Abstract
Intestinal inflammation fuels the transmission of Salmonella Typhimurium (S.Tm). However, a substantial fitness cost is associated with virulence expression. Mutations inactivating transcriptional virulence regulators generate attenuated variants profiting from inflammation without enduring virulence cost. Such variants interfere with the transmission of fully virulent clones. Horizontal transfer of functional regulatory genes (HGT) into attenuated variants could nevertheless favor virulence evolution. To address this hypothesis, we cloned hilD, coding for the master regulator of virulence, into a conjugative plasmid that is highly transferrable during intestinal colonization. The resulting mobile hilD allele allows virulence to emerge from avirulent populations, and to be restored in attenuated mutants competing against virulent clones within-host. However, mutations inactivating the mobile hilD allele quickly arise. The stability of virulence mediated by HGT is strongly limited by its cost, which depends on the hilD expression level, and by the timing of transmission. We conclude that robust evolution of costly virulence expression requires additional selective forces such as narrow population bottlenecks during transmission.
Subject terms: Pathogens, Experimental evolution, Bacterial evolution
Salmonella Typhimurium virulence is costly and can be lost by mutation during infection. Bakkeren et al. show that virulence restoration via horizontal gene transfer is only transient while transmission bottlenecks promote long-term virulence stability.
Introduction
Bacteria often exist in dense communities. Therefore, many aspects of bacterial lifestyle are governed by social interactions1. This has also been observed for pathogens, which often infect hosts through collective actions2–4. For example, they can secrete extracellular metabolites or enzymes to assist in growth (e.g. iron-scavenging siderophores)3, produce toxins to compete with other species5,6, establish and survive within biofilms7, or use virulence factors to modulate the host immune response to create a favorable environment8. These collective actions function through public goods, which are costly to produce. Hence, cheater mutants can emerge by profiting from the public good without enduring the cost of its production. In extreme cases, the overgrowth by cheaters can lead to population collapse due to the total breakdown of public good production2. Gene regulation, phenotypic heterogeneity, population structure, and ecological factors9,10 likely contribute, if not to their emergence, at least to the stability of cooperative traits by altering the cost/benefit ratio2,7,11–13.
The enteric pathogen Salmonella enterica serovar Typhimurium (S.Tm) favors its own growth, but also that of related Enterobacteriaceae by actively triggering gut inflammation via its Type Three Secretion System-1 (TTSS-1) and secreted effectors8,14. Production of TTSS-1 and co-regulated functions controlled by HilD15–18 is associated with a cost both in vitro19 and in vivo11, which favors the emergence of attenuated cheaters during infection11. The virulence of S.Tm is therefore a cooperative trait. The target of selection is the transcriptional regulation of virulence expression via HilD11. Mutants in hilD have been isolated from patients20 and swine21, and are under niche-specific positive selection according to comparative genomic analyses of more than 100,000 natural isolates of Salmonella enterica22. When co-transmitted with virulent clones, high frequencies of cheaters prevent the disease in recipient hosts23. This suggests that artificial introduction of cheaters in a population of hosts could be a viable biocontrol strategy against Salmonella spp. However, horizontal gene transfer (HGT), known to accelerate the evolution of virulence in pathogenic bacteria4, could potentially restore virulence in cheaters24,25. Nevertheless, over time, cheating should re-occur by mutation at the level of the vector26, in the same way that mutations in hilD occur on the chromosome turning cooperators into cheaters11,22. Here, we show that this prediction is correct. Using the mouse model to study the emergence of cooperative (i.e., cheatable) virulence and its stability in S.Tm during intestinal colonization and transmission, we demonstrate the rise and fall of cooperative virulence mediated by HGT during within-host evolution. This context captures the complexity of the host-pathogen interaction in which cheaters can naturally evolve11,27. We hypothesized that although HGT may favor cooperative virulence, host-to-host transmission timing and associated population bottlenecks should stabilize cooperative virulence in the long run, since virulent clones trigger disease and promote shedding whereas cheating clones do not23.
Results
Evolution of cooperative virulence in an avirulent population mediated by HGT in vivo
We created a tractable model to address the role of HGT in the evolution of cooperative virulence by cloning hilD coupled to a chloramphenicol resistance cassette into the conjugative IncI1 plasmid P2 (aka. pCol1B9), which is native to S.Tm SL134414. The resulting construct was named pVir. We have previously shown that P2 spreads efficiently into S.Tm 14028S and some E. coli strains in vivo14,28–31. This system allows robust HGT independently of intestinal inflammation, which is not the case for other vectors such as temperate bacteriophages like SopEΦ30. As a donor strain, we conjugated pVir into an S.Tm 14028S derivative that lacks both a chromosomal copy of hilD and a functional SPI-1 locus (Fig. 1a and Table 1). As a recipient strain, we used a kanamycin-resistant derivative of S.Tm 14028S that also lacks a chromosomal copy of hilD, but has all necessary genes to produce a functional TTSS-1 (Fig. 1a). Both the donor and the recipient are genetically avirulent (i.e. they do not elicit overt gut inflammation), but conjugation of pVir to the recipient should produce a transconjugant able to trigger inflammation (Fig. 1a). Both the donor and the recipient lack a functional TTSS-2 (ssaV mutant) to exclude inflammation triggered through TTSS-2 at a later stage in mouse gut infections32,33.
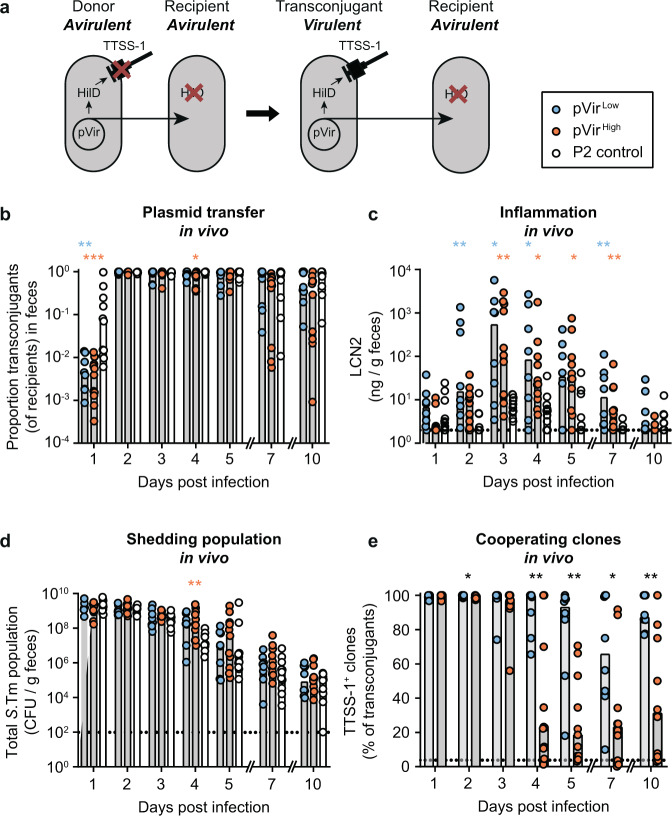
Fig. 1. Virulence can emerge through HGT in a population of cheaters in vivo, but is unstable.
a Experimental system to measure maintenance of cooperative virulence by HGT. Donors contain pVir encoding hilD, but cannot produce a functional TTSS-1 (invG mutant), making them avirulent. Recipients contain all genes for a functional TTSS-1 but do not have a functional copy of hilD (cheaters), preventing TTSS-1 expression and virulence. Transfer of pVir from the donor to the recipient forms a transconjugant that contains both functional TTSS-1-encoding genes and a copy of hilD from pVir, allowing TTSS-1-mediated virulence. Transconjugants can transfer pVir to additional recipients. b–e pVir is transferred to cheater recipients and allows cooperative virulence to emerge. Ampicillin pretreated mice were sequentially infected orally with donors (14028S ΔinvG ΔhilD ΔssaV; CmR, AmpR) harboring pVirLow (blue; n = 8), pVirHigh (orange; n = 11), or P2 lacking hilD (control; white; n = 11), and recipients (14028 S ΔhilD ΔssaV; KanR, AmpR). Each replicate is shown and bars indicate the median. Source data are provided as a Source Data file. b–d Statistics compare pVirLow (blue asterisks) and pVirHigh (orange asterisks) to the control on each day; Kruskal–Wallis test with Dunn’s multiple test correction (p > 0.05 not significant and not indicated, *p < 0.05, **p < 0.01, ***p < 0.001. Dotted line represents the detection limit. b Plasmid transfer was measured by selective and/or replica plating. The proportion of transconjugants is calculated by dividing the transconjugant population by the sum of recipients and transconjugants. c Inflammation was measured by a Lipocalin-2 ELISA on fecal samples. d Total population determined by summing all subpopulations. Donor, recipient, and transconjugant populations are presented in Fig. S3. e Transconjugants carrying pVirLow (blue) and pVirHigh (orange) were analyzed by colony western-blot and compared using a two-tailed Mann–Whitney U test (p > 0.05 not significant and not indicated, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). The percentage of colonies that expressed SipC are reported out of the total transconjugant population. Bars indicate the median. The black dotted line indicates the conservative detection limit, which is dependent on the number of colonies on the plate (values can therefore appear below the detection limit).
Table 1.
Strains used in this study.
| Strain name | Strain number | Relevant genotypea | Resistanceb | Reference |
|---|---|---|---|---|
| SL1344 | SB300 | Wild type | Sm | 65 |
| ATCC 14028S | 14028S | Wild type | None | 66 |
| E. coli CC118 λpir | – | λpir; used for R6K ori replication (e.g. in pKD3) | None | 67,68 |
| SB300 ΔhilD | M3101 | ΔhilD | Sm | 11 |
| SB300 ΔhilD pVirLow | Z2325 | ΔhilD pVirLow | Sm, Cm | This work |
| SB300 ΔhilD pVirHigh | Z2326 | ΔhilD pVirHigh | Sm, Cm | This work |
| 14028S ΔhilD ΔinvG ΔssaV pM975 | Z2327 | 14028S ΔhilD ΔinvG ΔssaV pM975 | Amp | This work |
| Low cost pVir donor | Z2317 | 14028S ΔhilD ΔinvG ΔssaV pVirLow pM975 | Amp, Cm | This work |
| High cost pVir donor | Z2236 | 14028S ΔhilD ΔinvG ΔssaV pVirHigh pM975 | Amp, Cm | This work |
| Control donor | Z2318 | 14028S ΔhilD ΔinvG ΔssaV P2cat pM975 | Amp, Cm | This work |
| Cheater recipient | Z2235 | 14028S ΔhilD ssaV::aphT pM975 | Amp, Kan | This work |
| Wild-type pM972 | Z2319 | 14028S ssaV::aphT pM972 | Amp, Kan | This work |
| ΔhilD recipient pM972 | Z2320 | 14028S ΔhilD ssaV::aphT pM972 | Amp, Kan | This work |
| 14028S ΔhilD ΔssaV pM975 | T144 | 14028S ΔhilD ΔssaV pM975 | Amp | This work |
| 14028S ΔhilD ΔssaV pM975 pVirLow | T1 | 14028S ΔhilD ΔssaV pVirLow pM975 | Amp, Cm | This work |
| Cheater recipient P2 | T154 | 14028S ΔhilD ΔssaV P2aphT pM975 | Amp, Kan | This work |
| ΔhilD pVirLow transconjugant pM972 −1 | Z2321 | 14028S ΔhilD ssaV::aphT pM972 pVirLow | Amp, Kan, Cm | This work |
| ΔhilD pVirLow transconjugant pM972 −2 | Z2322 | 14028S ΔhilD ssaV::aphT pM972 pVirLow | Amp, Kan, Cm | This work |
| ΔhilD pVirHigh transconjugant pM972 −1 | Z2323 | 14028S ΔhilD ssaV::aphT pM972 pVirHigh | Amp, Kan, Cm | This work |
| ΔhilD pVirHigh transconjugant pM972 −3 | T292 | 14028S ΔhilD ssaV::aphT pM972 pVirHigh | Amp, Kan, Cm | This work |
| ΔhilD pVirHigh transconjugant pM972 −4 | T293 | 14028S ΔhilD ssaV::aphT pM972 pVirHigh | Amp, Kan, Cm | This work |
| ΔhilD pVirHigh transconjugant pM972 −5 | T294 | 14028S ΔhilD ssaV::aphT pM972 pVirHigh | Amp, Kan, Cm | This work |
| ΔhilD pVirHigh transconjugant pM972 −6 | T295 | 14028S ΔhilD ssaV::aphT pM972 pVirHigh | Amp, Kan, Cm | This work |
| ΔhilD pVirHigh transconjugant pM972 −7 | T296 | 14028S ΔhilD ssaV::aphT pM972 pVirHigh | Amp, Kan, Cm | This work |
| Evolved transconjugant Low cost TTSS-1+ −1 | Z2296 | 14028S ΔhilD ssaV::aphT pM975 pVirLow | Amp, Kan, Cm | This work |
| Evolved transconjugant Low cost TTSS-1+ −2 | Z2306 | 14028S ΔhilD ssaV::aphT pM975 pVirLow | Amp, Kan, Cm | This work |
| Evolved transconjugant Low cost TTSS-1+ −3 | Z2310 | 14028S ΔhilD ssaV::aphT pM975 pVirLow | Amp, Kan, Cm | This work |
| Evolved transconjugant Low cost TTSS-1+ −4 | Z2299 | 14028S ΔhilD ssaV::aphT pM975 pVirLow | Amp, Kan, Cm | This work |
| Evolved transconjugant Low cost TTSS-1+ −5 | Z2302 | 14028S ΔhilD ssaV::aphT pM975 pVirLow | Amp, Kan, Cm | This work |
| Evolved transconjugant Low cost TTSS-1+ −6 | Z2308 | 14028S ΔhilD ssaV::aphT pM975 pVirLow | Amp, Kan, Cm | This work |
| Evolved transconjugant Low cost TTSS-1- −1 | Z2298 | 14028S ΔhilD ssaV::aphT pM975 pVirLow | Amp, Kan, Cm | This work |
| Evolved transconjugant Low cost TTSS-1- −2 | Z2305 | 14028S ΔhilD ssaV::aphT pM975 pVirLow | Amp, Kan, Cm | This work |
| Evolved transconjugant Low cost TTSS-1- −3 | Z2301 | 14028S ΔhilD ssaV::aphT pM975 pVirLow | Amp, Kan, Cm | This work |
| Evolved transconjugant Low cost TTSS-1- −4 | Z2304 | 14028S ΔhilD ssaV::aphT pM975 pVirLow | Amp, Kan, Cm | This work |
| Evolved transconjugant Low cost TTSS-1- −5 | Z2309 | 14028S ΔhilD ssaV::aphT pM975 pVirLow | Amp, Kan, Cm | This work |
| Evolved transconjugant High cost TTSS-1+ −1 | Z2238 | 14028S ΔhilD ssaV::aphT pM975 pVirHigh | Amp, Kan, Cm | This work |
| Evolved transconjugant High cost TTSS-1+ −2 | Z2253 | 14028S ΔhilD ssaV::aphT pM975 pVirHigh | Amp, Kan, Cm | This work |
| Evolved transconjugant High cost TTSS-1+ −3 | Z2246 | 14028S ΔhilD ssaV::aphT pM975 pVirHigh | Amp, Kan, Cm | This work |
| Evolved transconjugant High cost TTSS-1+ −4 | Z2242 | 14028S ΔhilD ssaV::aphT pM975 pVirHigh | Amp, Kan, Cm | This work |
| Evolved transconjugant High cost TTSS-1+ −5 | Z2244 | 14028S ΔhilD ssaV::aphT pM975 pVirHigh | Amp, Kan, Cm | This work |
| Evolved transconjugant High cost TTSS-1+ −6 | Z2312 | 14028S ΔhilD ssaV::aphT pM975 pVirHigh | Amp, Kan, Cm | This work |
| Evolved transconjugant High cost TTSS-1− −1 | Z2239 | 14028S ΔhilD ssaV::aphT pM975 pVirHigh | Amp, Kan, Cm | This work |
| Evolved transconjugant High cost TTSS-1− −2 | Z2243 | 14028S ΔhilD ssaV::aphT pM975 pVirHigh | Amp, Kan, Cm | This work |
| Evolved transconjugant High cost TTSS-1− −3 | Z2311 | 14028S ΔhilD ssaV::aphT pM975 pVirHigh | Amp, Kan, Cm | This work |
| Evolved transconjugant High cost TTSS-1− −4 | Z2245 | 14028S ΔhilD ssaV::aphT pM975 pVirHigh | Amp, Kan, Cm | This work |
| Evolved transconjugant High cost TTSS-1− −5 | Z2247 | 14028S ΔhilD ssaV::aphT pM975 pVirHigh | Amp, Kan, Cm | This work |
| Evolved transconjugant High cost TTSS-1− −6 | Z2252 | 14028S ΔhilD ssaV::aphT pM975 pVirHigh | Amp, Kan, Cm | This work |
| Evolved transconjugant High cost TTSS-1− −7 | Z2254 | 14028S ΔhilD ssaV::aphT pM975 pVirHigh | Amp, Kan, Cm | This work |
| Evolved transconjugant High cost TTSS-1− −8 | Z2255 | 14028S ΔhilD ssaV::aphT pM975 pVirHigh | Amp, Kan, Cm | This work |
To determine to which extent cooperative virulence evolution depends on the fitness cost of the cooperative allele34, we constructed two variants of pVir. The “low cost” variant (pVirLow) contains 648 bp of the regulatory region upstream of hilD (and all four transcriptional start sites characterized in Kroger et al.15), while the “high cost” variant (pVirHigh) contains 279 bp of upstream regulatory region (only two transcriptional start sites of hilD;15 Fig. S1A). To test the difference in cost associated with each pVir variant, we performed in vitro experiments comparing growth rate with TTSS-1 expression, which is associated with a cost19 and thus inversely correlated to growth (Fig. S1B, C). We confirmed that transconjugants harboring pVirLow expressed less TTSS-1 and grew better than those harboring pVirHigh, confirming the difference in cost associated with TTSS-1 expression induced by these constructions (Fig. S1B, C).
To address the conditions that may support the evolution of cooperative virulence, we performed conjugation experiments in an antibiotic pretreated mouse model (modified from Barthel et al.35). We introduced the donor and recipient strains sequentially into ampicillin pretreated mice at low inoculum size (102 CFU donors; 104 CFU recipients) to ensure that conjugation occurred only in the gut. By 2 days post infection, 97% of recipients (median of all mice) obtained the plasmid, although spread of pVirLow and pVirHigh proceeded slower than the P2 control plasmid (pVir lacking hilD; i.e. P2cat; Fig. 1b, day 1 p.i.). The plasmid was also maintained in the majority of mice for the entire course of the experiment with a median of 45% recipient cells carrying the plasmid. To test if transfer of pVir could allow the emergence of cooperative virulence in a population of avirulent recipients, we measured fecal lipocalin-2 (LCN2) as a readout for the inflammatory status of the gut. Inflammation was progressively triggered as more pVir transconjugants were formed, leading to a maximum at 3 days post infection (Fig. 1b, c). Mice containing S.Tm with either pVirLow or pVirHigh were significantly more inflamed than mice infected with control S.Tm donors (day 2–7 post infection; Fig. 1d). This shows that virulence can evolve within a host, since neither the donor, nor the recipient were virulent prior to conjugation (Fig. S2). However, intestinal inflammation was not sustained (Fig. 1c), and mice began to recover leading to exclusion of S.Tm from the gut likely by the re-growing microbiota (Figs. 1d and S3)36. Furthermore, mice harboring virulent S.Tm did not excrete significantly more S.Tm than control mice (Fig. 1d and Supplementary discussion). This observation led to two questions: (1) what makes emerged cooperative virulence short lived, and (2) can HGT of a cooperative allele favor the transmission of the disease?
HGT-mediated cooperative virulence is short lived, characterized by cost-dependent inactivation of the mobile cooperative allele
We hypothesized that the waning inflammation was a result of insufficient TTSS-1 expression. Inflammation started to decrease after day 3 post infection, while the proportion of transconjugants in the feces of mice infected with pVir-harboring S.Tm remained 84% at day 4 post infection (median of all mice with pVirHigh or pVirLow; Fig. 1b). Therefore, plasmid loss could not explain cooperative virulence loss. However, cheating could have occurred by mutations on the plasmid, as previously suggested25,26.
To address this, we performed a western blot on transconjugant colonies isolated from feces (“colony blot”)11,27 to probe for TTSS-1 expression. As expected, cooperative virulence emergence by HGT was transient, since clones that do not express TTSS-1 arose (Fig. 1e). We performed whole-genome sequencing on evolved clones that were either TTSS-1+ or TTSS-1− as determined by the colony blot (n = 11 for pVirLow; n = 14 clones for pVirHigh). It showed that cheating was a result of mutations or deletions in either the coding sequence or regulatory regions of hilD on pVir, and not due to chromosomal changes (Tables S1–4). HGT-mediated cooperation is therefore short lived, since cheating now occurred at the level of the mobile genetic element (MGE). As expected, the loss of TTSS-1+ clones was slower with pVirLow compared to pVirHigh, leading to a higher proportion of cooperating clones bearing pVirLow by the end of the experiment (Fig. 1e). This indicates that cost influences the maintenance of cooperative virulence mediated by HGT (as predicted by theory24,26), extending our previous in vivo work on the cost-dependent cheating dynamics of S.Tm with the chromosomal copy of hilD11.
However, since both the cheating dynamics and the total population size dictate the size of the population able to trigger inflammation, we multiplied the proportion of transconjugants able to trigger inflammation (from Fig. 1e) by the transconjugant population size (Fig. S3A, B) to obtain the effective size of the cooperative population (i.e., the TTSS-1+ population; Fig. S3D). We observed a rise in the cooperative population due to plasmid transfer correlating with the onset of inflammation (day 1–2 p.i., Fig. S3D), followed by a drop in the cooperative population associated with the waning inflammation between days 5–10 p.i., Fig. S3D). This supported the hypothesis that the loss of inflammation could be driven by the loss of the cooperative population. As predicted by analyzing the proportion of transconjugants able to produce TTSS-1 (Fig. 1e), the cooperative population was higher in mice infected with pVirLow harboring cells compared to those infected with pVirHigh harboring cells at the end of the experiment (Day 10; Fig. S3D).
The cost of virulence expression drives the proportion of cooperators shed over generations
In our model system, HGT allowed cooperative virulence to emerge, however it remains unstable within-host. In the case of S.Tm, TTSS-1-triggered inflammation has two important consequences that can nevertheless promote cooperative virulence: it favors transmission of S.Tm8,37 and fosters pathogen blooms in the next host. Therefore, HGT may influence the evolution of S.Tm by increasing the duration of shedding of sufficient virulent clones to colonize and to trigger inflammation in a new host. Selection for cooperative virulence should be a result of increased benefit after transmission. To address this hypothesis, we took fecal suspensions from mice in Fig. 1 on day 2 p.i. (the maximum population size of TTSS-1-producing clones; Fig. S3D) and day 10 p.i. (the minimum population size of TTSS-1-producing clones; Fig. S3D) and transferred them into new ampicillin pretreated mice (Fig. 2a).
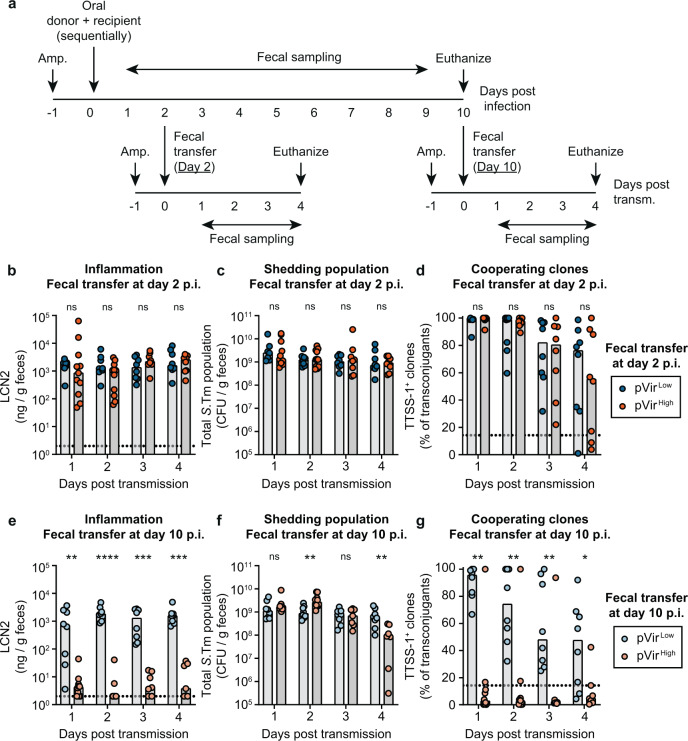
Fig. 2. Successful infections in new hosts depend on the proportion of transmitted cooperators.
a Experimental scheme for transmission experiments. Feces from mice in Fig. 1 collected on day 2 and day 10 post infection were suspended in PBS and given to new ampicillin (Amp.) pretreated mice. b–g Mice orally given fecal resuspensions with S.Tm harboring pVirLow (blue; dark shade for day 2 transmission; light shade for day 10 transmission; n = 8 for both groups) are compared to pVirHigh (orange; dark shade for day 2 transmission (n = 11); light shade for day 10 transmission (n = 10)) using a two-tailed Mann–Whitney U test (p > 0.05 (ns), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). All data points are shown and medians are represented by bars. Source data are provided as a Source Data file. b–d Mice given feces from day 2 post infection. e–g Mice given feces from day 10 post infection. b, e Inflammation was quantified using a LCN2 ELISA. The dotted lines indicate the detection limit. c, f The shedding population was enumerated by summing all populations determined by selective plating. Donor, recipient, and transconjugant populations are presented in Fig. S4. d, g MacConkey plates containing colonies of transconjugants were analyzed for expression of SipC as a proxy for TTSS-1 expression using a colony western blot; the percentage of colonies that expressed SipC are reported out of the total transconjugant population. The black dotted line indicates the conservative detection limit for the colony blot, which is dependent on the number of colonies on the plate (values can therefore appear below the detection limit).
When fecal populations taken from day 2 p.i. were transferred into new mice, inflammation was triggered, and there were no significant differences between mice infected with pVirLow or pVirHigh S.Tm carriers (Fig. 2b). This led to consistent shedding over the course of the experiment in all the recipient mice (Figs. 2c and S4A–C). This is likely attributed to the high proportion of cooperating clones transferred with samples from day 2 p.i. (Fig. 1). However, in all mice, cheaters arose (Fig. 2d), further supporting the instability of cooperative virulence in this system. In contrast, when feces from day 10 p.i. were transferred, only the recipient mice infected with S.Tm harboring pVirLow became inflamed (Fig. 2e). This was reflected in the shedding population at day 4 post transmission, where mice infected with S.Tm harboring pVirLow contained significantly more S.Tm in the feces compared to mice infected with S.Tm containing pVirHigh (Figs. 2f and S4D–F). These differences were likely a result of the proportion of cooperative clones in the feces of the donor mice at day 10 p.i.: mice infected with S.Tm pVirLow had significantly more cooperators than mice infected with S.Tm pVirHigh (Figs. 1 and S3). Moreover, as in the fecal transfer at day 2 p.i., in all mice, cheaters arose and outgrew cooperators (Fig. 2g). Interestingly, three mice with S.Tm pVirHigh that contained a high proportion of cooperative clones at day 10 p.i. (Fig. 1e) did not lead to strong inflammation after transmission (Fig. 2e). This could indicate that additional cost-dependent factors influence the ability to trigger inflammation after transmission. Nevertheless, since transmission of feces containing pVirHigh S.Tm led to inflammation dependent on the proportion of cooperators (e.g. compare the fecal transfer at day 2 p.i. (Fig. 2b) to the transfer at day 10 p.i. (Fig. 2e)), we concluded that the proportion of cooperators do contribute to disease development post transmission.
Altogether, this confirms that triggering disease in the next host requires sufficient proportion of cooperators in the transmitted population. This is in accordance with previous observations that a population made of 99% cheaters cannot trigger the disease after transmission23 and that at least 10 to 50% of the gut luminal S.Tm population must encode a functional TTSS-1 to provoke host response38. The proportion of cheaters depends mainly on the cost of virulence expression and on the duration of evolution within the donor. Long-term colonization and high cost are detrimental to stability of virulence as cheaters are more likely to reach high frequency before transmission. Moreover, co-transmitted cheaters keep accumulating in the new host, eventually reaching fixation, which prevents disease and further transmission.
Genetic drift favors cooperative virulence
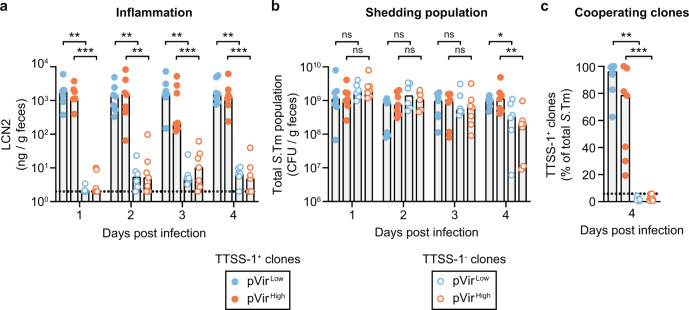
In our system, HGT can allow the re-emergence of cooperating clones. However, cooperation remained unstable within-host as mutations inactivating the cooperative allele on the plasmid arose quickly. Narrow transmission bottlenecks, that is, when few founding members lead to the establishment of new populations10, could nevertheless promote the stability of cooperative virulence by purging cheaters from the population. A population bottleneck at transmission can be the result of environmental stress between hosts, dilution of the bacterial population in the environment (followed by growth to reach the infective dose, e.g. in contaminated foods) or due to competition against the resident microbiota before establishing a favorable niche in the gut39. To address this, we performed a proof of principle experiment simulating an extreme population bottleneck by infecting new mice with single evolved clones from mice in Fig. 1. We used clones evolved in different mice, representing both cooperators (i.e., TTSS-1+ clones) and cheaters (i.e., TTSS-1− clones) in both the high cost and low cost variants (3 clones per group; 2-3 new mice infected per clone). For both pVirLow and pVirHigh, TTSS-1+ clones were able to trigger inflammation (LCN2 ≥ 102 ng/g feces) and TTSS-1− clones were not (Fig. 3a). Again, this was reflected in the shedding population, where mice infected with TTSS-1+ clones shed significantly more S.Tm on day 4 p.i. compared to mice infected with TTSS-1− clones (Fig. 3b). Note that the antibiotic pretreatment allows pure cheater populations to reach the same population size as cooperators for three days post-infection. As a control, we measured the proportion of cooperative clones in these mice on day 4 p.i. As expected, mice infected with cheater clones contained only TTSS-1− clones and mice infected with cooperative clones contained mostly TTSS-1+ clones, although cheaters began to emerge in these mice as well (Fig. 3c). Importantly, the proportion of cooperators in mice infected with single TTSS-1+ clones appeared higher than in mice that received a non-isogenic mixture of cooperators and cheaters (compare Fig. 3c to 2d, g). In accordance with theory and experimental works demonstrating that assortment favors cooperative traits3,40, monoclonal infections clearly promote cooperative virulence. To further support this point, we tested the correlation between the proportion of cooperators given to mice in the transmission experiments (Figs. 2 and 3) and inflammation, the shedding population, and the proportion of cooperators at day 4 post infection (Fig. S5). The proportion of cooperators in the input correlated with the resulting inflammation, shedding, and the final proportion of cooperative clones (slopes are significantly non-zero: Fig. S5Ap < 0.0001; Fig. S5Bp = 0.0002; Fig. S5Cp < 0.0001), in line with previous work comparing the proportion of the population able to express the TTSS-1+ phenotype and the resulting inflammation38.
Fig. 3. Genetic drift favors cooperative virulence.
Ampicillin pretreated mice were orally infected with evolved transconjugant clones isolated from day 7 or day 10 from mice in Fig. 1. Three cooperating clones (solid circles; TTSS-1+ clones) and cheating clones (hollow circles; TTSS-1− clones) were randomly chosen for each of pVirLow (blue) or pVirHigh (orange). Each clone was infected into 2–3 mice (~5 × 107 CFU inoculum), leading to a total of 6–7 mice per group. All clones were whole-genome sequenced (mutations and indels are summarized in Tables S1–4): TTSS-1+ pVirLow (Z2296 (3 mice), Z2306 (2 mice), Z2310 (2 mice); n = 7), TTSS-1+ pVirHigh (Z2238 (3 mice), Z2246 (2 mice), Z2253 (2 mice); n = 7), TTSS-1- pVirLow (Z2298, Z2301, Z2305; 2 mice per clone; n = 6), TTSS-1- pVirHigh (Z2239 (3 mice), Z2243 (2 mice), Z2311 (2 mice); n = 7). All data points are shown and medians are indicated by bars. Comparisons are made between TTSS-1+ and TTSS-1- clones (for each of pVirLow and pVirHigh) using a two-tailed Mann–Whitney U test (p > 0.05 (ns), *p < 0.05, **p < 0.01, ***p < 0.001). Source data are provided as a Source Data file. a Inflammation was quantified using a LCN2 ELISA. The dotted lines indicate the detection limit. b The shedding population was enumerated on MacConkey agar. c MacConkey plates containing colonies were analyzed for expression of SipC as a proxy for TTSS-1 expression using a colony western blot; the percentage of colonies that expressed SipC are reported out of the total transconjugant population. The black dotted line indicates the conservative detection limit for the colony blot, which is dependent on the number of colonies on the plate (values can therefore appear below the detection limit).
HGT can maintain cooperation in a virulent population invaded by cheaters
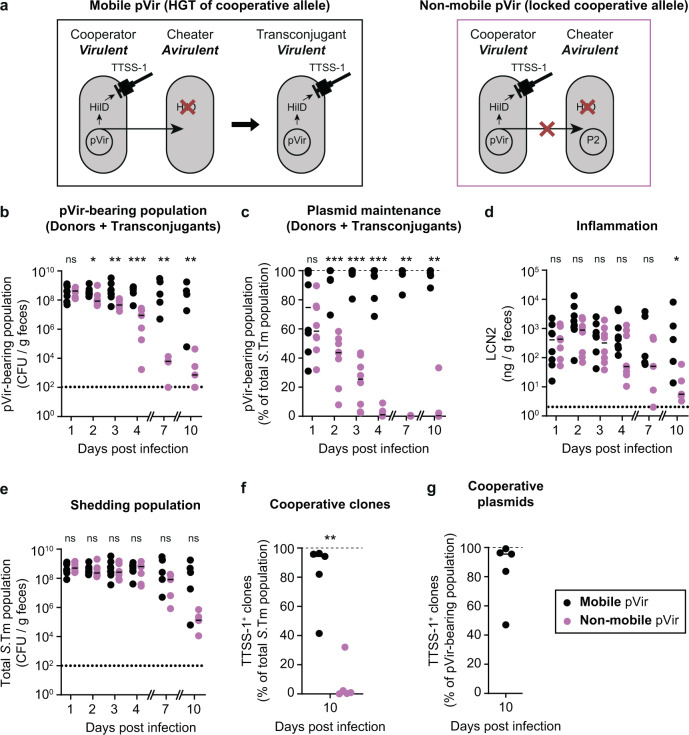
After addressing the emergence of cooperative virulence in an avirulent population and the role of transmission in its maintenance, we assessed the contribution of HGT in maintaining cooperation within-host in a population of cooperators competing against cheaters (as proposed in Smith24). In this experiment, the donor contained a functional invG allele, making it virulent (in comparison to the scenario in Fig. 1 where the donor is avirulent). We performed a competitive infection between a hilD mutant (i.e., cheater) and the donor containing pVirLow (i.e., cooperator) at an equal ratio (low inoculum size; ~102 CFU of each strain, introduced sequentially to avoid plasmid transfer in the inoculum). Importantly, we performed this experiment in two configurations (Fig. 4a): in the first group which we called “mobile pVir”, we used the same recipient cheater strain as in Fig. 1, which can obtain pVir from the cooperator; in the second group called “non-mobile pVir”, we used a cheater strain that carried P2 (P2 does not confer a fitness advantage to competing 14028 S strains14,31) labeled with a kanamycin resistance marker. In this case, since pVir and P2 are incompatible and have mechanisms for entry exclusion41, plasmid transfer cannot be detected (confirmed by selective plating). As expected, when pVir was mobile, the plasmid was maintained in the population for longer compared to the non-mobile scenario, in which the cooperating strain was outcompeted by the cheating strain (Figs. 4b, c and S6). Furthermore, inflammation was maintained for longer in mice with the mobile pVir scenario (Fig. 4d), which was reflected in a trend towards higher shedding populations (Fig. 4e). Importantly, in some mice with the mobile pVir scenario, the inflammation and the shedding population also diminished over time (Fig. 4d, e). Therefore, we measured the proportion of TTSS-1-expressing clones in the population at the end of the experiment. Although more TTSS-1-expressing clones were observed in the mobile pVir scenario compared to when pVir was not mobile (Fig. 4f), clones that did not express TTSS-1 were detected within the pVir-containing population (Fig. 4g).
Fig. 4. HGT can increase the duration of, but does not stabilize, cooperative virulence.
a Experimental system to determine the role of HGT in the stabilization of cooperative virulence. In both the mobile pVir and non-mobile pVir scenarios, the cooperator contains pVirLow and a functional TTSS-1, making it virulent. In the mobile scenario, pVir can be transferred to cheaters. In the non-mobile scenario, transfer of pVir is blocked because of an incompatible plasmid, P2, in the cheater strain. b–g Ampicillin pretreated mice were orally infected with ~102 CFU of cheater (14028 ΔhilD ΔssaV; KanR, AmpR) immediately followed by ~102 CFU of pVirLow donor (14028 ΔhilD ΔssaV pVir; CmR encoded on pVir, AmpR). The donor contained a functional invG allele, making it virulent (ssaV is deleted in both strains). Mice (n = 8 until day 4; n = 5 until day 10 for both groups) were given either a cheater with no plasmid (i.e., same strain used in Fig. 1; mobile pVir; black) or a cheater with P2 (incompatible with pVir; non-mobile pVir; pink). Dotted lines indicate detection limits. Medians are indicated by lines. Two-tailed Mann–Whitney U tests (p > 0.05 (ns), *p < 0.05, **p < 0.01, ***p < 0.001) are used to compare the mobile pVir and non-mobile pVir scenarios on each day. Donor, recipient, and transconjugant populations are presented in Fig. S6. Source data are provided as a Source Data file. b The pVir-bearing population was determined by selective plating on Cm-supplemented MacConkey agar. c the pVir-bearing population is reported as a percentage of the total population. Dashed line indicates 100% plasmid spread. d Inflammation was measured by a Lipocalin-2 ELISA on fecal samples. e Total S.Tm populations determined by the sum of Cm- and Kan-supplemented MacConkey agar. f, g Cm-supplemented MacConkey agar plates containing colonies from feces collected on day 10 post infection were analyzed for SipC expression as a proxy for TTSS-1 expression using a colony western blot and represented as the percentage of colonies that produced SipC are reported out of the total S.Tm population (f) or out of the pVir-containing population for the mobile pVir scenario (g).
Discussion
Using an experimental evolution model in vivo we show that HGT can facilitate restoration, and possibly the emergence of cooperative virulence. However, we observed that cheating re-occurred quickly in recipient bacteria via mutations inactivating the mobile cooperative allele (Tables S1–S4). Because both the mobile cooperative and mutated “cheating” alleles spread equally well in the population, here via conjugation, cooperation is only restored transiently. This has been previously shown in vitro and predicted from several theoretical scenarios25,26,42,43. Moreover, the concept that cooperative alleles are more often encoded on MGE because HGT could maintain cooperation26,44, has been recently re-visited and eventually ruled out by thorough comparative genetic analysis45. While likely rare it is possible that in nature, revertants could occur by other means than conjugation like generalized transduction of wild-type cooperative alleles in the place of mutant alleles. Our experimental work nevertheless strengthens the notion that, if HGT may allow for the emergence of cooperators, it is unlikely that it ensures the long-term stability of cooperative traits. The maintenance of cooperative virulence rather depends on additional factors, including managing the cost of virulence expression via tight regulation16,46, and the transmission dynamics of the pathogen (i.e., timing and population bottleneck size) between hosts.
What about a potential role of tissue invasion and the subsequent formation of intra-cellular Salmonella populations in these dynamics? In this study, we use TTSS-2 mutants to prevent deadly systemic spread of S.Tm in C57BL/6J Nramp knock-out mice47. The TTSS-2 mutation limits the intra-cellular population size of S.Tm32,47. In theory, this might diminish the frequency at which intra-cellular bacteria travel back from the tissues into the intestinal lumen48. However, we have previously demonstrated that, in the streptomycin pretreated mouse model, the intracellular reservoir of virulent clones is only relevant for stabilizing cooperation when the intestinal lumen is empty, for instance after treating mice with an antibiotic. This allows luminal growth of re-seeding bacteria from the tissues23. In the absence of antibiotic-mediated depletion of the gut-luminal S.Tm population, cheaters outcompete cooperators as observed in this study. Moreover, once cheaters are fixed in the luminal population, reseeding events of cells carrying the cooperative allele on MGE31 or on the chromosome23 will be too rare to restore virulence. Based on these considerations, it is reasonable to think that the choice of a TTSS-2 defective background strain does not fundamentally affect the general conclusion about the insufficiency of HGT to restore virulence in the long run. Higher fitness cost in our experimental system led to greater instability of cooperative virulence (Fig. 1), in line with the fact that a complex fine-tuned regulation is essential to ensure virulence stability in S.Tm11 and other “cooperative” pathogens49–51. Accordingly, hilD is integrated into the chromosome of S.Tm within SPI-1, which, compared to carriage on multi-copy plasmids, could favor the evolution of a regulation deeply entangled with the general physiology of the pathogen16,46,52.
Long-term intestinal colonization was detrimental for cooperative virulence as fast growing cheaters accumulate over generations within-host (Figs. 1–4)11,23. Theoretical work predicts that transmission likely plays a role in the epidemiological success of virulence4,24,53. In our case, early transmission ensures that enough cooperators can trigger the disease in the next host (Fig. 2). Moreover, beside timing, population bottlenecks at transmission influence this process10. S.Tm is a case where the benefit of cooperative virulence directly fuels transmission37. Cheaters alone cannot free-ride off of the inflammation normally triggered by cooperators that increases the carrying capacity in the gut and transmission of the pathogen. Hence, narrow population bottlenecks at transmission favor cooperation, as described in other experimental systems54,55, and could apply for other enteric pathogens such as Vibrio cholerae (encoding the cholera toxin on a phage)56, Shigella spp. (containing phage- and plasmid-encoded secreted virulence factors)57, or Yersinia spp. (encoding the Yop virulon on a plasmid)58, which all use secreted virulence factors to survive in the host and/or directly increase shedding. In the case of S.Tm, population fragmentation occurs when a subset of the population is released from a host through fecal pellets, and bottlenecks are likely to occur in harsh external environment or during colonization of a recipient host in which colonization resistance is mediated by the protective gut microbiota. Environmental factors such as diet perturbations28,59 or exposure to antibiotics35, that affect the composition of the microbiota and reduce colonization resistance, can widen transmission bottlenecks and should have profound implications on the evolution of virulence in S.Tm. Further work is needed to fathom this key aspect of the host/pathogen/microbiota interaction.
The current antibiotic crisis60 highlight the critical importance of understanding the role of bacterial sociality in the evolution of virulence, as this aids the discovery of antibiotic-free treatments to manage bacterial infections. It has inspired “anti-virulence compounds” that target extracellular virulence factors or their expression as a therapeutic avenue for minimizing resistance (reviewed in61. Alternatively, exploiting cheating behavior to destabilize cooperation has been suggested as a possible therapeutic strategy (coined “Hamiltonian medicine”)62. Previous work on S.Tm23 demonstrated that administering hilD mutants has the potential to prevent inflammation-mediated Salmonella blooms and transmission. The present study suggests that, in the absence of direct positive selective pressure within-host, HGT of functional hilD copies is rather unlikely to restore virulence in hilD mutants. Indeed, preventing inflammation and S.Tm bloom also prevents HGT in the gut4,14. Besides, providing that such cooperative alleles would naturally exist on MGEs, the cost of suboptimal virulence expression levels makes them prone to inactivation, without impairing their ability to spread in the recipient population once inactivated. Furthermore, strategies that slow down HGT, such as vaccination29–31, could be used in combination with avirulent competitors to further reduce the horizontal spread of cooperative alleles, thus ensuring evolutionary robustness of cheater-based biocontrol approaches.
Methods
Strains, plasmids, and primers used in this study
All the strains and plasmids used in this work are summarized in Tables 1 and 2. Bacteria were grown in lysogeny broth (LB) media containing the appropriate antibiotics (50 µg/ml streptomycin (AppliChem); 15 µg/ml chloramphenicol (AppliChem); 50 µg/ml kanamycin (AppliChem); 100 µg/ml ampicillin (AppliChem)) at 37 °C (or 30 °C if containing pKD46 or pCP20). Gene deletion mutants were performed using the λ red system63. Desired genetic constructs were transferred into the appropriate background strain using P22 HT105/1 int-201 phage transduction64. Antibiotic resistance cassettes were removed using the heat-inducible FLP recombinase encoded on pCP20, if desired63. Expression vectors (e.g. pM975 and pM972) were transformed into the desired strain using electroporation.
Table 2.
Plasmids used in this study.
| Plasmid name | Relevant genotype | Resistance | Ref. |
|---|---|---|---|
| pM975 | bla; used to confer ampicillin resistance | Amp | 32 |
| pCP20 | FLP recombinase | Amp, Cm | 63 |
| pKD46 | Arabinose-incudible λ red system | Amp | 63 |
| pM972 | PsicA-gfp; reporter for TTSS-1 | Amp | 19 |
| pKD3 | cat | Cm | 63 |
| pKD3-hilD-cat Low | hilD with 648 bp of upstream regulatory region, cat | Cm | This work |
| pKD3-hilD-cat High | hilD with 279 bp of upstream regulatory region, cat | Cm | This work |
| P2 | Wild-type | None | 14 |
| P2cat | cat | Cm | 14 |
| pVirLow | hilD with 648 bp of upstream regulatory region, cat | Cm | This work |
| pVirHigh | hilD with 279 bp of upstream regulatory region, cat | Cm | This work |
To create pVir donor strains, hilD was amplified with PCR using high-fidelity Phusion polymerase (ThermoFisher Scientific) from the chromosome of SL1344 with either 648 bp (low cost) or 279 bp (high cost) of regulatory region (Fig. S1) and cloned into pKD3 upstream of the chloramphenicol resistance cassette using Gibson Assembly (NEB). Primers to amplify hilD contained ~40 bp homology to the sites flanking a NdeI site in pKD3. pKD3 was digested with NdeI, purified, and mixed with the PCR amplicon in Gibson Assembly Master Mix (NEB; protocol as described by the manufacturer). The products were transformed into E. coli CC118 λpir, and colonies were verified to contain the desired plasmid through PCR and Sanger sequencing. The resulting hilD-cat construct was then amplified from cloned plasmid with Phusion PCR using primers with homology to the target site in P2 (upstream of the colicin Ib locus; cib) and introduced into SB300 ΔhilD using λ red63. Positive clones were determined by PCR, leading to pVirLow and pVirHigh. Lastly, the pVir plasmids were conjugated in vitro into the desired strain by mixing the 105 CFU from an overnight culture of the donor strain with the desired recipient, allowing conjugation overnight at 37 °C on a rotating wheel, and plating the cells on MacConkey agar to select for transconjugants. For in vivo experiments, pVir plasmids were conjugated into 14028 S ΔhilD ΔinvG ΔssaV pM975 or 14028 S ΔhilD ΔssaV pM975. For TTSS-1 expression analysis, pVir plasmids were conjugated into 14028 S ΔhilD ssaV::aphT pM972. All primers used for strain or plasmid construction and verification are listed in Table 3.
Table 3.
Primers used in this study.
| Primer name | Sequence (5′ to 3′) | Purpose | Ref. |
|---|---|---|---|
| HilD-31-F | GGAACACTTAACGGCTGACATGGGAATTAGCCATGGTCCATACAGGATAAGCAATTCACCG | Gibson Assembly of hilD into pKD3 (pVirLow) | This work |
| HilD-1-F | GGAACACTTAACGGCTGACATGGGAATTAGCCATGGTCCATAGCAGATTACCGCACAGGA | Gibson Assembly of hilD into pKD3 (pVirHigh) | This work |
| HilD-2-R |
AGAATAGGAACTTCGGAATAGGAACT AAGGAGGATATTCATAGTGTTAATGC GCAGTCTGA |
Gibson Assembly of hilD into pKD3 (both pVirLow and pVirHigh) | This work |
| HilD-3Po-F | AGGAACTTCGGAATAGGAAC | Verification of hilD in pKD3 | This work |
| HilD-4Po-R | AACACTTAACGGCTGACATG | Verification of hilD in pKD3 | This work |
| HilD-32-F | GCATGATAATAATAATCAATAACAATAAGCTGTGTCACGTTTACATCATCAGGATAAGCAATTCACCG | λ-red for hilD-cat in P2 downstream of cib (pVirLow) | This work |
| HilD-29-F | GCATGATAATAATAATCAATAACAATAAGCTGTGTCACGTTTACATCATGCAGATTACCGCACAGGA | λ-red for hilD-cat in P2 downstream of cib (pVirHigh) | This work |
| HilD-30-R | AAGGGTAATGGCGGAAGCCGGATACCCAGCCGCCAGAGAATGTGTAGGCTGGAGCTGCTTC | λ-red for hilD-cat in P2 downstream of cib (both pVirLow and pVirHigh) | This work |
| insert_ p2_up | GTA CCG GTG CGT GAT AAC | Verification of hilD-cat insert in P2 to create pVir (both pVirLow and pVirHigh) | 31 |
| insert_ p2_dw | CAA CAG CGT GAC CTG CC | Verification of hilD-cat insert in P2 to create pVir (both pVirLow and pVirHigh) | 31 |
| ver_hilD_up2 | TCTCGATAGCAGCAGATTAC | Verification of ΔhilD in the chromosome | 11 |
| ver_hilD_dw2 | CAGTATAAGCTGTCTTCCG | Verification of ΔhilD in the chromosome | 11 |
| ssaV-137F | GCAGCGTTCCAGGGTATTCC | Verification of ΔssaV in the chromosome | This work |
| ssaV + 155R | CAGCAAGTTCTTCTCCAGGC | Verification of ΔssaV in the chromosome | This work |
| invG-134F | GAAGGCCACGAGAACATCAC | Verification of ΔinvG in the chromosome | This work |
| invG + 112R | GCGGCCTGTTGTATTTCCGC | Verification of ΔinvG in the chromosome | This work |
In vitro growth and TTSS-1 expression
Subcultures were grown in LB with appropriate antibiotics for 6 h and subsequently diluted 200 times in 200 µl of media distributed in 96-well black side microplates (Costar). The lid-closed microplates were incubated at 37 °C with fast and continuous shaking in a microplate reader (Synergy H4, BioTek Instruments). Optical density at 600 nm and GFP fluorescence (491 nm excitation; 512 nm emission) were measured every 10 min for 14 h. OD and fluorescence values were corrected for the baseline value measured for sterile broth.
Infection experiments
All mouse experiment protocols are derived from the streptomycin pretreated mouse model described in Barthel et al.35. We used ampicillin rather than streptomycin since S.Tm 14028S is not naturally resistant to streptomycin. Ampicillin resistance is conferred by pM975 contained in all strains used in vivo. All experiments were performed in 8–12-week-old specified opportunistic pathogen-free (SOPF) C57BL/6J mice, which were given 20 mg of ampicillin by oral gavage to allow robust colonization of S.Tm. This ampicillin pretreatment model has been used previously to measure HGT in the gut29–31. All infection experiments were approved by the responsible authorities (Tierversuchskommission, Kantonales Veterinäramt Zürich, licenses 193/2016 and 158/2019). Sample size was not predetermined and mice were randomly assigned to treatment group.
Single infections of donors or recipients
Overnight cultures grown at 37 °C in LB with the appropriate antibiotics were diluted 1:20 and subcultured for 4 h in LB without antibiotics. Cells were centrifuged and resuspended in PBS before being diluted. Ampicillin pretreated mice were orally gavaged with ~5 × 107 CFU. Fecal samples were collected daily, homogenized in PBS with a steel ball at 25 Hz for 1 min, and bacterial populations were enumerated on selective MacConkey agar. Lipocalin-2 ELISA (R&D Systems kit; protocol according to manufacturer) was performed on feces to determine the inflammatory state of the gut. At day 4 post infection, mice were euthanized.
Plasmid transfer experiments
Donor and recipient strains (14028S derivatives; ssaV mutants) were grown overnight in LB with the appropriate antibiotics at 37 °C and subsequently diluted 1:20 and subcultured for 4 h in LB without antibiotics, washed in PBS, and diluted. Ampicillin pretreated mice were orally gavaged sequentially with ~102 CFU of donors followed by ~104 CFU of recipients. Feces were collected when needed, homogenized in PBS, diluted, and bacterial populations were enumerated on MacConkey agar containing the appropriate antibiotics (donors = Cm; recipients = Kan; transconjugants, i.e., a recipient (kanamycin resistant, kan) carrying the plasmid and with it an extra-resistance to chloramphenicol (Cm) (selection Cm+Kan); total population = populations of donors + recipients). Plating allows analyzing up to 200 clones. Therefore, when 99% of recipients are transconjugants, we count 2 KanR/CmS clones for 198 KanR/CmR clones, i.e., more transconjugants than recipients without plasmid. The detection limit was a 2-log difference between transconjugants and the remaining fraction of recipient cells. Replica plating was used if the CFUs on the Cm+Kan plates approached those on the Cm or Kan plates to determine an exact ratio of plasmid transfer, and the donor population size. At day 10 post infection, mice were euthanized. Lipocalin-2 ELISA was performed on feces to determine the inflammatory state of the gut. When needed, transconjugants on the Cm+Kan plates were kept at 4 °C until analysis by colony blot.
Competitions involving mobile versus non-mobile pVir
Cooperator (donor) and cheater (recipient) strains (14028S derivatives; ssaV mutants) were grown overnight in LB with the appropriate antibiotics at 37 °C and subsequently diluted 1:20 for a 4 h subculture in LB without antibiotics. Cells were resuspended in PBS and diluted. Ampicillin pretreated mice were sequentially orally gavaged with ~102 CFU of cooperators (pVirLow) immediately followed by ~102 CFU of cheaters. Feces were collected when needed, homogenized, diluted, and bacterial populations were enumerated on MacConkey agar containing the appropriate antibiotics as for the plasmid transfer experiments. At day 4 or 10 post infection, mice were euthanized. Lipocalin-2 ELISA was performed on feces to determine the inflammatory state of the gut. The colonies on the Cm plates from day 10 fecal plates were kept at 4 °C until analysis by colony blot.
Transmission experiments
Feces from mice given donor and recipient strains were collected on day 2 and day 10 p.i., resuspended in PBS, briefly centrifuged, and 100 µl of the suspension was given to ampicillin pretreated mice. These experiments occurred in parallel to the plasmid transfer experiments to ensure fresh fecal populations were transmitted into new mice. Bacterial populations and the state of inflammation were measured as for the plasmid transfer experiments. Mice were euthanized at day 4 post transmission.
Evolved transconjugant infections
Single clones from plasmid transfer experiments were isolated on day 7 or day 10, and stored in 20% LB + glycerol at −80 °C. Isolates were grown in LB containing the appropriate antibiotics (Cm, Kan, Amp) overnight at 37 °C and subsequently diluted 1:20 and subcultured for 4 hours in LB without antibiotics. Of note, loss of pM975 was observed for some clones (based on loss of ampicillin resistance), and could therefore not be used for infection. Subcultured cells were centrifuged, resuspended in PBS, and ~5 × 107 CFU were given to ampicillin pretreated mice by oral gavage. The shedding population was enumerated on MacConkey supplemented with chloramphenicol after suspension in PBS followed by dilution. On day 4 post infection, mice were euthanized and fecal samples were additionally enumerated on MacConkey supplemented with kanamycin, to ensure that plasmid loss did not contribute to the detected shedding population. The kanamycin-resistant colonies (KanR is encoded on the chromosome) were replica plated onto MacConkey supplemented with chloramphenicol to confirm that no pVir plasmid loss occurred. The MacConkey chloramphenicol plates were stored at 4 °C until analysis by colony blot. LCN2 ELISA was used to determine the inflammatory state of the mice over time.
Colony blots
To assess TTSS-1 expression at the clonal level (to determine the proportion of cooperators), a colony Western blot was performed. SipC was used as a proxy for TTSS-1 expression, since SipC is regulated by HilD. We have previously established this protocol to assess heterogeneously expressed phenotypes such as TTSS-1 in S.Tm11,27, since single-cell approaches would not differentiate cheaters from the phenotypically OFF subpopulation27. For a detailed protocol and an overview of applications, see27. Briefly, colonies on MacConkey agar were replica transferred to nitrocellulose membranes and placed face-up on LB agar without antibiotics and allowed to grow overnight. The original MacConkey plates are also allowed to re-grow and then stored at 4 °C. Colonies were lysed and cellular material was hybridized to the membrane by passing the membranes over a series of Whatman filter papers soaked with buffers: 10 minutes on 10% SDS, 10 minutes on denaturation solution (0.5 M NaOH, 1.5 M NaCl), twice for 5 minutes on neutralization solution (1.5 M NaCl, 0.5 M Tris-HCl, pH 7.4), and 15 minutes on 2× SSC (3 M NaCl, 0.3 M sodium citrate, pH 7). Membranes were washed twice with TBS (10 mM Tris-HCl, 150 mM NaCl, pH 7.4) and excess cellular debris was gently removed by scraping the surface with a folded Whatman paper. Membranes were blocked with TBS containing 3% BSA for 1 h at room temperature and then incubated with 5 ml of TBS with 3% BSA containing a 1:4000 dilution of anti-SipC rabbit antibody provided by Virotech Diagnostics GmbH (reference number: VT110712) overnight in a moist chamber at 4 °C on a rocking platform. Washing once with TBS-T (20 mM Tris-HCl, 500 mM NaCl, 0.05% Tween 20, 0.2% Triton X-100, pH 7.5) and twice with TBS removed non-specific binding. Secondary antibodies (1:2500 dilution of goat anti-rabbit IgG conjugated to HRP; Sigma; catalog number A0545-1ML) were then added to membrane in TBS with 3% BSA and incubated at room temperature on a rocking platform for 2-4 hours. Three more washing steps with TBS were performed before resolving the staining with 5 ml of substrate per membrane: a 30 mg tablet of 4-chloro-1-naphthol (Sigma) dissolved in 10 ml of methanol, mixed with H2O2 (0.06% w/v) in 50 ml of TBS. The reaction is stopped with water after the desired intensity is observed.
Clones of interest can be identified by changes in SipC abundance. Desired isolates were matched to the original MacConkey plate and inoculated in LB containing chloramphenicol and kanamycin. Isolates were then stored in 20% LB + glycerol at −80 °C until whole-genome bacterial sequencing was performed, or evolved clones were used for infection.
Whole-genome bacterial sequencing
Strains stored in 20% LB + glycerol at −80 °C were inoculated in LB with the appropriate antibiotics. Genomic DNA was extracted from 1 ml of overnight culture using a QIAamp DNA Mini Kit (Qiagen). Illumina MiSeq sequencing operated by the Functional Genomics Centre Zurich and Novogene (Cambridge) was performed to generate 150 bp paired end reads with at least 50× coverage across the genome. Bioinformatic analysis was performed using CLC Genomics Workbench 11.0. Reads were mapped to the 14028S chromosome reference (NCBI accession NC_016856.1 [https://www.ncbi.nlm.nih.gov/nuccore/NC_016856.1/]) and the pVir plasmids (the SL1344 P2 plasmid (NCBI accession NC_017718.1 [https://www.ncbi.nlm.nih.gov/nuccore/NC_017718.1/]) was modified by inserting the cloned hilD-cat regions to create pVirLow and pVirHigh reference sequences). Basic variant detection was performed to detect variants that occurred in a minimum of 70% of reads. Variants were excluded if they occurred in non-specific regions determined by read mapping in CLC (e.g. where reads could map equally well to another location in the genome). Small insertions or deletions (Indels) were also detected using software in CLC Genomics Workbench 12.0.2. This is summarized in Tables S1–4.
Statistical analysis
Data were collected using Microsoft Excel 2016 (16.0.5266.1000). Statistical tests on experimental data were performed using GraphPad Prism 8.0.2 for Windows.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We would like to thank members of the Hardt and Diard labs for valuable discussions, as well as the staff at RCHCI and EPIC animal facilities. We thank Stuart West, Kevin Foster and Ashleigh Griffin for helpful feedback and comments on this manuscript. We would also like to acknowledge the staff at Functional Genomics Centre Zurich and Novogene for whole-genome bacterial sequencing. We acknowledge grant funding from the Swiss National Science Foundation (NRP 72 407240_167121, 310030B_173338, and 310030_192567), the Gebert Rüf Foundation (GRS-060/18) and the Monique Dornonville de la Cour Foundation to WDH. MD is funded by an SNF professorship grant (PP00PP_176954) and a BRCCH multi-investigator grant, J.S.H. is funded by NRP72 grant 407240-167121 from the Swiss National Science Foundation, and EB received a Boehringer Ingelheim Fonds PhD fellowship.
Source data
Author contributions
E.B., W.D.H., and M.D. conceived the project and designed the experiments. E.B., E.G., Y.S., and A.R. carried out the experiments and analyzed data. E.B., W.D.H., and M.D. wrote the manuscript. E.G. and J.S.H. provided valuable input on experimental design, theoretical background, and the manuscript. All authors read, commented on, and approved this manuscript.
Peer review
Peer review information
Nature Communications thanks Wael Elhenawy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All source data to generate plots in the manuscript and p-values for statistical comparisons are available in the source data Excel sheet. Citation of the current paper should accompany any further publication based on these data. Figures 1–4 and S1–S6 are associated with raw data. All Illumina sequencing data are publicly available from the NCBI repository (BioProject accession number PRJNA817059). Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Wolf-Dietrich Hardt, Médéric Diard.
Contributor Information
Wolf-Dietrich Hardt, Email: wolf-dietrich.hardt@micro.biol.ethz.ch.
Médéric Diard, Email: mederic.diard@unibas.ch.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-29597-7.
References
- 1.Crespi BJ. The evolution of social behavior in microorganisms. Trends Ecol. Evol. 2001;16:178–183. doi: 10.1016/s0169-5347(01)02115-2. [DOI] [PubMed] [Google Scholar]
- 2.West SA, Griffin AS, Gardner A, Diggle SP. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 2006;4:597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- 3.Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430:1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 4.Diard, M. & Hardt, W. D. Evolution of bacterial virulence. FEMS Microbiol. Rev. 41, 679–697 (2017). [DOI] [PubMed]
- 5.Mavridou DAI, Gonzalez D, Kim W, West SA, Foster KR. Bacteria use collective behavior to generate diverse combat strategies. Curr. Biol. 2018;28:345–355.e344. doi: 10.1016/j.cub.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 6.Chao L, Levin BR. Structured habitats and the evolution of anticompetitor toxins in bacteria. Proc. Natl Acad. Sci. USA. 1981;78:6324–6328. doi: 10.1073/pnas.78.10.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadell CD, Drescher K, Foster KR. Spatial structure, cooperation and competition in biofilms. Nat. Rev. Microbiol. 2016;14:589–600. doi: 10.1038/nrmicro.2016.84. [DOI] [PubMed] [Google Scholar]
- 8.Stecher B, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black AJ, Bourrat P, Rainey PB. Ecological scaffolding and the evolution of individuality. Nat. Ecol. Evol. 2020;4:426–436. doi: 10.1038/s41559-019-1086-9. [DOI] [PubMed] [Google Scholar]
- 10.Cremer J, Melbinger A, Frey E. Growth dynamics and the evolution of cooperation in microbial populations. Sci. Rep. 2012;2:281. doi: 10.1038/srep00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diard M, et al. Stabilization of cooperative virulence by the expression of an avirulent phenotype. Nature. 2013;494:353–356. doi: 10.1038/nature11913. [DOI] [PubMed] [Google Scholar]
- 12.Foster KR, Shaulsky G, Strassmann JE, Queller DC, Thompson CR. Pleiotropy as a mechanism to stabilize cooperation. Nature. 2004;431:693–696. doi: 10.1038/nature02894. [DOI] [PubMed] [Google Scholar]
- 13.West SA, Cooper GA, Ghoul MB, Griffin AS. Ten recent insights for our understanding of cooperation. Nat. Ecol. Evol. 2021;5:419–430. doi: 10.1038/s41559-020-01384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stecher B, et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc. Natl Acad. Sci. USA. 2012;109:1269–1274. doi: 10.1073/pnas.1113246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroger C, et al. An infection-relevant transcriptomic compendium for Salmonella enterica Serovar Typhimurium. Cell Host Microbe. 2013;14:683–695. doi: 10.1016/j.chom.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Smith, C., Stringer, A. M., Mao, C., Palumbo, M. J. & Wade, J. T. Mapping the regulatory network for salmonella enterica serovar typhimurium invasion. MBio10.1128/mBio.01024-16 (2016). [DOI] [PMC free article] [PubMed]
- 17.Sobota, M. et al. The expression of virulence increases outer-membrane permeability and sensitivity to envelope stress in Salmonella Typhimurium. bioRxiv10.1101/2021.06.08.447568 (2021). [DOI] [PMC free article] [PubMed]
- 18.Colgan AM, et al. The impact of 18 ancestral and horizontally-acquired regulatory proteins upon the transcriptome and sRNA landscape of Salmonella enterica serovar Typhimurium. PLoS Genet. 2016;12:e1006258. doi: 10.1371/journal.pgen.1006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sturm A, et al. The cost of virulence: retarded growth of Salmonella Typhimurium cells expressing type III secretion system 1. PLoS Pathog. 2011;7:e1002143. doi: 10.1371/journal.ppat.1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzel A, et al. Persistent infections by nontyphoidal Salmonella in humans: epidemiology and genetics. Clin. Infect. Dis. 2016;62:879–886. doi: 10.1093/cid/civ1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tambassi M, et al. Mutation of hilD in a Salmonella Derby lineage linked to swine adaptation and reduced risk to human health. Sci. Rep. 2020;10:21539. doi: 10.1038/s41598-020-78443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherry JL. Selection-driven gene inactivation in Salmonella. Genome Biol. Evol. 2020;12:18–34. doi: 10.1093/gbe/evaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diard M, et al. Antibiotic treatment selects for cooperative virulence of Salmonella Typhimurium. Curr. Biol. 2014;24:2000–2005. doi: 10.1016/j.cub.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 24.Smith J. The social evolution of bacterial pathogenesis. Proc. Biol. Sci. 2001;268:61–69. doi: 10.1098/rspb.2000.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimitriu T, et al. Genetic information transfer promotes cooperation in bacteria. Proc. Natl Acad. Sci. USA. 2014;111:11103–11108. doi: 10.1073/pnas.1406840111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nogueira T, et al. Horizontal gene transfer of the secretome drives the evolution of bacterial cooperation and virulence. Curr. Biol. 2009;19:1683–1691. doi: 10.1016/j.cub.2009.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakkeren E, Dolowschiak T, Diard M. Detection of mutations affecting heterogeneously expressed phenotypes by colony immunoblot and dedicated semi-automated image analysis pipeline. Front. Microbiol. 2017;8:2044. doi: 10.3389/fmicb.2017.02044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wotzka SY, et al. Escherichia coli limits Salmonella Typhimurium infections after diet shifts and fat-mediated microbiota perturbation in mice. Nat. Microbiol. 2019;4:2164–2174. doi: 10.1038/s41564-019-0568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moor K, et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature. 2017;544:498–502. doi: 10.1038/nature22058. [DOI] [PubMed] [Google Scholar]
- 30.Diard M, et al. Inflammation boosts bacteriophage transfer between Salmonella spp. Science. 2017;355:1211–1215. doi: 10.1126/science.aaf8451. [DOI] [PubMed] [Google Scholar]
- 31.Bakkeren E, et al. Salmonella persisters promote the spread of antibiotic resistance plasmids in the gut. Nature. 2019;573:276–280. doi: 10.1038/s41586-019-1521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hapfelmeier S, et al. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar Typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J. Immunol. 2005;174:1675–1685. doi: 10.4049/jimmunol.174.3.1675. [DOI] [PubMed] [Google Scholar]
- 33.Coombes BK, et al. Analysis of the contribution of Salmonella pathogenicity islands 1 and 2 to enteric disease progression using a novel bovine ileal loop model and a murine model of infectious enterocolitis. Infect. Immun. 2005;73:7161–7169. doi: 10.1128/IAI.73.11.7161-7169.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton WD. The genetical evolution of social behaviour. I + II. J. Theor. Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 35.Barthel M, et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endt K, et al. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 2010;6:e1001097. doi: 10.1371/journal.ppat.1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawley TD, et al. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect. Immun. 2008;76:403–416. doi: 10.1128/IAI.01189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ackermann M, et al. Self-destructive cooperation mediated by phenotypic noise. Nature. 2008;454:987–990. doi: 10.1038/nature07067. [DOI] [PubMed] [Google Scholar]
- 39.Wotzka SY, Nguyen BD, Hardt WD. Salmonella Typhimurium diarrhea reveals basic principles of enteropathogen infection and disease-promoted DNA exchange. Cell Host Microbe. 2017;21:443–454. doi: 10.1016/j.chom.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Fletcher JA, Doebeli M. A simple and general explanation for the evolution of altruism. Proc. Biol. Sci. 2009;276:13–19. doi: 10.1098/rspb.2008.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcillan-Barcia MP, de la Cruz F. Why is entry exclusion an essential feature of conjugative plasmids? Plasmid. 2008;60:1–18. doi: 10.1016/j.plasmid.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Mc Ginty SÉ, Lehmann L, Brown SP, Rankin DJ. The interplay between relatedness and horizontal gene transfer drives the evolution of plasmid-carried public goods. Proc. R. Soc. B Biol. Sci. 2013;280:20130400. doi: 10.1098/rspb.2013.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mc Ginty SE, Rankin DJ, Brown SP. Horizontal gene transfer and the evolution of bacterial cooperation. Evolution. 2011;65:21–32. doi: 10.1111/j.1558-5646.2010.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nogueira T, Touchon M, Rocha EP. Rapid evolution of the sequences and gene repertoires of secreted proteins in bacteria. PLoS ONE. 2012;7:e49403. doi: 10.1371/journal.pone.0049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dewar AE, et al. Plasmids do not consistently stabilize cooperation across bacteria but may promote broad pathogen host-range. Nat. Ecol. Evol. 2021;5:1624–1636. doi: 10.1038/s41559-021-01573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erhardt M, Dersch P. Regulatory principles governing Salmonella and Yersinia virulence. Front. Microbiol. 2015;6:949. doi: 10.3389/fmicb.2015.00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hensel M, et al. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 48.Bakkeren, E. et al. Pathogen invasion-dependent tissue reservoirs and plasmid-encoded antibiotic degradation boost plasmid spread in the gut. Elife10.7554/eLife.69744 (2021). [DOI] [PMC free article] [PubMed]
- 49.Ronin, I., Katsowich, N., Rosenshine, I. & Balaban, N. Q. A long-term epigenetic memory switch controls bacterial virulence bimodality. Elife10.7554/eLife.19599 (2017). [DOI] [PMC free article] [PubMed]
- 50.Lin CK, Lee DSW, McKeithen-Mead S, Emonet T, Kazmierczak B. A primed subpopulation of bacteria enables rapid expression of the type 3 secretion system in Pseudomonas aeruginosa. mBio. 2021;12:e0083121. doi: 10.1128/mBio.00831-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czechowska K, McKeithen-Mead S, Al Moussawi K, Kazmierczak BI. Cheating by type 3 secretion system-negative Pseudomonas aeruginosa during pulmonary infection. Proc. Natl Acad. Sci. USA. 2014;111:7801–7806. doi: 10.1073/pnas.1400782111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Romero MA, Olivenza DR, Gutierrez G, Casadesus J. Contribution of DNA adenine methylation to gene expression heterogeneity in Salmonella enterica. Nucleic Acids Res. 2020;48:11857–11867. doi: 10.1093/nar/gkaa730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonhoeffer S, Nowak MA. Intra-host versus inter-host selection: viral strategies of immune function impairment. Proc. Natl Acad. Sci. USA. 1994;91:8062–8066. doi: 10.1073/pnas.91.17.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrison F, Browning LE, Vos M, Buckling A. Cooperation and virulence in acute Pseudomonas aeruginosa infections. BMC Biol. 2006;4:21. doi: 10.1186/1741-7007-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Leeuwen E, O’Neill S, Matthews A, Raymond B. Making pathogens sociable: the emergence of high relatedness through limited host invasibility. ISME J. 2015;9:2315–2323. doi: 10.1038/ismej.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson EJ, Harris JB, Morris JG, Jr., Calderwood SB, Camilli A. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat. Rev. Microbiol. 2009;7:693–702. doi: 10.1038/nrmicro2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin Q, et al. Genome sequence of Shigella flexneri 2a: insights into pathogenicity through comparison with genomes of Escherichia coli K12 and O157. Nucleic Acids Res. 2002;30:4432–4441. doi: 10.1093/nar/gkf566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cornelis GR, et al. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kreuzer, M. & Hardt, W.-D. How food affects colonization resistance against enteropathogenic bacteria. Annu. Rev. Microbiol.74, 787-813 (2020). [DOI] [PubMed]
- 60.WHO. Antimicrobial Resistance: Global Report On Surveillance (World Health Organization (WHO), 2014).
- 61.Allen RC, Popat R, Diggle SP, Brown SP. Targeting virulence: can we make evolution-proof drugs? Nat. Rev. Microbiol. 2014;12:300–308. doi: 10.1038/nrmicro3232. [DOI] [PubMed] [Google Scholar]
- 62.Foster KR. Biomedicine. Hamiltonian medicine: why the social lives of pathogens matter. Science. 2005;308:1269–1270. doi: 10.1126/science.1108158. [DOI] [PubMed] [Google Scholar]
- 63.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sternberg NL, Maurer R. Bacteriophage-mediated generalized transduction in Escherichia coli and Salmonella Typhimurium. Methods Enzymol. 1991;204:18–43. doi: 10.1016/0076-6879(91)04004-8. [DOI] [PubMed] [Google Scholar]
- 65.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella Typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 66.Jarvik T, Smillie C, Groisman EA, Ochman H. Short-term signatures of evolutionary change in the Salmonella enterica serovar Typhimurium 14028 genome. J. Bacteriol. 2010;192:560–567. doi: 10.1128/JB.01233-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herrero M, de Lorenzo V, Timmis KN. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bakkeren E, Diard M, Hardt WD. Evolutionary causes and consequences of bacterial antibiotic persistence. Nat. Rev. Microbiol. 2020;18:479–490. doi: 10.1038/s41579-020-0378-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All source data to generate plots in the manuscript and p-values for statistical comparisons are available in the source data Excel sheet. Citation of the current paper should accompany any further publication based on these data. Figures 1–4 and S1–S6 are associated with raw data. All Illumina sequencing data are publicly available from the NCBI repository (BioProject accession number PRJNA817059). Source data are provided with this paper.