However, the mean AUCtau and Cmax values for ZDV developed during the combination therapy phase are comparable to historical steady-state values (9). In the present study, the similarities of the mean AUCtau and Cmax values for ZDV across abacavir treatment cohorts indicate that increasing doses of abacavir (by as much as threefold) did not alter the kinetics of ZDV. Additionally, in cohort III, which also received 300 mg of ZDV BID during the combination therapy phase, pharmacokinetic parameter values for ZDV were observed to be in the expected range when adjustments were made for the 50% higher ZDV dose.
CSF abacavir concentrations in subjects who received 200 mg of abacavir TID exceeded the IC50 of abacavir (3). These results confirm the penetration of active abacavir concentrations into the central nervous system. However, determinations of ratios of abacavir concentrations in CSF to those in plasma revealed a wide coefficient of variability (154%), which is probably due to the use of a single fixed time point for analysis. CSF and plasma drug concentrations are frequently out of phase, such that this variability is not surprising (1).
After administration of the first dose and during steady-state conditions, mean AUCtau and Cmax values were higher for women than for men. Associations with body weight were sought but were not observed, so this apparent difference by gender does not appear to be related to gender body weight differences. While such a gender effect could be of clinical significance, the overall pharmacodynamic-pharmacokinetic profile of abacavir in women indicates acceptable safety for doses up to 600 mg TID. Furthermore, it should be noted that this possible gender effect is based on data for only 11 females and 68 males. A second abacavir multiple-dose study also examined a potential gender effect but did not find a significant difference in population pharmacokinetic results by gender (17). Additional confirmatory studies or pooled analyses are planned to further explore this possible gender-related difference.
Statistically significant but weak relationships between TD-AUC and Ctau versus a change in the log10 HIV-1 RNA load from the baseline to week 4 were observed, but relationships with a change in the CD4+ cell count were not apparent. As indicated by other pharmacodynamic analyses, the difficulty in demonstrating clear relationships between pharmacokinetic parameters and either increases in CD4+ cell counts or reductions in log10 HIV-1 RNA loads may be explained by the plateau in the dose-versus-effect curve, in which a large change in drug exposure corresponds to little change in effect (17). Drusano and Stein (5) and Weller et al. (17) have shown that with the protease inhibitor indinavir and with abacavir, the increase in the CD4+ cell count is poorly correlated with drug exposure, as it is linked to much lower drug exposures for 50% of maximal effect than for HIV-1 RNA loads.
In conclusion, the results of this pharmacokinetic assessment and other clinical evaluations (11, 13, 17) support the selection of a daily abacavir dose of 600 mg for further clinical investigation. In addition to providing highly effective antiviral activity, the 300-mg-BID regimen is convenient and should be easy for subjects to follow.
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of Michael O'Mara and William Mahony for performing the bioanalytical studies and Belinda Ha for manuscript preparation.
REFERENCES
- 1.Burger D M, Kraaijeveld C L, Meenhorst P L, Mulder J W, Koks C H W, Bult A, Beijnen J H. Penetration of zidovudine into the cerebrospinal fluid of patients infected with HIV. AIDS. 1993;7:1581–1587. doi: 10.1097/00002030-199312000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Chittick G E, Gillotin C, McDowell J A, Lou Y, Edwards K D, Prince W T, Stein D S. Abacavir (1592U89): absolute bioavailability and effect of food. Pharmacotherapy. 1999;19:932–942. doi: 10.1592/phco.19.11.932.31568. [DOI] [PubMed] [Google Scholar]
- 3.Daluge S M, Good S S, Faletto M B, Miller W H, St. Clair M H, Boone L R, Tisdale M, Parry N R, Reardon J E, Dornsife R E, Averette D R, Krenitsky T A. 1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity. Antimicrob Agents Chemother. 1997;41:1082–1093. doi: 10.1128/aac.41.5.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drusano G L, D'Argenio D Z, Symonds W, Bilello P A, McDowell J, Sadler B, Bye A, Bilello J A. Nucleoside analog 1592U89 and human immunodeficiency virus protease inhibitor 141W94 are synergistic in vitro. Antimicrob Agents Chemother. 1998;42:2153–2159. doi: 10.1128/aac.42.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drusano G L, Stein D S. Mathematical modeling of the interrelationship of CD4 lymphocyte count and viral load changes induced by the protease inhibitor indinavir. Antimicrob Agents Chemother. 1998;42:358–361. doi: 10.1128/aac.42.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faletto M B, Miller W H, Garvey E P, St. Clair M H, Daluge S M, Good S S. Unique intracellular activation of the potent anti-human immunodeficiency virus agent 1592U89. Antimicrob Agents Chemother. 1997;41:1099–1107. doi: 10.1128/aac.41.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes W, McDowell J A, Shenep J, Flynn P, Kline M W, Yogev R, Symonds W, Lou Y, Hetherington S. Safety and single-dose pharmacokinetics of abacavir (1592U89) in human immunodeficiency virus type 1-infected children. Antimicrob Agents Chemother. 1999;43:609–615. doi: 10.1128/aac.43.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar P N, Sweet D E, McDowell J A, Symonds W, Lou Y, Hetherington S, LaFon S. Safety and pharmacokinetics of abacavir (1592U89) following oral administration of escalating single doses in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother. 1999;43:603–608. doi: 10.1128/aac.43.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laskin O, Miranda P, Blum M R. Azidothymidine steady-state pharmacokinetics in patients with AIDS and AIDS-related complex. J Infect Dis. 1989;159:745–747. doi: 10.1093/infdis/159.4.745. [DOI] [PubMed] [Google Scholar]
- 10.McDowell J, Chittick G, Ravitch J, Polk R E, Kerkering T M, Stein D S. Pharmacokinetics of [14C]abacavir, a human immunodeficiency virus type 1 (HIV-1) reverse transcriptase inhibitor, administered in a single oral dose to HIV-1-infected adults: a mass balance study. Antimicrob Agents Chemother. 1999;43:2855–2861. doi: 10.1128/aac.43.12.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.McDowell J A, Chittick G E, Stevens C P, Edwards K D, Stein D S. Pharmcokinetic interaction of abacavir (1592U89) and ethanol in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2000;44:1686–1690. doi: 10.1128/aac.44.6.1686-1690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saag M S, Sonnerborg A, Torres R A, Lancaster D, Gazzard B G, Schooley R T, Romero C, Kelleher D, Spreen W, LaFon S the Abacavir Phase 2 Clinical Team. Antiretroviral effect and safety of abacavir and abacavir in combination with zidovudine in HIV-infected adults. AIDS. 1998;12:F203–F209. doi: 10.1097/00002030-199816000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Schuirmann D J. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharm Biopharm. 1987;15:657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- 13.Staszewski S, Katlama C, Harrer T, Massip P, Yeni P, Cutrell A, Tortell S M, Harrigan P R, Steel H, Lanier E R, Pearce G. A dose-ranging study to evaluate the safety and efficacy of three doses of abacavir (1592U89) alone or in combination with zidovudine and lamivudine in antiretroviral treatment naive subjects. AIDS. 1998;12:F197–F202. doi: 10.1097/00002030-199816000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Tadepalli S M, Puckett L, Jeal S, Kamocs L, Quinn R P. Differential assay of zidovudine and its glucuronide metabolite in serum and urine with a radioimmunoassay kit. Clin Chem. 1990;36:897–900. [PubMed] [Google Scholar]
- 15.Tisdale M, Alnadaf T, Cousens D. Combination of mutations in human immunodeficiency virus type 1 reverse transcriptase required for resistance to the carbocyclic nucleoside 1592U89. Antimicrob Agents Chemother. 1997;41:1094–1098. doi: 10.1128/aac.41.5.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L H, Chittick G E, McDowell J A. Single-dose pharmacokinetics and safety of abacavir (1592U89), zidovudine, and lamivudine administered alone and in combination in adults with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1999;43:1708–1715. doi: 10.1128/aac.43.7.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weller S, Radomski K M, Lou Y, Stein D S. Population pharmacokinetics and pharmacodynamic modeling of abacavir (1592U89) from a dose-ranging, double-blind, randomized monotherapy trial with human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2000;44:2052–2060. doi: 10.1128/aac.44.8.2052-2060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]