Abstract
The activity of gentamicin at various concentrations against two strains of Enterococcus faecalis was investigated in vitro and in a rabbit model of aortic endocarditis. In vitro, gentamicin at 0.5 to 4 times the MIC failed to reduce the number of bacteria at 24 h. Rabbit or human serum dramatically increased gentamicin activity, leading to a ≥3-log10 CFU/ml decrease in bacterial counts when the drug concentration exceeded the MIC. Susceptibility testing in the presence of serum was predictive of in vivo activity, since gentamicin alone significantly reduced the number of surviving bacteria in the vegetations if the peak-to-MIC ratio was greater than 1. However, gentamicin selected resistant mutants in rabbits. The intrinsic activity of gentamicin should be taken into account in evaluation of combinations of gentamicin and cell wall-active agents against enterococci.
Enterococci are intrinsically resistant to low levels of aminoglycosides due to inefficient active transport across the cytoplasmic membrane (13). Thus, aminoglycosides alone are considered inactive in the treatment of enterococcal infections and are usually combined with inhibitors of cell wall synthesis which may facilitate their uptake (16). Therapeutic regimens with once-daily doses achieving high peak levels of aminoglycosides in serum have been recommended since they provide increased activity, in comparison to more traditional administration of the drugs (1, 3, 4). Consistent with this notion, it was shown that the bactericidal activity of aminoglycosides is concentration dependent (6) and that high peak-to-MIC ratios could reduce the emergence of resistant mutants (5). We conducted the present study to determine if the higher peak levels of gentamicin in serum obtained with these new regimens affect the antienterococcal activity of the drug.
MATERIALS AND METHODS
Bacterial strains and media.
Enterococcus faecalis JH2-2 is susceptible to glycopeptides and β-lactams and is intrinsically resistant to low levels of aminoglycosides (12). E. faecalis BM4281 is a JH2-2 transconjugant harboring a 250-kb chromosomal genetic element conferring VanB-type resistance (21). BM4281 is significantly less resistant to gentamicin (MIC = 5 μg/ml) than JH2-2 (MIC = 16 μg/ml) (14).
In vitro susceptibility testing.
The MICs of gentamicin (Unilabo, Levallois Perret, France) were determined by the method of Steers et al. (23) with 105 CFU per spot on brain heart infusion (BHI) agar (Difco Laboratories, Detroit, Mich.) after 24 h of incubation. For time-kill curves, exponentially growing E. faecalis was diluted in glass tubes containing 10 ml of BHI broth (pH 7.0; Difco) or 5 ml of BHI broth and 5 ml of undiluted complement-intact rabbit serum (mixture pH 7.4; Sigma Chemical Co., St. Louis, Mo.) or 5 ml of BHI broth and 5 ml of undiluted complement-intact human serum (mixture pH 7.4; Sigma) to obtain 107 CFU/ml and was incubated with gentamicin at various concentrations (0.5 to 4 times the MIC). Aliquots were taken after 0, 3, 6, and 24 h of incubation and plated on BHI agar to enumerate the surviving bacteria. In the experiment using human serum, surviving bacteria were also enumerated at 48 h. A bactericidal effect was defined as a ≥3-log10 CFU/ml difference between the initial inoculum and the bacterial count after 24 h of incubation.
Experimental endocarditis.
Aortic endocarditis was induced in female New Zealand White rabbits (2.2 to 2.5 kg) by insertion of a polyethylene catheter through the right carotid artery into the left ventricle (2). Twenty-four hours after catheter insertion, the rabbits were inoculated by the ear vein with 108 CFU of E. faecalis JH2-2 or BM4281 in 1 ml of 0.9% NaCl. The catheter was left in place throughout the experiment. Forty-eight hours after inoculation, animals received gentamicin intramuscularly twice daily for 5 days at 3 or 6 mg/kg of body weight per injection. Control animals were sacrificed 48 h after inoculation (start of therapy) or at the same time as treated animals (end of therapy).
For determination of peak and trough gentamicin levels in serum, blood was sampled on day 5 of antimicrobial therapy 30 min and 12 h after the last injection, respectively. Antibiotic concentrations were measured in serum by fluorescence polarization immunoassay (AxSYM System; Abbott Diagnostics, Rungis, France).
The animals were killed by an intravenous injection of pentobarbital. At the time of sacrifice, the heart was removed and the chambers on the left side were examined to confirm vegetative endocarditis. For each rabbit, vegetations were excised, pooled, weighed, and homogenized in 1 ml of sterile distilled water. Vegetation homogenates were plated on agar to count surviving bacteria and on agar containing gentamicin at twice the MIC to enumerate mutants after 48 h of incubation. When mutants were recovered on gentamicin-containing agar, 10 colonies per rabbit were regrown on drug-free agar and then subcultured in antibiotic-free broth before testing the MICs. The results were expressed as log10 CFU per gram of vegetation.
Statistics.
Comparison of bacterial counts of the vegetations of rabbits treated with various regimens was performed by the Fisher test (22). A P value of <0.05 was considered significant.
RESULTS AND DISCUSSION
In vitro activity of gentamicin.
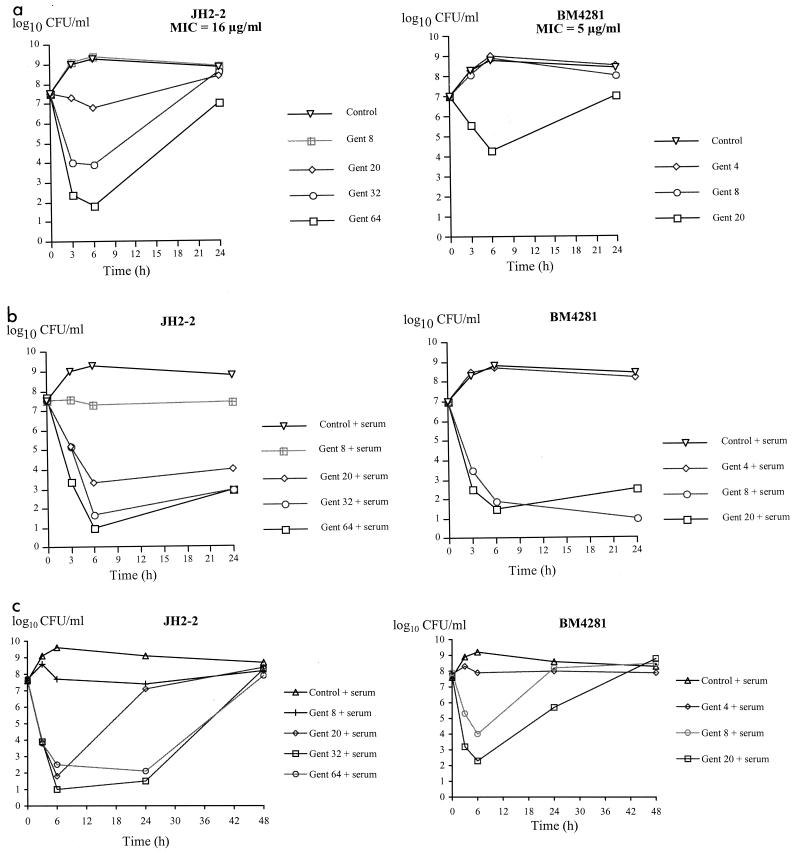
A concentration-dependent increase in bacterial killing of JH2-2 (MIC = 16 μg/ml) was observed during the first 6 h of incubation with gentamicin (Fig. 1a). However, the number of bacteria increased between 6 and 24 h for all gentamicin concentrations tested. Regrowth of JH2-2 was previously shown to result from the selection of mutants with moderate increases (two- to sixfold) in the level of gentamicin resistance (14). Rabbit serum enhanced the activity of gentamicin, leading to a bacteriostatic effect at 8 μg of gentamicin/ml (0.5 times the MIC) and a bactericidal effect at 24 h for gentamicin concentrations that were ≥20 μg/ml (≥1.2 times the MIC) (Fig. 1b). Similarly, killing of BM4281 (MIC = 5 μg/ml) was observed only in the presence of serum for gentamicin concentrations that were ≥8 μg/ml (≥1.6 times the MIC) (Fig. 1b). Thus, the supplementation of media with rabbit serum dramatically increased the activity of gentamicin against JH2-2 and BM4281, resulting in a bactericidal effect when the drug concentration exceeded the MIC. Time-kill curves performed in the presence of human serum also showed that adding serum increased the activity of gentamicin against the two strains, with a ≥3-log CFU/ml decrease in bacterial titers when the concentration of gentamicin exceeded the MIC (Fig. 1c). However, bacterial regrowth was observed at 48 h for all concentrations of gentamicin tested, suggesting the emergence of gentamicin-resistant mutants (14).
FIG. 1.
In vitro activity of gentamicin (Gent) against E. faecalis JH2-2 and BM4281 in BHI (a), 50% BHI and 50% rabbit serum (b), or 50% BHI and 50% human serum (c). Gentamicin concentrations are expressed in micrograms per milliliter.
Experimental endocarditis.
Treatment of rabbits with gentamicin at 3 mg/kg twice a day (b.i.d.) led to peak levels in serum of 7.0 ± 1.3 μg/ml (Table 1), similar to those previously recommended with traditional therapeutic regimens (18–20). Peak levels in serum obtained with a dosage of 6 mg/kg b.i.d. (17.7 ± 1.5 μg/ml) were within the range achieved with current schedules of gentamicin administration (8; D. R. McNamara, A. N. Nafziger, A. M. Menhinick, L. J. Cabelus, and J. S. Bertino, Jr., Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1009, 1999).
TABLE 1.
Gentamicin levels in serum and peak-to-MIC ratios in rabbits with experimental endocarditis due to E. faecalis JH2-2 or BM4281
| Gentamicin dose (mg/kg) | Level in serum (μg/ml)
|
Peak/MIC ratio
|
||
|---|---|---|---|---|
| Peaka | Trough | JH2-2 | BM4281 | |
| 3 | 7.0 ± 1.3 | <0.2 | 0.4 | 1.4 |
| 6 | 17.7 ± 1.5 | <0.2 | 1.1 | 3.5 |
Means ± standard deviations.
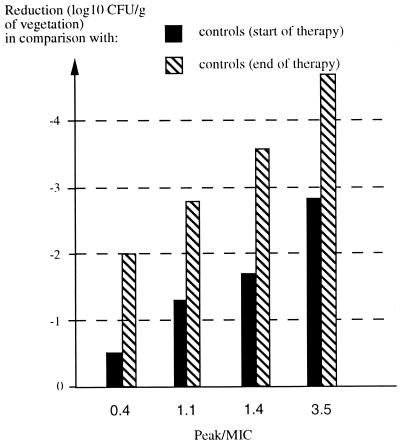
When bacterial titers in the vegetations of treated animals were compared to those in controls left untreated for the same time period, a statistically significant decrease was noted for both strains and both gentamicin regimens (3 or 6 mg/kg b.i.d.). Compared to the initial bacterial counts in the vegetations 48 h after inoculation of the bacteria, which provides an estimate of the actual reduction of the number of bacteria during therapy, the numbers of CFU were also significantly decreased, except for the most resistant strain (JH2-2, MIC = 16 μg/ml) and the low-dose gentamicin regimen (3 mg/kg b.i.d.), which generated a peak-to-MIC ratio of <1 (Tables 1 and 2). None of the regimens prevented the emergence of gentamicin-resistant mutants of JH2-2 or BM4281 (Table 2), as previously reported for low doses of gentamicin in the same experimental model (14) and as expected from in vitro time-kill curves in the presence of human serum that showed bacterial regrowth at 48 h, suggestive of the emergence of gentamicin-resistant mutants (Fig. 1c). Analysis of the same set of data in terms of reduction of the number of surviving bacteria as a function of the peak-to-MIC ratio revealed a dose-dependent in vivo killing of enterococci by gentamicin (Fig. 2).
TABLE 2.
Activity of gentamicin in experimental endocarditis due to E. faecalis JH2-2 or BM4281
| Regimen | Log10 CFU/g of vegetation (no. of rabbits with Gent-resistant mutants/total)a
|
|
|---|---|---|
| JH2-2 | BM4281 | |
| Controls (start of therapy) | 8.3 ± 1.2 (0/13) | 6.6 ± 1.7 (0/6) |
| Controls (end of therapy) | 9.8 ± 1.0 (0/20) | 8.5 ± 1.0 (0/10) |
| Gent, 3 mg/kg b.i.d. | 7.8 ± 1.7d (2/17) | 4.9 ± 1.7bd (6/17) |
| Gent, 6 mg/kg b.i.d. | 7.0 ± 1.0bd (3/11) | 3.8 ± 1.3cd (1/9) |
The mutants were selected on agar containing gentamicin (Gent) at twice the MIC. Values are means ± standard deviations.
P < 0.05 versus controls (start of therapy).
P < 0.01 versus controls (start of therapy).
P < 0.01 versus controls (end of therapy).
FIG. 2.
Activity of gentamicin in experimental endocarditis due to E. faecalis. Reductions of bacterial counts in the vegetations were plotted for different peak-to-MIC ratios of gentamicin.
A discrepancy was observed between the in vivo results and those obtained in vitro in BHI medium. As shown previously, rabbit and human serum enhanced the in vitro activity of gentamicin against E. faecalis (24, 26). Susceptibility testing performed in the presence of serum was predictive of in vivo activity in rabbits. The increase in the bactericidal activity of gentamicin in the presence of serum may result from several factors. First, it may be due to species-related factors, as suggested by the bactericidal activity of vancomycin against enterococci in the presence of rat serum but not rabbit or human serum (9). However, we noted in our study an increase in the activity of gentamicin not only with rabbit serum but also with human serum. Second, it was shown that the alkalinization of nutrient broth and modifications in the concentrations of cations (such as calcium or magnesium), the various components of complement, and specific immunoglobulins of the serum may interact with antibiotics in killing bacteria (7, 10, 25, 26). Finally, the increase in bactericidal activity may be due to the interaction between gentamicin and serum noncomplement cationic proteins, such as β-lysin (9, 15, 26). Indeed, Traub et al. showed that the antagonization of β-lysin abolished the augmentation of gentamicin antienterococcal activity induced by the addition of rabbit, bovine, or human serum (26).
Conclusions.
It is well established for aminoglycosides that the area under the concentration-time curve-to-MIC ratio and the peak-to-MIC ratio are two pharmacokinetic parameters that are strongly related and predictive of in vivo efficiency (6, 11, 17). These parameters have not been evaluated for gentamicin alone in enterococcal infections, since aminoglycosides are always used in combinations. In this study, gentamicin was found to be significantly active against E. faecalis in rabbit endocarditis, provided that the peak exceeded the MIC (Table 2 and Fig. 2). Peak-to-MIC ratios greater than 1 are achievable in humans infected with low-level gentamicin-resistant enterococci and treated with currently recommended once- or twice-daily dosing regimens. For example, a single perfusion of 7 mg of gentamicin/kg was shown to provide peak levels in serum of 28.3 ± 6.5 μg/ml (McNamara et al., 39th ICAAC). However, these observations do not imply that gentamicin can be used in monotherapy in the case of severe enterococcal infection, for two reasons. In clinical practice, the peak-to-MIC ratio of gentamicin may not reliably exceed 1, depending on the dosage used and susceptibility of the strain. In addition, gentamicin monotherapy selects gentamicin-resistant mutants that may be responsible for therapeutic failure (14). However, our study indicates that the intrinsic activity of gentamicin should be taken into account in studying combinations of aminoglycosides and agents active against the cell wall, such as ampicillin or vancomycin. Besides increasing the activity of aminoglycosides by facilitating their uptake into the cell (16), combinations may also prevent the emergence of gentamicin-resistant mutants (14). Conversely, the intrinsic activity of gentamicin may be crucial for preventing the emergence of resistance to cell wall-active agents, as shown for the emergence of teicoplanin resistance in VanB-type glycopeptide-resistant enterococci (2, 14). Finally, an evaluation of the synergistic effect of combinations of cell wall-active agents and aminoglycosides against enterococci should include a careful study of each drug separately.
ACKNOWLEDGMENT
A.L. was supported by the Fondation pour la Recherche Médicale.
REFERENCES
- 1.Ali M Z, Goetz M B. A meta-analysis of the relative efficacy and toxicity of single daily dosing versus multiple daily dosing of aminoglycosides. Clin Infect Dis. 1997;24:796–809. doi: 10.1093/clinids/24.5.796. [DOI] [PubMed] [Google Scholar]
- 2.Aslangul E, Baptista M, Fantin B, Depardieu F, Arthur M, Courvalin P, Carbon C. Selection of glycopeptide-resistant mutants of VanB-type Enterococcus faecalis BM4281 in vitro and in experimental endocarditis. J Infect Dis. 1997;175:598–605. doi: 10.1093/infdis/175.3.598. [DOI] [PubMed] [Google Scholar]
- 3.Bailey T C, Little J R, Littenberg B, Reichley R M, Dunagan W C. A meta-analysis of extended-interval dosing versus multiple daily dosing of aminoglycosides. Clin Infect Dis. 1997;24:786–795. doi: 10.1093/clinids/24.5.786. [DOI] [PubMed] [Google Scholar]
- 4.Blaser J, Konig C. Once-daily dosing of aminoglycosides. Eur J Clin Microbiol Infect Dis. 1995;14:1029–1038. doi: 10.1007/BF01590935. [DOI] [PubMed] [Google Scholar]
- 5.Blaser J, Stone B B, Groner M C, Zinner S H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987;31:1054–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 7.Davis S D, Iannetta A. Influence of serum and calcium on the bactericidal activity of gentamicin and carbenicillin on Pseudomonas aeruginosa. Appl Microbiol. 1972;23:775–779. doi: 10.1128/am.23.4.775-779.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demczar D J, Nafziger A N, Bertino J S., Jr Pharmacokinetics of gentamicin at traditional versus high doses: implications for once-daily aminoglycoside dosing. Antimicrob Agents Chemother. 1997;41:1115–1119. doi: 10.1128/aac.41.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold M J, Calmon J, Wendeler M, Levison M E, Johnson C C. Synergistic bactericidal activity of rat serum with vancomycin against enterococci. J Infect Dis. 1991;163:1358–1361. doi: 10.1093/infdis/163.6.1358. [DOI] [PubMed] [Google Scholar]
- 10.Harvey B S, Baker C J, Edwards M S. Contributions of complement and immunoglobulin to neutrophil-mediated killing of enterococci. Infect Immun. 1992;60:3635–3640. doi: 10.1128/iai.60.9.3635-3640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyatt J M, McKinnon P S, Zimmer G S, Schentag J J. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Focus on antibacterial agents. Clin Pharmacokinet. 1995;28:143–160. doi: 10.2165/00003088-199528020-00005. [DOI] [PubMed] [Google Scholar]
- 12.Jacob A E, Hobbs S J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974;117:360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leclercq R. Enterococci acquire new kinds of resistance. Clin Infect Dis. 1997;24(Suppl. 1):80–84. doi: 10.1093/clinids/24.supplement_1.s80. [DOI] [PubMed] [Google Scholar]
- 14.Lefort A, Baptista M, Fantin B, Depardieu F, Arthur M, Carbon C, Courvalin P. Two-step acquisition of resistance to the teicoplanin-gentamicin combination by VanB-type Enterococcus faecalis in vitro and in experimental endocarditis. Antimicrob Agents Chemother. 1999;43:476–482. doi: 10.1128/aac.43.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leggett J E, Craig W A. Enhancing effect of serum ultrafiltrate on the activity of cephalosporins against gram-negative bacilli. Antimicrob Agents Chemother. 1989;33:35–40. doi: 10.1128/aac.33.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moellering R C, Jr, Weinberg A N. Studies on antibiotic synergism against enterococci. II. Effect of various antibiotics on the uptake of 14C-labeled streptomycin by enterococci. J Clin Investig. 1971;50:2580–2584. doi: 10.1172/JCI106758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore R D, Lietman P S, Smith C R. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 18.Moore R D, Smith C R, Lietman P S. The association of aminoglycoside plasma levels with mortality in patients with gram-negative bacteremia. J Infect Dis. 1984;149:443–448. doi: 10.1093/infdis/149.3.443. [DOI] [PubMed] [Google Scholar]
- 19.Moore R D, Smith C R, Lietman P S. Association of aminoglycoside plasma levels with therapeutic outcome in gram-negative pneumonia. Am J Med. 1984;77:657–662. doi: 10.1016/0002-9343(84)90358-9. [DOI] [PubMed] [Google Scholar]
- 20.Noone P, Parsons T M, Pattison J R, Slack R C, Garfield-Davies D, Hughes K. Experience in monitoring gentamicin therapy during treatment of serious gram-negative sepsis. Br Med J. 1974;i:477–481. doi: 10.1136/bmj.1.5906.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quintiliani R, Jr, Courvalin P. Conjugal transfer of the vancomycin resistance determinant vanB between enterococci involves the movement of large genetic elements from chromosome to chromosome. FEMS Microbiol Lett. 1994;119:359–364. doi: 10.1111/j.1574-6968.1994.tb06913.x. [DOI] [PubMed] [Google Scholar]
- 22.Steel R G D, Tovrie J H. Principles and procedures of statistics: a biometrical approach. New York, N.Y: McGraw-Hill Book Co.; 1980. Multiple comparisons; pp. 172–194. [Google Scholar]
- 23.Steers E, Foltz E L, Graves B S, Riden J. An inocula replicating apparatus for routine testing of bacterial susceptibility to antibiotics. Antibiot Chemother (Basel) 1959;9:307–311. [PubMed] [Google Scholar]
- 24.Sullam P M, Täuber M G, Hackbarth C J, Sande M A. Antimicrobial activity of gentamicin in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1985;27:224–226. doi: 10.1128/aac.27.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traub W H, Sherris J C. Studies on the interaction between serum bactericidal activity and antibiotics in vitro. Chemotherapy. 1970;15:70–83. doi: 10.1159/000220669. [DOI] [PubMed] [Google Scholar]
- 26.Traub W H, Spohr M, Bauer D. Streptococcus faecalis: in vitro susceptibility to antimicrobial drugs, single and combined, with and without defibrinated human blood. Chemotherapy. 1986;32:270–285. doi: 10.1159/000238424. [DOI] [PubMed] [Google Scholar]